Abstract
Retrieving fear memories just prior to extinction has been reported to effectively erase fear memories and prevent fear relapse. The current study examined whether the type of retrieval procedure influences the ability of extinction to impair fear renewal, a form of relapse in which responding to a conditional stimulus (CS) returns outside of the extinction context. Rats first underwent Pavlovian fear conditioning with an auditory CS and footshock unconditional stimulus (US); freezing behavior served as the index of conditioned fear. Twenty-four hours later, the rats underwent a retrieval-extinction procedure. Specifically, 1 h prior to extinction (45 CS-alone trials; 44 for rats receiving a CS reminder), fear memory was retrieved by either a single exposure to the CS alone, the US alone, a CS paired with the US, or exposure to the conditioning context itself. Over the next few days, conditional freezing to the extinguished CS was tested in the extinction and conditioning context in that order (i.e., an ABBA design). In the extinction context, rats that received a CS+US trial before extinction exhibited higher levels of conditional freezing than animals in all other groups, which did not differ from one another. In the renewal context, all groups showed renewal, and none of the reactivation procedures reduced renewal relative to a control group that did not receive a reactivation procedure prior to extinction. These data suggest retrieval-extinction procedures may have limited efficacy in preventing fear renewal.
Keywords: Context, Extinction, Fear, Postretrieval Extinction, Rat, Reconsolidation, Relapse, Renewal
1. Introduction
Fear memories may last a lifetime (Bergstrom, 2016). Even with extensive clinical and pharmaceutical treatments, humans often exhibit relapse of pathological fear and anxiety (Borkovec & Costello, 1993; Hermans et al., 2006; Vervliet et al., 2013a, 2013b; Wicking et al., 2016). Fear relapse can be modeled in the laboratory using Pavlovian fear conditioning and extinction (Bouton, 1993, 2002, 2004, 2014; Bouton et al., 2006; Craske et al., 2014; Goode & Maren, 2014; Haaker et al., 2014; Hermans et al., 2006; Kim & Richardson, 2010; Maren & Holmes, 2016; Maren et al., 2013; Vervliet et al., 2013a, 2013b), which may contribute to and interact with fear and anxiety disorders (Careaga et al., 2016; Nees et al., 2015; Ribrough et al., 2016; Smith et al., 2017; Zuj et al. 2016). Specifically, Pavlovian fear conditioning consists of pairing a harmless conditioned stimulus (“CS”; e.g., auditory tone) with a noxious unconditioned stimulus (“US”; e.g., footshock) (Konorski, 1948; Pavlov & Anrep, 1927; Rescorla, 1988). Following one or more pairings in a conditioning chamber, animals will come to express conditioned fear responses (e.g., freezing behavior, autonomic activity) to the CS alone (Fanselow, 1994; Izquierdo et al., 2016; LeDoux, 2000; Maren, 2001). After conditioning, nonreinforced presentations of the CS result in the gradual reduction of fear responses to the CS, a process termed extinction (Bouton et al., 2006; Maren et al., 2013; Myers & Davis, 2007; Pavlov & Anrep, 1927). However, extinguished fear in humans and other animals is known to return under a variety of circumstances (Bouton, 1993, 2002, 2004, 2014; Bouton et al., 2006; Craske et al., 2014; Goode & Maren, 2014; Haaker et al., 2014; Hermans et al., 2006; Kim & Richardson, 2010; Maren & Holmes, 2016; Maren et al., 2013; Vervliet et al., 2013a, 2013b), including after encountering the CS outside of the environment or “context” in which extinction occurred (termed “renewal”; Bouton & Bolles, 1979). Thus, while fear responses to a CS generalize across contexts, extinguished fear responses are context-dependent. Renewal and other relapse phenomena (e.g., shock-induced reinstatement and time-dependent spontaneous recovery of fear) reveal that extinction is not typically a fear-erasing process, rather extinction results in a new competitive memory that is thought to suppress the expression of conditioned fears (Bouton, 1993, 2002, 2004, 2014; Bouton et al., 2006; Maren, 2011). Given that extinction learning is thought to be an important factor in common forms of cognitive-behavioral therapy (e.g., exposure therapy; Graham et al., 2011; Graham & Milad, 2011; Hermans et al., 2006; Kaplan et al., 2011; Wicking et al., 2016), there is considerable interest in identifying new methods to enhance fear extinction and erase pathological fear memories selectively (Dejean et al., 2015; Fitzgerald et al., 2014; Goode & Maren, 2014; Herry et al., 2010; LeDoux, 2015; Maren, 2011; Maren et al., 2013; Maren & Holmes, 2016; Morrison & Ressler, 2014; VanElzakker et al., 2014).
One possible method for the selective erasure of maladaptive fear memories involves disrupting memory reconsolidation. After conditioning, encountering fear conditioning-related stimuli (the CS, US, and/or conditioned context) can trigger the previously consolidated conditioned memory to enter a labile state that requires reconsolidation (Auber et al., 2013; Clem & Schiller, 2016; Kredlow et al., 2016; Schiller & Phelps, 2011). Behavioral, pharmacological, or neural manipulations during this postretrieval period allows for modification of the fear memory, including weakening or potentially erasing the memory (Auber et al., 2013; Giustino et al., 2016; Kindt et al., 2009; Kindt & van Emmerik, 2016; Lattal & Wood, 2013; Meir Drexler & Wolf, 2016b; Monfils et al., 2009; Nader, 2003, 2015; Nader et al., 2000; Quirk et al., 2010; Schiller et al., 2010; Schwabe et al., 2014; Soeter & Kindt, 2011). Of particular interest, it has been shown that reactivating or retrieving fear memories prior to extinction training can lead to a loss of responding to the CS that does not exhibit renewal, reinstatement, or spontaneous recovery (Monfils et al., 2009). This effect was time-dependent, such that the retrieval trial was found to enhance extinction only if it preceded normal extinction by 1 or 6 h but not 24 h (i.e., during the “reconsolidation window”; Monfils et al., 2009). Similarly, time-dependent postretrieval extinction has been shown to prevent relapse in humans (Schiller et al., 2010). In these studies, it has been proposed that the CS reminder engages a reconsolidation process that can be disrupted (and the labile memory erased) by extinction trials delivered shortly after memory enters a malleable state (Monfils et al., 2009; Schiller et al., 2010; Schiller & Phelps, 2011).
The possibility that fear memories can be erased has generated enormous excitement in the clinical community (Careaga et al., 2016; Kroes et al., 2016; Post & Kegan, 2017; Quirk et al., 2010; Smith et al., 2017), but the efficacy of “reconsolidation update” procedures in preventing fear relapse is mixed (Auber et al., 2013; Clem & Schiller, 2016; Kredlow et al., 2016; Schiller & Phelps, 2011). A critical variable that has not yet been fully explored might be the procedure used to reactivate the fear memory prior to extinction. For example, reconsolidation windows can be opened by the presentation of the CS alone, the US alone, a conditioned context, or even a conditioning trial (CS+US) and protein synthesis inhibitors delivered after these forms of reactivation lead to impaired retention of conditioned fear memories (Duvarci & Nader, 2004). Moreover, recent work in humans and rats indicates that weak US-alone exposure prior to extinction prevents fear reinstatement and spontaneous recovery (Liu et al., 2014; Thompson & Lipp, 2017). However, the relative efficacy of these manipulations in preventing relapse phenomena, including renewal, have not been explored.
In the present study, we examined the efficacy of four different retrieval procedures in preventing fear renewal after extinction. We hypothesized that retrieval procedures that produced prediction errors (CS-, US-, or shock-associated context-alone reminders; Rescorla & Wagner, 1972) would be more effective than a CS+US trial in promoting reconsolidation update and in preventing fear renewal (provided animals were sufficiently extinguished). This hypothesis is based on work by Sevenster and colleagues (2012, 2013, 2014), which highlight the importance of prediction error in engaging reconsolidation (Fernández et al., 2016). Accordingly, rats were conditioned and underwent extinction 1 h after brief or single exposure to the CS, US, a CS+US trial, or the conditioning context; another group of rats did not receive any retrieval procedure to serve as a control. To assess relapse, we tested animals to the extinguished CS outside of the extinction context (renewal). None of the retrieval procedures attenuated fear renewal—in fact, retrieval with a US-alone or CS+US trial facilitated fear expression during renewal. These results challenge the efficacy of retrieval-extinction procedures in preventing fear relapse.
2. Materials and Methods
2.1. Subjects
Subjects were sixty-four adult male Long-Evans (Blue Spruce) rats (200–225 g) obtained from Harlan Sprague-Dawley (Indianapolis, IN). Subjects were individually housed in a climate-controlled vivarium at the University of Michigan where the present experiment was conducted. Rats were kept on a reverse light (14 h)-dark (10 h) cycle. Food and water were accessible ad libitum. Rats were handled once a day for ~1 min for 5 consecutive days prior to the start of behavior. The University of Michigan Animal Care and Use Committee approved all experimental procedures.
2.2. Behavioral apparatuses
All training and testing procedures occurred in rodent observation chambers (MED-Associates, St. Albans, VT) of identical size (30 × 24 × 21 cm) and construction (Plexiglas ceilings, rear walls, and doors, aluminum side walls, and stainless steel grid floors). Observation chambers were contained within external sound-attenuating cabinets. Grid floors of the observation chambers (consisting of 19 stainless steel rods) were connected to shock sources and solid-state grid scramblers (MED-Associates) for delivering footshock (US). Small speakers were attached to the chambers and provided auditory tones (CS). The observation chambers were also fitted with 15-W house lights and ventilation fans. Each observation chamber rested upon a load-cell platform (connected to load-cell amplifiers) that would respond to cage displacement as a result of a rat’s movements (load-cell amplifiers were calibrated to a standardized degree of chamber displacement prior to behavioral training). Load-cell activity output (+/−10 V) was transformed into values of 0–100 and captured every 200 ms using Threshold Activity software (MED-Associates). Smaller values indicated less cage displacement and freezing was quantified as transformed load-cell activity values of ≤10 for 1 s or more.
Sensory features of the chambers were manipulated to obtain three unique contexts (A, B, and C) for the current study. For context A, 1% acetic acid was used to wipe down the chambers (grid floors were dried) and a small volume of the odor was poured into the pans beneath the grid floors. Chamber house lights remained lit, room lights were on, chamber fans were on, cupboard doors encasing the chambers were left open, and rats were transported to and from the chambers in white plastic transport boxes. For context B, 1% ammonium hydroxide was used for the context’s odor, chamber house lights were turned off, red room lights were used, chamber fans were turned off, the cupboard doors were closed, and rats were transported in black plastic transport containers. For context C, 10% ethanol served as the chambers’ odor, chamber house lights were on, red room lights were used, fans remained off, cupboard doors were left open, and rats were transported in white plastic buckets with a layer of bedding at the bottom.
2.3. Behavioral procedures
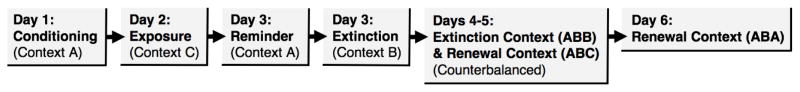
A summary of the behavioral procedures is shown in Figure 1. Subjects were randomly assigned to one of five groups: extinction only (“NO REMINDER”; n = 12), conditioned stimulus reminder (“CSR”; n = 13), conditioning context reminder (“CONTEXT ALONE”; n = 13), unconditioned stimulus reminder (“USR”; n = 13), or reinforced conditioned stimulus reminder (“CS+USR”; n = 13). Rats were trained and tested in squads of eight (counterbalanced by group assignment when possible).
Figure 1.
Behavioral design.
On day 1, rats were placed in context A and allowed to acclimate for 3 min before the onset of the first CS+US pairing (US onset immediately followed CS offset for all pairings). A 10 s, 2 kHz, 80 dB auditory tone served as the CS, and a 2 s, 1 mA footshock served as the US for both conditioning and retrieval, if involved. For conditioning, rats experienced 5 CS+US pairings, separated by 58 s interstimulus intervals (ISI’s). Rats remained in the chamber for 58 s after the final pairing before being returned to their homecages. On day 2, rats received nonreinforced exposure to context C for 35 min and 30 s (this context would later serve as a renewal context). On day 3, rats were exposed to context A for 5 min, during which rats experienced a retrieval trial based on their respective group assignments. Specifically, after 3 min in the chamber, CSR, USR, and CS+USR rats experienced a single nonreinforced CS, a single US trial alone, or a single CS+US pairing, respectively. CONTEXT ALONE rats remained in the chamber for 5 min without reinforcement. NO REMINDER rats remained in their homecages during the reminder phase. Rats were returned to their homecages following the reminder. 1 hr after the reminder phase, all rats were extinguished in context B. Extinction consisted of 45 nonreinforced CS alone trials separated by 30 s ISI’s. To equate CS exposure, rats that received a CS during the reminder phase received 44 extinction trials. Extinction trials started 3 min after rats were placed in the chambers. The entirety of the extinction session lasted 35 min and 30 s for all groups.
Twenty-four hours after extinction, half of the rats were tested to the CS in the extinction context (B), while the other half were tested to the CS in the familiar context (C). These testing assignments were counterbalanced across reminder conditions and were swapped out for testing on the following day (i.e., for the second day of testing, rats tested in context B were tested in Context C and vice versa). For each test, five CS-only trials (30 s ISI’s) began 3 min after rats were placed in the chambers. The entirety of each test lasted 8 min and 50 s. Twenty-four hours after testing in Context B and C, rats were tested to the CS (identical trials to the previous tests) back in the conditioning context (A).
2.4. Data analysis
Mean percentage freezing (±SEM) served as the dependent variable. Freezing data were collected continuously during each behavioral session. Freezing during the post-CS interstimulus interval served as the primary index of fear to each CS; statistical outcomes for freezing during the CS (not reported) were similar across tests as compared to ISI responding (ISI freezing is often a more reliable measure of fear than freezing during the CS, which is influenced by a head-jerk orienting response; e.g., Holland, 1977, 1980). Data were submitted to analysis of variance (ANOVA). Following a significant overall F ratio, post-hoc comparisons were tested using Fisher’s Protected Least Significant Difference (PLSD). Trials for repeated measures/factorial ANOVA correspond to the data shown in the figures, unless noted differently in the Results. No rats were excluded from the analysis.
3. Results
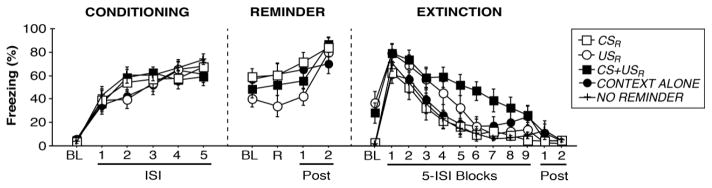
Results are depicted in Figures 2 and 3. Rats exhibited robust conditioning (Fig. 2, “CONDITIONING”), as revealed by a significant main effect of trial [F(5,295) = 81.978, p < 0.0001]. No group differences in freezing were observed (no main effect of group, no interaction [Fs < 1]), indicating the groups acquired similar levels of freezing. Data for the novel context exposure on day 2 is not shown, however no group differences were observed [Fs < 1]; freezing was low during the exposure session (mean = 11.638% [±0.735%]), suggesting robust discrimination of contexts A and C. For the reminder phase on day 3 (Fig. 2, “REMINDER”), a main effect of trial [F(3,144) = 18.368, p < 0.0001] revealed that fear increased throughout the 5 min session in the conditioning context. No group differences were detected [Fs < 2.5] (note: NO REMINDER rats not included).
Figure 2.
Effects of pre-extinction fear reactivation on fear extinction. CONDITIONING: Mean freezing (%) during a 3-min baseline period (‘BL’) followed by five interstimulus intervals (‘ISI’; 58 s each) separating conditioning trials (CS+US). REMINDER: Mean freezing (%) during a 3-min baseline period, during a 10-s window starting at the onset of a fear reminder (‘R’), followed by freezing across the final two minutes in the chamber (‘Post’; note: Post 2 is 50 s). EXTINCTION: Mean freezing (%) during a 3-min baseline period, during forty-five extinction (CS-no-US) trials (broken up into nine 5-ISI blocks with each ISI spanning 30 s each; note: the fifth ISI of block 9 is 1 min), and during each minute remaining in the chamber (‘Post’). Rats that received CS retrieval during the reminder phase experienced 44 extinction trials (freezing was measured across equivalent time points).
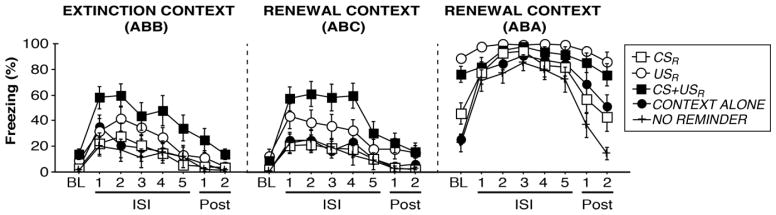
Figure 3.
Effects of pre-extinction fear reactivation on fear renewal. EXTINCTION CONTEXT (ABB): Mean freezing (%) during a 3-min baseline, during five ISI’s (ISI 1–4 are 30 s each, ISI 5 spans 1 min) separating testing trials (CS-), and during each minute remaining in the extinction context. RENEWAL CONTEXT (ABC): Mean freezing (%) across trials in the familiar context (trials are identical to testing in the extinction context). RENEWAL CONTEXT (ABA): Mean freezing (%) across trials in the conditioning context (trials are identical to testing in the extinction context).
During the extinction session (Fig. 2, “EXTINCTION”), there was a significant main effect of trial which reflected the significant decrease in freezing across extinction blocks [F(11,649) = 71.717, p < 0.0001]. A main effect of group [F(4,59) = 3.708, p < 0.01] and a group x trial interaction was observed for extinction [F(44,649) = 2.152, p < 0.0001]. Fisher’s PLSD revealed that CS+USR rats exhibited significantly more within-session freezing for extinction training as compared to CONTEXT ALONE [p < 0.05], CSR [p < 0.005], and NO REMINDER groups [p < 0.005]. Additionally, a separate factorial ANOVA of baseline freezing during extinction revealed a main effect of group [F(4,59) = 8.916, p < 0.0001]. Post-hoc comparisons showed USR rats exhibited significantly higher baseline fear as compared to CONTEXT ALONE [p < 0.0001], CSR [p < 0.0001], and NO REMINDER groups [p < 0.0001]. Similarly, CS+USR rats were higher at baseline as compared to CONTEXT ALONE [p < 0.005], CSR [p < 0.005], and NO REMINDER groups [p < 0.005]. These data indicate that reminder procedures that include a US significantly enhance freezing in subsequent sessions. However, the groups did not significantly differ during the final extinction block (separate factorial ANOVA: [Fs < 2.5]), suggesting that extinction was equivalent despite the higher baseline freezing.
On days 4 and 5, rats were tested to the CS in a counterbalanced manner in either the extinction context or in the familiar (previously novel; renewal) context. For the test in the extinction context (Fig. 3, “EXTINCTION CONTEXT (ABB)”), repeated measures ANOVA revealed a main effect of trial [F(7,413) = 27.670, p < 0.0001] as rats increased in freezing after the baseline period. A main effect of group [F(4,59) = 3.666 p < 0.01] was observed for the extinction test. Similar to extinction training sessions, post-hoc comparisons revealed that CS+USR rats exhibited significantly more within-session freezing as compared to CONTEXT ALONE [p < 0.01], CSR [p < 0.005], and NO REMINDER groups [p < 0.005]. Comparisons of mean post-CS freezing (a mean of ISI’s 1 through 5) during the extinction test vs. mean freezing during the final block of extinction training revealed a significant main effect of trial [F(1,59) = 10.055, p < 0.005] and group [F(4,59) = 3.178, p < 0.05]. Post-hoc comparisons indicated that rats exhibited significantly more freezing at test than at the end of extinction training (independent of group assignment; [p < 0.005]). Furthermore, CS+USR rats exhibited significantly more fear across this analysis as compared to CSR and NO REMINDER groups [ps < 0.005]. Collectively, these data indicate that rats (independent of group assignment) exhibited some spontaneous recovery when tested in the extinction context, although this recovery was mild: mean freezing across testing was significantly less for all groups (no main effect of group; no trial x group interaction [Fs < 2.5]) as compared to the first block of extinction training (repeated measures ANOVA; main effect of trial: [F(1,59) = 161.322, p < 0.0001]).
For ABC renewal testing (Fig. 3, “RENEWAL CONTEXT (ABC)”), a main effect of trial [F(7,413) = 38.799; p < 0.0001], a main effect of group [F(4,59) = 5.214, p < 0.005], and a group x trial interaction [F(28,413) = 1.929, p < 0.005] were detected. Fisher’s PLSD indicated that CS+USR rats exhibited significantly more freezing across the ABC renewal test as compared to CONTEXT ALONE [p < 0.005], CSR [p < 0.001], and NO REMINDER groups [p < 0.001]. To assess the extent of ABC renewal (i.e., greater freezing in the ABC vs. ABB conditions), mean freezing across ISI’s 1 through 5 of the extinction test and the ABC renewal test were compared using repeated measures ANOVA. A significant main effect of group [F(4,59) = 6.241, p < 0.0005] was revealed, with Fisher’s PLSD further indicating that CS+USR rats exhibited significantly more freezing across the ISI’s of both tests as compared to CONTEXT ALONE [p < 0.0005], CSR [p < 0.0001], and NO REMINDER groups [p < 0.0001]. However, we observed no significant main effect of trial or interactions for these comparisons [Fs < 0.5], suggesting that the ABC test produced weak renewal when compared to freezing during testing in the extinction context (rats failed to discriminate between context B and C). Although CSR rats did not exhibit ABC renewal, neither did CONTEXT ALONE, NO REMINDER, CS+USR, or USR rats, suggesting no fear erasure by the CSR procedure, in particular.
Twenty-four hours later, rats were tested to the CS in the conditioning context (ABA renewal; Fig. 3, “RENEWAL CONTEXT (ABA)”), a context in which renewal is more robust (Bouton and Bolles, 1979; Bouton and King, 1983; Harris et al., 2000; Bouton et al., 2006). For the ABA test, a main effect of trial [F(7,413) = 59.722, p < 0.0001], a main effect of group [F(4,59) = 10.279, p < 0.0001], and a trial x group interaction [F(28,413) = 4.987, p < 0.0001] were detected. Post-hoc comparisons showed that USR and CS+USR rats exhibited significantly higher fear overall as compared to CONTEXT ALONE, NO REMINDER, and CSR rats [ps < 0.05]. Interestingly, CSR and CONTEXT ALONE rats also exhibited significantly more fear across ABA testing as compared to NO REMINDER rats [ps < 0.05]. These differences in part may relate to levels of baseline fear in the conditioning context (however it should be noted that the main effect of trial across testing suggests fear increased generally after the CS). Factorial ANOVA of baseline fear showed a main effect of group [F(4,59) = 25.342, p < 0.0001]. Post-hoc analyses indicated that US and CS+USR rats exhibited significantly greater baseline fear than all other groups [ps < 0.0001], but not between each other. Interestingly, CSR rats also expressed significantly more freezing during baseline than CONTEXT ALONE or NO REMINDER rats [ps < 0.05].
A comparison of mean post-CS freezing (ISI’s 1 through 5) during ABA renewal testing vs. extinction retrieval revealed a main effect of trial [F(1,59) = 305.061, p < 0.0001], indicating significant renewal of fear (unlike ABC renewal). Additionally, a main effect of group was detected for this comparison [F(4,59) = 4.013, p < 0.01], however no trial x group interaction was revealed [F<2]. Similar to other sessions, post-hoc analyses indicated that CS+USR rats exhibited significantly more freezing across the extinction and ABA tests as compared to CSR, NO REMINDER, or CONTEXT ALONE groups [ps < 0.05]. Similarly, USR rats exhibited more fear as compared to NO REMINDER rats [p < 0.05]. Collectively, the results indicate that the pre-extinction fear reactivation procedures were not effective in erasing fear, as evident by renewal and lingering baseline fear. Furthermore, these reminders were not any more successful in reducing fear relapse than extinction alone.
4. Discussion
The present study examined the effects of various fear reactivation procedures (CS alone, US alone, a conditioning trial, or conditioned context alone) on extinction and relapse of conditioned fear, particularly renewal. In contrast to what has been reported previously for postretrieval extinction procedures (e.g., Monfils et al., 2009), the current study found that reactivating fear memory prior to extinction training did not prevent fear renewal. Indeed, the observation of robust ABA renewal in all of the groups receiving reminders suggests that the original fear memory was not eliminated by postretrieval extinction. The low levels of fear during retrieval testing and even during the ABC renewal test (while unusual and presumably due to weak discrimination of context B and C) indicate that our effects were not due to a failure of rats to acquire extinction. It is worth noting, the USR and the CS+USR reminder groups showed enhanced baseline freezing during the extinction session and in the ABA renewal test. This appears to be consistent with the work by Liu and colleagues (2014) in that a strong US reminder impeded context extinction. However, we now show that full extinction of a fear CS shortly after a strong US reminder (or an additional conditioning trial) does not ultimately prevent renewal. CS+USR rats also exhibited more freezing across trials for extinction retrieval and in the ABC renewal session as compared to other groups, but this did not specifically enhance the extent of ABA renewal for this group (however, this could be a ceiling effect). This suggests that CS+USR rats were not necessarily at asymptote following conditioning. Enhanced fear in USR and CS+USR rats may relate to the immediate extinction deficit (which is observed when animals are extinguished shortly after conditioning trials; Maren, 2014), however this may be due to the additional training trial itself. Interestingly, CSR rats also exhibited higher baseline fear during ABA renewal, at least when compared to NO REMINDER and CONTEXT ALONE rats (however, this increase was not as large as CS+USR or USR rats). It’s not fully clear why this may be the case (also, see Chan et al., 2010), but perhaps the isolated CS- retrieval trial in the conditioning context increased fear of the conditioning context by contributing to its ambiguity. All of this considered, the current data do not support the hypothesis that fear reactivation (with or without the original US) before extinction is an effective means of erasing fear and preventing renewal. Conversely, the current data support a model of fear learning in which fear memories are difficult to erase behaviorally, a finding that is consistent with numerous studies documenting relapse after extinction (Bouton, 1993, 2002, 2004, 2014; Bouton et al., 2006; Craske et al., 2014; Goode & Maren, 2014; Haaker et al., 2014; Hermans et al., 2006; Kim & Richardson, 2010; Maren & Holmes, 2016; Maren et al., 2013; Vervliet et al., 2013a, 2013b).
Failures to observe the postretrieval extinction effect have been reported in both animals (Chan et al., 2010; Ishii et al., 2012) and humans (Golkar et al., 2012; Kindt & Soeter, 2013; Klucken et al., 2016; Meir Drexler et al., 2014; Soeter & Kindt, 2011). A recent meta-analysis of these and similar studies found that the effects of postretrieval extinction on relapse in animals are typically small and often nonsignificant (Kredlow et al., 2016), which is in line with the current data. That said, in cases where postretrieval extinction was successful, Kredlow and colleagues (2016) found housing conditions and the timing of retention testing to be important factors in the effect. Specifically, the authors found that housing conditions were a significant moderator of postretrieval extinction, such that group housing appeared to reduce the efficacy of postretrieval extinction. It is not yet clear what may mediate this effect, although it may relate to social transfer of fear (Knapska et al., 2010; Nowak et al., 2013). Animals were individually housed in the present study. Kredlow and colleagues (2016) also found that postretrieval extinction was significantly more effective in studies with longer time intervals (roughly a week or more) between extinction’s end and testing for relapse. The current study examined relapse one to three days after extinction, so it is possible that a longer retention interval would have revealed a renewal deficit. Nonetheless, others have reported significant effects of postretrieval extinction when testing occurred 24 h after extinction (Auber et al., 2013; Kredlow et al., 2016). The timing following postretrieval extinction is an area of ongoing discussion (Auber et al., 2013).
Of course, it is possible that weaker fear memories are more susceptible to postretrieval extinction. In the current study, rats underwent five training trials with a 2 sec, 1 mA footshock US. Similarly, Ishii and colleagues (2012), who failed to see fear erasure using postretrieval extinction in mice, utilized six training trials with a 2 sec, 0.75 mA footshock US. Moreover, stress-enhanced fear learning has been found to be resistant to reconsolidation blockade techniques (Hoffman et al., 2015). To our knowledge, all of the currently published studies on the effects of postretrieval extinction on fear relapse in rats and mice—outside of the mouse study of Clem & Huganir (2010)—utilized fewer and weaker US intensities as compared to the present study. However, US intensities and trials are often weaker in studies involving mice. In humans, fear conditioning studies are often considered less aversive when compared to rodent studies. Human participants may experience fewer conditioning trials and select for the intensity of their own shock (Sehlmeyer et al. 2009). Interestingly, Kredlow and colleagues (2016) observed trending effects for US intensity (not duration) as a potential moderator of the postretrieval extinction effect in animals, however, this trended towards greater efficacy of postretrieval extinction with higher intensity USs. That said, once group housing was controlled for, this effect of US intensity was no longer predictive of the effect. Ultimately, it is not yet clear if strong US intensities (and stronger fear memories) account for the various effects of postretrieval extinction on relapse.
Another possible explanation for the current results is that the conditioning context prevented the animals from processing the reminders or that the reactivation procedure failed to produce reconsolidation (Sevenster et al., 2012, 2013, 2014). However, other groups have used the conditioning context during the reactivation phase (albeit some with success in preventing relapse and some without). It is worth noting that Chan and colleagues (2010) tested retrieval in the conditioning context or in a novel (but habituated) context and both ended without successful prevention of fear relapse (also, see Ishii et al. 2012).
As Kredlow and colleagues (2016) highlight, significant moderators of postretrieval extinction efficacy were not consistent across the domains of animal studies of fear and appetitive relapse and human fear relapse studies. In contrast to the animal work, they found significant effects (small to moderate) overall for postretrieval extinction in humans. However, no positive effect was observed in human studies of fear renewal. While these findings concord with the current data, it should be noted that there are fewer studies examining the effects of postretrieval extinction on fear renewal in humans as compared to fear reinstatement for example. Perhaps certain forms of relapse (such as reinstatement) are more amendable to the procedure.
Although others have demonstrated that postretrieval extinction can prevent fear relapse in animals (Auchter et al., 2017; Baker et al., 2013; Clem & Huganir, 2010; Flavell et al., 2011; Jones et al., 2013; Jones & Monfils, 2016; Monfils et al., 2009; Olshavsky et al., 2013; Rao-Ruiz et al., 2011; Shumake & Monfils, 2015) and in humans (Agren et al., 2012a, 2012b, 2017; Björkstrand et al., 2015; Golkar et al., 2017; Liu et al., 2014; Meir Drexler et al., 2014; Meir Drexler & Wolf, 2016a; Oyarzún et al., 2012; Schiller et al., 2010, 2013; Thompson & Lipp, 2017), it is important to consider that prevention of relapse may not equate to erasure of the fear memory. For example, a well-trained extinction memory may be able to outcompete a fear memory for expression, without the fear memory necessarily being eliminated (Lattal & Wood, 2013). Furthermore, it should be noted that there are existing studies demonstrating an increase in the degree of relapse as a result of postretrieval extinction (Chan et al., 2010; although that was not the case in the current data; also, see Auber et al., 2013 for comparison). Ultimately, we found that multiple forms of retrieval failed to generate postretrieval extinction impairments on renewal, suggesting that there are limits to the efficacy of these procedures in preventing fear relapse (Auber et al., 2013; Kredlow et al., 2016; Nader & Einarsson, 2010).
Highlights.
Conditioned stimulus (CS) retrieval 1h prior to extinction did not prevent fear renewal.
Pre-extinction exposure to the conditioning context did not disrupt fear renewal.
Unconditioned stimulus (US) exposure prior to extinction enhanced baseline fear but did not eliminate renewal.
A conditioning trial prior to extinction enhanced fear across training but did not block renewal.
Acknowledgments
Supported by grants from the National Institutes of Health (R01MH065961 to S.M. and F31MH107113 to T.D.G.) and a McKnight Foundation Memory and Cognitive Disorders Award to S.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agren T, Björkstrand J, Fredrikson M. Disruption of human fear reconsolidation using imaginal and in vivo extinction. Behavioural Brain Research. 2017;319:9–15. doi: 10.1016/j.bbr.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Agren T, Engman J, Frick A, Björkstrand J, Larsson EM, Furmark T, Fredrikson M. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012a;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- Agren T, Furmark T, Eriksson E, Fredrikson M. Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes. Translational Psychiatry. 2012b;2:e76. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology (Berl) 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchter A, Cormack LK, Niv Y, Gonzalez-Lima F, Monfils MH. Reconsolidation-extinction interactions in fear memory attenuation: the role of the inter-trial interval variability. Frontiers in Behavioral Neuroscience. 2017;11:2. doi: 10.3389/fnbeh.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, McNally GP, Richardson R. Memory retrieval before or after extinction reduces recovery of fear in adolescent rats. Learning & Memory. 2013;20:467–473. doi: 10.1101/lm.031989.113. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC. The neurocircuitry of remote cued fear memory. Neuroscience & Biobehavioral Reviews. 2016;71:409–417. doi: 10.1016/j.neubiorev.2016.09.028. [DOI] [PubMed] [Google Scholar]
- Björkstrand J, Agren T, Frick A, Engman J, Larsson EM, Furmark T, Fredrikson M. Disruption of memory reconsolidation erases a fear memory trace in the human amygdala: an 18-month follow-up. PLoS One. 2015;10:e0129393. doi: 10.1371/journal.pone.0129393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Why behavior change is difficult to sustain. Preventative Medicine. 2014;68:29–36. doi: 10.1016/j.ypmed.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Careaga MB, Girardi CE, Suchecki D. Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neuroscience & Biobehavioral Reviews. 2016;71:48–57. doi: 10.1016/j.neubiorev.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Chan WY, Leung HT, Westbrook FR, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learning & Memory. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Schiller D. New learning and unlearning: strangers or accomplices in threat memory attenuation? Trends in Neurosciences. 2016;39:340–351. doi: 10.1016/j.tins.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T, Herry C. Neuronal circuits for fear expression and recovery: recent advances and potential therapeutic strategies. Biological Psychiatry. 2015;78:298–306. doi: 10.1016/j.biopsych.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. Journal of Neuroscience. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fernández RS, Boccia MM, Pedreira ME. The fate of memory: reconsolidation and the case of prediction error. Neuroscience & Biobehavioral Reviews. 2016;68:423–441. doi: 10.1016/j.neubiorev.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of the pharmacological and behavioral findings. Brain Research Bulletin. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell CR, Barber DJ, Lee JL. Behavioural memory reconsolidation of food and fear memories. Nature Communications. 2011;2:504. doi: 10.1038/ncomms1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: Memory erasure or extinction enhancement? Neurobiology of Learning and Memory. 2016;130:26–33. doi: 10.1016/j.nlm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Bellander M, Olsson A, Ohman A. Are fear memories erasable?-reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Frontiers in Behavioral Neuroscience. 2012;6:80. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Tjaden C, Kindt M. Vicarious extinction learning during reconsolidation neutralizes fear memory. Behaviour Research and Therapy. 2017 doi: 10.1016/j.brat.2017.02.004. (in press) [DOI] [PubMed] [Google Scholar]
- Goode TD, Maren S. Animal models of fear relapse. ILAR Journal. 2014;55:246–258. doi: 10.1093/ilar/ilu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: preclinical models. British Journal of Pharmacology. 2011;164:1230–1247. doi: 10.1111/j.1476-5381.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. The American Journal of Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Golkar A, Hermans D, Lonsdorf TB. A review on human reinstatement studies: an overview and methodological challenges. Learning & Memory. 2014;21:424–440. doi: 10.1101/lm.036053.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. Journal of Experimental Psychology: Animal Learning and Cognition. 2000;26:174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biological Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP, Jr, Conrad CD. Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiology of Learning and Memory. 2015;120:61–68. doi: 10.1016/j.nlm.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Learning and Cognition. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. CS-US interval as a determinant of the form of Pavlovian appetitive conditioned responses. Journal of Experimental Psychology: Animal Learning and Cognition. 1980;6:155–174. [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Matsuda S, Tomizawa H, Sutoh C, Shimizu E. No erasure effect of retrieval-extinction trial on fear memory in the hippocampus-independent and dependent paradigms. Neuroscience Letters. 2012;523:76–81. doi: 10.1016/j.neulet.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiological Reviews. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Jones CE, Monfils MH. Post-retrieval extinction in adolescence prevents return of juvenile fear. Learning & Memory. 2016;23:567–575. doi: 10.1101/lm.043281.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Ringuet S, Monfils MH. Learned together, extinguished apart: Reducing fear to complex stimuli. Learning & Memory. 2013;20:674–685. doi: 10.1101/lm.031740.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacology Biochemistry and Behavior. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biological Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biological Psychology. 2013;92:43–50. doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nature Neuroscience. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kindt M, van Emmerik A. New avenues for treating emotional memory disorders: towards a reconsolidation intervention for posttraumatic stress disorder. Therapeutic Advances in Psychopharmacology. 2016;6:283–295. doi: 10.1177/2045125316644541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Kruse O, Schweckendiek J, Kuepper Y, Mueller EM, Hennig J, Stark R. No evidence for blocking the return of fear by disrupting reconsolidation prior to extinction learning. Cortex. 2016;79:112–122. doi: 10.1016/j.cortex.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learning & Memory. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Conditioned reflexes and neuron organization. Cambridge: Cambridge University Press; 1948. [Google Scholar]
- Kredlow MA, Unger LD, Otto MW. Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin. 2016;142:314–336. doi: 10.1037/bul0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MC, Schiller D, LeDoux JE, Phelps EA. Translational approaches targeting reconsolidation. Current Topics in Behavioral Neurosciences. 2016;28:197–230. doi: 10.1007/7854_2015_5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal MK, Wood MA. Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nature Neuroscience. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Anxious: using the brain to understand and treat fear and anxiety. New York: Viking; 2015. [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, Chai B, Yang C, Fang Q, Zhang Y, Bao Y, Pickens CL, Lu L. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biological Psychiatry. 2014;76:895–901. doi: 10.1016/j.biopsych.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Nature and causes of the immediate extinction deficit: a brief review. Neurobiology of Learning and Memory. 2014;113:19–24. doi: 10.1016/j.nlm.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir Drexler S, Merz CJ, Hamacher-Dang TC, Marquardt V, Fritsch N, Otto T, Wolf OT. Effects of postretrieval-extinction learning on return of contextually controlled cued fear. Behavioral Neuroscience. 2014;128:474–481. doi: 10.1037/a0036688. [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Wolf OT. Stress disrupts the reconsolidation of fear memories in men. Psychoneuroendocrinology. 2016a;77:95–104. doi: 10.1016/j.psyneuen.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Wolf OT. The role of glucocorticoids in emotional memory reconsolidation. Neurobiology of Learning and Memory. 2016b doi: 10.1016/j.nlm.2016.11.008. (in press) [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrsion FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depression and Anxiety. 2014;31:279–290. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends in Neurosciences. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K. Reconsolidation and the dynamic nature of memory. Cold Spring Harbor Perspectives in Biology. 2015;7:a021782. doi: 10.1101/cshperspect.a021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: an update. Annals of the New York Academy of Sciences. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nees F, Heinrich A, Flor H. A mechanism-oriented approach to psychopathology: the role of Pavlovian conditioning. International Journal of Psychophysiology. 2015;98:351–364. doi: 10.1016/j.ijpsycho.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Nowak A, Werka T, Knapska E. Social modulation in extinction of aversive memories. Behavioural Brain Research. 2013;238:200–205. doi: 10.1016/j.bbr.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Olshavsky ME, Jones CE, Lee HJ, Monfils MH. Appetitive behavioral traits and stimulus intensity influence maintenance of conditioned fear. Frontiers in Behavioral Neuroscience. 2013;7:179. doi: 10.3389/fnbeh.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún JP, Lopez-Barroso D, Fuentemilla L, Cucurell D, Pedraza C, Rodriguez-Fornells A, de Diego-Balaguer R. Updating fearful memories with extinction training during reconsolidation: A human study using auditory aversive stimuli. PLoS ONE. 2012;7:e38849. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP, Anrep GV. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Kegan R. Prevention of recurrent affective episodes using extinction training in the reconsolidation window: a testable psychotherapeutic strategy. Psychiatry Research. 2017 doi: 10.1016/j.psychres.2017.01.034. (in press) [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. Journal of Neuroscience. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nature Neuroscience. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It’s not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Ribrough VB, Glenn DE, Baker DG. On the road to translation for PTSD treatment: theoretical and practical considerations of the use of human models of conditioned fear for drug development. Current Topics in Behavioral Neurosciences. 2016;28:173–196. doi: 10.1007/7854_2015_5010. [DOI] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Phelps EA. Does reconsolidation occur in humans? Frontiers in Behavioral Neuroscience. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biology Psychiatry. 2014;76:274–280. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Monfils MH. Assessing fear following retrieval + extinction through suppression of baseline reward seeking vs. freezing. Frontiers in Behavioral Neuroscience. 2015;9:355. doi: 10.3389/fnbeh.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiology of Learning and Memory. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339:830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learning & Memory. 2014;21:580–584. doi: 10.1101/lm.035493.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NB, Doran JM, Sippel LM, Harpaz-Rotem I. Fear extinction and memory reconsolidation as critical components in behavioral treatment for posttraumatic stress disorder and potential augmentation of these processes. Neuroscience Letters. 2017 doi: 10.1016/j.neulet.2017.01.006. (in press) [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learning & Memory. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Thompson A, Lipp OV. Extinction during reconsolidation eliminates recovery of fear conditioned to fear-irrelevant and fear-relevant stimuli. Behavior Research and Therapy. 2017;92:1–10. doi: 10.1016/j.brat.2017.01.017. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning and Memory. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biological Psychology. 2013a;92:51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2013b;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Wicking M, Steiger F, Nees F, Diener SJ, Grimm O, Ruttorf M, Schad LR, Winkelmann T, Wirtz G, Flor H. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiology of Learning and Memory. 2016;136:116–126. doi: 10.1016/j.nlm.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Zuj DV, Palmer MA, Lommen MJ, Felmingham KL. The centrality of fear extinction in linking risk factors to PTSD: a narrative review. Neuroscience & Biobehavioral Reviews. 2016;69:15–35. doi: 10.1016/j.neubiorev.2016.07.014. [DOI] [PubMed] [Google Scholar]