Abstract
Commensal intestinal bacteria can prevent pathogenic infection; however, limited knowledge of the mechanisms by which individual bacterial species contribute to pathogen resistance has restricted their potential for therapeutic application. Here, we examined how colonization of mice with a human commensal Enterococcus faecium protects against enteric infections. We show that E. faecium improves host intestinal epithelial defense programs to limit Salmonella enterica serotype Typhimurium pathogenesis in vivo in multiple models of susceptibility. E. faecium protection is mediated by a unique peptidoglycan hydrolase, SagA, and requires epithelial expression of pattern recognition receptor components and antimicrobial peptides. Ectopic expression of SagA in non-protective and probiotic bacteria is sufficient to enhance intestinal barrier function and confer resistance against S. Typhimurium and Clostridium difficile pathogenesis. These studies demonstrate that specific factors from commensal bacteria can be used to improve host barrier function and limit the pathogenesis of distinct enteric infections.
One Sentence Summary
SagA from E. faecium triggers protective intestinal epithelial cell programs that limit S. Typhimurium and C. difficile pathogenesis.
Introduction
The human intestine is populated by approximately 1013 bacteria, as well as eukaryotic viruses, bacteriophages and fungi (1). In addition to playing a crucial role in physiological processes such as digestion of food and detoxification of bile acids, these microorganisms have been implicated in conditions as diverse as metabolic syndrome (2, 3), cardiovascular diseases (4) and autism (5, 6). Gut microbes provide low grade stimulation of the intestinal immune system (7), and colonization by the microbiota activates host defense pathways in the gut epithelium and underlying immune cells that restrict invasion of both commensals and pathogens (8, 9). Accordingly, antibiotic-induced dysbiosis and changes in the relative abundance of several bacterial taxa correlate with increased susceptibility to inflammatory bowel diseases and enteric pathogens (10, 11). Recent studies have shown several beneficial effects of microbial metabolites on regulation of intestinal pathogen resistance and immune function. For example, microbiota regulation of bile acid metabolism has been shown to restrict C. difficile growth (12), and the production of short chain fatty acids by specific commensal bacteria modulates colonic anti-inflammatory T cell populations (13, 14) and attenuates enteric infections by enhancing intestinal epithelial cell integrity (15). Nonetheless, the precise mechanisms of protection elicited by many intestinal bacterial species remain to be determined. Notably, E. faecium, a commensal bacterium found in the intestines of insects, fish, birds, and mammals (16), can enhance resistance against enteric pathogens and has been utilized as a probiotic in livestock for decades (17). However, the protective mechanisms of E. faecium are unknown, and its use as a probiotic in humans is limited by its potential drug-resistance and pathogenesis in the form of vancomycin-resistant Enterococcus (VRE) (18). Therefore, dissecting the mechanisms of E. faecium-mediated pathogen resistance is required to reveal novel means to trigger these protective programs without introducing a potential pathobiont.
To investigate the protective mechanisms of E. faecium, we employed three different murine models of microbiota manipulation and evaluated key components of host immunity. We observed that E. faecium does not engage adaptive immune mechanisms, but instead enhances intestinal epithelial barrier effectors through innate immune pathways to restrict Salmonella pathogenesis in vivo. Additionally, we found that SagA, a secreted peptidoglycan hydrolase from E. faecium, is sufficient to increase the epithelial expression of barrier effectors and antimicrobial peptides in vivo. The ectopic expression of SagA in non-protective bacteria not only protects against the Gram-negative bacterial pathogen, S. Typhimurium, but also confers resistance against a major hospital-acquired Gram-positive enteric pathogen, C. difficile. These studies reveal a major protective mechanism of E. faecium in mammals and show that a specific secreted factor from a commensal bacterium, SagA, can be used to restrict the pathogenesis of distinct enteric infections.
Results
E. faecium limits Salmonella pathogenesis in multiple models of susceptibility
To study the protective activity of E. faecium (human commensal isolate, strain Com15), we first compared it to another human commensal Enterococcus species, E. faecalis (strain OG1RF), in two non-typhoidal Salmonella enterica serotype Typhimurium infection models. Germ-free (GF) mice were mono-colonized with E. faecium or E. faecalis one week prior to oral infection with S. Typhimurium. Control GF mice with no pre-existing microbiota succumbed to infection within 6 days as did 50% of E. faecalis mono-colonized mice; however, E. faecium mono-colonized mice were significantly protected against early weight loss and exhibited decreased S. Typhimurium titers in the feces (Fig. 1, A and B and fig. S1). This corresponded to 100% survival at day 6 post-infection and an overall survival of 42% for E. faecium mono-colonized mice (Fig. 1C). Because antibiotics deplete normal microbiota and increase susceptibility to several enteric infections, including C. difficile and S. Typhimurium (11), we also treated conventional specific pathogen-free (SPF) mice with a broad-spectrum antibiotic cocktail prior to colonization with E. faecium or E. faecalis and infection with S. Typhimurium. While antibiotic-treated E. faecium-colonized mice no longer exhibited significant decreases in early weight loss or average S. Typhimurium titers, they displayed an overall survival benefit (Fig. 1D–F). In fact, survival was significantly improved despite sub-optimal E. faecium colonization, as E. faecium was not as well maintained in mice after antibiotics were discontinued and the normal microbiota began to return (fig. S2).
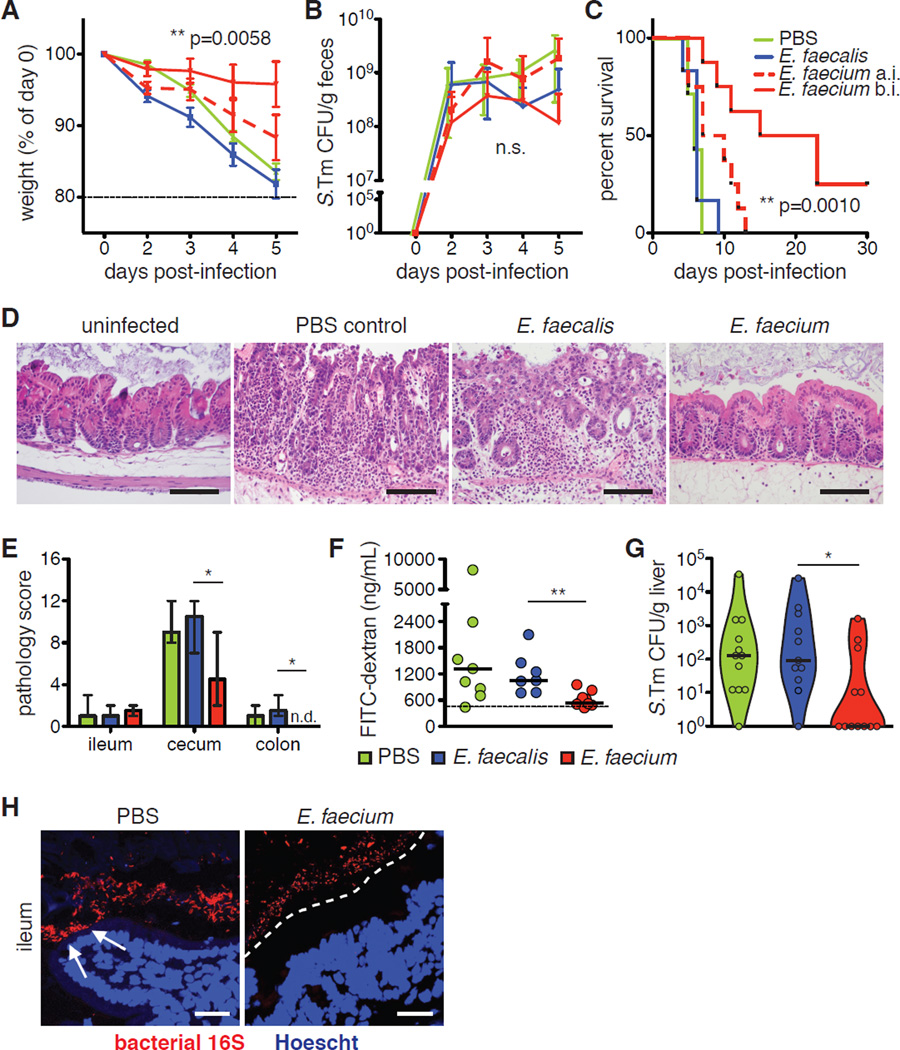
Fig. 1. E. faecium-mediated Salmonella resistance does not require an adaptive immune response.
(A–C) Germ-free (GF) C57BL/6 mice were orally gavaged with 108 CFU E. faecalis or E. faecium 7d before oral infection with 102 CFU S. Tm. (A) Weight loss, (B) S. Tm bacterial burden in feces, and (C) survival are shown. Pooled data from 4 independent experiments, n=12 mice/group. (D–F) C57BL/6 mice were orally gavaged with a broad-spectrum antibiotic cocktail of ampicillin, metronidazole, neomycin, and vancomycin (AMNV) daily for 7d prior to gavage with 108 CFU E. faecalis or E. faecium or PBS, followed by infection with 106 S. Tm. (D) Weight loss, (E) S. Tm bacterial burden in feces, and (F) survival are shown. Pooled data from 4 independent experiments, n=14 mice/group. (G) Mice were gavaged with AMNV daily for 7d prior to gavage with 108 CFU E. faecium (E. fm). 5d post-colonization, intraepithelial lymphocytes were isolated and analyzed by flow cytometry for relative frequency and cytokine expression of shown subpopulations. Pooled data from 2 independent experiments, n=5 mice/group. (H) Rag1−/− mice were gavaged with AMNV antibiotic cocktail daily for 7d prior to gavage with PBS or 108 CFU E. faecalis or E. faecium, followed by infection with 106 S. Tm. Pooled data from 4 independent experiments, n=13–14 mice/group. (A, B, D and E) mean±SEM, 2-way ANOVA, p-value shown comparing E. faecalis to E. faecium. n.s.=not significant. (C, F and H) Log-rank analysis, p-value shown comparing E. faecalis to E. faecium. (G) mean±SEM, Mann-Whitney. *p≤0.05, **p≤0.01, ***p≤0.001 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
These results suggested that protection was not dependent on secondary effects on the remaining microbiota, since E. faecium was protective in gnotobiotic mice and in antibiotic-treated mice from which no bacterial 16S rRNA could be amplified at the time of colonization (data not shown). Furthermore, although E. faecium treatment correlated in some cases with a delay in or clearance of infection (fig. S3), it did not eliminate S. Typhimurium colonization or influence expression of type III effectors involved in S. Typhimurium invasion of host cells (fig. S4). These results indicate that E. faecium does not act by direct bactericidal mechanisms, inhibiting S. Typhimurium virulence gene expression, or indirectly modulating the microbiota.
To investigate host mechanisms involved in E. faecium-mediated protection against S. Typhimurium pathogenesis in vivo, we analyzed intestinal immune cells after E. faecium colonization. E. faecium colonization did not induce significant changes in the relative frequency or cytokine production of intestinal myeloid cell and lymphocyte subsets (Fig. 1G and fig. S5). In addition, prolonged host survival in response to E. faecium colonization was preserved in Rag1−/− mice, which lack B and T cells (Fig. 1H). These data suggest that although adaptive immunity contributes to overall S. Typhimurium resistance, it is not a major component of E. faecium-mediated protection.
E. faecium colonization rapidly enhances epithelial function and bacterial segregation
Mice treated with a single dose of streptomycin are rendered highly susceptible to S. Typhimurium infection (19). To characterize the timing of E. faecium-mediated protection, we next utilized the streptomycin model to rapidly induce S. Typhimurium susceptibility. As expected, streptomycin-treated control and E. faecalis-colonized mice showed severe S. Typhimurium-induced pathogenesis, as evidenced by rapid weight loss and mortality within 10 days of infection (Fig. 2A–C and fig. S6). In contrast, streptomycin-treated mice pre-colonized with E. faecium 4 hours before infection displayed significantly reduced early weight loss and prolonged survival, without affecting pathogen colonization (Fig. 2A–C and fig. S7). Of note, administration of E. faecium 24 hours after S. Typhimurium infection also conferred some protective effects, albeit greatly reduced compared to E. faecium administered before infection (Fig. 2, A and C), indicating that E. faecium colonization induces a protective response that must be triggered prior to infection.
Fig. 2. E. faecium improves epithelial barrier function and bacterial segregation.
(A–C) C57BL/6 mice were orally gavaged with streptomycin and given 108 colony-forming units (CFU) E. faecalis or E. faecium 4h before (b.i.) or 24h after (a.i.) oral infection with 106 CFU S. Typhimurium. (A) Weight loss, (B) S. Typhimurium (S. Tm) bacterial burden in feces, and (C) survival are shown. Pooled data from 3 independent experiments, n=6–8 mice/group. (D) Cecum tissue stained with H&E, harvested at 48h post-infection (p.i.) from mice given streptomycin and left uninfected or treated before infection as in (A–C). Representative images, 40X objective, from 1 of 3 independent experiments, n=2–3 mice/group. Scale bar = 100 µm. (E) Ileum, cecum, and colon tissues were harvested and prepared as in (D) and scored for 4 pathology parameters. Pooled combined pathology scores from 3 independent experiments, n=6–7 mice/group. (F) Intestinal permeability measured by FITC-dextran in the serum 48h p.i. from mice treated before infection as in (A–C). Dashed line = background level (uninfected). Pooled data from 3 independent experiments, n=7–9 mice/group. (G) S. Tm bacterial burden 48h p.i. in the livers of mice treated before infection as in (A–C). Pooled data from 4 independent experiments, n=11–12 mice/group. (H) FISH staining for all bacteria (universal 16S probe) and epithelial nuclei (Hoechst) from intestinal tissues 48h p.i. Representative images, 40X objective, from 1 of 3 independent experiments, n=4–6 mice/group. White arrows indicate bacteria in contact with or invading through the epithelium, and white dashed line designates zone of segregation from bacteria. (A and B) mean±SEM, 2-way ANOVA, p-value shown comparing E. faecalis to E. faecium b.i., n.s.=not significant. (C) Log-rank analysis, p-value shown comparing E. faecalis to E. faecium b.i. (E) median±range, Mann-Whitney comparing E. faecalis to E. faecium (Wilcoxon for colon, n.d.=none detected, score of zero). (F and G) bar=median, Mann-Whitney comparing E. faecalis to E. faecium. *p≤0.05, **p≤0.01 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
S. Typhimurium produces several effector proteins that rapidly disrupt the intestinal epithelium and facilitate bacterial dissemination into the liver and other systemic tissues (20, 21). Accordingly, early (48h) after infection, control and E. faecalis-colonized mice exhibited severe edema, neutrophil infiltration, and destruction of intestinal epithelial integrity, while E. faecium pre-treated mice displayed markedly reduced S. Typhimurium-induced inflammation, pathology, and epithelial damage (Fig. 2, D and E). E. faecium colonization also attenuated S. Typhimurium-induced intestinal paracellular permeability, as measured by dissemination of FITC-dextran from the gut into the circulation (Fig. 2F). This corresponded to decreased S. Typhimurium invasion into peripheral organs (Fig. 2G). In addition, fluorescence in situ hybridization (FISH) using a universal bacterial probe showed improved bacterial segregation from the intestinal epithelium in E. faecium-colonized mice compared to controls (Fig. 2H), with bacterial contact much more apparent in untreated mice. These data show that E. faecium rapidly results in improved or maintained epithelial barrier function and S. Typhimurium resistance.
Intestinal epithelial cells upregulate barrier defenses upon E. faecium colonization
Given the beneficial effects of E. faecium on intestinal barrier integrity, we next examined E. faecium-induced changes in intestinal epithelial cell (IEC) gene expression. In the presence of microbial colonization, IECs produce numerous proteins that function to restrict bacterial contact and invasion of the epithelium, including antimicrobial peptides (AMPs), such as a-defensins (cryptdins in mice) and the C-type lectins RegIIIβ and RegIIIγ, and mucous components like mucins (22, 23). Expression of many AMP and mucin genes is markedly reduced in response to antibiotic treatment (24, 25); however, we observed that E. faecium colonization after broad-spectrum antibiotic treatment restored expression of both cryptdin 2 and mucin 2 to pre-antibiotic levels and strongly increased expression of the C-type lectin Reg3g (RegIIIγ) (Fig. 3A and fig. S8). The induction of Reg3g above steady-state levels suggests that E. faecium-derived signals are especially capable of prompting expression of certain AMPs. This increase was also present in streptomycin-treated, E. faecium-colonized mice as early as 4 hours post-colonization (Fig. 3B), consistent with the timing of protection observed against S. Typhimurium infection (Fig. 2, A and C). Using iDISCO+ tissue-clearing (26, 27) and light sheet microscopy to visualize three-dimensional mucin distribution in the intact intestine, we also observed enhanced MUC2 distribution along the epithelium in E. faecium-treated mice (Fig. 3C and Movies S1 and S2). This enhanced mucin distribution and the decreased bacterial contact with the epithelium observed by FISH (Fig. 2H) could be sufficient to limit S. Typhimurium invasion and dissemination to peripheral organs. Taken together, these data indicate that E. faecium stimulates protective programs in the intestinal epithelium that limit S. Typhimurium pathogenesis and result in prolonged host survival.
Fig. 3. E. faecium colonization increases anti-microbial peptide and mucin expression.
(A) C57BL/6 mice were orally gavaged with AMNV daily for 7d prior to gavage with 108 CFU E. faecalis or E. faecium. 4d post-colonization, intestinal epithelial cells (IECs) were isolated and analyzed by RQ-PCR for expression of shown genes vs unmanipulated specific pathogen-free (SPF) mice. Representative data from 1 of 3 independent experiments, n=2–3 mice/group. (B) Mice were given a single gavage of streptomycin followed by gavage with 108 CFU E. faecium 24h later. At indicated time-points post-colonization, IECs were isolated and analyzed by RQ-PCR. Representative data from 1 of 2 independent experiments, n=2 mice/group. (C) Captured images of tissue-cleared, MUC2- and Ep-CAM-stained colon from mice treated as in (J). Corresponding videos are Movies S1 and S2. Scale bar = 200 µm. (A and B) mean±SEM, 1-way ANOVA with Dunnett’s post-test comparing each to antibiotic-only controls. *p≤0.05, **p≤0.01, ***p≤0.001 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
RegIIIγ is required for E. faecium-induced bacterial segregation from the epithelium
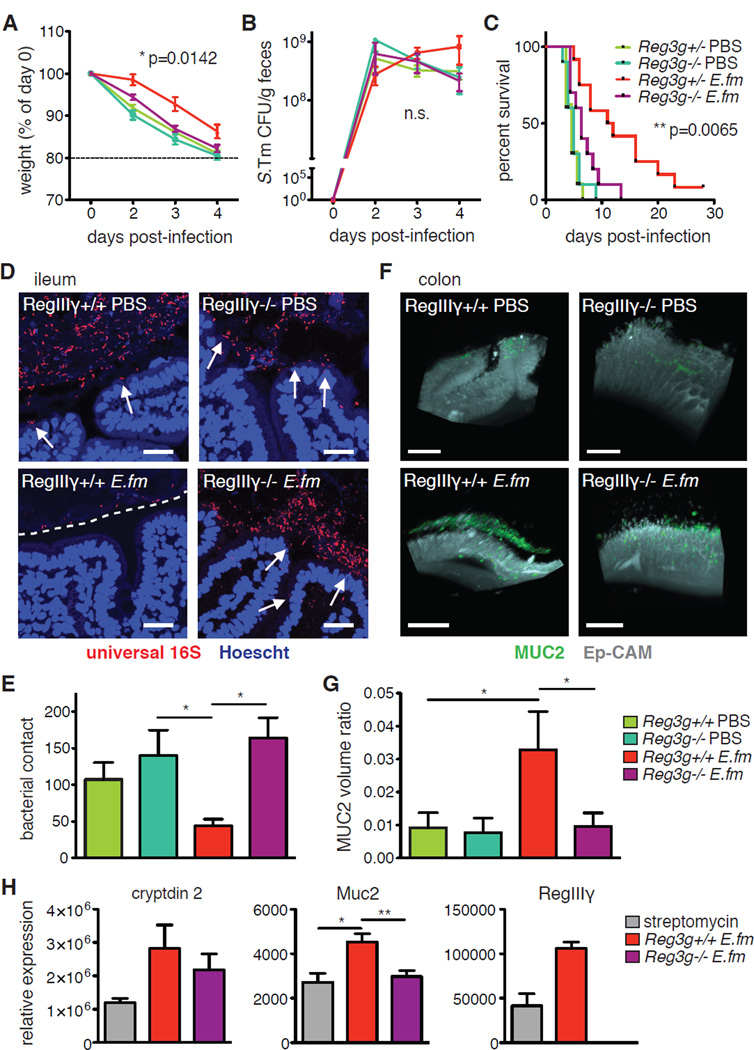
The secretion of AMPs, including the RegIII-family, has been shown to play an important role in excluding bacteria from the epithelial surface both by direct bactericidal activity and by affecting bacterial spatial organization (28, 29). Although neither RegIIIβ nor RegIIIγ are bactericidal toward S. Typhimurium, RegIIIγ has also been shown to contribute to normal mucin distribution and bacterial segregation (28, 30). To determine the relevance of the observed increase in expression of Reg3g to E. faecium-mediated protection, we colonized antibiotic-treated Reg3g-deficient (Reg3g−/−) mice and infected them with S. Typhimurium. Absence of Reg3g significantly abrogated E. faecium-mediated protection against S. Typhimurium pathogenesis compared to Reg3g+/− controls (Fig. 4A–C and fig. S9), while un-colonized Reg3g−/− and heterozygous littermate controls were equally susceptible to infection-induced weight loss and mortality. These observations indicate that RegIIIγ is a required component of an E. faecium-induced protective program.
Fig. 4. E. faecium-induced expression of Reg3g is required for protection against S. Typhimurium.
(A–C) Reg3g−/− mice or +/− littermate controls were given AMNV for 7d and colonized with 108 E. faecium (E. fm) prior to oral infection with 106 S. Tm. (A) Weight loss, (B) S. Tm bacterial burden in feces, and (C) survival are shown. Pooled data from 4 independent experiments, n=10–12 mice/group. (D–G) Reg3g−/− mice or +/+ controls were given streptomycin 24h before gavage with 108 E. fm prior to oral infection with 106 S. Tm. (D) FISH staining for all bacteria (universal 16S probe) and epithelial nuclei (Hoechst) from intestinal tissues 48h p.i. Representative images, 40X objective, from 1 of 3 independent experiments, n=4–6 mice/group. White arrows indicate bacteria in contact with or invading through the epithelium, and white dashed line designates zone of segregation from bacteria. (E) Quantitation of bacterial contact from 4 images per mouse from (F). (F) Captured images of tissue-cleared, MUC2- and Ep-CAM-stained colon from mice treated as in (F). Corresponding videos are Movies S3–6. Scale bar = 200 µm. (G) Quantitation of MUC2 volume as a ratio to volume of Ep-CAM from 4 sections per mouse from (H). (H) Reg3g−/− mice or +/+ controls were gavaged with streptomycin 24h before gavage with 108 E. fm. IECs were harvested 4h later and analyzed by RQ-PCR for expression of shown genes. Pooled data from 2 independent experiments, n=3–6 mice/group. (A and B) mean±SEM, 2-way ANOVA, p-value shown comparing Reg3g−/− E. fm to Reg3g+/− E. fm, n.s.=not significant. (C) Log-rank analysis, p-value shown comparing Reg3g−/− E. fm to Reg3g+/− E. fm. (E and G) Kruskal-Wallis with Dunn’s post-test comparing all groups to Reg3g+/+ E. fm. (H) 1-way ANOVA with a Bonferroni post-test comparing all groups to each other. RegIIIγ expression shown as a control, all groups significant compared to knock-out but not depicted on the graph. *p≤0.05, **p≤0.01, ***p≤0.001 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
S. Typhimurium dissemination to peripheral organs like the liver can occur by invasion across IECs, disruption of tight junctions, or by unimpeded transport through microfold (M) cells. However, bacteria that are sampled through M cells are likely to be taken up by mononuclear phagocytes in the underlying Peyer’s patches (PPs) and transported to the mesenteric lymph nodes (mLN) (31). In Reg3g+/− mice, colonization with E. faecium prevented early S. Typhimurium invasion into the liver, whereas Reg3g-deficient mice suffered liver invasion comparable to untreated mice (fig. S10A). However, translocation of bacteria to the mLN was not significantly affected by colonization or genotype, implying that active transport of S. Typhimurium is intact in E. faecium-treated mice, regardless of Reg3g expression (fig. S10B). In addition, we did not observe significant E. faecium-specific changes in IEC transcription of tight-junction protein genes in wild-type (Reg3g-sufficient) mice (fig. S11). Therefore, to investigate how RegIIIγ might be contributing to E. faecium-mediated protection, we examined bacterial spatial organization and MUC2 distribution along the intestinal epithelium. In the absence of Reg3g, E. faecium colonization failed to decrease bacterial contact with the epithelium (Fig. 4, D and E) or increase MUC2 protein distribution and mRNA expression (Fig. 4F–H and Movies S3–6). Collectively, these data indicate that RegIIIγ is required for E. faecium-mediated bacterial segregation from the epithelium. Because many bacterial pathogens, including S. Typhimurium, require direct cell contact to induce pathology (32–35), by decreasing bacterial contact with the epithelium, RegIIIγ may afford enhanced pathogen resistance that is in addition to and distinct from its bactericidal effects. Along with mucins and other AMPs, this likely contributes to decreased S. Typhimurium invasion of peripheral organs and subsequent morbidity.
Epithelial pattern-recognition receptors are required for E. faecium-mediated protection
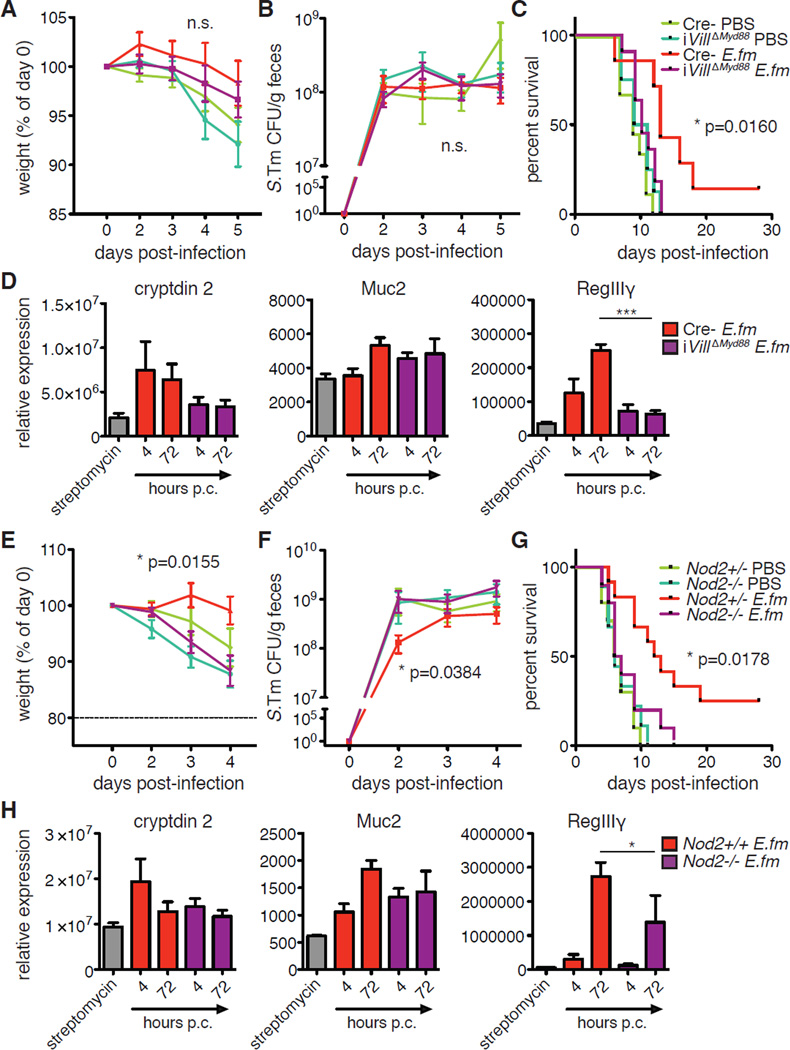
Expression of mucins and AMPs in the intestinal epithelium is upregulated in response to signaling through a number of pattern recognition receptors (PRRs) (36). Specifically, toll-like receptors (TLRs), and their signaling adapter, MyD88, have been shown to be required for IEC expression of Muc2, Reg3b, and Reg3g (37, 38). We therefore examined the requirement for MyD88 signaling in E. faecium-mediated protection by generating a tamoxifen-inducible, IEC-specific conditional Myd88 knockout mouse (VillinCre-ERT2xMyd88f/f (iVillΔMyd88)). We administered tamoxifen to iVillΔMyd88 and Cre− littermate controls one week prior to streptomycin treatment and followed this with E. faecium colonization and subsequent S. Typhimurium infection (fig. S12). We observed no significant difference in early weight loss or pathogen burden between iVillΔMyd88 and Cre− mice (Fig. 5A and B and fig. S13). However, E. faecium-mediated prolonged host survival was abrogated in the absence of epithelial MyD88 expression (Fig. 5C). Furthermore, E. faecium-induced expression of AMPs, particularly Defa2 (cryptdin 2) and Reg3g, was impaired in iVillΔMyd88 IECs (Fig. 5D). While macrophages are known to play a major role in S. Typhimurium pathogenesis and clearance (39), conditional deletion of Myd88 in myeloid cells, in particular macrophages, by interbreeding Myd88f/f with Lyz2Cre mice (LysMΔMyd88) still allowed for a significant survival benefit of E. faecium colonization prior to S. Typhimurium infection (fig. S14A and B). This benefit was evident despite a distinct increase in susceptibility to S. Typhimurium in mice harboring MyD88-deficient myeloid cells. These data indicate that although MyD88 expression in myeloid cells plays an important role in overall resistance to S. Typhimurium, E. faecium-mediated protection requires IEC, but not myeloid, MyD88 expression to improve barrier function and survival.
Fig. 5. MyD88 and NOD2 are required for AMP induction and E. faecium-mediated protection.
(A–C) iVillΔMyd88 or Cre− (MyD88f/f) littermate control mice were injected with tamoxifen 7 days prior to oral gavage with streptomycin. After 24h, mice were gavaged with 108 CFU E. fm followed 4h later by oral infection with 106 CFU S. Tm. (A) Weight loss, (B) S. Tm bacterial burden in feces, and (C) survival are shown. Pooled data from 3 independent experiments, n=7–11 mice/group. (D) Mice were treated and colonized as in (A–C). At 4 and 72h post-colonization (p.c.), IECs were isolated and analyzed by RQ-PCR for expression of shown genes. Pooled data from 2 independent experiments, n=2–4 mice/group. (E–G) Nod2−/− mice or +/− littermate controls were gavaged with streptomycin 24h prior to gavage with 108 CFU E. fm followed 4h later by oral infection with 106 CFU S. Tm. (E) Weight loss, (F) S. Tm bacterial burden in feces, and (G) survival are shown. Pooled data from 4 independent experiments, n=9–12 mice/group. (H) Mice were treated and colonized as in (E–G). At 4 and 72h post-colonization (p.c.), IECs were isolated and analyzed by RQ-PCR for expression of shown genes. Pooled data from 2 independent experiments, n=2–4 mice/group. (A, B, E, and F) mean±SEM, 2-way ANOVA, p-value shown comparing E. fm-colonized deficient to sufficient controls, n.s.=not significant. (C and G) Log-rank analysis, p-value shown comparing E. fm-colonized deficient to sufficient controls. (D and H) mean±SEM, 1-way ANOVA with Dunnett’s post-test comparing deficient to sufficient controls at the same time-point. *p≤0.05 and ***p≤0.001 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
In addition to MyD88, previous studies have shown that mice with a mutation in the Nod2 gene, another relevant PRR, also exhibit reduced intestinal production of cryptdins, MUC2, and RegIIIγ (40–42). To investigate the contribution of NOD2 to E. faecium-induced protection and AMP expression, we compared the efficacy of E. faecium colonization in streptomycin-treated Nod2-deficient mice (Nod2−/−) and heterozygous littermate controls. E. faecium-mediated protection was absent in Nod2−/− animals in terms of early weight loss, pathogen burden, and survival (Fig. 5E–G and fig. S15). While induction of Defa2 and Muc2 expression was mostly maintained, Reg3g upregulation was present but significantly impaired in Nod2−/− mice (Fig. 5H). This may indicate that other pathways, like MyD88, are able to contribute to upregulation of Reg3g in the absence of NOD2 signaling, and it implies that other NOD2-dependent, but RegIIIγ-independent, responses to E. faecium may also be required for protection. Together, these data show that IEC innate signaling adapters and receptors known to contribute to Reg3g and AMP expression, namely MyD88 and NOD2, are required for E. faecium-mediated protection and improvement of barrier function.
E. faecium-derived SagA enhances barrier function and resistance to S. Typhimurium
In parallel studies performed in C. elegans, we identified that a E. faecium-derived secreted NlpC/p60 peptidoglycan hydrolase, secreted antigen A (SagA), is sufficient for protection against S. Typhimurium in worms (43). Deletion of sagA has been shown to render E. faecium non-viable, and it is encoded in the genomes of all sequenced E. faecium strains (44, 45); however, sagA is absent in E. faecalis and other known commensal species (46). Additionally, introduction of SagA expression into non-protective E. faecalis significantly improved early weight loss and survival compared to wild-type E. faecalis in gnotobiotic mice infected with S. Typhimurium (43). To determine what aspects of E. faecium-induced protection were mediated by SagA, we examined the intestinal epithelium in mice colonized with E. faecalis, E. faecium and E. faecalis-sagA. Both E. faecium and E. faecalis-sagA, but not wild-type E. faecalis, significantly induced expression of Defa2, Muc2 and Reg3g in streptomycin-treated mice (Fig. 6A). Ectopic sagA expression in E. faecalis also abrogated S. Typhimurium-induced intestinal inflammation and pathology (Fig. 6, B and C), which was correlated with increased bacterial segregation from the epithelium and decreased liver invasion when compared to mice colonized with wild-type E. faecalis (Fig. 6, D and E). These results indicate that expression of sagA in Enterococcus is sufficient to increase epithelial barrier function and attenuate S. Typhimurium pathogenesis.
Fig. 6. The E. faecium protein SagA is sufficient to improve host intestinal barrier function and resistance to C. difficile.
Mice were given a single gavage of streptomycin followed 24h later by gavage with 108 CFU E. fm, E. fs, or E. fs-sagA. (A) At 72h postcolonization, IECs were isolated and analyzed by RQ-PCR. Pooled data from 2 independent experiments, n=2–4 mice/group. (B–E) Intestinal tissues 48h p.i. from mice colonized 4h prior to infection with 106 S. Tm. (B) Cecum tissue stained with H&E. Representative images, 40X objective, from 1 of 2 independent experiments, n=7 mice/group. (C) Ileum, cecum, and colon tissues were prepared as in (B) and scored for 4 pathology parameters. Pooled combined pathology scores from 2 independent experiments, n=7 mice/group. (D) FISH staining for all bacteria (universal 16S probe) and epithelial nuclei (Hoechst) from Representative images, 40X objective, from 1 of 2 independent experiments, n=7 mice/group. White arrows indicate bacteria in contact with or invading through the epithelium, and white dashed line designates zone of segregation from bacteria. (E) S. Tm bacterial burden in the livers of mice 48h p.i. Pooled data from 3 independent experiments, n=9–11 mice/group. (F–H) Mice were given AMNV for 7d and colonized with 108 CFU of indicated bacteria 2d prior to oral infection with 106 C. difficile. (F) Weight loss, (G) C. difficile bacterial burden in feces at day 1 p.i., and (H) survival are shown. Pooled data from 3 independent experiments, n=6–9 mice/group. (A) mean±SEM, 1-way ANOVA with Dunnett’s post-test comparing all to streptomycin-only controls. (C and E) Mann-Whitney. (F) mean±SEM, 2-way ANOVA, p-value shown comparing E. fm or sagA-expressing E. fs or L. pl to WT or vector controls, respectively. (G) 1-way ANOVA comparing all groups. n.s.=not significant. (H) Log-rank analysis, p-value shown comparing E. fm or sagA-expressing E. fs or L. pl to WT or vector controls, respectively. *p≤0.05, **p≤0.01, ***p≤0.001 for all analyses. Comparisons with no (*) or bar had p>0.05 and were not considered significant.
SagA-expressing bacteria improve barrier function and protect against C. difficile pathogenesis
Use of probiotic strains of bacteria has been proposed as an inexpensive strategy to decrease the incidence and severity of intestinal infection and dysbiosis without contributing to the increasing problem of antibiotic resistance (47). Although Enterococcus strains have been used as probiotics in livestock, their pathogenic potential makes them problematic for use in humans (18). We thus introduced sagA into a known non-pathogenic probiotic, Lactobacillus plantarum (48). Consistent with our findings with E. faecalis-sagA and E. faecium protection, sagA-expressing L. plantarum significantly prevented weight loss and improved survival upon S. Typhimurium infection compared to control L. plantarum (43). Bacterial segregation from the intestinal epithelium and MUC2 distribution were also maintained in L. plantarum-sagA-colonized mice relative to the prominent epithelial contact present in L. plantarum-vector controls (fig. S16 and Movies S7 and S8). These studies indicate that SagA protective activity is transferrable to other probiotic bacteria and can enhance epithelial barrier function to limit S. Typhimurium pathogenesis.
To determine if E. faecium and SagA protection was effective against other enteric pathogens, we investigated their activity against C. difficile. C. difficile is a leading cause of hospital-acquired infectious diarrhea that develops in antibiotic-treated patients. While existing antibiotics are effective in many patients, recurrent C. difficile infection is currently most effectively treated with fecal microbiota transplant (FMT) therapy (49). Following broad spectrum-antibiotic treatment, we found that mice colonized with E. faecium, E. faecalis-sagA or L. plantarum-sagA were protected against C. difficile-induced early weight loss when compared to non-sagA-expressing bacteria controls, despite similar colonization of each putative probiotic bacteria and similar early pathogen burden (Fig. 6, F and G and fig. S17). Further, the protection elicited by sagA-expressing bacteria led to significantly enhanced recovery and survival of mice (Fig. 6H and fig. S17). These data demonstrate that E. faecium and SagA provide effective protection against distinct enteric pathogens, suggesting broad efficacy of SagA-enhanced epithelial barrier function.
Discussion
The intestinal epithelium may be exquisitely responsive to modulation by metabolites and factors from commensal bacteria. In our studies, we characterize both epithelial and commensal microbial contributions to a protective mechanism in the mammalian intestine that reduces early pathogen invasion and tissue damage. Our results suggest that the commensal bacterium E. faecium triggers enhanced epithelial barrier function and pathogen resistance via its expression of a unique secreted peptidoglycan hydrolase, SagA. These observations complement our parallel studies that identified and biochemically characterized SagA as a protective factor in C. elegans, demonstrating extended worm survival following S. Typhimurium infection (43). Together, these studies depict a trans-phylum, microbiota-induced protective strategy that may be conserved among many species of animals and their cognate commensal bacteria (50, 51).
Our studies in mice indicate that E. faecium and SagA improve host pathogen resistance by increasing the expression of mucins and AMPs through MyD88 and NOD2 innate signaling pathways, facilitating bacterial segregation from the epithelium. Although the molecular mechanisms involved in activation of MyD88 and NOD2 via SagA remain to be determined, our data suggest that these innate signaling pathways have unequal and partially redundant contributions to upregulation of AMPs in response to E. faecium colonization. In particular, RegIIIg upregulation was abrogated in Myd88-deficient epithelial cells but only partially hindered in Nod2-deficient animals. This is consistent with studies showing that the low levels of RegIIIg expression observed in Nod2−/− mice can be boosted or recovered by colonization with certain cohorts of bacteria (42), and our observations suggest that this may be mediated by compensatory MyD88 signaling. Because S. Typhimurium is a Gram-negative pathogen whose cell wall lipid A and peptidoglycan are not readily accessible to RegIIIβ or RegIIIγ, respectively (52–54), these AMPs are not efficiently bactericidal toward S. Typhimurium, and this is evidenced by the ability of S. Typhimurium to colonize E. faecium and E. faecalis-SagA-treated mice despite robust RegIIIγ upregulation. However, in addition to its bactericidal activity, RegIIIγ has been shown to facilitate robust MUC2 distribution and proper bacterial spatial organization (28, 29), suggesting a mechanism by which SagA decreases both S. Typhimurium invasion and C. difficile toxin access to the epithelium (55, 56). In contrast to Reg3g, Muc2 transcription was not significantly affected by either Myd88- or Nod2-deficiency. This indicates that increased Muc2 expression is either redundantly induced by both MyD88 and NOD2 or via additional signaling pathways (57). Beyond transcriptional regulation, MUC2 proteins undergo disulfide-mediated polymerization to form complex net-like structures and are densely O-glycosylated, both of which contribute to the gel-forming properties of MUC2 upon secretion from goblet cells in the intestine (58). Additional pathways triggered by E. faecium and SagA may therefore contribute to host pathogen resistance by facilitating proper post-translational modification or secretion of mucins and other barrier effectors.
Beyond it’s benefit to the host and based on its conservation among all sequenced E.faecium strains (46), it is likely that SagA also contributes to E. faecium’s fitness and ability to colonize the intestines. Digestive tract microbiota are known to produce enzymes that facilitate bacterial access to nutrients (59, 60) and that can therefore influence bacterial survival in nutrient-poor conditions (61, 62). Determining whether and how SagA regulates the composition of gut microbes or influences niche competition in the intestines of mammals and other animals could provide important new insight into this complex host-commensal relationship.
The commensal intestinal microbiota have several potential beneficial modes of action that can be harnessed to fine-tune probiotics, including niche competition with pathogenic bacteria, activation of immune cells and augmentation of epithelial barrier function (63, 64). Our data demonstrate that SagA can be ectopically expressed in probiotic bacteria for potential prophylactic applications against diverse enteric infections, including clinically-challenging pathogens like C. difficile. There are nearly 500,000 new cases of C. difficile infection (CDI) each year in the U. S. alone, and approximately 20% of patients experience at least one recurrence (65). Use of non-CDI antimicrobials has been shown to be a significant risk factor for CDI recurrence, and the treatment strategy with the highest success rate for recurrent CDI is restoration of the healthy microbiota by FMT therapy (66). However, current probiotic formulations have shown only marginal or highly variable in vivo efficacy against C. difficile in patients (67). Future studies are warranted to determine if low endogenous levels of commensal E. faecium can serve as a biomarker of CDI susceptibility and if administration of SagA-expressing probiotics can counteract this susceptibility or serve as a treatment alternative to FMT therapy. In conclusion, our findings uncover both host and microbial components of a protective program in the mammalian intestinal epithelium that could have important ramifications for the design of better probiotics.
Materials and Methods
Animals
C57BL/6J (000664), Lyz2Cre (004781), MyD88f/f (008888), Nod2−/− (005763), Rag1−/−(002216) and Reg3g−/− (017480) mice were purchased from Jackson Laboratories and maintained in our facilities. VillinCreERT2 mice were developed by Silvie Robine and generously provided by David Artis. Several of these lines were interbred in our facilities to obtain the final strains described in the text. Genotyping was performed according to the protocols established for the respective strains by Jackson Laboratories. Mice were maintained at the Rockefeller University animal facilities under specific pathogen-free (SPF) or germ-free (GF) conditions. GF C57BL/6J mice were obtained from Sarkis Mazmanian and bred and maintained in germ-free isolators in our facilities. GF status was confirmed by plating feces as well as by qPCR analysis (16S rRNA). Mice were used at 8–10 weeks of age for most experiments. Animal care and experimentation were consistent with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee at the Rockefeller University.
Induction of MyD88 excision in VillinCreERT2xMyD88f/f (iVillΔMyd88) mice
Mice were injected i.p. with 0.6mg tamoxifen/mouse for female mice and 0.8mg/mouse for males. Primers were designed to check floxed MyD88 allele (primers 1&2) or tamoxifen-induced excision (primers 1&3) by PCR of genomic DNA, producing a ~357bp product for excised MyD88 and a ~737bp product for unexcised. For mRNA expression, primers were designed for RQ-PCR (see below). MyD88 primers used were:
| PCR: | 1 | AGGCTGAGTGCAAACTTGGT |
| 2 | GTTGTGTGTGTCCGACCGTG | |
| 3 | ATACCGGAAGCAGATGGATG | |
| RQ-PCR: | Forward | ACAGAAGCGACTGATTCCTATT |
| Reverse | TGGTGCAAGGGTTGGTATAG |
In pilot experiments, complete excision was detected at 5 days after a single dose of tamoxifen (fig. S10). For colonization and infection experiments, tamoxifen was injected once per day for two consecutive days, 7 days prior to streptomycin administration. In each experiment, intestinal tissue was collected post-mortem and genomic DNA and/or mRNA were isolated to check for correct MyD88 excision.
Bacteria
E. faecium (strain Com15) was provided by Michael Gilmore, and E. faecalis (strain OG1RF) and L. plantarum (strain WCFS1) were generously provided by Balfour Sartor. E. faecium and E. faecalis were cultured in BHI broth (BD 237500), S. Typhimurium (strain 14028) was cultured in Luria Broth (LB) and L. plantarum was cultured in de Man, Rogosa and Sharpe (MRS) broth. E. faecium, E. faecalis, and L. plantarum were grown overnight at 37°C at 230 rpm shaking to stationary phase growth prior to colonization. S. Typhimurium was grown overnight followed by a 3.5 hour subculture at 37°C at 230 rpm to log phase growth prior to infection. C. difficile (strain VPI 10463) was provided by Eric Pamer and cultured in an anaerobic chamber on BHIS agar or broth with 16mg/L cefoxitin, 250mg/L cycloserine and 10mL/L 10% (w/v) taurocholic acid (henceforth denoted BHIS++) as described below and previously (68).
Colonization and S. Typhimurium infection of mice
To ensure effective colonization and induce infection susceptibility, specific pathogen-free (SPF) mice were gavaged with either a single dose of 20mg streptomycin 24 hours prior to colonization or a daily dose of AMNV antibiotic cocktail (4mg ampicillin, 2mg metronidazole, 4mg neomycin, and 2mg vancomycin) for 7–14 days as indicated in the figure legends. AMNV treatment was ceased 2 days prior to colonization. Germ-free (GF) mice were exported from isolators into autoclaved cages containing autoclaved food, water and bedding. Bacterial cultures were washed and resuspended in sterile PBS at 109/mL for E. faecium, E. faecalis, and L. plantarum or at 107/mL (SPF) or 103/mL (GF) for S. Typhimurium. Mice were colonized by gavage with 100µL of the indicated bacterial suspension 4 hours before infection (for streptomycin-treated mice) or 2–7 days before infection (for AMNV-treated and GF gnotobiotic mono-colonized mice). Colonization was followed by infection with S. Typhimurium by oral gavage at the doses indicated above and in the figure legends. Weight loss was monitored from just before infection, and mice were euthanized when they reached 80% baseline weight or when they appeared hunched, moribund or exhibited a visibly-distended abdomen (indicative of peritoneal effusion), whichever occurred first. Death was not used as an end-point. Colony-forming units (CFU) in the feces were determined by plating 5 serial dilutions of feces suspended in sterile PBS on selective agars: Salmonella Shigella Agar (BD 211597) for S. Typhimurium, Enterococcosel Agar (BD 212205) for E. faecium and E. facealis, and MRS Agar with 8ug/mL chloramphenicol for L. plantarum-vector and –sagA. Resulting quantities were normalized to feces weight. Experiments in which putative probiotics did not colonize or colonize equally well by day 2 post-colonization were terminated and not included in our analyses.
C. difficile infection of mice
Mice were gavaged with AMNV antibiotic cocktail daily for 7 days, and colonized as indicated above. In an anaerobic chamber, C. difficile spores were streaked onto BHIS++ plates and incubated overnight at 37°C. The following day, a single colony from the plate was inoculated into 5mL BHIS++ broth and incubated overnight at 37°C. 5mL sterile PBS was added, and broth culture tubes were then parafilmed and exported from the anaerobic chamber for centrifugation at 2000 × g for 5 minutes. Tubes were returned to the anaerobic chamber, supernatant was removed, and the bacterial pellet was resuspended in 3mL sterile PBS.
This suspension was loaded into 1-mL syringes fitted with gavage needles and exported from the anaerobic chamber in plastic bags. C. difficile suspensions were immediately transported to animal facilities for oral gavage of 100µL/mouse. Leftover inocula were serially-diluted and plated in the anaerobic chamber to confirm the number of CFU administered. Weight loss was monitored from just before infection, and mice were euthanized when they reached 80% baseline weight or when they appeared hunched or moribund, whichever occurred first. Death was not used as an end-point. CFU in the feces were determined in an anaerobic chamber by plating 5 serial dilutions of feces suspended in sterile PBS on BHIS++ agar plates. Resulting quantities were normalized to feces weight. Feces suspensions were then exported from the anaerobic chamber for quantification of aerobic colonizing bacteria as described above.
Pathological scoring
Representative portions of ileum, cecum and colon were fixed in Methacarn (methanol-Carnoy) solution and embedded in paraffin according to standard protocols (see 16S FISH below). 5-µm sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) for blinded histologic evaluation. Quantitative measures of inflammation were then recorded according to the methods of Barthel et al. (19). Briefly, each tissue section was microscopically assessed for the extent of submucosal edema, preservation of epithelial integrity and goblet cell retention (each category scored from 0–3), as well as for polymorphonuclear cell infiltration into the lamina propria (scored from 0–4). The sum of these scores represents the combined pathological score reported for each tissue. Scores >9 are indicative of severe inflammation, while scores of 5–8 are consistent with moderate inflammation. Scores <4 are associated with minimal to mild inflammation and frequently appear histologically normal.
Intestinal permeability and peripheral organ invasion
Epithelial barrier integrity was assessed by a FITC-dextran leakage assay and by measurement of S. Typhimurium invasion to the liver and mesenteric lymph nodes (mLN). At 48 hours post-infection, mice were gavaged with FITC-dextran 4 kDa (600 µg/kg body weight, Sigma) dissolved in sterile PBS. After 4 hours, animals were anesthetized, and ~500µL blood were harvested by cardiac puncture. Serum was isolated using Z-Gel microtubes (Sarstedt), and FITC fluorescence (excitation 488nm, emission 518nm) was measured in triplicate on a FLUOstar Omega microplate reader (BMG Labtech). Concentration of FITC in serum (ng/mL) was determined using a standard curve. Background levels of FITC in serum from uninfected mice averaged ~450ng/mL, indicated by a dashed line in the figure. To measure Salmonella invasion, whole livers or mLN were homogenized in PBS with 0.1% Triton X-100 to release intracellular bacteria. 3 serial dilutions were plated on Salmonella Shigella Agar, and resulting quantities were normalized to organ weight prior to homogenization.
16S FISH
To visualize bacterial segregation from the intestinal epithelium, 16S rRNA FISH was performed with a universal probe (EUB388, Cy3-GCTGCCTCCCGTAGGAGT-Cy3) on sections fixed with Methacarn solution as described previously (69, 70). In brief, intestinal tissues (about 1 cm) including intestinal contents were fixed in Methacarn solution (60% methanol, 30% chloroform, 10% glacial acetic acid) for 6 hours at 4°C. After 3 washes with 70% ethanol tissues were embedded in paraffin. 5-µm sections were cut, mounted on glass slides, and de-paraffinized with xylene and subsequently dehydrated. Probe was diluted (5 ng/µL) in hybridization buffer ((0.9 M NaCl, 20 mM Tris-HCl (pH 7.2), 0.1% SDS)) and hybridization was carried out at 50°C for 3 hours. Nuclei were stained with Hoechst and tissues were imaged on an inverted LSM 780 laser scanning confocal microscope (Zeiss). Two 0.4mm2 images per tissue per mouse were acquired with four 2µm Z-stacks. Images were stacked, adjusted and analyzed in Image-J; bacterial contact was quantified as the number of 16S foci touching the epithelial brush border per mm of brush border length. All image analysis was performed blindly on randomized samples.
Tissue clearing - iDISCO
For 3D assessment of the colonic mucus layer, iDISCO tissue clearing and whole mount was performed as previously described ((26), updated at https://idisco.info/) with some modifications. Colonic tissues (about 1 cm) including contents were fixed in 4% PFA overnight at 4°C and then washed 3X in PBS. Samples were dehydrated in a series of methanol/PBS washes (30 minutes each: 20%, 40%, 60%, 80%, 100%; 1 hour: 100%) and then bleached in ice cold 5% H202/methanol overnight at 4°C. Samples were rehydrated in methanol/PBS (30 minutes each: 100%, 80%, 60%, 40%, 20%, 0%) and then washed 2X 1 hour in 0.2% Triton X-100/PBS. At this step, samples were opened longitudinally, and intestinal contents were gently washed out with PBS. Samples were then incubated in PTDG (1X PBS, 0.2% Triton X-100, 20% DMSO, 0.3 M glycine) for 2 days at 37°C, and then blocked in PTDD (1X PBS, 0.2% Triton X-100, 10% DMSO, 6% donkey serum) for 3 days at 37°C. After blocking, samples were washed 2X 1 hour in PTwH (1X PBS, 0.2% Tween-20, 10 µg/mL heparin) and then stained with rabbit anti-MUC2 (1:100 in PTwH; Santa Cruz Biotechnology sc-15334) for 3 days at 37°C. Samples were washed every 2 hours (or as often as possible) in PTwH for 2 days, stained with donkey anti-rabbit AF568 (1:200 in PTwH, Life Technologies A10042) and EpCAM AF647 (1:200 in PTwH, Biolegend 118212) for 3 days at 37°C, and then washed every 2 hours (or as often as possible) in PTwH for 2 days. Tissues were mounted in 1.5% agarose in TAE and then dehydrated in a series of methanol/water washes (30 minutes each: 20%, 40%, 60%, 80%, 100%, 100%). Dehydrated samples were incubated in 66% dichloromethane/methanol overnight at room temperature, followed by 1 hour in 100% dichloromethane. Samples were then cleared in 100% dibenzyl ether and imaged on a light-sheet UltraMicroscope II (LaVision Biotec). 3D reconstruction was done in Imaris (Bitplane) and MUC2 and EpCAM volumes were quantified using the Surfaces masking function. All iDISCO analyses were performed blindly on randomized samples.
Intestinal cell isolation
Small intestines and large intestines were excised and freed of mesentery and fat. Feces were removed and Peyer’s patches were excised from the small intestine. Both small and large intestines were opened longitudinally and washed in HBSS, followed by HBSS + 1mM DTT (Sigma-Aldrich). Tissue was cut into 1 cm pieces and incubated with 25 mL HBSS + 2% FBS + 5mM EDTA for 15 minutes at 37°C at 230 rpm. Supernatant containing intraepithelial lymphocytes was decanted and spun down (for RNA isolation from epithelial cells, see below). Tissue was washed with 25 mL HBSS + 2% FBS for 15 minutes at 37°C at 230 rpm. Supernatant containing additional intraepithelial lymphocytes was decanted and added to the previously-collected supernatant (IEL fraction). The gut tissues were then finely chopped and digested in HBSS + 2% FBS + 1X Sodium Pyruvate + 25mM HEPES + 50 µg/mL DNaseI + 0.05 mg/mL Collagenase VIII for 40 minutes at 37° C at 90 rpm followed by rigorous pipetting to dissociate lamina propria cells (LPL fraction). Cells were separated by discontinuous Percoll gradient (70%/35%) centrifugation. Hematopoietic cells were isolated from the interphase and stained for analysis by flow cytometry.
Lymph node and Peyer’s patch cell isolation
Lymph nodes and Peyer’s patches were mechanically disrupted and incubated with HBSS + 5% FBS + 0.5 mg/mL CollagenaseD (Roche) at 37 °C for 30 min. Cells were filtered through 100 µm cell strainers and washed. Erythrocytes were lysed with ACK Lysing buffer (Gibco). Resulting single-cell suspensions were then stained for analysis by flow cytometry.
Antibodies and flow cytometry analysis
Intestinal immune cell populations were characterized by flow cytometry. For analysis of cytokine-secreting cells, cells were incubated in the presence of 100ng/mL PMA (Sigma), 500ng/mL ionomycin (Sigma) and 10µg/mL brefeldin A (BFA) (Sigma) for 3 hours prior to staining. Cell populations were stained with antibodies against cell surface markers, followed by permeabilization in Fix/Perm buffer and intracellular staining in Perm/Wash buffer (BD Pharmingen). Fluorescent-dye-conjugated antibodies were purchased from BD-Pharmingen (anti-CD4, 550954; anti-CD45R, 557683) or eBioscience (anti-CD8α, 56-0081; anti-TCR-β, 47-5961; anti-IFN-γ, 25-7311; anti-TCRγδ, 46-5711; anti-CD8β, 46-0083; anti-TNF-α, 17-7321; B220 (RA3-6B2); F4/80 (BM8); Foxp3 (FJK-16s); Gr-1 (RB6-8C5); Ly6C (AL-21); Ly6G (1A8); MHCII (M5/114.15.2); NK1.1 (PK136); RORg(t) (B2D); Sca-1 (D7); Thy1.2 (53-2.1); CD3 (145-2C11); CD4 (RM4-5); CD8 (53-6.7); CD11b (M1/70); CD11c (N418); CD19 (1D3); CD44 (1M7); CD45 (30-F110); CD62L (MEL-14); CD64 (X54-5/7.1); CD103 (2E7); CD115 (AFS98). Flow cytometry data were acquired on an LSR-II flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Tree Star).
Real-time quantitative PCR (RQ-PCR)
Gene expression programs in intestinal epithelial cells were assessed by RQ-PCR. Epithelial cells were harvested as described above and resuspended in Trizol (Invitrogen), RNA was isolated according to the manufacturer’s protocol, and cDNA was generated using iScript cDNA Synthesis Kit (BioRad). RQ-PCR was performed using Power SYBR Green Master Mix (Agilent) in an ABI 7900HT system. Relative gene expression was quantified by the ΔΔCt method and normalized to HPRT and GAPDH gene expression. Primers used were:
| Forward | Reverse | |
|---|---|---|
| claudin-3 | CCTGTGGATGAACTGCGTG | GTAGTCCTTGCGGTCGTAG |
| cryptdin 1 | CTAGTCCTACTCTTTGCCCT | TTGCAGCCTCTTGATCTACA |
| cryptdin 2 | CTATTGTAGAACAAGAGGCT | TCTCCATGTTCAGCGACAGC |
| cryptdin 3 | GCGTTCTCTTCTTTTGCAGC | AAACTGAGGAGCAGCCAGG |
| cryptdin 4 | GTCCAGGCTGATCCTATCCA | GGGGCAGCAGTACAAAAATC |
| cryptdin 5 | GTCCAGGCTGATCCTATCCA | GATTTCTGCAGGTCCCAAAA |
| cryptdin 6 | GTCCCATTCATGCGTTCTCT | AGGACCAGGCTGTGTCTGTC |
| Muc1 | TGCTCCTACAAGTTGGCAGA | TACCAAGCGTAGCCCCTATG |
| Muc2 | ACAAAAACCCCAGCAACAAG | GAGCAAGGGACTCTGGTCTG |
| Muc4 | GGACATGGGTGTCTGTGTTG | CTCACTGGAGAGTTCCCTGG |
| Muc13 | CTTTGTACTGTGGTGGGAGGA | TCAAGCAAGAGCAGCTACCA |
| Muc17 | ATTCAGAGGCCCCAGGTAGT | TGGCTAAACACGCTTCTCCT |
| Muc20 | CAGGAAGGACATGTGGACAA | TTCCTCCTTGTACGGCTGAC |
| occludin | ATTCACTTTGCCATTGGAGG | ATTTATGATGAACAGCCCCC |
| Reg3g | TCCACCTCTGTTGGGTTCAT | AAGCTTCCTTCCTGTCCTCC |
| ZO-1 | TGCAATTCCAAATCCAAACC | AGAGACAAGATGTCCGCCAG |
S. Typhimurium Pathogenicity Gene RQ-PCR
Expression of Salmonella pathogenicity genes during infection was assessed by RQ-PCR as previously described (71) with some modifications. Mouse fecal samples and cecal contents were suspended in 200 µL PBS, vortex mixed extensively, and centrifuged at low speed (200 × g, 3 minutes). This process was repeated twice more for each sample, collecting supernatant each time. Pooled supernatant from the three washes was centrifuged at high speed (10,000 × g, 1 minute) and the resulting bacterial pellet was resuspended in 300 µL Trizol Max Bacterial Enhancement Reagent (Life Technologies) preheated to 95°C. Following a 4 minute incubation at 95°C, 1 mL of Trizol (Invitrogen) was added and RNA was extracted according to the manufacturer’s protocol. cDNA was generated using iScript cDNA Synthesis Kit (BioRad) and RQ-PCR was performed using Power SYBR Green Master Mix (Agilent) in an ABI 7900HT system. Primers for invA (72), hilA, hilD, invC, invF, invJ, orgA, prgH, prgK, rtsA, sicA, sipA, and sipC (73) have been previously described. Relative gene expression was quantified by the ΔCt method and normalized to invA gene expression.
Generation of E. faecalis-sagA and L. plantarum-sagA
E. faecalis-sagA and L. plantarum-sagA were generated as described in Rangan et al. (43).
Statistical Analyses
Comparisons and statistical tests were performed as indicated in each figure legend. Briefly, for comparisons of multiple groups over time or with 2 variables, a 2-way ANOVA was used with an appropriate Bonferroni post-test comparing all groups to each other, all groups to a control, or selected groups to each other. Survival data were analyzed using a log-rank (Mantel-Cox) test with a Bonferroni correction for the degrees of freedom based on the number of comparisons made. To compare two groups with non-normal distribution or low sample-size, the medians of the two groups were compared using a Mann-Whitney test unless one data set contained only zero values. In these cases, a Mann-Whitney could not be performed because all values in the group were identical, and a Wilcoxon test was performed instead. For comparisons of multiple groups with only one variable, a 1-way ANOVA or Kruskal-Wallis test was performed for data with underlying normal or non-normal distribution, respectively, with Bonferroni, Dunnett’s or Dunn’s post-tests where appropriate. Statistical analyses were performed in GraphPad Prism software. A P value of less than 0.05 was considered significant, denoted as *p≤0.05, **p≤0.01 and ***p≤0.001 for all analyses.
Supplementary Material
Acknowledgments
We are indebted to M. Nussenzweig, M.L. Miller, and members of our laboratory for discussions and critical reading and editing of the manuscript. We especially thank M. London, T. Sujino, and I. Gabanyi for assistance with experiments, P. Muller for help setting up iDISCO, D. Esterhazy for providing LysMΔMyd88 mice, B.C. Kim for help with L. plantarum-sagA, the New York University histology core for histological preparations, and members of the Nussenzweig lab and The Rockefeller University employees for continuous assistance. This work was supported by the NIH-NIGMS 1R01GM103593 and Robertson Therapeutic Development Fund to H.C.H and D. M. and a Center for Basic and Translational Research on Disorders of the Digestive System Pilot Award to V.A.P. through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust. H.C.H also thanks the Lerner Trust for support. K.J.R. received support from the David Rockefeller Graduate Program and a Helmsley Graduate Fellowship. V.P. designed the study, performed the experiments and wrote the manuscript; A.L. performed imaging experiments and scored bacterial contact; K.R. initiated SagA studies in C. elegans and generated sagA-expressing bacteria; J.C. analyzed and scored tissue pathology; J.L. helped with FISH and intestinal lymphocyte preps; A.R. established the germ-free mouse colony and helped with experiments; H.H. and D.M. initiated, designed and supervised the study and wrote the manuscript.
Footnotes
The authors declare no competing financial interests. Patent application PCT/US2016/028836, entitled "Modified microorganisms expressing SagA as anti-infective agents, probiotics and food components" was filed Apr. 22, 2016.
References and Notes
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. bioRxiv. 2016 doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell metabolism. 2016 Mar 8;23:413. doi: 10.1016/j.cmet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012 Mar 16;148:1258. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013 Dec 19;155:1451. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi GB, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016 Feb 26;351:933. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010 Mar;10:159. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 8.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009 May;9:313. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008 May 29;453:620. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 10.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB. Effects of antibiotics on human microbiota and subsequent disease. Annu Rev Microbiol. 2014;68:217. doi: 10.1146/annurev-micro-091313-103456. [DOI] [PubMed] [Google Scholar]
- 12.Buffie CG, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015 Jan 8;517:205. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013 Aug 2;341:569. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013 Aug 8;500:232. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011 Jan 27;469:543. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 16.Lebreton F, Willems RJL, Gilmore MS. In: Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Boston: 2014. [PubMed] [Google Scholar]
- 17.Franz CM, Huch M, Abriouel H, Holzapfel W, Galvez A. Enterococci as probiotics and their implications in food safety. Int J Food Microbiol. 2011 Dec 2;151:125. doi: 10.1016/j.ijfoodmicro.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Lund B, Edlund C. Probiotic Enterococcus faecium strain is a possible recipient of the vanA gene cluster. Clin Infect Dis. 2001 May 1;32:1384. doi: 10.1086/319994. [DOI] [PubMed] [Google Scholar]
- 19.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003 May;71:2839. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle EC, Brown NF, Finlay BB. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol. 2006 Dec;8:1946. doi: 10.1111/j.1462-5822.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009 Jul;77:2635. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015 Jan 20;42:28. doi: 10.1016/j.immuni.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012 Feb;15:57. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandl K, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008 Oct 9;455:804. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wlodarska M, et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011 Apr;79:1536. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renier N, et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014 Nov 6;159:896. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016 Jan 28;164:378. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loonen LM, et al. REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014 Jul;7:939. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- 29.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011 Oct 14;334:255. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stelter C, et al. Salmonella-induced mucosal lectin RegIIIbeta kills competing gut microbiota. PLoS One. 2011;6:e20749. doi: 10.1371/journal.pone.0020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson KG, Holden DW. Dynamics of growth and dissemination of Salmonella in vivo. Cell Microbiol. 2010 Oct;12:1389. doi: 10.1111/j.1462-5822.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 32.Kansal R, et al. Transcriptional modulation of enterotoxigenic Escherichia coli virulence genes in response to epithelial cell interactions. Infect Immun. 2013 Jan;81:259. doi: 10.1128/IAI.00919-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey AK, Bhagat A, Chowdhury R. Host cell contact induces expression of virulence factors and VieA, a cyclic di-GMP phosphodiesterase, in Vibrio cholerae. J Bacteriol. 2013 May;195:2004. doi: 10.1128/JB.02127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginocchio CC, Olmsted SB, Wells CL, Galan JE. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994 Feb 25;76:717. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 35.Armentrout EI, Rietsch A. The Type III Secretion Translocation Pore Senses Host Cell Contact. PLoS Pathog. 2016 Mar;12:e1005530. doi: 10.1371/journal.ppat.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010 Feb;10:131. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 37.Bhinder G, et al. Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses. Infect Immun. 2014 Sep;82:3753. doi: 10.1128/IAI.02045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dessein R, et al. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut. 2009 Jun;58:771. doi: 10.1136/gut.2008.168443. [DOI] [PubMed] [Google Scholar]
- 39.Valdez Y, Ferreira RB, Finlay BB. Molecular mechanisms of Salmonella virulence and host resistance. Curr Top Microbiol Immunol. 2009;337:93. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005 Feb 4;307:731. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 41.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014 Aug 21;41:311. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natividad JM, et al. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−; Nod2−/− mice. Inflamm Bowel Dis. 2012 Aug;18:1434. doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 43.Rangan KJ, Pedicord VA, Wang Y-C, Kim B-C, Lu Y, Shaham S, Mucida D, Hang HC. A secreted bacterial peptidoglycan hydrolase enhances host resistance to intestinal pathogens. Science. 2016 doi: 10.1126/science.aaf3552. accompanying submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng F, Kawalec M, Weinstock GM, Hryniewicz W, Murray BE. An Enterococcus faecium secreted antigen, SagA, exhibits broad-spectrum binding to extracellular matrix proteins and appears essential for E. faecium growth. Infect Immun. 2003 Sep;71:5033. doi: 10.1128/IAI.71.9.5033-5041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed JA, Teng F, Nallapareddy SR, Murray BE. Pleiotrophic effects of 2 Enterococcus faecalis sagA-like genes, salA and salB, which encode proteins that are antigenic during human infection, on biofilm formation and binding to collagen type i and fibronectin. J Infect Dis. 2006 Jan 15;193:231. doi: 10.1086/498871. [DOI] [PubMed] [Google Scholar]
- 46.Palmer KL, et al. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J Bacteriol. 2010 May;192:2469. doi: 10.1128/JB.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathog. 2009;1:19. doi: 10.1186/1757-4749-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dicks LM, Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes. 2010 Mar;1:11. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- 49.Leffler DA, Lamont JT. Clostridium difficile Infection. N Engl J Med. 2015 Jul 16;373:287. doi: 10.1056/NEJMc1506004. [DOI] [PubMed] [Google Scholar]
- 50.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 51.Rakoff-Nahoum S, Medzhitov R. Role of the innate immune system and host-commensal mutualism. Curr Top Microbiol Immunol. 2006;308:1. doi: 10.1007/3-540-30657-9_1. [DOI] [PubMed] [Google Scholar]
- 52.Miki T, Holst O, Hardt WD. The bactericidal activity of the C-type lectin RegIIIbeta against Gram-negative bacteria involves binding to lipid A. J Biol Chem. 2012 Oct 5;287:34844. doi: 10.1074/jbc.M112.399998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehotzky RE, et al. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proceedings of the National Academy of Sciences of the United States of America. 2010 Apr 27;107:7722. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miki T, Hardt WD. Outer membrane permeabilization is an essential step in the killing of gram-negative bacteria by the lectin RegIIIbeta. PLoS One. 2013;8:e69901. doi: 10.1371/journal.pone.0069901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson A, Diebel LN, Liberati DM. Effect of host defenses on Clostridium difficile toxin-induced intestinal barrier injury. J Trauma Acute Care Surg. 2013 Apr;74:983. doi: 10.1097/TA.0b013e3182858477. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Hirota SA. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol Immunol. 2015 Feb;63:193. doi: 10.1016/j.molimm.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HY, Crawley S, Hokari R, Kwon S, Kim YS. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB, PI3K/Akt/IkappaB/NF-kappaB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int J Oncol. 2010 Apr;36:941. doi: 10.3892/ijo_00000573. [DOI] [PubMed] [Google Scholar]
- 58.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011 Mar 15;108(Suppl 1):4659. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brune A, Dietrich C. The Gut Microbiota of Termites: Digesting the Diversity in the Light of Ecology and Evolution. Annu Rev Microbiol. 2015;69:145. doi: 10.1146/annurev-micro-092412-155715. [DOI] [PubMed] [Google Scholar]
- 60.Silver AC, Rabinowitz NM, Kuffer S, Graf J, Identification of Aeromonas veronii genes required for colonization of the medicinal leech. Hirudo verbana. J Bacteriol. 2007 Oct;189:6763. doi: 10.1128/JB.00685-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SM, et al. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013 Sep 19;501:426. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamada N, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012 Jun 8;336:1325. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Candela M, et al. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol. 2008 Jul 31;125:286. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013 Nov;13:790. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lessa FC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015 Feb 26;372:825. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent Y, Manji A, Gregory-Miller K, Lee C. A Review of Management of Clostridium difficile Infection: Primary and Recurrence. Antibiotics (Basel) 2015;4:411. doi: 10.3390/antibiotics4040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen SJ, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013 Oct 12;382:1249. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 68.Sorg JA, Dineen SS. Laboratory maintenance of Clostridium difficile. Curr Protoc Microbiol. 2009 Feb; doi: 10.1002/9780471729259.mc09a01s12. Chapter 9, Unit9A 1. [DOI] [PubMed] [Google Scholar]
- 69.Salzman NH, et al. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002 Nov;148:3651. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 70.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005 Jul;43:3380. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang S, Denman SE, Morrison M, Yu Z, McSweeney CS. An efficient RNA extraction method for estimating gut microbial diversity by polymerase chain reaction. Curr Microbiol. 2009 May;58:464. doi: 10.1007/s00284-008-9345-z. [DOI] [PubMed] [Google Scholar]
- 72.Zhang G, Brown EW, Gonzalez-Escalona N. Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl Environ Microbiol. 2011 Sep;77:6495. doi: 10.1128/AEM.00520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abernathy J, Corkill C, Hinojosa C, Li X, Zhou H. Deletions in the pyruvate pathway of Salmonella Typhimurium alter SPI1-mediated gene expression and infectivity. J Anim Sci Biotechnol. 2013;4:5. doi: 10.1186/2049-1891-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.