Abstract
Acute lung injury (ALI) is a devastating disorder of the lung that is characterized by hypoxemia, overwhelming pulmonary inflammation, and a high mortality in the critically ill. Adenosine has been implicated as an anti-inflammatory signaling molecule, and previous studies showed that extracellular adenosine concentrations are increased in inflamed tissues. Adenosine signaling is terminated by the uptake of adenosine from the extracellular into the intracellular compartment via equilibrative nucleoside transporters (ENTs). However, their role in controlling adenosine signaling during pulmonary inflammation remains unknown. After inflammatory in vitro experiments, we observed a repression of ENT1 and ENT2 that was associated with an attenuation of extracellular adenosine uptake. Experiments using short, interfering RNA silencing confirmed a significant contribution of ENT repression in elevating extracellular adenosine concentrations during inflammation. Furthermore, an examination of the ENT2 promoter implicated NF-κB as a key regulator for the observed ENT repression. Additional in vivo experiments using a murine model of inflammatory lung injury showed that the pharmacological inhibition of ENT1 and ENT2 resulted in improved pulmonary barrier function and reduced signs of acute inflammation of the lung. Whereas experiments on Ent1−/− or Ent2−/− mice revealed lung protection in LPS-induced lung injury, an examination of bone marrow chimeras for ENTs pointed to the nonhematopoetic expression of ENTs as the underlying cause of dampened pulmonary inflammation during ALI. Taken together, these findings reveal the transcriptional repression of ENTs as an innate protective response during acute pulmonary inflammation. The inhibition of ENTs could be pursued as a therapeutic option to ameliorate inflammatory lung injury.
Keywords: adenosine, lung injury, inflammation, equilibrative nucleoside transporter
Clinical Relevance
The results of the present research highlight a novel aspect of adenosine metabolism within the lung during an acute inflammatory process and its impact on the pathophysiology of lung injury. We highlight novel experimental approaches for the treatment of lung injury through the inhibition of the nucleoside transporters 1 and 2. On the basis of our findings, novel therapeutic options in the treatment of lung injury might arise in the future.
Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome, are devastating inflammatory disorders of the lung associated with high mortality (1). Hallmarks of ALI include the disruption of the alveolar–capillary barrier, the extravasation of intravascular fluid, and the infiltration of neutrophils into the alveolar space. Clinically, these changes result in bilateral pulmonary edema and severe hypoxia. At present, therapeutic approaches to patients suffering from lung injury are mainly symptomatic. Therefore, the search for novel and specific therapies for ALI entails an area of intense investigation.
Previous studies identified adenosine as an endogenous molecule driving an innate protective response during hypoxia-induced and endotoxin-induced lung injury (2, 3). Through the activation of specific adenosine receptors (ARs) such as A2BAR, for example, extracellular adenosine promotes alveolar–capillary barrier function, minimizing vascular leakage and reducing pulmonary inflammation (4, 5). Furthermore, previous studies implied that endogenous adenosine attenuates neutrophil recruitment during hypoxia-induced inflammation (6). Extracellular adenosine is generated by the ecto-Apyrase (CD39) and the ecto–5′-nucleotidase (CD73), which catalyze the serial dephosphorylation of adenosine triphosphate (ATP) into adenosine diphosphate (ADP), adenosine monophosphate (AMP), and finally adenosine. Once generated in the extracellular milieu, adenosine is subsequently cleared rapidly from the extracellular compartment through passive uptake by the equilibrative nucleoside transporters (ENTs) 1 and 2 (7, 8).
We recently demonstrated that hypoxia results in a transcriptional repression of both ectonucleotidases, amplifying the extracellular accumulation of adenosine (9, 10). In fact, some studies demonstrated that the transport of hypoxia-induced increased extracellular adenosine is directed predominantly inward, thereby shortening protective adenosine signaling (11). Yet we have also shown that the transcriptional repression of intestinal and vascular ENTs during hypoxia is an adaptive response aimed at increasing protective extracellular adenosine concentrations (12, 13).
Based on these observations, we pursued the hypothesis that pulmonary ENTs play an active role during the pathophysiology of lung injury. Our results provide the first evidence for the NF-κB–mediated transcriptional repression of endothelial and epithelial ENT1 and ENT2 as an innate mechanism aimed at increasing extracellular protective adenosine during inflammatory conditions. Furthermore, the pharmacological inhibition of the ENTs in vivo results in the reinforcement of the barrier function and an attenuated sequestration of neutrophils. Taken together, these studies provide a novel, promising therapeutic approach to dampening the inflammatory response during acute lung injury.
Materials and Methods
Cell Culture
Human microvascular endothelial (HMEC-1) cells and epithelial A549 cells were cultured as described previously (12).
Transcriptional Analysis
HMEC-1 cells were grown to confluence and stimulated with IL-6 (20 ng/ml), IL-1β (20 ng/ml), or TNF-α (100 ng/ml) for 2, 4, 8, and 24 hours. Cells were also stimulated with increasing concentrations of the described cytokines. Afterward, real-time PCR was performed as described previously (12, 13). For details about the primer sets used, please see the online supplement.
Protein Analysis
Cells or murine tissues were analyzed by Western blotting, as described previously (12, 13). For details about the antibodies used, please see the online supplement.
Differential Biotinylation of Apical or Basolateral Surface Proteins
Differentiation between the apical or basolateral expression of ENT1 and ENT2 was achieved as described previously (12).
ENT2 Reporter Assays
The promoter analysis and identification of the transcription start site, as well as of potential NF-κB binding sites, were performed using MatInspector by Genomatix (Munich, Germany), as described previously (14). The vector pGL4.17–expressing sequence corresponding to the full-length ENT2 promoter, either native (ENT2-FL, −500 to +331, 831 base pairs [bp]) or containing mutations in one of several potential NF-κB binding sites, namely, ENT2Δ+120 (bp +120 to +133), ENT2Δ−231 (bp −231 to −243), and ENT2Δ−331 (bp −331 to −343), were purchased from GeneArt AG (Regensburg, Germany). As a positive control for inflammation, cells were transfected with a poly (p)NF-κB–luciferase vector (Clontech, Mountain View, CA). After transfection with GeneJuice Transfection Reagent (EMD Biosciences, Inc., Darmstadt, Germany), cells were stimulated for 24 hours. Luciferase activity was assessed using a luciferase assay kit (Promega, Mannheim, Germany). All firefly luciferase activity was normalized with respect to total protein content.
ENT1 and ENT2 Suppression with Short, Interfering RNA
HMEC-1 cells were grown on semipermeable transwell inserts (Costar, Tewksbury, MA). In accordance with previous studies, we used sets of short, interfering RNA (siRNA) directed against human ENT1 or ENT2 from Ambion (Austin, TX). As controls, cells were transfected in parallel with a scrambled control siRNA (Ambion). HMEC-1 loading was accomplished using standard conditions of the GeneJuice Transfection Reagent (EMD Biosciences, Inc.) when cells had reached 40–60% confluence. After 24 hours of loading, functional assays were performed.
Measurement of Adenosine Uptake
The measurement of adenosine in supernatants was performed as described previously (12). For further details, please see the online supplement.
Murine LPS Inhalation Model
Wild-type (WT), ENT1-knockout (ENT1−/−), and ENT2-knockout (ENT2−/−) C57Bl/6 mice (Lexicon Pharmaceutical, The Woodlands, TX) were genotyped and matched according to sex, age, and weight. Animals were exposed either to 0.9% NaCl (control) or to aerosolized LPS (Escherichia coli B026, 0.5 mg/ml; Sigma-Aldrich, St. Louis, MO) in a cylindrical vacuum chamber connected to an air nebulizer (MicroAir; Omron Healthcare, Inc., Bannockburn, IL) for 30 minutes. Four hours later, the mice were killed and their organs were harvested for further analysis. In a subset of experiments, animals inhaled the ENT1/2 inhibitor dipyridamole (3.33 μg/kg · min; Sigma-Aldrich) or the specific ENT1 inhibitor S-(4-nitrobenzyl)-6-thioinosine (NBTI; 0.33 μg/kg · min; Sigma-Aldrich) for 30 minutes, right after NaCl or LPS inhalation. All animal protocols were in accordance with the German guidelines for the use of living animals, and were approved by the Institutional Animal Care and Use Committee of the Tübingen University Hospital and the Regierungspräsidium Tübingen.
Evaluation and Quantification of Pulmonary Inflammation
After animals were killed, bronchoalveolar lavage (BAL) was achieved by performing a tracheotomy and flushing the lungs three times with 0.6 ml PBS. The BAL was then analyzed as described previously (14, 15).
Immunohistochemistry
After NaCl/LPS inhalation, WT, ENT1−/−, and ENT2−/− mice were killed, and their lungs were fixed. For further details, please see the online supplement.
Generation of Bone Marrow Chimeric Animals
To define the contributions of myeloid-specific and tissue-specific ENT1 and ENT2, we generated bone marrow chimeric mice, as described previously (16).
Data Analysis
Data were compared according to the Student t test (two-tailed, α< 0.05) or by one-factor ANOVA, where appropriate. Values are expressed as the means ± SDs from at least three separate experiments.
Results
Adenosine Uptake Is Attenuated after Exposure to Inflammatory Stimuli
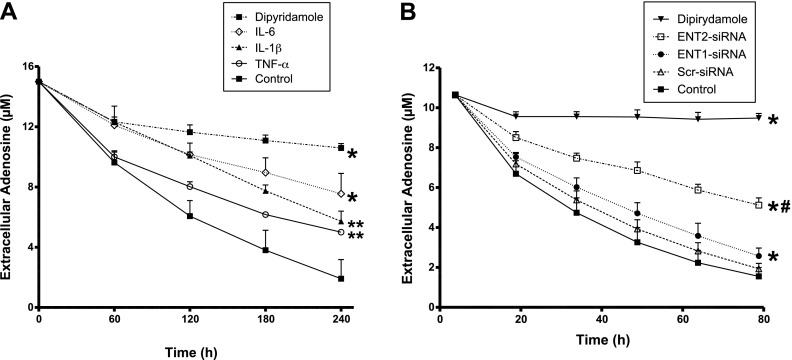
As a first step, we investigated whether the extracellular concentrations of adenosine are affected during periods of inflammation. For this purpose, we made use of a previously described model for adenosine uptake (12). Briefly, we grew HMEC-1 cells on semipermeable inserts until they were confluent, and exposed them to different proinflammatory stimuli for 24 hours. We then added 15 μM of adenosine to the upper compartment, and adenosine uptake was monitored by measuring the remaining adenosine in the supernatant at different time points. As shown in Figure 1A, adenosine uptake was significantly attenuated after interleukin exposure. Control experiments performed in the presence of the adenosine transport inhibitor dipyridamole revealed a robust reduction of adenosine uptake. Next, we sought to define the individual contributions of ENT1 and ENT2 to the apical transport of adenosine. To this end, we transiently silenced ENT1 or ENT2, using specific siRNAs, and performed functional studies of adenosine uptake (Figure 1B). Scrambled siRNA was used as a negative control. The results demonstrated a major decrease of adenosine uptake after the siRNA-mediated repression of ENT2, compared with the decrease obtained after silencing ENT1.
Figure 1.
Adenosine uptake during inflammatory conditions and specific contribution of equilibrative nucleoside transporters (ENTs). (A) Confluent human microvascular endothelial (HMEC-1) cells were exposed to 50 ng/ml IL-6, 50 ng/ml IL-1β, or 100 ng/ml TNF-α for 24 hours. We then added 15 μM of adenosine to the upper compartment, and the dissipation of adenosine in the supernatant was measured by high-performance liquid chromatography. *P < 0.001 and **P < 0.01 indicate differences regarding control cells, according to one-factor ANOVA. (B) HMEC-1 cells were loaded with 200 nM of specific short, interfering RNA (siRNA) against ENT1, ENT2, or scrambled control siRNA (Scr) for 24 hours. Adenosine uptake was then evaluated as before. *P < 0.001 indicates differences regarding control nontransfected cells. #P < 0.001 indicates differencea between ENT1 siRNA and ENT2 siRNA, according to one-factor ANOVA. In A and B, the addition of 30 μM dipyridamole was used as a control for complete adenosine transport inhibition.
Expression of Endothelial ENT1 and ENT2 Is Attenuated during Inflammation
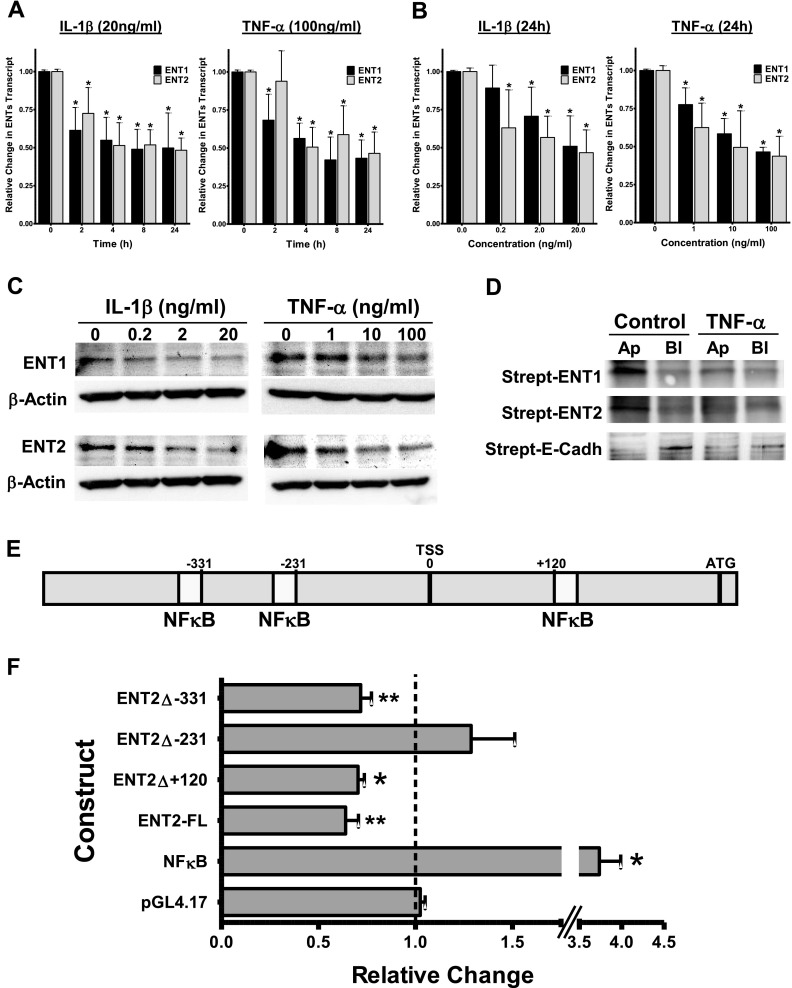
We next investigated whether exposure to proinflammatory stimuli influences the expression levels of ENT1 and ENT2. For this, we exposed confluent vascular endothelial HMEC-1 cells to different concentrations of the inflammatory cytokines IL-6, IL-1β, and TNF-α. The results demonstrated significantly reduced concentrations of ENT1 and ENT2 in response to inflammation in a time-dependent and concentration-dependent manner (Figures 2A and 2B and Figures E1A and E1B in the online supplement). Additional Western blotting analysis confirmed these findings at the protein level (Figures 2C and E1C). We also found a repression of both ENT1 and ENT2 in alveolar epithelial cells, confirming our results with the endothelial cells (Figure E2). The equilibrative nucleosides transporters have been reported to be located on the apical and the basolateral sides of intestinal and renal epithelia, respectively, but their polarization in vascular endothelium remains unknown. Given the mostly apical colocalization of the enzymes responsible for the generation of extracellular adenosine (CD39 and CD73), and the apical and basolateral colocalization of the adenosine A2AAR and A2BAR receptors (17, 18), we sought to ascertain specific localizations of ENT1 and ENT2. For this purpose, we biotinylated either the apical or the basolateral membranes of HMEC-1 cells. After immunoprecipitation and further Western blotting with streptavidin, the results confirmed a predominance of both transporters on the apical side (Figure 2D). Assessment of E-cadherin was used a positive control for the basolateral membrane (19). Together, these results implicate the inflammation-mediated repression of vascular ENT1, and more importantly ENT2, largely at the apical surface of the cells.
Figure 2.
NF-κB–mediated repression of ENTs during inflammation in vitro. Confluent HMEC-1 cells were exposed to IL-1β (20 ng/ml) or TNF-α (100 ng/ml) for 2, 4, 8, and 24 hours (A), or stimulated for 24 hours with increasing concentrations of IL-1β (0.2, 2, and 20 ng/ml) or TNF-α (1, 10, and 100 ng/ml) (B). Total RNA was isolated, and ENT1 and ENT2 mRNA concentrations were determined by RT-PCR. Data are expressed as fold change in transcript over nonstimulated control samples ± SD, and have been calculated relative to the internal housekeeping gene β-actin. Results are derived from three experiments under each condition. *P < 0.001, according to one-factor ANOVA. (C) Expression of ENT1 and ENT2 protein in endothelial HMEC-1 cells exposed to the indicated concentrations of IL-1β and TNF-α. (D) Confluent HMEC-1 cells grown on inserts were exposed to 100 ng/ml TNF-α, and either apical (Ap) or basolateral (Bl) surface proteins were biotinylated. Immunoprecipitates were resolved by Western blotting, and visualized using streptavidin–horseradish peroxidase (Strept). The immunoprecipitation of E-cadherin (E-Cadh) served as a positive control for the basolateral membrane. (E) Graphic representation of the putative ENT2 promoter. Three potential NF-κB binding sites were identified at positions +120 to +133, −231 to −243, and −331 to −343, relative to the transcription start site (TSS). (F) Full-length (ENT2-FL) and indicated constructs containing mutations in one of the potential NF-κB binding sites were transfected into HMEC-1 cells and assayed for luciferase activity after exposure to 50 ng/ml IL-1β for 24 hours. Results depict the fold change in luminescence relative to nonstimulated control samples. A NF-κB plasmid is shown as a positive control for inflammation. Data shown are pooled from n = 6/experiment and are normalized for background vector (empty pGL4.17) and total protein, and are presented as means ± SDs, where *P < 0.001 and **P < 0.01 indicate significance between individual plasmids and empty pGL4.17 plasmid, according to two-tailed Student t-test.
Role of NF-κB in Regulation of ENT2 during Inflammation
Next we aimed at gaining some insights into the mechanisms responsible for ENT2 repression under these conditions. For this purpose, we examined response pathways of the ENT2 promoter during inflammation. Using available public databases and after analysis of the full-length cDNA, the transcription start site of human ENT2 was identified at position −230, relative to the first codon (Figure 2E). Further analysis revealed the existence of the three potential consensus sequences required to constitute a binding site for NF-κB. Specifically, they were located at positions +120 to +133 (DNA consensus motif, 5′- CTGGGGAGACCCG -3′), −231 to −243 (5′- GGGAAGAGCCCCG -3′), and −331 to −343 (5′- GGGGACTCCGCAG -3′), relative to the transcription start site. To assess the binding of NF-κB to the ENT2 promoter, we performed a chromatin immunoprecipitation assay, and found that NF-κB binds to the ENT2 promoter region (Figure E3). To address the functional role of these NF-κB binding sites, we designed luciferase reporter constructs expressing the putative full-length ENT2 promoter (ENT2-FL, from −500 to +331, 831 bp). After transfection, HMEC-1 cells were stimulated with 50 ng/ml of IL-1β for 24 hours. Consistent with mRNA and protein analyses, luciferase activity was reduced in response to the inflammatory stimulus (Figure 2F). Furthermore, the use of additional promoter constructs containing site-directed mutations in one of the NF-κB binding sites (ENT2Δ+120, ENT2Δ−231, and ENT2Δ−331) identified the binding site at position −231 to −243 as directly responsible for the repression of the ENT2 promoter, given the lack of responsiveness to IL-1β by this specific construct (Figure 2F). Such results confirm a functional role of NF-κB in the repression of ENT2 during inflammation.
Regulation of ENT1 and ENT2 in a Murine Model of Pulmonary Inflammation
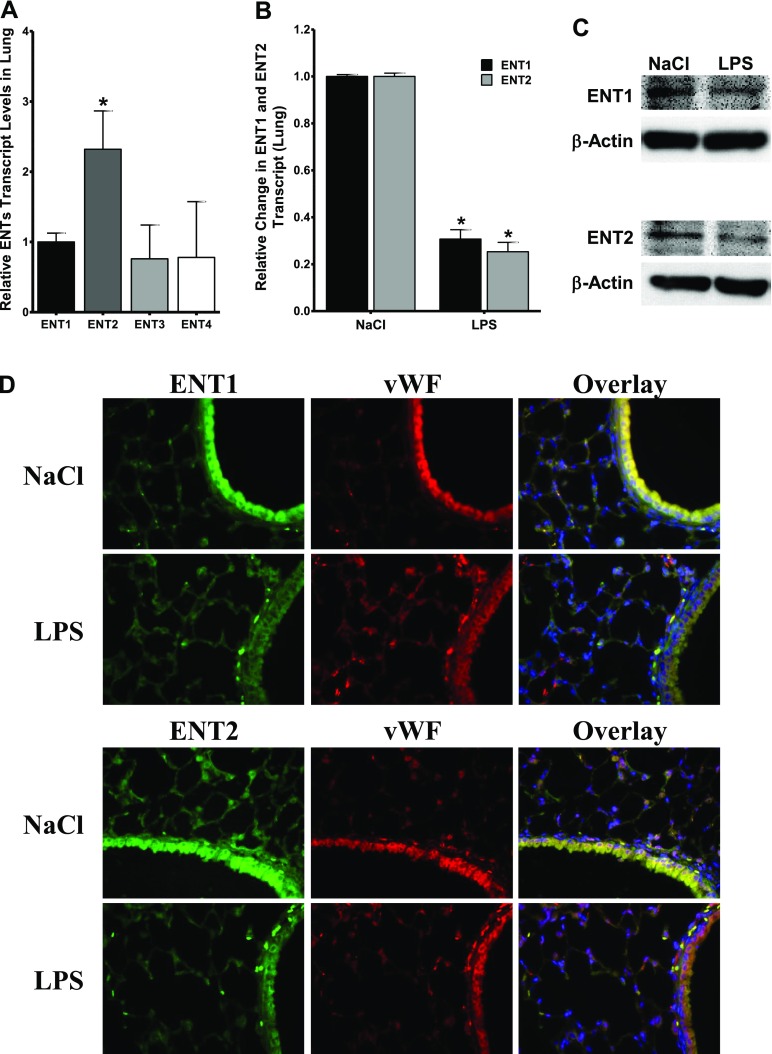
As proof of principle for these in vitro results, we extended our work into an in vivo model. First of all, we measured the relative expression levels of ENTs in lung tissue, and found ENT2 to be the predominant transporter (Figure 3A), which additionally supports the major role of ENT2 observed in vitro. Next, we made use of a murine model of acute pulmonary inflammation induced by LPS inhalation, and determined the expression levels of ENT1 and ENT2, 4 hours after triggering pulmonary injury. The result corroborated the attenuation of pulmonary ENT1 and ENT2 in WT animals, at both the mRNA and protein levels (Figures 3B and 3C). The immunohistochemistry of ENT1 and ENT2 in pulmonary tissue sections verified the diminished ENT1/ENT2-specific immunofluorescence after LPS inhalation (Figure 3D). Moreover, the endothelial location of ENT1 and ENT2 was demonstrated by co-immunostaining for the specific endothelial marker von Willebrand factor.
Figure 3.
Regulation of ENT1 and ENT2 during inflammation in vivo. (A) Relative concentrations of ENT1, ENT2, ENT3, and ENT4 were determined in lung tissue. Data are expressed as fold over ENT1 expression. *P < 0.001, according to one-factor ANOVA. (B) ENT1 mRNA and ENT2 mRNA concentrations were examined in lung from mice, 4 hours after NaCl or LPS inhalation. Data are pooled from two independent experiments, where n = 5 mice. *P < 0.001 indicates differences between NaCl and LPS inhalation, according to one-factor ANOVA. (C) Analysis of pulmonary ENT1 and ENT2 protein from mice, 4 hours after NaCl or LPS inhalation. Protein samples were pooled (n = 6) before being electrophoresed. (D) Pulmonary immunohistochemistry for murine ENT1, ENT2, and von Willebrand factor (vWF) during LPS inhalation. Lung from mice exposed to NaCl or LPS inhalation were harvested and embedded in paraffin. Sections were stained with specific antibodies against murine ENT1 or ENT2 (green) and the endothelial marker von Willebrand factor (red) or isotype controls (data not shown). The colocalization of ENTs with von Willebrand factor is seen as yellow in the merged image. We used 4′6-diamidino-2-phenylindole for a nuclear counterstain (blue) (magnification, ×200). A representative image from three different lung sections is displayed.
Attenuated Inflammatory Response in ENT1−/− and ENT2−/− Mice during ALI
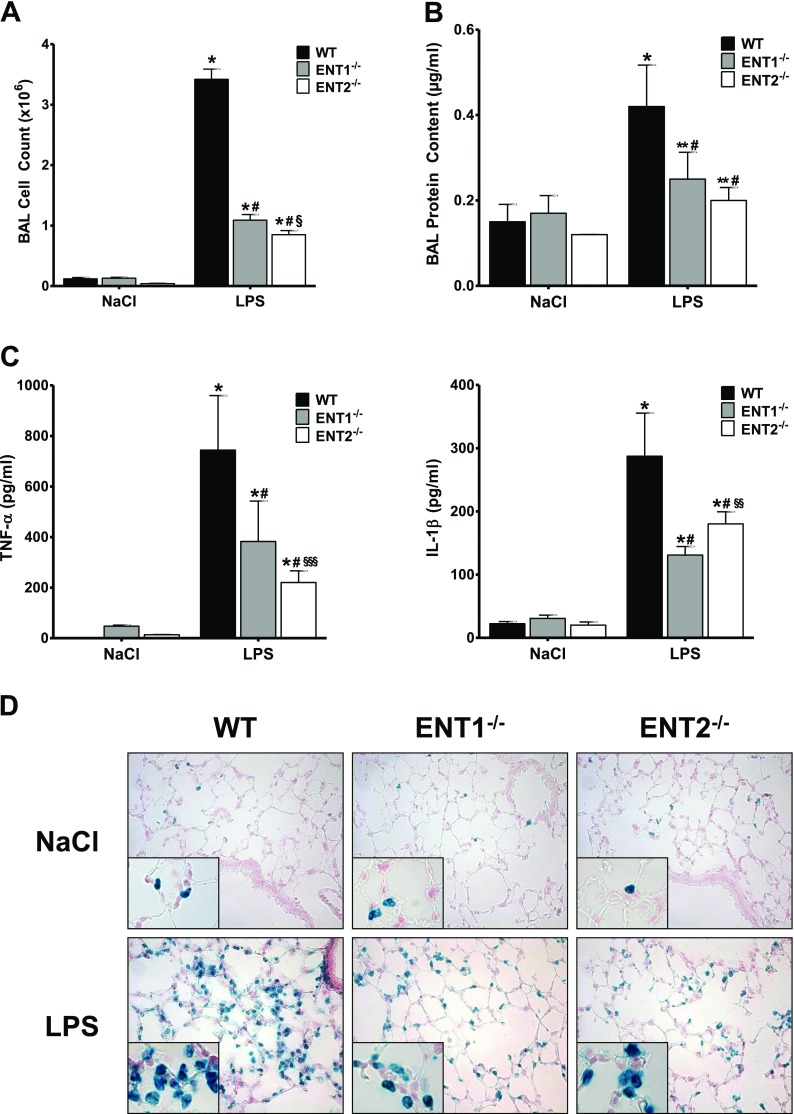
Guided by our in vitro results showing an accumulation of extracellular adenosine after the repression of ENT1 and ENT2 during inflammation, we investigated the functional consequences of that lack of these transporters during ALI. First we evaluated adenosine concentrations within the alveolar space of ENT1−/− and ENT2−/− animals, and found that ENT1−/− as well as ENT2−/− demonstrated increased concentrations of adenosine within the alveolar space (Figure E4). After exposing WT, ENT1−/−, and ENT2−/− mice to LPS inhalation, BAL was performed to screen a variety of parameters by which to evaluate and quantify the extent of pulmonary damage. As depicted in Figures 4A–4C and E2, WT animals that inhaled LPS demonstrated significantly increased cell numbers, protein content, myeloperoxidase (MPO) activity, and concentrations of inflammatory cytokines (TNF-α, IL-1β, IL-6, and macrophage inflammatory protein-1α) in their BAL, compared with the control animals, as expected. Conversely, lung injury turned out to be much lower in the ENT1−/− and ENT2−/− mice after LPS inhalation. In addition to the MPO assays, the accumulation of polymorphonuclear cells (PMNs) in the lungs was also evaluated by immunohistochemistry. Again, ENT1−/− and ENT2−/− mice presented a significant reduction in the influx of neutrophils in comparison with the WT control mice (Figure 4D).
Figure 4.
Attenuated pulmonary damage in ENT1 knockout and ENT2 knockout mice during acute lung injury (ALI). Cell count (A), protein content (B), and concentrations of TNF-α and IL-1β (C) were determined in the bronchoalveolar lavage (BAL) of ENT1−/− and ENT2−/− animals, 4 hours after LPS inhalation. Data are expressed as means ± SDs (n ≥ 6). *P < 0.001 and **P < 0.01 indicate significance between NaCl− and LPS inhalation. #P < 0.001 indicates significance between WT and ENT knockout mice. §P < 0.001, §§P < 0.01, and §§§P < 0.05 indicate significance between ENT1−/− and ENT2−/−, according to one-factor ANOVA. (D) Staining of polymorphonuclear cells (PMNs) in histological sections of pulmonary tissue of WT, ENT1−/−, and ENT2−/− mice, 4 hours after LPS challenge (magnification, ×400; insets, ×1,000). Representative images from three different lung sections are displayed.
Pharmacological Inhibition of ENT1 and ENT2 Dampens the Inflammatory Response during ALI In Vivo
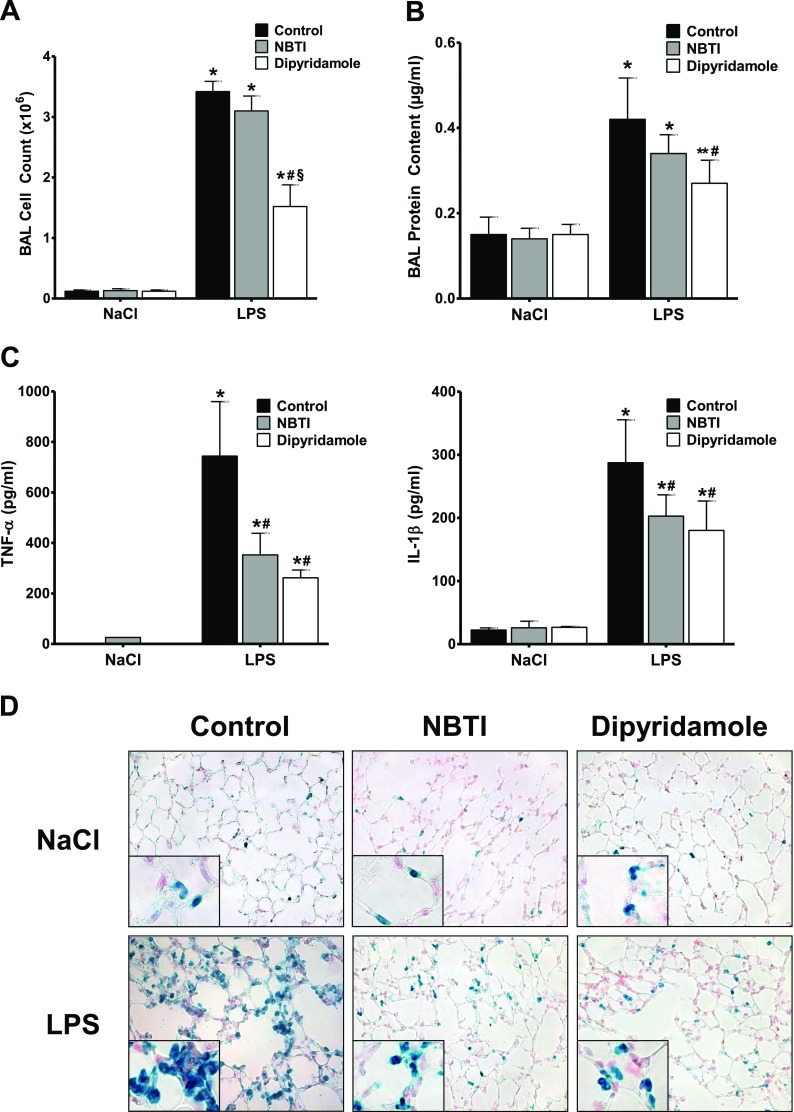
Encouraged by these promising results showing a robust reduction in pulmonary damage in ENT1−/− and ENT2−/− mice after LPS inhalation, we pursued the potential pharmacological inhibition of these transporters in WT animals as a new therapeutic approach to ALI. To this purpose, WT animals inhaled either the ENT1/2 inhibitor dipyridamole (3.33 μg/kg · min) or the specific ENT1 inhibitor NBTI (0.33 μg/kg · min) for 30 minutes, right after NaCl or LPS inhalation. Dipyridamole as well as NBTI inhalation resulted in a significant increase of adenosine concentrations within the alveolar space of the treated animals (Figure E4). The results after LPS inhalation demonstrated that the inhibition of ENT1 and ENT2 significantly diminished LPS-induced increased values for cell counts, protein content, MPO, and inflammatory cytokines (Figures 5A–5C and E5). As before, the reduced accumulation of PMNs into the lungs was further indicated by immunohistochemistry (Figure 5D). These findings provide strong evidence that the pharmacological inhibition of ENT1 and ENT2 dampens the extent of pulmonary damage during acute lung injury.
Figure 5.
Pharmacological inhibition of ENT1 and ENT2 during ALI. Right after NaCl or LPS inhalation, wild-type mice also inhaled either the specific ENT1 inhibitor S-(4-nitrobenzyl)-6-thioinosine (NBTI; 0.33 μg/kg · min) or the ENT1/2 inhibitor dipyridamole (3.33 μg/kg · min) for 30 minutes. Four hours later, BAL was performed, and cell counts (A), protein content (B), and concentrations of TNF-α and IL-1β (C) were assessed in the BAL. Data are expressed as means ± SDs (n ≥ 5). *P < 0.001 and **P < 0.01 indicate significance between NaCl− and LPS inhalation. #P < 0.001 indicates significance between treated and nontreated mice after LPS inhalation. §P < 0.001 indicates significance between treatment with NBTI and dipyridamole, according to one-factor ANOVA. (D) Staining of PMNs in histological sections of pulmonary tissue from the animals already described (magnification, ×400; insets, ×1,000). Representative images from three different lung sections are displayed.
Nonhematopoetic ENT1 and ENT2 Are Essential for the Resuscitation of Pulmonary Barrier Function during ALI In Vivo
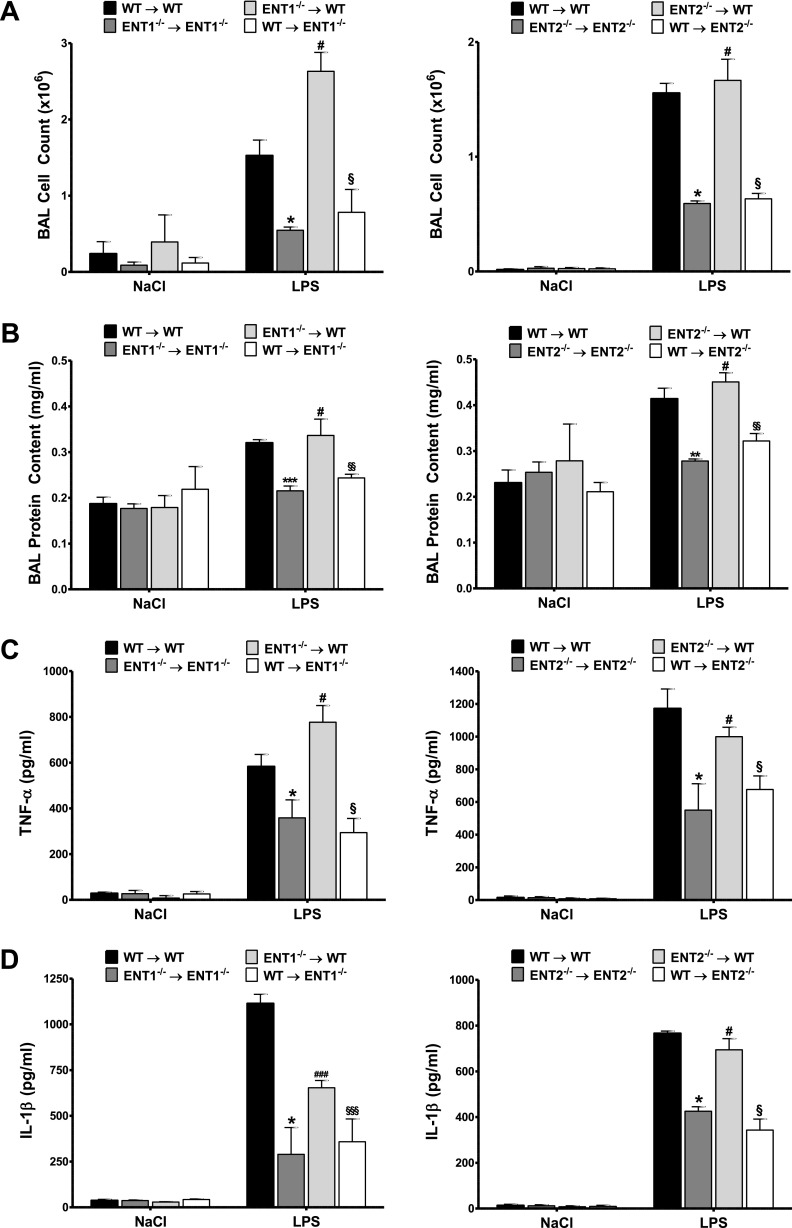
Based on our findings so far, we next investigated whether nonhematopoetic or hematopoetic ENT1 and ENT2 would be responsible for the observed results. For this, chimeric animals were created by transferring bone marrow from WT to ENT1−/− or ENT2−/− mice, and vice versa. The transplant of bone marrow from WT to WT, ENT1−/− to ENT1−/−, and ENT2−/− to ENT2−/− mice was used to generate control animals for the nonspecific effects caused by irradiation. Next, these chimeric animals were exposed to LPS inhalation, and pulmonary damage was evaluated as before. Consistent with the experimental results already described, chimeric WT mice receiving WT bone marrow presented notable lung injury, whereas chimeric ENT1−/− and ENT2−/− mice receiving bone marrow from ENT1−/− and ENT2−/− mice, respectively, showed a significant attenuation of LPS-elicited pulmonary inflammation, as reflected by the cell counts, protein content, MPO activity, and inflammatory cytokines (Figures 6 and E6). Concerning the crossover mice, the ENT-associated reduction in lung injury was only preserved in both ENT1−/− and ENT2−/− chimeric animals receiving WT bone marrow, revealing the attenuation of tissue-specific ENT1 and ENT2 as responsible for the resuscitation of pulmonary barrier function during acute lung injury in vivo (Figures 6 and E7).
Figure 6.
Tissue-specific expression of ENT1 and ENT2 is key for alveolar–capillary barrier function. Cell counts (A), protein content (B), and concentrations of TNF-α (C) and IL-1β (D) were determined in the BAL of chimeric animals, 4 hours after LPS inhalation. Data are expressed as means ± SDs (n ≥ 6). *P < 0.001, **P < 0.01, and ***P < 0.05 indicate significance between WT transplanted and knockout (KO) transplanted control mice. #P < 0.001 and ###P < 0.05 indicate significance between KO control mice and tissue-specific WT mice. §P < 0.001, §§P < 0.01, and §§§P < 0.05 indicate significance between tissue-specific WT mice and tissue-specific KO mice, according to one-factor ANOVA.
Discussion
In the present work, we explored the contributions of equilibrative nucleoside transporters in regulating protective adenosine signaling during an acute inflammatory process. We found that attenuated adenosine uptake correlates with a repression of the adenosine transporters ENT1 and ENT2. Furthermore, we identified significant contributions of ENT1 and ENT2 in the pathophysiology of lung inflammation, both in vitro and in vivo. Moreover, pharmacological experiments demonstrated that the in vivo inhibition of ENTs results in attenuated tissue injury during pulmonary inflammation. Finally, experiments in bone marrow chimeric animals identified the nonhematopoetic expression of ENTs to be responsible for the observed results.
We observed that the endothelial and epithelial expression of ENTs is decreased after inflammatory stimulation in vitro and in vivo. These findings are consistent with previous work showing the existence of an innate and coordinated protective response during inflammation and hypoxia-elicited inflammation, which is aimed at enhancing protective adenosine signaling by promoting the accumulation of extracellular adenosine. This response includes the induction of CD39 and CD73, the nucleotidases responsible for the generation of extracellular adenosine (20), as well as of the specific adenosine receptor A2B (21), whose activation triggers tissue protection. Furthermore, to preserve and increase the concentrations of adenosine in the extracellular milieu, we also describe two additional mechanisms. First, we reported on the down-regulation of the equilibrative nucleoside transporters during tissue hypoxia (12, 13), and second, we have described an inflammatory process in the present work. Such regulation attenuates the natural flux of extracellular adenosine along its concentration gradient toward the intracellular space (22, 23). Furthermore, the reduction of adenosine kinase expression during hypoxia results in the accumulation of intracellular adenosine and consequently a strong mitigation of its concentration gradient toward the cell that also decreases the influx of adenosine into cell (12). Together, these mechanisms prevent that the extracellular adenosine concentration decreases rapidly through the uptake of adenosine into the cellular compartment, and as a result this effect enhances the protective role of extracellular adenosine signaling.
A crucial step during the initial phase of lung injury involves the extravasation of neutrophils from the vascular compartment and their migration into the alveolar space (1, 24). This step in part defines the extent of subsequent lung injury and, as such, the extent of intraalveolar inflammation (25). Adenosine serves as stop signal for neutrophil migration and, as such, attenuates the extent of infiltration of inflammatory cells into pulmonary tissues and the resultant inflammatory changes (6, 26, 27). This explains why the pharmacological inhibition of ENTs we presented here results in a reduced infiltration of leukocytes into the alveolar space and, as such, reduces the extent of inflammatory tissue changes within the lung. However, several other defense mechanisms of the lung are impaired during lung injury. One of these involves the clearance of mucus within the airways as a primary innate defense mechanism against pathogens (28). This is dependent on an active sodium transport that produces the mucus and clears potential pathogens within it (29). Although adenosine was already known to modulate anion/cation transport in the human airways (30, 31), only in recent studies were the detail mechanisms identified (4). Factor and colleagues (29) demonstrated that extracellular adenosine preserves the alveolar fluid balance by regulating the activity of both the epithelial sodium channel and the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Such regulation is critically dependent on the concentration of extracellular adenosine in the vicinity of cell surface receptors. Whereas high physiological concentrations decrease alveolar fluid clearance (via adenosine A1 receptors), alveolar fluid clearance is increased by low doses of adenosine (via adenosine A2A receptors). Based on this, the infiltration of fluid into the airways during ALI would significantly decrease concentrations of extracellular adenosine, resulting in the transition from A1AR-mediated to A2AAR-mediated signaling pathways. Moreover, as shown in studies of ventilator-induced lung injury, A2BAR also contributes significantly to promoting fluid clearance from the alveolus, as suggested by Eckle and colleagues (4) The regulation of extracellular concentrations of adenosine is mainly performed by the equilibrative nucleoside transporters (12, 13). In the present work, we have demonstrated the transcriptional attenuation of ENTs as an innate novel protective response during ALI. Indeed, our experiments in vivo demonstrate that the pharmacological inhibition of ENTs dampens pulmonary damage. Consistent with our findings, inhibition of the equilibrative nucleoside transporters has been also shown to promote CFTR-mediated alveolar fluid clearance (31, 32). Specifically, the inhibition of adenosine transport prevents extracellular adenosine from being internalized, and therefore facilitates ther activation of CFTR via A2AAR-signaling and A2BAR-signaling pathways.
Through experiments in bone marrow chimeric animals, Reutershan and colleagues identified that myeloid cell–specific A2AAR prompted anti-inflammatory effects during LPS-induced lung injury (33). This specificity correlates with the fact that the A2AAR-mediated activation of CFTR in neutrophils attenuates proinflammatory responses during endotoxin-induced acute lung inflammation and injury (34). On the other hand, the A2BAR-mediated mitigation of the inflammatory response occurs through tissue-specific A2B adenosine receptors (5). Our experiments with ENT-chimeric animals clearly identified pulmonary nonhematopoietic nucleoside transporters as responsible for the alleviation of lung injury during ALI. This specificity could be explained by the finding that the inhibition of transport of adenosine in the endothelia likely provides their surroundings with more adenosine than an inhibition of ENTs in the myeloid cells (4).
Although extracellular adenosine responses appear to be protective during acute ischemic (35–37) or inflammatory diseases (9, 16, 38), including disease of the lungs (5, 39, 40), chronic elevations of adenosine may turn out to be detrimental during chronic disease states. For example, research by Zhou and colleagues (41), Eltzschig and colleagues (42), and Blackburn (43) suggests that although adenosine signaling is protective during the acute stage of bleomycin-induced lung injury, it can prove detrimental at a later point of the disease (41–43). For example, mice with chronically elevated concentrations of adenosine attributable to adenosine deaminase deficiency develop a pulmonary phenotype characterized by acute lung inflammation (44). Similarly, during disease conditions such as sickle-cell disease with chronically elevated adenosine concentrations, adenosine signaling through A2BAR may become detrimental (45). Therefore, it will be critically important to determine the time point when protective adenosine responses during ALI become detrimental and promote fibrosis or chronicity.
In conclusion, the present work implicates the transcriptional repression of ENTs during LPS-induced lung injury as an innate mechanism aimed not only at promoting an adenosine-mediated protective response in the vascular barrier function, but also at providing the tissue with a negative feedback to stop further harmful NF-κB signaling pathways. In addition, the pharmacological inhibition of ENTs with dipyridamole arises as a promising therapeutic approach to disorders involving excessive inflammatory responses.
Footnotes
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0457OC on April 3, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto–5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2b adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2b adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 7.Allen-Gipson DS, Jarrell JC, Bailey KL, Robinson JE, Kharbanda KK, Sisson JH, Wyatt TA. Ethanol blocks adenosine uptake via inhibiting the nucleoside transport system in bronchial epithelial cells. Alcohol Clin Exp Res. 2009;33:791–798. doi: 10.1111/j.1530-0277.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor A2–mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol. 2010;298:L755–L767. doi: 10.1152/ajplung.00330.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2b receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, Sato F, Miyamoto K, Kawakami Y. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. Am J Respir Crit Care Med. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- 12.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor–dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, et al. HIF-1–dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181:815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 15.Mirakaj V, Brown S, Laucher S, Steinl C, Klein G, Kohler D, Skutella T, Meisel C, Brommer B, Rosenberger P, et al. Repulsive guidance molecule–A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci USA. 2011;108:6555–6560. doi: 10.1073/pnas.1015605108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 17.Papanikolaou A, Papafotika A, Murphy C, Papamarcaki T, Tsolas O, Drab M, Kurzchalia TV, Kasper M, Christoforidis S. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280:26406–26414. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Kolachala V, Walia B, Balasubramanian S, Hall RA, Merlin D, Sitaraman SV. Agonist-induced polarized trafficking and surface expression of the adenosine 2b receptor in intestinal epithelial cells: role of snare proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1100–G1107. doi: 10.1152/ajpgi.00164.2004. [DOI] [PubMed] [Google Scholar]
- 19.Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane FCgamma-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2b adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2b receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 22.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 23.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia–reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 24.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 25.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 26.Flamand N, Bourdreault S, Picard S, Austin M, Surette M, Plante H, Krump E, Vallee M-J, Gilbert C, Naccache P, et al. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am J Respir Crit Care Med. 2000;161:88S–94. doi: 10.1164/ajrccm.161.supplement_1.ltta-18. [DOI] [PubMed] [Google Scholar]
- 27.Wakai A, Wang JH, Winter DC, Street JT, O’Sullivan RG, Redmond HP. Adenosine inhibits neutrophil vascular endothelial growth factor release and transendothelial migration via A2b receptor activation. Shock. 2001;15:297–301. doi: 10.1097/00024382-200115040-00008. [DOI] [PubMed] [Google Scholar]
- 28.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, et al. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA. 2007;104:4083–4088. doi: 10.1073/pnas.0601117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2b receptor–coupled pathway. Am J Physiol. 1999;276:C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- 31.Szkotak AJ, Ng AM, Sawicka J, Baldwin SA, Man SF, Cass CE, Young JD, Duszyk M. Regulation of K(+) current in human airway epithelial cells by exogenous and autocrine adenosine. Am J Physiol Cell Physiol. 2001;281:C1991–C2002. doi: 10.1152/ajpcell.2001.281.6.C1991. [DOI] [PubMed] [Google Scholar]
- 32.Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. Coupling of CFTR-mediated anion secretion to nucleoside transporters and adenosine homeostasis in Calu-3 cells. J Membr Biol. 2003;192:169–179. doi: 10.1007/s00232-002-1073-x. [DOI] [PubMed] [Google Scholar]
- 33.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 34.Su X, Looney MR, Su HE, Lee JW, Song Y, Matthay MA. Role of CFTR expressed by neutrophils in modulating acute lung inflammation and injury in mice. Inflamm Res. 2011;60:619–632. doi: 10.1007/s00011-011-0313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, et al. Adora2b-elicited PER2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eltzschig HK, Eckle T. Ischemia and reperfusion: from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine a2b receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1–dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 40.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A(2b) adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blackburn MR. Too much of a good thing: adenosine overload in adenosine–deaminase–deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 44.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]