Abstract
Artificial light at night has shown a remarkable increase over the past decades. Effects are reported for many species groups, and include changes in presence, behaviour, physiology and life-history traits. Among these, bats are strongly affected, and how bat species react to light is likely to vary with light colour. Different spectra may therefore be applied to reduce negative impacts. We used a unique set-up of eight field sites to study the response of bats to three different experimental light spectra in an otherwise dark and undisturbed natural habitat. We measured activity of three bat species groups around transects with light posts emitting white, green and red light with an intensity commonly used to illuminate countryside roads. The results reveal a strong and spectrum-dependent response for the slow-flying Myotis and Plecotus and more agile Pipistrellus species, but not for Nyctalus and Eptesicus species. Plecotus and Myotis species avoided white and green light, but were equally abundant in red light and darkness. The agile, opportunistically feeding Pipistrellus species were significantly more abundant around white and green light, most likely because of accumulation of insects, but equally abundant in red illuminated transects compared to dark control. Forest-dwelling Myotis and Plecotus species and more synanthropic Pipistrellus species are thus least disturbed by red light. Hence, in order to limit the negative impact of light at night on bats, white and green light should be avoided in or close to natural habitat, but red lights may be used if illumination is needed.
Keywords: light pollution, experimental light at night, bats, light colour
1. Introduction
Artificial light at night has shown a dramatic increase over the past few decades [1,2]. The use of artificial light will continue to grow and sky brightness increases with an estimated 6% per year [3]. The disappearance of the natural night-time darkness affects many species groups [4–6]; among these, bats are well represented [7–10]. A conspicuous and long known effect on some bat species is the attraction to light. This does not appear to be a direct effect, but the result of the accumulation of insects that are first attracted to the light sources [9,11–15]. The increase in density of insects, but also the impairment of defence mechanisms of moth species, facilitate bats' foraging [16–19]. This cascading effect of light was recently confirmed in an experimental set-up by Minnaar et al. [17], although other studies show that these species tend to avoid light in absence of tree cover [20,21], suggesting that the attraction to light is context dependent. However, not all bat species are recorded around light sources at night in high densities. For example, slow-flying Myotis and Plecotus species [9] are likely deterred by light. For these species, avoidance of experimental light sources has been shown for bats flying along commuting routes [22]. The most widely accepted hypothesis explaining why these bat species avoid light is the fear of predators capable of hunting bats by visual cues. Bats that do not need to forage early in the evening on crepuscular insect species tend to emerge later in the night when the light intensity has dropped further [23,24]. Heavier bats, which are less manoeuvrable, emerge later than lighter bats of the same species, and delayed emergence has likewise been reported for young bats that are still improving their flight skills [25,26]. In addition, a significant negative correlation has been shown between flight speed and emergence time, suggesting that slow-flying bats are more wary of predation [23]. When flying at an illuminated location, bats are observed to fly faster than in the dark [27].
How species react to light often varies with light colour. Spectrum-dependent responses are known for example in insects [28], birds [29–32], reptiles [33,34], toads [35] and mice [36]. The response of bats is also reported to be dependent on the light source spectrum: the activity of some non-light-shy bat species around low-pressure sodium (LPS) lamps, which produce monochromatic orange light, is much lower compared to light sources that contain shorter wavelengths [9,37]. This difference can most likely be attributed to the scarcity of insects around LPS lights [9,38]. How the response of light-averse bats varies with light spectrum is not well known. Light avoidance by bats may depend on how well bats are able to see different light colours. Bat eyes are adapted to a dark environment, with a high rod/cone ratio in the retina compared to diurnal mammal species [39–42]. Bat opsins are reported to be ultraviolet-sensitive and bat eyes may be generally more sensitive to the blue part of the spectrum [43]. However, bats may well be able to see red light as, unlike many other small mammals, the genetic code of the L opsins is preserved in temperate vespertilionid bats and several species are known to have functional long-wavelength-sensitive opsins [40,44,45].
The use of different spectra potentially can mitigate the impact of light on bats, and therefore we tested the effects of light with three different spectra (white, green and red) on slow-flying light-shy Myotis and Plecotus species, agile non-light shy Pipistrellus species, and the larger Nyctalus and Eptesicus species foraging in open habitat. We expected variation in the response of bats to the different light colours: as bats are thought to be most sensitive to long wavelengths, we hypothesized light-shy bats to be less often present in white and green light. Conversely, because insects are more strongly attracted to short wavelengths, we expected agile, non-light shy bats to be more active around green and white light. We did not have a clear expectation towards the presence of bat species foraging in open habitat.
For this study, we recorded bat activity using automatic bat detectors within the infrastructure of a long-term ecosystem-wide research project on the impact of experimental light in natural habitat [46], in which we illuminate natural habitat with white, red and green light. We included a simultaneous assessment of insect density, as this potentially explains differences in the response of opportunistic bats.
2. Methods
(a). Experimental set-up
At eight sites with natural habitat in dark areas in the Netherlands, we set up four 100 m long transects, each with five 4 m tall light posts (except for two sites with 50 m long transects with three light posts) emitting white, green and red light, and one transect was permanently left dark. Light post transects were placed perpendicular to a forest edge, with two light posts in open area, one light post at the forest edge, and two in the forest interior. The white lamps (Philips Fortimo White) emit broad-spectrum light. The green (Philips Fortimo ClearSky) and red (Philips Fortimo ClearField) lamps include low levels of all wavelengths in the visible spectrum, but the green lamp has an increased share of blue and green, and reduced levels of red light. Conversely, the red light has increased red and reduced levels of blue (see electronic supplementary material, figure S1 for the spectral composition). The lampposts have a realistic light intensity for the illumination of a countryside road according to CIE standards [47]. The light level for each spectrum is perceived by humans as equally intense, with a nominal flux of 1800 Lumen and 7.6 ± 1.2 Lux (1 s.e.m.) at ground level (see also electronic supplementary material, table S1). The lamps produce a negligible amount of UV light, and none of the three lamp types emits any sound between 0 and 120 kHz. The lights are on from sunset to sunrise throughout the year, except for a maximum of eight separate nights per year distributed over May–September for moth sampling, when the lights were kept off. A limited number of these dark nights coincided with bat activity measurements. In 2014, the lamps were deliberately switched off for one additional night for the simultaneous assessment of the response of insects and pipistrelle bats to experimental darkness. For a more detailed description of the set-up please see [46].
(b). Bat activity measurement
Bat activity was measured with Pettersson D500X detectors (Pettersson Elektronik AG, Sweden) powered by external 6 V lead-acid batteries. In order to protect the equipment from rain and wind, detectors were placed in Explorer Cases (GT Line srl, Bologna, Italy) with a Ø 40 mm opening for the microphone facing the open area. Cases were permanently mounted in a tree close to the middle light post in each transect (with a maximum distance of 6 m, i.e. at the forest's edge; see electronic supplementary material, figure S2). In each of the years 2012–2016, twice per year in June–July (early summer) and August–September (late summer), detectors were placed in the field for five to 15 nights. When triggered by sound above 20 kHz of sufficient amplitude, echolocation calls were recorded for 5 s and stored in wav files (see electronic supplementary material for a detailed description). Detectors often stopped recording before they were collected from the field because of an empty battery or full memory cards. To ensure all transects were sampled equally we always identified, for each site individually, the first night at which a detector stopped at one of the transects. Data collected during this night and all following nights were discarded for all transects of that specific site. Sound files were subsequently analysed with SonoChiro (Biotope Research & Development, Mèze, France; see electronic supplementary material, information). Because of the problematic identification of bats to the species level by echolocation sounds, especially for Myotis species, we limited identification to group level as provided by SonoChiro. At the experimental sites, the software identified the following groups: Plecotus sp., Myotis sp. and two Pipistrellus sp. groups with calls around 35 kHz and 50 kHz respectively, and bats belonging to a group with both Nyctalus and Eptesicus species. Calls assigned to other groups that are not known to be present in the Netherlands [48], such as Barbastella and Rhinolophus, were discarded. The results from SonoChiro for these groups were consequently pooled to three groups for analysis: Myotis and Plecotus species (group 1), Pipistrellus species (group 2) and Nyctalus and Eptesicus species (group 3; these two genera are directly clustered by SonoChrio). Bat activity was quantified as the number of bat passes for each group at each transect for each night. A bat pass of a group was added if a 5-second sound file contained two or more pulses that were recognized by SonoChiro as belonging to that group.
(c). Insect activity measurement
For two (at one site three) nights in June 2014, insect density was measured simultaneously with bat activity. During one additional night, we experimentally switched off the lights in order to assess the immediate response of bat and insect activity in darkness. Insect activity was measured with the use of sticky sheets placed approximately 50 cm below the lamps of the light posts in the forest edge (the light posts that were closest to the detectors). We used a thin aluminium frame to prevent bats from accidentally touching the sticky surface, and the sheets from getting stuck against the light post (electronic supplementary material, figure S3). Sticky sheets were custom made out of laminated white paper coated with insect glue (Andermatt Biocontrol, Grossdietwil, Switzerland) in order to avoid confounding effects by the standard yellow colour of commercially available sticky sheets. Sticky sheets were digitized and processed with ImageJ [49]. Non-insect material and Lepidoptera wings were digitally removed. The total area of all clusters with dark pixels larger than 0.5 mm2 was used as a proxy for insect availability (see electronic supplementary material for details).
(d). Statistical analysis
Statistical analysis was done with R v. 3.3.1 [50] with a significance level of 0.05. For all three bat groups, models following a negative binomial distribution with a logit link performed best, based on the model AIC value and normality of residual variance. For bat passes, we first tested for interactions between year and light treatment, and year period (early and late summer) and light treatment. With no interaction, data for all years and early and late summer were pooled for each group for each transect. We then fitted general linear models using the glm.nb routine in the R package MASS [51], with treatment (white, green and red light, and dark control) and site as fixed effects.
For testing the effects of insect density on the number of passes of group 2 bats (Pipistrellus species), we fitted similar models with log-transformed insect area and site as fixed effects. Models with and without treatment and log insect area were compared by calculating the likelihood ratio. The effect of treatment on insect density was modelled by fitting a linear mixed-effects model with a Gaussian distribution using the lmer routine in the R package lme4 [52], with treatment as fixed effect and site as a random term. Post hoc testing was done using the lsmeans package [53] with Bonferroni-corrected probabilities.
3. Results
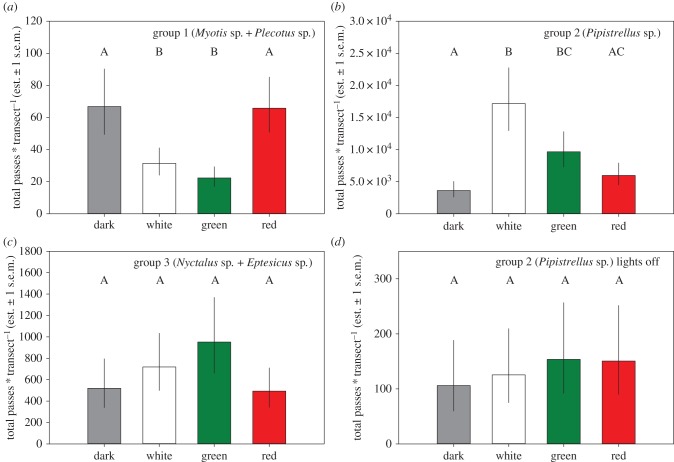
The total number of fully recorded nights (after excluding nights during which a detector was not active all night at one or more transects within a site) was 54.4 ± 3.8 nights per site (average ± 1 s.e.m.) during the years 2012–2016. Likewise, the number of fully recorded nights with lights off varied between one and seven nights, dependent on how often moth sampling coincided with bat recording (see electronic supplementary material, table S2). The number of bat passes per site per night strongly varied between groups, with 2.43 ± 0.8 passes for Myotis/Plecotus species (group 1), 608 ± 129 passes for Pipistrellus species (group 2), and 69 ± 26 (all average ± 1 s.e.m.) for Nyctalus/Eptesicus species (group 3). We did not find an interaction between year and (light) treatment, nor between year period and (light) treatment on bat passes in any of the three groups, so we used the total number of passes at each transect for further calculations. We found a highly significant effect of treatment on Myotis and Plecotus species (group 1; see figure 1a and table 1). Post hoc tests showed that, compared to dark control, there were significantly fewer Plecotus and Myotis species passing nearby white and green light, but not red light (see electronic supplementary material, table S3). For Pipistrellus species (group 2; figure 1b), light treatment was highly significant as well, however, the response to light colour was very different. Pipistrellus species passed significantly more often in the white and green light compared to dark control. In the red light, the number of passes was not different from dark control or the green light treatment. Group 3 bats did not respond to light (figure 1c, table 1). For group 2 bats, we further evaluated the response during the nights when the lights were switched off, and during those nights, the effect of treatment disappeared (figure 1d and electronic supplementary material, table S2).
Figure 1.
Total bat passes (summed over all nights measured per transect) during all years 2012–2016 (back-transformed treatment estimates from negative binomial generalized linear models with bat passes and site as fixed effects) for (a) group 1 (Myotis and Plecotus species), (b) group 2 (Pipistrellus species), (c) group 3 (Nyctalus and Eptesicus species) and (d) passes of group 2 bats during nights when the lights were off for moth sampling (electronic supplementary material, table S2). Capitals identify groups that significantly differ from each other in post hoc tests (electronic supplementary material, table S3).
Table 1.
Results of the negative binomial generalized linear model comparisons without and with light treatment and insect density as fixed effects, and Gaussian models on insect density with and without light treatment. loglik, log likelihood; l.r., likelihood ratio.
| d.f. | theta | 2 * loglik | d.f. | log l.r. | p | |
|---|---|---|---|---|---|---|
| Group 1: Myotis sp. + Plecotus sp. | ||||||
| site | 24 | 2.450 | −260.548 | |||
| treatment + site | 21 | 4.242 | −243.327 | 3 | 17.221 | <0.001 |
| Group 2: Pipistrellus sp. | ||||||
| site | 24 | 1.728 | −622.234 | |||
| treatment + site | 21 | 3.165 | −600.180 | 3 | 22.055 | <0.0001 |
| Group 3: Nyctalus sp. + Eptesicus sp. | ||||||
| site | 24 | 1.718 | −457.728 | |||
| treatment + site | 21 | 1.905 | −453.985 | 3 | 3.743 | 0.29 |
| Group 2: Pipistrellus sp. (during all nights with lights off) | ||||||
| site | 21 | 1.080 | −358.170 | |||
| treatment + site | 18 | 1.098 | −357.701 | 3 | 0.469 | 0.93 |
| Group 2: Pipistrellus sp. (during insect sampling with lights on) | ||||||
| site | 21 | 1.742 | −291.308 | |||
| treatment + site | 18 | 2.372 | −282.329 | 3 | 8.979 | <0.05 |
| Group 2: Pipistrellus sp. (during insect sampling with lights off) | ||||||
| site | 18 | 1.347 | −209.884 | |||
| treatment + site | 15 | 1.635 | −205.156 | 3 | 4.727 | 0.19 |
| Group 2: Pipistrellus sp. (with insects as independent variable with lights on) | ||||||
| site | 21 | 1.742 | −291.308 | |||
| insects + site | 20 | 1.967 | −287.713 | 1 | 3.595 | 0.06 |
| Group 2: Pipistrellus sp. (with insects as independent variable with lights off) | ||||||
| site | 18 | 1.347 | −209.884 | |||
| insects + site | 17 | 1.400 | −208.950 | 1 | 0.933 | 0.33 |
| Group 2: Pipistrellus sp. (with insects as independent variable, all nights) | ||||||
| site | 45 | 1.154 | −518.457 | |||
| insects + site | 44 | 1.488 | −503.985 | 1 | 14.471 | <0.0005 |
| d.f. | AIC | loglik | d.f. | χ2 | p | |
|---|---|---|---|---|---|---|
| insects (with lights on) | ||||||
| 1|site | 3 | 35.150 | −14.575 | |||
| treatment + 1|site | 6 | 5.974 | 3.013 | 3 | 35.175 | <0.00001 |
| insects (with lights off) | ||||||
| 1|site | 3 | 31.049 | −12.525 | |||
| treatment + 1|site | 6 | 29.890 | −8.945 | 3 | 7.159 | 0.07 |
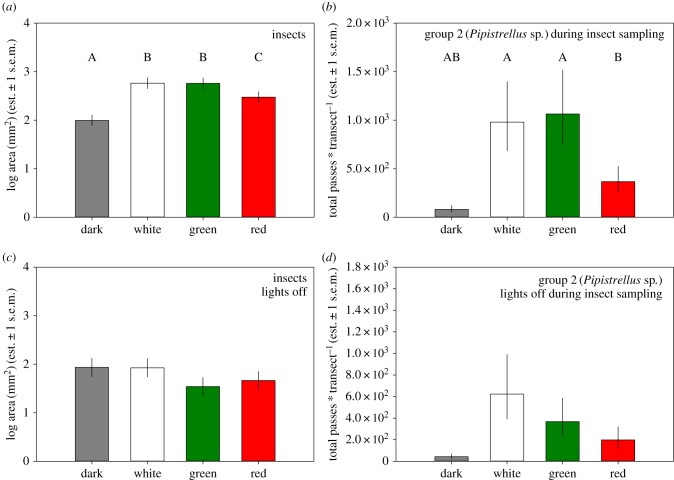
The data we collected simultaneously on insect activity and bat passes are limited to seven sites for the nights with light on, and six sites with the light off due to a malfunctioning detector. We found a highly significant effect of light on insect activity (table 1, figure 2a), and an almost significant effect (p = 0.057) of insect activity on bat passes (figure 2b). Like for all other nights with lights on, the treatment effect for the nights during which we collected insects is significant. During the nights sampled with lights off, the treatment effect disappeared for both insects and bats (table 1, figure 2c and d). When the data from both the nights with lights on and off are combined, insect activity significantly explains the number of Pipistrellus passes (p < 0.0005; table 1).
Figure 2.
Insect activity (treatment estimates from a linear mixed effect model with light treatment as fixed, and site as random effect) as measured with sticky sheets with lights on (a) and off (c), and (b) and (d) group 2 total bat passes (Pipistrellus species; back-transformed treatment estimates from negative binomial generalized linear models with bat passes and site as fixed effects) during the same nights. Capitals identify groups that significantly differ from each other in post hoc tests (electronic supplementary material, table S3).
4. Discussion
Following our hypothesis, both the Myotis and Plecotus species as well as the Pipistrellus species showed a marked response to the different spectra of the experimental light. The slow-flying Plecotus and Myotis species reduce their activity significantly in white and green, but retain activity in red light. Because measuring activity of free-ranging bats of these species is difficult, information on the response of these slow-flying species to experimental light has been limited to date. Although Myotis species may occasionally be present near lights in North America, bats of this genus are not reported to use insect concentrations near streetlights there [14,54] and are in Northern Europe only sparsely recorded in proximity to streetlights [55] or reported not to be present there at all [9]. However, forest-dwelling Myotis and Plecotus species are commonly present in low densities, and difficult to record due to their soft echolocation sounds. Although the density of Myotis and Plecotus species was probably very low at the experimental sites as well, measuring for multiple nights over five years at all eight sites eventually provided sufficient data to show an effect of light treatment and to compare the different spectra.
The absence of a response to the red light was significantly different from the responses to both white and green light by Myotis and Plecotus species, and clearly follows our hypothesis. The preservation of activity by these species may be caused by the relative high sensitivity of the bat eye to the blue part of the spectrum [43], which is attenuated in the experimental red light we used. However, the spectral sensitivity of the bat visual system may not necessarily determine the response of bats to light; further work on the dose–response relation of light with different spectra is therefore important. An alternative explanation for the reduced activity of Myotis and Plecotus species in the white and green light compared to red light, is the high abundance of Pipistrellus species in the white and green light. The possibility of an increase in abundance of non-light-shy species at the expense of light-shy species owing to the presence of light at night has been suggested earlier [56]. We tested for this by correlating the presence of Pipistrellus species and Myotis and Plecotus species in the data collected at the dark transects. In a negative binomial model with transect and night number as random terms, the numbers of Myotis and Plecotus passes binned in 30 min intervals were not dependent on the number of Pipistrellus passes (p = 0.38).
The agile Pipistrellus species are significantly more active in white and green light, which is the opposite of the response of Myotis and Plecotus species in group 1 bats. Like the Myotis and Plecotus species, the activity in the red light is comparable to dark control. The presence of pipistrelles is most likely explained by insect density; the near-significant relation (table 1) is probably caused by the number of nights sampled (two nights at most sites), and the link with insects is in line with many earlier studies [9,12,15]. The presence of equal insect activity in white and green light is somehow surprising, as our green light contains relatively more short wavelengths, and hence was expected to attract more insects and consequently bats ([9,57,58], but see [55] and [59]).
When the experimental lights were left off for just a single night for the monitoring of moths with small light traps (a routine not directly related to the study presented in this paper, but for which we had to switch off the lights separately for eight isolated nights per season, see §2), the effect of treatment (i.e. the colour of the light if the lamps had been on) on Pipistrellus species was absent. As these nights with lights off were always isolated in between nights with lights on, with at least 10 days of illuminated nights in between, the disappearance of the effect of light treatment on pipistrelles indicates a direct response. In 2014, the lights were switched off for one additional night during the insect activity measurements with sticky sheets beneath the light post lamps (which were intended to relate bat and insect activity with both lights on and off). The immediate disappearance of the effects of light treatment on insects during these nights suggests that the response of pipistrelle bats is a direct response to insect abundance.
In the third group with Nyctalus and Eptesicus species we observed no effect of the different spectra even though in earlier studies, both Nyctalus noctula and Eptesicus serotinus, the two species in this group that are common in the Netherlands, have been observed foraging around street lights [9,60,61]. The illumination conditions in the surroundings where these bats have been reported foraging may, however, be different from the relative small-scale lighting at the experimental sites in our study—for example, Nyctalus species are known to forage above brightly illuminated areas such as car parks and large road crossings [62]. Furthermore, Nyctalus noctula has very loud echolocation calls and may be recorded from afar (more than 100 m). During manual observations at the experimental sites, noctules were observed passing at a higher altitude over the site, without being exposed to the experimental light. The echolocation calls of Eptesicus serotinus are quieter compared to noctules, but still may be picked up by detectors when bats are flying relatively far away from the light posts. Although serotines have been recorded foraging around streetlights [60,61], the species does not use existing streetlights as often as pipistrelles.
To conclude, the reduction in activity of slow-flying light-shy species around white and green illumination implies a loss of habitat. The loss of habitat for light-shy bats at the experimental sites may be limited by the scale of the illumination in our set-up, and the dose–response relationship between bat activity and light intensity cannot be easily established for free-ranging bats in a natural habitat. However, in a situation with comparable lighting along full stretches of roads, there may be substantial effects at the population level. Conversely, the introduction of white and green light in natural habitat facilitates the presence of synanthropic species. Our findings show that bat activity in red light, which has less light of short wavelength and more light of long-wavelength, most resembles dark. This holds up for both light-shy species and more agile non-light shy species. Therefore, this finding opens the possibility for the mitigation of adverse consequences of artificial lighting for bats in situations where natural habitat has to be exposed to illumination.
Supplementary Material
Acknowledgements
We thank ZIUT for service and quick repairs on the experimental set-up when needed, and Staatsbosbeheer, Natuurmonumenten, the Dutch Ministry of Defence, Het Drentse Landschap and the municipality of Ede for allowing us to illuminate natural habitat and to work in their terrain. We thank the Dutch Mammal Society, Rob Koelman, Hugo Loning and Annemieke Kolvoort for their logistic support during the fieldwork, and Herman Limpens and Jasja Dekker for feedback on the set-up of the bat monitoring.
Data accessibility
The data collected during this study are available from the Dataverse Digital Depository, and can be accessed via the link http://hdl.handle.net/10411/20867 [63].
Authors' contributions
M.E.V., E.M.V., R.H.A.v.G., M.D. and K.S. designed the set-up of the study. R.H.A.v.G and K.S. established research sites. Data were collected and processed by J.J.C.R., K.B.F., T.R. and K.S., and analysed by K.S. K.S. wrote the paper, and all authors have commented on the manuscript.
Competing interests
M.D. is employed by Philips, the producer of the lamps used in this study and Philips provided financial support.
Funding
The set-up and maintenance of the research sites are financed by the Dutch Technology Foundation STW, part of the Netherlands Organization for Scientific Research (NWO). The project is supported by Philips and the Nederlandse Aardolie Maatschappij (NAM). K.B.F. and her contribution to this work were supported by a BCI (Bat Conservation International) Student Research Scholarship.
References
- 1.Bennie J, Duffy JP, Davies TW, Correa-Cano ME, Gaston KJ. 2015. Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sens. 7, 2715–2730. ( 10.3390/rs70302715) [DOI] [Google Scholar]
- 2.Kyba CCM, et al. 2015. Worldwide variations in artificial skyglow. Sci. Rep. 5, 1–6. ( 10.1038/srep08409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hölker F, et al. 2010. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 15, 13 ( 10.5751/ES-03685-150413) [DOI] [Google Scholar]
- 4.Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133 ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich C, Longcore T (eds). 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 6.Swaddle JP, et al. 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. ( 10.1016/j.tree.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 7.Downs NC, Beaton V, Guest J, Polanski J, Robinson SL, Racey PA. 2003. The effects of illuminating the roost entrance on the emergence behaviour of Pipistrellus pygmaeus. Biol. Conserv. 111, 247–252. ( 10.1016/S0006-3207(02)00298-7) [DOI] [Google Scholar]
- 8.Laidlaw GWJ, Fenton MB. 1971. Control of nursery colony populations of bats by artificial light. J. Wildl. Manag. 35, 843–846. ( 10.2307/3799798) [DOI] [Google Scholar]
- 9.Rydell J. 1992. Exploitation of insects around streetlamps by bats in Sweden. Funct. Ecol. 6, 744–750. ( 10.2307/2389972) [DOI] [Google Scholar]
- 10.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 11.Bell GP. 1980. Habitat use and response to patches of prey by desert insectivorous bats. Can. J. Zool. 58, 1876–1883. ( 10.1139/z80-256) [DOI] [Google Scholar]
- 12.Blake D, Hutson A, Racey P, Rydell J, Speakman J. 1994. Use of lamplit roads by foraging bats in southern England. J. Zool. 234, 453–462. ( 10.1111/j.1469-7998.1994.tb04859.x) [DOI] [Google Scholar]
- 13.Fenton MB, Merriam HG, Holroyd GL. 1983. Bats of Kootenay, Glacier, and Mount Revelstoke national parks in Canada: identification by echolocation calls, distribution, and biology. Can. J. Zool. 61, 2503–2508. ( 10.1139/z83-332) [DOI] [Google Scholar]
- 14.Furlonger CL, Dewar HJ, Fenton MB. 1987. Habitat use by foraging insectivorous bats. Can. J. Zool. 65, 284–288. ( 10.1139/z87-044) [DOI] [Google Scholar]
- 15.Rydell J, Racey P. 1995. Street lamps and the feeding ecology of insectivorous bats. In Symposia of the Zoological Society of London (eds PA Racey, SM Swift), pp. 291–307. London, UK: The Society. [Google Scholar]
- 16.Acharya L, Fenton MB. 1999. Bat attacks and moth defensive behaviour around street lights. Can. J. Zool. 77, 27–33. ( 10.1139/z98-202) [DOI] [Google Scholar]
- 17.Minnaar C, Boyles JG, Minnaar IA, Sole CL, McKechnie AE. 2014. Stacking the odds: light pollution may shift the balance in an ancient predator–prey arms race. J. Appl. Ecol. 52, 522–531. ( 10.1111/1365-2664.12381) [DOI] [Google Scholar]
- 18.Svensson AM, Rydell J. 1998. Mercury vapour lamps interfere with the bat defence of tympanate moths (Operophteraspp.; Geometridae). Anim. Behav. 55, 223–226. ( 10.1006/anbe.1997.0590) [DOI] [PubMed] [Google Scholar]
- 19.Wakefield A, Stone EL, Jones G, Harris S. 2015. Light-emitting diode street lights reduce last-ditch evasive manoeuvres by moths to bat echolocation calls. R. Soc. Open Sci. 2, 150291 (doi:10.1098/rsos.150291; ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hale JD, Fairbrass AJ, Matthews TJ, Davies G, Sadler JP. 2015. The ecological impact of city lighting scenarios: exploring gap crossing thresholds for urban bats. Glob. Change Biol. 21, 2467–2478. ( 10.1111/gcb.12884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews F, Roche N, Aughney T, Jones N, Day J, Baker J, Langton S. 2015. Barriers and benefits: implications of artificial night-lighting for the distribution of common bats in Britain and Ireland. Phil. Trans. R. Soc. B 370, 20140124 ( 10.1098/rstb.2014.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone EL, Jones G, Harris S. 2012. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Change Biol. 18, 2458–2465. ( 10.1111/j.1365-2486.2012.02705.x) [DOI] [Google Scholar]
- 23.Jones G, Rydell J. 1994. Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Phil. Trans. R. Soc. Lond. B 346, 445–455. ( 10.1098/rstb.1994.0161) [DOI] [Google Scholar]
- 24.Rydell J, Entwistle A, Racey PA. 1996. Timing of foraging flights of three species of bats in relation to insect activity and predation risk. Oikos 76, 243–252. ( 10.2307/3546196) [DOI] [Google Scholar]
- 25.Duvergé PL, Jones G, Rydell J, Ransome RD. 2000. Functional significance of emergence timing in bats. Ecography 23, 32–40. ( 10.1111/j.1600-0587.2000.tb00258.x) [DOI] [Google Scholar]
- 26.Speakman JR. 1991. Why do insectivorous bats in Britain not fly in daylight more frequently? Funct. Ecol. 5, 518–524. ( 10.2307/2389634) [DOI] [Google Scholar]
- 27.Polak T, Korine C, Yair S, Holderied MW. 2011. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zool. 285, 21–27. ( 10.1111/j.1469-7998.2011.00808.x) [DOI] [Google Scholar]
- 28.van Grunsven RHA, Donners M, Boekee K, Tichelaar I, van Geffen KG, Groenendijk D, Berendse F, Veenendaal EM. 2014. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 18, 225–231. ( 10.1007/s10841-014-9633-9) [DOI] [Google Scholar]
- 29.de Jong M, Ouyang JQ, Silva AD, van Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K. 2015. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Phil. Trans. R. Soc. B 370, 20140128 ( 10.1098/rstb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans WR, Akashi Y, Altman N, Manville A. 2007. Response of night-migrating songbirds in cloud to colored and flashing light. North Am. Birds 60, 476–488. [Google Scholar]
- 31.Ouyang JQ, de Jong M, Hau M, Visser ME, van Grunsven RHA, Spoelstra K. 2015. Stressful colours: corticosterone concentrations in a free-living songbird vary with the spectral composition of experimental illumination. Biol. Lett. 11, 20150517 ( 10.1098/rsbl.2015.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiltschko W, Wiltschko R. 1995. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J. Comp. Physiol. A 177, 363–369. ( 10.1007/BF00192425) [DOI] [Google Scholar]
- 33.Witherington BE. 1992. Behavioral responses of nesting sea turtles to artificial lighting. Herpetologica 48, 31–39. [Google Scholar]
- 34.Witherington BE, Bjorndal KA. 1991. Influences of artificial lighting on the seaward orientation of hatchling loggerhead turtles Caretta caretta. Biol. Conserv. 55, 139–149. ( 10.1016/0006-3207(91)90053-C) [DOI] [Google Scholar]
- 35.Van Grunsven RHA, Creemers R, Joosten K, Donners M, Veenendaal EM. 2016. Behaviour of migrating toads under artificial lights differs from other phases of their life cycle. Amphib.-Reptil. 38, 49–55. ( 10.1163/15685381-00003081) [DOI] [Google Scholar]
- 36.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 37.Stone EL, Wakefield A, Harris S, Jones G. 2015. The impacts of new street light technologies: experimentally testing the effects on bats of changing from low-pressure sodium to white metal halide. Phil. Trans. R. Soc. B 370, 20140127 ( 10.1098/rstb.2014.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenbeis G. 2006. Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In Ecological consequences of artificial night lighting (eds C Rich, T Longcore), pp. 281–304. Washington, DC: Island Press. [Google Scholar]
- 39.Jacobs GH. 1993. The distribution and nature of colour vision among the mammals. Biol. Rev. 68, 413–471. ( 10.1111/j.1469-185X.1993.tb00738.x) [DOI] [PubMed] [Google Scholar]
- 40.Feller KD, Lagerholm S, Clubwala R, Silver MT, Haughey D, Ryan JM, Loew ER, Deutschlander ME, Kenyon KL. 2009. Characterization of photoreceptor cell types in the little brown bat Myotis lucifugus (Vespertilionidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 154, 412–418. ( 10.1016/j.cbpb.2009.08.006) [DOI] [PubMed] [Google Scholar]
- 41.Kim T-J, Jeon Y-K, Lee J-Y, Lee E-S, Jeon C-J. 2008. The photoreceptor populations in the retina of the greater horseshoe bat Rhinolophus ferrumequinum. Mol. Cells 26, 373–379. [PubMed] [Google Scholar]
- 42.Peichl L. 2005. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 287A, 1001–1012. ( 10.1002/ar.a.20262) [DOI] [PubMed] [Google Scholar]
- 43.Müller B, Glösmann M, Peichl L, Knop GC, Hagemann C, Ammermüller J. 2009. Bat eyes have ultraviolet-sensitive cone photoreceptors. PLoS ONE 4, e6390 ( 10.1371/journal.pone.0006390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D. 2003. Molecular evolution of bat color vision genes. Mol. Biol. Evol. 21, 295–302. ( 10.1093/molbev/msh015) [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Rossiter SJ, Teeling EC, Li C, Cotton JA, Zhang S. 2009. The evolution of color vision in nocturnal mammals. Proc. Natl. Acad. Sci. USA 106, 8980–8985. ( 10.1073/pnas.0813201106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spoelstra K, van Grunsven RHA, Donners M, Gienapp P, Huigens ME, Slaterus R, Berendse F, Visser ME, Veenendaal E. 2015. Experimental illumination of natural habitat—an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Phil. Trans. R. Soc. B 370, 20140129 ( 10.1098/rstb.2014.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CIE Technical Committee 4–44. 2010. Lighting of roads for motor and pedestrian traffic. Vienna, Austria: Commission internationale de l’éclairage, CIE Central Bureau. [Google Scholar]
- 48.Broekhuizen S, Spoelstra K, Thissen JBM, Canters KJ, Buys JC (eds). 2016. Atlas van De Nederlandse Zoogdieren. Leiden, The Netherlands: Naturalis. [Google Scholar]
- 49.Rasband W. 2012. ImageJ: image processing and analysis in Java. Astrophys. Source Code Libr. l, 06013. [Google Scholar]
- 50.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 51.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer; See http://www.stats.ox.ac.uk/pub/MASS4. [Google Scholar]
- 52.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 53.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 54.Acharya L. 1995. Sex-biased predation on moths by insectivorous bats. Anim. Behav. 49, 1461–1468. ( 10.1016/0003-3472(95)90067-5) [DOI] [Google Scholar]
- 55.Rowse EG, Harris S, Jones G. 2016. The switch from low-pressure sodium to light emitting diodes does not affect bat activity at street lights. PLoS ONE 11, e0150884 ( 10.1371/journal.pone.0150884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arlettaz R, Godat S, Meyer H. 2000. Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biol. Conserv. 93, 55–60. ( 10.1016/S0006-3207(99)00112-3) [DOI] [Google Scholar]
- 57.Somers-Yeates R, Hodgson D, McGregor PK, Spalding A, ffrench-Constant RH. 2013. Shedding light on moths: shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 9, 20130376 ( 10.1098/rsbl.2013.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D. 2011. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 144, 2274–2281. ( 10.1016/j.biocon.2011.06.004) [DOI] [Google Scholar]
- 59.Wakefield A, Broyles M, Stone EL, Jones G, Harris S. 2016. Experimentally comparing the attractiveness of domestic lights to insects: do LEDs attract fewer insects than conventional light types? Ecol. Evol. 6, 8028–8036. ( 10.1002/ece3.2527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baagøe HJ. 1984. Summer occurrence of Vespertilio murinus Linné-1758 and Eptesicus serotinus (Schreber-1780)(Chiroptera, Mammalia) on Zealand, Denmark, based on records of roosts and registrations with bat detectors. Ann. Naturhistorischen Mus. Wien Ser. B Für Bot. Zool. 88, 281–291. [Google Scholar]
- 61.Catto CMC, Hutson AM, Raccey PA, Stephenson PJ. 1996. Foraging behaviour and habitat use of the serotine bat (Eptesicus serotinus) in southern England. J. Zool. 238, 623–633. ( 10.1111/j.1469-7998.1996.tb05419.x) [DOI] [Google Scholar]
- 62.Kronwitter F. 1988. Population structure, habitat use and activity patterns of the noctule bat, Nyctalus noctula Schreb., 1774 Chiroptera: Vespertilionidae revealed by radio-tracking. Myotis 26, 23–85. [Google Scholar]
- 63.Spoelstra K, van Grunsven RHA, Ramakers JJC, Ferguson KB, Raap T, Donners M, Veenendaal EM, Visser ME. 2017. Data from: Response of bats to light with different spectra: light-shy and agile bat presence is affected by white and green, but not red light. Dataverse Digital Repository. (http://hdl.handle.net/10411/20867) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Spoelstra K, van Grunsven RHA, Ramakers JJC, Ferguson KB, Raap T, Donners M, Veenendaal EM, Visser ME. 2017. Data from: Response of bats to light with different spectra: light-shy and agile bat presence is affected by white and green, but not red light. Dataverse Digital Repository. (http://hdl.handle.net/10411/20867) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data collected during this study are available from the Dataverse Digital Depository, and can be accessed via the link http://hdl.handle.net/10411/20867 [63].