Abstract
The bronchial epithelium is continuously exposed to a multitude of noxious challenges in inhaled air. Cellular contact with most damaging agents is reduced by the action of the mucociliary apparatus and by formation of a physical barrier that controls passage of ions and macromolecules. In conjunction with these defensive barrier functions, immunomodulatory cross-talk between the bronchial epithelium and tissue-resident immune cells controls the tissue microenvironment and barrier homeostasis. This is achieved by expression of an array of sensors that detect a wide variety of viral, bacterial, and nonmicrobial (toxins and irritants) agents, resulting in production of many different soluble and cell-surface molecules that signal to cells of the immune system. The ability of the bronchial epithelium to control the balance of inhibitory and activating signals is essential for orchestrating appropriate inflammatory and immune responses and for temporally modulating these responses to limit tissue injury and control the resolution of inflammation during tissue repair. In asthmatic patients abnormalities in many aspects of epithelial barrier function have been identified. We postulate that such abnormalities play a causal role in immune dysregulation in the airways by translating gene-environment interactions that underpin disease pathogenesis and exacerbation.
Key words: Asthma, tight junction, innate immunity, cytokine, homeostasis
Abbreviations used: AhR, Aryl hydrocarbon receptor; AJ, Adherens junction; BHR, Bronchial hyperresponsiveness; CDHR3, Cadherin-related family member 3; DC, Dendritic cell; DUOX1, Dual oxidase 1; EGFR, Epidermal growth factor receptor; eQTL, Expression quantitative trait locus; GC, Gene cluster; GST, Glutathione-S-transferase; GWAS, Genome-wide association study; ILC, Innate lymphoid cell; ILC2, Type 2 innate lymphoid cell; NK, Natural killer; ORMDL3, Orosomucoid-like 3; PCDH1, Protocadherin 1; SC, Subject cluster; SNP, Single nucleotide polymorphism; TJ, Tight junction; TLR, Toll-like receptor; TSLP, Thymic stromal lymphopoietin
Glossary.
CIGARETTE SMOKE
Gases, hydrocarbon vapors, and particulate matter generated by burning tobacco. Cigarette smoke contains around 4000 substances, more than 60 of which have been identified as carcinogens. Cigarette smoke promotes increased local elastase production, which contributes to lung tissue injury likely caused by increased epithelial permeability through loss of tight junction integrity.
DEFENSINS
A distinct family of antimicrobial peptides produced by epithelial cells of mucosal surfaces, as well as by neutrophils, natural killer cells, and cytotoxic T lymphocytes. Defensins have direct antimicrobial activity, as well as the ability to activate inflammatory responses.
EPISTASIS
The expression of one gene is influenced by the expression of 1 or more independently inherited (nonallelic) genes.
GLUTATHIONE
An intracellular antioxidant whose principal site of synthesis is the liver. Glutathione serves as a cofactor for glutathione peroxidase, which is responsible for detoxifying lipid peroxides.
INNATE LYMPHOID CELLS
Cells possessing lymphoid morphology but without antigen receptors. Their subpopulations are divided into groups that resemble T helper subsets. Group 2 innate lymphoid cells produce type 2 cytokines, such as IL-4, IL-5, IL-9, and IL-13, on stimulation with epithelium-derived cytokines, such as IL-33, IL-25, and TSLP.
LATE-ONSET ASTHMA
Often defined as asthma that develops in adolescence or adulthood. Most cases of asthma are diagnosed in childhood. Late-onset asthma tends to be more common in women than in men. Occupational asthma and aspirin-exacerbated respiratory disease are subtypes of late-onset asthma. Smoking and passive smoke exposure appear to be risk factors for late-onset asthma.
NOD-LIKE RECEPTORS (NLRs)
A family of cytoplasmic receptors that recognize bacterial products, such as bacterial peptidoglycan. NLRs include an N-terminal effector region and a central nucleotide oligomerization domain (NOD), and most have C-terminal leucine-rich repeats for ligand (PAMP) binding. Once activated, NODs assemble signaling proteins, resulting in nuclear factor κB and mitogen-activated protein kinase activation, and control the activation of inflammatory caspases.
PARTICULATE MATTER (PM)
PM is a term describing airborne dust particles originating from a range of sources and processes, such as fossil fuel combustion, waste incineration, cigarette smoking, and erosion, which can contain black carbon (soot), metals, polyaromatic hydrocarbons, and anions, such as sulfate and nitrate, among others. If sufficiently small to be inhaled (aerodynamic diameter <10 μm), PM can settle in the airways and exert a range of effects.
PATHOGEN-ASSOCIATED MOLECULAR PATTERN (PAMP)
Molecules associated with groups of pathogens that are recognized as “danger signals” by pattern recognition receptors, such as Toll-like receptors or NOD-like receptors. Many types of molecules can serve as PAMPs, including LPSs, peptidoglycans, lipoteichoic acid, nucleic acid variants, and flagellin.
POLYAROMATIC HYDROCARBONS (PAHs)
Also known as polycyclic aromatic hydrocarbons, PAHs are volatile substances produced by cooking oils and coal-burning or petroleum products. Indoor biomass burning generates PAHs and is associated with chronic obstructive pulmonary disease development in women.
PSEUDOSTRATIFIED
Although showing features of layering in an epithelium, all cells are still attached to the basement membrane.
TOLL-LIKE RECEPTORS (TLRs)
TLRs are a family of innate pattern recognition receptors that respond to a variety of structurally conserved molecules derived from pathogens (ie, PAMPs). They are single, membrane-spanning, noncatalytic receptors whose signaling pathways are finely regulated by Toll/IL-1 receptor homologous region (TIR) domain–containing adaptors. Differential use of these adaptor proteins provides specificity of individual TLR-mediated signaling pathways.
The editors wish to acknowledge Daniel Searing, MD, for preparing this glossary.
Asthma heterogeneity
Asthma is a common chronic inflammatory disorder of the conducting airways, which undergo distinct structural and functional changes leading to nonspecific bronchial hyperresponsiveness (BHR) and variable airflow obstruction. Recruitment and careful clinical characterization of large cohorts of asthmatic patients has established beyond doubt that asthma is a heterogeneous disease in terms of phenotype, endotype (ie, underlying pathogenic mechanism), response to treatment, and/or long-term clinical outcomes.1 Cluster analysis has enabled identification of 4 to 5 phenotypic clusters that have differences in sex, asthma onset, lung function, atopic status, asthma control, health care use, and exacerbation frequency.2, 3, 4, 5 Molecular phenotyping of blood, induced sputum, and epithelial brushings has identified additional heterogeneity, especially in patients with severe asthma,6, 7, 8, 9 who are a major economic burden on the health care system because of poor responses to traditional asthma medications. Some of the differences in asthma clusters might reflect underlying genetic differences; for example, there are differences in genetic risk in early-onset compared with late-onset asthma,10 whereas others might reflect differences in environment and lifestyle or, perhaps most likely, a combination of both gene and environment effects.11 Many, but not all, asthmatic patients have TH2 inflammation in their airways, and clinical trials with mAbs to IL-5, IL-13, or IL-4 receptor (α chain) have identified a type 2 endotype.12 Thus patient stratification with type 2 relevant biomarkers has enabled effective targeting of these treatments to subsets of patients with moderate and severe asthma.13, 14, 15, 16, 17 However, although clinical trials have shown that type 2 inflammation is an important disease modifier in some patients, they have also highlighted that non–type 2 inflammatory pathways must contribute to certain forms of asthma.18 These can include pathways associated with obesity or neutrophilia or with susceptibility to environmental factors, such as infection and air pollution, but disease mechanisms/endotypes are not well understood. We postulate that a dynamic interaction between a genetically susceptible epithelium and environmental risk factors for asthma is important for the development of asthma and its subphenotypes.19
Bronchial epithelial barrier structure and function
Given the multitude of challenges imposed on the airway epithelium, it is not surprising that it combines structural and functional protective mechanisms with innate immunologic mechanisms to maintain healthy barrier homeostasis and to minimize inflammation and cellular dysregulation. Structurally, the bronchial epithelium is pseudostratified, comprising mainly columnar multiciliated, secretory (goblet), and undifferentiated cells that overlie smaller basal cells with the capacity for self-renewal.20 Rare cell types include pulmonary neuroendocrine cells21, 22 and brush (tuft) cells23 that can have neurosensory or chemosensory functions, but information on these cells is limited.
On the epithelial surface, the mucociliary apparatus is a crucial primary innate defense mechanism that protects the lungs from deleterious effects of inhaled pollutants such as noxious gases and particulate matter, allergens, and pathogens. Surface epithelial cells and submucosal glands produce secretions comprising a superficial gel or mucus layer and a layer of periciliary fluid that contacts the epithelial surface. Mucus contains hydrated gel-forming mucins and a range of host defense and cytoprotective molecules, including defensins, IgA, lactoperoxidase, catalase, superoxide dismutase, and low-molecular-weight antioxidants.24 The viscoelastic properties of the mucus are dictated in large part by the oligomeric secreted mucins MUC5AC and MUC5B,25 multifunctional glycoproteins that provide the structural framework of the mucus barrier. These bronchial secretions shield the epithelial surface, detoxify noxious agents, and trap many inhaled particles, allowing clearance through the action of the mucociliary escalator. MUC5B might also contribute to immune homeostasis by means of direct regulation of leukocyte functions.26, 27
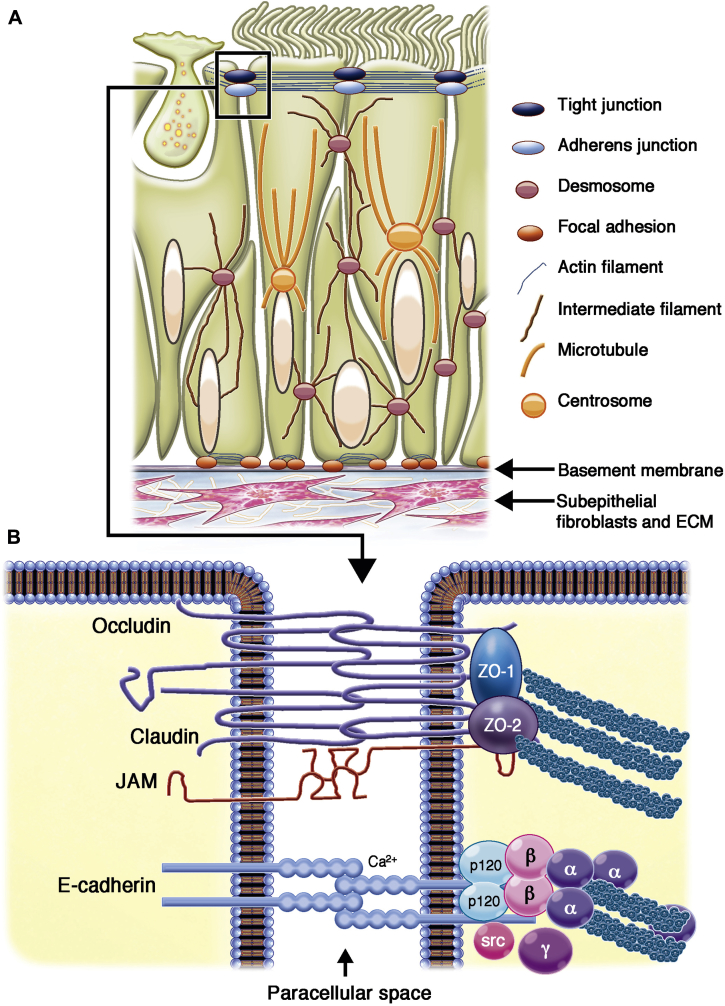
In addition to secreting mucus, the bronchial epithelium forms a sheet-like structure that acts as a physical barrier to protect the internal milieu of the tissue. Individual epithelial cells contact each other through a range of cell-cell adhesion complexes (tight junctions [TJs], adherens junctions [AJs], and desmosomes) that control the permeability of the epithelial sheet and link with the cytoskeleton to resist mechanical stress (Fig 1); in addition, gap junctions directly connect the cytoplasm of adjacent cells, allowing cell-cell communication.28, 29, 30 The apical-most adhesive complexes are the TJs, which are formed by transmembrane and intracellular proteins that link to the actin cytoskeleton (Fig 1, B).31 TJs seal the epithelium, regulating paracellular passage of ions, water, and various macromolecules. They also maintain cell polarity by preventing lateral diffusion and intermixing of molecules in the apical membrane with those in the lateral membrane. Proteins of the TJs include tricellulin and occludin, which regulate the passage of macromolecules through the TJs,32 and claudins, which are responsible for the size- and charge-selective conductance properties of the TJ paracellular pathway.33
Fig 1.
A, Schematic representation of a pseudostratified bronchial epithelial cell layer (comprising a goblet cell, 2 ciliated cells, and 2 basal cells) showing the junctional complexes and their interactions with the cytoskeleton or basement membrane to form a robust sheet-like structure. B, Illustration of the TJ and AJ complexes showing how they mediate cell-cell contact and interact with the actin cytoskeleton. ECM, Extracellular matrix; JAM, junctional adhesion molecule; ZO, zonula occludens.
Expression of barrier or sealing claudins that selectively decrease paracellular cation permeability has been reported in normal human adult lung (claudins 1, 3, 4, 5, 7, and 18),34 and the expression profile varies with anatomic location and function.35, 36 Claudin-2, a pore-forming claudin, is also detected in the lung, and its presence is thought to increase ionic permeability by acting as a cation-selective pore.36
Located below the TJs are the AJs, which link to the actin cytoskeleton37, 38; desmosomes, which link to the intermediate filaments39; and hemidesmosomes,40 containing α6β4 integrins that facilitate attachment to the basement membrane (Fig 1, A). AJs and desmosomes are critical for providing the adhesive force to ensure the integrity of the cell layer. Cadherin-catenin complexes comprise the core of the AJs, bridging neighboring cells and the actin-myosin cytoskeleton and contributing to mechanical coupling between cells. In addition to its adhesive function, E-cadherin physically interacts with several receptor tyrosine kinases and affects their signaling abilities. Similarly, β-catenin, which is an integral structural component of the AJs, is also the key nuclear effector of canonical Wnt signaling in the nucleus.41 This coupling of cell-cell adhesion with signaling functions ensures that AJs can be extremely plastic, allowing the cell to adapt rapidly to its changing environment. Like AJs, the TJ plaque also contains many signaling molecules,42, 43 allowing proteins involved in cell-cell and cell-matrix adhesion to integrate and coordinate epithelial responses.44 Therefore perturbation in the turnover and concentration of junctional proteins is likely to have important implications for the maintenance and stability of the epithelium and the permeability barrier.
Junctional adhesion molecules also serve as sites for interaction of the epithelium with cells involved in immune surveillance. For example, TJ proteins interact directly with dendritic cells (DCs) to allow them to sample the airway lumen without disruption of the epithelial barrier,45, 46 whereas E-cadherin is a ligand for αEβ7 integrin (CD103)–expressed T cells47, 48 and DCs.49 In addition to structural adhesion molecules, the bronchial epithelium expresses inducible adhesion molecules, such as intercellular adhesion molecules 1 and 2, which have essential functions in the clearance of T cells from the lung during resolution of inflammation.50
Airway epithelial cells express an array of pattern recognition receptors, including Toll-like receptors (TLRs), NOD-like receptors, retinoic acid–inducible gene I (RIG-I)–like receptors, and a variety of natural killer (NK) cell receptor ligands. These enable detection of a wide variety of microbial and nonmicrobial agents, resulting in production of many different soluble and cell-surface molecules, collectively termed the epimmunome (cytokines, chemokines, damage-associated molecular pattern molecules, and major histocompatibility complex [MHC] gene products),51 that recruit and activate cells, such as macrophages and neutrophils, involved in inflammation and induction of adaptive immunity. Together, these responses enable many infections to be controlled by the immune system with limited damage to host tissues; however, it is important to note that both innate and adaptive immune-signaling events are involved in mediating tissue damage.52 For example, macrophages, neutrophils, and eosinophils release a range of molecules, including cytotoxic cytokines, cationic proteins, lipid mediators, metalloproteinases, and reactive oxygen species, that induce tissue damage or malfunction. Therefore the ability of the epithelium to control the balance of inhibitory and activating signals is essential not only for initiating an appropriate immune response to environmental challenges, if required (Fig 2), but also for temporally orchestrating these responses to limit tissue injury and control the resolution of inflammatory reactions through cell-surface molecules and release of inhibitory cytokines and lipids during tissue repair.
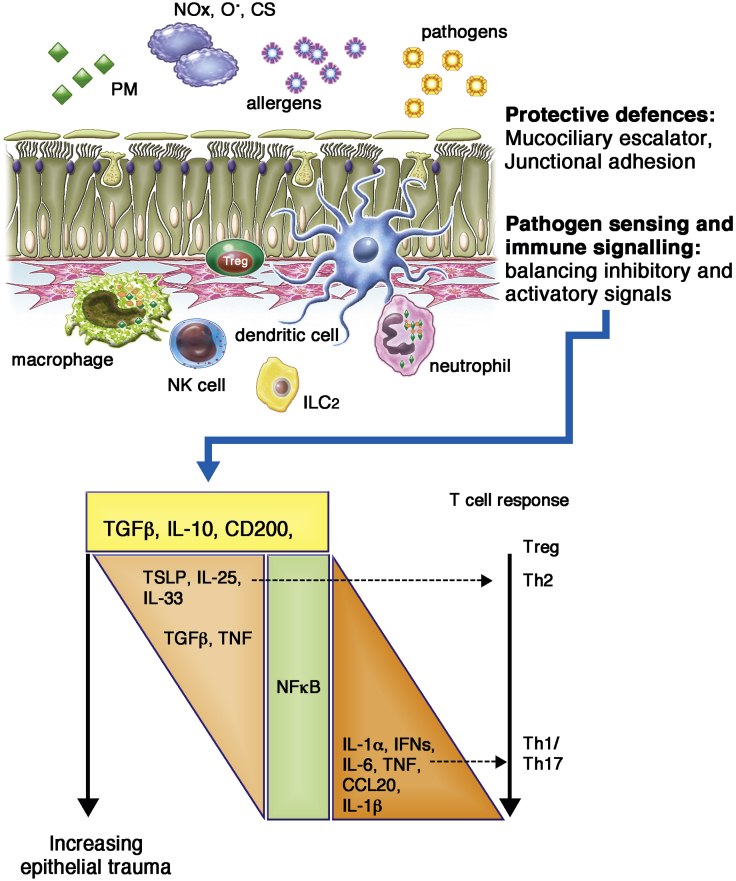
Fig 2.
Schematic representation of epithelial barrier function illustrating protective and immunoregulatory functions. Under basal conditions, the epithelium maintains homeostasis by limiting exposure of the airway tissue to components of the inhaled environment and by balancing immunoregulatory signals. However, when compromised, the epithelium responds by releasing innate cytokines that help to orchestrate appropriate innate and adaptive immune responses. CS, Cigarette smoke; NOx, nitrogen oxides; O·, oxygen radicals; PM, particulate matter; Treg, regulatory T cell.
In vitro and in vivo studies have shown that epithelial cells can modulate a variety of immune cells. For example, epithelium-derived TGF-β is chemoactive for innate lymphoid cells (ILCs),53 which might provide early defense against pathogens and intervene in repair of damaged tissues. TGF-β secreted by bronchial epithelial cells has a direct inhibitory effect on T-lymphocyte proliferation, and epithelial cell–conditioned T lymphocytes show increased differentiation toward IL-10–producing TR1 cells.54 Epithelial cell secretions also inhibit proinflammatory responses of monocytes, macrophages, and DCs; increase DC expression of the negative regulatory programmed death ligand 1 (CD274); decrease the ability of DCs to induce T-lymphocyte proliferation54; and suppress human lung mast cell histamine secretion.55 Epithelial cells express CD200, which binds to the inhibitory immune receptor CD200R, which is expressed at high levels on lung macrophages. This not only maintains a strong threshold for response in the context of inhaled nonpathogenic antigens56 but also dampens macrophage responses in the context of infection. Thus in CD200 knockout mice there is increased macrophage activity and severe immune-mediated lung damage after influenza infection.57
The activation status of NK cells is also controlled by the balance of various inhibitory and activation receptors.58, 59 For example, the NK cell–activating receptor NKG2D is ligated by molecules, such as MHC class I polypeptide–related sequences A and B or UL16-binding proteins, which are only expressed on stressed airway epithelial cells,60, 61 resulting in killing of the target cells and ultimately leading to protection from infection. The importance of NK cells and NKG2D in allergic airways responses has been suggested by the findings that mice lacking NKG2D are resistant to induction of allergic inflammation. Although adoptive transfer of wild-type NK cells was able to restore the response, granzyme B–deficient NK cells could not.62
One common link between both infectious and noninfectious triggers of type 2 immunity is that many induce some level of physical trauma that breaches the protective barrier of the body. Tissue damage, at least in the absence of strong type 1–promoting pathogen-associated molecular pattern signaling, appears to be a potent mechanism driving type 2 immunity. This involves rapid release of several epithelium-derived cytokine alarmins, such as IL-1, IL-33, thymic stromal lymphopoietin (TSLP), and IL-25, all of which can drive downstream type 2 immunity.63 These cytokines invoke an immune response, involving mast cells, basophils, eosinophils, type 2 innate lymphoid cells (ILC2s), and alternatively activated macrophages, which has evolved to respond to a parasitic infection by generating proinflammatory mediators, toxin-neutralizing enzymes, and helminth-killing toxins, which also have endogenous tissue-damaging properties. A number of studies have identified many environmental agents linked to asthma that have the potential to cause epithelial barrier disruption and tissue injury in the airways, including the house dust mite allergen Der p 1,64 fungal allergens,65 rhinovirus,66 cigarette smoke,67, 68 and air pollutants.69, 70
Nonetheless, a key question arising from these observations is the following: Why are the airways of asthmatic subjects more susceptible than normal to these relatively ubiquitous agents? As detailed below, it is likely that the explanation lies in a combination of (1) decreased epithelial barrier defenses reducing the threshold for epithelial damage, (2) dysregulated innate immune or immunoregulatory responses that contribute to ongoing barrier dysfunction, and (3) impaired epithelial barrier repair, leading to failure to resolve inflammatory responses.
Dysregulation of the epithelial barrier in asthmatic patients
Targeted studies of the bronchial epithelium have demonstrated a range of abnormalities at many levels of barrier function and innate immunity (Fig 3). However, unbiased transcriptomic approaches are now enabling in-depth analysis of epithelial gene expression profiles8, 9 to provide evidence of molecular mechanisms that might eventually define specific epithelial endotypes of asthma. We will first summarize key abnormalities identified in the epithelial barrier in asthmatic patients and then put these into the context of newer clusters that have been identified and how these relate to genetic susceptibilities.
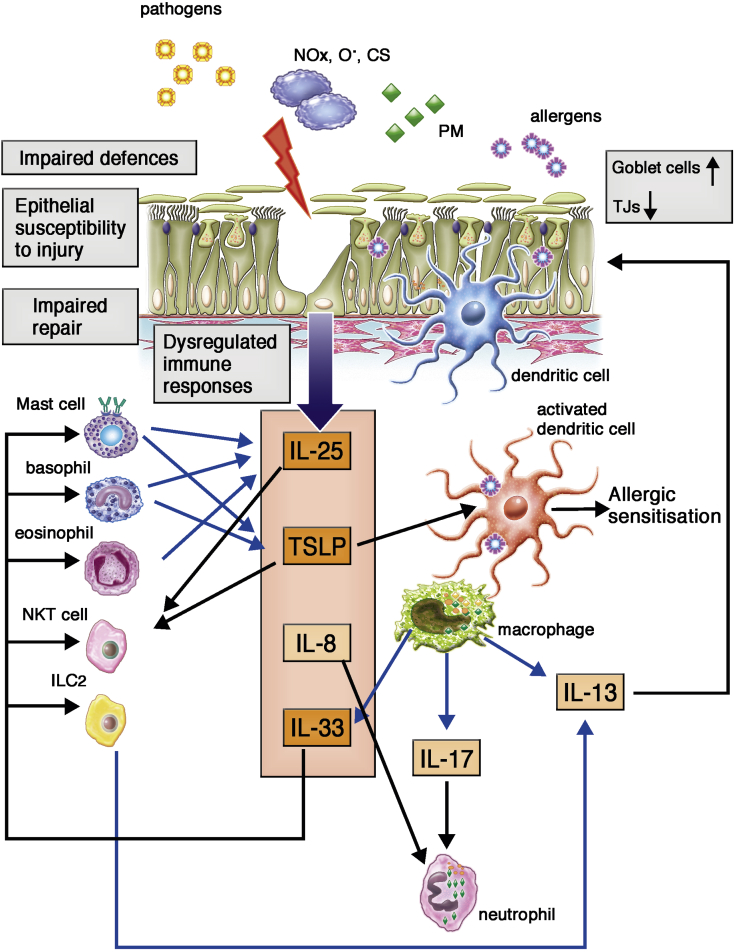
Fig 3.
Schematic representation of the epithelial barrier in asthmatic patients highlighting abnormalities in protective and immunoregulatory functions (gray boxes). Persistent airway inflammation most likely arises as a consequence of impaired barrier defenses (altered cytoprotective secretions and reduced cell-cell adhesion), leading to epithelial susceptibility to injury and dysregulated immune responses. In parallel, impaired repair might contribute to maintenance of epithelial activation and chronicity of responses. The relative contribution of each aspect of barrier dysfunction is likely to influence the overall phenotype of the epithelium and might manifest as distinct subgroups of asthma. CS, Cigarette smoke; NOx, nitrogen oxides; O·, oxygen radicals; PM, particulate matter.
The mucociliary apparatus is modified in asthmatic patients, as evidenced by an increase in the number of goblet cells with increased mucin gene expression, an increase in MUC5AC protein relative to MUC5B, and a reduction in ciliated cell numbers.71, 72, 73 In addition, decreased ciliary beat frequency, dyskinesia, and ciliary disorientation have been reported in patients with severe asthma.74 Together, mucus hypersecretion and ciliary dysfunction in asthmatic patients can result in stimulation of neural receptors that result in cough75 and mucous plugging, which, over time, can lead to severe airflow obstruction.
The increase in expression of MUC5AC relative to MUC5B seen in asthmatic patients has been postulated to affect mucus clearance, reduce eosinophil apoptosis,76 and/or contribute to abnormal innate immune responses.57 Reprogramming of epithelial differentiation toward a hypersecretory phenotype has been linked to increased expression of the epidermal growth factor receptor (EGFR)72 and to the activity of TH2 cytokines, including IL-13 and IL-9.77, 78 Consistent with this, patients with TH2-high asthma have significantly increased airway mucin gene expression.79 TH2 cytokines also significantly decrease epithelial expression of the antimicrobial peptide human β-defensin 2 in vitro, and mice with allergic airway inflammation have significantly more viable bacteria in their lungs after infection.80 In contrast, atopic asthmatic patients with type 2–high asthma have been reported to harbor significantly lower bronchial bacterial burden,81 and in patients with severe asthma, no taxa were associated with a TH2-related epithelial gene expression signature.82 These differences might reflect long-term changes and treatment effects and contrast with the acute responses seen after infection of mice with allergic airways inflammation.80
There is considerable evidence for an association between levels of particulate pollutants and asthma exacerbations,83, 84, 85 asthma pathogenesis, and poorer lung function outcomes.86, 87, 88 Exposure to air pollutants can lead to oxidative stress in the airways, and there is compelling evidence that asthmatic airways are deficient in antioxidant defenses.89 Furthermore, the antioxidant capacity of the lungs is inversely related to asthma severity.90 In addition to lower levels of superoxide dismutase and catalase,89 it has been shown that goblet cells express the high-affinity sodium ascorbate cotransporter, which is involved in vitamin C uptake into cells, and that expression of sodium ascorbate cotransporters is inversely related to lung lining fluid vitamin C levels.91 There is also considerable evidence that polymorphisms in glutathione-cycling enzymes can result in increased susceptibility to air pollution.92, 93, 94 Glutathione-S-transferase (GST)-pi is predominantly expressed in airway epithelial cells, and expression is decreased in the airways of children with asthma.95 In view of the increased susceptibility of the asthmatic bronchial epithelium to oxidant-induced apoptosis in vitro96 and the observation that increased levels of oxidants can reduce the anti-inflammatory effects of budesonide, an inability to control oxidative stress might not only drive epithelial damage but also confound treatment responses.97
Polyaromatic hydrocarbons are a key toxic component of air pollution. Polyaromatic hydrocarbon levels are increased in the plasma of asthmatic children and linked to a number of asthma markers.98 The aryl hydrocarbon receptor (AhR), which plays a key role in detoxification of environmental pollutants, also regulates multiciliogenesis.99 Importantly, although air exposure triggers AhR targeting of genes important for multiciliogenesis, toxic AhR ligands induce detoxifying cytochromes, with no overlap in target gene induction. These mutually exclusive responses suggest a potential pathophysiologic mechanism whereby AhR ligands in air pollutants disrupt AhR-mediated ciliogenesis to contribute to disruption of barrier defenses in asthmatic patients.99
Epithelial fragility100 and epithelial shedding101 in asthmatic patients have been recognized for many years, but this remains a controversial area.102 Nonetheless, through use of specific markers of response to injury, such as increased expression of EGFR, epithelial damage has been confirmed in bronchial biopsy specimens from asthmatic adults103 and children.104 Many studies have reported disruption of adhesive mechanisms in asthmatic patients, including loss of TJ proteins,67, 105, 106 reduction in AJ proteins,105 and reduction in desmosome length.107 Membrane expression of caveolin-1, a stabilizer of AJs, is significantly lower in airway epithelia of asthmatic patients, and in vitro loss of caveolin-1 causes loss of junctional E-cadherin and β-catenin expression and disrupted epithelial barrier function.108 Consistent with reduced adhesion, functional studies comparing epithelial cultures from asthmatic or healthy donors indicate that there is increased permeability and sensitivity to environmental stressors in asthmatic patients67 and increased susceptibility to oxidant stress.96 Increased barrier permeability might not only promote allergic sensitization but also reduce the threshold for epithelial damage and activation of a type 2 response, which itself might affect barrier function. Thus, in addition to their effects on goblet cell differentiation, TH2 cytokines have a disruptive effect on epithelial barrier function109 and lead to a distinct profile of epithelial gene expression, both in vitro and in TH2-high asthmatic patients in vivo.79
Claudin-18, a lung-specific barrier claudin, has been shown to be expressed in bronchial epithelium, and its levels are reduced in asthmatic patients, being lowest in patients with TH2-high asthma.106 In the same studies IL-13 downregulated claudin-18 in vitro, and targeted knockdown of claudin-18 increased epithelial permeability. Furthermore, claudin-18–null mice had significantly higher serum IgE levels and increased airway responsiveness after intranasal Aspergillus species sensitization, suggesting loss of claudin-18 can promote sensitization and airway hyperresponsiveness.106
Because mast cells are important sources of IL-13 and are in close proximity to the bronchial epithelium in asthma,110 it is noteworthy that IL-33–activated mast cells, as well as ILC2s, are able to drive a predominantly IL-13–regulated pattern of gene expression in normal human bronchial epithelial cells in vitro.111 Furthermore, ILC2s have been shown to directly impair epithelial barrier integrity through IL-13,112 whereas TH2 cells cause barrier leakiness through IL-4 and IL-13, an effect that can be prevented by inhibition of histone deacetylases.113
Consistent with the evidence of epithelial disruption in asthmatic patients, levels of epithelium-derived cytokine alarmins, such as IL-33, TSLP, and IL-25, are increased in asthmatic patients.114, 115 IL-33, a member of the IL-1 cytokine family, has gained prominence in type 2 immunity by virtue of the genetic association of both IL33 and its receptor, IL1RL1 (ST2), with asthma10, 116 and by its functional effects on ILC2 cells, TH2 cells, mast cells, basophils, and alternatively activated macrophages.117 IL-33 is normally localized in the nucleus, where it is a transcriptional regulator118 and can act as an extracellular cytokine by binding to its receptor, ST2.119 Full-length IL-33 binds ST2 and is biologically active, although activity can be increased after cleavage by inflammatory proteases,120 whereas caspase cleavage leads to inactivation.121 IL-33 can be released by nonprogrammed cell death, or it can be actively secreted through vesicular transport from the Golgi complex.122 Stimulation of bronchial epithelial cells with allergen or ATP results in active release of IL-33, which depends on the NADPH oxidase dual oxidase 1 (DUOX1)–mediated activation of Src and EGFR signaling through cysteine oxidation.123 Nasal epithelial cells from asthmatic patients display enhanced DUOX1 expression, as well as allergen-induced IL-33 secretion, compared with healthy control subjects, suggesting that increased expression and activation of DUOX1 might be an important feature of enhanced IL-33 secretion in asthmatic patients.123 In addition to full-length IL-33, alternative splicing of the IL-33 transcript can result in deletion of exons 3 and 4 (Δ exon 3,4) to confer cytoplasmic localization and facilitate extracellular secretion without cell death, while retaining signaling capacity. Analyses of epithelial brush RNA suggest that Δ exon 3,4 is strongly associated with airway type 2 inflammation, whereas full-length IL-33 is not.124 These results suggest that therapeutic IL-33 inhibitors will need to block all biologically active isoforms.
TSLP is an IL-7–like cytokine that can trigger DC-mediated TH2 inflammatory responses125 and TH2 cytokine production by mast cells.126 A variety of stimuli, including double-stranded RNA and allergens, stimulate TSLP expression in bronchial epithelial cells, and this is enhanced by inflammatory cytokines.127 Challenge of cultured epithelial cells from asthmatic donors with double-stranded RNA results in a skewed response favoring more TSLP and less type 1 interferon compared with healthy cells.128 Allergen-specific T cells also enhance TSLP production by epithelial cells from asthmatic donors, suggesting that T cell–airway epithelium interactions can lead to maintenance and amplification of allergic inflammation.129 In a double-blind, placebo-controlled study, treatment with a human mAb to TSLP resolved airway inflammation and attenuated allergen-induced bronchoconstriction, findings consistent with TSLP as a therapeutic target in patients with allergic asthma.130 However, in addition to its effects on immune cells, it is noteworthy that TSLP drives an IL-13–dependent increase in bronchial epithelial cell proliferation131 and increases TJ expression to enhance nasal epithelial barrier function, suggesting a role for TSLP in restoration of epithelial barrier integrity.132 In contrast, TSLP has been reported to disrupt TJs in 16HBE bronchial epithelial cells.133 Furthermore, a short and constitutively expressed form of TSLP has been detected in the skin and gut; this variant cannot activate signal transducer and activator of transcription (STAT) 5 but has potent antimicrobial activity.134 Recent studies suggest that the short and constitutively expressed form of TSLP can protect against bronchial epithelial barrier disruption in vitro and house dust mite– or toluene diisocyanate–induced airway inflammation in vivo.133, 135 Consequently, optimal therapeutic antibody targeting might need to be directed specifically to the long form of TSLP.
IL-25 belongs to the IL-17 cytokine family and is secreted by TH2 cells, mast cells, basophils, and eosinophils, as well as epithelial cells.136 It can drive airway remodeling in allergic models of airway inflammation,137 and, in combination with IL-33, can promote the development of ILC2s, which appear critical in early initiation of the TH2 response.138 Expression of IL-25 has been reported to be increased in epithelial cells from patients with asthma and can be induced further by rhinovirus infections.139 Others have found increased systemic levels of IL-25 in subgroups of patients with TH2-high asthma.140 Furthermore, the IL-25 receptor (IL-17RB) is upregulated on myeloid and plasmacytoid DCs in blood and sputum 24 hours after allergen challenge.141 IL-25 upregulated TLR9 expression by plasmacytoid DCs and orchestrated the responses to TLR9 ligation, suggesting that IL-25 can act as a link between the adaptive and innate immune responses.141
Viral respiratory tract infections, especially rhinovirus infection, are the main triggers of asthma exacerbations.142, 143 Several,144, 145, 146 but not all,147, 148 studies have shown that bronchial epithelial cells from asthmatic donors respond abnormally to rhinovirus infection involving an insufficiency of IFN-β and IFN-λ. This has been linked to increased TGF-β2 production by epithelial cells from asthmatic patients149 and suppression of cytokine signaling expression150; however, it is also of interest that rhinovirus-induced EGFR activation can suppress IFN-λ production and increase viral infection.151 The importance of decreased antiviral immunity in asthmatic patients has been tested in a clinical trial with inhaled IFN-β: the drug was found to improve asthma control and reduce exacerbations in patients with difficult-to-treat asthma.152
It is well known that mechanical forces are critical to lung development and that abnormal mechanical stresses can lead to pathologic lung injury.153 In asthmatic patients constriction of bronchial smooth muscle during an acute asthma attack causes the airway wall to buckle, resulting in folding and compression of the bronchial epithelium.153 In vitro studies have shown that airway epithelial cells respond rapidly and robustly to compressive stress with changes in goblet cell numbers and production of profibrogenic growth factors.154, 155 The relevance of these findings has been demonstrated in vivo, where induction of bronchoconstriction with methacholine caused airway remodeling involving goblet cell metaplasia and subepithelial fibrosis without evidence of inflammation.156 Although these changes might simply be due to the hyperresponsive properties of bronchial smooth muscle in asthmatic patients, there is evidence that bronchial epithelial cells from asthmatic donors respond abnormally to compression with increased release of TGF-β and GM-CSF,157 suggesting that bronchoconstriction can skew epithelial innate immune responses in asthmatic patients. Because the asthma susceptibility gene a disintegrin and metalloprotease 33 (ADAM33) has been linked to BHR158 and has been shown to cause bronchial smooth muscle contraction,159 there is the potential for multifactorial indirect genetic effects on epithelial barrier function.
Increased expression of the EGFR in bronchial biopsy specimens from asthmatic adults103 and children104 is consistent with an ongoing response to injury, and this is highly correlated with epithelial IL-8 expression.160 However, expression of the cyclin-dependent kinase inhibitor p21waf might be indicative of impaired proliferation or ongoing epithelial stress in asthmatic patients.104, 161 During epithelial repair, neighboring epithelial cells become migratory in response to growth factors, such as TGF-β or epidermal growth factor. This repair phenotype is characterized by downregulation of TJs and increased expression of matrix metalloproteases and extracellular matrix components, as observed in asthmatic patients. Studies with cultures of epithelial cells from asthmatic children suggest that the airway epithelium displays a dysregulated repair response, taking longer to repair mechanically induced wounds162 and undergoing a more extensive epithelial-mesenchymal transition in response to TGF-β than cultures from nonasthmatic donors.163 Recently, it has been reported that IL-22 can promote a repair phenotype in the presence of TGF-β1, causing a marked reduction in E-cadherin but only in cells obtained from donors with severe asthma.164
Epithelial clusters and asthma heterogeneity
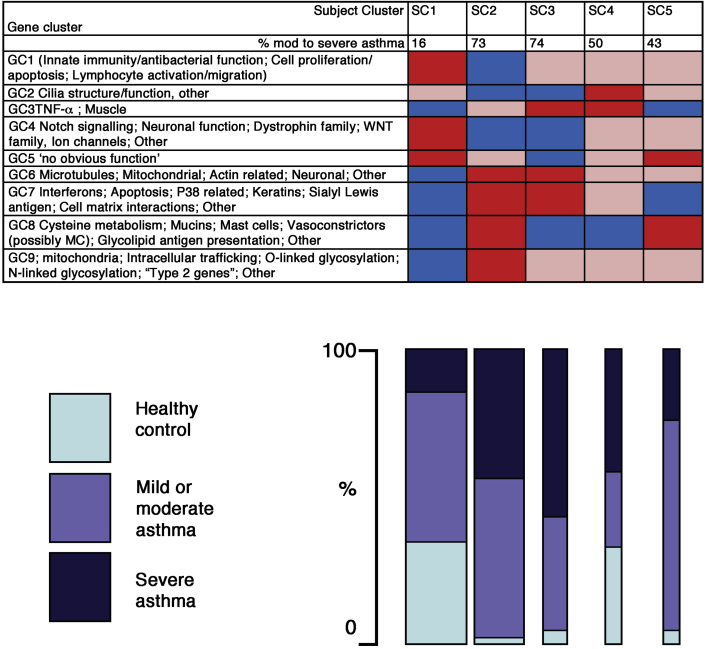
Use of large-scale transcriptomic approaches in large cohorts of well-characterized asthmatic and healthy control volunteers has enabled unbiased in-depth analysis of gene expression profiles in epithelial brushings and allowed clustering into distinct phenotypes. Analysis of transcriptomic data from 155 donors in combination with exhaled nitric oxide has identified 5 molecularly defined and clinically distinct subject clusters (SCs) with distinct expression of gene clusters (GCs),8 as summarized in Fig 4. The majority (73%) of all healthy control subjects were located in SC1, which was distinguished by high expression of GCs involved in processes including “innate immunity/antibacterial function” and “Notch signaling” and low expression of GCs, including “interferons/stress” and “type 2 immunity.” In contrast, the largest group of patients with severe asthma (SC2) showed a diametrically opposite pattern with low expression of both “innate immunity/antibacterial function” and “Notch signaling” GCs and high expression of “interferons/stress” and “type 2 immunity” GCs. In addition, “cilia structure and function” was low in SC2 with severe asthma. It is interesting to note an apparent paradox that gene signatures for both cilia-related genes and Notch signaling are reduced in SC2. Because Notch signaling inhibits ciliated cell differentiation in vitro by repressing multicilin and forkhead box J1,165 low Notch levels might suggest increased ciliogenesis, but this was not the case. However, it has been shown that IL-13 inhibits ciliated cell differentiation independent of Notch signaling,166 suggesting 2 distinct signaling pathways can affect ciliated cell differentiation, which might be of relevance in the different SCs of severe asthma. The other SCs showed some overlap with SC2, but each exhibited distinct profiles illustrating the heterogeneity of the epithelial gene signature across the spectrum of asthma severity. Further analysis of the same data using weighted gene coexpression network analysis (WGCNA) highlighted that genes in modules linked to epithelial growth and repair and neuronal function were markedly decreased in patients with severe asthma.9 Of particular note, low expression of epithelial growth and repair and neuronal function genes was more strongly associated with severe asthma than type 2 inflammation, suggesting that epithelial integrity and related processes are of primary importance to the development of asthma and severe asthma.
Fig 4.
Pictorial representation of the SCs and GCs found in a transcriptomic analysis of epithelial brushings from 155 donors. Red indicates high, pink indicates medium, and blue indicates low expression of genes within the cluster. The bar chart indicates the percentage of healthy control subjects and patients with mild, moderate, or severe asthma in each SC, and the width of the bar is proportional to the number of subjects in the cluster. Findings are summarized from Modena et al.8
Assuming that these phenotypes are stable rather than fluctuations because of disease activity, these data illustrate the complexity of the epithelial phenotype. Reinforcement of these findings with longitudinal studies should provide a basis for hypothesis-driven research that allows precise definition of epithelial endotypes in asthmatic patients. Nonetheless, based on the evidence to date, further consideration of strategies that promote epithelial repair and restore epithelial homeostasis might provide novel therapeutic approaches for the treatment of asthma.24 For example, the protective effects of growth factors, such as epidermal growth factor, have been recognized for many years (reviewed by Swindle et al24). However, novel strategies include potential use of the macrolide antibiotic azithromycin, which has been shown to decrease ionic permeability of human airway epithelia by changing the processing of TJ proteins,167 or histone deacetylase inhibition with JNJ-26481585, which has been shown to ameliorate the effects of TH2 cells on barrier function.113
From asthma genes to function
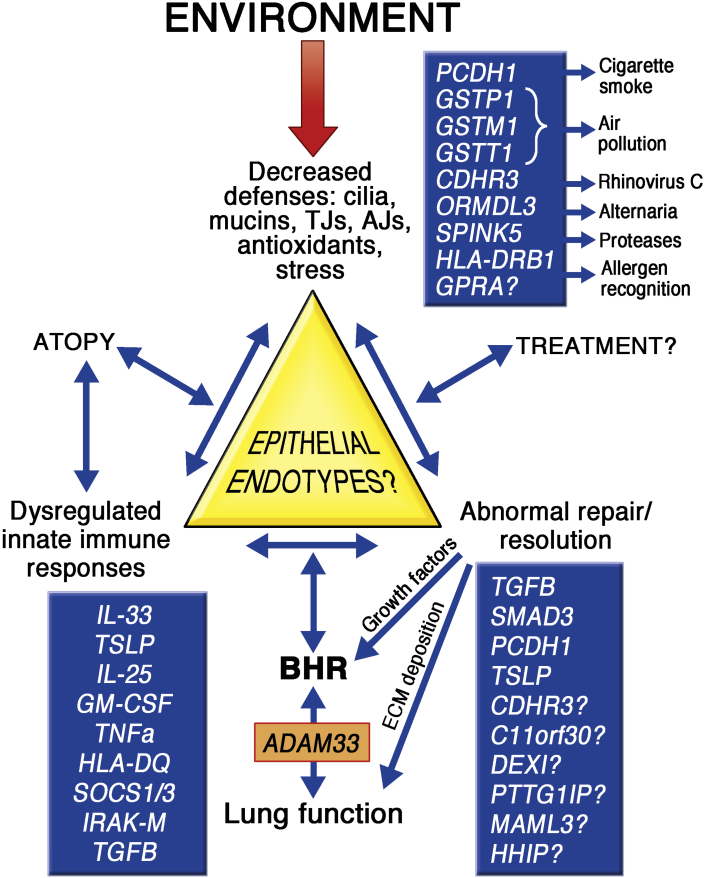
Genome-wide association studies (GWASs) of asthma have identified novel risk alleles and loci, with many of the asthma susceptibility genes being expressed in the airway epithelium.168 Among susceptibility factors for asthma, the genes IL1RL1/IL18R1, IL33, and TSLP have emerged as some of the most important associated with development of the disease,10 linking epithelium-derived cytokines to type 2 inflammation. Furthermore, a number of genes associated with epithelial homeostasis, differentiation, or barrier immunity have been identified, including protocadherin 1 (PCDH1),169 cadherin-related family member 3 (CDHR3),170 HLA-DQ,10 SPINK5,171 GPRA,172 and orosomucoid-like 3 (ORMDL3)/GSDMB10 at the 17q12-21 locus. However, it should be noted that asthma-associated alleles have small effect sizes and account for little of the prevalence of asthma, and it is likely that a significant portion of the genetic risk for asthma and its exacerbations results from genotype-specific responses to environmental exposures, including allergens, pollution, and viral infections, especially at particular stages of life.173, 174, 175, 176, 177 Here we have attempted to place some of the asthma susceptibility genes in the context of epithelial barrier dysregulation, with a view to highlighting potential epithelial endotypes of disease linked to reduced barrier defenses, dysregulated immune responses, and/or abnormal repair responses (Fig 5).
Fig 5.
Potential mechanisms of asthma defined by epithelial barrier dysfunction. Identification of potential links with asthma susceptibility genes and their interaction with environmental stimuli are shown.
Epidemiologic and genetic evidence have implicated epithelial susceptibility to environmental insults in asthma pathogenesis. However, clear functional relationships are not always easy to identify, perhaps reflecting the need for assessment in the context of an appropriate environmental trigger. For example, although 2 common deletion polymorphisms of the GST genes GSTM1 and GSTT1 and the GSTP1 Ile105Val polymorphism have been associated with asthma in children and adults, a meta-analysis has revealed extreme between-study heterogeneity,178 suggesting more focused study in the context of environmental oxidative exposures would be more informative.
Genes, such as the cadherin family members CDHR3170 and PCDH1,169 appear to play roles in adhesion. Several single nucleotide polymorphisms (SNPs) in PCDH1 have been linked to asthma and BHR. These include Ala750Ala and IVS3_116, which are localized in the 3′ untranslated region of exon 3 and might affect mRNA stability or splicing, whereas Ala514Thr is localized in the fifth cadherin repeat of the extracellular domain and can affect cell-cell adhesion169; however, the functional consequences of this mutation have not been explored. Protocadherin 1 (PCDH1) colocalizes with E-cadherin in airway epithelial cells, and it has been implicated in the barrier-enhancing properties of glucocorticoids179 and suppression of TGF-β signaling.180 Because gene–passive smoking interactions have been found to be relevant for the association of PCDH1 with asthma,169, 181 the contribution of PCDH1 gene variants to asthma might only become evident in the context of smoke exposure.182 CDHR3 was originally identified as an asthma susceptibility gene linked to childhood exacerbation.170 The asthma-associated SNP (rs6967330) causes a nonsynonymous mutation (G>A; C529Y) in the fifth cadherin repeat of CDHR3, which affects cellular localization.170 Subsequent studies showed that CDHR3 is a receptor for rhinovirus C, suggesting that the increased localization of Y529 CDHR3 on the bronchial epithelial cell surface increases susceptibility for rhinovirus C infection and replication.183 However, the normal cellular function of CDHR3 is still unknown.
ORMDL3 has been shown to be associated with early-onset asthma susceptibility in multiple independent genome-wide and candidate-gene association studies.173 It is regulated by STAT6 and can be induced by IL-13 or IL-4,184 and SNPs in ORMDL3 correlate with changes in TH2 cytokine levels.185 ORMDL3 is found in the endoplasmic reticulum and is involved in maintaining sphingolipid homeostasis and in the unfolded protein response,186 but in vitro studies involving underexpression or overexpression of ORMDL3 did not show a significant role in modulating innate immune responses and the unfolded protein response.187 However, in mice overexpression of ORMDL3 decreases serum sphingolipid levels and increases inflammatory markers, airway remodeling, and BHR in response to allergic stimuli.188 Furthermore, pulmonary epithelial expression of ORMDL3 is sufficient for induction of Alternaria species–induced allergic airways disease.189
As already described, polymorphisms in genes, including IL33, IL1RL1, and TSLP, have been linked to epithelial activation/damage and type 2 immunity, although detailed studies are still revealing new levels of complexity involving alternative splicing.124 In the case of TSLP, multiple SNPs are correlated with the expression levels of TSLP, and some alleles are protective.190 Of note, in subjects with 1 or more SPINK5 risk alleles, the absence of the TSLP protective minor alleles has been associated with a significant increase in asthma.191 Thus, in addition to gene-environment effects, epistasis adds another level of complexity to asthma pathogenesis. Other immune regulators might be relevant to exacerbation-prone asthma: these include suppressor of cytokine signaling 1 (SOCS1)192 and IL-1 receptor–associated kinase M (IRAK-M),193 both of which suppress IFN-β signaling and antiviral responses.150, 194
The focus on epithelial repair genes in asthmatic patients has been limited to date, but promoter variants in TGFB1 and TGFB2, which increase TGF-β expression, are associated with asthma195, 196 and airflow obstruction.197 It is also interesting to note that genes, such as hedgehog interacting protein (HHIP) and patched homolog 1 (PTCH1), which might play a role in epithelial repair have been identified through genetic association with reduced lung function,198 suggesting that impaired repair might drive extracellular matrix deposition and tissue remodeling.
Most of the asthma-associated SNPs identified by using GWASs are not coding-change variants. Therefore expression quantitative trait loci (eQTLs) analysis has been adopted to identify functional SNPs regulating expression levels of disease-associated genes in a cell type–specific fashion. Applying this analysis to bronchial epithelial cells has revealed SNPs in TSLP, GSDMB, IL33, HLA-DQB1, C11orf30, DEXI, CDHR3, and ZBTB10 that affect asthma risk by allowing cis-regulation of its gene expression in an epithelial specific manner.190 In the case of IL33, all asthma-associated SNPs in this region of the genome are located in the 5′ or first intron of IL33, and eQTL analysis has revealed that SNPs in the promoter region of IL33 are correlated with IL-33 expression in bronchial epithelial cells. The same study identified an eQTL SNP for CDHR3 (rs17152490) in bronchial epithelial cells, which is in linkage disequilibrium with the GWAS SNP (rs6967330, G>A; C529Y), suggesting cis-regulation of CDHR3 expression can also contribute to the asthma risk. SNPs in pituitary tumor–transforming 1 interacting protein (PTTG1IP) and mastermind-like 3 (MAML3) have been reported to be associated with BHR severity in adult asthma,199 and eQTL analyses indicate higher tissue expression with less severe BHR. These gene products might be particularly relevant to epithelial repair because PTTG1lP is coexpressed with vimentin and E-cadherin 1, whereas MAML3 is coexpressed with MAML2, both of which are involved in Notch signaling, a repair pathway that was deficient in the transcriptomic studies of severe asthma.
Concluding comments
Taken together, the evidence for epithelial dysregulation in asthmatic patients is compelling. Genomic studies have revealed the extent of epithelial heterogeneity in asthmatic patients and have provided considerable insight into expression profiles, pathways, and processes that can drive epithelial dysfunction. Further understanding of asthma endotypes will come from integration of findings from these large data sets with the function and regulation of asthma genes and how these are modified by interaction with environmental factors, including the airway microbiome. However, the stability of the asthma phenotypes identified in molecular studies still needs to be addressed in longitudinal studies. In addition, the appreciation that changes in gene expression are also evident in epithelial cells harvested from peripheral airways of patients with severe asthma raises new questions about gene dysregulation in the smaller airways, which comprise the majority of the airway surface area, and the need for better-targeted therapies for the peripheral airways.200 Furthermore, there is a lack of critical information about epithelial heterogeneity and its role in childhood asthma. Crucially, we still lack detailed information about the functions of many asthma genes and how genetic polymorphism of these genes drives asthma susceptibility. The high costs of transgenic and gene-deletion mouse models has restricted progress in this area. Thus it would be timely to investigate the potential of nonmammalian models, such as Drosophila species or zebrafish, as tools to investigate gene function because the genetic tractability and low cost of rearing these organisms are major advantages.201, 202 Better understanding of epithelial dysfunction and its interrelationship with airways inflammation and structural remodeling should help to define specific epithelial endotypes in asthmatic patients. Through development and use of therapeutic approaches that restore epithelial barrier homeostasis, it might be possible to prevent or modify the disease course by intervening close to the origin of the disease.
Footnotes
Supported by the Medical Research Council (MRC [UK]), the National Centre for Reduction Refinement and Replacement of Animals in Research (NC3Rs [UK]), Asthma UK, the Asthma Allergy and Inflammation Research Charity (AAIR), the National Institute for Health Research (NIHR [UK]), and the Biotechnology and Biological Sciences Research Council (BBSRC).
Disclosure of potential conflict of interest: M. Loxham received a fellowship from the Biotechnology and Biological Sciences Research Council (BBSRC) UK. D. E. Davies has received grants from the Medical Research Council UK, National Centre for Reduction, Refinement, and Replacement of Animals in Research (NC3Rs), Asthma Allergy and Inflammation Research Charity, Asthma UK, and the NIHR; has received consulting fees from Synairgen Research; has received travel support from the American Academy of Allergy, Asthma & Immunology and the World Immune Regulation Meeting; has a patent through the University of Southampton; received royalties from the University of Southampton; is cofounder and shareholder of Synairgen; and is chair of the Trustees of the Asthma Allergy and Inflammation Research Charity (AAIR).
Terms in boldface and italics are defined in the glossary on page 1737.
References
- 1.Muraro A., Lemanske R.F., Jr., Hellings P.W., Akdis C.A., Bieber T., Casale T.B. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137:1347–1358. doi: 10.1016/j.jaci.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loza M.J., Adcock I., Auffray C., Chung K.F., Djukanovic R., Sterk P.J. Longitudinally stable, clinically defined clusters of patients with asthma independently identified in the ADEPT and U-BIOPRED asthma studies. Ann Am Thorac Soc. 2016;13(suppl 1):S102–S103. doi: 10.1513/AnnalsATS.201508-519MG. [DOI] [PubMed] [Google Scholar]
- 4.Chang T.S., Lemanske R.F., Jr., Mauger D.T., Fitzpatrick A.M., Sorkness C.A., Szefler S.J. Childhood asthma clusters and response to therapy in clinical trials. J Allergy Clin Immunol. 2014;133:363–369. doi: 10.1016/j.jaci.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denlinger L.C., Phillips B.R., Ramratnam S., Ross K., Bhakta N.R., Cardet J.C. Inflammatory and co-morbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigler J., Boedigheimer M., Schofield J.P., Skipp P.J., Corfield J., Rowe A. A severe asthma disease signature from gene expression profiling of peripheral blood from U-BIOPRED cohorts. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201604-0866OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Lefaudeux D., De M.B., Loza M.J., Peffer N., Rowe A., Baribaud F. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.048. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Modena B.D., Tedrow J.R., Milosevic J., Bleecker E.R., Meyers D.A., Wu W. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modena B.D., Bleecker E.R., Busse W.W., Erzurum S.C., Gaston B.M., Jarjour N.N. Gene expression correlated to severe asthma characteristics reveals heterogeneous mechanisms of severe disease. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201607-1407OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vawda S., Mansour R., Takeda A., Funnell P., Kerry S., Mudway I. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol. 2014;179:432–442. doi: 10.1093/aje/kwt269. [DOI] [PubMed] [Google Scholar]
- 12.Fajt M.L., Wenzel S.E. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015;135:299–310. doi: 10.1016/j.jaci.2014.12.1871. [DOI] [PubMed] [Google Scholar]
- 13.Corren J., Busse W., Meltzer E.O., Mansfield L., Bensch G., Fahrenholz J. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 14.Corren J., Lemanske R.F., Hanania N.A., Korenblat P.E., Parsey M.V., Arron J.R. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 15.Piper E., Brightling C., Niven R., Oh C., Faggioni R., Poon K. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013;41:330–338. doi: 10.1183/09031936.00223411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 17.Haldar P., Brightling C.E., Hargadon B., Gupta S., Monteiro W., Sousa A. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahy J.V. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies D.E., Wicks J., Powell R.M., Puddicombe S.M., Holgate S.T. Airway remodelling in asthma—new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 20.Rock J.R., Randell S.H., Hogan B.L. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds S.D., Giangreco A., Power J.H., Stripp B.R. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosney J.R. Pulmonary neuroendocrine cell system in pediatric and adult lung disease. Microsc Res Tech. 1997;37:107–113. doi: 10.1002/(SICI)1097-0029(19970401)37:1<107::AID-JEMT11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Reid L., Meyrick B., Antony V.B., Chang L.Y., Crapo J.D., Reynolds H.Y. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med. 2005;172:136–139. doi: 10.1164/rccm.200502-203WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swindle E.J., Collins J.E., Davies D.E. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J Allergy Clin Immunol. 2009;124:23–34. doi: 10.1016/j.jaci.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Thornton D.J., Rousseau K., McGuckin M.A. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 26.Roy M.G., Livraghi-Butrico A., Fletcher A.A., McElwee M.M., Evans S.E., Boerner R.M. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen W.J., Stefanski A.L., Bochner B.S., Evans C.M. Control of lung defence by mucins and macrophages: ancient defence mechanisms with modern functions. Eur Respir J. 2016;48:1201–1214. doi: 10.1183/13993003.00120-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kast J.I., Wanke K., Soyka M.B., Wawrzyniak P., Akdis D., Kingo K. The broad spectrum of interepithelial junctions in skin and lung. J Allergy Clin Immunol. 2012;130:544–547. doi: 10.1016/j.jaci.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Rezaee F., Georas S.N. Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol. 2014;50:857–869. doi: 10.1165/rcmb.2013-0541RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georas S.N., Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 32.Krug S.M., Schulzke J.D., Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Krause G., Winkler L., Mueller S.L., Haseloff R.F., Piontek J., Blasig I.E. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Schlingmann B., Molina S.A., Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015;42:47–57. doi: 10.1016/j.semcdb.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niimi T., Nagashima K., Ward J.M., Minoo P., Zimonjic D.B., Popescu N.C. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21:7380–7390. doi: 10.1128/MCB.21.21.7380-7390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaarteenaho-Wiik R., Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009;57:187–195. doi: 10.1369/jhc.2008.951566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanov A.I., Naydenov N.G. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol. 2013;303:27–99. doi: 10.1016/B978-0-12-407697-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 38.Nelson W.J. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrod D., Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Nievers M.G., Schaapveld R.Q., Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18:5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 41.Gangl K., Reininger R., Bernhard D., Campana R., Pree I., Reisinger J. Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009;64:398–405. doi: 10.1111/j.1398-9995.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Mariscal L., Tapia R., Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Balda M.S., Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Tsukita S., Yamazaki Y., Katsuno T., Tamura A., Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- 45.Veres T.Z., Voedisch S., Spies E., Tschernig T., Braun A. Spatiotemporal and functional behavior of airway dendritic cells visualized by two-photon microscopy. Am J Pathol. 2011;179:603–609. doi: 10.1016/j.ajpath.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blank F., Wehrli M., Lehmann A., Baum O., Gehr P., Von G.C. Macrophages and dendritic cells express tight junction proteins and exchange particles in an in vitro model of the human airway wall. Immunobiology. 2011;216:86–95. doi: 10.1016/j.imbio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Pauls K., Schon M., Kubitza R.C., Homey B., Wiesenborn A., Lehmann P. Role of integrin alphaE(CD103)beta7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol. 2001;117:569–575. doi: 10.1046/j.0022-202x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- 48.Cepek K.L., Shaw S.K., Parker C.M., Russell G.J., Morrow J.S., Rimm D.L. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 49.Kim T.H., Lee H.K. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014;14:128–137. doi: 10.4110/in.2014.14.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter J.C., Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 2009;23:492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swamy M., Jamora C., Havran W., Hayday A. Epithelial decision makers: in search of the “epimmunome.”. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denney L., Byrne A.J., Shea T.J., Buckley J.S., Pease J.E., Herledan G.M. Pulmonary epithelial cell-derived cytokine TGF-beta1 is a critical cofactor for enhanced innate lymphoid cell function. Immunity. 2015;43:945–958. doi: 10.1016/j.immuni.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer A.K., Bartz H., Fey F., Schmidt L.M., Dalpke A.H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur J Immunol. 2008;38:1689–1699. doi: 10.1002/eji.200737936. [DOI] [PubMed] [Google Scholar]
- 55.Martin N., Ruddick A., Arthur G.K., Wan H., Woodman L., Brightling C.E. Primary human airway epithelial cell-dependent inhibition of human lung mast cell degranulation. PLoS One. 2012;7:e43545. doi: 10.1371/journal.pone.0043545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwyer F.E., Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012;2012:140937. doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snelgrove R.J., Goulding J., Didierlaurent A.M., Lyonga D., Vekaria S., Edwards L. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 58.Vivier E., Nunes J.A., Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 59.Bryceson Y.T., March M.E., Ljunggren H.G., Long E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obeidy P., Sharland A.F. NKG2D and its ligands. Int J Biochem Cell Biol. 2009;41:2364–2367. doi: 10.1016/j.biocel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Borchers M.T., Harris N.L., Wesselkamper S.C., Vitucci M., Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L222–L231. doi: 10.1152/ajplung.00327.2005. [DOI] [PubMed] [Google Scholar]
- 62.Farhadi N., Lambert L., Triulzi C., Openshaw P.J., Guerra N., Culley F.J. Natural killer cell NKG2D and granzyme B are critical for allergic pulmonary inflammation. J Allergy Clin Immunol. 2014;133:827–835. doi: 10.1016/j.jaci.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammad H., Lambrecht B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Wan H., Winton H.L., Soeller C., Tovey E.R., Gruenert D.C., Thompson P.J. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai H.Y., Tam M.F., Chou H., Peng H.J., Su S.N., Perng D.W. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 66.Sajjan U., Wang Q., Zhao Y., Gruenert D.C., Hershenson M.B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao C., Puddicombe S.M., Field S., Haywood J., Broughton-Head V., Puxeddu I. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 68.Olivera D.S., Boggs S.E., Beenhouwer C., Aden J., Knall C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal Toxicol. 2007;19:13–22. doi: 10.1080/08958370600985768. [DOI] [PubMed] [Google Scholar]
- 69.London N.R., Jr., Tharakan A., Rule A.M., Lane A.P., Biswal S., Ramanathan M., Jr. Air pollutant-mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J Allergy Clin Immunol. 2016;138:1736–1738. doi: 10.1016/j.jaci.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 70.Ghio A.J., Devlin R.B. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med. 2001;164:704–708. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- 71.Fahy J.V. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(suppl):320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 72.Takeyama K., Fahy J.V., Nadel J.A. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001;163:511–516. doi: 10.1164/ajrccm.163.2.2001038. [DOI] [PubMed] [Google Scholar]
- 73.Lachowicz-Scroggins M.E., Yuan S., Kerr S.C., Dunican E.M., Yu M., Carrington S.D. Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma. Am J Respir Crit Care Med. 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas B., Rutman A., Hirst R.A., Haldar P., Wardlaw A.J., Bankart J. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol. 2010;126:722–729. doi: 10.1016/j.jaci.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 75.Nadel J.A. Mucous hypersecretion and relationship to cough. Pulm Pharmacol Ther. 2013;26:510–513. doi: 10.1016/j.pupt.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Kiwamoto T., Katoh T., Evans C.M., Janssen W.J., Brummet M.E., Hudson S.A. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–1340. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kondo M., Tamaoki J., Takeyama K., Nakata J., Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002;27:536–541. doi: 10.1165/rcmb.4682. [DOI] [PubMed] [Google Scholar]
- 78.Vermeer P.D., Harson R., Einwalter L.A., Moninger T., Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–295. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- 79.Woodruff P.G., Modrek B., Choy D.F., Jia G., Abbas A.R., Ellwanger A. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beisswenger C., Kandler K., Hess C., Garn H., Felgentreff K., Wegmann M. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J Immunol. 2006;177:1833–1837. doi: 10.4049/jimmunol.177.3.1833. [DOI] [PubMed] [Google Scholar]
- 81.Durack J., Lynch S.V., Nariya S., Bhakta N.R., Beigelman A., Castro M. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang Y.J., Nariya S., Harris J.M., Lynch S.V., Choy D.F., Arron J.R. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz J., Slater D., Larson T.V., Pierson W.E., Koenig J.Q. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993;147:826–831. doi: 10.1164/ajrccm/147.4.826. [DOI] [PubMed] [Google Scholar]
- 84.Norris G., YoungPong S.N., Koenig J.Q., Larson T.V., Sheppard L., Stout J.W. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malig B.J., Green S., Basu R., Broadwin R. Coarse particles and respiratory emergency department visits in California. Am J Epidemiol. 2013;178:58–69. doi: 10.1093/aje/kws451. [DOI] [PubMed] [Google Scholar]
- 86.Clark N.A., Demers P.A., Karr C.J., Koehoorn M., Lencar C., Tamburic L. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McConnell R., Islam T., Shankardass K., Jerrett M., Lurmann F., Gilliland F. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young M.T., Sandler D.P., DeRoo L.A., Vedal S., Kaufman J.D., London S.J. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190:914–921. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erzurum S.C. New insights in oxidant biology in asthma. Ann Am Thorac Soc. 2016;13(suppl 1):S35–S39. doi: 10.1513/AnnalsATS.201506-385MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmad A., Shameem M., Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann Thorac Med. 2012;7:226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larsson N., Rankin G.D., Bicer E.M., Roos-Engstrand E., Pourazar J., Blomberg A. Identification of vitamin C transporters in the human airways: a cross-sectional in vivo study. BMJ Open. 2015;5:e006979. doi: 10.1136/bmjopen-2014-006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amaral A.F., Ramasamy A., Castro-Giner F., Minelli C., Accordini S., Sorheim I.C. Interaction between gas cooking and GSTM1 null genotype in bronchial responsiveness: results from the European Community Respiratory Health Survey. Thorax. 2014;69:558–564. doi: 10.1136/thoraxjnl-2013-204574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowatte G., Lodge C.J., Perret J.L., Matheson M.C., Dharmage S.C. Interactions of GST polymorphisms in air pollution exposure and respiratory diseases and allergies. Curr Allergy Asthma Rep. 2016;16:85. doi: 10.1007/s11882-016-0664-z. [DOI] [PubMed] [Google Scholar]
- 94.Bowatte G., Lodge C.J., Knibbs L.D., Lowe A.J., Erbas B., Dennekamp M. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol. 2017;139:122–129. doi: 10.1016/j.jaci.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Schroer K.T., Gibson A.M., Sivaprasad U., Bass S.A., Ericksen M.B., Wills-Karp M. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J Allergy Clin Immunol. 2011;128:539–548. doi: 10.1016/j.jaci.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bucchieri F., Puddicombe S.M., Lordan J.L., Richter A., Buchanan D., Wilson S.J. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 97.Heijink I., van O.A., Kliphuis N., Jonker M., Hoffmann R., Telenga E. Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax. 2014;69:5–13. doi: 10.1136/thoraxjnl-2013-203520. [DOI] [PubMed] [Google Scholar]
- 98.Al-Daghri N.M., Alokail M.S., Abd-Alrahman S.H., Draz H.M., Yakout S.M., Clerici M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environ Health. 2013;12:1. doi: 10.1186/1476-069X-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Villa M., Crotta S., Dingwell K.S., Hirst E.M., Gialitakis M., Ahlfors H. The aryl hydrocarbon receptor controls cyclin O to promote epithelial multiciliogenesis. Nat Commun. 2016;7:12652. doi: 10.1038/ncomms12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Naylor B. The shedding of the mucosa of the bronchial tree in asthma. Thorax. 1962;17:69–72. doi: 10.1136/thx.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laitinen L.A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 102.Ordonez C., Ferrando R., Hyde D.M., Wong H.H., Fahy J.V. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- 103.Puddicombe S.M., Polosa R., Richter A., Krishna M.T., Howarth P.H., Holgate S.T. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 104.Fedorov I.A., Wilson S.J., Davies D.E., Holgate S.T. Epithelial stress and structural remodelling in childhood asthma. Thorax. 2005;60:389–394. doi: 10.1136/thx.2004.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Boer W.I., Sharma H.S., Baelemans S.M., Hoogsteden H.C., Lambrecht B.N., Braunstahl G.J. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105–112. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]
- 106.Sweerus K., Lachowicz-Scroggins M., Gordon E., LaFemina M., Huang X., Parikh M. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139:72–81. doi: 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shahana S., Bjornsson E., Ludviksdottir D., Janson C., Nettelbladt O., Venge P. Ultrastructure of bronchial biopsies from patients with allergic and non-allergic asthma. Respir Med. 2005;99:429–443. doi: 10.1016/j.rmed.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Hackett T.L., de Bruin H.G., Shaheen F., Van Den Berge M., van Oosterhout A.J., Postma D.S. Caveolin-1 controls airway epithelial barrier function. Implications for asthma. Am J Respir Cell Mol Biol. 2013;49:662–671. doi: 10.1165/rcmb.2013-0124OC. [DOI] [PubMed] [Google Scholar]
- 109.Saatian B., Rezaee F., Desando S., Emo J., Chapman T., Knowlden S. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1:e24333. doi: 10.4161/tisb.24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bradding P., Walls A.F., Holgate S.T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 111.Nagarkar D.R., Ramirez-Carrozzi V., Choy D.F., Lee K., Soriano R., Jia G. IL-13 mediates IL-33-dependent mast cell and type 2 innate lymphoid cell effects on bronchial epithelial cells. J Allergy Clin Immunol. 2015;136:202–205. doi: 10.1016/j.jaci.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 112.Sugita K., Steer C.A., Martinez-Gonzalez I., Altunbulakli C., Morita H., Castro-Giner F. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions via IL-13 in asthma. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.02.038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Wawrzyniak P., Wawrzyniak M., Wanke K., Sokolowska M., Bendelja K., Ruckert B. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139:93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 114.Prefontaine D., Nadigel J., Chouiali F., Audusseau S., Semlali A., Chakir J. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 115.Shikotra A., Choy D.F., Ohri C.M., Doran E., Butler C., Hargadon B. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 116.Torgerson D.G., Ampleford E.J., Chiu G.Y., Gauderman W.J., Gignoux C.R., Graves P.E. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carriere V., Roussel L., Ortega N., Lacorre D.A., Americh L., Aguilar L. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith D.E. The biological paths of IL-1 family members IL-18 and IL-33. J Leukoc Biol. 2011;89:383–392. doi: 10.1189/jlb.0810470. [DOI] [PubMed] [Google Scholar]
- 120.Lefrancais E., Roga S., Gautier V., Gonzalez-de-Peredo A., Monsarrat B., Girard J.P. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cayrol C., Girard J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daniels M.J., Brough D. Unconventional pathways of secretion contribute to inflammation. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hristova M., Habibovic A., Veith C., Janssen-Heininger Y.M., Dixon A.E., Geiszt M. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol. 2016;137:1545–1556. doi: 10.1016/j.jaci.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gordon E.D., Simpson L.J., Rios C.L., Ringel L., Lachowicz-Scroggins M.E., Peters M.C. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci U S A. 2016;113:8765–8770. doi: 10.1073/pnas.1601914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W.L., Li H.Y., Zhang M.S., Gao P.S., He S.H., Zheng T. Thymic stromal lymphopoietin: a promising therapeutic target for allergic diseases. Int Arch Allergy Immunol. 2013;160:18–26. doi: 10.1159/000341665. [DOI] [PubMed] [Google Scholar]
- 126.Nagarkar D.R., Poposki J.A., Comeau M.R., Biyasheva A., Avila P.C., Schleimer R.P. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–232. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hallstrand T.S., Hackett T.L., Altemeier W.A., Matute-Bello G., Hansbro P.M., Knight D.A. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151:1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Uller L., Bedke N., Sammut D., Green N.G., Lau L., Howarth P.H. Disproportionate thymic stromal lymphopoietin versus interferon-beta production by asthmatic bronchial epithelial cells challenged with double-stranded RNA. Thorax. 2010;65:626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 129.Hui C.C., Murphy D.M., Neighbour H., Al-Sayegh M., O'Byrne S., Thong B. T cell-mediated induction of thymic stromal lymphopoietin in differentiated human primary bronchial epithelial cells. Clin Exp Allergy. 2014;44:953–964. doi: 10.1111/cea.12330. [DOI] [PubMed] [Google Scholar]
- 130.Gauvreau G.M., O'Byrne P.M., Boulet L.P., Wang Y., Cockcroft D., Bigler J. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 131.Semlali A., Jacques E., Koussih L., Gounni A.S., Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125:844–850. doi: 10.1016/j.jaci.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 132.Kojima T., Go M., Takano K., Kurose M., Ohkuni T., Koizumi J. Regulation of tight junctions in upper airway epithelium. Biomed Res Int. 2013;2013:947072. doi: 10.1155/2013/947072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dong H., Hu Y., Liu L., Zou M., Huang C., Luo L. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep. 2016;6:39559. doi: 10.1038/srep39559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bjerkan L., Sonesson A., Schenck K. Pharmaceuticals; Basel: 2016. Multiple functions of the new cytokine-based antimicrobial peptide thymic stromal lymphopoietin (TSLP) p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Le Y., Zhao W., Lin Y., Wu Y., Yu C. Short TSLP attenuates toluene diisocyanate (TDI)-induced airway inflammation and inhibits HMGB1-RAGE and long TSLP expression. Toxicol Sci. 2017 doi: 10.1093/toxsci/kfx043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 136.Angkasekwinai P., Park H., Wang Y.H., Wang Y.H., Chang S.H., Corry D.B. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gregory L.G., Jones C.P., Walker S.A., Sawant D., Gowers K.H., Campbell G.A. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68:82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saenz S.A., Noti M., Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 139.Beale J., Jayaraman A., Jackson D.J., Macintyre J.D., Edwards M.R., Walton R.P. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng D., Xue Z., Yi L., Shi H., Zhang K., Huo X. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tworek D., Smith S.G., Salter B.M., Baatjes A.J., Scime T., Watson R. IL-25 receptor expression on airway dendritic cells after allergen challenge in subjects with asthma. Am J Respir Crit Care Med. 2016;193:957–964. doi: 10.1164/rccm.201509-1751OC. [DOI] [PubMed] [Google Scholar]
- 142.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gern J.E. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112(suppl 6A):19S–27S. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 144.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 146.Kicic A., Stevens P.T., Sutanto E.N., Kicic-Starcevich E., Ling K.M., Looi K. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy. 2016;46:1441–1455. doi: 10.1111/cea.12767. [DOI] [PubMed] [Google Scholar]
- 147.Lopez-Souza N., Favoreto S., Wong H., Ward T., Yagi S., Schnurr D. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bochkov Y.A., Hanson K.M., Keles S., Brockman-Schneider R.A., Jarjour N.N., Gern J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bedke N., Sammut D., Green B., Kehagia V., Dennison P., Jenkins G. Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PLoS One. 2012;7:e44580. doi: 10.1371/journal.pone.0044580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gielen V., Sykes A., Zhu J., Chan B., Macintyre J., Regamey N. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol. 2015;136:177–188. doi: 10.1016/j.jaci.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ueki I.F., Min-Oo G., Kalinowski A., Ballon-Landa E., Lanier L.L., Nadel J.A. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon lambda and antiviral defense in airway epithelium. J Exp Med. 2013;210:1929–1936. doi: 10.1084/jem.20121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Djukanovic R., Harrison T., Johnston S.L., Gabbay F., Wark P., Thomson N.C. The effect of inhaled interferon-beta on worsening of asthma symptoms caused by viral infections: a randomised trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park J.A., Fredberg J.J., Drazen J.M. Putting the squeeze on airway epithelia. Physiology (Bethesda) 2015;30:293–303. doi: 10.1152/physiol.00004.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tschumperlin D.J., Shively J.D., Kikuchi T., Drazen J.M. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28:142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 155.Park J.A., Tschumperlin D.J. Chronic Intermittent Mechanical Stress Increases MUC5AC Protein Expression. Am J Respir Cell Mol Biol. 2009;41:459–466. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grainge C.L., Lau L.C., Ward J.A., Dulay V., Lahiff G., Wilson S. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 157.Grainge C., Dennison P., Lau L., Davies D., Howarth P. Asthmatic and normal respiratory epithelial cells respond differently to mechanical apical stress. Am J Respir Crit Care Med. 2014;190:477–480. doi: 10.1164/rccm.201401-0107LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Eerdewegh P., Little R.D., Dupuis J., Del Mastro R.G., Falls K., Simon J. Association of the ADAM-33 gene with asthma and bronchial hyper-responsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 159.Duan Y., Long J., Chen J., Jiang X., Zhu J., Jin Y. Overexpression of soluble ADAM33 promotes a hypercontractile phenotype of the airway smooth muscle cell in rat. Exp Cell Res. 2016;349:109–118. doi: 10.1016/j.yexcr.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Hamilton L.M., Torres-Lozano C., Puddicombe S.M., Richter A., Kimber I., Dearman R.J. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33:233–240. doi: 10.1046/j.1365-2222.2003.01593.x. [DOI] [PubMed] [Google Scholar]
- 161.Puddicombe S.M., Torres-Lozano C., Richter A., Bucchieri F., Lordan J.L., Howarth P.H. Increased expression of p21(waf) cyclin dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28:61–68. doi: 10.1165/rcmb.4715. [DOI] [PubMed] [Google Scholar]
- 162.Stevens P.T., Kicic A., Sutanto E.N., Knight D.A., Stick S.M. Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin Exp Allergy. 2008;38:1901–1910. doi: 10.1111/j.1365-2222.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- 163.Hackett T.L., Warner S.M., Stefanowicz D., Shaheen F., Pechkovsky D.V., Murray L.A. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 164.Johnson J.R., Nishioka M., Chakir J., Risse P.A., Almaghlouth I., Bazarbashi A.N. IL-22 contributes to TGF-beta1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res. 2013;14:118. doi: 10.1186/1465-9921-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gerovac B.J., Valencia M., Baumlin N., Salathe M., Conner G.E., Fregien N.L. Submersion and hypoxia inhibit ciliated cell differentiation in a notch-dependent manner. Am J Respir Cell Mol Biol. 2014;51:516–525. doi: 10.1165/rcmb.2013-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gerovac B.J., Fregien N.L. IL-13 inhibits multicilin expression and ciliogenesis via janus kinase/signal transducer and activator of transcription independently of Notch cleavage. Am J Respir Cell Mol Biol. 2016;54:554–561. doi: 10.1165/rcmb.2015-0227OC. [DOI] [PubMed] [Google Scholar]
- 167.Asgrimsson V., Gudjonsson T., Gudmundsson G.H., Baldursson O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 2006;50:1805–1812. doi: 10.1128/AAC.50.5.1805-1812.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y., Moffatt M.F., Cookson W.O. Genetic and genomic approaches to asthma: new insights for the origins. Curr Opin Pulm Med. 2012;18:6–13. doi: 10.1097/MCP.0b013e32834dc532. [DOI] [PubMed] [Google Scholar]
- 169.Koppelman G.H., Meyers D.A., Howard T.D., Zheng S.L., Hawkins G.A., Ampleford E.J. Identification of PCDH1 as a novel susceptibility gene for bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:929–935. doi: 10.1164/rccm.200810-1621OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bonnelykke K., Sleiman P., Nielsen K., Kreiner-Moller E., Mercader J.M., Belgrave D. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 171.Meng J.F., Rosenwasser L.J. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res. 2010;2:215–227. doi: 10.4168/aair.2010.2.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Laitinen T., Polvi A., Rydman P., Vendelin J., Pulkkinen V., Salmikangas P. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- 173.Ober C. Asthma Genetics in the Post-GWAS Era. Ann Am Thorac Soc. 2016;13(suppl 1):S85–S90. doi: 10.1513/AnnalsATS.201507-459MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moheimani F., Hsu A.C., Reid A.T., Williams T., Kicic A., Stick S.M. The genetic and epigenetic landscapes of the epithelium in asthma. Respir Res. 2016;17:119. doi: 10.1186/s12931-016-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gavala M.L., Bertics P.J., Gern J.E. Rhinoviruses, allergic inflammation, and asthma. Immunol Rev. 2011;242:69–90. doi: 10.1111/j.1600-065X.2011.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kelly F.J., Fussell J.C. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 177.Jackson D.J., Evans M.D., Gangnon R.E., Tisler C.J., Pappas T.E., Lee W.M. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Minelli C., Granell R., Newson R., Rose-Zerilli M.J., Torrent M., Ring S.M. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int J Epidemiol. 2010;39:539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kozu Y., Gon Y., Maruoka S., Kazumichi K., Sekiyama A., Kishi H. Protocadherin-1 is a glucocorticoid-responsive critical regulator of airway epithelial barrier function. BMC Pulm Med. 2015;15:80. doi: 10.1186/s12890-015-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Faura T.G., Vandepoele K., Brouwer U., Koning H., Elderman R.M., Hackett T.L. Protocadherin-1 binds to SMAD3 and suppresses TGF-beta1-induced gene transcription. Am J Physiol Lung Cell Mol Physiol. 2015;309:L725–L735. doi: 10.1152/ajplung.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mortensen L.J., Kreiner-Moller E., Hakonarson H., Bonnelykke K., Bisgaard H. The PCDH1 gene and asthma in early childhood. Eur Respir J. 2014;43:792–800. doi: 10.1183/09031936.00021613. [DOI] [PubMed] [Google Scholar]
- 182.Faura T.G., Nawijn M.C., Koppelman G.H. Protocadherin-1: epithelial barrier dysfunction in asthma and eczema. Eur Respir J. 2014;43:671–674. doi: 10.1183/09031936.00179713. [DOI] [PubMed] [Google Scholar]
- 183.Bochkov Y.A., Watters K., Ashraf S., Griggs T.F., Devries M.K., Jackson D.J. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qiu R., Yang Y., Zhao H., Li J., Xin Q., Shan S. Signal transducer and activator of transcription 6 directly regulates human ORMDL3 expression. FEBS J. 2013;280:2014–2026. doi: 10.1111/febs.12225. [DOI] [PubMed] [Google Scholar]
- 185.Schedel M., Michel S., Gaertner V.D., Toncheva A.A., Depner M., Binia A. Polymorphisms related to ORMDL3 are associated with asthma susceptibility, alterations in transcriptional regulation of ORMDL3, and changes in TH2 cytokine levels. J Allergy Clin Immunol. 2015;136:893–903. doi: 10.1016/j.jaci.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 186.Worgall T.S. Sphingolipids, ORMDL3 and asthma: what is the evidence? Curr Opin Clin Nutr Metab Care. 2016;20:99–103. doi: 10.1097/MCO.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 187.Hsu K.J., Turvey S.E. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin Immunol. 2013;9:4. doi: 10.1186/1710-1492-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miller M., Rosenthal P., Beppu A., Mueller J.L., Hoffman H.M., Tam A.B. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014;192:3475–3487. doi: 10.4049/jimmunol.1303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loser S., Gregory L.G., Zhang Y., Schaefer K., Walker S.A., Buckley J. Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li X., Hastie A.T., Ha wkins G.A., Moore W.C., Ampleford E.J., Milosevic J. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy. 2015;70:1309–1318. doi: 10.1111/all.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Biagini Myers J.M., Martin L.J., Kovacic M.B., Mersha T.B., He H., Pilipenko V. Epistasis between serine protease inhibitor Kazal-type 5 (SPINK5) and thymic stromal lymphopoietin (TSLP) genes contributes to childhood asthma. J Allergy Clin Immunol. 2014;134:891–899. doi: 10.1016/j.jaci.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Harada M., Nakashima K., Hirota T., Shimizu M., Doi S., Fujita K. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 193.Balaci L., Spada M.C., Olla N., Sole G., Loddo L., Anedda F. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007;80:1103–1114. doi: 10.1086/518259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu Q., van Dyk L.F., Jiang D., Dakhama A., Li L., White S.R. Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway. Virology. 2013;446:199–206. doi: 10.1016/j.virol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yao Y.S., Chang W.W., He L.P., Jin Y.L., Li C.P. An updated meta-analysis of transforming growth factor-beta1 gene: three well-characterized polymorphisms with asthma. Hum Immunol. 2016;77:1291–1299. doi: 10.1016/j.humimm.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 196.Hatsushika K., Hirota T., Harada M., Sakashita M., Kanzaki M., Takano S. Transforming growth factor-beta(2) polymorphisms are associated with childhood atopic asthma. Clin Exp Allergy. 2007;37:1165–1174. doi: 10.1111/j.1365-2222.2007.02768.x. [DOI] [PubMed] [Google Scholar]
- 197.Ueda T., Niimi A., Matsumoto H., Takemura M., Yamaguchi M., Matsuoka H. TGFB1 promoter polymorphism C-509T and pathophysiology of asthma. J Allergy Clin Immunol. 2008;121:659–664. doi: 10.1016/j.jaci.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 198.Li X., Howard T.D., Moore W.C., Ampleford E.J., Li H., Busse W.W. Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol. 2011;127:1457–1465. doi: 10.1016/j.jaci.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nieuwenhuis M.A., Vonk J.M., Himes B.E., Sarnowski C., Minelli C., Jarvis D. PTTG1IP and MAML3, novel genomewide association study genes for severity of hyperresponsiveness in adult asthma. Allergy. 2017;72:792–801. doi: 10.1111/all.13062. [DOI] [PubMed] [Google Scholar]
- 200.Singhania A., Rupani H., Jayasekera N., Lumb S., Hales P., Gozzard N. Altered epithelial gene expression in peripheral airways of severe asthma. PLoS One. 2017;12:e0168680. doi: 10.1371/journal.pone.0168680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roeder T., Isermann K., Kallsen K., Uliczka K., Wagner C. A Drosophila asthma model—what the fly tells us about inflammatory diseases of the lung. Adv Exp Med Biol. 2012;710:37–47. doi: 10.1007/978-1-4419-5638-5_5. [DOI] [PubMed] [Google Scholar]
- 202.Progatzky F., Cook H.T., Lamb J.R., Bugeon L., Dallman M.J. Mucosal inflammation at the respiratory interface: a zebrafish model. Am J Physiol Lung Cell Mol Physiol. 2016;310:L551–L561. doi: 10.1152/ajplung.00323.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]