The physiological mechanisms and skeletal muscle bioenergetics underlying all-out exercise performance are unclear. This study revealed an increase in oxidative ATP synthesis rate gain and the ATP cost of contraction during all-out exercise. Furthermore, peripheral fatigue was related to the perturbation in pH and deprotonated phosphate ion. These findings support the concept that the oxygen uptake slow component arises from within active skeletal muscle and that skeletal muscle force generating capacity is linked to the intramuscular metabolic milieu.
Keywords: ATP synthesis, ATP cost, magnetic resonance spectroscopy, muscle metabolism, neuromuscular fatigue
Abstract
Although all-out exercise protocols are commonly used, the physiological mechanisms underlying all-out exercise performance are still unclear, and an in-depth assessment of skeletal muscle bioenergetics is lacking. Therefore, phosphorus magnetic resonance spectroscopy (31P-MRS) was utilized to assess skeletal muscle bioenergetics during a 5-min all-out intermittent isometric knee-extensor protocol in eight healthy men. Metabolic perturbation, adenosine triphosphate (ATP) synthesis rates, ATP cost of contraction, and mitochondrial capacity were determined from intramuscular concentrations of phosphocreatine (PCr), inorganic phosphate (Pi), diprotonated phosphate (), and pH. Peripheral fatigue was determined by exercise-induced alterations in potentiated quadriceps twitch force (Qtw) evoked by supramaximal electrical femoral nerve stimulation. The oxidative ATP synthesis rate (ATPOX) attained and then maintained peak values throughout the protocol, despite an ~63% decrease in quadriceps maximal force production. ThusATPOX normalized to force production (ATPOX gain) significantly increased throughout the exercise (1st min: 0.02 ± 0.01, 5th min: 0.04 ± 0.01 mM·min−1·N−1), as did the ATP cost of contraction (1st min: 0.048 ± 0.019, 5th min: 0.052 ± 0.015 mM·min−1·N−1). Additionally, the pre- to postexercise change in Qtw (−52 ± 26%) was significantly correlated with the exercise-induced change in intramuscular pH (r = 0.75) and concentration (r = 0.77). In conclusion, the all-out exercise protocol utilized in the present study elicited a “slow component-like” increase in intramuscular ATPOX gain as well as a progressive increase in the phosphate cost of contraction. Furthermore, the development of peripheral fatigue was closely related to the perturbation of specific fatigue-inducing intramuscular factors (i.e., pH and concentration).
NEW & NOTEWORTHY The physiological mechanisms and skeletal muscle bioenergetics underlying all-out exercise performance are unclear. This study revealed an increase in oxidative ATP synthesis rate gain and the ATP cost of contraction during all-out exercise. Furthermore, peripheral fatigue was related to the perturbation in pH and deprotonated phosphate ion. These findings support the concept that the oxygen uptake slow component arises from within active skeletal muscle and that skeletal muscle force generating capacity is linked to the intramuscular metabolic milieu.
all-out exercise protocols are increasingly being utilized as an integral component of experimental designs (7, 22, 56, 58). The appeal of these protocols stems from the potential to determine multiple important physiological parameters in a single testing session. Indeed, maximal oxygen uptake (V̇o2max), a gold standard in human physiology (19, 48), is elicited during all-out cycling exercise lasting longer than 90 s (7, 58). Furthermore, the 3-min all-out cycling protocol allows for the determination of critical power (CP) (for review see Refs. 7, 53), a parameter with great utility in both health and disease (25, 44, 55). This protocol has been adapted to provide equivalent parameters for other exercise modalities (4, 6, 9, 42, 57), such as critical force (CF), during a 5-min all-out knee-extensor protocol (6). However, despite growing use of these protocols, the physiological mechanisms underlying performance during all-out exercise are still under investigation, and an in-depth assessment of skeletal muscle bioenergetics (i.e., sources and rates of ATP synthesis) is currently lacking.
Interestingly, all-out exercise exhibits an oxygen uptake (V̇o2) slow component (7, 18, 52, 56, 59). The V̇o2 slow component, originally described for constant-work-rate exercise above the lactate threshold, is manifest as additional V̇o2, predominantly arising from a loss of efficiency within the exercising skeletal muscle (43, 45, 47), and is closely related to exercise tolerance (40). During all-out exercise, the V̇o2 slow component likely arises as a result of all motor units being recruited at the onset of exercise and motor units dropping out as fatigue ensues (i.e., decreased force production), while V̇o2max is maintained (7, 18, 52, 56, 59). Importantly, these findings demonstrate that progressive motor unit recruitment is not requisite for the V̇o2 slow component and therefore provides an interesting approach to study the mechanisms by which the V̇o2 slow component originates. In particular, it remains to be determined whether the slow component during all-out exercise is still evident during small-muscle-mass exercise and actually arises from the exercising skeletal muscle.
Remarkably, the magnitudes of intramuscular metabolic perturbation during the 3-min all-out cycling and 5-min all-out knee-extensor protocols are very similar (8, 52). Specifically, intramuscular phosphocreatine concentration ([PCr]) decreased to ~20–25% of resting concentrations and muscle pH decreased to ~6.7, while muscle inorganic phosphate concentration ([Pi]) and blood lactate concentration increased to ~500% and ~1,200% of resting concentrations, respectively (8, 52). For high-intensity cycling exercise, the magnitude of intramuscular perturbation ([Pi] and pH) is closely related to peripheral fatigue (2). However, the robustness of this relationship across exercise modality and exercising muscle mass remains unknown. Of note, the 5-min all-out knee-extensor protocol both induces a high degree of peripheral fatigue [~50% reduction in potentiated quadriceps twitch force (Qtw)] (6) and allows for phosphorus magnetic resonance spectroscopy (31P-MRS) measurements of intramuscular metabolites (8), making this an ideal exercise paradigm to further assess the relationship between the magnitude of intramuscular perturbation and the development of peripheral fatigue.
Therefore, the purpose of this study was to quantitatively characterize skeletal muscle bioenergetics and peripheral fatigue during all-out exercise. Specifically, we utilized 31P-MRS to determine the rate and source of ATP production and electrical femoral nerve stimulation to determine peripheral fatigue for all-out intermittent isometric single-leg knee-extensor exercise. We hypothesized that during the all-out exercise 1) the oxidative ATP synthesis rate (ATPOX) would reach and then remain at peak values, while force production would progressively decrease, indicating an intramuscular V̇o2 slow component; 2) the ATP cost of contraction would progressively increase; and 3) in terms of fatigue, the reduction in quadriceps Qtw would be related to the change in both intramuscular [Pi] and pH.
METHODS
Subjects.
Eight healthy men (age 28 ± 5 yr, stature 178 ± 4 cm, and body mass 77 ± 8 kg) volunteered and provided written informed consent to participate in this investigation. All experimental procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Department of Veterans Affairs Medical Center. Subjects were instructed to abstain from vigorous activity during the 24 h preceding each visit to the laboratory and to arrive at the laboratory having abstained from food and caffeine during the preceding 3 h. Subjects were tested in the laboratory a minimum of twice with at least 72 h between visits.
Experimental design.
Single-leg intermittent isometric knee-extensor exercise (3-s contraction and 2-s relaxation) was performed for all exercise protocols. This was conducted in a semirecumbent position (~15° elevation of the trunk), with the knee of the leg to be exercised situated over a custom-built knee support (~45° knee joint angle), the ankle fixed to an immovable strain gauge (SSM-AJ-250, Interface), and nonelastic straps positioned over the hips and thigh. The choice of exercising leg was balanced for dominance across subjects. During the initial visit, subjects performed 60 maximal voluntary quadriceps contractions (MVCs) over 5 min with neuromuscular function assessments before and immediately after exercise (i.e., at the 60th MVC). This 60-MVC protocol with 5 min of recovery was then completed inside a whole body magnetic resonance imaging (MRI) system during the second visit. An audio recording cued the start and stop of each 3-s contraction, but no information regarding the duration of exercise was provided to the subjects. The integrated force, mean force, and peak force were determined for each of the 60 MVCs, and CF was defined as the mean force of the final 6 MVCs (6).
Neuromuscular function.
During the initial visit, neuromuscular function assessments were conducted before and immediately after exercise. The femoral nerve was stimulated with a constant-current stimulator (model DS7AH, Digitimer), with the anode placed in the femoral triangle and the cathode placed between the greater trochanter and the iliac crest. Low-intensity single-pulse stimuli (200-μs pulse width, 100–150 mA) were used to locate the optimal position of the stimulating electrode, defined as the location evoking the greatest force production. The electrodes were then fixed in position until all measurements for the visit were conducted. The stimulation intensity was increased in 20-mA increments until maximal force was obtained. The stimulator intensity was then set to 120% of this, to ensure supramaximality. For the evaluation of quadriceps function, Qtw was performed 2 s after 3-s MVCs both before exercise and then immediately after exercise (i.e., at the 60th MVC). For each Qtw, the contraction time (CTQtw) and half-relaxation time (0.5RTQtw) were determined. During each MVC, a superimposed twitch was delivered and voluntary activation of the quadriceps was calculated as VA = [1 − (superimposed twitch/Qtw)] × 100.
31P-MRS.
A clinical 2.9-T MRI system (Tim-Trio, Siemens Medical Systems, Munich, Germany) operating at 49.9 MHz (31P resonance) and a dual-tuned 31P-1H surface coil (110-mm 1H coil loop surrounded by 31P single-loop coil with a diameter of 125 mm) with linear polarization were utilized to acquire 31P-MRS data (RAPID Biomedical, Rimpar, Germany). The surface coil was secured over the midthigh with elastic straps, and advanced localized volume shimming was performed after a three-plane scout proton image was acquired to ensure that all major quadriceps muscles were sampled. Two fully relaxed spectra were acquired (3 averages per spectrum and a repetition time of 30 s) before the 60-MVC protocol commenced. Throughout exercise and recovery, MRS data acquisition was conducted with a free-induction decay pulse sequence with a 2.56-ms adiabatic-half-passage excitation RF pulse, a repetition time of 2.5 s, a receiver bandwidth of 5 kHz, 1,024 data points, and 2 averages per spectrum. Comparisons between fully relaxed and partially relaxed spectra were utilized to quantify saturation factors.
A time-domain fitting routine using the AMARES algorithm (51) incorporated into CSAIPO software (33–35) was utilized to determine absolute and relative concentrations of intramuscular PCr, Pi, , and ATP. Intracellular pH was calculated from the chemical shift difference between the Pi and PCr signals. The free cytosolic [ADP] was calculated from [PCr] and pH with the creatine kinase (CK) equilibrium constant (KCK = 1.66 × 109 M−1) and the assumption that PCr represents 85% of the total creatine content (23). Resting concentrations were calculated from the average peak areas of the two fully relaxed spectra and assuming a resting [ATP] of 8.2 mM (16). To account for Pi splitting, the pH corresponding to each Pi pool was calculated separately as pH1 and pH2 on the basis of the chemical shift of each peak relative to PCr, such that the overall pH was then calculated as
The concentration of was calculated as (32)
Free cytosolic adenosine monophosphate (AMP) was calculated based on the equilibrium of the adenylate kinase reaction corrected for the effects of pH and assuming a free magnesium concentration of 1 mM (15). Relative amplitudes were corrected for partial saturation due to the repetition time relative to T1 with the fully relaxed spectra acquired at rest.
ATP synthesis rates and ATP cost of contraction.
The rate of ATP production from the breakdown of PCr through the CK reaction (ATPCK, mM/min) was calculated from the change in [PCr] for each time point of the exercise period (27):
Based on the sigmoid relationship between ATPOX (mM/min) and free cytosolic [ADP], the rate of mitochondrial ATP production was calculated as
in which Km (the [ADP] at half-maximal oxidation rate) is ~30 μM in skeletal muscle (27), 2.2 is the Hill coefficient for a sigmoid function (24), and Vmax is the peak rate of in vivo oxidative ATP synthesis (see PCr recovery kinetics).
During exercise, changes in intramuscular pH result from glycogen breakdown to pyruvate and lactate, proton efflux, buffering capacity, protons produced by oxidative phosphorylation, and the consumption of protons by the CK reaction (27). Assuming that the glycogenolytic production of 1 mol of H+, when coupled to ATP hydrolysis, yields 1.5 mol of ATP, the ATP production from anaerobic glycolysis (ATPGLY) can be deduced from the total number of protons (P) produced throughout exercise (20, 27, 28):
where
(in mM/min) was calculated from the time-dependent changes in [PCr] and from the stoichiometric coefficient (γ):
where γ is the proton stoichiometric coefficient of the coupled Lohmann reaction as previously described (31). (in mM/min) was calculated from the apparent buffering capacity βtotal (in slykes, millimoles acid added/unit change in pH) and from the rate of pH changes:
where
where
in which βa was determined from the initial change in PCr (ΔPCri) and alkalinization of pH (ΔpH) (10):
was determined based on the dissociation constant of the buffer (K) according to the standard formula (11):
where K = 1.77 × 10−7. In agreement with previous studies and assuming that muscle is a closed system during exercise (11, 28), βbicarbonate was set to zero. (in mM/min) was calculated from the factor m = 0.16/[1 + 10(6.1 – pH)], which accounts for the amount of protons produced through oxidative ATP production (28, 29):
(in mM/min) was calculated for each time point of exercise using the proportionality constant λ relating proton efflux rate to ΔpH (28, 29):
This proportionality constant λ (in mM·min−1·pH unit−1) was calculated during the recovery period:
During the recovery period, PCr is regenerated throughout the CK reaction as the consequence of oxidative ATP production in mitochondria. Thus can be calculated from the rates of proton production from the CK reaction (, in mM/min) and mitochondrial ATP production (, in mM/min) on one side and the rate of pH changes on the other side. At this time, ATP production is exclusively aerobic and lactate production is considered negligible:
To improve precision, a modified version of this calculation was used (28, 29), in which the total proton disappearance (i.e., ∫Edt) is estimated cumulatively from the start of recovery and then fitted to an exponential function to obtain the initial recovery rate (Vefflux).
The total ATPase rate (ATPTOTAL, in mM/min) was calculated for each time point as
and the anaerobic ATPase rate (ATPANA, mM/min) was calculated for each time point as
The ATP cost of contraction (in mM/N) was calculated as the ratio between ATPTOTAL and the force integral. The ATPOX and ATPANA gains were determined as the respective ATP synthesis rate normalized to the force integral. All values were calculated for each minute of exercise.
PCr recovery kinetics.
The [PCr] kinetics during the recovery period were described by a monoexponential curve:
where [PCr](t) is the [PCr] at a given time t, [PCr]end is the [PCr] at end exercise, Δ[PCr] is the amount of PCr resynthesized during the recovery period, and τ represents the time constant of the PCr offset kinetics. The initial rate of PCr resynthesis (ViPCr) was calculated from the derivative of the monoexponential equation at the onset of recovery:
where Δ[PCr] is the amount of PCr resynthesized during the recovery period and the rate constant k = 1/ τ (27). The peak rate of in vivo oxidative ATP synthesis (Vmax) was calculated with ViPCr and the [ADP] at end exercise (50):
where Km (the [ADP] at half the highest oxidative rate) is ~30 μM in skeletal muscle (27).
Model variables were determined with an iterative process by minimizing the sum of squared residuals (RSS) between the fitted function and the observed values. Goodness of fit was assessed by visual inspection of the residual plot and the frequency plot distribution of the residuals, with the χ2 values and the coefficient of determination (r2) calculated as follows (39):
Statistical analysis.
Force, 31P-MRS, ATP synthesis, ATP cost of contraction, ATPOX gain, and ATPANA gain were analyzed by one-way ANOVAs with repeated measures. A two-way ANOVA with repeated measures was used to analyze the percent contribution of ATPOX and ATPANA to ATPTOTAL. Tukey’s post hoc analyses were conducted when significant main effects were detected. Preexerecise-to-postexercise comparisons for MVC, Qtw, and VA were made with Student’s paired t-tests. ATPOX was compared with the 95% confidence interval (CI) around Vmax to determine whether maximal oxidative ATP production was attained. The relationships between neuromuscular function measurements and intramuscular metabolic perturbations were assessed with Pearson product moment correlation coefficients. Significance for the statistical analysis was accepted at P < 0.05. Results are presented as means ± SD, except in figures, where SE is used for clarity.
RESULTS
Force production, intramuscular metabolites, and neuromuscular function.
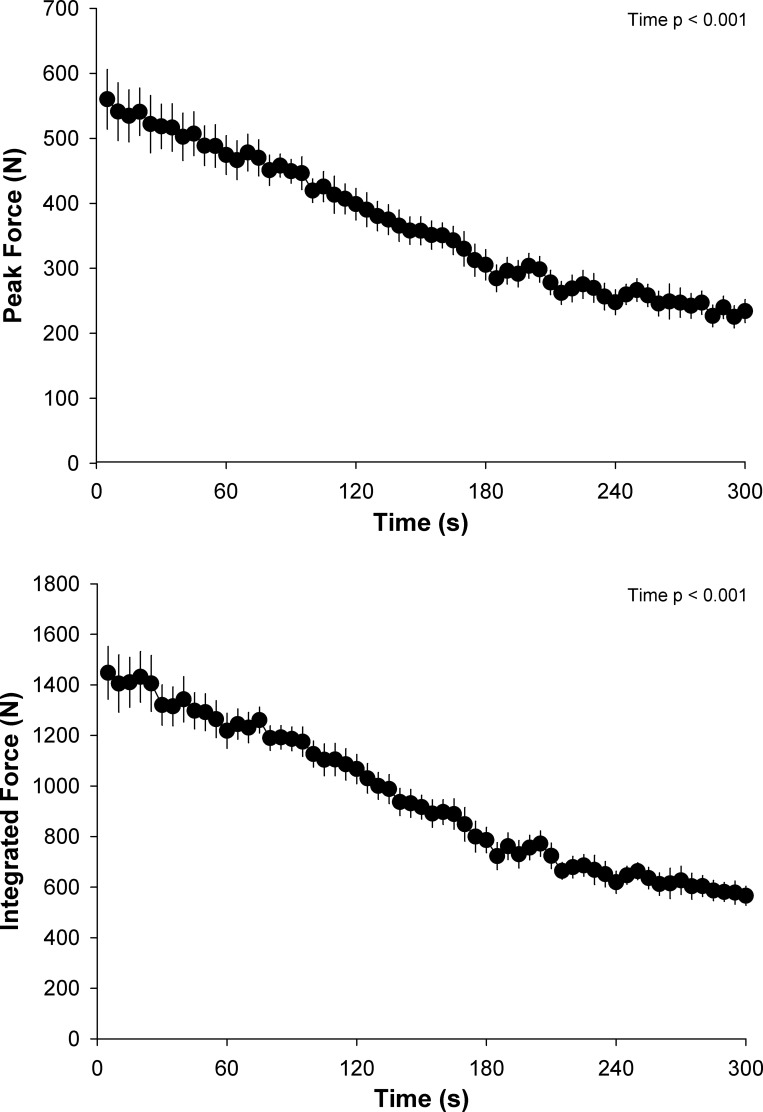
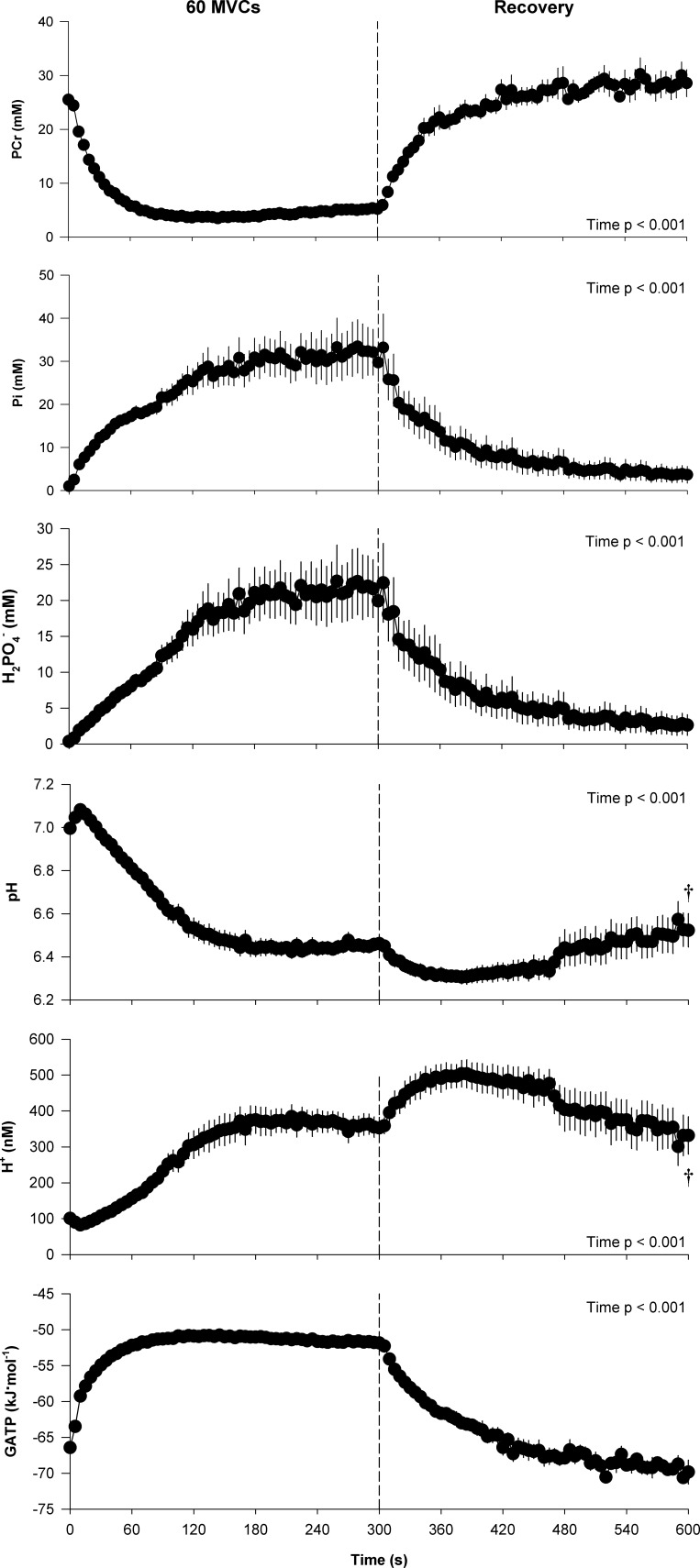
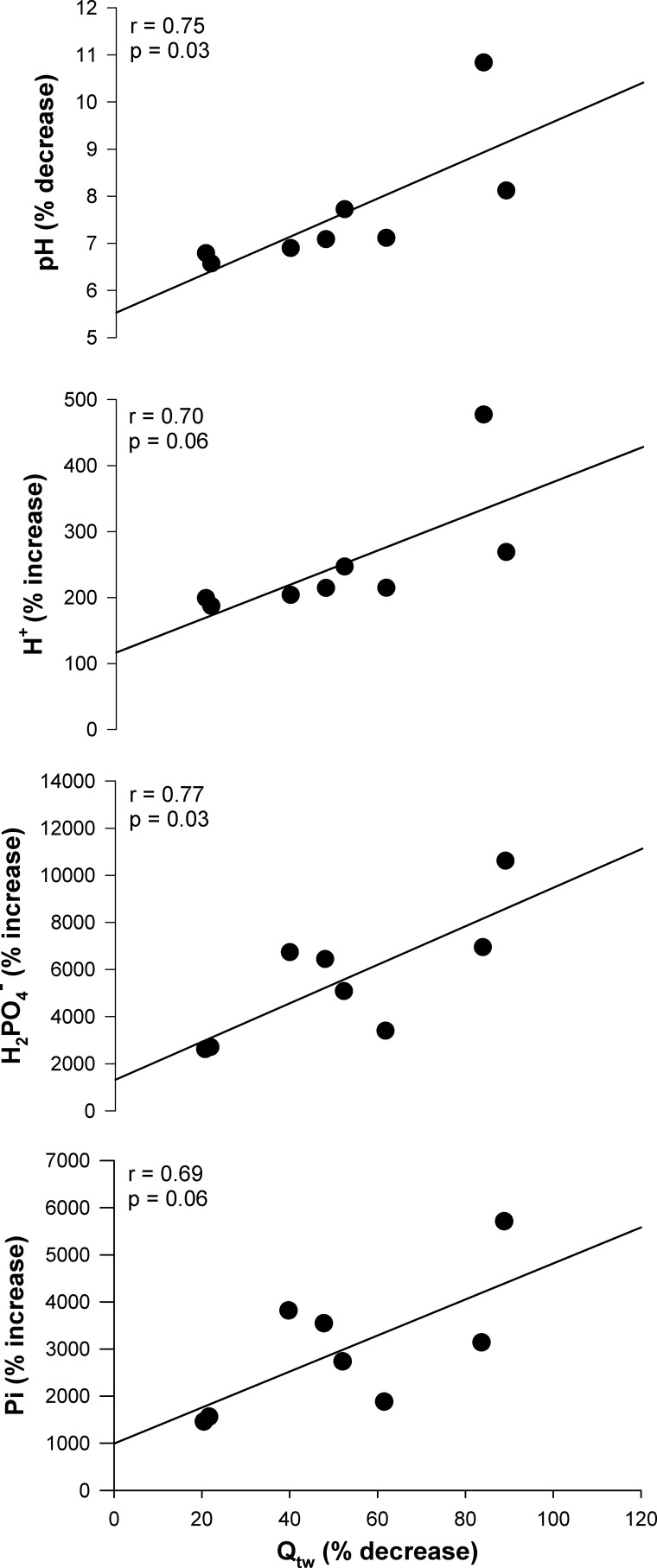
The peak MVC force attained during the protocol was 568 ± 126 N, which significantly decreased to 234 ± 50 N by the final MVC (Fig. 1). Throughout the majority of the exercise protocol both the peak force and the integrated force per MVC significantly decreased over time (Fig. 1). However, as illustrated, force did not significantly change over the final 30 s of the test and the resulting CF was 209 ± 41 N, which corresponded to 38 ± 10% of the peak force during the protocol. The intramuscular metabolic responses to the 60-MVC protocol and postexercise recovery are presented in Fig. 2. All variables changed significantly as a function of time during both the exercise and recovery periods. Intramuscular pH remained significantly below, and [H+] significantly above, baseline values for the entire duration of the 5-min recovery period, while [PCr], [Pi], [], and free energy of ATP hydrolysis [ΔGATP] were not significantly different from baseline values by the end of this period. The force generating capacity of the quadriceps was significantly reduced immediately after exercise, as measured by the pre- to postexercise reduction in MVC. This decrease was accompanied by significant central (ΔVA: −12 ± 11%) and peripheral (ΔQtw: −52 ± 26%) fatigue (Table 1). The fall in Qtw was significantly correlated with the decrease in intramuscular pH and increase in intramuscular [] (Fig. 3), while the correlations with the increase in intramuscular [H+] and [Pi] tended to be significantly related, with P values of 0.06.
Fig. 1.
Force development during the 5-min all-out intermittent isometric single-leg knee-extensor protocol. Subjects performed a series of 60 intermittent maximal voluntary contractions (3-s contraction, 2-s relaxation) over 5 min. Peak force and mean force were determined per maximal voluntary contraction.
Fig. 2.
Intramuscular metabolic perturbation during the 5-min all-out intermittent isometric single-leg knee-extensor protocol. Intramuscular metabolite concentrations were determined with phosphorus magnetic resonance spectroscopy. †Significantly different from baseline.
Table 1.
Changes in neuromuscular function with an all-out intermittent isometric single-leg knee-extensor exercise test
| Preexercise | Postexercise | %Δ Preexercise to Postexercise | |
|---|---|---|---|
| Qtw, N | 187 ± 26 | 89 ± 46† | −52 ± 26 |
| VA, % | 91 ± 5 | 80 ± 12† | −12 ± 11 |
| CTQtw, ms | 79 ± 9 | 69 ± 8† | −13 ± 28 |
| 0.5RTQtw, ms | 54 ± 7 | 49 ± 22 | −12 ± 6 |
Values are means ± SD. Qtw, potentiated quadriceps twitch force; VA, voluntary activation; CTQtw, contraction time; 0.5RTQtw, half-relaxation time.
Significantly different from preexercise.
Fig. 3.
Relationship between quadriceps fatigue and intramuscular metabolites. Data are expressed as % difference from baseline to end exercise for intramuscular pH and hydrogen ion ([H+]), inorganic phosphate ([Pi]), and diprotonated phosphate ([]) concentrations vs. preexercise-to-postexercise % difference for the potentiated quadriceps twitch force (Qtw).
ATP synthesis rates and ATP cost of contraction.
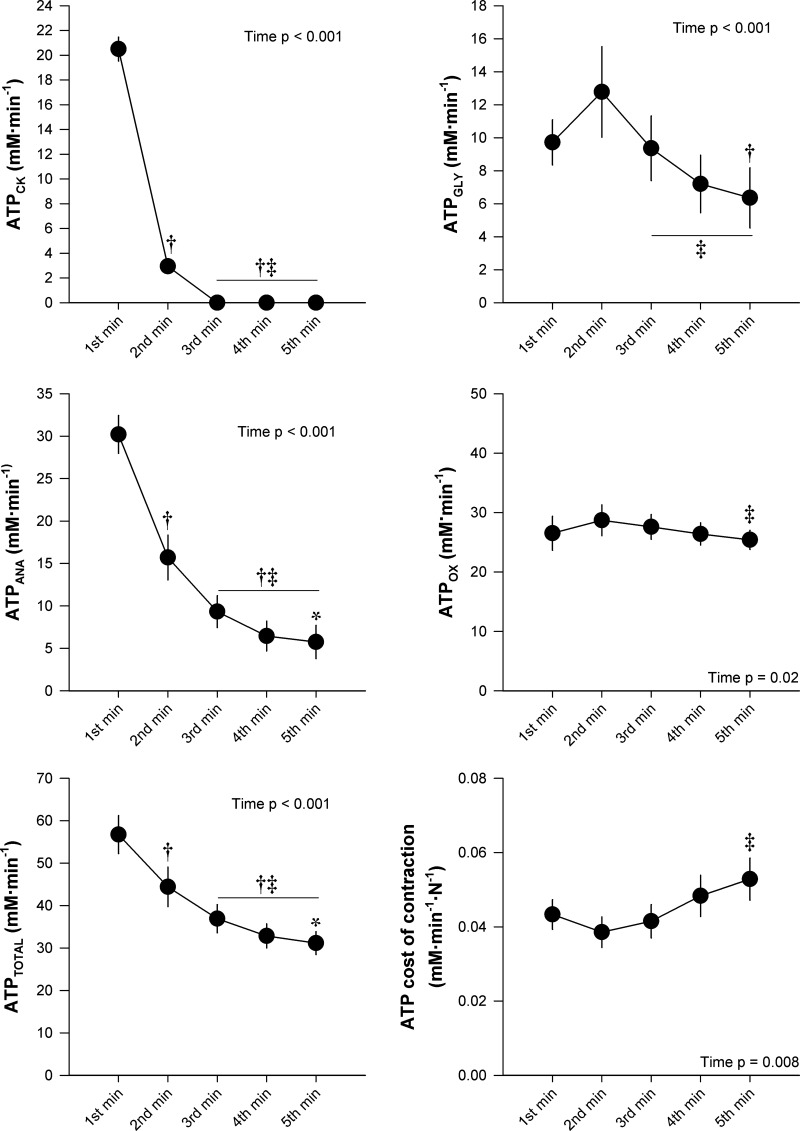
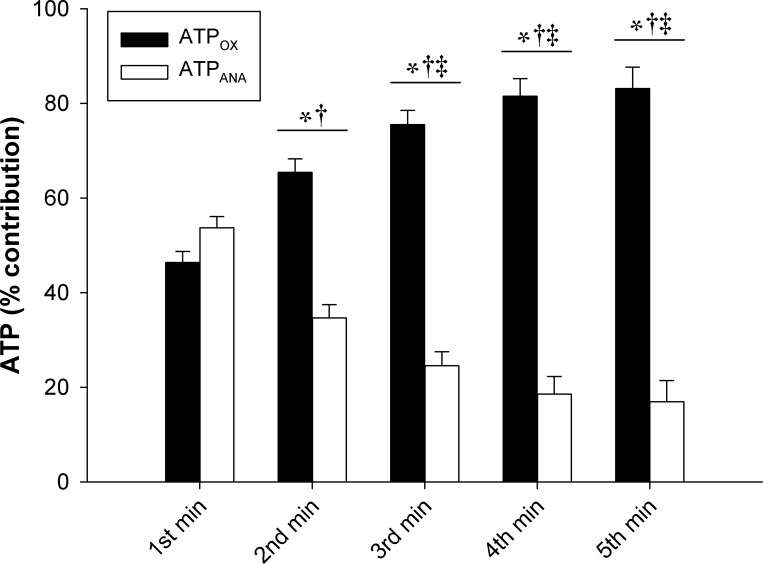
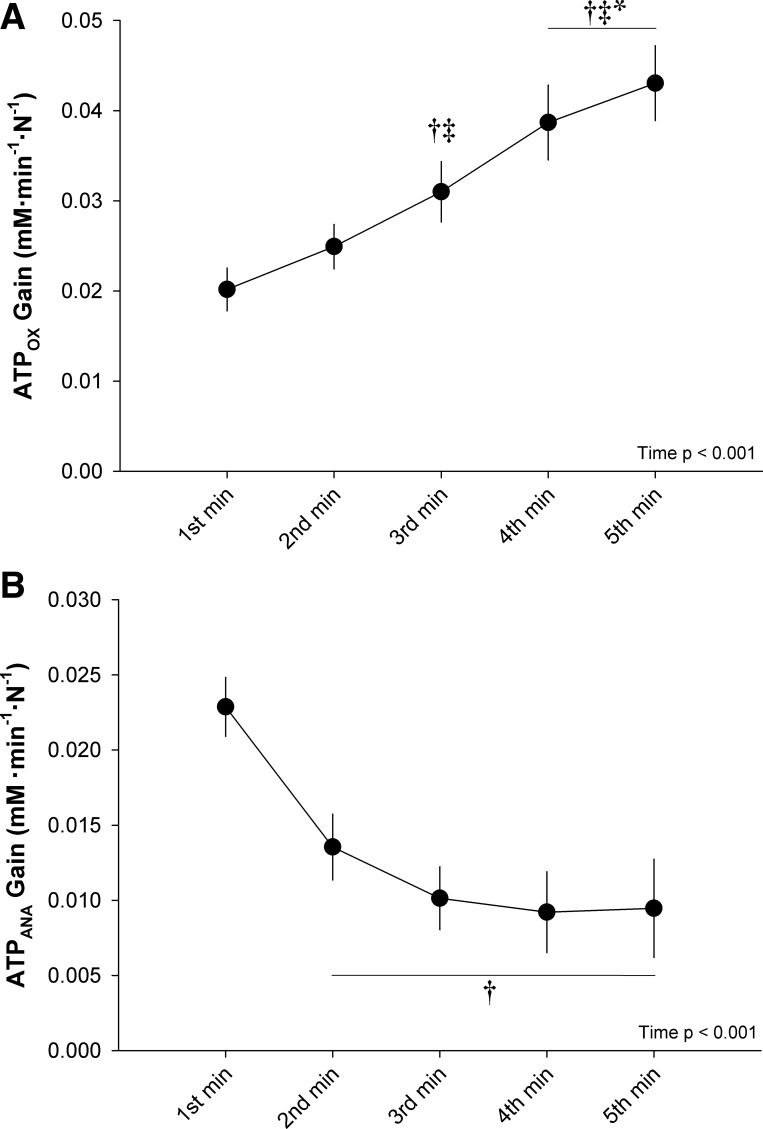
ATP synthesis rates and ATP cost of contraction data are presented in Fig. 4. ATPCK, ATPGLY, ATPANA, and ATPTOTAL significantly decreased throughout the exercise test. ATPOX remained within the Vmax 95% CI (30.8 ± 8.7 mM·min−1·N−1, 95% CI: 23.5–38.1 mM·min−1·N−1) throughout the entire protocol. There was a significant, but transient, increase in ATPOX during the protocol, with a small significant difference between the second and fifth minutes. The ATP cost of contraction significantly increased as a function of time during the exercise. The change in ATP cost of contraction was not significantly correlated with changes in any of the neuromuscular function measures. Data for the percent contribution of ATPOX and ATPANA to ATPTOTAL are presented in Fig. 5. The percentage of ATPTOTAL arising from ATPANA significantly decreased over the first 2 min, while the percentage arising from ATPOX significantly increased over the first 2 min, with no significant changes in either variable thereafter. The percent contribution from ATPOX was significantly greater than ATPANA for minutes 2–5 (Fig. 5). During exercise at CF (i.e., the final 30 s), the percentage of ATPTOTAL arising from ATPOX (83 ± 13%) was significantly greater than ATPANA (17 ± 13%). The ATPOX gain significantly increased throughout the exercise, while the ATPANA gain significantly decreased from the first minute to the second minute and did not significantly change thereafter (Fig. 6).
Fig. 4.
Adenosine triphosphate (ATP) synthesis rates and ATP cost of contraction during 5-min all-out intermittent isometric single-leg knee-extensor protocol. Rate of ATP synthesis through creatine kinase reaction (ATPCK), anaerobic glycolysis (ATPGLY), cumulative anaerobic metabolism (ATPANA), and oxidative phosphorylation (ATPOX), total ATPase rate (ATPTOTAL), and ATP cost of contraction were determined for each minute of exercise. Significantly different: †from 1st minute, ‡from 2nd minute, *from 3rd minute (P < 0.05).
Fig. 5.
Contribution of oxidative and anaerobic adenosine triphosphate (ATP) production during 5-min all-out intermittent isometric single-leg knee-extension protocol. Rates of ATP synthesis through oxidative phosphorylation (ATPOX) and anaerobic metabolism (ATPANA) are expressed relative to total ATPase rate (ATPTOTAL). Significantly different: *between conditions, †from 1st minute, ‡from 2nd minute (P < 0.05).
Fig. 6.
Oxidative and anaerobic adenosine triphosphate (ATP) gain during 5-min all-out intermittent isometric single-leg knee-extension protocol. Gains were determined as the rate of ATP synthesis through oxidative phosphorylation (ATPOX) and anaerobic metabolism (ATPANA) normalized to the integrated force. Significantly different: †from 1st minute, ‡from 2nd minute, *from 3rd minute (P < 0.05).
DISCUSSION
This study utilized 31P-MRS to quantitatively characterize skeletal muscle bioenergetics during all-out exercise. Consistent with our first hypothesis, ATPOX attained and remained at peak values, while force production progressively decreased throughout the all-out exercise. As a result, the ATPOX gain progressively increased in a “slow component-like” manner, similar to the V̇o2 slow component documented during all-out cycling exercise. Furthermore, the ATP cost of muscle contraction progressively increased throughout the all-out exercise, which is consistent with our second hypothesis. This provides evidence of an increased phosphate cost of force generation across time with such all-out exercise. In agreement with our third hypothesis, the exercise-induced reduction in Qtw was related to the changes in intramuscular [] and pH. This finding reinforces the link between the magnitude of intramuscular perturbation and peripheral fatigue that has previously been documented for large-muscle-mass cycling exercise. In combination, the findings of this study offer a novel, in-depth assessment of skeletal muscle bioenergetics during all-out exercise, while also providing mechanistic insight in the determinants of the V̇o2 slow component and neuromuscular fatigue during exercise.
Bioenergetics of all-out exercise.
The force profile and intramuscular metabolic perturbation (both magnitude and time course) in this study were similar to previous reports for both the 5-min all-out knee-extensor and 3-min all-out cycling protocols (6, 8, 52, 53). However, of importance, the present work builds upon and extends the findings of these previous studies by assessing the sources and rates of ATP synthesis throughout the all-out exercise (Fig. 4). Consistent with the role of PCr as an energy buffer, ATPCK peaked during the first minute of exercise and then rapidly decreased to no longer measurably contribute to ATPTOTAL over the third through fifth minutes of exercise. ATPGLY peaked during the second minute and then decreased, yet still contributed ~20% of the ATPTOTAL during the final 3 min of the exercise. Concomitantly, ATPOX attained maximal values (i.e., not different from Vmax) during the first minute of exercise, which were maintained throughout the remainder of the protocol. In concert, the time course changes in ATPANA and ATPOX resulted in a peak in ATPTOTAL during the first minute of exercise, which progressively decreased thereafter. This is consistent with the suggestion that, during fatiguing exercise, muscle force production declines so that intramuscular [ATP] is maintained within a certain range (21). Additionally, the proportion of ATPTOTAL arising from ATPANA and ATPOX progressively shifted from ~50/50% during the first minute to ~20/80% over the final 3 min of exercise (Fig. 5). These findings provide a novel, comprehensive characterization of skeletal muscle bioenergetics during all-out exercise.
ATP cost of contraction during all-out exercise.
Importantly, normalizing ATPTOTAL to force production unveiled a progressive increase in the ATP cost of contraction from the second minute of exercise onward (Fig. 4). For this exercise paradigm, the increased ATP cost of contraction is likely not related to a progressive recruitment of higher-order muscle fibers, as all motor units appear to be recruited at the onset of such all-out exercise (6, 36, 49, 56). Moreover, motor unit recruitment, assessed by electromyography, has been documented to progressively decrease during all-out cycling and knee-extension exercise (6, 56). The findings of the present study also demonstrate that the increased ATP cost of contraction is likely not related to changes in the contractile properties of the muscle fibers, as the change in ATP cost was not related to changes in neuromuscular function (i.e., Qtw, CTQtw, and 0.5RTQtw). Rather, the dramatic decline in MVC force (~63%) in the face of a moderate decrease in ATP synthesis (~40%) lends support to the hypothesis of persistent metabolism in muscle fibers that are contributing less to the overall force production as a result of fatigue. In this regard, it is interesting to note that the ATPANA gain attained a plateau after 3 min of exercise, while the ATPOX gain increased continuously throughout the exercise (Fig. 6). Although it is not possible to definitively determine the metabolic contribution of motor units comprising different muscle fiber types with the present experimental design, these results are consistent with findings in isolated single myocytes that fatigue-resistant muscle fibers exhibit a greater oxidative ATP cost of contraction during fatiguing exercise, whereas more fatigable muscle fibers reduce metabolic demand proportionally to the fall in tension (17).
Peripheral fatigue and intramuscular metabolic perturbation during all-out exercise.
The all-out exercise-induced central and peripheral fatigue and intramuscular metabolic perturbation were closely related in the present study (Fig. 2). Specifically, the reduction in Qtw was correlated to changes in [], [H+], [Pi], and pH. These findings are consistent with a previous report from our group that documented similar relationships during high-intensity cycling exercise (2). Collectively, these findings demonstrate that the link between exercise-induced peripheral fatigue and intramuscular metabolic perturbation is robust across exercise modalities involving quite different amounts of muscle. Mechanistically, the robustness of these relationships provides further, in vivo, evidence of an important role of these metabolites in the development of peripheral fatigue (1). Interestingly, while a low cellular pH or elevated [Pi] has been demonstrated to diminish skeletal muscle function (13, 30), it has recently been demonstrated that these metabolites can also interact synergistically in the development of peripheral fatigue (41). Moreover, the apparent differences in the time course changes between the intramuscular metabolites and force production in the present study further suggest that fatigue development is likely a complex process with synergistic mechanisms.
Implications for V̇o2 slow component.
The present data suggest that an intramuscular “V̇o2max” was attained throughout the all-out exercise, as ATPOX values were similar to the peak rate of in vivo oxidative ATP synthesis (i.e., Vmax). This finding is consistent with the attainment of pulmonary V̇o2max during whole body all-out exercise protocols (7, 9, 22, 52, 53). However, such findings, at the muscle level, bolster the use of the all-out exercise protocol as a simple and practical test to determine CF and muscle aerobic capacity in a single test. Interestingly, ATPOX maintained maximal values despite a ~63% reduction in force production in the present study, resulting in the progressive increase in ATPOX gain throughout the exercise (Fig. 6). Importantly, this reveals an intramuscular slow component in the rate of oxidative phosphorylation elicited during the all-out exercise that arose from a progressive loss of muscle efficiency within the exercising skeletal muscle. These findings further support that the V̇o2 slow component arises from the exercising skeletal muscle (45, 47) and that progressive recruitment of higher-order muscle fibers is not requisite to evoke the V̇o2 slow component (18, 52, 56, 59). These findings are also consistent with the expression of a pulmonary V̇o2 slow component during a 3-min all-out cycling test (52, 56).
Implications for CF.
From the outset of discussing the implications for CF, although it is recognized that CP and CF are different, as the implications of assessing both CP and CF are very similar, for clarity, only the term CF will be used here. The growing body of evidence supports that CF is predominantly determined by oxidative energy production (3, 5, 12, 14, 26, 37, 38, 46, 52, 54). This interpretation comes from assessing the alteration in CF as a result of manipulations in oxygen delivery. However, the intramuscular contribution of ATPOX and ATPANA to ATPTOTAL has not been quantified during exercise at CF. In this study, it was demonstrated that ATPOX and ATPANA contributed ~80% and ~20% to ATPTOTAL during exercise at CF (i.e., final 30 s), respectively (Fig. 5). These findings support the concept that CF is predominantly determined by oxidative energy production and explains why manipulating oxygen delivery has such a significant impact on CF. Importantly, however, the findings of this study demonstrate that CF is not fully independent from ATPANA (specifically ATPGLY), which contributed ~20% to ATPTOTAL. In light of the observation that intramuscular metabolites do not progressively change during exercise at or slightly below CF (26, 46, 52), this ~20% ATPANA contribution is likely such that energy utilization and replenishment are in equilibrium and, therefore, no net change occurs. In support of this interpretation, pH was constant across the latter portion of the exercise protocol in the present study, despite the continued ~20% contribution from ATPANA.
Conclusions.
The quantitative characterization of skeletal muscle bioenergetics during all-out exercise revealed that within this paradigm a small muscle mass exhibits an intramuscular V̇o2 slow component, as well as a progressive increase in the phosphate cost of force generation. Additionally, the intramuscular metabolic perturbation was closely related to the development of peripheral fatigue during the all-out exercise. Collectively, these findings provide direct evidence supporting the concept that the V̇o2 slow component arises from within the active skeletal muscle and that the force generating capacity of skeletal muscle is closely linked to the intramuscular metabolic milieu.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants (HL-103786, HL-116579, HL-091830, and K99 HL-125756); Department of Veterans Affairs Rehabilitation Research and Development Merit Awards (E6910-R and E1697-R), SPiRE Grants (E1572-P and E1433-P), and Senior Research Career Scientist Award (E9275-L); and the Flight Attendant Medical Research Institute (YFEL141011).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.B., G.L., T.J.H., M.A., and R.S.R. conceived and designed research; R.M.B., G.L., T.J.H., M.A., and R.S.R. performed experiments; R.M.B., G.L., T.J.H., M.A., and R.S.R. analyzed data; R.M.B., G.L., T.J.H., M.A., and R.S.R. interpreted results of experiments; R.M.B., G.L., T.J.H., M.A., and R.S.R. prepared figures; R.M.B., G.L., T.J.H., M.A., and R.S.R. drafted manuscript; R.M.B., G.L., T.J.H., M.A., and R.S.R. edited and revised manuscript; R.M.B., G.L., T.J.H., M.A., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was conducted at the Utah Vascular Research Laboratory housed in the Salt Lake City Department of Veterans Affairs Medical Center.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broxterman RM, Ade CJ, Craig JC, Wilcox SL, Schlup SJ, Barstow TJ. Influence of blood flow occlusion on muscle oxygenation characteristics and the parameters of the power-duration relationship. J Appl Physiol (1985) 118: 880–889, 2015. doi: 10.1152/japplphysiol.00875.2014. [DOI] [PubMed] [Google Scholar]
- 4.Broxterman RM, Ade CJ, Poole DC, Harms CA, Barstow TJ. A single test for the determination of parameters of the speed-time relationship for running. Respir Physiol Neurobiol 185: 380–385, 2013. doi: 10.1016/j.resp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Broxterman RM, Ade CJ, Wilcox SL, Schlup SJ, Craig JC, Barstow TJ. Influence of duty cycle on the power-duration relationship: observations and potential mechanisms. Respir Physiol Neurobiol 192: 102–111, 2014. doi: 10.1016/j.resp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Burnley M. Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol (1985) 106: 975–983, 2009. doi: 10.1152/japplphysiol.91474.2008. [DOI] [PubMed] [Google Scholar]
- 7.Burnley M, Doust JH, Vanhatalo A. A 3-min all-out test to determine peak oxygen uptake and the maximal steady state. Med Sci Sports Exerc 38: 1995–2003, 2006. doi: 10.1249/01.mss.0000232024.06114.a6. [DOI] [PubMed] [Google Scholar]
- 8.Burnley M, Vanhatalo A, Fulford J, Jones AM. Similar metabolic perturbations during all-out and constant force exhaustive exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95: 798–807, 2010. doi: 10.1113/expphysiol.2010.052688. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CF, Yang YS, Lin HM, Lee CL, Wang CY. Determination of critical power in trained rowers using a three-minute all-out rowing test. Eur J Appl Physiol 112: 1251–1260, 2012. doi: 10.1007/s00421-011-2081-2. [DOI] [PubMed] [Google Scholar]
- 10.Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol 273: C306–C315, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Conley KE, Kushmerick MJ, Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol 511: 935–945, 1998. doi: 10.1111/j.1469-7793.1998.935bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588: 5077–5087, 2010. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol 287: C673–C681, 2004. doi: 10.1152/ajpcell.00044.2004. [DOI] [PubMed] [Google Scholar]
- 14.Dekerle J, Mucci P, Carter H. Influence of moderate hypoxia on tolerance to high-intensity exercise. Eur J Appl Physiol 112: 327–335, 2012. doi: 10.1007/s00421-011-1979-z. [DOI] [PubMed] [Google Scholar]
- 15.Golding EM, Teague WE Jr, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198: 1775–1782, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Harris RC, Hultman E, Nordesjö LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974. doi: 10.3109/00365517409082477. [DOI] [PubMed] [Google Scholar]
- 17.Hepple RT, Howlett RA, Kindig CA, Stary CM, Hogan MC. The O2 cost of the tension-time integral in isolated single myocytes during fatigue. Am J Physiol Regul Integr Comp Physiol 298: R983–R988, 2010. doi: 10.1152/ajpregu.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández A, McDonald JR, Lai N, Gladden LB. A prior bout of contractions speeds V̇o2 and blood flow on-kinetics and reduces the V̇o2 slow-component amplitude in canine skeletal muscle contracting in situ. J Appl Physiol (1985) 108: 1169–1176, 2010. doi: 10.1152/japplphysiol.01318.2009. [DOI] [PubMed] [Google Scholar]
- 19.Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med 16: 135–171, 1923. doi: 10.1093/qjmed/os-16.62.135. [DOI] [Google Scholar]
- 20.Hochachka PW, Mommsen TP. Protons and anaerobiosis. Science 219: 1391–1397, 1983. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- 21.Hogan MC, Richardson RS, Kurdak SS. Initial fall in skeletal muscle force development during ischemia is related to oxygen availability. J Appl Physiol (1985) 77: 2380–2384, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Hureau TJ, Olivier N, Millet GY, Meste O, Blain GM. Exercise performance is regulated during repeated sprints to limit the development of peripheral fatigue beyond a critical threshold. Exp Physiol 99: 951–963, 2014. doi: 10.1113/expphysiol.2014.077974. [DOI] [PubMed] [Google Scholar]
- 23.Jeneson JA, Westerhoff HV, Brown TR, Van Echteld CJ, Berger R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physiol Cell Physiol 268: C1474–C1484, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem 271: 27995–27998, 1996. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 25.Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC. Critical power: implications for determination of V˙O2max and exercise tolerance. Med Sci Sports Exerc 42: 1876–1890, 2010. doi: 10.1249/MSS.0b013e3181d9cf7f. [DOI] [PubMed] [Google Scholar]
- 26.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294: R585–R593, 2008. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- 27.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994. [PubMed] [Google Scholar]
- 28.Kemp GJ, Taylor DJ, Styles P, Radda GK. The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR Biomed 6: 73–83, 1993. doi: 10.1002/nbm.1940060112. [DOI] [PubMed] [Google Scholar]
- 29.Kemp GJ, Thompson CH, Taylor DJ, Radda GK. Proton efflux in human skeletal muscle during recovery from exercise. Eur J Appl Physiol Occup Physiol 76: 462–471, 1997. doi: 10.1007/s004210050276. [DOI] [PubMed] [Google Scholar]
- 30.Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol 575: 887–899, 2006. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol 272: C1739–C1747, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Layec G, Bringard A, Vilmen C, Micallef JP, Fur YL, Perrey S, Cozzone PJ, Bendahan D. Accurate work-rate measurements during in vivo MRS studies of exercising human quadriceps. MAGMA 21: 227–235, 2008. doi: 10.1007/s10334-008-0117-3. [DOI] [PubMed] [Google Scholar]
- 34.Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK, Richardson RS. Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin Sci (Lond) 128: 213–223, 2015. doi: 10.1042/CS20140274. [DOI] [PubMed] [Google Scholar]
- 35.Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F. Grid-free interactive and automated data processing for MR chemical shift imaging data. MAGMA 23: 23–30, 2010. doi: 10.1007/s10334-009-0186-y. [DOI] [PubMed] [Google Scholar]
- 36.McCartney N, Heigenhauser GJ, Jones NL. Power output and fatigue of human muscle in maximal cycling exercise. J Appl Physiol Respir Environ Exerc Physiol 55: 218–224, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Monod H, Scherrer J. The work capacity of a synergic muscular group. Ergonomics 8: 329–338, 1965. doi: 10.1080/00140136508930810. [DOI] [Google Scholar]
- 38.Moritani T, Nagata A, deVries HA, Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics 24: 339–350, 1981. doi: 10.1080/00140138108924856. [DOI] [PubMed] [Google Scholar]
- 39.Motulsky HJ, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. New York: Oxford Univ. Press, 2004. [Google Scholar]
- 40.Murgatroyd SR, Ferguson C, Ward SA, Whipp BJ, Rossiter HB. Pulmonary O2 uptake kinetics as a determinant of high-intensity exercise tolerance in humans. J Appl Physiol (1985) 110: 1598–1606, 2011. doi: 10.1152/japplphysiol.01092.2010. [DOI] [PubMed] [Google Scholar]
- 41.Nelson CR, Debold EP, Fitts RH. Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am J Physiol Cell Physiol 307: C939–C950, 2014. doi: 10.1152/ajpcell.00206.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettitt RW, Jamnick N, Clark IE. 3-min all-out exercise test for running. Int J Sports Med 33: 426–431, 2012. doi: 10.1055/s-0031-1299749. [DOI] [PubMed] [Google Scholar]
- 43.Poole DC, Barstow TJ, Gaesser GA, Willis WT, Whipp BJ. VO2 slow component: physiological and functional significance. Med Sci Sports Exerc 26: 1354–1358, 1994. [PubMed] [Google Scholar]
- 44.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48: 2320–2334, 2016. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol (1985) 71: 1245–1260, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31: 1265–1279, 1988. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- 47.Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol 541: 991–1002, 2002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993, p. 162–325. [Google Scholar]
- 49.Sargeant AJ, Hoinville E, Young A. Maximum leg force and power output during short-term dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol 51: 1175–1182, 1981. [DOI] [PubMed] [Google Scholar]
- 50.Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve 33: 524–531, 2006. doi: 10.1002/mus.20484. [DOI] [PubMed] [Google Scholar]
- 51.Vanhamme L, van den Boogart A, Van Huffel S . Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 52.Vanhatalo A, Black MI, DiMenna FJ, Blackwell JR, Schmidt JF, Thompson C, Wylie LJ, Mohr M, Bangsbo J, Krustrup P, Jones AM. The mechanistic bases of the power-time relationship: muscle metabolic responses and relationships to muscle fibre type. J Physiol 594: 4407–4423, 2016. doi: 10.1113/JP271879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanhatalo A, Doust JH, Burnley M. Determination of critical power using a 3-min all-out cycling test. Med Sci Sports Exerc 39: 548–555, 2007. doi: 10.1249/mss.0b013e31802dd3e6. [DOI] [PubMed] [Google Scholar]
- 54.Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95: 528–540, 2010. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- 55.Vanhatalo A, Jones AM, Burnley M. Application of critical power in sport. Int J Sports Physiol Perform 6: 128–136, 2011. doi: 10.1123/ijspp.6.1.128. [DOI] [PubMed] [Google Scholar]
- 56.Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol 300: R700–R707, 2011. doi: 10.1152/ajpregu.00761.2010. [DOI] [PubMed] [Google Scholar]
- 57.Wakayoshi K, Yoshida T, Kasai T, Moritani T, Mutoh Y, Miyashita M. Validity of critical velocity as swimming fatigue threshold in the competitive swimmer. Ann Physiol Anthropol 11: 301–307, 1992. doi: 10.2114/ahs1983.11.301. [DOI] [PubMed] [Google Scholar]
- 58.Williams CA, Ratel S, Armstrong N. Achievement of peak VO2 during a 90-s maximal intensity cycle sprint in adolescents. Can J Appl Physiol 30: 157–171, 2005. doi: 10.1139/h05-112. [DOI] [PubMed] [Google Scholar]
- 59.Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of V̇o2 kinetics. J Appl Physiol (1985) 105: 575–580, 2008. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]