Abstract
Reactive oxygen species (ROS) are a family of oxygen molecules with an unpaired electron and play an important role in homeostasis and pathogenesis. The reactive molecules modify lipids, proteins and nucleic acids, and modulate a wide range of cellular functions. The importance of ROS in infection has been established through clinical and in vitro studies. Here we review the role of oxidative stress in HIV pathogenesis, the impact of ROS on immune responses in HIV patients, and ROS-mediated regulation of HIV infection. Future studies on the interplay between ROS and HIV infection may offer a new strategy for prevention and treatment.
Keywords: Reactive oxygen species, HIV, Immune response
Introduction
Reactive oxygen species (ROS), metabolic byproducts in both prokaryotic and eukaryotic cells, were thought to be detrimental to the host by damaging cellular macromolecules including DNA, proteins and lipid, leading to pathogenesis of diseases [1]. However, it has been well recognized that ROS are crucial for maintaining host physiological functions [2] and play an important role in control of infection [3–9]. The association of ROS with HIV pathogenesis and disease progression has been speculated since late 80’s [10,11]. Several clinical trials on modulation of ROS in HIV-infected patients have been conducted (reviewed in [12]) although the results have not been consistent. Considering the complex role of ROS in infection and immune response, understanding the balance between redox biology and oxidative stress will offer insights into prevention and treatment for infection and diseases.
ROS and their Functions
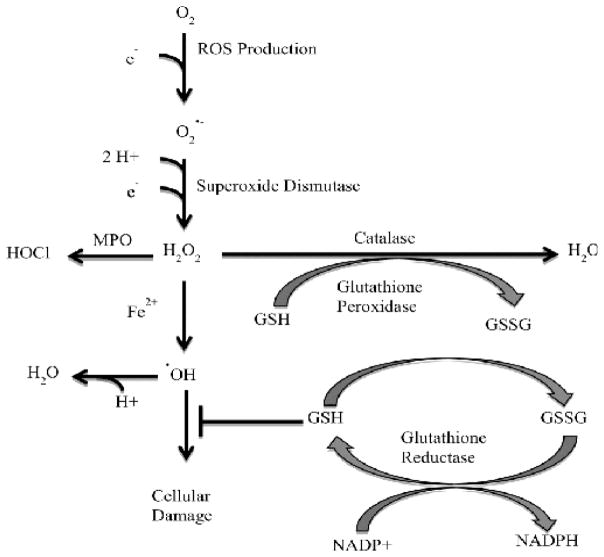
ROS are a family of reactive free radicals. Physiologically relevant ROS include superoxide (O2•−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2) phenoxyl radicals (PhO•), lipid peroxides (ROOH), hydroxyl radical (OH•), nitric oxide (•NO), nitrogen dioxide(•NO2), and peroxynitrite (ONOO−) [13,14]. ROS originate from specific intracellular locations and are generated by different mechanisms. ROS reactions are dependent on the kinetics, thermodynamics, and location (reviewed in [1,2,9]). The largest source of ROS is from the mitochondria during oxidative phosphorylation. The respiratory chain proteins complexes I and III can “leak” electrons which quickly react with mitochondrial O2; this reaction forms superoxide (O2•−) (reviewed in [15]). Superoxide is quickly converted into H2O2 by superoxide dismutase. H2O2 is less reactive and can act as a second messenger in mitochondrial signaling. ROS production is illustrated in (Figure 1).
Figure 1.
Catabolism of ROS. ROS are highly reactive and must be buffered to protect the cell from damage. ROS enzymatic sources, such as mitochondrial proteins and NOX, pass electrons to oxygen (O2); this results in superoxide (O2•-) formation. Superoxide can be converted to hydrogen peroxide (H2O2) by superoxide dismutase. Hydrogen peroxide is a less reactive than superoxide and stable enough to exist as a signaling molecule. The fate of hydrogen peroxide will be determined by location and the surrounding microenvironment. For example, in the phagosome myeloperoxidase (MPO) can convert H2O2 into hypochlorous acid (HOCL), yet in the cytosol H2O2 can be converted to water by catalase. H2O2 exposure to ferrous iron (Fe2+) results in Fenton’s reaction generating the very reactive hydroxyl radical •OH). Hydroxyl radical is buffered by single molecule antioxidants, such as glutathione. Antioxidants are enzymatically recycled.
Other major sources of ROS are enzymes located in specific organelles. NADPH oxidases (NOXs) are located at cellular membranes and generate superoxide. NOXs are important for both pathogen killing and cellular signaling. Xanthine oxidases are found in the peroxisomes and generate hydrogen peroxide for catabolic oxidation. Oxidants are also generated in the endoplasmic reticulum to facilitate protein folding and disulfide bond formation (reviewed in [3]).
ROS are highly reactive and must be regulated to prevent damage to cellular macromolecules including DNA, lipids and proteins. ROS are regulated by antioxidants and redox proteins. Single molecule antioxidants, such as glutathione or thioredoxin, are oxidized by ROS. Oxidized antioxidants are then recycled enzymatically to replenish the reduced form of the molecule; this makes the antioxidant available to reduce ROS again. An imbalanced reduction/oxidation ratio results in oxidative stress. Oxidative stress is also reduced by redox proteins such as catalase and glutathione peroxidase. These proteins catalytically convert ROS to harmless byproducts such as water (reviewed in [13–14]).
ROS are antimicrobial. Phagocytic immune cells, such as macrophages and neutrophils, contain NOX in the membrane of the phagosome. NOX pumps ROS into the phagosome where engulfed pathogens can be damaged. ROS target thiols for depletion and metal centers for •OH production, a highly reactive oxygen intermediate; this increases damage of nucleotide bases, proteins, and lipids (reviewed in [16]).
ROS play an important role in cellular signaling. The pleiotropic role of ROS is illustrated by the function of the NOX proteins. NOX signaling has been linked to apoptosis, proliferation, homeostasis and gene regulation.
ROS act as a second messenger in the MAP kinase cascade to control homeostasis and gene regulation. ROS can also directly modulate redox sensitive transcription factors such as NFκB, AP-1 and p53 (reviewed in [17]). Proteins that are also redox sensitive can be oxidized at cysteine, methionine, tyrosine, phenylalanine and tryptophan resides. These small redox reactions can have major implications for the cell. For example, latency associated peptide 1β (LAP-1β) is redox sensitive chaperone protein for transforming growth factor β1 (TGF-β1). When LAP-1β is oxidized at its amino acid 253 methionine residue, a conformational change is triggered that releases TGF-β1, leading to TGF-β1 activation [18]. Activated TGF-β1 is then free to bind tyrosine I and II receptors and initiate a signal cascade.
Recent studies on the involvement of ROS in inflammasome activation highlight the role of ROS in immune responses and immuneassociated diseases [19–21]. ROS triggered by mitophagy/autophagy blockade leads to ROS-generating mitochondria, resulting in NLRP3 inflammasome activation [22]. The crosstalk between ROS and toll-like receptor activation or cytokines such as TNFα or IL-6 modulates innate immune response and inflammation [6,7,23–25] that can alter the course of infection and disease progression.
ROS in HIV Infection
Oxidative stress was first observed in HIV pathology within a few years of the discovery of HIV. Glutathione (GSH) is depleted in plasma, lymphocytes, monocytes and lung epithelial lining in HIV-infected patients [26–29]. GSH depletion is inversely correlated to increased GSSG (glutathione disulfide) in plasma of HIV positive patients, suggesting that free radical defense was compromised [30,31]. Thioredoxin (TRX) is depleted in the dendritic cells of the lymph nodes but elevated in the plasma of HIV-infected patients [32,33]. Treitinger., et al. [34] have found reduced SOD levels in plasma and monocytes of HIV-infected patients although glutathione peroxidase levels are comparable compared to the control subjects. Spontaneous H2O2 production in monocytes of HIV-infected patients is associated with viral load. However, intracellular TRX levels are suppressed in asymptomatic patients while increased in patients with AIDS compared to healthy controls [35]. Clinical data are summarized in (Table 1).
Table 1.
Summary of Clinical Data Assessing the Impact of ROS on HIV Patients.
| Citation | ART | Sex | AIDS (CDC Classification) | CD4 Counts (Mean) | Oxidative Stress |
|---|---|---|---|---|---|
| van der Ven., et al. 1998 | Y | 30M; 5 W | 11 AIDS; 17 asymptomatic; 7 symptomatic | 232±224/mmE3 no-AIDS; 83±55/mmE3 AIDS | GSH Elevation |
| Allard., et al. 1998 | Y/N | 47M; 2W | 24 AIDS; 25 asymptomatic | 214±41 E6/L placebo; 269±46 E6/L supplement | Vitamin E & C decrease ROS levels |
| Masutani., et al. 1992 | NA | 6 M; 0W | 5 AIDS; 1 ARC | NA | TRX depletion in lymph nodes |
| Staal., et al. 1992 | NA | 121M; 11W; 2T | 71 AIDS; 31 asymptomatic; 32 ARC | 74±120/μl AIDS; 283±243/μl ARC; 516±318/μl asymptomatic | GSH and cysteine depletion |
| Malorni., et al. 1998 | N | 10M; 4W | 1 Acute HIV; 7 AIDS; 6 asymptomatic | 789/mmE3 acute HIV; 94±56/mmE3 AIDS; 423±92/mmE3 asymptomatic | Inverse correlation of ROS levels and CD4+ cell count |
| Nakamura., et al. 1996 | Y | M | 12 AIDS; 32 ARC; 40 asymptomatic | <500 cells/μl | TRX elevated |
| Lopez Galera., et al. 1996 | Y/N | 30 M; 4W | 16 AIDS; 4 ARC; 14 asymptomatic | NA | GSH depletion/ GSSG elevation |
| Aukrust., et al. 1995 | NA | 18 M; 4W | 12 symptomatic; 10 asymptomatic | 120 E6/L | GSH depletion/ GSSG elevation |
| Elbim., et al. 1999 | Y/N | 26M; 9W | 9 AIDS; 10 asymptomatic; 7 asymptomatic | <500/μl symptomatic; >500/μl asymptomatic; <500/μl asymptomatic | Enhanced ROS in monocytes |
| Mburu., et al. 2013 | N | 5M; 15W | 20 asymptomatic | 411/mmE3 | Vitamin C & NAC decreased early apoptosis and CD25 |
Abbreviations: Y: Yes On ART, N: No ART, Y/N: Cohorts Yes On ART and No ART, NA: Information Not Available, M: Men, W: Women, T: Transexual, ARC: AIDS-related Complex
The changes in ROS in HIV-infected patients provided the rationale for clinical studies to assess antioxidant regimens as treatment for patients with HIV. There was a decrease in viral load after three months of Vitamin E & C supplementation [36]; however these effects were not widely reproduced. While vitamin supplementation did have beneficial effects of reduced mitochondrial dysfunction, oxidative stress and metabolic complications in HIV-infected patients receiving HAART, vitamins failed to reduce viral loads or consistently preserve CD4+ T cell counts in patients with anti-retroviral therapy [37]. A clinical trial that aims to assess the impact of micronutrient and antioxidant supplementation on disease progression, decline of immunity, and the start of ART treatment in untreated asymptomatic HIV positive adults is currently in a Phase III trial [38].
The immunological profiles in HIV-infected patients are impacted by oxidative stress and possibly modulated by antioxidant treatment. High oxidative stress is found in serum of HIV-infected patients and is inversely associated with IL-7-mediated STAT5 activation in CD8+ T cell subsets and CD4+ central memory [39]. Oxidative stress-mediated apoptosis is enhanced in lymphocytes of asymptomatic HIV patients [40]; however, LPS-induced apoptosis is reduced in CD4+ T cells of asymptomatic HIV patients with combined Vitamin C and NAC treatment [41]. Vitamin C and NAC treatment also reduced LPS-induced up-regulation of activation marker, CD25 on CD4+ T cells in these patients. Lipid peroxidation, an indicator of oxidative stress, is inversely correlated with CD4+ T cell counts in HIV positive patients; however, pretreatment with NAC prevented altered morphology of PBMC in vitro [42]. In chronically infected monocytic cell line, U1, glutathione and NAC inhibited reverse transcription when stimulated with PMA, TNF, or IL-6 [43]. In Ul cells, NAC also showed significant suppression of HIV mRNA accumulation with PMA or TNF stimulation. Similar findings showed PMA reactivation of HIV-LTR was blocked by NAC in stably transfected cell lines 293.27.2 and H9 T cells [44]. NAC and GSH were also found to suppress reverse transcription in primary cord blood monocyte-derived-macrophage (MDM) and adult MDM treated post-infection [45,46].
HIV proteins have been shown to modulate ROS in vitro as summarized in (Table 2). HIV accessory protein TAT (trans-activator of transcription) increases H2O2 production. TAT-induced H2O2 signal along with a Ca2+ influx induces CD95L thereby, mediating activation induced cell death [47]. TAT also up-regulated NOX 2, a ROS-generating enzyme, in a phosphatidylinositol-3-kinase/Akt dependent manner, leading to NFkB activation [48]. TAT increases TNF-mediated cytotoxicity and NFkB transcription in a dose dependent manner by reducing mitochondrial SOD expression and function [49]. TAT also reduces the GSH:GSSG glutathione ratio, leading to decreased redox capacity of the cell [49]. Interestingly, TAT induces the master regulator of the antioxidant response elements (ARE), Nrf2 genes, which encode many antioxidant enzymes [50]. Viral accessory protein, Vpr, increases ROS thereby modulating IL-6 and mediating reactivation of latent virus in primary MDM [51]. HIV env glycoprotein gp41 can also increase ROS in bystander cells and is critical for HIV env-mediated apoptosis, which depends on ROS production [52].
Table 2.
Summary of Studies Showing HIV Proteins Enhance ROS Production. ↑ indicates induction
| HIV | ROS | Cellular Target | Cell type | Reference |
|---|---|---|---|---|
| TAT | ↑ | Increase NFkB Transcription | MAGI-CCR5 | Zhang, 2011 |
| TAT | ↑ | Increase TNF | Jurkat T cell & HeLa-tat | Westendorp, 1995 |
| TAT | ↑ | Decrease Mitochondrial SOD | Jurkat T cell & HeLa-tat | Westendorp, 1995 |
| TAT | ↑ | Increase Activation-Induced Cell Death (ACID) | Jurkat T cell & Primary T cells | Gulow, 2005 |
| gp41 | ↑ | HIV Env-mediated apoptosis | CHO & Primary T cells | Garg, 2005 |
| Vpr | ↑ | IL-6 mediated viral reactivation | Primary MDM & Ul monocytes | Hoshino, 2010 |
ROS modulates HIV infectivity in vitro. ROS has been shown to enhance HIV entry into Jurkat T cells at H2O2 concentrations between 1–10 μM, which is correlated with increased surface chemokine receptor CXCR4 expression [53]. However, HIV entry is decreased at H2O2 concentrations above 50 μM and CXCR4 expression is also decreased at higher H2O2 concentrations. Surface receptor modulation by ROS has also been shown in macrophage [54]. ROS increase viral replication by reactivating 5′LTR (long terminal repeat) in latently infected cells through canonical NFκB signaling [29, 55]. These studies are summarized in (Table 3).
Table 3.
Summary of Studies Illustrating ROS’s Modulation of HIV Infectivity. ↑ induction; ↓ inhibition
| ROS | Mechanism | HIV Infection | Cell Type | Reference |
|---|---|---|---|---|
| ↑ | Enhance HIV Entry | ↑ | Jurkat T cell | Lan, 2013 |
| ↑ | Increase LTR Induction | ↑ | Jurkat T cell | Staal, 1990 |
| ↑ | Increase CXCR4 Expression | ↑ | Primary Macrophage | Saccani, 2000 |
| ↓ | Decrease RT & viral protein synthesis | ↓ | U1 Monocytes | Kalebic, 1991 |
The role of ROS in the inflammasome activation of HIV is an exciting area and has not yet been thoroughly investigated. ROS production is required for NLRP3 inflammasome activation in both primary macrophage and THP-1 cells, a human monocytic cell line [56]. Basal levels of NLRP3 inflammasome genes in monocyte-derived dendritic cells from HIV+ patients was increased three fold compared to healthy controls [57]. Additionally, single nucleotide polymorphisms in the NLRP3 gene are associated with increased HIV susceptibility[58]. Inflammasome caspase-1 mediated pyroptosis has been found to cause 95% of CD4+ cell death in human lymphoid aggregate culture infected with HIV ex vivo [59]. Therefore, further studies on the interplay between ROS and inflammasome will offer insights into the dynamic of immune activation in response to HIV infection [60,61].
Conclusions
ROS are clearly involved in HIV pathogenesis and disease progression. It may also play a role in HIV transmission, which has not been thoroughly investigated. Because of their diverse species, locations and pleotropic functions in immune responses, it has been challenging to alter the symptoms or course of diseases in HIV-infected patients. Further understanding the interplay between ROS and immune response in context of HIV infection at the molecular level may help develop new strategies for HIV prevention and treatment.
Bibliography
- 1.Cross CE, et al. Oxygen radicals and human disease. Annals of Internal Medicine. 1987;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 2.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nature Reviews Immunology. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder K, et al. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 5.Marchi LF, et al. In vitro activation of mouse neutrophils by recombinant human interferon-gamma: increased phagocytosis and release of reactive oxygen species and pro-inflammatory cytokines. International Immunopharmacology. 2014;18(2):228–235. doi: 10.1016/j.intimp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 6.West AP, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West AP, et al. Mitochondria in innate immune responses. Nature Reviews Immunology. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupre-Crochet S, et al. ROS production in phagocytes: why, when, and where? Journal of Leukocyte Biology. 2013;94(4):657–670. doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- 9.Droge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 10.Papadopulos-Eleopulos E. Reappraisal of AIDS--is the oxidation induced by the risk factors the primary cause? Medical Hypotheses. 1988;25(3):151–162. doi: 10.1016/0306-9877(88)90053-9. [DOI] [PubMed] [Google Scholar]
- 11.Papadopulos-Eleopulos E, et al. An alternative explanation for the radiosensitization of AIDS patients. International journal of radiation oncology, biology, physics. 1989;17(3):695–697. doi: 10.1016/0360-3016(89)90128-4. [DOI] [PubMed] [Google Scholar]
- 12.Kashou A, Agarwal A. Oxidants and Antioxidants in the Pathogenesis of HIV/AIDS. The Open Reproductive Science Journal. 2011;3:154–161. [Google Scholar]
- 13.Winterbourn C. Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology. 2008;4(5):278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson B, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nature Chemical Biology. 2011;7(8):504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M. How miochonria produce reactive oxygen species. Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang F. Antimcrobial Reactive Oxygen and Nitrogen Species: Concepts and Controversies. Nature Reviews Microbiology. 2004;2(10):820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiology Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jobling M. Isoform-Specific Activation of Latent Transforming Growth Factor β (LTGF-β) by Reactive Oxygen Species. Radiation Research. 2006;166(6):839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 19.Gross O, et al. The inflammasome: an integrated view. Immunological Reviews. 2011;243(1):136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, et al. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 21.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature Reviews Immunology. 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 23.Kohchi C, et al. ROS and innate immunity. Anticancer Research. 2009;29(3):817–821. [PubMed] [Google Scholar]
- 24.Yang D, et al. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Experimental Eye Research. 2007;85(4):462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. The Journal of experimental medicine. 2011;208(3):417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eck HP. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1 infected patients. Biological Chemistry Hoppe Seyler. 1989;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 27.de Quay B. Glutathione depletion in HIV-inected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992;6(8):815–820. [PubMed] [Google Scholar]
- 28.Buhl R. System Glutatione Deficiency in Symptom-Free HIV-Seropositive Individuals. The Lancet. 1989;334(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- 29.Staal FJT. Intracellular thiols regulate activation of nuclear factor kB and transcription of human immunodeficiency virus. Proceedings of the National Academy of Sciences. 1990;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LopezGalera RM, et al. Glutathione and cysteine in HIV-infected hemophiliacs. Clinica Chimica Acta. 1996;254(1):63–72. doi: 10.1016/0009-8981(96)06366-8. [DOI] [PubMed] [Google Scholar]
- 31.Aukrust P, et al. Increased Levels of Oxidized Glutathione in CD4+ Lymphocytes Associated with Disturbed Intracellular Redox Balance n Human Immunodeficiency Virus Type 1 Infection. Blood. 1995;86(1):258–267. [PubMed] [Google Scholar]
- 32.Masutani H, et al. Dysregulation of Adult T-cell Leukeia-Derived Factor (ADF)/ Thioredoxin in HIV Infection: Loss of ADF High Producer Cells in Lymphoid Tissue of AIDS Patients. AIDS Research and Human Retroviruses. 1992;8(9):1707–1715. doi: 10.1089/aid.1992.8.1707. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, et al. Elevation of plasma thioredoxin levels in HIV-infected individuals. International Immunology. 1996;8(4):603–611. doi: 10.1093/intimm/8.4.603. [DOI] [PubMed] [Google Scholar]
- 34.Treitiner, et al. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. European Journal of Clinical Investigation. 2000;30(5):454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 35.Elbim C, et al. Redox and Activation Status of Monocytes from Human Immunodeficiency Virus-Infected Patients: Relationship with Viral Load. Journal of Virology. 1999;73(6):4561–4566. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allard JP, et al. Effects of vitamin E and C supplementation on oxidtive stress and viral load in HIV-infeced subjects. AIDS. 1998;12(13):1653–1659. doi: 10.1097/00002030-199813000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Drain P, et al. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. The American Journal of Clinical Nutrition. 2007;85(2):333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 38.Cameron W. Micronutrients and Antioxidnts in HIV Infection (MAINTAIN) ClinicalTrials.gov. 2015 [Google Scholar]
- 39.Kalinowska M. Decreased IL-7 Responsiveness is Related to Oxidative Stress in HIV Disease. PloS ONE. 2013;8(3):e58764. doi: 10.1371/journal.pone.0058764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobmeyer TS. Ex vivo induction of apoptosis in lymphocytes is mediated by oxidative stress: Role for lymphocyte loss in HIV infection. Free Radical Biology & Medicine. 1997;22(5):775–785. doi: 10.1016/s0891-5849(96)00403-0. [DOI] [PubMed] [Google Scholar]
- 41.Mburu S. Modulation of LPS-Induced CD4+ T-Cell Activation and Apoposis by Antioxidants in Untreated Asymptomatic HIV Infected Participants: An In Vitro Study. Clinical and Developmental Immunology. 2013:631063. doi: 10.1155/2013/631063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malorni W, et al. The Role of Oxidative Imbalance in Progression to AIDS: Effect of the Thiol Supplier N-Acetylcysteine. AIDS Research and Human Retroviruses. 1998;14(17):1589–1596. doi: 10.1089/aid.1998.14.1589. [DOI] [PubMed] [Google Scholar]
- 43.Kalebic T. Suppression of human immunodeficiency virus expression in chronically infeced monocytic cells by glutathione, glutathione eser, and N-acetylcysteine. Proceedings of the National Academy of Sciences. 1991;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roederer M. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proceedings of the National Academy of Sciences. 1990;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lioy J. Thiol suppression of immunodeficiency virus type 1 replication in primary cord blood monocyte-derived macrophages in vitro. Journal of Clinical Investigation. 1993;91(2):495–498. doi: 10.1172/JCI116227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho WZ, Douglas SD. Glutathione and N-acetylcysteine suppression of human imunodeficiency virus type1 replication in human monocyte/macrophages in vitro. AIDS Res Hum Retroviruses. 1992;8(7):1249–1253. doi: 10.1089/aid.1992.8.1249. [DOI] [PubMed] [Google Scholar]
- 47.Gulow K. HIV-1Trans-Activator of Transcription Substitues for Oxidative Signaling in Activation-Induced T Cell Death. The Journal of Immunology. 2005;174(9):5249–5260. doi: 10.4049/jimmunol.174.9.5249. [DOI] [PubMed] [Google Scholar]
- 48.Zhang HS. Akt/Nox2/NF-kB signaling pathway is invoved in Tat-induced HIV-1 Long terminal repeat (LTR) transactivation. Archives of Biochemistry and Biophysics. 2011;505(2):266–272. doi: 10.1016/j.abb.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Westendorp MO. HIV-1 Tat potentiates TNF-induced NF-kB activation and cytotoxicity by altering the cellular redox state. The EMBO Journal. 1995;14(3):546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HS. Nrf2 is involved in inhibiting Tat-induced HIV-1 long terminal repeat transactivation. Free Radical Biology & Medicine. 2009;47(3):261–268. doi: 10.1016/j.freeradbiomed.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 51.Hoshino S. HIV-1 Vpr induces TLR/MyD88-mediated IL-6 production and reactivates viral production from latency. Journal of Leukocyte Biology. 2010;87(6):1133–1143. doi: 10.1189/jlb.0809547. [DOI] [PubMed] [Google Scholar]
- 52.Garg H. HIV gp41-induced apoptosis is mediated by caspase-3-dependent mitochondrial depolarization, which is inhibited by HIV protease inhibior nelfinavir. Journal of Leukocyte Biology. 2005;79(2):351–362. doi: 10.1189/jlb.0805430. [DOI] [PubMed] [Google Scholar]
- 53.Lan X. High glucose enhances HIV entry into T cells through upregulation of CXCR4. Journal of Leukocyte Biology. 2013;94(4):769–777. doi: 10.1189/jlb.0313142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saccani A. Redox regulation of chemokine receptor expression. Proceedings of the National Academy of Sciences. 2000;97(6):2761–2766. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyo CW. Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-kB. Biochemical and Biophysical Research Communications. 2008;376(1):180–185. doi: 10.1016/j.bbrc.2008.08.114. [DOI] [PubMed] [Google Scholar]
- 56.Guo H. HIV-1 infection induces interleukin1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. Journal of Biological Chemistry. 2014;289(31):21716–21726. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pontillo A. HIV-1 induces NALP3-inflammasome expression and interleukin-1B secretion in dendritic cells from healthy individuals but not from HIV-posiive patients. AIDS. 2012;26(1):11–18. doi: 10.1097/QAD.0b013e32834d697f. [DOI] [PubMed] [Google Scholar]
- 58.Pontillo A. A 3′ UTR SNP in NLRP3 Gene is Associated with Susceptibiltiy to HIV-1 Infection. Journal of Aquired Immune Deficiency Syndrome. 2010;54(3):236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 59.Doitsh G. Cell death by pyroptosis drives CD4 T-cell depetion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staal F, et al. Intracellular Glutathione Levels in T Cell Subsets Decrease in HIV-infected Individuals. AIDS Research and Human Retroviruses. 1992;8(2):305–311. doi: 10.1089/aid.1992.8.305. [DOI] [PubMed] [Google Scholar]
- 61.van der Ven, et al. Glutathione homeostasis is disturbed in CD4-positive lymphocytes of HIV-seropositive individuals. European Journal of Clinical Investigation. 1998;28:187–193. doi: 10.1046/j.1365-2362.1998.00267.x. [DOI] [PubMed] [Google Scholar]