Abstract
Given the recent surge in functional neuroimaging studies on social exclusion, the current study employed activation likelihood estimation (ALE) based meta-analyses to identify brain regions that have consistently been implicated across different experimental paradigms used to investigate exclusion. We also examined the neural correlates underlying Cyberball, the most commonly used paradigm to study exclusion, as well as differences in exclusion-related activation between developing (7–18 years of age, from pre-adolescence up to late adolescence) and emerging adult (broadly defined as undergraduates, including late adolescence and young adulthood) samples. Results revealed involvement of the bilateral medial prefrontal and posterior cingulate cortices, right precuneus and left ventrolateral prefrontal cortex across the different paradigms used to examine social exclusion; similar activation patterns were identified when restricting the analysis to Cyberball studies. Investigations into age-related effects revealed that ventrolateral prefrontal activations identified in the full sample were driven by (i.e. present in) developmental samples, while medial prefrontal activations were driven by emerging adult samples. In addition, the right ventral striatum was implicated in exclusion, but only in developmental samples. Subtraction analysis revealed significantly greater activation likelihood in striatal and ventrolateral prefrontal clusters in the developmental samples as compared to emerging adults, though the opposite contrast failed to identify any significant regions. Findings integrate the knowledge accrued from functional neuroimaging studies on social exclusion to date, highlighting involvement of regulatory lateral prefrontal regions and midline structures involved in social cognitive and self-evaluative processes across experimental paradigms and ages, as well as limbic structures in developing samples specifically.
Keywords: functional MRI, social exclusion, meta-analysis, Cyberball, adolescence
1. Introduction
As human beings, we are strongly motivated to build social ties with one another, giving us a sense of belonging and connectedness. The experience of social rejection is highly distressing, and has been related to negative affectivity and mental health problems, including depression and anxiety (Nolan et al., 2003; Prinstein and Aikins, 2004; Rigby, 2003). It has also been argued that the neural correlates of social rejection may overlap with those of physical pain, including the dorsal anterior cingulate cortex (ACC) and insula (Eisenberger, 2012). While an earlier meta-analysis on fMRI studies of social exclusion provided some support for common brain activation patterns, minimal evidence was present for the role of the dorsal ACC; rather, a more rostral section of the ACC, along with the medial orbitofrontal cortex (OFC) and anterior insula, were implicated (Cacioppo et al., 2013). As a number of new empirical investigations have been conducted on this topic over the last few years, we aim to confirm the reliability of these results using a coordinate-based meta-analysis of a substantially larger sample of studies. We also extend on prior work by identifying neural correlates of social exclusion that are specific to developmental samples given the heightened sensitivity to peer rejection during adolescence (Sebastian, 2010b; Somerville, 2013.
With the onset of puberty, adolescents become more socially oriented than children, spending up to a third of their waking hours with peers (Hartup & Stevens, 1997). However, this developmental period can also be characterised by potentially unstable peer relationships, with research showing that only half of close friendships endure for longer than a year (Değirmencioğlu et al., 1998). There is also extensive antagonism between and within peer groups; higher-status members often ridicule lower-status members of the same group, and group members tend to make fun of those outside of their group (Adler and Adler, 1998). Moreover, these changes are accompanied by increased affective salience of social events (Steinberg and Morris, 2001), including greater sensitivity to rejection and acceptance by peers (Nelson et al., 2005). Along with these behavioral changes, there is significant neural development in regions sensitive to social exclusion listed above (rostral ACC, medial OFC, and anterior insula), as well as other regions which may be implicated in peer rejection. This includes changes in activation of subcortical regions to affective stimuli, cortical midline and temporo-parietal structures supporting social-cognitive processes, and lateral prefrontal cortices involved in inhibitory control (Crone and Dahl, 2012). As such, it seems likely that the neural correlates of social exclusion may differ in the developing brain compared to one that has reached adult-levels of maturity.
Functional neuroimaging research on social exclusion in both adults and adolescents has most commonly employed the Cyberball paradigm (Williams et al., 2000; Williams and Jarvis, 2006), where participants are excluded from a ball-tossing game by virtual players with pre-programmed actions. Most versions of this task lead participants to believe that virtual players are real individuals at other sites, though some researchers have not used deception given that feelings of distress are also reported when participants are aware that they are being excluded by computer players (Zadro et al., 2004). The traditional Cyberball design involves two runs: the first is an inclusion run where participants receive the ball approximately a third of the time (i.e. fair play), and the second is an exclusion run where, following a brief period of fair play, the other players stop throwing to the participant for the remainder of the run. This task, first used in the MRI scanner by Eisenberger and colleagues (2003) to examine the neural correlates of social exclusion in a convenience sample of undergraduates, has since been used to study neural responses to social exclusion in clinical populations, including autism (Bolling et al., 2011b; Masten et al., 2011a), depression (Groschwitz et al., 2016), alcohol dependence (Maurage et al., 2012), and schizophrenia (Gradin et al., 2012). Within non-clinical samples, researchers have examined individual differences in neural responses to social exclusion, including the effect of social support (Nishiyama et al., 2015; Onoda et al., 2009), attachment style (DeWall et al., 2012), and rejection sensitivity (Masten et al., 2013), to name a few examples. The paradigm has also been modified to compare neural responses to differing forms of rejection (i.e. micro- vs. sustained rejection; Kawamoto et al., 2012), as well as differing perpetrators (i.e. gender: Bolling et al., 2012, and in- vs out-group members: Masten et al., 2011d). While most of these studies have used undergraduate convenience samples aged approximately between 18 and 25 years, a number of studies have also focused specifically on developing (primarily adolescent) samples to examine developmental correlates of social exclusion (Bolling et al., 2011a; Falk et al., 2014; Puetz et al., 2014; Will et al., 2016).
Aside from Cyberball, the most commonly used paradigms in this field of research are social evaluation tasks, such as the Social Judgment and Chatroom tasks. The former paradigm was used to differentiate competing accounts of the dorsal ACC’s role in social pain and expectancy violation, given that greater activation of this region during exclusion as compared to fair play in Cyberball may relate to participants receiving the ball less than they expect (Somerville et al., 2006). In both the Social Judgment and Chatroom tasks, participants are asked to evaluate unfamiliar peers based on photographs and subsequently receive feedback about whether these same individuals are interested in them (Guyer et al., 2008; Somerville et al., 2006). Both paradigms are able to differentiate neural responses associated with social feedback (i.e. negative or positive) from those related to expectancy violation (when feedback matches participants’ initial judgments). Similarly, another study employed a virtual task of others accepting or rejecting the participant’s handshake (Lee et al., 2014). While all these paradigms attempt to directly exclude the participant, a few other tasks aim to elicit feelings of exclusion less directly, or even specifically examine the neural correlates of witnessing others being excluded. For example, studies have examined neural responses to rejection cues in the form of pictures and words related to social exclusion (Premkumar et al., 2012; Sebastian et al., 2010a), or used Cyberball to show others/peers being excluded (Beeney et al., 2011; Masten et al., 2013; 2011c; Meyer et al., 2013).
1.1 Aims/hypotheses
Given the number of recent neuroimaging studies using Cyberball and associated social exclusion paradigms, the current study employed coordinate-based meta-analytic methods to identify neural activation patterns of social exclusion in the functional MRI literature to date. We specifically used activation likelihood estimation (ALE) implemented in the GingerALE software (Eickhoff et al., 2009) to compare activation patterns across individual studies and identify brain regions that have been consistently associated with social exclusion. This technique is a widely used and validated method for quantitatively analyzing voxel-wise neuroimaging foci (Kober and Wager, 2010). We aimed to confirm and extend on the previous meta-analysis of social exclusion (Cacioppo et al., 2013) by incorporating a number of new empirical studies using Cyberball, as well as fMRI studies using other social exclusion paradigms. In addition, as the results of meta-analyses can be affected by variations in study design, we ran a separate analysis of Cyberball studies alone, specifically focusing on studies that involve the participant being excluded (as opposed to witnessing others being excluded – referred to as hereafter as “others-Cyberball”). Furthermore, we examined potential design considerations within the Cyberball literature by comparing the two most commonly used “traditional” (one period of inclusion followed by one period of exclusion) and “alternating” (interspersed periods of inclusion and exclusion) designs. Unfortunately, we were unable to run separate analyses for other paradigms due to insufficient sample size (i.e. there were too few neuroimaging studies employing each of these alternate non-Cyberball paradigms). Finally, this study also explored differences in the neural correlates of social exclusion during development, given the continued maturation of brain regions underlying social processes from late childhood into and through early adulthood (Crone and Dahl, 2012). We performed separate analyses for Cyberball studies using developing (defined as 7 to 18 year olds) and emerging adult samples (broadly defined as convenience undergraduate samples), as well as a subtraction analysis to identify differences between these two age groups in brain regions activated by social exclusion using Cyberball (following Silverman et al., 2015).
2. Method
2.1 Study selection
Studies investigating neural correlates of social exclusion were identified from PubMed and Medline using the following search terms: 1) social rejection OR social exclusion OR ostracism AND 2) MRI OR fMRI. These searches yielded a total of 216 non-replicated studies. Seven additional potentially relevant studies were identified from reference lists of other articles. Studies were deemed eligible for inclusion in the meta-analysis if they met all of the following criteria: 1) were empirical investigations (i.e. not review articles), 2) employed fMRI, 3) reported group main effects of an exclusion/rejection condition relative to an inclusion/acceptance condition, 4) studied healthy samples, and 5) reported peak activation coordinates from whole brain analyses (i.e. could not employ region-of-interest analyses alone).
Of the 216 studies, 116 did not meet the inclusion criteria based on the abstract alone. More in-depth review of the remaining articles found that 29 articles also did not meet the inclusion criteria for reasons such as being reviews, not employing social exclusion paradigms, not using fMRI, examining romantic rejection, and only employing a social exclusion paradigm for mood induction. Of the remaining 71 articles, 51 employed Cyberball paradigms (4 of which used the others-Cyberball paradigm), 16 employed Social Judgment/Chatroom paradigms, and 4 employed other tasks. Of these 71 studies, 24 did not meet inclusionary criteria due to failure to report main effects of a healthy group (i.e. only provided results comparing healthy vs. atypical samples) and 10 did not conduct whole brain analysis (these counts are not exclusive of each other – i.e., some studies failed both inclusionary standards). Overlapping samples were identified in two pairs of studies, either based on reported demographics or consultation with investigators. Masten and colleagues used the same sample to examine neural correlates of being socially excluded (Masten et al., 2011a) and, subsequently, witnessing others being excluded (Masten et al., 2013). Bolling and colleagues’ (2011a) sample of healthy adolescents were also used as controls in comparison to a clinical sample (Bolling et al., 2011b). Given that overlapping samples can introduce bias from non-independence of observations, in each case we chose to include the initial study alone. This resulted in a final set of 40 studies that met inclusionary criteria. Refer to Table 1 for an overview of these studies. Two Cyberball studies that provided main effects combining across both healthy control and clinical samples were included in this list (Domsalla et al., 2014; van Harmelen et al., 2014). However, we report findings from analyses that exclude these samples as well.
Table 1.
Social exclusion studies meeting inclusionary criteria
| Study | N (M) | Age | Task | Design | N (foci) | Developmental sub-analyses | Design sub-analyses |
|---|---|---|---|---|---|---|---|
| Beeney et al., 2011 | 20 (10) | 24.6 (5.8), 18 – 35 | Others-Cyberball | Block | 9 | ||
| Bolling et al., 2011a | 21 (15) | 12.90 (2.59), 7–18 | Cyberball | Block | 10 | Developmental | Alternating |
| Bolling et al., 2011c | 33 (11) | 24.0 (3.81) | Cyberball | Block | 12 | Emerging adult | Alternating |
| Bolling et al., 2012 | 20 (11) | 24.99 (3.91) | Cyberball | Block | 12 | Emerging adult | Alternating |
| Bolling et al., 2015a | 15 (9) | 11.88 (3.2), 7–18 | Cyberball | Block | 12 | Developmental | Alternating |
| Bolling et al., 2015b | 20 (10) | 12.61(2.5), 7–17 | Cyberball | Block | 12 | Developmental | Alternating |
| Bonenberger et al., 2015 | 31 (0) | 22.2 (3.38) | Cyberball | Block | 8 | Emerging adult | Traditional |
| DeWall et al., 2012 | 25 (9) | Undergraduate | Cyberball | Block | 15 | Emerging adult | Traditional |
| Domsalla et al., 2014 | 40 (0) | 1: 28.7 (7.8) 2: 29.2 (7.5) |
Cyberball | Block | 12 | Emerging adult | Alternating |
| Eisenberger et al., 2003 | 13 (4) | Undergraduate | Cyberball | Block | 4 | Emerging adult | Traditional |
| Falk et al., 2014 | 36 (36) | 16.8 (0.47), 16–17 | Cyberball | Block | 6 | Developmental | Traditional |
| Gonzalez et al., 2015 | 85 (40) | 24.5 (1.35) | Cyberball | Block | 6 | Emerging adult | Traditional |
| Gradin et al., 2012 | 16 (7) | 40.87 (11.72) | Cyberball | Block | 3 | Alternating | |
| Gunther Moor et al., 2010 | 57 (27) | 1: 9.7 (0.9), 8–10 2: 13.3 (0.8), 12–14 3: 17.1 (0.6), 16–17 4: 21.7 (1.9), 19–25 |
Social Judgment | Event | 0 | ||
| Gunther Moor et al., 2012 | 53 (22) | 1: 11.8(0.87), 10–12 2: 15.74(0.74), 14–16 3: 20.38(0.8), 19–21 |
Cyberball | Events | 9 | Traditional (events) | |
| Guyer et al., 2012 | 36 (20) | 13.54 (2.5), 9–18 | Chatroom | Event | 0 | ||
| Gyurak et al., 2012 | 33 (12) | 20.61 (1.77) | Viewing paintings | Block | 4 | ||
| Karremans et al., 2011 | 15 (5) | 22 (19–33) | Cyberball | Block | 13 | Emerging adult | Traditional |
| Kawamoto et al., 2012 | 22 (3) | 20.7 (1.7), 18–24 | Cyberball | Events | 12 | Emerging adult | Alternating (events) |
| Lee et al., 2014 | 16 (9) | 27.4 (6.9) | Virtual handshake | Event | 3 | ||
| Masten et al., 2009 | 23 (9) | 13.0, 12.4–13.6 | Cyberball | Block | 4 | Developmental | Traditional |
| Masten et al., 2011a | 17 (15) | 13.6 (2.5) | Cyberball | Block | 19 | Developmental | Traditional |
| Masten et al., 2011d | 18 (9) | 21.4 (19–28) | Cyberball | Block | 15 | Emerging adult | Traditional |
| Masten et al., 2011c | 16 (7) | 19.88, 18–24 | Others-Cyberball | Block | 5 | ||
| Masten et al., 2012 | 21 (7) | 17.77 (0.43) | Cyberball | Block | 25 | Developmental | |
| Meyer et al., 2013 | 16 (12) | 21.69 (2.12) | Others-Cyberball | Block | 7 | ||
| Nishiyama et al., 2015 | 46 (17) | 19.85 (no range) | Cyberball | Block | 14 | Emerging adult | |
| Novembre et al., 2015 | 23 (0) | 22.4 (2.0), 20–28 | Cyberball | Block | 13 | Emerging adult | |
| Onoda et al., 2009 | 26 (11) | 21.79 (1.3), 20–25 | Cyberball | Block | 2 | Emerging adult | |
| Preller et al., 2016 | 21 (12) | 26.48 (4.76) | Cyberball | Events | 27 | Emerging adult | Traditional (events) |
| Puetz et al., 2014 | 51 (25) | 1: 10.6 (1.75) 2: 10.38 (1.7) |
Cyberball | Block | 2 | Developmental | Traditional |
| Premkumar et al., 2012 | 26 (5) | 1: 30 (10.58) 2: 28.64 (6.07) |
Viewing IAPS pictures | Block | 9 | ||
| Sebastian et al., 2010a | 19 (0) | 14 – 28 | Rejection Stroop | Block | 8 | ||
| Sebastian et al., 2011 | 35 (0) | 1: 15.44 (0.81, 14–17 2: 28.70 (3.91), 24–39 |
Cyberball | Block | 12 | Alternating | |
| Silk et al., 2014 | 48 (14) | 15.48 (1.68), 11–17 | Chatroom | Event | 0 | ||
| Van Harmelen et al., 2014 | 46 (12) | 1: 18.31 (1.23) 2: 18.85 (1.90) |
Cyberball | Events | 8 | Emerging adult | Traditional (events) |
| Via et al., 2015 | 20 (0) | 28.15 (8.62) | Social Judgment | Block | 12 | ||
| Will et al., 2015 | 28 (12) | 20.7 (1.97) | Cyberball | Events | 13 | Emerging adult | Traditional (events) |
| Will et al., 2016 | 44 (26) | 14.0 (0.70) | Cyberball | Events | 5 | Developmental | Traditional (events) |
| Wudarczyk et al., 2015 | 24 (14) | 24.33 (2.91), 18–29 | Cyberball | Block | 3 | Emerging adult |
Note: N (M) = total sample size (number of males). “Others-Cyberball” involved participants watching others/peers playing Cyberball. Design sub-analyses all represent block designs unless noted otherwise (i.e. as event-related).
2.2 Statistical meta-analyses
GingerALE v2.3.6 was used to conduct the random effects ALE meta-analyses (Turkeltaub et al., 2002). In this procedure, the peak coordinates for relevant contrasts from the pooled studies are used to obtain activation likelihood estimates for each voxel using three main steps. Firstly, the spatial uncertainty of each individual activation focus is modeled using a three-dimensional Gaussian probability distribution, the width of which is determined from the number of subjects in the associated study. The three-dimensional probabilities of all activation foci in a given study are then combined for every voxel, producing a modeled activation map. The union of these study-specific maps produces the non-thresholded voxel-wise ALE map that represents the convergence of results from all the studies across the brain. The significance of this non-thresholded ALE map is then tested by comparison with an empirically defined null distribution map. As opposed to testing for above-chance clustering of individual foci, the current version of GingerALE assesses above-chance clustering of activated foci between studies, thus enabling random-effects inference. The significance of the statistical map of p values was determined using a cluster-level inference corrected threshold of p < 0.05, 5000 thresholding permutations, and an uncorrected p-value of < 0.001 (Note: this version of the software has addressed a previous error in GingerALE that resulted in inferences on uncorrected cluster-level p values as opposed to FWE corrected clusters; Eickhoff et al., 2016). All analyses were conducted in MNI space, and studies that reported coordinates in Talairach space were grouped together and transformed into MNI space using the icbm2tal transformation function in GingerALE (Lancaster et al., 2007).
2.3 Sub-analyses
Using these statistical methods, a series of ALE analyses were conducted on the final set of 40 studies, labeled: 1) Social Exclusion, 2) Cyberball, 3) Developmental and 4) Design.
We began by conducting the Social Exclusion analysis that incorporated all fMRI studies in this area of research. There were 40 studies that met the inclusion criteria, with a total of 1122 participants available for the overall ALE analysis. This number of studies is consistent with prior ALE analyses (Silverman et al., 2015; Wagner et al., 2014). We subsequently ran the Cyberball analysis that consisted of studies using this paradigm to induce feelings of being excluded in participants (omitting others-Cyberball studies). This analysis included 29 studies with a total of 857 participants. Given that only 10 studies meeting our inclusionary criteria used non-Cyberball paradigms, with only one or two studies using each specific task, we were unable to perform a separate analysis for each of these tasks. Considering that these tasks were highly varied, we did not run a separate sub-analysis to examine ALE results across the non-Cyberball paradigms. However, the analysis of Cyberball studies allowed us to examine whether neural correlates varied when studies using the other tasks were excluded.
Next, we ran the Developmental analysis that separates studies using late childhood up to late-adolescent samples (referred to as “developmental” samples; i.e. 7 to 18 year olds) from those using late-adolescent or young adult samples (referred to as “emerging adult” samples; i.e. undergraduate samples and/or 18 to late-20 year olds). Three studies included in the Cyberball analysis were not included in the Developmental analysis for the following reasons: two studies presented main effects across developing and emerging adult groups (Moor et al., 2012; Sebastian et al., 2011) and one study was comprised of older adults, with a mean age of 41 years (Gradin et al., 2012). This left a total of nine studies with 248 participants in the developmental sample, with a mean weighted age of 13.5 years and overall range of 7 to 18 years. In comparison, there were 17 studies with 516 participants in the emerging adult sample. Estimated weighted mean age in the emerging adult sample was 22.9 years from the 15 studies that reported the mean age of their sample. Two studies merely described their sample as being composed of undergraduate students, making it difficult to know the exact age range of the participants. However, we hypothesize the range as being between 18 and late-20s/early-30s.
Finally, we ran the Design analysis that separates Cyberball studies based on their use of the differing paradigms. The majority of Cyberball studies either employ the traditional design that involves one extended period of inclusion/fair play followed by an extended period of exclusion (usually one run for each condition) or an alternating design interspersing blocks of inclusion/fair play and exclusion. Five Cyberball studies were excluded for employing designs that did not clearly fit either of these categories (e.g., Novembre et al., (2015) presented 3 inclusion blocks followed by 5 exclusion blocks and another 2 inclusion blocks). This left a total of 16 studies with 530 participants in the traditional sample compared to 9 studies with 222 participants in the alternating sample.
A minimum of 8–10 studies is required to obtain valid results from ALE analysis (as reported in Wagner et al., 2014), and all our subsamples (i.e. developmental, emerging adult, traditional, alternating) meet this requirement. In addition, we ran subtraction analyses of developmental vs. emerging adult subsamples and traditional vs. alternating design subsamples to identify differences associated with age and paradigm design, respectively. However, it should be noted that a minimum of 15 studies in each group is recommended when using GingerALE’s subtraction analysis, and as such, our subtraction analyses may not have enough power to detect subtle differences due to the limited number of developmental and alternating design studies.
3. Results
3.1 Social exclusion analysis
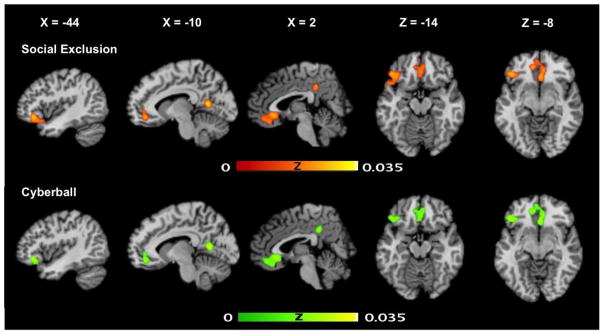
The ALE meta-analysis of all 40 studies on social exclusion revealed four clusters of convergence. As illustrated in Figure 1, the main brain regions that were reliably activated during conditions of social exclusion were located in one large medial bilateral cluster including the ACC (encompassing perigenual and subgenual portions) and extending into the ventromedial PFC and medial OFC. There was also activation within one left PFC cluster encompassing the ventrolateral PFC and lateral OFC, one cluster in the left posterior cingulate, and one in the right posterior cingulate extending into the precuneus (see Table 2 for corresponding MNI coordinates). When excluding the two studies that reported main effects for exclusion combined across clinical and healthy groups, the two posterior cingulate clusters were no longer present. However, one additional novel cluster was evident in the right dorsomedial PFC.
Fig 1.
Brain regions showing likelihood of brain activation in the overall social exclusion analysis (top panel) and Cyberball analysis (second panel).
Table 2.
Brain regions activated by all social exclusion studies
| Brain Region | Hemi | Extrema | x | y | z | Volume (mm3) | Cluster Number | N studies (foci) |
|---|---|---|---|---|---|---|---|---|
| ventral ACC | R | 0.03 | 4 | 32 | −6 | 5512 | 1 | 18(26) |
| vmPFC/medial OFC | L | 0.02 | −10 | 44 | −10 | |||
| medial OFC | 0.02 | 0 | 36 | −16 | ||||
| vmPFC | R | 0.02 | 6 | 38 | −6 | |||
| mPFC | L | 0.02 | −12 | 46 | 0 | |||
| vmPFC | R | 0.02 | 4 | 44 | −12 | |||
| vmPFC | L | 0.02 | −4 | 54 | −10 | |||
| inferior frontal gyrus | L | 0.03 | −46 | 32 | −10 | 2976 | 2 | 12(16) |
| inferior frontal gyrus | L | 0.02 | −50 | 18 | −12 | |||
| posterior cingulate | L | 0.03 | −8 | −56 | 12 | 1232 | 3 | 7(7) |
| posterior cingulate/precuneus | R | 0.02 | 6 | −40 | 40 | 912 | 4 | 6(6) |
3.2 Cyberball analysis
Meta-analysis of the 29 Cyberball studies that met inclusion criteria revealed the same four clusters of convergence (although with slightly varying peaks and extents) as the Social Exclusion analysis. The main brain regions that were reliably activated during conditions of exclusion relative to inclusion (or fair play) were located in the bilateral medial PFC, left ventrolateral PFC extending to lateral OFC, and bilateral posterior cingulate (see Table 3 for corresponding MNI coordinates). However, the right posterior cingulate cluster was no longer present when excluding the two studies with clinical samples. In addition, one cluster of convergence was present in the right precentral gyrus.
Table 3.
Brain regions activated by Cyberball studies
| Brain Region | Hemi | Extrema | x | y | z | Volume (mm3) | Cluster Number | N studies (foci) |
|---|---|---|---|---|---|---|---|---|
| ventral ACC | R | 0.03 | 4 | 32 | −6 | 5448 | 1 | 17(24) |
| vmPFC/OFC | L | 0.02 | −10 | 44 | −10 | |||
| ventral ACC/vmPFC | R | 0.02 | 6 | 38 | −6 | |||
| vmPFC/OFC | L | 0.02 | 0 | 36 | −16 | |||
| vmPFC | R | 0.02 | 4 | 44 | −12 | |||
| vmPFC/ventral ACC | L | 0.02 | −12 | 46 | 0 | |||
| vmPFC | L | 0.02 | −4 | 54 | −10 | |||
| inferior frontal gyrus | L | 0.03 | −46 | 32 | −10 | 1992 | 2 | 8(10) |
| lateral OFC | L | 0.02 | −38 | 32 | −12 | |||
| posterior cingulate | L | 0.03 | −8 | −56 | 12 | 1344 | 3 | 7(7) |
| posterior cingulate/precuneus | R | 0.02 | 4 | −40 | 40 | 1000 | 4 | 6(6) |
| precentral gyrus | R | 0.03 | 54 | −8 | 4 | 736 | 5 | 4(4) |
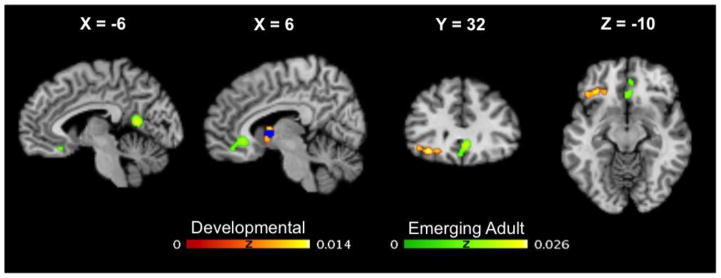
3.3 Developmental analysis
The meta-analysis of nine Cyberball studies using developmental samples identified two clusters of convergence, and analysis of the 17 studies using emerging adult samples also produced two clusters of convergence. As illustrated in Figure 2, clusters were located in the right ventral striatum (VS), and left ventrolateral PFC extending into the lateral OFC in the developmental sample. In emerging adults, activation was present within a large bilateral medial cluster encompassing the perigenual and subgenual ACC, ventromedial PFC and medial OFC and in the left posterior cingulate (see Table 4 for corresponding MNI coordinates). This latter cluster was not present when excluding studies that combined healthy and clinical samples.
Fig 2.
Brain regions showing likelihood of brain activation in the emerging adult and developmental samples in Cyberball studies. Regions with greater likelihood of activation in developmental compared to emerging adult samples are highlighted in blue.
Table 4.
Brain regions activated by developmental and emerging adult samples in Cyberball studies
| Brain Region | Hemi | Extrema | x | y | z | Volume (mm3) | Cluster Number | N studies (foci) |
|---|---|---|---|---|---|---|---|---|
| Developmental | ||||||||
| lateral OFC | L | 0.01 | −34 | 32 | −12 | 880 | 1 | 4(4) |
| ventrolateral PFC | L | 0.01 | −44 | 30 | −10 | |||
| lateral OFC | L | 0.01 | −26 | 32 | −12 | |||
| VS | R | 0.01 | 8 | 6 | 2 | 864 | 2 | 3(4) |
| Emerging Adult | ||||||||
| ventral ACC | R | 0.02 | 4 | 32 | −6 | 1752 | 1 | 8(12) |
| ventromedial PFC | R | 0.01 | 4 | 44 | −12 | |||
| medial OFC | L | 0.01 | −4 | 28 | −16 | |||
| medial OFC | R | 0.01 | 4 | 36 | −18 | |||
| posterior cingulate | L | 0.03 | −8 | −56 | 14 | 1096 | 2 | 5(5) |
| Subtraction* | ||||||||
| Developmental > Emerging Adult | ||||||||
| VS | R | 2.65 | 7.3 | 9.3 | 1.8 | 480 | 1 | 2(2) |
| VS | R | 2.56 | 8 | 6 | 4 | |||
| VS | R | 2.42 | 6.5 | 2 | 5.5 | |||
No clusters were identified when contrasting emerging adult > developmental studies
Subsequent subtraction analysis of the developmental and emerging adult convergence maps revealed significantly greater activation likelihood in the right VS in developmental compared to adults. Exclusion of the two adult studies that incorporated clinical samples did not change this result. No regions were found to be significantly more active in the inverse contrast (i.e. emerging adult > developmental).
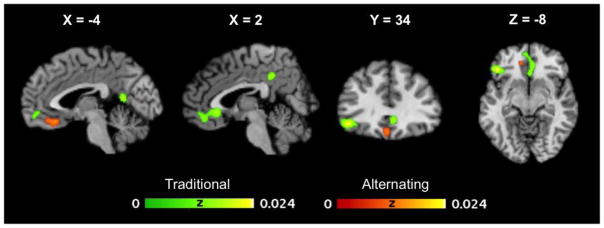
3.4 Design analysis
Finally, ALE meta-analysis of the 16 Cyberball studies with traditional designs identified similar clusters to those present in the full Cyberball sample, as highlighted in Figure 3. In comparison, only one cluster in the medial OFC extending towards ventromedial PFC was identified from the nine alternating design studies. Nevertheless, subtraction analysis comparing these two designs did not identify any significant differences. Given that variation in analytic strategy may have influenced these results, we re-ran the subtraction analysis excluding studies that used event-related analyses as opposed to the more frequently employed block designs (only one of the alternating and five of the traditional design studies employed event-related statistical analyses). The subtraction analyses still failed to identify any differences, suggesting that the absence of differences between traditional and alternating designs cannot be attributed to noise introduced by collapsing across different analytic strategies.
Fig 3.
Brain regions showing likelihood of brain activation in the traditional and alternating design samples in Cyberball studies.
4. Discussion
The current study employed a quantitative meta-analytic approach to identify neural correlates of social exclusion based on a synthesis of the literature to date. There were three main aims of the study: 1) identification of the neural correlates associated with social exclusion across the different paradigms employed in fMRI research, 2) investigation into neural correlates of Cyberball studies alone, and 3) exploration of differences in brain networks underlying social exclusion within developmental (i.e. late childhood up to late adolescence, 7–18 years) and emerging adult (i.e. late adolescence and young adults, 18–30 years approximately) samples. In studies examining the neural correlates of social exclusion using various paradigms, results revealed activation of the bilateral medial prefrontal and posterior cingulate cortices (extending into the right precuneus), as well as left ventrolateral PFC extending to lateral OFC. Similar activation patterns were identified when restricting the analysis to Cyberball studies, with the lateral prefrontal findings being driven by (i.e. present in) developmental samples and the medial prefrontal and posterior cingulate findings being driven by the emerging adult samples. In addition, the VS was implicated when the developmental samples experienced exclusion in Cyberball studies. We also explored whether the neural correlates of social exclusion differed depending on the design of the Cyberball paradigm, and failed to identify any significant differences between traditional and alternating designs.
Our main findings from the overall analysis of all fMRI studies on social exclusion (meeting our inclusionary criteria) revealed activation of a large bilateral medial prefrontal cluster encompassing the perigenual and subgenual ACC, ventromedial PFC, and medial OFC. There was also activation within a smaller cluster in the left lateral PFC, including the ventrolateral PFC and lateral OFC. Finally, activation was identified within the left posterior cingulate and right posterior cingulate cortex extending into the precuneus, although the latter cluster was not present when excluding two studies that incorporated clinical samples. Restricted analysis of Cyberball studies revealed a highly similar pattern of activation, which is not surprising given that 29 out of 40 studies used this paradigm. Therefore, while we speculate that similar neural correlates underlie exclusion as experienced by these different paradigms, we also acknowledge that our analyses were unlikely to reveal differences produced by non-Cyberball paradigms.
Activation within the perigenual and subgenual ACC is thought to be an important marker of sensitivity to social stressors (including peer rejection), having been found to be more active in individuals with lower rejection sensitivity (Burklund et al., 2007) and also more responsive to negative stimuli in depressed populations (Chen et al., 2007). Furthermore, activation within this region may be a marker of risk for future depressive symptoms (Masten et al., 2011b). While the exact mechanisms underlying this region’s sensitivity to social stress remains unknown, activation may be interpreted as: 1) altering subjective emotional experiences, resulting in more acute emotional responses and negative interpretations of social interactions, 2) reflecting an inability to adequately regulate emotions arising from negative events, and/or 3) producing greater mentalizing about negative peer interactions, potentially leading to chronic rumination. Also falling within our medial cluster of activation, ventromedial PFC and medial OFC activation likely reflects engagement in social cognitive processes, consistent with past research that has associated these regions with social and self-relevant evaluative processes (Burnett et al., 2011; Pfeifer et al., 2013; 2011).
In comparison, activation within the ventrolateral PFC/lateral OFC cluster is consistent with its hypothesized role in emotion regulation, thus potentially representing regulation of distress or negative affect following exclusion. Supporting this distinction between the medial and lateral prefrontal cortices, Masten and colleagues (2009) found that greater ACC activity was related to greater self-reported distress from exclusion during Cyberball, while greater ventrolateral PFC activation was related to less distress; this latter association (but not the former) was also supported by Kawamoto et al. (2012) and Bolling et al. (2012). The precuneus and posterior cingulate cortices, dominant hubs of the default mode network, are thought to support mentalizing processes, facilitating an individual’s ability to comprehend or interpret other people’s thoughts, feelings and intentions (Beer and Hughes, 2010; Frith and Frith, 2003; Hyatt et al., 2015). Engaging in such perspective taking is also aided by self-guided mental imagery, which has consistently been shown to involve the precuneus (Cavanna and Trimble, 2006). Within the context of experiencing social exclusion or rejection, activation within these regions could potentially reflect social cognitive processes involved in understanding the mental states of the perpetrators (e.g., considering their motives for committing the rejection).
An important extension of the current meta-analysis was examination of neural correlates of social exclusion during adolescence, given the surge in social cognitive research focused on developmental samples (Pfeifer and Blakemore, 2012). Our meta-analysis of Cyberball studies revealed different activation patterns associated with social exclusion during late childhood through mid/late adolescence compared to late-adolescence through early adulthood. While the medial PFC and posterior cingulate clusters identified in the above analysis were driven by (i.e. present in) emerging adult studies, the ventrolateral PFC cluster was driven by developmental samples. These differences may arise from regional heterogeneity in trajectories of maturation across the brain during adolescence. For example, mentalizing and self-evaluative processes, as well as their associated midline structures are thought to continue to mature throughout the adolescent period (Burnett et al., 2011; Pfeifer and Peake, 2012). While ventrolateral PFC and associated adaptive emotion regulatory capacities also show protracted development during this time (Crone and Ridderinkhof, 2011), structural neuroimaging suggests that medial and lateral prefrontal regions may exhibit different developmental trajectories and thus attain adult-levels of maturity at different ages (Mills et al., 2014; Shaw et al., 2008; Tamnes et al., 2010). As such, it is possible that younger adolescents are engaging in these higher order social cognitive and emotion regulatory processes differentially than emerging adults. However, it cannot be assumed that the latter group has completed maturation, as these processes likely continue to develop during early adulthood. We also acknowledge that only the ventrolateral PFC cluster was significantly different between developmental and emerging adult studies, and further, only when including two studies that incorporated both healthy and clinical samples. As such, further research (i.e. meta-analysis of a larger sample of empirical studies when more are conducted) is needed to corroborate these findings.
We also identified activation of the VS when children and younger adolescents experienced exclusion in the Cyberball paradigm, which was absent in emerging adults. This heightened VS activity during childhood and adolescence is interesting in relation to a recent meta-analysis that likewise found greater activity in VS during reward processing for children and adolescents (aged 8–19 years) than adults (Silverman et al., 2015). Our results may therefore suggest that adolescent hyper-reactivity of VS is not limited to reward processing, and that this region may not be selective for the learning and prediction of rewarding outcomes (McClure et al., 2003; O’Doherty et al., 2004; 2003). Consistent with our findings, studies have also reported activation of VS during cognitive reappraisal of negative stimuli (McRae et al., 2008; Ochsner et al., 2002; 2004; van Reekum et al., 2007). Although the exact mechanisms remain uncertain, Wager and colleagues (2008) found that ventrolateral PFC activation was positively correlated with that of the VS when engaging in cognitive reappraisal of aversive images, and VS activity mediated the relationship between ventrolateral PFC activation and reappraisal success (i.e. less negative emotional experience). We speculate that successful regulation of social exclusion, a type of aversive situation, may similarly involve the generation of positive appraisal via VS involvement, in addition to the traditional down-regulation of regions implicated in negative reactivity.
Analyses comparing the two most commonly employed Cyberball paradigms failed to reveal any significant differences between studies employing the traditional and alternating designs. Low power might have limited our ability to identify significant differences, given that differing results were present when examining the traditional and alternating studies separately. There are concerns in the field that these two paradigms might produce differing reactivity in relation to social exclusion. One hypothesis is that the alternating design might result in “spill-over” effects, such that reactivity associated with exclusion blocks might affect BOLD response in subsequent inclusion blocks (Sebastian et al., 2010a), potentially diminishing neural differences between these conditions. Indeed, social exclusion can affect psychological states for extended periods following the experience, including ratings of self-esteem and meaningful existence up to 30 minutes later (Buelow et al., 2015). On the other hand, there are also concerns that the traditional design might result in fluctuating levels of reactivity over the course of a prolonged period of exclusion, as highlighted by findings from electrophysiological studies (Kawamoto et al., 2013; Themanson et al., 2013). This may act to increase noise in the BOLD response associated with the exclusion condition. Additional empirical studies are required to appropriately address this question. These would also benefit from the use of a non-social baseline condition, as opposed to the typically employed fair play (or inclusion) condition, as this would enable studies to potentially differentiate whether variations in task design influence response to either inclusion or exclusion blocks.
It is interesting to note that there is minimal overlap between our findings and Cacioppo and colleagues’ (2013) prior meta-analysis of the Cyberball literature. Common activation was limited to one area, the left lateral OFC. While activation in this region was driven by the developmental samples in our analysis, the prior meta-analysis only included one such study on adolescents. Although both studies also identified activation within the ACC, our findings primarily lie within the subgenual portion, while their findings fall more rostrally (in what we consider to be primarily anterior rostral medial PFC). We hypothesise that these differences may be due in part to varying samples, as Cacioppo and colleagues only included 12 studies (nine of which overlapped with our sample), which can be attributed to the large number of studies published since their analysis (including almost 50% of studies in the current analyses). However, it is also possible that differences arose from the meta-analytic method employed, as they performed Multi-level Kernel Density Analysis (MKDA) that models activation with spheres and uses experiment counts (i.e. the count at each voxel represents number of overlapping studies) in comparison to ALE’s 3-D Gaussians used to evaluate the probability of activity localization (Eickhoff et al., 2012). We also failed to identify recruitment of two regions commonly associated in social exclusion: the anterior insula and dACC. While Cacioppo and colleagues (2013) found activation of the former region, neither meta-analysis identified involvement of the dACC despite the sizeable focus on the region within the literature on social pain. This perhaps speaks to the tendency for early findings to unduly influence future studies and interpretations, as potentially occurred with this region.
Indeed, the absence of dACC and anterior insula involvement in our results calls into question the hypothesis that the neural circuitry for physical pain was evolutionarily co-opted to increase the affective salience of social exclusion (Eisenberger et al., 2012). As another challenge to this hypothesis, research using multivariate pattern analyses suggests distinct neural representations of physical pain and social rejection in the dACC and anterior insula, which are not distinguishable using traditional task-based fMRI contrasts (Woo et al., 2014). While it is difficult to reconcile differing findings in the literature thus far, some studies continue to hypothesize activation of, particularly, the dACC in relation to social pain. Moreover, region-of-interest analyses are frequently conducted in the dACC without supplemental whole brain analyses. At certain times, this occurs using lowered statistical thresholds, which are further biased towards confirmatory findings. Although involvement of both the dACC and anterior insula was identified when we lowered the statistical threshold for significance, our findings suggest that these regions are not identified as reliably and robustly across studies relative to other regions. This does not exclude the possibility that there are meaningful individual differences pertaining to the involvement of these regions. Comparatively, it appears that regions commonly associated with social cognitive processing and emotion regulation are more likely to be activated during the experience of social exclusion.
The findings from the current study need to be considered within the context of methodological strengths and limitations. A major strength of this meta-analytic approach is that it weighs the contribution of each study based on sample size, thus minimizing sampling error. In addition, there is increased statistical power and sensitivity to identify effects when combining results across studies, as opposed to the findings of individual studies. However, we acknowledge that only the minimum number of studies necessary to obtain valid results were included in the developmental and alternating design analyses. This may be particularly problematic for identifying clusters of convergence in the developmental samples relative to the emerging adult samples, as well as in the alternating design samples as compared to the traditional design samples. Nevertheless, the fact that VS and ventrolateral PFC were consistently implicated in in younger adolescents suggests that it is likely a strong finding. While our analysis was constrained to dichotomizing sub-analyses into samples under and over 18 years of age given the characteristics of current studies, future meta-analyses would benefit from more detailed grouping (i.e. smaller age variance) once there is an adequate number of such studies, which would also enable us to investigate important developmental questions, such as the influence of puberty. Relatedly, we were unable to make inferences about potential similarities or differences between the neural correlates of Cyberball and other social exclusion paradigms given the limited number of studies employing these other tasks. Moreover, some of these other studies examined neural correlates underlying witnessing others being rejected (i.e. either using Cyberball variants, or viewing IAPS pictures or paintings of others being rejected). Although we included these studies in the Social Exclusion analysis, future meta-analyses should try to tease apart differences between being socially rejected and watching others being rejected when are there enough studies to examine these processes separately.
We were also unable to investigate gender differences because only two of the reviewed studies conducted such analyses. While some studies attempted to control for gender by recruiting an even number of males and females, among studies with an uneven gender distribution all but one failed to include this variable as a covariate in their analyses. This represents a valuable avenue for future investigation, particularly considering that sex differences have been identified in a range of social cognitive processes (Pavlova, 2016; Proverbio, 2017). Meta-analyses of the effect of individual differences related to social sensitivity are another important area for future investigation, given that a meta-analysis focusing on the ACC found that self-reported distress was associated with the location of peak BOLD response within this region (Rotge et al., 2015). It should also be highlighted that many studies met our inclusion criteria except for failing to report main effects. We emphasize the need for fMRI studies to report main effects, even if they are not of primary interest, to increase the statistical power of valuable meta-analytic approaches that improve our comprehension of the neuroimaging literature. Along the same lines, regular use of Neurovault (http://neurovault.org/) to share whole-brain statistical maps would enable us to engage in more sophisticated and powerful meta-analytic techniques, such as image-based meta-analysis, because they are not limited by varying thresholding methods (sometimes even within a single study) and reported peak coordinates (and can thus pick up on subthreshold effects that are consistently present across studies).
In conclusion, the current ALE meta-analysis of the neural correlates of social exclusion identified involvement of medial and lateral prefrontal cortices, as well as posterior cingulate cortices. We also identified varying patterns of brain activation in studies of younger adolescents compared to emerging adults, highlighting differences that may arise from continued maturation of the brain during adolescence. The use of such meta-analytic methodology helps bring together results in the literature to date, and empirically highlights the tendency of the field to focus on certain regions, such as the dACC, as playing an important role in social exclusion/rejection, despite other areas being more consistently implicated.
Supplementary Material
Video 1. Brain regions showing likelihood of brain activation in the overall social exclusion analysis.
Table 5.
Brain regions activated by traditional and alternating design samples in Cyberball studies
| Brain Region | Hemi | Extrema | x | y | z | Volume (mm3) | Cluster Number | N studies (foci) |
|---|---|---|---|---|---|---|---|---|
| Traditional | ||||||||
| ventral ACC | R | 0.02 | 4 | 34 | −4 | 2376 | 1 | 8(11) |
| ventral ACC | R | 0.02 | 2 | 28 | −4 | |||
| vmPFC | R | 0.01 | 4 | 44 | −12 | |||
| vmPFC | L | 0.01 | −2 | 52 | −6 | |||
| inferior frontal gyrus | L | 0.02 | −46 | 34 | −8 | 1488 | 2 | 6(7) |
| occipital lobe | L | 0.02 | −12 | −90 | 2 | 896 | 3 | 3(4) |
| occipital lobe | L | 0.02 | −12 | −94 | 4 | |||
| posterior cingulate | L | 0.02 | −6 | −56 | 12 | 880 | 4 | 4(4) |
| posterior cingulate/precuneus | R | 0.02 | 4 | −40 | 40 | 776 | 5 | 4(5) |
| Alternating | ||||||||
| medial OFC | L | 0.01 | −4 | 30 | −16 | 1448 | 1 | 6(7) |
| vmPFC/OFC | L | 0.01 | −10 | 42 | −10 | |||
| medial OFC | L | 0.01 | −12 | 44 | 0 | |||
Highlights.
Meta-analysis of studies examining the neural correlates of social exclusion.
Recruits medial and lateral prefrontal, and midline posterior parietal cortices.
Neural differences identified between developmental and emerging-adult samples.
Unique role of ventral striatum in developmental samples.
Acknowledgments
Funding
This work was supported by the grants P50 DA035763 (PIs: Chamberlain and Fisher) and R01 MH107418 (PI: Pfeifer).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PA, Adler P. Peer Power: Preadolescent Culture and Identity. Rutgers University Press; New Brunswick, NJ: 1998. [Google Scholar]
- Beeney JE, Franklin RGJ, Levy KN, Adams RBJ. I feel your pain: emotional closeness modulates neural responses to empathically experienced rejection. Soc Neurosci. 2011;6:369–376. doi: 10.1080/17470919.2011.557245. [DOI] [PubMed] [Google Scholar]
- Beer JS, Hughes BL. Neural systems of social comparison and the “above-average” effect. NeuroImage. 2010;49:2671–2679. doi: 10.1016/j.neuroimage.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Differential brain responses to social exclusion by one’s own versus opposite-gender peers. Soc Neurosci. 2012;7:331–346. doi: 10.1080/17470919.2011.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Trait-level temporal lobe hypoactivation to social exclusion in unaffected siblings of children and adolescents with autism spectrum disorders. Accident Analysis and Prevention. 2015a;13:75–83. doi: 10.1016/j.dcn.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Unlike adults, children and adolescents show predominantly increased neural activation to social exclusion by members of the opposite gender. Soc Neurosci. 2015b:1–12. doi: 10.1080/17470919.2015.1117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science. 2011a;14:1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Kaiser MD, Wyk BCV, Wu J, Mayes LC, Pelphrey KA. Enhanced neural responses to rule violation in children with autism: a comparison to social exclusion. Accident Analysis and Prevention. 2011b;1:280–294. doi: 10.1016/j.dcn.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011c;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenberger M, Plener PL, Groschwitz RC, Gron G, Abler B. Polymorphism in the micro-opioid receptor gene (OPRM1) modulates neural processing of physical pain, social rejection and error processing. Exp Brain Res. 2015;233:2517–2526. doi: 10.1007/s00221-015-4322-9. [DOI] [PubMed] [Google Scholar]
- Buelow MT, Okdie BM, Brunell AB, Trost Z. Stuck in a moment and you cannot get out of it: The lingering effects of ostracism on cognition and satisfaction of basic needs. Personality and Individual Differences. 2015;76:39–43. [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc Neurosci. 2007;2:238–253. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CHY, Merlo-Pich E, Bullmore E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR. The developing brain: From theory to neuroimaging and back. Accident Analysis and Prevention. 2011;1:101–109. doi: 10.1016/j.dcn.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Değirmencioğlu SM, Urberg KA, Tolson JM, Richard P. Adolescent friendship networks: Continuity and change over the school year. Merrill-Palmer Quarterly …. 1998;44:313–337. [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7:184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsalla M, Koppe G, Niedtfeld I, Vollstadt-Klein S, Schmahl C, Bohus M, Lis S. Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience. 2014;9:1789–1797. doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT. Implementation errors in the GingerALE Software: Description and recommendations. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Falk EB, Cascio CN, O’Donnell MB, Carp J, Tinney FJJ, Bingham CR, Shope JT, Ouimet MC, Pradhan AK, Simons-Morton BG. Neural responses to exclusion predict susceptibility to social influence. J Adolesc Health. 2014;54:S22–31. doi: 10.1016/j.jadohealth.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond, B, Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MZ, Beckes L, Chango J, Allen JP, Coan JA. Adolescent neighborhood quality predicts adult dACC response to social exclusion. Social Cognitive and Affective Neuroscience. 2015;10:921–928. doi: 10.1093/scan/nsu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Waiter G, Kumar P, Stickle C, Milders M, Matthews K, Reid I, Hall J, Steele JD. Abnormal neural responses to social exclusion in schizophrenia. PLoS ONE. 2012;7:e42608. doi: 10.1371/journal.pone.0042608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz RC, Plener PL, Groen G, Bonenberger M, Abler B. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Research. 2016;255:43–49. doi: 10.1016/j.pscychresns.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, Van Leijenhorst L, Rombouts SARB, Crone EA, van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc Neurosci. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Hooker CI, Miyakawa A, Verosky S, Luerssen A, Ayduk ON. Individual differences in neural responses to social rejection: the joint effect of self-esteem and attentional control. Social Cognitive and Affective Neuroscience. 2012;7:322–331. doi: 10.1093/scan/nsr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartup WW, Stevens N. Friendships and adaptation in the life course. Psychological Bulletin. 1997;121:355–370. [Google Scholar]
- Hyatt CJ, Calhoun VD, Pearlson GD, Assaf M. Specific default mode subnetworks support mentalizing as revealed through opposing network recruitment by social and semantic FMRI tasks. Hum Brain Mapp. 2015;36:3047–3063. doi: 10.1002/hbm.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PAM. Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. International Journal of Psychophysiology. 2011;81:44–50. doi: 10.1016/j.ijpsycho.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima K, Nittono H, Yamaguchi S, Ura M. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Front Evol Neurosci. 2012;4:11. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Nittono H, Ura M. Cognitive, affective, and motivational changes during ostracism: an ERP, EMG, and EEG study using a computerized Cyberball task. Neuroscience Journal, ID: 304674. 2013b doi: 10.1155/2013/304674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Wager TD. Meta-analysis of neuroimaging data. Wiley Interdiscip Rev Cogn Sci. 2010;1:293–300. doi: 10.1002/wcs.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ku J, Kim J, Jang DP, Yoon KJ, Kim SI, Kim JJ. Aberrant neural responses to social rejection in patients with schizophrenia. Soc Neurosci. 2014;9:412–423. doi: 10.1080/17470919.2014.907202. [DOI] [PubMed] [Google Scholar]
- Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Accident Analysis and Prevention. 2011a;1:260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Develop Psychopathol. 2011b;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Pfeifer JH, Dapretto M. Neural responses to witnessing peer rejection after being socially excluded: fMRI as a window into adolescents’ emotional processing. Developmental Science. 2013;16:743–759. doi: 10.1111/desc.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage. 2011c;55:381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An FMRI investigation of attributing negative social treatment to racial discrimination. J Cogn Neurosci. 2011d;23:1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, Wang C, Shi Z, Eisenberger NI, Han S. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience. 2013;8:446–454. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore SJ. Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience. 2014;9:123–131. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor BG, Guroglu B, Op de Macks ZA, Rombouts SARB, van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Nelson E, Leibenluft E, McClure E, Pine D. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Okamoto Y, Kunisato Y, Okada G, Yoshimura S, Kanai Y, Yamamura T, Yoshino A, Jinnin R, Takagaki K, Onoda K, Yamawaki S. fMRI Study of Social Anxiety during Social Ostracism with and without Emotional Support. PLoS ONE. 2015;10:e0127426. doi: 10.1371/journal.pone.0127426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan SA, Flynn C, Garber J. Prospective relations between rejection and depression in young adolescents. Journal of Personality and Social Psychology. 2003;85:745–755. doi: 10.1037/0022-3514.85.4.745. [DOI] [PubMed] [Google Scholar]
- Novembre G, Zanon M, Silani G. Empathy for social exclusion involves the sensory-discriminative component of pain: a within-subject fMRI study. Social Cognitive and Affective Neuroscience. 2015;10:153–164. doi: 10.1093/scan/nsu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Pavlova MA. Sex and gender affect the social brain: Beyond simplicity. Journal of Neuroscience Research. 2016;95(1–2):235–250. doi: 10.1002/jnr.23871. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social Cognitive and Affective Neuroscience. 2012;7:1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, Lieberman MD, Mazziotta JC, Dapretto M. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. The Journal of Neuroscience. 2013;33:7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Accident Analysis and Prevention. 2012;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stampfli P, Seifritz E, Scheidegger M, Vollenweider FX. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proceedings of the National Academy of Sciences. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P, Ettinger U, Inchley-Mort S, Sumich A, Williams SCR, Kuipers E, Kumari V. Neural processing of social rejection: the role of schizotypal personality traits. Hum Brain Mapp. 2012;33:695–706. doi: 10.1002/hbm.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Aikins JW. Cognitive moderators of the longitudinal association between peer rejection and adolescent depressive symptoms. J Abnorm Child Psychol. 2004;32:147–158. doi: 10.1023/b:jacp.0000019767.55592.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM. Sex differences in social cognition: The case of face processing. Journal of Neuroscience Research. 2017;95(1–2):222–234. doi: 10.1002/jnr.23817. [DOI] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schuppen A, Schultz RT, Heim CM, Fink GR, Herpertz-Dahlmann B, Konrad K. Neural response to social rejection in children with early separation experiences. JAAC. 2014;53:1328–1337. e8. doi: 10.1016/j.jaac.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Rigby K. Consequences of bullying in schools. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2003;48:583–590. doi: 10.1177/070674370304800904. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, et al. A meta-analysis of the anterior cingulate contribution to social pain. SCAN. 2015;10:19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GCY, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Behav. 2010a;9:628–637. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams K, Blakemore S-J. Social brain development and the affective consequences of ostracism in adolescence. Brain & Cognition. 2010b;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience. 2014;9:1798–1807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. Special issue on the teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Khatcherian SM, Ball AB, Rosen PJ. An eventrelated examination of neural activity during social interactions. SCAN. 2013;8(6):727–733. doi: 10.1093/scan/nss058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Van Harmelen AL, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, Elzinga BM. Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS ONE. 2014;9:e85107. doi: 10.1371/journal.pone.0085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Via E, Soriano-Mas C, Sanchez I, Forcano L, Harrison BJ, Davey CG, Pujol J, Martinez-Zalacain I, Menchon JM, Fernandez-Aranda F, Cardoner N. Abnormal Social Reward Responses in Anorexia Nervosa: An fMRI Study. PLoS ONE. 2015;10:e0133539. doi: 10.1371/journal.pone.0133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Sebastian A, Lieb K, Tüscher O, Tadić A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 2014;15:19. doi: 10.1186/1471-2202-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will GJ, Crone EA, Guroglu B. Acting on social exclusion: neural correlates of punishment and forgiveness of excluders. Social Cognitive and Affective Neuroscience. 2015;10:209–218. doi: 10.1093/scan/nsu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will GJ, van Lier PAC, Crone EA, Guroglu B. Chronic Childhood Peer Rejection is Associated with Heightened Neural Responses to Social Exclusion During Adolescence. J Abnorm Child Psychol. 2016;44:43–55. doi: 10.1007/s10802-015-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Woo C-W, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD. Separate neural representations for physical pain and social rejection. NatCommun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudarczyk OA, Kohn N, Bergs R, Gur RE, Turetsky B, Schneider F, Habel U. Chemosensory anxiety cues moderate the experience of social exclusion - an fMRI investigation with Cyberball. Front Psychol. 2015;6:1475. doi: 10.3389/fpsyg.2015.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Brain regions showing likelihood of brain activation in the overall social exclusion analysis.