Abstract
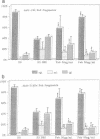
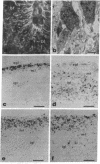
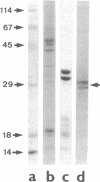
In the central nervous system, postmitotic neurons migrate along astrocytic processes to reach their adult position. The molecular mechanisms of this guided migration are not clearly defined, although some steps have been shown to involve proteases and cell adhesion molecules. We report that monovalent antibodies (Fab fragments) raised against an endogenous cerebellar soluble lectin (CSL) completely inhibit neuronal migration in cultures of cerebellar explants at concentrations as low as 50 micrograms/ml. A similar inhibition pattern was obtained with Fab fragments prepared against one of the endogenous glycoprotein ligands of CSL, the 31-kDa glycoprotein (this glycoprotein is a membrane-bound glycoprotein specifically occurring, in the cerebellum, at the surface of immature neurons). We propose that this lectin-glycoprotein interaction supports the adhesion between neurons and the astrocyte guide during the migration of cerebellar immature neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972 Aug;145(4):465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Chuong C. M., Crossin K. L., Edelman G. M. Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol. 1987 Feb;104(2):331–342. doi: 10.1083/jcb.104.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fressinaud C., Kuchler S., Sarlieve L. L., Vincendon G., Zanetta J. P. La prolifération des oligodendrocytes est stimulée après leur adhésion sur une matrice constituée par une lectine nerveuse, la CSL. C R Acad Sci III. 1988;307(20):863–868. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Joubert R., Kuchler S., Zanetta J. P., Bladier D., Avellana-Adalid V., Caron M., Doinel C., Vincendon G. Immunohistochemical localization of a beta-galactoside-binding lectin in rat central nervous system. I. Light- and electron-microscopical studies on developing cerebral cortex and corpus callosum. Dev Neurosci. 1989;11(6):397–413. doi: 10.1159/000111916. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Fressinaud C., Sarlieve L. L., Vincendon G., Zanetta J. P. Cerebellar soluble lectin is responsible for cell adhesion and participates in myelin compaction in cultured rat oligodendrocytes. Dev Neurosci. 1988;10(3):199–212. doi: 10.1159/000111970. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Herbein G., Sarlieve L. L., Vincendon G., Zanetta J. P. An endogenous lectin "CSL" interacts with glycoprotein components in peripheral nervous system myelin. Cell Mol Biol. 1989;35(5):581–596. [PubMed] [Google Scholar]
- Kuchler S., Joubert R., Avellana-Adalid V., Caron M., Bladier D., Vincendon G., Zanetta J. P. Immunohistochemical localization of a beta-galactoside-binding lectin in rat central nervous system. II. Light- and electron-microscopical studies in developing cerebellum. Dev Neurosci. 1989;11(6):414–427. doi: 10.1159/000111917. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Perraud F., Sensenbrenner M., Vincendon G., Zanetta J. P. An endogenous lectin found in rat astrocyte cultures has a role in cell adhesion but not in cell proliferation. Glia. 1989;2(6):437–445. doi: 10.1002/glia.440020606. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Rougon G., Marschal P., Lehmann S., Reeber A., Vincendon G., Zanetta J. P. Location of a transiently expressed glycoprotein in developing cerebellum delineating its possible ontogenetic roles. Neuroscience. 1989;33(1):111–124. doi: 10.1016/0306-4522(89)90315-1. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Vincendon G., Zanetta J. P. Localisation immunocytochimique au cours du développement d'une lectine endogène du cervelet de rat. C R Acad Sci III. 1987;305(8):317–320. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindner J., Guenther J., Nick H., Zinser G., Antonicek H., Schachner M., Monard D. Modulation of granule cell migration by a glia-derived protein. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4568–4571. doi: 10.1073/pnas.83.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner J., Orkand P. M., Schachner M. Histotypic pattern formation in cerebellar reaggregate cultures in the presence of antibodies to L1 cell surface antigen. Neurosci Lett. 1985 Apr 9;55(2):145–149. doi: 10.1016/0304-3940(85)90010-2. [DOI] [PubMed] [Google Scholar]
- Lindner J., Rathjen F. G., Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. 1983 Sep 29-Oct 5Nature. 305(5933):427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Lindner J., Zinser G., Werz W., Goridis C., Bizzini B., Schachner M. Experimental modification of postnatal cerebellar granule cell migration in vitro. Brain Res. 1986 Jul 9;377(2):298–304. doi: 10.1016/0006-8993(86)90872-3. [DOI] [PubMed] [Google Scholar]
- Mallet J., Huchet M., Shelanski M., Changeux J. P. Protein differences associated with the absence of granule cells in the cerebella from the mutant weaver mouse and from x-irradiated rat. FEBS Lett. 1974 Sep 15;46(1):243–246. doi: 10.1016/0014-5793(74)80378-9. [DOI] [PubMed] [Google Scholar]
- Marschal P., Reeber A., Neeser J. R., Vincendon G., Zanetta J. P. Carbohydrate and glycoprotein specificity of two endogenous cerebellar lectins. Biochimie. 1989 May;71(5):645–653. doi: 10.1016/0300-9084(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Moonen G., Grau-Wagemans M. P., Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982 Aug 19;298(5876):753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Perraud F., Kuchler S., Gobaille S., Labourdette G., Vincendon G., Zanetta J. P. Endogenous lectin CSL is present on the membrane of cilia of rat brain ependymal cells. J Neurocytol. 1988 Dec;17(6):745–751. doi: 10.1007/BF01216703. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Reeber A., Vincendon G., Zanetta J. P. Isolation and immunohistochemical localization of a "Purkinje cell specific glycoprotein subunit from rat cerebellum. Brain Res. 1981 Dec 14;229(1):53–65. doi: 10.1016/0006-8993(81)90745-9. [DOI] [PubMed] [Google Scholar]
- Sidman R. L., Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973 Nov 9;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Changeux J. P. Bergmann fibers and granular cell migration in the cerebellum of homozygous weaver mutant mouse. Brain Res. 1974 Sep 13;77(3):484–491. doi: 10.1016/0006-8993(74)90636-2. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Changeux J. P. Transsynaptic degeneration 'en cascade' in the cerebellar cortex of staggerer mutant mice. Brain Res. 1974 Mar 8;67(3):519–526. doi: 10.1016/0006-8993(74)90499-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetta J. P., Dontenwill M., Reeber A., Vincendon G., Legrand C., Clos J., Legrand J. Con A-binding glycoproteins in the developing cerebellum of control and hypothyroid rats. Brain Res. 1985 Jul;353(1):1–6. doi: 10.1016/0165-3806(85)90018-5. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Meyer A., Kuchler S., Vincendon G. Isolation and immunochemical study of a soluble cerebellar lectin delineating its structure and function. J Neurochem. 1987 Oct;49(4):1250–1257. doi: 10.1111/j.1471-4159.1987.tb10017.x. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Roussel G., Ghandour M. S., Vincendon G., Gombos G. Postnatal development of rat cerebellum: massive and transient accumulation of concanavalin A binding glycoproteins in parallel fiber axolemma. Brain Res. 1978 Feb 24;142(2):301–319. doi: 10.1016/0006-8993(78)90637-6. [DOI] [PubMed] [Google Scholar]