Abstract
Since the beginning of the millennium, research in primary cilia has revolutionized our way of understanding how cells integrate and organize diverse signaling pathways during vertebrate development and in tissue homeostasis. Primary cilia are unique sensory organelles that detect changes in their extracellular environment and integrate and transmit signaling information to the cell to regulate various cellular, developmental, and physiological processes. Many different signaling pathways have now been shown to rely on primary cilia to function properly, and mutations that lead to ciliary dysfunction are at the root of a pleiotropic group of diseases and syndromic disorders called ciliopathies. In this review, we present an overview of primary cilia-mediated regulation of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Further, we discuss how defects in the coordination of these pathways may be linked to ciliopathies.
Primary cilia help coordinate a cell's response to changes in the extracellular environment. In mammals, the RTK and TGF-β pathways are involved. Defects in these pathways are linked to ciliopathies.
Cellular signaling pathways form complex networks that converge signals from many different receptors to regulate a range of cellular and physiological processes during development and in tissue homeostasis (Lage et al. 2010; Kirouac et al. 2012). These networks operate in a spatiotemporal manner to balance the capacity of cells to perceive and transmit signals and process information. It is now evident that primary cilia take center stage in the ability of cells to register extracellular cues and coordinate the activity of multiple signaling pathways (Satir et al. 2010), which when defective may lead to ciliopathies (Hildebrandt et al. 2011; Waters and Beales 2011; Norris and Grimes 2012).
Cilia are membrane-bound, microtubule (MT)-based organelles that extend from a modified centriole (basal body) at the surface of many eukaryotic cells ranging from single-celled organisms, such as Chlamydomonas and Trypanosoma, to complex multicellular organisms, such as human (Satir and Christensen 2007). The MT core of the cilium, the axoneme, can vary in structure and composition among cell types and organisms, and cilia are, therefore, classified based on axoneme structure and presence or absence of proteins important for motility (i.e., inner and outer dynein arms, nexins and radial spokes). Motile cilia typically have axonemes comprised by nine outer doublets of MTs surrounding a central MT pair (9+2 cilia) and are usually 5 to 10 µm long and present in large numbers per cell, such as in the epithelium of the tracheal and nasal cavity, the oviduct, and the brain ventricles (Satir and Christensen 2007). Flagella, which are present in a single copy in sperm cells and one or more copies in many protists, may be longer than cilia but are otherwise similar in axonemal construction. Primary cilia, on the other hand, are thought to be nonmotile, usually display axonemes with 9+0 MT configuration, and are present in a single copy on the surface of most nondividing vertebrate cell types, depending on their lineage and differentiation stage (Blitzer et al. 2011; Bangs et al. 2015). The typical primary cilium ranges in length from about 1 to 20 µm with a diameter of roughly 200 nm, similar to that of motile cilia.
The basal body of primary cilia is derived from the centrosomal mother centriole (Fig. 1) (Paintrand et al. 1992; Kobayashi and Dynlacht 2011), whereas in multiciliated cells basal body formation requires the production of many new centrioles (Al et al. 2014). Importantly, primary cilia can be found in different shapes and structures, depending on their function, which primarily is associated with the ability to organize and integrate receptors and cellular signaling pathways during development and in tissue homeostasis (Satir and Christensen 2007). Modified primary cilia comprise those present at dendritic endings of sensory neurons, including the outer segment of photoreceptor cells and cilia on olfactory sensory neurons. As an example, the outer segment of rod photoreceptors corresponds to the ciliary shaft of a prototypic cilium and is composed of a cylinder-shaped stack of membrane disks that detect photons through the activation of the dim light receptor Rhodopsin (Roepman and Wolfrum 2007). Motile cilia in mammalian cells also have sensory capacities, such as those in the oviduct epithelium, in which transient receptor potential (TRP) ion channels as well as angiopoietin and progesterone receptors may localize to the cilia in a temporal manner during ovulation (Teilmann and Christensen 2005; Teilmann et al. 2005; Teilmann et al. 2006). Further, members of the bitter taste receptors in the class A/rhodopsin family of G-protein-coupled receptors (GPCRs) localize to motile cilia in the mammalian airways to support sinonasal innate immunity and prevent lung inflammation (Shah et al. 2009).
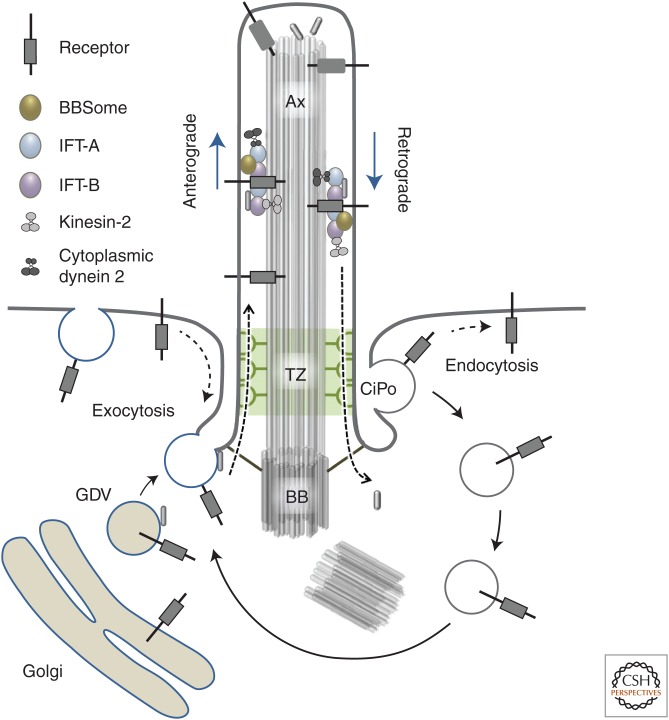
Figure 1.
Overview on trafficking pathways involved in ciliary assembly, homeostasis, and signaling. IFT-A/B, Intraflagellar transport complexes A/B; Ax, axoneme; TZ, ciliary transition zone; BB, basal body; GDV, Golgi-derived vesicle; CiPo, ciliary pocket. Please see text for further details and references.
For most classes of cilia, axonemes are assembled and maintained by intraflagellar transport (IFT). IFT is characterized by kinesin-2 and cytoplasmic dynein 2-mediated movement of trains of IFT particles, with associated ciliary cargo, from the ciliary base toward the tip and back along the axonemal outer doublet MTs (Fig. 1) (Pedersen et al. 2008; Bhogaraju et al. 2013; Lechtreck 2015). Importantly, the IFT system also participates in ciliary transport and compartmentalization of selected membrane receptors and other signaling proteins. For example, during Sonic hedgehog (SHH) signaling retrograde IFT and the BBSome (a complex of eight Bardet–Biedl syndrome proteins) (Nachury et al. 2007; Loktev et al. 2008) mediate ciliary export of the SHH receptor Patched1 (PTCH1) and the class F GPCR Smoothened (SMO) (Keady et al. 2012; Eguether et al. 2014), as well as ciliary trafficking and processing of the GLI transcription factors (Haycraft et al. 2005), which are the main downstream effectors of the pathway. This dynamic IFT-mediated compartmentalization of signaling proteins is fundamental for ciliary function, exemplified by the fact that mutations that specifically inhibit the association of signaling receptors (e.g., PTCH1 and SMO) with the BBSome or retrograde IFT machinery lead to disease-associated signaling defects (Seo et al. 2011; Keady et al. 2012; Marion et al. 2012b; Eguether et al. 2014; Schaefer et al. 2014). For a recent review on IFT and regulation of ciliary signaling, see Mourão et al. (2016). In addition to IFT-mediated compartmentalization of ciliary signaling components, the transition zone (TZ) located between the basal body and cilium proper also plays an essential role in establishment and maintenance of the cilium as a compartmentalized signaling organelle by regulating selective passage of proteins and lipids into and out of the cilium (Fig. 1) (Garcia-Gonzalo et al. 2011; Szymanska and Johnson 2012; Jensen et al. 2015; Takao and Verhey 2015). Ultrastructurally, the TZ is characterized by champagne-glass-like structures that connect the axonemal outer doublet MTs to the ciliary/TZ membrane and which may organize rows of intramembrane particles known as the ciliary necklace (Gilula and Satir 1972). Furthermore, the TZ membrane appears to be enriched in condensed lipids that may function as a fence to separate the ciliary membrane from the adjacent periciliary membrane domain (Vieira et al. 2006). Importantly, a large number of genes that are mutated in ciliopathies such as nephronophthisis (NPHP) and Meckel–Gruber syndrome (MKS) code for proteins that localize to the TZ (Reiter et al. 2012; Takao and Verhey 2015), highlighting the functional importance of this region.
The region between the TZ membrane and the plasma membrane, referred to as the periciliary membrane, is often infolded to produce a ciliary pocket (CiPo) that comprises an active site for exocytosis and clathrin-mediated endocytosis (CME) of ciliary components (Fig. 1) (Benmerah 2013). These processes are essential not only for ciliary membrane formation and maintenance, but also for regulation of trafficking and activation/inactivation of ciliary receptors and downstream signaling components (Dwyer et al. 2001; Field and Carrington 2009; Hu et al. 2007; Kaplan et al. 2012; Clement et al. 2013a; Bauss et al. 2014).
In summary, multiple components along the centrosome–cilium axis contribute to the establishment and maintenance of the cilium as a dynamic, compartmentalized signaling organelle, which in a spatiotemporally controlled manner can detect, integrate, and transmit a range of different signals to regulate fundamental processes at various levels from cell to organism. Consequently, mutations that impair the structure or function of either subdomain along the centrosomal–ciliary axis are associated with diseases and syndromic disorders (ciliopathies) that include cystic diseases of kidney, pancreas and liver, blindness, congenital heart disease, craniofacial and skeletal patterning defects, neurological disorders and obesity, which may be manifested in pleiotropic syndromes such BBS, NPHP, and MKS as well as Alström (AS) and Joubert (JS) syndromes (Hildebrandt et al. 2011; Koefoed et al. 2014; Valente et al. 2014). In the following, we first present a brief, general overview of signaling pathways that are known to be coordinated by primary cilia. Next, we provide a more in-depth description of cilia-mediated regulation of RTK and TGF-β signaling, including a discussion on how defects in the coordination of these pathways may be linked to ciliopathies.
OVERVIEW OF SIGNALING IN PRIMARY CILIA
The sensory capacity of primary cilia is reflected by the enriched localization of specified receptors in the ciliary membrane, where ligand binding activates downstream signaling events for transmission of information to the cell. Some of the best described examples of ciliary receptors include the 12 transmembrane SHH receptor PTCH1, which when bound to ligand exits the cilium concomitantly with ciliary entry of SMO, thereby initiating a series of downstream events to activate GLI-mediated target gene expression during development and in the adult (Mukhopadhyay and Rohatgi 2014; Pedersen et al. 2016), class A and B GPCRs that regulate various behavioral responses and tissue homeostasis (Schou et al. 2015; Hilgendorf et al. 2016), RTKs that function in cell migration and proliferation (Christensen et al. 2012), and TGF-β receptors (TGF-βRs) that are important in heart development (Koefoed et al. 2014). The latter two classes of receptors will be described in more detail below. Further, the membrane of primary cilia has been associated with receptors for extracellular matrix (ECM) proteins (McGlashan et al. 2006; Seeger-Nukpezah and Golemis 2012), purinergic receptors (Masyuk et al. 2008), as well as multiple types of ion channels of the TRP family, which similar to ciliary receptors for ECM proteins (McGlashan et al. 2006; Seeger-Nukpezah and Golemis 2012) were proposed to function in flow sensing and/or mechanosensation (Phua et al. 2015). Finally, Notch receptors, which are activated by the δ-like and Jagged families of transmembrane ligands expressed in trans on neighboring cells (Hori et al. 2013), were shown to localize to primary cilia to control skin development (Ezratty et al. 2011).
The multiplicity of ciliary receptor systems opens up a whole realm of possibilities for the primary cilium to coordinate the cross talking between different signaling pathways, which in a concerted action balances the biological output. To illustrate this, primary cilia are associated with numerous pathways that are well known to form complex signaling networks. These include mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)-AKT, Hippo, nuclear factor κ light-chain enhancer of activated B cells (NF-κB), and mammalian target of rapamycin (mTOR) pathways (Boehlke et al. 2010; Christensen et al. 2012; Wann et al. 2014; Hansen et al. 2015; Umberger and Caspary 2015). In addition, primary cilia were proposed to play a critical role in coordinating the balanced regulation of WNT/β-catenin versus WNT/PCP (planar cell polarity) pathways (Lienkamp et al. 2012; Oh and Katsanis 2013; Veland et al. 2013; Li et al. 2015; Saito et al. 2015), which extensively cross talk with SHH, TGF-β, RTK, MAPK, Hippo, and Notch signaling (Bernascone and Martin-Belmonte 2013; Zhang et al. 2014; Borggrefe et al. 2016; Zhang et al. 2016).
The sensory capacity of primary cilia evidently relies on regulation of ciliary formation and maintenance as well as dynamic localization of receptors and their downstream signaling components along the cilium–centrosome axis. Although much of the existing work on ciliary signaling has focused on discrete pathways and isolated cellular processes, there is an increasing interest in understanding how these pathways cooperate in the context of primary cilia, for instance, how HH signaling is modulated by Notch signaling (Kong et al. 2015; Stasiulewicz et al. 2015). Recent advances in proteome-wide analyses of ciliary protein networks (Mick et al. 2015) may prove helpful in profiling the mechanisms that set up and translate ciliary pathway interactions and how perturbation of these mechanisms causes disease.
PRIMARY CILIA AND RTK SIGNALING
Receptor tyrosine kinases comprise a large family of cell-surface receptors that play key roles in regulating cell proliferation, differentiation, survival, metabolism, migration, and cell-cycle control. The human genome encodes 58 known RTKs that are grouped into 20 distinct subfamilies, which all share a similar structural organization comprising an extracellular ligand-binding domain, a single transmembrane helix, a cytoplasmic region harboring the protein tyrosine kinase domain, as well as additional carboxy-terminal and juxta-membrane regulatory regions. On ligand binding, RTKs undergo phosphorylation at intracellular tyrosine residues, which triggers recruitment and binding of signaling proteins at these sites followed by activation of downstream signaling pathways (Lemmon and Schlessinger 2010). Interestingly, different RTKs generally signal via the same downstream signaling pathways (e.g., the MAPK, PI3K-AKT, and phospholipase Cγ [PLCγ]) pathways, yet produce distinct outcomes in many different cell types, most likely because of differences in signal timing, magnitude, and duration (Vasudevan et al. 2015). Ligand binding usually also induces receptor dimerization although some RTKs form dimers or higher-order oligomers even in the absence of ligand. Many RTKs are able to form both homodimeric as well as heterodimeric species with different ligand-binding and signaling properties, thereby expanding their functional repertoire. Moreover, RTKs cross talk extensively with each other as well as with other types of signaling receptors, thereby contributing to the formation of highly complex signaling networks (Lemmon and Schlessinger 2010). Given their importance in regulating a range of basic cellular processes, it is not surprising that mutations in genes coding for specific RTKs have been linked to numerous diseases, including many cancers, and the structure and function of many RTKs have, therefore, been intensely investigated over the years (for recent reviews, see Lemmon and Schlessinger 2010; Fantauzzo and Soriano 2015; McDonell et al. 2015). Here we will limit our discussion to RTKs that have been found to localize to primary cilia and/or that have been functionally coupled to this organelle.
Primary Cilia and Regulation of PDGFRαα Signaling
One of the first RTKs that was shown to localize to primary cilia is platelet-derived growth factor receptor α (PDGFRα) (Fig. 2) (Schneider et al. 2005), which is broadly required during embryogenesis (e.g., during development of the central nervous system, neural crest, and neural crest mesenchyme-derived structures, such as the cardiac outflow tract, the thymus, and skeletal components of the facial region). Consequently, targeted disruption of Pdgfra in the mouse is embryonic lethal and results in embryos with a range of developmental phenotypes (Soriano 1997; Andrae et al. 2008; Fantauzzo and Soriano 2015). Similar abnormalities (e.g., isolated cleft palate) have been observed in human patients harboring PDGFRA mutations (Rattanasopha et al. 2012). Moreover, dysregulation of PDGFRα signaling has been linked to various cancers and fibrotic diseases in humans (Andrae et al. 2008; Corless et al. 2011). In addition to PDGFRα, the mammalian PDGF signaling network includes a related RTK, PDGFRβ, as well as four ligands, PDGFA–D, that are known to bind as dimers to homodimeric PDGFR species with different affinities. For example, PDGF-AA specifically binds and activates homodimeric PDGFRαα, whereas PDGF-DD solely activates PDGFRββ (Andrae et al. 2008; Fantauzzo and Soriano 2015). Although a heterodimeric species of PDGFRαβ is known to exist (Rupp et al. 1994), its specificity and function remains unclear (Andrae et al. 2008; Gerhardt et al. 2013). Following ligand binding and receptor dimerization the PDGFR dimers undergo autophosphorylation to activate downstream signaling via the MAPK, PI3K-AKT, and PLCγ pathways (Andrae et al. 2008).
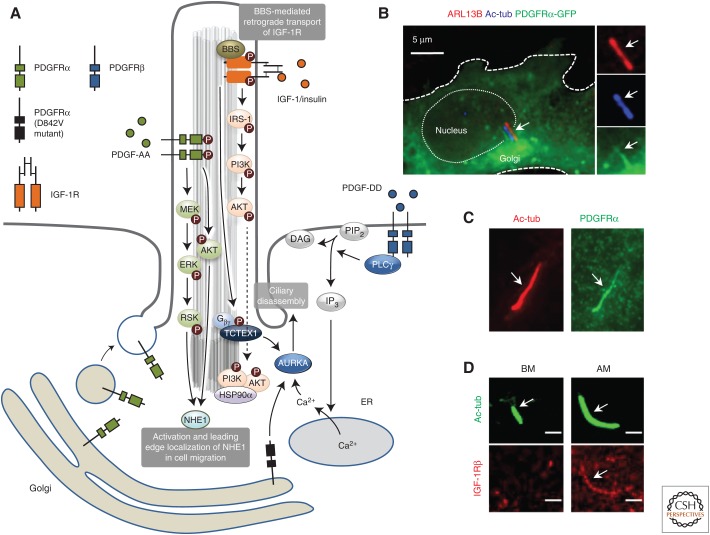
Figure 2.
Overview on receptor tyrosine kinase (RTK) signaling in the primary cilium. (A) Cartoon illustrating signaling pathways regulated by platelet-derived growth factor receptor (PDGFR)αα and insulin-like growth factor (IGF)-1R in the primary cilium as well as extraciliary RTK signaling in ciliary disassembly. (B) Fluorescence microscopy analysis on the localization of green fluorescent protein (GFP)-PDGFRα (green) to the Golgi and the primary cilium in retinal pigment epithelium (RPE) cells. Cilia (arrows) were costained with anti-ARL13B (red) and antiacetylated α-tubulin (Ac-tub, blue). (Panel from Nielsen et al. 2015; reprinted, with permission, from Company of Biologists.) (C) Fluorescence microscopy analysis on the localization of endogenous PDGFRα (green) to the primary cilium (Ac-tub, red) in mouse embryonic fibroblasts. (Panel from Schneider et al. 2005; reprinted, with permission, from Elsevier.) (D) Fluorescence microscopy analysis on ciliary length and localization of endogenous IGF-1Rβ (red) to primary cilia (Ac-tub, green) in human mesenchymal stem cells (hMSCs) cultured in basal media (BM), adipogenic media (AM), and basal medium (BM), and after 5 days. Scale bar, 10 µm. (Panel from Dalbay et al. 2015; reprinted under the terms of the Creative Commons Attribution License.)
Using specific antibodies and expression of GFP-tagged PDGFRα, Schneider and colleagues first showed that PDGFRα localizes to the primary cilium of mouse NIH3T3 cells and mouse embryonic fibroblasts (MEFs) (Fig. 1C), whereas PDGFRβ is largely absent from this compartment and predominantly distributed to patches on the plasma membrane (Schneider et al. 2005, 2010). In line with this result, analysis of PDGFRα mRNA and protein levels showed that PDGFRα is highly upregulated during serum deprivation in cultured cells, concomitantly with formation of the primary cilium (Lih et al. 1996; Schneider et al. 2005). Subsequent studies have confirmed cilia-specific localization of PDGFRα in a range of additional cell types, including rat astrocytes and neuroblasts (Danilov et al. 2009), mouse heart ventricular cells (Gerhardt et al. 2013), human embryonic stem cells (Awan et al. 2010), ovarian surface epithelial cells (Egeberg et al. 2012), and mouse osteoblasts (Noda et al. 2016). Of note, in some ciliated cell types, such as rat oligodendrocytes (Falcon-Urrutia et al. 2015) and mouse heart atrial cells (Gerhardt et al. 2013), PDGFRα seems to be conspicuously absent from the organelle, whereas in retinal pigment epithelial (RPE) cells, which are commonly used in ciliary studies, PDGFRα seems hardly to be expressed at all (Lei et al. 2011; Nielsen et al. 2015). Nevertheless, when GFP-tagged PDGFRα was expressed in RPE cells, the fusion protein was found to localize to the primary cilium (Fig. 2B) (Nielsen et al. 2015), suggesting that these cells indeed contain the machinery for targeting the receptor to this organelle. Moreover, careful examination of newly divided, cultured NIH3T3 cells revealed that PDGFRα localizes asynchronously to cilia of two sister cells, with the receptor preferentially accumulating in the cilium emanating from the cell with the oldest mother centriole (Anderson and Stearns 2009). It is possible that such age-dependent recruitment of PDGFRα to the cilium also operates in vivo, which may explain why the receptor is absent from cilia in certain cell types such as rat oligodendrocytes (Falcon-Urrutia et al. 2015) and mouse heart atrial cells (Gerhardt et al. 2013). The molecular determinants that confer cell-type- and/or age-dependent ciliary targeting of PDGFRα, as well as the mechanisms regulating its cilium- and/or cell-type-dependent expression are interesting avenues for future research.
Although studies using antibody staining of endogenous receptor or heterologous expression of GFP-tagged receptor have now confirmed localization of PDGFRα to the primary cilium in a variety of cell types (see above), cell-based assays in a range of mutant cells with specific ciliary assembly defects have substantiated a requirement for primary cilia in regulating PDGFRα-mediated signaling. First, in MEFs that contain a hypomorphic mutation in the gene encoding IFT-B complex protein IFT88 (Tg737orpk) (Murcia et al. 2000; Pazour et al. 2000), PDGFRαα-mediated signaling via the MEK1/2-ERK1/2 and PI3K-AKT pathways is ablated (Fig. 2A) (Schneider et al. 2005, 2010), and this is associated with impaired directional cell migration and wound healing by a mechanism involving the Na+/H+ exchanger NHE1 (Schneider et al. 2009; Clement et al. 2013b; for review, see Christensen et al. 2012). Mutations affecting other components of the IFT machinery also lead to impaired PDGFRαα signaling in MEFs in a manner that depends on the nature of the mutation and how it affects IFT and ciliary structure (Umberger and Caspary 2015). For example, mutant cells with defects in anterograde IFT components generally display low PDGFRα levels, whereas retrograde IFT mutants display normal levels of the receptor. Nevertheless, both classes of mutant cells fail to respond properly to PDGF-AA stimulation, presumably caused by dysregulated mTORC1 signaling and PP2A activity, which affect PDGFRα expression levels and PDGF-AA mediated signaling (Umberger and Caspary 2015). Furthermore, in heart ventricles from Ftm−/− mutant mice embryos, which lack the ciliary TZ protein RPGRIP1L, ciliary enrichment of PDGFRα and expression of the downstream target gene of PDGFRα signaling, Hif1α, are reduced (Gerhardt et al. 2013). These studies highlight the importance of cilia integrity for regulating PDGFRαα signaling at both the cellular as well as organ level, and are in line with the known roles of PDGFRα during development (Soriano 1997; Andrae et al. 2008; Fantauzzo and Soriano 2015). In addition, specific kinase-activating mutations in PDGFRα that are associated with increased PLCγ signaling and development of gastrointestinal stromal tumors (GISTs), such as the PDGFRα D842V mutation (Olson and Soriano 2009; Corless et al. 2011; Bahlawane et al. 2015), may lead to cilia loss by triggering calmodulin and Aurora A kinase-dependent cilia disassembly (Fig. 2A) (Pugacheva and Golemis 2006; Plotnikova et al. 2012; Nielsen et al. 2015). However, it remains to be determined whether cilia loss contributes to disease progression in GIST patients with the PDGFRα D842V mutation, although precursor cells of GIST are known to possess primary cilia (Castiella et al. 2013).
Primary Cilia and Regulation of Insulin and IGF-1 Signaling
In their inactive state the insulin and insulin-like growth factor (IGF-1) receptors (IR and IGF-1R) are unlike most RTKs covalent dimers composed of two extracellular α subunits and two transmembrane β subunits containing the tyrosine kinase domains (Hubbard 2013). The IR further comes in two isoforms, IR-A and IR-B, which can form receptor hybrids with IGF-1R that have different affinities for insulin and insulin-like growth factors (IGF-1 and IGF-2) (Belfiore et al. 2009). On ligand binding the dimers alter receptor conformation, which allows the catalytic domains to become activated followed by autophosphorylation at tyrosine residues beyond the catalytic site for recruitment of adaptor and effector proteins in signal transduction, including insulin receptor substrate 1 (IRS-1), which activates the PI3K/AKT pathway (Hubbard 2013; Cabail et al. 2015). In their activated forms, receptors of the insulin family play an essential role in the control of diverse cellular and physiological processes, including cell-cycle control, cell survival, programmed cell death, cell migration, cell differentiation, as well as metabolism (Taniguchi et al. 2006; Belfiore et al. 2009; Cohen and LeRoith 2012).
A link between primary cilia and IGF-1 signaling was first shown in 3T3-L1 preadipocytes, where cilia-mediated IGF-1R signaling was found to be important for induction of preadipocyte differentiation to adipocytes. Specifically, when ciliogenesis was ablated by knockdown of IFT88 or the anterograde IFT motor subunit Kif3a, the ability of the cells to respond to insulin was reduced; this was accompanied by reduced phosphorylation of IGF-1R and AKT at the base of the primary cilium (Fig. 2A), as well as decreased cellular expression of adipocyte transcription factors C/EBPα and PPARγ. Furthermore, a fraction of the cellular pool of IGF-1R was detected in primary cilia of the differentiating preadipocytes, and it was proposed that the receptors localized in the cilium are more sensitive to insulin stimulation than those present in the plasma membrane (Zhu et al. 2009). Similarly, in human mesenchymal stem cells (hMSCs), adipogenic differentiation was shown to involve cilia elongation and recruitment of IGF-1Rβ onto cilia (Fig. 2D), which was accompanied by increased nuclear accumulation of the early adipogenesis marker PPARγ (Dalbay et al. 2015). Interestingly, downstream transmission of ciliary IGF-1R signaling appears to rely on formation of a signaling platform at the ciliary base, composed of a complex of HSP90α, IRS-1, and AKT (Fig. 2A) (Wang et al. 2015). Ciliary compartmentalization of IGF-1/insulin signaling components may furthermore involve active intraciliary transport by the BBSome and IFT system (Fig. 2A), because a recent proteomics analysis indicated that IGF-1R accumulates dramatically in photoreceptor outer segments from Bbs17/Lztfl1 mutant mice (Datta et al. 2015), in which association of the BBSome with the retrograde IFT machinery is impaired (Seo et al. 2011; Lechtreck 2015). Furthermore, studies using additional Bbs mutant animal models as well as BBS patient cells have confirmed a role for BBS proteins in regulating trafficking and function IGF-1/insulin signaling components, specifically the IR-B isoform (Gerdes et al. 2014; Starks et al. 2015), which in turn is important for controlling whole body insulin action and glucose metabolism. However, the precise mechanisms by which BBS proteins control insulin signaling and metabolism are likely to be complex, and may involve cilium-dependent as well as cilium-independent functions of BBS proteins (Marion et al. 2012a; Gerdes et al. 2014; Starks et al. 2015). Finally, in addition to regulating cilia/BBS-dependent adipocyte differentiation and metabolism, IGF-1/insulin signaling components have also been implicated in ciliary disassembly and cell cycle progression. Specifically, activation of IGF-1R localized on the cilia of mouse fibroblasts and RPE cells was found to accelerate cilia resorption and G1-S phase progression via a noncanonical Gβγ signaling pathway culminating in recruitment of phospho(T94)Tctex-1 to the ciliary TZ (Fig. 2A) (Yeh et al. 2013). This pathway also seems to operate in neuronal progenitor cells such as radial glia where TZ localized phospho(T94)Tctex-1 may control cell fate choice by activating the ciliary disassembly factors HDAC6 and Aurora A kinase (Li et al. 2011; Yeh et al. 2013). Interestingly, patients with mutations in IGF-1 or IGF-1R were reported to suffer from microcephaly and mental retardation (Walenkamp et al. 2005; Walenkamp and Wit 2006), which may be caused by defects in cilia disassembly of neural progenitor cells (Gabriel et al. 2016). Of note, additional RTKs may cooperate with IGF-1R to promote cilia disassembly in a cell- or tissue-dependent manner, because in some cell types (e.g., mouse fibroblasts) significant IGF-1-induced ciliary disassembly was observed only in combination with other RTK ligands such as PDGF-AA (Jacoby et al. 2009; Nielsen et al. 2015). Moreover, because activation of nonciliary RTKs such as PDGFRβ induces robust ciliary disassembly, at least in some cell types (Nielsen et al. 2015), the signaling network(s) involved in control of ciliary disassembly and cell-cycle control is likely to be quite complex.
Additional RTKs Associated with Primary Cilia
Although PDGFRα and insulin/IGF-1 receptors are the most studied RTKs from a ciliary perspective, a number of other RTKs have also been linked to primary cilia in various ways (reviewed in Christensen et al. 2012). For example, epidermal growth factor (EGF) receptors were reported to localize to primary cilia in kidney epithelial cells (Ma et al. 2005), astrocytes, and neuroblasts (Danilov et al. 2009), as well as in airway smooth muscle cells where EGF signaling may contribute to mechanosensation and directed cell migration in cooperation with integrins and polycystins 1 and 2 (Wu et al. 2009). Polycystin signaling may also involve interaction with another RTK, c-Met, which was shown in mouse kidney cells to rely on polycystin 1 for appropriate ubiquitylation and signaling down-regulation in response to stimulation with the c-Met ligand, hepatocyte growth factor (HGF) (Qin et al. 2010). Interestingly, another member of the HGF receptor subfamily of RTKs, recepteur d’origine nantais (RON), was shown to localize to motile cilia in human airway epithelial cells where it may participate in regulation of ciliary beat frequency (Manzanares et al. 2007). Functional links to cilia have also been reported for fibroblast growth factor receptors (FGFRs), which seem to regulate ciliogenesis by promoting transcription of ciliogenic genes (Christensen et al. 2012). Further, FGFRs were reported to localize to motile cilia in the airways of the rhesus monkey (Evans et al. 2002) as well as to the basal body of primary cilia in mouse neural progenitor cells (Garcia-Gonzalez et al. 2016). Finally, angiopoietin receptors Tie1 and Tie2 were shown to localize to cilia of the murine female reproductive organs, including primary cilia of the surface epithelium of the ovary, bursa, and extra-ovarian rete ducts, as well as to motile cilia of the oviduct (Teilmann and Christensen 2005).
In summary, a growing number of RTKs have now been shown to localize to primary (and/or motile) cilia in a variety of cell types and tissues where they control important cellular and physiological processes in a context-dependent fashion. Ciliary RTKs are likely to cross talk extensively with each other, as well as with other ciliary signaling systems such as SHH signaling (Parathath et al. 2008; McGowan and McCoy 2013) to ensure balanced signaling outputs.
PRIMARY CILIA AND TGF-β SIGNALING
The superfamily of TGF-β signaling provides one of the most fascinating systems of cellular communication, in which the cellular response greatly relies on the cellular context. This means that the effects of the same ligand can be quite different depending on the cell type and the conditions. The superfamily comprises more than 30 different ligand types of the TGF-β–activin–Nodal and bone morphogenetic protein (BMP) subfamilies, which act in paracrine or autocrine manners to activate receptor serine/threonine kinases of types I and II (TGF-βRI/II and BMP-RI/II, respectively) (Massague 2012). Canonical signaling is propagated through the activation of SMAD transcription factors (R-SMADs) and a plethora of so-called noncanonical pathways, which together with other receptor systems form extensive circuits of cross talking. In canonical signaling, the type I receptor is responsible for phosphorylation of R-SMADs, which comprise SMAD2/3 in TGF-β–activin–Nodal signaling and SMAD1/5/8 in BMP signaling. The R-SMADs then interact with SMAD4 for nuclear translocation as well as with different DNA-binding transcription factors, which are activated by other receptor pathways, such as in RTK, HH, Hippo, and WNT signaling (Guo and Wang 2009; Varelas and Wrana 2012; Zhang et al. 2016). Signaling includes the expression of SMAD7, which antagonizes TGF-β signaling through multiple mechanisms in the cytoplasm and in the nucleus, including SMURF1/2-mediated ubiquitination and degradation of TGF-βRI and R-SMADs (Yan and Chen 2011). SMAD7 also suppresses WNT/β-catenin signaling (Han et al. 2006), illustrating another layer of cross talking based on feedback inhibition.
Likewise, both type I and II receptors are able to regulate a series of non-R-SMAD pathways that impinge on multiple cellular and physiological processes. As an example, TGF-βRI-mediated activation of TGF-β-activated kinase 1 (TAK1) plays a critical role in activation of MAPKs, RhoA/Cdc42 GTPases, and NF-κB signaling, which defines a critical step in balancing diverse signaling networks (Yan and Chen 2011). In NF-κB signaling, TAK1 and TAK1 bindings proteins (TAB1/2) mediate the phosphorylation of the IKKα/β/λ complex, which in turn phosphorylates inhibitor of NF-κB (I-κB), leading to its proteasomal degradation followed by nuclear translocation of NF-κB and activation of NF-κB target genes (Kim and Choi 2012). Further, TGF-βRII directly phosphorylates the cell polarity protein PAR6, which controls axon formation in neocortical neurons (Yi et al. 2010) as well as cell polarity processes, which when aberrantly regulated are associated with both tumor promoting and premetastatic effects (Heldin et al. 2012). Indeed, the extensive cross talking between different pathways from both canonical and noncanonical signaling pathways are responsible for the balanced and cell-context-dependent expression of thousands of different target genes to control a myriad of cellular processes (Ranganathan et al. 2007).
A critical step in the balancing of diverse pathways in TGF-β/BMP signaling includes the internalization of receptors by CME (Balogh et al. 2013; Ehrlich 2016). A well-described example includes activation of SMAD2/3 signaling, in which internalization of activated receptors into early endosomes (EEs) greatly enhances the phosphorylation of the R-SMADs. Here, SARA (SMAD anchor for receptor activation) binds to the PtdIns3P-enriched membrane of the endosomes via its FYVE zinc finger domain to enable the association between TGF-βRI and the R-SMADs (Sorkin and von-Zastrow 2009). Conversely, caveolae-mediated endocytosis has been proposed to enhance noncanonical signaling through MAPKs and PI3K-AKT (Zuo and Chen 2009). However, this view has been challenged by the recent findings that clathrin-coated and caveolae vesicles may fuse to form multifunctional compartments in TGF-β signaling (He et al. 2015). Because the CiPo is a site for extensive CME, this has led to the suggestion that the primary cilium may play a role in coordination of R-SMAD signaling (Clement et al. 2013a) as well as other pathways, which rely on receptor internalization, including HH (Pal et al. 2016) and tumor necrosis factor α (TNF-α) signaling (Rattner et al. 2010). In the following, we will give an overview on the association between TGF-β signaling and primary cilia and how this coupling is linked to the regulation of cellular and physiological processes.
Coupling Primary Cilia to TGF-β Signaling
Ciliopathies comprise a series of syndromic disorders, which extensively overlaps with phenotypes associated with aberrant TGF-β/BMP signaling. Prominent examples include structural heart defects associated with congenital heart disease (CHD) (Koefoed et al. 2014), suggesting that cilia may play a critical role in heart development through the coordination of TGF-β/BMP signaling. To some extent, this is true for nodal cilia, which are motile units that create a leftward flow of fluid across the embryonic node for defining lateral asymmetry (Hirokawa et al. 2012). In this scenario, the flow creates a gradient of Nodal ligands that accumulate at the left side of the node to activate SMAD2/3 signaling, which regulates specified gene expression profiles for asymmetric morphogenesis (Shiratori and Hamada 2014). Consequently, defects in nodal cilia cause heterotaxy and isolated CHD in humans that that arise from abnormal looping and remodeling of the heart tube into a multichambered organ (Chen et al. 2010). Further, primary cilia are present throughout the embryonic heart, and, in some cases, these cilia are expressed and distributed in a spatiotemporal manner during the developmental stages of the heart development and morphogenesis. Therefore, cardiac primary cilia may also contribute to cellular events regulated by TGF-β/BMP signaling morphogenetic events during heart development.
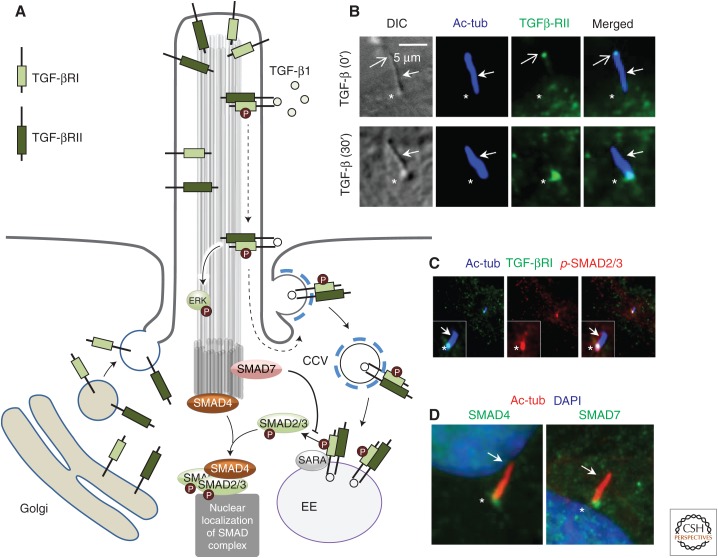
In support of a function of cardiac cilia in TGF-β signaling during heart development, the formation of primary cilia was reported to suppress SMAD2/3 signaling in endothelial cells thereby preventing shear stress-mediated EndoMT, which is required to populate the endocardial cushions for proper development into fibrous valves (Egorova et al. 2011). Although these studies implicated a suppressive function of primary cilia in canonical TGF-β signaling, other studies have shown that primary cilia in conjunction with CME at the CiPo are required to maintain an operative level of TGF-β signaling in fibroblasts as well as in stem cells undergoing in vitro cardiomyogenesis (Clement et al. 2013a). In fibroblasts, different components of the TGF-β signaling machinery, including TGF-βRI, TGF-βRII, SMAD2/3, SMAD4, and SMAD7 are present at the cilia–centrosome axis as evidenced by immunolocalization with specific antibodies (Fig. 3), and upon TGF-β1 stimulation the receptors accumulate at the CiPo for CME-mediated activation of SMAD2/3 (Fig. 3A–C). Similarly, TGF-β receptors localize to primary cilia of human embryonic stem cells (Vestergaard et al. 2016), and TGF-β-mediated in vitro differentiation of mouse stem cells into cardiomyocytes is linked to a dramatic buildup of these receptors at the ciliary base where SMAD2/3 is activated. Stimulation with TGF-β1 ligand in fibroblasts also leads to increased activation of the ERK1/2 (Fig. 3A) at the ciliary base, but this seems to be independent of CME, because phosphorylation of ERK1/2 is not affected by inhibition of clathrin-mediated processes. In support of a function of primary cilia in TGF-β signaling, proteomics of primary cilia in IMCD3 cells based on proximity labeling identified SMAD2/3 and SMAD4 as well as several proteins in noncanonical signaling, such as TAB1, ERK1/2, AKT, and Rho GTPases (Mick et al. 2015).
Figure 3.
Overview of transforming growth factor β (TGF-β) signaling in the primary cilium. (A) Cartoon illustrating TGF-β signaling at the primary cilium. (B) Fluorescence microcopy analysis on the localization of endogenous TGF-βRII (green) at the primary cilium before (0′) and 30 min after TGF-β1 stimulation (30′). Primary cilia are shown with differential interference contrast microcopy (DIC) and stained with antiacetylated α-tubulin (Ac-tub, blue, closed arrows). The ciliary base region is marked with asterisks and the ciliary tip with open arrows. (C) Fluorescence microcopy analysis on the accumulation of TGF-βRI (green) and phospho-SMAD2/3 (p-SMAD2/3, red) at the ciliary base region after 30 min of TGF-β1 stimulation. (D) Fluorescence microscopy analysis of localization of SMAD4 (green, left panel) and SMAD7 (green, right panel) to the ciliary base region (asterisks). The cilium (Ac-tub, red) is marked with closed arrows, and nuclei were stained with DAPI (blue). All images were obtained from cultures of human foreskin fibroblasts. (From Clement et al. 2013a; reprinted, with permission, from Elsevier.)
Interestingly, Tg737orpk mutant fibroblasts, which contain a hypormorphic mutation in the IFT88 gene, show reduced TGF-β1-mediated SMAD2/3 signaling at the base of stunted primary cilia (Clement et al. 2013a). Although this observation is in line with the idea that cilia formation is required for proper TGF-β signaling, further studies showed that mutant cells display reduced CME at the ciliary base region (Clement et al. 2013a). This may indicate that IFT88 plays a role in setting up the CiPo for endocytic events that control TGF-β signaling. In support of this idea, IFT88 was shown to be essential for organization, orientation, and function of the flagellar pocket in Trypanosoma brucei (Absalon et al. 2008), which roots the flagellum and defines a unique microdomain for exocytosis and receptor-meditated endocytosis at the posterior end of this organism (Field and Carrington 2009). Indeed, cells of Trypanosoma brucei subjected RNAi-mediated depletion of IFT88 display perturbations of vesicular trafficking, including a drastic reduction in endocytosis (Absalon et al. 2008).
Finally, TGF-β signaling has been linked to length control and TZ function in both primary and motile cilia. As an example, inhibition of SMAD2 signaling reduces the length of motile cilia at the gastrocoel roof plate, at the Xenopus left–right (LR) organizer, and at the neural tube and the epidermis, and blockage of TGF-β signaling was suggested to impair the structure and/or function of the ciliary TZ as evidenced by lack of the TZ protein B9D1/MSKR-1 in cilia emerging from the epidermis (Tozser et al. 2015). In chondrocytic cells, TGF-β signaling was reported to suppress the levels of Ift88 mRNA stability, leading to a reduced average length and number of primary cilia (Kawasaki et al. 2015), and in the disorganized growth plate of Smad1/5CKO mutant mice, the orientation of projecting primary cilia is disturbed (Ascenzi et al. 2011). These results suggest that TGF-β signaling plays multiple roles in cilia, and future studies should focus on the mechanisms by which the CiPo is organized and regulated to control endo- and exocytic events in ciliary signaling, and how TGF-β signaling itself impacts on ciliary length and TZ organization. Further, it will be important to address the mechanisms that control the movement of TGF-β receptors into and out of the cilium proper and how this impinges on the balanced regulation of diverse signaling pathways to control cellular and physiological processes during development and in tissue homeostasis.
CONCLUDING REMARKS
Primary cilia play a critical role in the coordination of multiple cellular signaling pathways that control diverse cellular and physiological processes, and when aberrantly regulated may be the cause of syndromic disorders and disease. Here we have presented an overview of our current understanding of the connection between primary cilia and regulation of RTK and TGF-β signaling in mammalian cells, which offer a platform from which to understand complex signaling machineries during development and in function of tissues and organs in the adult. Clearly, many questions still need to be resolved; especially in terms of the molecular mechanisms of ciliary targeting and dynamic trafficking of RTKs and TGF-β receptors into and out of the cilium proper, and how the spatiotemporal regulation of these events contributes to the balanced regulation of signaling networks. Furthermore, it will be of interest to investigate how endocytic events as well as recycling of receptors to the cilium may contribute to these networks.
ACKNOWLEDGMENTS
We apologize to our colleagues whose work we could not cite because of space limitations. This work is supported by grants from the Danish Council for Independent Research (1331-00254; 6108-004578), the Novo Nordisk Foundation (NNF15OC0016886), and the University of Copenhagen Excellence Programme for Interdisciplinary Research (2016 Funds). S.K.M. and J.B.M. are partially supported by PhD fellowships from the Department of Biology, University of Copenhagen.
Footnotes
Editors: Wallace Marshall and Renata Basto
Additional Perspectives on Cilia available at www.cshperspectives.org
REFERENCES
- Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, Toutirais G, Buisson J, Robinson D, Bastin P. 2008. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J Cell Sci 121: 3704–3716. [DOI] [PubMed] [Google Scholar]
- Al JA, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. 2014. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516: 104–107. [DOI] [PubMed] [Google Scholar]
- Anderson CT, Stearns T. 2009. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol 19: 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. 2008. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascenzi MG, Blanco C, Drayer I, Kim H, Wilson R, Retting KN, Lyons KM, Mohler G. 2011. Effect of localization, length and orientation of chondrocytic primary cilium on murine growth plate organization. J Theor Biol 285: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan A, Oliveri RS, Jensen PL, Christensen ST, Andersen CY. 2010. Immunoflourescence and mRNA analysis of human embryonic stem cells (hESCs) grown under feeder-free conditions. Methods Mol Biol 584: 195–210. [DOI] [PubMed] [Google Scholar]
- Bahlawane C, Eulenfeld R, Wiesinger MY, Wang J, Muller A, Girod A, Nazarov PV, Felsch K, Vallar L, Sauter T, et al. 2015. Constitutive activation of oncogenic PDGFRα-mutant proteins occurring in GIST patients induces receptor mislocalisation and alters PDGFRα signalling characteristics. Cell Commun Signal 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh P, Katz S, Kiss AL. 2013. The role of endocytic pathways in TGF-β signaling. Pathol Oncol Res 19: 141–148. [DOI] [PubMed] [Google Scholar]
- Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. 2015. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol 17: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauss K, Knapp B, Jores P, Roepman R, Kremer H, Wijk EV, Marker T, Wolfrum U. 2014. Phosphorylation of the Usher syndrome 1G protein SANS controls Magi2-mediated endocytosis. Hum Mol Genet 23: 3923–3942. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. 2009. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30: 586–623. [DOI] [PubMed] [Google Scholar]
- Benmerah A. 2013. The ciliary pocket. Curr Opin Cell Biol 25: 78–84. [DOI] [PubMed] [Google Scholar]
- Bernascone I, Martin-Belmonte F. 2013. Crossroads of Wnt and Hippo in epithelial tissues. Trends Cell Biol 23: 380–389. [DOI] [PubMed] [Google Scholar]
- Bhogaraju S, Engel BD, Lorentzen E. 2013. Intraflagellar transport complex structure and cargo interactions. Cilia 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C. 2011. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci 108: 2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, et al. 2010. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F, Giaimo BD. 2016. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGF-β/BMP and hypoxia pathways. Biochim Biophys Acta 1863: 303–313. [DOI] [PubMed] [Google Scholar]
- Cabail MZ, Li S, Lemmon E, Bowen ME, Hubbard SR, Miller WT. 2015. The insulin and IGF1 receptor kinase domains are functional dimers in the activated state. Nat Commun 6: 6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiella T, Munoz G, Luesma MJ, Santander S, Soriano M, Junquera C. 2013. Primary cilia in gastric gastrointestinal stromal tumours (GISTs): An ultrastructural study. J Cell Mol Med 17: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Norris D, Bhattacharya S. 2010. Transcriptional control of left–right patterning in cardiac development. Pediatr Cardiol 31: 371–377. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Clement CA, Satir P, Pedersen LB. 2012. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 226: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, et al. 2013a. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 3: 1806–1814. [DOI] [PubMed] [Google Scholar]
- Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, Pedersen SF, Christensen ST. 2013b. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci 126: 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DH, LeRoith D. 2012. Obesity, type 2 diabetes, and cancer: The insulin and IGF connection. Endocr Relat Cancer 19: F27– F45. [DOI] [PubMed] [Google Scholar]
- Corless CL, Barnett CM, Heinrich MC. 2011. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer 11: 865–878. [DOI] [PubMed] [Google Scholar]
- Dalbay MT, Thorpe SD, Connelly JT, Chapple JP, Knight MM. 2015. Adipogenic differentiation of hMSCs is mediated by recruitment of IGF-1R onto the primary cilium associated with cilia elongation. Stem Cells 33: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov AI, Gomes-Leal W, Ahlenius H, Kokaia Z, Carlemalm E, Lindvall O. 2009. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia 57: 136–152. [DOI] [PubMed] [Google Scholar]
- Datta P, Allamargot C, Hudson JS, Andersen EK, Bhattarai S, Drack AV, Sheffield VC, Seo S. 2015. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet–Biedl syndrome. Proc Natl Acad Sci 112: E4400–E4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. 2001. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31: 277–287. [DOI] [PubMed] [Google Scholar]
- Egeberg DL, Lethan M, Manguso R, Schneider L, Awan A, Jorgensen TS, Byskov AG, Pedersen LB, Christensen ST. 2012. Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten DP, Poelmann RE, Hierck BP. 2011. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, et al. 2014. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell 31: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. 2016. Endocytosis and trafficking of BMP receptors: Regulatory mechanisms for fine-tuning the signaling response in different cellular contexts. Cytokine Growth Factor Rev 27: 35–42. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Fanucchi MV, Van Winkle LS, Baker GL, Murphy AE, Nishio SJ, Sannes PL, Plopper CG. 2002. Fibroblast growth factor-2 during postnatal development of the tracheal basement membrane zone. Am J Physiol Lung Cell Mol Physiol 283: L1263–L1270. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. 2011. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145: 1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon-Urrutia P, Carrasco CM, Lois P, Palma V, Roth AD. 2015. Shh signaling through the primary cilium modulates rat oligodendrocyte differentiation. PLoS ONE 10: e0133567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantauzzo KA, Soriano P. 2015. Receptor tyrosine kinase signaling: Regulating neural crest development one phosphate at a time. Curr Top Dev Biol 111: 135–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Carrington M. 2009. The trypanosome flagellar pocket. Nat Rev Microbiol 7: 775–786. [DOI] [PubMed] [Google Scholar]
- Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F, et al. 2016. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J 35: 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez D, Murcia-Belmonte V, Esteban PF, Ortega F, Diaz D, Sanchez-Vera I, Lebron-Galan R, Escobar-Castanondo L, Martinez-Millan L, Weruaga E, et al. 2016. Anosmin-1 over-expression increases adult neurogenesis in the subventricular zone and neuroblast migration to the olfactory bulb. Brain Struct Funct 221: 239–260. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Christou-Savina S, Xiong Y, Moede T, Moruzzi N, Karlsson-Edlund P, Leibiger B, Leibiger IB, Ostenson CG, Beales PL, et al. 2014. Ciliary dysfunction impairs β-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat Commun 5: 5308. [DOI] [PubMed] [Google Scholar]
- Gerhardt C, Lier JM, Kuschel S, Ruther U. 2013. The ciliary protein Ftm is required for ventricular wall and septal development. PLoS ONE 8: e57545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Satir P. 1972. The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53: 494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang XF. 2009. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res 19: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten DP, Lin X, et al. 2006. Smad7-induced β-catenin degradation alters epidermal appendage development. Dev Cell 11: 301–312. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Moroishi T, Guan KL. 2015. YAP and TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol 25: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. 2005. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Yan X, Li N, Dang S, Xu L, Zhao B, Li Z, Lv Z, Fang X, Zhang Y, et al. 2015. Internalization of the TGF-β type I receptor into caveolin-1 and EEA1 double-positive early endosomes. Cell Res 25: 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Vanlandewijck M, Moustakas A. 2012. Regulation of EMT by TGF-β in cancer. FEBS Lett 586: 1959–1970. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. 2011. Ciliopathies. N Engl J Med 364: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf KI, Johnson CT, Jackson PK. 2016. The primary cilium as a cellular receiver: Organizing ciliary GPCR signaling. Curr Opin Cell Biol 39: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y. 2012. Cilia, KIF3 molecular motor and nodal flow. Curr Opin Cell Biol 24: 31–39. [DOI] [PubMed] [Google Scholar]
- Hori K, Sen A, Artavanis-Tsakonas S. 2013. Notch signaling at a glance. J Cell Sci 126: 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wittekind SG, Barr MM. 2007. STAM and Hrs down-regulate ciliary TRP receptors. Mol Biol Cell 18: 3277–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR. 2013. The insulin receptor: Both a prototypical and atypical receptor tyrosine kinase. Cold Spring Harb Perspect Biol 5: a008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. 2009. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Jensen VL, Li C, Bowie RV, Clarke L, Mohan S, Blacque OE, Leroux MR. 2015. Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. EMBO J 34: 2537–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan OI, Doroquez DB, Cevik S, Bowie RV, Clarke L, Sanders AA, Kida K, Rappoport JZ, Sengupta P, Blacque OE. 2012. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr Biol 22: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Ezura Y, Hayata T, Notomi T, Izu Y, Noda M. 2015. TGF-β suppresses Ift88 expression in chondrocytic ATDC5 cells. J Cell Physiol 230: 2788–2795. [DOI] [PubMed] [Google Scholar]
- Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. 2012. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell 22: 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Choi ME. 2012. TGF-β-activated kinase-1: New insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res Clin Pract 31: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac DC, Saez-Rodriguez J, Swantek J, Burke JM, Lauffenburger DA, Sorger PK. 2012. Creating and analyzing pathway and protein interaction compendia for modelling signal transduction networks. BMC Syst Biol 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Dynlacht BD. 2011. Regulating the transition from centriole to basal body. J Cell Biol 193: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koefoed K, Veland IR, Pedersen LB, Larsen LA, Christensen ST. 2014. Cilia and coordination of signaling networks during heart development. Organogenesis 10: 108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JH, Yang L, Dessaud E, Chuang K, Moore DM, Rohatgi R, Briscoe J, Novitch BG. 2015. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev Cell 33: 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage K, Mollgard K, Greenway S, Wakimoto H, Gorham JM, Workman CT, Bendsen E, Hansen NT, Rigina O, Roque FS, et al. 2010. Dissecting spatio-temporal protein networks driving human heart development and related disorders. Mol Syst Biol 6: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF. 2015. IFT-cargo interactions and protein transport in cilia. Trends Biochem Sci 40: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Rheaume MA, Velez G, Mukai S, Kazlauskas A. 2011. Expression of PDGFRα is a determinant of the PVR potential of ARPE19 cells. Invest Ophthalmol Vis Sci 52: 5016–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. 2011. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol 13: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BI, Matteson PG, Ababon MF, Nato AQ Jr, Lin Y, Nanda V, Matise TC, Millonig JH. 2015. The orphan GPCR, Gpr161, regulates the retinoic acid and canonical Wnt pathways during neurulation. Dev Biol 402: 17–31. [DOI] [PubMed] [Google Scholar]
- Lienkamp S, Ganner A, Walz G. 2012. Inversin, Wnt signaling and primary cilia. Differentiation 83: S49–S55. [DOI] [PubMed] [Google Scholar]
- Lih CJ, Cohen SN, Wang C, Lin-Chao S. 1996. The platelet-derived growth factor α-receptor is encoded by a growth-arrest-specific (gas) gene. Proc Natl Acad Sci 93: 4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. 2008. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell 15: 854–865. [DOI] [PubMed] [Google Scholar]
- Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. 2005. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D, Monzon ME, Savani RC, Salathe M. 2007. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol 37: 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Mockel A, De MC, Obringer C, Claussmann A, Simon A, Messaddeq N, Durand M, Dupuis L, Loeffler JP, et al. 2012a. BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell Metab 16: 363–377. [DOI] [PubMed] [Google Scholar]
- Marion V, Stutzmann F, Gerard M, De MC, Schaefer E, Claussmann A, Helle S, Delague V, Souied E, Barrey C, et al. 2012b. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet–Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet 49: 317–321. [DOI] [PubMed] [Google Scholar]
- Massague J. 2012. TGF-β signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, LaRusso NF. 2008. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295: G725–G734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell LM, Kernohan KD, Boycott KM, Sawyer SL. 2015. Receptor tyrosine kinase mutations in developmental syndromes and cancer: Two sides of the same coin. Hum Mol Genet 24: R60–R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. 2006. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem 54: 1005–1014. [DOI] [PubMed] [Google Scholar]
- McGowan SE, McCoy DM. 2013. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol 305: L229–L239. [DOI] [PubMed] [Google Scholar]
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV. 2015. Proteomics of primary cilia by proximity labeling. Dev Cell 35: 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourão A, Christensen ST, Lorentzen E. 2016. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol 41: 98–108. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Rohatgi R. 2014. G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin Cell Dev Biol 33: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. 2000. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left–right axis determination. Development 127: 2347–2355. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Malinda RR, Schmid FM, Pedersen SF, Christensen ST, Pedersen LB. 2015. PDGFRβ and oncogenic mutant PDGFRα D842V promote disassembly of primary cilia through a PLCγ- and AURKA-dependent mechanism. J Cell Sci 128: 3543–3549. [DOI] [PubMed] [Google Scholar]
- Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y. 2016. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc Natl Acad Sci 113: E2589–E2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DP, Grimes DT. 2012. Mouse models of ciliopathies: The state of the art. Dis Model Mech 5: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Katsanis N. 2013. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol 24: 10–18. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P. 2009. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M, Moudjou M, Delacroix H, Bornens M. 1992. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol 108: 107–128. [DOI] [PubMed] [Google Scholar]
- Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S. 2016. Smoothened determines β-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol 212: 861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parathath SR, Mainwaring LA, Fernandez L, Campbell DO, Kenney AM. 2008. Insulin receptor substrate 1 is an effector of sonic hedgehog mitogenic signaling in cerebellar neural precursors. Development 135: 3291–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. 2000. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Veland IR, Schroder JM, Christensen ST. 2008. Assembly of primary cilia. Dev Dyn 237: 1993–2006. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Mogensen JB, Christensen ST. 2016. Endocytic control of cellular signaling at the primary cilium. Trends Biochem Sci 10.1016/j.tibs.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Phua SC, Lin YC, Inoue T. 2015. An intelligent nano-antenna: Primary cilium harnesses TRP channels to decode polymodal stimuli. Cell Calcium 58: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. 2012. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell 23: 2658–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Golemis EA. 2006. HEF1-Aurora A interactions: Points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle 5: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Taglienti M, Nauli SM, Contrino L, Takakura A, Zhou J, Kreidberg JA. 2010. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest 120: 3617–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Agrawal A, Bhushan R, Chavalmane AK, Kalathur RK, Takahashi T, Kondaiah P. 2007. Expression profiling of genes regulated by TGF-β: differential regulation in normal and tumour cells. BMC Genomics 8: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanasopha S, Tongkobpetch S, Srichomthong C, Siriwan P, Suphapeetiporn K, Shotelersuk V. 2012. PDGFRa mutations in humans with isolated cleft palate. Eur J Hum Genet 20: 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner JB, Sciore P, Ou Y, van der Hoorn FA, Lo IK. 2010. Primary cilia in fibroblast-like type B synoviocytes lie within a cilium pit: A site of endocytosis. Histol Histopathol 25: 865–875. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Blacque OE, Leroux MR. 2012. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 13: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepman R, Wolfrum U. 2007. Protein networks and complexes in photoreceptor cilia. Subcell Biochem 43: 209–235. [DOI] [PubMed] [Google Scholar]
- Rupp E, Siegbahn A, Ronnstrand L, Wernstedt C, Claesson-Welsh L, Heldin CH. 1994. A unique autophosphorylation site in the platelet-derived growth factor α receptor from a heterodimeric receptor complex. Eur J Biochem 225: 29–41. [DOI] [PubMed] [Google Scholar]
- Saito S, Tampe B, Muller GA, Zeisberg M. 2015. Primary cilia modulate balance of canonical and noncanonical Wnt signaling responses in the injured kidney. Fibrogenesis Tissue Repair 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. 2007. Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. 2010. The primary cilium at a glance. J Cell Sci 123: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E, Lauer J, Durand M, Pelletier V, Obringer C, Claussmann A, Braun JJ, Redin C, Mathis C, Muller J, et al. 2014. Mesoaxial polydactyly is a major feature in Bardet–Biedl syndrome patients with LZTFL1 (BBS17) mutations. Clin Genet 85: 476–481. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. 2005. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15: 1861–1866. [DOI] [PubMed] [Google Scholar]
- Schneider L, Stock CM, Dieterich P, Jensen BH, Pedersen LB, Satir P, Schwab A, Christensen ST, Pedersen SF. 2009. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-α in the primary cilium. J Cell Biol 185: 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, et al. 2010. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem 25: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou KB, Pedersen LB, Christensen ST. 2015. Ins and outs of GPCR signaling in primary cilia. EMBO Rep 16: 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger-Nukpezah T, Golemis EA. 2012. The extracellular matrix and ciliary signaling. Curr Opin Cell Biol 24: 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. 2011. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet 7: e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. 2009. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. 2014. TGF-β signaling in establishing left-right asymmetry. Semin Cell Dev Biol 32: 80–84. [DOI] [PubMed] [Google Scholar]
- Soriano P. 1997. The PDGF α receptor is required for neural crest cell development and for normal patterning of the somites. Development 124: 2691–2700. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von-Zastrow M. 2009. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starks RD, Beyer AM, Guo DF, Boland L, Zhang Q, Sheffield VC, Rahmouni K. 2015. Regulation of insulin receptor trafficking by Bardet–Biedl syndrome proteins. PLoS Genet 11: e1005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiulewicz M, Gray SD, Mastromina I, Silva JC, Bjorklund M, Seymour PA, Booth D, Thompson C, Green RJ, Hall EA, et al. 2015. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development 142: 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska K, Johnson CA. 2012. The transition zone: An essential functional compartment of cilia. Cilia 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao D, Verhey KJ. 2015. Gated entry into the ciliary compartment. Cell Mol Life Sci 73: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. 2006. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96. [DOI] [PubMed] [Google Scholar]
- Teilmann SC, Christensen ST. 2005. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int 29: 340–346. [DOI] [PubMed] [Google Scholar]
- Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, Christensen ST. 2005. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev 71: 444–452. [DOI] [PubMed] [Google Scholar]
- Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. 2006. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol 191: 525–535. [DOI] [PubMed] [Google Scholar]
- Tozser J, Earwood R, Kato A, Brown J, Tanaka K, Didier R, Megraw TL, Blum M, Kato Y. 2015. TGF-β signaling regulates the differentiation of motile cilia. Cell Rep 11: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberger NL, Caspary T. 2015. Ciliary transport regulates PDGF-AA/αα signaling via elevated mammalian target of rapamycin signaling and diminished PP2A activity. Mol Biol Cell 26: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Rosti RO, Gibbs E, Gleeson JG. 2014. Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Wrana JL. 2012. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol 22: 88–96. [DOI] [PubMed] [Google Scholar]
- Vasudevan HN, Mazot P, He F, Soriano P. 2015. Receptor tyrosine kinases modulate distinct transcriptional programs by differential usage of intracellular pathways. eeLife 4: e07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veland IR, Montjean R, Eley L, Pedersen LB, Schwab A, Goodship J, Kristiansen K, Pedersen SF, Saunier S, Christensen ST. 2013. Inversin/nephrocystin-2 is required for fibroblast polarity and directional cell migration. PLoS ONE 8: e60193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard ML, Awan A, Warzecha CB, Christensen ST, Andersen CY. 2016. Immunofluorescence microscopy and mRNA analysis of human embryonic stem cells (hESCs) including primary cilia associated signaling pathways. Methods Mol Biol 1307: 123–140. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. 2006. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin–Darby canine kidney (MDCK) cells. Proc Natl Acad Sci 103: 18556–18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM. 2006. Genetic disorders in the growth hormone–insulin-like growth factor-I axis. Horm Res 66: 221–230. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van DJ, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, et al. 2005. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab 90: 2855–2864. [DOI] [PubMed] [Google Scholar]
- Wang H, Zou X, Wei Z, Wu Y, Li R, Zeng R, Chen Z, Liao K. 2015. Hsp90α forms a stable complex at the cilium neck for the interaction of signalling molecules in IGF-1 receptor signalling. J Cell Sci 128: 100–108. [DOI] [PubMed] [Google Scholar]
- Wann AK, Chapple JP, Knight MM. 2014. The primary cilium influences interleukin-1β-induced NFκB signalling by regulating IKK activity. Cell Signal 26: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. 2011. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. 2009. Characterization of primary cilia in human airway smooth muscle cells. Chest 136: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Chen YG. 2011. Smad7: Not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J 434: 1–10. [DOI] [PubMed] [Google Scholar]
- Yeh C, Li A, Chuang JZ, Saito M, Caceres A, Sung CH. 2013. IGF-1 activates a cilium-localized noncanonical Gβγ signaling pathway that regulates cell-cycle progression. Dev Cell 26: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. 2010. TGF-β signaling specifies axons during brain development. Cell 142: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pizzute T, Pei M. 2014. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 358: 633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tian XJ, Xing J. 2016. Signal transduction pathways of EMT Induced by TGF-β, SHH, and WNT and their crosstalks. J Clin Med 5: E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Shi S, Wang H, Liao K. 2009. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci 122: 2760–2768. [DOI] [PubMed] [Google Scholar]
- Zuo W, Chen YG. 2009. Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell 20: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]