Abstract
Voltage-gated sodium (Nav) channels are essential contributors to neuronal excitability, making them the most commonly targeted ion channel family by toxins found in animal venoms. These molecules can be used to probe the functional aspects of Nav channels on a molecular level and to explore their physiological role in normal and diseased tissues. This chapter summarizes our existing knowledge of the mechanisms by which animal toxins influence Nav channels as well as their potential application in designing therapeutic drugs.
Keywords: Sodium channel, Animal toxin, Pore-blocker, Gating-modifier, Venom
1 Introduction
Voltage-gated sodium (Nav) channels regulate the Na+ permeability of the cell membrane, thereby generating electrical signals that encode and propagate vital information across long distances (Catterall 2000; Hille 2001; Hodgkin and Huxley 1952; Ashcroft 1999). Venomous animals and poisonous plants readily exploit this excitatory role by producing toxins that modify Nav channel opening or closing (e.g., gating) with the goal of incapacitating prey or as defense against predators (Bosmans and Swartz 2010; Catterall et al. 2007; Billen et al. 2008; Smith and Blumenthal 2007; Mebs 2002; Escoubas et al. 2002; de la Vega et al. 2005). Historically, toxins from spider, scorpion, sea anemone, cone snail, insect venoms, and plant extracts have been used to describe diverse receptor sites in particular regions of the nine Nav channel isoforms that have been identified in various mammalian tissues (Nav1.1–Nav1.9) (Catterall 1980; Martin-Eauclaire and Couraud 1992; Catterall et al. 2005; Terlau and Olivera 2004; Honma and Shiomi 2006; Hanck and Sheets 2007). Based on their primary amino acid sequence, all Nav channel isoforms seem to be similarly configured and consist of four domains (I–IV) that each contains a voltage sensor and a portion of the structure that forms the Na+-selective pore which can open upon activation of the four voltage sensors in response to changes in membrane potential (Catterall 2000; Bosmans et al. 2008; Cha et al. 1999; Chanda and Bezanilla 2002; Horn et al. 2000; Sheets et al. 1999, 2000). The voltage sensors in domains I–III are essential for channel opening, whereas domain IV movement is crucial for terminating the Na+ flux after the channel has opened by initiating fast inactivation (Billen et al. 2008; Cha et al. 1999; Chanda and Bezanilla 2002; Horn et al. 2000; Sheets et al. 1999, 2000; Campos et al. 2008; Capes et al. 2012). Similar to voltage-activated potassium (Kv) channels (Bosmans et al. 2008; Alabi et al. 2007; Jiang et al. 2003; Long et al. 2007; Phillips et al. 2005; Ruta and MacKinnon 2004; Swartz 2007; Swartz and MacKinnon 1997a, b; Xu et al. 2013), each Nav channel voltage sensor possesses a conserved S3b-S4 motif, or paddle motif, which drives activation of the voltage sensor in each domain and opening of the pore (Billen et al. 2008; Bosmans et al. 2011; Xiao et al. 2008; Campos et al. 2007, 2008). As is the case with Kv channels, each of the four Nav channel paddle motifs is a key pharmacological target for a range of animal toxins (Bosmans and Swartz 2010; Bosmans et al. 2008, 2011; Xiao et al. 2008; Rogers et al. 1996).
Overall, toxins that influence Nav channel function do so through two distinct mechanisms. Pore-blocking toxins inhibit the flow of Na+ by binding to the outer vestibule or inside the ion conduction pore (Hille 2001; Terlau and Olivera 2004) whereas gating-modifier toxins interact with a region of the channel that changes conformation during channel opening to alter the gating mechanism (Cahalan 1975; Koppenhofer and Schmidt 1968a, b). Although certain gating-modifier toxins interact with the voltage-sensing domains as well as the pore region (Quandt and Narahashi 1982), their subsequent effect on Nav channel gating can usually be associated with their ability to stabilize a voltage sensor in a particular state. As such, in the next sections we will refer to the interaction site of animal toxins within mammalian Nav channels as either the voltage-sensing domains or the pore region and we will further refine this division using the primary functional effects of toxins on channel gating.
2 Toxins Influencing Nav Channel Function by Interacting with the Pore Region
2.1 Non-Peptidic Toxins
2.1.1 Tetrodotoxin and Saxitoxin
The naturally occurring marine toxins tetrodotoxin (TTX) and saxitoxin (STX) interact strongly with the Nav channel pore region to occlude the Na+ permeation pathway (Fig. 1) (Hille 1975). Both toxins are of great historical and medical value and were originally isolated more than five decades ago from the Japanese Puffer fish (TTX) and marine dinoflagellates (STX) (Furukawa et al. 1959; Moore et al. 1967; Narahashi 1974; Narahashi et al. 1964). STX is perhaps most notorious for its role in paralytic shellfish poisoning, caused by the consumption of toxic shellfish such as clams and mussels. TTX and STX have been used extensively in studying the structural and functional properties of Nav channels. Early work by Narahashi and others revealed that TTX affects neuronal Na+ currents only when applied to the extracellular surface of the cell and not when perfused inside axons (Narahashi et al. 1964, 1967). After resolving the crystal structure of TTX in 1964 (Woodward 1964) and STX in 1975 (Schantz et al. 1975), this information was used by Hille to predict the diameter of the Nav channel pore (Hille 1975), thereby providing unique insights into the molecular structure of this ion channel family that resonate until this day (Payandeh et al. 2011, 2012; Zhang et al. 2012; McCusker et al. 2012). Later, the low-nanomolar affinity of [3H]TTX contributed to the isolation of the Nav channel pore-forming subunit from the electroplaque organ of the electric eel, Electrophorus electricus (Miller et al. 1983; Agnew et al. 1978). Similarly, [3H]STX played a vital role in the purification of the neuronal Nav channel from rat brain (Hartshorne and Catterall 1981) and skeletal muscle (Barchi et al. 1980), thereby also revealing the existence of two associated auxiliary subunits (Hartshorne et al. 1982). Nowadays, TTX sensitivity is extensively used to divide the Nav channel family into two groups; TTX-sensitive channels (Nav1.1–Nav1.4, Nav1.6–Nav1.7) are inhibited by nanomolar amounts (Fig. 1), whereas Nav1.8 and Nav1.9 require millimolar quantities to be fully blocked (Catterall 2000). Although Nav1.5 inhibition requires an intermediate micromolar concentration, TTX response can be markedly increased by substituting a cysteine in the domain I S5–S6 loop with a hydrophobic or aromatic residue such as a tryptophan of phenylalanine (Lipkind and Fozzard 1994; Leffler et al. 2005; Sivilotti et al. 1997). The latter amino acids support nanomolar blockade through the formation of a cation–pi interaction with TTX, possibly via an interaction with the charged guanidinium groups within the toxin (Lipkind and Fozzard 1994). These cationic groups also interact with anionic residues within the pore region of the channel to prevent Na+ conductance. As such, experiments geared toward finding residues that diminished Nav channel TTX sensitivity aided in the discovery of amino acids that create the Nav channel selectivity filter (Terlau et al. 1991), namely an aspartate (DI S5–S6 loop), glutamate (DII S5–S6 loop), lysine (DIII S5–S6 loop), and alanine (DIV S5–S6 loop) (Lipkind and Fozzard 2008). Even though this region of the selectivity filter plays a role in STX interaction as well, other important extracellular residues have now been implicated in forming the STX receptor site (Fozzard and Lipkind 2010), most likely due to supplementary interactions with the additional guanidinium group present within the toxin.
Fig. 1.
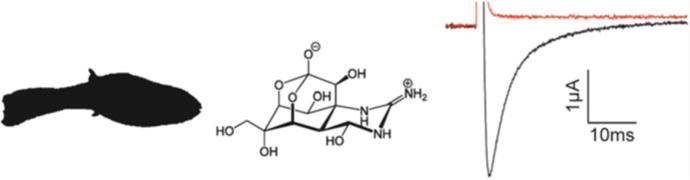
Tetrodotoxin inhibits Nav channel opening. TTX is found in the Japanese puffer fish (silhouette of Takifugu rupripes on the left) and has a complex molecular structure (middle). At 10 nM, TTX inhibits rNav1.2a currents evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 10 nM TTX (right)
Despite its potential toxicity, clinical trials in which TTX was used to treat various pain disorders have been conducted (Nieto et al. 2012). However, the results are ambiguous and further research is essential. Of note is the peculiar protein saxiphilin, a transferrin-like molecule that binds STX with nanomolar affinity (Morabito and Moczydlowski 1994). Saxiphilin is found across a variety of aquatic and terrestrial species, including fish, reptiles, amphibians, and even arthropods. Notwithstanding its ancient character and global distribution, the physiological role of saxiphilin is not fully understood. A possible contribution to neutralizing dietary or environmental STX seems most obvious, but many animals expressing saxiphilin do not occupy biomes in which they will encounter STX (Llewellyn et al. 1997). Regardless, saxiphilin may still find use as an alternative to animal testing or be incorporated into more efficient STX assays (Llewellyn et al. 1998).
2.1.2 Ciguatoxin and Brevetoxin
Aside from TTX and STX, the cyclic polyether ciguatoxins (Michio et al. 1989) and brevetoxins (Yong-Yeng et al. 1981) found in algae can also cause seafood poisoning. Produced by the dinoflagellates Gambierdiscus toxicus (Bagnis et al. 1980) and Karenia brevis (Yong-Yeng et al. 1981), respectively, these molecules can accumulate in the food chain in fish that are consumed by humans or marine mammals in which they disrupt Nav channel function (Lewis et al. 1991). Although ciguatoxins research has been hindered by the small quantities available from marine fauna (Legrand et al. 1989), this hurdle was recently overcome by Hirama et al. (2001) when they synthesized CTX3C, thereby permitting easier access to large quantities of purified toxin. Initial forays into algaetoxin pharmacology indicated that ciguatoxin (Bidard et al. 1984) as well as brevetoxin (Lombet et al. 1987), albeit with a much lower affinity (Lombet et al. 1987), can potentiate opening of multiple Nav channel isoforms in addition to altering their Na+ permeability. As a result, both molecules are capable of inducing spontaneous firing in neurons which eventually leads to paralysis (Benoit et al. 1986). Interestingly, the Nav1.8 isoform found in the periphery seems particularly susceptible to CTX3C, resulting in the appearance of a large persistent current upon toxin exposure, an observation that may in part explain some of the neurological sequelae seen in ciguatera victims (Yamaoka et al. 2009). Although the exact working mechanism is unclear, photolabeling experiments on Nav channels indicate that the S6 segment within domain I and the S5 segment of domain IV are involved in brevetoxin binding (Trainer et al. 1994). Moreover, a study utilizing chimeric Nav1.4 and Nav1.8 channels in combination with CTX3C suggest an interaction site of this toxin within domains I and II (Yamaoka et al. 2009). Finally, it has been suggested that these ladder-like polyether toxins may partition in the membrane to complement a structural motif within the Nav channel (e.g., α-helix) by means of a hydrogen bond network, which may lead to their biological activity (Ujihara et al. 2008).
2.1.3 Batrachotoxin
Well known in popular culture is the image of the jungle cannibal dispatching his prey with poison-tipped darts launched from a blowgun. The poison used here is often found in the secretions of “poison dart frogs” (family Dendrobatidae) endemic to Central and South America. While all members of the Dendrobatidae secrete toxins, only the Phyllobates genus is known to produce batrachotoxin (BTX), a potent Nav channel toxin with an unusual steroidal alkaloid structure (Fig. 2) (Tokuyama et al. 1969). Frogs reared in captivity are not toxic, leading to the conclusion that they acquire and concentrate the toxin from their environment. Similarly, select species of birds from New Guinea (Ifrita kowaldi and members of the Pitohui genus) are found to secrete BTX onto their skin and feathers (Dumbacher et al. 2000). Dumbacher and coworkers propose that Melyrid beetles may be the dietary source of BTX for both poison-dart frogs and Pitohui birds; Choresine beetles are known to be rich sources of BTX alkaloids and related species are found in the New World (Dumbacher et al. 2004).
Fig. 2.
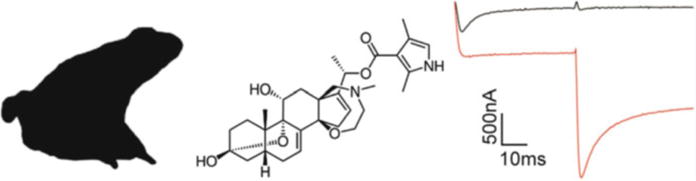
Batrachotoxin disrupts Nav channel gating. BTX is found in particular frog species (silhouette of Phyllobates bicolor on the left) and has a complex molecular structure (middle). At 5 μM, BTX completely inhibits fast inactivation of rNav1.8 currents evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. During the second voltage step to −30 mV, channels normally close; however, BTX-bound channels are still deactivating. Black is control and red is after addition of TTX (right)
Although the exact working mechanism has yet to be elucidated, the Nav channel receptor site for BTX has been shown to involve a cluster of residues located in the S6 pore-segments of domains I (Wang and Wang 1998), III (Wang et al. 2000; Du et al. 2011), and IV (Linford et al. 1998) and may partially overlap with the binding site of local anesthetics (Wang et al. 1998). Unlike lidocaine, which blocks Na+ currents, BTX may partially occlude the ion permeation pathway, thereby leaving room for a fraction of Na+ to pass through the pore. Although BTX may bind within the pore region, the toxin also (1) abolishes fast and slow inactivation (Huang et al. 1982) (Fig. 2) and (2) shifts Nav channel opening to voltages where it is normally closed (Quandt and Narahashi 1982; Bosmans et al. 2004), thereby leading to repeated depolarizations in vivo and eventual paralysis as muscles become insensitive to neuronal signals (Wang and Wang 1998; Linford et al. 1998; Bosmans et al. 2004; Wasserstrom et al. 1993; Catterall 1975).
Even though BTX is unsuitable for drug design, radioactive BTX has been used extensively for Nav channel identification in tissues and vesicles and in screening potential therapeutics (Cooper et al. 1987; Gill et al. 2003). A functional relative of BTX is veratridine, an alkaloid toxin found in Liliaceae plants, which causes persistent opening of Nav channels while reducing single-channel conductance (Ulbricht 1998). Although veratridine is thought to interact with the pore-forming S6 segments in open Nav channels, the exact mechanism by which the toxin causes persistent Nav channel opening remains uncertain. Nevertheless, due to its ability to open Nav channels, veratridine is used extensively in drug screening essays for which controlling the membrane voltage is impractical (Felix et al. 2004).
2.2 Peptidic Toxins
2.2.1 Cone Snail Toxins
The pore-blocking μ-conotoxin peptides found in cone snail venom are among the best-characterized Nav channel antagonists. Identifying cone snail venoms likely to contain novel Nav channel pore-blocking peptide toxins was made possible by using the molecular phylogeny framework established for the 700 species of cone snails (Fig. 3). The biological rationale for the presence of these toxins in the venom of a specific subset of Conus species is presented here.
Fig. 3.
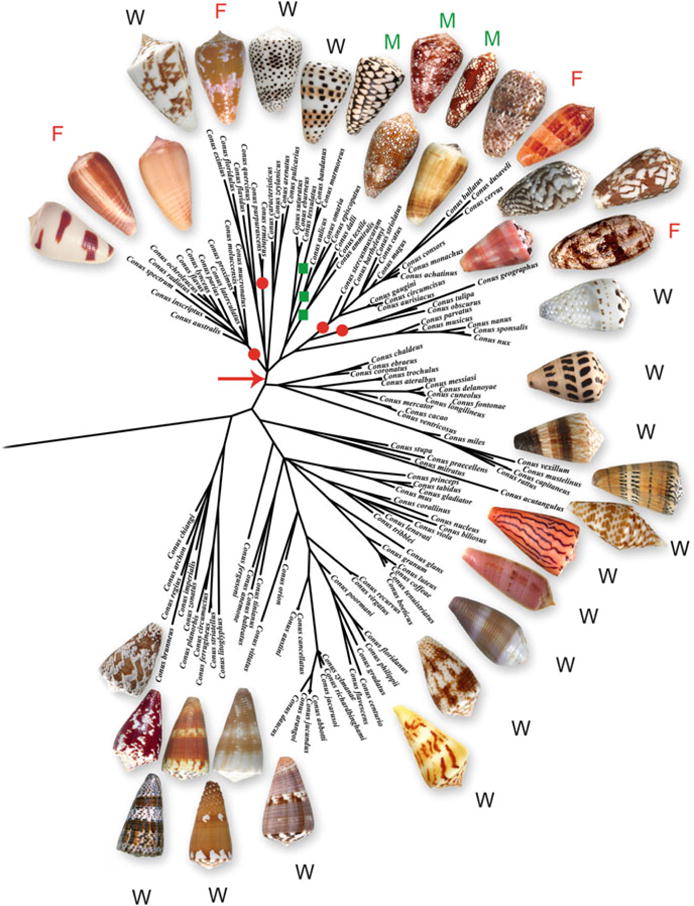
Phylogenetic tree of a variety of Conus species. Fish-hunting clades are labeled with a red dot, mollusc-hunting clades with a green square; others are labeled “W” and are believed to be worm-hunting Conus. μ-conotoxins are derived from venoms of fish-hunting clades, whereas the μO-conotoxins are from the venom of a snail-hunting species. The tree is based on 12S mitochondrial rRNA markers; data generated by M. Watkins; tree constructed by J. P. Ownby
Cone snails (Fig. 4) are venomous predatory marine gastropods that are slow moving, unable to swim, and have little in the way of mechanical weaponry for catching their prey. As such, their successful adaptive radiation throughout tropical marine environments required the evolution of venoms that provide the only effective arsenal for capturing their victims. All pore-blocking Nav channel targeting conopeptides characterized to date are found in the venom of cone snail species that specialize in hunting fish as their primary prey (approximately 100 different species of cone snails are believed to be fish-hunting) (Olivera 1997). In order to block neuromuscular transmission, cone snails evolved groups of peptides that act coordinately (called “cabals”) but each on a different molecular target. Among those are the functionally linked signal components of the neuromuscular transmission circuitry, i.e., the nicotinic receptor at the postsynaptic terminus, the presynaptic voltage-gated calcium channels that control neurotransmitter release, and Nav channels that mediate action potential transmission on the muscle membrane (Terlau and Olivera 2004). The most abundantly expressed pore-blocking toxins within cone snail venoms, the μ-conotoxins, primarily interact with Nav1.4, a Nav channel isoform found in skeletal muscle. Nonetheless, the affinity of each peptide for other Nav channel subtypes can vary considerably. By systematically investigating μ-conotoxins from diverse clades of fish-hunting cone snails [see (Baldomero et al. 2014) for a discussion of different lineages of fish-hunting cone snails], a series of peptides with varying neuronal Nav channel selectivity profiles has been obtained. To summarize this work, μ-conotoxins from Conus species in five lineages are shown in Table 1 (Wilson et al. 2011) which demonstrates the striking affinity differences of a range of peptides for neuronal Nav channel subtypes.
Fig. 4.

Conus shells are remarkably diverse. Shells of fish-hunting Conus species listed in Table 1. Top row, left to right: C. consors; C. magus; C. tulipa; C. aurisiacus; C. geographus; and C. striatus. Bottom row, left to right: C. bullatus; C. stercusmuscarum; C. purpurascens; and C. striolatus. Different clades of fish-hunting Conus are represented. Conus purpurascens belongs to the subgenus Textilia, Conus tulipa and Conus geographus to the subgenus Gastridium, and all other species shown to the subgenus Pionoconus. Figure prepared by My Hyunh
Table 1.
Affinities of m-conopeptides (mM) for Nav channel subtypes
| Peptide | Conus species (Fish-hunting clade) | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| SmIIIA | C. stercusmuscarum (1) | 0.0038 | 0.0013 | 0.035 | 0.00022 | 1.3aa | 0.16a | 1.3a | >100a | West et al. (2002) |
| KIIIA | C. kinoshitai (2) | 0.29 | 0.005 | 8a | 0.09a | 287a | 0.24a | 0.29 | >100a | Bulaj et al. (2005) |
| SIIIA | C. striatus (1) | 11a | 0.05 | 11a | 0.13a | 251a | 0.76a | 65a | >100a | Bulaj et al. (2005) |
| CnIIIA | C. consors (1) | 14.2a | 0.25 | 11a | 0.27a | 7.4a | 7.1a | >100a | >100a | Zhang et al. (2006) |
| MIIIA | C. magus (1) | 22.6a | 0.45 | 7.7 | 0.33 | >100a | 21.6a | 97a | >100a | Zhang et al. (2006) |
| GIIIA | C. geographus (3) | 0.26 | 17.8a | >100a | 0.019a | >100a | 0.68a | >100a | >100a | Cruz et al. (1985) |
| PIIIA | C. purpurascens (4) | 0.12a | 0.62a | 3.2a | 0.036a | >100a | 0.1a | >100a | >100a | Shon et al. (1998) |
| SxIIIA | C. striolatus (1) | 0.37a | 1a | >100a | 0.007a | >100a | 0.57a | >100a | >100a | Walewska et al. (2008) |
| BuIIIA | C. bullatus (5) | 0.35 | 0.012 | 0.35 | 0.013 | 13.8a | 4.4a | >100a | >100a | Holford et al. (2009) |
| BuIIIB | C. bullatus (5) | 0.36 | 0.013 | 0.2 | 0.0036 | 9a | 1.8a | >100a | >100a | Kuang et al. (2013) |
| TIIIA | C. tulipa (2) | 0.9a | 0.045 | 7.9a | 0.005 | >100a | 25a | >100a | >100a | Lewis et al. (2007) |
Fish-hunting clades: (1) Pionoconus; (2) Alfonsoconus; (3) Gastridium; (4) Chelyconus; (5) Textilia
IC50 values; all other values are KDs. All Nav channel clones were from rat except Nav1.6, which is from mouse
The importance of μ-conotoxins for the Nav channel field stems from the fact that at the present time, a sufficient diversity of toxins have been characterized to assemble a pharmacological kit for distinguishing various Nav channel isoforms. This toolkit takes advantage of individual μ-conotoxin selectivity for the various neuronal Nav channel subtypes shown in Table 1 (Wilson et al. 2011; Bulaj et al. 2005; Leipold et al. 2011; Zhang et al. 2006). For example, μ-conotoxin SxIIIA is highly selective for Nav1.4 while having at least a 50-fold lower affinity for all other subtypes, whereas μ-conotoxin KIIIA and CnIIIA predominantly interact with neuronal Nav channel isoforms. Structure–function studies on KIIIA analogs (Bulaj et al. 2005; McArthur et al. 2011; Van Der Haegen et al. 2011; Zhang et al. 2007, 2009, 2010) demonstrate that the binding sites of the μ-conotoxin and TTX overlap but not coincidence. Consequently, it was shown that KIIIA interacts with additional amino acid residues within the pore vestibule (McArthur et al. 2011; Van Der Haegen et al. 2011; Zhang et al. 2007). Thus, different μ-conotoxins presumably interact with a particular subset of residues within the pore, thereby accounting for the diverse targeting selectivity for the various neuronal Nav channel isoforms. This diversity makes it possible to list which μ-conotoxins are most effective for discriminating between any pair of Nav channel isoforms (see Table 2). Zhang et al. (2013a) employed μ-conotoxins to identify particular Nav channel isoforms in native neuronal cell preparations and demonstrated that a combination of three μ-conotoxins is sufficient to determine which TTX-sensitive Nav channel isoform contributes to the Na+ current in the different neuronal subclasses present in the dorsal root ganglion. As such, the standard Nav channel classification into TTX-sensitive and TTX-resistant classes can be more finely parsed into the individual subtypes, using the appropriate combination of different μ-conotoxins. Zhang and coworkers (Zhang et al. 2013b) also found that auxiliary β-subunits can influence the kinetics of toxin block thereby raising the possibility of employing μ-conotoxins to discriminate different combinations of α- and β-subunits present in native neurons.
Table 2.
m-Conopeptide activity matrix: peptides that best discriminate between given pairs of Nav1-isoforms, as revealed by their discrimination index (DI) values
| Nav | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 |
|---|---|---|---|---|---|---|---|
| 1.1 | SIIIA | MIIIA | TIIIA | CnIIIA | SIIIA | KIIIA | |
| 2.3 | 0.5 | 2.3 | 0.3 | 1.2 | 0.0 | ||
| 1.2 | GIIIA | PIIIA | GIIIA | GIIIA | GIIIA | GIIIA | |
| 1.8 | −0.7 | 3.0 | −0.8 | 1.4 | −0.8 | ||
| 1.3 | GIIIA | KIIIA | SxIIIA | CnIIIA | SxIIIA | KIIIA | |
| 2.6 | 3.2 | 4.2 | 0.2 | 2.2 | 1.4 | ||
| 1.4 | KIIIA | KIIIA | MIIIA | CnIIIA | KIIIA | KIIIA | |
| −0.5 | 1.3 | −1.4 | −1.4 | −0.4 | −0.5 | ||
| 1.5 | KIIIA | KIIIA | BuIIIB | TIIIA | KIIIA | KIIIA | |
| 3.0 | 4.8 | 1.7 | 4.3 | 3.1 | 3.0 | ||
| 1.6 | SmIIIA | TIIIA | BuIIIA | TIIIA | CnIIIA | KIIIA | |
| 1.6 | 2.7 | 1.1 | 3.7 | 0.0 | −0.1 | ||
| 1.7 | PIIIA | BuIIIB | BuIIIB | BuIIIB | CnIIIA | PIIIA | |
| 2.9 | 3.9 | 2.7 | 4.4 | 1.1 | 3.0 |
2.2.2 Spider Toxins
Although a few spider toxins have been shown to inhibit Na+ flux without altering channel gating, the mechanism through which they act remains elusive. For example, the binding site of Tx1 from the Phoneutria nigriventer spider may overlap with that of the μ-conotoxin GIIIA, but not that of TTX (Silva et al. 2012). However, additional experiments are needed to clarify the state-dependent binding of Tx1, an observation that suggests an interaction with one or more Nav channel voltage-sensing domains. An additional example is Hainantoxin-I from the Selenocosmia hainana tarantula (Li et al. 2003), a toxin that weakly inhibits mammalian Nav channel currents without affecting channel opening or fast inactivation. However, a potential competition with cone snail toxins, TTX, or an interaction with the Nav channel pore region or its voltage sensors has not yet been investigated.
3 Toxins Influencing Nav Channel Gating by Interacting with the Voltage Sensors
3.1 Sea Anemone Toxins
Sea anemones are primitive aquatic animals that possess tentacles containing cells bearing a specialized structure called the nematocyst which, when triggered, injects a mix of toxins into the target. While this venom contains a diversity of chemical components, it is primarily the peptides that have been of great interest to neuroscientists. The majority of peptide toxins that target mammalian Nav channels have been isolated from the venom of the genii Anemonia, Anthopleura, and Heteractis (Kem et al. 1989). Despite this seemingly limited distribution, there are untold numbers of anemone species whose toxic components have not yet been explored, leaving open the possibility of many more bioactive peptides remaining to be discovered (Honma and Shiomi 2006). Sea anemone toxins that act on mammalian Nav channels are typically basic proteins, sharing a conserved arginine at position 14 (Khera and Blumenthal 1996) and a lysine residue around position 37 (Benzinger et al. 1998). The lysine is considered to be crucial for mammalian toxicity (Gallagher and Blumenthal 1994; Norton 2009; Barhanin et al. 1981) and flexibility of the loop containing this residue is important for Nav channel isoform discrimination by the toxin (Gooley et al. 1984; Seibert et al. 2003).
Due to their intimate interaction with the paddle motif in the domain IV voltage sensor (Rogers et al. 1996), sea anemone toxins can (1) inhibit Nav channel fast inactivation at nanomolar concentrations (Fig. 5) (Alsen et al. 1981; Romey et al. 1976; Catterall and Beress 1978), albeit with exceptionally slow on- and off-rates; and (2) enhance recovery from inactivation without affecting Nav channel activation, deactivation, or closed-state inactivation (Hanck and Sheets 2007; Sherif et al. 1992; Richard Benzinger 1999). Although careful studies on various Nav channel isoforms with ATX-II and Ap-B (Fig. 5) reveal that sea anemone toxins exert their effect by delaying domain IV voltage sensor activation, Oliveira and colleagues observed that ATX-II is more potent on Nav1.2 when compared to Nav1.6 (Oliveira et al. 2004). Since the domain IV paddle motif in these two channel isoforms is very similar, their results suggest that ATX-II may interact with regions other than the domain IV voltage sensor. In contrast to Kv channels and acid-sensing ion channels for which sea anemone venoms have proven to be a valuable source of therapeutic leads (Chandy et al. 2004; Baron et al. 2013), a molecule with similar potential that targets Nav channels has yet to emerge.
Fig. 5.
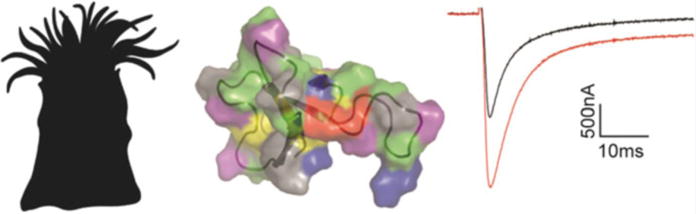
ATX-II inhibits Nav channel fast inactivation. ATX-II is found in the Anemonia sulcata sea anemone and amino acids important to toxin functionality have been identified throughout the related peptide Ap-a (middle figure: protein backbone is shown together with the electrostatic surface of the protein. Blue are basic residues, red acidic, green hydrophobic, yellow cysteine, and purple polar.). At 500 nM, ATX-II inhibits fast inactivation of rNav1.2a currents evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 500 nM ATX-II (right)
3.2 Scorpion Toxins
Throughout some 400 million years of evolution, scorpions have perfected peptide toxins to specifically and potently target mammalian or insect ion channel families. As such, classic studies on scorpion venom recognized the presence of toxins capable of interfering with Nav channel voltage sensor function (Martin-Eauclaire and Couraud 1992; Cahalan 1975; Koppenhofer and Schmidt 1968a, b). In general, scorpion toxins interact with extracellular loops between S3 and S4 to stabilize the voltage sensors of Nav channels in particular states. Based on their functional effects, two classes of Nav channel scorpion toxins have been defined (Couraud et al. 1982).
First, α-scorpion toxins act on Nav channels in a manner similar to sea anemone toxins and as such were found to compete for binding at the same location in the domain IV voltage sensor (Fig. 6) (Jover et al. 1978). Nonetheless, early antibody and photo-affinity-labeling studies indicated an interaction of LqTX (isolated from Leiurus quinquestriatus venom) with the S5–S6 loops of domains I (Tejedor and Catterall 1988) and IV (Thomsen and Catterall 1989) in rat neuronal Nav channels. Later, mutagenesis experiments revealed a strong interaction with the S3–S4 paddle motif within domain IV (Rogers et al. 1996), as well as with the domain I S5–S6 loop and domain IV S1–S2 loop (Wang et al. 2011). This secondary binding site in domain I may help to position the α-scorpion toxin in such a way that the crucial hydrophobic residues within the toxin are positioned correctly for their interaction with the domain IV voltage sensor. Recently, a study by Campos et al. (2004) used Ts3 from Tityus serrulatus in concert with fluorescently labeled voltage-sensing domains demonstrating an inhibitory effect on voltage-sensing domain IV movement resulting in the inhibition of fast inactivation as well as speeding recovery from fast inactivation. Interestingly, Ts3 also affected the voltage-dependent gating of domain I suggesting the notion of an allosteric coupling between the adjacent domains I and IV.
Fig. 6.
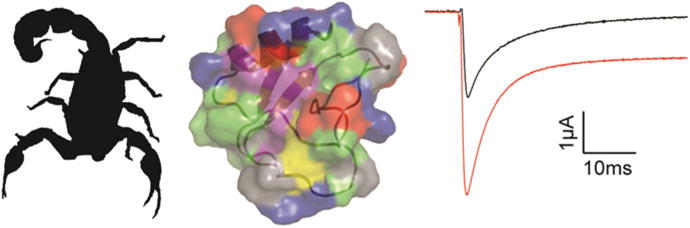
α-scorpion toxins hamper Nav channel fast inactivation. The α-scorpion toxin AaHII is produced by the Androctonus australis Hector scorpion (silhouette on the left) and amino acids important to toxin functionality have been identified in both the hydrophobic patch as well as charged residues surrounding it (middle figure: protein backbone is shown together with the electrostatic surface of the protein). At 100 nM, AaHII inhibits fast inactivation of rNav1.2a currents evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 100 nM AaHII (right)
A second class of Nav channel toxins present in scorpion venom are the β-scorpion toxins which promote subthreshold channel opening by shifting the voltage dependence of activation to more negative membrane potentials (Fig. 7). Distinct from α-scorpion toxins and sea anemone toxins, β-scorpion toxins are thought to primarily target the paddle motif within the domain II voltage sensor and trap it in an activated state (Cestèle et al. 1998, 2006). With the voltage sensor trapped, further depolarizations require the transition of fewer voltage-sensing domains, resulting in the hyperpolarized voltage-dependent activation observed in toxin-bound channels (Fig. 7). A common practice to examine the functional effects of β-scorpion toxins is to apply a short depolarizing pre-pulse in order to initiate their effect on Nav channel gating (Cestèle et al. 1998; Leipold et al. 2012). Although it is conceivable that this pre-pulse facilitates an interaction with the activated domain II voltage sensor, not every β-scorpion toxin requires this additional depolarization. For example, TsVII from Tityus serrulatus (Marcotte et al. 1997) and Tz1 from the Tityus zulianus scorpion (Leipold et al. 2006) do not need such a pulse to influence Nav channel gating, possibly because these toxins are capable of influencing regions other than the one in domain II (Bosmans et al. 2008).
Fig. 7.
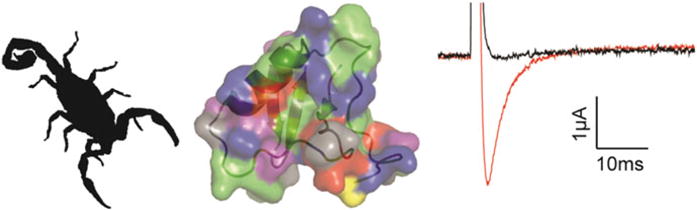
β-scorpion toxins promote Nav channel opening. The β-scorpion toxin CssIV is found in the venom of the Centruroides suffusus suffusus scorpion (silhouette on the left) and amino acids important to toxin functionality have been identified in both the hydrophobic region and a ring of charged residues a (middle figure: protein backbone of the related β-scorpion toxin TsVII is shown together with the electrostatic surface of the protein). At 1 μM, CssIV opens rNav1.2a at voltages where the channel is normally closed. Current trace shown was evoked from a holding potential of −90 mV to a voltage of −40 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 1 μM CssIV (right)
Despite the tedious nature of purifying scorpion toxins from complex venom mixtures, researchers have been successful in identifying peptides that show promise for designing drugs to treat disorders such as erectile dysfunction (Nunes et al. 2013), autoimmune diseases (Norton et al. 2004), and cancer (Stroud et al. 2011). The advent of advanced recombinant (Vita et al. 1995) and synthetic production techniques (M’Barek et al. 2004) in combination with genetic approaches may soon yield Nav channel drug leads as well.
3.3 Spider Toxins
In contrast to toxins from sea anemone, scorpion, and cone snail venoms, the exploration of the mechanism by which spider toxins interact with mammalian Nav channel voltage sensors has only recently begun. Structure–function studies on Magi 5 from the hexathelid spider Macrothele gigas with Nav channels (Corzo et al. 2007) and the Kv channel toxin SGTx1 from the Scodra griseipes tarantula (Lee et al. 2004) reveal the functional importance of a hydrophobic residue cluster surrounded by charged amino acids. As a result of this ubiquitous amphipathic character, spider toxins may access their receptor site within Nav channel and Kv channel voltage-sensing domains after partitioning into the membrane (Bosmans and Swartz 2010; Milescu et al. 2007, 2009; Swartz 2008). Depending on which voltage sensors are targeted and how they couple to the overall Nav channel-gating process, spider toxins can have three diverse effects on Nav channel function. The most commonly observed is for the toxin to inhibit channel opening in response to membrane depolarization (Fig. 8) (Bosmans et al. 2008; Edgerton et al. 2008; Middleton et al. 2002; Smith et al. 2005, 2007; Sokolov et al. 2008). Another outcome is for the toxin to delay fast inactivation by impairing domain IV movement (Wang et al. 2008). Finally, as uniquely observed with Magi 5, the toxin can facilitate channel opening by shifting the activation voltage to more hyperpolarized values (Corzo et al. 2007). Recently, the identification of an S3b–S4 paddle motif within each of the four Nav channel voltage sensors significantly contributed to our understanding of the multifaceted working mechanism of spider toxins. As a result of this discovery, it was demonstrated that the paddle motif in each of the four Nav channel voltage sensors can interact with spider toxins and that multiple paddle motifs are often targeted by a single toxin (Bosmans et al. 2008). These novel insights also led to the identification of the first tarantula toxin (ProTx-I) active on Nav1.9, a Nav channel isoform predominantly expressed in nociceptive DRG neurons (Bosmans et al. 2011; Cummins et al. 1999). Interestingly, ProTx-I potentiates Nav1.9-mediated currents in rat DRG neurons, whereas the other TTX-resistant channel Nav1.8 is inhibited (Bosmans et al. 2011). The related tarantula toxin ProTx-II selectively targets Nav1.7, a Nav channel isoform implicated in various pain syndromes, with an affinity that is at least 100-fold higher when compared to other Nav channel isoforms that were examined (Schmalhofer et al. 2008). Although C-fiber compound action potential in de-sheathed cutaneous nerves can be completely inhibited, ProTx-II is not efficacious in rodent models of acute or inflammatory pain. As is the case with HWTX-IV from the Chinese bird spider Selenocosmia huwena, ProTx-II preferentially targets the Nav1.7 domain II voltage sensor (Xiao et al. 2010). Yet, ProTx-II also binds to the paddle motif in domain IV of this particular isoform, thereby resulting in a noticeable slowing of fast inactivation, a phenomenon that is not visible with Nav1.2 (Fig. 8) (Bosmans et al. 2008; Xiao et al. 2011). Several tarantula toxins have also been shown to possess a certain degree of selectivity toward Nav1.5, a Nav channel isoform known to be involved in cardiovascular function. For example, CcoTx3, JzTx-I, and JzTx-III modulate cardiac Na+ currents; however, the isoform specificity of JzTX-I and JzTx-III has not yet been examined (Bosmans et al. 2006, 2009; Liao et al. 2007; Xiao et al. 2005). Overall, toxins isolated from spider venom have proven to be valuable tools to probe the structure and functional mechanisms of Nav channels. As such, future opportunities may arise for using these peptides as therapeutic drugs.
Fig. 8.
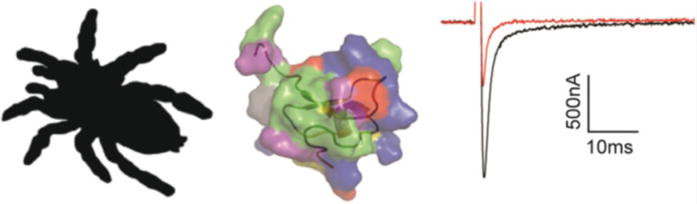
Hanatoxin is a tarantula toxin that inhibits Nav channel opening. HaTx is found in the venom of the Chilean Rose tarantula (Grammostola spatulata) (silhouette of the related tarantula Brachypelma smithi is shown on the left) and amino acids important to toxin functionality have been identified in both the hydrophobic dimple as well as the ring of charged residues surrounding it (middle: protein backbone is shown together with the electrostatic surface of the protein). At 100 nM, HaTx inhibits rNav1.2a opening when currents are evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 100 nM HaTx (right)
3.4 Wasp Toxins
The two wasp toxins that have been thoroughly examined so far, α-pompilidotoxin from Anoplius samariensis and β-pompilidotoxin from Batozonellus maculifrons (Konno et al. 1998), are short peptides with no disulfide bonds or homology to toxins isolated from other organisms (Sahara et al. 2000). Both peptides slow fast inactivation of neuronal Nav channels but not Nav1.4 and Nav1.5, doing so in a manner similar to α-scorpion and sea anemone toxins (Fig. 9). Single amino acid mutagenesis of Nav1.2 has revealed an important role for a glutamate residue in the paddle motif of the domain IV voltage sensor in forming the toxin receptor site (Kinoshita et al. 2001). Moreover, similar to α-scorpion and sea anemone toxins, cationic residues within these peptides were found to be critical for toxin activity (Konno et al. 2000). Mandaratoxin, a 19 kDa protein isolated from the hornet Vespa mandarinia venom, irreversibly reduces Na+ current (Abe et al. 1982). Little else is known about this toxin, yet it serves as a reminder that many toxins in nature remain to be discovered, some of which with novel mechanisms of action that may be exploited for therapeutic or research use.
Fig. 9.
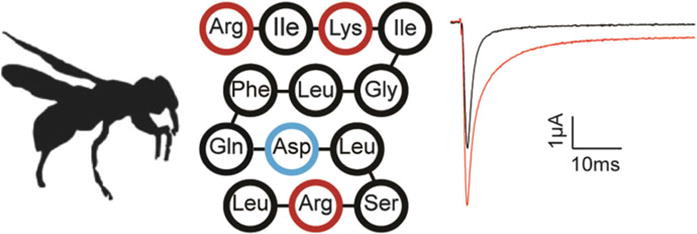
Pompilidotoxins inhibit Nav channel fast inactivation. β-pompilidotoxin from Batozonellus maculifrons (silhouette of related wasp Vespula germanica on the left—peptide sequence in the middle) inhibits rNav1.2a fast inactivation when currents are evoked from a holding potential of −90 mV to a voltage of −20 mV when expressed in Xenopus oocytes. Black is control and red is after addition of 1 μM toxin (right)
3.5 Cone Snail Toxins
Several distinct groups of cone snail toxins are thought to influence Nav channel gating by interacting with the voltage-sensing domains. The biological backdrop resulting in the evolution of these peptides is different from that of the cone snail pore-blocking toxins. In a number of fish-hunting cone snail clades, the ability to rapidly elicit muscle paralysis was insufficient for success. As a result, these snails have evolved to make prey capture even more efficient through a mechanism that is functionally equivalent to electrocuting their victims. In essence, at the venom injection site, a combination of peptides that block Kv channels but keep Nav channels open, elicits a massive depolarization of the axons first encountered by the venom. This causes the affected axons to fire action potentials uncontrollably, analogous to applying a Taser at the point of injection. The result is an almost immediate tetanic paralysis after a successful strike by the cone snail. The group of toxins that cause this very rapid prey immobilization is called the “lighting strike cabal.” The first characterized group of Nav channel targeting cone snail toxins that belong to this lightning strike cabal are called δ-conopeptides and inhibit channel fast inactivation (Terlau et al. 1996) resulting in a continuous entry of Na+ into the affected axons followed by a massive depolarization of the targeted plasma membranes. Consistent with their effect on Nav channel fast inactivation, it was shown that δ-conotoxins target the paddle motif in the domain IV voltage sensor (Leipold et al. 2005). δ-conotoxins are expressed in a wide range of cone snail venoms and are typically between 25 and 35 amino acids in length. Despite the fact that they are ubiquitous in cone snail venoms, the hydrophobic character of these peptides makes them a challenge to synthesize chemically thereby rendering them a less well-characterized family when compared to the μ-conotoxins (Figs. 3 and 4). δ-conotoxins belong to the O-superfamily, are genetically unrelated to the pore-blocking μ-conotoxins (which belong to the M-superfamily), and are primarily found in several clades of fish-hunting cone snails (Barbier et al. 2004; Bulaj et al. 2001; West et al. 2005; Fainzilber et al. 1995; Shon et al. 1995), and in at least one clade of mollusc-hunting snails (Hasson et al. 1993; Hillyard et al. 1989; Shon et al. 1994; Sudarslal et al. 2003). Interestingly, the first δ-conotoxin was discovered in the molluscivorous species Conus textile and was called the King Kong peptide because injection of the toxin into lobsters caused the animal to continuously assume a dominant posture (Hillyard et al. 1989).
A second group of cone snail toxins that contribute to the lightning strike cabal are the ι-conotoxins, which shift Nav channel activation to more hyperpolarized potentials thereby causing these channels to open at voltages where they are normally closed. These peptides differ from the μ-conotoxins and the δ-conotoxins in their mechanism of action, the gene superfamily to which they belong, and the presence of unusual posttranslational modifications. So far, these peptides have been found in only one species of fish-hunting cone snail, Conus radiatus, and belong to the I1 gene superfamily which is typically defined by four disulfide crosslinks and a length of about 40–50 amino acids. A most unusual feature of many native ι-conotoxins is the presence of a single posttranslationally modified D-amino acid toward the C-terminal end. The first biochemically characterized ι-conotoxin, r11a was found to contain a single D-Phe44 (Buczek et al. 2005a). The NMR structure of this peptide revealed that three of the disulfide linkages are consistent with an inhibitory cysteine knot (ICK) motif, with one additional disulfide linkage. The modified D-Phe residue is present beyond the last disulfide crosslink in the C-terminal region, and the NMR structure suggests that this region of the peptide is disordered in solution. Functionally, r11a target selectivity with respect to Nav channel isoforms expressed in Xenopus oocytes is Nav1.6 > Nav1.2 > Nav1.7, whereas other channel subtypes tested are insensitive to the toxin (Fiedler et al. 2008). Substitution of L-Phe for D-Phe has two major functional consequences: (1) a twofold lower affinity and twofold faster off-rate can be observed for the Nav1.6 subtype and (2) the L-Phe analog is inactive on Nav1.2. So far, four ι-toxins have been purified from Conus radiatus and chemically synthesized, whereas only one peptide (r11a) has been identified and characterized from the worm-hunting cone snail, Conus arenatus (Buczek et al. 2005a, b, 2008; Jimenez et al. 2003). While all of the ι-conotoxins from the fish-hunting species Conus radiatus cause severe excitotoxic symptoms when injected into mice, the Conus arenatus ι-conotoxin only causes mild symptoms even at high doses. Furthermore, it was demonstrated that three of the peptides from Conus radiatus (r11a, r11b, and r11c) have a posttranslationally modified amino acid at the predicted site as opposed to only one peptide from Conus radiatus (r11d) and one from Conus arenatus (r11a) in which all amino acids are in the L configuration. Despite their unique characteristics, the receptor site to which ι-conotoxins bind remains to be determined, yet it seems likely that one of the three voltage sensors involved in channel opening may be stabilized in an activated state.
Similar to a variety of other polypeptide toxins, the μO-conotoxins target Nav channel voltage sensors, yet they have distinctive properties (Daly et al. 2004; Leipold et al. 2007; Zorn et al. 2006). Unlike other conotoxins, the μO-conotoxins have not been found in fish-hunting cone snails. Instead, they were discovered in the molliscivorous Conus marmoreus. After this Conus species stings other snails, their envenomated prey become progressively more flaccid; thus, like fish-hunting cone snails, some molluscivorous Conus may have the equivalent of a “motor cabal” to block neuromuscular transmission and would likely have evolved Nav channel antagonists. In contrast to the μ-conotoxins that physically occlude the pore region, the μO-conotoxins are gating modifiers capable of antagonizing Nav channel opening (Leipold et al. 2007; Zorn et al. 2006). It has been hypothesized that they inhibit conductance by preventing the domain II voltage sensor from activating, a feature known to prevent channel opening. The two μO-conotoxins that have been characterized thus far, μO-MrVIA and MrVIB (Fainzilber et al. 1995; Daly et al. 2004; McIntosh et al. 1995), were shown to inhibit Na channel conductance without competing with the pore-blocking μ-conotoxins (Terlau et al. 1996). The analysis carried out by Heinemann and collaborators (Leipold et al. 2007; Zorn et al. 2006) demonstrates that the peptide interacts with both domains II and III, and competition with the β-scorpion toxin Ts1 suggests that the receptor site of μO-conotoxins may overlap with that of β-scorpion toxins (Leipold et al. 2006). Part of the intense interest in μO-conotoxins (Bulaj et al. 2006; Ekberg et al. 2006) stems from their ability to inhibit TTX-resistant Nav channel isoforms, some of which are thought to be involved in nociception (Gilchrist et al. 2012; Knapp et al. 2012). Subsequently, μO-conotoxins have been assessed for analgesic activity and were shown to be anti-nociceptive in various animal models of pain (Teichert et al. 2012).
Conclusions
Notwithstanding their limited availability, animal toxins have been successfully employed for many decades to investigate Nav channel function. Now more than ever, the ability to construct cDNA libraries from animal venom glands in concert with recombinant and synthetic production techniques will allow researchers to fully exploit animal peptides and probe the functional aspects of various Nav channel isoforms on a molecular level as well as investigate their physiological role in healthy and diseased tissues. Moreover, novel synthetic methods such as minimization, cyclization, and the use of diselenide bridges (Bulaj 2008) can address the challenges that may exist between the initial drug discovery phase and the clinical application of animal toxins, which will expand the use of these peptides as therapeutic drugs.
Acknowledgments
We would like to thank Kenton J Swartz and Marie-France Martin-Eauclaire for hanatoxin, and AaHII/CssIV, respectively, and Thomas Gilchrist for making silhouettes that were adapted from photographs of Takifugu rupripes (Emőke Dénes/CC-BY-SA 2.5), Phyllobates bicolor (Luis Miguel Bugallo Sánchez/CC-BY-SA 3.0), Brachypelma smithi (Fir0002/CC-BY-SA 3.0), Centruroides suffusus (Pedro Sánchez/CC-BY-SA 3.0), Androctonus australis (Kmo5ap/CC-BY-SA 3.0), Vespula germanica (Richard Bartz/CC-BY-SA 2.5), and a diagram of an anemone (Hans Hillewaert/CC-BY-SA 3.0). TTX and β-pompilidotoxin were acquired from Alomone labs, ATX-II from Sigma Aldrich, and BTX was obtained from Latoxan through Fisher Scientific.
Contributor Information
John Gilchrist, Department of Physiology, Johns Hopkins University, School of Medicine, Baltimore, MD 21205, USA.
Baldomero M. Olivera, Department of Biology, University of Utah, Salt Lake City, UT 84112, USA
Frank Bosmans, Department of Physiology, Johns Hopkins University, School of Medicine, Baltimore, MD 21205, USA; Department of Neuroscience, Solomon H. Snyder, Johns Hopkins University, School of Medicine, Baltimore, MD 21205, USA.
References
- Abe T, Kawai N, Niwa A. Purification and properties of a presynaptically acting neurotoxin, mandaratoxin, from hornet (Vespa mandarinia) Biochemistry. 1982;21(7):1693–1697. doi: 10.1021/bi00536a034. [DOI] [PubMed] [Google Scholar]
- Agnew WS, et al. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978;75(6):2606–10. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi AA, et al. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450(7168):370–5. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsen C, Harris J, Tesseraux I. Mechanical and electrophysiological effects of sea anemone (Anemonia sulcata) toxins on rat innervated and denervated skeletal muscle. Br J Pharmacol. 1981;74(1):61–71. doi: 10.1111/j.1476-5381.1981.tb09955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. Ion channels and disease. Vol. 1. Elsevier; Amsterdam: 1999. p. 481. [Google Scholar]
- Bagnis R, et al. Origins of ciguatera fish poisoning: a new dinoflagellate, Gambierdiscus toxicus Adachi and Fukuyo, definitively involved as a causal agent. Toxicon. 1980;18(2):199–208. doi: 10.1016/0041-0101(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Baldomero MO, Patrice SC, Maren W, Alexander F. Biodiversity of cone snails and other venomous marine gastropods: evolutionary success through neuropharmacology. Annu Rev Anim Biosci. 2014;2:487–513. doi: 10.1146/annurev-animal-022513-114124. [DOI] [PubMed] [Google Scholar]
- Barbier J, et al. A d-conotoxin from Conus ermineus venom inhibits inactivation in vertebrate neuronal Na+ channels but not in skeletal and cardiac muscles. J Biol Chem. 2004;279:4680–4685. doi: 10.1074/jbc.M309576200. [DOI] [PubMed] [Google Scholar]
- Barchi R, Cohen S, Murphy L. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, et al. Structure-function relationships of sea anemone toxin II from Anemonia sulcata. J Biol Chem. 1981;256(11):5764–5769. [PubMed] [Google Scholar]
- Baron A, et al. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon. 2013;75:187–204. doi: 10.1016/j.toxicon.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Benoit E, Legrand A, Dubois J. Effects of ciguatoxin on current and voltage clamped frog myelinated nerve fibre. Toxicon. 1986;24(4):357–364. doi: 10.1016/0041-0101(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Benzinger G, et al. A specific interaction between the cardiac sodium channel and site-3 toxin anthopleurin B. J Biol Chem. 1998;273(1):80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- Bidard J, et al. Ciguatoxin is a novel type of Na+ channel toxin. J Biol Chem. 1984;259(13):8353–8357. [PubMed] [Google Scholar]
- Billen B, Bosmans F, Tytgat J. Animal peptides targeting voltage-activated sodium channels. Curr Pharm Des. 2008;14(24):2492–502. doi: 10.2174/138161208785777423. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Escoubas P, Nicholson GM. Animal toxins: state of the art. In: De Lima ME, editor. Perspectives in health and biotechnology. Editora UFMG; Belo Horizonte: 2009. [Google Scholar]
- Bosmans F, Swartz KJ. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol Sci. 2010;31(4):175–182. doi: 10.1016/j.tips.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans F, et al. The poison Dart frog’s batrachotoxin modulates Nav1.8. FEBS Lett. 2004;577(1–2):245–8. doi: 10.1016/j.febslet.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Bosmans F, et al. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol Pharmacol. 2006;69(2):419–29. doi: 10.1124/mol.105.015941. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclaire MF, Swartz KJ. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456(7219):202–8. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans F, et al. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J Gen Physiol. 2011;138(1):59–72. doi: 10.1085/jgp.201110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek O, et al. Characterization of D-amino-acid-containing excitatory conotoxins and redefinition of the I-conotoxin superfamily. FEBS J. 2005a;272(16):4178–88. doi: 10.1111/j.1742-4658.2005.04830.x. [DOI] [PubMed] [Google Scholar]
- Buczek O, Bulaj G, Olivera BM. Conotoxins and the posttranslational modification of secreted gene products. CMLS. 2005b;62(24):3067–79. doi: 10.1007/s00018-005-5283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek O, et al. I(1)-superfamily conotoxins and prediction of single D-amino acid occurrence. Toxicon. 2008;51(2):218–29. doi: 10.1016/j.toxicon.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Bulaj G. Integrating the discovery pipeline for novel compounds targeting ion channels. Curr Opin Chem Biol. 2008;12(4):441–7. doi: 10.1016/j.cbpa.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulaj G, et al. d-Conotoxin structure/function through a cladistic analysis. Biochemistry. 2001;40:13201–13208. doi: 10.1021/bi010683a. [DOI] [PubMed] [Google Scholar]
- Bulaj G, et al. Novel conotoxins from Conus striatus and Conus kinoshitai selectively block TTX-resistant sodium channels. Biochemistry. 2005;44:7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- Bulaj G, et al. Synthetic μO-conotoxin MrVIB blocks TTX-resistant sodium channel Nav1.8 and has a long-lasting analgesic activity. Biochemistry. 2006;45:7404–7414. doi: 10.1021/bi060159+. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975;244(2):511–34. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Coronas F, Beirão P. Voltage-dependent displacement of the scorpion toxin Ts3 from sodium channels and its implication on the control of inactivation. Br J Pharmacol. 2004;142(7):1115–1122. doi: 10.1038/sj.bjp.0705793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, et al. beta-Scorpion toxin modifies gating transitions in all four voltage sensors of the sodium channel. J Gen Physiol. 2007;130(3):257–68. doi: 10.1085/jgp.200609719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, et al. Alpha-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J Gen Physiol. 2008;132(2):251–63. doi: 10.1085/jgp.200809995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes DL, et al. Gating transitions in the selectivity filter region of a sodium channel are coupled to the domain IV voltage sensor. Proc Natl Acad Sci U S A. 2012;109(7):2648–53. doi: 10.1073/pnas.1115575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975;250(11):4053–4059. [PubMed] [Google Scholar]
- Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall W, Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J Biol Chem. 1978;253(20):7393–7396. [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49(2):124–41. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Cestèle S, et al. Voltage sensor-trapping: enhanced activation of sodium channels by betascorpion toxin bound to the S3–S4 loop in domain II. Neuron. 1998;21(4):919–931. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Cestèle S, et al. Structure and function of the voltage sensor of sodium channels probed by a beta-scorpion toxin. J Biol Chem. 2006;281(30):21332–21344. doi: 10.1074/jbc.M603814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, et al. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 1999;22(1):73–87. doi: 10.1016/s0896-6273(00)80680-7. [DOI] [PubMed] [Google Scholar]
- Chanda B, Bezanilla F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J Gen Physiol. 2002;120(5):629–45. doi: 10.1085/jgp.20028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25(5):280–9. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Tomiko SA, Agnew WS. Reconstituted voltage-sensitive sodium channel from Electrophorus electricus: chemical modifications that alter regulation of ion permeability. Proc Natl Acad Sci U S A. 1987;84(17):6282–6. doi: 10.1073/pnas.84.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo G, et al. Solution structure and alanine scan of a spider toxin that affects the activation of mammalian voltage-gated sodium channels. J Biol Chem. 2007;282(7):4643–52. doi: 10.1074/jbc.M605403200. [DOI] [PubMed] [Google Scholar]
- Couraud F, et al. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, et al. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- Cummins TR, et al. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 1999;19(24):RC43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly NL, et al. Structures of muO-conotoxins from Conus marmoreus. I nhibitors of tetrodotoxin (TTX)-sensitive and TTX-resistant sodium channels in mammalian sensory neurons. J Biol Chem. 2004;279(24):25774–82. doi: 10.1074/jbc.M313002200. [DOI] [PubMed] [Google Scholar]
- de la Vega R, Vega RC, Possani LD. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure-function relationships and evolution. Toxicon. 2005;46(8):831–44. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Du Y, et al. Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel. J Biol Chem. 2011;286(15):13151–60. doi: 10.1074/jbc.M110.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbacher J, Spande T, Daly J. Batrachotoxin alkaloids from passerine birds: a second toxic bird genus (Ifrita kowaldi) from New Guinea. Proc Natl Acad Sci U S A. 2000;97(24):12970–12975. doi: 10.1073/pnas.200346897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbacher J, et al. Melyrid beetles (Choresine): a putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds. Proc Natl Acad Sci U S A. 2004;101(45):15857–15860. doi: 10.1073/pnas.0407197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton GB, Blumenthal KM, Hanck DA. Evidence for multiple effects of ProTxII on activation gating in Na(V)1.5. Toxicon. 2008;52(3):489–500. doi: 10.1016/j.toxicon.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg J, et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci U S A. 2006;103(45):17030–5. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, et al. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol. 2002;62(1):48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- Fainzilber M, et al. New sodium channel blocking conotoxins also affect calcium currents in Lymnaea neurons. Biochemistry. 1995;34:5364–5371. doi: 10.1021/bi00016a007. [DOI] [PubMed] [Google Scholar]
- Felix JP, et al. Functional assay of voltage-gated sodium channels using membrane potential-sensitive dyes. Assay Drug Dev Technol. 2004;2(3):260–8. doi: 10.1089/1540658041410696. [DOI] [PubMed] [Google Scholar]
- Fiedler B, et al. Specificity, affinity and efficacy of iota-conotoxin RXIA, an agonist of voltage-gated sodium channels Na(V)1.2, 1.6 and 1.7. Biochem Pharmacol. 2008;75(12):2334–44. doi: 10.1016/j.bcp.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard HA, Lipkind GM. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Marine Drugs. 2010;8(2):219–34. doi: 10.3390/md8020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Sasaoka T, Hosoya Y. Effects of tetrodotoxin on the neuromuscular junction. Jpn J Physiol. 1959;9(2):143–52. doi: 10.2170/jjphysiol.9.143. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Blumenthal K. Importance of the unique cationic residues arginine 12 and lysine 49 in the activity of the cardiotonic polypeptide anthopleurin B. J Biol Chem. 1994;269(1):254–259. [PubMed] [Google Scholar]
- Gilchrist J, Bosmans F. Animal toxins can alter the function of Nav1.8 and Nav1.9. Toxins. 2012;4(8):620–32. doi: 10.3390/toxins4080620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, et al. Flux assays in high throughput screening of ion channels in drug discovery. Assay Drug Dev Technol. 2003;1(5):709–17. doi: 10.1089/154065803770381066. [DOI] [PubMed] [Google Scholar]
- Gooley P, Blunt J, Norton R. Conformational heterogeneity in polypeptide cardiac stimulants from sea anemones. FEBS Lett. 1984;174(1):15–19. doi: 10.1016/0014-5793(84)81068-6. [DOI] [PubMed] [Google Scholar]
- Hanck D, Sheets M. Site-3 toxins and cardiac sodium channels. Toxicon. 2007;49(2):181–193. doi: 10.1016/j.toxicon.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R, Catterall W. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R, et al. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J Biol Chem. 1982;257(23):13888–13891. [PubMed] [Google Scholar]
- Hasson A, et al. Alteration of sodium currents by new peptide toxins from the venom of a molluscivorous Conus snail. Eur J Neurosci. 1993;5(1):56–64. doi: 10.1111/j.1460-9568.1993.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Hille B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys J. 1975;15(6):615–619. doi: 10.1016/S0006-3495(75)85842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd. Vol. 1. Sinauer Associates; Sunderland, MA: 2001. p. 814. [Google Scholar]
- Hillyard DR, et al. A molluscivorous Conus toxin: conserved frameworks in conotoxins. Biochemistry. 1989;28(1):358–61. doi: 10.1021/bi00427a049. [DOI] [PubMed] [Google Scholar]
- Hirama M, et al. Total synthesis of ciguatoxin CTX3C. Science (New York, NY) 2001;294(5548):1904–1907. doi: 10.1126/science.1065757. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Propagation of electrical signals along giant nerve fibers. Proc R Soc Lond B Biol Sci. 1952;140(899):177–83. doi: 10.1098/rspb.1952.0054. [DOI] [PubMed] [Google Scholar]
- Holford M, et al. Pruning nature: Biodiversity-derived discovery of novel sodium channel blocking conotoxins from Conus bullatus. Toxicon. 2009;53(1):90–8. doi: 10.1016/j.toxicon.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Marine Biotechnol. 2006;8(1):1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Ding S, Gruber HJ. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116(3):461–76. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Moran N, Ehrenstein G. Batrachotoxin modifies the gating kinetics of sodium channels in internally perfused neuroblastoma cells. Proc Natl Acad Sci U S A. 1982;79(6):2082–2085. doi: 10.1073/pnas.79.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423(6935):33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, et al. Novel excitatory Conus peptides define a new conotoxin superfamily. J Neurochem. 2003;85(3):610–21. doi: 10.1046/j.1471-4159.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- Jover E, et al. Scorpion toxin: specific binding to rat synaptosomes. Biochem Biophys Res Commun. 1978;85(1):377–382. doi: 10.1016/s0006-291x(78)80053-9. [DOI] [PubMed] [Google Scholar]
- Kem W, et al. Isolation, characterization, and amino acid sequence of a polypeptide neurotoxin occurring in the sea anemone Stichodactyla helianthus. Biochemistry. 1989;28(8):3483–3489. doi: 10.1021/bi00434a050. [DOI] [PubMed] [Google Scholar]
- Khera P, Blumenthal K. Importance of highly conserved anionic residues and electrostatic interactions in the activity and structure of the cardiotonic polypeptide anthopleurin B. Biochemistry. 1996;35(11):3503–3507. doi: 10.1021/bi9528457. [DOI] [PubMed] [Google Scholar]
- Kinoshita E, et al. Novel wasp toxin discriminates between neuronal and cardiac sodium channels. Molecular pharmacology. 2001;59(6):1457–1463. [PubMed] [Google Scholar]
- Knapp O, McArthur JR, Adams DJ. Conotoxins targeting neuronal voltage-gated sodium channel subtypes: potential analgesics? Toxins. 2012;4(11):1236–60. doi: 10.3390/toxins4111236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno K, et al. Isolation and structure of pompilidotoxins, novel peptide neurotoxins in solitary wasp venoms. Biochem Biophys Res Commun. 1998;250(3):612–616. doi: 10.1006/bbrc.1998.9299. [DOI] [PubMed] [Google Scholar]
- Konno K, et al. Molecular determinants of binding of a wasp toxin (PMTXs) and its analogs in the Na+ channels proteins. Neurosci Lett. 2000;285(1):29–32. doi: 10.1016/s0304-3940(00)01017-x. [DOI] [PubMed] [Google Scholar]
- Koppenhofer E, Schmidt H. Effect of scorpion venom on ionic currents of the node of Ranvier. I. The permeabilities PNa and PK. Pflugers Arch. 1968a;303(2):133–49. doi: 10.1007/BF00592631. [DOI] [PubMed] [Google Scholar]
- Koppenhofer E, Schmidt H. Effect of scorpion venom on ionic currents of the node of Ranvier. II. Incomplete sodium inactivation. Pflugers Arch. 1968b;303(2):150–61. doi: 10.1007/BF00592632. [DOI] [PubMed] [Google Scholar]
- Kuang Z, Zhang M-M, Gupta K, Gajewiak J, Gulyas J, Balaram P, Rivier JE, Olivera BM, Yoshikami D, Bulaj G, Norton RS. Mammalian neuronal sodium channel blocker μ-conotoxin BuIIIB has a structured N-terminus that influences potency. ACS Chem Biol. 2013;8(6):1344–1351. doi: 10.1021/cb300674x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, et al. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry. 2004;43(4):890–7. doi: 10.1021/bi0353373. [DOI] [PubMed] [Google Scholar]
- Leffler A, et al. Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue. Pflugers Archiv. 2005;451(3):454–63. doi: 10.1007/s00424-005-1463-x. [DOI] [PubMed] [Google Scholar]
- Legrand AM, et al. Isolation and some properties of ciguatoxin. J Appl Phycol. 1989;1:183–188. [Google Scholar]
- Leipold E, et al. Molecular interaction of delta-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005;579(18):3881–4. doi: 10.1016/j.febslet.2005.05.077. [DOI] [PubMed] [Google Scholar]
- Leipold E, et al. Subtype specificity of scorpion beta-toxin Tz1 interaction with voltage-gated sodium channels is determined by the pore loop of domain 3. Mol Pharmacol. 2006;70(1):340–347. doi: 10.1124/mol.106.024034. [DOI] [PubMed] [Google Scholar]
- Leipold E, et al. μO conotoxins inhibit NaV channels by interfering with their voltage sensors in domain-2. Channels (Austin) 2007;1(4):253–62. doi: 10.4161/chan.4847. [DOI] [PubMed] [Google Scholar]
- Leipold E, et al. Molecular determinants for the subtype specificity of mu-conotoxin SIIIA targeting neuronal voltage-gated sodium channels. Neuropharmacology. 2011;61(1–2):105–11. doi: 10.1016/j.neuropharm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Leipold E, Borges A, Heinemann SH. Scorpion beta-toxin interference with NaV channel voltage sensor gives rise to excitatory and depressant modes. J Gen Physiol. 2012;139(4):305–19. doi: 10.1085/jgp.201110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R, et al. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae) Toxicon. 1991;29(9):1115–1127. doi: 10.1016/0041-0101(91)90209-a. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, et al. Isolation and structure-activity of mu-conotoxin TIIIA, a potent inhibitor of tetrodotoxin-sensitive voltage-gated sodium channels. Mol Pharmacol. 2007;71(3):676–85. doi: 10.1124/mol.106.028225. [DOI] [PubMed] [Google Scholar]
- Li D, et al. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana. FEBS Lett. 2003;555(3):616–22. doi: 10.1016/s0014-5793(03)01303-6. [DOI] [PubMed] [Google Scholar]
- Liao Z, et al. Solution structure of Jingzhaotoxin-III, a peptide toxin inhibiting both Nav1.5 and Kv2.1 channels. Toxicon. 2007;50(1):135–43. doi: 10.1016/j.toxicon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Linford N, et al. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci U S A. 1998;95(23):13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind G, Fozzard H. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J. 1994;66(1):1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Voltage-gated Na channel selectivity: the role of the conserved domain III lysine residue. J Gen Physiol. 2008;131(6):523–9. doi: 10.1085/jgp.200809991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn L, Bell P, Moczydlowski E. Phylogenetic survey of soluble saxitoxin-binding activity in pursuit of the function and molecular evolution of saxiphilin, a relative of transferrin. Proc R Soc. 1997;264(1383):891–902. doi: 10.1098/rspb.1997.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn L, Doyle J, Negri A. A high-throughput, microtiter plate assay for paralytic shellfish poisons using the saxitoxin-specific receptor, saxiphilin. Anal Biochem. 1998;261(1):51–56. doi: 10.1006/abio.1998.2707. [DOI] [PubMed] [Google Scholar]
- Lombet A, Bidard J, Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987;219(2):355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Long SB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–82. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Marcotte P, et al. Effects of Tityus serrulatus scorpion toxin gamma on voltage-gated Na+ channels. Circ Res. 1997;80(3):363–9. doi: 10.1161/01.res.80.3.363. [DOI] [PubMed] [Google Scholar]
- Martin-Eauclaire MF, Couraud F. Scorpion neurotoxins: effects and mechanisms. In: Chang LW, Dyer RS, editors. Handk neurotoxicology. Marcel-Dekker; New York, NY: 1992. [Google Scholar]
- M’Barek S, et al. First chemical synthesis of a scorpion alpha-toxin affecting sodium channels: the Aah I toxin of Androctonus australis hector. J Peptide Sci. 2004;10(11):666–677. doi: 10.1002/psc.582. [DOI] [PubMed] [Google Scholar]
- McArthur JR, et al. Interactions of key charged residues contributing to selective block of neuronal sodium channels by mu-conotoxin KIIIA. Mol Pharmacol. 2011;80(4):573–84. doi: 10.1124/mol.111.073460. [DOI] [PubMed] [Google Scholar]
- McCusker EC, et al. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun. 2012;3:1102. doi: 10.1038/ncomms2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, et al. A new family of conotoxins that blocks voltage-gated sodium channels. J Biol Chem. 1995;270:16796–16802. doi: 10.1074/jbc.270.28.16796. [DOI] [PubMed] [Google Scholar]
- Mebs D. Venomous and poisonous animals: a handbook for biologists, toxicologists and toxinologists, physicians and pharmacists. 1st. Medpharm; Lyttelton, New Zealand: 2002. p. 360. [Google Scholar]
- Michio M, et al. Structures of ciguatoxin and its congener. J Am Chem Soc. 1989;111:8289–8931. [Google Scholar]
- Middleton RE, et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry. 2002;41(50):14734–47. doi: 10.1021/bi026546a. [DOI] [PubMed] [Google Scholar]
- Milescu M, et al. Tarantula toxins interact with voltage sensors within lipid membranes. J Gen Physiol. 2007;130(5):497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu M, et al. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat Struct Mol Biol. 2009;16(10):1080–1085. doi: 10.1038/nsmb.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Agnew W, Levinson S. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Moore JW, et al. Basis of tetrodotoxin’s selectivity in blockage of squid axons. J Gen Physiol. 1967;50(5):1401–11. doi: 10.1085/jgp.50.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito M, Moczydlowski E. Molecular cloning of bullfrog saxiphilin: a unique relative of the transferrin family that binds saxitoxin. Proc Natl Acad Sci U S A. 1994;91(7):2478–2482. doi: 10.1073/pnas.91.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974;54(4):813–89. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol. 1964;47:965–74. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Anderson N, Moore J. Comparison of tetrodotoxin and procaine in internally perfused squid giant axons. J Gen Physiol. 1967;50(5):1413–1428. doi: 10.1085/jgp.50.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FR, et al. Tetrodotoxin (TTX) as a therapeutic agent for pain. Marine Drugs. 2012;10(2):281–305. doi: 10.3390/md10020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton R. Structures of sea anemone toxins. Toxicon. 2009;54(8):1075–1088. doi: 10.1016/j.toxicon.2009.02.035. [DOI] [PubMed] [Google Scholar]
- Norton RS, Pennington MW, Wulff H. Potassium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and other autoimmune diseases. Curr Med Chem. 2004;11(23):3041–52. doi: 10.2174/0929867043363947. [DOI] [PubMed] [Google Scholar]
- Nunes KP, et al. New insights on arthropod toxins that potentiate erectile function. Toxicon. 2013;69:152–9. doi: 10.1016/j.toxicon.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Oliveira J, et al. Binding specificity of sea anemone toxins to Nav 1.1–1.6 sodium channels: unexpected contributions from differences in the IV/S3–S4 outer loop. J Biol Chem. 2004;279(32):33323–33335. doi: 10.1074/jbc.M404344200. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus venom peptides, receptor and ion channel targets and drug design: 50 million years of neuropharmacology (E.E. Just Lecture, 1996) Mol Biol Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, et al. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–8. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, et al. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486(7401):135–9. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LR, et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature. 2005;436(7052):857–60. doi: 10.1038/nature03873. [DOI] [PubMed] [Google Scholar]
- Quandt F, Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982;79(21):6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard Benzinger G, Tonkovich G, Hanck D. Augmentation of recovery from inactivation by site-3 Na channel toxins. A single-channel and whole-cell study of persistent currents. J Gen Physiol. 1999;113(2):333–346. doi: 10.1085/jgp.113.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, et al. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem. 1996;271(27):15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Romey G, et al. Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta V, MacKinnon R. Localization of the voltage-sensor toxin receptor on KvAP. Biochemistry. 2004;43(31):10071–9. doi: 10.1021/bi049463y. [DOI] [PubMed] [Google Scholar]
- Sahara Y, et al. A new class of neurotoxin from wasp venom slows inactivation of sodium current. Eur J Neurosci. 2000;12(6):1961–1970. doi: 10.1046/j.1460-9568.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- Schantz E, et al. Letter: The structure of saxitoxin. J Am Chem Soc. 1975;97(5):1238. doi: 10.1021/ja00838a045. [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, et al. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74(5):1476–84. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- Seibert A, et al. Arg-14 loop of site 3 anemone toxins: effects of glycine replacement on toxin affinity. Biochemistry. 2003;42(49):14515–14521. doi: 10.1021/bi035291d. [DOI] [PubMed] [Google Scholar]
- Sheets MF, et al. The Na channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys J. 1999;77(2):747–57. doi: 10.1016/S0006-3495(99)76929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Kyle JW, Hanck DA. The role of the putative inactivation lid in sodium channel gating current immobilization. J Gen Physiol. 2000;115(5):609–20. doi: 10.1085/jgp.115.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif N, Fozzard H, Hanck D. Dose-dependent modulation of the cardiac sodium channel by sea anemone toxin ATXII. Circul Res. 1992;70(2):285–301. doi: 10.1161/01.res.70.2.285. [DOI] [PubMed] [Google Scholar]
- Shon KJ, et al. Delta-conotoxin GmVIA, a novel peptide from the venom of Conus gloriamaris. Biochemistry. 1994;33(38):11420–5. doi: 10.1021/bi00204a003. [DOI] [PubMed] [Google Scholar]
- Shon K, et al. Purification, characterization and cloning of the lockjaw peptide from Conus purpurascens venom. Biochemistry. 1995;34:4913–4918. doi: 10.1021/bi00015a002. [DOI] [PubMed] [Google Scholar]
- Shon K, et al. μ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J Neurosci. 1998;18:473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AO, et al. Inhibitory effect of the recombinant Phoneutria nigriventer Tx1 toxin on voltage-gated sodium channels. Biochimie. 2012;94(12):2756–63. doi: 10.1016/j.biochi.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, et al. A single serine residue confers tetrodotoxin insensitivity on the rat sensory-neuron-specific sodium channel SNS. FEBS Lett. 1997;409(1):49–52. doi: 10.1016/s0014-5793(97)00479-1. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Blumenthal KM. Site-3 sea anemone toxins: molecular probes of gating mechanisms in voltage-dependent sodium channels. Toxicon. 2007;49(2):159–70. doi: 10.1016/j.toxicon.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Smith JJ, et al. Differential phospholipid binding by site 3 and site 4 toxins. Implications for structural variability between voltage-sensitive sodium channel domains. J Biol Chem. 2005;280(12):11127–33. doi: 10.1074/jbc.M412552200. [DOI] [PubMed] [Google Scholar]
- Smith JJ, et al. Molecular interactions of the gating modifier toxin ProTx-II with NaV 1.5: implied existence of a novel toxin binding site coupled to activation. J Biol Chem. 2007;282(17):12687–97. doi: 10.1074/jbc.M610462200. [DOI] [PubMed] [Google Scholar]
- Sokolov S, et al. Inhibition of sodium channel gating by trapping the domain II voltage sensor with protoxin II. Mol Pharmacol. 2008;73(3):1020–8. doi: 10.1124/mol.107.041046. [DOI] [PubMed] [Google Scholar]
- Stroud MR, Hansen SJ, Olson JM. In vivo bio-imaging using chlorotoxin-based conjugates. Curr Pharmaceut Design. 2011;17(38):4362–71. doi: 10.2174/138161211798999375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarslal S, et al. Sodium channel modulating activity in a delta-conotoxin from an Indian marine snail. FEBS letters. 2003;553(1–2):209–12. doi: 10.1016/s0014-5793(03)01016-0. [DOI] [PubMed] [Google Scholar]
- Swartz KJ. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 2007;49(2):213–30. doi: 10.1016/j.toxicon.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KJ. Sensing voltage across lipid membranes. Nature. 2008;456(7224):891–7. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KJ, MacKinnon R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997a;18(4):665–73. doi: 10.1016/s0896-6273(00)80306-2. [DOI] [PubMed] [Google Scholar]
- Swartz KJ, MacKinnon R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron. 1997b;18(4):675–82. doi: 10.1016/s0896-6273(00)80307-4. [DOI] [PubMed] [Google Scholar]
- Teichert RW, et al. Characterization of two neuronal subclasses through constellation pharmacology. Proceedings of the National Academy of Sciences. 2012 Jul 9; doi: 10.1073/pnas.1209759109. PNAS Early Edition: p. Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F, Catterall W. Site of covalent attachment of alpha-scorpion toxin derivatives in domain I of the sodium channel alpha subunit. Proc Natl Acad Sci U S A. 1988;85(22):8742–8746. doi: 10.1073/pnas.85.22.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84(1):41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Terlau H, et al. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991;293(1–2):93–96. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- Terlau H, et al. Strategy for rapid immobilization of prey by a fish-hunting cone snail. Nature. 1996;381:148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- Thomsen W, Catterall W. Localization of the receptor site for alpha-scorpion toxins by antibody mapping: implications for sodium channel topology. Proc Natl Acad Sci U S A. 1989;86(24):10161–10165. doi: 10.1073/pnas.86.24.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama T, Daly J, Witkop B. The structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, Phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs. J Am Chem Soc. 1969;91(14):3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]
- Trainer V, Baden D, Catterall W. Identification of peptide components of the brevetoxin receptor site of rat brain sodium channels. J Biol Chem. 1994;269(31):19904–19909. [PubMed] [Google Scholar]
- Ujihara S, et al. Interaction of ladder-shaped polyethers with transmembrane alpha-helix of glycophorin A as evidenced by saturation transfer difference NMR and surface plasmon resonance. Bioorg Med Chem Lett. 2008;18(23):6115–8. doi: 10.1016/j.bmcl.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev Physiol Biochem Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- Van Der Haegen A, Peigneur S, Tytgat J. Importance of position 8 in μ-conotoxin KIIIA for voltage-gated sodium channel selectivity. FEBS J. 2011;278:3408–3418. doi: 10.1111/j.1742-4658.2011.08264.x. [DOI] [PubMed] [Google Scholar]
- Vita C, et al. Scorpion toxins as natural scaffolds for protein engineering. Proc Natl Acad Sci U S A. 1995;92(14):6404–6408. doi: 10.1073/pnas.92.14.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walewska A, et al. NMR-based mapping of disulfide bridges in cysteine-rich peptides: application to the mu-conotoxin SxIIIA. J Am Chem Soc. 2008;130(43):14280–6. doi: 10.1021/ja804303p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang G. Point mutations in segment I-S6 render voltage-gated Na+ channels resistant to batrachotoxin. Proc Natl Acad Sci U S A. 1998;95(5):2653–2658. doi: 10.1073/pnas.95.5.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang SY. Local anesthetic block of batrachotoxin-resistant muscle Na+ channels. Mol Pharmacol. 1998;54(2):389–96. doi: 10.1124/mol.54.2.389. [DOI] [PubMed] [Google Scholar]
- Wang S, Nau C, Wang G. Residues in Na(+) channel D3–S6 segment modulate both batrachotoxin and local anesthetic affinities. Biophys J. 2000;79(3):1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. JZTX-IV, a unique acidic sodium channel toxin isolated from the spider Chilobrachys jingzhao. Toxicon. 2008;52(8):871–80. doi: 10.1016/j.toxicon.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor. Proc Natl Acad Sci U S A. 2011;108(37):15426–15431. doi: 10.1073/pnas.1112320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstrom J, et al. Modification of cardiac Na+ channels by batrachotoxin: effects on gating, kinetics, and local anesthetic binding. Biophys J. 1993;65(1):386–395. doi: 10.1016/S0006-3495(93)81046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West PJ, et al. μ-Conotoxin SmIIIA, a potent inhibitor of TTX-resistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41:15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- West PJ, Bulaj G, Yoshikami D. Effects of delta-conotoxins PVIA and SVIE on sodium channels in the amphibian sympathetic nervous system. J Neurophysiol. 2005;94(6):3916–24. doi: 10.1152/jn.01304.2004. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, et al. μ-conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proc Natl Acad Sci U S A. 2011;108(25):10302–7. doi: 10.1073/pnas.1107027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. The structure of tetrodotoxin. Pure Appl Chem. 1964;9:49–74. [Google Scholar]
- Xiao Y, et al. Jingzhaotoxin-I, a novel spider neurotoxin preferentially inhibiting cardiac sodium channel inactivation. J Biol Chem. 2005;280(13):12069–76. doi: 10.1074/jbc.M411651200. [DOI] [PubMed] [Google Scholar]
- Xiao Y, et al. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain ii voltage sensor in the closed configuration. J Biol Chem. 2008;283(40):27300–13. doi: 10.1074/jbc.M708447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, et al. The tarantula toxins ProTx-II and huwentoxin-IV differentially interact with human Nav1.7 voltage sensors to inhibit channel activation and inactivation. Mol Pharmacol. 2010;78(6):1124–34. doi: 10.1124/mol.110.066332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, et al. Common molecular determinants of tarantula huwentoxin-IV inhibition of Na+ channel voltage sensors in domains II and IV. J Biol Chem. 2011;286(31):27301–10. doi: 10.1074/jbc.M111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, et al. Energetic role of the paddle motif in voltage gating of Shaker K(+) channels. Nat Struct Mol Biol. 2013;20(5):574–81. doi: 10.1038/nsmb.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K, et al. Synthetic ciguatoxins selectively activate Nav1.8-derived chimeric sodium channels expressed in HEK293 cells. J Biol Chem. 2009;284(12):7597–7605. doi: 10.1074/jbc.M806481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Yeng L, et al. Isolation and structure of brevetoxin B from the “red tide” dinoflagellate Ptychodiscus brevis (Gymnodinium breve) J Am Chem Soc. 1981;103:6773–6776. [Google Scholar]
- Zhang MM, et al. Structural and functional diversities among mu-conotoxins targeting TTX-resistant sodium channels. Biochemistry. 2006;45(11):3723–32. doi: 10.1021/bi052162j. [DOI] [PubMed] [Google Scholar]
- Zhang MM, et al. Structure/function characterization of micro-conotoxin KIIIA, an analgesic, nearly irreversible blocker of mammalian neuronal sodium channels. J Biol Chem. 2007;282(42):30699–706. doi: 10.1074/jbc.M704616200. [DOI] [PubMed] [Google Scholar]
- Zhang MM, et al. Synergistic and antagonistic interactions between tetrodotoxin and mu-conotoxin in blocking voltage-gated sodium channels. Channels (Austin) 2009;3(1):32–8. doi: 10.4161/chan.3.1.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, et al. Cooccupancy of the outer vestibule of voltage-gated sodium channels by micro-conotoxin KIIIA and saxitoxin or tetrodotoxin. J Neurophysiol. 2010;104:88–97. doi: 10.1152/jn.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486(7401):130–4. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, et al. Co-expression of Na(V) beta-subunits alters the kinetics of inhibition of voltage-gated sodium channels by pore-blocking mu-conotoxins. Br J Pharmacol. 2013a;168:1597–1610. doi: 10.1111/bph.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Wilson MJ, Gajewiak J, Rivier JE, Bulaj G, Olivera BM, Yoshikami D. Pharmacological fractionation of tetrodotoxin-sensitive sodium currents in rat dorsal root ganglion neurons by μ-conotoxins. Br J Pharmacol. 2013;169(1):102–14. doi: 10.1111/bph.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn S, et al. The muO-conotoxin MrVIA inhibits voltage-gated sodium channels by associating with domain-3. FEBS Lett. 2006;580(5):1360–4. doi: 10.1016/j.febslet.2006.01.057. [DOI] [PubMed] [Google Scholar]


