Abstract
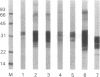
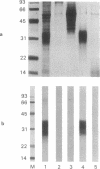
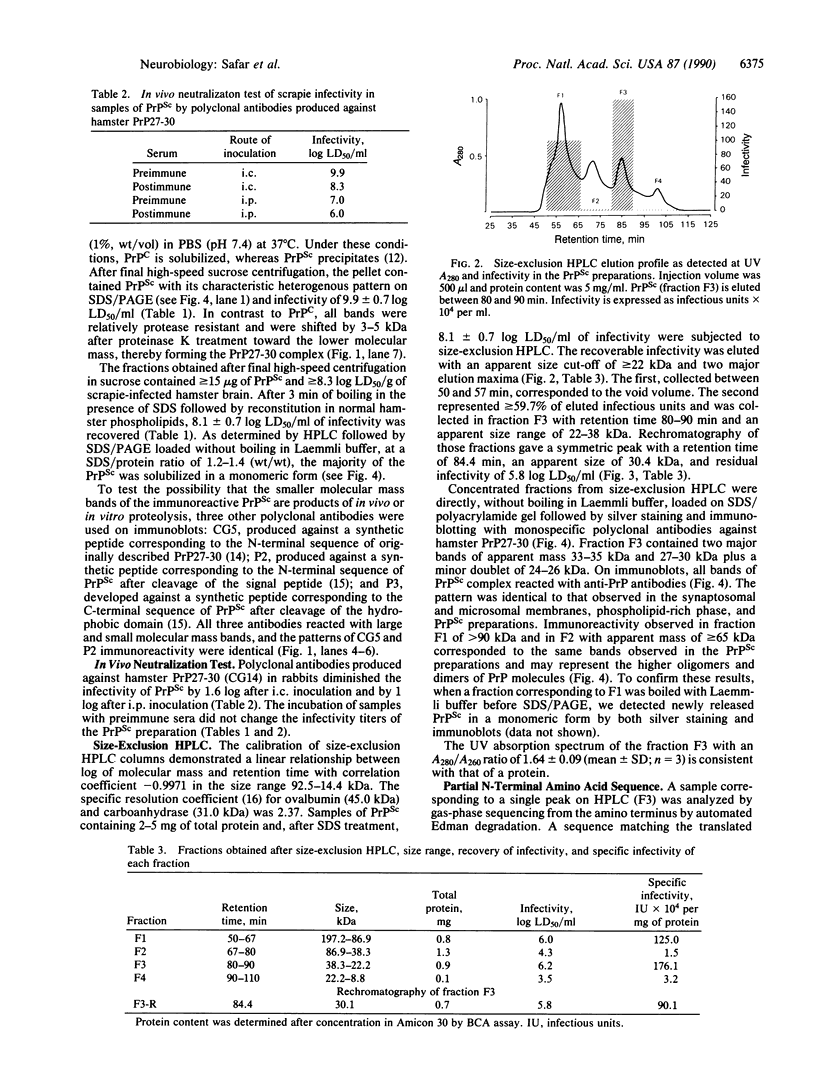
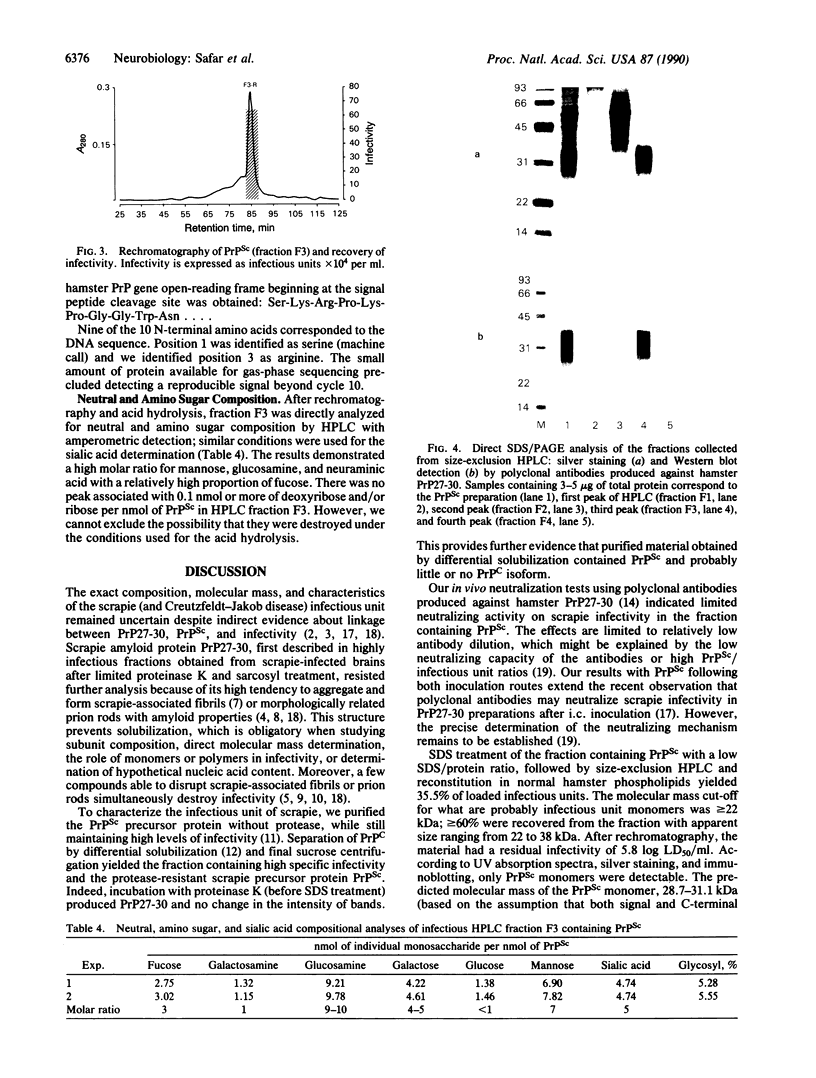
A highly purified fraction obtained from scrapie (263-K strain)-infected hamsters' brains by an alternative procedure without proteinase K treatment contained a protease-resistant form of the scrapie precursor protein (PrPSc) and infectivity of 9.9 +/- 0.7 log LD50/ml. Polyclonal antibodies produced against hamster scrapie amyloid protein (PrP27-30) and used in a neutralization test diminished infectivity of the PrPSc preparations by 1.6 log after intracerebral inoculation and by 1 log after intraperitoneal inoculation. PrPSc was subjected to size-exclusion HPLC; greater than or equal to 60% of the eluted infectious units were recovered from the peak with an apparent mass of 30.4 +/- 0.6 kDa. Characterization by UV absorption spectra, SDS/PAGE, immunoblots, N-terminal amino acid sequence, and neutral sugar and amino sugar analyses demonstrated homogeneity of the infectious units. The neutral sugar and amino sugar compositional analyses revealed high mannose, glucosamine, fucose, and sialic acid content. This demonstrated an extensive posttranslational modification by the complex type of N-linked glycosylation and glycane core of C-terminal glycolipid of PrPSc. The results correspond to the predicted size, composition, and sequence of PrPSc and indicate that this protein may be the only component of scrapie infectious unit or the infectious form of scrapie precursor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Vincent M. T., Kent S. B., Hood L. E., Prusiner S. B. Characterization of prion proteins with monospecific antisera to synthetic peptides. J Immunol. 1988 Feb 15;140(4):1188–1193. [PubMed] [Google Scholar]
- Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. Isolation and structural studies of the intact scrapie agent protein. Arch Biochem Biophys. 1987 Nov 1;258(2):579–590. doi: 10.1016/0003-9861(87)90380-8. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D. F., Kenaga L., Prusiner S. B. Properties of scrapie prion protein liposomes. J Biol Chem. 1988 Apr 5;263(10):4950–4955. [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D., Prusiner S. B. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6617–6621. doi: 10.1073/pnas.85.18.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W., Anand R., Williams A. F. Complete structure of the glycosyl phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein. Nature. 1988 May 19;333(6170):269–272. doi: 10.1038/333269a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., van Renswoude J., Rivnay B. Reconstitution of membrane proteins. Methods Enzymol. 1984;104:340–347. doi: 10.1016/s0076-6879(84)04100-8. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Meng H., Burleigh B. D., Kelly G. M. Reduction studies on bacterial recombinant somatomedin C/insulin-like growth factor-1. J Chromatogr. 1988 Jun 29;443:183–192. doi: 10.1016/s0021-9673(00)94792-7. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup G., Diringer H., Hilmert H., Prinz H., Heukeshoven J., Beyreuther K. The protein component of scrapie-associated fibrils is a glycosylated low molecular weight protein. EMBO J. 1985 Jun;4(6):1495–1501. doi: 10.1002/j.1460-2075.1985.tb03808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Piccardo P., Safar J., Ceroni M., Gajdusek D. C., Gibbs C. J., Jr Immunohistochemical localization of prion protein in spongiform encephalopathies and normal brain tissue. Neurology. 1990 Mar;40(3 Pt 1):518–522. doi: 10.1212/wnl.40.3_part_1.518. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Takahashi N. Structural characterization of glycoproteins. J Chromatogr. 1988 Jun 29;443:267–284. doi: 10.1016/s0021-9673(00)94799-x. [DOI] [PubMed] [Google Scholar]
- Safar J., Ceroni M., Piccardo P., Gajdusek D. C., Gibbs C. J., Jr Scrapie-associated precursor proteins: antigenic relationship between species and immunocytochemical localization in normal, scrapie, and Creutzfeldt-Jakob disease brains. Neurology. 1990 Mar;40(3 Pt 1):513–517. doi: 10.1212/wnl.40.3_part_1.513. [DOI] [PubMed] [Google Scholar]
- Safar J., Ceroni M., Piccardo P., Liberski P. P., Miyazaki M., Gajdusek D. C., Gibbs C. J., Jr Subcellular distribution and physicochemical properties of scrapie-associated precursor protein and relationship with scrapie agent. Neurology. 1990 Mar;40(3 Pt 1):503–508. doi: 10.1212/wnl.40.3_part_1.503. [DOI] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Welling-Wester S., Kazemier B., Orvell C., Welling G. W. Effect of detergents on the structure of integral membrane proteins of Sendai virus studied with size-exclusion high-performance liquid chromatography and monoclonal antibodies. J Chromatogr. 1988 Jun 29;443:255–266. doi: 10.1016/s0021-9673(00)94798-8. [DOI] [PubMed] [Google Scholar]
- Welling G. W., Slopsema K., Welling-Wester S. Size-exclusion high-performance liquid chromatography of Sendai virus membrane proteins in different detergents. A comparison of different columns. J Chromatogr. 1986 May 30;359:307–314. doi: 10.1016/0021-9673(86)80084-x. [DOI] [PubMed] [Google Scholar]