Abstract
Mammals synthesize, cell-type specifically, the diastereomeric hexosylceramides, β-galactosylceramide (GalCer) and β-glucosylceramide (GlcCer), which are involved in several diseases, such as sphingolipidosis, diabetes, chronic kidney diseases, or cancer. In contrast, Bacteroides fragilis, a member of the human gut microbiome, and the marine sponge, Agelas mauritianus, produce α-GalCer, one of the most potent stimulators for invariant natural killer T cells. To dissect the contribution of these individual stereoisomers to pathologies, we established a novel hydrophilic interaction chromatography-based LC-MS2 method and separated (R > 1.5) corresponding diastereomers from each other, independent of their lipid anchors. Testing various bacterial and mammalian samples, we could separate, identify (including the lipid anchor composition), and quantify endogenous β-GlcCer, β-GalCer, and α-GalCer isomers without additional derivatization steps. Thereby, we show a selective decrease of β-GlcCers versus β-GalCers in cell-specific models of GlcCer synthase-deficiency and an increase of specific β-GlcCers due to loss of β-glucoceramidase 2 activity. Vice versa, β-GalCer increased specifically when cerebroside sulfotransferase (Gal3st1) was deleted. We further confirm β-GalCer as substrate of globotriaosylceramide synthase for galabiaosylceramide synthesis and identify additional members of the human gut microbiome to contain immunogenic α-GalCers. Finally, this method is shown to separate corresponding hexosylsphingosine standards, promoting its applicability in further investigations.
Keywords: hydrophilic interaction chromatography, electrospray ionization-tandem mass spectrometry, glucosylceramide, glucocerebroside, galactosylceramide, cerebroside, glucosylsphingosine, glucopsychosine, galactosylsphingosine, psychosine, α-galactosylceramide, KRN7000, liver, kidney, Bacteroides fragilis, microbiota, glucosidase beta 2, fatty acid 2-hydroylase, glucosylceramide synthase, globotriaosylceramide synthase
Mammals produce a large variety of glycosphingolipid (GSL) structures, based on the initial synthesis of either glucocerebrosides [the term is used here only for β-glucosylceramides (GlcCers)] or cerebrosides [the term is used here only for β-galactosylceramides (GalCers)] by β-GlcCer synthase (UGCG) or β-GalCer synthase (UGT8A), respectively (1–3). Due to the Golgi and ER location of their synthesis and the topology of the corresponding enzymes, β-GlcCer and β-GalCer are in contrast to more complex GSLs also located on the cytosolic side of cell membranes (4–6), by that widening their terrain of biological action to cytosolic binding partners. Whereas glucocerebrosides appear to be omnipresent in almost all cell types of the mammalian body, cerebrosides have mainly been associated to brain, especially myelin structures, and to kidneys (7). Likewise, systemic deficiency of β-GlcCer-based GSLs due to mutation of Ugcg is embryonically lethal at E6.5 (8) and organ-specific functions of β-GlcCer, including its derivatives, have been addressed with cell-specific deletion of the Ugcg-gene function (9–19). In contrast, mice with a systemic deletion of Ugt8a pass embryogenesis. However, Ugt8a-deficiency results in severe demyelination and motor dysfunction before most of the mice die by the age of 5 weeks (20, 21).
The catabolism of GSLs includes the lysosomal degradation of β-GlcCer and β-GalCer by β-glucosylceramidase (GBA) and galactosylceramidase (GALC) with the help of activator proteins (22, 23). Mutations of GBA and of GALC resulting in substantial loss of corresponding enzyme activities cause Gaucher disease and Krabbe disease, respectively (24–28). Symptoms of defective cerebroside catabolism caused by GALC mutations are motor deterioration, cognitive decline, and the presence of numerous, often multinucleated, globoid cells. The lysosomal storage of glucocerebroside especially affects phagocytic cells (“Gaucher cells”) and goes along with hepatosplenomegaly, which is currently treated with enzyme replacement and/or substrate reduction (29–32). Some patients on enzyme replacement therapy have been reported to develop type 2 diabetes or metabolic syndrome, malignancies, and central nervous system disorders (33), which underlines the biological activity of glucocerebroside and its metabolites. Likewise, increased levels of glucocerebroside correlate multidrug resistance in several tumor cell lines and tumor specimens (34, 35). In general, sphingolipid metabolism defects further promote complications like cardiovascular events via oxidant stress in chronic kidney diseases (36) and downregulation of GSL synthesis appears to help reverse symptoms/phenotypes (37), including polycystic kidney disease (38).
Enzymes catalyzing the production of corresponding α-anomers in mammals have not been described so far, although recently it has been proposed that, at very low levels, α-GalCer is endogenously present and necessary for invariant natural killer T (iNKT) cell homeostasis (39). Furthermore, α-GalCer, which was described initially in the marine sponge, Agelas mauritianus (40), is a highly potent activator of iNKT cells, when presented via CD1d (structurally related to the major histocompatibility complex proteins) by antigen-presenting cells (41). Currently, the potential of α-GalCer-stimulated iNKT cells in tumor treatment is under investigation (42–45). The natural stimulation of iNKT cells via CD1-presented α-GalCer may derive from members of the gut microbiome, such as Bacteroides fragilis, which was shown to produces α-GalCer and thereby probably influences host immune homeostasis (46). Although this is, so far, the only strain investigated to produce α-GalCer, the majority of bacteroides strains were described to produce precursor Cers and Cer phospholipids (47).
Differentiation of β-GlcCer and β-GalCer from complex biological samples has, so far, been achieved by normal phase TLC in the presence of borate (48), by normal phase HPLC with (49) or without derivatization (50, 51), and quite recently by isocratic or zwitterionic hydrophilic interaction chromatography (HILIC)-coupled MS2 (52, 53). On the contrary, discrimination of β-GalCer from α-GalCer with high sensitivity from complex samples, so far, is based on TLC-immuno-overlays with antibodies specific for the α-glycosidic linkage (39).
To set up a method capable of quantitatively and sensitively distinguishing the four diastereomeric hexosylceramides (HexCers), β-GlcCer, β-GalCer, α-GlcCer, and α-GalCer (Fig. 1A), we used a synthetic mixture of α- and β-GlcCer and a synthetic mixture of α- and β-GalCer, both with an α/β-ratio of about 15/85 and with an identical Cer backbone containing nervonic acid amid-bound C18-sphingosine, i.e., HexCer(d18:1/24:1). Here we show separation of these α/β-HexCer mixtures with HILIC using propionitrile as a major component of solvent A. Further investigations demonstrate that β-HexCers with α-hydroxy fatty acid moieties, which are either of 2R- or 2S-configuration, separated from each other. We transferred the method to biological samples. Addressing murine organs, we identified and quantified complex mixtures of β-GlcCers and β-GalCers and successfully showed specific changes of either β-GlcCers or β-GalCers in mice lacking either β-glucosylceramidase 2 (Gba2, systemically), Ugcg (cell specifically), globotriaosylceramide synthase (Gb3S; A4galt, systemically), or cerebroside sulfotransferase (CST; Gal3st1, cell specifically). Whereas these murine samples did not contain detectable amounts of α-GalCer, we confirmed the presence of α-GalCers in B. fragilis and identified low levels of identical α-GalCers in at least two more bacterial strains of the human gut microbiome.
Fig. 1.
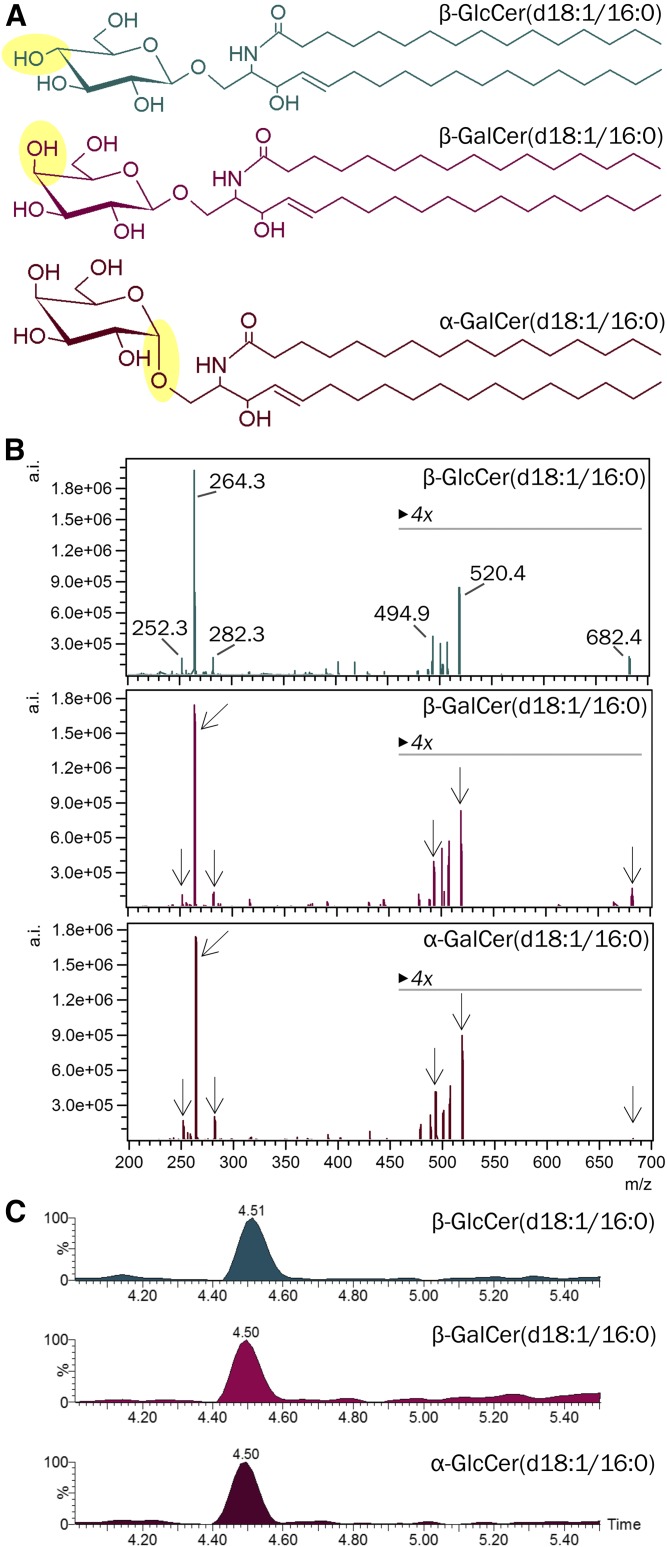
A: Stereochemical structures of β-GlcCer, β-GalCer, and α-GalCer containing a C18-sphingosine and an N-linked palmitic acid [HexCer(d18:1/16:0)]. B: Product ion spectra of corresponding HexCers obtained at 25 eV collision energy by ultra-performance LC-ESI-triple quadrupole MS2. Note, abundant product ions are detected in all three compounds. C: Extracted ion chromatograms from reversed phase chromatography (CSH C18) for the detection of HexCer(d18:1/16:0) from the three diastereomers, β-GlcCer, β-GalCer, and α-GalCer. Note the identical retention time of all three compounds.
MATERIALS AND METHODS
HexCer standards
β-GlcCer(d18:1/16:0), β-GlcCer[d18:1/24:1(15Z)], α-GalCer(d18:1/16:0), α-GalCer[d18:1/24:1(15Z)], β-GalCer(d18:1/16:0), β-GalCer[d18:1/24:1(15Z)], β-GalCer[d18:1/α(R)h18:0], β-GalCer[d18:1/α(S)h18:0], and synthetic mixtures of α/β-GlcCer[d18:1/24:1(15Z)] and of α/β-GalCer[d18:1/24:1(15Z)] with an α/β-ratio of about 15/85, and α-galactosylsphingosine (GalSph) (d18:1) were purchased from Avanti Polar Lipids. β-GalSph(d18:1) was obtained from Abcam. β-glucosylsphingosine (GlcSph) (d18:1), kerasin [N-nonhydroxy acyl sphingosine (NS)-type β-GalCers from bovine brain], and phrenosin [N-α-hydroxy (AS)-type β-GalCers from bovine brain] were purchased from Matreya. KRN7000/α-GalCer(t18:0/26:0) was obtained from AdipoGen Life Sciences. A previously published mix of β-GlcCer(d18:1/14:0, 19:0, 25:0) and β-GalCer(d18:1/31:0) was used as internal standard (10).
Solvents and additives
Chloroform, methanol, isopropanol, water, all LC-MS Chromasolv®-grade, and 2-butanol (≥99.5%) for GC were from Honeywell Specialty Chemicals Seelze GmbH; propionitrile for synthesis was from Merck Darmstadt; formic acid (99–100%) was from VWR International; and ammonium formate (≥99%) for MS-analytics was from Sigma-Aldrich.
Mice
Tissue was isolated from euthanized mice of recently published mouse strains: Gba2−/− (54), Gb3S−/− (55), Ugcgf/fAlbCre (11), Ugcgf/fPax8Cre, CSTf/fPax8Cre and (Ugcgf/f+CSTf/f)Pax8Cre (16), and fatty acid 2-hydroxylase (Fa2h)−/− (56). All animal procedures were approved and performed in accordance with federal laws.
Bacteria
B. fragilis (DSM-2151), Bacteroides vulgatus (DSM-1447), Bacteroides ovatus (DSM-1896), Bacteroides thetaiotaomicron (DSM-2079), Bacteroides intestinalis (DSM-17393), Bacteroides caccae (DSM-19024), Bacteroides uniformis (DSM-6597), Prevotella copri (DSM-18205), Lactobacillus reuteri (DSM-20016), and Bifidobacterium longum subsp. infantis (DSM-20088) were obtained from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures.
Bacteroides culture
According to a previous publication (57), Bacteroides were grown anaerobically on Columbia agar plates with 5% sheep blood and Bifidobacterium on MRS medium plates containing L-cysteine-HCl (0.5 g/l) for 48 h. Lactobacillus was grown on MRS medium plates in an aerobic overnight culture. One culture plate was harvested and transferred in a tube containing 200 μl methanol.
Lipid extraction from mouse tissue
Mouse tissue was homogenized in water on ice using an Ultra Turrax T25 and homogenates were lyophilized. GSLs were isolated as previously described (58). Briefly, dried tissue samples were extracted three times with mixtures of chloroform/methanol/water. Supernatants were pooled, dried, and subjected to methanolic mild alkaline hydrolysis (0.1 M potassium hydroxide) for 2 h at 37°C to eliminate glycerophospholipids and subsequently were neutralized with acetic acid. Saponified extracts were then desalted using reverse phase C-18 columns.
Lipid extraction from bacteria
Dried bacteria samples were homogenized in methanol using the Quiagen TissueLyser II. One stainless steel bead (5 mm) per tube was agitated for 2 min at 25 Hz. Lysates were extracted in 1 ml chloroform/methanol (2/1) at 37°C for 15 min with occasional sonication. Lipid-containing supernatants were collected following centrifugation at 2,000 g for 10 min and pellets were then re-extracted once with chloroform/methanol/water (10/10/1) and once more with chloroform/methanol/water (30/60/8). Supernatants were pooled and dried.
HILIC- and reversed phase LC-MS2 analysis of HexCers
Aliquots corresponding to 2 mg mammalian tissue wet weight or, rather, 2 mg dried bacteria were mixed with internal lipid standards for analysis by LC-MS2 using an Aquity I-class ultra-performance LC and a Xevo TQ-S “triple-quadrupole” instrument, both from Waters. Using a CSH C18 column (2.1 × 100 mm, 1.7 μm; Waters), lipids were measured in reversed phase LC mode with a gradient between 57% solvent A (50% methanol) and 99% solvent B (1% methanol, 99% isopropanol), both containing 0.1% formic acid and 10 mM ammonium formate as additives (supplemental Table S1). In HILIC mode, lipids were separated on a CORTECS HILIC column (2.1 × 150 mm, 1.6 μm; Waters) using a gradient between 100% solvent A (97% propionitrile, 2% 2-butanol, and 1% water) and 100% solvent B (97% methanol, 2% 2-butanol, 1% water, and 10 mM ammonium formate), both containing 0.1% formic acid as additive (supplemental Table S2). The lipids analyzed are listed in supplemental Table S3 and were detected by multireaction monitoring (supplemental Table S4). To increase the sensitivity of the mass spectrometric detection, a solution of 120 mM ammonium formate in methanol was co-injected with the eluent of the column for ESI at a flow rate of 10 μl/min. GlcCer(d18:1;14:0), GlcCer(d18:1;19:0), GlcCer(d18:1;25:0), and GalCer(d18:1;31:0) were used as internal standards to quantify α- and β-anomers of GlcCer and GalCer with NS-, AS-, N-nonhydroxy acyl phytosphingosine (NP)-, and N-α-hydroxy acyl phytosphingosine (AP)-type Cer anchors. For quantification of NP- and AP-sphingolipids with the NS-type standard, the previously determined empirical factor of 12.2 was taken into account (12). Student’s t-tests were conducted with GraphPad Prism (GraphPad Software, San Diego, CA) and P ≤ 0.05 was considered to be statistically significant. Data are presented as the mean ± standard deviation.
RESULTS
Separation of β-GlcCer, β-GalCer, and α-GalCer by HILIC
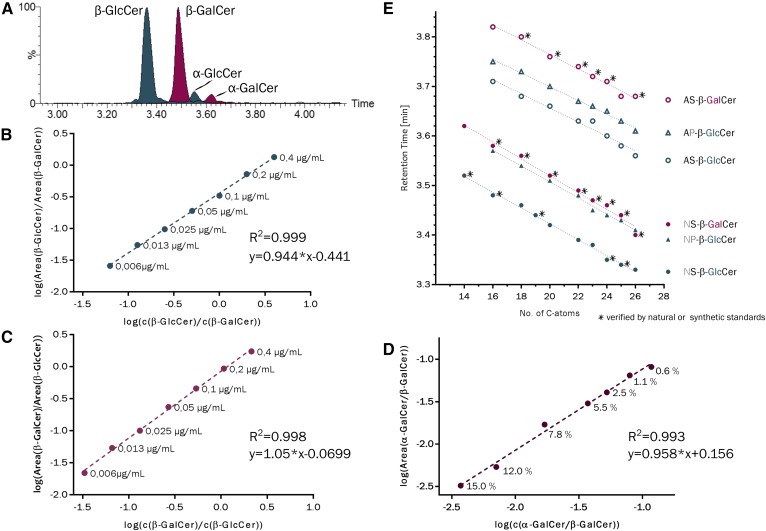
The diastereomeric GSLs, β-GlcCer, β-GalCer, α-GlcCer (not shown), and α-GalCer are not distinguishable by their fragmentation patterns in triple quadrupole-based MS2 (Fig. 1A, B) (59). Furthermore, they are not separated by C18-reversed phase mode with LC (Fig. 1C). The latter is predictable, as the structural differences of these glycolipids are within the hydrophilic moiety and not within the hydrophobic part, which interacts with the reversed phase. With normal-phase high-performance TLC (HPTLC), all four diastereomeric HexCer standards can be separated, if the HPTLC plate is impregnated prior to separation with borate (39, 48) and if they contain identical Cer anchors (supplemental Fig. S1). To quantitatively differentiate all four isomers in biological samples, chromatography, therefore, has to be combined with information about the Cer anchor. Therefore, we set up a method for HILIC coupled MS2. Initially, we used solvent gradient systems starting with 95–97% acetonitrile, but did not observe separation of our diastereomeric HexCer mixtures on HILIC columns used in our laboratory (data not shown). Consequently, we increased the hydrophobicity of the starting solvent (solvent A): acetonitrile was exchanged by propionitrile and 2% of water by 2% of 2-butanol (because methanol in solvent A widened the peaks). In a gradient of increasing polarity, solvent A was replaced successively by increasing proportions of solvent B (97% methanol, 2% 2-butanol, 1% water) in a nonlinear fashion with increasing steepness. As a result, β-GlcCer eluted first followed by β-GalCer, α-GlcCer, and finally α-GalCer, all of them containing the identical Cer anchor, Cer(d18:1/24:1). Importantly, β-GlcCer and β-GalCer (R = 1.7), as well as β-GalCer and α-GalCer (R = 2.3), separated by baseline (R ≥ 1.5, Fig. 2A). Keeping β-GalCer constant at a concentration of 0.1 μg/ml, a dilution series of β-GlcCer followed a linear decreasing peak area and β-GlcCer was detected with a LOD of 2 ng/ml (25 fmol injected), corresponding to 1% of the abundant β-GalCer (Fig. 2B). Vice versa, keeping β-GlcCer constant (0.1 μg/ml), a dilution series of β-GalCer was detected down to a LOD of 7 ng/ml (86 fmol injected), corresponding to 5% of the abundant β-GlcCer (Fig. 2C). Furthermore, α-GalCer (75 ng/ml) could be quantified in the presence of a 110-fold concentration of the β-anomer (8 μg/ml) (Fig. 2D).
Fig. 2.
A: HILIC separation of a synthetic α/β-anomeric mixture of GlcCer(d18:1/24:1) (dark cyan) and a synthetic α/β-anomeric mixture of GalCer(d18:1/24:1) (red), each with approximately 15% α-content. B: Dilution of β-GlcCer(d18:1/24:1) in the presence of constant amounts of β-GalCer(d18:1/24:1). C: Dilution of β-GalCer(d18:1/24:1) in the presence of constant amounts of β-GlcCer(d18:1/24:1). D: Constant amounts of α-GalCer(d18:1/24:1) in the presence of increasing amounts of β-GalCer(d18:1/24:1). E: Retention times of standard HexCers marked with an asterisk and endogenous β-HexCers, which have been described in literature and were identified based on the behavior of standard compounds and on the molecular ion size. Note the relatively strong shift from nonhydroxy (NS) to α-hydroxy fatty acid containing compounds (AS), while introduction of phytosphingosine (NP) instead of sphingosine (NS) as well as decreasing acyl chain length contributed in a minor way to later elution. Additional double bonds as in HexCer[d18:1/24:1(15Z)] did not contribute to a significant retention time shift.
Identifying HexCers of different Cer compositions
Next, we compared retention times of endogenous HexCers from mouse organs with our initial and some further HexCer-standards: β-GlcCer[d18:1/(14 or 16 or 19 or 25:0)], kerasin [a mixture of NS-type β-GalCers with nonhydroxy fatty acids (N) and sphingosine (S)], and phrenosin [a mixture of AS-type β-GalCers with α(R)-hydroxy fatty acids (A) and sphingosine (S)]. Liver contains mainly NS-type β-GlcCers (60); stomach contains, in addition, substantial amounts of AS- and NP-type β-GlcCers (61); and intestine contains predominantly the AP-type besides AS- and NP-type β-GlcCers (12, 62, 63). Kidney is also enriched in β-GalCers (49, 64) of the NS- and AS-type (60, 65).
Combining data of retention times and associated MS2 information (revealing the type of Cer anchor) for standards and biological samples, we observed decreasing retention times with longer acyl chain length (roughly −0.016 ± 0.005 min/CH2-unit). As compared with NS-β-HexCer, corresponding AS-β-HexCers were delayed by +0.242 ± 0.009 min, NP-HexCer by +0.084 ± 0.004 min, and AP-HexCer by +0.282 ± 0.008 min (Fig. 2E).
Comparison of the fatty acid chain length distribution in β-GlcCer with that in β-GalCers of various mouse tissues
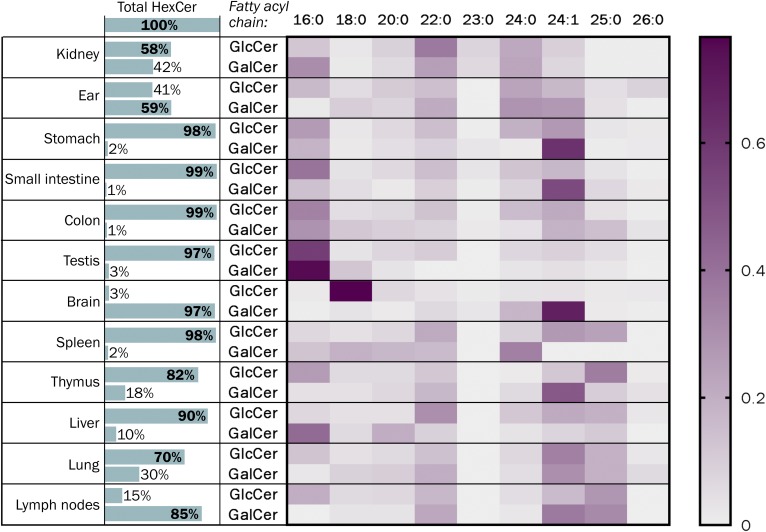
Applying the HILIC-MS2-based separation of β-GlcCer and β-GalCer, we determined the distribution of both compounds in various mouse tissues and, by that, confirming enrichment of β-GalCer mainly in brain (97%) and kidney (42%), and additionally revealing its abundance in lymph nodes (85%) and auricles (59%) (Fig. 3). Furthermore, results confirmed enrichment of nervonic acid within brain cerebrosides, whereas corresponding glucocerebrosides were enriched in stearic acid. The latter is the main acyl chain of neuronal gangliosides. Although not always as clear cut, the acyl chain length distribution of cerebrosides and glucocerebrosides was not identical in any tissue investigated.
Fig. 3.
Relative distribution of β-GlcCer and β-GalCer in various mouse organs. NS-, AS-, NP-, and AP-HexCers were determined, which contained a C18-sphingoid base and N-bound fatty acids with the chain length C16 up to C26 (as annotated). Special structures with ultra-long acyl chains as they occur in epidermis (ultra-long omega hydroxyl fatty acids) or in male germ cells (ultra-long polyunsaturated fatty acids) as well as with different sphingoid bases (e.g., C20-sphingosine in brain or kidney papillae or C17-sphingosine in epidermis) were not considered in this study. Note, nervonic acid is mainly incorporated into brain cerebrosides, whereas corresponding glucocerebrosides are enriched in stearic acid, which is the typical acyl chain of neuronal gangliosides.
The method confirms pathological changes of β-GlcCer and β-GalCer in mouse organs
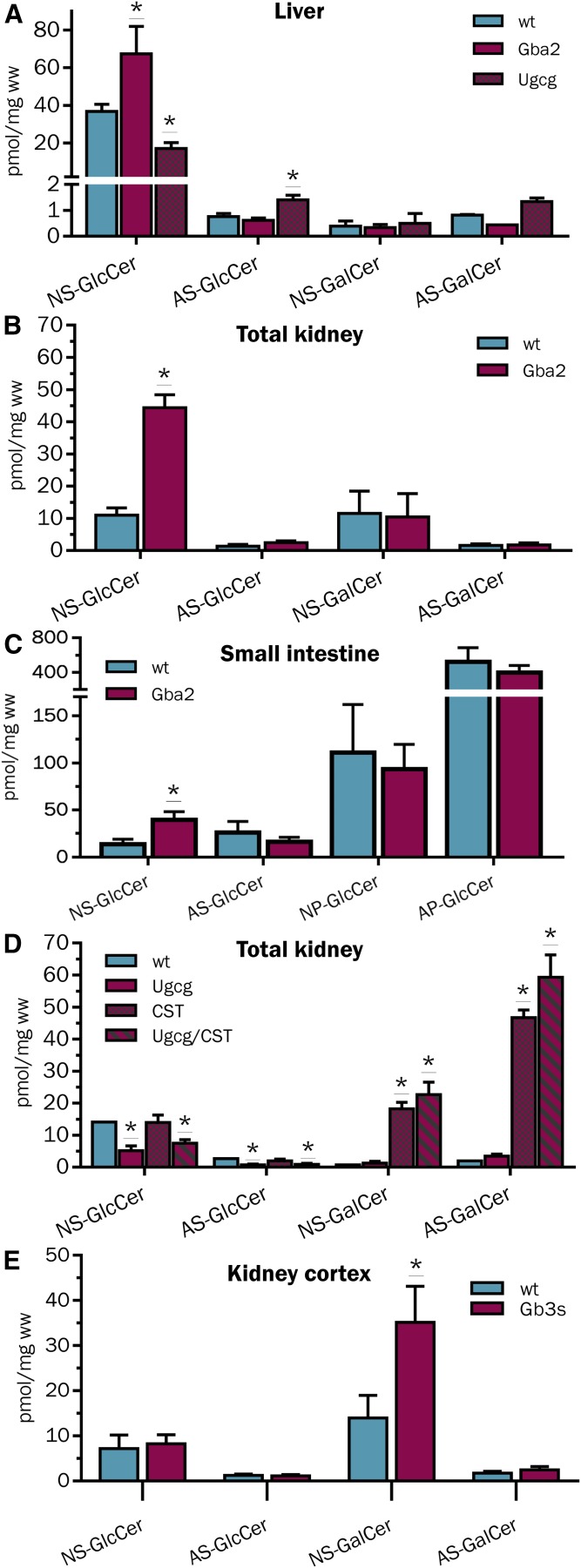
Gba2 catalyzes the hydrolysis of β-GlcCer to glucose and Cer at the cytosolic side of intracellular membranes (66). Mice lacking Gba2 activity were reported with 2- to 3-fold increased levels of β-GlcCer in liver (67, 68). Analysis of HexCers from liver, kidney, and intestine of WT and Gba2-deficient mice revealed an increase of β-GlcCers, specifically, in Gba2-deficient mice, but not corresponding β-GalCers (Fig. 4A–C). Interestingly, the increase of intestinal β-GlcCers was restricted to the rather minor NS-type, whereas the AS-type, NP-type, and dominant AP-type of β-GlcCers were not affected. Furthermore, HILIC-MS2 signals corresponding to β-GlcCers, but not to β-GalCers, were significantly reduced in liver and kidney samples of mice with a hepatocyte-specific and renal proximal tubulus-specific deficiency of the UGCG (Ugcgf/fAlbCre and Ugcgf/fPax8Cre), respectively (Fig. 4A, D). However, mainly NS-type β-GlcCers were decreased and AS-type β-GlcCers even increased a little bit in hepatocyte-Ugcg-deficient liver samples. Vice versa, β-GalCer accumulated more than 10-fold in kidneys of CST-deficient mice due to a block in further conversion to sulfatides (Fig. 4D). Here, β-GlcCer concentrations did not change. Likewise, analysis of male kidney cortex samples from Gb3S-deficient mice revealed a more than 2-fold increase of β-GalCers, which was almost exclusively due to an increase of NS-type cerebrosides (Fig. 4E). Levels of β-GlcCers were also not affected by this model. We expected increased levels of cerebrosides (β-GalCer) in kidneys of this model, as murine Gb3S also catalyzes the conversion of β-GalCer into galabiaosylceramide (Ga2Cer/Galα4Galβ1Cer/GaOse2Cer) (55), a gala-series GSL enriched in male kidneys (69); altogether, these data supported the correct association of chromatographic peaks to cerebrosides (β-GalCers) and glucocerebrosides (β-GlcCers), respectively.
Fig. 4.
HILIC-MS2-based quantification of β-GlcCers and β-GalCers in liver of WT, Gba2−/−, and Ugcgf/fAlbCre mice (A); kidney of WT and Gba2−/− mice (B); small intestine of WT, and Gba2−/− mice (C); kidney of WT, Ugcgf/fPax8Cre, CSTf/fPax8Cre, (Ugcg and CST)f/fPax8Cre mice (D); and in kidney cortex of WT and Gb3s−/− mice. Note the specific increase of NS-β-GlcCer in liver, kidney and small intestine of Gba2−/− mice, the selective decrease of NS-GlcCer in liver and of NS- and AS-GlcCer in kidney of Ugcgf/fAlbCre and Ugcgf/fPax8Cre [and (Ugcg and CST)f/fPax8Cre] mice, respectively (E). Vice versa, NS- and AS-β-GalCer accumulates in kidneys with CST-deficiency [CSTf/fPax8Cre and (Ugcg and CST)f/fPax8Cre] as well as NS-β-GalCer in cortex of Gb3s−/− mice. n ≥ 3, except for (D) WT = 1.
AS-type cerebrosides with 2R-hydroxyacyl chains are separated from those with 2S-hydroxyacyl chains
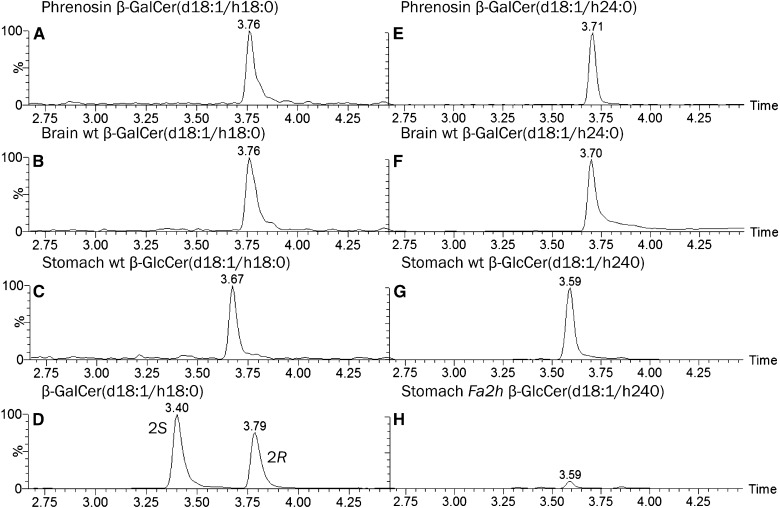
Cerebrosides from brain are enriched in cerebrosides of the AS-type (70), which contain 2R-hydroxy fatty acids (71) and depend on FA2H (56, 72), which specifically incorporates acyl chains with an α-hydroxy group in R-configuration (73). Here, we compared the retention times of AS-type cerebrosides with corresponding synthetic standards containing a 2-hydroxyacyl chain with either R- or S-stereochemistry. The β-GalCer standard with a 2R-hydroxy-stearoyl chain separated from the corresponding compound with 2S-hydroxy configuration and eluted together with corresponding AS-type cerebrosides from murine brain about 0.38 ± 0.02 min later than the 2S-isomer (Fig. 5). By that, cerebrosides with 2S-hydroxy fatty acids eluted significantly earlier (−0.27 ± 0.02 min) than AS-type glucocerebrosides (β-GlcCer) with 2R-configuration at the α-hydroxy group and, thus, could be distinguished by this HILIC method. In contrast to brain, stomach contains AS-type β-GlcCer. Normal levels depend on synthesis by FA2H as corresponding enzyme deficiency (Fig. 5H) reduces the signal to about one tenth. Due to the specificity of the enzyme (73), this dependence implies the α-hydroxy acyl group of AS-β-GlcCers from stomach to be of 2R-configuration.
Fig. 5.
HILIC-MS2-based separation of AS-type β-GalCers with 2R- and 2S-hydroxy stearic acid. Extracted ion chromatogram (EIC) for AS-HexCer(d18:1/h18:0) and (d18:1/h24:0) from a purified mixture of brain AS-type β-GalCers [phrenosin (A, E)], the synthetic standards β-GalCer[d18:1/(2S)h18:0] and β-GalCer[d18:1/(2R)h18:0] (D), a mouse brain lipid extract enriched in neutral GSLs (B, F), and a stomach lipid extract from WT (C, G) and Fa2h−/− (H) mice. The intensity in (H) is normalized to that of the corresponding WT signal in (G). In compliance with a report that AS-type β-GalCer from brain contain 2R-hydroxy fatty acids, the AS-HexCer(d18:1/h18:0) from phrenosin and from mouse brain migrate together with the β-GalCer[d18:1/(2R)h18:0] standard. Note, the decrease of β-GalCer(d18:1/h24:0) with 2R-hydroxy configuration in Fa2h−/− stomach.
The α-GalCer of B. fragilis is identified by the HILIC-MS2 method
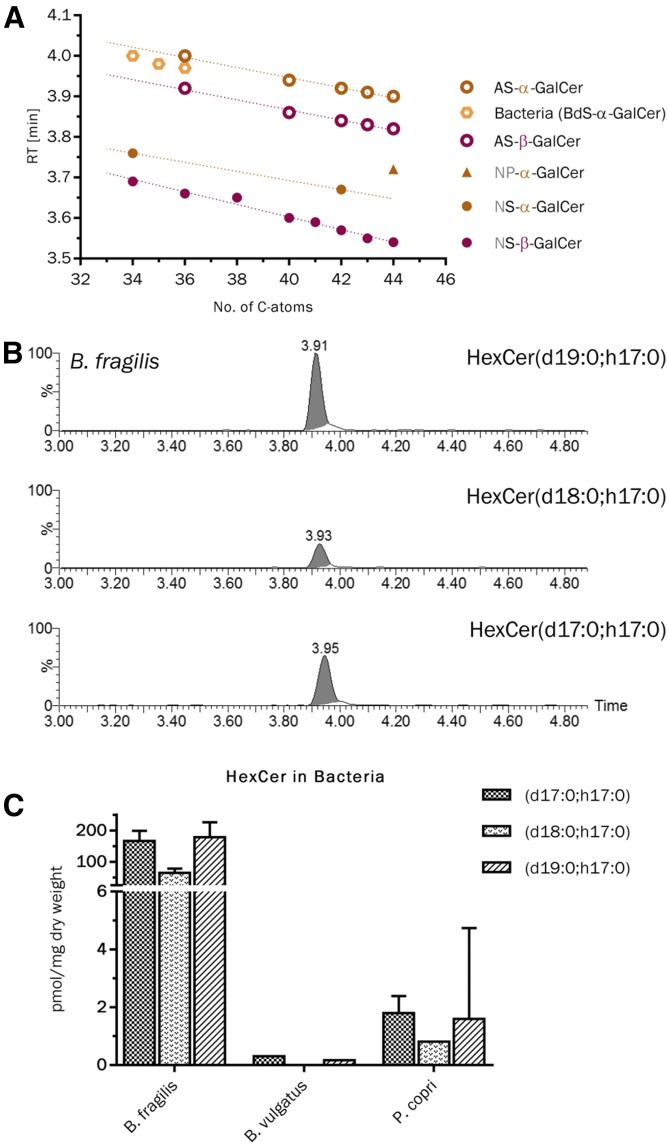
B. fragilis has been reported to produce Cers and α-GalCers containing an iso- or anteiso-branched C17-, C18-, and C19-sphinganin and a β(R)-hydroxylated and iso-branched C17-fatty acid (46, 74). Here, we observed α-GalCers from B. fragilis to elute 0.03 min before our calculated retention times (tR = 4.00–4.03 min) for corresponding AS-type α-GalCers, but definitely beyond AS-type β-GalCers (tR = 3.92–3.95 min, Δ = 0.05–0.06 min). This slight forward shift may be due to the hydroxy group of the acyl chain, which is, for B. fragilis, in the 3(β)-position, but not in the 2(α)-position, of the fatty acid. This shift was consistent for all three compounds reported by B. fragilis (Fig. 6A, B).
Fig. 6.
HILIC-MS2-based detection of α-GalCers from B. fragilis and identification of equivalent compounds in B. vulgatus and P. copri. A: Comparison of the determined retention times of α-GalCers from B. fragilis with those of synthetic α-GalCer and β-GalCer standards and predicted values for AS-type α-GalCers. B: Extracted ion chromatograms for individual HexCer from B. fragilis. C: Quantification of the three α-GalCers in B. fragilis, B. vulgatus, and P. copri. Note the 100-fold amount of α-GalCers in B. fragilis. n = 3.
Corresponding α-GalCers are common to further bacteria of the human gut microbiome
In addition to B. fragilis, we analyzed lipid extracts of nine further bacteria of the human gut microbiome. In P. copri and B. vulgatus, we detected the identical HexCer peaks as for the N-β-hydroxy acyl sphingosine (BS)-type α-GalCers of B. fragilis (Fig. 6C). However, overall levels of these compounds were at least 100-fold lower than in B. fragilis, respectively. The other seven species investigated did not contain quantifiable amounts of α-GalCer. Nevertheless, B. ovatus, B. thetaiotaomicron, and B. caccae contained signals for α-GalCers, which were above the LOD in both the HILIC and the reversed phase LC method (supplemental Table S5).
Diastereomeric hexosylsphingosines elute differently from the HILIC column
Finally, we addressed the elution behavior of hexosylsphingosines (HexSphs) with standards for glucopsychosine (β-GlcSph), psychosine (β-GalSph), and α-GalSph, all with a C18-sphingosine as long chain base. Although not baseline separated, the β-GlcSph eluted before β-GalSph (R = 1.0) and α-GalSph came off the column last (R = 0.7) in the same order as the corresponding HexCers (Fig. 7). Hence, this method may also be useful to identify the type of HexSph present.
Fig. 7.
HILIC-MS2-based detection of diastereomeric HexSph standards with a C18-sphingosine (d18:1). β-GlcSph elutes first and separates from β-GalSph with Δt = 0.12 ± 0.005 min. After these compounds, α-GalSph elutes with Δt = 0.07 ± 0.005 min significantly behind β-GalSph.
DISCUSSION
Lipidomics is a fast growing field of interest impacting more and more medical research. Often all-in-one analysis is demanded and high throughput methods like direct infusion into high resolution mass spectrometers with MSall-scans and subsequent data processing software try to fulfill these requests (75). These MS-only techniques, however do not distinguish stereoisomeric compounds with qualitatively identical fragmentation behaviors. Separation and quantification of such stereoisomers therefore requires combination of MS with orthogonal methods, such as chromatography or ion mobility. LC, especially, is a widespread technique, often coupled to MS to increase specificity and sensitivity of detection.
Here, we present a HILIC-based method, which enables the separation of α- and β-anomers of GlcCer and GalCer comprising identical Cer anchors, which was not possible with reversed phase LC. HexCers are amphipathic molecules with their structural differences buried in the polar head group, but not the hydrophobic anchor, the latter interacting with the stationary phase in reversed phase LC and the former in HILIC. Coupling to MS2 allows identification of differences in Cer anchor composition to unambiguously ascribe NS-, AS-, NP-, and AP-type HexCer-derived HILIC-peaks of biological samples to either GlcCer or GalCer with either α- or β-glycosidic linkage. The method can be used to analyze complex biological lipid extracts, as demonstrated by the application to various mouse organs and bacteria.
The correct assignment of peaks was supported by measuring a selective increase or decrease of β-GlcCer over β-GalCer in various tissues with GBA2 or UGCG deficiencies, respectively. Loss of GBA2 activity leads to the accumulation of the substrate β-GlcCer. In contrast, the lack of UGCG activity either in hepatocytes or in renal tubular cells omitted corresponding production of β-GlcCer, thereby reducing the overall β-GlcCer concentration of liver or kidney, as expected (11, 16, 67, 68, 76). Further confirmation was obtained by detecting a selective increase of β-GalCer over β-GlcCer in organs lacking activity of either CST or Gb3S, for both of which β-GalCer is the substrate to produce either sulfatide SM4s or galabiaosylceramide, respectively (16, 55, 77).
Interestingly, however, the levels of not all β-GlcCer species went up with Gba2-deficiency and the levels of AS-type β-GlcCers remained unchanged. There are two possible explanations, which are: i) GBA2 may not be expressed in cells that express the AS-type; or ii) the AS-type β-GlcCer resides in lipid layers with no access to the cytosolic activity of GBA2. Whereas loss of UGCG activity in renal tubular epithelial cells caused a decrease of NS- and AS-type β-GlcCers in kidney, the hepatocyte-specific loss of UGCG activity, causing NS-types to go down, even led to an increase of minor AS-type β-GlcCers. Because tissues are multi cell-type structures, the loss of enzyme activity in one specific cell type will always lead only to a decrease of a product and not to complete disappearance, if this product is synthesized by several cell types of the respective organ. An increase of a subspecies may imply either an increased relative abundance of the corresponding synthesizing cell type in mutant tissue or a reactive increased production or decreased catabolism of these compounds within the corresponding cells.
The method on top separates AS-type cerebrosides containing the α-hydroxy group of the acyl chain either in 2R- or 2S-configuration. Both configurations may appear in biological samples and AS-type cerebrosides of the brain were reported to contain basically the 2R-configuration (71), which is confirmed with this HILIC-MS2 method.
Screening different mouse tissues with this method, we further confirmed high abundance of cerebrosides in brain and kidney, whereas most other tissues, including liver and spleen, are dominated by the presence of glucocerebrosides. Two exceptions were identified: auricles and, especially, lymph nodes are relatively abundant in cerebrosides, as compared with glucocerebrosides, and further investigations need to address from which cell types they originate.
As this method delivers data for individual species, it allows detection of differences in Cer anchor compositions between cerebrosides and glucocerebrosides or following of changes in these compositions upon aging, activation, or disease progression. Here, we plotted the relative acyl chain length distributions of cerebrosides against those of glucocerebrosides found in WT mouse tissues. As expected, brain cerebrosides are rich in nervonic acid, a major component of myelin sheaths (60, 78, 79). In contrast, the small amount of brain glucocerebrosides is synthesized mainly with stearic acid. This points to neuronal cell origin, as these cells convert this compound to complex gangliosides, such as GM1a, GD1a, GD1b, or GT1b, all of which are known to basically contain stearic acid in their lipid anchor (80, 81). Likewise, nervonic acid was the major fatty acid incorporated into cerebrosides of colon, small intestine, thymus, and lymph nodes, whereas glucocerebrosides of these organs were enriched with the shorter palmitic acid. In summary, this points to different cell types or differentiation stages of cells forming either cerebrosides or glucocerebrosides and expressing different pattern of Cer synthases, which remains to be investigated. It may be noted that this investigation did not include HexCers with other sphingoid bases than C18-sphingosine and C18-phytosphingosine or acyl chains shorter than C16 or longer than C26, excluding, thereby, compounds such as those with C20-sphingosine that are described for aging brain (82) or renal papillae (65) or the well-known sphingolipids with ultra-long acyl chains (>C26) specific for epidermis or male germ cells (83).
In contrast to the afore-discussed cerebrosides and glucocerebrosides, iNKT cells are activated when recognizing GalCer with an α-glycosidic linkage presented on the cell surface receptor, CD1d, of antigen-presenting cells (41, 84). One natural source of this compound in contact with the human body is B. fragilis, a bacterial member of the human gut microbiome (46). Here, we show detection of α-GalCers from B. fragilis by HILIC-MS2 with unique retention times, different from the β-glycosidic mammalian counterparts. How many other bacteria of the human gut microbiome may also express these immune stimulatory compounds has to be elucidated, as well as their potential role in colitis, when the intestinal barrier gets leaky for bacteria or in sepsis. To elucidate the use of HILIC-MS2 for screening of bacteria that express α-GalCer, we analyzed an initial set of 10 bacteria of the human gut microbiome. At least two other bacteria were detected with quantifiable amounts of these compounds and another three strains delivered signals corresponding to α-GalCer, which were above the lower detection limit. Although concentration of α-GalCer in all five of these strains was at least 100-fold lower than in B. fragilis, the results show that there might be a remarkably high number of bacteria present in our gut, which is capable of synthesizing the immunogenic α-anomeric variant of GalCer. These results underline the power of the method to screen for such compounds and further support the idea that α-GalCer is a natural CD1d-ligand for iNKT cell activation.
Very low amounts of endogenously produced α-GalCer (and eventually α-GlcCer) have also been discussed to contribute to successive iNKT cell maturation (39). In this context, endolysosomal catabolism of α-GalCer has been discussed to regulate levels of α-GalCer on antigen-presenting cells. Cerebrosidase and GBA are specific for β-glycosylceramides and seem not to turn over α-GalCer. Catabolism rather appears to be initiated by acidic ceramidase releasing the lyso-compound, α-GalSph, which, in contrast to α-GalCer, is a substrate for α-galactosidase. Either pharmacological inhibition of these enzymes or defective activity in Fabry disease thereby induces a pro-inflammatory phenotype of iNKT cells (39, 85–87). Under pathological conditions of Krabbe disease, metachromatic leukodystrophy, or Gaucher disease, when either cerebrosidase or GBA is dysfunctional, significant amounts of β-GalSph or β-GlcSph accumulate due to turnover by acidic ceramidase, and these compounds contribute to the pathology of the disease (25, 26, 88, 89). Here, significantly different retention times were achieved for the three types of HexSphs, making the HILIC-MS2 method a tool to identify the type of HexSph appearing and to study concentration changes of these cell-toxic or immune-stimulatory compounds.
In summary, the HILIC method presented here in combination with detection by MS2 is useful to selectively identify and quantify small amounts of the diastereomers, β-GlcCer, β-GalCer, α-GalCer, and corresponding lyso-compounds from complex biological lipid extracts, without any prior derivatization step.
Supplementary Material
Acknowledgments
The authors thank Benita von Tümpling-Radosta for excellent technical assistance and Sinan Demirci for his support during his Bachelor thesis.
Footnotes
Abbreviations:
- AP
- N-α-hydroxy acyl phytosphingosine
- AS
- N-α-hydroxy acyl sphingosine
- BS
- N-β-hydroxy acyl sphingosine
- Cer
- ceramide
- CST
- cerebroside sulfotransferase (Gal3st1)
- Fa2h
- fatty acid 2-hydroxylase
- GALC
- galactosylceramidase
- GalCer
- galactosylceramide
- GalSph
- galactosylsphingosine
- GBA
- glucosylceramidase
- Gba2
- β-glucosylceramidase 2
- Gb3S
- globotriaosylceramide synthase
- GlcCer
- glucosylceramide
- GlcSph
- glucosylsphingosine
- GSL
- glycosphingolipid
- HexCer
- hexosylceramide
- HexSph
- hexosylsphingosine
- HILIC
- hydrophilic interaction chromatography
- HPTLC
- high-performance TLC
- iNKT
- invariant natural killer T
- NP
- N-nonhydroxy acyl phytosphingosine
- NS
- N-nonhydroxy acyl sphingosine
- UGCG
- glucosylceramide synthase
- Ugt8a
- β-galactosylceramide synthase
- CSTf/fPax8Cre
- deletion of cerebroside sulfotransferase by Cre (cyclization recombinase) under control of the paired box 8 (Pax8)-promotor in renal tubular epithelial cells
- (Hex)Cer(d18:1/24:1)
- (hexosyl)ceramide with a C18-sphingosine (d18:1) and nervonic acid (24:1) (i.e., a N-nonhydroxy acyl sphingosine-type hexosylceramide)
- Ugcgf/fAlbCre or Ugcgf/fPax8Cre
- deletion of Ugcg (UDP-glucose ceramide glucosyltransferase) by Cre (cyclization recombinase) under control of the albumin (Alb)-promotor in hepatocytes or the paired box 8 (Pax8)-promotor in renal tubular epithelial cells
This work was supported by assignments to R.S. within a joint grant (“ZAFH ABIMAS” Zentrum für angewandte Forschung an Hochschulen, Applied Biomedical Mass Spectrometry) from ZO IV by the “Landesstiftung Baden-Württemberg” and the “Europäischer Fond für regionale Entwicklung” to Carsten Hopf and by Bundesministerium für Bildung und Forschung Grant 01DL13008 to R.S.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Schulte S., and Stoffel W.. 1993. Ceramide UDPgalactosyltransferase from myelinating rat brain: purification, cloning, and expression. Proc. Natl. Acad. Sci. USA. 90: 10265–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichikawa S., Nakajo N., Sakiyama H., and Hirabayashi Y.. 1994. A mouse B16 melanoma mutant deficient in glycolipids. Proc. Natl. Acad. Sci. USA. 91: 2703–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolter T., and Sandhoff K.. 1999. Sphingolipids—their metabolic pathways and the pathobiochemistry of neurodegenerative diseases. Angew. Chem. Int. Ed. 38: 1532–1568. [DOI] [PubMed] [Google Scholar]
- 4.Jeckel D., Karrenbauer A., Burger K. N., van Meer G., and Wieland F.. 1992. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell Biol. 117: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futerman A. H., and Pagano R. E.. 1991. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem. J. 280: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger K. N., van der Bijl P., and van Meer G.. 1996. Topology of sphingolipid galactosyltransferases in ER and Golgi: transbilayer movement of monohexosyl sphingolipids is required for higher glycosphingolipid biosynthesis. J. Cell Biol. 133: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makita A., and Taniguchi N.. 1985. Glycosphingolipids. In Glycolipids. H. Wiegandt, editor. Elsevier, Amsterdam. 1–99. [Google Scholar]
- 8.Yamashita T., Wada R., Sasaki T., Deng C., Bierfreund U., Sandhoff K., and Proia R. L.. 1999. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA. 96: 9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennemann R., Sandhoff R., Wang S., Kiss E., Gretz N., Zuliani C., Martin-Villalba A., Jager R., Schorle H., Kenzelmann M., et al. 2005. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc. Natl. Acad. Sci. USA. 102: 12459–12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennemann R., Sandhoff R., Langbein L., Kaden S., Rothermel U., Gallala H., Sandhoff K., Wiegandt H., and Grone H. J.. 2007. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J. Biol. Chem. 282: 3083–3094. [DOI] [PubMed] [Google Scholar]
- 11.Jennemann R., Rothermel U., Wang S., Sandhoff R., Kaden S., Out R., van Berkel T. J., Aerts J. M., Ghauharali K., Sticht C., et al. 2010. Hepatic glycosphingolipid deficiency and liver function in mice. Hepatology. 51: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 12.Jennemann R., Kaden S., Sandhoff R., Nordstrom V., Wang S., Volz M., Robine S., Amen N., Rothermel U., Wiegandt H., et al. 2012. Glycosphingolipids are essential for intestinal endocytic function. J. Biol. Chem. 287: 32598–32616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amen N., Mathow D., Rabionet M., Sandhoff R., Langbein L., Gretz N., Jackel C., Grone H. J., and Jennemann R.. 2013. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Hum. Mol. Genet. 22: 4164–4179. [DOI] [PubMed] [Google Scholar]
- 14.Nordström V., Willershauser M., Herzer S., Rozman J., von Bohlen Und Halbach O., Meldner S., Rothermel U., Kaden S., Roth F. C., Waldeck C., et al. 2013. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLoS Biol. 11: e1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabionet M., Bayerle A., Jennemann R., Heid H., Fuchser J., Marsching C., Porubsky S., Bolenz C., Guillou F., Grone H. J., et al. 2015. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 24: 4792–4808. [DOI] [PubMed] [Google Scholar]
- 16.Stettner P., Bourgeois S., Marsching C., Traykova-Brauch M., Porubsky S., Nordström V., Hopf C., Koesters R., Sandhoff R., Wiegandt H., et al. 2013. Sulfatides are required for renal adaptation to chronic metabolic acidosis. Proc. Natl. Acad. Sci. USA. 110: 9998–10003. [Erratum. 2013. Proc. Natl. Acad. Sci. USA. 110: 14813.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T., Allende M. L., Kalkofen D. N., Werth N., Sandhoff K., and Proia R. L.. 2005. Conditional LoxP-flanked glucosylceramide synthase allele controlling glycosphingolipid synthesis. Genesis. 43: 175–180. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S., Endo S., Oshima E., Hoshi T., Higashi H., Yamada K., Tohyama K., Yamashita T., and Hirabayashi Y.. 2010. Glycosphingolipid synthesis in cerebellar Purkinje neurons: roles in myelin formation and axonal homeostasis. Glia. 58: 1197–1207. [DOI] [PubMed] [Google Scholar]
- 19.Seito N., Yamashita T., Tsukuda Y., Matsui Y., Urita A., Onodera T., Mizutani T., Haga H., Fujitani N., Shinohara Y., et al. 2012. Interruption of glycosphingolipid synthesis enhances osteoarthritis development in mice. Arthritis Rheum. 64: 2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosio A., Binczek E., and Stoffel W.. 1996. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA. 93: 13280–13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coetzee T., Fujita N., Dupree J., Shi R., Blight A., Suzuki K., and Popko B.. 1996. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 86: 209–219. [DOI] [PubMed] [Google Scholar]
- 22.Sandhoff K. 2013. Metabolic and cellular bases of sphingolipidoses. Biochem. Soc. Trans. 41: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 23.Sandhoff K. 2016. Neuronal sphingolipidoses: membrane lipids and sphingolipid activator proteins regulate lysosomal sphingolipid catabolism. Biochimie. 130: 146–151. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E., and Grabowski G. A.. 2001. Gaucher disease. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, et al., editors. McGraw-Hill, New York: 3635–3668. [Google Scholar]
- 25.Grabowski G. A., Petsko G. A., and Kolodny E. H.. 2017. Gaucher disease. In The Online Metabolic and Molecular Bases of Inherited Disease. Valle D., Beaudet A. L., Vogelstein B., et al., editors. McGraw-Hill Education, New York. [Google Scholar]
- 26.Wenger D. A., Escolar M., Luzi P., and Rafi M. A.. 2017. Krabbe disease (globoid cell leukodystrophy) In The Online Metabolic and Molecular Bases of Inherited Disease. Valle D., Beaudet A. L., Vogelstein B., et al., editors. McGraw-Hill Education, New York. [Google Scholar]
- 27.Wenger D. A., Suzuki K., Suzuki Y., and Suzuki K.. 2001. Galactosylceramide lipidosis. Globoid cell leukodystrophy (Krabbe disease) In The Metabolic and Molecular Bases of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., et al., editors. McGraw-Hill, New York: 3669–3694. [Google Scholar]
- 28.Sidransky E. 2004. Gaucher disease: complexity in a “simple” disorder. Mol. Genet. Metab. 83: 6–15. [DOI] [PubMed] [Google Scholar]
- 29.Thomas A. S., Mehta A., and Hughes D. A.. 2014. Gaucher disease: haematological presentations and complications. Br. J. Haematol. 165: 427–440. [DOI] [PubMed] [Google Scholar]
- 30.Smid B. E., Ferraz M. J., Verhoek M., Mirzaian M., Wisse P., Overkleeft H. S., Hollak C. E., and Aerts J. M.. 2016. Biochemical response to substrate reduction therapy versus enzyme replacement therapy in Gaucher disease type 1 patients. Orphanet J. Rare Dis. 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deegan P., Fernandez-Sasso D., Giraldo P., Lau H., Panahloo Z., and Zimran A.. Treatment patterns from 647 patients with Gaucher disease: an analysis from the Gaucher Outcome Survey. Blood Cells Mol. Dis. Epub ahead of print. October 20, 2016; doi:10.1016/j.bcmd.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Futerman A. H., and Platt F. M.. 2017. The metabolism of glucocerebrosides - from 1965 to the present. Mol. Genet. Metab. 120: 22–26. [DOI] [PubMed] [Google Scholar]
- 33.Ilan Y., Elstein D., and Zimran A.. 2009. Glucocerebroside: an evolutionary advantage for patients with Gaucher disease and a new immunomodulatory agent. Immunol. Cell Biol. 87: 514–524. [DOI] [PubMed] [Google Scholar]
- 34.Lucci A., Cho W. I., Han T. Y., Giuliano A. E., Morton D. L., and Cabot M. C.. 1998. Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Res. 18: 475–480. [PubMed] [Google Scholar]
- 35.Astudillo L., Therville N., Colacios C., Segui B., Andrieu-Abadie N., and Levade T.. 2016. Glucosylceramidases and malignancies in mammals. Biochimie. 125: 267–280. [DOI] [PubMed] [Google Scholar]
- 36.Ueda N. 2017. Sphingolipids in genetic and acquired forms of chronic kidney diseases. Curr. Med. Chem. Epub ahead of print. January 12, 2017; doi:10.2174/0929867324666170112114525. [DOI] [PubMed] [Google Scholar]
- 37.Shayman J. A. 2016. Targeting glycosphingolipid metabolism to treat kidney disease. Nephron. 134: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natoli T. A., Smith L. A., Rogers K. A., Wang B., Komarnitsky S., Budman Y., Belenky A., Bukanov N. O., Dackowski W. R., Husson H., et al. 2010. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 16: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kain L., Webb B., Anderson B. L., Deng S., Holt M., Costanzo A., Zhao M., Self K., Teyton A., Everett C., et al. 2014. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity. 41: 543–554. [Erratum. 2014. Immunity. 41: 867.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natori T., Koezuka Y., and Higa T.. 1993. Agelasphins, novel α-galactosylceramides from the marine sponge Agelas mauritianus. Tetrahedron Lett. 34: 5591–5592. [Google Scholar]
- 41.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278: 1626–1629. [DOI] [PubMed] [Google Scholar]
- 42.Chang D. H., Osman K., Connolly J., Kukreja A., Krasovsky J., Pack M., Hutchinson A., Geller M., Liu N., Annable R., et al. 2005. Sustained expansion of NKT cells and antigen-specific T cells after injection of {alpha}-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 201: 1503–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watarai H., Yamada D., Fujii S., Taniguchi M., and Koseki H.. 2012. Induced pluripotency as a potential path towards iNKT cell-mediated cancer immunotherapy. Int. J. Hematol. 95: 624–631. [DOI] [PubMed] [Google Scholar]
- 44.Fujii S., Shimizu K., Okamoto Y., Kunii N., Nakayama T., Motohashi S., and Taniguchi M.. 2013. NKT cells as an ideal anti-tumor immunotherapeutic. Front. Immunol. 4: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassiri H., Das R., Guan P., Barrett D. M., Brennan P. J., Banerjee P. P., Wiener S. J., Orange J. S., Brenner M. B., Grupp S. A., et al. 2014. iNKT cell cytotoxic responses control T-lymphoma growth in vitro and in vivo. Cancer Immunol. Res. 2: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wieland Brown L. C., Penaranda C., Kashyap P. C., Williams B. B., Clardy J., Kronenberg M., Sonnenburg J. L., Comstock L. E., Bluestone J. A., and Fischbach M. A.. 2013. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 11: e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato M., Muto Y., Tanaka-Bandoh K., Watanabe K., and Ueno K.. 1995. Sphingolipid composition in Bacteroides species. Anaerobe. 1: 135–139. [DOI] [PubMed] [Google Scholar]
- 48.Kean E. L. 1966. Separation of gluco- and galactocerebrosides by means of borate thin-layer chromatography. J. Lipid Res. 7: 449–452. [PubMed] [Google Scholar]
- 49.Kaye E. M., and Ullman M. D.. 1984. Separation and quantitation of perbenzoylated glucocerebroside and galactocerebroside by high-performance liquid chromatography. Anal. Biochem. 138: 380–385. [DOI] [PubMed] [Google Scholar]
- 50.Merrill A. H. Jr., Sullards M. C., Allegood J. C., Kelly S., and Wang E.. 2005. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 36: 207–224. [DOI] [PubMed] [Google Scholar]
- 51.Shaner R. L., Allegood J. C., Park H., Wang E., Kelly S., Haynes C. A., Sullards M. C., and Merrill A. H. Jr. 2009. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 50: 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boutin M., Sun Y., Shacka J. J., and Auray-Blais C.. 2016. Tandem mass spectrometry multiplex analysis of glucosylceramide and galactosylceramide isoforms in brain tissues at different stages of Parkinson disease. Anal. Chem. 88: 1856–1863. [DOI] [PubMed] [Google Scholar]
- 53.Nakajima K., Akiyama H., Tanaka K., Kohyama-Koganeya A., Greimel P., and Hirabayashi Y.. 2016. Separation and analysis of mono-glucosylated lipids in brain and skin by hydrophilic interaction chromatography based on carbohydrate and lipid moiety. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1031: 146–153. [DOI] [PubMed] [Google Scholar]
- 54.Yildiz Y., Matern H., Thompson B., Allegood J. C., Warren R. L., Ramirez D. M., Hammer R. E., Hamra F. K., Matern S., and Russell D. W.. 2006. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Invest. 116: 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porubsky S., Speak A. O., Salio M., Jennemann R., Bonrouhi M., Zafarulla R., Singh Y., Dyson J., Luckow B., Lehuen A., et al. 2012. Globosides but not isoglobosides can impact the development of invariant NKT cells and their interaction with dendritic cells. J. Immunol. 189: 3007–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zöller I., Meixner M., Hartmann D., Bussow H., Meyer R., Gieselmann V., and Eckhardt M.. 2008. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 28: 9741–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eley A., Greenwood D., and O’Grady F.. 1985. Comparative growth of Bacteroides species in various anaerobic culture media. J. Med. Microbiol. 19: 195–201. [DOI] [PubMed] [Google Scholar]
- 58.Rabionet M., van der Spoel A. C., Chuang C. C., von Tumpling-Radosta B., Litjens M., Bouwmeester D., Hellbusch C. C., Korner C., Wiegandt H., Gorgas K., et al. 2008. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J. Biol. Chem. 283: 13357–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu F. F., and Turk J.. 2001. Structural determination of glycosphingolipids as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisional-activated dissociation on a triple stage quadrupole instrument. J. Am. Soc. Mass Spectrom. 12: 61–79. [DOI] [PubMed] [Google Scholar]
- 60.Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Gieselmann V., Sandhoff K., and Willecke K.. 2009. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284: 33549–33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouhours D., and Bouhours J. F.. 1985. Developmental changes of the lipidic part of the neutral glycosphingolipids of the rat stomach. J. Biol. Chem. 260: 2172–2177. [PubMed] [Google Scholar]
- 62.Umesaki Y., Suzuki A., Kasama T., Tohyama K., Mutai M., and Yamakawa T.. 1981. Presence of asialo GM1 and glucosylceramide in the intestinal mucosa of mice and induction of fucosyl asialo GM1 by conventionalization of germ-free mice. J. Biochem. 90: 1731–1738. [DOI] [PubMed] [Google Scholar]
- 63.Okabe K., Keenan R. W., and Schmidt G.. 1968. Phytosphingosine groups as quantitatively significant components of the sphingolipids of the mucosa of the small intestines of some mammalian species. Biochem. Biophys. Res. Commun. 31: 137–143. [DOI] [PubMed] [Google Scholar]
- 64.Adams E. P., and Gray G. M.. 1968. The carbohydrate structures of the neutral ceramide glycolipids in kidneys of different mouse strains with special reference to the ceramide dihexosides. Chem. Phys. Lipids. 2: 147–155. [DOI] [PubMed] [Google Scholar]
- 65.Marsching C., Rabionet M., Mathow D., Jennemann R., Kremser C., Porubsky S., Bolenz C., Willecke K., Grone H. J., Hopf C., et al. 2014. Renal sulfatides: sphingoid base-dependent localization and region-specific compensation of CerS2-dysfunction. J. Lipid Res. 55: 2354–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Körschen H. G., Yildiz Y., Raju D. N., Schonauer S., Bonigk W., Jansen V., Kremmer E., Kaupp U. B., and Wachten D.. 2013. The non-lysosomal beta-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi. J. Biol. Chem. 288: 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yildiz Y., Hoffmann P., Vom Dahl S., Breiden B., Sandhoff R., Niederau C., Horwitz M., Karlsson S., Filacamo M., Elstein D., et al. 2013. Functional and genetic characterization of the non-lysosomal glucosylceramidase 2 as a modifier for Gaucher disease. Orphanet J. Rare Dis. 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Carmona M. A., Sandhoff R., Tacke F., Vogt A., Weber S., Canbay A. E., Rogler G., Sauerbruch T., Lammert F., and Yildiz Y.. 2012. Beta-glucosidase 2 knockout mice with increased glucosylceramide show impaired liver regeneration. Liver Int. 32: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 69.Coles L., Hay J. B., and Gray G. M.. 1970. Factors affecting the glycosphingolipid composition of murine tissues. J. Lipid Res. 11: 158–163. [PubMed] [Google Scholar]
- 70.Kishimoto Y., and Radin N. S.. 1963. Occurrence of 2-hydroxy fatty acids in animal tissues. J. Lipid Res. 4: 139–143. [PubMed] [Google Scholar]
- 71.Hammarström S. 1969. Configuration of 2-hydroxy acids from brain cerebrosides determined by gas chromatography. FEBS Lett. 5: 192–195. [DOI] [PubMed] [Google Scholar]
- 72.Eckhardt M., Yaghootfam A., Fewou S. N., Zoller I., and Gieselmann V.. 2005. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 388: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo L., Zhang X., Zhou D., Okunade A. L., and Su X.. 2012. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J. Lipid Res. 53: 1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyagawa E., Azuma R., Suto T., and Yano I.. 1979. Occurrence of free ceramides in Bacteroides fragilis NCTC 9343. J. Biochem. 86: 311–320. [DOI] [PubMed] [Google Scholar]
- 75.Simons B., Kauhanen D., Sylvanne T., Tarasov K., Duchoslav E., and Ekroos K.. 2012. Shotgun lipidomics by sequential precursor ion fragmentation on a hybrid quadrupole time-of-flight mass spectrometer. Metabolites. 2: 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walden C. M., Sandhoff R., Chuang C. C., Yildiz Y., Butters T. D., Dwek R. A., Platt F. M., and van der Spoel A. C.. 2007. Accumulation of glucosylceramide in murine testis, caused by inhibition of beta-glucosidase 2: implications for spermatogenesis. J. Biol. Chem. 282: 32655–32664. [DOI] [PubMed] [Google Scholar]
- 77.Ogawa D., Shikata K., Honke K., Sato S., Matsuda M., Nagase R., Tone A., Okada S., Usui H., Wada J., et al. 2004. Cerebroside sulfotransferase deficiency ameliorates L-selectin-dependent monocyte infiltration in the kidney after ureteral obstruction. J. Biol. Chem. 279: 2085–2090. [DOI] [PubMed] [Google Scholar]
- 78.Kishimoto Y., and Radin N. S.. 1959. Composition of cerebroside acids as a function of age. J. Lipid Res. 1: 79–82. [Google Scholar]
- 79.Radin N. S., and Akahori Y.. 1961. Fatty acids of human brain cerebrosides. J. Lipid Res. 2: 335–341. [Google Scholar]
- 80.Ginkel C., Hartmann D., Vom Dorp K., Zlomuzica A., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Rabionet M., Dere E., et al. 2012. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin associated glycoprotein in oligodendrocytes. J. Biol. Chem. 287: 41888–41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kishimoto Y., and Radin N. S.. 1966. Determination of brain gangliosides by determination of ganglioside stearic acid. J. Lipid Res. 7: 141–145. [PubMed] [Google Scholar]
- 82.Rosenberg A., and Stern N.. 1966. Changes in sphingosine and fatty acid components of the gangliosides in developing rat and human brain. J. Lipid Res. 7: 122–131. [PubMed] [Google Scholar]
- 83.Sandhoff R. 2010. Very long chain sphingolipids: tissue expression, function and synthesis. FEBS Lett. 584: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 84.Brossay L., Chioda M., Burdin N., Koezuka Y., Casorati G., Dellabona P., and Kronenberg M.. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira C. S., Azevedo O., Maia M. L., Dias A. F., Sa-Miranda C., and Macedo M. F.. 2013. Invariant natural killer T cells are phenotypically and functionally altered in Fabry disease. Mol. Genet. Metab. 108: 241–248. [DOI] [PubMed] [Google Scholar]
- 86.Darmoise A., Teneberg S., Bouzonville L., Brady R. O., Beck M., Kaufmann S. H., and Winau F.. 2010. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 33: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., Kim J. H., and Winau F.. 2014. Thinking inside the box: endogenous alpha-anomeric lipid antigens. Immunity. 41: 505–506. [DOI] [PubMed] [Google Scholar]
- 88.Gieselmann V., and Krägeloh-Mann I.. 2017. Metachromatic leukodystrophy. In The Online Metabolic and Molecular Bases of Inherited Disease. Valle D., Beaudet A. L., Vogelstein B., et al., editors. McGraw-Hill Education, New York. [Google Scholar]
- 89.Toda K., Kobayashi T., Goto I., Ohno K., Eto Y., Inui K., and Okada S.. 1990. Lysosulfatide (sulfogalactosylsphingosine) accumulation in tissues from patients with metachromatic leukodystrophy. J. Neurochem. 55: 1585–1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.