Abstract
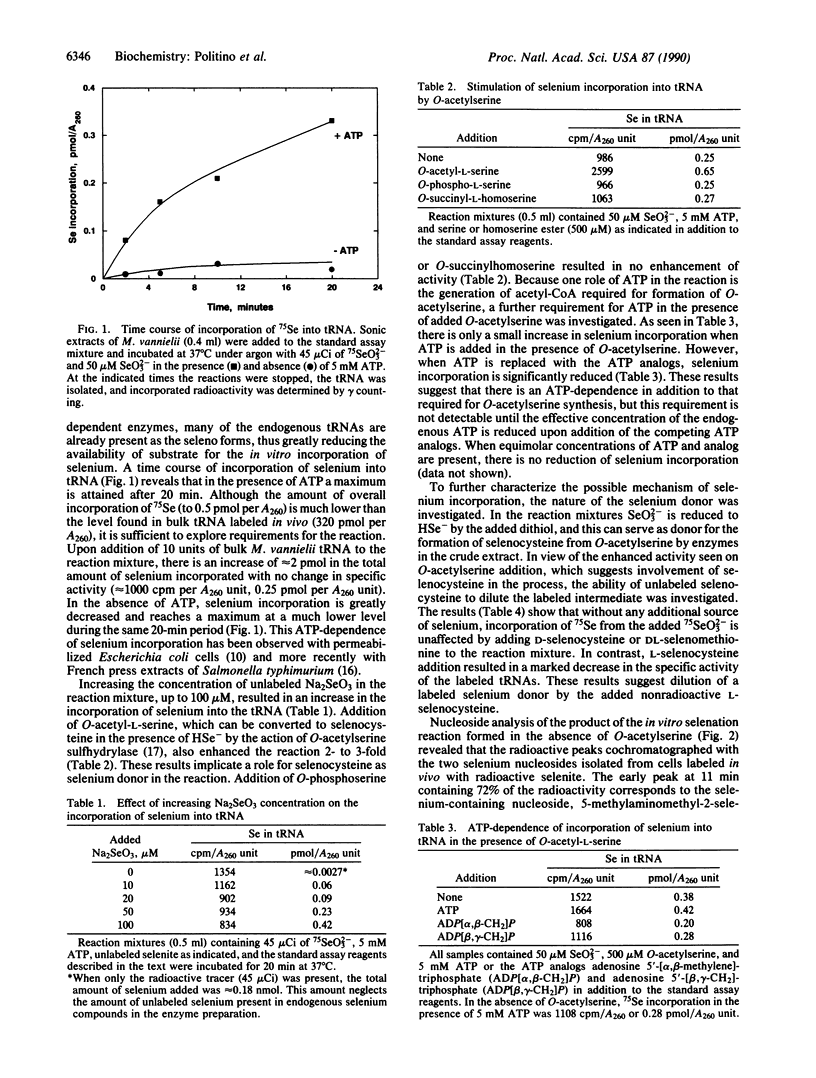
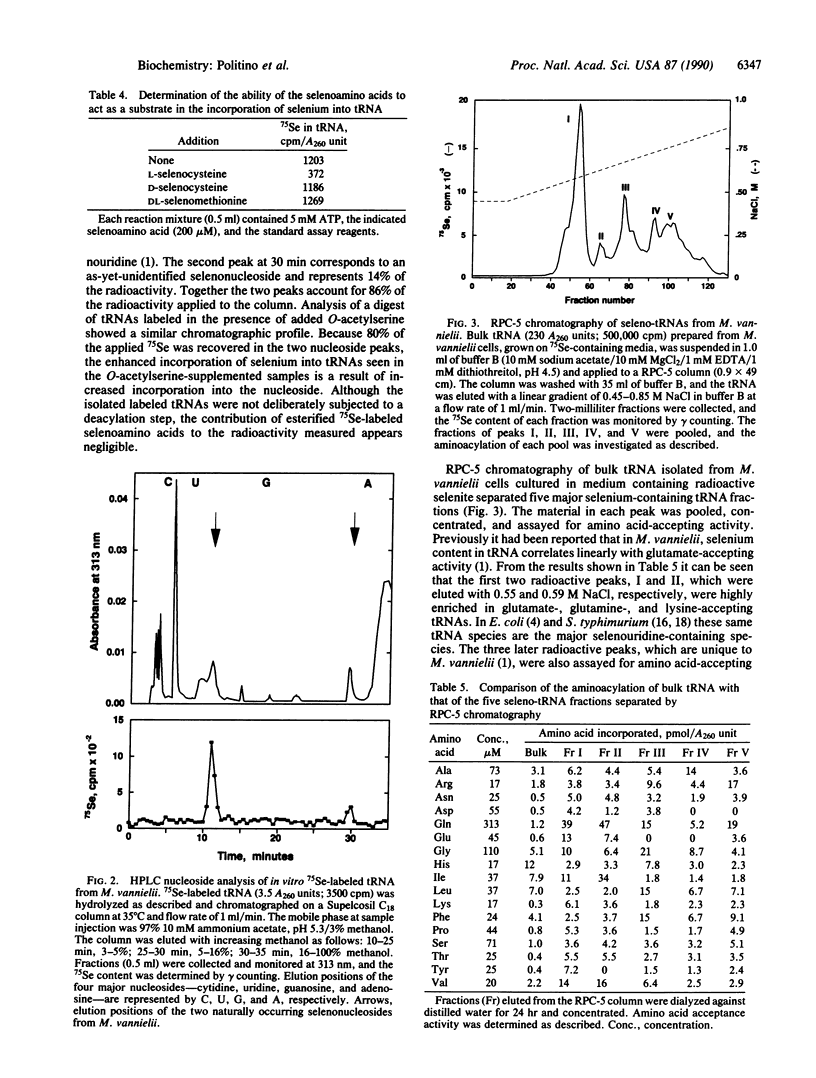
Selenium-containing nucleosides are natural components of several tRNA species in Methanococcus vannielii. In the present study, the incorporation of selenium from 75SeO3(2-) into these macromolecules was investigated in sonic extracts of M. vannielii. Nucleoside analysis of the 75Se-labeled tRNAs from these in vitro reaction mixtures demonstrated that the selenium was present in 75Se-labeled nucleosides identical to the two naturally occurring 2-selenouridines produced in vivo. Incorporation of selenium into these nucleosides was ATP-dependent and was maximal after 20 min. Addition of O-acetylserine enhanced the activity 2- to 3-fold, implicating a role for selenocysteine in the reaction. Added L-selenocysteine could function as a selenium donor, but the D isomer and DL-selenomethionine were inactive. RPC-5 chromatography of bulk tRNA isolated from M. vannielii grown on 75SeO3(2-) separated five major species of seleno-tRNAs. The amino acid-accepting activity of these tRNAs was investigated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Alzner-DeWeerd B., Stadtman T. C. A selenium-containing nucleoside at the first position of the anticodon in seleno-tRNAGlu from Clostridium sticklandii. Proc Natl Acad Sci U S A. 1985 Jan;82(2):347–350. doi: 10.1073/pnas.82.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M. Occurrence of selenium-containing tRNAs in mouse leukemia cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3010–3013. doi: 10.1073/pnas.81.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Stadtman T. C. Selenium-containing tRNAGlu from Clostridium sticklandii: correlation of aminoacylation with selenium content. Proc Natl Acad Sci U S A. 1982 Jan;79(2):374–377. doi: 10.1073/pnas.79.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Wittwer A. J., Tsai L., Stadtman T. C. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc Natl Acad Sci U S A. 1984 Jan;81(1):57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Selenium-dependent and selenium-independent formate dehydrogenases of Methanococcus vannielii. Separation of the two forms and characterization of the purified selenium-independent form. J Biol Chem. 1981 Jan 25;256(2):656–663. [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Novelli G. D., Stulberg M. P. Separation of transfer ribonucleic acids by reverse phase chromatography. J Biol Chem. 1965 Oct;240(10):3979–3983. [PubMed] [Google Scholar]
- Kramer G. F., Ames B. N. Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):736–743. doi: 10.1128/jb.170.2.736-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T. S., Cler J. A., Mick S. J., Dilworth V. M., Contreras P. C., Iyengar S., Wood P. L. Neurochemical characterization of dopaminergic effects of opipramol, a potent sigma receptor ligand, in vivo. Neuropharmacology. 1990 Dec;29(12):1191–1197. doi: 10.1016/0028-3908(90)90044-r. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Soda K. Selenocysteine. Methods Enzymol. 1987;143:240–243. doi: 10.1016/0076-6879(87)43045-0. [DOI] [PubMed] [Google Scholar]
- Veres Z., Tsai L., Politino M., Stadtman T. C. In vitro incorporation of selenium into tRNAs of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6341–6344. doi: 10.1073/pnas.87.16.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J., Ching W. M. Selenium-containing tRNA(Glu) and tRNA(Lys) from Escherichia coli: purification, codon specificity and translational activity. Biofactors. 1989 Mar;2(1):27–34. [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]
- Wittwer A. J., Stadtman T. C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch Biochem Biophys. 1986 Aug 1;248(2):540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]


