Therapeutic hypothermia is now a class I recommendation for resuscitation from cardiac arrest. This study determined that hypothermia preserves gap junction coupling as well as Na+ channel function during acute cardiac ischemia, attenuating conduction slowing and preventing conduction block, suggesting that induced hypothermia may be a novel antiarrhythmic strategy in resuscitation.
Keywords: ischemia, arrhythmias, ventricular fibrillation, hypothermia, connexin43
Abstract
Acute cardiac ischemia induces conduction velocity (CV) slowing and conduction block, promoting reentrant arrhythmias leading to sudden cardiac arrest. Previously, we found that mild hypothermia (MH; 32°C) attenuates ischemia-induced conduction block and CV slowing in a canine model of early global ischemia. Acute ischemia impairs cellular excitability and the gap junction (GJ) protein connexin (Cx)43. We hypothesized that MH prevented ischemia-induced conduction block and CV slowing by preserving GJ expression and localization. Canine left ventricular preparations at control (36°C) or MH (32°C) were subjected to no-flow prolonged (30 min) ischemia. Optical action potentials were recorded from the transmural left ventricular wall, and CV was measured throughout ischemia. Cx43 and Na+ channel (NaCh) remodeling was assessed using both confocal immunofluorescence (IF) and/or Western blot analysis. Cellular excitability was determined by microelectrode recordings of action potential upstroke velocity (dV/dtmax) and resting membrane potential (RMP). NaCh current was measured in isolated canine myocytes at 36 and 32°C. As expected, MH prevented conduction block and mitigated ischemia-induced CV slowing during 30 min of ischemia. MH maintained Cx43 at the intercalated disk (ID) and attenuated ischemia-induced Cx43 degradation by both IF and Western blot analysis. MH also preserved dV/dtmax and NaCh function without affecting RMP. No difference in NaCh expression was seen at the ID by IF or Western blot analysis. In conclusion, MH preserves myocardial conduction during prolonged ischemia by maintaining Cx43 expression at the ID and maintaining NaCh function. Hypothermic preservation of GJ coupling and NaCh may be novel antiarrhythmic strategies during resuscitation.
NEW & NOTEWORTHY Therapeutic hypothermia is now a class I recommendation for resuscitation from cardiac arrest. This study determined that hypothermia preserves gap junction coupling as well as Na+ channel function during acute cardiac ischemia, attenuating conduction slowing and preventing conduction block, suggesting that induced hypothermia may be a novel antiarrhythmic strategy in resuscitation.
sudden cardiac arrest (SCA) is a leading cause of death in the United States, most commonly due to ischemia-induced reentrant ventricular arrhythmias (31). Targeted temperature management (TTM) is now an American Heart Association (AHA) class I recommendation for all patients comatose after resuscitation from arrest as it has shown both survival and neurological benefits (8). As most SCA is secondary to ischemia-induced ventricular fibrillation (VF), the effect of temperature on arrhythmias (or arrhythmia substrates) during acute ischemia is of significant importance. Severe hypothermia (<30°C) has been shown to cause reentrant ventricular arrhythmias (15, 33). However, the effect of milder hypothermic temperatures on arrhythmia substrates is unknown and the literature is conflicting, suggesting harmful effects, no effect, or beneficial effects on arrhythmia susceptibility, highlighting the need to understand the effects of TTM on arrhythmias during and after resuscitation (28, 32).
We previously found that mild hypothermia (MH), consistent with temperatures used during TTM, was antiarrhythmic during early global ventricular ischemia by attenuating ischemia-induced conduction slowing and conduction block, prerequisites for reentry (17, 24, 25). However, the mechanisms by which MH attenuates conduction slowing and conduction block remain unknown. Myocardial conduction is established by cellular excitability [determined primarily by Na+ channel (NaCh) current (INa)] and cell-to-cell communication [determined by gap junctions (GJs)]. Acute ischemia affects both connexin (Cx)4, the primary ventricular GJ protein, and NaCh function (9, 17). Specifically, ischemia induces time-dependent increases in intracellular resistance and conduction, which after the first 10–15 min of ischemia, directly correlate with decreases in GJ coupling (20, 29) and can be attributed to Cx43 dephosphorylation and/or lateralization away from the intercalated disk (ID), which is the first step in Cx43 degradation (7, 19, 30). Additionally, decreased expression of Cx43 at the ID further contributes to conduction slowing (1). Ischemia-induced Cx43 remodeling has been closely associated with the development of ventricular arrhythmias after the onset of electrical uncoupling, termed phase 1b arrhythmias (7, 29). Changes in NaCh function occur during acute ischemia as well, resulting in conduction slowing where resting membrane potential becomes depolarized during an ischemic insult, reducing NaCh availability (9).
Cx43 may respond to temperature-dependent regulation. Hibernating animals gain cardioprotective effects through upregulation of Cx43 expression (13). In addition, hypothermia can induce alterations in Cx43 localization (16). As Cx43 expression and localization are responsive to hypothermia, this suggests that MH may provide antiarrhythmic benefit by attenuating ischemia-induced Cx43 remodeling. However, NaCh function also is temperature dependent, as in Brugada syndrome, where arrhythmias due to reduced INa occur during fever (4, 26).
We sought to determine the mechanisms by which MH attenuates ischemia-induced conduction slowing. Our hypothesis was that MH prevents ischemia-induced conduction slowing by preserving GJ expression and specifically localization at the ID.
METHODS
Experimental preparation.
Experiments were carried out in accordance with Public Health Service guidelines for the care and use of laboratory animals. The study protocol was approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Twelve purpose-breed male mongrel dogs (Oak Hill, ~15–20 kg) were used in this study. Organ harvest by right lateral thoracotomy was performed under pentobarbital (30 mg/kg) general anesthesia, and the intact heart was rapidly excised. The details of our system for high-resolution transmural optical mapping of the arterially perfused canine wedge preparation, as initially described by Yan and Antzelevitch, were as previously described (2, 3, 12, 14, 34). Briefly, wedges of myocardium surrounding secondary branches of the left anterior descending artery and circumflex were harvested from the left ventricle (~3 × 2 × 1.5 cm). The corresponding branch of coronary artery was cannulated and Langendorff perfused with Tyrode solution (140 mM NaCl, 4.03 mM KCl, 1.8 mM CaCl2, 5.5 mM dextrose, 0.5 mM MgSO4, 0.9 mM NaH2PO4, and 10 mM HEPES and NaOH titrated to a pH 7.41 and oxygenated with 100% O2). The wedge was then placed in the chamber with the transmural surface against the glass imaging plate and perfused with a pressure of 40–50 mmHg and a perfusion rate of 12–20 ml/min. Pressure and rate were monitored throughout the experiment. Wedges were then perfused with a voltage-sensitive dye (di-4-ANNEPS, 15 μM; Sigma-Aldrich, http://www.sigmaaldrich.com/). Voltage-sensitive dye fluorescence (excited by 514 ± 5-nm light) was long-pass filtered at 610 nm and focused onto an array with 256 sites recording simultaneously with high spatial (0.89 mm), temporal (0.5 ms), and voltage (0.5mv) resolution. Optical magnification of 1−1.2× was used. Blebbistatin (5 μM, Enzo Life Sciences) was used in optically mapped wedges (n = 6 wedges/group) to eliminate motion artifacts (23, 24) and for microelectrode recordings. We have previously validated a method for precise homogeneous temperature manipulation in the canine wedge preparation (23, 24). The imaging chamber was encased in a water insulated circuit, and temperature was measured using a digital temperature probe (Omega) in the water bath, allowing for a temperature precision of ±0.1°C. Temperature was controlled at 36°C [control (CT) group] or 32°C (MH group) before the induction of ischemia so as to achieve and maintain specific temperature during the ischemia protocol. We specifically chose to achieve target temperature before the ischemia protocol to independently assess the effect of temperature on conduction and Cx43 without the potentially confounding effects of cooling during the dynamic electrophysiological changes that occur and are induced during ischemia. Target temperature was achieved over 20–30 min and stable for at least 10 min before the start of the ischemia protocol in all preparations.
CV measurements were analyzed using previously validated algorithms using custom software specifically designed for the analysis of optically recorded action potentials (6, 14). Activation times, defined as the maximal first derivative of phase 0 of the action potential, and action potential duration were measured directly from all optical action potentials. Cells were classified as epicardial, midmyocardial, or endocardial according to previously established criteria (2, 3). Conduction velocity (CV) vectors, which represent the magnitude and the direction of the CV at each recording site, were calculated by fitting the activation time to a parabolic surface (6). The components of the CV vector were then derived from the gradient of the surface at each site (6, 12). Isochronal maps that represent the positions of the wave front in 10-ms intervals were generated from activation maps and used to depict the spread of activation. CV vectors were averaged across sites located within 660 μm of the line that defined each transmurally oriented conduction path. Average velocities were calculated for conduction paths spreading from the endocardial stimulus site toward the epicardium. If there was epicardial conduction block, velocity vectors were averaged from those demonstrating transmurally oriented propagation of conduction.
Ischemia protocol.
We developed a model of global ischemia in the canine wedge preparation to evaluate the electrophysiologic effects of ischemia as seen in SCA. To induce global ischemia, the flow of oxygenated Tyrode solution was stopped. Measurements were made at 0, 5, 10, 15, 20, 25, and 30 min of no-flow ischemia to model prolonged ischemia. In a separate set of wedge preparations that were not optically mapped, with the use of the identical protocol and conditions but without blebbestatin perfusion, tissue was quickly frozen in liquid nitrogen for Western blot analysis or fixed in 10% formalin for confocal immunofluorescence (IF) at 15 or 30 min of ischemia. We similarly collected timed controlled nonischemic CT or MH wedge preparations at 15 min (early) or 30 min (prolonged). As conduction slowing and block were observed preferentially in the epicardium, all Western blot and IF studies were performed in epicardial tissue.
Western blot analysis and Triton X fractionation.
Western blot analysis was performed using conventional methods to isolate total protein Cx43 (CT: n = 8 and MH: n = 10) or Triton-X-insoluble (a.k.a. ID) Cx43 (n = 7 per group) or ID NaCh (n = 4 per group) using Triton-X (22). Total lysates were homogenized in RIPA buffer [150 mM NaCl, 1% Triton-X, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), 1 mM NaF, and 100 μM NaVO4 with Complete Mini protease inhibitor cocktail]. Tissue was homogenized on ice and lysed for 1 h at 4°C in Triton-X isolation buffer [50 mM Tris (pH 7.4), 1% Triton X-100, 2 mM EDTA, 2 mM EGTA, 250 mM NaCl, 1 mM NaF, and 0.1 mM Na3VO4 with Complete Mini protease inhibitor cocktail]. Lysates were spun at 13,000 g for 20 min at 4°C. The resulting supernatant was collected as the “soluble” fraction (cytosolic and free membrane Cx43) and added 1:1 to Triton-X isolation buffer with 8 M urea and 2 M thiourea (Sigma-Aldrich). The pellet or Triton-X-insoluble fraction (ID Cx43) was resuspended in Triton-X isolation buffer with 4 M urea and 1 M thiourea. Bradford Reagents were used for protein quantification (Bio-Rad, http://www.bio-rad.com/). Lysates were loaded with sample XT buffer and reducing agent (Bio-Rad). Samples were heated at 70°C for 10 min for Cx43 analysis or 37°C for 30 min for NaCh expression and run on 10% bis-Tris gels (Criterion-XT, Bio-Rad). Blots were probed for total Cx43 (1:1,000, rabbit, no. 71-0700, ThermoFisher Scientific, http://www.thermofisher.com/us/en/home.html), N-cadherin (1:1,000, mouse, no. 33-3900, ThermoFisher Scientific), NaCh (1:1,000, Sigma-Aldrich), or α-tubulin (1:6,000, mouse, T5168, Sigma-Aldrich) overnight. Western blots were developed using SuperSignal ECL (ThermoFisher-Scientific). Densitometry was performed using ImageJ software (https://imagej.nih.gov/ij/). Cx43 and NaCh were normalized to N-cadherin or α-tubulin.
Immunohistochemistry.
Paraffin-embedded tissues were coded for blind analysis, sliced (3 μm thick), and prepared for confocal IF. Two images per subject were analyzed when available for Cx43 (CT baseline: n = 8 images, CT ischemia: n = 11 images, MH baseline: n = 12 images, and MH ischemia: n = 12 images for Cx43). Sections were washed two times in xylene (Sigma-Aldrich) followed by a series of ethanol washes (100–50%). Sections were finally washed in double-distilled H20. For antigen retrieval, sections were boiled in citrate buffer (0.1 M sodium citrate and 0.1 M citric acid) for 30 min and cooled to room temperature. Sections were blocked [1% normal goat serum (Jackson ImmunoResearch, https://www.jacksonimmuno.com/), 0.1% Triton-X, 1% BSA, and PBS] for 90 min at room temperature. Sections were stained for total Cx43 (1:1,000, no. 71-0700, ThermoFisher Scientific) and N-cadherin (1:1,000, no. 333900, ThermoFisher Scientific) or NaCh (gift from the laboratory of R. Gourdie) and N-cadherin overnight at 4°C. After three washes with PBS, Cy-3 anti-rabbit (1:400; Jackson ImmunoResearch) and Alexa 488 anti-mouse (1:800, Jackson ImmunoResearch) were placed for 1.5 h at room temperature. Sections were washed three times in PBS and mounted with mounting media (Vectashield, Vector Laboratories, https://vectorlabs.com/; or ProLong Diamond Antifade Mountant with DAPI, ThermoFisher Scienticfic). Laser scanning confocal microscopy obtained images shown using a ×40 oil immersion objective (Leica).
For the detection of NaCh at the ID, the above methods were used with the following differences. One image per subject was obtained (CT baseline: n = 3 images, CT ischemia: n = 3 images, MH baseline: n = 2 images, and MH ischemia: n = 3 images). For primary antibodies, sections were stained for NaCh (1:100, from the laboratory of R. Gourdie) and N-cadherin (1:1,000, no. 333900, ThermoFisher Scientific) overnight at 4°C. Imaging was performed described as above.
ImageJ software was used for the quantification of Cx43 or NaCh expression at the ID. Background was subtracted from images. N-cadherin was used as a marker for ID and to set the regions of interests. Minimally 40 IDs were analyzed per image for Cx43 analysis and minimally 3 to 25 IDs per image for NaCh. Cy3 intensity was measured in the regions of interest, normalized to N-cadherin expression, and considered as ID Cx43 or NaCh.
Resting membrane potential and upstroke dV/dtmax recordings.
To measure resting membrane potential (RMP), action potentials were recorded in an additional set of samples with floating microelectrodes using previously validated techniques (CT baseline: n = 7, CT ischemia: n = 8, MH baseline: n = 7, and MH ischemia: n = 6 for dV/dtmax, 36–43 recordings/group for RMP analysis) (34). Briefly, glass pipettes were filled with 3M KCl with a tip impedance of 10–15 MΩ. Electrodes were connected through an Ag/AgCl wire to a head stage and signal conditioning amplifier (Axoprobe-1A, Axon Instruments). Microelectrode signals were amplified and displayed on a digital oscilloscope, allowing real-time determination of action potential characteristics including maximum velocity (dV/dtmax) of phase 0 of the action potential and RMP.
Canine myocyte isolation.
Myocytes were isolated enzymatically from a wedge of canine hearts using enzymatic dispersion techniques. Briefly, the canine wedge was cannulated in Langendorff (at 37°C) and perfused with Ca2+-free Tyrode solution for 2.5 min and then switched to enzyme solution (collagenase type II, 1.5 mg/ml) for ~50 min. After perfusion with high-K+ solution for 5 min, tissue was trimmed and myocytes were filtered into culture tubes. After 2 h, resting myocytes were centrifuged and resuspended in medium 199, stored at room temperature, and used within 24 h of isolation.
INa activity.
Paired INas were recorded by ruptured-patch whole cell voltage clamp at 32°C and then 36°C (n = 6) or 36°C and then 32°C (n = 15). Microelectrodes were filled with a solution of the following (in mM): 120 CsF, 2 MgCl2, 10 HEPES, and 11 EGTA and brought to a pH of 7.2. Canine myocytes placed in the solution containing the following (in mM): 25 NaCl, 105 N-methyl d-glucamine, 5.4 CsCl, 1.8 MgCl2, 1.8 CaCl2, 10 glucose, 10 HEPES, and 0.001 nisodipine, pH 7.4. INa was elicited from a holding potential of −80 mV with depolarizing voltage pulses from −60 to 45 mV for 16 ms starting after a minimum of 5 min for current stabilization. Ionic current density (in pA/pF) was calculated from the ratio of current amplitude to cell capacitance.
Data analysis and statistics.
For 15-min ischemia confocal IF and RMP analysis, univariate ANOVA was used to identify differences between samples. Post hoc analysis was subsequently performed using Tukey’s honestly significant difference test. For 30-min ischemia confocal IF, Student’s t-test was performed between the two groups. For optical mapping of CV (n = 6 per group) and Western blot analysis (CT: n = 8 and MH: n = 10), samples from each group (CT baseline, CT ischemia, MH baseline, and MH ischemia) were paired for statistical analysis. One experiment was missing a CT baseline sample for Western blot analysis and therefore was removed from analysis. When comparing continuous variables such as CV throughout ischemia time, repeated measures was performed for temperature (36 vs. 32°C). Student’s t-tests were then used when comparing a continuous variable between temperatures at a single ischemia time. The χ2-test was used for dichotomous variables (conduction block, Fig. 1B). All mean data are presented as means ± SE. SPSS Statistics (23.0, IBM) was used to perform statistical analysis.
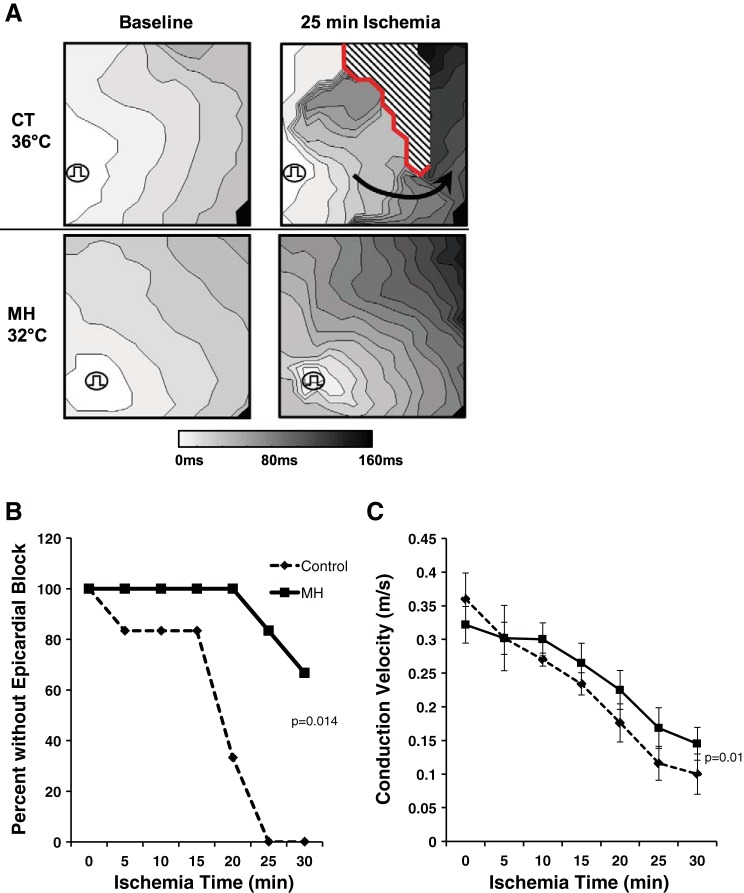
Fig. 1.
Mild hypothermia (MH) prevents conduction failure during ischemia. A: transmural conduction maps are shown with pacing from the endocardium. Top, conduction slowing and epicardial conduction block (red line) seen under control (CT) temperature. Note the development of reentrant conduction (arrow), which is expected to increase arrhythmia susceptibility. Bottom, MH attenuated conduction slowing and block during ischemia. B: summary data of preparations with conduction block (n = 6, P = 0.014 by χ2-test at 30-min ischemia). C: summary data of conduction velocity during ischemia. MH attenuated conduction slowing over ischemia time compared with CT (n = 6, P = 0.01 by repeated-measures ANOVA).
RESULTS
MH attenuates ischemia-induced myocardial conduction slowing and epicardial conduction block during 30 min of ischemia.
MH prevented ischemia-epicardial conduction block during 30 min of prolonged ischemia (Fig. 1, A and B), where 6 of 6 CT preparations developed subepicardial conduction block compared with 2 of 6 MH preparations by 30 min of ischemia (P = 0.014). MH also attenuated ischemia induced CV slowing by 22% by 30 min of ischemia in MH versus CT (P = 0.01; Fig. 1C). No difference in CV was seen at baseline between groups. These data demonstrate that MH prevents epicardial conduction block and improves conduction through prolonged (30 min) of ischemia.
Cx43 expression and localization are maintained with MH.
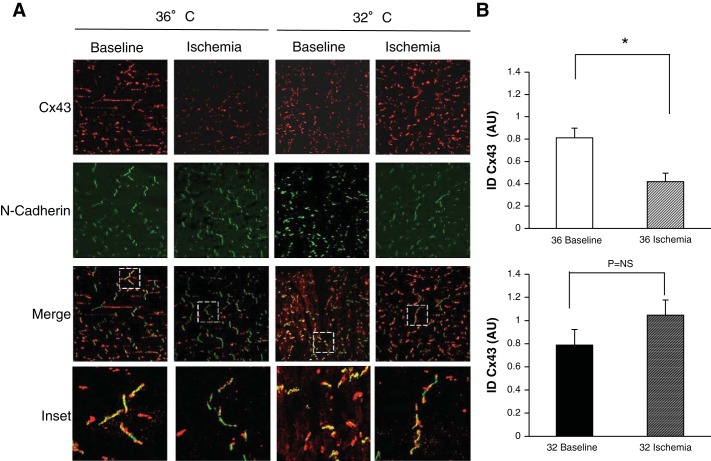
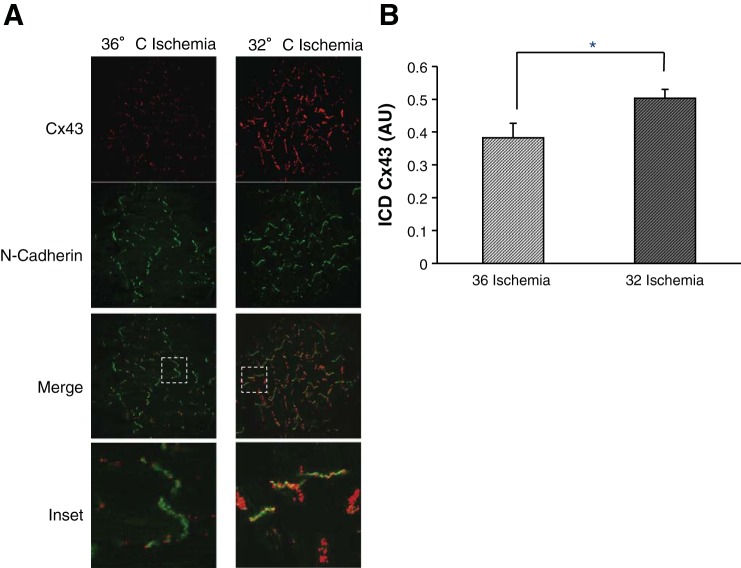
To determine the mechanisms that underlie hypothermic preservation of CV during ischemia, we examined the effects of MH on Cx43. ID localization of Cx43 largely contributes to normal maintenance of myocardial conduction. Therefore, maintained ID Cx43 expression during ischemia would be expected to maintain GJ communication and myocardial conduction. We analyzed Cx43 localization in the ID with the ID protein N-cadherin using confocal IF. Figure 2A shows CT and MH groups during nonischemia (baseline) and 15 min of ischemia conditions stained for Cx43 (red) and N-cadherin (green) with overlapped images below showing localization of Cx43 at the ID (yellow). At baseline, there was no difference in fluorescence between temperatures. At 15 min of ischemia, fluorescence of Cx43 in the ID was reduced by 52% (P = 0.004). In contrast, there was no reduction in Cx43 in the MH group. Post hoc analysis demonstrated a significant difference between CT and MH at 15 min of ischemia (P = 0.002). Summary data are shown in Fig. 2B. This result was maintained after 30 min of ischemia (n = 4 subjects, 8 images/group, P = 0.036; Fig. 3).
Fig. 2.
MH preserves connexin (Cx)43 at the intercalated disk (ID). A: canine wedges under CT nonischemic (36° baseline) or 15 min of ischemia (36° ischemia) conditions and MH nonischemic (32° baseline) or 15 min of ischemia (32° ischemia) conditions were stained for total Cx43 (red) and the ID protein N-cadherin (green). N-cadherin marked regions of interest for Cx43 quantification at the ID. Merged images show N-cadherin overlapped with total Cx43 images, with dashed white boxes shown as enlarged inset images (bottom row). Ischemia reduced Cx43 localization in the ID expression as shown by mostly green ID (inset, second image). MH attenuated Cx43 loss from the ID as shown by yellow IDs (inset, fourth image). B: summary data. CT (n = 4 subjects, 8 images) significantly reduced ID Cx43 during ischemia (n = 6 subjects, 11 images, *P = 0.002), whereas MH did not significantly change ID Cx43 between CT (n = 6 subjects, 12 images) and ischemia (n = 6 subjects, 12 images). AU, arbitrary units.
Fig. 3.
MH preserves Cx43 at the ID (ICD) at 30 min of ischemia. A: canine wedges after 30 min of ischemia at CT temperature (36° ischemia) or MH (32° ischemia) stained for total Cx43 (red) and the ID protein N-cadherin (green). N-cadherin marked regions of interest for Cx43 quantification at the ID. Merged images show N-cadherin overlapped with total Cx43 images, with dashed white boxes shown as enlarged inset images (bottom row). Ischemia reduced Cx43 localization in the ID expression as shown by mostly green ID (left inset). MH attenuated Cx43 loss from the ID as shown by yellow IDs (right inset). B: summary data. During CT, there was significantly reduced ID Cx43 compared with MH (*P = 0.036) during ischemia (n = 4 subjects, 8 images/group).
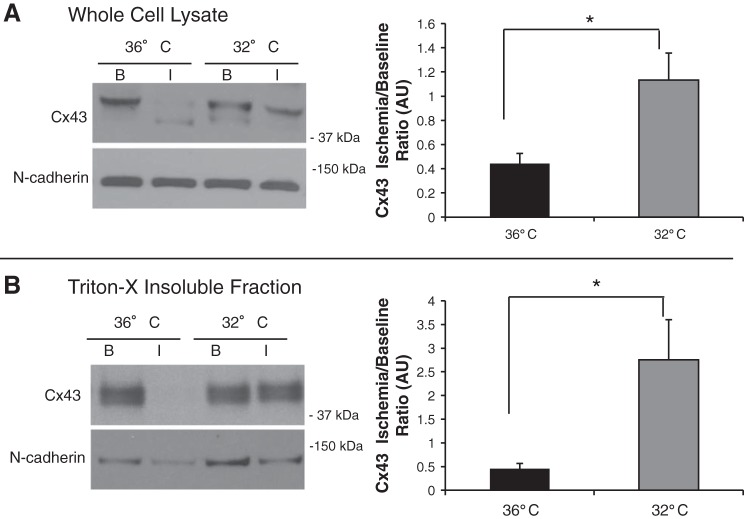
We quantified total Cx43 and ID Cx43 protein expression using Western blot analysis. Figure 4 (example on left, summary data of ratio of ischemia to baseline on right) shows total Cx43 (Fig. 4A) and Triton-X-insoluble Cx43 (Fig. 4B). At baseline, there was a lower amount of Cx43 in the MH group compared with the CT group (by 39%, P = 0.016), similar to previous observations (16). However, there was no difference between Cx43 at the ID (P = 0.12) at baseline. At 30 min of ischemia, total Cx43 expression in the CT group was reduced by 56% versus no change in the MH group (P = 0.02). ID Cx43 expression was similarly reduced during ischemia in the CT group by 55% versus a 2.7-fold increase in the MH group (P = 0.006) Taken together, these data suggest that MH maintains and promotes total and Triton-X-insoluble Cx43 expression during myocardial ischemia, preserving GJ communication and therefore myocardial CV.
Fig. 4.
MH attenuates ischemia-induced total and ID Cx43 protein expression. A: total Cx43 protein expression. Left, representative Western blot analysis under CT baseline and 30 min of ischemia (36° B and 36° I) and MH baseline and 30 min of ischemia (32° B and 32° I). Right, summary data for the Cx43 ischemia-to-baseline ratio (CT: n = 8 and MH: n = 10). During ischemia, there was decrease in total Cx43 expression versus baseline at 36°C (*P = 0.002). During ischemia, MH attenuated the ischemia-induced total Cx43 reduction (P = 0.018 vs. CT). B: Triton-X-insoluble “ID” Cx43 expression. Left, representative example of ID expression in 36° B, 36° I, 32° B, and 32° I groups. Right, summary data (CT: n = 7 and MH: n = 7). No difference was seen at baseline in ID Cx43. MH attenuated the ischemia-induced ID Cx43 reduction (P = 0.020 vs. CT).
Effect of MH on cellular excitability.
Cellular excitability is heavily dependent on NaCh function. As the phase 0 action potential upstroke is primarily an indicator of NaCh function and to examine NaCh function in ischemic tissue rather than simulated ischemic cells, we measured phase 0 upstroke velocity (dV/dtmax) using microelectrodes in wedge preparations (Fig. 5A). Before ischemia, there was no difference between CT and MH baseline dV/dtmax (164.1 ± 8.9 vs. 180.2 ± 13.9 V/S, P = not significant; Fig. 5B). During ischemia, in the CT group, dV/dtmax decreased by 86% (P < 0.01) compared with the MH group by 70% (P < 0.01, CT ischemia vs. MH ischemia). These data suggest that NaCh function is partially maintained during ischemia by MH.
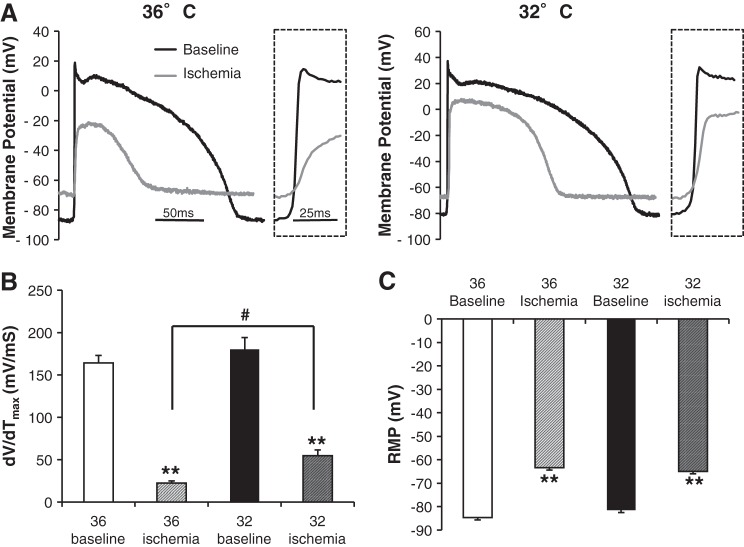
Fig. 5.
MH attenuates ischemia-induced dV/dtmax slowing. A: glass microelectrodes were used to record action potentials in canine wedges during 36 or 32°C under baseline (black) or ischemic conditions (gray, 25–30 min). Inset: phase 0 upstroke. B: summary data (CT baseline: n = 7, CT ischemia: n = 8, MH baseline: n = 7, and MH ischemia: n = 6). dV/dtmax significantly decreased during ischemia in CT and MH (P < 0.001), and MH attenuated ischemia-induced dV/dtmax slowing (P < 0.001 vs. 36° I). C: resting membrane potential (RMP) significantly depolarized during ischemia (P = 0.004 vs. baseline at 36 or 32°C, n as stated above). No difference was identified between temperatures at baseline or during ischemia.
NaCh function is highly dependent on NaCh availability, which is largely determined by RMP. To assess the effect of MH on RMP during ischemia, floating microelectrodes were also used to directly determine RMP in CT or MH groups at baseline and during ischemia. At baseline, there was no difference in RMP between CT and MH groups (−84.6 ± 2.2 vs. −81.5 ± 2.6 mV, respectively, P = 0.37). As expected, ischemia induced RMP depolarization (P < .001); however, this was not affected by MH (−63.4 ± 2.9 vs. −65 ± 2 mV, respectively, P = 0.65; Fig. 5C).
To determine whether hypothermic temperatures affected NaCh function, we measured INa in isolated canine myocytes. Increased INa was observed with hypothermic temperature (32°C) in patched cells, where the observation was consistent regardless of whether current was measured initially at 32 or 36°C before warming or cooling myocytes and subsequently measuring current again at 36 or 32°C, respectively (P < 0.001; Fig. 6, A–D). These data suggest that MH increased INa, thereby preserving phase 0 of the action potential, contributing to maintained myocardial CV during MH.
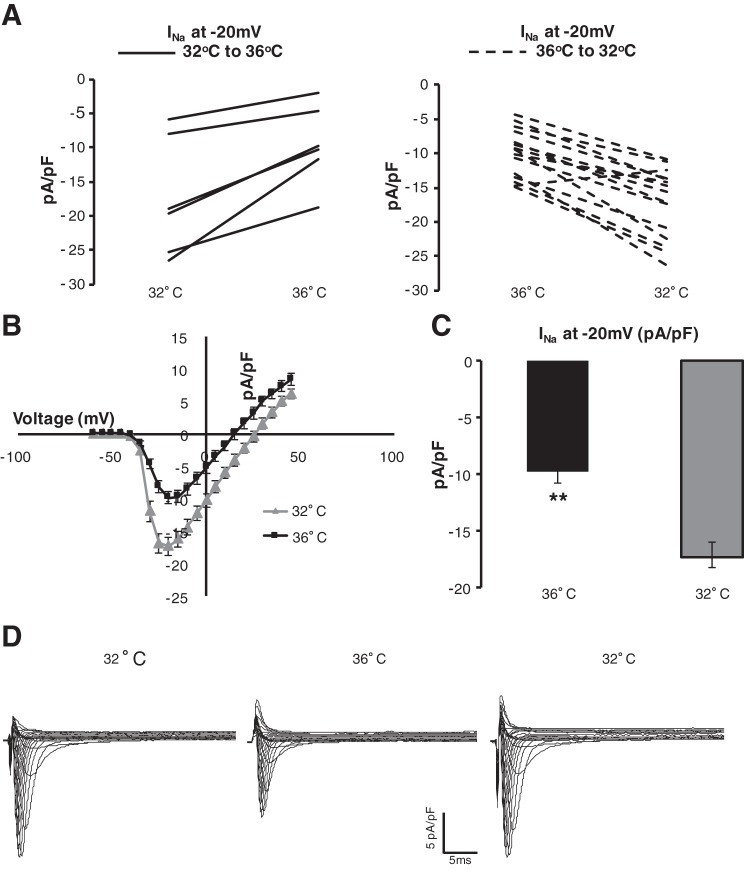
Fig. 6.
MH enhances Na+ channel (NaCh) current (INa). A: isolated canine myocytes were patched at 36°C (dashed, n = 15) or 32°C (solid, n = 6) and then either warmed or cooled to 32 or 36°C, respectively. Each cell patch is shown. B: current-voltage relationship of NaCh at 36°C (black) or 32°C (gray). C: MH resulted in significantly increased INa at −20 mV (P < 0.001). D: example tracings of an isolated myocyte patched at 32°C, warmed to 36°C, and then cooled to 32°C. Increased INa can be observed at 32°C.
Effect of MH on NaCh expression and localization at the ID.
We performed parallel experiments to those with Cx43 to evaluate NaCh expression using Western blot analysis during ischemia with or without MH (n = 4). Unlike Cx43, over 15–30 min of ischemia we did not observe differences in NaCh expression in Triton-X-insoluble fractions (Fig. 7). Furthermore, we analyzed NaCh localization in the ID with the ID protein N-cadherin using confocal IF at 15 min (Fig. 8) and 30 min of ischemia. We observed no difference in NaCh localization during 15 or 30 min of ischemia, nor did we observe differences between 36 or 32°C. Taken together with our data on INa and action potential dV/dtmax, this suggests that maintenance of excitability during ischemia by MH is mediated by changes in NaCh function during MH.
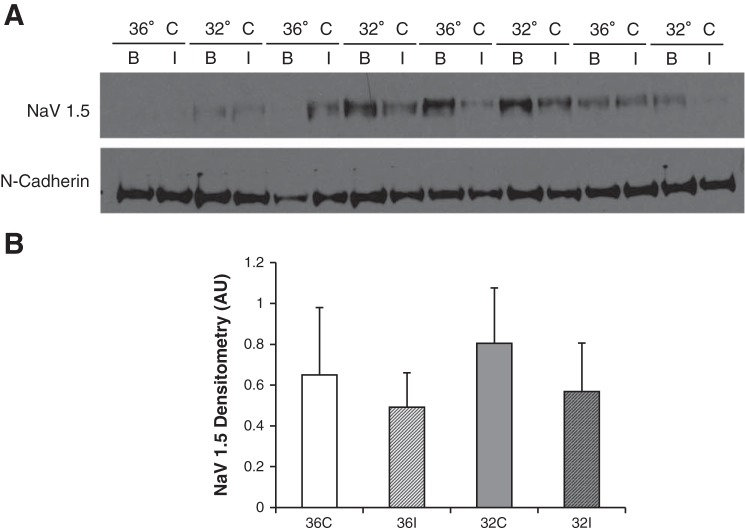
Fig. 7.
NaCh expression at the ID at 30 min of ischemia. A: Western blots of the Triton-X-insoluble fraction in CT (36°C) at baseline and 30 min of ischemia (36° B and 36° I) and MH (32°C) at baseline and 30 min of ischemia (32° B and 32° I). Blots were probed for NaCh (Nav1.5) and ID protein N-cadherin. B: summary data (n = 4 per group) showing no significant changes in NaCh expression in either group during ischemia.
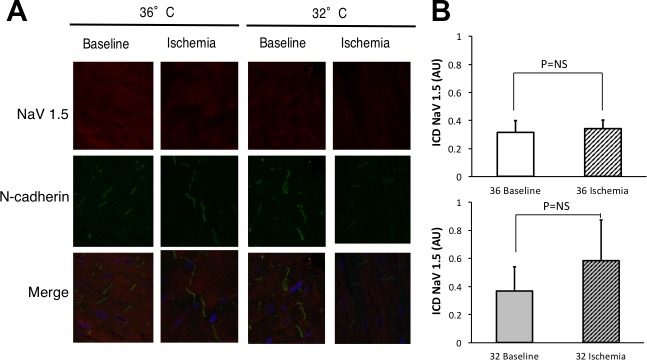
Fig. 8.
NaCh at the ID during ischemia. A: canine wedges under CT nonischemic (36° baseline, n = 3 subjects) or 15 min of ischemia (36° ischemia, n = 3 subjects) conditions and MH nonischemic (32° baseline, n = 2 subjects) or 15 min of ischemia (32° ischemia, n = 3 subjects) conditions were stained for total Nav1.5 (red) and the ID protein N-cadherin (green). Merged images show N-cadherin overlapped with total Nav1.5 images. B: summary data. There was no difference in NaCh at the ID either during ischemia (P = not significant) and no difference between temperatures (P = not significant).
DISCUSSION
Overall, we establish that MH maintains Cx43 expression and localization at the ID during 30 min of ischemia, which is associated with maintained myocardial conduction and reduced susceptibility to conduction block. MH also maintained dV/dtmax during ischemia and increased INa, suggesting that MH also maintains cellular excitability. This suggests that MH’s improvement of CV and conduction block during ischemia is likely secondary to a combined effect of improved Cx43 at the ID and maintenance of cellular excitability.
Prevention of arrhythmias during acute cardiac ischemia is of significant clinical importance. By maintaining Cx43 expression, MH reduced an important arrhythmogenic substrate, conduction slowing leading to conduction block. Clinically, it has been observed that MH may reduce arrhythmias in patients (28). By better understanding the mechanisms by which MH affects cardiac conduction and arrhythmia susceptibility, we can develop more clinically relevant targeted therapies for ischemia-induced arrhythmias.
The progression of ischemic Cx43 remodeling is well established. Cx43 is remodeled as early as 7 min of ischemia (5) and decreased cardiac conduction and susceptibility to arrhythmias related to cellular uncoupling become prominent after 10–15 min of ischemia, correlating with increases in tissue resistance and Cx43 dephosphorylation (7, 20, 29). Consistent with these observations, we showed by confocal IF that Cx43 ID localization is significantly reduced as early as 15 min of ischemia in CT, which was attenuated by MH. This is consistent with data from showing decreased Cx43 expression and lateralization of Cx43 away from the ID by 30 min of ischemia (7, 19). Although out of the scope of the current work, it is likely that hypothermic modulation of Cx43 phosphorylation during ischemia is involved in the mechanism of preserved Cx43 at the ID. During ischemia, Cx43 dephosphorylating and altered phosphorylation at specific sites are observed (7, 21, 27, 30). Our data are consistent with these observations (Fig. 4) that Cx43 dephosphorylation appears to be attenuated by MH. We are the first to show that MH attenuates these detrimental Cx43 remodeling events. In line with our findings, hypothermic hibernating animals have shown upregulated Cx43 expression, which maintains myocardial CV during hypothermia with potentially antiarrhythmic properties (13). Hseih et al. (16) reported in a rabbit model without ischemia that Cx43 lateralization increased, particularly at more severe hypothermic temperatures. However, we established by both confocal and Western blot analysis that Cx43 localized in the ID was higher with MH than CT during myocardial ischemia, when we observed that MH preserved CV. Although performed in separate experiments, there seems to be a further reduction in the signal localized to the ID as determined by confocal IF between 15 and 30 min of ischemia (Figs. 2, 3, and 7). This suggests that MH may slow, but not prevent, relocalization and degradation of Cx43 from ID GJs. This observation is consistent with CV data, where CV is further slowed between 15 and 30 min of ischemia during MH (Fig. 1C).
During an ischemic insult, RMP becomes depolarized, which reduces cellular excitability. Markers of NaCh excitability including action potential amplitude and upstroke velocity are also reduced during acute ischemia (9). During MH, we observed attenuation of ischemia-induced slowing of dV/dtmax using microelectrodes in canine wedges. Similarly, we observed MH directly augmented INa in isolated myocytes. Although direct INa measurements were not performed with simulated ischemia, we chose to measure surrogates of NaCh function (dV/dtmax) under truly ischemic conditions, the same conditions during which Cx43 localization/expression and CV were measured. We did not observe a change in ID NaCh expression. However, pairing direct INa measurements in canine myocytes with upstroke velocity measurements under truly ischemic conditions in the wedge preparation at both temperatures strongly suggests that MH improves cellular excitability during ischemia.
Limitations in our studies should be considered in interpreting our findings. We chose to examine transmural changes in CV because transmural conduction block is well described to be mechanistically related to ischemia-induced reentrant arrhythmias (9, 18). However, with this preparation, we are not able to evaluate potential additional effects of MH on CV in the whole heart. In addition, CV is somewhat nonspecific and does not provide direct information on relative contributions of cellular excitability, intracellular coupling, or ischemia-induced edema in explaining our results. Cooling was done before an ischemic insult in contrast to clinical settings, where cooling is done after return of spontaneous circulation in the hospital, where global reperfusion has occurred but regional ischemia may be ongoing. Ischemia alone induces many different electrophysiological effects, which are dynamic and dependent on the duration and degree of ischemia. The effect of inducing hypothermia during ischemia is likely complex and expected to variably effect multiple ischemia-induced electrophysiological derangements during induction of hypothermia. Our aim was to first understand the mechanisms of MH in the most basic model, where temperature is controlled before any ischemia-induced remodeling, as a first step before examining its effect in more complex, but clinically relevant, models of clinical TTM and ischemia-reperfusion. MH appears to significantly decrease total but not Triton-X-insoluble Cx43 at baseline. Despite a total baseline decrease, our data show that MH clearly prevents ischemia-induced decreases in Cx43 at the ID, which promotes significant preservation of transmural conduction. The relative contributions of preservation of Cx43 at the ID versus maintained INa during ischemia in maintaining CV and preventing conduction block remains to be determined.
Blebbestatin used in optical mapping and floating microelectrode experiments is expected to effect the rate of ischemic metabolism. However, given that these functional experiments were carefully matched between groups and those observations were consistent with molecular and confocal IF studies that were done without blebbestatin, it is unlikely that it altered our primary observations. A potential concern is that maintaining GJ coupling during acute ischemia may increase infarct size, but others have shown that MH reduces infarct size, thereby minimizing downstream arrhythmogenic substrates (10, 11). Finally, although altered Cx43 banding patterns (Fig. 4) in our Western blot analysis suggest that there may be differences in phosphorylation patterns of Cx43 during ischemia and hypothermia, we did not formally evaluate at this time the effect of MH on Cx43 phosphorylation. Furthermore, the multiple band pattern in the Triton-X-insoluble fraction Western blot analysis is less clear. It is possible that the phosphorylation pattern at the ID is different and likely highly phosphorylated. Determining the molecular mechanisms by which MH preserves Cx43 at the ID is out of the scope of the current work, as both ischemia and hypothermia alter Cx43 phosphorylation (16, 27); future studies examining how hypothermic modulation of Cx43 phosphorylation during ischemia might explain our results would be a next important step.
In conclusion, MH provides preserves myocardial conduction and prevents conduction block during ischemia through two mechanisms: 1) preserving Cx43 localization and expression at the intercalated disk and 2) preserving cellular excitability through maintaining NaCh current and upstroke velocity. Understanding the mechanisms by which ischemic Cx43 remodeling is prevented and NaCh function is maintained may provide novel targeted therapies during ischemia and resuscitation.
GRANTS
This work was supported by a MetroHealth Medical Center Department of Emergency Medicine Foundation Grant (to L. D. Wilson and J. S. Piktel) and National Heart, Lung, and Blood Institute Grants R01-HL-096962 and R01-HL-094450 (to I. Deschênes).
DISCLOSURES
J. S. Piktel received a grant from the ZOLL Foundation not related to this research. J. S. Piktel and L. D. Wilson receive compound only from Zealand Pharma.
AUTHOR CONTRIBUTIONS
M.M.N., L.D.W., and J.S.P. conceived and designed research; M.M.N., X.W., Z.D., and J.S.P. performed experiments; M.M.N., X.W., Z.D., and J.S.P. analyzed data; M.M.N., X.W., Z.D., I.D., L.D.W., and J.S.P. interpreted results of experiments; M.M.N., X.W., Z.D., and L.D.W. prepared figures; M.M.N. and L.D.W. drafted manuscript; M.M.N., Z.D., I.D., L.D.W., and J.S.P. edited and revised manuscript; M.M.N., Z.D., I.D., L.D.W., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert Gourdie for the generous gift of the NaCh antibody developed in the laboratory, Gary Pawlowski for expertise in animal preparations, Ken Laurita for expertise in experimental setup, Michael O’Neil for assistance in experiments, and Haiyan Liu for expertise in Western blot analysis.
REFERENCES
- 1.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 2.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res 93: 638–645, 2003. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 3.Akar FG, Yan GX, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of M cells underlies reentrant mechanism of torsade de pointes in the long-QT syndrome. Circulation 105: 1247–1253, 2002. doi: 10.1161/hc1002.105231. [DOI] [PubMed] [Google Scholar]
- 4.Amin AS, Klemens CA, Verkerk AO, Meregalli PG, Asghari-Roodsari A, de Bakker JM, January CT, Wilde AA, Tan HL. Fever-triggered ventricular arrhythmias in Brugada syndrome and type 2 long-QT syndrome. Neth Heart J 18: 165–169, 2010. doi: 10.1007/BF03091755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjølbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123). J Mol Cell Cardiol 40: 790–798, 2006. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng 45: 563–571, 1998. doi: 10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 7.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 87: 656–662, 2000. doi: 10.1161/01.RES.87.8.656. [DOI] [PubMed] [Google Scholar]
- 8.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132, Suppl 2: S465–S482, 2015. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79: 917–1017, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dai WHM, Hale SH, Kloner RA. Transient hypothermia during the acute phase of the myocardial infarction dramatically reduces scar size, prevents left ventricular dilation and remodeling, and preserves cardaic function (Abstract). Circulation 120: A158, 2014. [Google Scholar]
- 11.Dash RMY, Holt B, Tachibana A, Lyons J, Ikeno F, McConnell M, Yeung A, Illindala U, Yang P. Moderate hypothermia reduces infarct/injury size and preserves myocatdial function following acute ischemia-reperfusion injury (Abstract). Circulation 120: A19073, 2014. [Google Scholar]
- 12.Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 51: 681–690, 2001. doi: 10.1016/S0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 13.Fedorov VV, Li L, Glukhov A, Shishkina I, Aliev RR, Mikheeva T, Nikolski VP, Rosenshtraukh LV, Efimov IR. Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: possible role of Cx43 and Cx45 up-regulation. Heart Rhythm 2: 966–975, 2005. doi: 10.1016/j.hrthm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7: 1024–1038, 1996. doi: 10.1111/j.1540-8167.1996.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauty MG, Esrig BC, Hill JG, Long WB. Prognostic factors in severe accidental hypothermia: experience from the Mt. Hood tragedy. J Trauma 27: 1107–1112, 1987. doi: 10.1097/00005373-198710000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh YC, Yeh HI, Lin SF, Hsu YW, Huang JL, Lin TC, Ting CT, Wu TJ. Short-duration therapeutic hypothermia causes prompt connexin43 gap junction remodeling in isolated rabbit hearts. Circ J 75: 1706–1716, 2011. doi: 10.1253/circj.CJ-10-1001. [DOI] [PubMed] [Google Scholar]
- 17.Janse MJ, van Capelle FJ, Morsink H, Kléber AG, Wilms-Schopman F, Cardinal R, d’Alnoncourt CN, Durrer D. Flow of “injury” current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ Res 47: 151–165, 1980. doi: 10.1161/01.RES.47.2.151. [DOI] [PubMed] [Google Scholar]
- 18.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69: 1049–1169, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res 104: 1103–1112, 2009. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kléber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res 61: 271–279, 1987. doi: 10.1161/01.RES.61.2.271. [DOI] [PubMed] [Google Scholar]
- 21.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci 119: 3435–3442, 2006. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115: 1357–1374, 1991. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piktel JS, Jeyaraj D, Said TH, Rosenbaum DS, Wilson LD. Enhanced dispersion of repolarization explains increased arrhythmogenesis in severe versus therapeutic hypothermia. Circ Arrhythm Electrophysiol 4: 79–86, 2011. doi: 10.1161/CIRCEP.110.958355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piktel JS, Rosenbaum DS, Wilson LD. Mild hypothermia decreases arrhythmia susceptibility in a canine model of global myocardial ischemia. Crit Care Med 40: 2954–2959, 2012. doi: 10.1097/CCM.0b013e31825fd39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pogwizd SM, Corr PB. Reentrant and nonreentrant mechanisms contribute to arrhythmogenesis during early myocardial ischemia: results using three-dimensional mapping. Circ Res 61: 352–371, 1987. doi: 10.1161/01.RES.61.3.352. [DOI] [PubMed] [Google Scholar]
- 26.Porres JM, Brugada J, Urbistondo V, García F, Reviejo K, Marco P. Fever unmasking the Brugada syndrome. Pacing Clin Electrophysiol 25: 1646–1648, 2002. doi: 10.1046/j.1460-9592.2002.01646.x. [DOI] [PubMed] [Google Scholar]
- 27.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res 108: 1459–1466, 2011. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagalyn E, Band RA, Gaieski DF, Abella BS. Therapeutic hypothermia after cardiac arrest in clinical practice: review and compilation of recent experiences. Crit Care Med 37, Suppl: S223–S226, 2009. doi: 10.1097/CCM.0b013e3181aa5c7c. [DOI] [PubMed] [Google Scholar]
- 29.Smith WT, Fleet WF, Johnson TA, Engle CL, Cascio WE. The 1b phase of ventricular arrhythmias in ischemic in situ porcine heart is related to changes in cell-to-cell electrical coupling. Circulation 60: 397–403, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419: 261–272, 2009. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA; Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators . Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 352: 2581–2588, 2005. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 32.Tiainen M, Parikka HJ, Mäkijärvi MA, Takkunen OS, Sarna SJ, Roine RO. Arrhythmias and heart rate variability during and after therapeutic hypothermia for cardiac arrest. Crit Care Med 37: 403–409, 2009. doi: 10.1097/CCM.0b013e31819572c4. [DOI] [PubMed] [Google Scholar]
- 33.White JD. Hypothermia: the Bellevue Experience. Ann Emerg Med 11: 417–424, 1982. doi: 10.1016/S0196-0644(82)80038-3. [DOI] [PubMed] [Google Scholar]
- 34.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation 93: 372–379, 1996. doi: 10.1161/01.CIR.93.2.372. [DOI] [PubMed] [Google Scholar]