Abstract
In association with increasing diabetes prevalence, it is desirable to develop new glucose sensing systems with low cost, ease of use, high stability and good portability. Boronic acid is one of the potential candidates for a future alternative to enzyme-based glucose sensors. Boronic acid derivatives have been widely used for the sugar recognition motif, because boronic acids bind adjacent diols to form cyclic boronate esters. In order to develop colorimetric sugar sensors, boronic acid-conjugated azobenzenes have been synthesized. There are several types of boronic acid azobenzenes, and their characteristics tend to rely on the substitute position of the boronic acid moiety. For example, o-substitution of boronic acid to the azo group gives the advantage of a significant color change upon sugar addition. Nitrogen-15 Nuclear Magnetic Resonance (NMR) studies clearly show a signaling mechanism based on the formation and cleavage of the B–N dative bond between boronic acid and azo moieties in the dye. Some boronic acid-substituted azobenzenes were attached to a polymer or utilized for supramolecular chemistry to produce glucose-selective binding, in which two boronic acid moieties cooperatively bind one glucose molecule. In addition, boronic acid-substituted azobenzenes have been applied not only for glucose monitoring, but also for the sensing of glycated hemoglobin and dopamine.
Keywords: azobenzene, boronic acid, glucose sensor, sugar sensor, 15N NMR
1. Introduction
The growing number of people with diabetes is a serious issue worldwide. It has increased from 153 million in 1980 to 347 million in 2008 [1]. The blood glucose level of diabetes patients is abnormally high, and this leads to severe complications, such as cardiovascular disease, chronic renal failure and diabetic retinopathy. Usually, full recovery from diabetes is difficult; however, early treatment of diabetes will prevent complications. Thus, early recognition of diabetes is important. For this purpose, urine test strips have been widely used. They are easy to use, painless and low cost, and there is no need for electronic devices. This would be continuously important, because more than 80% of diabetes deaths occur in low- and middle-income countries [2].
The self-monitoring of blood glucose is recommended for diabetes patients who control their blood glucose level with medicines [3–6]. They monitor their glucose level to ensure their proper medication. In addition, some diabetes patients have to monitor their blood glucose level to adjust their applied dose of insulin injections to avoid hypoglycemia. Widely used enzyme-based glucose sensors can only provide a single datum at the time of measurement. However, the glucose level fluctuates throughout the day. In general, glucose level is lowest in the morning and rises after meals. Accordingly, it is recommended that type 1 diabetes patients, who have defects in insulin production, perform self-blood glucose monitoring at least three times a day [3].
Currently, practical glucose sensing systems have been designed with enzyme reactions. Usually, a test strip contains glucose oxidase, peroxidase and chromogen. For a blood glucose sensing system, glucose oxidase or glucose dehydrogenase is immobilized on the surface of electrodes. The enzymes selectively bind glucose, even in a mixture of sugars, and catalyze the oxidation of glucose. Recently, continuous monitoring systems based on enzymes have been developed and used practically. However, they need improvement with some problems in accuracy, the need for calibration, the invasiveness, the short usable period and the difficulties associated with sterilization [4–6].
In order to compensate for the imperfection of glucose monitoring systems, chemists are trying to replace the enzymes with synthetic chemical ligands, because they are considered to be more stable, easier to handle and low cost compared to enzyme-based systems. Several approaches are ongoing [7–12], and the most promising chemical ligand is boronic acid, which reversibly forms a cyclic ester with the cis-1,2- or 1,3-diol structures of sugars. This review provides a brief overview of the sugar binding ability of boronic acids, and the applications of them in optical sensing systems, and summarizes recent studies for a colorimetric sugar sensing system using boronic acid-substituted azobenzene derivatives.
2. Binding Ability of Boronic Acids
Boronic acid is a kind of Lewis acid. Its sp2 boron center has a vacant p orbital that can accept a lone pair of a Lewis base. In aqueous media, boronic acids interact with a hydroxide ion, which results in a conformational change to sp3 hybridized boronates. The formation of boronates is dependent on the concentration of the hydroxide ion. In other words, the boronate formation is pH dependent. To express the acidity of boronic acids, an acid dissociation constant, Ka, and a logarithmic constant, pKa, are usually used. When the pH of the aqueous solution is adjusted to the pKa of boronic acids, the concentration of the boronic acid form is equal to the concentration of the boronate form. The acidity of boronic acid tends to conform to Hammett equation-like carboxylic acids [13]. It is widely known that sugar addition induces a decrease of the apparent pKa. This phenomenon is explained by using a theory demonstrated by Lorand and Edwards [14]. They have concluded that the boronate form plays the major role for binding diol structures compared to the boronic acid form (Figure 1). It is reported that the pKa of phenylboronic acid is approximately nine [13]. In neutral or weak alkaline solution, a certain degree of the boronate form exists. Upon sugar addition, the boronate form binds the hydroxyl groups of sugar to form cyclic ester, resulting in the consumption of the boronate form. To maintain the acid-base equilibrium between the boronic acid form and the boronate form, the boronic acid form changes to the boronate form. Accordingly, sugar addition induces the decrease of the boronic acid form, leading to a decrease of apparent pKa. This structural change upon sugar addition is very important for signaling the mechanism of a chemical sugar sensor based on boronic acids.
Figure 1.
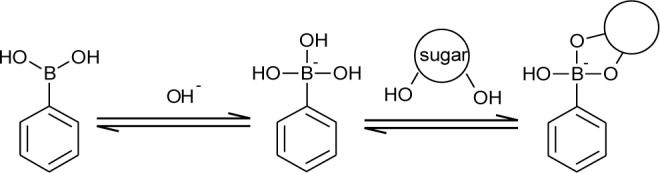
Equilibriums of phenylboronic acid and sugar.
It is generally recognized that mono-boronic acid derivatives show higher affinity for D-fructose over D-glucose (Table 1) [15].
Table 1.
The binding constants of phenylboronic acid to polyol compounds in 0.1 M of phosphate buffer.
| Polyol | K in pH 6.5/M−1 | K in pH 7.4/M−1 | K in pH 8.5/M−1 |
|---|---|---|---|
| D-glucose | 0.84 | 4.6 | 11 |
| D-fructose | 29 | 160 | 560 |
| sorbitol | 47 | 370 | 1000 |
| catechol | 150 | 830 | 3300 |
James et al. pointed out that this is due to the fact that boronic acids prefer the diol of the furanose form [16]. D-Fructose has some configurations, and the relative percentage of the β-D-fructofuranose form is 25% in D2O at 27 °C [17]. In contrast, D-glucose shows only 0.14% of α-D-glucofuranose in D2O at 31 °C [18]. They proposed that the difference in affinity is strongly associated with this difference of the amount of the furanose form.
A post-meal glucose level of less than 10 mM and a fasting plasma glucose of 3.9 to 7.2 mM [19] are recommended. Table 1 shows that the binding constant of phenylboronic acid to D-glucose is 4.6 M−1, which means that the dissociation constant of that is 217 mM. Compared to the blood glucose level, the dissociation constant is too high, which suggests that the practical use of phenylboronic acid for blood glucose monitoring is difficult.
For a stronger binding for D-glucose, bis-boronic acids have been developed in synthetic approaches. In 1995, James et al. first reported that a fluorescent sensor containing two boronic acid moieties shows a selective and higher affinity for D-glucose [20,21]. The fluorescent sensor covered the clinical range of the blood glucose level. Norrild et al. have demonstrated that all five hydroxyl groups of α-D-glucofuranose in the furanose form are bound by bis-boronic acid [22,23].
It is possible to verify the selectivity to a specific sugar other than glucose by a proper arrangement of two boronic acid groups [24–26]. Furthermore, some bis-boronic acids are able to discriminate the chirality of target molecules [26–29].
3. Optical Sugar Sensor Based on Boronic Acid
Glucose oxidase in a blood sugar sensor binds D-glucose and catalyzes its oxidation, which is the trigger of the electrochemical signal. In other words, glucose oxidase serves as both the molecular recognition motif and transducer motif. The dual role of the enzyme is very useful for fabricating sensors. In contrast, boronic acid works as only a molecular recognition motif, and it should be combined with a transducer motif that produces a signal change upon sugar binding. Fluorescent dyes are most widely used as a transducer, because fluorescent dyes provide a signal change in various signaling mechanisms, such as photoinduced electron transfer (PET), internal charge transfer (ICT), fluorescence resonance energy transfer (FRET), excimer and so on [30]. There are many reports about a fluorescent sugar sensor based on boronic acids, and they are summarized in some excellent reviews [31–34]. Using hydrogel containing fluorescent boronic acid sensors, Shibata et al. have succeeded in continuous monitoring in vivo [35,36]. Fluorescent sensors have made a great contribution for the development of the molecular recognition chemistry of boronic acids, and it has a great potential to become alternatives for enzyme-based glucose sensors.
On the other hand, colorimetric sensing systems are suitable for practical use. Although the most widely used glucose meter is based on electrochemistry, there is some commercial, handy-sized blood glucose meters based on colorimetric measurements, which show a performance equivalent to electrochemical sensors [37]. As for urine test strips, we can detect the color change without any devices.
4. Colorimetric Sugar Sensor Using Boronic Acid and Azobenzene
The number of colorimetric sugar sensors based on boronic acids is less compared to that of fluorescence sensors [31–34]. The reason for this may be due to the difficulty of the development of a signaling mechanism for the color change of synthetic dyes containing boronic acid.
There has been an interesting approach to produce a color signal without dyes, which uses smart hydrogels that exhibit a change in volume in the response of boronic acid to sugars [38–51]. Sugar-sensitive smart hydrogels with crystalline colloidal arrays or holographic grating reflect light and give a visible narrow band with a wavelength governed by the spacing of the crystal colloid lattice or the holographic fringe. Sugar addition induced a volume change of the smart hydrogels, which results in the shift of the wavelength. These approaches have been summarized in a review [52].
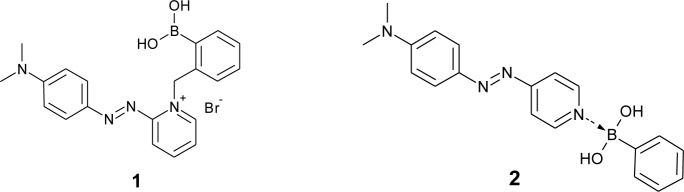
For the development of colorimetric sugar sensors with synthetic dyes, azobenzene has been most widely used. In the 1990s, some boronic acid-appended azobenzene derivatives were synthesized for sugar sensing. Some of them show a sugar response based on a circular dichroism analysis or a change in aggregate formations [53,54]. In 1997, Takeuchi et al. developed a conjugate of azo dye and phenylboronic acid (1, Figure 2) as a dye that shows an absorption spectral change upon an accompanying structural change [55]. The dye 1 shows an absorption maximum at 558 nm, and the absorption maximum shifted to 568 nm upon the addition of the nucleoside containing cis-diol of the ribose moiety. For a clear color change, Koumoto et al. used a boron-nitrogen (B–N) interaction between two molecules (2, Figure 2) [56]. The original color of the azo dye is yellow in aqueous solution, and the color changed to orange due to the B–N interaction. The color of the solution turned red upon sugar addition.
Figure 2.
Chemical structures of dye 1 and 2.
This is a pioneering work using intermolecular interactions between boronic acid and dyes. The concept of the combination of two molecules has become a growing trend. Many researchers used dyes containing catechol structures, like alizarin red S and pyrocatechol violet [57–60]. These dyes form a cyclic ester with boronic acid, which accompanies a change in the color or fluorescence of the dyes. The sugar addition induced the displacement of dyes from the cyclic ester, which results in a recovery of the original signal of the dyes. These combinations of boronic acids and dyes will offer some interesting applications.
Ideally, a simple system using one constituent would be suitable for practical glucose sensing to avoid interferences in complex biological fluids. In 2000, Ward et al. reported a series of dyes with a basic skeleton [61,62]. In particular, dye 3 shows a large color change upon sugar addition in aqueous MeOH at pH 11 (Figure 3). The wavelength shifted ca. 55 nm to a shorter wavelength upon sugar complexation, corresponding to the color change from purple to red. They proposed that a key structure of the signaling mechanism is an intramolecular B–N interaction between the boronic acid moiety and the nitrogen of the aniline moiety. They applied the concept to another dye, 4, which shows a color change in a neutral aqueous solution [63]. In aqueous MeOH at pH 8.2, the addition of sugar induced a shape change in the absorption spectrum, which is recognized as a color change from purple to pink.
Figure 3.
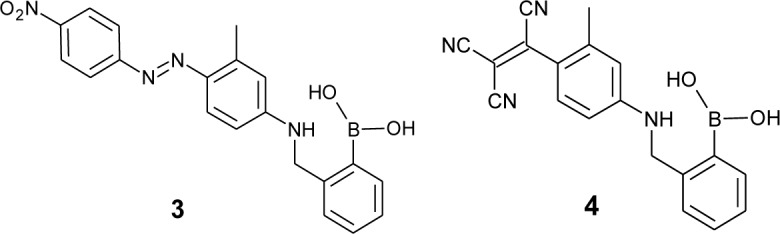
Chemical structures of dye 3 and 4.
DiCesare and Lakowicz have demonstrated that a dye, 5, works as a visible color sensor in a complete water system at pH 7.0 (Figure 4) [64]. The sugar addition induces a red-shift, which results in a change from orange to a purple-reddish color. They proposed that this color change is due to the conformational change of the boron atom from the neutral sp2 form to the anionic sp3 form.
Figure 4.
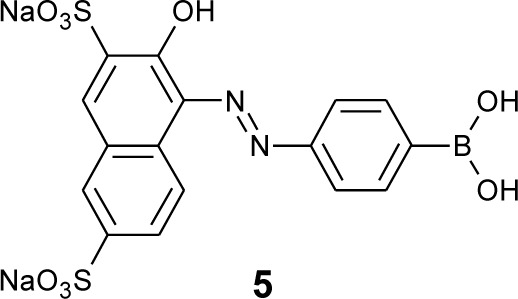
Chemical structure of dye 5.
5. o-Boronic Acid Substituted Azobenzene
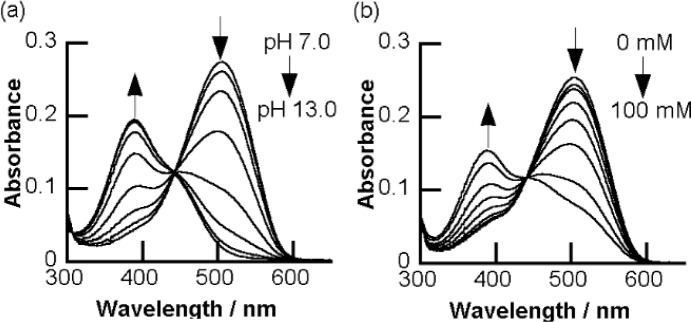
In order to fabricate sugar sensors that show a significant color change, we have developed a strategy to arrange a boronic acid group adjacent to a chromophore. We expected that a structural change of the boronic acid group upon sugar addition would directly affect the adjacent chromophore. We introduced a boronic acid group to the o-position of the azo group. Some o-boronic acid substituted azobenzenes were successfully synthesized with diazo-coupling reactions [65,66]. An azo dye, 6 (Figure 5), which has a basic skeleton of a series of o-boronic acid substituted azobenzenes, shows an absorption maximum at 505 nm in aqueous MeOH, which is significantly red-shifted compared to that of 4-aminoazobenzene (365 nm) [67]. Figure 6 shows the effect of pH and sugar on the UV-visible absorption spectra of 6. A pH increase induced a decrease in the absorption maximum at 505 nm and an increase in a new band at 386 nm. Sugar addition induced a similar spectral change. To our knowledge, the dyes containing 6 as a basic skeleton show the largest color change among boronic acid-based sugar sensors. In patents, Russell and Zepp have shown a synthesis of boronic acid azo dyes using a diazo-coupling reaction [68,69]. Although the patents do not contain accurate structures of the dyes, the obtained structure would be similar to 6.
Figure 5.
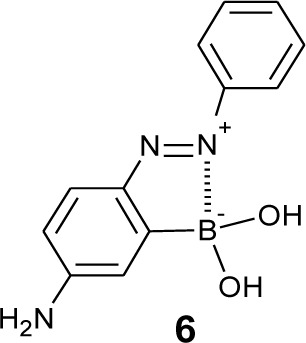
Chemical structure of dye 6.
Figure 6.
(a) UV-visible absorption spectra of dye 6 (10 μM) in different pH solutions (pH 7.0, 10.0, 10.5, 11.0, 11.5, 12.0, 12.5 and 13.0), measured in a methanol/water mixture (1/1, v/v) containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, 5.0 mM); (b) the UV-visible absorption spectra of dye 6 (10 μM) in the presence and absence of D-fructose (0, 1, 2, 5, 10, 20, 50 and 100 mM), measured in a methanol/water mixture (1/1, v/v) containing N-cyclohexyl-2-aminoethanesulfonic acid (CHES, 5.0 mM), pH 10.0. Reprinted with permission from [66]. Copyright 2010 The Chemical Society of Japan.
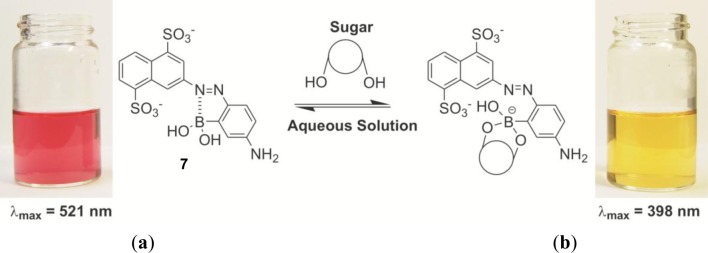
In order to improve the solubility in water, two sulfonyl groups were introduced to the azo dye. The dye, 7, works as a sugar sensor with completely aqueous system at pH 10, and it showed a drastic changed from red to yellow upon sugar addition (Figure 7), which corresponds to a significant change of the absorption maximum from 521 nm to 398 nm. The binding constants are calculated to be 110 M−1 and 6.2 M−1 for D-fructose and D-glucose, respectively.
Figure 7.
Solutions of dye 7 (20 μM) in CHES buffer (10 mM, pH 10.0), (a) in the absence of sugar; and (b) in the presence of 100 mM of D-fructose. Reprinted with permission from [65]. Copyright 2007 Elsevier Besloten Vennootschap.
6. Investigation of B–N Interactions Using 15N NMR
We postulated that the large spectral change of o-boronic acid substituted azobenzenes could be explained by B–N interactions between the boronic acid and azo group. In order to gain insight into the B–N interaction, we used 15N NMR spectroscopy, because the formations of coordination bonds are sensitively reflected in the 15N chemical shifts [70,71]. We synthesized a 15N-labelled azo dye (8, Figure 8), which corresponds to dye 6 [66].
Figure 8.
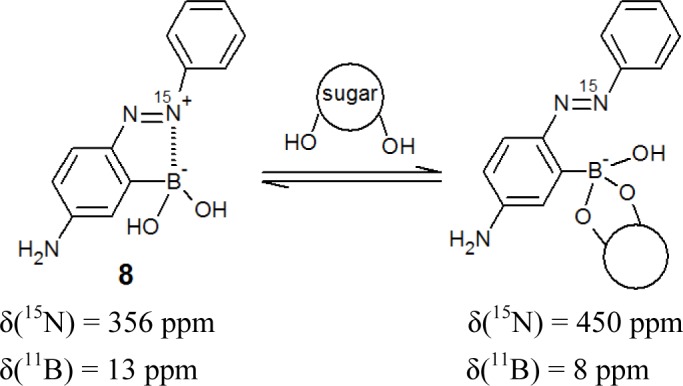
The equilibrium of dye 8 and sugar, and their chemical shifts in multinuclear NMR.
Figure 9a shows the 15N NMR spectra of 8 in D2O. The 15N chemical shift was observed at 339 ppm in D2O; this value is strongly upfield shifted, because the 15N chemical shifts of azo groups are generally observed at around 500 ppm [70]. In contrast, the 15N chemical shift of 8 in a 1.0 M NaOD D2O solution was a normal value (450 ppm) (Figure 9b).
Figure 9.
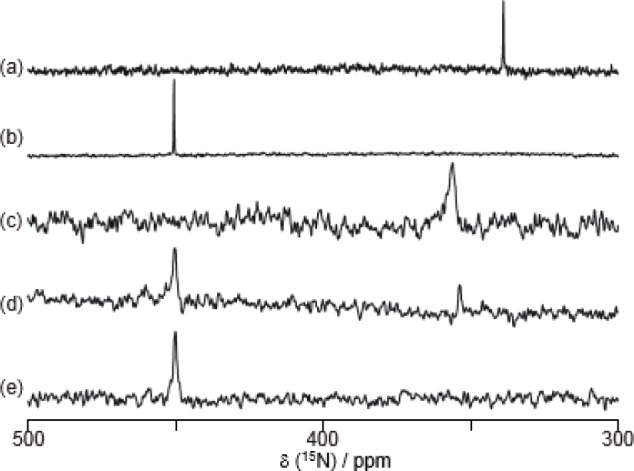
The 15N NMR spectra of 8 (20 mM) under various conditions: (a) in D2O; (b) in a 1.0 M NaOD·D2O solution; (c–e) in a mixed solvent (100 mM CHES buffer, pH 10.0/DMSO-d6 = 3/1, v/v); (c) without D-fructose; (d) in the presence of 0.10 M D-fructose; and (e) in the presence of 1.0 M D-fructose. The 15N-frequency (0 ppm) is 81.07646745 MHz. Reprinted with permission from [66]. Copyright 2010 The Chemical Society of Japan.
We used quantum chemical calculations based on density functional theory to optimize the conformation and to predict the value of the 15N chemical shift. Based on the value of actual measurements and calculations, we have concluded that dye 8 in neutral solutions has the B–N dative bond, which is responsible for the upfield value of the 15N chemical shift around 350 ppm.
Figure 9c–e shows the effect of sugar in the 15N NMR spectra of 8. Sugar addition induced a decrease of the peak at around 350 ppm and an increase of the peak at around 450 ppm. These results demonstrate that adding sugar induces a B–N dative bond cleavage, which results in a recovery of the 15N chemical shift in the normal range.
In the UV-visible absorption spectrum (Figure 6), the B–N dative bond causes the significant red-shift of the absorption maximum, and the B–N dative bond is cleaved with sugar addition, which results in a large spectral change.
Boron-11 NMR is a common method to investigate B–N interactions in solution [72–77]. It is known that the 11B chemical shifts of sp3 and sp2 appeared at about zero and 30 ppm, respectively. Figure 10 shows the 11B NMR spectra of the same conditions as that for the 15N NMR spectra in Figure 9. The addition of D-fructose induced a disappearance of the peak at around 13 ppm and an appearance of the peak at around 8 ppm. Only with this result of 11B NMR would it be very difficult to describe the structural change of the B–N motif in 8, because both the 11B chemical shifts at around 13 and 8 ppm correspond to quasi-tetrahedral boron.
Figure 10.
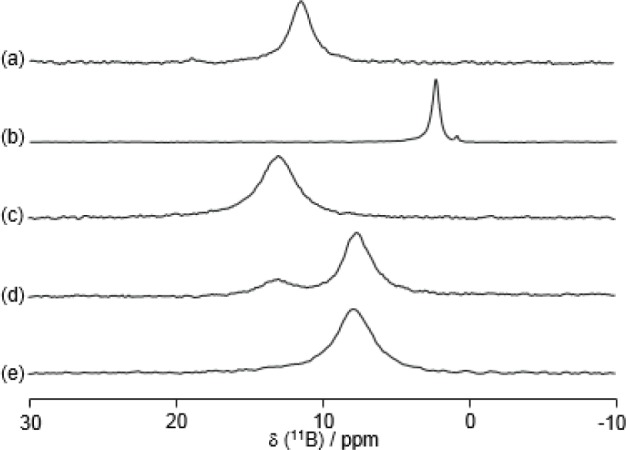
11B NMR spectra of 8 (20 mM) under various conditions: (a) in D2O; (b) in 1.0 M NaOD·D2O solution; (c–e) in a mixed solvent (100 mM CHES buffer, pH 10.0/DMSO-d6 = 3/1, v/v); (c) without D-fructose; (d) in the presence of 0.10 M D-fructose; and (e) in the presence of 1.0 M D-fructose. The 11B-frequency (0 ppm) is Et2O·BF3 in toluene-d8. Reprinted with permission from [66]. Copyright 2010 The Chemical Society of Japan.
We have clearly showed the motion of the B–N motif that plays a key role for the signaling mechanism of o-boronic acid-substituted azobenzene. This signaling mechanism corresponds to a solvolysis mechanism, which is proposed by Wang’s group [76,77]. They questioned the signaling mechanism of a fluorescent sugar sensor containing a B–N motif. The B–N motif has been widely used not only in fluorescence sensors, but also in electrochemical and colorimetric sensors [20,21,61–63,78,79]. Previously, 11B NMR was the only method to investigate the B–N motif in the dissolved state. Accordingly, it was hard to understand the role of the B–N motif. In fact, the B–N interactions of sugar sensors have been investigated and debated for a long time [72–77]. We have demonstrated that 15N NMR will provide a clear picture of the B–N motif, which will contribute to the further development of sugar sensors with the B–N motif.
7. Polymers Containing o-Boronic Acid-Substituted Azobenzene
It is known that mono-boronic acid shows a higher affinity for D-fructose compared to D-glucose. Springsteen and Wang calculated the binding constant between phenylboronic acid and sugar. To obtain a higher affinity for D-glucose, we synthesized an azo dye containing carboxylic acid (9) and attached it to poly(ethyleneimine) (10) (Figure 11) [80], which is a simple and effective strategy for multivalent binding [81,82]. The addition of D-glucose induced a significant change in the UV-visible absorption spectra of polymer dye 10 solutions. In aqueous solution (pH 9.0), the binding constants for D-glucose (Kglu) and D-fructose (Kfru) were calculated to be 54 M−1 and 110 M−1, respectively (Table 2). The selectivity for D-glucose of dye 10 was increased compared with that of monomeric dye 9 (Kglu = 1.2 M−1, Kfru = 17 M−1).
Figure 11.
Chemical structures of dye 9 and 10.
Table 2.
The binding constants of dye 9, 10 and multilayered films in 10 mM CHES buffer (pH 9.0). PVS, poly(vinyl sulfate); CMC, carboxymethylcellulose.
| Dye | Kglu/M−1 | Kfru/M−1 | Kglu/Kfru |
|---|---|---|---|
| 9 | 1.2 | 17 | 0.071 |
| 10 | 54 | 110 | 0.49 |
| (PVS/10)10 | 1.7 | 28 | 0.061 |
| (CMC/10)5 | 18 | 42 | 0.43 |
Dye 10 has advantage of a good solubility in water, due to the positive charges of the polymer chain. In addition, it can be used in the preparation of multilayered films on the solid surface using layer-by-layer techniques. This technique is based on the alternate adsorption of polyelectrolytes on a solid surface through electrostatic interactions (Figure 12). It is very useful to build functional multilayered films in nanometer-order [83–85]. We fabricated multilayered films composed of dye 10 and polyanions (poly(vinyl sulfate) (PVS), carboxymethylcellulose (CMC)) using the technique. In a (PVS/10)10 film, a film composed of ten bilayers of PVS and 10, the affinity for D-glucose was relatively low (Kglu = 1.7 M−1, Kfru = 28 M−1). In contrast, (CMC/10)5 film, a film composed of five bilayers of CMC and 10, showed a high affinity for D-glucose (Kglu = 18 M−1, Kfru = 42 M−1). We proposed that the loosely packed structure of the (CMC/10)5 film probably led to a high affinity for D-glucose [86–88].
Figure 12.
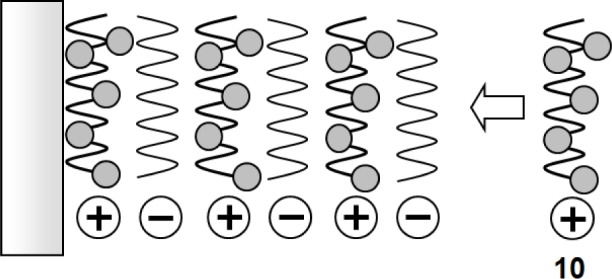
Schematic illustration of fabrication of layer-by-layer films using dye 10.
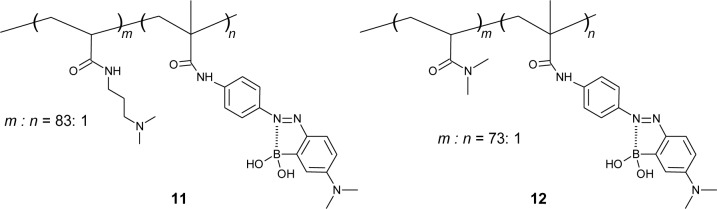
Okasaka and Kitano synthesized an o-boronic acid substituted azobenzene with the vinyl group and use it to prepare copolymers (Figure 13) [89]. They presented the azo polymer containing a tertiary amine in the side chain (11), which shows a good sugar response in pH 8.0 solution. On the other hand, the azo polymer containing amide (12) shows a sugar response in a more alkaline region (pH > 9). Interestingly, the copolymer showed a change in the fluorescence emission spectra, although azobenzene derivatives usually do not show fluorescence. They proposed that the unordinary fluorescence of the copolymer is derived from the B–N bond, which restricted the photo-isomerization of azobenzene.
Figure 13.
Chemical structures of 11 and 12.
8. Supramolecular Approaches for Glucose Sensing
Shimpuku et al. used a unique strategy using supramolecular chemistry to increase D-glucose selectivity [90]. γ-Cyclodextrin (γ-CyD) is a cyclic oligosaccharide composed of eight glucose units, and it works as a host to include guest molecules through hydrophobic interactions. Two molecules of boronic acid-appended azo dye (BA-Azo) are included in the cavity of γ-CyD. In the presence of γ-CyD, BA-Azo exhibits a selective response for D-glucose by forming a dye-D-glucose complex in 2:1 stoichiometry inside the γ-CyD cavity (13, Figure 14). They compared some azo dyes and suggested that the difference in the hydrophobic spacer significantly affects the sensing ability for D-glucose.
Figure 14.
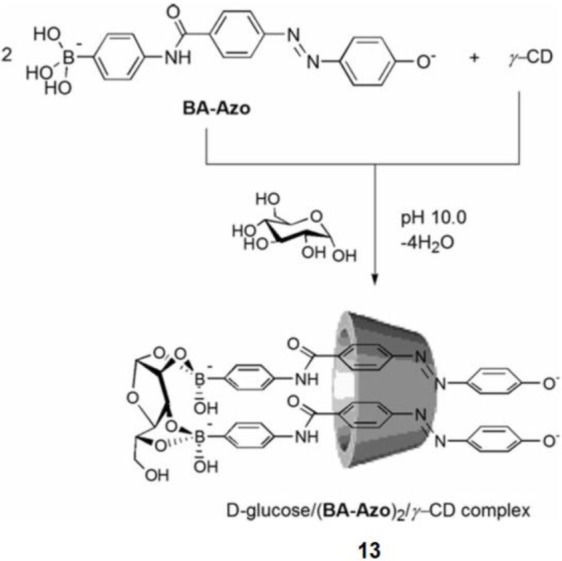
The 2:1 inclusion complex formation of boronic acid-appended azo dye (BA-Azo) with γ-CyD in the presence of D-glucose. Reprinted with permission from [90]. Copyright 2009 The Royal Society of Chemistry.
For a supramolecular approach, we used anionic dye 5 and poly(allylamine) (PAA), which interact through electrostatic force [91]. In pH 7.0 solution, the absorption spectrum of dye 5 was changed upon the addition of PAA, which indicates the formation of aggregates of 5 on the PAA. In the presence of PAA, the binding constant of 5 to D-glucose was improved to 130 M−1, which was 93-times higher than the original binding constant, while the binding constant to D-fructose was increased only 3.3-times (Table 3). These results show that the coexistence of PAA can selectively enhance the binding ability of dye 5 to D-glucose. It is expected that two molecules of dye 5 on the surface of PAA cooperatively bind one molecule of D-glucose.
Table 3.
The binding constants of dye 5 (20 μM) to sugars in the absence and presence of poly(allylamine) (PAA) (10 μg/mL) in 25 mM HEPES buffer (pH 7.0).
| Dye | Kglu/M−1 | Kfru/M−1 | Kglu/Kfru |
|---|---|---|---|
| 5 | 1.4 | 75 | 0.018 |
| 5 + PAA | 130 | 250 | 0.52 |
9. Other Target Molecules for Boronic Acid Azobenzenes
Human blood glucose level naturally fluctuates throughout the day. In contrast, measuring glycated hemoglobin (HbA1c) is useful for assessing the effectiveness of therapy by monitoring long-term serum glucose regulation. HbA1c is a marker for average blood glucose levels over four weeks to three months. As the average concentration of plasma glucose increases, the fraction of HbA1c increases. HbA1c is formed in the Amadori rearrangement, in which glucose and the N-end of the β chain form a Schiff base, and it is converted to 1-deoxyfructose. Boronic acids show affinity for this glycated moiety, and boronate affinity chromatographies are practically used for the determination of HbA1c [92–94]. Kim et al. have developed a method for the detection of glycated protein on the basis of the spectral shifting of dye 5 [95]. The measurements revealed that the method has a dynamic detection range of 3%–15% (HbA1c/total hemoglobin), which covers the required clinical reference range.
Boronic acids have been used to recognize a catechol structure, because its 1,2-diol on the aromatic ring can form a cyclic ester with boronic acids [96–102]. Dopamine is a catecholamine neurotransmitter, and it is associated with several neurological diseases, e.g., Parkinson’s disease and schizophrenia. Monitoring dopamine concentration could be useful for diagnosis and medication treatment for the diseases. Hashimoto et al. have designed a boronic acid azo dye (14, Figure 15) containing boronic acid and crown ether, which enable two point recognitions through boronate-diol and crown ether-cation interactions [103]. At pH 8, the solution of dye 14 changed its color from red to orange upon the addition of dopamine. The binding constant was 111 M−1 in pH 8.0. They showed that dye 14 has a phenolate structure in neutral solution.
Figure 15.
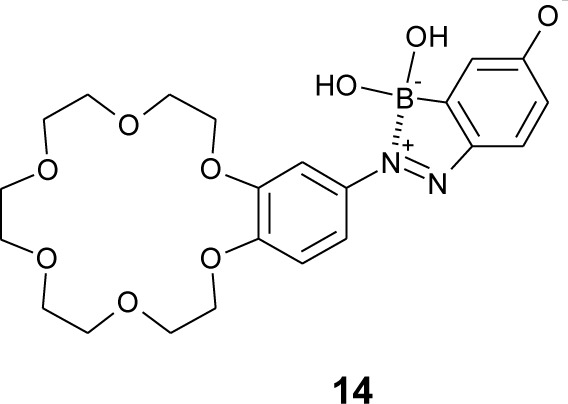
Chemical structure of dye 14.
10. Conclusions
The combination of boronic acid and azo dye has been successfully used for the development of color sensors for a compound containing adjacent diol structure. We developed a series of o-boronic acid substituted azobenzenes that shows a drastic color change upon sugar addition. The signaling mechanism was investigated with multinuclear NMR. Especially, 15N NMR demonstrated the existence of a B–N dative bond between boronic acid and azo groups well. The B–N dative bond causes a significant red-shift of the absorption maximum, and it is cleaved upon sugar addition, which results in a significant color change. Interestingly, the azo dye containing the B–N dative bond shows fluorescence, which means that the dyes can be a dual colorimetric and fluorescent sensor. The dyes with a B–N dative bond were combined with polymer or crown ether. Polymer appended azo dye 10 shows a high glucose affinity in solutions and in a multilayered film on the surface of a solid. The combination of crown ether and azo dye shows a selective binding ability for dopamine.
The p-boronic acid-substituted azobenzenes developed by DiCesare and Lakowicz have an advantage in that they work in a neutral solution, and they were applied for glucose selective binding and HbA1C measurements.
The systems showed in this review have their own advantages, i.e., a drastic color change, a high affinity for D-glucose and working in a neutral solution. An ideal colorimetric sensor should include these advantages, and we expect that further study will integrate these advantages. Additionally, it would have great potential to contribute to medical sensors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J., Lin J.K., Farzadfar F., Khang Y.-H., Stevens G.A., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks D.B., Bruns D.E., Goldstein D.E., Maclaren N.K., McDonald J.M., Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 2002;48:436–472. [PubMed] [Google Scholar]
- 4.Kovatchev B.P., Cox D.J., Gonder-Frederick L.A., Clarke W.L. Evaluating the accuracy of continuous mathematical model. Diabetes Care. 2004;27:1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 5.Vaddiraju S., Burgess D.J., Tomazos I., Jain F.C., Papadimitrakopoulos F. Technologies for continuous glucose monitoring: Current problems and future promises. J. Diabetes Sci. Technol. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo E.-H., Lee S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors. 2010;10:4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis A.P., Wareham R.S. Carbohydrate recognition through noncovalent interactions: A challenge for biomimetic and supramolecular chemistry. Angew. Chem. Int. Ed. Engl. 1999;38:2978–2996. [PubMed] [Google Scholar]
- 8.Mazik M., Cavga H. Carboxylate-based receptors for the recognition of carbohydrates in organic and aqueous media. J. Org. Chem. 2006;71:2957–2963. doi: 10.1021/jo052479p. [DOI] [PubMed] [Google Scholar]
- 9.Ferrand Y., Crump M.P., Davis A.P. A synthetic lectin analog for biomimetic disaccharide recognition. Science. 2007;318:619–622. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]
- 10.Kubik S. Synthetic lectins. Angew. Chem. Int. Ed. Engl. 2009;48:1722–1725. doi: 10.1002/anie.200805497. [DOI] [PubMed] [Google Scholar]
- 11.Rauschenberg M., Bomke S., Karst U., Ravoo B.J. Dynamic peptides as biomimetic carbohydrate receptors. Angew. Chem. Int. Ed. Engl. 2010;49:7340–7345. doi: 10.1002/anie.201002847. [DOI] [PubMed] [Google Scholar]
- 12.Ke C., Destecroix H., Crump M.P., Davis A.P. A simple and accessible synthetic lectin for glucose recognition and sensing. Nat. Chem. 2012;4:718–723. doi: 10.1038/nchem.1409. [DOI] [PubMed] [Google Scholar]
- 13.Yan J., Springsteen G., Deeter S., Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 14.Lorand J.P., Edwards J.O. Polyol complexes and structure of the benzeneboronate ion. J. Org. Chem. 1959;268:769–774. [Google Scholar]
- 15.Springsteen G., Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 16.James T., Phillips M., Shinkai S. Boronic Acids in Saccharide Recognition. The Royal Society of Chemistry; Cambridge, UK: 2006. The Importance of Pyranose to Furanose Interconversion; pp. 77–82. [Google Scholar]
- 17.Angyal S.J. The composition of reducing sugars in solution. Adv. Carbohydr. Chem. Biochem. 1984;42:15–68. [Google Scholar]
- 18.Angyal S.J. The composition of reducing sugars in solution: Current aspects. Adv. Carbohydr. Chem. Biochem. 1991;49:19–35. [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes–2006. Diabetes Care. 2006;29:S4–S42. [PubMed] [Google Scholar]
- 20.James T.D., Sandanayake K.R.A.S., Iguchi R., Shinkai S. Novel saccharide-photoinduced electron transfer sensors based on the interaction. J. Am. Chem. Soc. 1995;117:8982–8987. [Google Scholar]
- 21.James T.D., Sandanayake K.R.A.S., Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angew. Chem. Int. Ed. Engl. 1996;35:1910–1922. [Google Scholar]
- 22.Norrild J.C., Egged H. Evidence for mono- and bisdentate boronate complexes of glucose in the furanose form. Application of 1J c-c coupling constants as a structural probe. J. Am. Chem. Soc. 1995;117:1479–1484. [Google Scholar]
- 23.Bielecki M., Eggert H., Norrild J.C. A fluorescent glucose sensor binding covalently to all five hydroxy groups of α-D-glucofuranose. A reinvestigation. J. Chem. Soc. Perkin Trans. 2. 1999:449–455. [Google Scholar]
- 24.Kondo K., Shiomi Y., Saisho M., Harada T., Shinkai S. Specific complexation of disaccharides with diphenyl-3,3′-diboronic acid that can be detected by circular dichroism. Tetrahedron. 1992;48:8239–8252. [Google Scholar]
- 25.Swamy K.M.K., Jang Y.J., Park M.S., Koh H.S., Lee S.K., Yoon Y.J., Yoon J. A sorbitol-selective fluorescence sensor. Tetrahedron Lett. 2005;46:3453–3456. [Google Scholar]
- 26.Wu Y., Guo H., Zhang X., James T.D., Zhao J. Chiral donor photoinduced-electron-transfer (d-PET) boronic acid chemosensors for the selective recognition of tartaric acids, disaccharides, and ginsenosides. Chem. Eur. J. 2011;17:7632–7644. doi: 10.1002/chem.201100033. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J., Fyles T.M., James T.D. Chiral binol-bisboronic acid as fluorescence sensor for sugar acids. Angew. Chem. Int. Ed. Engl. 2004;43:3461–3464. doi: 10.1002/anie.200454033. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J., Davidson M.G., Mahon M.F., Kociok-Köhn G., James T.D. An enantioselective fluorescent sensor for sugar acids. J. Am. Chem. Soc. 2004;126:16179–16186. doi: 10.1021/ja046289s. [DOI] [PubMed] [Google Scholar]
- 29.Han F., Chi L., Liang X., Ji S., Liu S., Zhou F., Wu Y., Han K., Zhao J., James T.D., et al. 3,6-Disubstituted carbazole-based bisboronic acids with unusual fluorescence transduction as enantioselective fluorescent chemosensors for tartaric acid. J. Org. Chem. 2009;74:1333–1336. doi: 10.1021/jo8025669. [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Liu W., Ge J., Zhang H., Wang P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011;40:3483–3495. doi: 10.1039/c0cs00224k. [DOI] [PubMed] [Google Scholar]
- 31.Mader H.S., Wolfbeis O.S., Cao H., Heagy M.D. Fluorescent chemosensors for carbohydrates: A decade’s worth of bright spies for saccharides in review. J. Fluoresc. 2004;14:569–584. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 32.Mader H.S., Wolfbeis O.S. Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim. Acta. 2008;162:1–34. [Google Scholar]
- 33.Steiner M.-S., Duerkop A., Wolfbeis O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011;40:4805–4839. doi: 10.1039/c1cs15063d. [DOI] [PubMed] [Google Scholar]
- 34.Hansen J., Christensen J., Petersen J.F., Hoeg-Jensen T., Norrild J.C. Arylboronic acids: A diabetic eye on glucose sensing. Sens. Actuator B Chem. 2012;161:45–79. [Google Scholar]
- 35.Shibata H., Heo Y.J., Okitsu T., Matsunaga Y., Kawanishi T., Takeuchi S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA. 2010;107 doi: 10.1073/pnas.1006911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heo Y.J., Takeuchi S. Towards smart tattoos: Implantable biosensors for continuous glucose monitoring. Adv. Healthc. Mater. 2013;2:43–56. doi: 10.1002/adhm.201200167. [DOI] [PubMed] [Google Scholar]
- 37.Yamada S. Historical achievements of self-monitoring of blood glucose technology development in Japan. J. Diabetes Sci. Technol. 2011;5:1300–1306. doi: 10.1177/193229681100500541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asher S.A., Alexeev V.L., Goponenko A.V., Sharma A.C., Lednev I.K., Wilcox C.S., Finegold D.N. Photonic crystal carbohydrate sensors: Low ionic strength sugar sensing. J. Am. Chem. Soc. 2003;125:3322–3329. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 39.Kabilan S., Marshall A.J., Sartain F.K., Lee M.-C., Hussain A., Yang X., Blyth J., Karangu N., James K., Zeng J., et al. Holographic glucose sensors. Biosens. Bioelectron. 2005;20:1602–1610. doi: 10.1016/j.bios.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Dean K.E.S., Horgan A.M., Marshall A.J., Kabilan S., Pritchard J. Selective holographic detection of glucose using tertiary amines. Chem. Commun. 2006:3507–3509. doi: 10.1039/b605778k. [DOI] [PubMed] [Google Scholar]
- 41.Horgan A.M., Marshall A.J., Kew S.J., Dean K.E.S., Creasey C.D., Kabilan S. Crosslinking of phenylboronic acid receptors as a means of glucose selective holographic detection. Biosens. Bioelectron. 2006;21:1838–1845. doi: 10.1016/j.bios.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Sartain F.K., Yang X., Lowe C.R. Holographic lactate sensor. Anal. Chem. 2006;78:5664–5670. doi: 10.1021/ac060416g. [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Lee M.-C., Sartain F., Pan X., Lowe C.R. Designed boronate ligands for glucose-selective holographic sensors. Chemistry. 2006;12:8491–8497. doi: 10.1002/chem.200600442. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Moshe M., Alexeev V.L., Asher S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006;78:5149–5157. doi: 10.1021/ac060643i. [DOI] [PubMed] [Google Scholar]
- 45.Worsley G.J., Tourniaire G.A., Medlock K.E.S., Sartain F.K., Harmer H.E., Thatcher M., Horgan A.M., Pritchard J. Continuous blood glucose monitoring with a thin-film optical sensor. Clin. Chem. 2007;53:1820–1826. doi: 10.1373/clinchem.2007.091629. [DOI] [PubMed] [Google Scholar]
- 46.Kuzimenkova M.V., Ivanov A.E., Thammakhet C., Mikhalovska L.I., Galaev I.Y., Thavarungkul P., Kanatharana P., Mattiasson B. Optical responses, permeability and diol-specific reactivity of thin polyacrylamide gels containing immobilized phenylboronic acid. Polymer. 2008;49:1444–1454. doi: 10.1002/jmr.873. [DOI] [PubMed] [Google Scholar]
- 47.Pan X., Yang X., Lowe C.R. Evidence for a cross-linking mechanism underlying glucose-induced contraction of phenylboronate hydrogel. J. Mol. Recognit. 2008;21:205–209. doi: 10.1002/jmr.885. [DOI] [PubMed] [Google Scholar]
- 48.Sartain F.K., Yang X., Lowe C.R. Complexation of L-lactate with boronic acids: A solution and holographic analysis. Chemistry. 2008;14:4060–4067. doi: 10.1002/chem.200701911. [DOI] [PubMed] [Google Scholar]
- 49.Yang X., Pan X., Blyth J., Lowe C.R. Towards the real-time monitoring of glucose in tear fluid: Holographic glucose sensors with reduced interference from lactate and pH. Biosens. Bioelectron. 2008;23:899–905. doi: 10.1016/j.bios.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Muscatello M.M.W., Stunja L.E., Asher S.A. Polymerized crystalline colloidal array sensing of high glucose concentrations. Anal. Chem. 2009;81:4978–4986. doi: 10.1021/ac900006x. [DOI] [PubMed] [Google Scholar]
- 51.Ayyub O.B., Ibrahim M.B., Briber R.M., Kofinas P. Self-assembled block copolymer photonic crystal for selective fructose detection. Biosens. Bioelectron. 2013;46:124–129. doi: 10.1016/j.bios.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Egawa Y., Seki T., Takahashi S., Anzai J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C. 2011;31:1257–1264. [Google Scholar]
- 53.Shinmori H., Takeuchi M., Shinkai S. A novel light-gated sugar receptor, which shows high glucose selectivity. J. Chem. Soc. Perkin Trans. 1998;2:847–852. [Google Scholar]
- 54.Kimura T., Takeuchi M., Shinkai S. Saccharide induction of chiral orientation of the aggregate formed from boronic-acid-appended amphiphiles. Bull. Chem. Soc. Jpn. 1998;71:2197–2204. [Google Scholar]
- 55.Takeuchi M., Taguchi M., Shinmori H., Shinkai S. Molecular design of boronic acid-based dye receptors for nucleosides. Bull. Chem. Soc. Jpn. 1996;69:2613–2618. [Google Scholar]
- 56.Koumoto K., Takeuchi M., Shinkai S. Design of a visualized sugar sensing system utilizing a boronic acid-azopyridine interaction. Supramol. Chem. 1998;9:203–210. [Google Scholar]
- 57.Springsteen G., Wang B. Alizarin red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates. Chem. Commun. 2001:1608–1609. doi: 10.1039/b104895n. [DOI] [PubMed] [Google Scholar]
- 58.Boduroglu S., El Khoury J.M., Venkat Reddy D., Rinaldi P.L., Hu J. A colorimetric titration method for quantification of millimolar glucose in a pH 7.4 aqueous phosphate buffer. Bioorg. Med. Chem. Lett. 2005;15:3974–3977. doi: 10.1016/j.bmcl.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 59.Ma W.M.J., Pereira Morais M.P., D’Hooge F., van den Elsen J.M.H., Cox J.P.L., James T.D., Fossey J.S. Dye displacement assay for saccharide detection with boronate hydrogels. Chem. Commun. 2009;7345:532–534. doi: 10.1039/b814379j. [DOI] [PubMed] [Google Scholar]
- 60.Kitamura M., Shabbir S.H., Anslyn E.V. Guidelines for pattern recognition using differential receptors and indicator displacement assays. J. Org. Chem. 2009;74:4479–4489. doi: 10.1021/jo900433j. [DOI] [PubMed] [Google Scholar]
- 61.Ward C.J., Ashton P.R., James T.D., Patel P. A molecular colour sensor for monosaccharides. Chem. Commun. 2000:229–230. [Google Scholar]
- 62.Ward C.J., Patel P., James T.D. Boronic acid appended azo dyes-colour sensors for saccharides. J. Chem. Soc. Perkin Trans. 2002;1:462–470. [Google Scholar]
- 63.Ward C.J., Patel P., James T.D. Molecular color sensors for monosaccharides. Org. Lett. 2002;4:477–479. doi: 10.1021/ol016923w. [DOI] [PubMed] [Google Scholar]
- 64.DiCesare N., Lakowicz J.R. New color chemosensors for monosaccharides based on azo dyes. Org. Lett. 2001;3:3891–3893. doi: 10.1021/ol016813p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Egawa Y., Gotoh R., Niina S., Anzai J. Ortho-azo substituted phenylboronic acids for colorimetric sugar sensors. Bioorg. Med. Chem. Lett. 2007;17:3789–3792. doi: 10.1016/j.bmcl.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 66.Egawa Y., Tanaka Y., Gotoh R., Niina S., Kojima Y., Shimomura N., Nakagawa H., Seki T., Anzai J. Nitrogen-15 NMR spectroscopy of sugar sensor with B–N interaction as a key regulator of colorimetric signals. Chem. Lett. 2010;39:1188–1189. [Google Scholar]
- 67.Liu Y., Zhao Y., Chen Y., Guo D. Assembly behavior of inclusion complexes of β-cyclodextrin with 4-hydroxyazobenzene and 4-aminoazobenzene. Org. Biomol. Chem. 2005;2:584–591. doi: 10.1039/b415946b. [DOI] [PubMed] [Google Scholar]
- 68.Russell A. Method for Detecting Polyhydroxyl Compounds. Aug 11, 1992. US Patent 5,137,833.
- 69.Russell A., Zepp C. Method and Means for Detecting Polyhydroxyl Compounds. Apr 30, 1996. US Patent 5,512,246.
- 70.Lambert J.B., Binsch G., Roberts J.D. Nitrogen-15 magnetic resonance spectroscopy, I. Chemical shifts. Proc. Natl. Acad. Sci. USA. 1964;51:735–737. doi: 10.1073/pnas.51.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy G.C., Lichter R.L. Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy. John Wiley & Sons, Inc; New York, NY, USA: 1979. [Google Scholar]
- 72.Wiskur S.L., Lavigne J.J., Ait-Haddou H., Lynch V., Chiu Y.H., Canary J.W., Anslyn E.V. pKa values and geometries of secondary and tertiary amines complexed to boronic acids-implications for sensor design. Org. Lett. 2001;3:1311–1314. doi: 10.1021/ol0156805. [DOI] [PubMed] [Google Scholar]
- 73.Zhu L., Shabbir S.H., Gray M., Lynch V.M., Sorey S., Anslyn E.V. A structural investigation of the N–B interaction in an o-(N,N-dialkylaminomethyl)arylboronate system. J. Am. Chem. Soc. 2006;128:1222–1232. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 74.Collins B.E., Sorey S., Hargrove A.E., Shabbir S.H., Lynch V.M., Anslyn E.V. Probing intramolecular B–N interactions in ortho-aminomethyl arylboronic acids. J. Org. Chem. 2009;74:4055–4060. doi: 10.1021/jo900187a. [DOI] [PubMed] [Google Scholar]
- 75.Larkin J.D., Fossey J.S., James T.D., Brooks B.R., Bock C.W. A computational investigation of the nitrogen-boron interaction in o-(N,N-dialkylaminomethyl)arylboronate systems. J. Phys. Chem. A. 2010;114:12531–12539. doi: 10.1021/jp1087674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ni W., Kaur G., Springsteen G., Wang B., Franzen S. Regulating the fluorescence intensity of an anthracene boronic acid system: A B–N bond or a hydrolysis mechanism? Bioorg. Chem. 2004;32:571–581. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Franzen S., Ni W., Wang B. Study of the mechanism of electron-transfer quenching by boron-nitrogen adducts in fluorescent sensors. J. Phys. Chem. B. 2003;107:12942–12948. [Google Scholar]
- 78.Arimori S., Ushiroda S., Peter L.M., Jenkins A.T.A., James T.D. A modular electrochemical sensor for saccharides. Chem. Commun. 2002:2368–2369. doi: 10.1039/b207643h. [DOI] [PubMed] [Google Scholar]
- 79.Arimori S., Bell M.L., Oh C.S., Frimat K.A., James T.D. Modular fluorescence sensors for saccharides. J. Chem. Soc. Perkin Trans. 2002;1:803–808. [PubMed] [Google Scholar]
- 80.Egawa Y., Gotoh R., Seki T., Anzai J. Sugar response of boronic acid-substituted azobenzene dye-modified polymer. Mater. Sci. Eng. C. 2009;29:115–118. [Google Scholar]
- 81.Yamamoto T., Omote M., Miyazaki Y., Kashiwazaki A., Lee B., Kanbara T., Osakada K., Inoue T., Kubota K. Poly (thiophene-2,5-diyl)s with a crown ethereal subunit. Preparation, optical properties, and n-doped state stabilized against air. Macromolecules. 1997;30:7158–7165. [Google Scholar]
- 82.Price G.J., Drake P.L. Potassium selective acrylic copolymers: Synthesis and application to chemical sensors. React. Funct. Polym. 2006;66:109–121. [Google Scholar]
- 83.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 84.Sato K., Takahashi S., Anzai J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012;28:929–938. doi: 10.2116/analsci.28.929. [DOI] [PubMed] [Google Scholar]
- 85.Sato K., Anzai J. Dendrimers in layer-by-layer assemblies: Synthesis and applications. Molecules. 2013;18:8440–8460. doi: 10.3390/molecules18078440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ball V., Hübsch E., Schweiss R., Voegel J.-C., Schaaf P., Knoll W. Interactions between multivalent ions and exponentially growing multilayers: Dissolution and exchange processes. Langmuir. 2005;21:8526–8531. doi: 10.1021/la050866o. [DOI] [PubMed] [Google Scholar]
- 87.Picart C., Mutterer J., Richert L., Luo Y., Prestwich G.D., Schaaf P., Voegel J.-C., Lavalle P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA. 2002;99:12531–12535. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noguchi T., Anzai J. Redox properties of the ferricyanide ion on electrodes coated with layer-by-layer thin films composed of polysaccharide and poly(allylamine) Langmuir. 2006;22:2870–2875. doi: 10.1021/la053226u. [DOI] [PubMed] [Google Scholar]
- 89.Okasaka Y., Kitano H. Direct spectroscopic observation of binding of sugars to polymers having phenylboronic acids substituted with an ortho-phenylazo group. Colloids Surf. B Biointerfaces. 2010;79:434–439. doi: 10.1016/j.colsurfb.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Shimpuku C., Ozawa R., Sasaki A., Sato F., Hashimoto T., Yamauchi A., Suzuki I., Hayashita T. Selective glucose recognition by boronic acid azoprobe/γ-cyclodextrin complexes in water. Chem. Commun. 2009:1709–1711. doi: 10.1039/b819938h. [DOI] [PubMed] [Google Scholar]
- 91.Egawa Y., Niina S., Anzai J. Effects of poly(allylamine) on the sugar-binding properties of a phneylboronic acid-appended azo dye. Bunseki Kagaku. 2006;55:1003–1006. [Google Scholar]
- 92.Hage D.S. Affinity chromatography: A review of clinical applications. Clin. Chem. 1999;45:593–615. [PubMed] [Google Scholar]
- 93.Hage D.S., Anguizola J.A., Bi C., Li R., Matsuda R., Papastavros E., Pfaunmiller E., Vargas J., Zheng X. Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. J. Pharm. Biomed. Anal. 2012;69:93–105. doi: 10.1016/j.jpba.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., Liu Z. Recent advances in monolithic column-based boronate-affinity chromatography. Trends Anal. Chem. 2012;37:148–161. [Google Scholar]
- 95.Kim E., Song S., Lee B., Yoon H. Determination of glycated hemoglobin on the basis of spectral shifting from protein-dye interaction. BioChip J. 2010;4:16–21. [Google Scholar]
- 96.Fabre B., Taillebois L. Poly(aniline boronic acid)-based conductimetric sensor of dopamine. Chem. Commun. 2003:2982–2893. doi: 10.1039/b311198a. [DOI] [PubMed] [Google Scholar]
- 97.Ali S.R., Parajuli R.R., Ma Y., Balogun Y., He H. Interference of ascorbic acid in the sensitive detection of dopamine by a nonoxidative sensing approach. J. Phys. Chem. B. 2007;111:12275–12281. doi: 10.1021/jp073705x. [DOI] [PubMed] [Google Scholar]
- 98.Hagihara S., Tanaka H., Matile S. Boronic acid converters for reactive hydrazide amplifiers: Polyphenol sensing in green tea with synthetic pores. J. Am. Chem. Soc. 2008;130:5656–5657. doi: 10.1021/ja801094p. [DOI] [PubMed] [Google Scholar]
- 99.Freeman R., Bahshi L., Finder T., Gill R., Willner I. Competitive analysis of saccharides or dopamine by boronic acid-functionalized CdSe-ZnS quantum dots. Chem. Commun. 2009:764–766. doi: 10.1039/b820112a. [DOI] [PubMed] [Google Scholar]
- 100.Belitsky J.M. Aryl boronic acid inhibition of synthetic melanin polymerization. Bioorg. Med. Chem. Lett. 2010;20:4475–4478. doi: 10.1016/j.bmcl.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y.-J., Jiang Y.-B., Fossey J.S., James T.D., Marken F. Assembly of N-hexadecyl-pyridinium-4-boronic acid hexafluorophosphate monolayer films with catechol sensing selectivity. J. Mater. Chem. 2010;20:8305–8310. [Google Scholar]
- 102.Xia N., Deng D., Zhang L., Yuan B., Jing M., Du J., Liu L. Sandwich-type electrochemical biosensor for glycoproteins detection based on dual-amplification of boronic acid-gold nanoparticles and dopamine-gold nanoparticles. Biosens. Bioelectron. 2013;43:155–159. doi: 10.1016/j.bios.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 103.Hashimoto T., Oyaidu S., Hayashita T. Design and function of novel azoprobe possessing multipoint binding sites for dopamine recognition. Bunseki Kagaku. 2012;61:213–219. [Google Scholar]