Abstract
Mutations in bone morphogenetic protein 1 (BMP1) in humans or deletion of BMP1 and related protease tolloid like 1 (TLL1) in mice lead to osteogenesis imperfecta (OI). Here, we show progressive periodontal defects in mice in which both BMP1 and TLL1 have been conditionally ablated, including malformed periodontal ligament (PDL) (recently shown to play key roles in normal alveolar bone formation), significant loss in alveolar bone mass (P < 0.01), and a sharp reduction in cellular cementum. Molecular mechanism studies revealed a dramatic increase in the uncleaved precursor of type I collagen (procollagen I) and a reduction in dentin matrix protein 1 (DMP1), which is partially responsible for defects in extracellular matrix (ECM) formation and mineralization. We also showed a marked increase in the expression of matrix metallopeptidase 13 (MMP13) and tartrate-resistant acid phosphatase (TRAP), leading to an acceleration in periodontal breakdown. Finally, we demonstrated that systemic application of antibiotics significantly improved the alveolar bone and PDL damage of the knockdown phenotype, which are thus shown to be partially secondary to pathogen-induced inflammation. Together, identification of the novel roles of BMP1 and TLL1 in maintaining homeostasis of periodontal formation, partly via biosynthetic processing of procollagen I and DMP1, provides novel insights into key contributions of the extracellular matrix environment to periodontal homeostasis and contributes toward understanding of the pathology of periodontitis.
Keywords: periodontal tissues/periodontium, ECM, matrix biology, craniofacial biology/genetics, mineralized tissue/development, inflammation
Introduction
Periodontal disease is initiated by pathogenic microorganisms in the biofilm, although lifestyle risk factors and systemic diseases play roles in modifying progress of the disease. There is also a genetic component to periodontal disease, as people in certain families are more likely to develop periodontal disease despite aggressive oral care habits (Meng et al. 2011; Genco and Borgnakke 2013).
Bone morphogenetic protein 1 (BMP1) and tolloid-like 1 (TLL1), which are encoded by 2 different genes and belong to a small family of extracellular metalloproteinases, share a similar structure, distribution of expression and some redundancy of function (Ge and Greenspan 2006). For example, these proteinases enhance extracellular matrix (ECM) assembly and accelerate collagen fibril formation by removing the C-propeptides from procollagen precursors (Kessler et al. 1996). Studies have also demonstrated that the BMP1-like proteinases are capable of activating transforming growth factor (TGF)–β1 (Vadon-Le Goff et al. 2015), a powerful signaling molecule that is involved in upregulation of ECM synthesis. Importantly, mutations in the gene BMP1 on chromosome 8p21 have been shown to be causal in development of osteogenesis imperfecta (OI) type XIII (OMIM 614856) (Valadares et al. 2014).
Conventional knockout (KO) of the BMP1 gene Bmp1 (Suzuki et al. 1996) or TLL1 gene Tll1 (Clark et al. 1999) in mice leads to embryonic death, preventing study of postnatal functions. Thus, we generated a compound conditional KO mouse line in which both Bmp1 and Tll1 are together deleted postnatally via tamoxifen-induced, ubiquitous Cre expression in tissues (Muir et al. 2014). This approach avoids both the barriers of lethality and issues of functional redundancy. These null mice displayed an OI-like phenotype, including poorly formed bone (weakened and brittle) with spontaneous fractures, osteomalacia, thinned/porous cortical bone, reduced processing of procollagen and dentin matrix protein 1 (DMP1), remarkably high bone turnover, and defective osteocyte maturation accompanied by decreased expressions of osteocyte markers (Muir et al. 2014). Furthermore, we have recently shown severe tooth phenotypes in these doubly knocked-out mice, including short and thin root dentin, defects in dentin mineralization, and delayed tooth eruption (Wang et al. 2017). Together, these studies documented vital roles of BMP1 and TLL1 in postnatal bone and tooth development.
In the present study, we attempted to investigate the roles of BMP1 and TLL1 in contributions to periodontal ligament (PDL) formation and remodeling using multiple techniques that combined X-ray, micro–computed tomography (µCT), histological stain, and immunohistochemistry at 2 developmental stages: 8 wk (reflecting an early stage of PDL formation) and 17 wk (reflecting the PDL remodeling stage) of age. Our data revealed progressively developing periodontal defects (such as irregular PDL fibers, alveolar bone loss, poor mineralization, reduced cementum masses, and an acceleration in periodontal breakdown) in the double knockout (dKO) mice. Mechanism studies revealed a dramatic decrease in DMP1 levels related to mineralization defects and a decrease in the biosynthetic processing of procollagen I (the precursor molecule to type I collagen) related to defects in ECM structure. Importantly, these phenotypes were significantly improved by a 3-wk period of antibiotic treatment. Thus, we conclude not only that BMP1/TLL1 activities are essential for normal periodontium formation and maintenance but that deficits in such activities can be the basis for susceptibility to bacterially induced periodontitis as well.
Materials and Methods
Mice Breeding
BMP1/TLL1 dKO mice were generated as previously described (Muir et al. 2014). Briefly, mice homozygous for both Bmp1flox and Tll1flox alleles were crossed with mice with the Cre-ERT2 transgene under control of the human ubiquitin C promoter (Ruzankina et al. 2007) to obtain mice in which tamoxifen treatment induced ubiquitous KO of BMP1 and TLL1. Tamoxifen-treated Bmp1flox/flox; Tll1flox/flox mice lacking the Cre transgene were used as controls. Tamoxifen was administered to both experimental and control mice via intraperitoneal (IP) injection (100 mg/kg body weight) starting at 4 wk of age (injections every day for 5 d in the fourth week and for 5 d in the fifth week of age and then twice a week after that until sacrifice) and harvested at 8 wk and 17 wk of age, respectively. A subset of the tamoxifen-treated dKO and control mice was given the antibiotics sulfamethoxazole (8 mg/mL) and trimethoprim (1.6 mg/mL) in their drinking water for 3 wk, from 5 wk of age to 8 wk of age. Water with fresh antibiotics was changed daily (Delima et al. 2001). In addition, the oral cavities of these mice were rinsed with chlorhexidine gluconate during antibiotics treatment. All animal protocols were approved by the Animal Care and Use Committees at Texas A&M College of Dentistry and at the University of Wisconsin School of Medicine and Public Health. This study conformed with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for preclinical animal studies.
Radiography and µCT
Mandibles were analyzed using X-ray radiography (Faxitron X-Ray LLC) and by a µ-CT35 imaging system. For the µCT analyses, we initially used a medium-resolution scan (7.0-µm slice increments) of the whole mandible to obtain an overall assessment of the tooth shape and structure. We then took a high-resolution scan (3.5-µm slice increments) of the mandible with 400 slices selected for analyses of the alveolar bone around the first molar. Of note, the tooth and PDL were excluded when contouring. The bone volume (BV) to total volume (TV) ratio was analyzed using Scanco Medical software. We quantified the distance between the cementum-enamel junction (CEJ) and the alveolar crest using ImageJ (National Institutes of Health) to evaluate the role of antibiotic treatment in maintaining the height of the alveolar crest.
Sample Preparation and Histochemistry
Right mandibles were fixed in 70% ethanol and used for radiographs and embedded in methylmethacrylate (MMA; Buehler) (Sun et al. 2011) for radiographs, µCT, Masson-Goldner staining, fluorescein isothiocyanate (FITC), and scanning electron microscopy (SEM), and 10-µm undecalcified mesiodistal sections were stained using Masson-Goldner staining, as previously described (Ma et al. 2011). Left mandibles were fixed in buffered 4% paraformaldehyde, decalcified in ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin using standard histological procedures as previously described (Fen et al. 2002), and 4-µm-thick mesiodistal serial sections were used for hematoxylin and eosin (H&E), tartrate-resistant acid phosphatase (TRAP), Sirius red, and immunohistochemistry stains. For the latter, 1:100 BMP1 (Abcam), 1:400 DMP1 (Baba et al. 2004), 1:200 bone sialoprotein (BSP) (Sun et al. 2010) (generously donated by Dr. Chunlin Qin, Texas A&M College of Dentistry), 1:100 matrix metallopeptidase 13 (MMP13) (MilliporeSigma), 1:100 osteopontin (OPN) (Santa Cruz), 1:1,000 periostin (Innovative Research), 1:1,000 collagen I C-telopeptide (LF-68), and 1:1,000 collagen I C-propeptide (LF-41) (kindly provided by Dr. Larry Fisher, National Institutes of Health) antibodies were employed. ImageJ was used to calculate the number of osteoclasts per bone perimeter (N. Oc/B. Pm).
FITC Stain and Confocal Imaging
Jaw bones were dehydrated through a series of ethanol solutions from 70% to 100% and acetone solution, followed by FITC stain (Sigma) overnight, with additional dehydration and MMA embedding. A cross section (300–400 µm thick) was cut with a diamond-bladed saw (Buehler), and the plastic sections were then sanded and ground to a final thickness of 30 to 50 µm for confocal imaging (Ren et al. 2014).
Statistical Analysis
Statistical significance was determined by an independent-sample t test using SPSS 19.0 (SPSS, Inc.). A P value of <0.05 was considered statistically significant.
Results
DKO Mice Displayed Progressive Defects in Periodontium
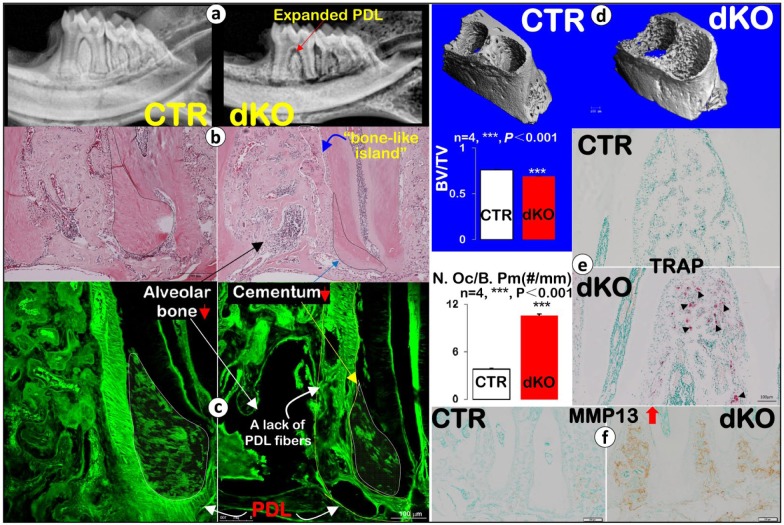
We have previously shown the tamoxifen treatment regimens similar to those used here to yield highly efficient excision of Bmp1 and Tll1 sequences in this mouse model system (Muir et al. 2014, 2016). In the present study, we also show sharply reduced BMP1 protein expression levels in the dKO PDL and alveolar bone areas (Appendix Fig. 1: a, 8 wk; and b, 17 wk; right panels). Next, using radiographs, we documented an overall reduction in alveolar BV and a great increase in PDL areas in the 8-wk dKO mice (Fig. 1a, right panel). Employing H&E staining, we confirmed a decrease in alveolar BV and an expanded PDL (Fig. 1b). We also found that many PDL areas were filled by islands of bone-like cells, with few signs of PDL fibrous cells in the dKO (Appendix Fig. 2a′). Furthermore, the dKO cementum volume was greatly reduced, with almost half of the cellular cementum area showing an unusually “pale” appearance (Appendix Fig. 2b′). Similarly, H&E staining images showed a lack of pink color in the dKO bone, dentin, and cementum (Appendix Fig. 2b′), indicating a relative lack of mineralization and aberrant formation of these hard tissues. The Masson-Goldner staining assay in non-decalcified tissues (Appendix Fig. 2c′) further confirmed the “bony islands (red color)” in the dKO PDL matrix were osteoid. Detailed analyses of the non-decalcified periodontium by the FITC stain method under a confocal microscope revealed irregularly organized PDL fibers and reduced cementum mass with fewer cementocytes (Fig. 1c). In addition, quantitative data, based on µCT images, showed alveolar bone loss in dKO samples that was statistically significant (Fig. 1d, n = 4; ***P < 0.001). Furthermore, the 8-wk dKO mice showed remarkable increases in TRAP-positive cells via both qualitative observation and quantitative analysis (Fig. 1e, n = 4; ***P < 0.001), with a similarly large increase in the expression of MMP13 (Fig. 1f, the key enzyme released from osteoclasts and responsible for breakdown of extracellular matrices). In addition, TRAP-positive cells were observed on the surfaces of cellular and acellular cementum (Appendix Fig. 3a). It is highly likely that these increases in TRAP-positive cells and MMP13 levels contribute to alveolar bone loss. Furthermore, the dKO acellular cementum, a critical component of the periodontium, especially in its attachment function, also displayed pathological changes (poorly formed ECM with irregular and rough borders) (Appendix Fig. 3b).
Figure 1.
Eight-wk-old Bmp1/Tll1 double knockout (dKO) mice develop periodontal defects. (a) Representative X-ray images revealed alveolar bone loss and expanded periodontal ligament (PDL) in dKO mice (red arrow). (b) Hematoxylin and eosin staining showed alveolar bone loss (black arrow), a bone-like island (outlined in white), and much reduced cementum mass (black dashed line) with expanded PDL in dKO mice. (c) Confocal FITC images showed dKO alveolar bone loss, a lack of PDL fibers (yellow line), and reduced cementum mass with fewer cementocytes (white line). (d) Data from micro–computed tomography (µCT) analysis confirmed the dKO alveolar bone loss; quantitative analysis of bone volume (BV)/total volume (TV) of alveolar bone showed a statistically significant difference between dKO and control (CTR) samples (n = 4, ***P < 0.001). (e) Tartrate-resistant acid phosphatase (TRAP) stain images showed many more positive osteoclasts (black arrowheads) in dKO samples, which was statistically different from the control group (n = 4, ***P < 0.001), and (f) immunohistochemistry stains showed dramatic increases in matrix metallopeptidase 13 (MMP13) in dKO samples.
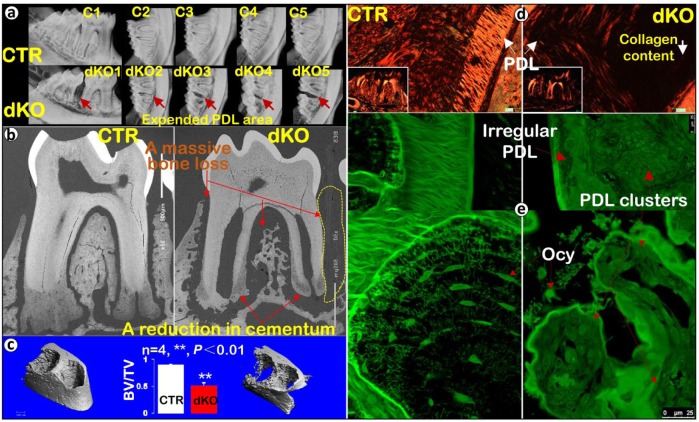
To analyze the extent of continuing roles for BMP1/TLL1 in periodontal homeostasis during adulthood, we deleted both genes at 4 wk of age and sacrificed the dKO mice with their age-matched controls at 17 wk of age. X-ray images showed more severe alveolar bone loss in the 17-wk-old dKO mice (Fig. 2a, lower panel) than had appeared at 8 wk of age (Figs. 1a and d). This assessment was in agreement with data from acid-etched SEM (Appendix Fig. 4a, right panel). In fact, in the area between the first and second molars, there was essentially a complete lack of alveolar bone, as observed via radiographic images (Fig. 2a), backscattered SEM (Fig. 2b), and H&E staining (Appendix Fig. 5a). The backscattered SEM image further confirmed a much reduced cementum mass in dKO mice (Fig. 2b), in which there were fewer cementocytes compared with the age-matched controls in the acid-etched SEM image (Appendix Fig. 4a, right panel). In addition, µCT data demonstrated a severe alveolar bone loss (~50% loss), which was statistically significant (Fig. 2c, n = 4, **P < 0.01). TRAP staining revealed a marked increase in osteoclasts in 17-wk dKO alveolar bone compared with controls (Appendix Fig. 5b). Taken together, the above data documented a progressive loss in dKO periodontal tissues (including PDL, alveolar bone, and cellular cementum) and a sustained increase in osteoclast numbers in the dKO mice, supporting critical roles for BMP1/TLL1 in maintaining periodontal homeostasis.
Figure 2.
The 17-wk-old Bmp1/Tll1 double knockout (dKO) mice display advanced periodontal defects. (a) X-ray images showed massive loss of alveolar bone between the dKO first and second molars. (b) Backscattered scanning electron microscopy images confirmed a great reduction in both bone and cementum masses in dKO mice. (c) Micro–computed tomography data showed advanced dKO alveolar bone loss, qualitatively and quantitatively (n = 4, **P < 0.01). (d) Sirius red staining imaged via polarized microscopy displayed an expanded dKO periodontal ligament (PDL) area with sharply reduced collagen fibers. (e) Images of fluorescein isothiocyanate staining displayed an irregularly organized dKO PDL layer with sparse, rounded osteocytes (Ocy). In contrast, in control samples, fibers filled in the entire PDL area and osteocytes were spindle shaped and well organized. BV, bone volume; CTR, control; TV, total volume.
Morphological and Molecular Changes in dKO Periodontal ECM
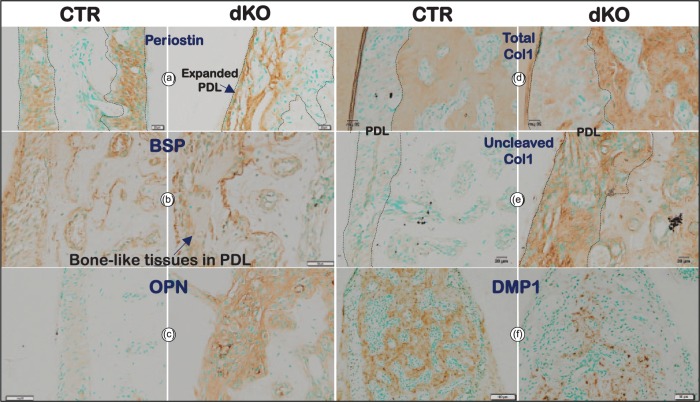
Sirius red stain images obtained by polarized light microscopy showed a great reduction of collagen fibers in PDL (Fig. 2d, right panel). Further changes were noted via FITC staining, which displayed an irregular PDL area, numerous PDL clusters with few signs of PDL fibers, a great reduction in osteocyte number, and a change in osteocyte shape from spindle shaped to rounded (Fig. 2e, right panels) compared with the age-matched controls (Fig. 2e, left panels). In contrast to these changes, levels of periostin (a matrix protein essential for PDL function) were similar in dKO and control mice (Fig. 3a and Appendix Fig. 6a), suggesting that deficits in this protein do not contribute to the dKO abnormal PDL phenotype.
Figure 3.
Bmp1/Tll1 deletion results in dramatic changes in molecular markers at 8 wk of age. (a) Periostin immunostaining revealed expanded double knockout (dKO) periodontal ligaments (PDLs) with irregular fibers. (b–e) Immunostaining showed marked increases in the levels of bone cell markers bone sialoprotein (BSP) (b) and osteopontin (OPN) (c) in dKO PDLs and in the levels of Col I in dKO PDLs (antibody against the α1(I) chain C-telopeptide recognized all forms of Col I [Total Col I]; antibody against the α1(I) chain C-propeptide only recognized uncleaved immature forms of Col I, d–e), whereas (f) dentin matrix protein 1 (DMP1) immunostaining displayed greatly reduced levels in dKO alveolar bone. CTR, control.
To define bone marker changes in dKO mice, we examined levels of BSP (an early marker for osteoblasts; Fig. 3b and Appendix Fig. 7a) and OPN (a late marker for osteoblasts; Fig. 3c and Appendix Fig. 7b). A great increase was noted in both markers in dKO PDL and alveolar bone, indicative of an active bone formation status. To define whether a reduction in the biosynthetic processing of procollagen I into its mature form might contribute to reduced Col I deposition in tissues, we used 2 different antibodies against the C-telopeptide (staining all forms of Col I, including procollagen, processing intermediates, and mature Col I, thus reflecting total Col I content; Fig. 3d) and C-propeptide (recognizing only uncleaved procollagen I, the processing intermediate pC-Col I, which retains the C-propeptide, but not mature Col I, thus reflecting levels of cleavage enzyme activity; Fig. 3e), respectively. Signal from staining with both antibodies was low in the control (Fig. 3d–e, left panels), whereas signal was high in dKO PDL and alveolar bone (especially for the C-propeptide-specific antibody), indicating large accumulations of uncleaved procollagen I in both PDL and alveolar bone (Fig. 3d–e, right panels). Finally, we examined levels of DMP1, a critical marker of mature osteocytes (the most abundant cell in bone), and found this marker to be dramatically reduced (Fig. 3f and Appendix Fig. 6b). This reduction thus indicated a differentiation defect in most bone cells in dKO samples. In summary, the above morphological and molecular changes present a complex combination of deficits in periodontium formation and remodeling.
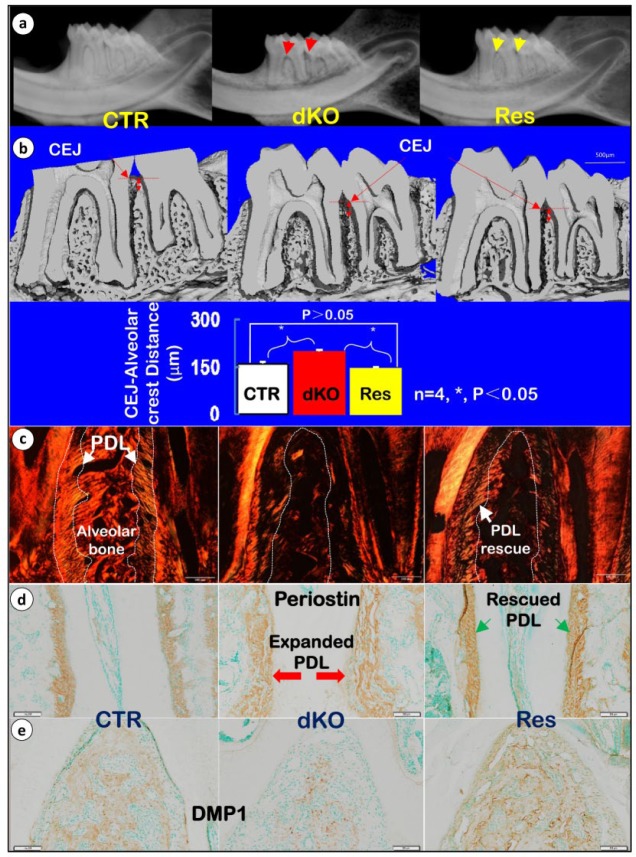
Antibiotic Treatment Partially Improved Periodontal Defects in dKO Mice
To investigate the potential roles of inflammation in periodontal defects, we employed a combined approach (improved oral hygiene and 3-wk systemic applications of antibiotics) that was originally developed by Delima et al. (2001) to attempt rescue of periodontal defects. This regimen was started at 5 wk of age and ended at 8 wk of age. Representative X-ray images show a great improvement in overall alveolar bone loss in response to these treatments (Fig. 4a). In addition, µCT data demonstrated restoration of the distance between the CEJ and alveolar crest, with ImageJ software showing such restoration to be statistically significant (Fig. 4b, n = 4, *P < 0.05). Remarkably, antibiotic treatment partly restored dKO PDL integrity, as evidenced by the appearance of some regularly organized PDL fibers, documented by polarized microscopy imaging (Fig. 4c) and as evidenced by periostin immunohistochemistry (IHC) images that showed improved PDL morphology (Fig. 4d and Appendix Fig. 6a). Furthermore, alveolar BV and bone quality were notably improved in the treatment group, as reflected by a restoration of DMP1 expression (Fig. 4e and Appendix Fig. 6b). In addition, we also found a decrease in expressions of BSP and OPN after antibiotic treatment (Appendix Fig. 7a, b) compared with the alveolar bone of untreated dKO mice. Together, the above data clearly demonstrate the causative role of inflammation in development of the dKO periodontal phenotype, with susceptibility toward inflammation likely caused in large part by loss of PDL integrity.
Figure 4.
Antibiotics treatment can partially rescue periodontal defects in double knockout (dKO) mice. (a) Representative radiographs reveal restoration (Res) of alveolar bone loss in dKO mice. (b) Micro–computed tomography data show a restoration of alveolar bone, especially in maintaining the height of alveolar crest. (c) Sirius red staining imaged by polarized microscopy is shown for the 3 groups (control [CTR], dKO, and Res mice treated with antibiotics and chlorhexidine gluconate). (d) Restoration of periodontal ligament (PDL) fiber morphology, as detected by periostin immunostaining. (e) Recovery of dentin matrix protein 1 (DMP1) levels in antibiotics-treated mice, as detected by immunostaining. CEJ, cementum-enamel junction.
Discussion
Recent studies have identified mutations in BMP1, a proteinase involved in ECM formation, in cases of OI, which also show some dentin defects and alveolar bone loss (Asharani et al. 2012; Syx et al. 2015). In addition, deletions of Bmp1/Tll1 have been shown to result in defects in bone (Muir et al. 2014) and tooth dentin formation (Wang et al. 2017). To determine the extent to which the overlapping activities of BMP1 and TLL1 might contribute to the ECM homeostasis essential for normal periodontium development and maintenance, we studied the effects of deleting these 2 related proteinases postnatally in periodontium in 2 age groups: 8 and 17 wk of age. Salient phenotypic features included severely disorganized PDL fibers, poorly formed bone matrices and extreme alveolar bone loss, and sharply increased numbers of osteoclasts. Detailed morphological and molecular mechanism analyses uncovered a loss of PDL integrity (Figs. 1–3 and Appendix Fig. 6a) that indicated likely roles for BMP1/TLL1 in the control of ECM formation via regulating formation of the mature Col I form.
The dKO mice displayed poorly formed alveolar bone and cellular cementum with hypomineralization (Figs. 1–2 and Appendix Fig. 2). An abnormally large amount of osteoid was found in alveolar bone, as well as sharp increases in osteoblast markers (BSP and OPN) and uncleaved procollagen I, but greatly reduced levels of osteocyte marker (DMP1) (Fig. 3). There were also dramatic reductions in osteocyte and cementocyte numbers (Fig. 2b, e and Appendix Fig. 4). Thus, BMP1/TLL1 are shown to have profound roles in the formation/maintenance of alveolar bone and cementum. Also observed in dKO jaws were dramatic increases in TRAP-positive cells (osteoclasts) and MMP13 (Fig. 1e, f and Appendix Fig. 5b), key elements responsible for the breakdown of periodontium. Importantly, we demonstrated that improving oral hygiene and systemic applications of antibiotics significantly improved the aberrant dKO oral phenotype and protein expression patterns (Fig. 4). This finding evidences the key roles that BMP1/TLL1 play in maintaining PDL integrity via proteolytic processing to produce the mature form of Col I and active DMP1, which is in turn essential to protecting periodontium from microbial infection, and also supports the conclusion that inflammation was an important contributing factor in breakdown of dKO periodontium. Of note, the hygiene/antibiotics treatments could not totally ameliorate the dKO periodontal phenotype, underscoring the critical roles of BMP1/TLL1 in periodontium formation and ECM homeostasis, which extend beyond their roles in preventing microbial infection.
DMP1, an acidic phosphorylated extracellular matrix protein, was first isolated from dentin and later found in bone and cementum (Qin et al. 2007). Several studies have revealed that DMP1 may promote differentiation of mesenchymal cells into odontoblast-like cells, thereby enhancing mineralization (Narayanan et al. 2001). In fact, our previous study showed an essential role for DMP1 in maintaining periodontium. For example, deletion of the DMP1 gene Dmp1 leads to defects in PDL, alveolar bone, and cementum, resulting in increased susceptibility to bacterial infection (Ye et al. 2008; Ren et al. 2016). In the present study, tissue levels of DMP1 are shown to dramatically decrease in dKO periodontium but to increase in response to hygiene/antibiotics treatment, accurately reflecting alterations of bone quality. Thus, results here are in agreement with previous studies, supporting the importance of DMP1 in periodontal health.
It is well documented that both bone resorption and formation are greatly increased in periodontitis patients and animal models, although bone resorption is dominant, leading to bone losses (Graves et al. 2011). In search of the progenitors responsible for alveolar bone formation, we recently demonstrated, via cell lineage tracing and mineral double-labeling approaches, that PDL progenitor cells serve as the major initiators of alveolar bone formation (Ren et al. 2015). The current study supports this hypothesis, as many immature “bone-like islands” were observed to develop in dKO PDL (Fig. 1b and Appendix Fig. 2), which also showed elevated levels of bone cell markers (BSP and OPN), although mature bone marker DMP1 was absent in this tissue (Fig. 3).
On the basis of the above findings, we propose that BMP1 and TLL1, 2 extracellular proteinases with overlapping functions, maintain matrix homeostasis in periodontium (especially in PDL) via provision of mature Col I and active DMP1. An accumulation of uncleaved procollagen I, a lack of the mature Col I, and reductions in active DMP1 lead to severe periodontal defects. Inflammation resulting from periodontal defects may contribute to a more serious periodontal destruction (Fig. 5).
Figure 5.
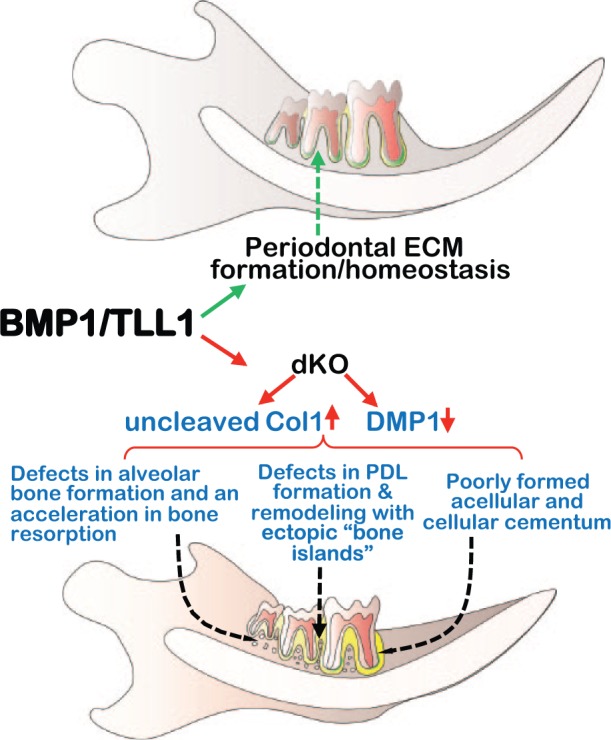
A model depicting critical roles for bone morphogenetic protein 1 (BMP1)/tolloid like 1 (TLL1) in formation and maintenance of periodontium. Here we propose that the closely related proteinases BMP1/TLL1 are required for maintaining matrix homeostasis in periodontium. Ablation of these proteinases led to periodontal ligament (PDL) and cementum defects with severe alveolar bone loss. DCM1, dentin matrix protein 1; dKO, double knockout; ECM, extracellular matrix.
Author Contributions
J. Wang, D. Massoudi, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; Y. Ren, A.M. Muir, contributed to data acquisition, drafted the manuscript; S.E. Harris, D.S. Greenspan, and J.Q. Feng, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This study was partially supported by NIH grants AR047746 to D.S. Greenspan, DE024797 to S.E. Harris, and DE025014 to J.Q. Feng.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, Yigit G, Pohl E, Becker J, Frommolt P, et al. 2012. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 90(4):661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. 2004. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 23(6):371–379. [DOI] [PubMed] [Google Scholar]
- Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, Hogan BL, Greenspan DS. 1999. The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development. 126(12):2631–2642. [DOI] [PubMed] [Google Scholar]
- Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, Graves DT. 2001. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol. 28(3):233–240. [DOI] [PubMed] [Google Scholar]
- Fen JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. 2002. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 17(10):1822–1831. [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. 2006. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today. 78(1):47–68. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Borgnakke WS. 2013. Risk factors for periodontal disease. Periodontol 2000. 62(1):59–94. [DOI] [PubMed] [Google Scholar]
- Graves DT, Li J, Cochran DL. 2011. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 90(2):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. 1996. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 271(5247):360–362. [DOI] [PubMed] [Google Scholar]
- Ma D, Zhang R, Sun Y, Rios HF, Haruyama N, Han X, Kulkarni AB, Qin C, Feng JQ. 2011. A novel role of periostin in postnatal tooth formation and mineralization. J Biol Chem. 286(6):4302–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Ren X, Tian Y, Feng X, Xu L, Zhang L, Lu R, Shi D, Chen Z. 2011. Genetic study of families affected with aggressive periodontitis. Periodontol 2000. 56(1):87–101. [DOI] [PubMed] [Google Scholar]
- Muir AM, Massoudi D, Nguyen N, Keene DR, Lee SJ, Birk DE, Davidson JM, Marinkovich MP, Greenspan DS. 2016. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biol. 56:114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AM, Ren Y, Butz DH, Davis NA, Blank RD, Birk DE, Lee SJ, Rowe D, Feng JQ, Greenspan DS. 2014. Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice. Hum Mol Genet. 23(12):3085–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. 2001. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci U S A. 98(8):4516–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, D’Souza R, Feng JQ. 2007. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 86(12):1134–1141. [DOI] [PubMed] [Google Scholar]
- Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, Qin C, Ke H, Liu M, Feng JQ. 2015. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J. 29(7):2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Han X, Jing Y, Yuan B, Ke H, Liu M, Feng JQ. 2016. Sclerostin antibody (Scl-Ab) improves osteomalacia phenotype in dentin matrix protein 1(Dmp1) knockout mice with little impact on serum levels of phosphorus and FGF23. Matrix Biol. 52–54:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Lin S, Jing Y, Dechow PC, Feng JQ. 2014. A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect Tissue Res. 55(Suppl 1):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 1(1):113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Gandhi V, Prasad M, Yu W, Wang X, Zhu Q, Feng JQ, Hinton RJ, Qin C. 2010. Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the condylar cartilage of rat mandible. Int J Oral Maxillofac Surg. 39(3):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lu Y, Chen L, Gao T, D’Souza R, Feng JQ, Qin C. 2011. DMP1 processing is essential to dentin and jaw formation. J Dent Res. 90(5):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. 1996. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to drosophila tolloid. Development. 122(11):3587–3595. [DOI] [PubMed] [Google Scholar]
- Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, Hermanns-Le T, Coucke PJ, De Paepe A, Malfait F. 2015. Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J Bone Miner Res. 30(8):1445–1456. [DOI] [PubMed] [Google Scholar]
- Vadon-Le Goff S, Hulmes DJ, Moali C. 2015. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol. 44–46:14–23. [DOI] [PubMed] [Google Scholar]
- Valadares ER, Carneiro TB, Santos PM, Oliveira AC, Zabel B. 2014. What is new in genetics and osteogenesis imperfecta classification? J Pediatr (Rio J). 90(6):536–541. [DOI] [PubMed] [Google Scholar]
- Wang J, Muir AM, Ren Y, Massoudi D, Greenspan DS, Feng JQ. 2017. Essential roles of bone morphogenetic protein-1 and mammalian tolloid-like 1 in postnatal root dentin formation. J Endod. 43(1):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zhang S, Ke H, Bonewald LF, Feng JQ. 2008. Periodontal breakdown in the Dmp1 null mouse model of hypophosphatemic rickets. J Dent Res. 87(7):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.