Abstract
Natural selection has favoured specialization in anthropophilic mosquito host choice, yet in the absence of human hosts, females feed on a selected range of vertebrates. For host recognition, we hypothesize that mosquitoes primarily rely on generic host volatiles. Detection and perception of such compounds would provide the mosquito with a flexible, yet constrained, odour coding system that could delineate host preference. In this study, we show that the quintessential generic volatile for host-seeking, carbon dioxide, activates and attracts the malaria mosquito, Anopheles coluzzii, and the arbovirus vectors, Aedes aegypti and Culex quinquefasciatus, within boundaries set by the dynamic range and coding capacity of the CO2-sensitive olfactory receptor neurons. These boundaries are sufficiently broad to elicit behavioural responses to various hosts within their preferred host range. This study highlights the significance of the sensitivity of the carbon dioxide detection system and its regulation of host seeking and recognition.
Keywords: carbon dioxide, behaviour, electrophysiology, host recognition
1. Background
Mosquitoes that transmit infectious diseases often express a marked, inherent host preference [1–6]. Host preference studies of the African malaria vector, Anopheles gambiae sensu lato, and the arbovirus vectors, Aedes aegypti and Culex quinquefasciatus, show that natural selection favours a restricted host breadth [3]. Despite this, there remains sufficient plasticity in host preference to provide a mechanism by which mosquitoes can adapt to different environmental conditions [1,3,5], which is an important variable regulating disease transmission by predominantly anthropophilic mosquitoes [6]. This indicates that there is both a cost and benefit to maintaining plasticity [1] and/or that these species are physiologically limited in the capacity to be plastic.
Olfaction is the principal sense by which mosquitoes locate their hosts [3,7]. Host discrimination and selection is a sequence of behaviours that includes activation, long- and short-range attraction, and landing on the host [8]. Initial recognition of an upwind host relies on the detection of minute fluctuations in carbon dioxide (CO2) concentration, which elicits activation and subsequent attraction in host-seeking mosquitoes [8–10], even in the absence of other host odours [8]. Emitted by all vertebrates, CO2 also gates the attraction to host odours over a range of distances in host-seeking mosquitoes [11,12]. Observed interspecific variation in the behavioural response to CO2 may be attributed to differences in the dynamic range of the CO2-chemosensory system for each mosquito species [9,13,14], and the underlying mechanism regulating the CO2 dynamic range is one of sensory constraint [15,16].
We hypothesize that such limitations could be generated by a series of constraints on the sensory system used to detect and discriminate between potential host species at different distances, from activation and attraction (this study) to short-range acceptance (see companion paper: [17]). Here, we test the hypothesis that host preference correlates with the receptive range of the CO2-chemosensory system, and suggest that the behavioural response of anthropophilic mosquitoes to CO2 is constrained by limits in sensory acuity.
2. Methods
2.1. Insects
Aedes aegypti (Rockefeller strain), Anopheles coluzzii (Suakoko strain; previously Anopheles gambiae M molecular form) and Culex quinquefasciatus (Thai strain) were reared at 27 ± 2°C, 70 ± 2% relative humidity (RH) under a 12 h : 12 h light : dark period, as previously described [17,18]. For all experiments, 4- to 10-day post-emergence sugar-fed adult female mosquitoes were used.
2.2. Single sensillum recordings
The maxillary palps of Ae. aegypti, An. coluzzii and Cx. quinquefasciatus are covered with capitate peg sensilla, variously described as peg sensilla or basiconic sensilla, each housing three olfactory receptor neurons (ORNs) [16,18–20]. In all species, the ORN with the largest amplitude is, by convention, referred to as the A cell, and has previously been shown to be an absolute detector of CO2 below 1200 ppm [15,16,19,20]. Electrophysiological recordings from this neuron were made and analysed as previously described [16].
A continuous humidified stream of synthetic air (Strandmöllen AB, Ljungby, Sweden), lacking CO2, was passed over the maxillary palp (2 l min−1) via a glass tube (7 mm i.d.). Carbon dioxide was introduced into the air stream through a hole (2 mm i.d.) in the glass tube, 11 cm upstream of the maxillary palps. Delivery of CO2 was regulated by two-way Teflon solenoid valves (Teddington, Skogås, Sweden), controlled via the digital output of an IDAC-4 (Syntech, Germany). Each valve was connected to separate gas cylinders containing metered amounts of CO2 (150, 300, 600, 1200, 2400, 4800 ppm) and oxygen (20%), balanced by nitrogen (Strandmöllen AB). A pulsed stimulus train of CO2 was used, with stimulation for 1 s and an interstimulus interval of 1 s.
2.3. Flight tube bioassay
Behavioural responses to pulsed CO2 stimuli were assessed in a glass flight tube bioassay (80 × 9.5 cm i.d.), as previously described [16], with minor modifications (figure 3b). Briefly, the tube assay was illuminated from above with white light at 280 lux for the diurnal Ae. aegypti, while red light (40 lux) was used for the nocturnal An. coluzzii and Cx. quinquefasciatus. Experiments for each species were conducted during their period of peak host-seeking activity [17]. Charcoal filtered humidified air (25 ± 2°C, RH 65 ± 2%) flowed through the flight tube at 30 cm s−1. To ensure a laminar flow and a homogeneous plume structure, the air passed through a series of stainless steel mesh screens prior to entering the flight tube (figure 3b). A pulsed flow of pure CO2 (Strandmöllen AB), regulated by a stimulus controller (SEC-2/b, Syntech, Germany), was introduced into a pulse generator. Homogenized CO2 pulses were delivered, using the same pulsation protocol as for the physiological experiments above, at the desired concentration (600, 1200, 2400 or 4800 ppm) through mixing pure CO2 with pressurized air at 4.5 l min−1 in the pulse generator, as previously described [16]. The concentration of CO2 in the flight tube was measured using a CO2 analyser (LI-820, LICOR Biosciences, Lincoln, NE, USA). Controls consisting of exposing individual mosquitoes in the flight tube to non-pulsed ambient CO2 levels (385.4 ± 6.28 ppm) that varied minimally over the duration of the experiment (1.3 ± 0.60 ppm) were run daily.
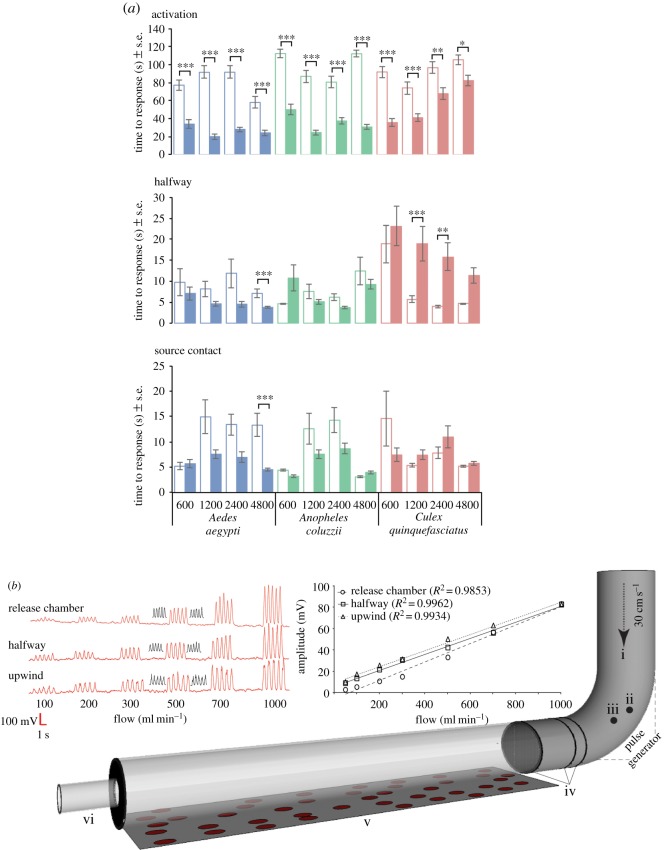
Figure 3.
The behavioural response of female Aedes aegypti, Anopheles coluzzii and Culex quinquefasciatus to pulsed CO2 stimuli, over increasing concentrations. (a) The time to activation, halfway and source contact of female mosquitoes in the flight tunnel towards constant ambient CO2 (control; open bars) and pulsed stimuli of the indicated concentrations of CO2 (filled bars; n = 30 each species). Asterisks indicate the significant differences among treatments and control (two-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001). Vertical bars represent the standard error of means ± SE. (b) Behaviour was assessed in a flight tube assay: (i) charcoal-filtered and humidified air, (ii) pressurized air inlet, (iii) stimulus inlet into which CO2 was injected, (iv) stainless-steel mesh plume diffusers, (v) glass flight tube, and (vi) release chamber. The upper panels demonstrate that the pulsed stimuli (here shown as five cycles of 1 s on and 1 s off) maintain their amplitude and shape throughout the flight tube and at all tested flow rates. The upper left panel shows the consistent and distinct pulsed stimuli at ascending flow rates of known concentration of acetone in the flight tube. Discrete pulsed stimuli were measured in the centre (in red) and at the lateral sides (in black) of the release chamber, at halfway and at the source. The upper right panel presents a graphical representation of the distinct pulsed stimuli, which shows the average amplitude of each of the five distinct pulses (N = 10) at different positions and the regression correlation coefficients (R2) that demonstrate the consistency of the stimulus amplitude at the different positions within the flight tube with increasing flow rates.
Individual mosquitoes were kept in glass release chambers (7 × 2.6 cm i.d.), covered with stainless steel mesh on one side and a cotton plug on the other, in the bioassay room for 24 h prior to the experiments [16]. The following times were measured: the time after opening the release chamber to take-off (flight activation), the time from take-off to upwind flight directed towards the odour source (halfway, 40 cm), and the time from halfway to source contact. The maximum time recorded was 120 s. Thirty individuals of each species were observed at each concentration of CO2. To minimize the effect of daily variation in baseline activity and responses to odours, an equal number of test and control individuals were observed each day.
2.4. Statistical analysis
Repeated measures 2-way ANOVA, followed by a Bonferroni post hoc test was performed to compare the physiological activity among the species. The behavioural data were treated in two ways. Two-way ANOVAs, followed by a Tukey post hoc test, were used to compare the time to response among treatments and controls, as well as across species, using GraphPad Prism v. 5.01 for Mac (GraphPad Software, La Jolla California, USA). The number of mosquitoes responding were analysed with nominal logistic regression, comparing treatments and controls for each species and concentration of CO2 (JMP®, Version 12.0.1, SAS Institute Inc., Cary, NC, 1989--2007).
3. Results
3.1. Physiological response to carbon dioxide
Stimulation with single pulses of CO2 with increasing concentrations elicited a dose-dependent response in the A cell of the capitate peg sensilla of all mosquito species (figure 1). The threshold of neuronal response to CO2, in a 0 ppm CO2 background, was lowest for An. coluzzii. At concentrations of 150 and 300 ppm CO2, the ORN activity was significantly higher in An. coluzzii than in Ae. aegypti and Cx. quinquefasciatus (t = 3.12, d.f. = 8, p < 0.05; t = 4.80, d.f. = 8, p < 0.001 and t = 3.09, d.f. = 8, p < 0.05; t = 2.92, d.f. = 8, p < 0.05, respectively). However, at concentrations exceeding 300 ppm, i.e. above ambient CO2 levels (350–400 ppm; indicated in figure 1), the ORN activity in Cx. quinquefasciatus was significantly higher than that of the other species (t = 10.61, d.f. = 8, p < 0.001).
Figure 1.
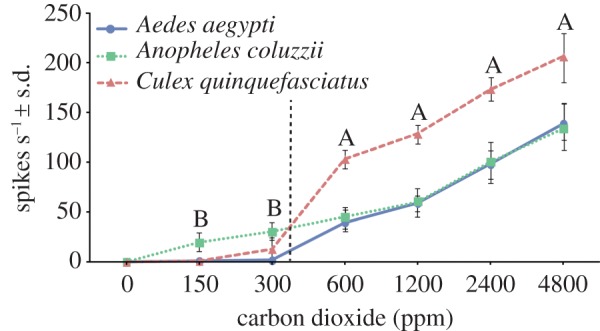
The A cell (n = 10) in the maxillary palps of Aedes aegypti, Anopheles coluzzii and Culex quinquefasciatus is differentially sensitive to carbon dioxide. ‘A’ represents a significant difference in neuronal firing rate in Cx. quinquefasciatus compared to An. coluzzii and Ae. aegypti (p < 0.0001); whereas ‘B’ depicts a significant difference in firing in An. coluzzii compared to Ae. aegypti and Cx. quinquefasciatus, respectively (two-way repeated-measures ANOVA, p < 0.05). Black dashed line indicates the average ambient CO2 concentration.
Pulsed stimuli of CO2 induced a phasic-tonic response from the A cell that remained unaltered and dependent on the stimulation. At concentrations of 600 and 1200 ppm CO2, the A cell of all species was able to detect and track the pulsed stimuli (figure 2). While, at higher concentrations all species were able to detect the pulses, only Ae. aegypti and An. coluzzii were able to track the stimuli, i.e. fire in response to CO2 throughout each pulse (figure 2). The A cell of Cx. quinquefasciatus, while detecting pulse onset rapidly, adapted to each CO2 pulse and with subsequent pulses affecting its ability to disadapt, thus limiting its capacity to track the stimuli at the higher concentrations.
Figure 2.
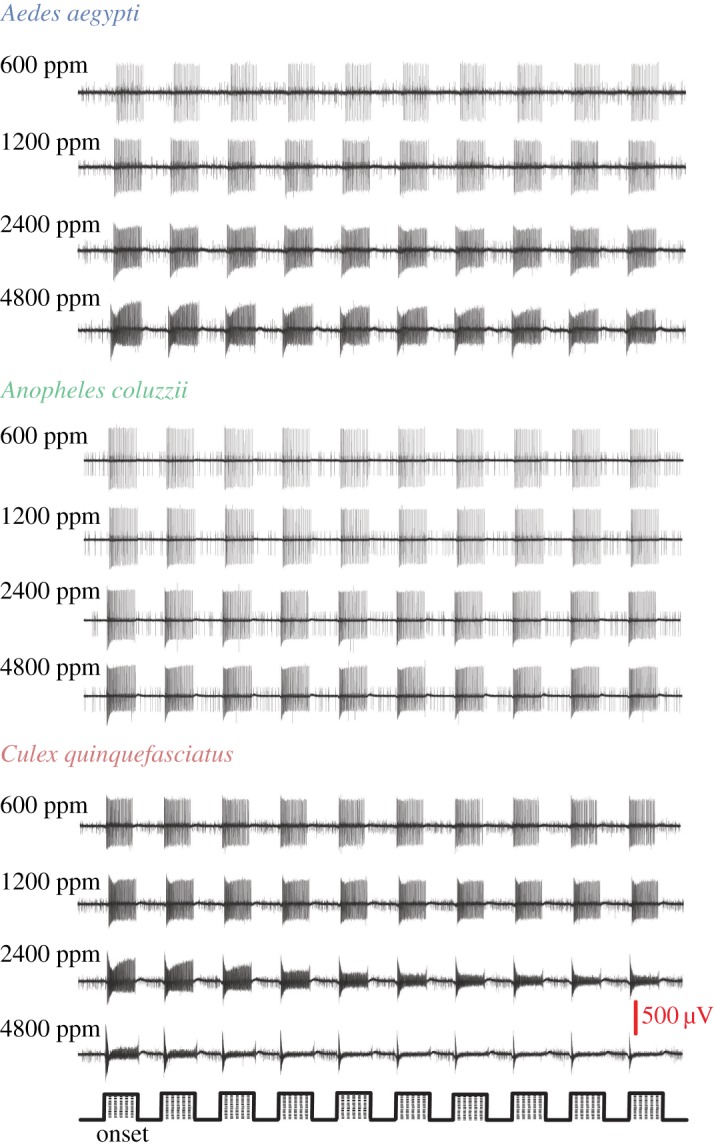
The temporal coding capacity of the CO2-sensitive neuron in female Aedes aegypti, Anopheles coluzzii and Culex quinquefasciatus, over increasing concentrations of CO2. The CO2 stimuli were delivered in trains of ten pulses, one second on and one second off, as indicated below the response traces (bars). Scale bar indicates spike amplitude (µV).
3.2. Behavioural response to carbon dioxide
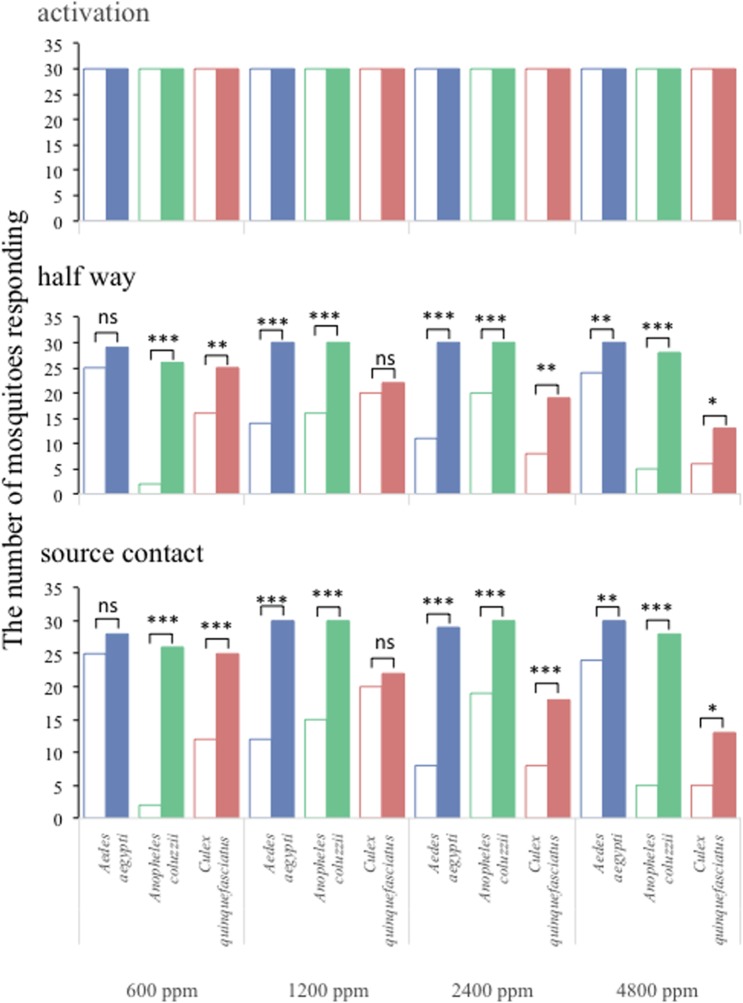
The behavioural responses to pulsed CO2 stimuli differed between species. The time to activation decreased in the presence of pulsed CO2 compared with the controls (figure 3a; upper panel). Comparing among the species, the time to activation was increased in Cx. quinquefasciatus compared to Ae. aegypti and An. coluzzii, at concentrations above 1200 ppm (2400 ppm: t-ratio −4.88, p < 0.0001; t-ratio −8.48, p < 0.0001; 4800 ppm: t-ratio −3.72, p = 0.0035; t-ratio −7.53, p < 0.001, respectively; figure 3a; upper panel). All mosquitoes of each of the three species were activated in both the presence of pulsed CO2 and the constant ambient CO2 control experiments (figure 4; upper panel).
Figure 4.
The numbers of female Aedes aegypti, Anopheles coluzzii and Culex quinquefasciatus responding to constant ambient CO2 (control; open bars) and pulsed stimuli of the indicated concentrations of CO2 (filled bars). Asterisks indicate the significant differences among treatments and control (nominal logistic regression; *p < 0.05, **p < 0.01, ***p < 0.001).
In response to pulsed CO2, the observed trend was a decrease in time to reach halfway along the flight tube (halfway) compared to the controls, for both Ae. aegypti and An. coluzzii as the concentration of CO2 increased, resulting in a significant difference at 4800 ppm for Ae. aegypti (figure 3a; middle panel). The opposite trend was observed for Cx. quinquefasciatus. Females of Cx. quinquefasciatus took significantly longer time to reach halfway at 1200 and 2400 ppm compared to controls (figure 3a; middle panel). In addition, to reach halfway took significantly less time for Ae. aegypti and An. coluzzii, compared to Cx. quinquefasciatus, at concentrations exceeding 600 ppm CO2 (1200 ppm: t-ratio −4.72, p < 0.0001; t-ratio −4.55, p = 0.0002; 2400 ppm: t-ratio −4.03, p = 0.0014; t-ratio −4.32, p = 0.0005; 4800 ppm: t-ratio −3.47, p = 0.0098, respectively; figure 3a; middle panel), with one exception. At the highest CO2 concentration tested, An. coluzzii took as long as Cx. quinquefasciatus to reach halfway, which was significantly slower than Ae. aegypti (4800 ppm: t-ratio −3.16, p = 0.0248; figure 3a; middle panel). In general, the time to make source contact did not differ between the controls and the pulsed CO2 stimuli for all three species (figure 3a; lower panel). There was one exception, Ae. aegypti reached the source faster in the presence of 4800 ppm CO2 than to the control (figure 3a; lower panel).
In general, the average number of mosquitoes that reached halfway (figure 4; middle panel) and made source contact (figure 4; lower panel) in the flight tube significantly increased in the presence of pulsed CO2 as compared with the control experiments. The proportion of mosquitoes reaching halfway in the flight tube was between 93% to 100% of the tested individuals of Ae. aegypti and An. coluzzii for all concentrations tested, whereas that of Cx. quinquefasciatus declined from 100% to 70% as concentration increased (figure 4; middle panel). Similarly, 87% to 100% of Ae. aegypti and An. coluzzii made source contact after flying upwind to pulsed CO2 (figure 4; lower panel), whilst 83% to 43% of the tested Cx. quinquefasciatus made source contact to increased concentrations of pulsed CO2 (figure 4; lower panel).
4. Discussion
Host choice by mosquitoes is, in part, regulated through senses that have been adapted to preferred hosts, and sensory constraint is a mechanism by which host breadth is regulated [7,21]. Here, we support the previous finding that detection and perception of CO2 by the olfactory system play a vital role in the activation of host-seeking behaviour [8–16]. The response characteristics of the CO2-detecting ORNs differ, however, among mosquito species, correlating with differential behavioural outputs. The data provided here emphasize that CO2 affords host recognition cues to mosquitoes, and that the detection and perception of CO2 provide mosquitoes with a dynamic, yet constrained, coding system for host finding.
4.1. Constraint in detection limits the responsiveness to CO2
The physiological and behavioural responses of the studied mosquito species to CO2 differed. In Cx. quinquefasciatus, the limited sensory ability to continuously respond throughout the pulses of CO2 at concentrations exceeding 1200 ppm, approximating that emitted by a large mammal [13], constrained the behavioural response, particularly activation. The concentration at which the first response was detected when stimulated with single pulses, above ambient CO2 levels, and the slope of the dose-response curve of the CO2-sensitive ORN in Cx. quinquefasciatus, indicate that CO2 sensing is more acute at lower ecologically relevant concentrations in this species. The increased sensitivity and reduced dynamic range of the CO2-sensitive ORNs in Cx. quinquefasciatus, compared with Ae. aegypti and An. coluzzii, when challenged with multiple pulses of high CO2 concentrations, correlates with differences in host preference breadth. Whereas all three species feed on human hosts, they also demonstrate plasticity in feeding behaviour. The hosts of Ae. aegypti and An. coluzzii include a range of mammals, whereas Cx. quinquefasciatus shifts between humans and birds, depending on host availability [3]. It appears that the preference of Cx. quinquefasciatus for birds has exerted a selective pressure on the CO2-chemosensory system to activate in response to and to follow intermittent contacts with CO2 filaments, which, because of the size of the birds, are smaller, of lower average concentration and probably rarer [22]. Having such an acute CO2-chemosensory system may have put restrictions on the dynamic range of the CO2-sensitive ORNs, as the neurons rapidly adapt, and disadapt more slowly, when stimulated with intermittent pulses of CO2 at high (greater than or equal to 2400 ppm) concentrations. It is likely that the CO2-sensory machinery has become saturated, reducing the sensitivity to repetitive stimulation. Similar restrictions have been described for pheromone-responsive ORNs in moths, showing that the tracking ability of single ORNs and the behavioural response to repeated stimuli is dependent on adaptation-disadaptation mechanics (for review see [23]). The constrained response of ORNs to high CO2 concentrations, when provided in multiple pulses, does not impede the activation and attraction of Cx. quinquefasciatus to CO2 emitted by humans, as they readily enter houses and tents with a sleeping person, where the measured CO2 concentration at the entrance of the tents was at or below 1500 ppm [24], which is within the dynamic range of their CO2-chemosensory system (this study). However, the time to activation and halfway, described in this study, increased in the presence of CO2 at or above 1200 ppm compared to controls, emphasizing that the CO2-chemosensory system is constrained at elevated CO2 concentrations over the short range. The CO2-sensitive ORNs of Ae. aegypti and An. coluzzii are less acute at concentrations above ambient CO2 levels, which is in agreement with previous studies [15,19], indicating that these highly anthropophilic mosquitoes are less dependent on CO2 alone in favour of other host cues as a basis for host selection. The dynamic range of their CO2-sensitive ORNs is wide, with a threshold at or below ambient concentration (350–400 ppm) and the neurons do not reach their maximal response at the highest dose tested (4800 ppm). Behavioural analysis also shows that both species are activated and attracted to the full concentration range of CO2 tested, which is in line with previous reports [14,25]. Thus, the CO2-chemosensory systems of Ae. aegypti and An. coluzzii are equipped to detect a wide range of CO2 emissions, from rare and intermittent CO2 signals at a distance of several metres from a potential host [26–28] to amounts equivalent to that emitted by a human or other large mammalian host [27]. In conclusion, host selection by Cx. quinquefasciatus is constrained by the dynamic range of their CO2-sensitive ORNs dictating the behavioural response, in particular activation, of this species. This may define the breadth of intrinsic preference and allow for behavioural plasticity within these limits.
5. Conclusion
While the role of CO2 in activating and attracting mosquitoes to potential hosts is well characterized [11,12], this study highlights the importance of CO2, within natural release rates, in regulating host seeking and recognition. The cross-species comparison revealed the importance of analysing the response properties and tuning of CO2-sensitive ORNs together with how this may affect the behavioural output. From a vector control perspective, this is essential when developing lures for optimal attraction of specific mosquito species in efforts to control and monitor populations.
Acknowledgements
We thank Professor Göran Birgersson for his critical reading and comments on the manuscript.
Data accessibility
All data that are relevant to the study are reported within the article.
Authors' contributions
R.I., S.R.H. and T.D. contributed to the conception and design of this study. S.M. acquired the data. All authors were involved in the analysis and interpretation of the data. R.I., S.R.H. and S.M. wrote and T.D. critically revised the manuscript. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This study was supported by the Linnaeus initiative ‘Insect Chemical Ecology, Ethology and Evolution, IC-E3’ (Formas, Swedish University of Agricultural Sciences) and a PhD scholarship to S.M. from the High Education Commission (HEC) of Pakistan.
References
- 1.Lyimo IN, Ferguson HM. 2009. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 25, 189–196. (doi:10.1016/j.pt.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 2.Scott TW, Takken W. 2012. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 28, 114–121. (doi:10.1016/j.pt.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 3.Takken W, Verhulst NO. 2013. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453. (doi:10.1146/annurev-ento-120811-153618) [DOI] [PubMed] [Google Scholar]
- 4.Dekker T, Takken W, Braks MAH. 2001. Innate preference for host-odor blends modulates degree of anthropophagy of Anopheles gambiae sensu lato (Diptera: Culicidae). J. Med. Entomol. 38, 868–871. (doi:10.1603/0022-2585-38.6.868) [DOI] [PubMed] [Google Scholar]
- 5.Lefèvre T, Gouagna L-C, Dabiré KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F. 2009. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae ss when humans are not readily accessible. Am. J. Trop. Med. Hyg. 81, 1023–1029. (doi:10.4269/ajtmh.2009.09-0124) [DOI] [PubMed] [Google Scholar]
- 6.Govella NJ, Chaki PP, Killeen GF. 2013. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar. J. 12, 124 (doi:10.1186/1475-2875-12-124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takken W, Knols BGJ. 1999. Odor-mediated behavior of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131–157. (doi:10.1146/annurev.ento.44.1.131) [DOI] [PubMed] [Google Scholar]
- 8.Cardé RT. 2015. Multi-cue integration: how female mosquitoes locate a human host. Curr. Biol. 25, R793–R795. (doi:10.1016/j.cub.2015.07.057) [DOI] [PubMed] [Google Scholar]
- 9.Geier M, Bosch OJ, Boeckh J. 1999. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J. Exp. Biol. 202, 1639–1648. [DOI] [PubMed] [Google Scholar]
- 10.Dekker T, Takken W, Cardé RT. 2001. Structure of host-odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiol. Entomol. 26, 124–134. (doi:10.1046/j.1365-3032.2001.00225.x) [Google Scholar]
- 11.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. (doi:10.1016/j.cell.2013.12.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster B, Lacey ES, Cardé RT. 2015. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J. Chem. Ecol. 41, 59–66. (doi:10.1007/s10886-014-0542-x) [DOI] [PubMed] [Google Scholar]
- 13.Dekker T, Takken W. 1998. Differential responses of mosquito sibling species Anopheles arabiensis and An. quadriannulatus to carbon dioxide, a man or a calf. Med. Vet. Entomol. 12, 136–140. (doi:10.1046/j.1365-2915.1998.00073.x) [DOI] [PubMed] [Google Scholar]
- 14.Dekker T, Cardé RT. 2011. Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. J. Exp. Biol. 214, 3480–3494. (doi:10.1242/jeb.055186) [DOI] [PubMed] [Google Scholar]
- 15.Grant AJ, Wigton BE, Aghajanian JG, O'Connell RJ. 1995. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. A 177, 389–396. (doi:10.1007/BF00187475) [DOI] [PubMed] [Google Scholar]
- 16.Majeed S, Hill SR, Ignell R. 2014. Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. J. Exp. Biol. 217, 598–604. (doi:10.1242/jeb.092718) [DOI] [PubMed] [Google Scholar]
- 17.Majeed S, Hill SR, Birgersson G, Ignell R. 2016. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R. Soc. Open Sci. 3, 160467 (doi:10.1098/rsos.160467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook JI, Majeed S, Ignell R, Pickett JA, Birkett MA, Logan JG. 2011. Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull. Entomol. Res. 101, 541–550. (doi:10.1017/s0007485311000162) [DOI] [PubMed] [Google Scholar]
- 19.Lu T, et al. 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544. (doi:10.1016/j.cub.2007.07.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syed Z, Leal WS. 2007. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem. Senses 32, 727–738. (doi:10.1093/chemse/bjm040) [DOI] [PubMed] [Google Scholar]
- 21.Bowen M. 1991. The sensory physiology of host-seeking behavior in mosquitoes. Annu. Rev. Entomol. 36, 139–158. (doi:10.1146/annurev.en.36.010191.001035) [DOI] [PubMed] [Google Scholar]
- 22.Mboera L, Takken W. 1997. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Rev. Med. Vet. Entomol. 85, 355–368. [Google Scholar]
- 23.Todd JL, Baker TC. 1999. Function of peripheral olfactory organs. In Insect olfaction (ed. Hansson BS.), pp. 67–96. Heidelberg, Germany: Springer. [Google Scholar]
- 24.Mboera LEG, Knols BGJ, Takken W, Torre Ad. 1997. The response of Anopheles gambiae s.l. and A. funestus (Diptera: Culicidae) to tents baited with human odour or carbon dioxide in Tanzania. Bull. Entomol. Res. 87, 173–178. (doi:10.1017/S0007485300027322) [Google Scholar]
- 25.Geier M, Sass H, Boeckh J. 1996. A search for components in human body odour that attract females of Aedes. In Olfaction in mosquito-host interactions (eds Bock GR, Cardew G), p. 132 Chichester, UK: Ciba Foundation Symposium 200, John Wiley & Sons. [DOI] [PubMed] [Google Scholar]
- 26.Zöllner GE, Torr SJ, Ammann C, Meixner FX. 2004. Dispersion of carbon dioxide plumes in African woodland: implications for host-finding by tsetse flies. Physiol. Entomol. 29, 381–394. (doi:10.1111/j.0307-6962.2004.00399.x) [Google Scholar]
- 27.Costantini C, Gibson G, Sagnon NF, Torre AD, Brady J, Coluzzi M. 1996. Mosquito responses to carbon dioxide in B West African Sudan savanna village. Med. Vet. Entomol. 10, 220–227. (doi:10.1111/j.1365-2915.1996.tb00734.x) [DOI] [PubMed] [Google Scholar]
- 28.Gillies MT. 1980. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull. Entomol. Res. 70, 525–532. (doi:10.1017/S0007485300007811) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that are relevant to the study are reported within the article.