Abstract
Fragile-X syndrome is the most common form of inherited mental retardation accompanied by other phenotypes, including macroorchidism. The disorder originates with mutations in the Fmr1 gene coding for the FMRP protein, which, with its paralogs FXR1 and FXR2, constitute a well-conserved family of RNA-binding proteins. Drosophila melanogaster is a good model for the syndrome because it has a unique fragile X-related gene: dFmr1. Recently, in addition to its confirmed role in the miRNA pathway, a function for dFmr1 in the piRNA pathway, operating in Drosophila gonads, has been established. In this review we report a summary of the piRNA pathways occurring in gonads with a special emphasis on the relationship between the piRNA genes and the crystal-Stellate system; we also analyze the roles of dFmr1 in the Drosophila gonads, exploring their genetic and biochemical interactions to reveal some unexpected connections.
Keywords: FMRP/dFmr1, fragile-X syndrome, piRNA pathway, crystal-Stellate system, dFmr1 interactors
1. Introduction
1.1. The Fragile-X Mental Retardation Gene
dFmr1 is the Drosophila homolog of the gene responsible for fragile-X mental retardation syndrome, one of the most frequent inherited causes of human mental retardation. Humans have three homologous genes coding for the Fragile-X Mental Retardation Proteins: Fmr1, FXR1, FXR2, with high sequence similarity; all of them code for RNA-binding proteins [1,2,3]. Mutations in Fmr1, located on the X chromosome and coding for FMRP proteins, are responsible for the syndrome. FMRP and its autosomal paralogs, the Fragile X-Related proteins FXR1P and FXR2P, constitute a conserved, small family of RNA-binding proteins. FMRP has been studied in humans, mice, and Drosophila, principally in relation to its role in the nervous system, at the synapses, as a translational regulator working with different hypothesized mechanisms: (i) by repressing translational initiation [4,5,6]; (ii) by the miRNA pathway binding to the 3′ end of target mRNAs [7,8,9,10,11,12]; and (iii) by direct interaction with translating ribosomes [8,12,13,14,15]. The human FMRP is expressed in different tissues during development, with a preference for the gonads and the brain and, as expected, the main effects of its loss are in the brain and the testes [16].
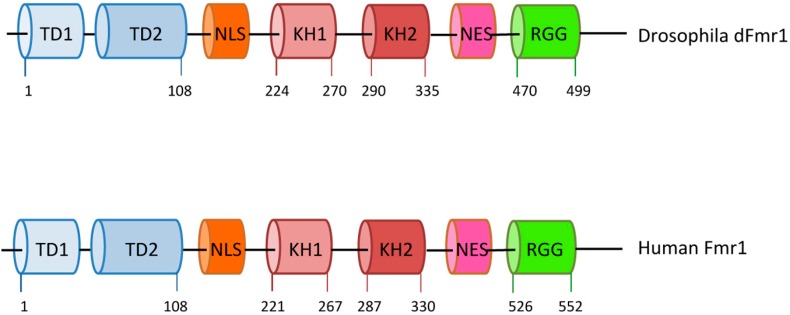
Drosophila melanogaster is considered a good model for fragile-X syndrome [17] because it has a single, well-conserved fragile X-related gene named dFmr1 [18,19], exhibiting high sequence similarity with all three human genes, and is predicted to be ancestral to the three mammalian members. dFmr1 mutant flies exhibit defects in neuronal structure and function, and in germline development, resembling those observed in Fmr1 mutations in mice and humans [19,20]. The human FMRP and Drosophila dFmr1 exhibit similar structures and domains [21]; they contain two KH domains, two Tudor domains, and an RGG box, in addition to a nuclear localization signal (NLS) and a nuclear export signal (NES) [2,3,21,22,23,24] (Figure 1). The high conservation between the two proteins suggests a common molecular role that is also supported by the phenotypes in the mutants.
Figure 1.
Domain organization of Drosophila and human Fmr1. The drawings are not to scale; the exact positions of the amino acids are indicated. TD1 and TD2 are tudor domains; NLS stands for nuclear localization signal; NES stands for nuclear export signal; KH1, KH2, and RGG motif are indicated.
FMRP has a wide spectrum of functions; however, its main role is exerted in different small RNA-mediated pathways in humans and in the mouse and Drosophila models. The majority of the studies regarding the small RNA pathways in Drosophila have been conducted in the ovaries, leading to the identification of specific molecular functions of dFmr1.
1.2. Drosophila Small RNA Pathways: An Overview
Different classes of small RNAs have been described and characterized in the last two decades; they have different biogenesis and functions and belong to different small RNA pathways depending on which Argonaute protein they interact with. Small RNAs bind to specific Argonaute proteins, forming different RISC complexes that initiate pathways leading to different post-transcriptional and transcriptional target regulation [25]. The three main small RNA pathways, also called RNA interference (RNAi) pathways, are: (i) the siRNA pathway (and the related endo-siRNA pathway) that uses a small RNA molecules of 21–23 nucleotides and Ago2 as its dedicated Argonaute protein [26]; (ii) the miRNA pathway that uses small RNAs as long as the siRNAs, and exhibit a very peculiar biogenesis; in Drosophila the miRNAs are generated, via a two-step process, from endogenously transcribed primary miRNAs. miRNAs guide Ago1, the Argonaute protein predominantly involved in this pathway, to obtain a translational repression of the mRNA targets [27,28]; (iii) the piRNA pathway that was first discovered in the gonads, and was predominantly considered the gonadal-specific pathway [29,30,31,32] even though, recently, the piRNA pathway has also been found in the nervous system not only in Drosophila but also in mice [33,34]. Piwi-interacting RNAs (piRNAs) are longer than the other classes of small RNAs (23–32 nt long) and Piwi, Aubergine (Aub), and Ago3, defined as the Piwi subfamily proteins, are the Argonaute proteins involved in this silencing pathway. The piRNAs protect animal cells from the de-regulation of transposons and other repetitive genetic elements, preserving genome stability [35,36]. Although the three main RNAi pathways exhibit specific features and components, there is some evidence that they share some components or functions with other pathways related to the RNA metabolism [37,38,39,40,41,42].
1.3. piRNA Genes Are Conserved during Evolution
Piwi proteins and the piRNA genes have been identified in model organisms and also in humans. They are deeply conserved in evolution even though the molecular mechanisms in which they are involved are not completely understood. The mouse is the animal in which many studies have been conducted, demonstrating that the piRNA-mediated silencing of transposons is active in the male germline to ensure fertility [43]. The role of piRNAs and the conservation of peculiar elements belonging to the piRNA pathway, such as the Piwi proteins, Vasa, Maelstrom, MOV10L1/Armitage, Tudor domain proteins, and others, suggest that they may act to protect animal cells from transposable elements, keeping them silenced and ensuring genome stability in the gonads [36,44,45,46,47,48]. A comprehensive analysis and annotation of human piRNAs in adult human testes has revealed a relationship between the small RNAs and transposons [49]. However, the direct role of the piRNA pathway in the silencing of TEs has not been clarified so far. A link between the Piwi human genes (PIWIL1, PIWIL2, and PIWIL4) and the transposons has been indirectly demonstrated in the tumorigenesis of human testes. In particular, the epigenetic inactivation of the Piwi proteins and the Tudor protein TDRD1 causes a reduction in piRNA expression in addition to the DNA hypomethylation of LINE1, an active human transposon [50].
2. The piRNA Pathway in Drosophila Gonads
The piRNA-mediated pathway is involved in the transcriptional and post-transcriptional silencing of the transposable and repetitive elements of the genome. It occurs predominantly in the germ cells and in their somatic precursors in the gonads of both sexes and is required for fertility not only in flies but also in mammals [35,51]. Most of what has been found on the piRNAs biogenesis, their roles, and the piRNA-related genes in Drosophila comes mainly from a combination of genetics and deep sequencing approaches. Genome-wide screens together with transcriptomic analyses have been performed with the aim to identify as many piRNA-related genes as possible [52,53,54]. Both germ cells and their somatic precursors use the piRNA pathway to silence transposable elements (TE). The biogenesis of piRNAs starts with the transcription of specific genomic clusters located at different positions in the genome; they are composed of repetitive, non-functional relicts of transposable elements [29]. Two main types of piRNA clusters have been described, both transcribed by RNA Polymerase II: uni-strand clusters, like AT-X1 and cluster 2 (germline specific) and flamenco (somatic specific), transcribed as single-strand precursors; and dual-strand clusters, like 42AB and 80EF (germline-specific) [55,56,57], transcribed from both strands [29,31,51]. Clusters produce RNA precursors that are processed into piRNAs in the perinuclear region of the gonadal cells called “nuage”, which surrounds the nuclear envelope [58,59,60,61,62]. Although the roles of the piRNA-related genes are almost the same in the gonads of both sexes, some differences exist [63,64].
2.1. The piRNA Pathway in the Ovary
Most of the knowledge about the piRNA pathway comes from studies in the Drosophila ovary. After their transcription, the piRNA precursors enter different pathways depending on which piRNA will be produced and in which type of cell it will function, somatic or germline. In the somatic primary piRNA pathway occurring in the follicle cells, the piRNA precursors undergo a cleavage by the endonuclease Zucchini, which seems to have a role in generating both the 5′ and the 3′ end of the primary piRNAs [29,40,65,66,67]. They possess a strong 1U bias and, after their production, they are bound to Piwi and to other proteins (Helicases and Tudor domain proteins) involved in the pathway and located in the structure called the Yb-body [68]. After that, mature piRNAs enter the nucleus to exert TE transcriptional silencing [65,66,69,70,71,72]. The interaction between the Tudor protein Yb, one of the key components of the Yb body, and the RNA helicase Armitage have a fundamental role in the entrance of Piwi into the nucleus [72], where it is involved in the H3K9me3-mediated transcriptional silencing of transposons. After their transcription, the primary piRNA intermediates place themselves in a well-defined structure called a flam-body or Dot Com adjacent to the Yb-body [73,74].
The primary pathway also occurs in the germ cells, nurse cells, and oocyte, producing primary piRNAs. The immature piRNAs transcripts are transported by the DEAD box helicase UAP56 [75,76], to the perinuclear region of the cytoplasm, the nuage, where a multi-protein perinuclear complex operates. The Piwi clade proteins Aubergine (Aub) and Argonaute-3 (Ago3) are then bound to the primary piRNAs and enter the germline specialized “ping-pong” amplification cycle with these two proteins as protagonists in the nuage to produce the secondary piRNAs that silence TEs [29,35,51,77,78]. The piRNAs produced by the ping-pong amplification loop show conserved specific signatures, like A in 10th position of the RNA (10A bias) [51]. Piwi seems not to be involved in the germline-specific primary pathway [51,79,80,81,82].
Many proteins involved in piRNAs biogenesis and transposon silencing, such as Aubergine, Ago3, Vasa, Krimper, Tudor, Spindle-E, Tejas, and Kumo/Qin are localized in the nuage [51,61,83,84,85,86,87,88]. Although for some of these proteins the molecular mechanism of the piRNA pathway is not completely clarified, the complex framework of interactions and actions continues to be enriched with new discoveries. The Tudor protein Krimper, for instance, has a key role in the formation of the nuage, leading to an interaction between Aub and Ago3 [87,89], even though its role seems to be different in the piRNA pathways of ovaries and testes, where it is required in association with Aubergine for the proper localization of itself and Aubergine at the nuage [63,64].
2.2. The piRNA Pathway in the Testes and Its Role in the crystal-Stellate Regulation
The “crystal-Stellate system” was first described as an example of heterochromatin–euchromatin interaction [90,91]. Stellate and crystal or Su(Ste) are homologous repetitive sequences located mainly at three different positions on the sexual chromosomes: Stellate repeats are located on the X chromosome, at the euchromatic 12E region and at the heterochromatic h26 region; crystal is on the Y at h11 region. All of them are repetitive homologous sequences differing in some features [91,92,93,94,95]. Males lacking the crystal region exhibit crystalline aggregates in their spermatocytes [96,97]. In addition to crystals, these males also show defects in chromosome condensation and segregation. The Stellate and crystal loci are normally silent; however, deficiencies in the crystal region lead to the de-repression of the Stellate sequences in the germline. At the molecular level, the loss of the crystal region results in the production of a testes-specific Stellate mRNA of 750 bases in length [93,98,99]. In 1995 we discovered that the Stellate protein, produced by the Stellate mRNA, is the main component of crystalline aggregates [100]. An early genetic screening permitted us to identify some modifiers of the crystal–Stellate interaction among which are aubsting and hsp83scratch [98,99,101]. In 2001 it became clear that the crystal-Stellate regulation occurs by the small-RNA-mediated pathway [30,102,103], which, later, was identified as the specialized piRNA pathway [29,35,51,64,83,95]. In fly testes the most abundant piRNAs associated with Aubergine and Ago3 correspond to “crystal” piRNAs [83]. This finding has reinforced the relationship between crystal-Stellate regulation and the piRNA pathway. Indeed, the de-regulation of the crystal–Stellate interaction represents a simple readout to identify genes involved in the piRNA pathway in testes [95,98,99,101,104,105]. Many genes have been assigned to the piRNA pathway, and the majority of them came from studies in ovaries [51,77,106,107]. Even though no system-wide screening has been performed to date in searching for piRNA-mediated genes in testes, our group and others analyzed a wide variety of mutations of piRNA genes in relation to their role in the crystal–Stellate interaction. The main features of several piRNA genes identified during the years are summarized in Table 1, where their domains, functions, localization, cellular sub-localization, and especially their relationship with the crystal-Stellate regulation are highlighted [32,35,38,53,57,61,64,65,66,69,70,71,73,75,76,78,81,82,83,84,85,86,88,95,99,101,104,105,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128]. From the analysis of the table and from Figure 2, where a Venn-like diagram is reported, showing the distribution of the genes listed in Table 1, it is clear that all the crystal-Stellate modifiers are piRNA-related genes, although the opposite is not true. A crucial point regards Piwi that is not involved in the piRNA-mediated silencing of Stellate in the testes: nonetheless, it is fundamental to piRNA biogenesis in the ovaries. Since Piwi seems not to be required in the germline primary pathway, at least in the ovary [64,79,80,81,82,129], it is conceivable that the silencing of the Stellate sequences mainly requires the germline primary pathway; this is also supported by the observation that the Stellate-related piRNAs share some signatures of the ping-pong pathway [64,83,95,128]. However, many genes required for the ping-pong amplification in the ovary are also required for the silencing of the Stellate sequences and of the transposons in testes (Table 1). Furthermore, the observations that Piwi and Rhino are required in the first step of the piRNA biogenesis in the ovaries [82] and that both proteins are not required for Stellate-related piRNA biogenesis in testes indicate that distinct pathways occur in the gonads of both sexes (the mechanism of primary piRNA production in the germline could be distinct from the piRNA production in the ovary) [63,87,89].
Table 1.
piRNA-related genes and the crystal-Stellate regulation. The best-known piRNA genes, with their domains and localizations reported. Abbreviations: N = nuclear; C = cytoplasmic; ND = not determined; the asterisks (*) indicate that the protein is located at the piNG bodies; “+” indicates the presence of the protein and/or of the crystals; “++” indicates a high presence of the protein and/or of the crystals; “++++” indicates a very high presence of the protein and/or of the crystals, “++”, where indicated the information are on the RNA coding for crystals; “?” indicates that the information are not available.
| Mutants | Protein Domains | Protein Localization | Testes | Ovaries | Protein Function | Transposons | Stellate-Made Crystals | References |
|---|---|---|---|---|---|---|---|---|
| Argonaute Proteins | ||||||||
| aubergine | PAZ, MID, PIWI | germline (C-nuage); germ granules | + * | + | ping-pong pathway, primary pathway | germinal | + | [35,38,78,83,88,108] |
| ago3 | PAZ, MID, PIWI | germline (C-nuage); germ granules | + * | + | ping-pong pathway | germinal | + | [35,70,71] |
| piwi | PAZ, MID, PIWI | soma + germline (nuclear) | + | + | primary pathway | germinal + somatic | - | [83,84,109] |
| ago1 | PAZ, PIWI | soma, germline (C-nuage) | + * | + | piRNA pathway, miRNA pathway | somatic | + | [105,111] |
| Helicases | ||||||||
| armitage | RNA helicase (SDE3) | soma(C-Yb bodies) + germline (C) | + | + | primary pathway | germinal + somatic | + | [70,71,112,113,114] |
| vasa | DEAD RNA helicase | germline (Amplifier Complex-C-nuage); germ granules | + * | + | primary + ping-pong pathway | germinal + somatic | ++ (RNA) | [84,86] |
| UAP56 | DEAD RNA helicase | germline (N) + N/C boundary | ND | + | primary + ping-pong pathway | germinal + somatic | ND | [73,75] |
| Tudor Proteins | ||||||||
| Yb | DEAD RNA helicase + Tudor domain | soma (C-Yb bodies) | ND | + | primary pathway (single strand clusters (flamenco)) | somatic | - | [32,70,71,113,114] |
| spindle-E | DEAD RNA helicase + Tudor domain | germline (C-nuage) | + * | + | primary pathway | germinal | + | [61,84,104] |
| krimper | Tudor domain | germline (C-nuage) + soma (krimper body) | + | + | germinal | ++++ | [61,115] | |
| tudor | Tudor domain | germline (C-nuage) soma; germ granules | + * | + | germinal | - | [116,117,118] | |
| tapas | Tudor domain + Lotus | germline (C-nuage) | + | + | ping-pong?, primary? | germinal | ++++ (RNA) | [113,119] |
| tejas | Tudor domain + Lotus domain | germline (C-nuage) | + | + | ping-pong?, primary? | germinal | ++++ | [84] |
| zucchini | Tudor domain + nuclease | soma(C) + germline (C-nuage) mitocondrial | + | + | primary pathway | germinal + somatic | + | [65,66,69,71,81] |
| squash | Tudor domain + nuclease | germline (C-nuage); ND in the soma | + * | + | germinal + somatic | + | [69,81] | |
| vreteno | Tudor domain + RRM domai | soma (C) + germline (C-nuage) | + | + | primary pathway | germinal (seen only in ovaries) + somatic | ND | [113,114] |
| qin/kumo | Tudor domain + RING domain | soma (N) + germline (Amplifier Complex-C-nuage) | + | + | primary pathway (dual strand clusters) | germline + somatic | + | [75,85,120] |
| dFmr1 | Tudor domain, KH, RGG, NLS, NES | soma + germline (N + C) | + | + | germinal + somatic | + | [105] | |
| papi | Tudor domain + KH domain | soma (N) + germline (C-nuage) | ND | + | primary + ping-pong pathway | germinal + somatic | ND | [121] |
| eggless (dSETDB1) | Tudor domain + SET domain | germline (N) + soma (N) | ND | + | H3K9 methyl transferase (TE regulation) | germinal + somatic | ND | [122] |
| Non Tudor Proteins | ||||||||
| rhino | chromo/shadow domain | germline + soma (N) | ND | + | primary + ping-pong pathway (dual strand clusters) | germinal | - | [57,76,82] |
| cutoff | DXO/Dom3Z family | germline (C-nuage) + N | ND | + | primary + ping-pong pathway (dual strand clusters) | germinal | - | [57,76] |
| maelstrom | Maelstrom domain (DNA/RNA binding); HMG protein | germline + soma: shuttle nucleus-cytoplasm (nuage) | + | + | primary + ping-pong pathway | germinal + somatic | ND | [123] |
| capsuleen | PRMT | germline (C-nuage) | + | + | arginine methyltransferase | germline | + | [124,125] |
| shutdown | FKBP6 family (co-chaperone) | soma (YB-bodies) + germline (N + C) | + | + | primary pathway + ping-pong | germinal + somatic | + | [115,127] |
| hsp83 | heat shock protein | soma + germline (N + C) | + | + | primary pathway + ping-pong | germinal + somatic | + | [70,78,99,101] |
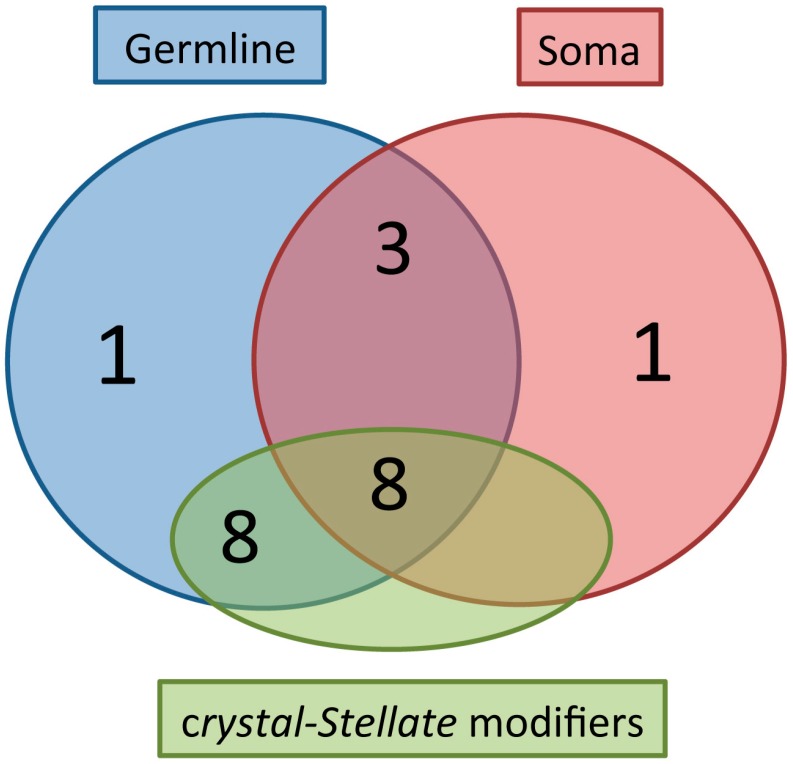
Figure 2.
The majority of the germline and somatic piRNA genes are crystal-Stellate modifiers. A Venn-like diagram is reported, showing the distribution of the 26 piRNA genes listed in Table 1, in three groups: the “Germline” group represents the piRNA genes with a localization in the germline; the “Soma” group represents the piRNA genes with a localization in the somatic part of the gonad; the “crystal-Stellate modifiers” group represents the genes with a role in the silencing of the Stellate sequences. The distribution of the piRNA genes in the three groups is reported below: Germline (1) -> cutoff; Soma (1) -> Yb; Germline + Soma (3) -> piwi, tudor, rhino; Germline + crystal-Stellate modifiers (8) -> aubergine, ago3, vasa, spindle-E, tapas, tejas, squash, capsuleen. (UAP56 has not been analyzed in relation to the crystal-Stellate regulation). Germline + Soma + crystal-Stellate modifiers (8) -> ago1, armitage, krimper, zucchini, qin/kumo, dFmr1, shutdown, hsp83. (vreteno, papi, eggless, rhino, maelstrom) have not been analyzed in relation to the crystal-Stellate regulation.
3. dFmr1 Participates in the piRNA Pathway Occurring in Gonads
Recently our group has demonstrated that dFmr1 plays a role in the piRNA pathway in testes and ovaries of Drosophila melanogaster [105]. This study originates from the observation that dFmr1, a protein involved in RNA metabolism, was found to be associated in a complex with components of the RNA interference pathway [7,8]. In addition, the complexity of the symptoms exhibited by fragile-X patients and by Drosophila mutants in the gonads and in the nervous system, along with the discovery of piRNAs in the nervous system of Drosophila and humans [33,34,130,131,132,133], allowed us to hypothesize that much more remains to be discovered about the multiple functions of the FMRP protein.
3.1. dFmr1’s Role in the Testes
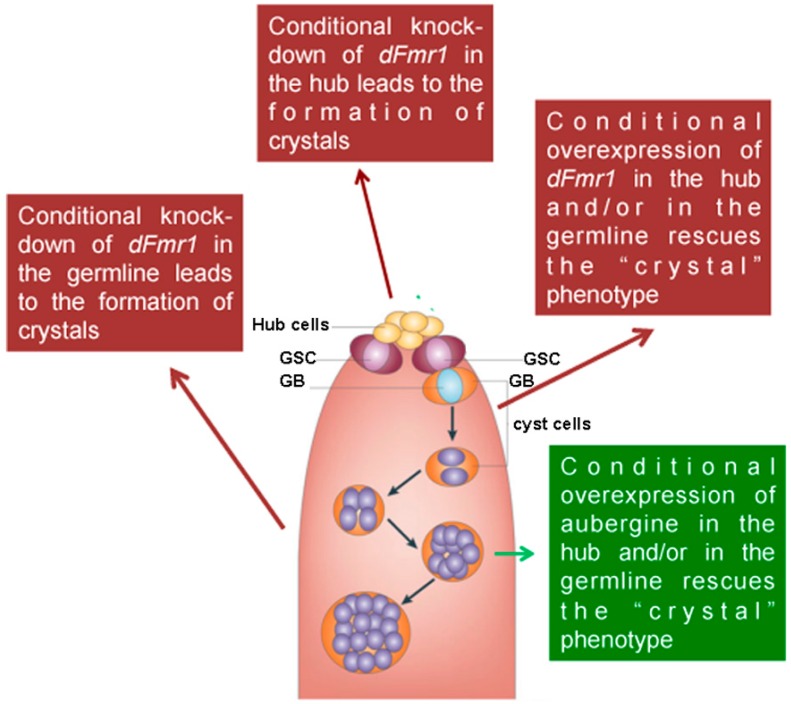
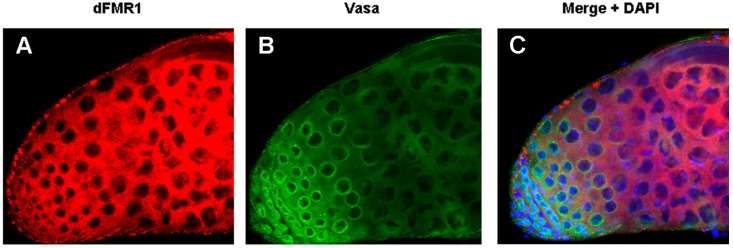
The role of dFmr1 in the piRNA pathway was demonstrated at first, by the presence of the Stellate-made crystalline aggregates in spermatocytes of dFmr1∆50, dFmr1∆113 mutant males and in RNAi-dFmr1 males in which the function of the protein was selectively reduced in different cells of the gonads (germinal or somatic) (Figure 3, red boxes). The presence of crystals was accompanied by the drastic reduction of the Stellate-related piRNAs. The confirmation of the role of dFmr1 in the piRNA pathway in testes, came from the observation that in dFmr1 mutants and dFmr1-RNAi males, several transposable elements are activated with effects on the fertility of the individuals [105]. dFmr1 is expressed in the germ cells of adult testes as concluded from its colocalization with Vasa, a germline-specific marker [86,134], as previously described [105]. It is also present in the piNG bodies (Figure 4A–C), representing specific giant bodies of the nuage, where some piRNA components perform their function [135]. dFmr1 localization is predominantly cytoplasmic, even though a nuclear localization and function have been reported [136,137].
Figure 3.
Germline and somatic requirement of dFMR1 in the testes. A scheme of the apical part of a testis is reported. Red arrows and boxes indicate the effect of the knockdown and/or the overexpression of dFmr1 in the hub and in the germline obtained using updGal4 (hub) and nanosGal4 (germline) drivers. The green arrow and box indicates the effects of the overexpression of aubergine. GSC stands for germ stem cells; GB stands for gonialblasts.
Figure 4.
dFmr1 and Vasa immunolocalization in wt adult testes. Max intensity of triple confocal sections (40×) of a wild type testis labeled with (A) anti-dFMR1, (B) anti-Vasa, (C) Merge with the DAPI channel.
dFmr1 was also found to interact genetically and biochemically with two Argonaute proteins: Aubergine (a Piwi protein) (Figure 3, green box) and Ago1 (an Ago protein), both of which are located at the nuage of the germ cells. The genetic interaction with Aubergine and Ago1 also occurs in the nervous system at the neuromuscolar junctions [105].
The presence of the Tudor/Agenet domain in dFmr1 suggests that it may bind the Argonaute proteins by their symmetrically di-methylated arginines (sDMAs), located at the N-terminal site to activate them [109,121,138]. However, in specific cases, the interaction between Argonaute proteins and some Tudor proteins is independent from the sDMAs, as demonstrated for the interaction between Aubergine and Kumo [120] or for Ago3 and Krimper [89]. In the case of dFmr1 and Aubergine, it seems more likely that the interaction occurs independently from the sDMAs residues of Aubergine because it has been demonstrated that the biochemical interaction of the two proteins is not limited to the N-terminal domain of Aubergine [105].
3.2. dFmr1’s Role in the Ovary
Recently, dFmr1 has emerged as a member of piRNA-mediated pathways in ovaries [105,139]. The first indication of the involvement of dFmr1 in the piRNA pathway of the ovary was the activation of transposons caused by the loss of dFmr1. The level of transcription of specific transposons increases significantly in the dFmr1 mutant ovaries accompanied, as expected, by the reduction of fertility. Moreover, it is interesting to note that the expression patterns of dFmr1 and the piRNA-related protein Aubergine co-localize in specific territories of the ovary [105].
The Drosophila ovary is composed of 12 to 16 ovarioles. In each one, the most apical structure is the germarium, where stem cells are located. The developing egg chamber passes through the germarium and proceeds through its development, forming a linear chain. dFmr1 and Aubergine co-localize in the germarium, where they accumulate at the position of the stem cells. The two proteins also co-localize in the nuage of the nurse cells and, in the later stages, they overlap in the cytoplasm of the oocyte [105]. The relationship between dFmr1 and the piRNA pathway is also confirmed by the interaction between dFmr1 and Piwi, which act together in the heterochromatic gene silencing in somatic cells and the transposon silencing in germ cells [140]. In particular, it has been demonstrated that dFmr1 is essential for the correct localization of HP1, in the heterochromatin of follicle cells [139]. It is known that HP1 is a highly conserved protein identified as a crucial component of the heterochromatin with a role in the Position Effect Variegation (PEV), now also recognized as playing roles in many other processes [141].
In the ovary, dFmr1 is also involved in the microRNA-mediated pathway. The tight link between dFMR1 and the miRNA pathway has emerged from the interaction between the Fragile-X protein and the bantam miRNA to control germline stem cells in the ovary of Drosophila [142]. In human cell lines, FMRP interacts with components of the miRNA pathway, such as Dicer1 and Ago1 [143]. In Drosophila, Ago1 is also required for the biological function of dFmr1 in neural development and synaptogenesis [9].
4. Genetic and Biochemical Interactors of dFmr1
In the last part of this review we will examine the multiple roles and functions of the dFmr1 protein, following the threads of its genetic and biochemical/physical interactors in the gonads and in the germ granules representing a bridge from oocyte to embryos in the fly and other animals (mice and humans).
dFmr1 has recently been associated with the piRNA pathway in the gonads of both sexes [105,139] and the interaction with some of the Argonaute proteins supports these findings. dFmr1 interacts with Aubergine, allowing the correct silencing of the transposons and repetitive sequences [105], and with Piwi [139], cooperating in the piRNA-mediated translational silencing of the transposons. These two proteins belong to the Piwi-subfamily of the Argonaute proteins, and whereas Aubergine is cytoplasmic as well as the most common localization of dFmr1, Piwi is predominantly nuclear, confirming a nuclear function and localization of dFmr1. Ago1, belonging to the Ago-subfamily of the Argonaute proteins, is a biochemical and genetic interactor of dFmr1 in the piRNA pathway [105], as well as in the miRNA pathway in the ovary [9,142,143,144,145,146,147].
The DEAD box RNA helicase Vasa is also considered a key component of the piRNA pathway; it is located at the nuage of ovaries and testes [35,51,83,134,148,149,150,151,152] and has been considered a ‘‘biochemical platform’’ to put together the key components of the piRNA amplification machinery (the Amplifier complex) in nuage [86]. dFmr1 has been demonstrated to co-localize with Vasa in the testes and ovaries [105]. The function of the dFmr1 gene is also required for pole cells formation, located at the posterior pole of the early embryo, in which the Vasa protein is one of the main components of the polar granules [134,153,154]. These findings support the hypothesis that dFmr1 and Vasa may exert their role in the same pathway from the germ cells in the ovary to the pole cells in the embryo in the silencing of the transposable elements maintaining genome stability in these tissues.
Another component of the germ granules at the pole plasm, where the pole cells will form, is the RNA binding protein Cup [155,156,157]. The gene coding for Cup has been found in a genetic screening for interactors of dFmr1 [158]. It codes for an RNA binding protein promoting the accumulation of the germ plasm components like Oskar, Vasa and Staufen at the posterior pole of the oocyte and it is required for the correct polar granules deposition [157,159]. During the development of the early stages of oogenesis, Cup co-localizes and interacts with Hsp83 [157]. Hsp83 has a well-demonstrated role in the silencing of transposons and repetitive sequences related to the genome stability [95,101,160,161,162].
The P-bodies component, Me31B DEAD box helicase, represents a very intriguing link between Cup, Hsp83 and dFmr1 [163]. Me31B co-localizes with dFmr1 and Cup in these cytoplasmic structures implicated in RNA metabolism like storage, translational repression, and RNA degradation. Me31B also interacts with Aubergine and Vasa, and is required to recruit dFmr1 to the neuronal granules [164,165,166,167], which may be considered a neuronal counterpart of the polar granules.
An emerging function which has been demonstrated for the mammalian form of Fmr1 protein is related to its capacity to recognize the G-quadruplets, a specific structure in RNAs [168]. It is interesting to note that the G-quadruplets have been shown to be present in the precursors of the piRNAs [47]. The connection between the Drosophila dFmr1 and the G-quadruplets can be supported by the finding that one of the targets of dFmr1 in the nervous system is the protein Futsch, the homolog of the mammalian MAP1B, exhibiting a putative G-quadruplets in its 5′ end [19,144,153]. This specific aspect has not yet been completely elucidated in Drosophila.
Nuclear Interactors in the Small RNA Pathway (in the Gonads)
In addition to the cytoplasmic function of dFmr1, a nuclear function has been suggested due not only to the NLS and NES domains in the protein (Figure 1) [1,24,169], but also to some of its nuclear interactors. One of the known nuclear interactors in mammals is NUFIP (Nuclear Fmr1 Interacting Protein), a nucleo-cytoplasmic shuttling protein related to the ribonucleoprotein (RNP) complex formation [170,171,172]. Recently dFmr1 and Nufip have been identified in a Zfrp8 complex in the Drosophila ovary [137]. Zfrp8 participates in the piRNA pathway, influencing the correct localization of Maelstrom in the nuage [173], and is required for maintaining follicle and germline stem cells (GSCs) [174]; it has been proposed that Zfrp8 is required in order to localize FMRP properly. Interestingly Hsp83, Hop (Hsp70/Hsp90 Organizing Protein Homolog), and Piwi have been identified in the same complex with a hypothesized role in piRNA-mediated canalization [137,175,176].
The emerging role of dFmr1 in the nucleus is strongly supported by its connection with HP1. In the Drosophila ovary, the loss of dFmr1 causes an incorrect localization of HP1 in the heterochromatin, suggesting a role for dFmr1 in chromatin state maintenance [139].
5. Conclusions
dFmr1 is an RNA binding protein with multiple domains and multiple demonstrated functions. Some of these functions may be related to the different interactors in different tissues or in different subcellular populations, or both. In this review, following its interactors in the gonads, cells, and subcellular compartments, we have analyzed dFmr1’s roles in small RNA pathways: in regulating the localization of some nuage components, in shuttling between nucleus and cytoplasm, in chromatin remodeling, in binding the G-quadruplets and, finally, in potentially regulating mRNA translation in the ovary as well as at the synapses. All these studies indicate a conservation of the pathways involved in the regulation of transposons from the animal models to humans, and furthermore emphasize the importance of the Drosophila model for the discovery of new components of the piRNA pathway as well as for molecular studies of the piRNA process to be applied in mammals.
It will be interesting, in the future, to further investigate the different roles of FMRP arising from its multiple interactors in different types of cells and tissues, in order to gain insights into its apparently different molecular roles that have a significant impact on human Fragile-X disease.
Acknowledgments
We acknowledge the financial support of Telethon-Italy [grant number GG14181] and MIUR [grant number RBFR10V8K6].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siomi M.C., Siomi H., Sauer W.H., Srinivasan S., Nussbaum R.L., Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J. 1995;14:2401–2408. doi: 10.1002/j.1460-2075.1995.tb07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., O’Connor J.P., Siomi M.C., Srinivasan S., Dutra A., Nussbaum R.L., Dreyfuss G. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J. 1995;14:5358–5366. doi: 10.1002/j.1460-2075.1995.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams-Cioaba M.A., Guo Y., Bian C., Amaya M.F., Lam R., Wasney G.A., Vedadi M., Xu C., Min J. Structural studies of the tandem Tudor domains of fragile X mental retardation related proteins FXR1 and FXR2. PLoS ONE. 2010;5:e13559. doi: 10.1371/journal.pone.0013559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenck A., Bardoni B., Langmann C., Harden N., Mandel J.L., Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–898. doi: 10.1016/S0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 5.Napoli I., Mercaldo V., Boyl P.P., Eleuteri B., Zalfa F., de Rubeis S., di Marino D., Mohr E., Massimi M., Falconi M., et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Aitken C.E., Lorsch J.R. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 2012;19:568–576. doi: 10.1038/nsmb.2303. [DOI] [PubMed] [Google Scholar]
- 7.Caudy A.A., Myers M., Hannon G.J., Hammond S.M. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizuka A., Siomi M.C., Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin P., Zarnescu D.C., Ceman S., Nakamoto M., Mowrey J., Jongens T.A., Nelson D.L., Moses K., Warren S.T. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 10.Hou L., Antion M.D., Hu D., Spencer C.M., Paylor R., Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Xu X.L., Li Y., Wang F., Gao F.B. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J. Neurosci. 2008;28:11883–11889. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siomi M.C., Zhang Y., Siomi H., Dreyfuss G. Specific sequences in the fragile X syndrome protein FMR1 and the FXR proteins mediate their binding to 60S ribosomal subunits and the interactions among them. Mol. Cell. Biol. 1996;16:3825–3832. doi: 10.1128/MCB.16.7.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamanini F., Meijer N., Verheij C., Willems P.J., Galjaard H., Oostra B.A., Hoogeveen A.T. FMRP is associated to the ribosomes via RNA. Hum. Mol. Genet. 1996;5:809–813. doi: 10.1093/hmg/5.6.809. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y., Absher D., Eberhart D.E., Brown V., Malter H.E., Warren S.T. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell. 1997;1:109–118. doi: 10.1016/S1097-2765(00)80012-X. [DOI] [PubMed] [Google Scholar]
- 15.Darnell J.C., Fraser C.E., Mostovetsky O., Stefani G., Jones T.A., Eddy S.R., Darnell R.B. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19:903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro M.R., Bray S.M., Warren S.T. Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annu. Rev. Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 17.Zarnescu D.C., Jin P., Betschinger J., Nakamoto M., Wang Y., Dockendorff T.C., Feng Y., Jongens T.A., Sisson J.C., Knoblich J.A., et al. Fragile X protein functions with Lgl and the PAR complex in flies and mice. Dev. Cell. 2005;8:43–52. doi: 10.1016/j.devcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Wan L., Dockendorff T.C., Jongens T.A., Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol. Cell. Biol. 2000;20:8536–8547. doi: 10.1128/MCB.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y.Q., Bailey A.M., Matthies H.J., Renden R.B., Smith M.A., Speese S.D., Rubin G.M., Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/S0092-8674(01)00589-X. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y.Q., Matthies H.J., Mancuso J., Andrews H.K., Woodruff E., 3rd, Friedman D., Broadie K. The Drosophila fragile X-related gene regulates axoneme differentiation during spermatogenesis. Dev. Biol. 2004;270:290–307. doi: 10.1016/j.ydbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Chen E., Joseph S. Fragile X mental retardation protein: A paradigm for translational control by RNA-binding proteins. Biochimie. 2015;114:147–154. doi: 10.1016/j.biochi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siomi H., Choi M., Siomi M.C., Nussbaum R.L., Dreyfuss G. Essential role for KH domains in RNA binding: Impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994;77:33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 23.Siomi H., Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardoni B., Sittler A., Shen Y., Mandel J.L. Analysis of domains affecting intracellular localization of the FMRP protein. Neurobiol. Dis. 1997;4:329–336. doi: 10.1006/nbdi.1997.0142. [DOI] [PubMed] [Google Scholar]
- 25.Ghildiyal M., Zamore P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czech B., Malone C.D., Zhou R., Stark A., Schlingeheyde C., Dus M., Perrimon N., Kellis M., Wohlschlegel J.A., Sachidanandam R., et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L., Hannon G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 28.Carthew R.W., Agbu P., Giri R. MicroRNA function in Drosophila melanogaster. Semin. Cell Dev. Biol. 2016;66:29–37. doi: 10.1016/j.semcdb.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennecke J., Aravin A.A., Stark A., Dus M., Kellis M., Sachidanandam R., Hannon G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Vagin V.V., Sigova A., Li C., Seitz H., Gvozdev V., Zamore P.D. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 31.Senti K.A., Brennecke J. The piRNA pathway: A fly’s perspective on the guardian of the genome. Trends Genet. 2010;26:499–509. doi: 10.1016/j.tig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki Y.W., Siomi M.C., Siomi H. PIWI-interacting RNA: Its biogenesis and functions. Annu. Rev. Biochem. 2015;84:405–433. doi: 10.1146/annurev-biochem-060614-034258. [DOI] [PubMed] [Google Scholar]
- 33.Lee E.J., Banerjee S., Zhou H., Jammalamadaka A., Arcila M., Manjunath B.S., Kosik K.S. Identification of piRNAs in the central nervous system. RNA. 2011;17:1090–1099. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrat P.N., DasGupta S., Wang J., Theurkauf W., Weng Z., Rosbash M., Waddell S. Transposition-driven genomic heterogeneity in the Drosophila brain. Science. 2013;340:91–95. doi: 10.1126/science.1231965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Vagin V.V., Lee S., Xu J., Ma S., Xi H., Seitz H., Horwich M.D., Syrzycka M., Honda B.M., et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell. 2009;137:509–521. doi: 10.1016/j.cell.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson T., Lin H. The biogenesis and function of PIWI proteins and piRNAs: Progress and prospect. Annu. Rev. Cell Dev. Biol. 2009;25:355–376. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forstemann K., Horwich M.D., Wee L., Tomari Y., Zamore P.D. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287–297. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Specchia V., Benna C., Mazzotta G.M., Piccin A., Zordan M.A., Costa R., Bozzetti M.P. aubergine gene overexpression in somatic tissues of aubergine(sting) mutants interferes with the RNAi pathway of a yellow hairpin dsRNA in Drosophila melanogaster. Genetics. 2008;178:1271–1282. doi: 10.1534/genetics.107.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi R., Schnabl J., Handler D., Mohn F., Ameres S.L., Brennecke J. Genetic and mechanistic diversity of piRNA 3′-end formation. Nature. 2016;539:588–592. doi: 10.1038/nature20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golden R.J., Chen B., Li T., Braun J., Manjunath H., Chen X., Wu J., Schmid V., Chang T.C., Kopp F., et al. An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature. 2017;542:197–202. doi: 10.1038/nature21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo P.K., Huang Y.C., Poulton J.S., Leake N., Palmer W.H., Vera D., Xie G., Klusza S., Deng W.M. RNA helicase Belle/DDX3 regulates transgene expression in Drosophila. Dev. Biol. 2016;412:57–70. doi: 10.1016/j.ydbio.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng W., Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–830. doi: 10.1016/S1534-5807(02)00165-X. [DOI] [PubMed] [Google Scholar]
- 44.Aravin A., Gaidatzis D., Pfeffer S., Lagos-Quintana M., Landgraf P., Iovino N., Morris P., Brownstein M.J., Kuramochi-Miyagawa S., Nakano T., et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 45.Aravin A.A., Hannon G.J., Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- 46.Reuter M., Chuma S., Tanaka T., Franz T., Stark A., Pillai R.S. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat. Struct. Mol. Biol. 2009;16:639–646. doi: 10.1038/nsmb.1615. [DOI] [PubMed] [Google Scholar]
- 47.Vourekas A., Zheng K., Fu Q., Maragkakis M., Alexiou P., Ma J., Pillai R.S., Mourelatos Z., Wang P.J. The RNA helicase MOV10L1 binds piRNA precursors to initiate piRNA processing. Genes Dev. 2015;29:617–629. doi: 10.1101/gad.254631.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soper S.F., van der Heijden G.W., Hardiman T.C., Goodheart M., Martin S.L., de Boer P., Bortvin A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell. 2008;15:285–297. doi: 10.1016/j.devcel.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha H., Song J., Wang S., Kapusta A., Feschotte C., Chen K.C., Xing J. A comprehensive analysis of piRNAs from adult human testis and their relationship with genes and mobile elements. BMC Genom. 2014;15:545. doi: 10.1186/1471-2164-15-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira H.J., Heyn H., Garcia del Muro X., Vidal A., Larriba S., Munoz C., Villanueva A., Esteller M. Epigenetic loss of the PIWI/piRNA machinery in human testicular tumorigenesis. Epigenetics. 2014;9:113–118. doi: 10.4161/epi.27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malone C.D., Brennecke J., Dus M., Stark A., McCombie W.R., Sachidanandam R., Hannon G.J. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137:522–535. doi: 10.1016/j.cell.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czech B., Preall J.B., McGinn J., Hannon G.J. A transcriptome-wide RNAi screen in the Drosophila ovary reveals factors of the germline piRNA pathway. Mol. Cell. 2013;50:749–761. doi: 10.1016/j.molcel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handler D., Meixner K., Pizka M., Lauss K., Schmied C., Gruber F.S., Brennecke J. The genetic makeup of the Drosophila piRNA pathway. Mol. Cell. 2013;50:762–777. doi: 10.1016/j.molcel.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muerdter F., Guzzardo P.M., Gillis J., Luo Y., Yu Y., Chen C., Fekete R., Hannon G.J. A genome-wide RNAi screen draws a genetic framework for transposon control and primary piRNA biogenesis in Drosophila. Mol. Cell. 2013;50:736–748. doi: 10.1016/j.molcel.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Thomas A., Toth K.F., Aravin A.A. To be or not to be a piRNA: Genomic origin and processing of piRNAs. Genome Biol. 2014;15:204. doi: 10.1186/gb4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Thomas A., Stuwe E., Li S., Du J., Marinov G., Rozhkov N., Chen Y.C., Luo Y., Sachidanandam R., Toth K.F., et al. Transgenerationally inherited piRNAs trigger piRNA biogenesis by changing the chromatin of piRNA clusters and inducing precursor processing. Genes Dev. 2014;28:1667–1680. doi: 10.1101/gad.245514.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohn F., Sienski G., Handler D., Brennecke J. The rhino-deadlock-cutoff complex licenses noncanonical transcription of dual-strand piRNA clusters in Drosophila. Cell. 2014;157:1364–1379. doi: 10.1016/j.cell.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 58.Eddy E.M. Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat. Rec. 1974;178:731–757. doi: 10.1002/ar.1091780406. [DOI] [PubMed] [Google Scholar]
- 59.Eddy E.M. Germ plasm and the differentiation of the germ cell line. Int. Rev. Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- 60.Snee M.J., Macdonald P.M. Live imaging of nuage and polar granules: Evidence against a precursor-product relationship and a novel role for Oskar in stabilization of polar granule components. J. Cell Sci. 2004;117(Pt 10):2109–2120. doi: 10.1242/jcs.01059. [DOI] [PubMed] [Google Scholar]
- 61.Lim A.K., Kai T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2007;104:6714–6719. doi: 10.1073/pnas.0701920104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klattenhoff C., Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 63.Nagao A., Sato K., Nishida K.M., Siomi H., Siomi M.C. Gender-Specific Hierarchy in Nuage Localization of PIWI-Interacting RNA Factors in Drosophila. Front. Genet. 2011;2:55. doi: 10.3389/fgene.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malone C.D., Lehmann R., Teixeira F.K. The cellular basis of hybrid dysgenesis and Stellate regulation in Drosophila. Curr. Opin. Genet. Dev. 2015;34:88–94. doi: 10.1016/j.gde.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishimasu H., Ishizu H., Saito K., Fukuhara S., Kamatani M.K., Bonnefond L., Matsumoto N., Nishizawa T., Nakanaga K., Aoki J., et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–287. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- 66.Ipsaro J.J., Haase A.D., Knott S.R., Joshua-Tor L., Hannon G.J. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–283. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunawardane L.S., Saito K., Nishida K.M., Miyoshi K., Kawamura Y., Nagami T., Siomi H., Siomi M.C. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 68.Szakmary A., Reedy M., Qi H., Lin H. The Yb protein defines a novel organelle and regulates male germline stem cell self-renewal in Drosophila melanogaster. J. Cell Biol. 2009;185:613–627. doi: 10.1083/jcb.200903034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haase A.D., Fenoglio S., Muerdter F., Guzzardo P.M., Czech B., Pappin D.J., Chen C., Gordon A., Hannon G.J. Probing the initiation and effector phases of the somatic piRNA pathway in Drosophila. Genes Dev. 2010;24:2499–2504. doi: 10.1101/gad.1968110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olivieri D., Sykora M.M., Sachidanandam R., Mechtler K., Brennecke J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 2010;29:3301–3317. doi: 10.1038/emboj.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saito K., Ishizu H., Komai M., Kotani H., Kawamura Y., Nishida K.M., Siomi H., Siomi M.C. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 2010;24:2493–2498. doi: 10.1101/gad.1989510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi H., Watanabe T., Ku H.Y., Liu N., Zhong M., Lin H. The Yb body, a major site for Piwi-associated RNA biogenesis and a gateway for Piwi expression and transport to the nucleus in somatic cells. J. Biol. Chem. 2011;286:3789–3797. doi: 10.1074/jbc.M110.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dennis C., Zanni V., Brasset E., Eymery A., Zhang L., Mteirek R., Jensen S., Rong Y.S., Vaury C. “Dot COM”, a nuclear transit center for the primary piRNA pathway in Drosophila. PLoS ONE. 2013;8:e72752. doi: 10.1371/journal.pone.0072752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murota Y., Ishizu H., Nakagawa S., Iwasaki Y.W., Shibata S., Kamatani M.K., Saito K., Okano H., Siomi H., Siomi M.C. Yb Integrates piRNA intermediates and processing factors into perinuclear bodies to enhance piRISC assembly. Cell Rep. 2014;8:103–113. doi: 10.1016/j.celrep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 75.Zhang F., Wang J., Xu J., Zhang Z., Koppetsch B.S., Schultz N., Vreven T., Meignin C., Davis I., Zamore P.D., et al. UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell. 2012;151:871–884. doi: 10.1016/j.cell.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Wang J., Schultz N., Zhang F., Parhad S.S., Tu S., Vreven T., Zamore P.D., Weng Z., Theurkauf W.E. The HP1 homolog rhino anchors a nuclear complex that suppresses piRNA precursor splicing. Cell. 2014;157:1353–1363. doi: 10.1016/j.cell.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siomi M.C., Sato K., Pezic D., Aravin A.A. PIWI-interacting small RNAs: The vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 78.Palazzo A., Marconi S., Specchia V., Bozzetti M.P., Ivics Z., Caizzi R., Marsano R.M. Functional characterization of the Bari1 transposition system. PLoS ONE. 2013;8:e79385. doi: 10.1371/journal.pone.0079385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y., Pane A., Schupbach T. Cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila. Curr. Biol. 2007;17:637–642. doi: 10.1016/j.cub.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klattenhoff C., Bratu D.P., McGinnis-Schultz N., Koppetsch B.S., Cook H.A., Theurkauf W.E. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev. Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Pane A., Wehr K., Schupbach T. zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev. Cell. 2007;12:851–862. doi: 10.1016/j.devcel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klattenhoff C., Xi H., Li C., Lee S., Xu J., Khurana J.S., Zhang F., Schultz N., Koppetsch B.S., Nowosielska A., et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137–1149. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishida K.M., Saito K., Mori T., Kawamura Y., Nagami-Okada T., Inagaki S., Siomi H., Siomi M.C. Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad. RNA. 2007;13:1911–1922. doi: 10.1261/rna.744307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patil V.S., Kai T. Repression of retroelements in Drosophila germline via piRNA pathway by the Tudor domain protein Tejas. Curr. Biol. 2010;20:724–730. doi: 10.1016/j.cub.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z., Xu J., Koppetsch B.S., Wang J., Tipping C., Ma S., Weng Z., Theurkauf W.E., Zamore P.D. Heterotypic piRNA Ping-Pong requires qin, a protein with both E3 ligase and Tudor domains. Mol. Cell. 2011;44:572–584. doi: 10.1016/j.molcel.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiol J., Spinelli P., Laussmann M.A., Homolka D., Yang Z., Cora E., Coute Y., Conn S., Kadlec J., Sachidanandam R., et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell. 2014;157:1698–1711. doi: 10.1016/j.cell.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 87.Webster A., Li S., Hur J.K., Wachsmuth M., Bois J.S., Perkins E.M., Patel D.J., Aravin A.A. Aub and Ago3 are recruited to nuage through two mechanisms to form a ping-pong complex assembled by krimper. Mol. Cell. 2015;59:564–575. doi: 10.1016/j.molcel.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahin H.B., Karatas O.F., Specchia V., Tommaso S.D., Diebold C., Bozzetti M.P., Giangrande A. Novel mutants of the aubergine gene. Fly. 2016;10:81–90. doi: 10.1080/19336934.2016.1174355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sato K., Iwasaki Y.W., Shibuya A., Carninci P., Tsuchizawa Y., Ishizu H., Siomi M.C., Siomi H. Krimper enforces an antisense bias on piRNA pools by binding AGO3 in the Drosophila germline. Mol. Cell. 2015;59:553–563. doi: 10.1016/j.molcel.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 90.Gatti M., Pimpinelli S., Santini G. Characterization of Drosophila heterochromatin. I. Staining and decondensation with Hoechst 33258 and quinacrine. Chromosoma. 1976;57:351–375. doi: 10.1007/BF00332160. [DOI] [PubMed] [Google Scholar]
- 91.Palumbo G., Bonaccorsi S., Robbins L.G., Pimpinelli S. Genetic analysis of Stellate elements of Drosophila melanogaster. Genetics. 1994;138:1181–1197. doi: 10.1093/genetics/138.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Livak K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Livak K.J. Detailed structure of the Drosophila melanogaster stellate genes and their transcripts. Genetics. 1990;124:303–316. doi: 10.1093/genetics/124.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shevelyov Y.Y. Copies of a Stellate gene variant are located in the X heterochromatin of Drosophila melanogaster and are probably expressed. Genetics. 1992;132:1033–1037. doi: 10.1093/genetics/132.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bozzetti M.P., Fanti L., Di Tommaso S., Piacentini L., Berloco M., Tritto P., Specchia V. The “Special” crystal-Stellate System in Drosophila melanogaster Reveals Mechanisms Underlying piRNA Pathway-Mediated Canalization. Genet. Res. Int. 2012;2012:5. doi: 10.1155/2012/324293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meyer G.F., Hess O., Beermann W. Phase specific function structure in spermatocyte nuclei of Drosophila melanogaster and their dependence of Y chromosomes. Chromosoma. 1961;12:676–716. doi: 10.1007/BF00328946. [DOI] [PubMed] [Google Scholar]
- 97.Hardy R.W., Lindsley D.L., Livak K.J., Lewis B., Siversten A.L., Joslyn G.L., Edwards J., Bonaccorsi S. Cytogenetic analysis of a segment of the Y chromosome of Drosophila melanogaster. Genetics. 1984;107:591–610. doi: 10.1093/genetics/107.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt A., Palumbo G., Bozzetti M.P., Tritto P., Pimpinelli S., Schafer U. Genetic and molecular characterization of sting, a gene involved in crystal formation and meiotic drive in the male germ line of Drosophila melanogaster. Genetics. 1999;151:749–760. doi: 10.1093/genetics/151.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tritto P., Specchia V., Fanti L., Berloco M., D’Alessandro R., Pimpinelli S., Palumbo G., Bozzetti M.P. Structure, regulation and evolution of the crystal-Stellate system of Drosophila. Genetica. 2003;117:247–257. doi: 10.1023/A:1022960632306. [DOI] [PubMed] [Google Scholar]
- 100.Bozzetti M.P., Massari S., Finelli P., Meggio F., Pinna L.A., Boldyreff B., Issinger O.G., Palumbo G., Ciriaco C., Bonaccorsi S., et al. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. USA. 1995;92:6067–6071. doi: 10.1073/pnas.92.13.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Specchia V., Piacentini L., Tritto P., Fanti L., D’Alessandro R., Palumbo G., Pimpinelli S., Bozzetti M.P. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463:662–665. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 102.Aravin A.A., Naumova N.M., Tulin A.V., Vagin V.V., Rozovsky Y.M., Gvozdev V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 2001;11:1017–1027. doi: 10.1016/S0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 103.Aravin A.A., Lagos-Quintana M., Yalcin A., Zavolan M., Marks D., Snyder B., Gaasterland T., Meyer J., Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev. Cell. 2003;5:337–350. doi: 10.1016/S1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 104.Stapleton W., Das S., McKee B.D. A role of the Drosophila homeless gene in repression of Stellate in male meiosis. Chromosoma. 2001;110:228–240. doi: 10.1007/s004120100136. [DOI] [PubMed] [Google Scholar]
- 105.Bozzetti M.P., Specchia V., Cattenoz P.B., Laneve P., Geusa A., Sahin H.B., Di Tommaso S., Friscini A., Massari S., Diebold C., et al. The Drosophila fragile X mental retardation protein participates in the piRNA pathway. J. Cell Sci. 2015;128:2070–2084. doi: 10.1242/jcs.161810. [DOI] [PubMed] [Google Scholar]
- 106.Ishizu H., Siomi H., Siomi M.C. Biology of PIWI-interacting RNAs: New insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siomi M.C., Miyoshi T., Siomi H. piRNA-mediated silencing in Drosophila germlines. Semin. Cell Dev. Biol. 2010;21:754–759. doi: 10.1016/j.semcdb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Specchia V., Bozzetti M.P. Different aubergine alleles confirm the specificity of different RNAi pathways in Drosophila melanogaster. Fly. 2009;3:170–172. doi: 10.4161/fly.8054. [DOI] [PubMed] [Google Scholar]
- 109.Kirino Y., Kim N., de Planell-Saguer M., Khandros E., Chiorean S., Klein P.S., Rigoutsos I., Jongens T.A., Mourelatos Z. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat. Cell Biol. 2009;11:652–658. doi: 10.1038/ncb1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang L., Chen D., Duan R., Xia L., Wang J., Qurashi A., Jin P. Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development. 2007;134:4265–4272. doi: 10.1242/dev.009159. [DOI] [PubMed] [Google Scholar]
- 111.Mugat B., Akkouche A., Serrano V., Armenise C., Li B., Brun C., Fulga T.A., van Vactor D., Pelisson A., Chambeyron S. MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells. PLoS Genet. 2015;11:e1005194. doi: 10.1371/journal.pgen.1005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cook H.A., Koppetsch B.S., Wu J., Theurkauf W.E. The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell. 2004;116:817–829. doi: 10.1016/S0092-8674(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 113.Handler D., Olivieri D., Novatchkova M., Gruber F.S., Meixner K., Mechtler K., Stark A., Sachidanandam R., Brennecke J. A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 2011;30:3977–3993. doi: 10.1038/emboj.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zamparini A.L., Davis M.Y., Malone C.D., Vieira E., Zavadil J., Sachidanandam R., Hannon G.J., Lehmann R. Vreteno, a gonad-specific protein, is essential for germline development and primary piRNA biogenesis in Drosophila. Development. 2011;138:4039–4050. doi: 10.1242/dev.069187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olivieri D., Senti K.A., Subramanian S., Sachidanandam R., Brennecke J. The cochaperone shutdown defines a group of biogenesis factors essential for all piRNA populations in Drosophila. Mol. Cell. 2012;47:954–969. doi: 10.1016/j.molcel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arkov A.L., Wang J.Y., Ramos A., Lehmann R. The role of Tudor domains in germline development and polar granule architecture. Development. 2006;133:4053–4062. doi: 10.1242/dev.02572. [DOI] [PubMed] [Google Scholar]
- 117.Nishida K.M., Okada T.N., Kawamura T., Mituyama T., Kawamura Y., Inagaki S., Huang H., Chen D., Kodama T., Siomi H., Siomi M.C. Functional involvement of Tudor and dPRMT5 in the piRNA processing pathway in Drosophila germlines. EMBO J. 2009;28:3820–3831. doi: 10.1038/emboj.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anne J. Targeting and anchoring Tudor in the pole plasm of the Drosophila oocyte. PLoS ONE. 2010;5:e14362. doi: 10.1371/journal.pone.0014362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patil V.S., Anand A., Chakrabarti A., Kai T. The Tudor domain protein Tapas, a homolog of the vertebrate Tdrd7, functions in piRNA pathway to regulate retrotransposons in germline of Drosophila melanogaster. BMC Biol. 2014;12:61. doi: 10.1186/s12915-014-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anand A., Kai T. The tudor domain protein kumo is required to assemble the nuage and to generate germline piRNAs in Drosophila. EMBO J. 2012;31:870–882. doi: 10.1038/emboj.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu L., Qi H., Wang J., Lin H. PAPI, a novel TUDOR-domain protein, complexes with AGO3, ME31B and TRAL in the nuage to silence transposition. Development. 2011;138:1863–1873. doi: 10.1242/dev.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rangan P., Malone C.D., Navarro C., Newbold S.P., Hayes P.S., Sachidanandam R., Hannon G.J., Lehmann R. piRNA production requires heterochromatin formation in Drosophila. Curr. Biol. 2011;21:1373–1379. doi: 10.1016/j.cub.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pek J.W., Lim A.K., Kai T. Drosophila maelstrom ensures proper germline stem cell lineage differentiation by repressing microRNA-7. Dev. Cell. 2009;17:417–424. doi: 10.1016/j.devcel.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 124.Anne J., Mechler B.M. Valois, a component of the nuage and pole plasm, is involved in assembly of these structures, and binds to Tudor and the methyltransferase Capsuleen. Development. 2005;132:2167–2177. doi: 10.1242/dev.01809. [DOI] [PubMed] [Google Scholar]
- 125.Anne J., Ollo R., Ephrussi A., Mechler B.M. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development. 2007;134:137–146. doi: 10.1242/dev.02687. [DOI] [PubMed] [Google Scholar]
- 126.Tomari Y., Du T., Zamore P.D. Sorting of Drosophila small silencing RNAs. Cell. 2007;130:299–308. doi: 10.1016/j.cell.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Preall J.B., Czech B., Guzzardo P.M., Muerdter F., Hannon G.J. shutdown is a component of the Drosophila piRNA biogenesis machinery. RNA. 2012;18:1446–1457. doi: 10.1261/rna.034405.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagao A., Mituyama T., Huang H., Chen D., Siomi M.C., Siomi H. Biogenesis pathways of piRNAs loaded onto AGO3 in the Drosophila testis. RNA. 2010;16:2503–2515. doi: 10.1261/rna.2270710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox D.N., Chao A., Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 130.Baillie J.K., Barnett M.W., Upton K.R., Gerhardt D.J., Richmond T.A., de Sapio F., Brennan P.M., Rizzu P., Smith S., Fell M., et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature. 2011;479:534–537. doi: 10.1038/nature10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rajasethupathy P., Antonov I., Sheridan R., Frey S., Sander C., Tuschl T., Kandel E.R. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomas C.A., Paquola A.C., Muotri A.R. LINE-1 retrotransposition in the nervous system. Annu. Rev. Cell Dev. Biol. 2012;28:555–573. doi: 10.1146/annurev-cellbio-101011-155822. [DOI] [PubMed] [Google Scholar]
- 133.Reilly M.T., Faulkner G.J., Dubnau J., Ponomarev I., Gage F.H. The role of transposable elements in health and diseases of the central nervous system. J. Neurosci. 2013;33:17577–17586. doi: 10.1523/JNEUROSCI.3369-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lasko P. The DEAD-box helicase Vasa: Evidence for a multiplicity of functions in RNA processes and developmental biology. Biochim. Biophys. Acta. 2013;1829:810–816. doi: 10.1016/j.bbagrm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 135.Kibanov M.V., Egorova K.S., Ryazansky S.S., Sokolova O.A., Kotov A.A., Olenkina O.M., Stolyarenko A.D., Gvozdev V.A., Olenina L.V. A novel organelle, the piNG-body, in the nuage of Drosophila male germ cells is associated with piRNA-mediated gene silencing. Mol. Biol. Cell. 2011;22:3410–3419. doi: 10.1091/mbc.E11-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okray Z., de Esch C.E., Van Esch H., Devriendt K., Claeys A., Yan J., Verbeeck J., Froyen G., Willemsen R., de Vrij F.M., et al. A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function. EMBO Mol. Med. 2015;7:423–437. doi: 10.15252/emmm.201404576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan W., Schauder C., Naryshkina T., Minakhina S., Steward R. Zfrp8 forms a complex with fragile-X mental retardation protein and regulates its localization and function. Dev. Biol. 2016;410:202–212. doi: 10.1016/j.ydbio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirino Y., Vourekas A., Sayed N., de Lima Alves F., Thomson T., Lasko P., Rappsilber J., Jongens T.A., Mourelatos Z. Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. RNA. 2010;16:70–78. doi: 10.1261/rna.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang F., Lu F., Li P., Liu W., Zhao L., Wang Q., Cao X., Zhang L., Zhang Y.Q. Drosophila Homolog of FMRP Maintains Genome Integrity by Interacting with Piwi. J. Genet. Genom. 2016;43:11–24. doi: 10.1016/j.jgg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Megosh H.B., Cox D.N., Campbell C., Lin H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr. Biol. 2006;16:1884–1894. doi: 10.1016/j.cub.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 141.Fanti L., Pimpinelli S. HP1: A functionally multifaceted protein. Curr. Opin. Genet. Dev. 2008;18:169–174. doi: 10.1016/j.gde.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y., Xu S., Xia L., Wang J., Wen S., Jin P., Chen D. The bantam microRNA is associated with drosophila fragile X mental retardation protein and regulates the fate of germline stem cells. PLoS Genet. 2009;5:e1000444. doi: 10.1371/journal.pgen.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jin P., Alisch R.S., Warren S.T. RNA and microRNAs in fragile X mental retardation. Nat. Cell Biol. 2004;6:1048–1053. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- 144.Menon L., Mader S.A., Mihailescu M.R. Fragile X mental retardation protein interactions with the microtubule associated protein 1B RNA. RNA. 2008;14:1644–1655. doi: 10.1261/rna.1100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pillai R.S. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Behm-Ansmant I., Rehwinkel J., Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant. Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 147.Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hay B., Jan L.Y., Jan Y.N. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–433. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 149.Lasko P.F., Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 150.Liang L., Diehl-Jones W., Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- 151.Findley S.D., Tamanaha M., Clegg N.J., Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 152.Khurana J.S., Theurkauf W. piRNAs, transposon silencing, and Drosophila germline development. J. Cell Biol. 2010;191:905–913. doi: 10.1083/jcb.201006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deshpande G., Calhoun G., Schedl P. The drosophila fragile X protein dFMR1 is required during early embryogenesis for pole cell formation and rapid nuclear division cycles. Genetics. 2006;174:1287–1298. doi: 10.1534/genetics.106.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dehghani M., Lasko P. In vivo mapping of the functional regions of the DEAD-box helicase Vasa. Biol. Open. 2015;4:450–462. doi: 10.1242/bio.201410579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Verrotti A.C., Wharton R.P. Nanos interacts with cup in the female germline of Drosophila. Development. 2000;127:5225–5232. doi: 10.1242/dev.127.23.5225. [DOI] [PubMed] [Google Scholar]
- 156.Zappavigna V., Piccioni F., Villaescusa J.C., Verrotti A.C. Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc. Natl. Acad. Sci. USA. 2004;101:14800–14805. doi: 10.1073/pnas.0406451101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pisa V., Cozzolino M., Gargiulo S., Ottone C., Piccioni F., Monti M., Gigliotti S., Talamo F., Graziani F., Pucci P., Verrotti A.C. The molecular chaperone Hsp90 is a component of the cap-binding complex and interacts with the translational repressor Cup during Drosophila oogenesis. Gene. 2009;432:67–74. doi: 10.1016/j.gene.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 158.Cziko A.M., McCann C.T., Howlett I.C., Barbee S.A., Duncan R.P., Luedemann R., Zarnescu D., Zinsmaier K.E., Parker R.R., Ramaswami M. Genetic modifiers of dFMR1 encode RNA granule components in Drosophila. Genetics. 2009;182:1051–1060. doi: 10.1534/genetics.109.103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ottone C., Gigliotti S., Giangrande A., Graziani F., Verrotti di Pianella A. The translational repressor Cup is required for germ cell development in Drosophila. J. Cell Sci. 2012;125(Pt 13):3114–3123. doi: 10.1242/jcs.095208. [DOI] [PubMed] [Google Scholar]
- 160.Sawarkar R., Sievers C., Paro R. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell. 2012;149:807–818. doi: 10.1016/j.cell.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 161.Karam J.A., Parikh R.Y., Nayak D., Rosenkranz D., Gangaraju V.K. Co-chaperone Hsp70/Hsp90 organizing protein (Hop) is Required for Transposon Silencing and piRNA Biogenesis. J. Biol. Chem. 2017;292:6039–6046. doi: 10.1074/jbc.C117.777730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piacentini L., Fanti L., Specchia V., Bozzetti M.P., Berloco M., Palumbo G., Pimpinelli S. Transposons, environmental changes, and heritable induced phenotypic variability. Chromosoma. 2014;123:345–354. doi: 10.1007/s00412-014-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomson T., Liu N., Arkov A., Lehmann R., Lasko P. Isolation of new polar granule components in Drosophila reveals P body and ER associated proteins. Mech. Dev. 2008;125:865–873. doi: 10.1016/j.mod.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hillebrand J., Barbee S.A., Ramaswami M. P-body components, microRNA regulation, and synaptic plasticity. Sci. World J. 2007;7:178–190. doi: 10.1100/tsw.2007.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barbee S.A., Estes P.S., Cziko A.M., Hillebrand J., Luedeman R.A., Coller J.M., Johnson N., Howlett I.C., Geng C., Ueda R., et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 168.Darnell J.C., Jensen K.B., Jin P., Brown V., Warren S.T., Darnell R.B. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/S0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 169.Chen X., Wang J., Xie H., Zhou W., Wu Y., Qin J., Guo J., Gu Q., Zhang X., Ji T., et al. Fragile X syndrome screening in Chinese children with unknown intellectual developmental disorder. BMC Pediatr. 2015;15:77. doi: 10.1186/s12887-015-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bardoni B., Willemsen R., Weiler I.J., Schenck A., Severijnen L.A., Hindelang C., Lalli E., Mandel J.L. NUFIP1 (nuclear FMRP interacting protein 1) is a nucleocytoplasmic shuttling protein associated with active synaptoneurosomes. Exp. Cell Res. 2003;289:95–107. doi: 10.1016/S0014-4827(03)00222-2. [DOI] [PubMed] [Google Scholar]
- 171.Cabart P., Chew H.K., Murphy S. BRCA1 cooperates with NUFIP and P-TEFb to activate transcription by RNA polymerase II. Oncogene. 2004;23:5316–5329. doi: 10.1038/sj.onc.1207684. [DOI] [PubMed] [Google Scholar]
- 172.Rothe B., Saliou J.M., Quinternet M., Back R., Tiotiu D., Jacquemin C., Loegler C., Schlotter F., Pena V., Eckert K., et al. Protein Hit1, a novel box C/D snoRNP assembly factor, controls cellular concentration of the scaffolding protein Rsa1 by direct interaction. Nucleic Acids Res. 2014;42:10731–10747. doi: 10.1093/nar/gku612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Minakhina S., Changela N., Steward R. Zfrp8/PDCD2 is required in ovarian stem cells and interacts with the piRNA pathway machinery. Development. 2014;141:259–268. doi: 10.1242/dev.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Minakhina S., Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gangaraju V.K., Yin H., Weiner M.M., Wang J., Huang X.A., Lin H. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nat. Genet. 2011;43:153–158. doi: 10.1038/ng.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peng J.C., Lin H. Beyond transposons: The epigenetic and somatic functions of the Piwi-piRNA mechanism. Curr. Opin. Cell Biol. 2013;25:190–194. doi: 10.1016/j.ceb.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]