Abstract
The tongue is one of the major structures involved in human food intake and speech. Tongue malformations such as aglossia, microglossia, and ankyloglossia are congenital birth defects, greatly affecting individuals' quality of life. However, the molecular basis of the tissue-tissue interactions that ensure tissue morphogenesis to form a functional tongue remains largely unknown. Here we show that ShhCre-mediated epithelial deletion of Wntless (Wls), the key regulator for intracellular Wnt trafficking, leads to lingual hypoplasia in mice. Disruption of epithelial Wnt production by Wls deletion in epithelial cells led to a failure in lingual epidermal stratification and loss of the lamina propria and the underlying superior longitudinal muscle in developing mouse tongues. These defective phenotypes resulted from a reduction in epithelial basal cells positive for the basal epidermal marker protein p63 and from impaired proliferation and differentiation in connective tissue and paired box 3 (Pax3)- and Pax7-positive muscle progenitor cells. We also found that epithelial Wnt production is required for activation of the Notch signaling pathway, which promotes proliferation of myogenic progenitor cells. Notch signaling in turn negatively regulated Wnt signaling during tongue morphogenesis. We further show that Pax7 is a direct Notch target gene in the embryonic tongue. In summary, our findings demonstrate a key role for the lingual epithelial signals in supporting the integrity of the lamina propria and muscular tissue during tongue development and that a Wnt/Notch/Pax7 genetic hierarchy is involved in this development.
Keywords: connective tissue, craniofacial development, mouse, myogenesis, Wnt signaling, muscle progenitor cells
Introduction
The vertebrate tongue, a complex muscular organ located in the oral cavity, plays a crucial role in food intake and is one of the important structures involved in human speech (1). The mammalian tongue is composed of a stratified, squamous, non-keratinized epithelium and the underlying cranial neural crest cell (CNCC)4-derived connective tissue and striated muscle (2–4). The fibrous connective tissue beneath the lingual epithelium is termed lamina propria, which provides support and nutrition to the epithelium and binds epithelium to other tissues. Previous studies have shown that tongue development is regulated by reciprocal interactions between CNCC and myogenic cells (2, 3, 5–7). Epithelial signaling also plays important roles in regulating tongue development (2, 8); however, the intrinsic mechanism remains largely unexplored, and the roles of lingual epithelium in the process of tongue development still remain elusive.
Tongue development begins at E10.5 in mice by formation of the median lingual swelling of the first branchial arch, followed by the formation and fusion of lateral lingual swellings. As early as this stage, CNCCs populate the tongue primordium. At E11.5, the myogenic progenitors enter the tongue primordium and establish intimate contact with the CNCC (3, 5). The myogenic progenitor cells expressing Pax3 or Pax7, the paired-box transcription factors, are maintained as a proliferating population in embryonic and fetal muscles of the trunk and limb throughout development (3, 9, 10). Moreover, ectopic expression of either Pax3 or Pax7 is able to stimulate cell proliferation in myoblasts (11, 12). Pax3 and Pax7 have distinct and overlapping functions in muscle development (13, 14). Pax3 knock-out embryos exhibit defective limb and tongue muscle development, and Pax7 knock-out mice grow with small skeletal muscles displaying a complete absence of functional satellite cells, whereas in Pax3/Pax7 double-knock-out mice, only the primary myotome forms, with all of the subsequent phases of myogenesis compromised (10, 15–17). In vitro studies have identified Pax7 as a direct target gene of the Notch signaling pathway, which plays critical roles in the quiescence/activation, proliferation, and differentiation of satellite cells and myoblasts and the maintenance of embryonic myogenic progenitor cells (18–20). Conditional deletion of RBP-J, the principle effector of Notch signaling, leads to reduced Pax7-positive muscle cells in developing mouse embryos followed by decreased myogenic cell proliferation and differentiation (19). It is clear that Notch signaling is required for maintenance of Pax7-expressing stem cells; however, it still remains to be investigated how Notch/Pax7 signaling is regulated in particular organ development.
The Wnt signaling pathway is an evolutionarily conserved pathway that plays critical roles in cell proliferation, cell polarity, and cell fate determination during embryonic development and tissue homeostasis (21–23). The biologic function of Wnt signaling in regulating tongue development has been largely focused on its function in tongue taste papillae development. Activation of canonical Wnt signaling has been shown to initiate lingual taste papillae development (24–26). Here, we find that epithelial deletion of Wntless (Wls), the key regulator of Wnt trafficking, leads to the formation of a hypoplastic tongue. This result suggests that epithelial Wnt ligand production mediated by Wls plays an important role in regulating tongue development. By using the conditional knock-out mouse model of Wls, we demonstrate that epithelial Wnt ligand production is required for the maintenance of epithelial p63-positive basal cells to form a stratified oral epithelium, the lamina propria, and the underlying muscle. Furthermore, our results reveal a genetic loop in which Wnt signaling regulates the proliferation of Pax7-positive myogenic cells through positive regulation of the Notch signaling pathway that negatively feeds back to regulate Wnt signaling activity to promote embryonic tongue development. Our findings thus provide a mechanism for patterning the tongue lamina propria and muscle in which a Wnt/Notch/Pax7 genetic hierarchy is involved.
Results
Epithelial Wnt production is essential for embryonic tongue development
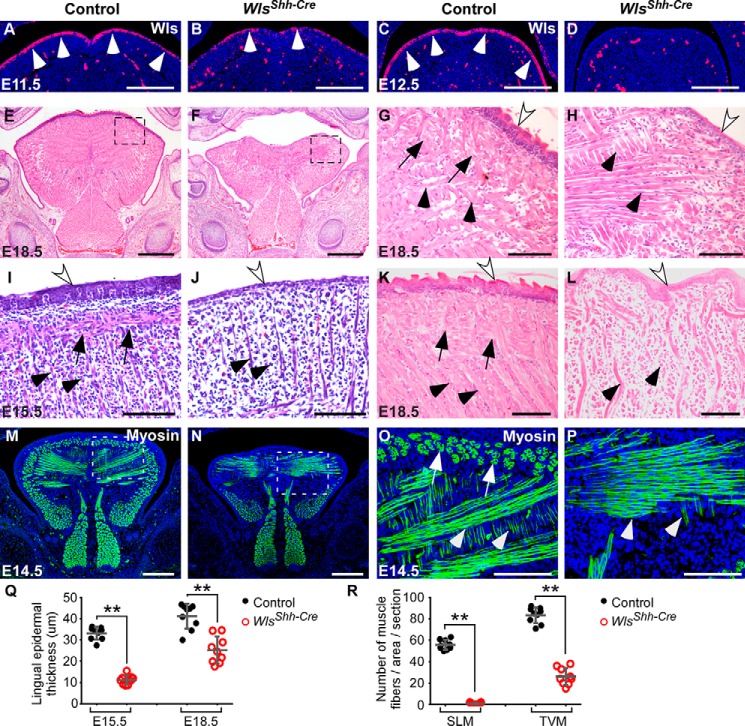
To investigate the role of epidermal Wnt production in developmental regulation of tongue composition, we deleted Wls, the gene encoding the main regulator of Wnt protein trafficking, in the mandibular arch using an Shh-Cre mouse in which Cre is active in the dorsal epithelium of the tongue (24, 27) (supplemental Fig. S1). Immunohistochemical staining on sections of tongue showed that Wls protein level is greatly reduced in the lingual epithelium of Wlsfx/fx;Shh-Cre (WlsShh-Cre) mutant at E11.5 and is completely deleted at E12.5 (Fig. 1, A–D). Histological analyses show that tongues lacking epidermal Wls were much smaller that the control tongues and displayed severe deformities in both the epithelium and the underlying muscle (Fig. 1, E–L). The epithelium of Wls mutant tongues were not stratified compared with the control tongues (Fig. 1, G–L, white arrows), and their epidermal thickness was significantly reduced (Fig. 1Q). In the control tongues, the muscle fibers were well organized and interwoven with each other (Fig. 1, G, I, and K). By contrast, muscle fibers in mutant embryos were severely reduced in number and disorganized (Fig. 1, H, J, and L), which was also revealed by immunostaining of myosin antibody and quantification of myofiber numbers (Fig. 1, M–P and R). It is noteworthy that the superior longitudinal muscle (SLM) was completely absent from tongues of WlsShh-Cre embryos (Fig. 1, black arrows in G–L and white arrows in O). Taken together, these data indicate that epithelial Wls-mediated production of Wnt plays a critical role in maintaining the integrity of the epithelium and the underlying muscle during tongue organogenesis.
Figure 1.
Epithelial Wnt production regulates embryonic tongue development. A–D, immunohistochemical staining analysis for Wls expression in the dorsal epithelium (arrowheads) and mesenchyme of embryonic tongues at E11.5 and E12.5. E–L, histological analyses of embryonic tongues show that deletion of epithelial Wls leads to microglossia (E and F), epithelial hypoplasia (white arrows), and compromised muscle formation. SLM and transverse and vertical myofibers (TVM) are indicated by arrows and arrowheads, respectively. Boxed regions in E and F are shown magnified in G and H. E–H, frontal sections; I–L, sagittal sections. M–P, immunostaining of myosin (green) for muscle fibers in E14.5 tongues. Boxed regions in M and N are shown magnified in O and P. Q, statistical analysis of lingual epidermal thickness from histological sections (I–L). R, comparison of the number of SLM and transverse and vertical myofibers in the designated area (O and P) of control and Wls mutant tongues. Data are shown as scatter plots. **, p < 0.01. Scale bars, 200 μm (A–D, M, and N), 500 μm (E and F), and 100 μm (G–L, O, and P).
Epithelial Wls regulates epithelial cell proliferation and lamina propria formation in the embryonic tongue
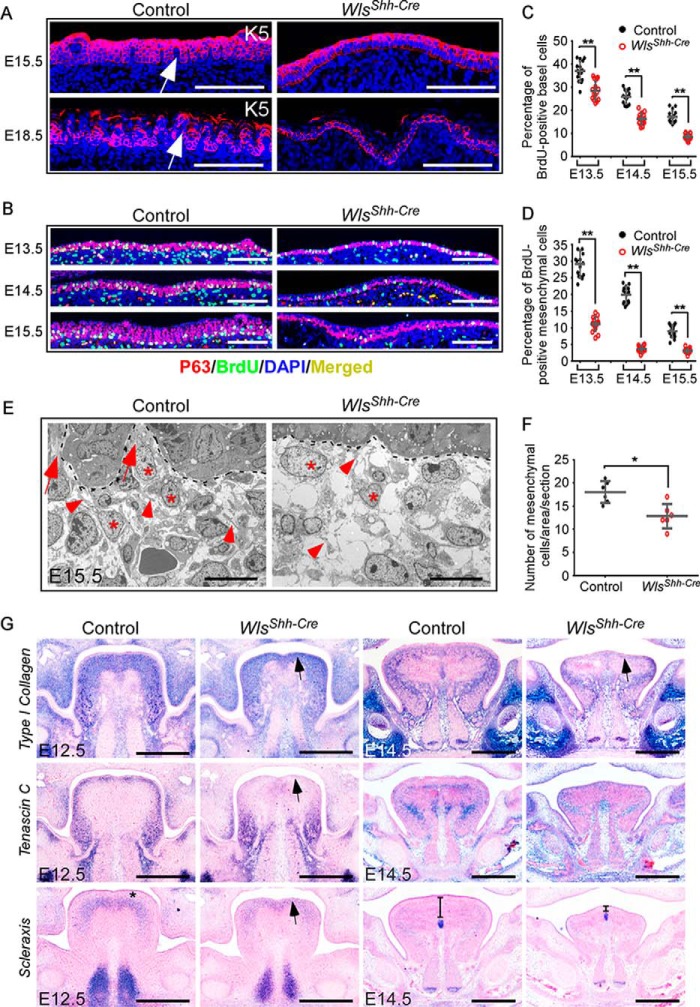
To decipher the role of Wls-mediated epithelial Wnt production in regulating embryonic tongue development, we first investigated lingual epithelial abnormalities. Because epithelial stratification requires proper proliferation of basal layer cells, we examined whether basal cell division was disrupted in epithelial Wls mutant tongues. Immunofluorescence analysis showed that the number of K5-labeled lingual epithelial basal layer cells was significantly decreased in tongues lacking epidermal Wls (Fig. 2A and supplemental Fig. S2A). Next, we examined whether cell proliferation in the dorsal epithelium of Wls-deficient tongues is compromised using sections double-stained with BrdU and p63, a transcription factor expressed in epidermal basal cells required for epidermal stem cell maintenance and regulation. Consistent with the K5 immunostaining result, the number of p63-positive basal cells was decreased in WlsShh-Cre tongues (Fig. 2B and supplemental Fig. S2B). The ratio of epithelial BrdU-positive cells in p63-positive basal cells in tongues of WlsShh-Cre embryos at E13.5–E15.5 was significantly reduced (Fig. 2C), suggesting that epithelial Wnt production is required for the proliferative potential of lingual epithelial basal cells, similar to that of the embryonic skin (28).
Figure 2.
Loss of epithelial Wls impairs the epithelial basal cell proliferation and the lamina propria formation in epithelial Wls mutant tongues. A, immunofluorescence (red) of K5 expression for basal cells in dorsal lingual epithelium at E15.5 and E18.5. Prominent lamina propria cores are indicated by arrows. B, immunostaining of BrdU (green) and p63 (red) shows BrdU-labeled cells in basal cells from E13.5 to E15.5. C and D, percentage of BrdU-labeled cells in p63-positive basal cells (C) and underlying tissue (D) in a designated area of the tongue (B). E, TEM micrographs show connective tissue cells (*) and fibers (arrowheads) in E15.5 control and WlsShh-Cre tongues. Lamina propria cores are indicated by arrows. F, quantitation of connective tissue cells from TEM micrographs of E15.5 control and Wls mutant tongues. G, in situ hybridization for the type I collagen, tenascin C, and scleraxis transcripts at E12.5 and E14.5. Data are shown as scatter plots. *, p < 0.05; **, p < 0.01. Scale bars, 100 μm (A and B), 10 μm (E), and 400 μm (G).
The development of multiple organs, including the skin, requires reciprocal interactions between the epithelium and the underlying mesenchyme (29–32). Thus, the development of the lamina propria, the dense connective tissue beneath the epithelium, is probably affected in Wls mutants. We noted that the lamina propria cores (Fig. 2A, arrows) and the epithelial undulations protruding into the lamina propria were missing in WlsShh-Cre tongues (Fig. 2A). In addition, mesenchymal cell proliferation just beneath the epithelium was also significantly decreased (Fig. 2, B and D). Transmission electron microscopy (TEM) micrographs of embryonic tongues at E15.5 reveal the presence of the lamina propria, which consists of abundant connective tissue cells (asterisks) and fibers (arrowheads), in control tongues (Fig. 2E). By contrast, the number of connective tissue cells and fibers was greatly reduced in the tissue just beneath the epithelium in tongues lacking epithelial Wls (Fig. 2, E and F), suggesting that the development of the lamina propria is severely inhibited due to a lack of epithelial Wnt production.
In situ hybridization showed that the expression of type I collagen and tenascin C, main extracellular matrix proteins in connective tissue, is greatly decreased in WlsShh-Cre embryos at E12.5 and E14.5 (Fig. 2G). Of note, type I collagen was expressed in the lamina propria and the underlying tissue in control embryos at E12.5, whereas its expression region was narrowed down in Wls mutants. At E14.5, type I collagen was expressed surrounding and intertwining with the SLM in the control, but not in the WlsShh-Cre tongues. Transcripts of tenascin C were found in the lingual lamina propria in control embryos at E12.5; however, no expression of tenascin C was detected in the comparable region in WlsShh-Cre embryos (Fig. 2G, arrow). Scleraxis is a transcription factor that plays critical roles in condensation, determination, and differentiation of tendon progenitors (33). Scleraxis was expressed in the tissue beneath the lamina propria in the control tongues at E12.5 (Fig. 2G, asterisk). By contrast, in Wls mutant tongues at E12.5, scleraxis was expressed immediately beneath the lingual epithelium (Fig. 2G, arrow). The distance between the scleraxis-expressing region and the epithelium was also dramatically reduced in E14.5 WlsShh-Cre tongues (Fig. 2G). These results suggest that the lamina propria in tongues lacking epithelial Wls is not properly formed.
Cell proliferation is significantly reduced but apoptosis is unaffected in tongues of WlsShh-Cre mice
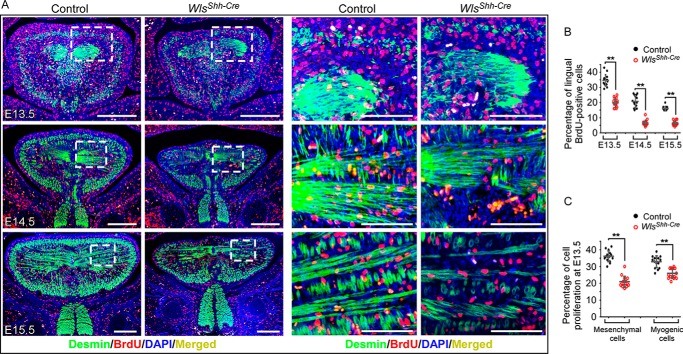
To investigate cellular processes underlying the defective tongue development in WlsShh-Cre mice, we performed BrdU labeling assays. The samples were double-stained with BrdU and Desmin, a muscle-specific intermediate filament protein, to distinguish connective tissue and muscle in the embryonic tongue. The number of BrdU-positive cells in the tongue was greatly reduced in WlsShh-Cre tongues between E13.5 and E15.5 compared with the control littermates (Fig. 3, A and B). Quantification of BrdU-positive cells in the Desmin-marked cells and in non-Desmin-positive cells delineated a significant reduction of cell proliferation in both myogenic and CNC-derived mesenchyme cells (Fig. 3C). In parallel, we found by TUNEL assay no evidence for increased cell death in WlsShh-Cre tongues (supplemental Fig. S3). Taken together, the loss of epithelial Wnt production causes a globally decreased cell proliferation in tongues of WlsShh-Cre mice and leads to severe defects in tongue development.
Figure 3.
Epithelial Wnt production mediated by Wls regulates cell proliferation in embryonic tongues. A, immunofluorescence with antibodies against desmin (green) and BrdU (red) shows BrdU-labeled cells in muscle (desmin-positive) and the surrounding tissue. Boxed areas are enlarged on the right. B, percentage of BrdU-positive nuclei in the total cell population in tongues from E13.5 to E15.5. C, percentage of BrdU-positive nuclei in desmin-positive cells (myogenic cells) and desmin-negative mesenchyme cell in E13.5 tongues. Data are shown as scatter plots. **, p < 0.01. Scale bars, 100 μm (enlarged images of boxed regions in A); 250 μm (others).
Epithelial Wls is required for maintaining myogenic progenitor cells in the embryonic tongue
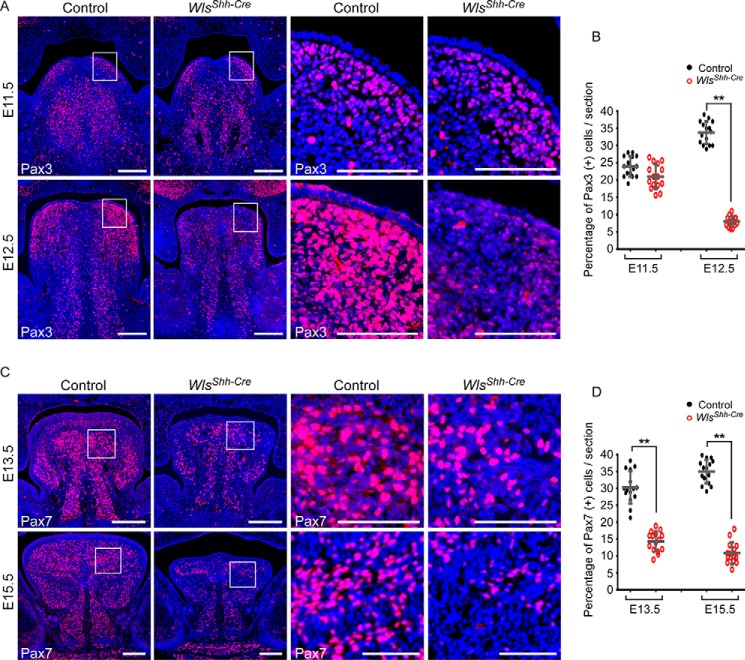
Given that the cell proliferation rate is reduced in tongue muscle, we investigated whether the expression of Pax3 and Pax7, two transcription factors critical for proliferation of myoblasts, was affected in the developing embryonic tongues. Immunostaining analysis shows that Pax3-positive myogenic progenitors reached the bilateral upper-tongue region (lamina propria and the lateral superior longitudinal muscle region) at E11.5 in both control and Wls mutant tongues (Fig. 4A). The number of Pax3-positive cells was comparable in both control and WlsShh-Cre tongues at E11.5 (Fig. 4, A and B). These data suggest that the migration of muscle precursor cells is not affected in Wls mutant tongues. In contrast, the number of Pax3-positive cells was significantly reduced in the E12.5 WlsShh-Cre tongue, in comparison with the wild type tongue, where the number of Pax3-positive muscle precursor cells was dramatically increased and the most abundant Pax3-positive cells were found in bilateral upper-tongue regions in control embryos (Fig. 4, A and B). Immunofluorescent analysis using a Pax7-specific antibody at later embryonic stages revealed that Pax7-positive muscle progenitor cells are also severely reduced in epithelial Wls mutant tongues (Fig. 4, C and D). We have also examined whether myogenic determination and differentiation processes are disrupted in WlsShh-Cre tongues. MyoD1 and myogenin were expressed in WlsShh-Cre tongues during E12.5–E16.5; however, the expression level was relatively lower than those of the control tongues (supplemental Fig. S4). Taken together, these data suggest that epithelial Wnt production is critical for the regulation of proliferation and differentiation processes of tongue muscle development.
Figure 4.
Significant decrease in the number of muscle progenitor cells in the absence of the epithelial Wls. A, immunofluorescence of Pax3 expression (red) in embryonic tongues at E11.5 and E12.5. B, percentage of Pax3-positive cells in the total cell population in E11.5 and E12.5 tongues. C, immunostaining of Pax7 for myogenic progenitor cells in E13.5 and E15.5 tongues. D, quantitation of Pax7-expressing cells in E13.5 and E15.5 tongues. Boxed areas are enlarged on the right. Data are shown as scatter plots. **, p < 0.01. Scale bars, 200 μm (A and C) and 100 μm (boxed regions in A and C).
Wls-mediated canonical Wnt signaling regulates Notch signaling activity
Reciprocal interaction between Wnt and Notch signaling pathways plays important roles in regulating many aspects of metazoan development (34). Notch signaling is required for maintaining myogenic progenitor cells during mouse fetal development (19). Thus, the decrease of myogenic cells in WlsShh-Cre tongues might be attributed to inhibition of the Notch signaling pathway. To test this possibility, we examined the effect of Wnt signaling on the activity of Notch signaling in WlsShh-Cre tongues.
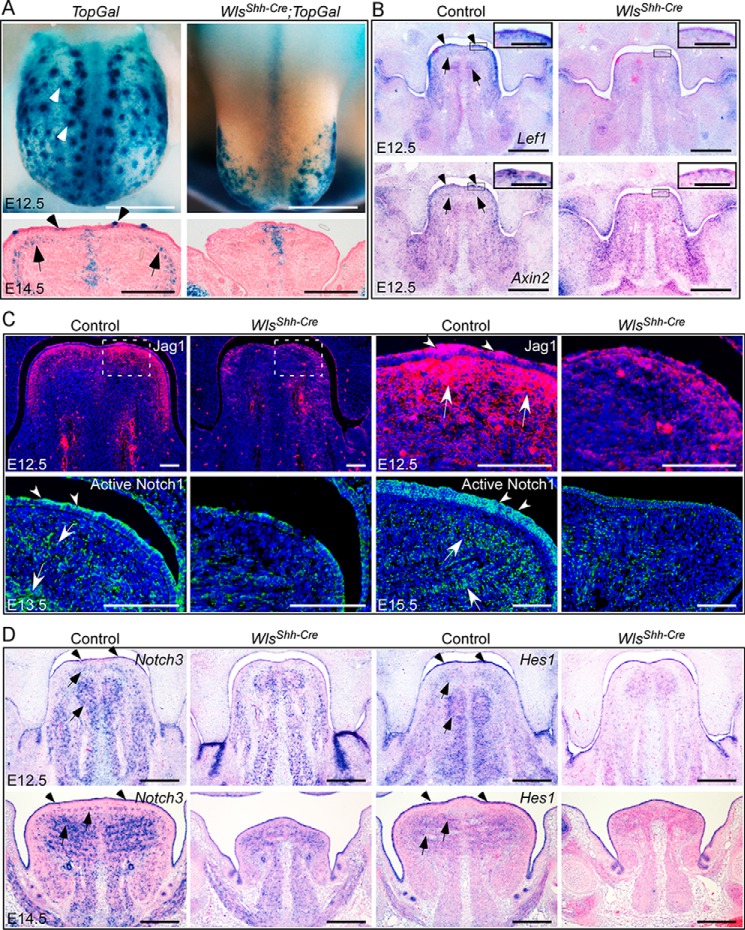
First, we tested the effect of Wls loss on Wnt signaling during tongue development. TopGal staining showed that deletion of epidermal Wls significantly impaired canonical Wnt signaling in E12.5 WlsShh-Cre tongues (Fig. 5A). The canonical Wnt signaling was manifest in both the epithelium and the superior longitudinal muscle region in the E14.5 wild-type control, but not in the WlsShh-Cre tongues (Fig. 5A). The expression of Axin2 and Lef1, two downstream targets of canonical Wnt signaling, was also significantly decreased in tongues lacking epidermal Wls at E12.5 (Fig. 5B), especially in the lingual epithelium and the underlying tissue (Fig. 5B, insets). These results suggest that epithelial Wnt ligands production is required for canonical Wnt signaling activation in the lingual epithelium and the underlying tissue.
Figure 5.
Wls-dependent canonical Wnt signaling regulates Notch signaling activity. A, TopGal staining for canonical Wnt activity in E12.5 and E14.5 tongues. B, in situ hybridization for Lef1 and Axin2 expression in E12.5 tongues. C, immunostaining of Jag1 and active Notch1 in embryonic tongues. D, in situ hybridization for the expression of Notch3 and Hes1 in E12.5 and E14.5 tongues. Arrowheads and arrows indicate positive immunostaining or gene expression in epithelium and the underlying tissue, respectively. Scale bars, 250 μm (A and D); 400 μm (B); and 100 μm (insets for B and C).
Next, we examined the activity of the Notch signaling pathway in response to epithelial Wnt deficiency. Immunofluorescent analysis of Jag1, one of the Notch ligands, shows that the most abundant Jag1 protein was expressed in the lingual epithelium and the adjacent tissue in control tongues at E12.5 (Fig. 5C). However, the Jag1 protein level was severely reduced in mutant tongues lacking epithelial Wls (Fig. 5C). As revealed by immunostaining of the active form of Notch1, activation of Notch signaling was present in the lingual epithelium, the adjacent mesenchyme, and muscles in E13.5 control embryos but was inhibited in Wls mutant tongues, especially in the epithelium and the underlying tissue (Fig. 5C). At E15.5, active Notch signaling was obviously detected in the lingual epithelium and muscle in control embryos; however, its level is greatly reduced in WlsShh-Cre tongues (Fig. 5C). In situ hybridization analyses showed that Notch3 and Hes1, the major Notch target genes, were expressed in the epithelium, the CNC-derived mesenchyme, and the muscle of E12.5 control tongues and in muscle and epithelium of E14.5 control tongues (Fig. 5D). In contrast, expression of both Notch3 and Hes1 was decreased in Wls mutant tongues (Fig. 5D), indicating the repression of Notch signaling activity in Wls mutant tongues. Taken together, these results suggest that canonical Wnt signaling mediated by epithelial Wls is required for activation of Notch signaling during embryonic tongue development.
Notch is involved in embryonic tongue muscle development by regulating Pax7
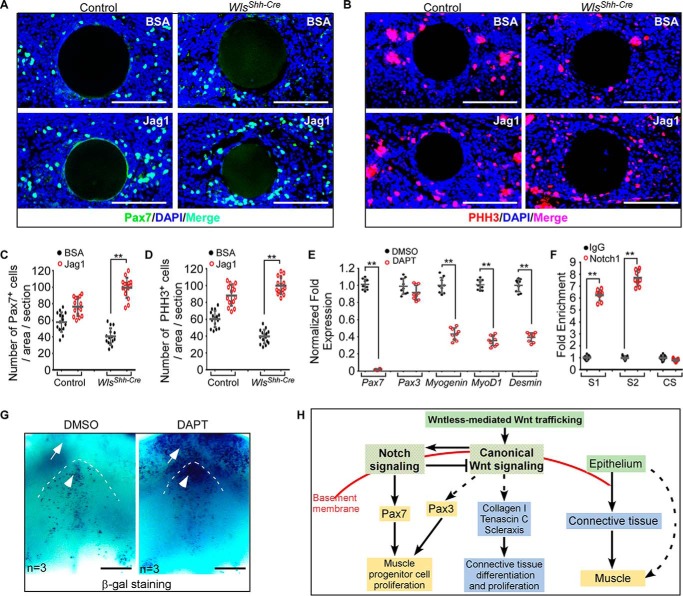
To ask the question of whether impaired Notch signaling accounts for the decrease in number of myogenic progenitor cells in Wls mutant tongues, we investigated whether the expression of myogenic progenitor marker Pax7 can be induced in response to activation of Notch signaling pathway in developing tongues. A bead implantation experiment revealed that exogenously applied active Jag1 peptide was able to induce Pax7 expression in WlsShh-Cre tongue explants (Fig. 6, A and C). Meanwhile, cell proliferation in Wls mutant tongue explants was successfully restored with implantation of Jag1-soaked beads (Fig. 6, B and D). Next, we investigated the effect of Notch inhibition on Pax7 expression in cultured tongue explants. The addition of DAPT, the Notch inhibitor, to the culture medium led to significant down-regulation of Notch target genes, including Hes1, Hes5, Hes7, and Notch3 (supplemental Fig. S5), suggesting that the Notch signaling activity was efficiently inhibited by DAPT. Expression of Pax7 was strikingly down-regulated in response to DAPT treatment, associated with decreased expression of myogenin, MyoD1, and desmin (Fig. 6E). By contrast, the expression of Pax3, the paralogue of Pax7 with partially overlapping functions during development, is almost unaffected in DAPT-treated tongue explants (Fig. 6E). Analysis of the genome sequence of Pax7 indicates two consensus sites of RBP-J present within the 8-kb promoter region of the Pax7 gene (supplemental Fig. S6). In an effort to test whether Pax7 is a Notch target gene in the developing tongue, we performed a ChIP assay using embryonic tongue samples with an antibody against active Notch1, which functions together with RBP-J to regulate expression of Notch target genes. Both binding sites are significantly enriched by > 5-fold in the ChIP assay (Fig. 6F), suggesting that Notch signaling regulates Pax7 expression directly by binding these two sequences in the Pax7 promoter region. Taken together, these results support the notion that the Notch signaling pathway plays a critical role in embryonic tongue development by regulating Pax7.
Figure 6.
Reciprocal interaction of Wnt and Notch signaling regulates embryonic tongue development. A and B, immunostaining analysis for Pax7 (A) and pHH3 (B) expression in the tongue explants implanted with Jag1 or BSA-soaked beads. C and D, quantitation of the number of Pax7-positive (C) and pHH3-positive (D) cells in the tongue explants. E, quantitative analysis of the myogenic gene expression in tongue explants after incubation with or without DAPT. F, -fold enrichment of RBP-J-binding sites (S1 and S2) and control site (CS) of the Pax7 promoter region following ChIP with a Notch1 antibody. G, BATGAL staining for canonical Wnt activity in tongue explant cultured with or without DAPT. H, schematic showing a genetic hierarchy that regulates embryonic tongue development. Data are shown as scatter plots. **, p < 0.01. Scale bars, 100 μm (A and B) and 200 μm (G).
Notch signaling pathway feeds back to negatively regulate Wnt signaling activity during tongue development
To further investigate the cross-talk between the Notch and Wnt signaling pathways in the embryonic tongue, we examined readouts of Wnt signaling in tongue explants with inhibited Notch signaling. We performed organ culture experiments using tongues from BATGAL transgenic mice, a reporter strain for canonical Wnt signaling activity. E11.5 BATGAL mandibles were cultured in the presence of DAPT to inhibit Notch signaling and then subjected to X-gal staining analysis. Upon DAPT treatment, the canonical Wnt signaling activity in both mandibles (arrows) and tongues (arrowheads) was greatly increased (Fig. 6G), indicating that Notch signaling feeds back to negatively regulate Wnt signaling during tongue morphogenesis.
Discussion
In the present study, we address the question of how the Wnt production of the lingual epithelium is involved in the developmental regulation of lingual lamina propria and muscle during tongue morphogenesis. Based on our results, we propose a genetic hierarchy model that integrates Wnt and Notch signaling between the lingual epithelium and the internal tongue tissues in tongue development (Fig. 6H). In this model, Wnt ligands from the tongue epithelium play an inductive role in regulating the embryonic tongue development by activating the canonical Wnt signaling pathway. Wnt signaling acts upstream of Notch signaling to maintain the proliferation of muscle progenitor cells by Pax7. Notch in turn represses Wnt signaling for normal tongue development. This model suggests a regulatory network from the epithelium to the CNC-derived connective tissue and muscle in ensuring the integrity of the epidermis, connective tissue, and underlying muscular tissues in tongue organogenesis.
It is well known that the development of the skin and its derivatives, such as the hair follicle, depend on reciprocal epidermal-dermal interactions (35–37). In the process of embryonic skin stratification, an essential BMP-FGF signaling axis in the dermis responds to the epidermal Wnts and feeds back to regulate basal progenitors marked by p63 (28). Similar to skin development, the development and homeostatic maintenance of the mucosa, which consists of a superficial epithelium and the underlying lamina propria, also require interactions between the epithelium and the lamina propria (29, 30, 38, 39). During intestinal mucosa development, epithelium-derived growth factor Hh plays a critical role in the formation of the lamina propria mesenchymal cells, which in turn contribute to the supportive microenvironment of the epithelial stem cell niche (38). In the epithelium of Wls mutant tongues, the number of p63-positive basal cells is decreased, and they are associated with loss of lamina propria. Together with previous reports, it is reasonable to deduce that defective lamina propria in turn inhibits normal development of the tongue epithelium in Wls mutants.
The tongues of Pax3 knock-out mice lack both intrinsic and extrinsic muscles. In Pax3neo/neo mutants, where Pax3 is partially reduced, intrinsic tongue muscles are partially absent, whereas extrinsic muscles are intact (40). In tongues lacking myogenic RBP-J, the development of limb and tongue muscles are also affected and are associated with decreased Pax7+ cells (19). In Wls mutant tongues, Pax7-positive myogenic progenitor cells are significantly reduced and associated with a reduction of intrinsic muscle fibers. These results suggest that the muscle defects in Wls mutant tongues directly result from the ablation of myogenic progenitor cells.
The Pax7-marked stem cells regulated by Notch signaling are satellite cells crucial for muscle regeneration after fetal development (9, 10, 12, 15, 18, 20). In WlsShh-Cre tongues, the number of Pax7+ cells greatly decreased due to epithelial Wnt deficiency, indicating the requirement of epithelial Wnt signaling for the maintenance of the lingual myogenic satellite cell pool. Our results have shown that Pax7 is downstream of Wnt-mediated Notch signaling during tongue development. Unlike Pax7, the expression of Pax3 seems not to be regulated by the Notch signaling pathway in developing embryonic tongue because the inhibition of Notch signaling pathway does not affect the Pax3 level. These results indicate the existence of distinct mechanisms that are involved in the regulation of Pax7- and Pax3-positive myogenic progenitors during tongue development.
The ability of the tongue to move flexibly depends on the unique arrangement of its eight interwoven muscles. The four intrinsic muscles act to change the shape of the tongue, while the four extrinsic muscles function to change the position of the tongue (4). It is intriguing to address how these lingual muscles are formed during tongue development. It has been well elucidated that extrinsic signals from adjacent tissues orchestrate the myogenic initiation, differentiation, movement, proliferation, and survival processes of trunk muscle precursors (9, 41–43). During tongue organogenesis, genes along various signaling pathways in CNCC-derived mesenchyme are implicated in the regulation of tongue muscle development. SLM is a thin stratum of oblique and longitudinal fibers appearing as a sheet covering the dorsal surface of the tongue just beneath the lamina propria. In WlsShh-Cre tongues, inhibition of epithelial signals significantly disrupted the SLM and lamina propria. As discussed above, a decrease of Pax3 expression affects the formation of the SLM and intrinsic muscle rather than the extrinsic muscle (40). This notion, together with our results, suggests that the function of epithelial Wnt production in the tongue to sustain Pax3+ myogenic cells is required for formation of the SLM. Given that the connective tissue plays a critical role in the regulation of muscle development, the lamina propria might integrate the epithelial signals to the formation of SLM. Thus, our results may suggest a regulation hierarchy of epithelium, lamina propria, and SLM during tongue morphogenesis and provide insight into the mechanism underlying the developmental regulation of SLM formation.
In the Pax3 and Pax7 double-knock-out mice, all of the subsequent processes of myogenesis are compromised (10, 13). It has also been reported that myogenic differentiation requires myogenic cell expansion stimulated by Lbx1 and Pax3 in chicken embryos (44). In Wls mutant tongues, the myogenic differentiation process is apparently impaired, as revealed by down-regulated expression of MyoD1 and myogenin. We also found that expression of MyoD1, myogenin, and desmin is decreased, associated with Pax7 decrease in response to Notch inhibition in cultured tongue explants. Of note, the inhibition of myogenic differentiation is associated with significant inhibition of myogenic proliferation and reduction of myogenic progenitors. Together with previous reports, our results suggest that myogenic cell differentiation requires the proliferation of myogenic progenitors during embryonic tongue development.
Activities of Wnt and Notch signaling pathways are closely intertwined during embryonic development and disease (34, 45–47). In Drosophila, Wnt and Notch signal act iteratively to regulate the development and patterning of the wing (46, 48). In mouse somitogenesis, expression of the Notch ligand Delta-like1 (Dll1) is regulated by the Wnt signaling pathway (49). Another Notch ligand, Jag1, is a Wnt target gene in ectopic hair follicle formation in the adult epidermis (35). The cross-talk between Wnt and Notch signaling also plays a critical role in normal muscle regeneration of the postnatal skeletal muscle (50). In addition to the interaction of Notch and Wnt in development, Notch has been linked to β-catenin-dependent tumorigenesis, where Jag1 has been suggested as the pathological link between the Wnt and Notch pathways (45, 51). Our results show that Wnt signaling activates Notch signaling, which in turn feeds back to negatively regulate Wnt signaling activity during embryonic tongue development. These results reveal the existence of cross-talk between Notch and Wnt signaling pathways during tongue organogenesis. Of note, the expression of Notch ligand Jag1 is down-regulated in WlsShh-Cre tongues, suggesting that Jag1 might be the pivotal molecule in coordinating the Notch and Wnt signaling pathways in developing tongues.
In summary, our results reveal that epidermal production of Wnt ligands in the tongue is essential for preserving lingual epithelium integrity, promoting lamina propria development, and maintaining muscle progenitor cells. Our results illustrate a mechanism of how the epithelium is involved in tissue-tissue interactions to regulate tongue development by serving as a pool of Wnt ligands. These findings highlight a Wnt/Notch/Pax7 genetic hierarchy during embryonic tongue muscle development.
Experimental procedures
Animals
The Wls conditional knock-out mice (Wlsfx/fx) were crossed with ShhCreGFP mice to generate mice with lingual epidermal loss of function of Wls (WlsShh-Cre). ShhCreGFP, R26R-LacZ, TopGal, and BATGAL mouse lines were purchased from the Jackson Laboratory (Bar Harbor, ME). Wlsfx/fx mice were crossed with TopGal or BATGAL mice to obtain Wlsfx/+ mice carrying the TopGal or BATGAL allele, which were back-crossed with Wlsfx/fx to produce Wlsfx/fx mice carrying the TopGal or BATGAL allele. In all experiments, the respective controls were Wlsfx/+;Shh-Cre littermates. The animal experimental protocols involved in this report were approved by the Animal Users Committee of Hangzhou Normal University, China.
Histology, in situ hybridization, X-gal staining, and TEM
Standard hematoxylin/eosin staining and nonradioactive in situ hybridization were performed on paraffin sections as described previously. X-gal staining was performed on cryostat sections as described previously (24). For TEM analysis, embryonic tongues were dissected, fixed with 2.5% glutaraldehyde, and processed according to standard protocols.
Immunohistochemistry
For immunohistochemistry, embryonic heads were fixed in 4% paraformaldehyde for 15–30 min, washed in PBS, and then processed for paraffin sections. Immunostaining was performed with 7-μm paraffin samples using antibodies against myosin (Sigma, M4276), Wls (31), Jag1 (Santa Cruz Biotechnology, Inc., sc-6011), active Notch1 (Abcam, ab8925), Pax3 (DSHB, AB_528426), Pax7 (DSHB, AB_528428), and phospho-histone H3 (Cell Signaling Technology, 3458S).
Cell proliferation and apoptosis assays
For the cell proliferation assay, timed pregnant mice were injected intraperitoneally with BrdU (Sigma) solution (3 mg/100 g of body weight) 1 h before embryo collection. For immunofluorescent analysis, paraffin sections were co-stained with antibody against p63 (Abcam, ab53039) or desmin (Abcam, ab185033) and anti-BrdU antibody (Roche Applied Science, 1170376001). Cell apoptosis was detected with the TUNEL BrightGreen apoptosis detection kit (Vazyme) according to the manufacturer's instructions. At least three embryonic heads for each genotype were used for data analysis according to the manufacturer's instructions.
Tongue organ culture
Embryonic tongues from timed pregnant mice were microdissected, placed on a Nucleopore Track-Etch membrane in a Trowell-type organ culture dish, and cultured as described previously. For the bead implantation experiment, explanted tongues at E14.5 were implanted with Affi-Gel blue agarose beads carrying Jag1 protein active peptide fragment (AnaSpec), harvested after 1 day in culture, and processed for paraffin sections for immunohistochemistry analysis. For the DAPT treatment experiment, E11.5 mandibles were cultured for 3 days in the presence of 23 μm DAPT (Sigma) with or without 10 mm LiCl, harvested, and processed for quantitative RT-PCR analysis or X-gal staining. The culture medium was changed daily with the fresh addition of chemicals as described above. Three or four embryos were used for each group in experiments.
ChIP
Tongues from E14.5 mouse embryos were cut into small pieces and processed for ChIP analysis as described previously. ChIP was performed with antibody against active Notch1 (Abcam, ab8925) or normal rabbit IgG (Beyotime, A7016) using the Magna ChIP G tissue kit (Millipore) according to the user manual. For the detection of the enriched Pax7 promoter region, eluted DNA was used as template for quantitative real-time PCR analysis with primers specific for RBP-J binding sites as described previously (18).
Quantitative real-time PCR
For analysis of gene expression, quantitative real-time PCR was performed in triplicate using SsoFast EvaGreen Supermix with the CFX96 real-time PCR detection system (Bio-Rad) as described previously. Briefly, total RNA was isolated from explanted tongues using an RNAqueous-4PCR kit (Ambion), and cDNA was synthesized and used as template for real-time PCR. The real-time PCR primer sequences were obtained from PrimerBank (52): Hes1, 5′-CCAGCCAGTGTCAACACGA-3′ and 5′AATGCCGGGAGCTATCTTTCT-3′; Hes5, 5′-AGTCCCAAGGAGAAAAACCGA-3′ and 5′-GCTGTGTTTCAGGTAGCTGAC-3′; Hes7, 5′-CGGGAGCGAGCTGAGAATAG-3′ and 5′-CACGGCGAACTCCAGTATCT-3′; Notch3, 5′-TGCCAGAGTTCAGTGGTGG-3′ and 5′-CACAGGCAAATCGGCCATC-3′; Pax7, 5′-TCTCCAAGATTCTGTGCCGAT-3′ and 5′-CGGGGTTCTCTCTCTTATACTCC-3′; myogenin, 5′-GAGACATCCCCCTATTTCTACCA-3′ and 5′-GCTCAGTCCGCTCATAGCC-3′; MyoD1, 5′-CCACTCCGGGACATAGACTTG-3′ and 5′-AAAAGCGCAGGTCTGGTGAG-3′; desmin, 5′-GTGGATGCAGCCACTCTAGC-3′ and 5′-TTAGCCGCGATGGTCTCATAC-3′; 18S, 5′-TAGAGGGACAAGTGGCGTTC-3′ and 5′-CGCTGAGCCAGTCAGTGT-3′. 18S rRNA was used as a reference gene.
Statistical analysis
For quantification of proliferation, BrdU-positive cells within a defined area were counted from ≥ 15 consecutive fields from three samples for both control and mutant embryos. Cells labeled with other antibodies were counted in a way similar to that described above. Numbers of total cells were calculated as DAPI-positive cells within a defined area. Student's t test was used to determine statistical significance. A p value < 0.05 was considered statistically significant. Data are represented as scatter plots with the average and S.D.
Author contributions
X.-J. Z. contributed to design, data acquisition, and analysis and drafted and critically revised the manuscript. X. Yuan contributed to design, data acquisition, and analysis and critically revised the manuscript. M. W., Y. F., Y. L., and X. Z. contributed to data acquisition and analysis and critically revised the manuscript. X. Yang and Y. L. contributed to data acquisition and critically revised the manuscript. J. L., F. L., Z. M. D., M. Q., and Ze Zhang contributed to data analysis and critically revised the manuscript. Zunyi Zhang contributed to conception, design, data acquisition, analysis, and interpretation and drafted and critically revised the manuscript.
Supplementary Material
Acknowledgments
We thank all members of the Zhang laboratory at the Institute of Life Sciences, Hangzhou Normal University for suggestions during the generation of these data.
This work was supported by Natural Scientific Foundation of China Grants 31371471 and 81670971; Science and Technology Plan Program of Zhejiang Province Grant 2017C37163; and Natural Scientific Foundation of Zhejiang Province Grants LY16C120002, LY15C090008, and LY15C120001. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S6.
- CNCC
- cranial neural crest cell
- CNC
- cranial neural crest
- SLM
- superior longitudinal muscle(s)
- DAPT
- N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- E
- embryonic day.
References
- 1. Iwasaki S. (2002) Evolution of the structure and function of the vertebrate tongue. J. Anat. 201, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parada C., and Chai Y. (2015) Mandible and tongue development. Curr. Top. Dev. Biol. 115, 31–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parada C., Han D., and Chai Y. (2012) Molecular and cellular regulatory mechanisms of tongue myogenesis. J. Dent. Res. 91, 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey E. F., and Fregosi R. F. (2004) Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J. Appl. Physiol. 96, 440–449 [DOI] [PubMed] [Google Scholar]
- 5. Han D., Zhao H., Parada C., Hacia J. G., Bringas P. Jr., and Chai Y. (2012) A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139, 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hosokawa R., Oka K., Yamaza T., Iwata J., Urata M., Xu X., Bringas P. Jr, Nonaka K., and Chai Y. (2010) TGF-β mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue-tissue interactions during tongue morphogenesis. Dev. Biol. 341, 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwata J., Suzuki A., Pelikan R. C., Ho T. V., and Chai Y. (2013) Noncanonical transforming growth factor β (TGFβ) signaling in cranial neural crest cells causes tongue muscle developmental defects. J. Biol. Chem. 288, 29760–29770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin C., Fisher A. V., Yin Y., Maruyama T., Veith G. M., Dhandha M., Huang G. J., Hsu W., and Ma L. (2011) The inductive role of Wnt-β-catenin signaling in the formation of oral apparatus. Dev. Biol. 356, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokoyama S., and Asahara H. (2011) The myogenic transcriptional network. Cell. Mol. Life Sci. 68, 1843–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Relaix F., Rocancourt D., Mansouri A., and Buckingham M. (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953 [DOI] [PubMed] [Google Scholar]
- 11. Padilla-Benavides T., Nasipak B. T., and Imbalzano A. N. (2015) Brg1 controls the expression of Pax7 to promote viability and proliferation of mouse primary myoblasts. J. Cell. Physiol. 230, 2990–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins C. A., Gnocchi V. F., White R. B., Boldrin L., Perez-Ruiz A., Relaix F., Morgan J. E., and Zammit P. S. (2009) Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One 4, e4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Messina G., and Cossu G. (2009) The origin of embryonic and fetal myoblasts: a role of Pax3 and Pax7. Genes Dev. 23, 902–905 [DOI] [PubMed] [Google Scholar]
- 14. Relaix F., Rocancourt D., Mansouri A., and Buckingham M. (2004) Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 18, 1088–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oustanina S., Hause G., and Braun T. (2004) Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 23, 3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansouri A., Pla P., Larue L., and Gruss P. (2001) Pax3 acts cell autonomously in the neural tube and somites by controlling cell surface properties. Development 128, 1995–2005 [DOI] [PubMed] [Google Scholar]
- 17. Seale P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P., and Rudnicki M. A. (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 [DOI] [PubMed] [Google Scholar]
- 18. Wen Y., Bi P., Liu W., Asakura A., Keller C., and Kuang S. (2012) Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 32, 2300–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasyutina E., Lenhard D. C., Wende H., Erdmann B., Epstein J. A., and Birchmeier C. (2007) RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc. Natl. Acad. Sci. U.S.A. 104, 4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conboy I. M., and Rando T. A. (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409 [DOI] [PubMed] [Google Scholar]
- 21. Nayak L., Bhattacharyya N. P., and De R. K. (2016) Wnt signal transduction pathways: modules, development and evolution. BMC Syst. Biol. 10, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacDonald B. T., Tamai K., and He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komiya Y., and Habas R. (2008) Wnt signal transduction pathways. Organogenesis 4, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu X., Liu Y., Zhao P., Dai Z., Yang X., Li Y., Qiu M., and Zhang Z. (2014) Gpr177-mediated Wnt signaling is required for fungiform placode initiation. J. Dent. Res. 93, 582–588 [DOI] [PubMed] [Google Scholar]
- 25. Liu F., Thirumangalathu S., Gallant N. M., Yang S. H., Stoick-Cooper C. L., Reddy S. T., Andl T., Taketo M. M., Dlugosz A. A., Moon R. T., Barlow L. A., and Millar S. E. (2007) Wnt-β-catenin signaling initiates taste papilla development. Nat. Genet. 39, 106–112 [DOI] [PubMed] [Google Scholar]
- 26. Iwatsuki K., Liu H. X., Grónder A., Singer M. A., Lane T. F., Grosschedl R., Mistretta C. M., and Margolskee R. F. (2007) Wnt signaling interacts with Shh to regulate taste papilla development. Proc. Natl. Acad. Sci. U.S.A. 104, 2253–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li F., Fu G., Liu Y., Miao X., Li Y., Yang X., Zhang X., Yu D., Gan L., Qiu M., Chen Y., Zhang Z., and Zhang Z. (2017) ISLET1-dependent β-catenin/Hedgehog signaling is required for outgrowth of the lower jaw. Mol. Cell. Biol. 10.1128/MCB.00590-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu X. J., Liu Y., Dai Z. M., Zhang X., Yang X., Li Y., Qiu M., Fu J., Hsu W., Chen Y., and Zhang Z. (2014) BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 10, e1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santosh A. B., and Jones T. J. (2014) The epithelial-mesenchymal interactions: insights into physiological and pathological aspects of oral tissues. Oncol. Rev. 8, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ribatti D., and Santoiemma M. (2014) Epithelial-mesenchymal interactions: a fundamental developmental biology mechanism. Int. J. Dev. Biol. 58, 303–306 [DOI] [PubMed] [Google Scholar]
- 31. Zhu X., Zhao P., Liu Y., Zhang X., Fu J., Ivy Yu H. M., Qiu M., Chen Y., Hsu W., and Zhang Z. (2013) Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J. Biol. Chem. 288, 12080–12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thesleff I. (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648 [DOI] [PubMed] [Google Scholar]
- 33. Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., and Schweitzer R. (2007) Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708 [DOI] [PubMed] [Google Scholar]
- 34. Collu G. M., Hidalgo-Sastre A., and Brennan K. (2014) Wnt-Notch signalling crosstalk in development and disease. Cell. Mol. Life Sci. 71, 3553–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Estrach S., Ambler C. A., Lo Celso C., Hozumi K., and Watt F. M. (2006) Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 [DOI] [PubMed] [Google Scholar]
- 36. Kishimoto J., Ehama R., Wu L., Jiang S., Jiang N., and Burgeson R. E. (1999) Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc. Natl. Acad. Sci. U.S.A. 96, 7336–7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blanpain C., Horsley V., and Fuchs E. (2007) Epithelial stem cells: turning over new leaves. Cell 128, 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powell D. W., Pinchuk I. V., Saada J. I., Chen X., and Mifflin R. C. (2011) Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 73, 213–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J., Mao J. J., and Chen L. (2011) Epithelial-mesenchymal interactions as a working concept for oral mucosa regeneration. Tissue Eng. Part B Rev. 17, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou H. M., Wang J., Rogers R., and Conway S. J. (2008) Lineage-specific responses to reduced embryonic Pax3 expression levels. Dev. Biol. 315, 369–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cisternas P., Henriquez J. P., Brandan E., and Inestrosa N. C. (2014) Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol. Neurobiol. 49, 574–589 [DOI] [PubMed] [Google Scholar]
- 42. von Maltzahn J., Chang N. C., Bentzinger C. F., and Rudnicki M. A. (2012) Wnt signaling in myogenesis. Trends Cell Biol. 22, 602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bentzinger C. F., Wang Y. X., and Rudnicki M. A. (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mennerich D., and Braun T. (2001) Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J. 20, 7174–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rodilla V., Villanueva A., Obrador-Hevia A., Robert-Moreno A., Fernández-Majada V., Grilli A., López-Bigas N., Bellora N., Albà M. M., Torres F., Duñach M., Sanjuan X., Gonzalez S., Gridley T., Capella G., et al. (2009) Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. U.S.A. 106, 6315–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayward P., Kalmar T., and Arias A. M. (2008) Wnt/Notch signalling and information processing during development. Development 135, 411–424 [DOI] [PubMed] [Google Scholar]
- 47. Cheng X., Huber T. L., Chen V. C., Gadue P., and Keller G. M. (2008) Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development 135, 3447–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klein T., and Arias A. M. (1999) The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development 126, 913–925 [DOI] [PubMed] [Google Scholar]
- 49. Galceran J., Sustmann C., Hsu S. C., Folberth S., and Grosschedl R. (2004) LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 18, 2718–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brack A. S., Conboy I. M., Conboy M. J., Shen J., and Rando T. A. (2008) A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59 [DOI] [PubMed] [Google Scholar]
- 51. Wang R., Sun Q., Wang P., Liu M., Xiong S., Luo J., Huang H., Du Q., Geller D. A., and Cheng B. (2016) Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 7, 5754–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X., Spandidos A., Wang H., and Seed B. (2012) PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 40, D1144–D1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.