Abstract
After its discovery as a key controller of metabolic function, leptin has been later extensively implicated in additional function including important modulatory activities on the innate and adaptive immune responses. This review analyzes the known implications of leptin in multiple inflammatory conditions, including autoimmune diseases, and how this knowledge could be instrumental in the design of leptin-based manipulation strategies to help restoration of abnormal immune responses.
Keywords: Leptin, inflammation, autoimmunity
1. Introduction
Leptin is a 16 kDa non-glycosylated protein that is mainly produced by adipocytes, for which reason it belongs to the group of cytokines that are commonly called adipocytokines or adipokines [1]. Leptin has a dual role as a hormone and a cytokine. As hormone, it influences multiple endocrine functions and bone metabolism, in addition to the key function of modulating energy homeostasis through mechanisms that include thermoregulation. As a cytokine, leptin promotes inflammatory responses. It derives that elevated levels of circulating leptin in obese patients contribute significantly to the low-grade inflammatory state that makes those individuals more susceptible to develop cardiovascular diseases, type II diabetes, or degenerative disease [2], in addition to autoimmune disease [3]. Conversely, reduced levels of leptin such as those found in malnourished individuals have been linked to increased risk of infection and reduced cell-mediated immunity [3], likely secondarily to an insufficient immune cell effector activity that cannot allow a proper control of pathogens (possibly concomitantly with an enhanced function of CD4+ regulatory T cells (Tregs) that suppress effector immune responses) [4]. These data point to the fact that leptin, together with its role in the regulation of food intake and energy expenditure [5], also exerts a strong proinflammatory activity likely linked to its resemblance to IL-6 [6].
Leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice not only have metabolic abnormalities and morbid obesity but also a series of marked abnormalities in neuroendocrine functions, angiogenesis, reproduction, insulin secretion, wound healing, hematopoiesis and lymphopoiesis, suggesting an involvement of leptin in the regulation of multiple important physiologic functions [7]. In addition to those disturbances, genetic deficiency in the leptin axis in both mice and humans associates with thymus atrophy and immune deficiency, in addition to an increased susceptibility to opportunistic infection [8–9]. Importantly, the impaired T-cell proliferation, reduced number of circulating CD4+ T cells (particularly naïve T cells) and impaired cytokine production (lack of IFN-γ secretion and reduced IL-4 and IL-10 production) present in leptin-deficient subjects can all be reversed by the administration of recombinant leptin, demonstrating an important role of leptin on the physiology of the immune response [7].
2. Immune effects of leptin
Innate immune responses and inflammation are interwoven processes. As a first line of defense against infection, innate immunity operates to fight against pathogens to which it had no prior exposure. However, this limited lack of specificity in the effector immune response can lead to the production of inflammatory mediators that may harm the host, particularly if the response is prolonged or insufficiently controlled by the host’s homeostatic mechanisms. The activation of innate immune cells including polymorphonuclear leukocytes (PMN) such as neutrophils, eosinophils, and basophils induced by proinflammatory insults favors the migration of these cells towards chemotactic stimuli produced at sites of inflammation. Once there, these cells produce powerful local mediators that cause harm to the pathogen but at the meantime can damage the host tissue. Together with the soluble mediators released by innate immune cells, macrophage phagocytosis, and dendritic cell (DC) presentation of antigens derived from the pathogen, adaptive immune responses ensue, with the recruitment of B and T lymphocytes. It derives that longer timeframes are required to mount an adaptive immune response (yet characterized by higher specificity and great efficacy also for the possibility to generate long-lasting immune responses available in case of subsequent encounters with the same pathogen). Interestingly, leptin provides a contribution to both the innate and adaptive immune response. Specifically, in monocytes/macrophages, leptin stimulates the expression of CD39, CD69, CD25, CD71 and IL-1Rα [10], and the production of proinflammatory cytokines IL-6 and TNF-α [11]. Moreover, it favors proliferation, phagocytosis through phospholipase activation [10], and the production of eicosanoids, nitric oxide, leukotriene B4 (LTB4) and cyclooxygenase 2 (COX-2) from these cells [12–13]. In neutrophils, leptin promotes chemotaxis and the release of reactive oxygen species [14]. It also protects neutrophils from apoptosis involving PI3K- and MAPK-dependent pathways, delays cleavage of Bid and Bax and mitochondrial release of cytochrome c and the activation of caspases [15], and upregulates the expression of several adhesion molecules and chemotaxis induced by the eosinophil chemoattractant RANTES [16].
In NK cells, leptin contributes to cell development, proliferation and cytotoxicity (this last function occurs via an upregulation of IL-2 and perforin expression through activation of STAT3) [17]. In DC, leptin promotes maturation and survival [18].
In general, leptin has proinflammatory properties and activities similar to other acute phase reactants, and upregulates the secretion of multiple inflammatory cytokines including TNF-α, IL-6, and IL-12 [6]. Moreover, after exposure to inflammatory stimuli such as LPS, TNF-α, and IL-1, the levels of circulating leptin and leptin expression in the adipose tissue increase [19–20]. Conversely, TNF-α and IL-1β increase the expression of leptin mRNA in the adipose tissue, creating a loop whose components influence each other in promoting inflammation [20]. Thus, proinflammatory mediators such as TNF-α and IL-1 - which upregulate leptin expression – can in turn contribute to the production of acute phase reactants that influence each other in promoting chronic inflammation, underscoring leptin’s promoting effect on low-grade inflammation.
As mentioned before and as shown in Fig. 1, leptin affects both innate and adaptive immunity. In the adaptive immune response, leptin promotes T cell survival [21] by modulating the expression of anti-apoptotic proteins [22], partly explaining the reduced T cell numbers during fasting, where a significant drop in circulating leptin levels is observed [23].
Figure 1.
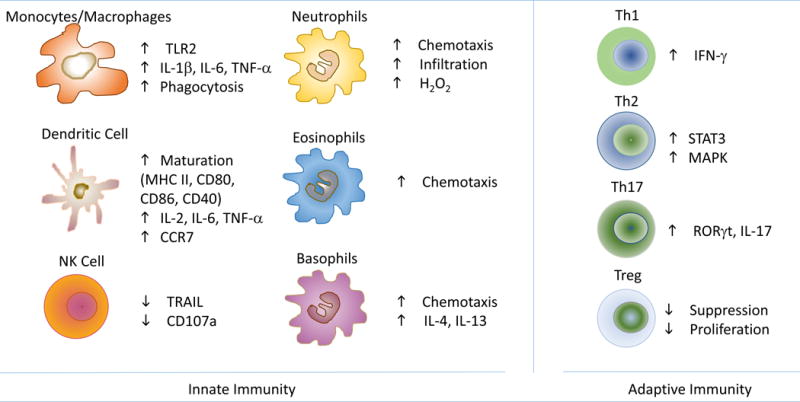
Schematic representation of the multiple effects of leptin on different types of immune cells of the innate and adaptive immune systems.
While leptin enhances the proliferation and activation of T cells, it is noteworthy that it can exert differential effects on proliferation of naïve vs memory T cells or T effector cells (Teffs) vs. Tregs [24–25]. Specifically, leptin promotes naїve T cell survival and the production of IFN-γ and IL-2, and facilitates the differentiation and activity of Th1 cells while inhibiting Th2 cells [26]. It also promotes IgG2a switch and inhibits IgG1 switch in B cells [6]. The activity of leptin on T cells also extends to proinflammatory Th17 cells, in which it promotes the expression of the master regulator transcription factor RAR-related orphan receptor (ROR)γt and leptin neutralization inhibits Th17 responses [27]. At the meantime, leptin represents a negative signal for the expansion of Tregs [24]. Leptin neutralization causes Tregs to proliferate and better suppress autoimmune responses, both in vitro and in vivo [24]. It is therefore expected that ob/ob and db/db mice display elevated numbers of Tregs [24].
Of note, freshly isolated Tregs produce leptin and express the leptin receptor, which is also expressed on additional immune cell subsets [28]. At the molecular level, leptin activates the mTOR pathway to control Tregs responsiveness [29] and Teffs functions [30]. More specifically, leptin inhibits rapamycin-induced proliferation of Tregs by increasing activation of the mTOR pathway [29]. Interestingly, leptin exerts opposite effects on Teffs, where it enhances their proliferation also through modulation of the mTOR pathway [30].
2.1 Leptin signaling in immune cells
Leptin receptor expression on multiple immune cell types makes the activity of leptin influential on different immune functions both in mice [31] and in humans [32]. The pleiotropic biological effects of leptin can be explained by the wide distribution of leptin receptors on different types of cells, not last those ones found in non-neural tissues and that take part in the immune response. In this context, the study of the biochemical events that follow the binding of leptin to its cognate receptors have been studied extensively and have been well characterized. Leptin receptors are class I cytokine receptors encoded by the db gene that vary in length due to alternative splicing. In mice, there are six different isoforms that share a common extracellular and transmembrane domain, but differ in the length of their intracellular domain. They have characteristic extracellular motifs of four cysteine residues and contain the aminoacid sequence WSXWS (Trp-Ser-Xaa-Trp-Ser) and fibronectin type III domains [33]. Leptin receptors include a soluble form (Ob-Re), four short forms (Ob-Ra, Ob-Rc, Ob-Rd and Ob-Rf) and one long isoform (Ob-Rb) [34]. All have a common extracellular domain of more than 800 amino acids, a transmembrane domain of 34 amino acids, and a variable intracellular domain characteristic of each isoform. The main differences are in the intracellular amino acids: 302 for OB-Rb, and 34, 32 and 40, respectively, for the short forms OB-Ra, OB-Rc and OB-Rd. Signaling through Ob-Rb is considered the most important in conveying leptin effects, as this long isoform has the longest intracellular domain with 302 amino acids and is the only isoform containing functional Janus kinase (Jak)2 and signal transducers and activators of transcription (STAT) binding sites which are essential for signal transduction and transmission of leptin function [28]. On the other hand, the lack of cytoplasmic (short forms) or transmembrane components (soluble receptor) results in receptors that contribute to the regulation of plasma leptin levels by binding leptin without transducing intracellular signals [35]. From a biochemical standpoint, Ob-R does not have an intrinsic tyrosine kinase domain but a proline-rich box 1 motif that can bind Jak2. Leptin binding to Ob-Rb causes homo-oligomerization and binding to Jak which leads to autophosphorylation of Jak2 and the phosphorylation of intracellular Tyr985, Tyr1077, and Tyr1138 [36–37]. The phosphorylation of Tyr1138 is important for the recruitment of STAT proteins to the Ob-Rb/Jak2 complex, leading to transcription activation of target genes including the suppressor of cytokine signaling (SOCS)3 [38–42]. In addition to SOCS3, protein tyrosine phosphatase 1B (PTP1B) inhibits leptin-mediated signaling and acts by dephosphorylating Jak2 [43], whereas phosphorylated Jak2 activates the mitogen-activated protein kinase (MAPK) cascade via recruitment of src homology 2-containing tyrosine phosphatase (SHP2) to OB-Rb, which binds to GRB2 to signal through RAS, RAF and MEK1 for the activation of extracellular signal-regulated kinase (ERK)1/2, p38 MAPK and p42/44 pathways [37, 44–46]. An alternative pathway involves the interaction of SHP2 and GRB2 on Jak2 [47–48]. In any case, autophosphorylated Jak2 can phosphorylate insulin receptor substrate1/2 (IRS1/2) and activate phosphatidylinositol 3-kinase (PI3K)/Akt [49].
In humans, four splice variants of leptin receptor have been identified: a long OB-R isoform and three short isoforms designed huB219.1–huB219.3 [50]. Of those, the long isoform responsible for the anorexigenic effects of leptin is abundant in the hypothalamic centers regulating food intake and can also be found on immune cells [51–53], being capable to stimulate proliferation of peripheral blood mononuclear cells [54].
3. Leptin in non-autoimmune inflammation
Alone or in combination with IL-1, leptin promotes the expression of inducible nitric oxide (NO) synthase (iNOS) and COX-2 and the production of NO, prostaglandin E, IL-6, and IL-8. The effects of leptin are mediated through activation of the transcription factor nuclear factor-κB (NF-κB) and c-Jun NH2-terminal protein kinase (JNK) (with subsequent phospholipase C (PLC) and protein kinase C (PKC) activation), having NO as a key mediator of the increased synthesis of those proinflammatory mediators [55–56]. These events are responsible for leptin’s stimulation of oxidative stress, and at the cardiovascular level they result in vascular inflammation and vascular smooth muscle hypertrophy – all factors that contribute to atherosclerosis, hypertension, coronary heart disease and thrombosis [57]. In other system, these events produce as well proinflammatory states that are discussed below.
3.1. Leptin and atherosclerosis
Leptin can contribute to atherogenesis [58–59] by promoting the recruitment of monocytes to the intima, eliciting foam cell formation, and favoring secretion of proinflammatory and atherogenic cytokines [60–61], in addition to altering cardiomyocyte structure and function [62]. This is because leptin receptors are present within lesions of atherosclerosis patients [63], and in fact; eptin-deficient ob/ob mice are resistant to atherosclerosis [64]. Also, low-density lipoprotein (LDL) receptor-deficient ob/ob (LDL-R−/−/ob/ob) mice show a considerable reduction in atherosclerotic lesions as compared to LDL-R−/− mice with intact leptin signaling [65]. Leptin levels are also positively correlated with levels of fibrinogen, plasminogen activator inhibitor-1, von Willebrand factor, and factor VIIa, and negatively correlated with protein C and tissue plasminogen activator, thus affecting fibrinolysis and favoring arterial thrombosis [66–70].
3.2. Leptin and type 2 diabetes
Since leptin modulates glucose homeostasis and inhibits insulin synthesis and secretion, the development of type 2 diabetes in ob/ob and db/db mice is dramatically attenuated by leptin administration [71–72]. In addition to decreasing insulin secretion via a direct action on the pancreatic β cells, leptin also enhances glucose uptake and oxidation in skeletal muscle and suppresses the hepatic production of glucose [73–75]. At the molecular level, leptin signals in pancreatic β cells through Jak-STAT and interferes with cAMP and the activation of PI3 kinase [76]. A role of leptin in diabetic nephropathy is as well suggested by the observation that leptin deficient ob/ob mice are relatively resistant to the development of diabetic glomerulopathy, and by the finding leptin levels are increased in albuminuric type 2 diabetes patients (where urinary leptin positively correlates with urinary albumin-creatinine ratio and inversely correlate with glomerular filtration rate) [77].
In the metabolic syndrome - a combination of abdominal obesity, high blood pressure, high blood glucose, low HDL, elevated cholesterol and high triglycerides that increase the risk of heart disease and diabetes, high leptin levels predict disease worsening independently of obesity [78]. Those patients may also be affected by non-alcoholic steatohepatitis (a liver disease characterized by fat accumulation in hepatocytes), where leptin could contribute to fat accumulation through a reduced hepatic oxidation and an increased synthesis of free fatty acids that would cause liver inflammation and fibrosis through local lipid accumulation and peroxidation [79–81].
3.3. Renal inflammation
Leptin is mainly cleared by the kidney [82], which is an organ targeted by leptin activities [83]. Indeed, leptin-deficient ob/ob mice are protected from renal inflammation and nephrotoxic nephritis [84].
The direct and indirect effects of leptin on the kidney include natriuresis, increased sympathetic nervous activity, and the stimulation of reactive oxygen species [85]. Moreover, leptin is involved in the development of glomerulosclerosis through a paracrine TGF-β pathway (between glomerular endothelial and mesangial cells) that promotes deposition of extracellular matrix and proteinuria [86].
3.4. Chronic obstructive pulmonary disease (COPD)
Among the inflammatory conditions characterized by a strong tendency to chronicity, leptin has been considered an inflammatory marker of airway inflammation and a possible contributor to severity of COPD, a chronic inflammatory disease of the lung [87].
3.5. Behçet’s disease
Also in Behçet’s disease (a chronic, systemic inflammatory disorder with generalized vasculitis), the levels of serum leptin are increased, particularly in patients with long disease duration, where they correlate with disease activity [88].
3.6. Pelvic endometriosis
Leptin elevation in the peritoneal fluid of patients with endometriosis positively correlates with the clinical stage of the disease [89–90], although no differences are found in serum leptin concentration of endometriosis patients and controls [91]. Leptin’s contribution to pelvic endometriosis could be related to the ability of leptin to promote neoangiogenesis and the production of proinflammatory cytokines [92].
3.7. Leptin and cancer
Given the immunomodulatory properties of leptin, considerations have been made on the possibility that leptin could influence tumorigenesis, particularly in hepatocellular carcinoma and prostate cancer [93]. The idea is that leptin might facilitate cancer cell progression by promoting inflammation, together with cell proliferation and migration while inhibiting apoptosis. Additionally, tumors and metastasis depend on angiogenesis, and leptin influences angiogenesis significantly [6]. However, insufficient information is currently available to draw conclusions, and further research and longitudinal studies are required to delineate both specific and additive effects of leptin in cancer [94].
3.8. Leptin and infection
In the course of acute inflammation, infection and sepsis, leptin levels increase favored by the presence of bacterial lipopolysaccharide and the increase in cytokines such as TNF-α, IL-6, and IL-1β [95]. In humans, plasma leptin is elevated in septic patients and positively correlates with patients’ survival [96–97]. However, in some acute inflammatory conditions such as acute experimental endotoxemia and newborn sepsis, no increased levels of serum leptin are found [98–99].
In tuberculosis and pediatric HIV patients, leptinemia is reduced [100–101], and in HIV infection, highly active anti-retroviral therapy (HAART) associates with increased leptinemia, together with the amelioration of the clinical picture and improvement of CD4+ T cell counts [102].
In bacterial infection, leptin proinflammatory activities (through TNF-α and IL-6 induction) seem to contribute to pathogens clearance. Leptin-deficient ob/ob mice develop severe disease and die of Klebsiella infection more rapidly than wild-type mice, and their elevated susceptibility to LPS-induced lethality can be reversed by leptin administration [103–107].
4. Leptin and autoimmunity
Elevated levels of circulating leptin have been associated with multiple autoimmune diseases, both in humans and in animal models of human autoimmunity [108], as discussed below.
4.1. Leptin and type-1 diabetes (T1D)
Leptin accelerates T1D onset and progression in nonobese diabetic (NOD) mice by stimulating autoimmune destruction of β-cells and by increasing IFN-γ production [109]. Also, a spontaneous mutation of ObR (LepRdb5J) in T1D-prone NOD mice inhibits T1D development [110]. Moreover, in ob/ob diabetic rats, systemic administration of leptin reversed ketoacidosis and normalizes blood glucose concentration by decreasing the delivery of glycerol and fatty acids to the liver, also reducing availability of acetyl-CoA (an inhibitor of the conversion of pyruvate to glucose) [111]. Although these data suggest a promoting role of leptin in T1D, also suggested by the finding of elevated circulating levels of leptin in T1D patients [112], recent data indicated that leptin treatment could reverse hyperglycemia in animal models of poorly controlled T1D (possibly through the suppression of glucagon production and/or responsiveness to this hormone) [111].
4.2. Leptin and multiple sclerosis
Leptin deficient ob/ob mice and leptin receptor-deficient db/db mice are resistant to the development of several experimentally-induced autoimmune diseases [6], among which experimental autoimmune encephalomyelitis (EAE) [113] – an animal model of human multiple sclerosis (MS). Resistance to EAE is reversed by leptin replacement while administration of leptin worsens EAE, indicating a key role of this adipokine in the disease susceptibility [113]. Furthermore, leptin neutralization improved clinical score and delayed EAE progression [114]. In MS patients, leptin production is increased in both serum and cerebrospinal fluid, and inversely correlates with the numbers of circulating Tregs [115].
4.3 Leptin and inflammatory bowel disease (IBD)
Although experimental colitis in rats resulted in increased leptin levels in association with weight loss [116], and ob/ob mice displayed resistance to acute and chronic intestinal inflammation [117], conflicting data and insufficient clinical studies may not allow at present to draw unequivocal conclusions on a possible role of leptin in IBD.
4.4 Leptin and autoimmune arthritis
ob/ob and db/db mice develop a milder form of antigen-induced arthritis than wild-type controls, and have markedly reduced antigen-specific autoreactive T cell proliferation and proinflammatory cytokine production [118]. In chondrocytes, leptin induces NOS activation, in synergy with IFN-γ and IL-1 [119], in addition to metalloproteases activation, apoptosis, and chondrocyte phenotype loss [120].
However, the role of leptin in the pathogenesis of rheumatoid arthritis (RA) is controversial. High levels of leptin both systemically and in the joints have been reported by some authors - also correlating with disease activity [121] - but other studies found no changes or even low levels of leptin and no correlations with disease biomarkers or activity scores [122].
4.5 Leptin and systemic lupus erythematosus
Leptin increase in SLE does not correlate with disease activity but it can contribute to elevated cardiovascular risk [123] by inducing proinflammatory high-density lipoproteins and atherosclerosis [124]. The propathogenic effects of leptin in SLE can be ascribed, at last in part, to its promoting activities on proinflammatory Th17 cells [27] and the facilitated autoantibody production that is concomitant to the inhibition of immunoregulatory responses [125]. Other mechanisms include leptin’s promotion of the activity and survival of autoreactive T cell via an increased expression of the anti-apoptotic molecule Bcl-2 [126–127]. Notably, leptin neutralization in lupus-prone mice inhibits proinflammatory responses and SLE manifestations [27, 125]. Similarly, a reduction in circulating leptin levels induced by fasting protects lupus mice from SLE via mechanisms that include an expansion of peripheral Tregs [128].
4.6. Leptin in psoriasis
Although an increase in leptin in psoriasis patients seems to parallel the severity of the disease, the data on a promoting role of leptin in the disease remain controversial [129–130].
5. Conclusions
Given the contribution of the proinflammatory activity of leptin to autoimmune and non-autoimmune inflammation, it has been suggested to limit leptin bioavailability in those conditions through antibody-based antagonism [3]. In non-autoimmune inflammatory conditions such cardiovascular and metabolic diseases, leptin inhibition could negatively influence the development and progression of the disease, whereas in autoimmunity it could reduce immune hyperactivity and slow the progression of chronic inflammation leading to target tissue damage.
It has nonetheless to be taken into account that lowered leptin levels could negatively affect effector immune responses during infection, and thus targeted approaches considering selective targeting of the leptin pathway might be considered to reduce this possibility. For example, useful leptin-based targets could be SOCS3 (an important factor of leptin resistance and negative feedback), PTP1B (which dephosphorylates Jak2 on OB-R), and SHP2 (which is critical for leptin signaling through downregulation of the ObRb-STAT3 pathway and the promotion of ERK signaling) [131–132].
Highlights.
The cytokine activity of leptin is responsible for effects of this adipokine on both innate and adaptive immune responses
Because of its pro-inflammatory actions, leptin contributes to the generation and maintenance of low-grade inflammation
Leptin levels are elevated in multiple autoimmune diseases, and leptin blockade in experimental animal models has proven as beneficial in reducing autoimmune reactivity
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health grant AI109677.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med. 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 2.La Cava A. Proinflammatory activities of leptin in non-autoimmune conditions. Inflamm Allergy Drug Targets. 2012;11:298–302. doi: 10.2174/187152812800959031. [DOI] [PubMed] [Google Scholar]
- 3.Matarese G, La Cava A, Sanna V, Lord GM, Lechler RI, Fontana S, Zappacosta S. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23:182–187. doi: 10.1016/s1471-4906(02)02188-9. [DOI] [PubMed] [Google Scholar]
- 4.Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 2010;16:247–256. doi: 10.1016/j.molmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 6.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard JK, Lord GM, Matarese G, Vendetti MA, Ghatei S, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3895. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 12.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJ. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287:L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 14.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–8096. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37:2337–2348. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 18.Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–3130. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- 19.Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Warldaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab. 2003;88:1285–1291. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- 20.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1β mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–8. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Riejos P, Goberna R, Sánchez-Margalet V. Leptin promotes cell survival and activates Jurkat T lymphocytes by stimulation of mitogen-activated protein kinase. Clin Exp Immunol. 2008;151:505–518. doi: 10.1111/j.1365-2249.2007.03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amarilyo G, Iikuni N, Shi FD, Liu A, Matarese G, La Cava A. Leptin promotes lupus T-cell autoimmunity. Clin Immunol. 2013;149:530–533. doi: 10.1016/j.clim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 24.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D, Qian K, Auvinen P, Calì G, Stallone G, Formisano L, La Cava A, Matarese G. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol. 2012;189:2941–2953. doi: 10.4049/jimmunol.1200935. [DOI] [PubMed] [Google Scholar]
- 26.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Liu Y, Shi FD, Zou H, Matarese G, La Cava A. Cutting edge: Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol. 2013;190:3054–3058. doi: 10.4049/jimmunol.1203275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Procaccini C, Lourenço EV, Matarese G, La Cava A. Leptin signaling: a key pathway in immune responses. Curr Signal Transduct Ther. 2009;4:22–30. doi: 10.2174/157436209787048711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath A, La Cava TL, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D, Qian K, Auvinen P, Calì G, Stallone G, Formisano L, La Cava A, Matarese G. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol. 2012;189:2941–2953. doi: 10.4049/jimmunol.1200935. [DOI] [PubMed] [Google Scholar]
- 31.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 32.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 33.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fei H, Okano JJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 36.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG., Jr Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 37.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 38.Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 39.Cui H, Cai F, Belsham DD. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J. 2006;20:2654–2656. doi: 10.1096/fj.06-5989fje. [DOI] [PubMed] [Google Scholar]
- 40.Mansour E, Pereira FG, Araújo EP, Amaral ME, Morari J, Ferraroni NR, Ferreira DS, Lorand-Metze I, Velloso LA. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–5479. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- 41.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 42.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 43.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:385–387. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 44.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Rec Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 45.van den Brink GR, O’Toole T, Hardwick JC, van den Boogaardt DE, Versteeg HH, van Deventer SJ, Peppelenbosch MP. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol Cell Biol Res Commun. 2000;4:144–150. doi: 10.1006/mcbr.2001.0270. [DOI] [PubMed] [Google Scholar]
- 46.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 47.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 48.Stofega MR, Herrington J, Billestrup N, Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol Endocrinol. 2000;14:1338–1350. doi: 10.1210/mend.14.9.0513. [DOI] [PubMed] [Google Scholar]
- 49.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 50.Dam J, Jockers R. Hunting for the functions of short leptin receptor isoforms. Mol Metab. 2013;2:327–328. doi: 10.1016/j.molmet.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 52.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Margalet V, Martin-Romero C, Gonzalez-Yanes C, Goberna R, Rodriguez-Bano J, Muniain MA. Leptin receptor (Ob-R) expression is induced in peripheral blood mononuclear cells by in vitro activation and in vivo in HIV-infected patients. Clin Exp Immunol. 2002;129:119–124. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulatory of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takekoshi K, Ishii K, Nanmoku T, Shibuya S, Kawakami Y, Isobe K, Nakai T. Leptin stimulates catecholamine synthesis in a PKC-dependent manner in cultured porcine adrenal medullary chromaffin cells. Endocrinology. 2001;142:4861–4871. doi: 10.1210/endo.142.11.8484. [DOI] [PubMed] [Google Scholar]
- 56.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, Moilanen E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage-mediator role of NO in leptin-induced PGE 2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matarese G, Mantzoros C, La Cava A. Leptin and adipocytokines: bridging the gap between immunity and atherosclerosis. Curr Pharm Des. 2007;13:3676–3680. doi: 10.2174/138161207783018635. [DOI] [PubMed] [Google Scholar]
- 58.Brennan AM, Lee JH, Tsiodras S, Chan JL, Doweiko J, Chimienti SN, Wadhwa SG, Karchmer AW, Mantzoros CS. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160:173–176. doi: 10.1530/EJE-08-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 61.O’Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557–42562. doi: 10.1074/jbc.M202151200. [DOI] [PubMed] [Google Scholar]
- 62.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 63.Matarese G, Mantzoros C, La Cava A. Leptin and adipocytokines: bridging the gap between immunity and atherosclerosis. Curr Pharm Des. 2007;13:3676–3680. doi: 10.2174/138161207783018635. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Navarro H, Burks DJ, Andres V. Murine models to investigate the influence of diabetic metabolism on the development of atherosclerosis and restenosis. Front Biosci. 2007;12:4439–4455. doi: 10.2741/2400. [DOI] [PubMed] [Google Scholar]
- 65.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 66.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 67.Konstantinides S, Schäfer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–1540. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Mitrio V, De Pergola G, Vettor R, Marino R, Sciaraffia M, Pagano C, Scaraggi FA, Di Lorenzo L, Giorgino R. Plasma plasminogen activator inhibitor-I is associated with plasma leptin irrespective of body mass index, body fat mass, and plasma insulin and metabolic parameters in premenopausal women. Metabolism. 1999;48:960–964. doi: 10.1016/s0026-0495(99)90190-7. [DOI] [PubMed] [Google Scholar]
- 69.Soderberg S, Olsson T, Eliasson M, Johnson O, Ahrén B. Plasma leptin levels are associated with abnormal fibrinolysis in men and postmenopausal women. J Intern Med. 1999;245:533–543. doi: 10.1046/j.1365-2796.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 70.Chu NF, Spiegelman D, Hotamisligil GS, Rifai N, Stampfer M, Rimm EB. Plasma insulin, leptin, and soluble TNF receptors levels in relation to obesity-related atherogenic and thrombogenic cardiovascular disease risk factors among men. Atherosclerosis. 2001;157:495–503. doi: 10.1016/s0021-9150(00)00755-3. [DOI] [PubMed] [Google Scholar]
- 71.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 72.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 73.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 74.Wang JL, Chinookoswong N, Scully S, Qi M, Shi ZQ. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology. 1999;140:2117–2124. doi: 10.1210/endo.140.5.6681. [DOI] [PubMed] [Google Scholar]
- 75.Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272:27758–27763. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 76.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fruehwald-Schultes B, Kern W, Beyer J, Forst T, Pfützner A, Peters A. Elevated serum leptin concentrations in type 2 diabetic patients with microalbuminuria and macroalbuminuria. Metabolism. 1999;48:1290–1293. doi: 10.1016/s0026-0495(99)90270-6. [DOI] [PubMed] [Google Scholar]
- 78.Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, Halsall I, O’Rahilly S, Wareham NJ. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [DOI] [PubMed] [Google Scholar]
- 79.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 80.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 81.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 82.Cumin F, Baum HP, Levens N. Leptin is cleared from the circulation primarily by the kidney. Int J Obes Relat Metab Disord. 1996;20:1120–1126. [PubMed] [Google Scholar]
- 83.Serradeil-Le Gal C, Raufaste D, Brossard G, Pouzet B, Marty E, Maffrand JP, Le Fur G. Characterization and localization of leptin receptors in the rat kidney. FEBS Lett. 1997;404:185–191. doi: 10.1016/s0014-5793(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 84.Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM. Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385–390. doi: 10.1016/S0002-9440(10)63128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175–183. doi: 10.1159/000095328. [DOI] [PubMed] [Google Scholar]
- 86.Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA. Leptin stimulates proliferation and TGF-β expression in renal glomerular endothelial cells: potential role in glomerulosclerosis. Kidney Int. 1999;56:860–872. doi: 10.1046/j.1523-1755.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- 87.Broekhuizen R, Vernooy JH, Schols AM, Dentener MA, Wouters EF. Leptin as local inflammatory marker in COPD. Respir Med. 2005;99:70–74. doi: 10.1016/j.rmed.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 88.Evereklioglu C, Inalöz HS, Kirtak N, Doganay S, Bülbül M, Ozerol E, Er H, Ozbek E. Serum leptin concentration is increased in patients with Behçet’s syndrome and is correlated with disease activity. Br J Dermatol. 2002;147:331–336. doi: 10.1046/j.1365-2133.2002.04703.x. [DOI] [PubMed] [Google Scholar]
- 89.Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab. 2000;85:2483–2487. doi: 10.1210/jcem.85.7.6703. [DOI] [PubMed] [Google Scholar]
- 90.Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A. Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Hum Reprod. 2003;18:1205–1209. doi: 10.1093/humrep/deg233. [DOI] [PubMed] [Google Scholar]
- 91.Wertel I, Gogacz M, Polak G, Jakowicki J, Kotarski J. Leptin is not involved in the pathophysiology of endometriosis-related infertility. Eur J Obstet Gynecol Reprod Biol. 2005;119:206–209. doi: 10.1016/j.ejogrb.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 93.Cai C, Shi FD, Matarese G, La Cava A. Leptin as clinical target. Rec Pat Inflamm Allergy Drug Discov. 2009;3:160–166. doi: 10.2174/187221309789257379. [DOI] [PubMed] [Google Scholar]
- 94.Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 95.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnalich F, Lopez J, Codoceo R, Jimenez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survival in sepsis and septic shock. J Infect Dis. 1999;180:908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 97.Bornstein SR, Licinio J, Tauchnitz R, Engelmann L, Negrão AB, Gold P, Chrousos GP. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280–283. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 98.Bornstein SR, Preas HL, Chrousos GP, Suffredini AF. Circulating leptin levels during acute experimental endotoxemia and antiinflammatory therapy in humans. J Infect Dis. 1998;178:887–890. doi: 10.1086/515349. [DOI] [PubMed] [Google Scholar]
- 99.Koç E, Ustündağ G, Aliefendioğlu D, Ergenekon E, Bideci A, Atalay Y. Serum leptin levels and their relationship to tumor necrosis factor-α and interleukin-6 in neonatal sepsis. J Pediatr Endocrinol Metab. 2003;16:1283–1287. doi: 10.1515/jpem.2003.16.9.1283. [DOI] [PubMed] [Google Scholar]
- 100.Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metabolism. 1997;46:303–305. doi: 10.1016/s0026-0495(97)90258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, van der Meer JW. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87:758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 102.Pinzone JJ, Fox ML, Sastry MK, Parenti DM, Simon GL. Plasma leptin concentration increases early during highly active antiretroviral therapy for acquired immunodeficiency syndrome, independent of body weight. J Endocrinol Invest. 2005;28:RC1–3. doi: 10.1007/BF03345372. [DOI] [PubMed] [Google Scholar]
- 103.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150:332–339. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wieland CW, Stegenga ME, Florquin S, Fantuzzi G, van der Poll T. Leptin and host defense against Gram-positive and Gram-negative pneumonia in mice. Shock. 2006;25:414–419. doi: 10.1097/01.shk.0000209524.12873.da. [DOI] [PubMed] [Google Scholar]
- 105.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–42. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 106.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao E, Xia-Zhang L, Vulliemoz NR, Ferin M, Wardlaw SL. Leptin modulates inflammatory cytokine and neuroendocrine responses to endotoxin in the primate. Endocrinology. 2003;144:4350–4353. doi: 10.1210/en.2003-0532. [DOI] [PubMed] [Google Scholar]
- 108.Hutcheson J. Adipokines influence the inflammatory balance in autoimmunity. Cytokine. 2015;75:272–279. doi: 10.1016/j.cyto.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Matarese G, Sanna V, Lechler RI, Sarvetnick N, Fontana S, Zappacosta S, La Cava A. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 110.Lee CH, Chen YG, Chen J, Reifsnyder PC, Serreze DV, Clare-Salzler M, Rodriguez M, Wasserfall C, Atkinson MA, Leiter EH. Novel leptin receptor mutation in NOD/LtJ mice suppresses type 1 diabetes progression: II. Immunologic analysis Diabetes. 2006;55:171–178. [PubMed] [Google Scholar]
- 111.Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, Rothman DL, Shulman GI. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iacobellis G, Diaz S, Mendez A, Goldberg R. Increased epicardial fat and plasma leptin in type 1 diabetes independently of obesity. Nutr Metab Cardiovasc Dis. 2014;24:72572–9. doi: 10.1016/j.numecd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 113.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Rosa V, Procaccini C, La Cava A, Chieffi P, Nicoletti GF, Fontana S, Zappacosta S, Matarese G. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barbier M, Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut. 1998;43:783–790. doi: 10.1136/gut.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 118.Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, Gabay C. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–82. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 119.Otero M, Gomez Reino JJ, Gualillo O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-γ in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 2003;48:404–409. doi: 10.1002/art.10811. [DOI] [PubMed] [Google Scholar]
- 120.Otero M, Lago R, Lago F, Reino JJ, Gualillo O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res Ther. 2005;7:581–591. doi: 10.1186/ar1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Toussirot É, Michel F, Binda D, Dumoulin G. The role of leptin in the pathophysiology of rheumatoid arthritis. Life Sci. 2015;140:29–36. doi: 10.1016/j.lfs.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 122.Procaccini C, Pucino V, Mantzoros CS, Matarese G. Metabolism. 2015;64:92–104. doi: 10.1016/j.metabol.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 123.McMahon M, Skaggs BJ, Sahakian L, Grossman J, FitzGerald J, Ragavendra N, Charles-Schoeman C, Chernishof M, Gorn A, Witztum JL, Wong WK, Weisman M, Wallace DJ, La Cava A, Hahn BH. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis. 2011;70:1619–1624. doi: 10.1136/ard.2010.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hahn BH, Lourenço EV, McMahon M, Skaggs B, Le E, Anderson M, Iikuni N, Lai CK, La Cava A. Pro-inflammatory high-density lipoproteins and atherosclerosis are induced in lupus-prone mice by a high-fat diet and leptin. Lupus. 2010;19:913–917. doi: 10.1177/0961203310364397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lourenço EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci USA. doi: 10.1073/pnas.1607101113. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amarilyo G, Iikuni N, Liu A, Matarese G, La Cava A. Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PLoS One. 2014;9:e112826. doi: 10.1371/journal.pone.0112826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amarilyo G, Iikuni N, Shi FD, Liu A, Matarese G, La Cava A. Leptin promotes lupus T-cell autoimmunity. Clin Immunol. 2013;149:530–533. doi: 10.1016/j.clim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol. 2012;188:2070–2073. doi: 10.4049/jimmunol.1102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cerman AA, Bozkurt S, Sav A, Tulunay A, Elbaşi MO, Ergun T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br J Dermatol. 2008;159:820–826. doi: 10.1111/j.1365-2133.2008.08742.x. [DOI] [PubMed] [Google Scholar]
- 130.Takahashi H, Tsuji H, Takahashi I, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Plasma adiponectin and leptin levels in Japanese patients with psoriasis. Br J Dermatol. 2008;159:1207–1208. doi: 10.1111/j.1365-2133.2008.08823.x. [DOI] [PubMed] [Google Scholar]
- 131.Feng S, Zhang GS, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–381. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 132.Feng GS. Shp2 as a therapeutic target for leptin resistance and obesity. Expert Opin Ther Targets. 2006;10:135–142. doi: 10.1517/14728222.10.1.135. [DOI] [PubMed] [Google Scholar]


