Abstract
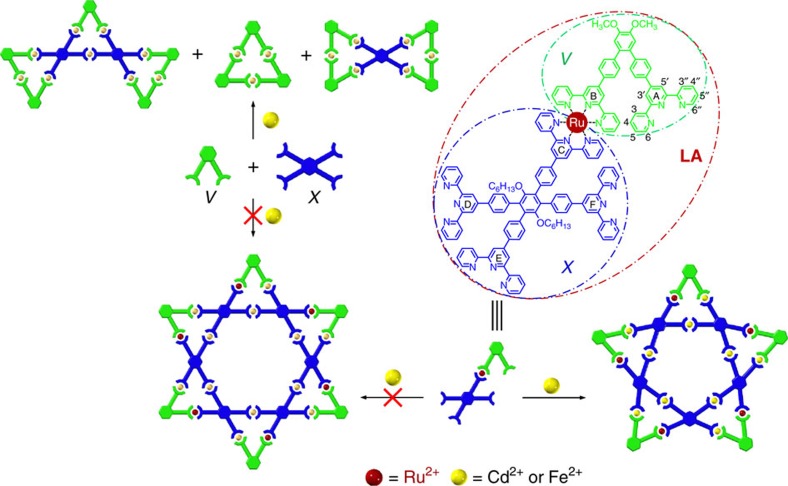
Five- and six-pointed star structures occur frequently in nature as flowers, snow-flakes, leaves and so on. These star-shaped patterns are also frequently used in both functional and artistic man-made architectures. Here following a stepwise synthesis and self-assembly approach, pentagonal and hexagonal metallosupramolecules possessing star-shaped motifs were prepared based on the careful design of metallo-organic ligands (MOLs). In the MOL design and preparation, robust ruthenium–terpyridyl complexes were employed to construct brominated metallo-organic intermediates, followed by a Suzuki coupling reaction to achieve the required ensemble. Ligand LA (VRu2+X, V=bisterpyridine, X=tetraterpyridine, Ru=Ruthenium) was initially used for the self-assembly of an anticipated hexagram upon reaction with Cd2+ or Fe2+; however, unexpected pentagonal structures were formed, that is, [Cd5LA5]30+ and [Fe5LA5]30+. In our redesign, LB [V(Ru2+X)2] was synthesized and treated with 60° V-shaped bisterpyridine (V) and Cd2+ to create hexagonal hexagram [Cd12V3LB3]36+ along with traces of the triangle [Cd3V3]6+. Finally, a pure supramolecular hexagram [Fe12V3LB3]36+ was successfully isolated in a high yield using Fe2+ with a higher assembly temperature.
Star motifs are ubiquitous throughout nature and in man-made architectures, but their molecular level design remains challenging. Here the authors present a stepwise approach to self-assemble pentagonal and hexagonal star-shaped metallo-architectures through the careful design of metallo-organic ligands.
Supramolecular self-assembly and recognition have become particularly appealing in recent years on scales ranging from relatively small and simple structures to complex, highly ordered architectures1. Garnering much of this attention, Lehn2,3, Stang4,5, Stoddart6,7, Fujita8,9,10,11,12,13, Raymond14,15, Newkome16,17,18,19, Leigh20,21,22,23,24,25, Nitschke26,27,28,29 and others30,31,32,33 have designed and constructed numerous unprecedented metallo-architectures with precisely geometry controlled by using predesigned organic ligands through self-assembly procedures. Among the supramolecular assemblies with a variety of topology, mimicking the Star of David, a historical Hebrew symbol known as the Shield of David or Magen David, has proven to be a fascinating challenge in supramolecular chemistry arena. In one of Lehn's pioneering studies, tetra-, penta- and hexameric cyclic helicates were assembled by a linear organic tris(bipyridine) motif with Fe2+ (refs 2, 3). On the basis of hexameric circular helicates, Leigh et al. constructed an interlocking Star of David catenane through the connection of adjacent terminal organic bipyridine residues after self-assembly20,21,22. A similar strategy was also used to create the pentafoil knot23 and Solomon's Knot34, based on pentameric and tetrameric cyclic helicates, respectively.
In the field of terpyridine (tpy)-based supramolecular chemistry35,36, precise control over the shape and size of the architectures is regarded as a tremendous challenge. Recent noteworthy accomplishments with tpy-based architectures include Sierpinski hexagonal gasket, spoked wheel, ring-in-ring and cage-like macromolecules16,17,18,19,37,38,39,40,41,42,43. Because of the ligand flexibility and deviation from the coordination geometry predicated by thermodynamic or kinetic stabilities38 of several possible coordinated isomeric products, the assembly might produce a mixture and/or polymer, especially when targeting large and discrete assemblies (molecular weight>10,000 Da). Therefore, appropriate ligand design and synthetic strategy play critical roles in the self-assembly process regarding the shape, size and complexity of the ultimate metallosupramolecule. To date, the multiple bipyridine-based one-step, pentagonal and hexagonal molecular assemblies have been reported in reasonable yields and could be shape-exchanged by using different counterions, such as Cl− and SO42+ (refs 20, 21, 22). The prospect of using octahedral <tpy–M2+–tpy> connectivity is considered to simplify the structural design and further, to introduce functionality for potential applications in sensing44, magnetics45, biomedicine46,47, photonics48,49 and catalysis15,30,50,51.
Herein, we present the synthesis and self-assembly of planar supramolecular pentagram and hexagram structures, using precisely predesigned tpy-based metallo-organic ligands (MOLs).
Results
The initial attempt to construct a supramolecular hexagram (Fig. 1) was performed by direct self-assembly of 1,2-bisterpyridine (V) and 1,2,4,5-tetrakisterpyridine (X) ligands, with metal ions in a precise stoichiometric ratio of 1:1:3 via one-pot procedure (Supplementary Figs 1 and 20–26). Unfortunately, direct self-assembly of V and X with Cd2+ cations generated a thermodynamically stable small metallo-triangle [Cd3V3]6+ and an unidentified metallo-polymer. Note that with Cd2+, however, the resultant complex could not be separated by column chromatography because of its structural lability. Using Fe2+ cations with strong coordination, we observed the formation of a mixture of small triangle, bowtie-like double-triangle and triple-triangle along with a small amount of unidentified metallo-polymer due to the self-sorting of polyterpyridines with metal ions (Fig. 2; Supplementary Information). With careful flash column chromatography, both small triangle [Fe3V3]6+ and bowtie [Fe6V4X]12+ were isolated and fully characterized by nuclear magnetic resonance (NMR) and electro-spray ionization mass spectrometry (ESI-MS; Supplementary Figs 3.1 and 4–16). A trace amount of triple-triangle [Fe9V5X2]18+ was only characterized with ESI-MS (Supplementary Figs 15 and 16) to confirm the proposed structure.
Figure 1. Chemical structures of supramolecular pentagram and hexagram.
Star-shaped pentagram and hexagram contained five or six 1,2-bisterpyridines, five or six 1,2,4,5-tetraterpyridines and fifteen or eighteen metals, respectively.
Figure 2. Illustration of an initial molecular hexagram designation.
Self-assembly of unexpected pentagram and multiple triangular assemblies in the initial design of hexagram.
A stepwise strategy for constructing a MOL was considered to avoid the self-sorting of the same type of organic polyterpyridinyl ligands (Supplementary Fig. 2). In theory, bridging X and V with Ru2+ would block the undesired assembly between the individual polyterpyridine ligands, for example, the dimer and trimer of V. Therefore, a stable <tpy–Ru2+–tpy> complex was chosen as the connector to bridge X and V as MOL for the purpose of creating the desired star-shaped pattern. The supramolecular Star of David was expected to create upon coordination of LA with Fe2+ or Cd2+ cations based on the advantage of high degree of reversible coordination, consequently leading to the desired architecture. This stepwise strategy would prevent the formation of triangle, for example, [Cd3V3]6+, from the self-sorting coordination of V with Cd2+ and metallo-polymers from multiple complexations. In this approach, the self-assembly could be forced into desired six star-shaped motifs. When direct mixing Ru2+ with V and X (that is, V+RuCl3+X≠[VRuX]2+, or LA) only generated a complex mixture (Supplementary Methods) in which the isolation and purification were particularly challenging due to their significant polarity and poor solubility. Subsequently, a coupling on the complex strategy was applied to construct LA based on the remarkable stability of the <tpy–Ru2+–tpy> connector. As shown in Supplementary Fig. 2, a monobromoterpyridineruthenium trichloride adduct was attached to one tpy of X to generate a key intermediate, L-Br (Supplementary Figs 17 and 27–32). The Suzuki cross-coupling of L-Br with tpy–C6H4–B(OH)2 introduced another free tpy to form LA (Supplementary Figs 18 and 33–36), with <tpy–Ru2+–tpy> connector (45%) after column chromatography (Al2O3, CHCl3/MeOH).
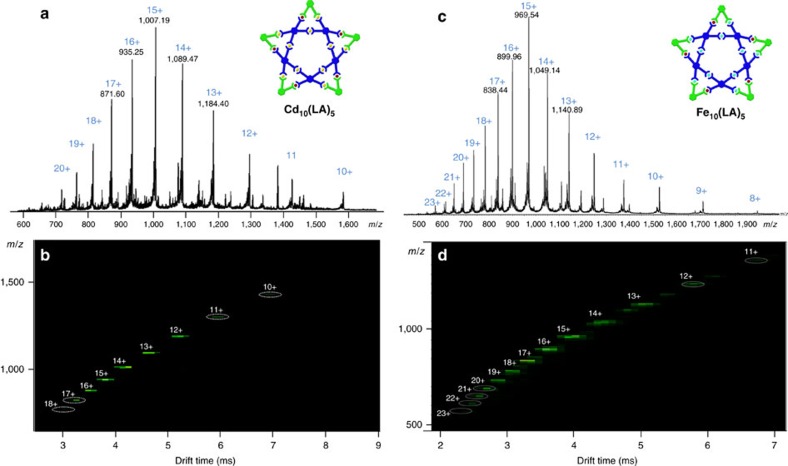
The asymmetric metallo-ligand LA containing four uncomplexed tpy moieties was mixed with Cd2+ or Fe2+ in 1:2 ratio (Supplementary Methods), respectively. After counterions exchange using excess NH4PF6, the resultant reddish powder was obtained in nearly quantitative yield (>95%). From geometrical and topological points of view, the one-step assembly of supramolecular building block LA with Cd2+ or Fe2+ should result in the highly ordered, six-pointed hexagram (Fig. 2). Surprisingly, the assembled products were shown to be the pure pentagrams with composition of [Cd10LA5]30+·30PF6− and [Fe10LA5]30+·30PF6−, respectively, after careful analysis of its high-resolution ESI-MS data (Fig. 3a,c). Therefore, the selectivity of this monomer LA favoured five-membered supramolecular structures. The mass spectrum of [Cd10LA5]30+ revealed a series of peaks with charge states from 10+ to 18+ derived by the successive loss numbers of PF6− counterions. Similarly, [Fe10LA5]30+ displayed similar peaks with charge states from 11+ to 23+. Both were analysed on the basis of the mass-to-charge ratios of molecular weights of 17,282 and 16,716 Da for [Cd10LA5]30+ and [Fe10LA5]30+, respectively. The isotope patterns for each charge state were in excellent agreement with the corresponding simulated isotope peaks (Supplementary Figs 48–51). Furthermore, the ESI-travelling wave ion mobility-mass spectrometry (ESI-TWIM-MS)37 data (Fig. 3b,d) supported the pentagonal structural assignment by exhibiting a single band with a narrow drift time distribution for each charge state, indicating that no other structural conformers, isomers or other components were detected. The experimental collision cross-section calculated from ion mobility was also compared to theoretical CCS obtained from molecular modelling for additional support52,53. The experimental CCS of each charge state of [Cd10LA5]30+ and [Fe10LA5]30+ gave an average CCS at 2,369 and 2,345 Å2, which are slightly smaller than the average theoretical CCS of one hundred energy-minimized modelling structures at 2,458 and 2,422 Å2, respectively.
Figure 3. ESI/TWIM-MS spectrum.
(a) ESI-MS and (b) TWIM-MS of pentagonal [Cd10LA5)]30+; (c) ESI-MS and (d) TWIM-MS of pentagonal [Fe10LA5]30+.
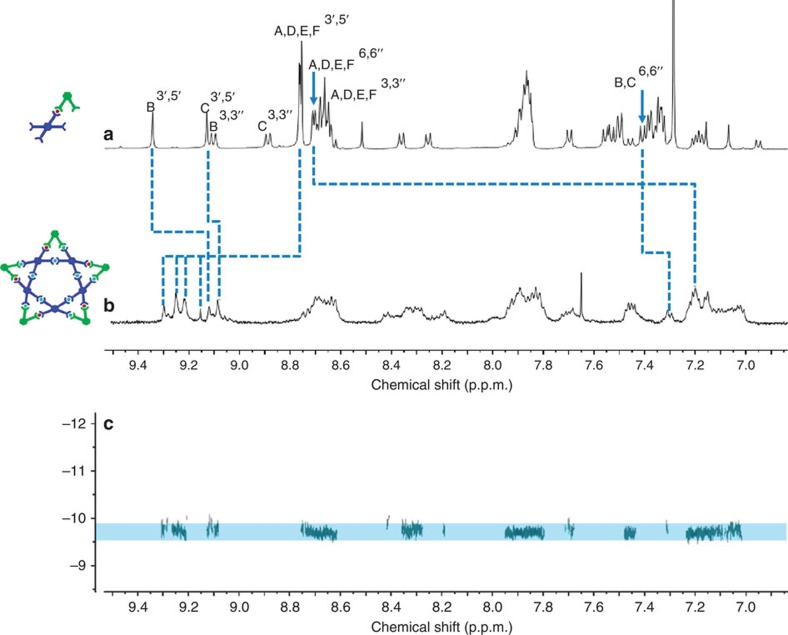
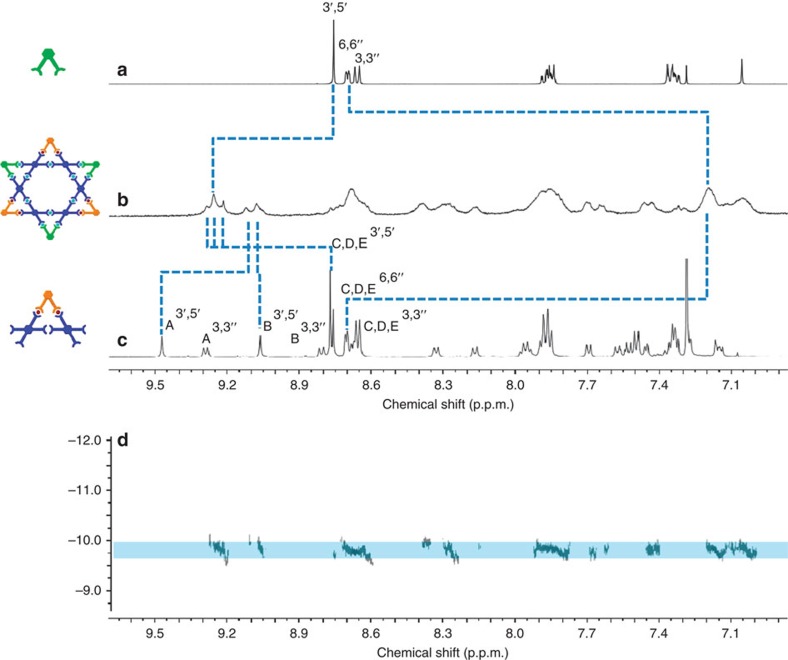
The 1H NMR spectra of [Fe10LA5)30+ in Fig. 4b and [Cd10LA5)30+ (Supplementary Figs 41–44) were complicated due to multiple overlaping polyterpyridine environments. In the 1H NMR spectrum of LA (Fig. 4a), two singlets of [Fe10LA5]30+ ∼9.25 and 9.30 p.p.m. were assigned to the tpyH3′,5′ protons for the <tpy–Ru2+–tpy> moieties, which only showed the small shifts; the tpyH3′,5′ of newly formed <tpy–Fe2+–tpy> linkages exhibited the expected large downfield shifts, as a result of the lower electron density upon complexation. Furthermore, the upfield shift in the tpyH6,6″ protons is attributed to the shielding effect of the metal centres. These observed chemical shifts and ultraviolet–visible (UV–vis) measurements (Supplementary Fig. 56) indicated the expected <tpy–Fe2+–tpy> linkages were formed. UV–vis spectroscopies of [Cd10LA5]30+, [Fe10LA5]30+ and [Fe12V3(LB)3]36+ in CH3CN (for PF6– as the anions) showed the expected absorbance patterns at ca. 495 and 575 nm for the tpy–Ru2+–tpy and tpy–Fe2+–tpy units, respectively. Moreover, six signals of tpyH3′,5′ were consistent with the desired structure. Peak assignments were confirmed using two-dimensional 2D-NOESY NMR spectra (Supplementary Figure 47) NMR spectra. The diffusion-ordered NMR spectroscopy (DOSY) was further applied to confirm the structure, which showed [Fe10LA5]30+ as a single component with the diffusion coefficients of 1.99 × 10−10 m2 s−1 at 298 K (Fig. 4c). The experimental hydrodynamic radius (rH=2.99 nm) was calculated and it agrees well with the diameter of molecular modelling.
Figure 4. 1H NMR spectrum.
(a) LA and (b) molecular pentagram [Fe10LA5)]; (c) DOSY of [Fe10LA5)] (500 MHz, 25 °C, CDCl3 for ligands and CD3CN for supramolecule).
The formation of pentagram instead of hexagram is most likely attributed to the slight flexibility of the extended terpyridines and deviation from typical coordination with the metal ions54. The different side lengths of each individual dinuclear triangle in the pentagram may play a vital role in determination of the formation of the five- versus six-pointed star's supramolecular architecture. LA reacted as an asymmetrical piece in which the edge lengths (<tpy–Ru–tpy>, <tpy–Cd–tpy> or <tpy–Fe–tpy>) in each identical triangle (Fig. 2) should have a slight variation. This is confirmed through the molecular modelling or reported crystal structures (18.004, 18.672 and 17.821 Å for <tpy–Ru2+–tpy>, <tpy–Cd2+–tpy> and <tpy–Fe2+–tpy>, respectively; Supplementary Fig. 57). Nevertheless, this subtle difference of lengths between <tpy–Ru2+–tpy> and <tpy–Fe2+–tpy> or <tpy–Cd2+–tpy> could generate different structural outcomes.
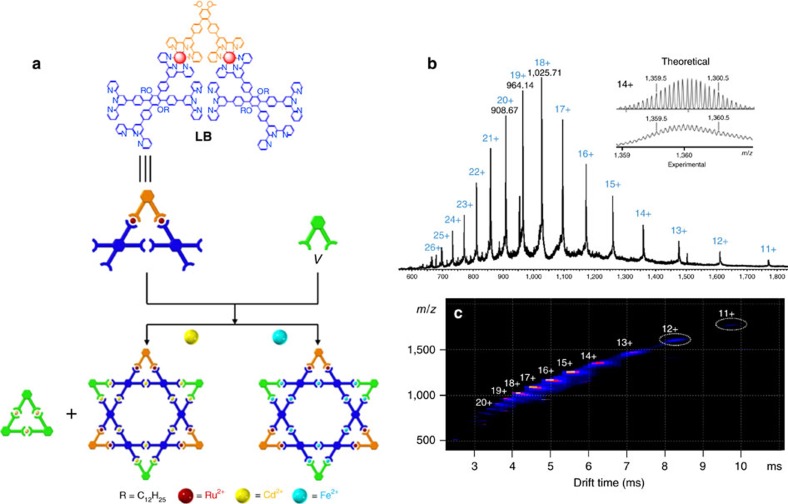
To assemble a six-pointed Star of David architecture, an alternative synthetic strategy was developed based on a new metallo-ligand (LB; Fig. 5a; Supplementary Figs 3,19 and 37–40). When a bistpy-RuCl3 adduct, S5, coordinated with 3.0 equiv. of tetraterpyridine X, a symmetric metallo-ligand LB was isolated via column chromatography (Al2O3, eluting with CHCl3/MeOH) in ca. 31% yield. The first attempt at assembling the supramolecular Star of David was by directly mixing LB and V with Cd2+ in a precise 1:1:4 stoichiometric ratio under conventional conditions. After counterion exchange with PF6−, a pale red precipitate was formed and then washed with deionized water and MeOH to afford (90%) a reddish product. Unfortunately, 1H NMR and DOSY measurements indicated the presence of two distinct products (Supplementary Fig. 45). In addition, this mixture was analysed by high-resolution ESI-MS (Supplementary Figs 52 and 53), which revealed the major assembled product was the desired metallo-hexagram with molecular composition of [Cd12V3LB3]36+·36PF6− and along with a small amount of metallo-triangle [Cd3V3]6+·6PF6− derived from the assembly of V with Cd2+. Although the self-assembly of LB, V with Cd2+ had been optimized to avoid the metallo-triangular formation of self-sorting in terms of different temperatures, solvents, counterions and starting material ratio, it was difficult to prevent the formation of small triangle. Moreover, we were not able to remove the small triangle from the Star of David mixture through conventional column chromatography because of the weak coordination of <tpy–Cd2+–tpy> during separation process.
Figure 5. Self-assembly of supramolecular hexagram [Fe12V3LB3]36+.
(a) Schematic illustration of synthesizing molecular Star of David by using metallo-ligand LB, V and metals. (b) ESI-MS and (c) TWIM-MS of metallo-hexagram.
Considering the greater stability of <tpy–Fe2+–tpy> coordination comparison to <tpy–Cd2+–tpy>, the combination of LB, V and Fe2+ in a stoichiometric ratio of 1:1:4 at a higher temperature condition (∼140 °C) in an ethylene glycol solution overnight was performed. Following the processes of the anion exchange with PF6−, rinse with distilled water and MeOH, dry in vacuo for 12 h; the product was left to yielding a reddish powder. With a short-path column chromatography (MeCN/H2O/NaNO3), the metallo-hexagram [Fe12V3LB3] was successfully isolated as the major product, along with a small fraction (∼5%) of metallo-triangle [Fe3V3], which is identical to the one isolated in our initial self-assembly attempt (Fig. 2). The high-resolution ESI-MS spectrum (Fig. 5b; Supplementary Figs 54 and 55) demonstrated a series of adequate peaks with charge states from 11+ to 25+ identified by the loss of PF6− counterions. All of the peaks' isotopic patterns corresponded to the calculated isotopic distributions of metallo-pentagram, [Fe12V3LB3]36+·36PF6− (molecular weight=21,069 Da). In ESI-TWIM-MS (Fig. 5c), a series of signals with narrow drift times were observed, indicating that a discrete and rigid molecular metallo-pentagram was assembled. The average experimental CCS was calculated as 2,843 Å2, which is close to the average theoretical CCS at 2,980 Å2 from molecular modelling.
Discussion
The 1H NMR spectra revealed the broad peaks for polyaromatic regions (Supplementary Figs 46 and 47); however, the specific chemical shifts upon complexation could be distinguished when compared with both precursors, that is, V and LB. The 1H NMR spectrum of molecular metallo-pentagram showed two sets of characteristic shifts for tpyH3′,5′. The first set of peaks is for the coordination of <tpy–Fe2+–tpy>, which shows a downfield shift to ca. 9.25 p.p.m. The second set was assigned for the original <tpy–Ru2+–tpy> at ca. 9.1 p.p.m. The proton of uncomplexed free tpyH6,6″ in V and LB shifted upfield to 7.2 p.p.m. from ca. 8.7 p.p.m. after self-assembly due to the electron shielding effects. In the nonaromatic region, there is only one set of signals, which can be assigned to the –OMe moieties at ca. 4.0 p.p.m. and alkyoxy chain for –OCH2– at 3.5 p.p.m. The full assignment of ligands and assembled architectures were verified and confirmed by 2D-COSY and 2D-NOESY experiments. DOSY unambiguously showed [Fe12V3LB3] as a single component with the diffusion coefficients of 1.58 × 10−10 m2 s−1 at 298 K (Fig. 6d). The experimental hydrodynamic radius (rH=3.77 nm) was approximately consistent with the theoretical molecular diameter.
Figure 6. Comparison of 1H NMR spectrum.
(a) bisterpyridine ligand (500 MHz, 25 °C, CDCl3), (b) molecular Star of David [Fe12V3LB3] (500 MHz, 25 °C, CD3CN), (c) metallo-ligand LB (500 MHz, 25 °C, CD3CN) and (d) DOSY of [Fe12V3LB3] (500 MHz, 25 °C, CD3CN).
The anions of PF6−, Cl−, NO3− and BF4− had been used to investigate the structural influence during the anion exchanges by means of high-resolution ESI-MS measurements. We found that there is no molecular structure transformation when ultilizing different anions as the counterions (Supplementary Fig. 59), the structure is stable even in a mixed counterion systems. It may due to the bigger molecular central holes (>3.5 nm) and larger molecular weight (close to 20,000 Da)36.
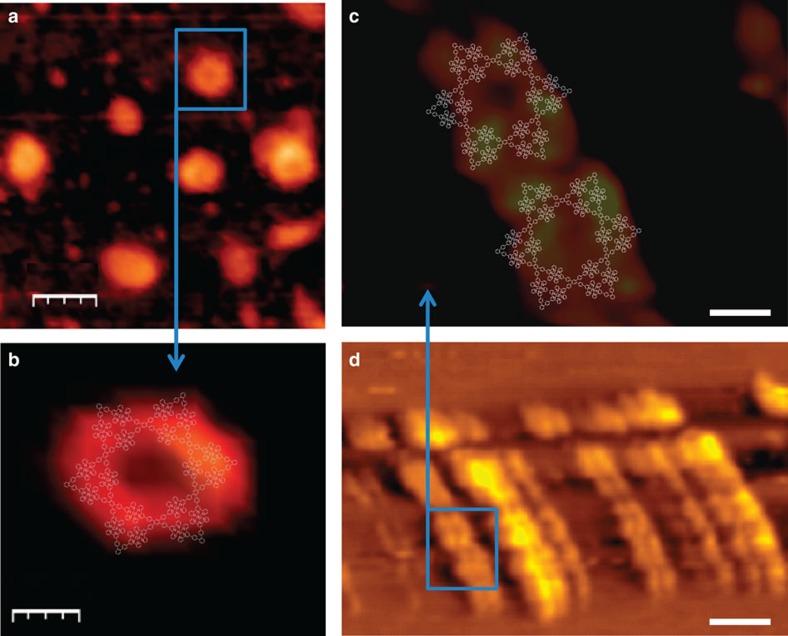
Furthermore, a droplet of hexagram (Fig. 7) or pentagram (Supplementary Fig. 58) solutions in acetonitrile (∼10−7 M) were deposited on the surface of newly cleaved mica or highly ordered pyrolytic graphite (HOPG), for atomic force microscopy (AFM) or room temperature scanning tunnelling microscopy (STM). The AFM image showed individual hexagonal shape and central hole (Fig. 7a,b). In the STM imaging, hexagram supramolecules were assembled into ribbon-like pattern on HOPG (Fig. 7d). The individual molecule (Fig. 7c) was also distinguished with more details than the AFM images. However, the exact shape of the molecules has a little distortion, which is common in room temperature STM imaging.
Figure 7. AFM and STM images of metallo-hexagram.
(a,b) AFM images on mica (scale bar, 40 and 9.3 nm, respectively). (c,d) STM images on HOPG at ambient condition (scale bar, 5 and 25 nm, respectively).
In summary, we have constructed a supramolecular pentagram and a supramolecular hexagram using metal–organic building blocks with strong <tpy–Ru2+–tpy> connectivities through stepwise strategies. Introducing Ru-polyterpyridyl moieties in the self-assembly with the weak coordination metal ions, that is, Cd2+ or Fe2+, successfully blocked the self-sorting of individual organics, and thus achieved the self-assembly of giant discrete star-shaped metallo-architectures. The fractal-like pentagonal and hexagonal architectures were fully characterized by high-resolution ESI-MS, TWIM-MS, NMR, 2D-NOESY and DOSY spectroscopies as well AFM and STM measurements. Using well-established coordination-mediated programmable assemblies and stepwise strategies, we may pave a new avenue towards a new series of ruthenium-based multi-nuclear metallosupramolecules with precisely controlled architecture, increasing complexity and ultimately tailored functionality.
Methods
Sample preparation
All starting materials were purchased from Aldrich and Alfa Aesar, and were used without further purification. Complex [Fe3V3]6+ is consistent with the results published by Newkome36. Column chromatography was conducted by using basic Al2O3 (sinopharm chemical reagents co., Ltd, 200–300 mesh) or SiO2 (Qingdao Haiyang Chemical co., Ltd, 200–300 mesh). The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400-, 500- and 600-MHz NMR spectrometer in CDCl3, DMSO-D6 and CD3CN with tetramethylsilane (TMS) as the inner standard. UV–vis absorption spectra were recorded with an Agilent 8453 UV–vis Spectrometer. Photoluminescence spectra were recorded on a Hitachi 2500 Luminescence spectrometer. ESI mass spectra were recorded with a Waters Synapt G2 tandem mass spectrometer, using solutions of 0.01-mg sample in 1 ml of CHCl3/CH3OH (1:3, v/v) for ligand or 0.5 mg in 1 ml of MeCN, MeOH or MeCN/MeOH (3:1, v/v) for complex.
TWIM-MS
TWIM MS experiments were performed under the following conditions: ESI capillary voltage, 3 kV; sample cone voltage, 30 V; extraction cone voltage, 3.5 V; source temperature 100 °C; desolvation temperature, 100 °C; cone gas flow, 10 l h−1; desolvation gas flow, 700 l h−1 (N2); source gas control, 0 ml min−1; trap gas control, 2 ml min−1; helium cell gas control, 100 ml min−1; ion mobility (IM) cell gas control, 30 ml min−1; sample flow rate, 5 μl min−1; IM travelling wave height, 25 V; and IM travelling wave velocity, 1,000 m s−1. Q was set in rf-only mode to transmit all ions produced by ESI into the tri-wave region for the acquisition of TWIM-MS data.
Molecular modelling
Energy minimization of the macrocycles was conducted with the Materials Studio version 6.0 program, using Anneal and Geometry Optimization tasks in the Materials Studio Forcite module (Accelrys, Inc.).
Microscopy analysis
Transmission electron microscopy was obtained on JEOL 2010. STM: the sample was dissolved in DMF or CH3CN at a concentration of 5.0 mg ml−1. Solution (5 μl) was dropped on HOPG surface. After 30 s, surface was washed slightly with water for three times and totally dried in room temperature in air. The STM images were taken with a PicoPlus SPM system using a PicoScan 3000 Controller. The obtained STM images were processed by WSxM software. AFM: the 40 N m−1 rectangle AFM tip was used to make markers on the surface of the sample with nanoshaving technique. The size of the markers is 10 × 10 μm and the distances between two closest markers are 30 μm. The loading forces used are 6 μN and shaving speed is ∼5 μm s−1.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Additional information
How to cite this article: Jiang, Z. et al. Self-assembly of a supramolecular hexagram and a supramolecular pentagram. Nat. Commun. 8, 15476 doi: 10.1038/ncomms15476 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Methods and Supplementary References
Acknowledgments
This research was supported by the National Natural Science Foundation of China (21274165 for P.W.; 21305098 for X.L.), the Distinguished Professor Research Fund from Central South University of China (for P.W.), the Fundamental Research Funds for the Central Universities from Central South University (2013zzts014 for P.W.), NSF (CHE-1506722 and DMR-1205670 for X.L.; ECCS-1609788 for B.X.; CHE-1151991 for G.R.N.), the Research Corporation for Science Advancement (23224 for X.L.) and the ACS Petroleum Research Fund (55013-UNI3 for X.L.). We gratefully acknowledge Dr Carol D. Shreiner for her professional consultation. We also acknowledge the NMR measurements from The Modern Analysis and Testing Center of CSU.
Footnotes
The authors declare no competing financial interests.
Author contributions All authors have given approval to the final version of the manuscript. Z.J., X.L. and P.W. designed the experiments. Z.J., M.W. and J.Y. completed the synthesis. Z.J., Y.L., M.W., B.S., K.W., X.L., M.S., B.X., D.L., M.C., Y.G., T.Z. and X.Y. carried out the characterization studies. Z.J., Y.L., C.N.M., G.R.N., B.X., X.L. and P.W. analysed the data and wrote the manuscript. All the authors discussed the results and commented on and proofread the manuscript.
References
- Lehn J.-M. Toward self-organization and complex matter. Science 295, 2400–2403 (2002). [DOI] [PubMed] [Google Scholar]
- Hasenknopf B., Lehn J.-M., Kneisel B. O., Baum G. & Fenske D. Self-assembly of a circular double helicate. Angew. Chem. Int. Ed. 35, 1838–1840 (1996). [Google Scholar]
- Hasenknopf B. et al. Self-assembly of tetra- and hexanuclear circular helicates. J. Am. Chem. Soc. 119, 10956–10962 (1997). [Google Scholar]
- Olenyuk B., Whiteford J. A., Fechtenkotter A. & Stang P. J. Self-assembly of nanoscale cuboctahedra by coordination chemistry. Nature 398, 796–799 (1999). [DOI] [PubMed] [Google Scholar]
- Yan X., Cook T. R., Wang P., Huang F. & Stang P. J. Highly emissive platinum(II) metallacages. Nat. Chem. 7, 342–348 (2015). [DOI] [PubMed] [Google Scholar]
- Chichak K. S. et al. Molecular borromean rings. Science 304, 1308–1312 (2004). [DOI] [PubMed] [Google Scholar]
- Pentecost C. D. et al. A molecular solomon link. Angew. Chem. Int. Ed. 46, 218–222 (2007). [DOI] [PubMed] [Google Scholar]
- Sun Q.-F. et al. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation. Science 328, 1144–1147 (2010). [DOI] [PubMed] [Google Scholar]
- Takeda N., Umemoto, K., Yamaguchi K. & Fujita M. A nanometre-sized hexahedral coordination capsule assembled from 24 components. Nature 398, 794–796 (1999). [Google Scholar]
- Fujita M. et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469–471 (1995). [Google Scholar]
- Fujita M., Ibukuro F., Hagihara H. & Ogura K. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature 367, 720–723 (1994). [Google Scholar]
- Fujita M., Fujita N., Ogura K. & Yamaguchi K. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature 400, 52–55 (1999). [Google Scholar]
- Sun Q.-F., Sato S. & Fujita M. An M18L24 stellated cuboctahedron through post-stellation of an M12L24 core. Nat. Chem. 4, 330–333 (2012). [DOI] [PubMed] [Google Scholar]
- Pluth M. D., Bergman R. G. & Raymond K. N. Acid catalysis in basic solution: a supramolecular host promotes orthoformate hydrolysis. Science 316, 85–88 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z. J., Clary K. N., Bergman R. G., Raymond K. N. & Toste F. D. A supramolecular approach to combining enzymatic and transition metal catalysis. Nat. Chem. 5, 100–103 (2013). [DOI] [PubMed] [Google Scholar]
- Newkome G. R. et al. Nanoassembly of a fractal polymer: a molecular ‘Sierpinski hexagonal gasket'. Science 312, 1782–1785 (2006). [DOI] [PubMed] [Google Scholar]
- Wang J.-L. et al. Stoichiometric self-assembly of shape-persistent 2D complexes: a facile route to a symmetric, supramacromolecular spoked wheel. J. Am. Chem. Soc. 133, 11450–11453 (2011). [DOI] [PubMed] [Google Scholar]
- Lu X. et al. Self-assembly of a supramolecular, three-dimensional, spoked, bicycle-like wheel. Angew. Chem. Int. Ed. 52, 7728–7731 (2013). [DOI] [PubMed] [Google Scholar]
- Xie T.-Z. et al. Precise molecular fission and fusion: quantitative self-assembly and chemistry of a metallo-cuboctahedron. Angew. Chem. Int. Ed. 127, 9356–9361 (2015). [DOI] [PubMed] [Google Scholar]
- Leigh D. A., Pritchard R. G. & Stephens A. J. A Star of David catenane. Nat. Chem. 6, 978–982 (2014). [DOI] [PubMed] [Google Scholar]
- Lehn J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 36, 151–160 (2007). [DOI] [PubMed] [Google Scholar]
- Alexandropoulos D. I. et al. Dodecanuclear 3d/4f-metal clusters with a ‘Star of David' topology: single-molecule magnetism and magnetocaloric properties. Chem. Commun. 52, 1693–1696 (2016). [DOI] [PubMed] [Google Scholar]
- Ayme J.-F. et al. A synthetic molecular pentafoil knot. Nat. Chem. 4, 15–20 (2012). [DOI] [PubMed] [Google Scholar]
- Marcos V. et al. Allosteric initiation and regulation of catalysis with a molecular knot. Science 352, 1555–1559 (2016). [DOI] [PubMed] [Google Scholar]
- Danon J. J. et al. Braiding a molecular knot with eight crossings. Science 355, 159–162 (2017). [DOI] [PubMed] [Google Scholar]
- Wood C. S., Ronson T. K., Belenguer A. M., Holstein J. J. & Nitschke J. R. Two-stage directed self-assembly of a cyclic [3]catenane. Nat. Chem. 7, 354–358 (2015). [DOI] [PubMed] [Google Scholar]
- Riddell I. A. et al. Anion-induced reconstitution of a self-assembling system to express a chloride-binding Co10L15 pentagonal prism. Nat. Chem. 4, 751–756 (2012). [DOI] [PubMed] [Google Scholar]
- Mal P., Breiner B., Rissanen K. & Nitschke J. R. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science 324, 1697–1699 (2009). [DOI] [PubMed] [Google Scholar]
- Bolliger J. L., Belenguer A. M. & Nitschke J. R. Enantiopure water-soluble [Fe4L6] cages: Host–guest chemistry and catalytic activity. Angew. Chem. Int. Ed. 52, 7958–7962 (2013). [DOI] [PubMed] [Google Scholar]
- Cullen W., Misuraca M. C., Hunter C. A., Williams N. H. & Ward M. D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 8, 231–236 (2016). [DOI] [PubMed] [Google Scholar]
- Schulze M., Kunz V., Frischmann P. D. & Würthner F. A supramolecular ruthenium macrocycle with high catalytic activity for water oxidation that mechanistically mimics photosystem II. Nat. Chem. 8, 576–583 (2016). [DOI] [PubMed] [Google Scholar]
- Guo J., Mayers P. C., Breault G. A. & Hunter C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2, 218–222 (2010). [DOI] [PubMed] [Google Scholar]
- Li J.-R. & Zhou H.-C. Bridging-ligand-substitution strategy for the preparation of metal–organic polyhedra. Nat. Chem. 2, 893–898 (2010). [DOI] [PubMed] [Google Scholar]
- Beves J. E., Campbell C. J., Leigh D. A. & Pritchard R. G. Tetrameric cyclic double helicates as a scaffold for a molecular solomon link. Angew. Chem. Int. Ed. 52, 6464–6467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild A., Winter A., Schlütter F. & Schubert U. S. Advances in the field of π-conjugated 2,2′:6′,2″-terpyridines. Chem. Soc. Rev. 40, 1459–1511 (2011). [DOI] [PubMed] [Google Scholar]
- Newkome G. R. & Moorefield C. N. From 1→3 dendritic designs to fractal supramacromolecular constructs: understanding the pathway to the Sierpiński gasket. Chem. Soc. Rev. 44, 3954–3967 (2015). [DOI] [PubMed] [Google Scholar]
- Chan Y.-T. et al. Self-assembly and traveling wave ion mobility mass spectrometry analysis of hexacadmium macrocycles. J. Am. Chem. Soc. 131, 16395–16397 (2009). [DOI] [PubMed] [Google Scholar]
- Lu X. et al. Probing a hidden world of molecular self-assembly: Concentration-dependent, three-dimensional supramolecular interconversions. J. Am. Chem. Soc. 136, 18149–18155 (2014). [DOI] [PubMed] [Google Scholar]
- Xie T.-Z. et al. Construction of a highly symmetric nanosphere via a one-pot reaction of a tristerpyridine ligand with Ru(II). J. Am. Chem. Soc. 136, 8165–8168 (2014). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. From trigonal bipyramidal to Platonic solids: self-assembly and self-sorting study of terpyridine-based 3D architectures. J. Am. Chem. Soc. 136, 10499–10507 (2014). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. Hexagon wreaths: Self-assembly of discrete supramolecular fractal architectures using multitopic terpyridine ligands. J. Am. Chem. Soc. 136, 6664–6671 (2014). [DOI] [PubMed] [Google Scholar]
- Fu J.-H., Lee Y.-H., He Y.-J. & Chan Y.-T. Facile self-assembly of metallo-supramolecular ring-in-ring and spiderweb structures using multivalent terpyridine ligands. Angew. Chem. Int. Ed. 54, 6231–6235 (2015). [DOI] [PubMed] [Google Scholar]
- Wang S.-Y. et al. Metallo-supramolecular self-assembly of a multicomponent ditrigon based on complementary terpyridine ligand pairing. J. Am. Chem. Soc. 38, 3651–3654 (2016). [DOI] [PubMed] [Google Scholar]
- Kumar A., Sun S.-S. & Lees A. J. Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 252, 922–939 (2008). [Google Scholar]
- Dul M.-C. et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 254, 2281–2296 (2010). [Google Scholar]
- Fujita D. et al. Protein encapsulation within synthetic molecular hosts. Nat. Commun. 3, 1093 (2012). [DOI] [PubMed] [Google Scholar]
- Grishagin I. V. et al. In vivo anticancer activity of rhomboidal Pt(II) metallacycles. Proc. Natl Acad. Sci. USA 111, 18448–18453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn D. C. et al. Ultrafast optical excitations in supramolecular metallacycles with charge transfer properties. J. Am. Chem. Soc. 132, 1348–1358 (2010). [DOI] [PubMed] [Google Scholar]
- Pollock J. B., Schneider G. L., Cook T. R., Davies A. S. & Stang P. J. Tunable visible light emission of self-assembled rhomboidal metallacycles. J. Am. Chem. Soc. 135, 13676–13679 (2013). [DOI] [PubMed] [Google Scholar]
- Lee S. J., Hu A. & Lin W. The first chiral organometallic triangle for asymmetric catalysis. J. Am. Chem. Soc. 124, 12948–12949 (2002). [DOI] [PubMed] [Google Scholar]
- Wang Q.-Q. et al. Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions. Nat. Chem. 8, 225–230 (2016). [DOI] [PubMed] [Google Scholar]
- Bleiholder C., Dupuis N. F., Wyttenbach T. & Bowers M. T. Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat. Chem. 3, 172–177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker E. R., Anderson S. E., Northrop B. H., Stang P. J. & Bowers M. T. Structures of metallosupramolecular coordination assemblies can be obtained by ion mobility spectrometry−mass spectrometry. J. Am. Chem. Soc. 132, 13486–13494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzen J. et al. Self-assembly of M24L48 polyhedra based on empirical prediction. Angew. Chem. Int. Ed. 51, 3161–3163 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Methods and Supplementary References
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.