Abstract
Background
The advent of highly active antiretroviral therapy (ART) has reduced the morbidity and mortality due to HIV infection. The World Health Organization (WHO) ART guidelines focus on three classes of antiretroviral drugs, namely nucleoside or nucleotide reverse transcriptase inhibitors (NRTI), non‐nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors. Two of the most common medications given as first‐line treatment are the NNRTIs, efavirenz (EFV) and nevirapine (NVP). It is unclear which NNRTI is more efficacious for initial therapy. This systematic review was first published in 2010.
Objectives
To determine which non‐nucleoside reverse transcriptase inhibitor, either EFV or NVP, is more effective in suppressing viral load when given in combination with two nucleoside reverse transcriptase inhibitors as part of initial antiretroviral therapy for HIV infection in adults and children.
Search methods
We attempted to identify all relevant studies, regardless of language or publication status, in electronic databases and conference proceedings up to 12 August 2016. We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov to 12 August 2016. We searched LILACS (Latin American and Caribbean Health Sciences Literature) and the Web of Science from 1996 to 12 August 2016. We checked the National Library of Medicine (NLM) Gateway from 1996 to 2009, as it was no longer available after 2009.
Selection criteria
We included all randomized controlled trials (RCTs) that compared EFV to NVP in people with HIV without prior exposure to ART, irrespective of the dosage or NRTI's given in combination.
The primary outcome of interest was virological success. Other primary outcomes included mortality, clinical progression to AIDS, severe adverse events, and discontinuation of therapy for any reason. Secondary outcomes were change in CD4 count, treatment failure, development of ART drug resistance, and prevention of sexual transmission of HIV.
Data collection and analysis
Two review authors assessed each reference for inclusion using exclusion criteria that we had established a priori. Two review authors independently extracted data from each included trial using a standardized data extraction form. We analysed data on an intention‐to‐treat basis. We performed subgroup analyses for concurrent treatment for tuberculosis and dosage of NVP. We followed standard Cochrane methodological procedures.
Main results
Twelve RCTs, which included 3278 participants, met our inclusion criteria. None of these trials included children. The length of follow‐up time, study settings, and NRTI combination drugs varied greatly. In five included trials, participants were receiving concurrent treatment for tuberculosis.
There was little or no difference between EFV and NVP in virological success (RR 1.04, 95% CI 0.99 to 1.09; 10 trials, 2438 participants; high quality evidence), probably little or no difference in mortality (RR 0.84, 95% CI 0.59 to 1.19; 8 trials, 2317 participants; moderate quality evidence) and progression to AIDS (RR 1.23, 95% CI 0.72 to 2.11; 5 trials, 2005 participants; moderate quality evidence). We are uncertain whether there is a difference in all severe adverse events (RR 0.91, 95% CI 0.71 to 1.18; 8 trials, 2329 participants; very low quality evidence). There is probably little or no difference in discontinuation rate (RR 0.93, 95% CI 0.69 to 1.25; 9 trials, 2384 participants; moderate quality evidence) and change in CD4 count (MD −3.03; 95% CI −17.41 to 11.35; 9 trials, 1829 participants; moderate quality evidence). There may be little or no difference in treatment failure (RR 0.97, 95% CI 0.76 to 1.24; 5 trials, 737 participants; low quality evidence). Development of drug resistance is probably slightly less in the EFV arms (RR 0.76, 95% CI 0.60 to 0.95; 4 trials, 988 participants; moderate quality evidence). No studies were found that looked at sexual transmission of HIV.
When we examined the adverse events individually, EFV probably is associated with more people with impaired mental function (7 per 1000) compared to NVP (2 per 1000; RR 4.46, 95% CI 1.65 to 12.03; 6 trials, 2049 participants; moderate quality evidence) but fewer people with elevated transaminases (RR 0.52, 95% CI 0.35 to 0.78; 3 trials, 1299 participants; high quality evidence), fewer people with neutropenia (RR 0.48, 95% CI 0.28 to 0.82; 3 trials, 1799 participants; high quality evidence), and probably fewer people withrash (229 per 100 with NVP versus 133 per 1000 with EFV; RR 0.58, 95% CI 0.34 to 1.00; 7 trials, 2277 participants; moderate quality evidence). We found that there may be little or no difference in gastrointestinal adverse events (RR 0.76, 95% CI 0.48 to 1.21; 6 trials, 2049 participants; low quality evidence), pyrexia (RR 0.65, 95% CI 0.15 to 2.73; 3 trials, 1799 participants; low quality evidence), raised alkaline phosphatase (RR 0.65, 95% CI 0.17 to 2.50; 1 trial, 1007 participants; low quality evidence), raised amylase (RR 1.40, 95% CI 0.72 to 2.73; 2 trials, 1071 participants; low quality evidence) and raised triglycerides (RR 1.10, 95% CI 0.39 to 3.13; 2 trials, 1071 participants; low quality evidence). There was probably little or no difference in serum glutamic oxaloacetic transaminase (SGOT; MD 3.3, 95% CI ‐2.06 to 8.66; 1 trial, 135 participants; moderate quality evidence), serum glutamic‐ pyruvic transaminase (SGPT; MD 5.7, 95% CI ‐4.23 to 15.63; 1 trial, 135 participants; moderate quality evidence) and raised cholesterol (RR 6.03, 95% CI 0.75 to 48.78; 1 trial, 64 participants; moderate quality evidence).
Our subgroup analyses revealed that NVP slightly increases mortality when given once daily (RR 0.34, 95% CI 0.13 to 0.90; 3 trials, 678 participants; high quality evidence). There were little or no differences in the primary outcomes for patients who were concurrently receiving treatment for tuberculosis.
Authors' conclusions
Both drugs have similar benefits in initial treatment of HIV infection when combined with two NRTIs. The adverse events encountered affect different systems, with EFV more likely to cause central nervous system adverse events and NVP more likely to raise transaminases, cause neutropenia and rash.
11 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (12 Aug, 2016) were included
Plain language summary
Effectiveness of EFV compared to NVP in the suppression of HIV infection when used as part of initial three‐drug combination
Research question
For people living with HIV who have never received antiretroviral therapy (ART), which drug is more effective in suppressing HIV infection in combination with two nucleoside reverse transcriptase inhibitors (NRTI): efavirenz (EFV) or nevirapine (NVP)?
Background
The introduction of highly active ART as treatment for HIV infection has greatly reduced mortality and morbidity for adults and adolescents living with HIV around the world. The recommended initial treatments for HIV infection include two drugs from a class of drugs known as NRTI and one from a related class of drugs called non‐nucleoside reverse transcriptase inhibitors (NNRTI). The two NNRTIs most commonly used are NVP and EFV. However, NVP can cause liver damage and severe rash, both of which can be fatal. EFV may also cause a rash, impair mental function, and cause foetal malformations.
Main results
Cochrane researchers examined the available literature up to 12 August 2016 and identified 12 randomized controlled trials, with a total of 3278 people, that met the inclusion criteria of this review. None of the included trials included children. Four trials included people who were also receiving treatment for tuberculosis. There was little or no difference in suppression of HIV infection (high quality evidence), probably little or no difference in mortality, progression to AIDS, stopping treatment early and changes in blood cells affected by HIV (moderate quality evidence). There may be little or no difference in treatment failure (low quality evidence). We are uncertain whether there is a difference in side‐effects (very low quality evidence). No studies were found that looked at sexual transmission of HIV. Development of drug resistance is probably slightly less in the EFV group (moderate quality evidence). When the side effects were examined individually, EFV probably caused more impaired mental function (6% in the EFV group and 2% in the NVP group; moderate quality evidence), while NVP probably caused more people to have a rash (3% in the EFV group and 6% in the NVP group; moderate quality evidence), caused more people to have reduced white blood cells (2% in the EFV group and 5% in the NVP group; high quality evidence), and signs of liver damage (6% in the EFV group and 11% in the NVP group; high quality evidence). There was probably little or no difference in increases in liver enzymes and levels of cholesterol (moderate quality evidence). There may be little or no difference in digestive side‐effects, fever, enzymes from the liver and pancreas, and fat in the blood (low quality evidence). People on NVP were probably more likely to die when given a once‐daily regimen (2% in the EFV group and 4% in the NVP group; moderate quality evidence). In people who were taking treatment for tuberculosis compared to those who were not, there was probably little or no difference in suppression of HIV, deaths, progression to AIDS or stopping treatment early (moderate to high quality evidence).
Conclusion
EFV and NVP are similarly effective in viral suppression, preventing HIV progression and reducing mortality. EFV is more likely to affect mental function, while NVP is more likely to cause signs of liver damage, reduced white blood cells and rash.
Summary of findings
Background
Description of the condition
A total of 36.7 million people were living with HIV in 2015. This is an increase from previous years, mostly due to the use of antiretroviral therapy (ART) (UNAIDS 2016). In many countries, ART has reduced hospitalization, morbidity, and mortality among people living with HIV (Gilks 2006; Hogg 1997; Mocroft 1998).
Significant public and private resources have been devoted to rapidly scale up efforts in low‐ and middle‐income countries (LMICs) to provide access to first‐line ART. In 2014, only 40% of eligible people in LMICs were receiving ART. These efforts to scale‐up access to ART should be accompanied by initiatives to determine the most effective first‐line therapy (UNAIDS 2016), which can be used in diverse populations.
ART guidelines were first published by the World Health Organization (WHO) in 2002 (WHO 2002), and were updated in 2006, 2010, 2013, 2014, and 2015 (WHO 2015b). For countries with limited resources, the WHO recommends a public health approach to ART to improve access, simplify clinical decision making, standardize regimens, and standardize the monitoring and management of toxicity and drug interactions (Gilks 2006). For any initial regimen, the potency, durability of efficacy, ease of administration and storage, tolerability, and toxicity need to be balanced with cost and availability (Gilks 2006). These guidelines provide a framework for choice of medication in most countries. However, when the recommended drugs have different costs and toxicity profiles, head‐to head comparisons are necessary to determine which medication should be the choice of preference for clinicians. The more recent guidelines integrate more evidence and are in favour of an earlier start to ART (CD4 cell count of 500 cells/mm3 or less as opposed to the previous threshold of 350 cells/mm3) in active tuberculosis, hepatitis B co‐infection with severe liver disease, pregnant and breastfeeding women, children under five years of age, and sero‐discordant couples (WHO 2014).
Description of the intervention
The WHO Model List of Essential Medicines describes three classes of antiretroviral drugs for treatment and prevention of HIV infection: Nucleoside reverse transcriptase inhibitors (NRTI), non‐nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (WHO 2015a). In 2006 the WHO recommended that initial ART should be with one of three regimens: two NRTIs plus efavirenz (EFV), two NRTIs plus nevirapine (NVP), or two NRTIs plus abacavir (ABC) (Gilks 2006; WHO 2006). ABC is not a NNRTI and didn't qualify for evaluation in this review. The NRTI combination drugs could either be zidovudine (AZT) plus lamivudine (3TC), or stavudine (d4T) plus 3TC. Stavudine is no longer recommended as a first‐line regimen, given its known metabolic toxicities and should be used only when no other drug can be offered (WHO 2015b). The current recommendations suggest that the preferred first‐line regimen be composed of tenofovir (TDF) and 3TC or emcitrabine (FTC) with EFV. TDF could be replaced with AZT and EFV with NVP in the event that drugs in the preferred regimen are unavailable or contraindicated (WHO 2015b). Protease inhibitors can also be used in special circumstances (WHO 2015b).
How the intervention might work
Protease inhibitors cost more, have higher pill burdens, and have dietary constraints associated with their use. Protease inhibitors are also linked to serious long‐term metabolic disorders, most notably an increased risk of lipodystrophy and hyperlipidaemia (Moyle 2000; BHIVA 2001). Moreover, a meta‐analysis of 12 trials revealed that NNRTI‐based regimens were better than protease inhibitor‐based regimens for virologic suppression (Chou 2006).
NNRTIs have a more favourable adverse effect profile than protease inhibitors, are cheaper, and are easier to administer. They are also more cost‐effective (Beck 2008). Their main disadvantage is that a single mutation may confer resistance to the entire class of NNRTIs, since cross‐resistance among agents of this class is nearly universal (Deeks 2001; Dybul 2002).
NVP may be responsible for severe or fatal hepatotoxicity, and a rash which may present in severe form as Stevens‐Johnson syndrome. Nevertheless, NVP is the NNRTI of choice for pregnant women because EFV may be teratogenic(DHHS 2001a). EFV may cause a rash and central nervous system symptoms such as dizziness, somnolence, insomnia, drowsiness, nightmares, hallucinations, and poor concentration (DHHS 2001b).
Why it is important to do this review
Providing evidence on the more appropriate choice of NNRTI with respect to efficacy, durability, and tolerability, is important to patients, caregivers, and policymakers worldwide. In the previous version of our review we found that EFV and NVP had similar efficacies, but different toxicity profiles (Mbuagbaw 2010).
The current review update represents a collaborative effort between the Cochrane Infectious Diseases Group, the University of California, San Francisco (UCSF), the School of Public Health of the University of Minnesota, the U.S. Centers for Disease Control and Prevention (CDC), the University of Cape Town, and the WHO to address questions through systematic reviews regarding the optimum first‐line ART regimen in patients living with HIV in low‐ and middle‐income countries. The previous review was used in the development of the 2009 WHO ART treatment guidelines (WHO 2009).
In the past five years, the body of evidence on NNRTI's has grown, especially among people co‐infected with tuberculosis. This Cochrane Review update responds to the need for evidence‐based recommendations for managing HIV and tuberculosis co‐morbidity.
Objectives
To determine which non‐nucleoside reverse transcriptase inhibitor, either EFV or NVP, is more effective in suppressing viral load when given in combination with two nucleoside reverse transcriptase inhibitors as part of initial antiretroviral therapy for HIV infection in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs)
Types of participants
We included adults and children infected with HIV and without prior exposure to antiretroviral therapy (ART), and women who had received short courses of NNRTIs for the prevention of mother‐to‐child transmission. We excluded pregnant or lactating women and children under five years of age.
Relevant subpopulations of interest were participants with the following:
concurrent hepatitis B virus infection;
concurrent hepatitis C virus infection;
concurrent tuberculosis.
Types of interventions
We considered triple‐drug antiretroviral combination regimens for initial therapy containing two NRTIs plus either EFV or NVP at any dose (EFV + 2NRTIs versus NVP + 2NRTIs). The NRTIs in each combination did not need to be specified, but if they were specified, they must have been the same in both the EFV and NVP arms, such that the only difference in the regimens was the NNRTI. We included trials with additional trial arms, but we only evaluated the EFV‐containing and NVP‐containing trial arms in this review.
We compared EFV‐containing and NVP‐containing triple‐drug regimens with regard to therapeutic efficacy, using plasma HIV ribonucleic acid (RNA) concentration as a surrogate marker for clinical progression. Plasma HIV RNA has been demonstrated to be a reliable predictor of HIV disease progression (Lau 2007; Mellors 2007).
An earlier Cochrane review of stavudine (d4T), lamivudine (3TC) and NVP, Siegfried 2006, analysed studies that compared this regimen to any other available regimen used in the treatment of HIV/AIDS in treatment‐naïve or previously‐treated adults and adolescents. We included one trial in this review, van Leth 2004, which was also included in Siegfried 2006 as it compared this regimen to another that contained d4T, 3TC, and EFV in participants who had never received ART.
We planned to extract data from trials that included participants irrespective of their exposure to ART, but provided separate reporting and analysis of the ART‐naïve group. By so doing, we could analyse the data for participants of interest from papers that included both ART‐naïve and ART‐exposed populations.
Types of outcome measures
Primary outcomes
The percentage of participants achieving undetectable plasma HIV RNA concentration (viral load) over time (virological success). For this outcome we used the lower limit of HIV RNA detection, and the time frame reported by the trial authors.
Mortality.
Progression to AIDS (clinical). We assessed clinical progression by the proportion of participants that progressed either to the Centers for Disease Control and Prevention (CDC)‐defined AIDS (stage III to stage IV disease) or who developed a second opportunistic infection or malignancy.
All severe adverse events. We classified these according to grade 1 to 4 of the Adverse Event Toxicity Scale (NIAID/NIH 2004), and reported them as the proportion of participants that experienced grade 3 and 4 clinical or laboratory adverse events. Using this scale, grade 1 and 2 denote mild to moderate symptoms, grade 3 denotes serious symptoms, and grade 4 denotes life‐threatening events requiring significant clinical intervention.
Discontinuation rate. We defined this variable as the proportion of study participants who either stopped their treatment regimens totally or switched for any reason associated with the regimen.
Secondary outcomes
Change in mean CD4 cell count (immunological response).
Treatment failure. We defined this variable as the proportion of participants with incomplete viral load suppression or who experienced a virological rebound in the time frame reported by the trial authors.
Prevention of sexual transmission of HIV. We defined this as the risk of sexual partners not acquiring HIV from the study participant.
Development of ART drug resistance. We defined this as the acquisition of major genotypic resistance mutations as reported by the trial authors.
Individual adverse events
Search methods for identification of studies
We performed the literature searches with the assistance of the HIV/AIDS Review Group Information Specialist. We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
Our initial search included the following electronic databases.
MEDLINE from 1996 to 12 August 2016 (Appendix 1).
Embase from 1996 to 12 August 2016 (Appendix 2).
The Cochrane Central Register of Controlled Trials (CENTRAL) from 1996 to 12 August 2016 (Appendix 3).
National Library of Medicine (NLM) Gateway from 1996 to 2009 (Appendix 4).
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for ongoing trials (inception to 12 August 2016).
LILACS (Latin American and Caribbean Health Sciences Literature) from 1996 to 12 August 2016.
Web of Science from 1996 to 12 August 2016.
The search strategy included text terms such as efavirenz, EFV, EFZ , Sustiva, Stocrin, nevirapine, NVP, Viramune, Nevimune, non‐nucleoside reverse transcriptase inhibitor, NNRTI, protease inhibitor‐sparing, non‐protease inhibitor‐ containing.
Searching other resources
We handsearched the reference lists of all pertinent reviews and studies found. We contacted research organizations and experts in the field for unpublished and ongoing studies. We conducted literature searches from 1996 to 2016 the years during which NNRTIs have been approved and been available on the market.
Limits
We performed the literature searches without limits to language or setting. The searches were limited to human studies published from 1996 (start of the triple‐drug combination ART era) to the present.
Inclusion criteria
RCTs
Trials evaluating first‐line ART regimens that compared EFV to NVP as part of a three‐drug treatment regimen.
Trials that provided sufficient regimen‐specific information (dosage, presentation, NRTI combination drugs) about first‐line drugs to compare regimens and outcomes of interest.
Exclusion criteria
Non‐RCTs.
Studies evaluating first‐line single or double antiretroviral regimens.
Studies evaluating first‐line ART with more than three antiretroviral drugs.
Data collection and analysis
Selection of studies
At least two review authors screened all identified citations from the literature search results by title/abstract to identify articles for inclusion in the review (LM, SM, JI, AS, NS). We retrieved the full‐text articles of citations that potentially met the inclusion criteria of the review. We assessed the articles for inclusion based on study design, types of participants, interventions, and outcome measures. We resolved any disagreements by discussion or by consulting a third review author. If we were unable to resolve disagreements because we required further information, we allocated the study to the list of studies awaiting classification. We listed all excluded studies and the reasons for exclusion in a 'Characteristics of excluded studies' table. In addition, we constructed a PRISMA flow diagram to illustrate the study selection process (Figure 1).
1.
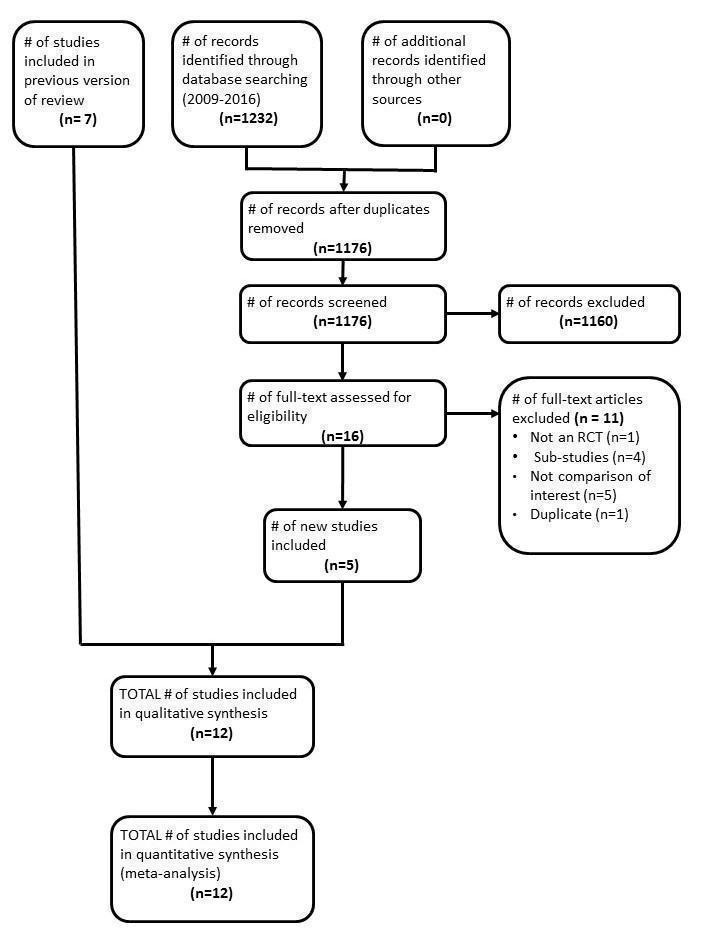
Flow diagram of study screening and selection
Data extraction and management
We designed and tested a data extraction form. Two review authors independently extracted data from each included trial using the data extraction form (AS, LM, SM). Both review authors verified the extracted data, which included methods, participant characteristics, interventions, and outcomes. Both review authors then compared the extracted data and resolved any discrepancies by discussion. In the event that the review authors disagreed on the abstraction of study details, we contacted a third review author to resolve the disagreement. We attempted to contact the principal investigators of the included trials in the case of any missing data or if we required clarification about the included trials.
Assessment of risk of bias in included studies
We assessed the risk of bias by the following criteria.
Sequence generation: how the allocation sequence was generated and whether it was adequate.
Allocation concealment: how the allocation sequence was concealed and whether it was adequate.
Blinding of participants, personnel, and outcome assessors.
The description of the completeness of outcome data for each main outcome.
Selective outcome reporting.
Other potential sources of bias (for example, funding).
Baseline data reported.
We rated studies as being at either high, low, or unclear risk of bias. At least two review authors independently completed the 'Risk of bias' tables (see Appendix 5).
Measures of treatment effect
We used Review Manager (RevMan) 5 for statistical analyses (RevMan 2014). We presented the results with 95% confidence intervals (CIs). We calculated the risk ratio (RR) and the odds ratio (OR) for binary data, the weighted mean difference (WMD) for continuous data measured on the same scale, and the standardized mean difference (SMD) for continuous data measured on different scales.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
We analysed the data according to the intention‐to‐treat (ITT) principle, with participants analysed in the groups to which they were randomized. We did not make any assumptions regarding the outcomes of participants who were lost to follow‐up and we conducted complete case analyses. We also attempted to contact the trial authors for missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity by inspecting the forest plots for overlap in the confidence intervals (CIs) and by applying the Chi2 test of homogeneity (P < 0.10 was the threshold for statistical significance) and the I2 statistic, with values of less than 50% denoting moderate heterogeneity.
Assessment of reporting biases
We used funnel plots to explore publication bias.
Data synthesis
We analysed the data using RevMan 5 (RevMan 2014). We used a random‐effect model to account for heterogeneity.
Subgroup analysis and investigation of heterogeneity
First we assessed the included trials for clinical heterogeneity. If we found that trials were similar enough to combine, we performed a meta‐analysis and assessed statistical heterogeneity. If there was significant unexplained statistical heterogeneity, we conducted a meta‐analysis using a random‐effects model.
If there was clinical heterogeneity and the data were available, we planned to explore this using the following subgroup analyses: age (children/adolescents/adults), sex (male/ female), baseline CD4 count, dosage, concurrent illness (hepatitis, tuberculosis) and study design. The efficacy of NVP may be associated with dosage (Veldkamp 2001).
For the purposes of this Cochrane Review, undetectable plasma HIV RNA (viral load) served as the primary endpoint. For the meta‐analyses, we defined an undetectable viral load as less than 500 copies/mL cut‐off, in order to include as many trials as possible.
Sensitivity analysis
We pooled the results from the included trials to determine the RR of achieving undetectable viral load. We planned to perform a sensitivity analysis to evaluate bias introduced by variability in study design, threshold of undetectable viral load, and specification of the two NRTIs. Finally, we conducted a test for homogeneity to ensure that the differences among the results of each trial could be expected by chance. We also performed a sensitivity analysis for studies with a high risk of bias.
When interventions and study populations were sufficiently similar across different studies, we pooled the outcomes and examined the differences between the two models using both fixed‐effect and random‐effects models. Since there were no significant differences between the two models, we presented the final results using a random‐effects model.
Quality of the evidence
We assessed the quality of the body of evidence using the GRADE approach (Guyatt 2008), which defines the quality of evidence for each outcome as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest (Higgins 2008). The quality rating across studies has four levels: high, moderate, low, or very low. RCTs are categorized as high quality but can be downgraded; similarly, other types of controlled trials and observational studies are categorized as low quality but can be upgraded. Factors that decrease the quality of evidence include limitations in design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results, or high probability of publication bias. Factors that can increase the quality level of a body of evidence include having a large magnitude of effect, whether plausible confounding would reduce a demonstrated effect, and if there is a dose‐response gradient. We used GRADEpro Guideline Development Tool (GDT) to construct 'Summary of findings' tables (GRADEpro 2014).
Results
Description of studies
Results of the search
We conducted the literature searches up to 12 August 2016, which yielded an additional 1232 titles. Three review authors (LM, AS, and GR) independently screened the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches to identify potentially relevant studies. We discarded reports that were irrelevant to this Cochrane Review, and we obtained the full‐text articles of all potentially relevant or uncertain reports. Three review authors (LM, AS and GR) independently assessed the full‐text articles. A fourth review author, NS, acted as arbiter where there was disagreement. The review authors LM, AS, GR, and JI independently extracted data from trials that met the inclusion criteria. Finally, where resolution was not possible because we required further information, we assigned the study to the list of those awaiting classification. We attempted to contact the trial authors for further clarification of data. We had previously identified seven randomized trials as meeting inclusion criteria. From the updated searches, we screened 1176 articles for eligibility after removal of duplicates. Agreement on screening for full text appraisal in this update was moderate (Ƙ 0.52; 95% CI 0.26 to 0.76; P < 0.001). We selected 16 full‐text articles for detailed appraisal, of which we identified six new studies. Agreement on inclusion/exclusion in this update was very good (Ƙ 0.86; 85% CI 0.59 to 1.00; P < 0.001) One of these was the full text publication of a study previously included as an abstract (Swaminathan 2011), so only five trials were newly published studies, giving a total of 12 studies. We have presented a PRISMA diagram, which illustrates the study selection process, in Figure 1.
Included studies
Twelve trials met the inclusion criteria of this Cochrane Review. We included seven RCTs in Mbuagbaw 2010, the previous version of this review (Ayala Gaytán 2004; Manosuthi 2009a; Núñez 2002; Sow 2006; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). In this review update five articles that reported on five trials met the inclusion criteria (Bonnet 2013a; Landman 2014; Mateelli 2013; Sinha 2013; Wester 2010). The findings reported here are from published papers.
See the 'Characteristics of included studies' table.
Locations
One trial was a multinational trial that included 17 countries (van Leth 2004). Five trials were conducted in Africa: one in Botswana (Wester 2010), one in Mozambique (Bonnet 2013a), one in Burkina Faso (Mateelli 2013), one in Senegal (Sow 2006), and one in both Senegal and Cameroon (Landman 2014). There were two trials from India (Sinha 2013; Swaminathan 2011) and one trial each: from Mexico (Bonnet 2013a), Spain (Núñez 2002), Thailand (Manosuthi 2009a), and the USA (van den Berg‐Wolf 2008).
Interventions
All included trials used EFV 600 mg and compared it to either NVP 400 mg once daily (Núñez 2002; Swaminathan 2011; van Leth 2004), or NVP 200 mg twice daily (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Mateelli 2013; Sinha 2013; Sow 2006; van den Berg‐Wolf 2008; van Leth 2004; Wester 2010). The 2NN trial, van Leth 2004, had trial arms that used NVP 400 mg once daily and NVP 200 mg twice daily.
Outcomes
Ten trials reported virological success (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Sinha 2013; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). Eight trials reported mortality (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Sinha 2013; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). Five trials reported progression to AIDS (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; van den Berg‐Wolf 2008; van Leth 2004). Eight trials reported adverse events (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Núñez 2002; Sinha 2013; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). Nine trials reported a discontinuation rate (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Núñez 2002; Sinha 2013; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). Nine trials reported change in CD4 count (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Sow 2006; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). Five trials reported treatment failure (Landman 2014; Núñez 2002; Sinha 2013; Swaminathan 2011; van Leth 2004), and four reported development of drug resistance (Bonnet 2013a; Landman 2014; van den Berg‐Wolf 2008; Wester 2010). None of the included trials reported on sexual transmission of HIV.
Co‐morbidities
In five included trials, the participants were concurrently receiving treatment for tuberculosis (Bonnet 2013a; Manosuthi 2009a; Mateelli 2013; Sinha 2013; Swaminathan 2011). Only one trial reported baseline co‐infection with hepatitis B and C virus (van Leth 2004).
Length of follow‐up
The shortest length of follow‐up was 24 weeks (Swaminathan 2011), and the longest was 156 weeks (Wester 2010). Five trials ran for 48 weeks (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Mateelli 2013; van Leth 2004), two trials for 96 weeks (Landman 2014; Sinha 2013), one for 36 weeks (Sinha 2013), and one for 72 weeks (Sow 2006).
We have provided further details on the included studies in an additional table (Table 3).
1. Additional characteristics of included studies.
| Trial ID | Location | NVP dosage | NRTI combination drugs | Co‐infection with tuberculosis | Virological success cut‐off point |
| Ayala Gaytán 2004 | Mexico | 200 mg twice daily | AZT 300 mg and 3TC 150 mg | No | < 400 copies/mL |
| Bonnet 2013a | Mozambique | 200 mg twice daily | 3TC and d4T/AZT1 | No | < 50 copies/mL |
| Landman 2014 | Cameroon and Senegal | 200 mg twice daily | TDF 300 mg and FTC 200 mg | No | < 50 copies/mL |
| Manosuthi 2009a | Thailand | 400 mg once daily | 3TC 150 mg and D4T 30 or 40 mg | Yes | < 50 copies/mL |
| Mateelli 2013 | Burkina Faso | 200 mg twice daily | D4T and 3TC1 | Yes | Not reported |
| Núñez 2002 | Spain | 400 mg once daily | D4T 40 mg and DDI 400 mg | No | < 50 copies/mL |
| Sinha 2013 | India | 200 mg twice daily | AZT, d4T, 3TC1 | Yes | < 400 copies/mL |
| Sow 2006 | Senegal | 200 mg twice daily | AZT 300 mg and 3TC 150 mg | No | Not reported |
| Swaminathan 2011 | India | 400 mg once daily | DDI 250 mg or 400 mg and 3TC 300 mg | Yes | Not reported |
| van den Berg‐Wolf 2008 | USA | 200 mg twice daily | ABC/3TC, DDI/d4T, AZT/3TC, d4T/3TC1 | No | < 50 copies/mL |
| van Leth 2004 | North/South America, Australia, Europe, South Africa, and Thailand | 200 mg twice daily and 400 mg twice daily | D4T 40 mg and 3TC 150 mg | No | < 50 copies/mL |
| Wester 2010 | Botswana | Not reported | AZT or d4T/3TC or DDI1 | No | Not reported |
1Dosage not specified. Abbreviations: NRTI: Nucleoside Reverse Transcriptase Inhibitor; ART: antiretroviral therapy; AZT: zidovudine; d4T: stavudine; 3TC: lamivudine; NVP: nevirapine; DDI: didanosine; ABC: abacavir; FTC: Emtricitabine; TDF: Tenofovir.
Excluded studies
We have provided our reasons for excluding 26 potentially relevant studies in the 'Characteristics of excluded studies' table. In this update, 11/26 studies were excluded: 5 did not have the comparison of interest (Antela 2004; He 2011; Musiime 2012; PENPACT 2011; Prendergast 2011), 4 were sub‐studies of already included studies (Bonnet 2013b; Mankhatitham 2011; Mankhatitham 2012; Padmapriyadarsini 2013), 1 was not an RCT(Puthanakit 2009b) and another was a duplicate (Swaminathan 2009).
Risk of bias in included studies
We assessed the risk of bias in each included study using the Cochrane 'Risk of bias' assessment tool (Appendix 5). We assessed the risk of bias in individual trials across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases. See the 'Risk of bias' summary (Figure 2) and 'Risk of bias' graph (Figure 3).
2.
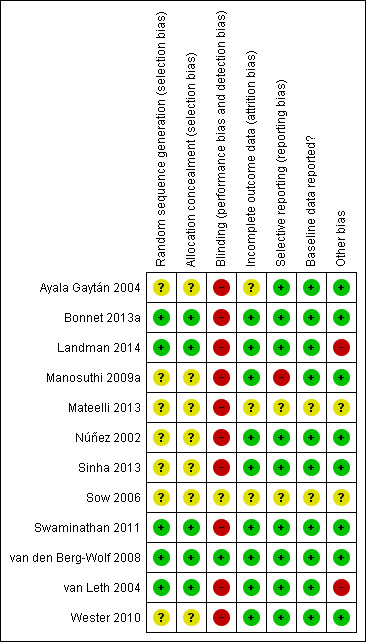
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
3.
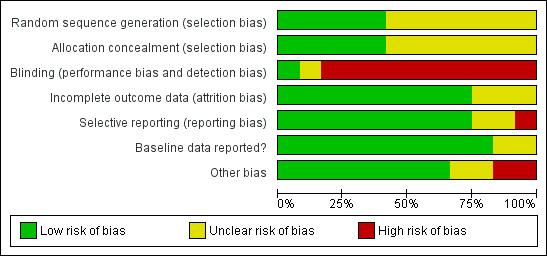
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Generation of allocation sequence
Only five trials reported how the allocation sequence was generated. (Bonnet 2013a; Landman 2014; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). The other included trials did not report how they generated the allocation sequence (Ayala Gaytán 2004; Sinha 2013; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Sow 2006; Wester 2010).
Allocation concealment
Only five trials reported that allocation was concealed (Bonnet 2013a; Landman 2014; Swaminathan 2011; van den Berg‐Wolf 2008; van Leth 2004). In seven trials, allocation concealment was unclear (Ayala Gaytán 2004; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Sinha 2013; Sow 2006; Wester 2010).
Blinding
Ten trials were reported as open‐label studies and we judged them to be at high risk of bias (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Mateelli 2013; Núñez 2002; Sinha 2013; Swaminathan 2011; van Leth 2004; Wester 2010; Manosuthi 2009a). Only one was blinded (van den Berg‐Wolf 2008). Sow 2006 did not report blinding and therefore we considered it to be at unclear risk of bias.
Incomplete outcome data
We judged three trials as having unclear risk of attrition bias (Ayala Gaytán 2004; Mateelli 2013; Sow 2006). The other nine included trials were at low risk of bias.
Selective reporting
One trial did not report all outcomes (Manosuthi 2009a). Two studies did not provide sufficient information to enable us to make a judgement (Mateelli 2013; Sow 2006).
Reporting of baseline data
Two trials, Sow (Sow 2006) and Mateelli (Mateelli 2013,) did not report baseline data, and we considered them as being at unclear risk of bias.
Other potential sources of bias
Funding
Eight trials received funding from governmental sources (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Núñez 2002; Sinha 2013; Swaminathan 2011; van den Berg‐Wolf 2008; Wester 2010) and were judged as low risk of bias. The 2NN study, van Leth 2004, was funded by Boehringer‐Ingelheim. Landman 2014 also received funding from Gilead Sciences, Merck, Sharp & Dome, and Abbott Laboratories. We judged them as high risk of bias. Mateelli 2013 and Sow 2006 did not report any source of funding. We judged them as unclear risk of bias.
Publication bias
We designed our search strategy to detect both published and unpublished studies. We appraised publication bias for our primary outcome of virologic suppression using a funnel plot and found no evidence of publication bias (Egger 1997). See Figure 4.
4.
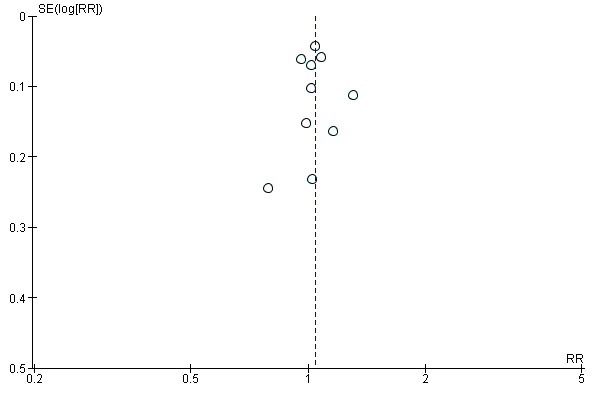
Funnel plot of comparison: 1 Efavirenz 600 mg versus Nevirapine all doses, outcome: 1.1 Virological success.
Effects of interventions
Summary of findings for the main comparison. 'Summary of findings' table 1.
| Efavirenz (600 mg) versus nevirapine (all doses) for three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïveindividuals | |||||
| Patient or population: antiretroviral‐naïve individuals Setting: all settings Intervention: efavirenz 600 mg Comparison: nevirapine all doses (400 mg once daily and 400 mg twice daily) as part of a three‐drug combination therapy with two NRTIs | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (RCTs) | Quality of the evidence (GRADE) | |
| Risk with nevirapine all doses | Risk with efavirenz 600 mg | ||||
| Virological success | 688 per 1000 | 715 per 1000 (681 to 750) | RR 1.04 (0.99 to 1.09) | 2438 (10 RCTs) | ⊕⊕⊕⊕ high1,2,3 |
| Mortality | 64 per 1000 | 54 per 1000 (38 to 76) | RR 0.84 (0.59 to 1.19) | 2317 (8 RCTs) | ⊕⊕⊕⊝ moderate4,5 |
| Progression to AIDS | 41 per 1000 | 50 per 1000 (29 to 86) | RR 1.23 (0.72 to 2.11) | 2005 (5 RCTs) | ⊕⊕⊕⊝ moderate5,6 |
| All severe adverse events | 192 per 1000 | 216 per 1000 (162 to 285) | RR 0.91 (0.71 to 1.18) | 2329 (8 RCTs) | ⊕⊝⊝⊝ very low5,7,8 |
| Discontinuation rate | 176 per 1000 | 164 per 1000 (122 to 220) | RR 0.93 (0.69 to 1.25) | 2384 (9 RCTs) | ⊕⊕⊕⊝ moderate5 |
| Change in CD4 count | The mean change in CD4 count was 0 | MD 3.03 lower (17.41 lower to 11.35 higher) | — | 1829 (9 RCTs) | ⊕⊕⊕⊝ moderate5,9,10,11 |
| Treatment failure | 249 per 1000 | 242 per 1000 (189 to 309) | RR 0.97 (0.76 to 1.24) | 737 (5 RCTs) | ⊕⊕⊝⊝ low12,13 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio; RCT: randomized controlled trial; GRADE: Grading of Recommendations Assessment, Development and Evaluation; MD: mean difference. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1Three trials used a cut‐off point of 400 copies/mL (Ayala Gaytán 2004; Swaminathan 2011; Sinha 2013), but we did not downgrade for this. 2Nine trials were open‐label (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Sinha 2013; Swaminathan 2011; van Leth 2004), but we did not downgrade for this. 3Two trials were industry‐funded (Landman 2014; van Leth 2004), but we did not downgrade for this. 4Seven trials were open‐label but we did not downgrade for this (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Sinha 2013; Swaminathan 2011; van Leth 2004). 5We downgraded by 1 for imprecision due to wide CIs including appreciable harm or benefit. 6Four trials were open‐labelled but we did not downgrade for this (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; van Leth 2004) 7Six trials were open‐label (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Núñez 2002; Swaminathan 2011; van Leth 2004). We downgraded by 1 for this. 8Trials did not report the same adverse events and used different severity scales. We downgraded by 1 for this. 9Seven of the trials were open‐label but we did not downgrade for this (Ayala Gaytán 2004; Bonnet 2013a; Manosuthi 2009a; Mateelli 2013; Núñez 2002; Swaminathan 2011; van Leth 2004). 10In one trial, the risk of bias was unclear (Sow 2006). 11One trial had industry funding (van Leth 2004), but we did not downgrade for this. 12All trials were open‐labelled but we did not downgrade for this. 13Each included trial that reported this outcome defined treatment failure differently. We downgraded by 2.
Summary of findings 2. 'Summary of findings' table 2.
| Efavirenz (600 mg) versus nevirapine (all doses): adverse events for three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïveindividuals | |||||
| Patient or population: antiretroviral‐naïve individuals Setting: all settings Intervention: efavirenz 600 mg Comparison: nevirapine all doses (400 mg once daily and 400 mg twice daily) as part of a three‐drug combination therapy with two NRTIs | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (RCTs) | Quality of the evidence (GRADE) | |
| Risk with nevirapine all doses: adverse events | Risk with efavirenz 600 mg | ||||
| Severe adverse events: central nervous system | 2 per 1000 | 7 per 1000 (2 to 18) | RR 4.46 (1.65 to 12.03) | 2049 (6 RCTs) | ⊕⊕⊕⊝ moderate1 |
| Severe adverse events: gastrointestinal | 18 per 1000 | 14 per 1000 (9 to 22) | RR 0.76 (0.48 to 1.21) | 2049 (6 RCTs) | ⊕⊕⊝⊝ low1,2 |
| Severe adverse events: pyrexia | 0 per 1000 | 0 per 1000 (0 to 0) | RR 0.65 (0.15 to 2.73) | 1799 (3 RCTs) | ⊕⊕⊝⊝ low2,3 |
| Severe adverse events: raised transaminases | 257 per 1000 | 134 per 1000 (90 to 201) | RR 0.52 (0.35 to 0.78) | 1299 (3 RCTs) | ⊕⊕⊕⊕ high4 |
| Severe adverse events: raised alkaline phosphatase | 12 per 1000 | 7 per 1000 (2 to 29) | RR 0.65 (0.17 to 2.50) | 1007 (1 RCT) | ⊕⊕⊝⊝ low2,5 |
| Severe adverse events: raised amylase | 14 per 1000 | 20 per 1000 (10 to 38) | RR 1.40 (0.72 to 2.73) | 1071 (2 RCTs) | ⊕⊕⊝⊝ low2,6,7 |
| Severe adverse events: raised triglycerides | 7 per 1000 | 7 per 1000 (3 to 21) | RR 1.10 (0.39 to 3.13) | 1071 (2 RCTs) | ⊕⊕⊝⊝ low2,6,7 |
| Severe adverse events: neutropenia | 38 per 1000 | 18 per 1000 (11 to 31) | RR 0.48 (0.28 to 0.82) | 1799 (3 RCTs) | ⊕⊕⊕⊕ high3,8 |
| Severe adverse events: rash | 229 per 1000 | 133 per 1000 (78 to 229) | RR 0.58 (0.34 to 1.00) | 2277 (7 RCTs) | ⊕⊕⊕⊝ moderate2,9 |
| Severe adverse events: serum glutamic oxaloacetic transaminase (SGOT) | The mean severe adverse events: SGOT was 0 | MD 3.3 higher (2.06 lower to 8.66 higher) | — | 135 (1 RCT) | ⊕⊕⊕⊝ moderate2 |
| Severe adverse events: serum glutamic‐ pyruvic transaminase (SGPT) | The mean severe adverse events: SGPT was 0 | MD 5.7 higher (4.23 lower to 15.63 higher) | — | 135 (1 RCT) | ⊕⊕⊕⊝ moderate2 |
| Raised cholesterol | 29 per 1000 | 172 per 1000 (21 to 1000) | RR 6.03 (0.75 to 48.78) | 64 (1 RCT) | ⊕⊕⊕⊝ moderate2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio; RCT: randomized controlled trial; GRADE: Grading of Recommendations Assessment, Development and Evaluation; MD: mean difference; SGOT: glutamic oxaloacetic transaminase; SGPT: serum glutamic‐ pyruvic transaminase | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1Five trials (Manosuthi 2009a; Núñez 2002; Swaminathan 2011; van Leth 2004; Wester 2010) were open‐label. One trial did not report blinding (Sow 2006). We downgraded by 1 for this. 2We downgraded by 1 for very wide CIs. 3All trials were open‐label. 4Two trials (Núñez 2002; van Leth 2004) were open label. van Leth 2004 was industry‐funded. We did not downgrade for this. 5Data from one open‐label industry‐funded study (van Leth 2004). We downgraded by 1 for this. 6Both trials were open‐label (Núñez 2002; van Leth 2004), but we did not downgrade for this. 7Most data came from one industry‐funded trial (van Leth 2004). We downgraded one point for this. 8We upgraded by 1 due to the large effect. 9Six studies were open‐label (Manosuthi 2009a; Núñez 2002; Sow 2006; Swaminathan 2011; van Leth 2004; Wester 2010). We did not downgrade for this.
All included trials compared EFV 600 mg once daily to NVP 200 mg twice daily or 400 mg once daily.
We performed a primary meta‐analysis to compare EFV 600 mg versus all dosages of NVP. We then conducted subgroup analyses to investigate the effect of NVP dosage (200 mg twice daily versus 400 mg once daily) and concurrent treatment for tuberculosis.
Efavirenz 600 mg versus nevirapine at any dosage
These results are summarized in 'Summary of findings' table 1 (Table 1).
Virological success
Virological success was comparable in both treatment groups (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.99 to 1.09; 10 trials, 2438 participants; P = 0.11; Analysis 1.1).
1.1. Analysis.
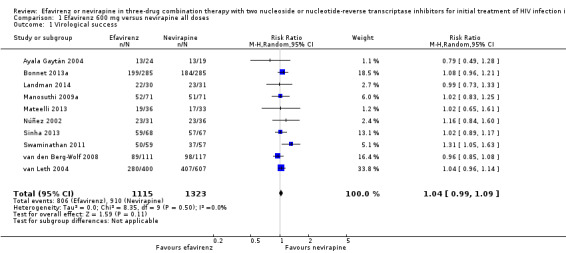
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 1 Virological success.
Mortality
There were no differences in mortality between the EFV‐ and NVP‐containing regimens (RR 0.84, 95% CI 0.59 to 1.19; 8 trials, 2317 participants; P = 0.32; Analysis 1.2).
1.2. Analysis.
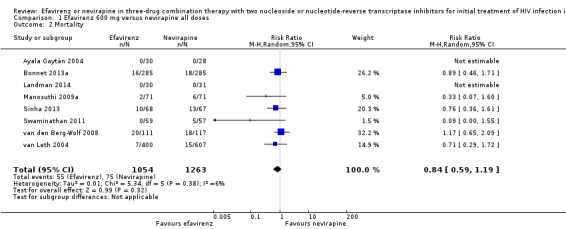
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 2 Mortality.
Progression to AIDS
In both EFV‐ and NVP‐containing regimens progression to AIDS was comparable (RR 1.23, 95% CI 0.72 to 2.11; 5 trials, 2005 participants; P = 0.46). Statistical heterogeneity was moderate (I2 statistic = 33%, P = 0.22; Analysis 1.3).
1.3. Analysis.
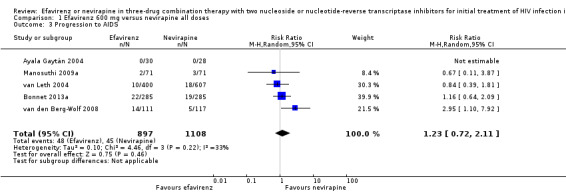
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 3 Progression to AIDS.
All severe adverse events
Severe adverse events were comparable in both treatment groups (RR 0.91, 95% CI 0.71 to 1.18; 8 trials, 2329 participants; P = 0.48). Statistical heterogeneity was moderate (I2 statistic = 43%, P = 0.11; Analysis 1.4).
1.4. Analysis.
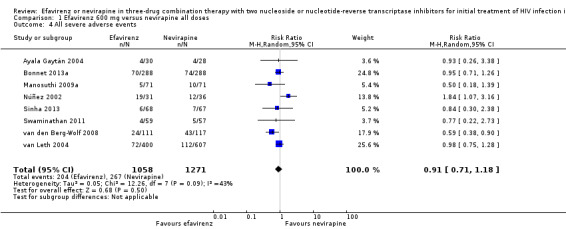
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 4 All severe adverse events.
Discontinuation rate
There was no difference in discontinuation rate between treatment groups (RR 0.93, 95% CI 0.69 to 1.25; 9 trials, 2384 participants; P = 0.62). Statistical heterogeneity was moderate (I2statistic = 35%, P = 0.14; Analysis 1.5).
1.5. Analysis.
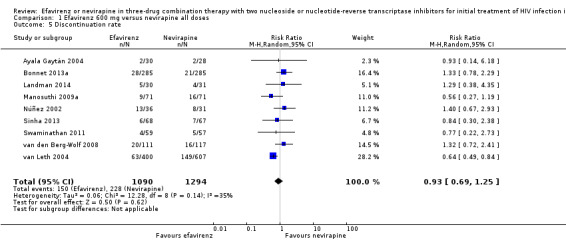
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 5 Discontinuation rate.
Change in CD4 count/immunological response
Change in CD4 count was comparable in both EFV‐ and NVP‐containing regimens (MD −3.03; 95% CI −17.41 to 11.35; 9 trials, 1829 participants; P = 0.68). Statistical heterogeneity was moderate (I2 statistic = 29%, P = 0.19; Analysis 1.6).
1.6. Analysis.
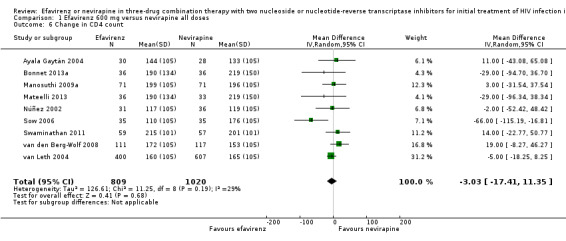
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 6 Change in CD4 count.
Treatment failure
Treatment failure was comparable in both treatment groups (RR 0.97, 95% CI 0.76 to 1.24; 5 trials, 737 participants; P = 0.82; Analysis 1.7).
1.7. Analysis.
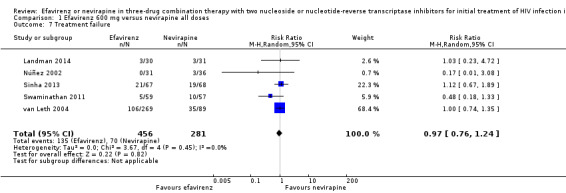
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 7 Treatment failure.
Sexual transmission of HIV
None of the included studies reported on this outcome.
Development of drug resistance
Four studies (988 participants) reported this outcome. Development of drug resistance was lower in the EFV arm (RR 0.76, 95% CI 0.60 to 0.95; 4 trials, 988 participants; P = 0.02; Analysis 1.8).
1.8. Analysis.
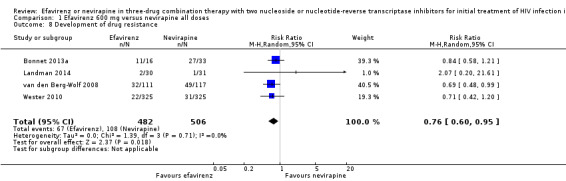
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 8 Development of drug resistance.
Individual adverse events
Individual comparisons for each of the adverse events: gastrointestinal (Analysis 2.2), pyrexia (Analysis 2.3), raised alkaline phosphatases (Analysis 2.5), elevated amylase (Analysis 2.6), elevated triglycerides (Analysis 2.7), elevated SGOT (Analysis 2.10), elevated SGPT (Analysis 2.11) and elevated cholesterol (Analysis 2.12) did not reveal any differences. Central nervous system adverse events were higher in the EFV arm (RR 4.46, 95% CI 1.65 to 12.03; 6 trials, 2049 participants; P = 0.003). Statistical heterogeneity was moderate (I2 statistic = 44%, P = 0.13; Analysis 2.1). Participants in the EFV arm were less likely to have raised transaminases than those in the NVP arm (RR 0.52, 95% CI 0.35 to 0.78; 3 trials, 1799 participants; P = 0.001; Analysis 2.4). Participants in the EFV arm were less likely to have neutropenia (RR 0.48, 95% CI 0.28 to 0.82; 3 trials, 1799 participants; P = 0.007; Analysis 2.8). Participants in the EFV arms were also less likely to have a rash (RR 0.58, 95% CI 0.34 to 1.00; 7 trials, 2277 participants; P = 0.05; Analysis 2.9). We have summarized the findings for the individual adverse events in 'Summary of findings' table 2 (Table 2).
2.2. Analysis.
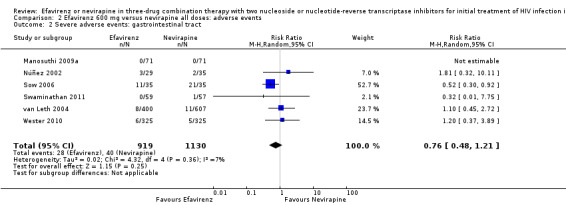
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 2 Severe adverse events: gastrointestinal tract.
2.3. Analysis.
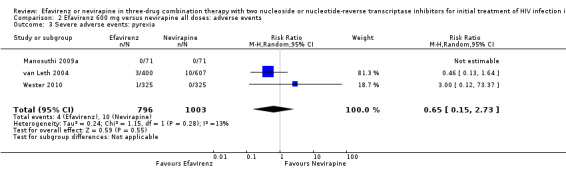
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 3 Severe adverse events: pyrexia.
2.5. Analysis.
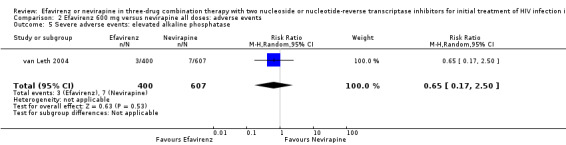
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 5 Severe adverse events: elevated alkaline phosphatase.
2.6. Analysis.
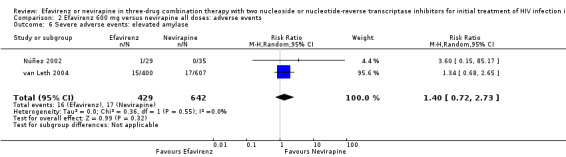
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 6 Severe adverse events: elevated amylase.
2.7. Analysis.
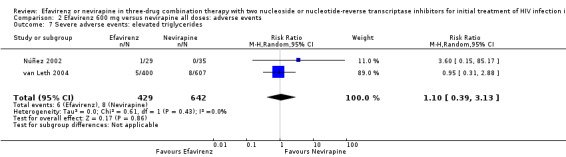
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 7 Severe adverse events: elevated triglycerides.
2.10. Analysis.
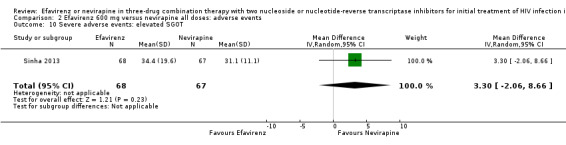
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 10 Severe adverse events: elevated SGOT.
2.11. Analysis.
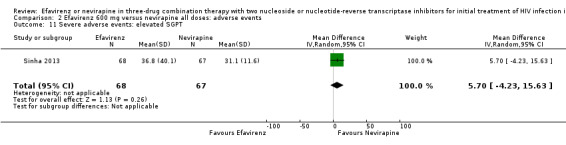
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 11 Severe adverse events: elevated SGPT.
2.12. Analysis.
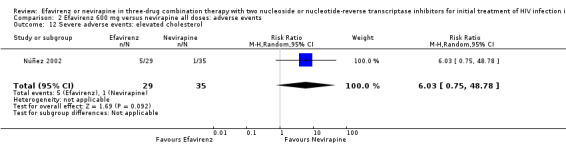
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 12 Severe adverse events: elevated cholesterol.
2.1. Analysis.
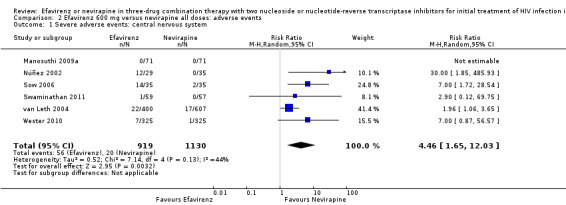
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 1 Severe adverse events: central nervous system.
2.4. Analysis.
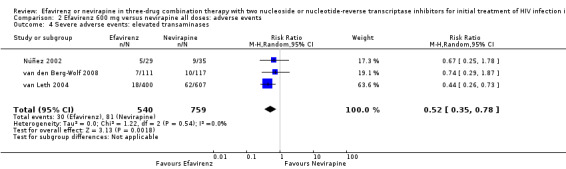
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 4 Severe adverse events: elevated transaminases.
2.8. Analysis.
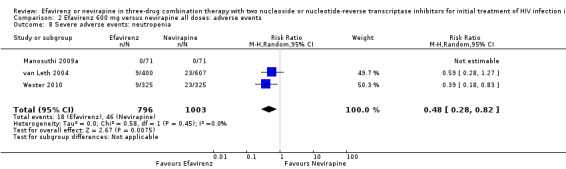
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 8 Severe adverse events: neutropenia.
2.9. Analysis.
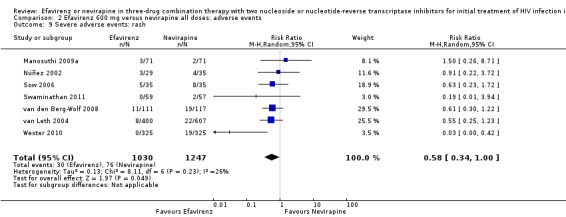
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 9 Severe adverse events: rash.
Subgroup analyses
Hepatitis co‐morbidity
There were insufficient data to explore this subgroup.
Concurrent treatment for tuberculosis
There were no significant subgroup effects for tuberculosis treatment: virological success (Analysis 3.1), mortality (Analysis 3.2), progression to AIDS (Analysis 3.3), discontinuation rate (Analysis 3.4).
3.1. Analysis.
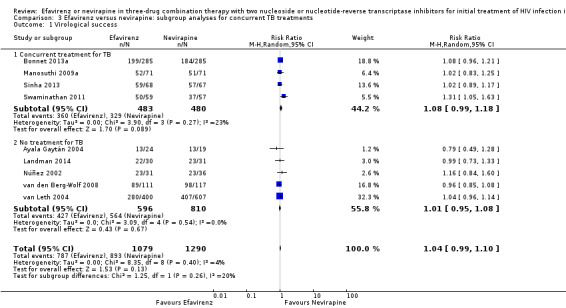
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 1 Virological success.
3.2. Analysis.
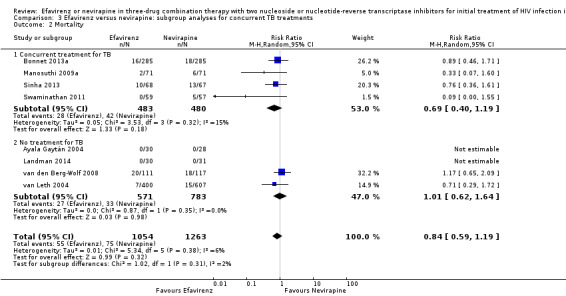
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 2 Mortality.
3.3. Analysis.
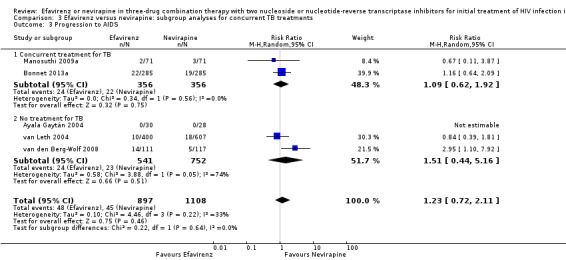
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 3 Progression to AIDS.
3.4. Analysis.
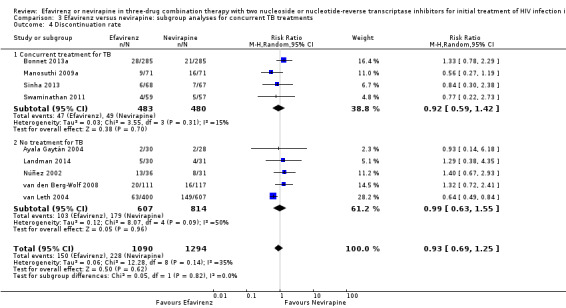
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 4 Discontinuation rate.
Dosage
We found that mortality was lower in the EFV arm than in the NVP 400 mg subgroup (RR 0.34, 95% CI 0.13 to 0.90; P = 0.03; Analysis 4.2). One study did not report the dosage of NVP used (Wester 2010), and we excluded it from this analysis. Virological success (Analysis 4.1), progression to AIDS (Analysis 4.3) and discontinuation rate (Analysis 4.4) were similar for both dosages.
4.2. Analysis.
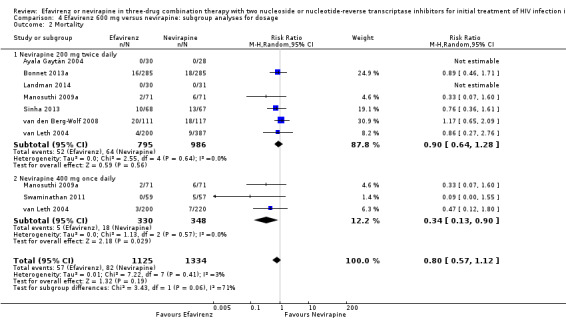
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 2 Mortality.
4.1. Analysis.
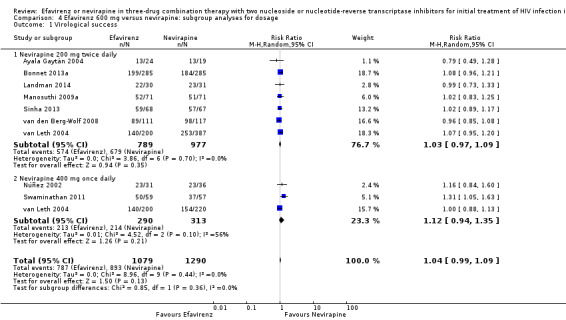
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 1 Virological success.
4.3. Analysis.
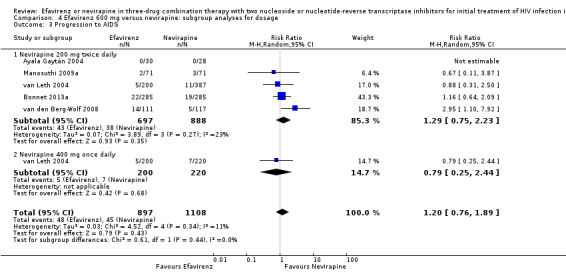
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 3 Progression to AIDS.
4.4. Analysis.
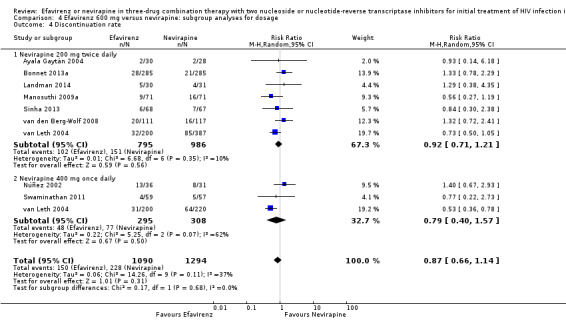
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 4 Discontinuation rate.
Discussion
Summary of main results
Twelve randomized controlled trials (RCTs) that included 3278 participants met the inclusion criteria of this Cochrane Review.
There was little or no difference between EFV and NVP in virological success (high quality evidence), probably little or no difference in mortality (moderate quality evidence) and progression to AIDS (moderate quality evidence). We are uncertain whether there is a difference in all severe adverse events (very low quality evidence). There is probably little or no difference in discontinuation rate (moderate quality evidence) and change in CD4 count (moderate quality evidence). There may be little or no difference in treatment failure (low quality evidence). Development of drug resistance is probably slightly less in the EFV arms (moderate quality evidence). No studies were found that looked at sexual transmission of HIV.
When we examined the adverse events individually, EFV probably increased impaired mental function (moderate quality evidence) but reduced elevated transaminases (high quality evidence), reduced cases of neutropenia (high quality evidence), and probably reduced cases of rash (moderate quality evidence). We found that there may be little or no difference in gastrointestinal adverse events (low quality evidence), pyrexia (low quality evidence), raised alkaline phosphatase (low quality evidence), raised amylase (low quality evidence) and raised triglycerides (low quality evidence). There was probably little or no difference in serum glutamic oxaloacetic transaminase (moderate quality evidence), serum glutamic‐ pyruvic transaminase (moderate quality evidence) and raised cholesterol (moderate quality evidence). NVP slightly increases mortality when given once daily (high quality evidence). There were little or no differences in the primary outcomes for patients who were concurrently receiving treatment for tuberculosis.
This literature is dominated by the landmark 2NN study, van Leth 2004, which found no difference between EFV and NVP in a non‐inferiority randomized open‐label, industry‐funded, four‐arm trial. Overall van Leth 2004 accounted for 1007 (31%) of the 3278 participants randomized.
We did not conduct subgroup analyses by NRTI combination drugs even though of the nine trials that had a NVP 200 mg twice daily arm (Ayala Gaytán 2004; Bonnet 2013a; Landman 2014; Manosuthi 2009a; Mateelli 2013; Sinha 2013; Sow 2006; van den Berg‐Wolf 2008; van Leth 2004); five used a 3TC/AZT (Ayala Gaytán 2004; Manosuthi 2009a; Sow 2006; van den Berg‐Wolf 2008; van Leth 2004), one trial used the 3TC/d4Tand switched to the 3TC/AZT backbone in the last year (Bonnet 2013a), and three trials (Sinha 2013; van den Berg‐Wolf 2008; Wester 2010) used at least two NRTI combinations including 3TC/AZT, 3TC/ABC, 3TC/d4T, or ddI/d4T. Moreover, all of the trials using NVP 400 mg once daily had different NRTI combination drugs. None of the included trials reported the outcome of sexual transmission of HIV. The length of follow‐up time, cut‐off point for undetectable viral load, dosage of NVP, and study settings varied greatly. We did not find any statistically significant heterogeneity for any of the key outcomes, and the I2 statistic value ranged from 0% to 40%.
Overall completeness and applicability of evidence
We identified literature that met the inclusion criteria of this Cochrane Review that clearly highlights the clinical equivalence of EFV and NVP based on RCTs.
This update includes studies from a wide variety of settings including a large multicentre trial with participants from the USA, Europe, Australia, Thailand, and South Africa (van Leth 2004), but also trials with participants from Mexico (Ayala Gaytán 2004), Senegal (Landman 2014; Sow 2006), Cameroon (Landman 2014), Thailand (Manosuthi 2009a), Spain (Núñez 2002), India (Sinha 2013; Swaminathan 2011), USA (van den Berg‐Wolf 2008), Mozambique (Bonnet 2013a) and Botswana (Mateelli 2013). These diverse populations support the applicability and generalizability of our findings.
Given optimal adherence, EFV, NVP 200 mg, and NVP 400 mg once daily may result in comparable virological suppression. However, there is a increased risk of mortality in the patients receiving the once‐daily NVP regimen. There is insufficient evidence to recommend the use of once daily NVP in regular clinical practice (Cooper 2007), and our findings do not support its use.
Quality of the evidence
This body of evidence includes twelve RCTs (3278 participants). The main methodological limitation in the included studies was the lack of blinding. Only one study was blinded (van den Berg‐Wolf 2008). In most instances, this did not affect our rating of the quality of evidence for outcomes unlikely to be affected by a lack of blinding such as virological success, mortality and progression to AIDS. In two studies reported as abstracts, risk of bias was unclear in almost all the domains (Mateelli 2013; Sow 2006). The cut‐off point used to define virological success also differed across studies, but this was related to the quality of the equipment available and did not seem to introduce any heterogeneity in measures of virological success. We did not downgraded for this. We downgraded when adverse events were graded using different scales, the definition of treatment failure varied across studies, industry funded studies contributed most of the data for certain outcomes and confidence intervals were too wide. Overall the quality of the evidence ranged from high to very low.
Potential biases in the review process
We minimized biases in the review process by not limiting the literature search by language, by performing a comprehensive search of databases and conference proceedings, and by contacting experts in the field for unpublished and ongoing studies. However, we were unable to fully appraise the trials published only as abstracts and is it unclear what methodological or data items were not captured in this review. We used a funnel plot and found no evidence of publication bias.
Agreements and disagreements with other studies or reviews
A systematic review that compared EFV to NVP in patients co‐infected with TB found superior virologic suppression in the EFV arm at the 400 copies/mL cut‐off point (but not at the 50 copies/mL cut‐off point). Mortality was comparable, but more participants in the NVP arm discontinued treatment (Jiang 2014). We found comparable effective of EFV and NVP (using all cut‐off points), comparable mortality and no differences in our subgroup analysis of participants on treatment for TB. Another systematic review found EFV to be less likely to lead to virological failure and more likely to induce virological success (Pillay 2013). A systematic review of adverse events found EFV to be less likely to be associated with hepatic and cutaneous adverse events, but more likely to be associated with central nervous system adverse events (Shubber 2013). The main difference between these systematic reviews and ours is their use of non‐randomized studies which may lead to differences in estimates. More so, the apparent poorer performance of NVP might also be induced by the once daily 400 mg regimen, which we found to be inferior to EFV with regard to mortality.
Authors' conclusions
Implications for practice.
EFV and NVP provide comparable levels of viral load suppression, but have different side‐effects. Clinicians need to determine which is the more appropriate for their patients by weighing other factors like availability, pill burden, cost, and concomitant medication. They must also consider individual tolerability and watch carefully for side‐effects, some of which can be fatal.
While subtle differences in risk of toxicity, discontinuation, and resistance may exist, we found that EFV and NVP have similar clinical efficacies. NVP given at the once daily dose of 400 mg led to higher mortality rates than EFV.
The use of NVP or EFV in paediatric populations has not been examined in randomized controlled trials (RCTs), and all inferences need to be drawn from trials conducted in adults.
Implications for research.
Although more trials would provide a more robust body of evidence, it is unlikely that additional trials will be conducted, at least in adults and adolescents. Prospective cohort studies are the most likely source of improved data on side effects, discontinuation, and development of resistance. One particular population of interest is women who have received single‐dose NVP for prevention of mother‐to‐child transmission of HIV, although the World Health Organization (WHO) no longer recommends it.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2016 | New citation required but conclusions have not changed | The review was updated with five new studies. |
| 18 November 2016 | New search has been performed | The review was updated with five new studies. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 6 January 2011 | Amended | We amended the sources of support. |
Acknowledgements
Dr Lawrence Mbuagbaw was awarded a Reviews for Africa Programme Fellowship, funded by a grant from the Nuffield Commonwealth Programme, through The Nuffield Foundation.
We acknowledge the assistance of the South African Cochrane Centre and the Cochrane HIV/AIDS Review Group Mentoring Programme. James Irlam provided mentorship to Lawrence Mbuagbaw for the first version of this review (Mbuagbaw 2010).
We gratefully acknowledge the contributions of Nancy Santesso and Holger Schünemann for their consultation and technical expertise with GRADEpro and GRADE tables presented in the prior versions of this review. We also thank Hacsi Horvath and Joy Oliver for their assistance with the literature searches. We thank Gail Kennedy, Dr Eliza Humphreys, Dr Larry Chang, and Dr Jamal Harris for their collaboration and support.
We also acknowledge the trial authors who shared their data.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this review do not necessarily reflect UK government policy.
Appendices
Appendix 1. MEDLINE search strategies
| Search | Most recent queries | Result |
| #11 | Search #8 AND #9 Limits: Publication Date from 1996 to 2009 | 645 |
| #10 | Search #8 AND #9 | 656 |
| #9 | Search NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING) | 13943 |
| #8 | Search #3 AND #4 AND #7 | 1744 |
| #7 | Search #5 OR #6 | 4073 |
| #6 | Search NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE | 2872 |
| #5 | Search EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ | 1794 |
| #4 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 3082417 |
| #3 | Search #1 OR #2 | 283409 |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | 97848 |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | 250716 |
| Search | Query | Items found |
| #9 | Search ((#3 AND #4 AND #7)) AND ("2009/05/01"[Date ‐ Publication] : "2014/02/07"[Date ‐ Publication]) | 236 |
| #8 | Search (#3 AND #4 AND #7) | 607 |
| #7 | Search (#5 AND #6) | 893 |
| #6 | Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | 4505 |
| #5 | Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | 2763 |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | 2838401 |
| #3 | Search (#1 AND #2) | 85427 |
| #2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab]))) | 135207 |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 307747 |
| Search | Query | Items found |
| #9 | Search (((#3 AND #4 AND #7))) AND ("2014/02/07"[Date ‐ Publication] : "2015/03/13"[Date ‐ Publication]) | 33 |
| #8 | Search (#3 AND #4 AND #7) | 675 |
| #7 | Search (#5 AND #6) | 997 |
| #6 | Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | 4901 |
| #5 | Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | 3120 |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | 3038686 |
| #3 | Search (#1 AND #2) | 91917 |
| #2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) | 145438 |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) | 324200 |
| Search | Query | Items found |
| #9 | Search (((#3 AND #4 AND #7))) AND ("2015/03/13"[Date ‐ Publication] : "2016/08/12"[Date ‐ Publication]) | 54 |
| #8 | Search (#3 AND #4 AND #7) | 767 |
| #7 | Search (#5 AND #6) | 1101 |
| #6 | Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | 5337 |
| #5 | Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | 3552 |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | 3326892 |
| #3 | Search (#1 AND #2) | 100806 |
| #2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) | 159959 |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) | 348268 |
Appendix 2. Embase search strategies
| No. | Query | Results | Date |
| #1 | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) | 292,932 | 22 May 2009 |
| #2 | ('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine') OR ('anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab) OR ('anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab) OR ('anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab) OR ('anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab) OR ('anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab) OR ('anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab) OR ('anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab) OR ('anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab) OR ('anti hiv':ti OR 'anti hiv':ab) OR (antiretrovir*:ti OR antiretrovir*:ab) OR ('anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab) OR (haart:ti OR haart:ab) OR ('aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab) OR (('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent')) OR (('antiretrovirus agent'/de OR 'antiretrovirus agent')) OR (('antivirus agent'/de OR 'antivirus agent')) OR (('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy')) | 92,810 | 22 May 2009 |
| #3 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/de OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/de OR 'randomised controlled trial'))))) | 865,259 | 22 May 2009 |
| #4 | #1 OR #2 | 321,839 | 22 May 2009 |
| #5 | ('efavirenz'/de OR 'efavirenz') OR ('sustiva'/de OR 'sustiva') OR ('stocrin'/de OR 'stocrin') OR efv OR efz | 7,680 | 22 May 2009 |
| #6 | ('nevirapine'/de OR 'nevirapine') OR nvp OR ('viramune'/de OR 'viramune') OR nevimune | 9,696 | 22 May 2009 |
| #7 | #5 OR #6 | 12,972 | 22 May 2009 |
| #8 | #3 AND #4 AND #7 | 1,088 | 22 May 2009 |
| #9 | ('nnrti'/de OR 'nnrti') OR 'non‐nucleoside reverse transcriptase inhibitor' OR 'non nucleoside reverse transcriptase inhibitor' OR ('nonnucleoside reverse transcriptase inhibitor'/de OR 'nonnucleoside reverse transcriptase inhibitor') OR 'non‐nucleoside reverse transcriptase inhibitors' OR 'non nucleoside reverse transcriptase inhibitors' OR 'nonnucleoside reverse transcriptase inhibitors' OR 'pi sparing' OR 'protease inhibitor sparing' OR 'protease‐inhibitor sparing' OR 'non‐protease inhibitor containing' OR 'non‐pi containing' OR 'non protease inhibitor containing' | 13,324 | 22 May 2009 |
| #10 | #8 AND #9 AND [1996‐2009]/py | 523 | 22 May 2009 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [embase]/lim AND [1‐5‐2009]/sd NOT [7‐2‐2014]/sd | 554 |
| #13 | #3 AND #9 AND #12 | 1115 |
| #12 | #10 AND #11 | 7336 |
| #11 | 'nevirapine'/de OR nevirapine OR 'viramune'/de OR viramune OR 'nevimune'/de OR nevimune OR nvp | 16047 |
| #10 | 'efavirenz'/de OR efavirenz OR 'sustiva'/de OR sustiva OR 'stocrin'/de OR stocrin OR efv | 13719 |
| #9 | #4 NOT #8 | 1551893 |
| #8 | #5 NOT #7 | 4886059 |
| #7 | #5 AND #6 | 1247730 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 14535586 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6133789 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1782714 |
| #3 | #1 AND #2 | 116833 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy':ab,ti | 165003 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 384563 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [7‐2‐2014]/sd NOT [13‐3‐2015]/sd | 85 |
| #13 | #3 AND #9 AND #12 | 796 |
| #12 | #10 AND #11 | 7647 |
| #11 | 'nevirapine'/de OR nevirapine:ab,ti OR 'viramune'/de OR viramune:ab,ti OR 'nevimune'/de OR nevimune:ab,ti OR nvp:ab,ti | 16066 |
| #10 | 'efavirenz'/de OR efavirenz:ab,ti OR 'sustiva'/de OR sustiva:ab,ti OR 'stocrin'/de OR stocrin:ab,ti OR efv:ab,ti | 14587 |
| #9 | #4 NOT #8 | 1438290 |
| #8 | #5 NOT #7 | 5079643 |
| #7 | #5 AND #6 | 1341085 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 15656505 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6420728 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1617084 |
| #3 | #1 AND #2 | 126453 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine' OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent' OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent' OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy' OR 'highly active antiretroviral therapy':ab,ti | 178964 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 410883 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [13‐3‐2015]/sd NOT [12‐8‐2016]/sd | 113 |
| #13 | #3 AND #9 AND #12 | 907 |
| #12 | #10 AND #11 | 8277 |
| #11 | 'nevirapine'/de OR nevirapine:ab,ti OR 'viramune'/de OR viramune:ab,ti OR 'nevimune'/de OR nevimune:ab,ti OR nvp:ab,ti | 17485 |
| #10 | 'efavirenz'/de OR efavirenz:ab,ti OR 'sustiva'/de OR sustiva:ab,ti OR 'stocrin'/de OR stocrin:ab,ti OR efv:ab,ti | 16217 |
| #9 | #4 NOT #8 | 1648816 |
| #8 | #5 NOT #7 | 5394456 |
| #7 | #5 AND #6 | 1491807 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 17407367 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6886263 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1847056 |
| #3 | #1 AND #2 | 140484 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy':ab,ti | 198729 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 448264 |
Appendix 3. CENTRAL search strategies
| ID | Search | Hits |
| #1 | HIV INFECTIONS explode all trees (MESH) OR HIV explode all trees (MeSH) OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME OR LYMPHOMA AIDS‐RELATED single term (MeSH) OR SEXUALLY TRANSMITTED DISEASES VIRAL single term (MeSH) | 8223 |
| #2 | ANTIRETROVIRAL THERAPY HIGHLY ACTIVE single term (MeSH) OR ANTI‐HIV AGENTS explode all trees (MeSH) OR ANTIVIRAL AGENTS single term (MeSH) OR AIDS VACCINES single term (MeSH) OR ANTI HIV OR ANTIRETROVIRAL* OR ANTI RETROVIRAL* OR AIDS VACCIN* | 3577 |
| #3 | (#1 OR #2) | 8432 |
| #4 | NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE | 315 |
| #5 | EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ | 227 |
| #6 | (#4 OR #5) | 487 |
| #7 | NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING) | 493 |
| #8 | (#3 AND #6 AND #7), from 1996 to 2009 | 146 |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 7543 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2495 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 12832 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 21 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 20 |
| #6 | #1 or #2 or #3 or #4 or #5 | 12913 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 961 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 2570 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3288 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 312 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 5678 |
| #12 | #7 or #8 or #9 or #10 or #11 | 8955 |
| #13 | #6 and #12 from 1980 to 2014, in Trials (Word variations have been searched) | 4943 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 562 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 523 |
| #16 | #14 and #15 | 121 |
| #17 | #13 and #16 from2009 to 2014, in Trials | 61 |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 8172 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2638 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 14285 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 21 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 23 |
| #6 | #1 or #2 or #3 or #4 or #5 | 14369 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 1056 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 2753 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3434 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 336 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 6380 |
| #12 | #7 or #8 or #9 or #10 or #11 | 9803 |
| #13 | #6 and #12 in Trials (Word variations have been searched) | 5538 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 633 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 634 |
| #16 | #14 and #15 | 148 |
| #17 | #13 and #16 Publication Year from 2014 to 2015, in Trials | 16 |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 8983 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2834 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 16377 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 23 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 25 |
| #6 | #1 or #2 or #3 or #4 or #5 | 16462 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 1164 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 3041 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3784 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 375 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 7445 |
| #12 | #7 or #8 or #9 or #10 or #11 | 11216 |
| #13 | #6 and #12 in Trials (Word variations have been searched) | 6006 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 767 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 859 |
| #16 | #14 and #15 | 226 |
| #17 | #13 and #16 Publication Year from 2015 to 2016, in Trials | 0 |
Appendix 4. NLM Gateway search strategy
| Search Number | Search | Items Found |
| #8 | Search: ((((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND ((EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE))) AND ((NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS)) OR ((NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING))) Limit: 1996:2009 | 260 |
| #7 | Search: (NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS)) OR ((NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING)) | 16334 |
| #6 | Search: (((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND ((EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE)) | 756 |
| #5 | Search: (EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE) | 6894 |
| #4 | Search: (((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) | 5030795 |
| #3 | Search: ((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw]))))) | 390683 |
| #2 | Search: (("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) | 122073 |
| #1 | Search: (((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL))) | 366953 |
Appendix 5. Cochrane 'Risk of bias' assessment tool
| Domain | Support for judgement | Review authors’ judgement |
| Selection bias. | ||
| Random sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Performance bias. | ||
| Blinding of participants and personnelAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Detection bias. | ||
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Attrition bias. | ||
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to amount, nature or handling of incomplete outcome data. |
| Reporting bias. | ||
| Selective reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other bias. | ||
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
Bias due to problems not covered elsewhere in the table. |
Data and analyses
Comparison 1. Efavirenz 600 mg versus nevirapine all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Virological success | 10 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 2 Mortality | 8 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 3 Progression to AIDS | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.72, 2.11] |
| 4 All severe adverse events | 8 | 2329 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 5 Discontinuation rate | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.25] |
| 6 Change in CD4 count | 9 | 1829 | Mean Difference (IV, Random, 95% CI) | ‐3.03 [‐17.41, 11.35] |
| 7 Treatment failure | 5 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 8 Development of drug resistance | 4 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.95] |
Comparison 2. Efavirenz 600 mg versus nevirapine all doses: adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe adverse events: central nervous system | 6 | 2049 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.65, 12.03] |
| 2 Severe adverse events: gastrointestinal tract | 6 | 2049 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.48, 1.21] |
| 3 Severe adverse events: pyrexia | 3 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.73] |
| 4 Severe adverse events: elevated transaminases | 3 | 1299 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.78] |
| 5 Severe adverse events: elevated alkaline phosphatase | 1 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.17, 2.50] |
| 6 Severe adverse events: elevated amylase | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.72, 2.73] |
| 7 Severe adverse events: elevated triglycerides | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.39, 3.13] |
| 8 Severe adverse events: neutropenia | 3 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.82] |
| 9 Severe adverse events: rash | 7 | 2277 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 10 Severe adverse events: elevated SGOT | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 3.30 [‐2.06, 8.66] |
| 11 Severe adverse events: elevated SGPT | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 5.70 [‐4.23, 15.63] |
| 12 Severe adverse events: elevated cholesterol | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [0.75, 48.78] |
Comparison 3. Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Virological success | 9 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.10] |
| 1.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.99, 1.18] |
| 1.2 No treatment for TB | 5 | 1406 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 2 Mortality | 8 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 2.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.19] |
| 2.2 No treatment for TB | 4 | 1354 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.62, 1.64] |
| 3 Progression to AIDS | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.72, 2.11] |
| 3.1 Concurrent treatment for TB | 2 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.62, 1.92] |
| 3.2 No treatment for TB | 3 | 1293 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.44, 5.16] |
| 4 Discontinuation rate | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.25] |
| 4.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.42] |
| 4.2 No treatment for TB | 5 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.63, 1.55] |
Comparison 4. Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Virological success | 9 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 1.1 Nevirapine 200 mg twice daily | 7 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.09] |
| 1.2 Nevirapine 400 mg once daily | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.35] |
| 2 Mortality | 8 | 2459 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 2.1 Nevirapine 200 mg twice daily | 7 | 1781 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.28] |
| 2.2 Nevirapine 400 mg once daily | 3 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.90] |
| 3 Progression to AIDS | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.76, 1.89] |
| 3.1 Nevirapine 200 mg twice daily | 5 | 1585 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.75, 2.23] |
| 3.2 Nevirapine 400 mg once daily | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.25, 2.44] |
| 4 Discontinuation rate | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.14] |
| 4.1 Nevirapine 200 mg twice daily | 7 | 1781 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.21] |
| 4.2 Nevirapine 400 mg once daily | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.57] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ayala Gaytán 2004.
| Methods | A prospective, open, randomized trial in the department of infectiology of the Hospital de Especialidades in Moterry, Nuevo Leon, Mexico. | |
| Participants | 58 participants. Inclusion criteria: at least 18 years old, of either gender, HIV‐positive, antiretroviral‐naïve. Exclusion criteria: patients with contraindications to either nevirapine (NVP) or efavirenz (EFV), pregnant women, diminished renal or liver functions. |
|
| Interventions | Zidovudine (AZT) 300 mg and Lamivudine (3TC) 150 mg with either NVP 200 mg twice daily (N = 28) or EFV 600 mg at night (N = 30). | |
| Outcomes | Viral load (< 400 copies/mL), CD4 count, adverse events, AIDS‐defining conditions, death. Follow‐up was for 48 weeks. | |
| Notes | All participants provided informed consent to participate in the study. Published in Spanish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on methods allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. The trial authors conducted intention‐to‐treat (ITT) analyses but loss to follow‐up was quite high and reasons for drop‐outs were not reported. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest. |
| Baseline data reported? | Low risk | The trial authors reported demographic characteristics, clinical stage, CD4 count, and viral load. |
| Other bias | Low risk | The Mexican Ministry of Health funded this trial (according to author communication). |
Bonnet 2013a.
| Methods | An open‐label non‐inferiority randomized trial in 3 trial sites in Maputo, Mozambique (Jose Macamo Hospital, Mavalane Hospital and Alto Mae Health Centre). | |
| Participants | 570 participants. Inclusion criteria: adults (> 18 years) , treatment naïve, treatment for tuberculosis for less then 4 weeks, Karnofsky score of 60% or more, CD4 count < 250 cells, negative pregnancy test, alanine aminotransferase(ALAT) and bilirubin less then 5 times upper limit of normal (ULN), absence of grade 4 clinical or biological adverse events. Exclusion criteria: not stated. |
|
| Interventions | 3TC + d4T + EFZ 600 mg (N = 285) versus 3TC + d4T + NVP 200 mg twice daily (N = 285) | |
| Outcomes | Virological success (< 50 copies/mL), change in CD4, mortality, progression to AIDS, discontinuation rate, adverse events | |
| Notes | All participants provided informed consent to participate in the study. This study was funded by the French Research Agency for HIV AIDS and hepatitis (ANRS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial randomly allocated participants to treatment. |
| Allocation concealment (selection bias) | Low risk | Central location randomization was conducted and communicated to site investigators. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were balanced between the trial groups, and the trial authors reported the reasons for losses to follow‐up. The trial authors used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest. |
| Baseline data reported? | Low risk | The trial authors reported demographic characteristics, clinical stage, CD4 count, and viral load. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Landman 2014.
| Methods | A multicenter, open‐label randomized trial conducted in 2 centres in Dakar, Senegal and Yaoundé, Cameroon. | |
| Participants | 120 participants. Inclusion criteria: adults (≥ 18 years in Senegal or ≥ 21 years in Cameroon), anti‐retroviral treatment naïve, and CD4+ T‐cell count > 50 cells/mm3 Exclusion criteria: ongoing opportunistic infection, serious disease, ongoing treatment with rifampin, severe renal or hepatic disorder, Cockcroft‐Gault calculated creatinine clearance ≤ 50 mL/min, hepatitis B surface antigen‐positive, haemoglobin < 8 g/dL, neutrophil count < 500 cells/mm3, pregnant, breastfeeding, treated with any contraindicated drugs. |
|
| Interventions | TDF/FTC 300/200 mg once daily and NVP 200 mg once daily for first 2 weeks and twice daily thereafter (N = 31) or TDF/FTC/EFV 300/200/600 mg once daily (N = 30) | |
| Outcomes | Virological efficacy (< 50 copies/mL), discontinuation rate, adherence rate, treatment failure, mortality, and adverse events | |
| Notes | Written informed consent was obtained from each participant. Trial number NCT00573001. Funding for the study was provided by the ANRS. Gilead Sciences, Merck Sgaro & Dhome, and Abbott Laboratories provided funding for some of the antiretroviral regimens. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial randomized participants to treatment. |
| Allocation concealment (selection bias) | Low risk | Through a centralized web site, participants were randomized to 1 of the 4 treatment groups at an allocation ratio of 1:1:1:1. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were balanced between groups, and the trial authors reported the reasons for dropouts. The trial authors used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes of interest. |
| Baseline data reported? | Low risk | The trial authors reported demographic characteristics, clinical stage, CD4 count, and viral load. |
| Other bias | High risk | Private funding. |
Manosuthi 2009a.
| Methods | Prospective open‐label randomized, comparative trial in Nonthaburi, Thailand from December 2006 to October 2007 | |
| Participants | Inclusion criteria: HIV‐1 infection in individuals aged 18 to 60 years; active TB diagnosed by clinical features plus acid‐fast stain or culture positive for M. tuberculosis, or both; receipt of treatment with a rifampicin‐ containing anti‐TB regimen 4 to 16 weeks before enrolment,naïve to ART; and CD4+ cell count, < 350 cells/mm3. Exclusion criteria: aspartate aminotransferase and alanine aminotransferase levels > 5 times the upper limit of normal range;serum creatinine level > 12 mg/dL; receipt of a medication that has drug‐drug interactions with nevirapine or efavirenz; receipt of immunosuppressive drugs; and pregnancy or lactation. |
|
| Interventions | Efavirenz 600 mg or nevirapine 200 mg twice daily with 3TC 150 mg/D4T 30 or 40 mg BID. Follow‐up was for 48 weeks. 142 participants with 71 in each trial arm. |
|
| Outcomes | Primary outcome: proportion of participants achieving a plasma HIV‐RNA level < 50 copies/mL after 48 weeks of ART. Secondary outcomes: proportion of participants with concentrations of NNRTI at 12 hours after dosing, lower than the recommended minimal level, CD4 cell count at week 48 of ART, incidence of NNRTI‐associated adverse reactions. |
|
| Notes | Written consent was obtained from the participants. This is also referred to as the N2R study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Primary, but not all secondary outcomes were reported. |
| Baseline data reported? | Low risk | The trial authors reported age, sex, body weight, body mass index, site of tuberculosis, time from tuberculosis diagnosis to initiation of ART, CD4 cell count, plasma HIV‐1 RNA level, haemoglobin concentration, serum alkaline phosphatase, alanine aminotransferase, albumin, creatinine, hepatitis B virus antigen, hepatitis C antibody, cholesterol, triglycerides. |
| Other bias | Low risk | Yes, this study was funded by the Thailand Ministry of Public Health, Thailand Research Fund, and Bamrasnaradura Infectious Diseases Institute. |
Mateelli 2013.
| Methods | A randomized, open‐label, parallel group study in Burkina Faso | |
| Participants | People with TB/HIV co‐infection | |
| Interventions | Stavudine (d4T) and lamivudine (3TC) with either EFV 600 mg once daily or NVP 200 mg twice daily | |
| Outcomes | The outcomes reported were: mean CD4 increase, viral success, TB treatment success. | |
| Notes | This study was reported in abstract form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report this. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report this. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial authors did not report this. |
| Selective reporting (reporting bias) | Unclear risk | The trial authors did not report all outcomes. There was no usable data on mortality. |
| Baseline data reported? | Unclear risk | The trial authors did not report baseline data. |
| Other bias | Unclear risk | It is unclear whether other sources of bias may exist. |
Núñez 2002.
| Methods | A randomized, open‐label, pilot study in Hospital Carlos III in Madrid Span from March 1999 to January 2002 | |
| Participants | Eligibility criteria: HIV‐infected antiretroviral‐naïve adults, aged above 18 years old with CD4 counts > 100 cells/mm3 and detectable plasma HIV RNA below 100,000 copies/mL, no major organ failure, use of standard of care prophylaxis for opportunistic infections, negative pregnancy test in women of child‐bearing age, and no current high alcohol intake or substance abuse. N = 67 (NVP = 36, EFV = 31). | |
| Interventions | d4T and ddI with either NVP or EFV at the following doses: NVP 400 mg once a day, d4T 40 mg twice a day, ddI 400 mg once a day, and EFV 600 mg once a day. Follow‐up was for 48 weeks. | |
| Outcomes | Primary: the proportion of individuals achieving plasma HIV RNA < 50 copies/mL and the proportion developing drug‐related toxicities, which caused cessation of the NNRTI. Secondary: mean changes in CD4+ lymphocyte counts, overall safety, degree of adherence, and adverse events. |
|
| Notes | All participants provided informed consent to participate in the trial. This trial is referred to as the SENC trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report this information. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no missing outcome data. Three participants were lost to follow‐up right after enrolment. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported primary and secondary outcomes. |
| Baseline data reported? | Low risk | The trial authors reported on age, gender, HIV transmission, plasma HIV RNA, absolute CD4 count, number of participants with AIDS, positive anti‐HCV antibody, positive HBsAg. |
| Other bias | Low risk | This trial was not funded by industry. It was funded by the Asociacíon Investigacíon y Educación en SIDA (AIES) and Comunidad Autónoma de Madrid. |
Sinha 2013.
| Methods | A randomized, open label, trial conducted at All India Institute of Medical Sciences, New Delhi, India. | |
| Participants | 142 participants Inclusion criteria: positive for HIV by ELISA, ART‐naïve and presenting with concomitant TB, CD4 count < 200 cells/mm3 and normal renal and hepatic function. Exclusion criteria: positive in hepatitis B and C serologies, taking antiepileptic drugs, immunosuppressant, and other drugs that induce liver microsomal enzyme systems, and pregnant. |
|
| Interventions | Zidovudine or Stavudine and Lamivudine/NVP once daily for the first 14 days and twice daily thereafter (200mg) or Zidovudine or Stavudine and Lamivudine/EFV (600 mg) daily. | |
| Outcomes | Virological response (< 400 copies/mL), discontinuation rate, treatment failure, mortality, and adverse events | |
| Notes | All participants gave signed informed consent to participate in this study. Funding was provided by the National AIDS Control Organization, Ministry of Health & Family Welfare, and the Government of India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report this information. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were balanced between treatment groups. The trial authors used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes of interest. |
| Baseline data reported? | Low risk | The trial authors reported demographic characteristics, clinical stage, CD4 count, viral load, and type of TB. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Sow 2006.
| Methods | A RCT to compare AZT+3TC+NVP versus AZT+3TC+EFV among 70 HIV‐infected patients in Senegal Age limits not given. |
|
| Participants | 70 ART treatment‐naïve patients from Senegal | |
| Interventions | AZT 300 mg, 3TC 150 mg and NVP 200 mg (N = 35) on one hand versus AZT 300 mg, 3TC 150 mg, and EFV 600 mg (N = 35) | |
| Outcomes | Decrease in viral burden, side‐effects and change in CD4 count. Follow‐up was for 76 weeks. | |
| Notes | This trial was reported in abstract form. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report this information. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report this information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial authors did not report this information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial authors did not report this information. |
| Selective reporting (reporting bias) | Unclear risk | The trial authors did not report this information. |
| Baseline data reported? | Unclear risk | The trial authors did not report this information. |
| Other bias | Unclear risk | The trial authors did not report this information. |
Swaminathan 2011.
| Methods | An open‐label, parallel arm, randomized controlled clinical trial conducted at 3 sites of the Tuberculosis Research Centre in Chennai, Vellore, and Madurai, located in southern India. | |
| Participants | 564 participants Inclusion criteria: People living with HIV who were at least 18 years of age with newly diagnosed TB, not pregnant and CD4+ cell counts < 250 cells/mm3. Exclusion criteria: previous ATT or ART for > 1month, HIV‐2 infection, major psychiatric illness, aspartate aminotransferase and alanine aminotransferase levels > 2.5 times the upper limit of normal and having a severe non‐HIV related disease. |
|
| Interventions | Didanosine (250/400 mg) + lamivudine (300 mg) + nevirapine (400mg after 14 days of 200 mg) or didanosine (250/400 mg) + lamivudine (300 mg) + efavirenz (600 mg) | |
| Outcomes | Change in CD4 count, discontinuation rate, adherence rate, treatment failure, mortality, and adverse events. | |
| Notes | Funded by the National AIDS Control Organization (New Delhi, India) and Indian Council of Medical Research (New Delhi, India). NCT00332306 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors performed permuted block randomisation. |
| Allocation concealment (selection bias) | Low risk | The trial conducted randomization centrally and statisticians prepared allocation codes in sealed and opaque envelopes for each site. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were balanced between groups and the trial authors reported reasons for losses to follow‐up. The trial authors used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest. |
| Baseline data reported? | Low risk | Demographic characteristics, CD4 count, and viral load. |
| Other bias | Low risk | We did not identify any other sources of bias. |
van den Berg‐Wolf 2008.
| Methods | The FIRST study randomized participants to 3 strategy arms, one of which was NNRTI+NRTI. NNRTI was determined by optional randomisation (NVP or EFV) or by choice. | |
| Participants | 228 antiretroviral‐naïve, HIV‐positive participants, aged at least 13 years. | |
| Interventions | There were 111 participants in the EFV arm (EFV 600 mg once daily) and 117 in the NVP arm (NVP 200 mg twice daily). We obtained information on dosing from the trial authors. They used 4 different NRTI combination drugs (ABC/3TC, ddI/d4T, AZT/3TC, d4T/3TC). | |
| Outcomes | HIV RNA > 50 copies/mL, change in CD4 count or death. Follow‐up was for 32 weeks. | |
| Notes | All participants provided informed consent to participate in the study aka FIRST or CPCRA study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial authors provided no information on methods of sequence generation |
| Allocation concealment (selection bias) | Low risk | Participants called a hotline to be assigned a treatment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes, in the FIRST paper (2001) the trial team was blinded to interm results so for this sub‐study we assumed they were blinded to treatment as well. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest. |
| Baseline data reported? | Low risk | Socio‐demographic data, CD4 count, viral load, prior AIDS event, hepatitis B or C and history of injection drug use. |
| Other bias | Low risk | This study was sponsored by non‐industry funding (NIH). |
van Leth 2004.
| Methods | Multicentre, open‐label, randomised trial of 1216 ARV naïve participants in North/South America, Australia, Europe, South Africa, and Thailand | |
| Participants | Inclusion criteria: ARV naïve participants of either sex, aged at least 16 years, with plasma RNA > 5000 copies per mL Exclusion criteria: pregnancy, lactation, HB < 6.3 mmol/L in males and 5.7 mmol/L in females, neutrophils < 1 x 109, platelets < 75 x 109, serum amylases > 2.0 times the upper limit of normal in combination with serum lipase < 1.5 times the upper limit of normal; aspartate aminotransferase < 5.0 times the upper limit of normal; or bilirubin < 2.5 times the upper limit of normal; history of clinical pancreatitis or neuropathy within the previous 6 months; renal failure necessitating dialysis; radiotherapy, cytotoxic, or immunomodulating treatment within the month preceding the start of study or the expected need for it; infection with HIV‐2; or likely non‐adherence as judged by the trial investigator. NVP once daily N = 220, NVP twice daily N = 387, EFV N = 400. |
|
| Interventions | Four arms; only 3 of interest: d4T 40 mg BID and 3TC 150 mg BID with either NVP 400 mg once daily, NVP 200 mg twice daily or EFV 600 mg once daily. Follow‐up for 48 weeks. | |
| Outcomes | Primary: proportion of participants with treatment failure Secondary: proportion of participants with virological failure (never having a plasma HIV‐1 RNA concentration < 50 copies/mL, or two consecutive measurements 50 copies/mL after having had a concentration below the cut‐off), the proportion of participants with plasma HIV‐1 RNA concentrations below 50 copies/mL at each study week; the change in CD4‐positive cells between the start of treatment and week 48; and the frequencies of clinical and laboratory adverse events. |
|
| Notes | Ethics: approved by the ethics review bodies in the participating countries, and all participants gave written informed consent. This study was industry funded (Boehringer‐Ingelheim) and is also known as the 2NN study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A treatment allocation sequence was generated by use of the minimisation variables CD4‐positive T‐cell count (350 vs >350 cells per μL) and study region. Treatment allocation was stratified by baseline plasma HIV‐1 RNA concentration (30 000 copies per mL vs >30 000 copies per mL)". |
| Allocation concealment (selection bias) | Low risk | The trial authors conducted centralized study allocation, which was concealed from the investigator. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "There was no masking after treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All analyses were done for the intention‐to‐treat population, including all randomised patients (n=1216)." |
| Selective reporting (reporting bias) | Low risk | The trial authors analyzed and reported all outcome measurements. |
| Baseline data reported? | Low risk | The trial authors reported on gender, age, body mass index, geographical region, HIV risk behaviour, CDC class C, CD4 cell count, HIV RNA, and co‐infection (hepatitis B, hepatitis C viruses). |
| Other bias | High risk | Some of the trial authors had received travel grants and honoraria from the sponsors. |
Wester 2010.
| Methods | Open‐label, randomized, factorial design study conducted at Princes Marina Hospital in Gaborone, Botswana. | |
| Participants | 898 participants
Inclusion criteria: haemoglobin value greater than 8.0 g/dL; absolute neutrophil count 1.0 x 103/μL or greater; aminotransferase levels less than 5 times the upper limit of normal; and for women of child‐bearing potential, a willingness to maintain active contraception throughout the duration of the study and a negative during pregnancy test within 14 days of study enrolment. Exclusion criteria: Karnofsky performance score (40 or below); an AIDS‐related malignancy other than mucocutaneous Kapsosi's sarcoma grade 2 or higher peripheral neuropathy; major psychiatric illness and for women, actively breastfeeding or less than 6 months postpartum. |
|
| Interventions | Zidovudine or stavudine/lamivudine or didanosine/nevirapine or Zidovudine or stavudine/lamivudine or didanosine/efavirenz | |
| Outcomes | Change in CD4 count, treatment failure (defined as > 5000 copies/mL and later as > 400 copies/mL i.e. undetectable plasma HIV RNA), mortality, and adverse events. | |
| Notes | Funding from the following research grant from the National Institute of Allergy and Infectious Diseases (NIAID), grant evaluating the risk factors for the Development of Lactic Acidosis and Pancreatitis Among HAART treated Adults in Botswana and Harvard Center for AIDS research grant evaluating the risk factors for the development of nevirapine‐associated toxicity in Southern Africa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors provided data on losses to follow‐up reasons and used ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes of interest. |
| Baseline data reported? | Low risk | The trial authors reported demographic characteristics, CD4 count, clinical stage, and HIV RNA. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Abbreviations: ITT: intention‐to‐treat; RCT: randomized controlled trial; NRTI: Nucleoside Reverse Transcriptase Inhibitor; ART: antiretroviral therapy; AZT: zidovudine; d4T: stavudine; 3TC: lamivudine; NVP: nevirapine; DDI: didanosine; ABC: abacavir; FTC: Emtricitabine; TDF: Tenofovir; ALAT: alanine aminotransferase; ULN: Upper Limit of Normal; HIV: human immune‐deficiency virus; RNA: Ribonucleic acid; NNRTI: non‐nucleoside reverse transcriptase inhibitors; SENC: Spanish efavirenz vs. nevirapine comparison; ELISA: Enzyme‐linked immunosorbent assay.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antela 2004 | Doesn't have the comparison of interest. |
| Bannister 2008 | Not a randomized clinical trial, but a retrospective cohort study from the EUROSIDA database. |
| Bonnet 2013b | A sub‐study of Bonnet 2013a, and not a randomized clinical trial. |
| Brück 2008 | Not a randomized clinical trial. |
| de Beaudrap 2008 | Not a randomized clinical trial, a retrospective cohort study from 'Initiative Senegalaise d'Acces aux Medicaments Antiretroviraux' (ISAARV) prospective cohort of which the EFV arm was a clinical trial. |
| Han 2005 | Not a randomized clinical trial. |
| Hartmann 2005a | Not a randomized clinical trial, but a prospective cohort study. |
| Hartmann 2005b | Not a randomized clinical trial, but a prospective cohort study. |
| He 2011 | Not a comparison of efavirenz (EFV) to nevirapine (NVP). |
| Lapphra 2008 | Not a randomized clinical trial, but a retrospective cohort study from medical records. |
| Manfredi 2004 | Not a randomized clinical trial, but an observational study. |
| Manfredi 2005 | Not a randomized clinical trial, but an observational study. |
| Manfredi 2006 | Not a randomized clinical trial, but an observational study. |
| Mankhatitham 2011 | A sub‐study of Manosuthi 2009a. |
| Mankhatitham 2012 | A sub‐study of Manosuthi 2009a. |
| Manosuthi 2004 | Not a randomized clinical trial, but a retrospective cohort study from medical records. |
| Manosuthi 2009b | A sub‐study of Manosuthi 2009a. |
| Musiime 2012 | Not a comparison of EFV to NVP. |
| Nachega 2008 | Not a randomized clinical trial, but a retrospective cohort study from the Aid for AIDS prospective database in southern Africa. |
| Negredo 2004 | Not a randomized clinical trial. |
| Padmapriyadarsini 2013 | A sub‐study of Swaminathan 2011. |
| PENPACT 2011 | Not a comparison of EFV to NVP. |
| Prendergast 2011 | Not a comparison of EFV to NVP. |
| Puthanakit 2009a | Not a randomized clinical trial, but a prospective cohort study. |
| Puthanakit 2009b | Not a randomized clinical trial, but a prospective cohort study. Data collected from 2 treatment cohorts. |
| Swaminathan 2009 | A duplicate of Swaminathan 2011 |
Abbreviations: EFV: efavirenz; NVP: nevirapine; ISAARV: Initiative Senegalaise d'Acces aux Medicaments Antiretroviraux' (ISAARV).
Differences between protocol and review
AIDSearch was no longer available at the time of the literature searches.
We searched the National Library of Medicine Gateway in 2009, but this was unavailable in 2016.
In the protocol we stated that we would include randomized controlled trials and observational cohorts (Mbuagbaw 2009). We did not include any observational cohorts in this review because we found sufficient evidence for randomized trials.
In the protocol, we defined undetectable viral load as less than 500 copies/mL in order to enable inclusion of as many trials as possible and 50 copies/mL limit of detection for subgroup analyses. Six included trials used the 50 copies/mL cut‐off (Bonnet 2013a; Landman 2014; Manosuthi 2009a; Núñez 2002; van den Berg‐Wolf 2008; van Leth 2004) and two trials used a cut‐off of 400 copies/mL (Ayala Gaytán 2004; Sinha 2013). The other included trials did not specify what cut‐off point they used (Mateelli 2013; Sow 2006; Swaminathan 2011; Wester 2010).
We did not extract any time‐to‐event data.
Contributions of authors
All review authors contributed to the design and conduct of this review, as well as with manuscript drafting and submission. LM, SM, JI, AS and NS screened articles for inclusion. AS, LM and SM extracted data and assessed risk of bias. LM, AS, GR and NS worked on the summary of findings tables.
Sources of support
Internal sources
South African Cochrane Centre, Medical Research Council, South Africa.
Cochrane HIV/AIDS Mentoring Programme, South Africa.
Liverpool School of Tropical Medicine, UK.
External sources
-
Centers for Disease Control and Prevention (CDC), USA.
Cooperative Agreement #U2GPS001468 "Atlanta HQ UCSF Technical Assistance to Support the President's Emergency Plan for AIDS Relief" from the CDC, with funds from the National Center for HIV, Viral Hepatitis, STDs and TB Prevention (NCHSTP). Its contents are solely the responsibility of the review authors and do not necessarily represent the official views of the CDC.
-
World Health Organization (WHO), Switzerland.
WHO #200106621, Systematic reviews and development of GRADE profiles, based on the new WHO GRC guidelines, for the "WHO Guidelines on antiretroviral therapy for HIV infection in adults and adolescents ‐ 2009 revision."
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
Disclaimer: we prepared this article while Alicen Spaulding was employed at the University of Minnesota. The opinions expressed in this article are those of the review authors' and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. Alice Spaulding received salary support from the WHO for this project. Lawrence Mbuagbaw, James Irlam, Sara Mursleen and George Rutherford have no known conflicts of interest. Nandi Siegfried provides technical consultation on the efficacy of drugs for a managed care organization (MEDSCHEME), for which she receives an honorarium.
Unchanged
References
References to studies included in this review
Ayala Gaytán 2004 {published data only}
- Ayala Gaytán JJ, Zapata de La Garza ER, Chávez García M, Valdovinos Chávez SB. [Nevirapine or efavirenz in combination with two nucleoside analogues in HIV‐infected antiretroviral‐naïve patients]. Medicina Interna de México 2004;20(1):24‐33. [CN‐00641209] [Google Scholar]
Bonnet 2013a {published data only}
- Bonnet M, Bhatt N, Baudin E, Silva C, Michon C, Taburet AM, et al. Nevirapine versus efavirenz for patients co‐infected with HIV and tuberculosis: a randomised non‐inferiority trial. Lancet Infectious diseases 2013;13(4):303‐12. [PUBMED: 23433590] [DOI] [PubMed] [Google Scholar]
Landman 2014 {published data only}
- Landman R, Koulla‐Shiro S, Sow PS, Ngolle M, Diallo MB, Guèye NF, et al. Evaluation of four tenofovir‐containing regimens as first‐line treatments in Cameroon and Senegal: the ANRS 12115 DAYANA Trial. Antiviral Therapy 2014;19(1):51‐9. [PUBMED: 23970206] [DOI] [PubMed] [Google Scholar]
Manosuthi 2009a {published data only}
- Manosuthi W, Sungkanuparph S, Tantanathip P, Lueangniyomkul A, Mankatitham W, Prasithsirskul W, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse‐transcriptase inhibitor‐based regimens in HIV‐infected patients receiving rifampicin: the N2R Study. Clinical Infectious Diseases 2009;48(12):1752‐9. [PUBMED: 19438397] [DOI] [PubMed] [Google Scholar]
Mateelli 2013 {unpublished data only}
- Mateelli A, Kouanda S, Saleri N, Ouedraogo G. Efficacy and safety of nevirapine‐ vs efavirenz based ART in TB/HIV patients in Burkina Faso: a clinical and pharmacokinetic study. 20th Conference on Retroviruses and Opportunistic Infections. 3‐16 March, 2013. Atlanta, USA, 2013:Abstract 47462.
Núñez 2002 {published data only}
- Núñez M, Soriano V, Martin‐Carbonero L, Barrios A, Barreiro P, Blanco F, et al. SENC (Spanish efavirenz vs. nevirapine comparison) trial: a randomized, open‐label study in HIV‐infected naïve individuals. HIV Clinical Trials 2002;3(3):186‐94. [PUBMED: 12032877] [DOI] [PubMed] [Google Scholar]
Sinha 2013 {published data only}
- Sinha S, Raghunandan P, Chandrashekhar R, Sharma SK, Kumar S, Dhooria S, et al. Nevirapine versus efavirenz‐based antiretroviral therapy regimens in antiretroviral‐naïve patients with HIV and tuberculosis infections in India: a pilot study. BMC Infectious Diseases 2013;13:482. [PUBMED: 24134449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sow 2006 {published data only}
- Sow PG, Badiane M, Diallo PD, Lo I, Ndiaye B, Gaye AM. Efficacy and safety of lamivudine+zidovudine+efavirenz and lamivudine+zidovudine+nevirapine in treatment HIV1 infected patients. A cross study analysis [Abstract CDB0584]. XVI International AIDS Conference; 13‐18 August, 2006. Toronto, Canada, 2006.
Swaminathan 2011 {published data only}
- Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Efficacy and safety of once‐daily nevirapine‐ or efavirenz‐based antiretroviral therapy in HIV‐associated tuberculosis: a randomized clinical trial. Clinical infectious disease 2011;53(7):716‐724. [DOI] [PubMed] [Google Scholar]
van den Berg‐Wolf 2008 {published data only}
- Berg‐Wolf M, Hullsiek KH, Peng G, Kozal MJ, Novak RM, Chen L, et al. Virologic, immunologic, clinical, safety, and resistance outcomes from a long‐term comparison of efavirenz‐based versus nevirapine‐based antiretroviral regimens as initial therapy in HIV‐1‐infected persons. HIV Clinical Trials 2008;9(5):324‐36. [PUBMED: 18977721] [DOI] [PubMed] [Google Scholar]
van Leth 2004 {published data only}
- Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first‐line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open‐label trial, the 2NN Study. Lancet 2004;363(9417):1253‐63. [PUBMED: 15094269] [DOI] [PubMed] [Google Scholar]
Wester 2010 {published data only}
- Wester CW, Thomas AM, Bussmann H, Moyo S, Makhema JM, Gaolathe T, et al. Non‐nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy‐treated adults in Botswana. AIDS (London, England) 2010;24 Suppl 1:S27‐36. [PUBMED: 20023437] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Antela 2004 {published data only}
- Antela A, Iribarren JA, Mahillo B, Santos I, Ribera E, Gutierrez C, et al. Final analysis of a prospective, randomized, open‐label, multicentre trial in naïve, HIV‐1‐infected patients, comparing ZDV/3TC vs d4T/ddI, plus efavirenz, nevirapine or indinavir/ritonavir (AMADEUS 01 study). International Conference on AIDS; 11‐16 July, 2004; Bangkok, Thailand. 2004; Vol. 15:Abstract no. B11920.
Bannister 2008 {published data only}
- Bannister WP, Ruiz L, Cozzi‐Lepri A, Mocroft A, Kirk O, Staszewski S, et al. Comparison of genotypic resistance profiles and virological response between patients starting nevirapine and efavirenz in EuroSIDA. AIDS (London, England) 2008;22(3):367‐76. [PUBMED: 18195563] [DOI] [PubMed] [Google Scholar]
Bonnet 2013b {published data only}
- Bonnet M, Baudin E, Jani IV, Nunes E, Verhoustraten F, Calmy A, et al. Incidence of paradoxical tuberculosis‐associated immune reconstitution inflammatory syndrome and impact on patient outcome. PLOS ONE 2013;8(12):e84585. [PUBMED: 24367678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brück 2008 {published data only}
- Brück S, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Hepatotoxicity in patients prescribed efavirenz or nevirapine. European Journal of Medical Research 2008;13(7):343‐8. [PubMed] [Google Scholar]
de Beaudrap 2008 {published data only}
- Beaudrap P, Etard JF, Guèye FN, Guèye M, Landman R, Girard PM, et al. Long‐term efficacy and tolerance of efavirenz‐ and nevirapine‐containing regimens in adult HIV type 1 Senegalese patients. AIDS Research and Human Retroviruses 2008;24(6):753‐60. [DOI: 10.1089/aid.2007.0295] [DOI] [PubMed] [Google Scholar]
Han 2005 {published data only}
- Han XX, Zhang M, Cui WG, Liu BG, Wang Y, Zhang ZN, et al. Efficacy of anti‐HIV treatment and drug‐resistance mutations in some parts of China. Zhonghua Yi Xue Za Zhi 2005;85(11):760‐4. [PubMed] [Google Scholar]
Hartmann 2005a {published data only}
- Hartmann M, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Comparison of efavirenz and nevirapine in HIV‐infected patients (NEEF Cohort). International Journal of STD & AIDS 2005;16(6):404‐9. [PUBMED: 15969773] [DOI] [PubMed] [Google Scholar]
Hartmann 2005b {published data only}
- Hartmann M, Brust J, Schuster D, Mosthaf F, Procaccianti M, Rump JA, et al. Rashes in HIV‐infected patients undergoing therapy with nevirapine or efavirenz [Arzneimittelexantheme bei Therapie der HIV‐Infektion mit Efavirenz und Nevirapin]. Der Hautarzt 2005;56(9):847‐53. [DOI] [PubMed] [Google Scholar]
He 2011 {published data only}
- He Y, Luo Y, Ding YL, Zheng YH, Li J, Huang J, et al. [Effect of highly active anti‐retroviral therapy on prevention of mother to child transmission of HIV and on infant growth and development]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2011;45(10):912‐5. [PUBMED: 22321592] [PubMed] [Google Scholar]
Lapphra 2008 {published data only}
- Lapphra K, Vanprapar N, Chearskul S, Phongsamart W, Chearskul P, Prasitsuebsai W, et al. Efficacy and tolerability of nevirapine‐ versus efavirenz‐containing regimens in HIV‐infected Thai children. International Journal of Infectious Diseases 2008;12(6):e33‐8. [PUBMED: 18573672] [DOI] [PubMed] [Google Scholar]
Manfredi 2004 {published data only}
- Manfredi R, Calza L, Chiodo F. Efavirenz versus nevirapine in current clinical practice: a prospective, open‐label observational study. Journal of Acquired Immune Deficiency Syndromes 2004;35(5):492‐502. [DOI] [PubMed] [Google Scholar]
Manfredi 2005 {published data only}
- Manfredi R, Calza L, Chiodo F. Prospective, open‐label comparative study of liver toxicity in an unselected population of HIV‐infected patients treated for the first time with efavirenz or nevirapine. HIV Clinical Trials 2005;6(6):302‐11. [DOI] [PubMed] [Google Scholar]
Manfredi 2006 {published data only}
- Manfredi R, Calza L. Nevirapine versus efavirenz in 742 patients: no link of liver toxicity with female sex, and a baseline CD4 cell count greater than 250 cells/microl. AIDS 2006;20(17):2233‐6. [DOI] [PubMed] [Google Scholar]
Mankhatitham 2011 {published data only}
- Mankhatitham W, Lueangniyomkul A, Manosuthi W. Hepatotoxicity in patients co‐infected with tuberculosis and HIV‐1 while receiving non‐nucleoside reverse transcriptase inhibitor‐based antiretroviral therapy and rifampicin‐containing anti‐tuberculosis regimen. Southeast Asian Journal of Tropical Medicine and Public Health 2011;42(3):651‐8. [PUBMED: 21706943] [PubMed] [Google Scholar]
Mankhatitham 2012 {published data only}
- Mankhatitham W, Luaengniyomkul A, Manosuthi W. Lipid profile changes in Thai HIV and tuberculosis co‐infected patients receiving non‐nucleoside reverse transcriptase inhibitors‐based antiretroviral therapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2012;95(2):163‐9. [PUBMED: 22435244] [PubMed] [Google Scholar]
Manosuthi 2004 {published data only}
- Manosuthi W, Sungkanuparph S, Vibhagool A, Rattanasiri S, Thakkinstian A. Nevirapine‐ versus efavirenz‐based highly active antiretroviral therapy regimens in antiretroviral‐naïve patients with advanced HIV infection. HIV Medicine 2004;5(2):105‐9. [DOI] [PubMed] [Google Scholar]
Manosuthi 2009b {published data only}
- Manosuthi W, Mankatitham W, Lueangniyomkul A, Thongyen S, Prommool V, Likanonsakul S, et al. Serial monitoring of drug concentrations while on and off rifampicin between standard doses of nevirapine based and efavirenz‐based antiretroviral regimens. HIV Medicine 2009;10(Suppl 2):45‐221. [Google Scholar]
Musiime 2012 {published data only}
- Musiime V, Cook A, Kayiwa J, Zangata D, Nansubuga C, Arach B, et al. Differences in body circumferences, skin‐fold thicknesses and lipid profiles among HIV‐infected African children on and not on stavudine. Journal of the International AIDS Society 2012;15(Suppl 4):18290. [Google Scholar]
Nachega 2008 {published data only}
- Nachega JB, Hislop M, Dowdy DW, Gallant JE, Chaissona RE, Regensberg L, et al. Efavirenz versus nevirapine‐based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS 2008;22(16):2117‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negredo 2004 {published data only}
- Negredo E, Paredes R, Peraire J, Pedrol E, Côté H, Gel S, et al. Alteration of antiretroviral drug regimens for HIV infection. Efficacy, safety and tolerability at week 96 of the Swatch Study. Antiviral Therapy 2004;9(6):889‐93. [PubMed] [Google Scholar]
Padmapriyadarsini 2013 {published data only}
- Padmapriyadarsini C, Bhavani PK, Tang A, Kumar H, Ponnuraja C, Narendran G, et al. Early changes in hepatic function among HIV‐tuberculosis patients treated with nevirapine or efavirenz along with rifampin‐based anti‐tuberculosis therapy. International Journal of Infectious Diseases 2013;17(12):e1154‐9. [PUBMED: 24120216] [DOI] [PMC free article] [PubMed] [Google Scholar]
PENPACT 2011 {published data only}
- PENPACT‐1 (PENTA 9/PACTG 390) Study Team, Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, et al. First‐line antiretroviral therapy with a protease inhibitor versus non‐nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV‐infected children: an open‐label, randomised phase 2/3trial. Lancet Infect Diseases 2011;11(4):273‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prendergast 2011 {published data only}
- Prendergast A, Bwakura‐Dangarembizi MF, Cook AD, Bakeera‐Kitaka S, Natukunda E, Nahirya Ntege P, et al. Hospitalization for severe malnutrition among HIV‐infected children starting antiretroviral therapy. AIDS 2011;25(7):951‐6. [PUBMED: 21487251] [DOI] [PubMed] [Google Scholar]
Puthanakit 2009a {published data only}
- Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Efficacy of non‐nucleoside reverse transcriptase inhibitor‐based highly active antiretroviral therapy in Thai HIV‐infected children aged two years or less. Pediatric Infectious Disease Journal 2009;28(3):246‐8. [PUBMED: 19165130] [DOI] [PubMed] [Google Scholar]
Puthanakit 2009b {published data only}
- Puthanakit T, Kerr SJ, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus‐infected children receiving non‐nucleoside reverse transcriptase inhibitor‐based highly active antiretroviral therapy. Pediatric Infectious Disease Journal 2009;28(6):488‐92. [DOI] [PubMed] [Google Scholar]
Swaminathan 2009 {published data only}
- Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Once‐daily nevirapine vs efavirenz in the treatment of HIV‐infected patients with TB: a randomized clinical trial. 16th conference on retroviruses and opportunistic infections. Montreal, Canada, February 8‐11, 2009.. 2009. [PUBMED: 21890776]21890776
Additional references
Beck 2008
- Beck EJ, Mandalia S, Youle M, Brettle R, Fisher M, Gompels M, et al. Treatment outcome and cost‐effectiveness of different highly active antiretroviral therapy regimens in the UK (1996‐2002). International Journal of STD & AIDS 2008;19(5):297‐304. [DOI] [PubMed] [Google Scholar]
BHIVA 2001
- BHIVA Writing Committee, BHIVA Executive Committee. Guidelines for the treatment of HIV‐infected adults with antiretroviral therapy. HIV Medicine 2001;2(4):276‐313. [DOI] [PubMed] [Google Scholar]
Chou 2006
- Chou R, Rongwei F, Huffman LH, Korthuis PT. Initial highly‐active antiretroviral therapy with a protease inhibitor versus a non‐nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta‐analyses. Lancet 2006;368(9546):1503‐15. [DOI] [PubMed] [Google Scholar]
Cooper 2007
- Cooper CL, Heeswijk RPG. Once‐daily nevirapine dosing: a pharmacokinetics, efficacy and safety review. HIV Medicine 2007;8(1):1‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. Journal of Acquired Immune Deficiency Syndromes 2001;26 Suppl 1:S25‐33. [DOI] [PubMed] [Google Scholar]
DHHS 2001a
- Department of Health and Human Sciences. AIDSinfo Drug Database. https://aidsinfo.nih.gov/drugs/116/nevirapine/0/professional (accessed 10 November 2016).
DHHS 2001b
- Department of Health and Human Sciences. AIDSinfo Drug Database. https://aidsinfo.nih.gov/drugs/269/efavirenz/0/professional (accessed 10 November 2016).
Dybul 2002
- Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK, Panel on Clinical Practices for the Treatment of HIV. Guidelines for using antiretroviral agents among HIV‐infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. Morbidity and Mortality Weekly Report 2002;51(RR‐7):1‐55. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilks 2006
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public‐health approach to antiretroviral treatment against HIV in resource‐limited settings. Lancet 2006;368(9534):505‐10. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (assessed 03 November 2016). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editor(s). In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Hogg 1997
- Hogg RS, O'Shaughnessy MV, Gataric N, Yip B, Craib K, Schechter MT, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet 1997;349(9061):1294. [DOI] [PubMed] [Google Scholar]
Jiang 2014
- Jiang HY, Zhang MN, Chen HJ, Yang Y, Deng M, Ruan B. Nevirapine versus efavirenz for patients co‐infected with HIV and tuberculosis: a systematic review and meta‐analysis. International Journal of Infectious Diseases 2014;25:130‐5. [PUBMED: 24911886] [DOI] [PubMed] [Google Scholar]
Lau 2007
- Lau B, Gange S, Kirk G, Mehta S, Merriman B, Moore R. Predictive value of plasma HIV RNA levels for rate of CD4 decline and clinical disease progression. 14th Conference on Retroviruses and Opportunistic Infections (CROI); 25‐28 February, 2007; Los Angeles, California. 2007, issue Abstract 140 (oral).
Mellors 2007
- Mellors J, Margolick J, Phair J, Rinaldo C, Detels R, Jaconson L, et al. Comparison of plasma HIV‐1 RNA, CD4 cell count, and CD38 expression on CD8 T cells as predictors of progression to AIDS and CD4 cell decline among untreated participants in the multicenter AIDS cohort study. 14th Conference on Retroviruses and Opportunistic Infections; 25‐28 February, 2007; Los Angeles, California. 2007.
Mocroft 1998
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV‐1. EuroSIDA Study Group. Lancet 1998;352(9142):1725‐30. [DOI] [PubMed] [Google Scholar]
Moyle 2000
- Moyle GJ. Considerations in the choice of protease inhibitor‐sparing regimens in initial therapy for HIV‐1 infection. Current Opinion in Infectious Diseases 2000;13(1):19‐25. [DOI] [PubMed] [Google Scholar]
NIAID/NIH 2004
- Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Table for Grading the Severity of Adult and Pediatric Adverse Events. Bethesda, Maryland: National Institutes of Health, 2004. [Google Scholar]
Pillay 2013
- Pillay P, Ford N, Shubber Z, Ferrand RA. Outcomes for efavirenz versus nevirapine‐containing regimens for treatment of HIV‐1 infection: a systematic review and meta‐analysis. PLOS ONE 2013;8(7):e68995. [PUBMED: 23894391] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shubber 2013
- Shubber Z, Calmy A, Andrieux‐Meyer I, Vitoria M, Renaud‐Théry F, Shaffer N, et al. Adverse events associated with nevirapine and efavirenz‐based first‐line antiretroviral therapy: a systematic review and meta‐analysis. AIDS (London, England) 2013;27(9):1403‐12. [PUBMED: 23343913] [DOI] [PubMed] [Google Scholar]
Siegfried 2006
- Siegfried NL, Deventer PJU, Mahomed FA, Rutherford GW. Stavudine, lamivudine and nevirapine combination therapy for treatment of HIV infection and AIDS in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2016
- UNAIDS. AIDS by the numbers 2015. http://www.unaids.org/sites/default/files/media_asset/AIDS‐by‐the‐numbers‐2016_en.pdf (accessed 03 November 2016).
Veldkamp 2001
- Veldkamp AI, Weverling GJ, Lange JM, Montaner JS, Reiss P, Cooper DA, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV‐1‐infected individuals. AIDS 2001;15(9):1089‐95. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Scaling up antiretroviral therapy in low in resource‐limited settings. Guidelines for a public health approach. June 2002. http://www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf (accessed 03 November 2016).
WHO 2006
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006 revision. http://whqlibdoc.who.int/publications/2006/9789241594677_eng.pdf (accessed 25 September 2008). [PubMed]
WHO 2009
- World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. November 2009. http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf (accessed 03 November 2016).
WHO 2014
- World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. July 2014. http://www.who.int/hiv/pub/guidelines/keypopulations/en/ (accessed 09 September 2016). [PubMed]
WHO 2015a
- World Health Organization. WHO Model List of Essential Medicines. 19th List. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1 (accessed 10 September 2008).
WHO 2015b
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second edition. http://www.who.int/hiv/pub/arv/arv‐2016/en/ (accessed 03 November 2016). [PubMed]
References to other published versions of this review
Mbuagbaw 2009
- Mbuagbaw LC, Irlam JH. Efavirenz versus nevirapine as a non‐nucleoside reverse transcriptase inhibitor in initial combination antiretroviral therapy for HIV infection. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004246.pub2] [DOI] [PubMed] [Google Scholar]
Mbuagbaw 2010
- Mbuagbaw LC, Irlam JH, Spaulding A, Rutherford GW, Siegfried N. Efavirenz or nevirapine in three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïve individuals. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD004246.pub3] [DOI] [PubMed] [Google Scholar]
Neuwelt MD 2002
- Neuwelt MD. Efavirenz versus nevirapine as a non‐nucleoside reverse transcriptase inhibitor in initial combination antiretroviral therapy for HIV infection. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD004246] [DOI] [Google Scholar]


