Abstract
Background:
Breast cancer is the commonest cancer in Egyptian females. Nrf2 is involved in oxidative stress while P73 functions in response to DNA damage. This study aimed to assess the role of Nrf2 promoter and P73 G4C14 to A4T14 SNPs in breast cancer in Egypt.
Patients:
Eighty-five female patients with breast tumours (41 malignant, 44 benign) were included. Nrf2 (rs6721961) and p73 (G4A) SNPs were determined by PCR- CTPP assay.
Results:
Genotype frequencies of the Nrf2 promoter SNP were 34.2% and 37.9% for AA in benign and malignant groups respectively, and 43.9% and 40.5% for CC and, 21.9 % and 21.6% for CA. Genotype frequencies for the P73 G4A SNP were 52.9% and 44.7% for GA in benign and malignant groups respectively, and 47.1% and 55.3% for GG.
Discussion:
Nrf2 genotypes in pre - and post-menopausal patients, showed significantly different distributions in the 2 patient groups, the AA genotype being significantly more common in pre-menopausal patients. The P73 G4A SNP showed no relation to age of disease onset.
Conclusion:
The Nrf2 (rs6721961) AA genotype might be related to early breast cancer onset. In contrast the P73 G4A polymorphism showed no relation to either disease risk or age at presentation.
Keywords: Breast cancer, polymorphisms, Nrf2, P73, genotype
Introduction
Breast cancer is the second cancer in mortality affecting mostly females. It is the most frequent malignancy with high morbidity and mortality among women worldwide (Gomes et al.,2012). It accounts for 22.9% of all female cancers worldwide (Ferlay et al., 2010). Mortality in breast cancer patients is mostly caused by metastasis which is related to poor prognosis of breast cancer patients (Fang et al., 2013). In Egypt, breast cancer is the commonest site of cancer in females as it represents (38.8%) of all female cancers (Ibrahim et al., 2014). Pathogenesis and progression of breast cancer are multifactorial processes affected by genetic, biological, and environmental factors, as well as lifestyle (Porter et al., 2009). Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (hER-2) are the most important prognostic and predictive markers in breast cancer. Triple-negative breast cancer (TN), does not express ER, PR or hER-2. TN breast cancer cases are about 15–26% of all breast cancer cases. Women with TN breast cancer usually show poor prognosis (Pal et al., 2011). Nrf2 is a transcriptional regulator of cytoprotective gene involved in the cellular defence mechanisms against electrophilic and oxidative stress. Activation of Nrf2 defense response has been shown to protect against neurodegenerative diseases, aging, diabetes, photo-oxidative stress, cardiovascular disease, pulmonary fibrosis and cancer (Motohashi and Yamamoto, 2014; Jeong et al.,2006; Zhang, 2006; Kensler et al.,2007; LAU, at al.,2008). Nuclear NRF2 protein plays important roles in the proliferation and/or progression of breast carcinoma, and nuclear NRF2 immunoreactivity is therefore considered a potent prognostic factor in breast cancer patients (Onodera at al.,2013). However, accumulation of Nrf2 in cancer cells has been shown to create an environment helpful for cell growth and protects against oxidative stress, chemotherapeutic agents, and radiotherapy (LAU et al.,2008; Wang et al.,2008; Jarmillo and Zhang, 2013). Nrf2/HO-1 stress response mechanism is a promising target for anticancer treatment which is able to overcome resistance to therapies (Furfaro et al.,2016).
An association between Nrf2 accumulation and adverse outcome of the patients has been reported in the lung (Solis et al.,2010; Inoue et al., 2013), gallbladder (Wang et al., 2010) and ovarian (Konstantinopoulos et al.,2011) carcinomas. These findings suggest that Nrf2 is possibly involved in the growth and/or progression of these carcinomas. Many single nucleotide polymorphisms (SNP) have been identified in the Nrf2 gene. The 3 promoter SNPs rs35652124 (A→G) and rs6721961 (C→A) were found to reduce the transcription activity of Nrf-2, decreasing Nrf-2-dependent gene transcription (Shimoyama et al., 2014).
P73, which is a member of P53 family of transcription factors, has similar cellular activities to those of P53, including binding and transactivation of P53-responsive genes and induction of apoptosis and cell cycle arrest, wherefore; P73 has tumor-suppressive activities (Dotsch et al., 2010). Also P73 plays unique roles in neuronal development and differentiation, metabolic control, and spermatogenesis and maintenance of male fertility (Dotsch et al., 2010; Cutruzzola et al., 2013; Inoue et al., 2014). P73 have several isoforms with different actions. Several SNPs of P73 were found to be related to cancer and could help in predicting cancer risk and chemotherapeutic outcome (Chen et al., 2008). P73 SNPs include G4C14-to-A4T14 which is a functional dinucleotide polymorphism at positions 4 (G→A) and 14 of the 50- untranslated region (50-UTR) of exon 2 of the P73 gene (C→T) (G4C14-to-A4T14, simply designed as G4A hereafter)(Gali et al., 2009; Lee, 2010).
The aim of this work is to assess the role of Nrf-2 promoter and P73 G4C14-to-A4T14 polymorphisms in breast cancer and the potential relation to the onset of the disease.
Materials and Methods
Ethical approval
The project and data forms were approved by the Regional Research and Ethics Committee at the National Cancer Institute (NCI), Cairo University, Egypt. Written informed consent was obtained from all participants involved in our study.
Subjects
This study included 85 female patients with breast tumor, they were admitted at the Department of Surgery in National Cancer Institute, Cairo University, where they were divided into two groups, first group includes 41 patients with malignant breast tumors, their age ranged from 28 to 78 (49.77±12.84), and 44 age matched patients with benign breast tumors.
Methods
Five ml blood sample was collected after overnight fasting and divided into 2 tubes; one for DNA extraction and the other tube for serum separation for biochemical parameters assay using standard laboratory methods. ER, PR and hER-2 were done on 10% formalin-fixed paraffin embedded blocks for each patient. DNA extraction was done by commercially available kit (KAPA Express Extract kit, cat. KK 7101, USA).
Genotyping of SNP of Nrf2 and P73: Genotyping was performed using (PCR-CTPP) PCR with confronting 2 -pair primers assay (Hamajima et al., 2000) using KAPA2G fast PCR kit (KAPA2G Fast PCR kit, cat. # KK 5008, USA) following the manufacturer instructions with some modifications.
1- Nrf2 promoter polymorphism: In this assay, the genotyping of Nrf2 (rs6721961) was performed using confronting pairs of primers (Wang et al., 2010) as shown in table (1) Region containing the polymorphism of Nrf2 was amplified by PCR with those primers with the initial denature at 95°C for 10 min followed by 30 cycles at 95°C for 1 min, at 58°C for 1 min, at 72°C for 1 min and additionally at 72°C for 5 min. PCR products were visualized on a 2% agarose gel with ethidium bromide staining. Genotyping was performed as follows; 282, 113 bp for CC genotype, 282, 205, 113 bp for CA genotype, and 282, 205 bp for AA genotype.
Table 1.
Nrf2 Genotype Distribution in Post and Pre-Menopausal with Malignant Breast Cancer Patients.
| Gene | SNP | Genotype | Post- menopause | Pre- menopause | P |
|---|---|---|---|---|---|
| (%) | (%) | ||||
| Nrf2 | rs6721961 | CC | 41.2 | 40 | < 0.05 |
| CA | 41.2 | 5 | |||
| AA | 17.6 | 55 | |||
| P73 | G4A | GG | 50 | 57.9 | |
| GA | 50 | 42.1 |
2- P73 exon 2 polymorphism: In this assay, the genotyping of P73 G4A polymorphism was performed using confronting pairs of primers(Ibrahim et al., 2014) as shown in table (1). For amplification, an initial denaturation step at 95°C for 10 min was followed by 35 cycles of 95°C for 1 min, 62°C for 45 seconds, and 72°C for 1 min, and a final extension step at 72°C for 5 min. The amplified DNA was visualized on a 2% agarose gel with ethidium bromide staining. 5 The P73 G4A polymorphism was genotyped as a 193 base pair band for the G allele, a 270 base pair band for the A allele, and a 428 base pair common band.
Statistical analysis
Data were assessed with Graph Pad prism software using Student t- test and Z- test. Fisher exact test was used to calculate the significance between genotype distributions in different studied group. Results were expressed as means ± standard deviation and p value less than 0.05 was considered statistically significant.
Results
The genotype frequencies of Nrf2 promoter SNP were 34.2% and 37.9% for AA in benign and malignant groups respectively, 43.9% and 40.5% for CC in benign and malignant groups respectively, 21.9 % and 21.6% for CA in benign and malignant group respectively (Figure 1).
Figure 1.
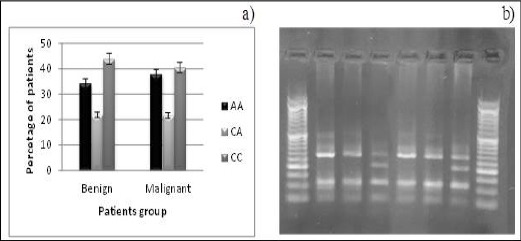
a) Nrf2 Promotor Genotype Distribution among Patients with benign and Malignant Breast Tumors. b) Gel Showing Genotype for Promotor SNP of Nrf2 Gene. Lanes (Left to Right) 3, 6 CA Genotype (282, 205, 113 bp), and Lanes 1, 2, 4, 5 CC Genotype (282, 113 bp).
Genotype frequencies for P73 G4A SNP were 52.94% and 44.73% for GA in benign and malignant groups respectively, 47.06% and 55.26% for GG in benign and malignant group respectively. AA genotype was not found in any case (Figure 2).
Figure 2.
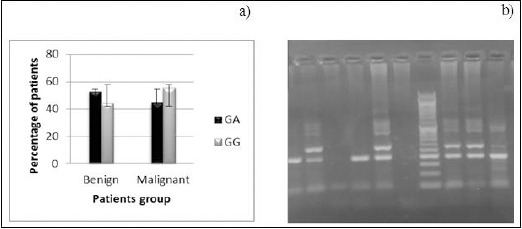
a) P73 G4A Genotype Distribution among Patients with benign and Malignant Breast Tumors. b) Gel Showing Genotype for G4C14-to-A4T14 SNP of P73 Gene. Lanes (Left to Right) 2,5, 8, 9 GA Genotype (428, 270, 193 bp), Lanes 1,4, 10 GG Genotype (428, 193 bp).
Regarding the disease onset, the three Nrf2 genotypes in pre - and post-menopausal patients, showed that the distribution differ significantly in the 2 patient’s groups (p < 0.05). Nrf2 rs6721961 SNP shows different genotype distribution between pre- and post- menopausal breast cancer patients. CA genotype is significantly higher in post-menopausal patients compared to pre-menopausal patients (p < 0.05). P73 G4A SNP showed no significant difference in genotype frequencies or distribution in pre- and post-menopausal breast cancer patients (Table 1). Nrf2 genotype distribution showed significant difference (p < 0.05) in post and pre-menopausal with malignant breast cancer patients.
It also noted that the frequency of AA genotype is significantly lower in post-menopausal breast cancer cases compared to the C allele carrier genotypes (CC & CA) (P< 0.05). On the other 6 hand, the genotype AA is significantly related to premenopausal cases when compared with genotypes CC+CA (p<0.05) (Figure 3).
Figure 3.
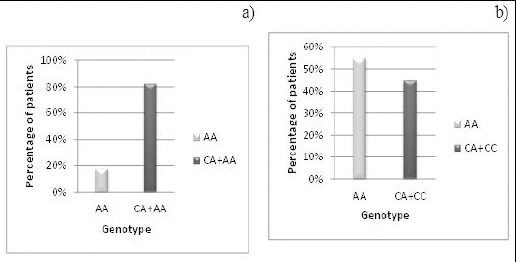
Genotype Distribution of Nrf2 rs6721961 SNP in: a) Post-Menopausal Patients with Malignant Breast Cancer. b) Pre-Menopausal Patients with Malignant Breast Cancer.
Lymph nodes involvement showed no significant association with genotype distribution of both Nrf -2 and P73 G4A. Regarding triple negative breast cancer patients, they were all of homozygous Nrf2 genotype (5o% were CC, 50% were AA). The heterozygous genotype (CA) is absent in this group. TN group shows marked (although statistically non-significant) predominance of the heterozygous genotype of P73 G4A (80% were GA).
Discussion
Breast cancer is one of the most malignant threats against women worldwide. Patient phenotype is closely associated with tumor behaviour, progression and treatment response (von Minckwitz et al., 2010). In Egyptian women, breast cancer is a challenging health problem coming on top of all malignancies with poor outcome compared to international figures (Ferlay et al., 2010). It was shown that age at diagnosis of breast cancer in Arab countries is a decade younger than that in Western countries (El Saghir et al., 2006; El Saghir et al., 2007). Defective Nrf2 signaling pathway may increase cancer susceptibility. Targeting Nrf2 is shown to effectively enhance chemotherapeutic agent in suppression of tumor growth in several animal models (Manandhar et al., 2012). The genetic polymorphism in the human Nrf2gene is considered as one of prognosis markers for cancer therapy (Ishikawa, 2014).
Genetic polymorphisms of Nrf2 on several SNPs (including rs6721961) were associated with breast cancer risk (Hartikainen et al., 2012). In the current study, Nrf2 rs6721961 genotype CC was found in more than 40% of Egyptian patients with benign or malignant breast tumours. Different ethnicities might have different genotype distribution, for example, it is reported that CC is the least common Nrf2 rs6721961 genotype in Japanese people in general (Shimoyama et al., 2014). 7 Nrf2 promoter rs6721961 (C→A) polymorphism was found to reduce the transcription activity of Nrf2, possibly resulting in decreased Nrf2-dependent gene transcription. This promoter polymorphism was shown to have functional significance affecting basal Nrf2 expression and function (Marzec et al., 2007). Nrf2 gene transcription activity was significantly high in rs6721961 C wild-type compared to rs6721961 A variant. Decreased Nrf2 transcription is shown to be related to some types of cancer, and Nrf2 inducers show cancer preventive effect. Thus, the presence of the A allele might explain the occurrence of breast cancer earlier (premenopausal) in the Nrf2 rs6721961 AA patients in the current study. In the current study, TN patients were all homozygous to Nrf-2 (50% were CC, 50% were AA). The heterozygous genotype is absent in this group. Two SNPs at position 4 (G to A) and 14 (C to T) in P73 gene have been identified. Functional analysis implies that this common p73 polymorphism may contribute to cancer development and progression (Li et al., 2006). In the current study, no patient had the AA genotype, which is the least common genotype in P73 G4A polymorphisms (Li et al., 2006). The current study results showed no statistically significant difference in P73 G4A between malignant and benign breast tumor patients groups. This suggests that the A allele might be not related to breast cancer risk. A previous study reported that the A allele isn’t related to gastric cancer risk (Liu et al., 2014). Further study is required to assess a potential effect of ethnicity on P73 G4A relation to breast cancer risk in Egyptians. Such effect was reported in Caucasians (Wang et al., 2012). TN group shows marked (although statistically non-significant) predominance of the A allele carriers genotype of P73 G4A (80% were GA). It was reported that the GG genotype is related to increased risk of TN breast cancer (Zhou and Wu, 2012). This point requires further investigations. 8 In conclusion, Nrf2 (rs6721961) AA genotype might be related to early breast cancer onset. P73 G4A polymorphism shows no relation to both disease risk and disease onset. Therefore, Nrf2 (rs6721961) promoter genotyping could be used to predict the risk of pre-menopausal breast cancer.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Chen J, Li D, Killary A, et al. Polymorphisms of p16, p27, p73, and MDM2 modulate response and survival of pancreatic cancer patients treated with preoperative chemoradiation. Ann Surg Oncol. 2008;16:431–39. doi: 10.1245/s10434-008-0220-8. [DOI] [PubMed] [Google Scholar]
- Cutruzzolà F, Avigliano L, Candi E. p73 keeps metabolic control in balance. Cell Cycle. 2013;13:179–80. doi: 10.4161/cc.27301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotsch V, Bernassola F, Coutandin D, et al. p63 and p73, the Ancestors of p53. Cold Spring Harb Perspect Biol. 2010;2:a004887–a004887. doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Saghir N, Khalil M, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Surg. 2007;5:225–33. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- El Saghir N, Seoud M, Khalil M, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Chen Y, Yu L, et al. Inhibition of breast cancer metastases by a novel inhibitor of TGF Receptor 1. JNCI J. Natl Cancer Inst. 2012;105:47–58. doi: 10.1093/jnci/djs485. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin H, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Furfaro A, Traverso N, Domenicotti C, et al. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med Cell Longev 2016. 2016:1–14. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallì P, Cadoni G, Volante M, et al. A case-control study on the combined effects of p53 and p73 polymorphisms on head and neck cancer risk in an Italian population. BMC Cancer. 2009;9:137. doi: 10.1186/1471-2407-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L, Terra L, Wailemann R, et al. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima N, Saito T, Matsuo K, et al. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res. 2000;91:865–68. doi: 10.1111/j.1349-7006.2000.tb01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen J, Tengstrom M, Kosma V, et al. Genetic polymorphisms and protein expression of NRF2 and sulfiredoxin predict survival outcomes in breast cancer. Cancer Res. 2012;72:5537–46. doi: 10.1158/0008-5472.CAN-12-1474. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Khaled H, Mikhail N, et al. Cancer Incidence in Egypt: Results of the National Population-Based Cancer Registry Program. J Cancer Epidemiol 2014. 2014:1–18. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Suzuki T, Mitsuishi Y, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Science. 2012;103:760–66. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Tomasini R, Rufini A, et al. TAp73 is required for spermatogenesis and the maintenance of male fertility. Proc Natl Acad Sci USA. 2014;111:1843–48. doi: 10.1073/pnas.1323416111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T. Yokohama, Japan: Personalized medicine research institute, NGO personalized medicine and healthcare, Yokohama, Japan RIKEN center for life science technologies; 2014. Mini Review Article. [Google Scholar]
- Jaramillo M, Zhang D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–91. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Jun M, Kong A. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- Kensler T, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos P, Spentzos D, Fountzilas E, et al. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081–89. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- Lau A, Villeneuve N, Sun Z, et al. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–70. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. p73 G4C14 to A4T14 polymorphism is associated with colorectal cancer risk and survival. World J Gastroenterol. 2010;16:4448. doi: 10.3748/wjg.v16.i35.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yao L, Ouyang T, et al. Association of p73 G4C14-to-A4T14 (GC/AT) polymorphism with breast cancer survival. Carcinogenesis. 2006;28:372–77. doi: 10.1093/carcin/bgl153. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong W, Mou Q, et al. Impact of p73 gene polymorphism on cancer susceptibility: a meta-analysis. Int J Clin Exp Pathol. 2014;7:6820–25. [PMC free article] [PubMed] [Google Scholar]
- Manandhar S, Choi B, Jung K, et al. NRF2 inhibition represses ErbB2 signaling in ovarian carcinoma cells: Implications for tumor growth retardation and docetaxel sensitivity. Free Radic Biol Med. 2012;52:1773–85. doi: 10.1016/j.freeradbiomed.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Marzec J, Christie J, Reddy S, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–46. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1–Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:135. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Motohashi H, Takagi K, et al. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr Relat Cancer. 2013;21:241–52. doi: 10.1530/ERC-13-0234. [DOI] [PubMed] [Google Scholar]
- Pal S, Childs B, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2010;125:627–36. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P. Global trends in breast cancer incidence and mortality. Salud Pública Méx. 2009;51:141–46. doi: 10.1590/s0036-36342009000800003. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Mitsuda Y, Tsuruta Y, et al. Polymorphism of Nrf2, an antioxidative gene, is associated with blood pressure and cardiovascular mortality in hemodialysis patients. Nagoya J Med Sci. 2014;11:726–31. doi: 10.7150/ijms.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis L, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–53. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Minckwitz G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2010;125:145–56. doi: 10.1007/s10549-010-1228-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang M, Zhang L, Cai H, et al. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res. 2010;164:99–105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Wang L, Gao R, Yu L. Combined analysis of the association between p73 G4C14-to-A4T14 polymorphisms and cancer risk. Mol Biol Rep. 2011;39:1731–38. doi: 10.1007/s11033-011-0913-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun Z, Villeneuve N, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–43. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wu C. Association of p73 G4C14-A4T14 polymorphisms with genetic susceptibilities to breast cancer: a case–control study. Med Oncol. 2012;29:3216–21. doi: 10.1007/s12032-012-0240-x. [DOI] [PubMed] [Google Scholar]


