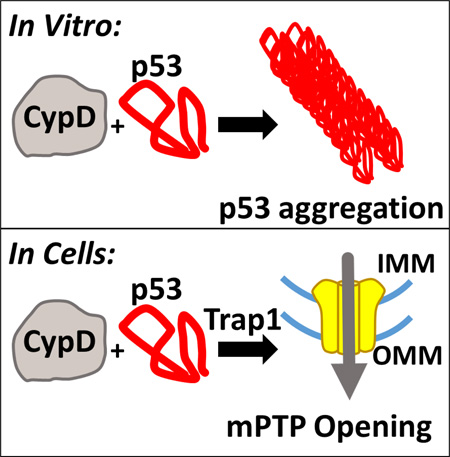
Abstract
Tissue necrosis as a consequence of ischemia-reperfusion injury and oxidative damage is a leading cause of permanent disability and death worldwide. The complete mechanism by which cells undergo necrosis upon oxidative stress is not understood. In response to an oxidative insult, wildtype p53 has been implicated as a central regulatory component of the mitochondrial permeability transition (mPT), triggering necrosis. This process is associated with cellular stabilization and translocation of p53 into the mitochondrial matrix. Here, we probe the mechanism by which p53 activates the key mPT regulator cyclophilin D (CypD). We explore the involvement of Trap1, an Hsp90-related mitochondrial matrix protein and member of the mitochondrial unfolded protein response (mtUPR), and its ability to suppress mPT in a p53-dependent manner. Our study finds that catalytically active CypD causes strong aggregation of wildtype p53 protein (both full length and isolated DNA binding domain) into amyloid-type fibrils in vitro. The responsible CypD residues for this activity were mapped by NMR to the active site amino acids R55, F60, F113 and W121. The data also present a new proline-isomerization assay for CypD by monitoring the aggregation of p53 as an indicator of CypD activity. Moreover, we find that inhibition of Trap1 by the mitochondria-specific HSP90 ATPase antagonist gamitrinib strongly sensitizes primary mouse embryonic fibroblasts (MEFs) to mPT and permeability transition pore (mPTP) opening in a p53- and CypD-dependent manner. We propose a mechanism by which influx of unfolded p53 into the mitochondrial matrix in response to oxidative stress indirectly activates the normally inhibited CypD by displacing it from Trap1 complexes. This activates CypD’s isomerase activity. Liberated CypD then isomerizes multiple proteins including p53 (causing p53 aggregation) and the structural components of the mPTP pore, inducing pore opening. This working model can now be tested in the future.
Keywords: ischemia/reperfusion, necrosis, cyclophilin D, gamitrinib, mitochondrial heat-shock proteins
Graphical Abstract
1. Introduction
Ischemia/Reperfusion (IR) injury can cause irreversible tissue damage and necrosis in vital organs such as brain (ischemic or hemorrhagic stroke) and heart (myocardial infarction), and reperfusion injury is a leading cause of permanent disability and death worldwide[1]. Ischemia/Reperfusion injury leads to increased intracellular concentrations of reactive oxygen species (ROS) and increased cytosolic Ca2+ influx into the mitochondrial matrix[2]. Together, these events lead to dissipation and collapse of the actively maintained electrochemical proton gradient (ΔΨm) across the inner mitochondrial membrane due to the sudden opening of the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane. The mPTP is an elusive non-selective pore for small solutes and water that under normal physiologic conditions is impermeable but opens abruptly in response to prolonged oxidative stress, an event called mitochondrial permeability transition (mPT)[2], [3]. This causes ion influx that dissipates ΔΨm and shuts down oxidative phosphorylation and ATP production, ending in catastrophic energetic failure[3]. Concomitantly, water influx causes matrix swelling, rupture of the rigid outer mitochondrial membrane (OMM) and release of all sequestered cell death factors from the intermembranous space. Recent studies identified several key players involved in mitochondrial permeability transition. They include the central regulatory protein cyclophilin D (CypD, gene name PPIF, UniProt: P30405, PDB: 2Z6W, not to be confused with the larger 40 kDa nuclear and cytosolic CypD otherwise known as CyP40 (Supplemental Figure 1a)), an exclusively mitochondrial matrix prolyl-isomerase whose enzymatic activity is essential to trigger mPT [4],[5], [6] , and the stress sensor p53 (TP53) under conditions of oxidative damage [7]–[12]. Structural pore components encompass the c-subunit ring[13],[14] and the oligomycin-sensitivity conferring protein (OSCP)[15] of the F1Fo-ATP synthase, and more recently the product of the Spastic Paraplegia 7 gene (SPG7) [16]. Despite these advances, the full assembly of the mPTP is not understood and more importantly, the mechanism by which pore opening is triggered remains unknown.
p53 is an extensively studied nuclear transcription factor that acts as a potent tumor suppressor by rapidly and broadly responding to DNA damage signals via activating genes involved in cell cycle arrest or senescence, DNA repair and apoptosis as effective anti-cancer mechanisms. Moreover, wildtype p53 protein has additional pro-death functions directly at the mitochondria, including driving transcription-independent apoptosis[17].
Recent work has shown that a fraction of stress-induced p53 protein translocates to mitochondria to interact with multiple members of the Bcl2 family and directly drive apoptosis through permeabilization of the outer mitochondrial membrane[18]. A long-standing paradigm had been that p53 controls apoptosis but plays no role in necrosis. However, in response to oxidative stress we recently established a necrotic mitochondrial p53 program[7]. We showed that upon hypoxia and oxidative stress, cytoplasmic p53 translocates into the mitochondrial matrix and participates in necrosis by triggering mPT through interaction with CypD[7]. While the apoptotic p53 cell death program requires Bax and Bak, the necrotic p53 cell death program requires CypD: purified p53 protein opens the PTP pore in isolated mitochondria independent of Bax and Bak but dependent on CypD[7]. Conversely, p53−/− MEFs are protected from oxidative stress-induced mPT and mPTP opening. Direct targeting of p53 to mitochondrial matrix induces mPT and necrosis in a CypD-dependent manner, and oxidative stress-induced PTP opening and necrosis is largely transcription-independent. Intriguingly, a robust p53-CypD complex forms during mouse brain ischemia/reperfusion injury (stroke model). In contrast, reduction of p53 levels or cyclosporine A pretreatment of mice prevents this complex and correlates with effective stroke protection [7]. However, details of the p53•CypD interaction and the mechanism by which p53 triggers opening of the mPTP have yet to be investigated.
The major event during ischemia/reperfusion injury leading to sudden collapse of mitochondrial function and energy catastrophe is the formation and prolonged opening of the mPTP following the insult by ROS and/or Ca2+. While it has been shown that the mPTP can be transiently opened and closed to serve as a physiologic Ca2+ release channel[19], it is the prolonged opening of the pore that is required for necrotic cell death[20]. The exact proteins that make up the mPTP have evaded researchers for over two decades and the only genetically proven and consistently indispensable component has been the obligatory regulatory factor CypD [4],[5],[6]. Genetic deletion of CypD[21]–[23] or inhibition of CypD with the highly specific pan-cyclophilin inhibitor cyclosporine A (CsA)[7], [24] prevents mPTP opening and mPT in vivo. It is thought that the structural pore component of the mPTP itself is composed of the c-subunit ring of the F1Fo-ATP synthase[14] and that CypD binds distally to the OSCP subunit of the F1Fo-ATP synthase[13]. Binding of CypD to OSCP is hypothesized to increase the PTP’s apparent affinity for Ca2+, in turn sensitizing the pore to opening[20].
Over 1500 different proteins can be found in mammalian mitochondria[25]. However, the mitochondrial genome only harbors 13 protein-coding genes[26], indicating that the vast majority of mitochondrial proteins including p53 and CypD are encoded in the nucleus and imported from the cytosol. As such, mitochondria heavily depend on chaperone proteins to maintain proper protein-folding homeostasis[27]. These mitochondrial chaperones are also regulators of mitochondrial permeability transition, contributing to a cytoprotective chaperone network that antagonizes CypD-dependent cell death[28]–[32]. Conversely, and consistent with this vital role, mitochondrial chaperones are involved in the mitochondrial unfolded protein response (mtUPR)[33] that is triggered by a variety of mitochondrial stressors including ROS[34]. Of note, both CypD and p53 interact with members of the mtUPR including mitochondrial mtHSP60[28], [35], mtHSP90[36] and the mitochondrial HSP90 homologue Trap1[29]. These interactions beckon the question of whether or not mitochondrial chaperones may also be involved in p53-mediated mPT.
To better understand the mechanism how p53 is involved in oxidative stress-induced opening of the mPTP, complementary biophysical methods were employed here to explore the p53•CypD interaction. We find that CypD and p53 engage in a transient enzyme-substrate interaction and not in a stable protein-protein complex. Rather, the isomerase activity of CypD leads to the aggregation of its substrate, p53. NMR and biochemical methods were used to identify catalytic CypD residues directly involved in aggregation of p53. We monitor CypD induced p53 aggregation in a continuous spectrophotometric assay and by co-aggregation assays. We explored the nature of the insoluble p53 aggregates formed by interaction with CypD using transmission electron microscopy (TEM). To explore the involvement of Trap1 in p53-mediated mPT, mitochondrial calcein retention assays were used to directly monitor opening of the mPTP in WT, p53−/− and CypD−/− MEFs by FACS in the presence or absence of H2O2 stress and gamitrinib, a specific Trap1 inhibitor. We find that CypD causes aggregation of p53 into amyloid-like fibrils and that inhibition of Trap1 sensitizes cells to mPT in a p53-dependent manner. This suggests that stress-imported matrix p53 is responsible for modulating mPT by cooperating with members of the mtUPR, possibly activating CypD by its competitive displacement from chaperone interaction.
Results
NMR chemical shift perturbations reveal that catalytic CypD residues are responsible for p53 aggregation
We previously showed by pulldown of GST-tagged proteins and co-immunoprecipitation experiments that p53 and CypD interact in mouse embryonic fibroblasts (MEFs), human HCT116 colon cancer cell lines and in acutely infarcted mouse brain tissue[7]. Mapping experiments by using a truncation series of p53 proteins in pulldown experiments with CypD showed that CypD interacts with p53 residues 80–220, corresponding to the N-terminal portion of the DNA Binding Domain of p53 (p53DBD, residues 94–312)[7]. To further characterize the interaction and identify the key residues of CypD responsible for interacting with p53DBD, we compared the chemical shift changes of isotopically-labeled CypD upon addition of p53DBD purified from Sf9 cells using 1H- 15N HSQC NMR spectroscopy (Figure 1a–b). Since NMR chemical shifts are sensitive to changes in the chemical environment, chemical shift perturbations (CSP) can be used to determine the binding interface. Upon addition of p53DBD to 15N-CypD, NMR crosspeaks shifted, peaks split and new crosspeaks emerged (summarized in Figure 1a–b). These CSP are due to p53DBD binding to CypD (Supplemental Figures 1b–d). Of note, upon completion of the 4-hour NMR experiment, we observed severe visible aggregation in the NMR tube. We found that p53DBD was quantitatively precipitated from the NMR sample, while no CypD was found among the aggregated fraction (Supplemental Figures 1e).
Figure 1. NMR reveals CypD active site residues required for interaction with p53.
a) 1H, 15N HSQC spectra containing 50 µM isotopically labeled CypD in the presence of 100 µM p53DBD at 25 °C. Titration of p53DBD to CypD shows that there is an interaction between the two proteins as indicated by CSP. Key active-site residues are highlighted and enlarged. b) Map of the significant chemical shift perturbations (>2 standard deviations) and residues with split peaks in the structure of CypD (PDB:2Z6W). c) Pulldown of endogenous wildtype p53+/+ from H2O2-stressed and unstressed HCT116 cells by wildtype (WT) CypD and the CypD active-site mutants R55A, F60A, F113A and W121A expressed as GST-fusion proteins and immobilized on glutathione beads
The NMR experiment reveals that CypD active-site residues R55, F60, F113 and the side chain of W121 exhibit chemical shift perturbations upon titration of p53 (Supplemental Figure 1d). These residues interact directly with the inhibitor CsA in the co-crystal structure of CypD and CsA[37]. The NMR titration of CsA to CypD confirms that these active-site residues are involved in binding CsA (Supplemental Figure 1f). The observed CSPs upon titration of either p53DBD or CsA are on similar scales (Supplemental Figures 1g–h) but exhibit different behavior. While titration of CsA to CypD results mainly in peak shifts, titration of p53DBD to CypD results in peak splitting as well as the emergence of new peaks. Peak splitting can be caused by NMR slow exchange behavior indicating dynamic processes commonly observed in prolyl isomerases [38], [39]. This would be consistent with a transient enzyme-substrate interaction between CypD and p53. Alternatively, p53 aggregation during the time course of the NMR experiment could deplete p53DBD and result in a second subset of peaks. In either case, the NMR experiments indicate that select CypD active site residues interact transiently with p53DBD.
To validate the importance of the CypD active site residues for p53 interaction, we individually mutated them to alanines and confirmed in a thermal shift assay that all mutants were folded at room temperature (Supplemental Figures 2c–f).We tested the ability of CypD active site mutants to pulldown endogenous p53 from HCT116 cells (Figure 1c). Mutant proteins F60A and W121A had lost all ability to pull down p53, while R55A and F113A showed severely reduced ability to pull down p53. Next, we tested the enzymatic activity of CypD variants using a fluorescent peptide cis/trans prolyl isomerization assay[40]. We found the active-site mutants R55A, F60A, F113A and W121A had completely lost isomerization activity (Supplemental Figures 2a–b) as has been observed previously for similar active site mutations [41]. Taken together, these results show that CypD enzymatic activity is necessary for the aggregation of p53DBD.
p53 aggregates in the presence of active CypD
In light of the propensity of p53 to aggregate in the presence of active CypD, we modified the pulldown assay. Previously, we immobilized GST-CypD on glutathione beads as the bait to capture soluble p53. However, in this assay, it is impossible to distinguish whether p53 is bound to CypD or whether insoluble p53 aggregates and sediments together with the glutathione beads. We therefore compared whether both the initial pulldown condition, i.e. immobilized GST-CypD, and a control with soluble CypD could capture soluble p53. In the latter condition, one would expect that if CypD was binding p53, the soluble co-complex would be washed away in subsequent washing steps and we would not detect p53 in the pellet. However, if CypD was causing p53 to form insoluble aggregates, we would be able to detect p53 in the pellet since the insoluble p53 aggregates will sediment with the glutathione beads (Supplemental Figure 1i). Indeed, CypD causes both highly purified full-length human p53 as well as recombinant Sf9-expressed p53DBD to aggregate and sediment with the beads (Figure 2a). Moreover, endogenous p53 from H2O2-stressed and unstressed human HCT116 cells also aggregates and sediments with the glutathione beads in the presence of soluble CypD, but fails to aggregate when CypD is inhibited by CsA (Figure 2b).
Figure 2. p53 is aggregated by active soluble CypD and sediments in pulldown experiment.
a) Co-precipitation of commercial WT full-length p53 and SF9 p53DBD by GST-CypD and soluble CypD (between dashed lines) in the presence of GST but not GST alone. Whether CypD is soluble (between dashed lines) or GST-CypD is immobilized on glutathione beads, commercially purified WT-full-length-p53 or recombinant SF9 p53DBD will co precipitate with the glutathione beads only in the presence of CypD. b) Co-precipitation of endogenous p53 from H2O2 stressed and unstressed HCT116 cells by GST-CypD and soluble CypD in the presence of GST but not GST alone. Whether CypD is soluble or GST-CypD is immobilized on glutathione beads, endogenous WT-p53 from H2O2- stressed or unstressed HCT116 cells will co-precipitates with glutathione beads in the presence of CypD. c) Pulldown of common cancer-associated p53DBD missense mutant proteins. Mutant proteins R175H, R248Q, R249S and R273H are structurally destabilized[42] (Supplementary Table 1), yet can be readily precipitated by CypD, indicating that proper folding of p53 is not required for interaction with CypD.
To further characterize the CypD-dependence of p53DBD aggregation, we used an absorbance-based assay to monitor the formation of insoluble aggregates. The results indicate that active CypD leads to the formation of insoluble p53DBD aggregates and that inhibition of CypD by CsA prevents aggregation of p53DBD (Figure 3 a–b). Taken together, these results indicate that the enzymatic activity of CypD is required to precipitate p53 even at the low concentrations of the pulldown assays. This supports a model where CypD transiently interacts with p53 as its substrate.
Figure 3. CypD cause p53 to aggregate in vitro.
a) Aggregation of p53DBD measured by absorbance at 350 nm under NMR conditions. Over the course of 4 hours, the duration of the NMR experiment described in Figure 1, 25 µM CypD leads to the aggregation of 50 µM SF9 p53DBD (red and blue curves) but not when CypD is inhibited by 50 µM CsA (black and grey curves). This indicates that CypD indeed acts as an enzyme and aggregates p53. b) Coomassie staining of mixed, soluble and precipitated fractions of absorbance assay samples from Figure 3a. It can be seen that little p53DBD remains in solution after interaction with active CypD and that CypD does not aggregate with p53DBD. Conversely, inhibition of CypD by CsA strongly suppresses the aggregation of p53DBD.
CypD aggregates thermodynamically destabilized p53
To better understand how the stability and folded state of p53DBD affects the interaction with CypD, we generated a set of common cancer-associated p53 missense mutations which were previously shown to be completely unstructured in the p53DBD at 25 °C [42]. Mutant p53 proteins R175H, L194V, R248Q, R249S, R273H and R282P were expressed in p53−/− HCT cells in the absence of stress and standard biochemical pulldowns were performed (Figure 2c). This is consistent with our previous finding that the p53 minimal construct (amino acids 80–220[7]) that interacts with CypD is likely unfoled since it lacks three β-strands from the core of the p53DBD which are critical for p53 stability (Supplemental Figure 2g). Structural destabilization of the p53DBD does not reduce p53’s ability to interact with CypD; on the contrary it appears to enhance it, indicating that p53 does not require a folded state in order to interact transiently with CypD.
To further address the role of thermodynamic stability on p53 aggregation by CypD we compared p53DBD protein expressed in Sf9 cells and in E.coli. Equilibrium urea denaturation experiments show that the Sf9 p53DBD protein has a thermodynamic stability of −3.78 kcal/mol while E.coli p53DBD protein has a thermodynamic stability of −5.91 kcal/mol (Supplemental Figure 2h–j, Supplemental Table 1). Though the two protein sequences are identical and of similar purity as judged by SDS-PAGE, mass spectrometry indicates heterogeneous post translational modifications (PTM) for Sf9-expressed p53DBD. Here, PTM may be responsible for the drastic reduction in thermodynamic stability of Sf9-expressed p53DBD compared to E.coli-expressed p53DBD.
Importantly, CypD causes aggregation of thermodynamically destabilized p53DBD from Sf9 cells but not of p53DBD from E.coli (Supplemental Figures 2j). This suggests that CypD preferentially interacts with posttranslationally modified, destabilized or unfolded forms of p53DBD as would be found in the mitochondrial matrix.
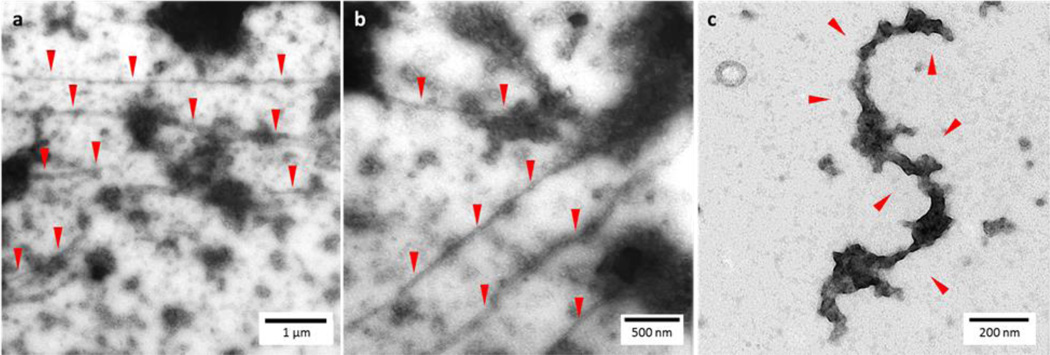
CypD causes p53 to self-aggregate into amyloid-like fibrils in vitro
There has been extensive research into the formation of p53 amyloid-like, fibrillar self-aggregates brought on by stress or mutation[43]–[46]. It is thought that p53 residues 252–258 within the p53DBD are highly aggregation prone and a recent study has shown that targeting this region with the small residue 252–258-derived peptide ReACp53 can prevent aggregation and amyloid formation of p53 in vivo[47], [48] . Thus, we investigated the nature of the p53DBD aggregates that were generated by CypD during our NMR experiments. It has been shown that thermal denaturation of p53DBD causes non-specific granular aggregation of p53DBD. In contrast, mild denaturation, such as the use of pressure, causes the formation of amyloid-like fibrils[43]. We generated p53DBD aggregates by either incubating 50 µM CypD with 100 µM p53DBD overnight at 25 °C, or by thermally unfolding 100 µM p53DBD at 55°C for 30 minutes in the absence of CypD. Following negative staining of aggregates by 2% uranyl acetate, visualization by transmission electron microscopy (TEM) revealed that p53DBD aggregates generated in the presence of CypD readily formed numerous elongated amyloid-like fibrillar structures, as well as non-specific granular aggregates (Figure 4 a–c and Supplemental Figure 3 a–c). It is generally accepted that amyloid fibrils begin as diffuse aggregates that transform into more stable fibrillary forms with time, suggesting that the fibrils act as a slow kinetic trap[49], [50]. In contrast, thermally denatured p53DBD aggregates formed only non-specific large granular precipitates but no fibrillar amyloid-like structures, as expected (Supplemental Figure 3d–e). As additional control, p53DBD that had been incubated overnight at 25 °C in the absence of CypD formed some granular aggregates albeit to a lesser degree than thermally denatured p53DBD, and, importantly, no amyloid-like fibrils were detected (Supplemental Figure 3f–g). Finally, p53DBD was incubated overnight at 25 °C with the catalytically dead CypD mutants F60A and W121A. Again, the degree of precipitation was much less than when p53DBD was incubated with WT CypD and importantly, no amyloid-like fibrils were observed (Supplemental Figure 3h–k). These results support a model where CypD acts as an enzyme and its activity toward its substrate, p53, leads to the formation of p53 amyloid-like fibrils.
Figure 4. TEM of p53DBD incubated with WT CypD ~16 hours at 25 °C under conditions identical to NMR experiments, negative staining with 2% uranyl acetate. Red arrows indicate amyloid-like fibrils among large diffuse aggregates.
a) Elongated fibrils at 30,000x magnification. b) Elongated fibrils at 68,000x magnification. c) Short, individual fibril at 98,000x magnification.
Inhibition of Trap1 sensitizes MEFs to mPT and mPTP pore opening in a p53-dependent and CypD-dependent manner
It has been shown that aggregation of p53 upregulates the expression of heat-shock proteins including HSP70[51], [52] and HSP90[36], [48], [52], [53]. Inhibition of HSP70 using the small inhibitor pifithrin-µ prevents translocation of p53 into the mitochondria[54]–[56]. We and others have previously shown that hypoxic and other stress-induced mitochondrial p53 physically associates with the mitochondrial matrix-located protein import and folding chaperones mtHSP60[7], [28], [35], [57] and mtHSP70[7], [57]–[59] (also known as GRP75, UniProt: P38646). Furthermore, we and others have shown that in order to prevent aggregation, mutant p53 in cancer cells forms a stable interaction with the cytosolic HSP90 chaperone[60] and that the HSP90 inhibitor 17-AAG disrupts this complex[36], [59], [61]–[65]. Strikingly, the inhibitor gamitrinib, a specific mitochondria-targeted 17-AAG analogue, inhibits a mitochondrial HSP90 homologue, Trap1, as well as the mitochondrial pool of HSP90. This leads to sensitization of cells to mPTP opening in a CypD dependent manner[29], [66], [67]. Conversely, overexpression of Trap1 protects cardiomyocytes and rat brains from I/R injury and mPT[31], [32], [68]. Importantly, it has been demonstrated that CypD co-immunoprecipitates with both HSP90 and Trap1[29] as well as HSP60[28].
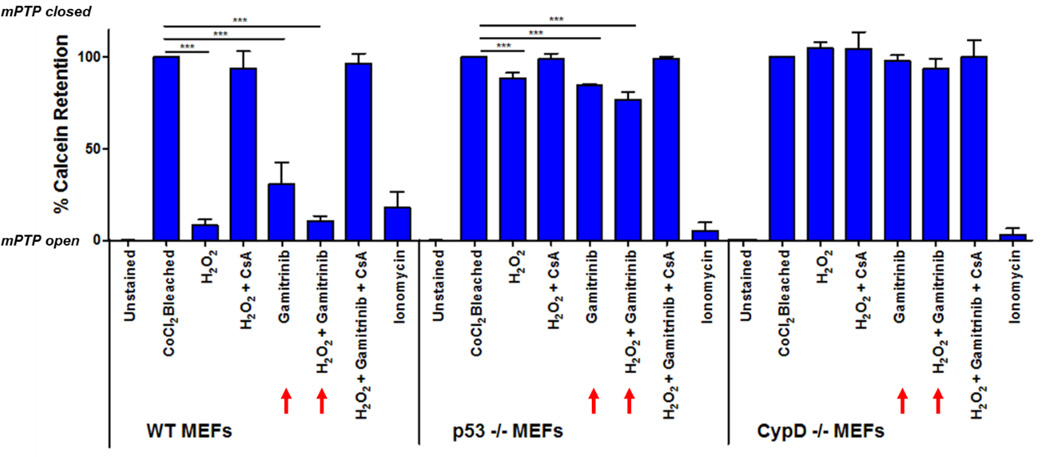
In order to understand the role of p53 in the context of Trap1-mediated protection from mPT, we performed calcein release assays in WT, p53−/− and CypD−/− MEFs using FACS. In this assay, healthy, mitochondria retain green fluorescence from calcein dye in the presence of the quencher CoCl2 which can only enter the mitochondria and quench calcein fluorescence upon opening of the mitochondrial permeability transition pore. Experiments were performed in the presence or absence of H2O2 stress, with and without the selective Trap1 inhibitor Gamitrinib and in the presence or absence of the CypD inhibitor CsA (Figure 5). Inhibition of Trap1 in unstressed WT MEFs caused marked mPTP opening, and further addition of H2O2 stress caused WT MEFs to undergo complete mPT opening. As expected, no mPT could be detected in WT MEFs in the presence of the CypD inhibitor CsA or in CypD−/− MEFs under any condition (except for the positive control with the calcium ionophore ionomycin), confirming the role of CypD as an indispensable component of mPT and the mPTP. Importantly, however, inhibition of Trap1 only minimally sensitized p53−/− MEFs to mPTP, and addition of H2O2 stress failed to yield further sensitization. These results indicate that p53 plays a major role in triggering mPT opening. Moreover, p53 acts through a novel mechanism involving Trap1, a member of the mitochondrial unfolded protein response (mtUPR) pathway.
Figure 5. Inhibition of Trap1 sensitizes MEFs to mPT in a p53-dependent and CypD-dependent manner.
Retention of calcein fluorescence within the mitochondrial matrix in MEFs is a direct reflection of mPTP opening, measured by FACS in the presence or absence of H2O2 oxidative stress, with and without the Trap1 inhibitor gamitrinib and in the presence or absence of the CypD inhibitor CsA. High values of retained calcein fluorescence indicate that the mPTP is closed while low values of retained calcein fluorescence indicate opening of the mPTP. H2O2 and gamitrinib greatly sensitize WT MEFs (red arrows) to mPT but only minimally sensitize in p53−/− MEFs. mPT does not occur in the absence of CypD. Data from 3 independent biological replicas, mean +/− SD are shown.
Discussion
There is a great interest in uncovering the full structural and functional details of the mitochondrial permeability transition pore, and particularly the regulation of this process. Modulation of the mPTP has been implicated as a therapeutic target for multiple diseases including ischemia-reperfusion injury[3], [7], Alzheimer dementia [6] and cancer[66]. Here we investigated the role of p53 in mPT, which in this context acts as an oxidative stress signaling molecule locally in the mitochondrial matrix. Our results provide new details of the potential mechanism how p53 regulates mPTP opening. We previously showed that in response to oxidative stress and in I/R brain injury (stroke), accumulation of p53 in the mitochondrial matrix causes activation of CypD, triggering the opening of the mPTP and necrotic death[4]. This novel effector pathway of necrotic rather than apoptotic death via the mitochondrial p53-mPTP axis, mediated by CypD interaction, was subsequently confirmed in the context of oxidative damage-, dexamethasone-, cisplatin- and doxorubicin-induced necrotic death in cancer cells, osteoblasts and neurons [5–9]. Proteins translocated across the outer and inner mitochondrial membranes need to be refolded when they arrive in the matrix, a process requiring one or more molecular chaperones[69]. Upon translocation, mitochondrial matrix p53 is stabilized by a variety of molecular chaperones including HSP60, HSP70 and HSP90 under stress and non-stress conditions [59], [62], [70]. Notably, in unstressed healthy cells CypD can also interact with the matrix chaperones HSP60, HSP90 and Trap1[5], [29], [70], [71]. Moreover, CypD can assemble into a higher-order complex consisting of CypD, HSP60, and either HSP90 or Trap1[28], [29].
Our in vitro experiments reveal that a dynamic interaction occurs between CypD and the DNA binding domain of p53. Multiple complementary biochemical assays including NMR, TEM, absorbance-based aggregation assay and pulldowns highlight the key involvement of CypD enzymatic activity in CypD’s interaction with p53. More importantly, we show that WT p53 interaction with CypD causes robust aggregation of WT p53 derived from various sources, including recombinant baculoviral human p53DBD, endogenous full-length human p53 from H2O2 -stressed and unstressed HCT116 colon cancer cells and purified human full-length p53. These results suggest that CypD isomerizes the imported unfolded p53, which leads to its irreversible aggregation in the matrix. The fact that CypD retains its ability to interact with structurally destabilized mutant p53 proteins supports the hypothesis that CypD interacts with unfolded WT p53 entering the mitochondrial matrix upon stress. Intriguingly, we find that the insoluble p53 aggregates formed by interaction with CypD are not amorphic but tend to be long, amyloid-like fibrillary structures. It should be noted that the absorbance, NMR and TEM assays are all conducted in the absence of beads and p53 aggregation is still observed in a CypD-dependent manner. This implies that, CypD activity is in fact responsible for aggregating p53 and not causing p53 to non-specifically bind pulldown beads. Importantly, this indicates that CypD and p53 engage in a transient and dynamic enzyme-substrate interaction.
The physiological significance of stress-induced p53 aggregation in the mitochondrial matrix is not yet clear. However, there is growing literature about the role of the mitochondrial unfolded protein response[27], [33], [72]. It has been shown that the refolding of p53DBD is slow[42], [73] which makes it vulnerable to aggregation, off-target interactions and processes[52] including proline cis-trans isomerization. mtUPR could play an important role in stabilizing the influx of the unfolded p53, since p53 will require refolding upon entry into the matrix. We had shown previously that CypD and p53 from cellular extracts interact with each other in immunoprecipitations[7], but it was not clear whether this is a direct interaction or whether this interaction between p53 and CypD is mediated by a bridging factor. Here, based on our biochemical and cellular data we propose a model where p53 translocates into the mitochondrial matrix upon cellular stress. This influx of unfolded p53 overloads the Trap1/HSP60/HSP90 chaperone proteins. These chaperone complexes are present in the mitochondrial matrix of unstressed cells and keep CypD inactive with respect to mPT[29], [35], [66], [67]. According to this model, transient binding of unfolded p53 to Trap1/HSP60/HSP90 induces dissociation and release of CypD from the chaperones. We expect that the affinity of CypD for chaperones is modest since CypD does not have any specific domains for interaction with chaperones. The ensuing CypD liberation activates its enzymatic isomerase activity. Active CypD then acts on structural mPTP proteins as isomerization substrates, thereby inducing opening of the mPT pores. Importantly, active CypD also isomerizes p53 (and possibly other proteins), thereby causing p53 aggregation (Figure 6). The driving force for a change in the prolyl cis/trans isomerization of p53 could be that the aggregates and fibrils are enriched with cis-prolyl p53. Preferential aggregation of a cis-prolyl p53 would remove cis-prolyl p53 from the equilibrium of soluble p53 and drive the isomerization[74]. For example, Soragni and coworkers showed recently that p53DBD which contains 13 prolines comprises multiple highly aggregation prone peptides [47]. Moreover, it is possible that aggregated p53 further sequesters Trap1/HSP60/HSP90, in turn leading to the activation of additional CypD. This would generate a positive feed-forward loop for CypD activation and the cell could undergo prolonged mPT, thereby reaching the ‘point-of-no-return’ towards necrotic death. Our FACS data support this model where the inhibition of Trap1 sensitizes p53-proficient WT MEFs to mPT and pore opening in a p53- and CypD-dependent manner. This implies that p53 serves as a major mitochondrial stress signal transducer that requires Trap1 to impinge on, thereby activating CypD. Our cell data suggest that p53 is necessary to mediate the bulk of mPTP opening. However, other proteins could also translocate into the matrix upon oxidative stress and displace CypD from Trap1, since our FACS data show that Gamitrinib-treated p53−/− MEFs are still partially sensitized to opening of the mPTP. This working model can now be tested in the future.
Figure 6. Proposed model of stress-induced p53 which activates CypD upon p53’s entry into the mitochondrial matrix. This triggers mPT.
1) Stress-induced p53 is imported into the mitochondrial matrix, unfolding p53 in the process. 2) p53 influx and refolding overloads the matrix chaperones Trap 1 and HSP60/90. This competes off CypD, which is normally held inactive by complexation with Trap1/HSP60/90. Release of CypD activates its isomerase activity. 3) Liberated CypD then isomerizes multiple proteins including p53, causing p53 aggregation which further sequesters Trap1/HSP60/HSP90, in turn leading to the activation of additional CypD in a positive feed-forward loop kind of manner. 4) CypD is recruited to the structural components of the mPTP pore and induces pore opening, leading to energetic breakdown and necrotic cell death.
Necrosis has long been considered a completely unregulated form of cell death. However, there is a growing body of evidence implicating a variety of regulated necrosis pathways. These pathways have taken on different names including the necrosome signaling complex, ferroptosis, oxytosis, NETosis, ETosis, parthanatos, pyroptosis, pyronecrosis and finally CypD-mediated necrosis[75], [76]. Globally, these various regulated necrosis pathways fall under the newly-coined term necroptosis. Our work supports the notion of a regulated necrosis in the condition of oxidative stress, and implicates p53 as an important signaling molecule within the CypD-mediated necrotic pathway. This work motivates additional research into modulation of p53-mediated necrosis as an inhibitory or activating target for the treatment of various diseases.
Materials and Methods
Purified WT full-length human p53 was purchased from OriGene (Cat # TP300003). The protein was expressed in HEK293 cells as a C-terminal MYC/DDK fusion protein and affinity purified. Protein purity is > 80% as determined by SDS-PAGE and Coomassie staining.
Expression and purification of recombinant human CypD and p53DBD
N-terminally His6 tagged human CypD (residues 45–206 of uniprot ID P30405) was expressed in E.coli BL21 DE3 cells, lysed by sonication and purified by FPLC (GE AKTA purifier) using an ion exchange column (GE HiTrapS) followed by a Ni-NTA affinity purification (GE HisTrap). 6xHIS tag was cleaved using tobacco etch virus (TEV) protease during overnight dialysis into 20 mM KHPO4 pH 7.7, 1 mM DTT for gel-filtration (GE HiLoad 16/60 Superdex 200) by FPLC.
WT GST-CypD and the GST-CypD mutants R55A, F60A, F113A, and W121A were cloned into expression vector 2GT (Addgene, Plasmid #29707), transformed into BL21DE3 cells and lysed using a high-pressure homogenizer (Avestin Emulsiflex C3). Proteins were purified by FPLC using a Ni-column (GE HisTrap) followed by an ion exchange column (GE HiTrapS). 6xHIS was cleaved using TEV during overnight dialysis into 20 mM TRIS pH 8.0, 50 mM NaCl, 1 mM DTT, 5% glycerol followed by gel filtration (GE HiLoad 16/60 Superdex 200) by FPLC.
15N CypD (residues 45–206) was expressed in BL21DE3 cells grown in M9 minimal media[77] and lysed by sonication. Protein was purified by FPLC with ion exchange column (GE HiTrapS) followed by Ni-column (GE HisTrap). 6xHIS tag was cleaved using TEV during overnight dialysis into 20 mM KHPO4 pH 7.7, 1 mM DTT for gel filtration (GE HiLoad 16/60 Superdex 200) by FPLC.
N-terminally tagged 6xHIS-p53DBD (residues 94–312) was expressed using the Bac-to-Bac Baculovirus Expression System (LifeTechnologies) and vector pFastBac HTb and purified with standard protocol. In brief, cells were lysed (50 mM Tris-HCl pH 8.5, 500 mM NaCl, 20 mM Imidazole, 5 mM 2-mercaptoethanol, 10% glycerol) and p53 was purified from cleared lysates by batch Ni-NTA purification. Protein 6xHIS tag was cleaved using TEV during overnight dialysis into 50 mM NaH2PO4 pH 7.4, 5 mM DTT. Protein purity is > 90% as determined by SDS-PAGE and Coomassie staining.
Tissue Culture and Lysate Generation
Mouse embryonic fibroblasts (MEFs) and human HCT116 cells were grown in DMEM+10%FBS. WT and p53−/− MEF cell lines were established from E13.5 embryos[7] and CypD−/− MEFs were purchased from ArtisOptimus[7]. HCT116 WT and isogenic HCT116 p53−/− cells were provided by Dr. Bert Vogelstein, Howard Hughes Medical Institute, Baltimore, MD. Lysates were generated by scraping cells into PBS with 1%TritonX100 and complete EDTA-free protease inhibitors (Sigma), lysed by 3x freeze-thaw and cleared of cell debris by centrifugation at 4000 g for 5 min. Cyclosporine A was purchased from Sigma (Cat #30024). Calcein and Ionomycin were purchased from LifeTechnologies (Cat #C3100MP and #I24222). Gamitrinib was kindly provided by Dr. Dario Altieri, Wistar Institute, Philadelphia, PA.
Pulldown Assay
GST pulldown experiments were conducted in PBS/1%TritonX100. GST, GST-CypD or GST-CypD mutants were bound to glutathione beads for 1 hr at 4 °C (ThermoScientific, Cat #15160), then blocked in PBS/1%TritonX100/1%BSA for 1 hr at 4 °C. Blocked beads were incubated with lysates for 16 hours at 4 °C in the presence of CsA where indicated. Beads were pelleted, thoroughly washed in PBS/1%TritonX100 and analyzed by immunoblot. For co-aggregation assays, the same pulldown conditions were used plus an additional condition containing bead-bound GST in the presence of soluble CypD (no GST tag).
NMR Experiments
The 1H, 15N HSQC spectra were obtained on 50 µM 15N CypD in the presence or absence of 100 µM p53DBD in 20 mM Tris pH8, 0.25 M KCl, 5 mM β-ME, 0.1 M Imidazole, 5% glycerol, 1% Triton X-100 and 10% D2O where indicated using a Bruker Avance3 700 MHz magnet. The spectra were obtained at 25 °C with 64 scans, TD2=2048, TD1=128 with spectral widths of 13,586.95 Hz (1H) and 3101.73 Hz (15N). Topspin 3.2 (Bruker) was used for processing the spectrum and CCPNMR analysis 2.1 was used to generate the figure. CypD crosspeak assignments were taken from Biological Magnetic Resonance Bank (BMRB) accession number 7310[78]. For SDS gel analysis, three samples were prepared: the sample was pipetted to uniformly mix the aggregates in solution and a 50 µl aliquot was taken (“mixed fraction”). The remainder of the sample was centrifuged at 14,000 g for 1 min, then a 50 µl aliquot from the upper soluble fraction was taken (“soluble supernatant”). The remaining supernatant was transferred to another tube and the aggregate was resuspended in 500 µl of fresh buffer and a 50 µl aliquot was taken (“aggregated pellet”). 10 µl of each sample were analyzed on a 15% SDS-PAGE gel at 200V for 1 hr followed by Coomassie staining.
CypD Activity Assay
Prolyl cis/trans isomerization of the fluorescent aminobenzoyl-Ala-Phe-Pro-Phe-4-nitroanilide (Abz-AFPF-pNA) peptide was monitored by changes in fluorescence emission at 416 nm upon excitation at 316 nm (FM4 Horriba-Jobin Yvon) in 50 mM NaH2PO4 + 5 mM DTT at 15 °C. Abz-AFPF-pNA maintains the fluorescence-quenched cis conformation in anhydrous trifuoroethanol (TFE) + 50 mM LiCl, but adopts the fluorescent trans conformation in aqueous buffer 50 mM NaH2PO4 + 5 mM DTT. CypD (0–15 nM) was pre-equilibrated and the isomerization reaction was started by addition 3 nM Abz-AFPF-pNA. The reaction was monitored for 400 sec and data points were recorded every second with an integration time of one second.
Urea Denaturation of p53DBD
Denaturation of SF9 p53DBD was monitored by changes in protein fluorescence using a Jobin Yvon Fluoromax-4 (Horiba) spectrofluorimeter exciting at 280 nm (bandpass 2.5 nm) and scanning emission 300–400 nm (bandpass 7 nm), integration time 0.1 sec. 15 µl of p53DBD was added to a final concentration of 2 µM in 900 µl of buffer containing 50 mM NaH2PO4 + 5 mM DTT and increasing amounts of urea from 0–8 M in 48 increments of 166.7 mM. The sample was incubated for 30 minutes at 25 °C before the fluorescence was recorded.
Aggregation Assay
Aggregation of p53 was monitored by changes in absorbance at 350 nm using a SpectraMax 340 PC Absorbance microplate reader (Molecular Devices) using a low-volume 96-well ELISA plate (Greiner). The experiment was monitored over the course of 4 hours at 25 °C, shaking the plate for 15 seconds before each measurement recorded every 30 seconds. Final protein concentrations were 25 µM CypD in the presence or absence of 50 µM SF9 p53DBD with or without 50 µM CsA. The experiment was carried out in a final volume of 100 µl of NMR buffer: 20 mM Tris pH 8, 0.25 M KCl, 5 mM β-ME, 0.1 M Imidazole, 5% glycerol, 1% Triton X-100.
TEM
Transmission electron microscopy images of p53DBD aggregates were obtained by letting aggregates of the different conditions settle on a copper grid for 30 seconds, wicking away remaining buffer followed by negative staining with 2% uranyl acetate. Images were taken on a Tecnai BioTwinG2 microscope (FEI)
Calcein Retention Assay
Mitochondrial permeability transition pore opening was assed using the calcein assay with CoCl2 quenching (LifeTechnologies) where loss of calcein fluorescence directly indicates opening of the mPTP. Where specified, MEFs were pre-treated with 0.4 mM H2O2 for 8 hr in the absence or presence of 2 µM CsA as previously described[7] and the absence or presence of 10 µM Gamitrinib. The cells were labeled with calcein for 30 min at 37 °C in Hanks’ balanced salt solution with 10 mM Hepes pH 7.4 and 0.1 µM calcein as described[79]. Fluorescence was detected by an EMD Millipore Amnis FlowSight Imaging Flow Cytometer using the manufacturer’s instructions and the supplied IDEAS analysis software. The fluorescent signal was normalized to CoCl2 bleached, unstressed untreated cells as maximum signal.
Supplementary Material
Highlights.
Catalytically active CypD drives insoluble aggregation of p53 in vitro
Active CypD causes the p53DBD to aggregate into amyloid-like fibrils in vitro
Inhibition of Trap1 sensitizes MEFs to mPT in a p53-dependent manner
Acknowledgments
This work has been supported by a grant from Stony Brook University School of Medicine and NIH R35GM119437 (MAS), R01CA176647 (UMM). We are grateful to Weibing Zhang and Sulan Xu for technical assistance and to Susan van Horn for help with acquiring TEM images. We are thankful to Drs. P. Schmidpeter and F.X. Schmid for providing us with Abz-AFPF-pNA peptide for CypD activity assays and for helpful discussion. We thank Dr. Dario Altieri of the Wistar Institute for kindly providing the Gamitrinib inhibitor. We thank Dr. R. Scott Powers of the Department of Pathology at Stony Brook University for providing the FACS instrumentation.
Abbreviations
- APP
amyloid precursor protein
- CsA
cyclosporine A
- CypD
mitochondrial cyclophilin D (gene name: PPIF, uniprot ID: P30405)
- HSP60
heat-shock protein 60
- HSP70
heat-shock protein 70
- HSP90
heat-shock protein 90
- HSQC
Heteronuclear Single-Quantum Correlation
- I/R
ischemia-reperfusion
- MCAO
middle cerebral artery occlusion
- MEF
mouse embryonic fibroblast
- mPT
mitochondrial permeability
- mPTP
mitochondrial permeability transition pore
- mtHSP70
mitochondrial heat-shock protein 70
- mtUPR
mitochondrial unfolded protein response
- NMR
nuclear magnetic resonance
- OSCP
oligomycin-sensitivity conferring protein
- p53
TP53
- p53DBD
p53 DNA Binding Domain
- PDB
protein databank
- ROS
reactive oxygen species
- SPG7
Spastic Paraplegia 7
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 Dec;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 2015 Mar;106(2):237–248. doi: 10.1093/cvr/cvv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005 Aug;102(34):12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005 Mar;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 6.Du H, Guo L, Fang F, Chen D, A Sosunov A, M McKhann G, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Du Yan S. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14(10):1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 Opens the Mitochondrial Permeability Transition Pore to Trigger Necrosis. Cell. 2012;149(7):1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen Y-F, Wang G-D, Zhu L-Q, Tan S-P, Zhang F-Y, Zhou X-Z, Wang X-D. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J. Cell. Physiol. 2014 Oct;229(10):1475–1483. doi: 10.1002/jcp.24589. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, Xu M, Zhang H, Wang J, Zheng P, Gong L, Wu G, Dai T. Cisplatin-induced non-apoptotic death of pancreatic cancer cells requires mitochondrial cyclophilin-D-p53 signaling. Biochem. Biophys. Res. Commun. 2013 Aug;437(4):526–531. doi: 10.1016/j.bbrc.2013.06.103. [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Sesaki H, Qi X. Drp1 stabilizes p53 on the mitochondria to trigger necrosis under oxidative stress conditions in vitro and in vivo. Biochem. J. 2014 Jul;461(1):137–146. doi: 10.1042/BJ20131438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L-P, Ji C, Lu P-H, Li C, Xu B, Gao H. Oxygen glucose deprivation (OGD)/re-oxygenation-induced in vitro neuronal cell death involves mitochondrial cyclophilin-D/P53 signaling axis. Neurochem. Res. 2013 Apr;38(4):705–713. doi: 10.1007/s11064-013-0968-5. [DOI] [PubMed] [Google Scholar]
- 12.Lu J-H, Shi Z-F, Xu H. The mitochondrial cyclophilin D/p53 complexation mediates doxorubicin-induced non-apoptotic death of A549 lung cancer cells. Mol. Cell. Biochem. 2014 Apr;389(1–2):17–24. doi: 10.1007/s11010-013-1922-1. [DOI] [PubMed] [Google Scholar]
- 13.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. 2013 Mar;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the F O ATP synthase in mitochondrial permeability transition. Cell Cycle. 2014 Oct;12(4):674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniel M, Giorgio V, Fogolari F, Glick G, Bernardi P, Lippe G. The Oligomycin-Sensitivity Conferring Protein of Mitochondrial ATP Synthase: Emerging New Roles in Mitochondrial Pathophysiology. Int. J. Mol. Sci. 2014 Apr;15(5):7513–7536. doi: 10.3390/ijms15057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S, Shaikh F, Cheung M, Leonard NJ, Stolakis RS, Wolfers MP, Ibetti J, Chuprun JK, Jog NR, Houser SR, Koch WJ, Elrod JW, Madesh M. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol. Cell. 2015 Oct;60(1):47–62. doi: 10.1016/j.molcel.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009 Apr;458(7242):1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.V Vaseva A, Moll UM. The mitochondrial p53 pathway. Biochim. Biophys. Acta. 2009 May;5:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardi P, von Stockum S. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium. 2012 Jul;52(1):22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front. Physiol. 2013 Jan;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parone PA, Da Cruz S, Han JS, McAlonis-Downes M, Vetto AP, Lee SK, Tseng E, Cleveland DW. Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. J. Neurosci. 2013 Mar;33(11):4657–4671. doi: 10.1523/JNEUROSCI.1119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barsukova A, Komarov A, Hajnóczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur. J. Neurosci. 2011 Mar;33(5):831–842. doi: 10.1111/j.1460-9568.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 2010 Oct;120(10):3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol Hear. Circ Physiol. 1992 Jun;262(6):H1699–H1704. doi: 10.1152/ajpheart.1992.262.6.H1699. [DOI] [PubMed] [Google Scholar]
- 25.Dudek J, Rehling P, van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta. 2013 Feb;1833(2):274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Taanman J-W. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta - Bioenerg. 1999 Feb;1410(2):103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 27.Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J. Cell Sci. 2010;123(22):3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010 Nov;70(22):8988–8993. doi: 10.1158/0008-5472.CAN-10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell. 2007 Oct;131(2):257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Kang BH. TRAP1 regulation of mitochondrial life or death decision in cancer cells and mitochondria-targeted TRAP1 inhibitors. BMB Rep. 2012 Jan;45(1):1–6. doi: 10.5483/bmbrep.2012.45.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Baqri RM, V Pietron A, Gokhale RH, Turner BA, Kaguni LS, Shingleton AW, Kunes S, Miller KE. Mitochondrial chaperone TRAP1 activates the mitochondrial UPR and extends healthspan in Drosophila. Mech. Ageing Dev. Jan;141–142:35–45. doi: 10.1016/j.mad.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Lu Y, Yu D, Zhang D, Hu W. TRAP1 Provides Protection Against Myocardial Ischemia-Reperfusion Injury by Ameliorating Mitochondrial Dysfunction. Cell. Physiol. Biochem. 2015 Jan;36(5):2072–2082. doi: 10.1159/000430174. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Q, Wang J, V Levichkin I, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002 Sep;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runkel ED, Liu S, Baumeister R, Schulze E. Surveillance-Activated Defenses Block the ROS-Induced Mitochondrial Unfolded Protein Response. PLoS Genet. 2013 Mar;9(3):e1003346. doi: 10.1371/journal.pgen.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008 Feb;283(8):5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- 36.Muller L, Schaupp A, Walerych D, Wegele H, Buchner J. Hsp90 Regulates the Activity of Wild Type p53 under Physiological and Elevated Temperatures. J. Biol. Chem. 2004 Sep;279(47):48846–48854. doi: 10.1074/jbc.M407687200. [DOI] [PubMed] [Google Scholar]
- 37.Kajitani K, Fujihashi M, Kobayashi Y, Shimizu S, Tsujimoto Y, Miki K. Crystal structure of human cyclophilin D in complex with its inhibitor, cyclosporin A at 0.96-Å resolution. Proteins Struct. Funct. Bioinforma. 2007 Dec;70(4):1635–1639. doi: 10.1002/prot.21855. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar P, Reichman C, Saleh T, Birge RB, Kalodimos CG. Proline cis-trans Isomerization Controls Autoinhibition of a Signaling Protein. Mol. Cell. 2007 Feb;25(3):413–426. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labeikovsky W, Eisenmesser EZ, Bosco DA, Kern D. Structure and Dynamics of Pin1 During Catalysis by NMR. J. Mol. Biol. 2007;367(5):1370–1381. doi: 10.1016/j.jmb.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoldák G, Aumüller T, Lücke C, Hritz J, Oostenbrink C, Fischer G, Schmid FX. A library of fluorescent peptides for exploring the substrate specificities of prolyl isomerases. Biochemistry. 2009 Nov;48(43):10423–10436. doi: 10.1021/bi9014242. [DOI] [PubMed] [Google Scholar]
- 41.Scholz C, Maier P, Dolinski K, Heitman J, Schmid FX. R73A and H144Q mutants of the yeast mitochondrial cyclophilin Cpr3 exhibit a low prolyl isomerase activity in both peptide and protein-folding assays. FEBS Lett. 1999 Jan;443(3):367–369. doi: 10.1016/s0014-5793(98)01735-9. [DOI] [PubMed] [Google Scholar]
- 42.Bullock aN, Henckel J, DeDecker BS, Johnson CM, V Nikolova P, Proctor MR, Lane DP, Fersht aR. Thermodynamic stability of wild-type and mutant p53 core domain. Proc. Natl. Acad. Sci. U. S. A. 1997;94(26):14338–14342. doi: 10.1073/pnas.94.26.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishimaru D, Andrade LR, Teixeira LSP, Quesado PA, Maiolino LM, Lopez PM, Cordeiro Y, Costa LT, Heckl WM, Weissmüller G, Foguel D, Silva JL. Fibrillar Aggregates of the Tumor Suppressor p53 Core Domain †. Biochemistry. 2003 Aug;42(30):9022–9027. doi: 10.1021/bi034218k. [DOI] [PubMed] [Google Scholar]
- 44.Silva JL, Vieira TCRG, Gomes MPB, Bom APA, Lima LMTR, Freitas MS, Ishimaru D, Cordeiro Y, Foguel D. Ligand binding and hydration in protein misfolding: insights from studies of prion and p53 tumor suppressor proteins. Acc. Chem. Res. 2010 Feb;43(2):271–279. doi: 10.1021/ar900179t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva JL, Gallo CVDM, Costa DCF, Rangel LP. Prion-like aggregation of mutant p53 in cancer. Trends Biochem. Sci. 2014;39(6):260–267. doi: 10.1016/j.tibs.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Rigacci S, Bucciantini M, Relini A, Pesce A, Gliozzi A, Berti A, Stefani M. The (1–63) region of the p53 transactivation domain aggregates in vitro into cytotoxic amyloid assemblies. Biophys. J. 2008 May;94(9):3635–3646. doi: 10.1529/biophysj.107.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soragni A, Janzen DM, Johnson LM, Lindgren AG, Thai-Quynh Nguyen A, Tiourin E, Soriaga AB, Lu J, Jiang L, Faull KF, Pellegrini M, Memarzadeh S, Eisenberg DS. A Designed Inhibitor of p53 Aggregation Rescues p53 Tumor Suppression in Ovarian Carcinomas. Cancer Cell. 2015 Dec;29(1):90–103. doi: 10.1016/j.ccell.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, Cornelis A, Rozenski J, Zwolinska A, Marine J-C, Lambrechts D, Suh Y-A, Rousseau F, Schymkowitz J. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7(5):285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 49.Bhak G, Choe Y-J, Paik SR. Mechanism of amyloidogenesis: nucleation-dependent fibrillation versus double-concerted fibrillation. BMB Rep. 2009 Sep;42(9):541–551. doi: 10.5483/bmbrep.2009.42.9.541. [DOI] [PubMed] [Google Scholar]
- 50.Uversky VN. Mysterious oligomerization of the amyloidogenic proteins. FEBS J. 2010 Jul;277(14):2940–2953. doi: 10.1111/j.1742-4658.2010.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiech M, Olszewski MB, Tracz-Gaszewska Z, Wawrzynow B, Zylicz M, Zylicz A. Molecular Mechanism of Mutant p53 Stabilization: The Role of HSP70 and MDM2. PLoS One. 2012 Dec;7(12):e51426. doi: 10.1371/journal.pone.0051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler JS, Loh SN. Kinetic partitioning during folding of the p53 DNA binding domain. J. Mol. Biol. 2005 Jul;350(5):906–918. doi: 10.1016/j.jmb.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 53.Park SJ, Borin BN, Martinez-Yamout MA, Dyson HJ. The client protein p53 adopts a molten globule-like state in the presence of Hsp90. Nat. Struct. Mol. Biol. 2011 May;18(5):537–541. doi: 10.1038/nsmb.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat. Chem. Biol. 2006 Sep;2(9):474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 55.Leu JI-J, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell. 2009 Oct;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.V Vaseva A, Marchenko ND, Moll UM. The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle. 2009 Jun;8(11):1711–1719. doi: 10.4161/cc.8.11.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchenko ND. Death Signal-induced Localization of p53 Protein to Mitochondria. A POTENTIAL ROLE IN APOPTOTIC SIGNALING. J. Biol. Chem. 2000 May;275(21):16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 58.Marchenko ND. Death Signal-induced Localization of p53 Protein to Mitochondria. A POTENTIAL ROLE IN APOPTOTIC SIGNALING. J. Biol. Chem. 2000 May;275(21):16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 59.Walerych D, Olszewski MB, Gutkowska M, Helwak A, Zylicz M, Zylicz A. Hsp70 molecular chaperones are required to support p53 tumor suppressor activity under stress conditions. Oncogene. 2009 Sep;28(48):4284–4294. doi: 10.1038/onc.2009.281. [DOI] [PubMed] [Google Scholar]
- 60.Hagn F, Lagleder S, Retzlaff M, Rohrberg J, Demmer O, Richter K, Buchner J, Kessler H. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat. Struct. Mol. Biol. 2011 Oct;18(10):1086–1093. doi: 10.1038/nsmb.2114. [DOI] [PubMed] [Google Scholar]
- 61.Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, Moll UM. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 2011 May;9(5):577–588. doi: 10.1158/1541-7786.MCR-10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller P, Hrstka R, Coomber D, Lane DP, Vojtesek B. Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene. 2008 Jan;27(24):3371–3383. doi: 10.1038/sj.onc.1211010. [DOI] [PubMed] [Google Scholar]
- 63.V Blagosklonny M, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. U. S. A. 1996 Aug;93(16):8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol. 1998 Mar;18(3):1517–1524. doi: 10.1128/mcb.18.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011 Dec;18(12):1904–1913. doi: 10.1038/cdd.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, Scroggins B, Neckers L, Altieri DC. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J. Clin. Invest. 2009 Mar;119(3):454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altieri DC. Hsp90 regulation of mitochondrial protein folding: from organelle integrity to cellular homeostasis. Cell. Mol. Life Sci. 2013 Jul;70(14):2463–2472. doi: 10.1007/s00018-012-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J. Cereb. Blood Flow Metab. 2009 Feb;29(2):365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin J. Molecular chaperones and mitochondrial protein folding. J. Bioenerg. Biomembr. 1997 Feb;29(1):35–43. doi: 10.1023/a:1022407705182. [DOI] [PubMed] [Google Scholar]
- 70.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 2005 Sep;280(39):33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 71.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002 Feb;512(1–3):1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 72.Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 2013 Dec;217(1):137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler JS, Loh SN. Structure, function, and aggregation of the zinc-free form of the p53 DNA binding domain. Biochemistry. 2003 Mar;42(8):2396–2403. doi: 10.1021/bi026635n. [DOI] [PubMed] [Google Scholar]
- 74.Smajlović A, Berbić S, Schiene-Fischer C, Tusek-Znidaric M, Taler A, Jenko-Kokalj S, Turk D, Zerovnik E. Essential role of Pro 74 in stefin B amyloid-fibril formation: dual action of cyclophilin A on the process. FEBS Lett. 2009 Apr;583(7):1114–1120. doi: 10.1016/j.febslet.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 75.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014 Feb;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 76.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010 Oct;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 77.Marley J, Lu M, Bracken C. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR. 20(1):71–75. doi: 10.1023/a:1011254402785. [DOI] [PubMed] [Google Scholar]
- 78.Schedlbauer A, Hoffmann B, Kontaxis G, Rüdisser S, Hommel U, Konrat R. Automated backbone and side-chain assignment of mitochondrial matrix cyclophilin D. J. Biomol. NMR. 2007 Jul;38(3):267. doi: 10.1007/s10858-006-9135-5. [DOI] [PubMed] [Google Scholar]
- 79.Wolff S, Erster S, Palacios G, Moll UM. p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008 Jul;18(7):733–744. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.