Abstract
Hydrogen sulfide (H2S) is a ubiquitous signaling molecule with important functions in many mammalian organs and systems. Observations in the 1990s ascribed physiological actions to H2S in the nervous system, proposing that this gasotransmitter acts as a neuromodulator. Soon after that, the vasodilating properties of H2S were demonstrated. In the past decade, H2S was shown to exert a multitude of physiological effects in the vessel wall. H2S is produced by vascular cells and exhibits antioxidant, antiapoptotic, anti-inflammatory, and vasoactive properties. In this concise review, we have focused on the impact of H2S on vascular structure and function with an emphasis on angiogenesis, vascular tone, vascular permeability and atherosclerosis. H2S reduces arterial blood pressure, limits atheromatous plaque formation, and promotes vascularization of ischemic tissues. Although the beneficial properties of H2S are well established, mechanistic insights into the molecular pathways implicated in disease prevention and treatment remain largely unexplored. Unraveling the targets and downstream effectors of H2S in the vessel wall in the context of disease will aid in translation of preclinical observations. In addition, acute regulation of H2S production is still poorly understood and additional work delineating the pathways regulating the enzymes that produce H2S will allow pharmacological manipulation of this pathway. As the field continues to grow, we expect that H2S-related compounds will find their way into clinical trials for diseases affecting the blood vessels.
Keywords: hydrogen sulfide, signaling, endothelium, vascular smooth muscle, blood vessels
hydrogen sulfide (H2S) is a ubiquitous second messenger molecule with important functions in the vessel wall (75, 161). Three enzymes have been shown to enzymatically generate H2S, cystathionine β-synthase (CBS), cystathionine γ-lyase (CTH or CSE) and 3-mercaptopyruvate sulfurtransferase (3MST) (85, 108, 158). CBS and CSE participate in the interconversion of homocysteine to cysteine, known as the transsulfuration pathway; both enzymes are pyridoxal-5 phosphate dependent (70, 75). It should, however, be kept in mind that CBS and CSE catalyze a number of additional reactions that do not yield H2S (70). CBS possesses unique features: it is the only known PLP-dependent enzyme with a heme prosthetic group and has a positive allosteric activator, S-adenosylmethionine (70). Under resting conditions, in many cell types, CBS and 3MST are found in both the mitochondria and the cytosol (132, 145, 153), while CSE is only present in the cytosol (48, 153).
All three H2S-producing enzymes have been reported to be expressed in vascular cells (115, 126, 131). However, little is known about the molecular pathways regulating their expression in vascular cells. Reactive oxygen species and laminar shear flow have been shown to enhance the expression of CSE and 3MST (61, 104), respectively, while elevations in calcium and specificity protein 1 (Sp1) have been shown to upregulate CSE in smooth muscle cells (165, 179). The majority of the vascular studies have focused on CSE. One factor that has contributed to this is the availability of better pharmacological inhibitors for CSE (116). Moreover, although CBS-deficient mice were available years before CSE knockouts (167), their severe phenotype resulting in death in the first weeks of life has limited their usefulness in experimental studies, leading investigators to use heterozygotes (2, 18, 106). In contrast, CSE knockout mice have no developmental abnormalities and appear to have a normal lifespan; they do, however, display a cardiovascular phenotype with elevated blood pressure and reduced endothelial-dependent responses (183). Although 3MST mice are available, no data have been published on their cardiovascular characteristics (110).
Acute vs. Delayed Effectors and Pathways in H2S Signaling
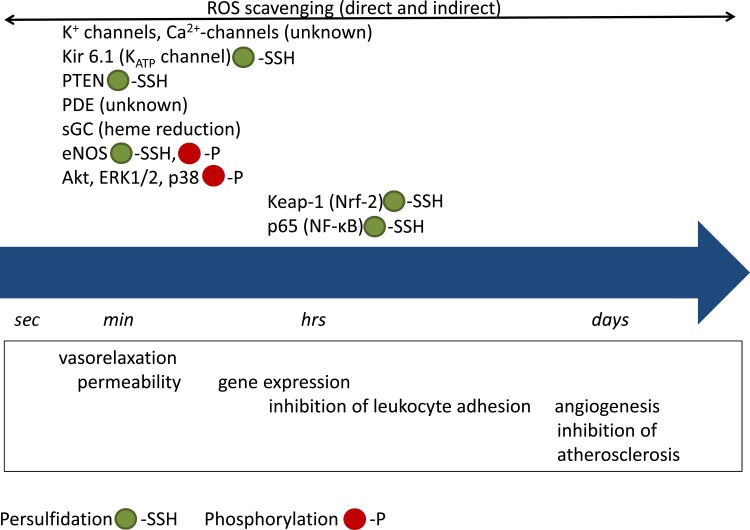
Hydrogen sulfide is highly soluble in water with a solubility of 80 mM at body temperature (71). It is also soluble in lipid membranes so that it has access to both intracellular and extracellular sites of target proteins (99, 122). Because H2S is a weak acid, it equilibrates with its anion HS− at body temperature (pKa of 7) with ~70% being present as HS− at physiological pH (103). The chemical nature of the molecule(s) responsible for the biological activity of H2S remains elusive; H2S itself, HS−, polysulfides, as well as S/N hybrid species have been shown to affect a variety of signaling pathways leading to biological responses (38, 76, 85, 87, 112). The targeted pathways include kinases and phosphatases, additional enzymes, ion channels, and transcription factors (Fig. 1). A primary mechanism through which H2S affects the activity of signaling proteins is persulfidation of reactive cysteine residues on target proteins to form a persulfide group (–SSH) (117, 127). Depending on the nature of the targeted protein, the effects of H2S might take from seconds to days to manifest. For example, persulfidation of ATP-sensitive (KATP) channels leads to hyperpolarization and relaxation of smooth muscle cells that occurs within seconds, while persulfidation of Keap-1 (185) allows activation of Nrf-2 and enhanced antioxidant gene expression, requiring hours to days for biological effects to become apparent.
Fig. 1.
Timescale of H2S effects. Acute effects of H2S include activation/inhibition of ion channels, kinases, and other enzymes. These events are observed within a few seconds and up to several minutes after exposure to H2S. Chronic effects are dependent on gene expression and involve altered transcription factor activity; although activation/inhibition of transcription factors might be observed within minutes to hours after H2S administration, the biological effects are not observed until after much later, requiring up to days to manifest. It should be noted that phosphorylation is secondary to activation of a kinase or inhibition of a phosphatase, through persulfidation or other mechanisms.
One of the earliest demonstrations of H2S sulfhydryl modification of cysteine residues leading to a functional change was a report by Mustafa et al. (107) that persulfidation on Cys150 augments glyceraldehyde dehydrogenase (GAPDH) activity. This study found that up to 25% of the proteins in liver homogenates were endogenously persulfidated, suggesting that sulfhydration is a widespread signaling paradigm. However, several years later Jarosz and coworkers were not able to reproduce the activation of GAPDH by persulfidation and instead observed a decrease in enzyme activity after modification of the protein by H2S (67a). Thus, the role of H2S modification of GAPDH in regulating cellular function is still unclear.
More recently, persulfidation of other targets has also been linked to changes in function. Persulfidation of inositol trisphosphate receptors (IP3R) was shown to inhibit Ca2+ release through these receptor channels, leading to H2S-induced airway relaxation (29). H2S modification of the mitogen activated protein kinase, MEK1, improved DNA damage repair and decreased senescence through PARP-1 activation (193). Both KIR and IKCa potassium channels (109), mitochondrial proteins (35, 140, 147), cytochrome P450 enzymes (164), NF-κB (128), and PTEN (111) have also been identified as targets of sulfhydration, suggesting that there is indeed widespread cellular regulation through this modification. For the majority of these modifications, persulfidation is inhibitory but there are notable exceptions including the early report by Mustafa et al. (107) for GAPDH and more recent descriptions of increased MEK1 activity after persulfidation in human umbilical vein endothelial cells (HUVECs; 193).
Evidence for the critical role of persulfidation in H2S signaling is largely dependent on observations that reversal of persulfide formation with dithiothreitol or another reducing agent reverses the effects of H2S treatment (29, 192). In addition, cysteine mutation studies further suggest that the H2S targets cysteine residues including Cys341 in MEK1 as the mediator of H2S-induced ERK1/2 phosphorylation and translocation (193) or Cys139 in the activation of PPAR-γ activity and translocation to the nucleus (25). In this latter study, mice fed a high-fat diet were protected from developing insulin resistance and liver injury by administration of a H2S donor, suggesting that H2S is protective from metabolic effects of a high-fat diet. Intriguingly, a high-fat diet downregulates the expression of CSE (51) and post hoc analysis of urinary sulfate concentrations in type 2 diabetic patients demonstrated a strong correlation with estimated glomerular filtration rate (eGFR), suggesting that renal H2S production is associated with protection from renal consequences of diabetes (155).
Persulfidation can be reversed by the thioredoxin system (168). Of interest, the thioredoxin system is upregulated in patients with sleep apnea (148) suggesting that upregulation of thioredoxin could modify H2S-regulated pathways during sleep apnea or other states of high oxidative stress. A recent study observed that thioredoxin cleavage of persulfide moieties leads to the release of H2S, suggesting that the persulfidated proteins could be an endogenous source of free H2S in the circulation (168).
The NO/cGMP pathway represents another major signaling mechanism through which H2S exerts its biological effects (16). H2S was shown to inhibit PDE activity and increase cGMP in smooth muscle cells (22). Additional ways through which H2S can affect the NO/cGMP pathway include 1) enhanced phosphorylation of the activator site S1177 of eNOS (36), 2) stabilization of eNOS in its dimeric active form (6), and 3) regulation of soluble guanylate cyclase (sGC) redox state shifting sGC towards the ferrous, NO-responsive form (200). Although, the effects of H2S on sGC and phosphodiesterase type 5 (PDE5) occur in the absence of persulfidation (16, 200), H2S-stimulated eNOS dimerization is mediated by persulfidation of C443 (6).
Vascular Tone
The primary action of H2S in the vasculature is vasodilatory (74, 85, 161) (Fig. 2). However, biphasic responses to H2S have been reported (40, 151). In addition, conflicting reports on the site and the vasorelaxant mechanism of action of H2S in the vasculature demonstrate that there is heterogeneity in the vascular responses to H2S. It is possible that much of the inconsistent data in the literature is due in part to species differences; more studies are needed to resolve the existing discrepancies.
Fig. 2.
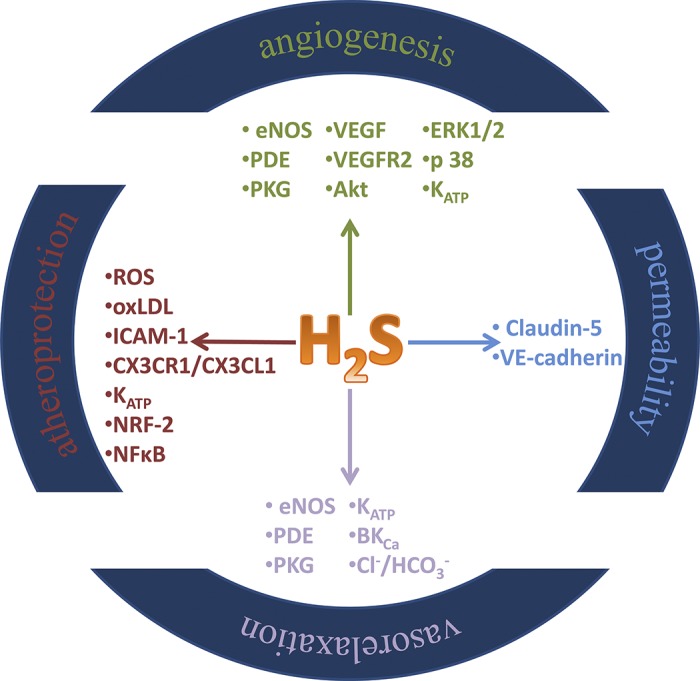
Summary of biological activity of H2S in vascular cells.
The earliest reports on vasoactive responses to endogenous H2S were from the laboratory of Hideo Kimura, who demonstrated in 1997 that several types of smooth muscle including aortic smooth muscle cells express H2S-synthesizing enzymes and generate H2S (60). This study also observed that relaxation of smooth muscle by H2S donors in rats was augmented in the presence of NO and H2S supplementation increased the dilatory response to NO donors. Based on its interaction with NO, one would expect that endothelium removal would reduce H2S-induced relaxation; however, several reports have shown that endothelial denudation does not significantly alter H2S responses (23, 60). Later studies, from Rui Wang’s group demonstrated the importance of KATP for H2S-triggered vasorelaxation (195). Based on 1) its ability to hyperpolarize endothelial and smooth cell membranes, 2) its biological activity on small and/or intermediate conductance KCa channels, and 3) its greater potency as a vasodilator in resistance versus conduit arteries, H2S has been proposed as a candidate for the elusive endothelium-derived hyperpolarizing factor (11, 109, 151).
Activation of KATP channels in isolated arteries from multiple species (89, 92, 109, 133) and in dissociated mouse colonic or rat cardiac myocytes (49, 199) requires millimolar concentrations of H2S donors, while endogenous levels of H2S are consistently reported in nanomolar (130) to micromolar (129) levels. In addition, infusion of H2S into a perfused mesenteric bed (31) or isolated porcine cerebral arteries (82, 89) causes dilation that is only partially inhibited by glibenclamide. In rat thoracic aorta, H2S at high micromolar concentrations caused vasorelaxation that was unaffected by the KATP channel blocker glibenclamide; this effect was reduced by a Cl−/ channel inhibitor and was associated with an overall metabolic suppression of the vascular tissue (77). Several groups have reported that nanomolar to micromolar concentrations of H2S activate large conductance Ca2+-activated potassium channels (BKCa) (63, 134) and the voltage-gated potassium channels (58, 95). A study in bovine retinal arteries demonstrated that the relaxation in response to the H2S donor NaHS was insensitive to KATP inhibition, but partially blocked by inhibition of KV and KIR inhibition (150). The physiological significance for H2S-induced dilation for some of the above-mentioned pathways remains unclear and there may be important species differences.
Other pathways implicated in H2S dilation include activation of nitric oxide synthase (NOS) and cyclooxygenase in human microvessels (81). Work in rodent vessels demonstrated that dilation to sulfide salts is attenuated in vessels from endothelial (e)NOS knockout (KO) mice (36). In line with the ability of NaHS and Na2S to increase cGMP levels in rodent smooth muscle cells (22, 23), Bucci et al. (23) demonstrated that sulfide salt-induced relaxations in mouse aorta were inhibited by DT-2, a cGMP-dependent protein kinase I (PKG-I) inhibitor, and in PKG-I KO animals. However, not all H2S donors cause vasodilation using the NO/cGMP pathway. For example, vasorelaxing responses in the bovine ciliary artery to the slow-releasing H2S donor compound GYY4137 were not blocked by nitro-l-arginine methyl ester (l-NAME; 34) and relaxations to the same H2S donor were not inhibited by DT-2 in the mouse aorta (23). Therefore, both exogenous and endogenous H2S can activate multiple second messenger systems, leading to relaxation of vascular smooth muscle. The pathways utilized seem to depend on the vascular bed studied, the species examined, and on the H2S source used.
Under certain conditions, H2S has also been found to enhance contraction of smooth muscle. In the rat mesenteric arterial bed, lower concentrations of H2S (up to 100 μΜ) promoted contraction, while higher concentrations elicited relaxations (40). Similar observations, with lower H2S concentrations eliciting contractions and higher concentrations exhibiting vasorelaxation, have been observed in the mouse aorta (151) and the rat gastric artery (80). In rat basilar arteries, Li and coworkers (88) observed vasoconstriction to NaHS at concentrations of 1.0 to 150 µM that was prevented by inhibition of adenylyl cyclase. The above studies, taken together, suggest that experimental and preexisting conditions influence the final functional response to increases in H2S. Clearly, additional studies are needed to discern the conditions under which H2S functions as a vasoconstrictor, rather than its more common role as a vasodilator.
In line with its ability to relax resistance arteries, H2S contributes to the maintenance of mean arterial blood pressure at physiological levels; pharmacological inhibition of H2S production was shown to increase blood pressure (124, 194). Important advances in understanding the role of endogenous H2S in vascular regulation were made in 2008 when it was reported by Yang and coworkers (183) that global deletion of CSE results in age-dependent increases in blood pressure and is accompanied by loss of endothelium-dependent dilation. On the other hand, administration of sulfide salts to anesthetized rats and mice caused a transient drop in blood pressure (23, 195). Moreover, administration of sulfide salts or the slowly releasing H2S donor GYY4137 reduced blood pressure in a genetic model of hypertension, as well as in rats rendered hypertensive by angiotensin-II or l-NAME administration (5, 86, 196).
Vascular Permeability
Endothelial cells are responsible for the formation of a barrier between the blood and the underlying tissues (9). The stringency of barrier function even under basal conditions varies considerably between vascular beds; the two extremes are exemplified by the blood brain barrier and fenestrated and sinusoidal endothelia (4). Continuous exchange of solutes between blood and the interstitial tissue occurs in the capillaries. While basal permeability is important for tissue homeostasis, hyperpermeability is associated with several pathological/pathophysiological processes including tissue remodeling and repair, inflammation, and tumorigenesis (9, 12). In a recent study, Geng and colleagues (52) reported that H2S inhalation decreased the permeability of the blood-brain barrier induced by cardiac arrest in rats. This effect of H2S was attributed to reduced expression of VEGF and matrix metalloproteinase-9 (MMP-9) with enhanced expression of the permeability-reducing growth factor angiopoietin-1. An earlier study had shown that NaHS attenuated the increase in lung endothelial barrier permeability triggered by particulate matter inhalation in mice (162). In this latter study, the protective action of H2S was mediated by reactive oxygen species (ROS) scavenging and activation of Akt. Based on the above, one would conclude that H2S limits permeability; however, in both studies mentioned above, the biological activity of H2S might well be indirect through antioxidant and anti-inflammatory effects attributed to H2S (161). Moreover, H2S is has been shown to be protective in ischemia-reperfusion injury (15, 67, 119). Therefore, H2S protection from increased permeability during lung inflammation and following cardiac arrest might be a secondary effect, resulting from suppression of the permeability trigger.
More recently, Yuan and colleagues (187) investigated the direct effects of H2S on vascular permeability in vitro and in vivo. Administration of diallyl trisulfide (DATS) and inorganic polysulfides increased permeability, leading to greater albumin flux and lower transendothelial resistance. Interestingly, the effect of H2S in this study was attributed to polysulfides, rather than H2S, as Na2S and GYY4137 that yield low levels of polysulfides had only minor effects compared with DATS and inorganic polysulfides. The increased permeability was accompanied by disruption of endothelial junction proteins claudin 5 and VE-cadherin, along with enhanced actin stress fiber formation. Cultured endothelial cells from CSE KO mice also displayed enhanced solute barrier function while CSE KO mice were resistant to the hyperpermeability triggered by VEGF. Taken together, the available data point towards a context-dependent effect of H2S on permeability. Clearly, additional studies are needed to dissect the direct and indirect effects on permeability that occur in physiological conditions, as well as during disease.
H2S in Angiogenesis
In vitro studies.
In healthy adult organisms, EC, although quiescent, retain their ability to form new blood vessels in pathological conditions or in response to injury (27). Upon activation, endothelial cells adopt an angiogenic program to contribute to wound healing and tissue remodeling (1). Increased angiogenesis is also observed in conditions such as psoriasis, arthritis, diabetic retinopathy, and cancer (27, 45, 46). A triad of cellular responses, namely proliferation, migration, and network formation, are crucial for EC angiogenic behavior (28). These responses are often studied in reductionist in vitro systems to predict the ability of a substance to drive angiogenesis in vivo. Several laboratories have confirmed that H2S stimulates EC growth, motility, and organization into vessel-like structures in a variety of EC types using common in vitro assays. Studies with exogenously administered H2S have been conducted exclusively with sulfide salts (Na2S and NaHS) (8, 17, 26, 36, 67, 115, 121, 152). Moreover, incubation of EC with substrates for H2S production (cysteine for CSE/CBS and 3-mercatopyruvate for 3MST) have also been shown to promote in vitro angiogenic responses (35, 36), while overexpression of CSE enhances EC growth and promotes vascular outgrowths in vitro (8, 36). In contrast, inhibition of H2S biosynthesis with pharmacological inhibitors or silencing of CSE, CBS, or 3MST reduces cell growth, migration, and tubelike network formation (8, 35, 36, 115, 126). In line with these observations, aortic rings from CSE KO mice generated fewer tubelike structures in an in vitro angiogenesis assay (8, 36). These data suggest that both exogenous and endogenously produced H2S are proangiogenic.
In vivo studies.
The first observation of exogenous H2S driving angiogenesis in vivo was made by Cai et al. (26), who reported that NaHS administration enhanced vascularization of Matrigel implants. The angiogenic response to sulfide salts in vivo is eNOS dependent, as suggested by the reduced responses observed in eNOS KO mice (17, 36). Evidence that endogenous H2S is crucial for angiogenesis in vivo came from studies in chicken chorioallantoic membranes (CAM). Treatment of CAM with the CSE inhibitors propargylglycine (PAG) and β-cyano-l-alanine attenuated vessel branching and length (115). More recently, CSE participation in VEGF-stimulated angiogenesis was confirmed in mice bearing Matrigel plug implants (73).
In line with the angiogenic role of CSE and CBS, supplementation with 3MP, the 3MST substrate, increased Matrigel plug neovascularization in mice (36). These observations taken together suggest that H2S, irrespectively of its enzymatic source (CSE, CBS, or 3MST), promotes new blood vessel formation. This redundancy might be explained by the relatively long half-life of this gasotransmitter (142) along with its ability to freely cross membranes and diffuse into cellular compartments and microenvironments (99). Moreover, it is possible that CSE, CBS, and 3MST each have the capability to drive angiogenesis, but individual enzymes might be preferentially utilized by specific angiogenic triggers.
Mechanisms of H2S-induced angiogenesis.
To promote angiogenesis in endothelial cells, H2S utilizes cyclic nucleotide-, kinase-, and ion channel-regulated pathways (73, 146, 161). H2S donors stimulate Akt, p38, and ERK1/2 phosphorylation while pharmacological inhibitors of PI-3K/Akt and MAPK block EC proliferation and migration (8, 26, 67, 115). Moreover, KATP channel openers mimic H2S responses, while KATP channel blockers reduce the angiogenic effects of H2S (8, 26, 115, 154). In a study using human EC, KATP channels were shown to act upstream of p38 (8, 26, 115). H2S additionally can interact with components of the NO/cGMP pathway at multiple levels (16). As expected, inhibition of eNOS, sGC, or cGMP-dependent protein kinase reduces or blunts H2S-stimulated angiogenic responses (8, 36).
VEGF-H2S interplay.
VEGF is a prototype angiogenic growth factor, regulating new blood vessel formation in physiological, as well as pathophysiological, conditions (8, 36, 44, 59). A number of studies have established extensive cross-talk between H2S and VEGF. Exogenously administered H2S upregulates VEGF expression (17, 67, 79, 160), and endogenous H2S is crucial for preserving VEGF responses; Saha and colleagues (126) demonstrated that silencing CBS in endothelial cells reduced VEGF signaling due to reduced expression of VEGF receptor 2 (VEGFR2) and neuropilin (NRP)-1. CBS-derived H2S stabilizes specificity protein 1 (Sp1) through Cys68 and Cys755 persulfidation that is required for Sp1-mediated VEGFR2 transcription. In addition to H2S regulation of VEGF expression, H2S participates in VEGF signaling. We have shown that short-term exposure of human EC to VEGF increased H2S production (115). The generated H2S contributes to activation of downstream effectors since CSE inhibition blocked VEGF-stimulated p38 and ERK1/2 activation (115). Thus, VEGF-stimulated angiogenesis in endothelial cells is attenuated by pharmacological inhibition or silencing of CSE/CBS (36, 115, 121, 126). Finally, H2S was shown to potentiate the activation of VEGFR2 after VEGF binding (152). Tao and colleagues (152) identified the existence of a disulfide bond between Cys1045 and Cys1024 of VEGFR2 that is inhibitory for the tyrosine kinase activity of the receptor. Nucleophilic attack of the disulfide bond by H2S leads to a disulfide reduction and boosts VEGFR2 tyrosine kinase activity.
Angiogenesis in the context of injury or disease.
After establishing a role for H2S in angiogenic responses in physiological conditions, the role of H2S as a proangiogenic substance was investigated in conditions such as tissue ischemia, heart failure, wound healing, and cancer (36, 78, 115, 118, 145). Two independent studies using sulfide salts (17, 160) and one using diallyl trisulfide (78) have shown H2S to increase angiogenesis and restore ischemic tissue function. The H2S donors have beneficial effects in hindlimb ischemia by enhanced NO production via eNOS-dependent and independent mechanisms and increased hypoxia-inducible factor-1α (HIF1α) expression and activity (17). In the same model, as well as in a model of myocardial ischemia, the H2S precursor S-propargyl-cysteine promoted angiogenesis and improved tissue perfusion (72). In rats with cerebral artery occlusion, treatment with a sulfide salt increased endothelial proliferation and angiogenesis in the peri-infarct area, improving the functional outcome (67). In a femoral artery ligation study, arteriogenesis was inhibited in the absence of CSE, with a significant reduction in mature vessel density, angiogenic indices, and blood flow in CSE KO mice compared with WT mice (78). Similarly, CBS+/− mice exhibited reduced arteriogenesis/angiogenesis that was due to impaired Akt phosphorylation associated with hyperhomocysteinemia (18).
The slow-releasing H2S donor GYY4137 was used to evaluate post-ischemia cardiac remodeling. GYY4137-treated animals exhibited reduced left ventricular (LV) size and preserved function (90). These beneficial effects coincided with greater vessel density in the LV area. Similarly, diallyl trisulfide (DATS), a naturally occurring H2S donor, improved LV remodeling and preserved LV function after aortic constriction (118). H2S donor administration shifted the angiogenic balance by increasing VEGF and reducing angiostatin expression. DATS treatment led to increased Ki67-stained EC and increased cardiac vascular density.
Increased angiogenesis is one of the hallmarks of cancer. A number of reports have indicated increased expression of H2S producing enzymes in cancer (142). The importance of elevated concentrations of H2S in tumor angiogenesis was highlighted in studies from three laboratories. Inhibition of CSE decreased vascularization of clear cell renal cell carcinoma (ccRCC) xenografts grown on CAMs (137). Moreover, the CBS/CSE inhibitor AOAA reduced CB31-positive vessel structures in colon cancer xenografts (145) and ovarian cancer cells with silenced CBS induced less angiogenesis in the tumor (14). It is likely that intratumor angiogenesis is stimulated both by tumor-derived as well as host-derived H2S production, although the relative contribution of the various potential sources remains to be further explored.
Antioxidant and Anti-Inflammatory Effects of H2S in the Vessel Wall
Enhanced oxidative stress is a key event for diseases affecting the vessel wall including hypertension, atherosclerosis, and vascular diabetic complications (84, 96, 135). H2S inhibits ROS production, but also eliminates ROS by direct scavenging, upregulation of GSH, and increased expression of antioxidant enzymes (112, 120, 144, 174). H2S would thus be expected to counteract many of the oxidative stress-related changes in the vessel wall. Indeed, administration of NaHS to mice rendered hypertensive by angiotensin II infusion reduced aortic NADPH-dependent superoxide generation and improved ACh-induced relaxation (5). Similarly, H2S reduced the levels of ROS in endothelial cells cultured in high glucose, preventing apoptosis and endothelial cell injury (53, 57). Adenovirus-mediated gene transfer of CSE or administration of a sulfide salt in hyperglycemic conditions reduced ROS production and improved endothelial-dependent vascular relaxation, while CSE knockdown led to a greater impairment in endothelial function (141). Moreover, pretreatment with NaHS reduced the intracellular reactive oxygen species levels, suppressed NF-κB activity, and inhibited the expression of intercellular adhesion molecule-1 (ICAM-1) in cells cultured in high glucose (56).
Antioxidant effects of H2S have also been demonstrated in the context of atherosclerosis, leading to delayed progression and/or restricting the severity of the disease. H2S administration was shown to inhibit lipid hydroperoxide formation in LDL and to protect against oxLDL cytotoxicity (68, 105). Moreover, H2S inhibited oxLDL-induced intracellular lipid accumulation and foam cell formation (173, 198). The mechanism through which H2S reduced OxLDL-uptake was KATP dependent and involved reduced expression of CD36, scavenger receptor A, and acyl-coenzyme A:cholesterol acyltransferase-1 (198). On the other hand, reducing H2S production through pharmacological inhibition of CSE led to enhanced oxLDL binding and uptake in macrophages, potentiating the accumulation of total and esterified cholesterol (198). Thus, maintaining H2S levels is important to offset the initial events of atheroma formation. The existence of an inverse relationship between H2S and oxLDL was reinforced by the observation that oxLDL upregulates DNA methyltransferase expression and activity, leading to hypermethylation of CpG rich regions in the CSE promoter and reduced CSE transcription (42).
Monocyte recruitment and accumulation is a key event in vascular inflammation and atherosclerosis and depends on the expression of the cellular adhesion molecules ICAM-1, VCAM-1, and P-selectin (123). ICAM-1 levels were reduced in aortas of ApoE KO mice following treatment with a sulfide salt (166). The mechanism of H2S-induced inhibition of ICAM-1 expression was addressed in cultured endothelial cells, where it was shown that NaHS limits the degradation of IκB-α, inhibiting NF-κB activation. The impact of NF-κB activation on adhesion molecule expression was also investigated in a study by Pan and colleagues (114), who observed that exogenous H2S blocked the adhesion of U937 cells to TNF-α-activated HUVECs. In the same study, NaHS also abrogated intracellular ROS triggered by TNF-α treatment (114). In agreement to the reduction in adhesion molecule expression observed after treatment with exogenous H2S, inhibition of CSE upregulated leukocyte function-associated antigen-1 and ICAM-1 on the cell surface and enhanced leukocyte adherence to the vessel wall (189). The anti-inflammatory effects of H2S on leukocytes are not restricted to interference with homing and transmigration, but extend to the production of proinflammatory mediators. H2S has been shown to reduce inflammatory cytokine levels, including IL-1β, TNF-α, and IL-6 from monocytes/macrophages; similar inhibitory effects on cytokine production have been noted in endothelial cells (98, 161).
Regulation of smooth muscle phenotype (synthetic vs. contractile) by environmental cues plays an important role in vascular pathologies (113). It has been proposed that H2S coordinates the expression of proliferative and contractile proteins favoring a differentiated smooth muscle cell phenotype (181). Smooth muscle cells from CSE KO animals display increased proliferation in vitro and in vivo, while H2S donors or CSE overexpression inhibits vascular smooth muscle cell growth and promotes apoptosis in cultured smooth muscle cells (43, 180, 182, 184). H2S also reduces smooth muscle migration (178). These observations are in line with findings that reduced H2S production leads to increased neointimal formation (101, 178). Thus, the above-mentioned effects of H2S on smooth muscle cell behavior contribute to its antiatherosclerotic effects and its ability to inhibit aberrant vascular remodeling in response to injury (98).
Alterations in H2S Levels and Signaling in the Vasculature During Disease
Cardiovascular disease is a contributing factor to the morbidity of many diseases, and damage to the vasculature mediates much of this pathology. It is now becoming clear that loss of H2S production contributes to at least some of the vascular dysfunction in cardiovascular disease (7, 21, 37, 94, 163). Dysregulation of H2S-related pathways has been reported in hypertension. CSE levels are reduced in the vessel wall of spontaneously hypertensive rats, and both CSE and CBS are reduced in the resistance vessels of rats rendered hypertensive after dexamethasone treatment (24, 39, 177). In addition, animals with salt-sensitive hypertension have lower levels of CBS (62). A causal link between low CSE levels and high blood pressure was established following the observation that CSE KO mice exhibit hypertension. Reduced H2S plasma levels have been confirmed in a human cohort of hypertensive patients (81, 139). In several studies, administration of H2S donors to hypertensive animals lowers mean arterial blood pressure and reverses vascular remodeling associated with hypertension (reviewed in refs. 100, 157).
Atherosclerosis is a disease with strong indications that loss of H2S contributes to the establishment and progression of the disease. Feeding CSE KO mice a high-fat diet leads to increased fatty streak formation, enhanced oxidative stress and expression of adhesion molecules and intimal proliferation (97). Atherosclerosis development in this model was reversed by exogenous H2S administration. In ApoE KO animals, inhibition of CSE increased the chemokine/chemokine receptor CX3CR1 and CX3CL1 and exacerbated atherosclerosis (190). Moreover, double CSE/ApoE KO animals displayed more extensive atherosclerotic lesions than ApoE KO mice; the double KO phenotype could be rescued by exogenous administration of NaHS. Administration of NaHS to high-fat-fed (HFD) ApoE KO mice not only reduces vascular Ο2− generation and lesion area, but also improves early signs of endothelial dysfunction as it improves endothelium-dependent vascular relaxations (47). However, H2S might have differential effects in developing versus established atherosclerosis. Intraplaque angiogenesis predisposes to plaque vulnerability (102) and H2S is a proven angiogenic stimulus. In a recent study, van den Born et al. (156) found high CSE expression in human atherosclerotic plaque microvessels. Thus, although CSE/H2S prevent lesion formation, it is likely that in established atheromas it could trigger plaque rupture.
Intervention studies in isolated cells and animal models of atherosclerosis offer potential mechanisms for this apparent protective effect of H2S. Dosing HFD ApoE KO mice with H2S-releasing aspirin, S-aspirin, decreased both chemokine receptor levels and aortic lesion size compared with mice receiving normal aspirin (191). Transgenic overexpression of CSE in ApoE KO mice ameliorated the lipid profile, downregulated NF-κB activation, and reduced lesion formation (33), further suggesting that the CSE/H2S pathway is a promising therapeutic target against atherosclerosis. In a study by Liu and colleagues (93), administration of an H2S donor GYY4137 to ApoE KO mice similarly decreased plaque size as well as the formation of proinflammatory cytokines and superoxide levels. A more recent study from this same group reported that treatment with an H2S donor reduces plaque formation by increasing translocation of the antioxidant transcription factor Nrf-2 with a resultant increase in hemeoxygenase-1 synthesis (173). The mechanism of H2S activation of Nrf-2 required sulfhydration of Cys151 in the Nrf-2 suppressor Keap-1, leading to dissociation of Keap-1 from Nrf-2 and subsequent activation of downstream antioxidant pathways. This pathway has also been implicated in the antiaging effects of H2S in embryonic fibroblasts from wild-type (WT) and CSE knockout mice (185). Alternatively, treatment with an H2S donor might also protect the vascular wall by increasing generation of NO. A 2016 study in ApoE KO mice on a western diet observed a reduction in plaque formation in mice treated with the H2S donor NaHS that was accompanied by increased plasma levels of NOx and increased protein nitrosylation. Furthermore, inhibition of CSE with propargylglycine (PAG) decreased plasma NOx and augmented lesion formation (91). Thus, H2S appears to activate antioxidant pathways in the vascular wall to preserve or increase the activity of NO and to decrease the generation and activity of proinflammatory cytokines.
In chronic hemodialysis patients, those with accelerated atherosclerosis had lower plasma levels of H2S than the healthier hemodialysis patients with less vascular disease (163), and in dialysis patients with diabetic nephropathy, the plasma level of H2S was negatively correlated with both the degree of atherosclerosis and the levels of the inflammatory marker MMP-12 (83). In healthy subjects, plasma H2S correlated positively with levels of the protective factors of adiponectin and HDL, but negatively with the atherosclerotic risk marker, LDL (65). This relationship of lower H2S levels in the most severely affected individuals is also reported in patients with coronary artery disease along with a negative correlation of H2S and chemokine receptors on circulating monocytes (50).
Similar to studies in atherosclerosis, diabetes in humans with or without the comorbidity of elevated lipids is generally associated with decreased plasma levels of H2S (64–66, 155). In addition, animal models of diabetes have also been reported to have decreased plasma or tissue levels of H2S (20, 32, 64, 69, 138, 141, 144, 159). However, there are several anomalous reports of elevated H2S synthesis in diabetes. For example, streptozotocin-induced diabetes in rats was associated with elevated levels of H2S in both the liver and the pancreas (188). This 2005 study reported that insulin therapy decreased expression of CBS and CSE, restoring enzyme expression and H2S to control levels and demonstrating that insulin may be an endogenous regulator of H2S production. In diabetic Zucker rats, increased production of H2S in pancreatic cells was associated with suppressed insulin levels (171). Suppression of H2S production with PAG increased circulating insulin and reduced hemoglobin A1c levels, leading the authors to conclude that excess pancreatic production of H2S in this model of type 1 diabetes suppresses insulin secretion leading to impaired glucose homeostasis. Thus excessive H2S production with subsequent activation of KATP channels in pancreatic islet cells might be one mechanism of insulin dysregulation. However, in human and animal studies of type 2 diabetes, it is increasingly apparent that obesity is associated with decreased production of H2S and this decreased production appears to contribute to diabetic nephropathy (3, 13, 125, 175). Thus future studies examining the sites and mechanisms of H2S regulation of renal function are needed to interrogate this system for potential new therapies to preserve renal function in diabetes.
Defective H2S production has also been shown to occur in other vascular pathologies. In a recent study, Gomez et al. (55) reported reduced CSE expression and H2S levels in abdominal aortic aneurisms in a small human cohort, extending observations made in animal models (181). Moreover, the CSE/H2S pathway has been shown to be downregulated in an animal model of vascular calcification in a NaHS-reversible manner (172, 186). The protective effect of H2S was reported to be dependent on endoplasmic reticulum (ER) stress inhibition (186). These observations are in agreement with the use of sodium thiosulfate, an agent that generates H2S (136), as a treatment for calciphylaxis in humans.
Conclusions and Future Directions
In conclusion, H2S, a ubiquitous gasotransmitter signaling molecule, exerts a multitude of beneficial effects in the vessel wall including suppression of oxidative stress, inhibition of inflammation, and enhancement of vasodilation. Suppression of H2S levels (either by increased H2S consumption and/or decreased H2S production) exacerbates a variety of cardiovascular diseases including atherosclerosis and diabetic vascular complications. Under such conditions, therapeutic replacement of H2S may be a future therapeutic option. The role of H2S in the context of angiogenesis, in some cases, is beneficial: in the context of post-ischemic revascularization, it promotes physiological angiogenesis so that stimulation of these responses by H2S donors may be of future therapeutic benefit. However, in other cases, pathological neovascularization may involve increased H2S production such as during the development of retinopathy (54) or in the context of cancer angiogenesis (142); in such cases, inhibition of H2S production may be a potential future therapeutic approach. Intensive efforts are under way to identify and optimize both donors of H2S (169, 170, 176, 197) and inhibitors of H2S biosynthesis (10, 19, 30, 41, 149); after successful preclinical to clinical translation, such compounds may enhance the therapeutic arsenal against multiple cardiovascular diseases. It is also worth emphasizing that the cardiovascular effects of H2S often require the functional integrity of the eNOS/cGMP pathway; when NO is not produced, many of the angiogenic and some of the vasodilatory effects of H2S are lost (reviewed in ref. 143); this interdependence of the two pathways should also be considered when designing H2S (or NO)-based therapeutic approaches in the future. Finally, the signaling pathways regulating acute activation of H2S-synthesizing enzymes is not well understood and defining upstream regulators of CSE, CBS, and 3MST will potentially allow the development of additional therapeutic agents to manipulate this pathway.
GRANTS
The work of C. Szabo on various aspects of H2S biology is supported by National Institutes of Health (NIH) Grants R01GM107846, R01CA178803, and R21TR00173401; the Shriners of North America Grant 85800; and the Cancer Prevention and Research Institute of Texas CPRIT) Grant DP150074. Support for A. Papapetropoulos's research on H2S was provided by an Excellence Award (1436; Hellenic Ministry of Education). N. L. Kanagy's research on H2S biology is funded by NIH Grants R01HL123301 and R41HL121871 and a Dialysis Clinics Incorporated Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.L.K., C.S., and A.P. drafted the manuscript; N.L.K., C.S., and A.P. edited and revised the manuscript; N.L.K., C.S., and A.P. approved final version of the manuscript.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A, Gerö D, Olah G, Szabo C. Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase. Int J Mol Med 38: 1683–1692, 2016. doi: 10.3892/ijmm.2016.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad FU, Sattar MA, Rathore HA, Abdullah MH, Tan S, Abdullah NA, Johns EJ. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren Fail 34: 203–210, 2012. doi: 10.3109/0886022X.2011.643365. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Magableh MR, Kemp-Harper BK, Hart JL. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res 38: 13–20, 2015. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 6.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 7.Altaany Z, Moccia F, Munaron L, Mancardi D, Wang R. Hydrogen sulfide and endothelial dysfunction: relationship with nitric oxide. Curr Med Chem 21: 3646–3661, 2014. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 8.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 17: 879–888, 2013. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amado-Azevedo J, Valent ET, Van Nieuw Amerongen GP. Regulation of the endothelial barrier function: a filum granum of cellular forces, Rho-GTPase signaling and microenvironment. Cell Tissue Res 355: 557–576, 2014. doi: 10.1007/s00441-014-1828-6. [DOI] [PubMed] [Google Scholar]
- 10.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169: 922–932, 2013. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baragatti B, Ciofini E, Sodini D, Luin S, Scebba F, Coceani F. Hydrogen sulfide in the mouse ductus arteriosus: a naturally occurring relaxant with potential EDHF function. Am J Physiol Heart Circ Physiol 304: H927–H934, 2013. doi: 10.1152/ajpheart.00718.2012. [DOI] [PubMed] [Google Scholar]
- 12.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84: 869–901, 2004. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 13.Bełtowski J. Hypoxia in the renal medulla: implications for hydrogen sulfide signaling. J Pharmacol Exp Ther 334: 358–363, 2010. doi: 10.1124/jpet.110.166637. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, Mukherjee P. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8: e79167, 2013. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibli S-I, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK, Papapetropoulos A. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res 106: 432–442, 2015. doi: 10.1093/cvr/cvv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bibli S-I, Yang G, Zhou Z, Wang R, Topouzis S, Papapetropoulos A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide 46: 7–13, 2015. doi: 10.1016/j.niox.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch-Marcé M, Pola R, Wecker AB, Silver M, Weber A, Luedemann C, Curry C, Murayama T, Kearney M, Yoon YS, Malinow MR, Asahara T, Isner JM, Losordo DW. Hyperhomocyst(e)inemia impairs angiogenesis in a murine model of limb ischemia. Vasc Med 10: 15–22, 2005. doi: 10.1191/1358863x05vm585oa. [DOI] [PubMed] [Google Scholar]
- 19.Brancaleone V, Esposito I, Gargiulo A, Vellecco V, Asimakopoulou A, Citi V, Calderone V, Gobbetti T, Perretti M, Papapetropoulos A, Bucci M, Cirino G. d-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-γ-lyase. Br J Pharmacol 173: 1556–1565, 2016. doi: 10.1111/bph.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol 155: 673–680, 2008. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucci M, Cirino G. Hydrogen sulphide in heart and systemic circulation. Inflamm Allergy Drug Targets 10: 103–108, 2011. doi: 10.2174/187152811794776204. [DOI] [PubMed] [Google Scholar]
- 22.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004, 2010. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 23.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7: e53319, 2012. doi: 10.1371/journal.pone.0053319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucci M, Vellecco V, Cantalupo A, Brancaleone V, Zhou Z, Evangelista S, Calderone V, Papapetropoulos A, Cirino G. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovasc Res 102: 138–147, 2014. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Shi X, Wang H, Fan J, Feng Y, Lin X, Yang J, Cui Q, Tang C, Xu G, Geng B. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim Biophys Acta 1861: 419–429, 2016. doi: 10.1016/j.bbalip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P. Angiogenesis in health and disease. Nat Med 9: 653–660, 2003. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 591: 5999–6015, 2013. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao C, Zatarain JR, Ding Y, Coletta C, Mrazek AA, Druzhyna N, Johnson P, Chen H, Hellmich JL, Asimakopoulou A, Yanagi K, Olah G, Szoleczky P, Törö G, Bohanon FJ, Cheema M, Lewis R, Eckelbarger D, Ahmad A, Módis K, Untereiner A, Szczesny B, Papapetropoulos A, Zhou J, Hellmich MR, Szabo C. Cystathionine-β-synthase inhibition for colon cancer: enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol Med 22: 361–379, 2016. doi: 10.2119/molmed.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D, Kishore R. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation 134: 1467–1483, 2016. doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung SH, Kwok WK, To KF, Lau JYW. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One 9: e113038, 2014. doi: 10.1371/journal.pone.0113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chitnis MK, Njie-Mbye YF, Opere CA, Wood ME, Whiteman M, Ohia SE. Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp Eye Res 116: 350–354, 2013. doi: 10.1016/j.exer.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Coletta C, Módis K, Szczesny B, Brunyánszki A, Oláh G, Rios ECS, Yanagi K, Ahmad A, Papapetropoulos A, Szabo C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by dl-α-lipoic acid. Mol Med 21: 1–14, 2015. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coletta C, Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr Vasc Pharmacol 11: 208–221, 2013. [PubMed] [Google Scholar]
- 38.Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 46: 14–24, 2015. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 39.d’Emmanuele di Villa Bianca R, Mitidieri E, Donnarumma E, Tramontano T, Brancaleone V, Cirino G, Bucci M, Sorrentino R. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide 46: 80–86, 2015. doi: 10.1016/j.niox.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 40.d’Emmanuele di Villa Bianca R, Sorrentino R, Coletta C, Mitidieri E, Rossi A, Vellecco V, Pinto A, Cirino G, Sorrentino R. Hydrogen sulfide-induced dual vascular effect involves arachidonic acid cascade in rat mesenteric arterial bed. J Pharmacol Exp Ther 337: 59–64, 2011. doi: 10.1124/jpet.110.176016. [DOI] [PubMed] [Google Scholar]
- 41.Druzhyna N, Szczesny B, Olah G, Módis K, Asimakopoulou A, Pavlidou A, Szoleczky P, Gerö D, Yanagi K, Törö G, López-García I, Myrianthopoulos V, Mikros E, Zatarain JR, Chao C, Papapetropoulos A, Hellmich MR, Szabo C. Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine β-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol Res 113, Pt A: 18–37, 2016. doi: 10.1016/j.phrs.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du H-P, Li J, You S-J, Wang Y-L, Wang F, Cao Y-J, Hu L-F, Liu C-F. DNA methylation in cystathionine-γ-lyase (CSE) gene promoter induced by ox-LDL in macrophages and in apoE knockout mice. Biochem Biophys Res Commun 469: 776–782, 2016. doi: 10.1016/j.bbrc.2015.11.132. [DOI] [PubMed] [Google Scholar]
- 43.Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, Tang C. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 19: 75–80, 2004. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 29: 789–791, 2009. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 438: 967–974, 2005. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 46.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6: 273–286, 2007. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 47.Ford A, Al-Magableh M, Gaspari TA, Hart JL. Chronic NaHS treatment is vasoprotective in high-fat-fed ApoE(−/−) mice. Int J Vasc Med 2013: 915983, 2013. doi: 10.1155/2013/915983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci USA 109: 2943–2948, 2012. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gade AR, Kang M, Akbarali HI. Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol Pharmacol 83: 294–306, 2013. doi: 10.1124/mol.112.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao L, Xu Z, Yin Z, Chen K, Wang C, Zhang H. Association of hydrogen sulfide with alterations of monocyte chemokine receptors, CCR2 and CX3CR1 in patients with coronary artery disease. Inflamm Res 64: 627–635, 2015. doi: 10.1007/s00011-015-0844-7. [DOI] [PubMed] [Google Scholar]
- 51.Geng B, Cai B, Liao F, Zheng Y, Zeng Q, Fan X, Gong Y, Yang J, Cui QH, Tang C, Xu GH. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PLoS One 8: e73892, 2013. doi: 10.1371/journal.pone.0073892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng Y, Li E, Mu Q, Zhang Y, Wei X, Li H, Cheng L, Zhang B. Hydrogen sulfide inhalation decreases early blood-brain barrier permeability and brain edema induced by cardiac arrest and resuscitation. J Cereb Blood Flow Metab 35: 494–500, 2015. doi: 10.1038/jcbfm.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gero″ D, Torregrossa R, Perry A, Waters A, Le-Trionnaire S, Whatmore JL, Wood M, Whiteman M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol Res 113: 186–198, 2016. doi: 10.1016/j.phrs.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gersztenkorn D, Coletta C, Zhu S, Ha Y, Liu H, Tie H, Zhou J, Szabo C, Zhang W, Motamedi M. Hydrogen sulfide contributes to retinal neovascularization in ischemia-induced retinopathy. Invest Ophthalmol Vis Sci 57: 3002–3009, 2016. doi: 10.1167/iovs.15-18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez I, Ozen G, Deschildre C, Amgoud Y, Boubaya L, Gorenne I, Benyahia C, Roger T, Lesèche G, Galardon E, Topal G, Jacob M-P, Longrois D, Norel X. Reverse regulatory pathway (H2S/PGE2/MMP) in human aortic aneurysm and saphenous vein varicosity. PLoS One 11: e0158421, 2016. doi: 10.1371/journal.pone.0158421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan Q, Wang X, Gao L, Chen J, Liu Y, Yu C, Zhang N, Zhang X, Zhao J. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 in endothelial cells. J Cardiovasc Pharmacol 62: 278–284, 2013. doi: 10.1097/FJC.0b013e31829875ef. [DOI] [PubMed] [Google Scholar]
- 57.Guan Q, Zhang Y, Yu C, Liu Y, Gao L, Zhao J. Hydrogen sulfide protects against high-glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol 59: 188–193, 2012. doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- 58.Hedegaard ER, Gouliaev A, Winther AK, Arcanjo DD, Aalling M, Renaltan NS, Wood ME, Whiteman M, Skovgaard N, Simonsen U. Involvement of potassium channels and calcium-independent mechanisms in hydrogen sulfide-induced relaxation of rat mesenteric small arteries. J Pharmacol Exp Ther 356: 53–63, 2016. doi: 10.1124/jpet.115.227017. [DOI] [PubMed] [Google Scholar]
- 59.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549–580, 2004. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 60.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 61.Huang B, Chen C-T, Chen C-S, Wang Y-M, Hsieh H-J, Wang DL. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun 464: 1254–1259, 2015. doi: 10.1016/j.bbrc.2015.07.115. [DOI] [PubMed] [Google Scholar]
- 62.Huang P, Chen S, Wang Y, Liu J, Yao Q, Huang Y, Li H, Zhu M, Wang S, Li L, Tang C, Tao Y, Yang G, Du J, Jin H. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 46: 192–203, 2015. doi: 10.1016/j.niox.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am J Physiol Heart Circ Physiol 304: H1446–H1454, 2013. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA Jr. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12: 1333–1337, 2010. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain SK, Kahlon G, Morehead L, Lieblong B, Stapleton T, Hoeldtke R, Bass PF III, Levine SN. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metab Syndr Relat Disord 10: 331–336, 2012. doi: 10.1089/met.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain SK, Manna P, Micinski D, Lieblong BJ, Kahlon G, Morehead L, Hoeldtke R, Bass PF III, Levine SN. In African American type 2 diabetic patients, is vitamin D deficiency associated with lower blood levels of hydrogen sulfide and cyclic adenosine monophosphate, and elevated oxidative stress? Antioxid Redox Signal 18: 1154–1158, 2013. doi: 10.1089/ars.2012.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang H, Oh M-Y, Kim Y-J, Choi I-Y, Yang HS, Ryu WS, Lee SH, Yoon BW. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res 92: 1520–1528, 2014. doi: 10.1002/jnr.23427. [DOI] [PubMed] [Google Scholar]
- 67a.Jarosz AP, Wei W, Gauld JW, Auld J, Özcan F, Aslan M, Mutus B. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is inactivated by S-sulfuration in vitro. Free Radic Biol Med 89: 512–521, 2015. doi: 10.1016/j.freeradbiomed.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Jeney V, Komódi E, Nagy E, Zarjou A, Vercellotti GM, Eaton JW, Balla G, Balla J. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S). Free Radic Biol Med 46: 616–623, 2009. doi: 10.1016/j.freeradbiomed.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin S, Pu SX, Hou CL, Ma FF, Li N, Li XH, Tan B, Tao BB, Wang MJ, Zhu YC. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid Med Cell Longev 2015: 758358, 2015. doi: 10.1155/2015/758358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kan J, Guo W, Huang C, Bao G, Zhu Y, Zhu YZ. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal 20: 2303–2316, 2014. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katsouda A, Bibli S-I, Pyriochou A, Szabo C, Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res 113: 175–185, 2016. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41: 4–10, 2014. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal 20: 783–793, 2014. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376, 2015. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiss L, Deitch EA, Szabó C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008. doi: 10.1016/j.lfs.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res 107: 590–600, 2015. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubo S, Kajiwara M, Kawabata A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology 15: 288–292, 2007. doi: 10.1007/s10787-007-1590-4. [DOI] [PubMed] [Google Scholar]
- 81.Kutz JL, Greaney JL, Santhanam L, Alexander LM. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J Physiol 593: 2121–2129, 2015. doi: 10.1113/JP270054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol (1985) 100: 1065–1076, 2006. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H, Feng SJ, Zhang GZ, Wang SX. Correlation of lower concentrations of hydrogen sulfide with atherosclerosis in chronic hemodialysis patients with diabetic nephropathy. Blood Purif 38: 188–194, 2014. doi: 10.1159/000368883. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 34: 313–319, 2013. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51: 169–187, 2011. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360, 2008. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 87.Li Q, Lancaster JR Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 35: 21–34, 2013. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Ping NN, Cao L, Mi YN, Cao YX. H2S induces vasoconstriction of rat cerebral arteries via cAMP/adenylyl cyclase pathway. Toxicol Appl Pharmacol 289: 389–396, 2015. doi: 10.1016/j.taap.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 89.Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol 300: H2088–H2095, 2011. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lilyanna S, Peh MT, Liew OW, Wang P, Moore PK, Richards AM, Martinez EC. GYY4137 attenuates remodeling, preserves cardiac function and modulates the natriuretic peptide response to ischemia. J Mol Cell Cardiol 87: 27–37, 2015. doi: 10.1016/j.yjmcc.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 91.Lin Y, Chen Y, Zhu N, Zhao S, Fan J, Liu E. Hydrogen sulfide inhibits development of atherosclerosis through up-regulating protein S-nitrosylation. Biomed Pharmacother 83: 466–476, 2016. doi: 10.1016/j.biopha.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Liu L, Liu H, Sun D, Qiao W, Qi Y, Sun H, Yan C. Effects of H2S on myogenic responses in rat cerebral arterioles. Circ J 76: 1012–1019, 2012. doi: 10.1253/circj.CJ-11-0890. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, Ji Y. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(−/−) mice. Br J Pharmacol 169: 1795–1809, 2013. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynn EG, Austin RC. Hydrogen sulfide in the pathogenesis of atherosclerosis and its therapeutic potential. Expert Rev Clin Pharmacol 4: 97–108, 2011. doi: 10.1586/ecp.10.130. [DOI] [PubMed] [Google Scholar]
- 95.Ma SF, Luo Y, Ding YJ, Chen Y, Pu SX, Wu HJ, Wang ZF, Tao BB, Wang WW, Zhu YC. Hydrogen sulfide targets the Cys320/Cys529 motif in Kv4.2 to inhibit the Ito potassium channels in cardiomyocytes and regularizes fatal arrhythmia in myocardial infarction. Antioxid Redox Signal 23: 129–147, 2015. doi: 10.1089/ars.2014.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127: 2523–2534, 2013. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 98.Mani S, Untereiner A, Wu L, Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal 20: 805–817, 2014. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 99.Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA 106: 16633–16638, 2009. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meng G, Ma Y, Xie L, Ferro A, Ji Y. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br J Pharmacol 172: 5501–5511, 2015. doi: 10.1111/bph.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol 170: 1406–1414, 2007. doi: 10.2353/ajpath.2007.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Michel J-B, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 32: 1977–1985, 2011. doi: 10.1093/eurheartj/ehr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishanina TV, Libiad M, Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol 11: 457–464, 2015. doi: 10.1038/nchembio.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mistry RK, Murray TVA, Prysyazhna O, Martin D, Burgoyne JR, Santos C, Eaton P, Shah AM, Brewer AC. Transcriptional regulation of cystathionine-γ-lyase in endothelial cells by NADPH oxidase 4-dependent signaling. J Biol Chem 291: 1774–1788, 2016. doi: 10.1074/jbc.M115.685578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muellner MK, Schreier SM, Laggner H, Hermann M, Esterbauer H, Exner M, Gmeiner BM, Kapiotis S. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J 420: 277–281, 2009. doi: 10.1042/BJ20082421. [DOI] [PubMed] [Google Scholar]
- 106.Muradashvili N, Tyagi R, Metreveli N, Tyagi SC, Lominadze D. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J Cereb Blood Flow Metab 34: 1472–1482, 2014. doi: 10.1038/jcbfm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal 2: re2, 2009. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagahara N, Nagano M, Ito T, Shimamura K, Akimoto T, Suzuki H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Sci Rep 3: 1986, 2013. doi: 10.1038/srep01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohno K, Okuda K, Uehara T. Endogenous S-sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem Biophys Res Commun 456: 245–249, 2015. doi: 10.1016/j.bbrc.2014.11.066. [DOI] [PubMed] [Google Scholar]
- 112.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, Fukuto JM. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic Biol Med 77: 82–94, 2014. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 114.Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One 6: e19766, 2011. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Papapetropoulos A, Whiteman M, Cirino G. Pharmacological tools for hydrogen sulphide research: a brief, introductory guide for beginners. Br J Pharmacol 172: 1633–1637, 2015. doi: 10.1111/bph.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paul BD, Snyder SH. H2S: a novel gasotransmitter that signals by sulfhydration. Trends Biochem Sci 40: 687–700, 2015. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 6: 1077–1086, 2013. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17: 119–140, 2012. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pupo E, Fioro Pla A, Avanzato D, Moccia F, Cruz JE, Tanzi F, Merlino A, Mancardi D, Munaron L. Hydrogen sulfide promotes calcium signals and migration in tumor-derived endothelial cells. Free Radic Biol Med 51: 1765–1773, 2011. doi: 10.1016/j.freeradbiomed.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Riahi S, Rowley CN. Why can hydrogen sulfide permeate cell membranes? J Am Chem Soc 136: 15111–15113, 2014. doi: 10.1021/ja508063s. [DOI] [PubMed] [Google Scholar]
- 123.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 124.Roy A, Khan AH, Islam MT, Prieto MC, Majid DS. Interdependency of cystathione γ-lyase and cystathione β-synthase in hydrogen sulfide-induced blood pressure regulation in rats. Am J Hypertens 25: 74–81, 2012. doi: 10.1038/ajh.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Safar MM, Abdelsalam RM. H2S donors attenuate diabetic nephropathy in rats: modulation of oxidant status and polyol pathway. Pharmacol Rep 67: 17–23, 2015. doi: 10.1016/j.pharep.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 126.Saha S, Chakraborty PK, Xiong X, Dwivedi SKD, Mustafi SB, Leigh NR, Ramchandran R, Mukherjee P, Bhattacharya R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J 30: 441–456, 2016. doi: 10.1096/fj.15-278648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J Mol Biol 429: 543–561, 2017. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, Metreveli N, Hayden MR, Tyagi SC. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology 82: 201–213, 2008. doi: 10.1159/000156486. [DOI] [PubMed] [Google Scholar]
- 130.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 146: 623–626, 2009. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 132.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 133.Siebert N, Cantré D, Eipel C, Vollmar B. H2S contributes to the hepatic arterial buffer response and mediates vasorelaxation of the hepatic artery via activation of KATP channels. Am J Physiol Gastrointest Liver Physiol 295: G1266–G1273, 2008. doi: 10.1152/ajpgi.90484.2008. [DOI] [PubMed] [Google Scholar]
- 134.Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch 459: 389–397, 2010. doi: 10.1007/s00424-009-0737-0. [DOI] [PubMed] [Google Scholar]
- 135.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 71: 40–56, 2015. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Snijder PM, Frenay AR, de Boer RA, Pasch A, Hillebrands JL, Leuvenink HG, van Goor H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol 172: 1494–1504, 2015. doi: 10.1111/bph.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sonke E, Verrydt M, Postenka CO, Pardhan S, Willie CJ, Mazzola CR, Hammers MD, Pluth MD, Lobb I, Power NE, Chambers AF, Leong HS, Sener A. Inhibition of endogenous hydrogen sulfide production in clear-cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential. Nitric Oxide 49: 26–39, 2015. doi: 10.1016/j.niox.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Streeter EY, Badoer E, Woodman OL, Hart JL. Effect of type 1 diabetes on the production and vasoactivity of hydrogen sulfide in rat middle cerebral arteries. Physiol Rep 1: e00111, 2013. doi: 10.1002/phy2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun NL, Xi Y, Yang SN, Ma Z, Tang CS. [Plasma hydrogen sulfide and homocysteine levels in hypertensive patients with different blood pressure levels and complications]. Zhonghua Xin Xue Guan Bing Za Zhi 35: 1145–1148, 2007. [PubMed] [Google Scholar]
- 140.Sun WH, Liu F, Chen Y, Zhu YC. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun 421: 164–169, 2012. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA 108: 13829–13834, 2011. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov 15: 185–203, 2016. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: mechanisms and implications. Am J Physiol Cell Physiol 312: C3–C15, 2017. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 17: 68–80, 2012. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA 110: 12474–12479, 2013. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szabó C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol 164: 853–865, 2011. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 171: 2099–2122, 2014. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takahashi K, Chin K, Nakamura H, Morita S, Sumi K, Oga T, Matsumoto H, Niimi A, Fukuhara S, Yodoi J, Mishima M. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxid Redox Signal 10: 715–726, 2008. doi: 10.1089/ars.2007.1949. [DOI] [PubMed] [Google Scholar]
- 149.Takano Y, Shimamoto K, Hanaoka K. Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. J Clin Biochem Nutr 58: 7–15, 2016. doi: 10.3164/jcbn.15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takır S, Ortaköylü GZ, Toprak A, Uydeș-Doğan BS. NaHS induces relaxation response in prostaglandin F(2α) precontracted bovine retinal arteries partially via K(v) and K(ir) channels. Exp Eye Res 132: 190–197, 2015. doi: 10.1016/j.exer.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 151.Tang G, Yang G, Jiang B, Ju Y, Wu L, Wang R. H2S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 19: 1634–1646, 2013. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 152.Tao BB, Liu SY, Zhang CC, Fu W, Cai W, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Teng H, Wu B, Zhao K, Yang G, Wu L, Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci USA 110: 12679–12684, 2013. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Umaru B, Pyriochou A, Kotsikoris V, Papapetropoulos A, Topouzis S. ATP-sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J Pharmacol Exp Ther 354: 79–87, 2015. doi: 10.1124/jpet.114.222000. [DOI] [PubMed] [Google Scholar]
- 155.van den Born JC, Frenay AR, Bakker SJ, Pasch A, Hillebrands JL, Lambers Heerspink HJ, van Goor H. High urinary sulfate concentration is associated with reduced risk of renal disease progression in type 2 diabetes. Nitric Oxide 55-56: 18–24, 2016. doi: 10.1016/j.niox.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 156.van den Born JC, Mencke R, Conroy S, Zeebregts CJ, van Goor H, Hillebrands JL. Cystathionine γ-lyase is expressed in human atherosclerotic plaque microvessels and is involved in micro-angiogenesis. Sci Rep 6: 34608, 2016. doi: 10.1038/srep34608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.van Goor H, van den Born JC, Hillebrands J-L, Joles JA. Hydrogen sulfide in hypertension. Curr Opin Nephrol Hypertens 25: 107–113, 2016. doi: 10.1097/MNH.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 158.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14: 329–345, 2015. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 159.Wang GG, Chen QY, Li W, Lu XH, Zhao X. Ginkgolide B increases hydrogen sulfide and protects against endothelial dysfunction in diabetic rats. Croat Med J 56: 4–13, 2015. doi: 10.3325/cmj.2015.56.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang M-J, Cai W-J, Li N, Ding Y-J, Chen Y, Zhu Y-C. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 161.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 162.Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, Garcia JGN. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 47: 491–496, 2012. doi: 10.1165/rcmb.2011-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang W, Feng SJ, Li H, Zhang XD, Wang SX. Correlation of lower concentrations of hydrogen sulfide with activation of protein kinase CβII in uremic accelerated atherosclerosis patients. Chin Med J (Engl) 128: 1465–1470, 2015. doi: 10.4103/0366-6999.157653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X, Han A, Wen C, Chen M, Chen X, Yang X, Ma J, Lin G. The effects of H2S on the activities of CYP2B6, CYP2D6, CYP3A4, CYP2C19 and CYP2C9 in vivo in rat. Int J Mol Sci 14: 24055–24063, 2013. doi: 10.3390/ijms141224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y, Wang X, Liang X, Wu J, Dong S, Li H, Jin M, Sun D, Zhang W, Zhong X. Inhibition of hydrogen sulfide on the proliferation of vascular smooth muscle cells involved in the modulation of calcium sensing receptor in high homocysteine. Exp Cell Res 347: 184–191, 2016. doi: 10.1016/j.yexcr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 166.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 29: 173–179, 2009. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 167.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA 92: 1585–1589, 1995. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wedmann R, Onderka C, Wei S, Szijártó IA, Miljkovic JL, Mitrovic A, Lange M, Savitsky S, Yadav PK, Torregrossa R, Harrer EG, Harrer T, Ishii I, Gollasch M, Wood ME, Galardon E, Xian M, Whiteman M, Banerjee R, Filipovic MR. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem Sci (Camb) 7: 3414–3426, 2016. doi: 10.1039/C5SC04818D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Whiteman M, Perry A, Zhou Z, Bucci M, Papapetropoulos A, Cirino G, Wood ME. Phosphinodithioate and phosphoramidodithioate hydrogen sulfide donors. Handb Exp Pharmacol 230: 337–363, 2015. doi: 10.1007/978-3-319-18144-8_17. [DOI] [PubMed] [Google Scholar]
- 170.Wu D, Hu Q, Zhu Y. Therapeutic application of hydrogen sulfide donors: the potential and challenges. Front Med 10: 18–27, 2016. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 171.Wu L, Yang W, Jia X, Yang G, Duridanova D, Cao K, Wang R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest 89: 59–67, 2009. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- 172.Wu SY, Pan CS, Geng B, Zhao J, Yu F, Pang YZ, Tang CS, Qi YF. Hydrogen sulfide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats. Acta Pharmacol Sin 27: 299–306, 2006. doi: 10.1111/j.1745-7254.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 173.Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y, Meng G, Han Y, Wang Y, Liu G, Moore PK, Wang X, Wang H, Zhang Z, Yu Y, Ferro A, Huang Z, Ji Y. Hydrogen sulfide induces keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 65: 3171–3184, 2016. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie ZZ, Liu Y, Bian JS. Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev 2016: 6043038, 2016. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xue H, Yuan P, Ni J, Li C, Shao D, Liu J, Shen Y, Wang Z, Zhou L, Zhang W, Huang Y, Yu C, Wang R, Lu L. H(2)S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS One 8: e74366, 2013. doi: 10.1371/journal.pone.0074366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yagdi E, Cerella C, Dicato M, Diederich M. Garlic-derived natural polysulfanes as hydrogen sulfide donors: friend or foe? Food Chem Toxicol 95: 219–233, 2016. doi: 10.1016/j.fct.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 177.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 313: 22–27, 2004. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 178.Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, Xu C, Wang R. Increased neointimal formation in cystathionine gamma-lyase deficient mice: role of hydrogen sulfide in α5β1-integrin and matrix metalloproteinase-2 expression in smooth muscle cells. J Mol Cell Cardiol 52: 677–688, 2012. doi: 10.1016/j.yjmcc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 179.Yang G, Pei Y, Teng H, Cao Q, Wang R. Specificity protein-1 as a critical regulator of human cystathionine γ-lyase in smooth muscle cells. J Biol Chem 286: 26450–26460, 2011. doi: 10.1074/jbc.M111.266643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18: 1782–1784, 2004. [DOI] [PubMed] [Google Scholar]
- 181.Yang G, Wang R. H2S and Blood Vessels: An Overview. In: Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide, edited by Moore PK, Whiteman M. Cham: Springer International Publishing, 2015, p. 85–110. doi: 10.1007/978-3-319-18144-8_4. [DOI] [PubMed] [Google Scholar]
- 182.Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res 86: 487–495, 2010. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 183.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J 20: 553–555, 2006. [DOI] [PubMed] [Google Scholar]
- 185.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 186.Yang R, Teng X, Li H, Xue H-M, Guo Q, Xiao L, Wu Y-M. Hydrogen sulfide improves vascular calcification in rats by inhibiting endoplasmic reticulum stress. Oxid Med Cell Longev 2016: 9095242, 2016. doi: 10.1155/2016/9095242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan S, Pardue S, Shen X, Alexander JS, Orr AW, Kevil CG. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol 9: 157–166, 2016. doi: 10.1016/j.redox.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun 333: 1146–1152, 2005. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 189.Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 190.Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, Wang C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS One 7: e41147, 2012. doi: 10.1371/journal.pone.0041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang H, Guo C, Zhang A, Fan Y, Gu T, Wu D, Sparatore A, Wang C. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur J Pharmacol 697: 106–116, 2012. doi: 10.1016/j.ejphar.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 192.Zhang R, Sun Y, Tsai H, Tang C, Jin H, Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One 7: e37073, 2012. doi: 10.1371/journal.pone.0037073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao K, Ju Y, Li S, Altaany Z, Wang R, Yang G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep 15: 792–800, 2014. doi: 10.1002/embr.201338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848–853, 2003. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- 195.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res 31: 1619–1630, 2008. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 197.Zhao Y, Pacheco A, Xian M. Medicinal chemistry: insights into the development of novel H2S donors. Handb Exp Pharmacol 230: 365–388, 2015. doi: 10.1007/978-3-319-18144-8_18. [DOI] [PubMed] [Google Scholar]
- 198.Zhao ZZ, Wang Z, Li GH, Wang R, Tan JM, Cao X, Suo R, Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 236: 169–176, 2011. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 199.Zhong GZ, Li YB, Liu XL, Guo LS, Chen ML, Yang XC. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology 115: 120–126, 2010. doi: 10.1159/000260073. [DOI] [PubMed] [Google Scholar]
- 200.Zhou Z, Martin E, Sharina I, Esposito I, Szabo C, Bucci M, Cirino G, Papapetropoulos A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol Res 111: 556–562, 2016. doi: 10.1016/j.phrs.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]