Abstract
Pathogenic human coronaviruses such as the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) cause acute respiratory illness. Epidemiological data from the 2002-2003 SARS epidemic and recent MERS indicate that there may be sex-dependent differences in disease outcomes. To investigate these differences, we infected male and female mice of different age groups with SARS-CoV and analyzed their susceptibility to the infection. Our results showed that male mice were more susceptible to SARS-CoV infection compared to age matched females. The degree of sex-bias to SARS-CoV infection increased with advancing age such that middle-aged mice showed much more pronounced differences compared to young mice. Enhanced susceptibility of male mice to SARS-CoV was associated with elevated virus titers, enhanced vascular leakage and alveolar edema. These changes were accompanied by increased accumulation of inflammatory monocyte macrophages (IMMs) and neutrophils in the lungs of male mice and depletion of IMMs partially protected these mice from lethal SARS. Moreover, the sex-specific differences were independent of T and B cell responses. Furthermore, ovariectomy or treating female mice with an estrogen receptor antagonist increased mortality indicating a protective effect for estrogen receptor signaling in mice infected with SARS-CoV. Together, these data suggest that sex differences in susceptibility to SARS-CoV in mice parallel those observed in patients and also identify estrogen receptor signaling as critical for protection in females.
Keywords: SARS-CoV, sex differences, respiratory diseases, rodent model, estrogen signaling
Introduction
Pathogenic coronaviruses such as SARS-CoV and MERS-CoV and newly identified SARS- and MERS-like coronaviruses pose a significant threat to public health (1-7). SARS-CoV and MERS-CoV infect airway and alveolar epithelial cells and cause acute respiratory illnesses (8, 9). High initial virus loads and increased number of IMMs and neutrophils in the lungs associated with elevated pro-inflammatory cytokine/chemokine levels caused lung damage in SARS patients (8, 10-12). The deleterious clinical manifestations of SARS likely stem from exuberant innate immune responses and virus-induced direct cytopathic effects (6, 13-15). Recent studies from our laboratory showed that robust virus replication accompanied by delayed type I interferon (IFN-I) signaling orchestrated inflammatory responses and lung immunopathology with diminished survival in susceptible BALB/c mice (16).
Studies from the 2002-2003 SARS epidemic showed that individuals below 25 years of age experienced mild to moderate illness. In contrast, elderly individuals aged 60 and above suffered worse outcomes with > 50% mortality rate (8, 14). Similarly, young (6- to 10-week-old) B6 mice were completely resistant to infection with mouse-adapted SARS-CoV (MA15); however, as mice aged, there was a steep increase in the susceptibility such that mice older than 5 months of age were highly susceptible to MA15 infection (17-19). In addition to age-dependent disease susceptibility, epidemiological studies showed sex-specific differences in incidence and case fatality rates (CFR) in humans after SARS-CoV infection, with males experiencing higher CFRs compared to females (20, 21). Similarly, data from recent MERS outbreaks showed high incidence and CFR rates among men (22). This sex-dependent increase in disease severity after pathogenic coronavirus infection was more pronounced with advancing age (20, 22).
Males and females respond differently to many RNA and DNA virus infections (23). In general, males generate less robust immune responses and are more susceptible to a variety of infectious agents (23-27). In contrast, females mount stronger innate and adaptive immune responses and are relatively resistant to virus infections (23, 28-30). However, robust immune response in females may also lead to immunopathology resulting in fatal outcomes (23, 29, 31). Sex-specific disease outcomes following virus infections are attributed to sex-dependent production of steroid hormones, different copy numbers of immune response X-linked genes, and the presence of disease susceptibility genes in males and females (23, 32). While testosterone suppresses innate immune responses, hormones such as estrogens have disparate functions, with an immune-suppressive effect at high concentrations and immunostimulatory activity at low concentrations (23, 24, 33, 34). Estrogen signaling also promotes adaptive T cell response in female mice by increasing neutrophil accumulation (30). Additionally, a recent study demonstrates a direct role for estrogen signaling in limiting influenza virus replication in nasal epithelial cells derived from humans by modulating genes that regulate the metabolic functions of cells (35).
While the epidemiological data from SARS and MERS outbreaks show male bias in disease susceptibility (20, 22), the basis for the differential susceptibility has not been established. Using a mouse model of SARS-CoV infection, we show that male mice are more susceptible to SARS-CoV infection than female mice. The enhanced susceptibility of male mice to SARS-CoV correlates with a moderate increase in virus titer and extensive IMMs and neutrophil accumulation in the lungs. Furthermore, while gonadectomy did not affect disease outcome in male mice, ovariectomy or treating female mice with estrogen receptor antagonist ICI 182, 780 resulted increased mortality to SARS-CoV infection, suggesting that estrogen signaling protects female mice from a lethal MA15 infection.
Materials and Methods
Mice and viruses
Specific pathogen-free male and female C57BL/6 and BALB/c mice (8-9 weeks, 5 months and 8-10 months) (Charles River) and 18-20 month male and female C57BL/6 mice (NIA aging colony) were bred and maintained in the University of Iowa animal care facility. The University of Iowa Institutional Animal Care and Use Committee (IACUC) approved all animal experiments. Mouse-adapted SARS-CoV (MA15), a kind gift from Kanta Subbarao (NIH, Bethesda, MD), was propagated on Vero E6 cells. Mice were lightly anesthetized using isoflurane and were intranasally infected with different doses of SARS-CoV in 50 μL DMEM. In preliminary experiments we showed that use of isoflurane, an inhalant and ketamine/xylazine, an injectable anesthetic gave similar results after SARS-CoV infection (Figure S1A). All work with infectious SARS-CoV was performed in CDC/BSAT-registered BSL-3 and ABSL-3 laboratories.
Virus titers in the lungs and preparation of lung cells for FACS analyses
Lung virus titers were determined on Vero E6 cell line as described earlier (17). FACS staining for lung cells was carried out as previously described (16, 17). Briefly, mice were sacrificed at the indicated time points. The lungs were perfused via the right ventricle with 10 ml PBS, and lungs were then removed, cut into small pieces and digested in HBSS buffer containing 2% FCS, 25 mM HEPES, 1 mg/ml Collagenase D (Roche), and 0.1 mg/ml DNase (Roche) for 30 minutes at room temperature. Digested lungs were minced and cell suspension was passed through 70μm strainer. Cells were then incubated with CD16/32, washed and stained with flurochrome-conjugated cell surface antibodies.
Vascular Leakage
Infected and control male and female mice were intravenously injected with 200 μL of Evan's blue dye (1.0 % in PBS) on day 4 post infection (p.i.). After 30 min, mice were anesthetized and lungs were perfused with 10 mL intracardial injection of PBS (36).
Antibodies and flow cytometry
For surface/intracellular staining, cells were incubated with fluorochrome-labeled antibodies specific for mouse: PECy7-anti-CD45 (30-F11), FITC-anti-Ly6G (1A8), PE/PerCp-Cy5.5-anti-Ly6C (AL-21), V450-anti-CD11b (M1/70), APC-anti-F4/80 (BM8), FITC/PE-anti-CD11c (HL3), anti-CD80 (16-10A1), anti-CD4 (RM4-5), anti-CD8α (53-6.7), APC-anti-TNF-α (MP6-XT22), APC-anti-IL-6 (MP5-20F3), APC anti- IL-β (NJTEN3) and APC-anti-iNOS (CXNFT) were procured from BD Biosciences or eBiosciences. Intracellular cytokine staining was carried out as per described protocol (16).
RNA preparation from lungs and cytokine/chemokine estimation by qPCR
RNA was extracted from the lungs of BALB/c and Ifnar-/- mice (Trizol, Invitrogen) mRNA levels were determined after normalizing each sample to HPRT. Specific primer sets used for qPCR were previously described (16).
Lung histology and Immunohistochemistry
Lungs removed were fixed in zinc formalin, paraffin embedded and tissue sections were stainined with hematoxylin and eosin. Lung tissues were analyzed using the post-examination method of masking on tissue sections. Tissues were scored based on the extent of edema in lung air spaces using the following grades: 0 – absence of edema; 1 – edema detected in <33% of lung; 2 – edema detected in 34-66% of lung; and 3 – edema detected in >66% of lung. Tissues were also scored for the extent of perivascular inflammation using the following scale: 0 – absence of perivascular inflammation (PI); 1 – mild PI aggregates around rare vessels (<3 vessels); 2– moderate PI aggregates around vessels (3-5 vessels); and 3 – moderate to expansile PI aggregates around vessels (>5 vessels). Scores were evaluated using Prism software and the Mann-Whitney test with P=0.05 as a threshold for significance.
Viral antigen was detected using rabbit anti-N protein (1:1,000) (IMG548; IMGENEX) followed by labeling with biotinylated goat anti-rabbit IgG (1:200). Samples were developed with 3,3′-diaminobenzidine for 3 min.
IMM depletion studies
8-10 month male and female C57BL/6 mice were treated intraperitoneally (i.p.) with anti-CCR2 antibody (Clone: MC21, 25 μg/mouse, i.p. in 250 μl PBS) at -6 hrs and day 1 p.i. (16).
Treatment with Flutamide, Tamoxifen and ICI 182, 780
Male mice (8-9 months) were treated with flutamide (20 mg/kg, in corn oil, subcutaneous) on days -6, -4, -2 and 0 of MA15 infection. Female mice (8-9 months) were treated with tamoxifen (1 mg/mouse, i.p.) or ICI 182, 780 (1 mg/mouse, i.p.) in corn oil on days -6, -4, -2 and 0 p.i. Equal volumes of corn oil were used as vehicle control (37). Mice were infected with 5000 pfu of MA15. Of note, ICI182, 780 treatment in naïve female mice did not change the serum estradiol levels (Figure S1B).
Quantitation of serum estrogen levels
Serum estrodiol concentration was measured by ELISA as per manufacturers instructions (Enzo Life Sciences).
Statistical Analysis
Statistical significance for survival studies was calculated using the log-rank (Mantel-Cox) test with 95% confidence interval (CI). Statistical analyses for the rest of figures (for Figures 2, 3, 4A, 4C, 7A, and 7C) fall under the generalized linear modeling (GLM) framework. Similar to a two-way ANOVA, all models considered contained two main effects variables along with their interaction term. Due to the outcome variables generally following a right-skewed distribution, we used a log-link function so that the modeling assumptions are appropriately satisfied by the data. In all models, time period was one of the two main effects. The other variable is the comparison of interest (i.e., sex, titer, or group). In some analyses, data sets cover multiple strata, so we fit each model to obtain estimates for each level of the variable of interest for each time point within each stratum. The comparisons at each time point are estimated and have corresponding p-values, which can be used to determine statistical significance by comparing to the cutoff value α = 0.05/n (n is the number of comparisons made within a time point and stratum). Statistical significance was determined based on p values of *P≤0.05, ** P≤0.01 and *** P ≤0.001.
Figure 2. Virus titers and lung pathology in MA15 infected mice.

9 month male and female mice were infected with 5000 PFU of MA15 and lungs were analyzed for titer (A), viral antigen staining in the lungs at different times p.i. (B), gross pathology and vascular leakage in lungs of naïve and MA15 infected male and female mice on day 4 p.i. (C) and histology in naïve and MA15 infected male and female mice on day 4 p.i. (D). Lung inflammation and edema scores were determined at day 4 p.i. (E). These data are derived from 4-5 mice per group. (A) Data are representative of two independent experiments. Statistical significance was determined as described in Materials and Methods. *P<0.05.
Figure 3. Increased IMM accumulation in the lungs of MA15-infected male mice.
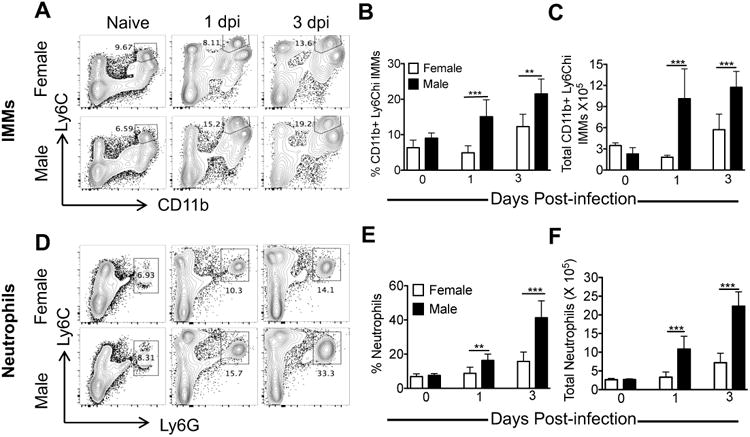
Mice were infected with 5000 PFU of MA15 and analyzed for IMMs (A, B) or neutrophils (C, D) in the lungs on days 0, 1 and 3 p.i. Representative FACS plots show percentage of IMMs (A) and neutrophils (D) in the lings. Bar graphs show percentage and total number of IMMs (B-C) and neutrophils (E-F) at different time p.i. Data are representative of 2 independent experiments with 4 mice/group/experiment. Statistical significance was determined as described in Materials and Methods. *P<0.05, **P<0.01, ***P < 0.001.
Figure 4. Enhanced proinflammatory cytokines/chemokines in the lungs of MA15 challenged male mice.

8-9 month-old male and female mice were infected with 5000 PFU of MA15. A) mRNA levels of antiviral and proinflammatory cytokines/chemokines in the lungs were measured at different times p.i. (B-C) FACS plots and bar graphs show percentage and number of cytokine producing IMMs in the lungs on day 3 p.i. following 7 hr ex-vivo incubation in the presence of Brefeldin A in male and female mice. (D) Nine-month old female and male mice were treated with control Ig or MC21 antibody (anti-CCR2, depletes IMMs) at -6hr and day 1. These mice were infected with 4000 PFU of MA15 and survival was recorded. (A) Data were obtained from 4-5 mice/group. (B-D) Data represent 2 independent experiments with 4-5 mice/group/experiment. Statistical significance was determined as described in Materials and Methods. *P<0.05, **P<0.01, ***P < 0.001.
Figure 7. Virus titer and IMM accumulation in ovariectomized female mice.

Nine-month old control or ovariectomized mice were infected with 5000 PFU of MA15 and virus titers and inflammatory cell accumulation were analyzed. (A) Lung virus titers in 9-month old male and female mice at days 2 and 4 p.i. (B, C) IMM and neutrophil accumulation in the lungs at day 2 p.i. Data are derived from 2 independent experiments with 3-4 mice/group/experiment (A) or are representative of 2 independent experiments with 3-4 mice/group/experiment (B-C). Statistical significance was determined as described in Materials and Methods. *P<0.05, **P<0.01, ***P < 0.001.
Results
Male mice are highly susceptible to SARS-CoV infection
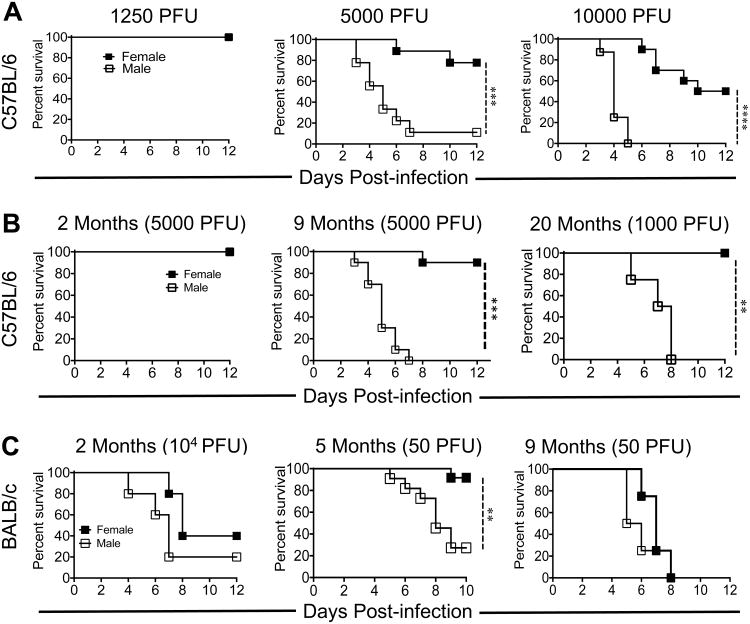
To examine sex-specific differences following SARS-CoV infection, we initially infected 8-10 month old male and female C57BL/6 mice with different doses of MA15 and monitored morbidity and mortality (Figure 1A). Both male and female mice infected with 1250 PFU of MA15 were completely protected from SARS disease. However, increasing the MA15 infection dose to 5000 PFU resulted in ∼90% mortality in male mice compared to ∼20% mortality in females (Figure 1A). Further, at 104 PFU, all male mice died while approximately 40% of the females survived MA15 infection (Figure 1A). Since case fatality rates of men and women varied with age following SARS-CoV and MERS-CoV infection (20, 22), we then investigated whether sex-specific differences in disease outcomes were age dependent. For these experiments male and female mice of different age groups were challenged with 5000 pfu of MA15 virus. As shown in figure 1B, young 2 month-old male and female mice were completely resistant to developing SARS. Conversely, all 8-9 month-old male mice succumbed to MA15 infection while only 10% of females died from the infection. While all aged (20 months) mice succumbed to infection with 5000 PFU MA15, male mice succumbed to infection with 1000 PFU MA15 whereas all female mice survived (Figure 1B).
Figure 1. Male mice are more susceptible to MA15 infection than female mice.
(A) 9 month male and female mice were infected with 1250 PFU, 5000 PFU and 104 PFU of MA15 and survival was monitored for 12 days. (B) 2 and 9 month-old B6 male and female mice were infected with 5000 PFU and 18-20 months B6 mice were challenged with 1000 PFU MA15. Survival was monitored for 12 dpi. (C) 2, 5 and 9 month-old BALB/c were infected with 104 and 50 PFU, respectively, of MA15 and were monitored for morbidity and mortality. A and B, Data are derived from 2 independent experiments with of 4-5 mice/group/experiment. C, Data are derived from 2-3 independent experiments with 3-5 mice/group/experiment (left and center panels) and one experiment with 4-5 mice/group (right panel). Statistical significance was determined using the log-rank test with 95% CI. *P<0.05, **P<0.01, ***P < 0.001.
To determine if sex-specific disease outcomes after MA15 challenge were limited to B6 mice, we infected young (8-9 weeks), adult (5 months) and middle aged (8-9 months) BALB/c mice with MA15 (Figure 1C). BALB/c mice exhibit striking age-dependent differences in morbidity and mortality so we used lower doses of MA15 in older mice to identify sex-specific disease outcomes. Similar to B6 mice, there was no difference in susceptibility of young male and female BALB/c mice to MA15 challenge. However, 5 month old adult male BALB/c mice were highly susceptible in comparison to age matched female mice when infected with 50 PFU of MA15. Sex-specific differences in 9 month old mice could not be discerned because both male and female mice were highly susceptible to MA15 infection, even at a low dose (50 PFU) of MA15 infection (Figure 1C). Together, these results show that males are more susceptible to MA15 infection than female mice.
Enhanced virus replication and lung pathology in male mice
Since sex-specific disease outcomes were most pronounced in middle-aged mice, we used 8-9 month old male and female B6 mice for further studies. We first determined virus titers in the lungs of MA15-infected male and female mice. Total virus loads were nearly identical in the lungs at 16 hr p.i. However, we observed a modest increase in MA15 titer (2-3 fold) in the lungs of male mice compared to females at days 1 and 3 p.i. (Figure 2A). SARS-CoV-N antigen was detected in airway epithelial cells and in type I and II pneumocytes at days 2 and 4 p.i, in both male and female mice. Moderately greater viral antigen staining was noted in the lung airways and parenchyma of male mice (Figure 2B). Gross examination of the lungs revealed extensive hyperemia and congestion in male mice at day 4 p.i, while those from female mice appeared nearly normal (Figure 2C). Additionally, lung vascular leakage was much more prominent in SARS-CoV-infected male mice as assessed in an Evan's blue extravasation assay at day 4 p.i. (Figure 2C, right panel). Further, histological examination of lungs revealed marked alveolar edema and terminal bronchiolar epithelial sloughing in male mice at day 4 p.i, while female lungs showed minimal alveolar edema with increased peribronchial-perivascular immune cell infiltration (Figure 2D-E). These results show a marginal but significant increase in virus replication with pronounced gross and microscopic pathological changes in male mice.
Increased accumulation of IMMs and neutrophils in the lungs of male mice
Next, we investigated if sex-specific disease susceptibility in males correlated with increased inflammatory cell recruitment. Total lung cells harvested from MA15 infected 8-9 month old male and female mice were analyzed for percentage and total number of different innate immune cells. Total numbers of alveolar macrophages (AMs), natural killer (NK) cells, and plasmacytoid dendritic cells (pDCs) were nearly the same in MA15-infected male and female lungs at days 1 and 3 p.i. (data not shown). In contrast, significantly increased numbers of Ly6ChiCD11b+ IMMs and neutrophils infiltrated into the lungs of male mice compared to females on day 1 and 3 p.i. (Figure 3). At day 3 p.i, 2-3 fold more IMMs were present in the lungs of male compared to female mice (Figure 3A-C). Similarly, a 4-5 fold increase in the total number of neutrophils was noted in the lungs of male mice at days 1 and 3 p.i. (Figure 3D-F).
Inflammatory cytokine and chemokine activity in the lungs of male and female mice
Increased mortality in male mice may result from an exuberant, but ineffective cytokine response. To evaluate this possibility, we analysed cytokine mRNA at different times post-MA15 infection. As shown in Figure 4A, transcript levels IFNβ were similar in male and female lungs at different time post-MA15 infection. In contrast, mRNA levels of pro-inflammatory cytokines (IL-6) and chemokines (CCL2 and CXCL-1), which were equivalent in both sexes early after infection, were upregulated in the lungs of male mice compared to females at day 3 p.i. (Figure 4A), suggesting a more robust inflammatory response. Next, to determine if the disparate pro-inflammatory cytokine/chemokine levels in the lungs were due to altered cytokine and chemokine production by IMMs, lung IMMs harvested from MA15 infected male and female mice at day 3 p.i. were stained directly ex vivo for intracellular TNF, IL-6 and IL-1-β without stimulation in vitro. Intracellular cytokine analyses showed that significantly higher frequencies and numbers of IMMs expressed pro-inflammatory cytokines in male mice compared to female mice (Figure 4B-C). To investigate if accumulating IMMs were in fact responsible for the increased mortality observed in 8-9 month male mice, we depleted IMMs in female and male mice using MC21 antibody as described previously (16). mAb MC21 targets CCR2 and specifically depletes IMMs. As expected, depletion of IMM did not affect disease outcome in female B6 mice (Figure 4D). However, mAb MC21 antibody treatment provided marginal but significant protection in 8-9 month male mice (Figure 4D), suggesting that IMMs contributed to lethal disease in male mice.
Sex-dependent SARS outcomes are independent of adaptive immunity
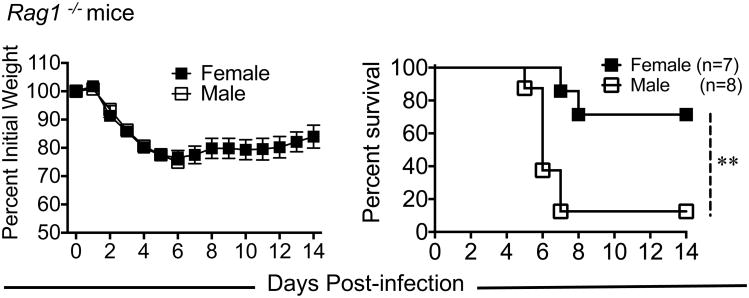
Most 8-9 month B6 male mice succumbed to MA15 infection between days 5 and 7 p.i., a time that correlates with peak T cell responses after MA15 infection. To examine whether differential adaptive immune response to MA15 infection contributed to increased disease in male mice, we examined MA15-induced morbidity and mortality in 8 month old male and female RAG1-/- mice, which lack mature T and B cells. As shown in figure 5, both male and female RAG1-/- mice lost equivalent weight after MA15 infection. However, the mortality rate was significantly higher in male RAG1-/- male compared to female mice suggesting that disease outcomes were independent of T cell and antibody responses.
Figure 5. Enhanced susceptibility of male mice to MA15 infection is independent of T and B cell response.
7-8 month old male and female RAG1-/- mice were challenged with 1750 PFU of MA15 and monitored for morbidity and mortality for 14 days. Data are derived from 7-8 mice/group. Statistical significance for survival curves was determined using the log-rank test with 95% CI. *P<0.05, **P<0.01 ***, P < 0.001
Estrogen signaling protects female mice from lethal MA15 infection
We next investigated the role of sex steroids in SARS-CoV pathogenesis by comparing gonadectomized and control counterparts after infection. Gonadectomy or treatment with flutamide, a non-steroidal anti-androgen did not affect morbidity and mortality in male mice following lethal MA15 infection, suggesting that androgens do not play a role in SARS-CoV pathogenesis (Figure 6A-B). However, a caveat of these experiments is that SARS-CoV infection significantly reduces serum testosterone levels (data not shown). In marked contrast, MA15-infected gonadectomized female mice showed progressive weight loss and death of approximately 85% of mice by day 8 p.i., while only 10-20% mortality was observed in control female mice (Figure 6C). Furthermore, female mice treated with the estrogen receptor antagonist ICI 182, 780 were more susceptible to MA15 infection compared to female mice treated with estrogen receptor agonist/modulator, tamoxifen or vehicle control (Figure 6D). Notably, female mice treated with tamoxifen showed significantly reduced weight loss compared to vehicle control treated mice after MA15 infection (Figure 6D). These results demonstrated that estrogen receptor signaling protected female mice from MA15 infection, while androgens did not influence disease outcome in males.
Figure 6. Estrogen receptor signaling protects female mice from lethal MA15 infection.
9-10 month-old male and female and their gonadectomized counterparts mice were infected with 5000 PFU of MA15. A) Nine-month old gonadectomized (n=8) or non-gonadectomized (n=8), and (B) control (n=6) or flutamide treated (n=7) male mice were monitored for disease severity. (C) Percentage weight loss and survival among control (n=12) or ovariectomized female (n=12) mice. (D) Female mice treated with vehicle (corn oil) (N=9), or Tamoxifen (1 mg/mouse, n=9-10) or ICI 182, 780 (1 mg/mouse, n=10) in 100μl corn oil were infected with 5000 PFU of MA15 and monitored for morbidity and mortality. Data are derived from 2-3 independent experiments with 3-5 mice/group/experiment. Statistical significance for survival curves was determined using the log-rank test with 95% CI. *P<0.05, **P<0.01, ***P < 0.001
Increased IMM accumulation in ovariectomized female mice
To determine if the lack of estrogen signaling was sufficient to cause the same changes in the immune response that was observed in males, we examined viral titers, and IMM and neutrophil accumulation in control and ovariectomized female mice at different time post MA15 infection. As shown in Figure 7A, total lung MA15 titers were identical in both groups of mice at day 2 p.i, but, marginally increased virus titers was noted on day 4 p.i. Additionally, lungs of ovariectomized mice showed an increased percentage and numbers of IMMs, but not neutrophils at day 2 p.i. (Figure 7B-C), suggesting a role for lung IMMs in disease severity, as we observed previously in another model of severe SARS (16). Thus, decreased estrogen signaling accounted for some, but not all of the immune changes observed in MA15-infected male mice.
Discussion
Our results show that male mice are highly susceptible to SARS-CoV infection compared to age-matched females. These results are consistent with SARS and MERS studies in humans that showed a trend towards sex-specific disease outcomes in middle-aged compared to younger individuals (20, 22). In agreement with the human studies, our results demonstrate that sex-specific differences in disease severity are prominent in middle-aged mice and less so in young and aged mice (Figure 1). Of note, differences were less apparent in individuals aged 75 and above, possibly because of enhanced mortality in all elderly regardless of sex, due to dysregulated immune responses (38).
Multiple factors contribute to disparity in sex-specific disease outcomes following virus infections. Sex-specific steroids and activity of X-linked genes, both of which modulate innate and adaptive immune response to virus infection, influence immune response (23, 27, 39-41). High copy numbers of TLR7 (located on the X-chromosome) and elevated IRF-7 expression in females induce increased IFNβ production by plasmacytoid dendritic cells and provided protection against HIV infection (42, 43). Similarly, elevated type I IFN levels correlated with reduced mouse hepatitis virus titers in female compared to male mice, possibly through TLR7 activation (31). In the current study, mRNA levels of IFN-β were equivalent during all time points examined. However, while pro-inflammatory cytokine (IL-6) and chemokine (CCL-2 and CXCL-1) expression was similar in both sexes early (16 hrs, 24 hrs and 48 hrs p.i.) after SARS-CoV challenge, levels of these cytokines and chemokines remained elevated or even increased in the lungs of male mice compared to females at 72 hrs p.i. (Figure 4A), suggesting a prolonged inflammatory response in male mice.
Estrogens are known to suppress monocyte-macrophage recruitment by downregulating MCP-1 expression during inflammation and inhibiting TLR4 mediated NFκβ activation in macrophages via suppression of micro-RNAs such as let7a and miR125b (44, 45). Similarly, treating gonadectomized mice with estrogen reduced levels of TNF and CCL2 and thus protected these mice from influenza virus infection (29, 30). We recently showed that IMMs were the predominant source of these pro-inflammatory cytokines and chemokines during lethal SARS (16). In the current study, we observed increased numbers of IMMs and neutrophils in SARS-CoV infected males. Additionally, increased numbers of IMMs correlated with elevated levels of pro-inflammatory cytokines and chemokines in the lungs of male mice and these cells also produced more of these inflammatory mediators in male compared to female mice (Figure 4). Further, increased numbers of IMMs in ovariectomized mice compared to intact females mice suggest that estrogens signaling in females suppressed accumulation and function of IMMs in the lungs. We also identified a pathogenic role for type IFN-I during SARS-CoV infection (16). Rapid SARS-CoV replication accompanied by delayed type I IFN signaling promoted accumulation of pathogenic IMMs, resulting in elevated lung cytokine/chemokine levels, vascular leakage and impaired T cell response (16). However, no detectable differences in IFNβ levels in the lungs of male and female mice suggest that the basis for the differences is IFN-independent in this instance. Additionally, it is unlikely that impaired virus-specific T cell responses resulted in enhanced SARS in males, as sex-specific disease outcomes were evident in T and B cell-deficient RAG1-/- mice.
Another factor that could contribute to differential outcomes in males and females is the direct cytopathic effect due to higher virus loads in males (6, 15). Estrogen treatment of cultured nasal epithelial cells isolated from naïve female mice suppressed IAV replication by modulating genes associated with cellular metabolism. In contrast, treating nasal epithelial cells isolated from male mice with estrogen had no effect on virus replication (35). Since SARS-CoV predominantly replicates in airways and alveolar epithelial cells (46) and estradiol concentrations are higher in female mice (47), estrogen signaling in females may directly suppress SARS-CoV replication via effects on cellular metabolism. Moreover, high viral RNA levels in the lungs of male mice may stimulate TLR7 on IMMs resulting in elevated pro-inflammatory cytokines, and chemokines (48).
Our results highlight sex-specific differences in susceptibility to SARS-CoV and perhaps other coronavirus infections. Higher virus titers and increased IMM and neutrophil infiltration in the lungs suggest contribution of multiple factors to disease severity observed in male mice. Additionally, enhanced susceptibility of ovariectomized and estrogen receptor antagonist-treated female mice demonstrate the protective effect of estrogen receptor signaling in females. Overall, the results are consistent with the sex-bias observed in human CoV infections and provide mechanistic insight into the differences in disease severity in men and women.
Supplementary Material
Acknowledgments
We thank Dr. Anthony R Fehr for careful review of this manuscript.
Footnotes
This work was supported in part by grants from the N.I.H. (PO1 AI060699, RO1 AI091322).
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nature Med. 2004;10:S88–97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menachery VD, Yount BL, Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, Burbelo PD, de Wit E, Munster VJ, Hensley LE, Zalmout IS, Kapoor A, Epstein JH, Karesh WB, Daszak P, Mohammed OB, Lipkin WI. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884–00814. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stohr K, Peiris JS, Osterhaus AD. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, Yan KW, Chan KH, Tsang NC, Guan Y, Yuen KY, Peiris JS. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, Selbs E, McEvoy CP, Hayden CD, Fukuoka J, Taubenberger JK, Travis WD. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Human Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Xu J, Zhou C, Wu Z, Zhong S, Liu J, Luo W, Chen T, Qin Q, Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 13.van den Brand JM, Haagmans BL, van Riel D, Osterhaus AD, Kuiken T. The Pathology and Pathogenesis of Experimental Severe Acute Respiratory Syndrome and Influenza in Animal Models. J Comp Pathol. 2014 doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Subbarao K. The Immunobiology of SARS*. Ann Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 15.Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005;79:15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frieman M, Yount B, Agnihothram S, Page C, Donaldson E, Roberts A, Vogel L, Woodruff B, Scorpio D, Subbarao K, Baric RS. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol. 2012;86:884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong HN, Earnest A, Lim HH, Chin CF, Tan C, Puhaindran ME, Tan A, Chen MI, Leo YS. SARS in Singapore--predictors of disease severity. Ann Acad Med Singapore. 2006;35:326–331. [PubMed] [Google Scholar]
- 22.Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein SL, Flanagan KL. Sex differences in immune responses. Nature Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 24.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 25.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Gomez E, Gonzalez-Pedrajo B, Camacho-Arroyo I. Role of sex steroid hormones in bacterial-host interactions. BioMed Res Intl. 2013;2013:928290. doi: 10.1155/2013/928290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 28.Hannah MF, V, Bajic B, Klein SL. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain, Behav Immune. 2008;22:503–516. doi: 10.1016/j.bbi.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88:4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karnam G, Rygiel TP, Raaben M, Grinwis GC, Coenjaerts FE, Ressing ME, Rottier PJ, de Haan CA, Meyaard L. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathog. 2012;8:e1002710. doi: 10.1371/journal.ppat.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 34.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 35.Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am J Physiol Lung Cell Mol Physiol. 2016;310:L415–425. doi: 10.1152/ajplung.00398.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baccala R, Welch MJ, Gonzalez-Quintial R, Walsh KB, Teijaro JR, Nguyen A, Ng CT, Sullivan BM, Zarpellon A, Ruggeri ZM, de la Torre JC, Theofilopoulos AN, Oldstone MB. Type I interferon is a therapeutic target for virus-induced lethal vascular damage. Proc Natl Acad Sci. 2014;111:8925–8930. doi: 10.1073/pnas.1408148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield J, Littlewood T, Soucek L. Tamoxifen administration to mice. Cold Spring Harb Protoc. 2015;2015:269–271. doi: 10.1101/pdb.prot077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nature Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein SL. Implications of X-linked gene regulation for sex differences in disease pathogenesis (comment on DOI 10.1002/bies.201100047) BioEssays. 2011;33:789–790. doi: 10.1002/bies.201100128. [DOI] [PubMed] [Google Scholar]
- 40.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci and Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 41.Brownstein DG, Gras L. Chromosome mapping of Rmp-4, a gonad-dependent gene encoding host resistance to mousepox. J Virol. 1995;69:6958–6964. doi: 10.1128/jvi.69.11.6958-6964.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C, Arnal JF, Douin-Echinard V, Gourdy P, Guery JC. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 44.Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Wang L, Zhang H, Guo D, Qiao Z, Qiao J. Estrogen inhibits lipopolysaccharide-induced tumor necrosis factor-alpha release from murine macrophages. Methods Find Exp Clin Pharmacol. 2001;23:169–173. doi: 10.1358/mf.2001.23.4.634640. [DOI] [PubMed] [Google Scholar]
- 46.Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, Herman BD, Sheahan T, Heise M, Genrich GL, Zaki SR, Baric R, Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cousins SW, Marin-Castano ME, Espinosa-Heidmann DG, Alexandridou A, Striker L, Elliot S. Female gender, estrogen loss, and Sub-RPE deposit formation in aged mice. Invest Ophthalmol Vis Sci. 2003;44:1221–1229. doi: 10.1167/iovs.02-0285. [DOI] [PubMed] [Google Scholar]
- 48.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.