Abstract
Background
Obesity is linked to cardiovascular diseases and increasingly common in type 1 diabetes mellitus (T1DM) since the introduction of intensified insulin therapy. Our main aim was to explore associations between obesity and depression, anxiety, alexithymia and self-image measures and to control for lifestyle variables in a sample of persons with T1DM. Secondary aims were to explore associations between abdominal and general obesity and cardiovascular complications in T1DM.
Methods
Cross sectional study of 284 persons with T1DM (age 18–59 years, men 56%), consecutively recruited from one secondary care hospital diabetes clinic in Sweden. Assessments were performed with self-report instruments (Hospital Anxiety and Depression Scale, Toronto Alexithymia Scale-20 items and Structural Analysis of Social Behavior). Anthropometrics and blood samples were collected for this study and supplemented with data from the patients’ medical records. Abdominal obesity was defined as waist circumference men/women (meters): ≥1.02/≥0.88, and general obesity as BMI ≥30 kg/m2 for both genders. Abdominal obesity was chosen in the analyses due to the high association with cardiovascular complications. Different explanatory logistic regression models were elaborated for the associations and calibrated and validated for goodness of fit with the data variables.
Results
The prevalence of abdominal obesity was 49/284 (17%), men/women: 8%/29% (P < 0.001). Abdominal obesity was associated with women (AOR 4.9), physical inactivity (AOR 3.1), alexithymia (AOR 2.6) and age (per year) (AOR 1.04). One of the three alexithymia sub factors, “difficulty identifying feelings” (AOR 3.1), was associated with abdominal obesity. Gender analyses showed that abdominal obesity in men was associated with “difficulty identifying feelings” (AOR 7.7), and in women with use of antidepressants (AOR 4.3) and physical inactivity (AOR 3.6). Cardiovascular complications were associated with abdominal obesity (AOR 5.2).
Conclusions
Alexithymia, particularly the alexithymia subfactor “difficulty identifying feelings”, physical inactivity, and women, as well as cardiovascular complications were associated with abdominal obesity. As abdominal obesity is detrimental in diabetes due to its association with cardiovascular complications, our results suggest two risk factor treatment targets: increased emotional awareness and increased physical activity.
Keywords: Alexithymia, Anxiety, Cardiovascular complications, Depression, Emotions, Gender, Obesity, Physical activity, Self-image, Type 1 diabetes mellitus
Background
Obesity, particularly abdominal obesity, is an important risk factor for impaired glycemic control, cardiometabolic diseases and increased mortality [1–4]. The prevalence of obesity is increasing in Sweden as well as globally [5, 6]. In Sweden in 2009, when this study was conducted, the prevalence of general obesity (BMI ≥ 30 kg/m2) was 11% in men, and 10% in women [7]. Traditionally in type 1 diabetes mellitus (T1DM), people were lean, the prevalence of the metabolic syndrome was low, and cardiovascular complications were rare [8]. Since the introduction of intensified insulin therapy in order to optimize glycemic control and to reduce microvascular complications, the prevalence of obesity, other features of the metabolic syndrome and cardiovascular complications have increased dramatically [8–10]. Cardiovascular disease is now a leading cause of death for persons with T1DM [8]. Particularly girls/women with T1DM are at risk for developing overweight and obesity [10].
Obesity has been associated with emotional eating, the tendency to eat when experiencing negative emotions [11, 12]. Emotional eating can be both a conscious behaviour to ease emotional distress, as well as an automatic reaction to unrecognized negative feelings i.e. reflexive emotional eating [13]. The latter has been associated with alexithymia [13], which is defined by difficulty identifying feelings, difficulty describing feelings, externally oriented thinking and constricted imaginative processes [14]. Persons with alexithymia have difficulties distinguishing bodily sensations due to emotional arousal from bodily sensations of somatic origin [14]. Alexithymia has been linked to obesity [15–17], and to cardiovascular mortality in men [18].
Persons with T1DM have an increased risk of depression [19]. The impact of depression on weight is depending on depression type [20, 21]. Melancholic depression is characterized by weight loss, whereas atypical depression is characterized by weight gain [20–22]. Whether either of these two depression types is more common in persons with T1DM is not known. Depression in diabetes have been linked to life style factors such as less physical activity and unhealthy diet [23]. We have previously shown that self-reported depression in this population was associated with impaired glycemic control [3] and increased midnight cortisol secretion [24]. Even if there is evidence for a positive association between obesity and anxiety disorders [25, 26], the strength of the association is depending on anxiety subtype [26]. There are gender differences, women are more affected by depression and anxiety than men, which unfortunately often go unrecognized and untreated [27].
Since persons with obesity often are stigmatized and face prejudice and discrimination [28, 29], this may negatively impact self-identity and self-perception [29]. Obese persons have indeed described self-hate, shame, guilt, and disgust directed to themselves [29].
Diabetes emotional distress is an emotional response to a demanding health-related condition in diabetes [30]. Failure to achieve diabetes treatment goals, such as maintaining or achieving normal weight, might induce diabetes emotional distress [30], which could potentially be expressed as self-blame or self-hate.
Our main hypotheses were that psychological factors are linked to obesity, and that increased knowledge of these links might be beneficial for the development of new strategies to target obesity in persons with T1DM. Our main aim was to explore associations between obesity and depression, anxiety, alexithymia and self-image in a sample of persons with T1DM controlling for potential confounders such as smoking and physical inactivity. Secondary aims were to explore associations between abdominal and general obesity and cardiovascular complications in T1DM.
Methods
Participants and procedures
This report has a cross sectional design and is one of three baseline analyses [3, 24] for a randomized controlled trial (ClinicalTrials.gov: NCT01714986) where “Affect School with Script Analysis” was tried against “Basic Body Awareness Therapy” for persons with diabetes, inadequate glycemic control and psychological symptoms [31, 32]. The participants were outpatients, recruited from one secondary care hospital diabetes clinic with a catchment population of 125,000 in southern Sweden during, the period 03/25/2009 to 12/28/2009. The participants were recruited consecutively by specialist diabetes physicians or diabetes nurses at regular follow up visits. In this study 284 persons with T1DM were included, 66% of the eligible patients, see Fig. 1. Somatic exclusion criteria were cancer, hepatic failure, end-stage renal disease, stroke with cognitive deficiency, or visual impairment to such a degree that reading the questionnaires was impossible. Mental exclusion criteria were psychotic disorder, bipolar disorder, severe personality disorder, severe substance abuse, or mental retardation. Patients underwent assessments with self-report instruments at the regular follow up visits; anthropometrics and blood samples were collected. Data regarding patients’ diagnoses, cardiovascular complications, life style factors, and medication were collected from computerized medical records.
Fig. 1.
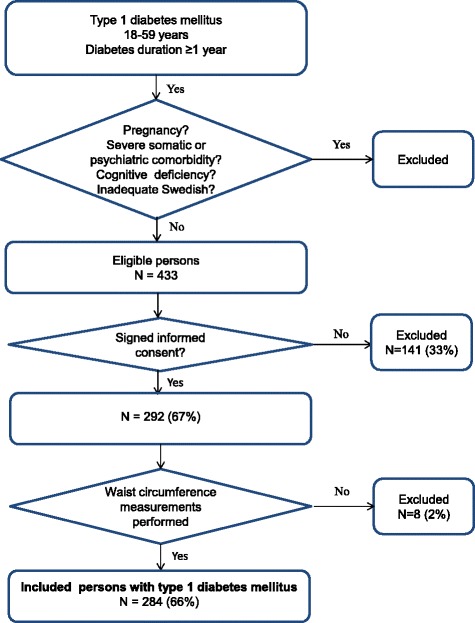
Description of criteria for inclusion in this study of obesity in persons with T1DM
Self-report instruments
Self-reported depression and anxiety were assessed by the Hospital Anxiety and Depression Scale (HADS) [33]. The depression (HADS-D) and anxiety (HADS-A) subscales, consists of 7 statements each, with 4 response alternatives from 0 to 3. The cut off level ≥ 8 points, recommended by the constructors, was used for both subscales to define self-reported depression and anxiety [33]. To try the validity of HADS, clinical psychiatric diagnosis and antidepressants use were tried against self-reported depression and self-reported anxiety.
Alexithymia was assessed by the Toronto Alexithymia Scale-20 items (TAS-20) which consists of 20 statements rated from 1 to 5 [11, 16, 17, 34, 35]. The cut off level ≥ 61 points for TAS-20 total scores, recommended by the constructors, was used to define alexithymia [17, 34, 35]. TAS-20 is based on three subscales “difficulty identifying feelings” (DIF), “difficulty describing feelings” (DDF), and “externally oriented thinking” (EOT). No normal values are presented for the subscales by the constructors [34, 35]. All three subscales were dichotomized at the 90th percentile.
Self-image measures were assessed by the Structural Analysis of Social Behavior assessment tool (SASB) [36, 37]. The questionnaire comprises 36 self-referential statements with response options on a scale from 0 to 100 with 10-point increments. The results were summarized into eight subscales (clusters): 1) self-emancipation, 2) self-affirmation, 3) self-love, 4) self-protection, 5) self-control, 6) self-blame, 7) self-hate, and 8) self-neglect. No normal values are presented for the subscales by the constructors [37]. Subscales 1, 6, 7 and 8 were dichotomized at the 90th percentile. Subscales 2, 3, 4 and 5 were dichotomized at the 10th percentile.
Anthropometrics
Waist circumference (WC), weight and length were measured by a nurse according to standard procedures. Abdominal obesity was defined as WC ≥ 1.02 m for men and as WC ≥ 0.88 m for women [1, 2, 4]. General obesity was defined as Body Mass Index (BMI) ≥ 30 kg/m2 for both genders [2].
Blood analyses
Venous HbA1c was analyzed with high pressure liquid chromatography, HPLC - variant II, Turbo analyzer (Bio – Rad®, Hercules, CA, USA) [38], at the department of Clinical Chemistry, Växjö Central Hospital. High HbA1c was defined as DCCT >8.6% (IFCC >70 mmol/mol) [3].
Data collection from medical records
Data regarding life style variables were collected. Smokers were defined as having smoked any amount of tobacco during the last year. Physical inactivity was defined as moderate activities, such as 30 min of walking, less than once a week.
Clinical psychiatric diagnoses were established prior to recruitment, and were dichotomized as having or not having a psychiatric diagnosis. Use of antidepressants was dichotomized as using or not using any type of antidepressant.
Diabetes specific treatment was divided into three groups: Multiple daily insulin injections (MDII); continuous subcutaneous insulin infusion (CSII); and MDII combined with oral antidiabetic agents (OAA).
Cardiovascular complications were defined as ischemic heart disease, stroke or transient ischemic attack.
Statistical analysis
Analysis of data distribution using histograms revealed that the test results for the self-report instruments, age and diabetes duration were not normally distributed. Data were presented as median (quartile (q)1, q3; min-max), or as median (10th, 90th percentile; min-max) and analyses were performed with Mann-Whitney U test. Fisher’s exact test (two-tailed) was used to analyze categorical data. A backward elimination multiple logistic regression analysis with cardiovascular complications as dependent variable showed that abdominal obesity had a higher association than general obesity. Thus, abdominal obesity was chosen in subsequent multiple regression modelling. Different explanatory logistic regression models were elaborated for the associations, and calibrated and validated for goodness of fit with the data variables. First crude odds ratios (CORs) were calculated. Then variables with P ≤ 0.10 were entered into multiple logistic regression models (Backward: Wald). The Hosmer and Lemeshow test for goodness-of-fit and Nagelkerke R2 were used to evaluate and calibrate the models. Confidence intervals (CIs) of 95% were used. P < 0.05 was considered statistically significant. SPSS® version 18 (IBM, Chicago, Illinois, USA) was used for all statistical analyses.
Results
Baseline data and test results for all and gender specified
In this study of obesity and psychological states and traits in TIDM (n = 284, age 18–59 years, men 56%), persons with abdominal obesity (n (%) = 49 (17)) were compared with persons without abdominal obesity (n (%) = 235 (83)). Baseline data are presented in Table 1. The women had 3.6 times higher prevalence of abdominal obesity than the men (29% compared to 8%, P < 0.001). None of the participants had been diagnosed with an eating disorder, according to their medical records.
Table 1.
Baseline characteristics for the 284 patients with T1DM presented for all and gender specified
| All | Men | Women | P a | |
|---|---|---|---|---|
| N | 284 | 159 | 125 | |
| Age (years) | 42 (32, 51; 18–59) | 43 (32, 52) | 41 (30, 50) | 0.12b |
| Diabetes duration (years) | 20 (11, 30; 1–55) | 21 (11–32) | 19 (11, 29) | 0.26b |
| Abdominal obesityc | 49 (17) | 13 (8) | 36 (29) | <0.001 |
| General obesityd | 34 (12) | 11 (7) | 23 (18) | 0.005 |
| High HbA1ce | 78 (28) | 39 (24) | 39 (31) | 0.23 |
| Smoking f | 28 (10) | 18 (12) | 10 (8) | 0.42 |
| Physical inactivityg | 31 (11) | 18 (12) | 13 (11) | 0.85 |
| Cardiovascular complications | 10 (4) | 6 (4) | 4 (3) | >0.99 |
| Clinical psychiatric diagnoses | 38 (13) | 16 (10) | 22 (17) | 0.079 |
| Antidepressants | 23 (8) | 11 (7) | 12 (10) | 0.51 |
| MDIIh and OAAi | 17 (6) | 6 (4) | 11 (9) | 0.15 |
| CSIIj | 26 (9) | 13 (8) | 13 (10) | |
| MDIIh | 241 (85) | 140 (88) | 101 (81) |
Data are n (%) or median (q1, q3; min-max)
aFisher’s exact test unless otherwise specified
bMann-Whitney U test
cAbdominal obesity: WC men/women (meters): ≥ 1.02/≥ 0.88
dGeneral obesity: BMI ≥ 30 kg/m2
eHigh HbA1c: >8.6% (>70 mmol/mol)
Missing values men/women: f n = 6/6; g n = 6/6
hMultiple daily insulin injections
iOral antidiabetic agents. j Continuous subcutaneous insulin infusion
The test results for the self-report instruments are presented in Table 2. Women had higher prevalence of self-reported anxiety (P < 0.001), high self- blame (P < 0.001), high self-hate (P = 0.001), low self-affirmation (P = 0.001), low self-love (P = 0.020), and high DIF (P = 0.036). Men had higher prevalence of high EOT (P < 0.035).
Table 2.
Test results for HADS, TAS-20 and SASB, for the 284 patients with T1DM presented for all and gender specified
| Scores a | Prevalenceb | ||||
|---|---|---|---|---|---|
| All | All | Men | Women | P c | |
| N | 284 | 284 | 159 | 125 | |
| HADS | |||||
| HADS-D scores/Depression (≥ 8p) | 3 (1, 8; 0–20) | 29 (10) | 15 (9) | 14 (11) | 0.70 |
| HADS-A scores/Anxiety (≥ 8p) | 5 (1, 11; 0–18) | 86 (34) | 39 (24) | 57 (46) | <0.001 |
| TAS-20 | |||||
| TAS-20 scores/Alexithymia (≥ 61p) | 46 (32, 64; 20–81) | 42 (15) | 19 (12) | 23 (18) | 0.13 |
| DIF scores/high DIF (≥ 23 p) | 13 (8, 23; 7–31) | 32 (11) | 12 (8) | 20 (16) | 0.036 |
| DDF scores /high DDF (≥ 18 p) | 12 (6, 18; 5–32) | 30 (11) | 17 (11) | 13 (10) | >0.99 |
| EOT scores /high EOT (≥ 27 p) | 21 (13, 27; 8–33) | 31 (11) | 23 (14) | 8 (6) | 0.035 |
| SASBd | |||||
| Self-emancipation scores /high self-emancipation (≥ 66 p)d | 50 (34, 66; 8–94) | 27 (10) | 18 (12) | 9 (7) | 0.31 |
| Self-affirmation scores /low self-affirmation (≤ 38 p)d | 70 (38, 88; 8–100) | 32 (12) | 9 (6) | 23 (19) | 0.001 |
| Self-love scores /low self-love (≤ 40 p)d | 64 (40, 86; 14–100) | 31 (11) | 11 (7) | 20 (16) | 0.020 |
| Self-protection scores /low self-protection (≤ 30 p)d | 52 (30, 75; 5–95) | 28 (10) | 19 (12) | 9 (7) | 0.23 |
| Self-control scores /low self-control (≤ 28 p)d | 52 (28, 74; 0–98) | 33 (12) | 18 (12) | 15 (12) | 0.85 |
| Self-blame scores /high self-blame (≥ 52 p)d | 18 (0, 52; 0–95) | 37 (13) | 9 (6) | 28 (23) | <0.001 |
| Self-hate scores /high self-hate (≥ 50 p)d | 16 (0, 50; 0–92) | 30 (11) | 8 (5) | 22 (18) | 0.001 |
| Self-neglect scores /high self-neglect (≥ 48 p)d | 22 (5, 48; 0–80) | 31 (11) | 18 (11) | 13 (10) | 0.85 |
aData are median (10th, 90th percentile; min-max)
b N (%)
cFisher’s exact test. dMissing values men/women: n = 3/4
Cardiovascular complications and obesity
Cardiovascular complications were associated with abdominal obesity (adjusted odds ratio (AOR) (CI) 5.2 (1.5–18.8), P = 0.011), whereas general obesity was not (AOR (CI) 0.9 (0.2–5.7), P = 0.95).
Comparisons between persons with and without abdominal obesity
The results of the comparisons are presented in Table 3. The 49 obese persons compared to the 235 non-obese had higher prevalence of high DIF (P = 0.005), self-reported anxiety (P = 0.007), cardiovascular complications (P = 0.016), high self-hate (P = 0.021), use of antidepressants (P = 0.038), and alexithymia (P = 0.046). The 13 obese men had higher prevalence of high DIF (P = 0.009) than the 146 non-obese men. The 36 obese women had higher prevalence of cardiovascular complications (P = 0.006) and use of antidepressants (P = 0.038) than the 89 non-obese women.
Table 3.
Comparisons between T1DM patients with and without abdominal obesity for all and gender specified
| All patients | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Obesitya | Non-obesity | P a | Obesitya | Non-obesity | P a | Obesitya | Non-obesity | P a | |
| N | 49 | 235 | 13 | 146 | 36 | 89 | |||
| Age (years) | 45 (35, 53) | 42 (31, 51) | 0.11b | 50 (34, 58) | 43 (32, 51) | 0.072 b | 45 (35–51) | 40 (30–48) | 0.18b |
| Diabetes duration (years) | 22 (14, 28) | 20 (11, 31) | 0.64b | 21 (8, 31) | 20 (12, 32) | 0.92 b | 23 (15–29) | 18 (10–29) | 0.27b |
| Depression (≥ 8p)c | 6 (12) | 23 (10) | 0.61 | 2 (15) | 13 (9) | 0.35 | 4 (11) | 10 (11) | >0.99 |
| Anxiety (≥ 8p)d | 25 (51) | 71 (30) | 0.007 | 3 (31) | 35 (24) | 0.74 | 21 (58) | 36 (40) | 0.078 |
| Alexithymia (≥ 61p)e | 12 (24) | 30 (13) | 0.046 | 3 (23) | 16 (11) | 0.19 | 9 (25) | 14 (16) | 0.31 |
| High DIF (≥23 p)e | 12 (24) | 20 (8) | 0.005 | 4 (31) | 8 (6) | 0.009 | 8 (22) | 12 (14) | 0.28 |
| High DDF (≥ 18 p)e | 4 (8) | 26 (11) | 0.80 | 1 (8) | 16 (11) | >0.99 | 3 (8) | 10 (11) | 0.72 |
| High EOT (≥ 27 p)e | 2 (4) | 29 (12) | 0.13 | 0 (0) | 23 (16) | 0.22 | 2 (6) | 6 (7) | >0.99 |
| High self-emancipation (≥ 66 p)f | 6 (12) | 21 (9) | 0.43 | 3 (23) | 15 (10) | 0.18 | 3 (9) | 6 (7) | 0.72 |
| Low self-affirmation (≤ 38 p)f | 9 (19) | 23 (10) | 0.13 | 1 (8) | 8 (6) | 0.55 | 8 (23) | 15 (17) | 0.61 |
| Low self-love (≤ 40 p)f | 8 (17) | 23 (10) | 0.21 | 2 (15) | 9 (6) | 0.23 | 6 (17) | 14 (16) | >0.99 |
| Low self-protection (≤ 30 p)f | 5 (10) | 23 (10) | >0.99 | 1 (8) | 18 (13) | >0.99 | 4 (11) | 5 (6) | 0.28 |
| Low self-control (≤ 28 p)f | 8 (17) | 25 (11) | 0.32 | 2 (15) | 16 (11) | 0.65 | 6 (17) | 9 (10) | 0.36 |
| High self-blame (≥ 52 p)f | 9 (19) | 28 (12) | 0.24 | 1 (8) | 8 (6) | 0.55 | 8 (23) | 20 (23) | >0.99 |
| High self-hate (≥ 50 p)f | 10 (21) | 20 (9) | 0.021 | 1 (8) | 7 (5) | 0.51 | 9 (26) | 13 (15) | 0.20 |
| High self-neglect (≥ 48 p)f | 7 (14) | 24 (10) | 0.45 | 1 (8) | 17 (12) | >0.99 | 6 (17) | 7 (8) | 0.19 |
| Smokingg | 4 (9) | 24 (11) | 0.80 | 1 (8) | 17 (12) | >0.99 | 3 (9) | 7 (8) | >0.99 |
| Physical inactivityh | 9 (19) | 22 (10) | 0.084 | 2 (15) | 16 (11) | 0.65 | 7 (20) | 6 (7) | 0.054 |
| Cardiovascular complications | 5 (10) | 5 (2) | 0.016 | 1 (8) | 5 (3) | 0.41 | 4 (11) | 0 | 0.006 |
| Clinical psychiatric diagnoses | 11 (22) | 27 (11) | 0.062 | 1 (8) | 15 (10) | >0.99 | 10 (28) | 12 (14) | 0.071 |
| Antidepressants | 8 (16) | 15 (6) | 0.038 | 1 (8) | 10 (7) | >0.99 | 7 (19) | 5 (6) | 0.038 |
| MDII and OAA | 13 (27) | 4 (2) | <0.001 | 3 (23) | 3 (2) | 0.009 | 10 (28) | 1 (1) | <0.001 |
| CSII | 1 (2) | 25 (10) | 0 | 13 (9) | 1 (3) | 12 (14) | |||
| MDII | 35 (71) | 206 (88) | 10 (77) | 130 (89) | 25 (69) | 76 (85) | |||
Obesity defined as men/women: WC ≥ 1.02/≥ 0.88 m. Data are n (%) or median (q1, q3)
aFisher’s exact test unless otherwise indicated
bMann-Whitney U test
cHADS-D
dHADS-A
eTAS-20
fSASB
Missing values for obese/non-obese men: f n = 0/3
g n = 0/6
h n = 0/6. Missing values for obese/non-obese women. f n = 1/3; g n = 2/4; h n = 1/5
Associations between psychological states and traits, life style variables and abdominal obesity
In Table 4 variables associated with abdominal obesity are presented in two models for all patients. In model 1, abdominal obesity was associated with women (AOR 4.9), physical inactivity (AOR 3.1), alexithymia (AOR 2.6), and age (per year) (AOR 1.04). In model 2, abdominal obesity was associated with women (AOR 4.9), physical inactivity (AOR 3.5), high DIF (AOR 3.1), and age (per year) (AOR 1.04). Treatment with the combination of MDII and OAA was strongly associated with obesity (COR 19.1).
Table 4.
Associations between abdominal obesity and psychological, somatic and life style variables in the T1DM patients
| Abdominal obesity (N = 265) |
||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| COR | P | AOR | P a | AOR | P a | |
| Gender (women) | 4.5 (2.3–9.0) | <0.001 | 5.0 (2.4–10.3) | <0.001 | 4.9 (2.4–10.2) | <0.001 |
| Depression (≥ 8p)b | 1.3 (0.5–3.3) | 0.61 | - | - | - | - |
| Anxiety (≥ 8p)c | 2.4 (1.3–4.5) | 0.006 | 1.5 (0.7–3.1) | 0.27 | 1.3 (0.6–2.8) | 0.51 |
| Alexithymia (≥ 61p)d | 2.2 (1.0–4.7) | 0.039 | 2.6 (1.1–6.0) | 0.028 | - | - |
| High DIF (≥23 p)d | 3.5 (1.6–7.7) | 0.002 | - | - | 3.1 (1.3–7.5) | 0.011 |
| High DDF (≥18 p)d | 0.7 (0.2–2.1) | 0.55 | - | - | - | - |
| High EOT (≥ 27 p)d | 0.3 (0.1–1.3) | 0.11 | - | - | - | - |
| High self-emancipation (≥66 p)e | 1.4 (0.5–3.7) | 0.48 | - | - | - | - |
| Low self-affirmation (≤38 p)e | 2.1 (0.9–4.8) | 0.091 | 1.0 (0.3–3.0) | 0.95 | 0.9 (0.3–2.6) | 0.85 |
| Low self-love (≤40 p)e | 1.8 (0.7–4.3) | 0.19 | - | - | - | - |
| Low self-protection (≤30 p)e | 1.0 (0.4–2.9) | 0.94 | - | - | - | - |
| Low self-control (≤28 p)e | 1.6 (0.7–3.9) | 0.27 | - | - | - | - |
| High self-blame (≥52 p)e | 1.7 (0.7–3.8) | 0.23 | - | - | - | - |
| High self-hate (≥50 p)e | 2.8 (1.2–6.3) | 0.017 | 1.1 (0.4–3.1) | 0.80 | 1.1 (0.4–3.5) | 0.85 |
| High self-neglect (≥48 p)e | 1.5 (0.6–3.6) | 0.41 | - | - | - | - |
| Smokingf | 0.8 (0.3–2.4) | 0.66 | - | - | - | - |
| Physical inactivityg | 2.1 (0.9–4.9) | 0.083 | 3.1 (1.2–8.1) | 0.023 | 3.5 (1.3–9.1) | 0.012 |
| Age (per year) | 1.02 (1.00–1.05) | 0.11 | 1.04 (1.00–1.07) | 0.024 | 1.04 (1.00–1.07) | 0.024 |
| Diabetes duration | 1.00 (0.98–1.03) | 0.77 | - | - | - | - |
| Cardiovascular complications | 5.2 (1.5–18.9) | 0.011 | - | - | - | - |
| Clinical psychiatric diagnoses | 2.2 (1.0–4.9) | 0.044 | 0.9 (0.2–3.6) | 0.86 | 0.9 (0.2–3.8) | 0.93 |
| Antidepressants | 2.9 (1.1–7.2) | 0.025 | 1.6 (0.6–4.7) | 0.38 | 1.6 (0.6–4.7) | 0.38 |
| MDII and OAA | 19.1 (5.9–62.0) | 0.001 | - | - | - | - |
| CSII | 0.2 (0.03–1.8) | 0.16 | - | - | - | - |
| MDII | 1 | - | - | - | - | |
N = 284 unless otherwise specified
a Multiple logistic regression analysis (Backward: Wald)
bHADS-D
cHADS-A
dTAS-20
eSASB
Missing values: e n = 7
f n = 12
g n = 12. Model 1/model 2: Hosmer and Lemeshow Test 0.685/0.735; Nagelkerke R Square 0.192/0.20
Variables associated with abdominal obesity are presented for each gender in Table 5. In men, high DIF (AOR 7.7) was associated with abdominal obesity. In women, use of antidepressants (AOR 4.3) and physical inactivity (AOR 3.6) were associated with abdominal obesity.
Table 5.
Associations between abdominal obesity and psychological and life style variables presented for each gender
| Abdominal obesity in men (N = 159) |
Abdominal obesity in women (N = 115) |
|||||||
|---|---|---|---|---|---|---|---|---|
| COR | P | AOR | P a | COR | P | AOR | P a | |
| Depression (≥ 8p) b | 1.9 (0.4–9.3) | 0.45 | - | - | 0.99 (0.29–3.4) | 0.98 | - | - |
| Anxiety (≥ 8p) b | 1.4 (0.4–4.9) | 0.51 | - | - | 2.1 (0.9–4.5) | 0.071 | 1.7 (0.8–4.0) | 0.20 |
| Alexithymia (≥ 61p) c | 2.4 (0.6–9.8) | 0.21 | - | - | 1.8 (0.7–4.6) | 0.23 | - | - |
| High DIF (≥23 p) c | 7.7 (1.9–30.4) | 0.004 | 7.7 (1.9–30.4) | 0.004 | 1.8 (0.7–5.0) | 0.23 | - | - |
| High DDF (≥18 p) c | 0.7 (0.1–5.6) | 0.72 | - | - | 0.7 (0.2–2.8) | 0.63 | - | - |
| High EOT (≥ 27 p) c | 0.00 | >0.99 | - | - | 0.8 (0.2–4.2) | 0.81 | - | - |
| High self-emancipation (≥66 p) d | 2.6 (0.6–10.3) | 0.19 | - | - | 1.2 (0.3–5.3) | 0.76 | - | - |
| Low self-affirmation (≤38 p) d | 1.4 (0.2–12.2) | 0.76 | - | - | 1.4 (0.5–3.7) | 0.49 | - | - |
| Low self-love (≤40 p) d | 2.7 (0.5–14.1) | 0.24 | - | - | 1.1 (0.4–3.0) | 0.91 | - | - |
| Low self-protection (≤30 p) d | 0.6 (0.1–4,7) | 0.61 | - | - | 2.1 (0.5–8.3) | 0.29 | - | - |
| Low self-control (≤28 p) d | 1.4 (0.3–7.1) | 0.65 | - | - | 1.8 (0.6–5.4) | 0.32 | - | - |
| High self-blame (≥52 p) d | 1.4 (0.2–12.2) | 0.76 | - | - | 1.0 (0.4–2.5) | 0.96 | - | - |
| High self-hate (≥50 p) d | 1.7 (0.2–14.3) | 0.66 | - | - | 1.9 (0.7–5.1) | 0.17 | - | - |
| High self-neglect (≥48 p) d | 0.6 (0.1–5.2) | 0.67 | - | - | 2.3 (0.7–7.5) | 0.15 | - | - |
| Smoking e | 0.6 (0.1–4.9) | 0.64 | - | - | 1.1 (0.3–4.4) | 0.92 | - | - |
| Physical inactivity f | 1.4 (0.3–6.9) | 0.67 | - | - | 3.2 (1.0–10.5) | 0.049 | 3.6 (1.1–11.8) | 0.037 |
| Clinical psychiatric diagnoses | 0.7 (0.1–6.0) | 0.77 | - | - | 2.5 (1.0–6.4) | 0.062 | 1.5 (0.4–6-7) | 0.57 |
| Age (per year) | 1.05 (0.99–1.11) | 0.091 | 1.04 (0.98–1.10) | 0.19 | 1.02 (0.99–1.06) | 0.19 | 1.02 (0.99–1.06) | 0.21 |
| Antidepressants | 1.1 (0.1–9.6) | 0.91 | - | - | 4.1 (1.2–13.8) | 0.025 | 4.3 (1.2–15.0) | 0.022 |
N (men/women) = 159/125 unless otherwise specified
aMultiple logistic regression analysis (Backward: Wald)
bHADS-D
cHADS-A
dTAS-20
eSASB
Missing values for men: d n = 3
e n = 6
f n = 6. Missing values for women
d n = 4
e n = 6
f n = 6. Men/women: Hosmer and Lemeshow Test 0.226/0.910; Nagelkerke R Square 0.10/0.105
Variables associated with women, alexithymia, DIF and physical inactivity - interaction tests
Associations with women: abdominal obesity (AOR 4.2 (2.0–8.7), P < 0.001), self-hate (AOR 3.1 (1.2–8.1), P = 0.022), age (per year) (AOR 0.98 (0.96–1.00), P = 0.046), and self-reported anxiety (AOR 1.8 (1.0–3.2), P < 0.050); and the p-values were higher than 0.36 for alexithymia, physical inactivity, low self-affirmation, physical inactivity, clinical psychiatric diagnosis, and use of antidepressants.
Associations with alexithymia: self-reported anxiety (AOR 3.6 (1.7–7.4), P = 0.001) and abdominal obesity (AOR 2.2 (1.0–4.9), P = 0.055); and the p-values were higher than 0.10 for physical inactivity, gender, low self-affirmation, high self-hate, physical inactivity, clinical psychiatric diagnosis, use of antidepressants, and age.
Associations with high DIF: self-reported anxiety (AOR 9.1 (3.2–26.0), P < 0.001), low self-affirmation (AOR 2.7 (1.0–7.0), P = 0.47) and abdominal obesity (AOR 2.5 (1.0–6.1), P = 0.055); the p-values were higher than 0.24 for physical inactivity, gender, high self-hate, physical inactivity, clinical psychiatric diagnosis, use of antidepressants, and age.
Associations with physical inactivity: abdominal obesity (AOR 2.6 (1.1–6.3), P = 0.034) and age (per year) (AOR 0.95 (0.92–0.99), P = 0.006); and the p-values were higher than 0.13 for alexithymia, gender, self-reported anxiety, low self-affirmation, high self-hate, clinical psychiatric diagnosis, and use of antidepressants. Significant associations with abdominal obesity, women, alexithymia and physical inactivity are presented in Fig. 2.
Fig. 2.
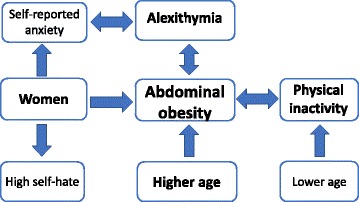
Illustration of significant associations found in this study
Validation of HADS
Clinical psychiatric diagnosis was associated with self-reported depression (COR 10.8 (4.6–25.2), P < 0.001) and self-reported anxiety (COR 2.8 (1.4–5.6), P = 0.003). Antidepressants were associated with self-reported depression (COR 9.8 (3.8–25.3), P < 0.001) and self-reported anxiety (COR 3.4 (1.4–8.2), P = 0.006).
Discussion
In this cross-sectional study of a population sample of 284 persons with T1DM, age 18–59 years, consecutively recruited from one hospital diabetes outpatient clinic, we found that female gender, physical inactivity and alexithymia were independently associated with abdominal obesity. The women had nearly 4 times higher prevalence of abdominal obesity than the men. High level of the alexithymia subfactor “difficulty identifying feelings” was responsible for the association between alexithymia and abdominal obesity. Gender analyses showed clear differences. In men, a high level of “difficulty identifying feelings” was strongly linked to abdominal obesity. In women, physical inactivity and the use of antidepressants were linked to abdominal obesity. The prevalence of general obesity in the women with T1DM was almost twice as high as in women in the Swedish population. Cardiovascular complications were strongly associated with abdominal obesity.
Strengths of our study are first that we systematically investigated the psychological states and traits in a well-defined population of persons with T1DM. Second, we explored interactions between the included variables. For example, at a first sight self-reported anxiety seemed to be associated with abdominal obesity which it was not in the further analyses. Instead self-reported anxiety was associated with both alexithymia and women which in turn were associated with abdominal obesity. Third, when the associations with abdominal obesity were tried the results were congruent. Clinical psychiatric diagnosis was not associated with abdominal obesity. Excess negative emotions, expressed as self-reported depression, anxiety, high levels of self-blame, self-hate, or self-neglect, were not linked to abdominal obesity; neither were low levels of positive emotions, expressed as low self-affirmation, low self-love or low self-protection. Fourth, we had access to both waist circumference measures and BMI, and we could choose in the analyses to use abdominal obesity, which showed the highest association with cardiovascular complications. Fifth, we validated the self-report instrument for depression and anxiety. We found strong associations with clinical psychiatric diagnosis and the use of antidepressants for both subscales of the self-report instrument.
The main limitation in our study was the small number of obese persons, particularly when the gender sub analyses were performed. There are several possible type two errors. The prevalence of alexithymia and high DIF were higher in both women and men with abdominal obesity compared to the non-obese. Also, the prevalence of physical inactivity was higher in both men and women with abdominal obesity compared to the non-obese. Second, there are difficulties connected to using the SASB and TAS-20 subscales as there are no normal values presented by the designers of these self-report instruments. We chose to compare the most extreme test results with the remaining, and all subscales were therefore dichotomized either at the 10th or 90th percentile. A third limitation is that we did not include any self-report instrument assessing diabetes related emotional distress.
This study is to our knowledge the first to demonstrate a link between alexithymia, mainly the subfactor “difficulty identifying feelings”, and abdominal obesity in persons with T1DM. However, an increased prevalence of alexithymia in persons with obesity but without diabetes has been shown [11, 15, 16]. Whether the direction of the association between abdominal obesity and alexithymia goes one-way or two-way cannot be concluded by this cross-sectional study. Alexithymia could lead to obesity, but if obesity is followed by a cognitive decline, the ability to process emotions could also decline. Persons with severe cognitive deficiencies were however excluded. The link between physical inactivity and obesity, which probably is two-ways, is in accordance with previous research in non-diabetic populations [39, 40]. The importance of obesity in T1DM in this study was demonstrated by the strong association between abdominal obesity and cardiovascular complications, as shown in previous research [1, 2, 8]. We found a gender difference with a higher obesity prevalence in the women than in the men, which also has been shown previously [10].
By this study, a new target in diabetes care emerges, “increased emotional awareness”, which today is not considered in conventional diabetes management. Persons with alexithymia have difficulties distinguishing bodily sensations due to emotional arousal from bodily sensations of somatic origin [14]. Difficulty identifying feelings might thus make it difficult to distinguish hunger from emotions involved in stress, anxiety or boredom, factors which serve as triggers for emotional eating [12]. One issue connected to diabetes emotional distress is that the affected persons do not know whether their mood and feelings are related to their diabetes or not [41]. This is probably particularly difficult for persons with alexithymia. The link between alexithymia and obesity might be one explanatory factor for the increased cardiovascular mortality found in men with alexithymia [18].
Treatment with a combination of multiple daily insulin injections and oral antidiabetic agents was associated with abdominal obesity in this study. Oral antidiabetic agents should however not be regarded as a cause of obesity, as they were added to insulin with the aim of reducing obesity and insulin resistance.
The women seemed to have more psychological symptoms than men, with higher prevalence of anxiety, high self-hate and self-blame, and low self-affirmation and self-love which also is in accordance with previous research [27]. However, these factors were not associated with abdominal obesity. The use of antidepressants was associated with abdominal obesity in women but not in men, though the frequency of antidepressant use did not differ between men and women. To adequately analyse why antidepressants were associated with obesity in women only, we would have needed to know whether women were prescribed different types of antidepressants than men, the prescribed daily dose, and the duration of medication.
There are several subjects for future research. The mechanism for the demonstrated higher obesity prevalence in women, compared both to the men with T1DM and to women in the general population, was not explained by this study and further research of this subject is necessary. Further, it would be of interest to explore whether alexithymia and diabetes emotional distress is associated, which to our knowledge has not been studied. Finally, we propose that interventions focusing on increased emotional awareness should be performed and evaluated for persons with T1DM, obesity and alexithymia [31, 32, 42–44].
Conclusions
Abdominal obesity in T1DM was associated with women, alexithymia, particularly the alexithymia subfactor “difficulty identifying feelings”, and physical inactivity. As abdominal obesity is detrimental in diabetes due to its association with cardiovascular complications, our results suggest two risk factor treatment targets: increased emotional awareness and increased physical activity.
Acknowledgments
We are grateful to Anna Lindgren, PhD, Lund University, Centre of Mathematics, Lund, Sweden, for her statistical skills.
Funding
This research was supported by the Research and Development Fund of Region Kronoberg, Växjö, Sweden. The funding source was not involved in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Availability of data and materials
All data are saved at SPSS files for 10 years at the department for Research and Development, Region Kronoberg, Växjö, Sweden. The data sets are not availably publicly as individual privacy could be compromised. The data set is available from the corresponding author on reasonable request.
Authors’ contributions
EOM, RS, MT, MH, HOT, and ML-O participated as investigators and reviewed, edited, and approved of the manuscript. All authors contributed to the study design, implementation and analysis. EOM was the initiator of this study, performed the statistical analysis, wrote the statistical methods and the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ information
EOM, MD and PhD, works as a GP in primary care and as a researcher at the Department for Research and Development, Region Kronoberg, Växjö, and is affiliated to Clinical Sciences, Lund University, Lund, Sweden.
RS, psychologist and psychotherapist, works at Linnaeus University, Department of Psychology, Växjö, Sweden.
MT, MD and PhD, works at the department of medicine at the Central Hospital, and at the department for Research and Development, Region Kronoberg, Växjö, and is affiliated to Clinical Sciences, Lund University, Lund, Sweden.
MH, PhD, works at BMC, Lund University, Lund, Sweden.
HOT, MD, PhD and assistant professor, works as a GP in primary care and as a researcher at the Department for Research and Development, Region Kronoberg, Växjö, and is affiliated to Clinical Sciences, Division of Family Medicine, Lund University, Malmö, Sweden.
ML-O, MD, PhD and professor, works at Lund University Hospital, Department of endocrinology and diabetology, Lund University, Lund, Sweden.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was conducted in accordance with the Code of Ethics of the World Medical Association, and was approved by the Regional Ethical Review Board of Linköping University, Linköping (Registration no. M120–07, T89–08). All participants provided written informed consent.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AOR
Adjusted odds ratio
- BMI
Body mass index
- CI
Confidence interval
- COR
Crude odds ratio
- CSII
Continuous subcutaneous insulin infusion
- DDF
Difficulty describing feelings
- DIF
Difficulty identifying feelings
- EOT
Externally oriented thinking
- HADS-A
Hospital Anxiety and Depression scale-anxiety-anxiety subscale
- HADS-D
Hospital Anxiety and Depression Scale-depression subscale
- MDII
Multiple daily insulin injections
- OAA
Oral antidiabetic agents
- SASB
Structural Analysis of Social Behavior assessment tool
- T1DM
Type 1 diabetes mellitus
- TAS-20
Toronto Alexithymia Scale-20 items
- WC
Waist circumference
Contributor Information
Eva O. Melin, Phone: +46703132827, Email: eva.melin@kronoberg.se
Ralph Svensson, Email: ralph.svensson@lnu.se.
Maria Thunander, Email: maria.thunander@kronoberg.se.
Magnus Hillman, Email: magnus.hillman@med.lu.se.
Hans O. Thulesius, Email: hansthulesius@gmail.com
Mona Landin-Olsson, Email: mona.landin-olsson@med.lu.se.
References
- 1.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and Cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity. 2007;15:1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 2.Tanamas SK, Permatahati V, Ng WL, Backholer K, Wolfe R, Shaw JE, et al. Estimating the proportion of metabolic health outcomes attributable to obesity: a cross-sectional exploration of body mass index and waist circumference combinations. BMC Obes. 2016;3:4. doi: 10.1186/s40608-016-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melin EO, Thunander M, Svensson R, Landin-Olsson M, Thulesius HO. Depression, obesity and smoking were independently associated with inadequate Glycemic control in patients with type 1 diabetes. Eur J Endocrinol. 2013;168:861–869. doi: 10.1530/EJE-13-0137. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen BK, Aars NA. Changes in waist circumference and the prevalence of abdominal obesity during 1994–2008 - cross-sectional and longitudinal results from two surveys: the Tromsø study. BMC Obes. 2016;3:41. doi: 10.1186/s40608-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neovius M, Janson A, Rössner S. Prevalence of obesity in Sweden. Obes Rev. 2006;7:1–3. doi: 10.1111/j.1467-789x.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCB. Undersökningarna av levnadsförhållanden år 2009. Sweden 2017. http://www.scb.se/ulf. Accessed 2 Apr 2017.
- 8.Chillarón JJ, Le-Roux JAF, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63 doi:10.1016/j.metabol.2013.10.002. [DOI] [PubMed]
- 9.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans R, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med. 2010;27:398–404. doi: 10.1111/j.1464-5491.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fröhlich-Reiterer EE, Rosenbauer J, Bechtold-Dalla Pozza S, Hofer SE, Schober E, Holl RW, et al. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child. 2014;99:738–743. doi: 10.1136/archdischild-2013-304237. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra H, van Middendorp H, Devaere L, Larsen JK, van Ramshorst B, Geenen R. Emotion processing and regulation in women with morbid obesity who apply for bariatric surgery. Psychol Health. 2011:1–13. doi:10.1080/08870446.2011.600761. [DOI] [PubMed]
- 12.Bennett J, Greene G, Schwartz-Barcott D. Perceptions of emotional eating behavior. A qualitative study of college students. Appetite. 2013;60:187–192. doi: 10.1016/j.appet.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Chesler BE. Emotional eating: a virtually untreated risk factor for outcome following bariatric surgery. ScientificWorldJournal. 2012;2012:365961. doi: 10.1100/2012/365961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor G, Bagby R, Parker J. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32:153–164. doi: 10.1016/S0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]
- 15.Clerici M, Albonetti S, Papa R, Penati G, Invernizzi G. Alexithymia and obesity. Psychother Psychosom. 1992;57:88–93. doi: 10.1159/000288580. [DOI] [PubMed] [Google Scholar]
- 16.Pinna F, Lai L, Pirarba S, Orru W, Velluzzi F, Loviselli A, et al. Obesity, alexithymia and psychopathology: a case-control study. Eat Weight Disord. 2011;16:e164–e170. doi: 10.3275/7509. [DOI] [PubMed] [Google Scholar]
- 17.Karukivi M, Jula A, Hutri-Kähönen N, Juonala M, Raitakari O. Is alexithymia associated with metabolic syndrome? A study in a healthy adult population. Psychiatry Res. 2016;236:58–63. doi: 10.1016/j.psychres.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Tolmunen T, Lehto SM, Heliste M, Kurl S, Kauhanen J. Alexithymia is associated with increased cardiovascular mortality in middle-aged Finnish men. Psychosom Med. 2010;72:187–191. doi: 10.1097/PSY.0b013e3181c65d00. [DOI] [PubMed] [Google Scholar]
- 19.Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia. 2011;54:2483–2493. doi: 10.1007/s00125-011-2240-3. [DOI] [PubMed] [Google Scholar]
- 20.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 21.Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, Beekman AT, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands study of depression and anxiety (NESDA) J Clin Psychiatry. 2010;71:1582–1589. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- 22.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18:692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 23.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 24.Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression, smoking, physical inactivity and season independently associated with midnight salivary cortisol in type 1 diabetes. BMC Endocr Disord. 2014;14:75. doi: 10.1186/1472-6823-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes. 2010;34:407–419. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 26.Mather AA, Cox BJ, Enns MW, Sareen J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J Psychosom Res. 2009;66:277–285. doi: 10.1016/j.jpsychores.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Stewart DE, Ashraf IJ, Munce SE. Women’s mental health: a silent cause of mortality and morbidity. Int J Gynaecol Obstet. 2006;94:343–349. doi: 10.1016/j.ijgo.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 29.Ogden J, Clementi C. The experience of being obese and the many consequences of stigma. J Obes. 2010;2010:9. doi:10.1155/2010/429098. [DOI] [PMC free article] [PubMed]
- 30.Fisher L, Gonzalez J, Polonsky W. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764–772. doi: 10.1111/dme.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin EO, Svensson R, Gustavsson S-Å, Winberg A, Denward-Olah E, Landin-Olsson M, et al. Affect school and script analysis versus basic body awareness therapy in the treatment of psychological symptoms in patients with diabetes and high HbA1c concentrations: two study protocols for two randomized controlled trials. Trials. 2016;17. doi:10.1186/s13063-016-1347-8. [DOI] [PMC free article] [PubMed]
- 32.Melin E. Thesis psychosomatic aspects on diabetes and chronic pain Alexithymia, depression and salivary cortisol the Affect school and script analysis therapy. Lund. Sweden: Lund University; 2014. [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 35.Bagby RM, Taylor GJ, Parker JD. The twenty-item Toronto Alexithymia scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 36.Björck C, Clinton D, Sohlberg S, Norring C. Negative self-image and outcome in eating disorders: results at 3-year follow-up. Eat Behav. 2007;8:398–406. doi: 10.1016/j.eatbeh.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin LS, Rothweiler JC, Critchfield KL. The use of structural analysis of social behavior (SASB) as an assessment tool. Annu Rev Clin Psychol. 2006;2:83–109. doi: 10.1146/annurev.clinpsy.2.022305.095337. [DOI] [PubMed] [Google Scholar]
- 38.Lavalard E, Szymezak J, Leroy N, Gillery P. Evaluation of variant II analyzer equipped with the new 270-2101 NU kit (bio-Rad) for HbA 1c assay. Ann Biol Clin. 2009;67:55–65. doi: 10.1684/abc.2008.0289. [DOI] [PubMed] [Google Scholar]
- 39.Koh-Banerjee P, Chu N-F, Spiegelman D, Rosner B, Colditz G, Willett W, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003;78:719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 40.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Intern Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Amsberg S, Wredling R, Lins P-E, Adamson U, Johansson U-B. The psychometric properties of the Swedish version of the problem areas in diabetes scale (Swe-PAID-20): scale development. Int J Nurs Stud. 2008;45:1319–1328. doi: 10.1016/j.ijnurstu.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Melin EO, Thulesius HO, Persson BA. Affect school for chronic benign pain patients showed improved alexithymia assessments with TAS-20. Biopsychosoc. 2010;4:1–10. doi: 10.1186/1751-0759-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergdahl J, Larsson A, Nilsson LG, Ahlstrom KR, Nyberg L. Treatment of chronic stress in employees: subjective, cognitive and neural correlates. Scand J Psychol. 2005;46:395–402. doi: 10.1111/j.1467-9450.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg LS, Pascual-Leone A. Emotion in psychotherapy: a practice-friendly research review. J Clin Psychol. 2006;62:611–630. doi: 10.1002/jclp.20252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are saved at SPSS files for 10 years at the department for Research and Development, Region Kronoberg, Växjö, Sweden. The data sets are not availably publicly as individual privacy could be compromised. The data set is available from the corresponding author on reasonable request.


