Abstract
Gram-negative bacterial endotoxin lipopolysaccharide (LPS) is implicated in acute and chronic liver injury; its effects are mediated predominantly via the membrane receptor Toll-like receptor 4 (TLR4). However, TLR4-independent effects of LPS may play important role in hepatic pathophysiology. We investigated carbon tetrachloride (CCl4)–induced fibrosis and LPS-induced acute liver injury in wild-type (WT) and B6.B10ScN-Tlr4lps-del/JthJ [TLR4-knockout (KO)] mice. Effects of LPS on fibrogenic hepatic stellate cells (HSCs) from WT and TLR4-KO mice were assessed in vitro. CCl4 produced similar fibrosis and necroinflammation and increased the mRNA and protein expression of cytokines and chemokines in WT and TLR4-KO mice. However, circulating LPS concentration did not increase in CCl4-treated mice. Interestingly, LPS down-modulated α-smooth muscle actin (activated HSC marker) and collagen 1 in both WT and TLR4-KO HSCs. LPS induced similar activation of NF-κB, and stimulated the expression of cytokines and chemokines in WT and TLR4-KO HSCs. Finally, LPS caused similar inflammation and injury in previously untreated WT and TLR4-KO mice. The results provide evidence of the TLR4/LPS-independent mechanisms of liver fibrosis and also indicate that TLR4 is not entirely critical to LPS-induced acute liver injury. The results further indicate that LPS signaling in activated HSCs might be a mechanism of limiting liver fibrosis.
Physiologically, the liver is exposed to very low concentrations of gut-derived lipopolysaccharide (LPS), a component of the outer wall of the gram-negative bacteria. LPS is efficiently cleared by the liver, but its blood levels increase during liver injury or on increased gut permeability.1, 2, 3 At elevated levels, LPS provokes inflammation and innate immune responses that are implicated in acute and chronic hepatic pathogenesis.4, 5 LPS binds to circulating LPS-binding protein (LBP) and soluble or membrane CD14 that lacks cytoplasmic domain; the ternary complex binds to Toll-like receptor 4 (TLR4) on the surfaces of various cell types, causing receptor dimerization and activation.6 LPS activates multiple signaling pathways, including NF-κB, nonreceptor tyrosine kinases, protein kinase C, and mitogen-activated protein kinases in various cell types, including monocytes, macrophages, and dendritic cells,7, 8, 9, 10 and induces secretion of several cytokines and chemokines, nitric oxide (NO), reactive oxygen species (ROS), and eicosanoids.11, 12, 13 The released mediators, including tumor necrosis factor (TNF)-α, IL-1β, interferon (IFN)-β, IFN-γ, and ROS, create a hepatic microenvironment that is damaging to hepatocytes.4, 5, 11, 13
Experiments with CD14-overexpressing,12 CD14-deficient,14 TLR4-mutant C57/HeJ,15 and LBP-deficient mice3 provided evidence for the role of CD14-LBP/TLR4 in the in vivo responses to endotoxin. However, LPS also causes liver injury in a CD14-independent manner.14 Furthermore, LPS-induced production of TNF-α and IL-6 in TLR4-knockout (TLR4-KO) and CD14-KO mice was found to be similar to that in wild-type (WT) mice after partial hepatectomy,16 and whereas bile duct ligation elicited similar liver injury in WT and TLR4-mutant (C3H/HeJ) mice, fibrosis was strongly reduced in the latter.17 Because TLR4 is essential for LPS actions on Kupffer cells and endothelial cells, TLR4-independent effects of LPS on other cell types are obviously important in hepatic pathophysiology.
During the last 15 years, perisinusoidal hepatic stellate cells (HSCs) have been investigated to assess their responses to LPS in hepatic inflammation, immune regulation, and fibrosis.18, 19 HSCs are quiescent physiologically and differentiate into an activated proliferating and fibrogenic myofibroblast–like phenotype during liver injury. LPS-stimulated quiescent and activated HSCs produce many cytokines and chemokines and influence the hepatic microenvironment.19, 20 Although the CD14-LBP/TLR4 axis can be an important mechanism of the LPS effects on HSCs, rat quiescent HSCs that express very low levels of TLR4 respond to LPS concentration as low as 1 ng/mL in serum-free medium (ie, in the absence of LBP) and produce TNF-α, IL-6, and NO.21 On activation, rat HSCs express increased levels of TLR421 and respond to LPS with robust time-dependent increases in numerous cytokines, chemokines, adhesion molecules, and molecules associated with hepatic fibrosis in serum (LBP)–free condition.21, 22, 23 Human quiescent HSCs also express low levels of TLR4 and increase its expression on activation,24 but mouse quiescent and activated HSCs express significantly higher TLR4.17 Thus, species-specific differences may have important implications in hepatic pathophysiology in which LPS contributes to acute and chronic liver injury. To address the TLR4-independent LPS effects, we determined carbon tetrachloride (CCl4)–induced liver fibrosis in TLR4-KO and paired WT mice and examined effects of LPS on HSCs isolated from the WT and TLR4-KO mice. CCl4-induced liver fibrosis was similar in WT and TLR4-KO mice, and LPS stimulated expression of cytokines and chemokines in both WT and TLR4-KO HSCs but down-regulated fibrogenic activity in them. The data indicate that TLR4-independent effects of LPS on HSCs are critical components of chronic liver injury.
Materials and Methods
Animals
B6.B10ScN-Tlr4lps-del/JthJ (TLR4-KO) and their control C57BL/6J (B6-WT) mice were purchased form the Jackson ImmunoResearch Laboratories (West Grove, PA), bred in the Laboratory Animal Facility at University of Cincinnati, and used at 8 to 10 weeks. All studies were approved by the Institutional Animal Care and Use Committee in accordance with NIH guidelines.
CCl4 and LPS Treatments
Six mice per group for CCl4 or LPS treatment and four mice per group as vehicle-treated controls were used. Liver fibrosis was produced by injecting 0.16 mL/kg of CCl4 in 100 μL of peanut oil i.p. (twice a week) for 4 weeks, and mice were euthanized at 20 to 24 hours after the last CCl4 administration; control mice received oil alone. LPS (serotype 0111:B4; 10 mg/kg i.p.; Sigma, St. Louis, MO) or phosphate-buffered saline carrier were administered to WT and TLR4-KO mice,25 which were euthanized at 6 hours. Serum was obtained from all animals, and portions of the liver were fixed in 10% neutral buffered formalin or snap frozen in liquid nitrogen.
Assessment of Liver Injury and Fibrosis
Formalin-fixed, paraffin-embedded liver sections were stained with hematoxylin and eosin for histopathologic examination, and collagen fibers were detected by staining with Sirius red in saturated picric acid as described previously.26 Neutrophil staining was performed on paraffin sections using an anti-neutrophil antibody (clone NIMP-R14; Abcam, Cambridge, MA). Quantitative assessment or scoring of the hematoxylin and eosin sections for hepatic inflammation and necro-inflammation (scale of 0 to 4) and fibrosis (scale of 0 to 6) was performed according to the Ishak System27 independently by two investigators (J.W. and S.K.S.) who were blinded to the samples. Serum alanine aminotransferase was measured using a kit from Catachem Inc. (Oxford, CT). Hepatic hydroxyproline was measured as described previously.26
Immunostaining
Formalin-fixed liver sections (5 μm) were deparaffinized with xylene and rehydrated through a series of graded alcohol treatment, then washed with phosphate-buffered saline. The sections were then treated overnight with anti-desmin antibody (at 1:500 dilution) (Abcam, Cambridge, MA) at 4°C. Alexa Fluor 594–conjugated goat anti-rabbit IgG secondary antibody (Thermo Fisher Scientific Inc., Waltham, MA) was used for detection and Vectashield mounting medium with DAPI for nuclear staining (Vector Laboratories, Burlingame, CA). A Zeiss Axioplan-2 imaging microscope was used to obtain the images.
Isolation and Culture of HSCs
HSCs were isolated as described previously.28 Briefly, livers were digested by perfusion with Hanks' balanced salt solution containing 816 μM CaCl2.2H2O, 0.25 mg/mL of collagenase 4 (Worthington, Lakewood, NJ), and 0.50 mg/mL of protease (Sigma), excised, washed, and minced in Hanks' balanced salt solution. After filtration through a 100-μm nylon filter, the suspension was centrifuged several times (50 × g for 2 minutes at 4°C) to remove hepatocytes and debris. After centrifugation at 600 × g for 7 minutes, the nonparenchymal cell pellet was washed and HSCs purified via Histodenz (Sigma) density gradient centrifugation. HSCs were suspended in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 10% horse serum and seeded in 6- or 24-well plates at a density of 0.5 × 106/cm2. Purity of the culture was >95% to 98% as determined by desmin and glial fibrillary acidic protein immunostaining (Supplemental Figure S1A), as well as vitamin A autofluorescence and flow cytometry.25, 28, 29 HSCs were used on days 5 to 7 of culture interval, and each experiment was performed in duplicate or triplicate using two to three independent cell preparations.
mRNA Analysis
RNA was extracted using Trizol Reagent (Life Technology, Carlsbad, CA) according to the manufacturer's protocol. After quantification, 1.5 μg of RNA was reverse transcribed into cDNA using a kit from Applied Biosystems (Foster City, CA). The expression of various genes was determined using primers (Integrated DNA Technology) (Table 1) and SYBER Green master mix in a 7300 Real Time PCR System (Applied Biosystems).
Table 1.
Quantitative PCR Mouse Primer Sequences
| Name | Type | Sequence |
|---|---|---|
| β-actin | Forward | 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| Reverse | 5′-CAATAGTGATGACCTGGCCGT-3′ | |
| BAMBI | Forward | 5′-TGAAGTGACTAGCAGGGAAATG-3′ |
| Reverse | 5′-CCTGGTCTCCACAATGACTTAC-3′ | |
| CCL2 | Forward | 5′-CTCACCTGCTGCTACTCATTC-3′ |
| Reverse | 5′-ACTACAGCTTCTTTGGGACAC-3′ | |
| Caspase-11 | Forward | 5′-GCCACTTGCCAGGTCTACGAG-3′ |
| Reverse | 5′-AGGCCTGCACAATGATGACTTT-3′ | |
| Collagen 1 | Forward | 5′-AGACCTGTGTGTTCCCTACT-3′ |
| Reverse | 5′-GAATCCATCGGTCATGCTCTC-3′ | |
| CXCL1 | Forward | 5′-CTGCACCCAAACCGAAGTC-3′ |
| Reverse | 5′-AGCTTCAGGGTCAAGGCAAG-3′ | |
| IFN-γ | Forward | 5′-CTCTTCCTCATGGCTGTTTCT-3′ |
| Reverse | 5′-TTCTTCCACATCTATGCCACTT-3′ | |
| IL-10 | Forward | 5′-CCCTGGGTGAGAAGCTGAAG-3′ |
| Reverse | 5′-CACTGCCTTGCTCTTATTTTCACA-3′ | |
| IL-6 | Forward | 5′-CCGGAGAGGAGACTTCACAG-3′ |
| Reverse | 5′-TCCACGATTTCCCAGAGAAC-3′ | |
| MMP-9 | Forward | 5′-TGTTCCCGTTCATCTTTGAG-3′ |
| Reverse | 5′-ATCCTGGTCATAGTTGGCTGT-3′ | |
| MMP-13 | Forward | 5′-GACACAGCAAGCCAGAATAAAG-3′ |
| Reverse | 5′-GGAAAGCAGAGAGGGATTAACA-3′ | |
| TGF-β1 | Forward | 5′-GGTGGTATACTGAGACACCTTG-3′ |
| Reverse | 5′-CCCAAGGAAAGGTAGGTGATAG-3′ | |
| TGFβR1 | Forward | 5′-CCTTGAGTCACTGGGTGTTATG-3′ |
| Reverse | 5′-CCACTTAGCTGTCACCCTAATC-3′ | |
| TIMP-1 | Forward | 5′-CGGTGGGTGGATGAGTAATG-3′ |
| Reverse | 5′-GGCTGCACAGTGGAGAATAA-3′ | |
| TIMP-2 | Forward | 5′-GGAATGACATCTATGGCAACC-3′ |
| Reverse | 5′-GGCCGTGTAGATAAACTCGAT-3′ | |
| TLR4 | Forward | 5′-GGGTATTTGACACCCTCCATAG-3′ |
| Reverse | 5′-CAAGAGTGCTGAGGGAATACAG-3′ | |
| TNF-α | Forward | 5′-CCCAGGTATATGGGCTCATACC-3′ |
| Reverse | 5′-GCCGATTTGCTATCTCATACCAGG-3′ |
BAMBI, bone morphogenic protein and activin membrane–bound inhibitor; CCL2, chemokine (C-C motif) ligand 2; IFN-γ, interferon γ; MMP, matrix metalloproteinase; TGF-β1, transforming growth factor 1; TGFβR1, TGF-β1 receptor; TIMP, tissue inhibitor of metalloproteinase; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α.
Determination of Cytokines and Chemokines
Cytokines and chemokines in the culture supernatants or liver extracts were measured using Millipore Luminex kit (Darmstadt, Germany) or enzyme-linked immunosorbent assay kits (eBioscience, Inc., San Diego, CA).
LPS Determination
LPS concentration in the serum was measured using Endpoint Chromogenic Limulus Amoebocyte Lysate test kit (Lonza, Walkersville, MD).
Western Blotting
Proteins were extracted using radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Dallas, TX) supplemented with protease inhibitor cocktail, phenylmethylsulfonyl fluoride, and sodium orthovanadate. After separation via 10% SDS-PAGE, proteins were transferred to polyvinylidene difluoride membranes (Sigma). The membranes were then blocked, incubated with primary antibodies, washed, and treated with appropriated secondary antibodies, and proteins were identified using Amersham ECL Select reagent (GE Health Care UK Ltd., Buckinghamshire, UK).
Determination of MMP Activity
The hepatic proteolytic activity of matrix metalloproteinases (MMPs) was measured as described.30 Briefly, 10 μg of liver lysates were incubated with activation buffer (0.1 mol/L 4-aminophenylmercuric acetate) for 1 hour at 37°C in Thermomixer, then transferred to 96-well, black-walled, clear-bottom microplates. Sixty-five microliters of 20 μmol/L fluorogenic MMP substrate Dnp-Pro-β-cyclohexyl-Ala-Gly-Cys(Me)-His-Ala-Lys(NMA)-NH2 (catalog number BML-P128-0001, Enzo Life Sciences, Ann Arbor, MI) in TCNB reaction buffer (50 mmol/L Tris, pH 7.5, containing 10 mmol/L CaCl2, 0.15 mol/L NaCl, and 0.05% Brij-35) (Bio-Rad Laboratories, Hercules, CA) was added to the wells and incubation continued for 1 hour. MMP activity was measured by monitoring fluorescence (excitation/emission = 360/455 nm) over time and expressed as arbitrary units.
Statistical Analysis
The results are presented as means ± SD. Statistical significance was derived by one-way analysis of variance (Tukey's multiple comparison test) or t-test. P < 0.05 was considered significant.
Results
Similar CCl4-Induced Liver Fibrosis in WT and TLR4-KO Mice
We confirmed absence of TLR4 mRNA expression in the liver and HSCs from all batches of TLR4-KO mice used in this study (Supplemental Figure S1B). Histopathologic examination revealed severe hepatocyte swelling and moderate to marked lobular inflammation that was more predominant in pericentral areas in association with pericentral necrosis and abundant pigment-laden macrophages in both CCl4-treated WT and TLR4-KO mice; the portal inflammation was mild in both groups (Figure 1A and Supplemental Figure S2A). The magnitude of inflammatory infiltration, hepatocyte ballooning degeneration, and necrosis in the CCl4-treated TLR4-KO mice were somewhat lower compared with the WT mice; these pathologic findings were observed primarily in the centrilobular area (Figure 1A; Supplemental Figure S2A). Consistent with these findings, serum alanine aminotransferase and necroinflammation scores were lower in magnitude in CCl4-treated TLR4-KO mice (Figure 1, B and C). The expression of CXCL1 increased similarly in WT and TLR4-KO mice and that of chemokine (C-C motif) ligand 2 (CCL2) increased only in WT mice (Supplemental Figure S2, B and C).
Figure 1.
Hepatic injury and cytokine expression in carbon tetrachloride (CCl4)– treated Toll-like receptor 4 knockout (TLR4-KO) mice. A: Hematoxylin and eosin–stained liver sections reveal marked hepatocyte swelling with predominant pericentral inflammation due to CCl4 treatment. Serum alanine aminotransferase (ALT) (B) and quantification of hepatic necroinflammation (C). D and E: mRNA and proteins were extracted from vehicle- or CCl4-treated wild-type (WT) and TLR4-KO mouse livers. mRNA expression and concentrations of tumor necrosis factor (TNF)-α and interferon (IFN)-γ were determined by quantitative real-time RT-PCR and enzyme-linked immunosorbent assay, respectively. Data are expressed as means ± SD (B–E). ∗P < 0.05, ∗∗P < 0.005. Scale bar = 50 μm (A).
In addition to being an inflammatory cytokine, TNF-α is an important mediator of the activation of HSCs from their physiologic quiescent to activated fibrogenic myofibroblast–like phenotype, the major mechanism of hepatic fibrosis of various origins.31, 32, 33 IFN-γ, on the other hand, is a negative regulator of liver fibrosis.34, 35 We found similarly increased hepatic levels of TNF-α in CCl4-treated WT and TLR4-KO mice (Figure 1D). In contrast, IFN-γ protein increased significantly in CCl4-treated WT mice despite reduced mRNA expression but not in TLR4-KO mice (Figure 1E).
TLR4/LPS interaction is reported to be a major component of CCl4-induced liver fibrosis.17 However, there was similar CCl4-induced fibrosis as assessed by Sirius red staining (Figure 2A). Although quantification revealed a slightly lower fibrosis score (Figure 2B) and collagen 1 mRNA expression (Figure 2C) in TLR4-KO mice than WT mice, the difference was not statistically significant. However, hydroxyproline content (Figure 2D) (a measure of collagen deposition) was similar. Furthermore, expression of α-SMA, an established marker of activated HSCs, increased similarly in CCl4-treated WT and TLR4-KO mice (Figure 2E). Immunostaining for desmin (expressed by normal and activated HSCs) revealed similarly increased hepatic accumulation of HSCs in the CCl4-treated WT and TLR4-KO mice (Supplemental Figure S3).
Figure 2.
Carbon tetrachloride (CCl4)–induced hepatic fibrosis in Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were subjected to 4 weeks of CCl4 treatment. A: Sirius red–stained sections of representative vehicle and CCl4-treated livers had similarly extensive bridging fibrosis in WT and TLR4-KO mice. B: Hepatic fibrosis as quantified by 0 to 6 Ishak Scoring System. C: Collagen 1a1 (Col1a1) mRNA expression. D: Hydroxyproline concentration. E: Western blot reveals hepatic α-smooth muscle actin (α-SMA) expression in vehicle- and CCl4-treated WT and TLR4-KO mice; bar graph shows densitometric quantification of α-SMA relative to β-actin (both antibodies from Sigma, St. Louis, MO). F–H: Total mRNA was extracted from vehicle- or CCl4-treated WT and TLR4-KO mouse livers. Bar graphs show quantitative real-time RT-PCR data for the mRNA expression of transforming growth factor (TGF)-β1, TGF-β1 receptor (TGFβR1), and bone morphogenic protein and activin membrane–bound inhibitor (BAMBI). Data are expressed as means ± SD (B–D, F–H). ∗P < 0.05, ∗∗P < 0.005. Scale bar = 100 μm (A).
The fibrogenic cytokine transforming growth factor (TGF)-β1, secreted in the injured liver by Kupffer cells, endothelial cell, and HSCs, has been implicated in HSC activation and subsequent liver fibrogenesis.17, 31, 32, 33 Hepatic expression of TGF-β1 increased in CCl4-treated WT and TLR4-KO mice, but the expression of the TGF-β1 receptor (TGFβR1) increased only in the WT mice (Figure 2, F and G). Interestingly, hepatic mRNA expression of the TGF-β pseudoreceptor bone morphogenic protein and activin membrane–bound inhibitor (BAMBI) was similar in control and CCl4-treated WT mice but decreased in TLR4-KO mice (Figure 2H). Because BAMBI is down-regulated by LPS,17 we determined its hepatic expression and that of TGF-β1 and TGFβR1 in LPS-treated mice and found that their expressions were lower in LPS-treated WT and TLR4-KO mice, the effect being much stronger for BAMBI (Supplemental Figure S4A). We reasoned that if LPS plays a major role in hepatic fibrosis, its circulating concentration should be elevated. However, the serum concentration of LPS in both CCl4-treated WT and TLR4-KO mice was essentially similar to the control levels (Supplemental Figure S4B). To examine whether TLR4 is activated in CCl4-treated mice, we determined the expression of TLR4 and Myd88 via Western analysis and compared that with the expression level in LPS-treated mice. LPS treatment increased TLR4 expression but not of MyD88; in contrast, CCl4 treatment increased the expression of both TLR4 and MyD88 (Supplemental Figure S5A).
Because noncanonical activation of inflammasome, especially caspase-11, is reported to mediate TLR4-independent effects of LPS,36 we examined hepatic caspase-11 expression in WT and TLR4-KO mice after LPS or CCl4 treatments. Although caspase-11 expression increased strongly in LPS-treated mice, no such effect was observed in CCl4-treated WT or TLR4-KO mice (Supplemental Figure S5B). These data suggest that although both TLR4-dependent and TLR4-independent pathways are involved in LPS-induced acute liver injury, LPS may not be critical to fibrosis development.
The net deposition of extracellular matrix is regulated by MMPs and tissue inhibitors of metalloproteinases (TIMPs). MMP13 (MMP1 in human) and MMP9 degrade collagens, whereas TIMP1 and TIMP2 inhibit the activities of the MMPs.37 We observed a significant increase in the expression of MMP9, MMP13, and TIMP1 in CCl4-treated WT mice but not in TLR4-KO mice; TIMP2 expression increased modestly in CCl4-treated WT and TLR4-KO mice, but the increase was not significant statistically (Supplemental Figure S6, A and B). The results suggested that unaltered expression of MMPs might be an important factor in fibrosis in TLR4-KO mice. Indeed, measurement of the enzymatic activity in vitro revealed no significant difference between vehicle and CCl4-treated mice (Supplemental Figure S6C). Because the gene expression of MMPs is regulated at the transcriptional level, activation of latent proenzyme by proteolytic cleavage, and inhibition by TIMPs,37 the data suggest that compromised cleavage of pro-MMPs and/or inhibition of MMP activity by contemporaneously increased TIMP1 in vivo may result in increased extracellular matrix deposition in CCl4-treated mice. Alternatively or additionally, the synthesis or deposition of the extracellular matrix is sufficiently excessive to allow significant MMP-induced degradation to be apparent.
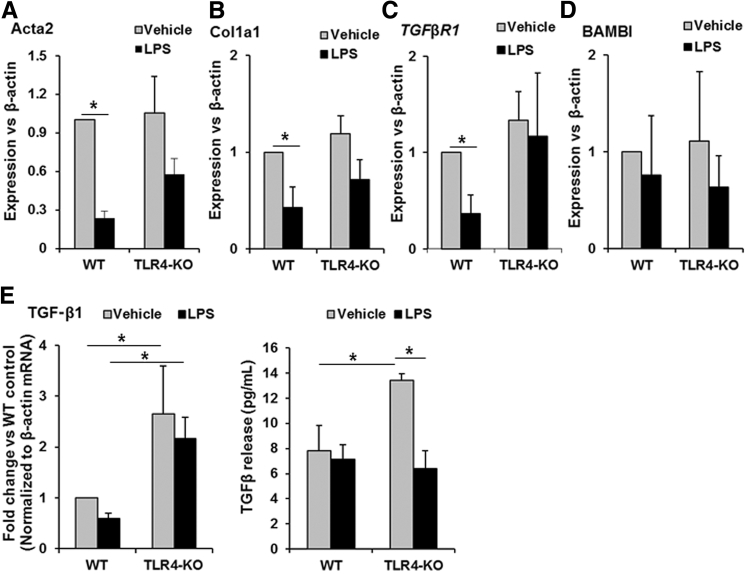
LPS Down-Regulates α-SMA and Collagen 1 Expression in Activated HSCs from WT and TLR4-KO Mice
We previously reported that rat HSCs increase TLR4 expression on activation21 and LPS down-regulates expression of α-SMA and collagen 1 in activated rat HSCs.23 Considering similar CCl4-induced fibrosis in WT and TLR4-KO mice (Figure 2), we examined the effects of LPS in culture-activated WT and TLR4-KO HSCs. LPS caused reduction in the mRNA expression of Acta2 (gene that encodes α-SMA) and Col1a1 in both WT and TLR4-KO HSCs (Figure 3, A and B). LPS down-regulated mRNA expression of TGFβR1, but not of BAMBI, in WT HSCs (Figure 3, C and D); LPS did not significantly reduce the mRNA expression of TGFβR1 or BAMBI in TLR4-KO HSCs, although the trend appears similar to that for WT HSCs. We also found that LPS did not significantly alter mRNA expression or release of TGF-β1 by WT HSCs; in contrast, TGF-β1 mRNA expression and protein release were relatively greater in TLR4-KO HSCs, and whereas LPS induced a insignificant decrease in mRNA expression of TGF-β1 in TLR4-KO HSCs, the protein release was significantly reduced (Figure 3E).
Figure 3.
Lipopolysaccharide (LPS) stimulation of hepatic stellate cells (HSCs) attenuates their fibrogenic markers. HSCs isolated from wild-type (WT) and Toll-like receptor 4 knockout (TLR4-KO) mice were treated with vehicle or 100 ng/mL of LPS for 24 hours. A–D: mRNA expression of the proteins associated with HSC activation [α-smooth muscle actin (α-SMA) or Acta2], and fibrogenic activity [collagen 1a1 (Col1a1), transforming growth factor (TGF)-β1 receptor (TGFβR1), and bone morphogenic protein and activin membrane–bound inhibitor (BAMBI)]. E: Expression of TGF-β1 mRNA and protein release by LPS-stimulated HSCs. Data are expressed as means ± SD. ∗P < 0.05.
Because LPS-induced down-regulation of BAMBI in quiescent HSCs occurred at 6 hours,17 a possibility that BAMBI may be down-regulated at an earlier time during LPS challenge was examined. In culture-activated WT or TLR4-KO HSCs, LPS did not down-regulate BAMBI at 6 hours (Supplemental Figure S7A). These findings and down-regulation of BAMBI in LPS-treated normal mice at 6 hours suggest that the effect may be distinct in quiescent and activated HSCs. We examined a possibility that TGF-β1 may reverse the inhibitory effect of LPS on α-SMA and collagen 1 expression in activated HSCs. However, TGF-β1-induced increase in α-SMA and collagen 1 expression were inhibited in presence of LPS (Supplemental Figure S7B) Furthermore, TGF-β1 slightly increased BAMBI expression in WT HSCs, but this effect was not apparent in the presence of LPS (Supplemental Figure S7A).
Cytokine and Chemokine Expression by LPS-Stimulated TLR4-KO HSCs
LPS-induced strong increase in the mRNA expression of TNF-α and IL-10 in WT HSCs; in TLR4-KO HSCs, however, LPS modestly increased the expression of TNF-α and IL-10 (Figure 4, A and B). The LPS-induced mRNA expression of the cytokines was consistent with similar increases in the corresponding proteins in culture supernatants of LPS-stimulated WT or TLR4-KO HSCs (Figure 4, A and B). No expression of IFN-γ, IL-2, IL-13, and IL-17A was detected in unstimulated or LPS-stimulated WT or TLR4-KO HSCs (data not shown). Because contaminating factors in LPS might mediate effects via other TLRs, such as TLR2, HSCs were stimulated with lipid A, the highly conserved component of LPS. Lipid A was found to produce equivalent effect as LPS on WT and TLR4-KO HSCs (Supplemental Figure S8).
Figure 4.
Lipopolysaccharide (LPS)–induced expression of cytokines and chemokines in Toll-like receptor 4 knockout (TLR4-KO) hepatic stellate cells (HSCs). HSCs were incubated in a medium that contained vehicle (grey bars) or 100 ng/mL of LPS (black bars) for 6 hours (left panels) or 24 hours (right panels). The mRNA expression of the cytokines (A and B) and chemokines (C and D) was measured by real-time quantitative PCR, and cytokines and chemokines released in the culture supernatants as measured by Luminex assay. Data are expressed as means ± SD. ∗P < 0.05. KC, keratinocyte chemoattractant; MCP-1, macrophage chemotactic protein-1.
In addition to the cytokines of hepatic injury and protection, LPS also stimulates the expression of several chemokines by mouse17, 28 and rat HSCs.23 We examined the TLR4 dependence of LPS-induced expression and release of neutrophil-, monocyte-, and T-cell–attractant KC (keratinocyte chemoattractant encoded by Cxcl1) and monocyte-, macrophage-, dendritic cell–, T-cell–attractant macrophage chemotactic protein-1 encoded by Ccl2. LPS increased the mRNA expression of CXCL1 and CCL2 by WT HSCs; the effect of LPS on TLR4-KO HSCs was almost similar for CXCL1 and modest for CCL2 in comparison to WT HSCs (Figure 4, C and D). The secretion of corresponding chemokines was consistent with the changes in mRNA expression (Figure 4, C and D).
LPS-Induced NFκB Activation and Its Coupling to Cytokine/Chemokine Expression in TLR4-KO HSCs
NFκB mediates LPS-induced synthesis of inflammatory cytokines in HSCs.23, 24, 38 Activation of NFκB involves phosphorylation-induced and proteasome-mediated degradation of the associated inhibitory component IκB; phosphorylated NFκB then translocates to nucleus and stimulates transcription of numerous cytokines, chemokines, and growth mediators.39 We observed constitutively expressed phosphorylated NFκB-p65 in WT and TLR4-KO HSCs, and LPS stimulation of both HSC phenotypes resulted in robust activation of NFκB accompanied by phosphorylation of IκBα (Figure 5A). We then assessed the effect of NFκB blockade on the key molecules in HSCs. Blocking NFκB caused inhibition of the expression of TNF-α, IL-10, and CXCL1 both in WT and TLR4-KO HSCs (Figure 5B). Together, the results indicate that TLR4 is not essential for LPS-induced NFκB activation or the synthesis of TNF-α, IL-10, or KC in activated mouse HSCs.
Figure 5.
Lipopolysaccharide (LPS)–induced activation of NF-κB and expression of tumor necrosis factor (TNF)-α, IL-10, and CXCL1 in Toll-like receptor 4 knockout (TLR4-KO) hepatic stellate cells (HSCs). A: HSCs were incubated in a medium that contained vehicle or 100 ng/mL LPS for 15 minutes. Western blot reveals phospho-IκBα (P-IκBα), phospho-NF-κB-p65 (P-p65), and β-actin (to ensure equal loading); bar graph shows densitometric quantification. B: HSCs were treated with 10 μmol/L NF-κB inhibitor (NFκB-i) BMS-345541 for 1 hour. The cells were then stimulated with 100 ng/mL of LPS for 6 hours. The bar graphs show mRNAs expression of TNF-α, IL-10, and CXCL1 as measured via quantitative real-time RT-PCR. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.0005.
TLR4-Independence of LPS-Induced Liver Injury
We recently reported a critical role of HSCs in LPS-induced hepatic inflammation and injury.25 On examining the role of TLR4, the livers of LPS-treated WT and TLR4-KO mice showed similar histological changes (Figure 6A), including mild hepatocyte swelling, and mild lobular and portal inflammation (Figure 6A; Supplemental Figure S9). The histological observations were consistent with similar necro-inflammatory scores (Figure 6B), and serum alanine aminotransferase levels in LPS-treated WT and TLR-KO mice (Figure 6C). The expression of CCL2 increased similarly in LPS-challenged WT and TLR4-KO mice, and the increase in CXCL1 was significant but of lower magnitude in TLR4-KO mice compared to WT mice (Figure 6, D and E).
Figure 6.
Lipopolysaccharide (LPS)–induced hepatic inflammation, injury, and chemokine expression in Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were administered vehicle or 10 mg/kg of LPS and sacrificed at 6 hours. A: Hematoxylin and eosin–stained sections showing mild lobular and portal inflammation in LPS-treated WT and TLR4-KO mice. Arrows indicate focal lobular inflammation; right panel shows inflammation in high-magnification images of LPS-treated mouse livers. B: Necroinflammatory scores as quantified according to Ishak Scoring System. C: Serum alanine aminotransferase (ALT) levels. D and E: Hepatic mRNA expression of chemokine (C-C motif) ligand (CCL2) and CXCL1 as determined by quantitative real-time RT-PCR. Data are expressed as means ± SD (B–E). ∗P < 0.05, ∗∗P < 0.005. Scale bar: 50 μm (left and middle panels); 20 μm (right panels).
Hepatic mRNA expression of TNF-α, IFN-γ, and IL-10 all showed strong increase in LPS-treated WT mice, and relatively small but significant increase in LPS-treated TLR4-KO mice (Figure 7A). The increase in the protein levels of these cytokines was consistent with the increase in mRNA expression, with the exception of TNF-α in LPS-treated TLR4-KO mice (Figure 7B). The basal hepatic concentrations of IFN-γ, and especially IL-10 were already somewhat higher in TLR4-KO mice compared to the WT mice.
Figure 7.
Lipopolysaccharide (LPS)–induced hepatic expression of cytokines in Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were administered vehicle (grey bars) or 10 mg/kg LPS (black bars) and sacrificed at 6 hours. Hepatic mRNA (A) and protein (B) levels of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-10 were measured by quantitative real-time RT-PCR and enzyme-linked immunosorbent assay, respectively. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.0005.
Discussion
LPS-induced signaling through LBP-CD14/TLR4 plays crucial role in acute liver injury and fibrosis, and LPS-HSC interactions can be important components of these pathologies. Here we found that TLR4-independent signaling may also be critical in CCl4-induced hepatic fibrosis and LPS-induced acute injury in mice. Although LPS stimulated the expression/release of TNF-α, IL-10, KC, and macrophage chemotactic protein-1 in TLR4-KO HSCs, the magnitude of the effect was somewhat lower than in WT HSCs, indicating the role of TLR4-dependent and -independent mechanisms. LPS also induced NF-κB activation similarly in WT and TLR4-KO HSCs, and its blockade caused inhibition of the cytokine and chemokine expression. These effects of LPS on HSCs are interesting because the macrophages obtained from Tlr2-, Tlr4-, Cd14-, and Myd88-KO mice do not produce TNF in response to LPS, and LPS does not increase circulating levels of the cytokine.14, 40, 41
If TLR4 is absolutely essential for hepatic effects of LPS in vivo, TLR4-KO mice should be resistant to hepatic inflammation and injury, given that LPS is a very potent inflammatory mediator. Thus, unlike the WT mice, TLR4-mutant C3H/HeJ mice did not have acute-phase protein response on LPS challenge; this effect was interpreted to be due to the inability of C3H/HeJ mice to produce significant levels of proinflammatory cytokines.14, 42 However, we observed similar level of inflammatory infiltration, necroinflammation, and liver injury in LPS-treated WT and the homozygous TLR4-KO B6.B10ScN-Tlr4lps-del/JthJ mice and relatively lower (but significant) increases in hepatic expression of TNF-α and CXCL1 in LPS-treated TLR4-KO mice. These findings are similar to the direct effect of LPS on WT and TLR4-KO HSCs in vitro. Clearly, the comparatively lower hepatic increase in the inflammatory mediators in TLR4-KO mice appears sufficient to induce similar injury as in WT mice.
CCl4-induced liver fibrosis was found to be strongly reduced in TLR4-mutant C3H/HeJ mice compared with WT mice.17 In contrast, CCl4-induced fibrosis in WT and TLR4-KO B6.B10ScN-Tlr4lps-del/JthJ mice was similar as determined by fibrosis score, collagen 1 expression, and hydroxyproline content. Furthermore, α-SMA expression (an established marker for activated HSCs, the major fibrogenic cell in the liver) and accumulation of desmin-positive cells (ie, HSCs) increased similarly in CCl4-treated WT and TLR4-KO mice. TGF-β1, the most potent fibrogenic cytokine, is produced at low levels in physiology by endothelial cells and Kupffer cells and mainly by HSCs during inflammation and fibrosis.43 TGF-β1 expression increased in both WT and TLR4-KO mice, but the expression of TGFβR1 increased only in WT mice. TNFα-induced increased surface kallikrein causes the release of TGF-β1 in its active form by activated HSCs.44 Previous work found that stimulation with bacterial cell wall products lipoteichoic acid or N-acetyl muramyl peptide, but not LPS, induced significant increase in TGF-β1 expression and release by BALB/c HSCs.45 Although the increase in hepatic TGF-β1 expression in CCl4-treated TLR4-KO mice was lower than in WT mice, it is plausible that most of the cytokine may be released in the active form and thus induces fibrogenic activity in HSCs.
It was suggested that LPS-induced down-regulation of BAMBI is critical to the activation and fibrogenic effect of TGF-β1 on HSCs.17 However, hepatic BAMBI expression was essentially unaltered in CCl4-treated WT and TLR4-KO mice. To gain an insight into these contrasting observations, we determined the effect of LPS on TGF-β1, TGFβR1, and BAMBI expression in previously normal mice. Interestingly, TGF-β1 and TGFβR1 expression decreased in LPS-treated WT and TLR4-KO mice, but the down-regulation of BAMBI expression was much stronger. In contrast, LPS did not affect BAMBI expression in culture-activated mouse HSCs (Figure 3D; Supplemental Figure S7A) and actually increased its expression in culture-activated rat HSCs.23 Furthermore, serum LPS levels were similar in WT and TLR4-KO mice and did not alter significantly after CCl4 treatment. These findings suggest that LPS may not regulate BAMBI expression during fibrosis progression. However, the possibility that during the early phase of acute liver injury a sudden increase in LPS in the portal circulation may transiently down-regulate BAMBI in HSCs (as seen with acute LPS treatment) (Supplemental Figure S4), thus allowing TGF-β1 to promote their activation and fibrogenic function in conjunction with inflammatory mediators, cannot be ruled out. It is worth noting that although long-term alcohol ingestion causes increase in LPS and TNF-α in rats and mice, the animals develop steatosis and steatohepatitis but not fibrosis.46, 47, 48
Free radicals, including CCl4-derived CCl3·, are considered to be primary stimuli of hepatocyte death.49, 50 The acute phase of CCl4-mediated liver fibrosis is characterized by activation of Kupffer cells, which can occur on engulfment of apoptotic bodies derived from dying hepatocytes.51 CCl4 thus elicits an inflammatory response, resulting in secretion of cytokines, chemokines, and other proinflammatory factors that attract and activate monocytes, neutrophils, and lymphocytes, which further contribute to liver cell death.49, 50 Apoptotic bodies52 and free radicals, as well as inflammatory mediators such as TNF-α, are also strong stimuli for HSC activation.31, 32 Thus, it can be concluded that TLR4-independent release of mediators from Kupffer cells and HSCs themselves are critically important in fibrosis development and an important mechanism of fibrosis in both WT and TLR4-KO mice.
The differential effects of CCl4 in causing fibrosis in C3H/HeJ17 and B6.B10ScN-Tlr4lps-del/JthJ (TLR4-KO) mice used in this investigation might be explained by the mode of the toxin administration. For example, C57BL/6J mice were found to be resistant to fibrosis when CCl4 was administered via gavage53 but not when administered i.p.54 It is possible that CCl4 administration by gavage or toxicity of thioacetamide metabolite derived from the effect of cytochrome P450 may increase gut permeability55, 56 and/or LPS release, resulting in its leakage into the portal blood.17 In addition, the bile duct ligation model used by Seki et al17 certainly disrupts gut homeostasis through the loss of bile acids in the gut, allowing LPS translocation.57, 58 Furthermore, Hillebrandt et al54 observed similar increase in hydroxyproline levels at 6 weeks of CCl4 administration in C3H/HeJ and BALB/c mice and intermediate increase in C57BL/6J mice. It will be of interest to determine what type(s) of compensatory mechanisms are instigated as a result of TLR4 mutation (C3H/HeJ) versus TLR4 deletion (B6.B10ScN-Tlr4lps-del/JthJ) to understand the discrepancy in distinct response to fibrogenic stimulation.
IFN-γ inhibits HSC activation and fibrogenesis,34, 35 and CCl4-induced (gavage) fibrosis was more pronounced in mice lacking IFN-γ than the WT mice.53 The net outcome of a pathologic process is determined by the balance of activity between the propathogenic and antipathogenic factor. Thus, it is reasonable to postulate that an increase in hepatic IFN-γ on treatment of WT mice with CCl4 (Figure 1) is an intrinsic mechanism of resisting fibrosis. The inability of the TLR4-KO mice to increase hepatic IFN-γ may be interpreted as a loss of such counterregulatory mechanism of fibrosis, resulting in the elevation of fibrosis development to the WT level.
We previously found that LPS inhibits DNA synthesis in activated HSCs.21, 22 We also observed that LPS down-regulates TGF-β1, TGFβR1, α-SMA, and collagen 1 expression in activated rat HSCs.23 Consistently, LPS strongly down-regulated the gene expression of Acta2, Col1a1, and Tgfβr1 not only in WT but also in TLR4-KO HSCs. These data suggest that LPS may act as a counterregulator of fibrogenic function of HSCs.
In summary, results of the present investigation provide evidence that during persistent inflammatory injury as that with CCl4 administrations, LPS-TLR4 interactions in activated HSCs may not be critical to hepatic fibrosis. The data, in fact, suggest that LPS may play an important role in limiting fibrosis by down-regulating TGF-β1, TGFβR1, and α-SMA in activated HSCs.
Footnotes
Supported by VA Merit Review award 1IO1BX001174 (C.R.G.) and NIH grants PO1AIO81678 and R21AA020846 (C.R.G.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.01.021.
Supplemental Data
Supplemental Figure S1.
A: Purity of stellate cells on day 3 after isolation as assessed via desmin (red) and glial fibrillary acid protein (green) immunostaining. Blue is DAPI staining of nucleus. B: The mRNA extracted from wild-type (WT) and Toll-like receptor 4 knockout (TLR4-KO) livers, and hepatic stellate cells (HSCs) were examined for TLR4 expression via quantitative PCR analysis.
Supplemental Figure S2.

Hepatic inflammation and cytokines in carbon tetrachloride (CCl4)–treated Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were subjected to 4 weeks of CCl4 treatment. A: Representative high-magnification hematoxylin and eosin–stained images show mild inflammation in portal tracts and marked hepatocyte swelling with predominant pericentral inflammation and abundant pigment-laden macrophages. Arrows indicate neutrophils. B and C: Hepatic CXCL1 and chemokine (C-C motif) ligand 2 mRNA expression as measured by quantitative real-time RT-PCR. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005. Scale bar = 20 μm (A).
Supplemental Figure S3.
Images showing desmin-expressing cells in the liver section as determined by immunohistochemistry. Bar graph shows quantification of desmin staining using ImageJ software (NIH, Bethesda, MD; http://imagej.nih.gov/ij). ∗P < 0.05. Scale bar = 100 μm. CCl4, carbon tetrachloride; TLR4-KO, Toll-like receptor 4 knockout; WT, wild type.
Supplemental Figure S4.

A: Expression of transforming growth factor (TGF)-β1 and related molecules in lipopolysaccharide (LPS)–treated Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were administered vehicle or 10 mg/kg of LPS and sacrificed at 6 hours. mRNA expression of TGF-β1, TGF-β1 receptor (TGFβ1R), and bone morphogenic protein and activin membrane–bound inhibitor (BAMBI) was determined by quantitative real-time RT-PCR, and TGF-β1 protein levels measured by enzyme-linked immunosorbent assay. B: Serum levels of LPS in carbon tetrachloride–treated mice. ∗P < 0.05, ∗∗P < 0.005.
Supplemental Figure S5.
Expression of Toll-like receptor 4 (TLR4), MyD88, and caspase-11 in lipopolysaccharide (LPS)– or carbon tetrachloride (CCl4)–treated TLR4-knockout (KO) mice. Wild-type (WT) and TLR4-KO mice were subjected to LPS- or CCl4 treatment as described in the Materials and Methods. A: Hepatic TLR4 and MyD88 expression was determined via Western analysis. Bar graph shows densitometric values for TLR4 or MyD88 using ImageJ software version 1.47v (NIH, Bethesda, MD; http://imagej.nih.gov/ij). Normalized to that of β-actin. B: mRNA expression of caspase-11 in LPS or CCl4-treated mice. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005.
Supplemental Figure S6.

A and B: Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in carbon tetrachloride (CCl4)–treated Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were subjected to 4 weeks of CCl4 treatment, and hepatic MMP13, MMP9, TIMP1, and TIMP2 mRNA expression was determined by quantitative real-time RT-PCR. C: MMP enzymatic activity in CCl4-treated TLR4-KO mice. ∗P < 0.05, ∗∗P < 0.005.
Supplemental Figure S7.
A and B: Expression of bone morphogenic protein and activin membrane–bound inhibitor (BAMBI), α-smooth muscle actin (α-SMA), and collagen 1a1 (Col1a1) in culture-activated hepatic stellate cells (HSCs) from wild-type (WT) and Toll-like receptor 4 knockout (TLR4-KO) mice. The HSCs were isolated from the livers of WT and TLR4-KO mice. Culture-activated HSCs were treated with 100 ng/mL of lipopolysaccharide (LPS) alone and/or with 1 ng/mL of recombinant transforming growth factor (TGF)-β for 6 hours, and the mRNA expression of indicated molecules was determined by quantitative real-time RT-PCR. ∗P < 0.05.
Supplemental Figure S8.
Hepatic stellate cells (HSCs) were stimulated with 100 ng/mL of lipopolysaccharide (LPS) or lipid A for 6 hours, and the mRNA expression of tumor necrosis factor (TNF)-α and IL-10 was measured via quantitative real-time RT-PCR.
Supplemental Figure S9.
Neutrophil infiltration in lipopolysaccharide (LPS)–treated Toll-like receptor 4 knockout (TLR4-KO) mice. Wild-type (WT) and TLR4-KO mice were administered vehicle or 10 mg/kg of LPS and sacrificed at 6 hours. Formalin-fixed, paraffin-embedded liver sections (5-μm thick) were deparaffinized with xylene and rehydrated through series of graded alcohol, then the heat-mediated antigen retrieval step was performed. Sections were then washed with phosphate-buffered saline (PBS) that contained 0.001% Tween 20, blocked with 10% goat and 5% bovine serum albumin for 1 hour, and incubated with anti-neutrophil antibody (clone NIMP-R14) for 2 hours at room temperature. After washing, the sections were incubated with horseradish peroxidase–conjugated goat anti-rat IgG (1:400) (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by 3,3′-diaminobezidine. Data are expressed as means ± SD of neutrophils counted in four to six high-power fields. ∗P < 0.05. Scale bar = 50 μm.
References
- 1.Triger D.R., Boyer T.D., Levin J. Portal and systemic bacteraemia and endotoxaemia in liver disease. Gut. 1978;19:935–939. doi: 10.1136/gut.19.10.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumsden A.B., Henderson J.M., Kutner M.H. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 3.Jack R.S., Fan X., Bernheiden M., Rune G., Ehlers M., Weber A., Kirsch G., Mentel R., Fürll B., Freudenberg M., Schmitz G., Stelter F., Schütt C. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature. 1997;389:742–745. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 4.Soares J.B., Pimentel-Nunes P., Roncon-Albuquerque R., Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liaskou E., Wilson D.V., Oo Y.H. Innate immune cells in liver inflammation. Mediators Inflamm. 2012;2012:949157. doi: 10.1155/2012/949157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton M.J., Golenbock D.T. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein S.L., Gold M.R., DeFranco A.L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991;88:4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J., Lee J.D., Bibbs L., Ulevitch R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 9.Shinji H., Akagawa K.S., Yoshida T. LPS induces selective translocation of protein kinase C-β in LPS-responsive mouse macrophages, but not in LPS-nonresponsive mouse macrophages. J Immunol. 1994;153:5760–5771. [PubMed] [Google Scholar]
- 10.Sanghera J.S., Weinstein S.L., Aluwalia M., Girn J., Pelech S.L. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 11.Su G.L. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero E., Jiao D., Tsuberi B., Tesio L., Rong G.W., Haziot A., Goyert S.M. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci U S A. 1993;90:2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurman R.G., Bradford B.U., Iimuro Y., Knecht K.T., Arteel G.E., Yin M., Connor H.D., Wall C., Raleigh J.A., Frankenberg M.V., Adachi Y., Forman D.T., Brenner D., Kadiiska M., Mason R.P. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13(Suppl):S39–S50. doi: 10.1111/jgh.1998.13.s1.39. [DOI] [PubMed] [Google Scholar]
- 14.Haziot A., Lin X.Y., Zhang F., Goyert S.M. The induction of acute phase proteins by lipopolysaccharide uses a novel pathway that is CD14-independent. J Immunol. 1998;160:2570–2572. [PubMed] [Google Scholar]
- 15.Poltorak A., He X., Smirnova I., Liu M.-Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 16.Campbell J.S., Riehle K.J., Brooling J.T., Bauer R.L., Mitchell C., Fausto N. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522–2528. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- 17.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi C.R. Hepatic stellate cells. In: Monga S.P., editor. Molecular pathology of liver diseases. Springer; New York, NY: 2010. pp. 53–80. [Google Scholar]
- 19.Gandhi C.R. Stellate cells in hepatic immunological tolerance. In: Gandhi C.R., Pinzani M., editors. Stellate cells in health and disease. Elsevier Press; Philadelphia, PA: 2015. pp. 227–249. [Google Scholar]
- 20.Marra F., Caligiuri A. Cytokine production and signaling in stellate cells. In: Gandhi C.R., Pinzani M., editors. Stellate cells in health and disease. Elsevier Press; Philadelphia, PA: 2015. pp. 63–86. [Google Scholar]
- 21.Thirunavukkarasu C., Uemura T., Wang L.F., Watkins S.C., Gandhi C.R. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–665. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- 22.Uemura T., Gandhi C.R. Inhibition of DNA synthesis in cultured hepatocytes by endotoxin-conditioned medium of activated stellate cells is transforming growth factor-β- and nitric oxide-independent. Br J Pharmacol. 2001;133:1125–1133. doi: 10.1038/sj.bjp.0704151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey S.A.K., Dangi A., Tandon A., Gandhi C.R. The transcriptomic response of rat hepatic stellate cells to endotoxin: implications for hepatic inflammation and immune regulation. PLoS One. 2013;8:e82159. doi: 10.1371/journal.pone.0082159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik Y.H., Schwabe R.F., Bataller R., Russo M.P., Jobin C., Brenner D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 25.Stewart R., Dangi A., Huang C., Murase N., Kimura S., Stolz D.B., Wilson G.C., Lentsch A.B., Gandhi C.R. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol. 2014;60:298–305. doi: 10.1016/j.jhep.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi C.R., Chaillet J.R., Nalesnik M.A., Kumar S., Dangi A., Demetris A.J., Ferrell R., Wu T., Divanovic S., Stankeiwicz T., Shaffer B., Stolz D.B., Harvey S.A.K., Wang J., Starzl T.E. Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology. 2015;148:379–391. doi: 10.1053/j.gastro.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F., Denk H., Desmet V., Korb G., MacSween R.N.M., Phillips M.J., Portmann B.G., Poulsen H., Scheuer P.J., Schmid M., Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 28.Dangi A., Sumpter T.L., Kimura S., Stolz D.B., Murase N., Raimondi G., Vodovotz Y., Huang C., Thomson A.W., Gandhi C.R. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S., Wang J., Thomson A., Gandhi C.R. Hepatic stellate cells increase the immunosuppressive function of natural Foxp3+ regulatory T cells via IDO-induced AhR activation. J Leukoc Biol. 2017;101:429–438. doi: 10.1189/jlb.2A0516-239R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessenbrock K., Brown M., Werb Z. Measuring matrix metalloproteinase activity in macrophages and polymorphonuclear leukocytes. Curr Protoc Immunol. 2011;Chapter 14:Unit14.24. doi: 10.1002/0471142735.im1424s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa D., Wallace M.C., Friedman S.L. Stellate cells and hepatic fibrosis. In: Gandhi C.R., Pinzani M., editors. Stellate cells in health and disease. Elsevier; San Diego, CA: 2015. pp. 41–62. [Google Scholar]
- 32.Koyama Y., Wang P., Brenner D.A., Kisseleva T. Stellate cells, portal myofibroblasts and epithelial-to-mesenchymal transition. In: Gandhi C.R., Pinzani M., editors. Stellate cells in health and disease. Elsevier; San Diego, CA: 2015. pp. 87–106. [Google Scholar]
- 33.Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H., Pradere J.P., Schwabe R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockey D.C., Maher J.J., Jarnagin W.R., Gabbiani G., Friedman S.L. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 35.Rockey D.C., Chung J.J. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994;42:660–670. [PubMed] [Google Scholar]
- 36.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszyński A., Forsberg L.S., Carlson R.W., Dixit V.M. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 37.Campana L., Iredale J. Matrix metalloproteinases and their inhibitors. In: Gandhi C.R., Pinzani M., editors. Stellate cells in health and disease. Elsevier Press; Philadelphia, PA: 2015. pp. 107–124. [Google Scholar]
- 38.Thirunavukkarasu C., Watkins S., Gandhi C.R. Mechanisms of endotoxin-induced nitric oxide, interleukin-6 and tumor necrosis factor-a production in activated rat hepatic stellate cells: role of p38MAPK. Hepatology. 2006;44:389–398. doi: 10.1002/hep.21254. [DOI] [PubMed] [Google Scholar]
- 39.Karin M. How NFκB is activated: the role of the IκB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 41.Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 42.Watson J., Largen M., McAdam K.P. Genetic control of endotoxic responses in mice. J Exp Med. 1978;147:39. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bissell D.M., Wang S.S., Jarnagin W.R., Roll F.J. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akita K., Okuno M., Enya M., Imai S., Moriwaki H., Kawada N., Suzuki Y., Kojima S. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002;123:352–364. doi: 10.1053/gast.2002.34234. [DOI] [PubMed] [Google Scholar]
- 45.Brun P., Castagliuolo I., Pinzani M., Palù G., Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 46.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandrekar P., Szabo G. Signaling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S., Wang J., Rani R., Gandhi C.R. Hepatic deficiency of augmenter of liver regeneration exacerbates alcohol-induced liver injury and promotes fibrosis in mice. PLoS One. 2016;11:e0147864. doi: 10.1371/journal.pone.0147864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams A.T., Burk R.F. Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Semin Liver Dis. 1990;10:279–284. doi: 10.1055/s-2008-1040483. [DOI] [PubMed] [Google Scholar]
- 50.Heindryckx F., Colle I., van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canbay A., Feldstein A.E., Higuchi H., Werneburg N., Gambihler A., Bronk S.F., Gores G. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 52.Canbay A., Higuchi H., Bronk S.F., Taniai M., Sebo T.J., Gores G.J. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 53.Shi Z., Wakil A.E., Rockey D.C. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillebrandt S., Goos C., Matern S., Lammert F. Genome-wide analysis of hepatic fibrosis in inbred mice identifies the susceptibility locus Hfib1 on chromosome 15. Gastroenterology. 2002;123:2041–2051. doi: 10.1053/gast.2002.37069. [DOI] [PubMed] [Google Scholar]
- 55.Ortega M.A., Torres M.I., Fernández M.I., Rios A., Sánchez-Pozo A., Gil A. Hepatotoxic agent thioacetamide induces biochemical and histological alterations in rat small intestine. Dig Dis Sci. 1997;42:1715–1723. doi: 10.1023/a:1018817600238. [DOI] [PubMed] [Google Scholar]
- 56.Harputluoglu M.M., Demirel U., Gul M., Temel I., Gursoy S., Selcuk E.B., Aladag M., Bilgic Y., Gunduz E., Seckin Y. Effects of rifaximin on bacterial translocation in thioacetamide-induced liver injury in rats. Inflammation. 2012;35:1512–1517. doi: 10.1007/s10753-012-9465-2. [DOI] [PubMed] [Google Scholar]
- 57.Parks R.W., Clements W.D.B., Smye M.G., Pope C., Rowlands B.J., Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–1349. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 58.Luyer M.D., Buurman W.A., Hadfoune M., Jacobs J.A., Dejong C.H., Greve J.W. High-fat enteral nutrition reduces endotoxin, tumor necrosis factor-alpha and gut permeability in bile duct-ligated rats subjected to hemorrhagic shock. J Hepatol. 2004;41:377–383. doi: 10.1016/j.jhep.2004.04.026. [DOI] [PubMed] [Google Scholar]