Abstract
Inflammatory bowel disease (IBD) is induced by multiple environmental factors, and there is still no known treatment capable of curing the disease completely. We propose a zeolite-containing mixture (Hydryeast®, HY)—a multi-component nutraceutical of which the main ingredients are Azumaceramics (mixture of zeolite and oyster shell burned under high temperature), citric acid, red rice yeast (monascus) and calcium stearate—as a nutraceutical intervention in IBD to ameliorate dextran sodium sulfate (DSS)-induced colitis. We show the mechanism through integrated omics using transcriptomics and proteomics. C57BL6 mice were given an AIN-93G basal diet or a 0.8% HY containing diet and sterilized tap water for 11 days. Colitis was then induced by 1.5% (w/v) DSS-containing water for 9 days. HY fed mice showed significantly improved disease activity index and colon length compared to DSS mice. Colonic mucosa microarray analysis plus RT-PCR results indicate HY supplementation may ameliorate inflammation by inhibiting the intestinal inflammatory pathway and suppress apoptosis by curbing the expression of genes like tumor protein 53 and epidermal growth factor receptor and by upregulating epithelial protection-related proteins such as epithelial cell adhesion molecule and tenascin C, thus maintaining mucosal immune homeostasis and epithelial integrity, mirroring the proteome analysis results. HY appears to have a suppressive effect on colitis.
Keywords: zeolite-containing mixture (Hydryeast®), Azumaceramics, DSS, inflammatory bowel disease, apoptosis, colon mucosa
1. Introduction
Inflammatory bowel diseases (IBD), which include Crohn’s disease (CD), ulcerative colitis (UC), and indeterminate colitis (IC), are characterized by chronic, remittent, and inflammatory-mediated disorders of the gastrointestinal tract [1]. The precise etiology of IBD is not fully understood, but it has gradually become clear that IBD involves the interactions of multiple environmental factors like foods and smoking [2], diverse properties of predisposing genetic factors, and immune system response [3] such as innate and adaptive immune abnormal regulation which results in responses to the intestinal microbiota in genetically susceptible hosts [4]. There is still no effective treatment that heals IBD completely, and affected patients continue to suffer from IBD symptoms along with a tendency to relapse.
Hydryeast® (HY, Azuma Chemical Co. Ltd., Tokyo, Japan) is a zeolite-containing mixture that is a multi-component nutraceutical with the following main ingredients: Azumaceramics (47.6%), citric acid (33.3%), red rice yeast (monascus) (16.7%), and calcium stearate (2.4%). The major component is the Azumaceramics, which is a mixture of zeolite and oyster shell burned under high temperature. Zeolite, a mineral with a microporous structure, is used mainly as a cation exchanger by a chemical/physical process in several industries and in the field of medicine [5], and zeolite is also added to the fodder of livestock and poultry, as it can improve the absorptivity and bioavailability of some minerals and vitamins [6,7]. Citric acid is a weak organic tribasic acid, the chemical formula of which is C6H8O7, and its main applications are as a food additive and in the fields of chemistry and textiles. Red rice yeast (monascus), produced from the fermentation of steamed rice using the fungus Monascus purpureus, is widely utilized in food coloring and preserving and wine making; moreover, in East Asia, red rice yeast has been used for centuries to promote food digestion and blood circulation [8]. Calcium stearate is an anticaking agent used in a wide variety of foods including salt, spices, confections, snack products, and dry mixes. To the best of our knowledge, little or no research has been conducted regarding the application of the components described above as a treatment for IBD.
In this study, we used a nutrigenomics method to gain a better understanding of HY as a dietary supplement for the improvement of colitis in a mouse model of dextran sodium sulfate (DSS)-induced colitis through transcriptome, proteome, and biochemical analyses using colon mucosa tissue.
2. Materials and Methods
2.1. Animals and Dietary Treatment
Seven-week-old male C57BL6 mice obtained from Charles River Japan (Tokyo, Japan) were housed in individual animal cages at controlled temperature (23 ± 2 °C), relative humidity (50–60%), and lighting condition (12-h light/dark cycle) throughout the entire experiment. After 3 days of acclimatization, the mice were assigned to four groups with equal mean body weights, with each group comprised of six to eight mice: (1) the HY8 group, which received the 0.8% HY powder added AIN-93G basal diet and sterilized tap water during the entire experiment; (2) the CON group, which received the AIN-93G basal diet and sterilized tap water for the course of the entire experiment; (3) the DSS group, which received the AIN-93G (American Institute of Nutrition in 1993) basal diet and sterilized tap water for 11 days, after which colitis was induced by 1.5% (w/v) DSS (DSS molecular weight, 40 kDa; MP Biomedicals, Irvine, CA, USA)-containing sterilized tap water for 9 days; (4) the DHY8 group, which received the 0.8% HY powder added AIN-93G basal diet during the entire experiment and sterilized tap water for 11 days, after which colitis was induced by 1.5% DSS-containing sterilized tap water for 9 days. The 0.8% HY added diet was adjusted with cornstarch to maintain the caloric balance, as shown in Table 1. The choice of 0.8% HY in this experiment was based on our preliminary dose-dependence experiment [9]. This research was approved by the Animal Care and Use Committee of the University of Tokyo (Approval No. P13-739).
Table 1.
Composition of the diets. HY, Hydryeast®.
| Ingredients | Control Diet (AIN-93G) | Zeolite-Containing Mixture 0.8% |
|---|---|---|
| Cornstarch | 39.75% | 38.95% |
| Casein (85.7% protein) | 20% | 20% |
| Detrinized cornstarch | 13.2% | 13.2% |
| Sucrose | 10.25% | 10.25% |
| Soybean oil (no additives) | 7% | 7% |
| Fiber | 5% | 5% |
| Mineral mix (AIN 93G-MX) | 3.5% | 3.5% |
| Vitamin mix (AIN 93G-MX) | 1% | 1% |
| L-Cystine | 0.3% | 0.3% |
| Zeolite-containing mixture (HY) | 0 | 0.8% |
2.2. Evaluation of the Disease Activity Index
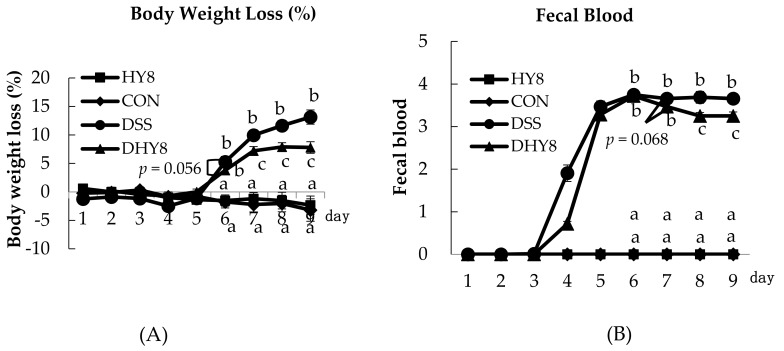
The Disease Activity Index (DAI) was the average of three scores (body weight loss, fecal blood, and stool consistency) after the DSS was fed to mice. These scores were determined as follows: body weight loss ratio (0: <1%, 1: 1–5%, 2: 5–10%, 3: 10–15%, 4: >15%), fecal blood (0: no fecal blood, 2: ++, 4: +++) and stool consistency (0: normal, 2: soft, 4: diarrhea) [10].
2.3. Blood Collection and Tissue Harvesting
Upon termination of the experiment, all of the mice were deeply anesthetized with pentobarbital sodium prior to euthanasia by bleeding from the carotid artery. The blood obtained was centrifuged at 1000× g for 15 min at 4 °C to obtain the plasma. The colon length was measured between the ileo-cecal junction and the proximal rectum, and this measurement was also used as one of the criteria for the extent of colitis. The tissues of liver, excised colon, mesenteric adipose, and retroperitoneal adipose were snap-frozen in liquid nitrogen and stored at −80 °C until further analysis.
2.4. Biochemical Assays
Colonic myeloperoxidase (MPO) activity was measured by the colorimetrical method using the MPO Activity Colorimetric Assay Kit (BioVision, Palo Alto, CA, USA) according to the manufacturer’s instructions.
2.5. Colon Histology
Each colon slice was embedded in OCT (Optimal Cutting Temperature) compound (Sakura Finetek, Torrance, CA, USA) and then snap-frozen in liquid nitrogen. Each 5-μm-thick slice of tissue was sectioned and stained by hematoxylin and eosin (H&E), then scanned by light microscopy (Olympus BX51 microscope, Olympus Optical, Tokyo, Japan).
2.6. Total RNA Extraction and Quality Assessment
Total RNA was extracted from the colon mucosa using the total RNA Isolation Kit, NucleoSpin® RNAΠ (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions. The concentration and purity of RNA were measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
2.7. Transcriptome Analysis
2.7.1. DNA Microarray Preparation
The colon mucosa RNA samples from individual mice in each group were pooled (n = 6–8), and a microarray analysis was then conducted using Affymetrix Mouse Genome 430 2.0 Array Genechips (Affymetrix, Santa Clara, CA, USA), which contains over 40,000 gene probe sets for genome-wide expression profiling.
2.7.2. Mapping and Functional Analysis
We compared gene expression ratios between the DSS and CON groups and between the DHY8 and DSS groups after the images were scanned by Microarray Suite ver. 5.0 software (Affymetrix, Santa Clara, CA, USA). An increase in an expression ratio between treatments that was >1.5-fold was regarded as significant expression.
2.8. Reverse Transcription-Polymerase Chain Reaction
To verify the expression of differentially expressed genes, we carried out a reverse transcription-polymerase chain reaction (RT-PCR), the primer sequences of which are shown in Table S1. The expression levels of respective genes were normalized against the expression of 60S acidic ribosomal protein p1 (Rplp1) in colon mucosa.
2.9. Protein Preparation, iTRAQ Labeling, and NanoLC-MS/MS Analysis for Proteome Analysis
Lysis buffer was used to extract protein from colon mucosa, which was then separated by centrifugation at 12,000× g for 30 min at 4 °C. Protein concentrations were determined by a Bradford assay. According to the manual of the 4-plex iTRAQ labeling kit (AB Sciex, Framingham, MA, USA), proteins (100 μg) were pooled for cysteine blocking and digested, then labeled with isobaric tags as follows for the further analysis by nanoscale liquid chromatography coupled to tandem mass spectrometry (NanoLC-MS/MS; AB Sciex, Tokyo, Japan): CON, 115 tag; DSS, 114 tag; DHY8, 116 tag.
2.10. Statistical Analysis
The data are presented as the mean value ± standard error (SE) and were analyzed by a two-way analysis of variance (ANOVA). Significant differences were evaluated with Tukey’s test at the level of p < 0.05.
3. Results
3.1. General Characteristics
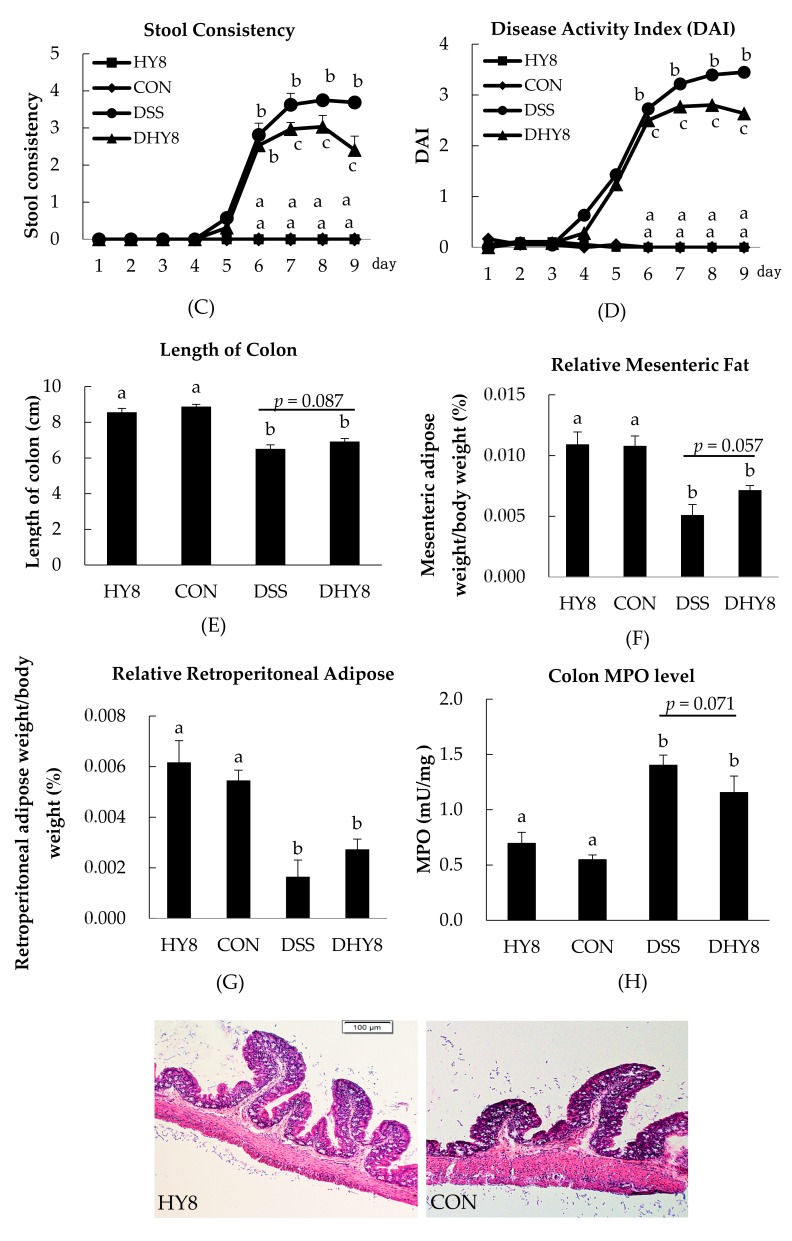
Some general characteristics were measured to investigate the effect of HY supplementation on colitis. No significant differences were observed in food intake between the DHY8 and DSS groups, but there was a significant difference in food intake between the CON and DSS groups (HY8: 76.7 ± 2.6; CON: 82.8 ± 3.5; DSS: 73.1 ± 1.7; DHY8: 76.4 ± 3.3 g). There was a significant difference in water intake between the HY8 and DSS groups (HY8: 84.8 ± 2.3; DSS: 70.3 ± 2.1; CON: 73.7 ± 1.9; DHY8: 72.2 ± 1.4 mL). Severe clinical phenomena occurred following the DSS treatment, including body weight loss and altered fecal blood and stool consistency, which led to deteriorated DAI values compared to the CON group (the clinical scores are shown in Table S4). HY supplementation ameliorated the body weight loss and ameliorated the pathological condition of colitis, which was also indicated by the DAI values on days 6–9 (Figure 1A–D). The DSS-induced decrease in the colon length and mesenteric fat weight as well as the increase of colon MPO activity tended to be improved by HY supplementation, while there was no change in the weight of retroperitoneal adipose tissue (Figure 1E–H).
Figure 1.
General characters of each groups. (A) Body weight loss; (B) Fecal blood; (C) Stool consistency; (D) Disease Activity Index (DAI); (E) Length of colon; (F) Relative mesenteric fat; (G) Relative retroperitoneal adipose; (H) Colon MPO level; (I) Hematoxylin and eosin (H&E) staining of colon. All values are mean ± SE (n = 6–8) by two-way ANOVA.
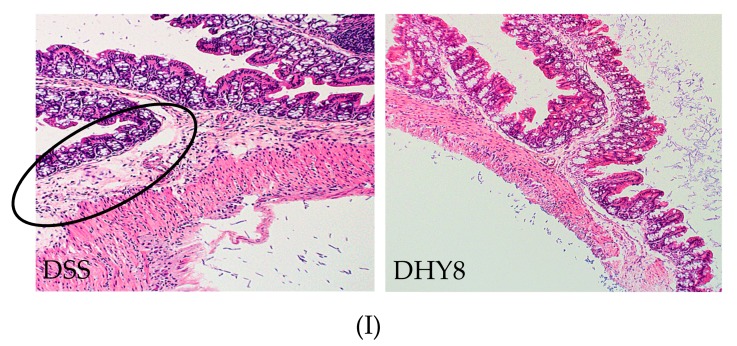
3.2. Colon Histology
Colon sections were made to directly confirm the effect of HY supplementation on colon tissue, especially on colon mucosa. DSS induced a structural disorder of mucosa epithelium cells, which may lead to an increased infiltration of inflammatory cells into the mucosa and submucosa. HY supplementation clearly ameliorated this disorder with a reduced infiltration of inflammation cells (Figure 1I).
3.3. Colonic Microarray Analysis
We also carried out the microarray analysis to identify the effect of HY supplementation on colitis from the gene level. A total of 5238 genes were differentially expressed, of which 2300 were significantly upregulated in the DHY8 mice compared to the DSS mice and downregulated in the DSS mice compared to the CON mice, whereas 2938 genes were notably downregulated in the DHY8 group compared to the DSS group and upregulated in the DSS group compared to the CON group. The list of relative gene changes is appended in Table S2.
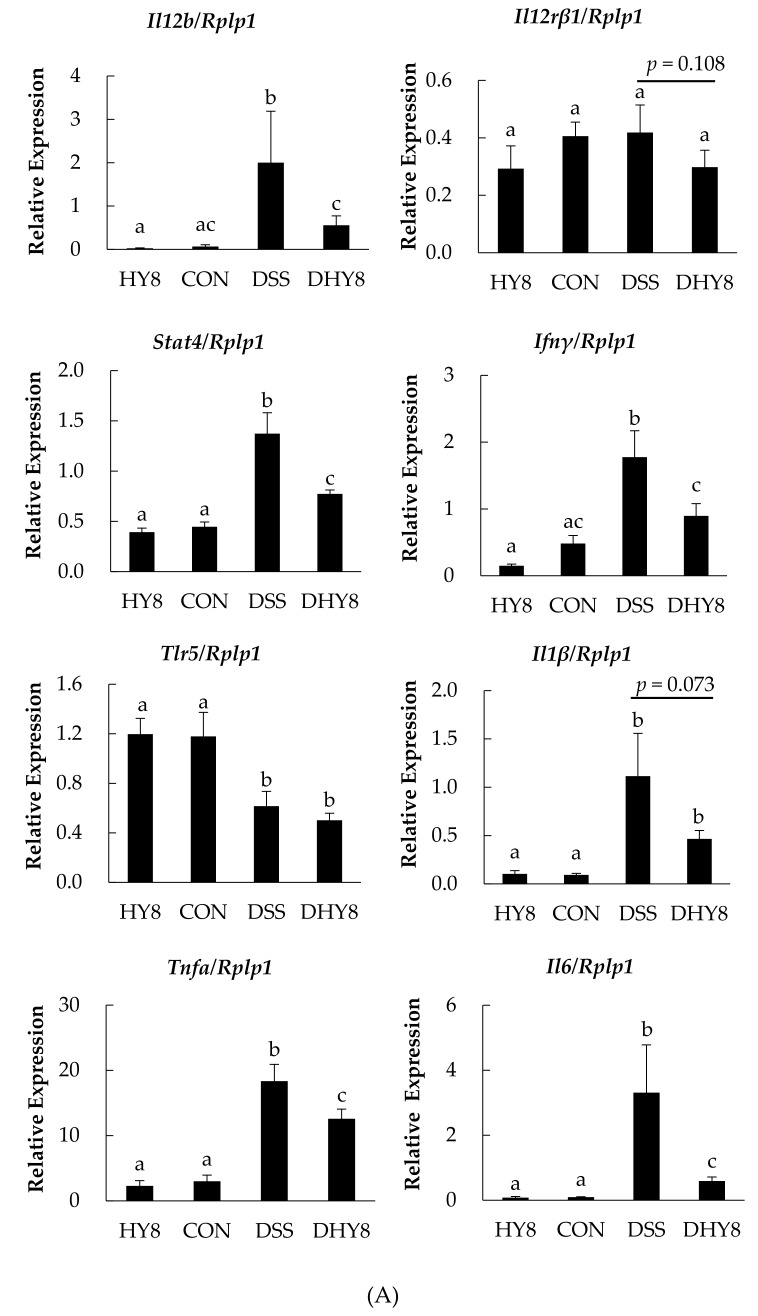
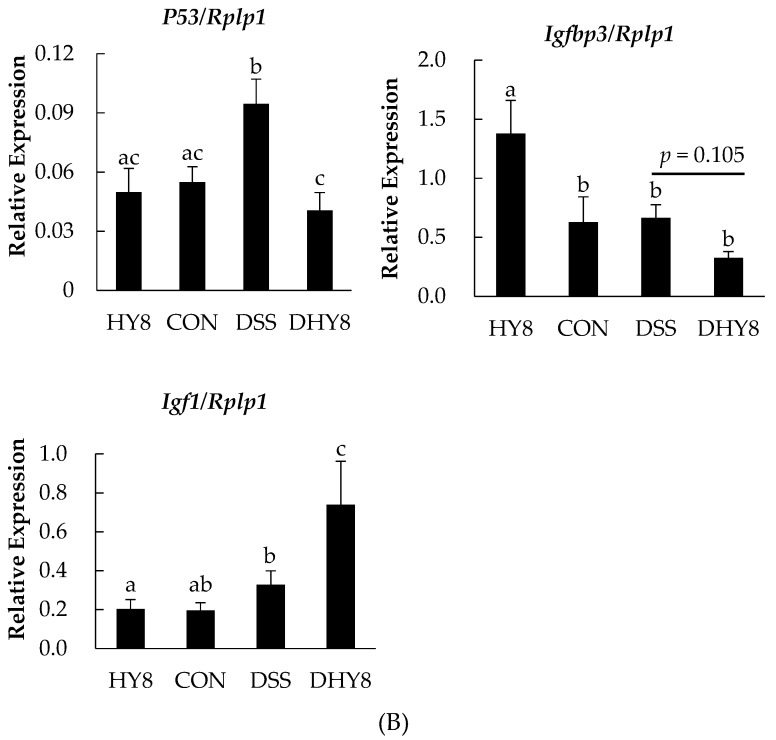
HY supplementation ameliorated the inflammation level in colon mucosa via suppressing the expression of genes related to the intestinal inflammatory pathway, including interleukin 12b (Il12b), signal transducer and activator of transcription 4 (Stat4), interferon gamma (Ifnγ), tumor necrosis factor-alpha (Tnfα), interleukin 6 (Il6); the expression of interleukin 12 receptor subunit beta 1 (Il12rβ1) and interleukin 1 beta (Il1β) showed a decreasing tendency (p = 0.108, p = 0.073) (Figure 2A). HY supplementation also downregulated the expression of the following genes: apoptosis-related tumor protein 53 (P53) (Figure 2B); members of the chemokine ligand family (Cxcl): Cxcl1, Cxcl2, Cxcl3, Cxcl10, Cxcl12, and Cxcl17, chemokine (C-C motif) ligand (Ccl): Ccl4 and Ccl24; and an epithelial maintenance-related gene, epidermal growth factor receptor (Egfr). Compared to the DSS group, trefoil family factor 2 (Tff2) and insulin-like growth factor 1 (Igf1) were upregulated in the DHY8 group (Figure 2B).
Figure 2.
(A) Colonic mucosa mRNA expression of genes related to the intestinal inflammatory pathway; (B) Apoptosis-related P53 pathway. The relative mRNA expressions of Il12b, Il12rβ1, Ifnγ, Stat4, Tlr5, Il1β, Tnfa, Il6, P53, Igfbp3, and Igf1 were measured by RT-PCR and normalized to Rplp1. All values are mean ± SE (n = 6–8) by two-way ANOVA.
3.4. Comparative Proteomic Analysis by iTRAQ
We then validated the effect of HY supplementation on colitis from the protein level by iTRAQ. More than 5000 proteins were identified by the comparative proteomic analysis by iTRAQ. We regarded the proteins that exhibited >1.5-fold changes as altered proteins, and 668 proteins were changed to this degree, including 332 proteins that were upregulated in the DHY8 mice compared to the DSS mice but downregulated in the DSS mice compared to the CON mice. There were 336 proteins that were downregulated in the DHY8 group compared to the DSS group but upregulated in the DSS group compared to the CON group (Table S3).
The 668 altered proteins were put into Ingenuity Pathway Analysis (IPA) [11] to further investigate the relative protein alteration associated with colitis. Supplementation with HY improved the inflammation, apoptosis, intestinal motility, and epithelia condition by upregulating the following proteins: a protein involved in cancer, periostin (Postn); the anti-inflammation-related proteins adenylate cyclase activating polypeptide (Perp1) and galectin 2 (Leg2); the anti-apoptosis-related proteins known as BCL2-associated athanogene 3 (Bag3), RNA-binding motif protein 3 (Rbm3), and ferritin heavy chain (Fhc); an intestinal motility-related protein, phosphatase 1 regulatory inhibitor subunit 14A (Pp14a); and some intestinal mucosal protection and epithelia homeostasis-related proteins, i.e., epithelial cell adhesion molecule (Epcam), tenascin C (Tena), and numb homolog (Numb) (Table 2).
Table 2.
Differentially expressed proteins in colonic mucosa.
| Accession No. | Protein Symbol | Protein Name | Gene Symbol | Fold Change | Fold Change |
|---|---|---|---|---|---|
| DSS vs. CON | DHY8 vs. DSS | ||||
| Q62009-5 | Postn | Isoform 5 of Periostin | Postn | 0.31 | 2.36 |
| Q9D8I1 | Perp1 | Plasma cell-induced resident endoplasmic reticulum protein | Pacap | 0.13 | 5.01 |
| Q9CQW5 | Leg2 | Galectin-2 | Lgals2 | 0.62 | 3.02 |
| Q9JLV1 | Bag3 | BAG family molecular chaperone regulator 3 | Bag3 | 0.67 | 2.31 |
| O89086 | Rbm3 | Putative RNA-binding protein 3 | Rbm3 | 0.43 | 4.25 |
| Q8VHX6-2 | Fhc | Ferritin heavy chain | Fhc | 0.37 | 2.99 |
| Q91VC7 | Pp14a | Protein phosphatase 1 regulatory subunit 14A | Ppp1r14a | 0.33 | 6.43 |
| Q99JW5 | Epcam | Epithelial cell adhesion molecule | Epcam | 0.32 | 3.19 |
| Q80YX1-5 | Tena | Isoform 5 of Tenascin | Tnc | 0.65 | 2.31 |
| Q9QZS3-3 | Numb | Isoform 71 kDa of Protein numb homolog | Numb | 0.44 | 4.17 |
| Q61646 | Hpt | Haptoglobin | Hp | 36.31 | 0.32 |
| P01027 | Co3 | Complement C3 | C3 | 6.55 | 0.34 |
| P11247 | Perm | Myeloperoxidase | Mpo | 5.50 | 0.55 |
| Q61316 | Hsp74 | Heat shock 70 kDa protein 4 | Hspa4 | 1.89 | 0.10 |
| Q9D312 | K1c20 | Keratin, type I cytoskeletal 20 | Krt20 | 1.98 | 0.50 |
| P28352 | Apex1 | DNA-(apurinic or apyrimidinic site) lyase | Apex1 | 1.92 | 0.07 |
| P70677 | Casp3 | Caspase-3 | Casp3 | 1.61 | 0.14 |
| P97864 | Casp7 | Caspase-7 | Casp7 | 6.61 | 0.42 |
| Q8VEK3 | Hnrpu | Heterogeneous nuclear ribonucleoprotein U | Hnrnpu | 1.92 | 0.34 |
CON (control group): AIN-93G basal diet and sterilized tap water; DSS (DSS (dextran sodium sulfate) group): AIN-93G basal diet and sterilized tap water for 11 days, after which colitis was induced by 1.5% (w/v) DSS -containing sterilized tap water for 9 days; DHY8 (DHY8 group), 0.8% HY powder added AIN-93G basal diet during the entire experiment and sterilized tap water for 11 days, after which colitis was induced by 1.5% DSS-containing sterilized tap water for 9 days.
Inflammation and apoptosis induced by DSS could also be proved to be improved by the decreased amounts of the following proteins: inflammatory degree-related proteins, i.e., haptoglobin (Hpt), complement component 3 (Co3), myeloperoxidase (Perm), Annexin A2 (Anxa2), and heat shock protein family A member 4 (Hsp74); proteins involved in apoptosis, i.e., keratin 20 (K1c20), apyrimidinic endodeoxyribonuclease 1 (Apex1), caspase-7 (Casp7), and caspase-3 (Casp3); and a protein of biomarker for colorectal carcinoma, heterogeneous nuclear ribonucleoprotein U (Hnrpu) (Table 2).
4. Discussion
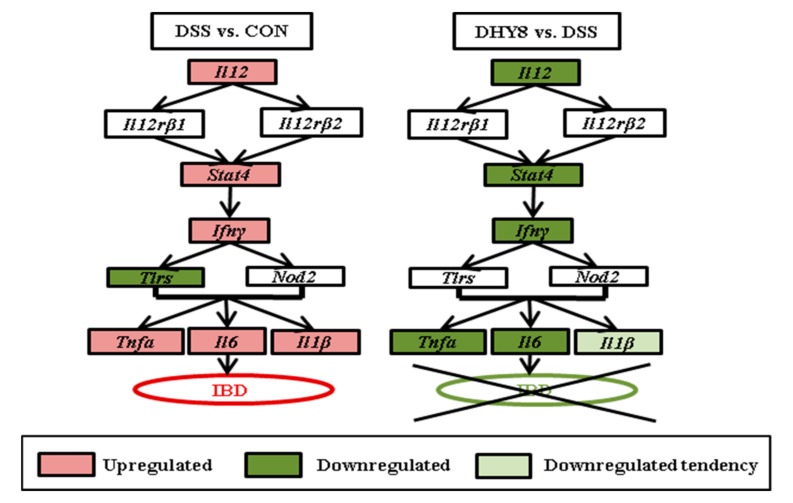
The microarray analysis revealed that HY supplementation ameliorated the inflammatory status in the mice by suppressing the expression of genes involved in the intestinal inflammatory pathway: Il12pos Il12rβ1pos Stat4pos Ifnγpos Tlr5pos Tnfα/Il6/Il1β. Figure 3 is a schematic representation of the intestinal inflammatory pathway expression altered by HY supplementation in DSS-induced colitis. In this pathway, Stat4 tyrosine phosphorylation is induced by IL-12; this is required for the T-cell-independent induction of the cytokine IFN-γ [12], and then in response to the TLR stimulation, both lymphoid and non-lymphoid cells can produce the pro-inflammatory cytokine IL6 [13], which together with TNFα and IL1β are considered to be the major pro-inflammatory cytokines. The downregulation of the intestinal inflammatory pathway led mainly to decreased levels of the pro-inflammatory cytokines induced by DSS, which by this point indicates the mitigated inflammatory degree by HY treatment.
Figure 3.
Schematic representation of the intestinal inflammatory pathway expression altered by Hydryeast supplementation (DHY8) in dextran sodium sulfate (DSS)-induced colitis.
The above-mentioned reduction of pro-inflammatory cytokine levels may also be reflected in the altered expression of the following three marker proteins. (1) The upregulation of Pp14a (Cpi-17), which is an inhibitor of smooth muscle myosin phosphatase and would be suppressed by the upregulation of IL1β or TNFα induced by chronic inflammatory bowel diseases and then lead to dysfunctional motility [14,15]; (2) The downregulation of Hpt, an acute-phase α-sialoglycoprotein with hemoglobin-binding capacity which could be induced by pro-inflammatory cytokines such as IL6, IL1β, and TNFα [16]; (3) The reduction of Hsp74, which could be induced by inflammation and might then result in the inhibition of apoptosis of inflammatory cells and enhance the immune response by increasing Bcl-2 and Il-12 expression [17].
The alteration of the following three proteins also indicated the amelioration of inflammation by HY supplementation. (1) The upregulation of Perp1, which plays a key role in anti-inflammation by controlling the balance of Th1/Th2 and reducing the secretion of pro-inflammatory cytokines such as IL6, IL1β, and TNFα. Perp1 is also associated with the maintenance of intestinal epithelial integrity, and a deficiency of Perp1 would thus result in damage to epithelial cells of intestine [18]; (2) The downregulation of Co3, which plays a key role in the activation of the complement system. The reduction of Co3 expression would lessen the number of infiltrating neutrophils in the lesions (which are the main producer of IL1β) and thus function as tumor suppression [19]; (3) The diminution of Anxa2 could downregulate another pro-inflammatory cytokine, TNFα, by the prevention of TNFα shedding in IBD [20]. Taking the findings regarding the significantly changed genes and relative proteins induced by HY supplementation together, we propose that HY supplementation might ameliorate DSS-induced inflammation by mainly suppressing the intestinal inflammatory pathway as well as relative genes and proteins, and then suppressing the expression of the pro-inflammatory cytokine genes Il6, Il1β, and Tnfα.
In addition to the anti-inflammation effect of HY treatment, we speculate that HY supplementation may alleviate DSS-induced colitis by ameliorating apoptosis, regulating the cell cycle, and preserving epithelial integrity, thus maintaining the barrier function of colon mucosa. In relation to the P53 gene, which was shown to be downregulated in microarray analysis, we investigated the expression of the pathway P53pos Igfbp3neg Igf1, the positive expression of which would lead to apoptosis. However, the expression of the gene Igfbp3 was not significantly different between the DSS and DHY8 groups. The upregulation of the matricellular protein Postn revealed by the proteome analysis also confirmed our hypothesis, as its overexpression may result in the downregulation of P53, the overexpression of which would lead to apoptosis [21]. We thus suspect that the addition of HY upregulated Postn and then inhibited the expression of P53, by which the apoptosis was suppressed and the cell growth and the cell cycle were improved. Moreover, the downregulation of P53 could also lead to a decrease in the production of DNA-damaging molecules, which could result in inflammation and then apoptosis and cell loss [22]. The reduced expression of P53 might not only indicate the anti-apoptosis function of HY but might also ameliorate the inflammatory status induced by DSS. From the results of the RT-PCR, we found that between the HY8 and CON groups there are no significant differences in the gene expressions of Il12b, Il12rβ1, Stat4, Ifnγ, Tnf-α, Il6, Il-1β, P53, and Igf1 except the result of Igfbp3. These findings indicate that HY might have no direct side effect on mice as compared to the CON group. However, future experiments are needed to confirm this.
In addition, the ameliorated apoptosis may be echoed by the upregulation of another three related proteins: Bag3, which is capable of suppressing apoptosis by Bcl-2 in vitro [23] and maintaining cell survival by restraining cell death and regulating gastrointestinal functions [24]; Rbm3, the lack of which could lead to mitotic catastrophe due to the absence of important proteins required for cell growth [25,26]; and Fhc, which may function as an essential mediator to antagonize apoptosis by inhibiting the activation of mitogen-activated protein kinase 8 (Mapk8) through iron sequestration (which is related to reactive oxygen species accumulation) and thereby suppressing the apoptosis induced by TNFα [27].
In light of the results of our microarray analysis showing downregulation of Mapk8 (DSS vs. CON: 1.49, DHY8 vs. DSS: 0.07) and the PCR result for Tnfα, we speculated that the supplementation of HY might also suppress apoptosis by promoting the pathway FhcnegMapk8posTnfα. In addition to the three upregulated proteins, the downregulation of another three apoptosis-related proteins may further confirm our speculation: Apex1, which could lead to the gathering of damaged DNA by affecting the DNA repair process via a reduction of the ability to renovate oxidative damage, thus promoting the rates of apoptosis and increasing the risk of UC [28,29]; Casp3, the overexpression of which is also reported to lead to increased apoptosis and a decline in proliferation capacity [30]; and K1c20, a marker of small intestinal goblet cells, which can be induced by apoptosis and tissue injury [31].
The expression of another two genes could be of great importance in the maintenance of epithelial cells and barrier function both directly and indirectly. The activation of Egfr—which is upregulated in AOM (azoxymethane)/DSS-induced colitis and also induced by colitis—plays a role in the amelioration of damaged epithelial cells and in the maintenance of the epithelial barrier function in UC [32]. The downregulation of Egfr by HY supplementation indicates the improvement of colitic status. Tff2, which is secreted and expressed by gastrointestinal epithelium and plays a vital role in the maintenance of epithelial integrity and mucosal surfaces [33], was upregulated by HY supplementation. Moreover, Tff2 is essential in the immune response in inflammatory conditions by controlling the expression of pro-inflammatory cytokines such as IL6 and IL1β [34].
In the present study, the changes of Egfr and Tff2 expression brought about by HY treatment indicated the amelioration of epithelial cells damaged by DSS, which might cause a lesion to the intestinal barrier and promote the permeability of barrier cells. The above-described findings might be supported by the colon histology results showing that the status of the goblet cells damaged by DSS was alleviated in the DHY8 mice compared to the DSS mice. In addition, TFF2 deficiency has been reported to lead to a loss of body weight [33], and the upregulation of TFF2 induced by HY supplementation seems consistent with improved body weight loss.
Based on the results of our proteome analysis, we speculate that HY treatment might have contributed to epithelial protection by upregulating the following proteins. Epcam, which plays a role in the shaping of the intestinal normal architectural structure and managing Ca2+-independent homotypic cell–cell adhesion, is downregulated in IBD patients, resulting in the loss of epithelial cell contact, increased permeability, and then the penetration of the bacterial products [35,36]. Tena, an extracellular matrix protein, may contribute to intestinal mucosal protection by promoting cell migration and remodeling, accelerating the recovery process in wound areas [37]; moreover, an anti-inflammatory effect of Tena was reported in a study in which the upregulation was shown to inhibit T-cell activation [38]. Numb, a membrane-bound protein that is predominantly expressed in intestinal mucosa and might determine cell fate, could lead to an improvement of intestinal epithelial barrier function via a modulation of the paracellular permeability by affecting the apical junctional complex assembly and myosin light chain phosphorylation; a Numb deficiency might result in barrier dysfunction [39].
In the present study, the upregulations of Epcam, Tena, and Numb indicated that the mucosal barrier repair or protection was enhanced and the dysfunction of epithelial adhesion and tight junctions induced by DSS were ameliorated. The above-described results are consistent with our observations of (1) the repaired epithelial cells from the colon histology analysis and (2) improved clinical symptoms, as the status of fecal blood and stool consistency was better in the DHY8 mice compared to the DSS mice.
Regarding the ingredients of HY, the RT-PCR results shown in Figures S1 and S2 suggested that zeolite and citric acid functioned independently, in that zeolite played a role in ameliorating apoptosis by decreasing the expression of P53 and upregulating the expression of Igf1, whereas citric acid significantly suppressed inflammation by inhibiting the intestinal inflammatory pathway. From this viewpoint, we believe that the functions of HY may be the result of interactions of the various HY ingredients.
5. Conclusions
Based on our observation of the downregulation of relative pro-inflammation cytokines and the increased expression of the anti-inflammatory factors through our colon transcriptome and proteome analyses, we propose that HY suppresses the expression of the intestinal inflammatory pathway, thus diminishing the degree of inflammation in DSS-induced colitis. HY may suppress apoptosis and maintain mucosal immune homeostasis by inhibiting relative genes such as P53; other epithelial maintenance-related genes and proteins were also greatly altered, which indicates that the cell-cycle disorder caused by DSS was improved to some extent. Collectively, the integrated omics analysis results indicate that the effects of HY on DSS-induced colitis are based mainly on ameliorating the inflammation status, improving the degree of apoptosis, and maintaining the epithelial integrity and barrier function of colon mucosa.
Acknowledgments
The authors would like to thank all of the subjects who took part in this study.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6643/9/5/467/s1.
Author Contributions
H.J. designed the study. H.J., C.D. and W.L. performed the experiments and collected the data. H.J., C.D. and W.L. analyzed the data. W.L. wrote and H.K. and H.J. revised the paper. The material was provided by S.Y. All authors reviewed the manuscript.
Conflict of Interest
We declare that we conducted the present study for scientific purposes only, with no financial or personal relationships with other people or organizations that would provide a conflict of interest.
References
- 1.Jakubowski A., Zagorowicz E., Kraszewska E., Bartnik W. Rising hospitalization rates for inflammatory bowel disease in Poland. Pol. Arch. Med. Wewn. 2014;124:180–190. doi: 10.20452/pamw.2188. [DOI] [PubMed] [Google Scholar]
- 2.Richman E., Rhodes J.M. Review article: Evidence-based dietary advice for patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013;38:1156–1171. doi: 10.1111/apt.12500. [DOI] [PubMed] [Google Scholar]
- 3.Owczarek D., Rodacki T., Domagala-Rodacka R., Cibor D., Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016;22:895–905. doi: 10.3748/wjg.v22.i3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 5.Tondar M., Parsa M.J., Yousefpour Y., Sharifi A.M., Shetab-Boushehri S.V. Feasibility of clinoptilolite application as a microporous carrier for ph-controlled oral delivery of aspirin. Acta Chim. Slov. 2014;61:688–693. [PubMed] [Google Scholar]
- 6.Li L., Li P., Chen Y., Wen C., Zhuang S., Zhou Y. Zinc-bearing zeolite clinoptilolite improves tissue zinc accumulation in laying hens by enhancing zinc transporter gene mrna abundance. Anim. Sci. J. 2015;86:782–789. doi: 10.1111/asj.12358. [DOI] [PubMed] [Google Scholar]
- 7.Katsoulos P.D., Zarogiannis S., Roubies N., Christodoulopoulos G. Effect of long-term dietary supplementation with clinoptilolite on performance and selected serum biochemical values in dairy goats. Am. J. Vet. Res. 2009;70:346–352. doi: 10.2460/ajvr.70.3.346. [DOI] [PubMed] [Google Scholar]
- 8.Ma J., Li Y., Ye Q., Li J., Hua Y., Ju D., Zhang D., Cooper R., Chang M. Constituents of red yeast rice, a traditional chinese food and medicine. J. Agric. Food Chem. 2000;48:5220–5225. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 9.Lyu W., Jia H., Kato H. (The University of Tokyo, Tokyo, Japan). 2015. Unpublished work.
- 10.McHenga S.S., Wang D., Li C., Shan F., Lu C. Inhibitory effect of recombinant il-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cell. Mol. Immunol. 2008;5:425–431. doi: 10.1038/cmi.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IPA. [(accessed on 13 April 2016)]; Available online: https://www.qiagenbioinformatics.com.
- 12.Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., et al. Requirement for stat4 in interleukin-12-mediated responses of natural killer and t cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 13.Wu W., Dietze K.K., Gibbert K., Lang K.S., Trilling M., Yan H., Wu J., Yang D., Lu M., Roggendorf M., et al. Tlr ligand induced il-6 counter-regulates the anti-viral cd8(+) t cell response during an acute retrovirus infection. Sci. Rep. 2015;5:10501. doi: 10.1038/srep10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohama T., Hori M., Momotani E., Iwakura Y., Guo F., Kishi H., Kobayashi S., Ozaki H. Intestinal inflammation downregulates smooth muscle cpi-17 through induction of tnf-alpha and causes motility disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1429–G1438. doi: 10.1152/ajpgi.00315.2006. [DOI] [PubMed] [Google Scholar]
- 15.Ohama T., Hori M., Sato K., Ozaki H., Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of cpi-17 and mypt-1 in intestinal smooth muscle. J. Biol. Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 16.Vanuytsel T., Vermeire S., Cleynen I. The role of haptoglobin and its related protein, zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1:e27321. doi: 10.4161/tisb.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi T., Sakurai T., Kashida H., Mine H., Hagiwara S., Matsui S., Yoshida K., Nishida N., Watanabe T., Itoh K., et al. Involvement of heat shock protein a4/apg-2 in refractory inflammatory bowel disease. Inflamm. Bowel Dis. 2015;21:31–39. doi: 10.1097/MIB.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma Y.T., Hagi K., Shintani N., Kuwamura M., Nakajima H., Hashimoto H., Baba A., Takeuchi T. Pacap provides colonic protection against dextran sodium sulfate induced colitis. J. Cell. Physiol. 2008;216:111–119. doi: 10.1002/jcp.21381. [DOI] [PubMed] [Google Scholar]
- 19.Ning C., Li Y.Y., Wang Y., Han G.C., Wang R.X., Xiao H., Li X.Y., Hou C.M., Ma Y.F., Sheng D.S., et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal il-1beta/il-17a axis. Mucosal Immunol. 2015;8:1275–1284. doi: 10.1038/mi.2015.18. [DOI] [PubMed] [Google Scholar]
- 20.Tanida S., Mizoshita T., Ozeki K., Katano T., Kataoka H., Kamiya T., Joh T. Advances in refractory ulcerative colitis treatment: A new therapeutic target, annexin a2. World J. Gastroenterol. 2015;21:8776–8786. doi: 10.3748/wjg.v21.i29.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B., Wang L., Chi B. Upregulation of periostin prevents p53-mediated apoptosis in sgc-7901 gastric cancer cells. Mol. Biol. Rep. 2013;40:1677–1683. doi: 10.1007/s11033-012-2218-3. [DOI] [PubMed] [Google Scholar]
- 22.Spehlmann M.E., Manthey C.F., Dann S.M., Hanson E., Sandhu S.S., Liu L.Y., Abdelmalak F.K., Diamanti M.A., Retzlaff K., Scheller J., et al. Trp53 deficiency protects against acute intestinal inflammation. J. Immunol. 2013;191:837–847. doi: 10.4049/jimmunol.1201716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.H., Takahashi T., Yasuhara N., Inazawa J., Kamada S., Tsujimoto Y. Bis, a bcl-2-binding protein that synergizes with bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y.D., Yoon J.S., Yoon H.H., Youn H.J., Kim J., Lee J.H. Expression of bis in the mouse gastrointestinal system. Anat. Cell Biol. 2012;45:160–169. doi: 10.5115/acb.2012.45.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjelm B., Brennan D.J., Zendehrokh N., Eberhard J., Nodin B., Gaber A., Ponten F., Johannesson H., Smaragdi K., Frantz C., et al. High nuclear rbm3 expression is associated with an improved prognosis in colorectal cancer. Proteom. Clin. Appl. 2011;5:624–635. doi: 10.1002/prca.201100020. [DOI] [PubMed] [Google Scholar]
- 26.Sureban S.M., Ramalingam S., Natarajan G., May R., Subramaniam D., Bishnupuri K.S., Morrison A.R., Dieckgraefe B.K., Brackett D.J., Postier R.G., et al. Translation regulatory factor rbm3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27:4544–4556. doi: 10.1038/onc.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham C.G., Bubici C., Zazzeroni F., Papa S., Jones J., Alvarez K., Jayawardena S., De Smaele E., Cong R., Beaumont C., et al. Ferritin heavy chain upregulation by nf-kappab inhibits tnfalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Bardia A., Tiwari S.K., Gunisetty S., Anjum F., Nallari P., Habeeb M.A., Khan A.A. Functional polymorphisms in xrcc-1 and ape-1 contribute to increased apoptosis and risk of ulcerative colitis. Inflamm. Res. 2012;61:359–365. doi: 10.1007/s00011-011-0418-2. [DOI] [PubMed] [Google Scholar]
- 29.Mort R., Mo L., McEwan C., Melton D.W. Lack of involvement of nucleotide excision repair gene polymorphisms in colorectal cancer. Br. J. Cancer. 2003;89:333–337. doi: 10.1038/sj.bjc.6601061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Q.J., Deng D.J., Che J., Zhao H.R., Yu J.J., Lu Y.Y. Hypothalamic paraventricular nucleus stimulation reduces intestinal injury in rats with ulcerative colitis. World J. Gastroenterol. 2016;22:3769–3776. doi: 10.3748/wjg.v22.i14.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q., Cadrin M., Herrmann H., Chen C.H., Chalkley R.J., Burlingame A.L., Omary M.B. Keratin 20 serine 13 phosphorylation is a stress and intestinal goblet cell marker. J. Biol. Chem. 2006;281:16453–16461. doi: 10.1074/jbc.M512284200. [DOI] [PubMed] [Google Scholar]
- 32.Shimoda M., Horiuchi K., Sasaki A., Tsukamoto T., Okabayashi K., Hasegawa H., Kitagawa Y., Okada Y. Epithelial cell-derived a disintegrin and metalloproteinase-17 confers resistance to colonic inflammation through egfr activation. EBioMedicine. 2016;5:114–124. doi: 10.1016/j.ebiom.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judd L.M., Chalinor H.V., Walduck A., Pavlic D.I., Dabritz J., Dubeykovskaya Z., Wang T.C., Menheniott T.R., Giraud A.S. Tff2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in dss colitis, and this cannot be rescued by wild-type bone marrow. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G12–G24. doi: 10.1152/ajpgi.00172.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurt-Jones E.A., Cao L., Sandor F., Rogers A.B., Whary M.T., Nambiar P.R., Cerny A., Bowen G., Yan J., Takaishi S., et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007;75:471–480. doi: 10.1128/IAI.02039-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furth E.E., Li J., Purev E., Solomon A.C., Rogler G., Mick R., Putt M., Zhang T., Somasundaram R., Swoboda R., et al. Serum antibodies to epcam in healthy donors but not ulcerative colitis patients. Cancer Immunol. Immunother. 2006;55:528–537. doi: 10.1007/s00262-005-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balzar M., Prins F.A., Bakker H.A., Fleuren G.J., Warnaar S.O., Litvinov S.V. The structural analysis of adhesions mediated by ep-cam. Exp. Cell Res. 1999;246:108–121. doi: 10.1006/excr.1998.4263. [DOI] [PubMed] [Google Scholar]
- 37.Islam M.S., Kusakabe M., Horiguchi K., Iino S., Nakamura T., Iwanaga K., Hashimoto H., Matsumoto S., Murata T., Hori M., et al. Pdgf and tgf-beta promote tenascin-c expression in subepithelial myofibroblasts and contribute to intestinal mucosal protection in mice. Br. J. Pharmacol. 2014;171:375–388. doi: 10.1111/bph.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruegg C.R., Chiquet-Ehrismann R., Alkan S.S. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc. Natl. Acad. Sci. USA. 1989;86:7437–7441. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y., Chen L., Tian Y., Ye J., Liu Y., Song L., Pan Q., He Y., Chen W., Peng Z., et al. Numb modulates the paracellular permeability of intestinal epithelial cells through regulating apical junctional complex assembly and myosin light chain phosphorylation. Exp. Cell Res. 2013;319:3214–3225. doi: 10.1016/j.yexcr.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.