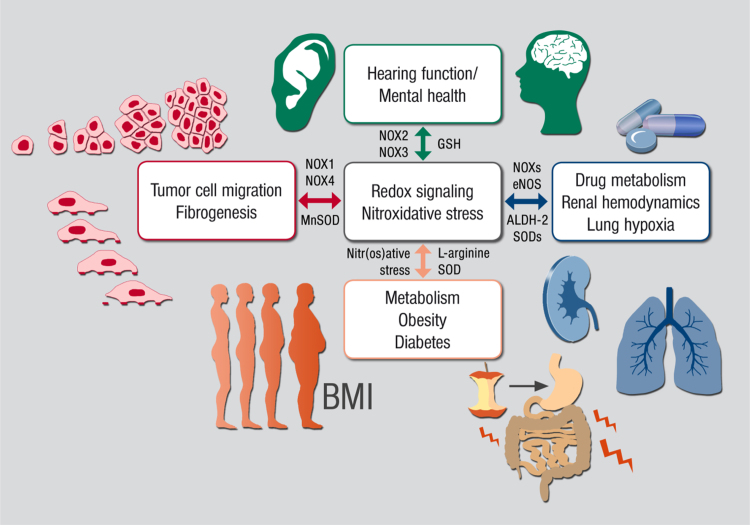
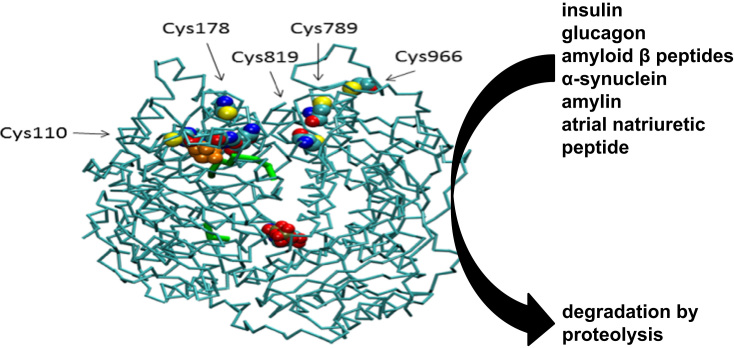
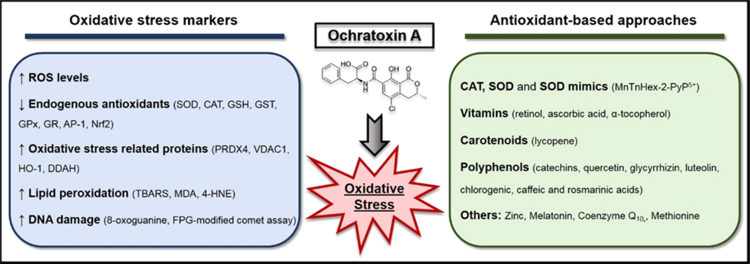
Abstract
The European Cooperation in Science and Technology (COST) provides an ideal framework to establish multi-disciplinary research networks. COST Action BM1203 (EU-ROS) represents a consortium of researchers from different disciplines who are dedicated to providing new insights and tools for better understanding redox biology and medicine and, in the long run, to finding new therapeutic strategies to target dysregulated redox processes in various diseases. This report highlights the major achievements of EU-ROS as well as research updates and new perspectives arising from its members. The EU-ROS consortium comprised more than 140 active members who worked together for four years on the topics briefly described below. The formation of reactive oxygen and nitrogen species (RONS) is an established hallmark of our aerobic environment and metabolism but RONS also act as messengers via redox regulation of essential cellular processes. The fact that many diseases have been found to be associated with oxidative stress established the theory of oxidative stress as a trigger of diseases that can be corrected by antioxidant therapy. However, while experimental studies support this thesis, clinical studies still generate controversial results, due to complex pathophysiology of oxidative stress in humans. For future improvement of antioxidant therapy and better understanding of redox-associated disease progression detailed knowledge on the sources and targets of RONS formation and discrimination of their detrimental or beneficial roles is required. In order to advance this important area of biology and medicine, highly synergistic approaches combining a variety of diverse and contrasting disciplines are needed.
Keywords: Reactive oxygen species, Reactive nitrogen species, Redox signaling, Oxidative stress, Antioxidants, Redox therapeutics
Graphical abstract
Highlights
-
•
RONS are chemical mediators and a communication tool.
-
•
RONS and disturbed redox balance play a role in a broad range of diseases and aging.
-
•
Bacteria and toxins are important stimulators of cellular RONS formation.
-
•
Drugs should preserve beneficial redox signaling and inhibit detrimental RONS sources.
-
•
Redox drugs may target the origin, identity, location and time of RONS formation.
1. Introduction
Andreas Daiber and Fabio Di Lisa.
1.1. Mission, structure and major achievements of EU-ROS consortium
The COST Action BM1203 (EU-ROS) is a research consortium of networking supported by the European Cooperation in Science and Technology (COST), which is embedded within the Biomedicine and Molecular Biosciences Domain. It covers areas of basic, preclinical and clinical research in biology, chemistry, physics and medicine, not only on mammalian cells but also plants, bacteria and other organisms. The mission of EU-ROS is to advance the field of redox biology and oxidative stress research by bringing together multi-disciplinary experts by organizing scientific meetings and providing funds for the exchange of researchers between laboratories (for more details see www.eu-ros.eu or www.cost.eu/COST_Actions/bmbs/Actions/BM1203). During the active funding period of EU-ROS (2013–2016) we organized ten major scientific meetings, supported 29 short-term scientific missions for research visits of postdoctoral fellows and Ph.D students, and co-organized three scientific symposia at European conferences of the Society for Free Radical Research Europe (SFRRe). In order to foster the next generation of redox biology scientists we also supported numerous early-stage researchers and invited them either to the six training schools that we (co)organized or to the young investigator sessions at our meetings. Among the major achievements of EU-ROS are three major successful grant applications within the European H2020 funding scheme as well as three major coordinated collections of position papers, reviews and original articles published by our members [1], [2], [3], [4]. The “small to medium sized enterprises” (SME) participating in EU-ROS filed three patents with the help of research collaborations established within our consortium. In order to disseminate the collected knowledge to a broader audience of non-expert scientists and the public we also opened a EU-ROS YouTube channel (https://www.youtube.com/channel/UCXFnyGD4uVFUTcLshvDtxcQ). The present collection of research updates and perspectives represents the final dissemination of COST Action BM1203 (EU-ROS).
In order to achieve the scientific objectives, as laid down in the memorandum of understanding of COST Action BM1203, we defined 6 working groups (WG) with elected leaders, each representing a taskforce for a specific area of research or organization/management: WG1 Sources of ROS (Ulla Knaus), WG2 Molecular Mechanisms (Agnes Görlach), WG3 Drugs & Tools (Tamara Seredenina), WG4 Biomarkers (Pietro Ghezzi/Paul Winyard), WG5 Imaging (Yves Frappart) and WG6 Technology Transfer & Funding (Vincent Jaquet).
1.2. Redox biology and oxidative stress: the EU-ROS approach
The fact that life requires oxygen, which per se represents a chemically aggressive molecule [5], bears the risk that biomolecules in all aerobic living species on earth are targets of oxidative modifications resulting from uncontrolled formation of reactive oxygen species (ROS2) and reactive nitrogen species (RNS3). In order to prevent oxidative damage, all aerobic organisms have developed highly efficient antioxidant strategies during evolution. However, these highly reactive species may be generated accidentally as a result of altered metabolism (e.g. during mitochondrial respiration) or can be formed deliberately (e.g. by professional ROS-producing enzymes at sites of inflammation). Hence, it is not surprising that most metabolic diseases, as well as those pathologies associated with low-grade inflammation, display increased patterns of biomarkers of oxidative stress [6]. It is well established that cardiovascular [7], [8], [9], neurodegenerative [10], [11], metabolic [12], [13] and inflammatory diseases [14], [15], [16] are associated with increased oxidative stress (some rare immune diseases are linked to insufficient ROS formation [17]). Despite the large body of evidence linking oxidative stress with many common diseases, which is supported by the significant correlation of redox biomarkers with cardiovascular and all-cause mortality [18], [19], [20], direct clinical proof is still lacking that oral therapy with antioxidants, such as vitamin C and E, helps to prevent the development or progression of these diseases. Most large-scale antioxidant clinical trials yielded disappointing results regarding all-cause mortality and in some cases oral antioxidants had detrimental effects [21], [22], [23] and to this date no “antioxidant” is admitted as a drug for clinical use [24]. More recent reports in publication channels dedicated to the non-expert or public readership highlighted the potential risks associated with excessive oral consumption of antioxidants (see online publications by Mustain [25] and Riley [26]). Antioxidant therapy was also mentioned among “the science myths that will not die” [27]. In contrast, a large number of small to intermediate size cohort studies with short-term and parenteral vitamin C therapy showed highly beneficial effects in various cardiovascular disease settings, and, for vitamin D, positive reports on oral therapy exist [9], [19], [28]. The most likely explanation for the antioxidant /oxidative stress paradox may be that reactive oxygen and nitrogen species (RONS) are not only injurious (oxidative stress) but also modulate important biological functions (redox signaling). Accordingly, chronic, systemic oral therapy with antioxidants will likely interfere with important ROS-mediated cellular processes, such as stress adaptation by ischemic preconditioning [19], [21], [28]. Additional reasons for the failure of large trials on oral antioxidant therapy might comprise the pro-oxidant effects of vitamin C and E radicals, reaction kinetics that are too slow, or lack of effective concentrations at sites of RONS formation. It is also possible that the patients included in these trials had already been exposed to drugs with pleiotropic antioxidant effects (e.g. ACE inhibitors or statins) (all reviewed in [19], [21], [28]). In addition, as shown by the EPIC Norfolk study, large antioxidant clinical trials suffer from suboptimal control of their potential efficacy, such as not measuring the patients’ plasma levels of antioxidants to verify compliance [29]. This study also showed that vitamin C concentrations in the blood inversely correlate with all-cause mortality in healthy volunteers. The inherent problems of vitamin C and E, that were mostly used in clinical trials, were also highlighted by Darley-Usmar and colleagues by a previous review [30]. Moreover, Forman, Davies and Ursini postulated that the nucleophilic tone and para-hormesis (paradoxic oxidative activation of intrinsic defence mechanisms such as the NRF2 pathway) is more important than free radical scavenging properties of antioxidants for their beneficial effects in vivo [31].
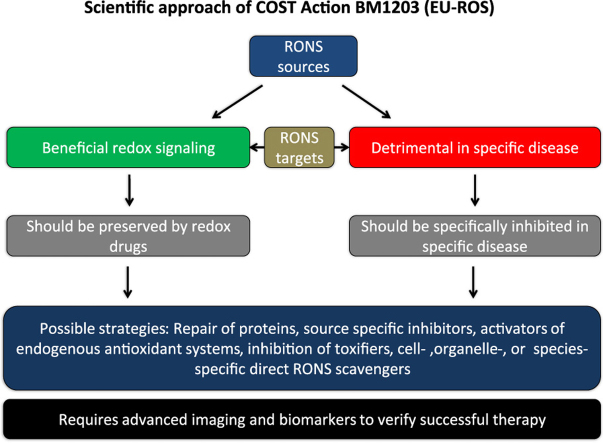
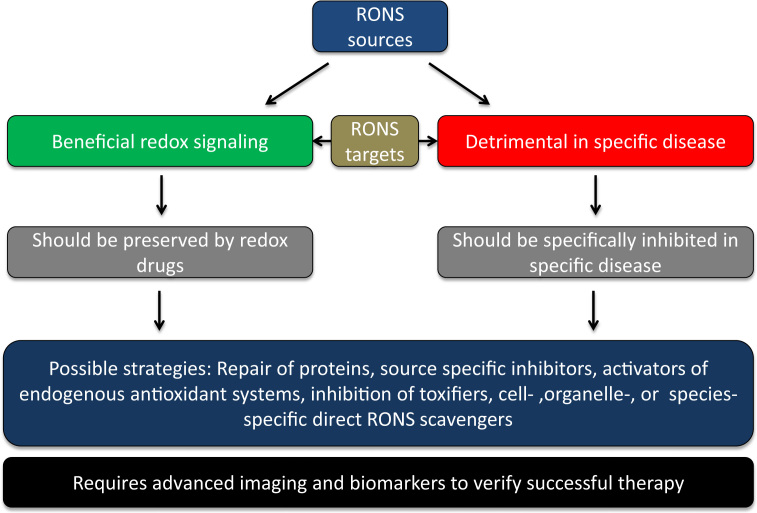
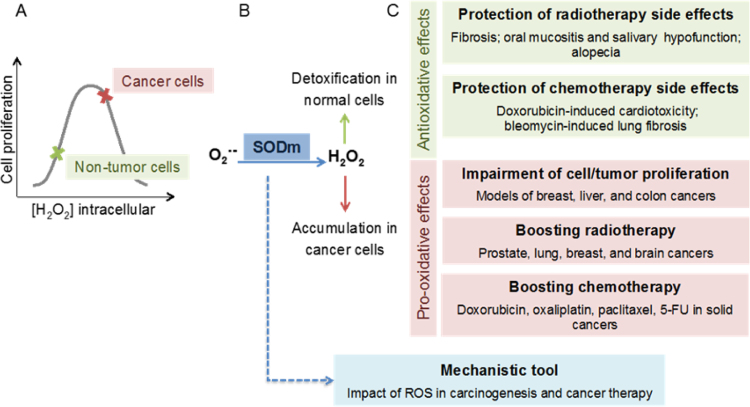
This obvious paradox in antioxidant therapy efficacy warrants better understanding of the role of RONS in physiology and pathophysiology. Our consortium put forward the concept that the failure of the traditional antioxidants such as vitamin C and E was predictable considering the above-mentioned limitations, but that excessive formation of RONS (mostly termed “oxidative stress”) plays a role in disease development and progression or, at least, leads to stable biomarkers (e.g. oxidatively modified biomolecules) that can be used for diagnostic aspects in various diseases (reviewed in full detail in the Forum issue by EU-ROS members [2]). In brief, we believe that activation of intrinsic antioxidant processes (e.g. NRF2-dependent pathways), inhibition of disease-relevant sources of RONS (e.g. isoform-specific NOX inhibitors), scavenging of disease-triggering RONS by site- and time-specific antioxidants or even repair of oxidatively inactivated enzymes (the most prominent examples being sGC activators) represent recently clinically established or promising future antioxidant strategies. The main concept of our biomedical approach and working scheme is shown in Fig. 1.1.
Fig. 1.1.
The main concept of our biomedical approach and working scheme within the EU-ROS consortium as explained in detail previously [32].
The present overview is an interdisciplinary forum of opinions from various experts in the field of redox biology and oxidative stress research participating in EU-ROS. We have separated the present work into 11 sections since the contributions cover a wide range of scientific topics (mammals, worms, bacteria, plants) and even the terminology of RONS varies substantially depending on whether they belong to rather theoretical and basic science oriented disciplines or biomedical and clinical research areas. RONS regulate not only hydrogen sulfide (H2S) but also nitric oxide (•NO) and carbon monoxide (CO), thereby affecting all major gasotransmitter systems [33], [34]. Also the concept of ROS-induced ROS formation (the crosstalk of different ROS-producing systems) is highlighted in the subsequent sections [35], [36]. These direct and indirect mechanisms based upon redox modifications or direct reactions with other messengers (e.g. •NO and H2S) underlie RONS involvement in receptor-dependent signaling pathways, also due to the modulation of expression and activity of transcription factors. The contributions below also highlight how RONS contribute to established signaling pathways by their redox modulation. The interactions of RONS with microparticles [37], [38], [39], protein aggregates [40], [41] and the gasotransmitter H2S [33], [42] represent important examples that illustrate how RONS as messengers indirectly influence other signaling systems. Therefore, far from being merely biological waste products, RONS represent highly active and tightly regulated signaling molecules.
2. Conceptual and mechanistic aspects of ROS and oxidative stress
Ulla G. Knaus (ulla.knaus@ucd.ie).
2.1. Background and terminology
“ROS is over”, a statement at the ESF-EMBO meeting (Spain 2015) by Fulvio Ursini, should probably be extended to “Oxidative Stress is over” (Ulla G. Knaus). There is no doubt that ROS exist and that the concept of oxidative stress summarizes certain conditions connected to a variety of diseases. However, the unspecific usage of these blanket terms, especially during recent years, does not adequately reflect the diversity of reactive oxygen metabolites being produced, the intricate regulation of redox signaling and the oxidant-antioxidant balance, or the causality of oxidative modifications versus detrimental outcome in biological systems. “ROS” is discussed below in detail, while oxidative stress is usually defined as an imbalance between production of ROS and antioxidants and the ensuing pathophysiological consequences of increased, unspecified ROS. If the term oxidative stress is used retrospectively via determination of oxidative modifications of proteins, lipids or DNA, it might instead be oxidative damage. Direct measurements of oxidative stress often use oxidized glutathione as readout, but Morgan et al. identified immediate glutathione disulfide removal pathways and antioxidant backup systems that ensured redox homeostasis [43]. In some circumstances oxidative stress is correlated with the presence or upregulation of an enzyme that can (but might not) generate superoxide or hydrogen peroxide, or the presence of cell types that produce these species when activated. However, the outcome in redox biology will always be dependent on the type of oxygen species generated over a certain time period at a certain location. One example is wound healing which requires ROS [44], but may progress to fibrosis when deregulation of the process occurs [45]. Another example is hypoxic tissue reconstitution facilitated by the neutrophil oxidative burst [46] versus neutrophil-mediated tissue injury in other circumstances [47]. What happens when the predominant superoxide source is missing is evident in chronic granulomatous disease (CGD), an inherited immunodeficiency caused by inactivating variants of the NOX2 NADPH oxidase complex. CGD patients present not only with life-threatening infections, but also with hyperinflammation, and Cybb-/- (Nox2) knockout mice show an increase in proinflammatory mediators and tissue damage in disease models [48], [49], [50].
As Carsten Berndt and Marcus Conrad note, ROS, a widely used umbrella term, urgently needs specification. Its use is misleading, as just some of the molecules encompassed under this term are indeed reactive species. This is especially true for the non-radical species, and it is another common misunderstanding that all ROS are radicals. Unlike the hydroxyl radical (HO•) or the superoxide anion radical (O2•-) singlet oxygen (1O2) or hydrogen peroxide (H2O2) are not radicals, which is underlined by the different reactivities (second order rate constant M−1 s−1) of ROS with a given substrate (methionine) that range from 2×10−2 (H2O2) to 7x109 (HO•) [51]. Another often neglected fact is the continuous formation of certain reactive species by enzymes under physiological conditions. Ero1α, for instance, generates one H2O2 molecule following each disulfide bridge formed in the endoplasmic reticulum. Therefore, reactive species cannot be considered solely a phenomenon of damage and disease. These species formed under physiological conditions can contribute to diverse cellular functions. For instance, reversible oxidative posttranscriptional thiol modifications are important during several processes in cells or even whole organisms including embryonic development [52], [53]. Moreover, the formation of reactive species at different subcellular sites distinguishes between cellular functions, such as stem cell maintenance or differentiation [54]. Not only posttranslational oxidative modifications, but also damage by certain types of reactive species can be a trigger for specific cellular functions: Ferroptosis after lipid peroxidation was recently shown to specifically impede reprogramming into neurons [55]. For these reasons, it is impossible to measure “ROS”, to induce “ROS”, to inhibit “ROS”, or to accumulate “ROS”. Besides scientific recognition, limited experimental tools are a problem to move research forward (or back) to the specific investigation of specific species. Promising in vivo tools are highly sensitive genetically encoded fluorescence probes [56] and genetic cell and animal models with targeted deficiencies in professional redox enzymes dealing with a select subset of distinct reactive species, such as the ferroptosis regulator glutathione peroxidase 4 [57]. Several research communities focus or have focused on ROS molecules, e.g. the Oxygen Club of California, the Society for Free Radical Research (SFRR), and EU-ROS. Hence, researchers working in the field of redox biology must, whenever possible, discriminate among the different partially reduced forms of oxygen being studied as this will allow us to acknowledge their different chemical features and/or biological functions as well as ultimately help to provide strong and solid mechanistic data on important biological processes.
2.2. ROS sources and their activation
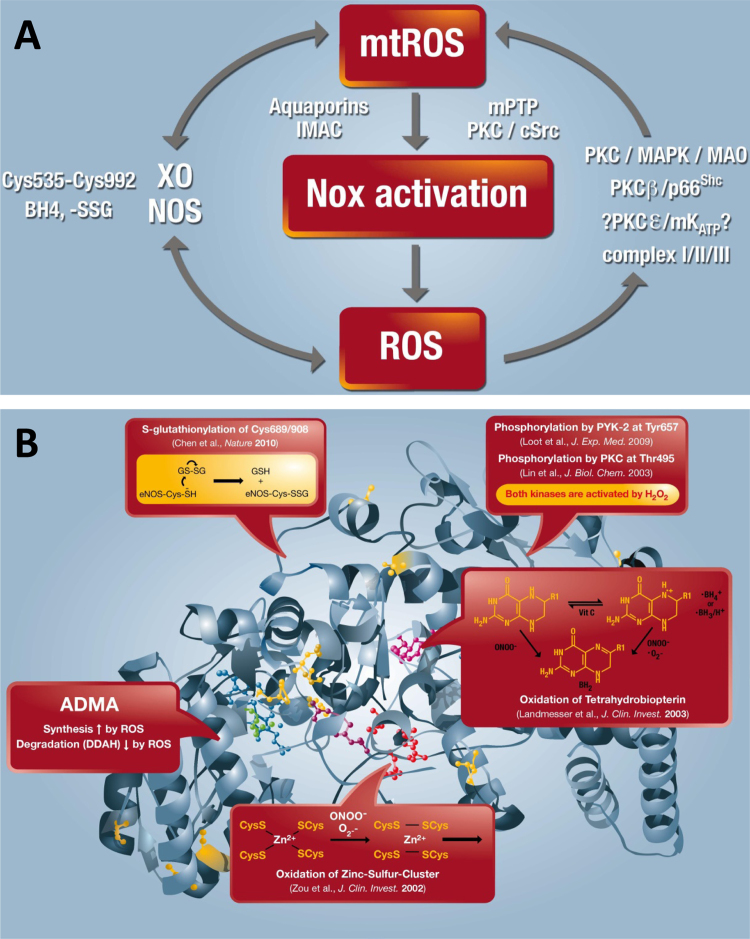
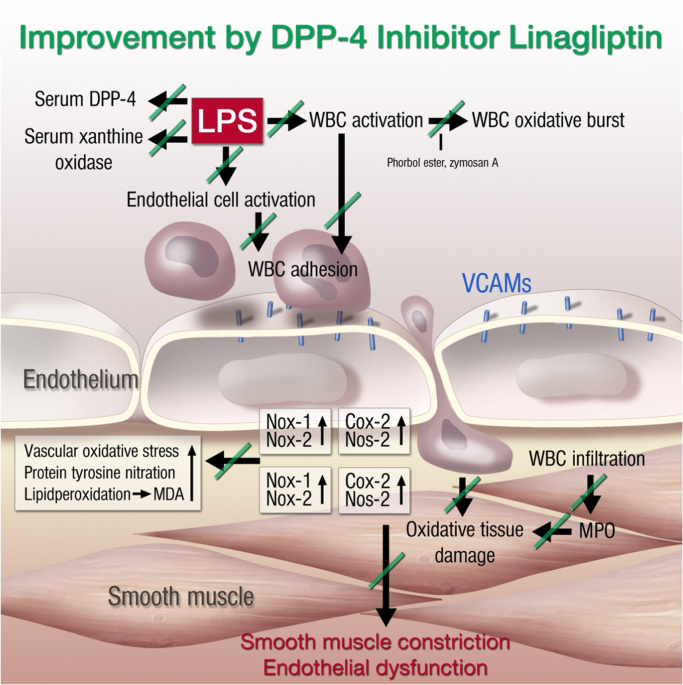
There are circumstances where the term “ROS” is unavoidable, mainly due to our inability to exactly measure the species generated in a spatiotemporal manner or to correctly identify or discriminate between species responsible for a certain biological event. Sometimes a mixture of oxygen metabolites is produced as several sources are stimulated or interact with each other. Thus, ROS sources should not be considered isolated enzymatic systems, and biological processes may involve ROS-induced ROS (Andreas Daiber and Matthias Oelze). There is increasing evidence that they can crosstalk with each other via reactive oxygen and nitrogen species signaling [35], [58]. The theory of so-called “kindling radicals” or also the “bonfire” hypothesis is based on the formation of a few primary ROS that “inflame” a cascade of ROS amplification by stimulating the sources of secondary ROS (Fig. 2.1A). This was first described for mitochondria [59] and involves several mitochondrial pores that are required for the release of mitochondrial ROS such as the mitochondrial permeability transition pore, aquaporins or the inner membrane anion channel as well as sources of oxidants (e.g. respiratory complexes, p66shc, monoamine oxidases) [18]. This crosstalk concept can be extended to almost all kinds of sources of oxidants. We have described “redox switches” that lead to uncoupling of endothelial nitric oxide synthase (eNOS), redirecting this enzyme from nitric oxide to superoxide production and thereby changing the entire vascular phenotype from a dilated, anti-thrombotic, anti-inflammatory state to a constricted, thrombotic and inflammatory state (Fig. 2.1B) [18], [20], [60]. Likewise, initially formed “kindling” ROS easily activate xanthine oxidase by a thiol oxidation-dependent and proteolytic conversion of xanthine dehydrogenase to the oxidase form [60] or activate NADPH oxidases (NOX), either by redox-sensitive activation of protein kinase C and translocation of cytosolic subunits (for NOX1 and NOX2) or by upregulation of all NOX isoforms by redox-sensitive transcription factors or changes in mRNA stability [60]. The most important crosstalk between different sources of oxidants was described for mitochondria and NOX, which was reviewed in full detail by us and others [18], [58]. We have observed this kind of crosstalk in nitroglycerin-induced endothelial dysfunction and oxidative stress [61], in models of aging-induced vascular dysfunction and oxidative stress [62], as well as in angiotensin-II induced hypertension and immune cell activation [63]. In conclusion, the redox crosstalk between different sources of oxidants may explain why multiple publications describe different ROS sources as the major pathological trigger in a certain disease (e.g. for the hypertension mitochondrial respiratory chain, NOX1, NOX2, NOX4 and xanthine oxidase) and that pharmacological or genetic blockade of one of these sources was enough to prevent the adverse phenotype [18]. If this concept can be translated to patients, it may be enough to target one specific source of ROS to prevent or retard the progression of a certain disease.
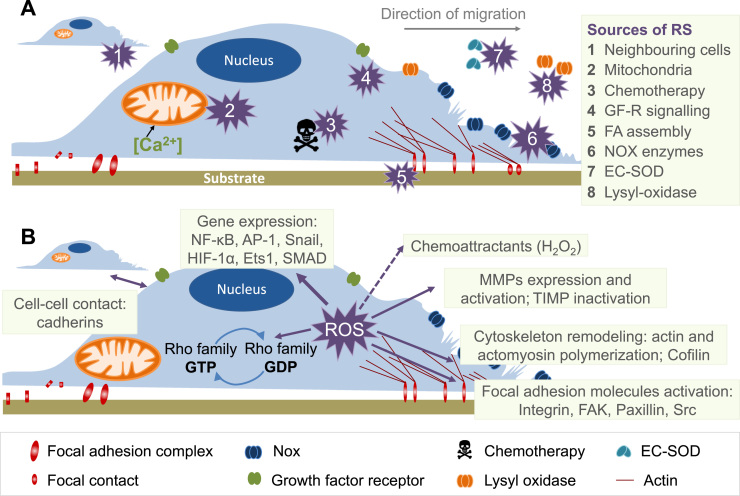
Fig. 2.1.
(A) Crosstalk between different sources of ROS and RNS (mitochondria, NADPH oxidases, xanthine oxidase and NO synthase). Xanthine oxidase (XO) originates from oxidative stress-mediated conversion of the xanthine dehydrogenase via oxidation of critical thiols in cysteine535/992. NO synthases (mainly eNOS) are uncoupled upon oxidative depletion of tetrahydrobiopterin (BH4), S-glutathionylation (-SSG) and other redox switches. Mitochondrial superoxide/hydrogen peroxide formation may be triggered by oxidative stress from all ROS sources (including other damaged/activated mitochondria) via redox-activation of PKC, MAPK, other kinase pathways and potential involvement of redox-sensitive mitochondrial ATP-sensitive potassium channels (mtKATP) with subsequent p66Shc, monoamine oxidase (MAO), respiratory complex activation or impairment of mitochondrial antioxidant defence. Mitochondrial superoxide/ hydrogen peroxide is released to the cytosol via mitochondrial pores and channels (e.g. redox-sensitive mitochondrial permeability transition pore (mPTP), inner membrane anion channel (IMAC) or aquaporins) or by diffusion due to increased mitochondrial permeability under pro-inflammatory conditions. In the cytosol these species (along with released calcium) cause activation of redox-sensitive protein kinases (PKC) and tyrosine kinases (cSrc) with subsequent activation of NADPH oxidases and amplification of the cellular oxidative stress. Modified from [35], [64]. With permission of Elsevier. Copyright 2010 & 2015. (B) Redox switches in endothelial nitric oxide synthase (eNOS). X-ray structure of human eNOS with the ironporphyrin (blue), the substrate L-arginine (green), the P450-forming axial iron-thiolate ligand from a cysteine residue (yellow), the cofactor tetrahydrobiopterin (BH4) (purple), the zinc-thiolate complex forming cysteines (red, two from each subunit), and the zinc ion (brown). The boxes represent the ‘‘redox switches’’ in eNOS, such as S-glutathionylation, PKC- and protein tyrosine kinase-2 (PYK-2)–dependent phosphorylation, oxidative BH4 depletion, disruption of the zinc-sulfur cluster, as well as asymmetric dimethylarginine (ADMA) synthesis/degradation, all of which contribute to the regulation of its enzymatic activity. GSH, glutathione; GSSG, glutathione disulfide. The crystal structure was rendered from the protein database entry 3NOS (DOI:10.2210/pdb3nos/pdb) using the PyMOL Molecular Graphics System Version 1.2r1 (DeLano Scientific LLC). Adapted from [60]. With permission of Mary Ann Liebert, Inc. Copyright 2014.
Determination of the ROS source(s) and their interactions is still challenging. The multitude of potential inputs range from NADPH oxidases (NOX1-5, DUOX1-2) and the mitochondrial electron chain, to xanthine oxidase, monoamino oxidase(s), cyclooxygenase(s), lipoxygenase(s), lysyl oxidase(s), cytochrome P450, or MICAL family members, to name a few. In all of the cases, the oxygen metabolite superoxide (O2•-) or H2O2 is primarily generated as a by-product of key enzymatic reactions of a particular enzyme. NADPH oxidases are the only enzymes solely dedicated to regulate O2- (NOX1-3, 5) and H2O2 (NOX4, DUOX1-2) production [65]. How certain NADPH oxidases can internally convert O2•- to H2O2 when using NADPH as the electron donor for the one-electron reduction of molecular oxygen is still unresolved. This feature discriminates the signaling input of NOX4 and DUOX1-2 from other family members due to the diffusibility and longer half-life of H2O2 versus O2•- or secondary reactive metabolites.
2.3. ROS sources in microbiota
O2•-/ H2O2 generation by enzymes and their consequences are mainly considered in the context of plant or animal hosts. However, hosts are colonized by an ecological community of commensal, symbiotic and pathogenic microorganisms, collectively termed microbiota. The presence and composition of the microbiota, which includes bacteria, fungi and viruses and resides in biofluids or on epithelial surfaces, is critical for health and disease. Bacteria represent the majority of the human microbiota (1014 bacterial cells) with estimates of 1000 or more bacterial species. Bacteria-host interactions in redox biology have been mostly defined by infections, where host defense relies on NOX2-mediated oxidative destruction of pathogens.
Yet an immediate and close relationship exists between commensals, pathogens and host epithelial surfaces, which is best characterized in the intestine. In the gut, feedback communication ensues with bacteria as inducers, targets and producers of ROS (Ulla G. Knaus and Gratiela G. Pircalabioru). The association of germ-free mice with microbiota induced the expression of epithelial DUOX2 [66]. Intestinal pathogens such as C. jejuni, K. pneumoniae, or L. monocytogenes triggered by a yet unknown mechanism the activation of NOX1 and DUOX2, resulting in O2•- generation and H2O2 release into the gut lumen [67], [68]. Enteropathogenic E. coli stimulated a NOX1-mediated pathway that included ASK1, p38 and AFT-2 and culminated in an over 20-fold upregulation of the DUOX2 complex [69]. Others reported that Lactobacillaceae activate NOX1, thereby promoting intestinal stem cell proliferation and wound healing responses [70]. While pathogens and segmented filamentous bacteria can gain access to the epithelium, lactobacilli usually colonize the further removed, loose mucus layer. However, any disruption of the barrier including changes in permeability or mucus composition/density will permit the interaction of commensals with host cells and may result in ROS signaling via NOX and/or mitochondria. For example, mitochondrial ROS is required for NLRP3 inflammasome activation by bacteria or bacterial products, and subsequent IL-1β and IL-18 production [71]. The bacteria-host interaction will also initiate release of H2O2 from the mucosal surface. Uptake of H2O2 by extracellular bacteria alters their transcriptional program and intrabacterial signaling. Although antioxidant defense genes will be upregulated, Fenton reaction-associated oxidations will decrease phosphotyrosine signaling and alter pathogenicity gene regulation [68], [69]. These oxidative modifications reduce the virulence of extracellular bacteria, which can then be eliminated more efficiently by the host.
Certain commensals, in particular Lactobacillus and Lactococcus strains, use endogenous H2O2 production as their own means of communication. The bacterial enzymes capable of generating H2O2 are largely unknown except for L. acidophilus [72], although the consequences to host physiology and niche protection have been reported. The antiinflammatory effects of lactobacilli are multifaceted, but lactobacilli-mediated killing of pathogens (e.g. S. enterica, E. coli) has been firmly associated with their H2O2 production [73]. Even some catalase-negative pathogens use endogenous H2O2 production for intra- and inter-bacterial signaling. Aerobic growth of Streptococcus pneumoniae leads to pyruvate oxidase (SpxB)-mediated H2O2 generation, which was required for fatty acid metabolism and inhibited replication of other microorganisms competing for the same environmental niche [74], [75]. In conclusion, bacteria need to be considered as endogenous sources and exogenous inducers of H2O2, thereby propagating intra-and interkingdom signaling.
This connection between bacteria and the host has been studied extensively in the nematode worm Caenorhabditis elegans [76], [77], [78], [79], but as Elizabeth A. Veal and Antonio Miranda-Vizuete comment, C. elegans can serve as a general model for redox biology and has already provided significant new insight into the interplay between ROS, ROS signaling and aging. Notably, genetic studies have failed to show that the ROS-detoxifying activities of any of C. elegans’ array of ROS-metabolizing enzymes protects against aging. However, these studies have revealed specific roles for several of these proteins in redox signaling, protein homeostasis or regulation of normal physiology. For example, glutathione reductase is essential for cell division during embryonic development [80], thioredoxin reductase or glutathione reductase activity for larval development to reduce disulfide bonds of cuticle components prior to molting [81] and the peroxiredoxin PRDX-2 for regulation of feeding behavior [82] and normal levels of insulin secretion [83]. The causative role of ROS-induced damage in animal aging has also been challenged by the unexpected discovery that increases in ROS can actually promote C. elegans longevity (for review see [84]). Nevertheless, stress-activated transcription factors DAF-16 and SKN-1 (orthologous to the mammalian FOXO and NRF2 transcription factors), which promote the expression of a range of defenses, including ROS-detoxifying and phase 2 metabolism enzymes, are vital for survival under stress conditions, during infection and the extended lifespan associated with inhibition of a variety of pathways. As the primary tissue encountering xenobiotics and pathogens that trigger increases in ROS, intestinal levels of these proteins seem particularly important for survival under stress conditions. However, studies with tissue-specific transgenes and RNAi have indicated that cell non-autonomous signals from neurons and germline cells play an important part in regulating these stress defenses (for reviews see [85], [86]). Moreover, there is increasing evidence that maintaining C. elegans on different strains of E. coli can profoundly influence ROS production, redox signaling, metabolism and longevity [87]. C. elegans transparency throughout its entire life cycle has enabled the development of a variety of fluorescent redox-sensitive probes that can be employed to monitor in vivo changes in redox status. For example, analysis of animals expressing a genetically encoded peroxide sensor has suggested that peroxide levels are higher during larval development than in adults but rise again following the reproductive period [88]. Recent advances in genome editing tools, NGS approaches to identify mutations obtained by traditional mutagenesis screens, redox proteomics, including OxICAT, metabolomics, and high throughput techniques for genome-wide and tissue-specific RNAi-screening and lifespan analyses have all added to the C. elegans toolbox that enhance the power of C. elegans as a model to advance the redox biology field. The simplicity, ease of manipulation, microscopic examination, short lifespan and vast range of genetic and post-genomic tools have established C. elegans as a powerful model for providing mechanistic insight into the role of redox changes in normal physiology and disease.
2.4. Redox regulation via redox modifications of biomolecules and altered phosphorylation
ROS, in particular H2O2, participate in redox regulation and signaling responses by modifying proteins, lipids and DNA. An emerging concept is redox control of protein-protein interactions (Andrew R. Pitt and Corinne M. Spickett). Signal transduction via proteins containing redox-sensitive cysteine residues is now a well-established concept, and the list of such proteins is growing rapidly [89]. The cysteine residues involved are most commonly ones with unusually low pKas, which therefore exist as thiolates under physiological conditions [90]. The oxidation of these residues to form a disulfide by reaction with a second, resolving cysteine alters the structure and activity of the protein. A fundamental requirement of redox regulation in signaling is that it must be reversible, and thioredoxin is a central enzyme that mediates the reduction of protein disulfides via formation of an intermolecular disulfide with the target protein. Covalent and non-covalent protein-protein interactions are known to be critical in signaling pathways and are thought to contribute to the commonly observed pleiotrophic effects of ligands and crosstalk between signaling pathways [91]. Advances in proteomic technologies have facilitated studies of the interactomes of an expanding number of proteins. Not surprisingly, these approaches have also been applied to redox proteins, and much attention has focused on members of the thioredoxin family. The covalent interactomes of thioredoxin from Plasmodium falciparum and tryparedoxin from Trypanosoma cruzi have been reported, driven by the need to identify novel drug targets for protozoan parasites, and in Escherichia coli, 268 substrates for Trx were reported from experiments involving strains engineered to optimize trapping of the covalent interaction [92]. The human glutathione-S-transferase P interactome has also been reviewed recently, and includes known key players in redox sensing such as STAT3 and NRF2 [93]. There are many reports of interactions of other redox proteins, and in an attempt to bring together all the available information, an “oxidative status interactome map” has been created of known interactions of human cellular-level oxidative status proteins [94]. Although all of these articles report the general interactions of redox-active proteins, the effect of the redox status of the individual proteins on their interactome, especially the non-covalent interactome, has largely been ignored. One study that has addressed this issue investigated the differential interactome of the tumor suppressor PTEN in native (reduced) and reversibly oxidized forms, and reported a number of proteins whose levels changed significantly depending on the redox state, including Anxa2, Trx and Prdx1 [95]. More studies of this kind would help to understand cell redox regulation, and would provide an additional dimension to oxidative status protein mapping.
Oxidative regulation of protein phosphorylation is not only an important part of efficient and essential signal propagation, but can also promote a deregulated state when the reversibility of the oxidation is compromised. This can happen by irreversible overoxidation of cysteines or by irreversible oxidative modification of other amino acids such as methionine or tyrosine. In balanced conditions H2O2 is required in the endoplasmic reticulum (ER) for oxidative protein folding, while overproduction of ROS results in ER stress. This two edged sword is discussed by Andreas Petry and Agnes Görlach using ER-localized NADPH oxidases as example [96], [97]. ER stress activates the unfolded protein response (UPR) in three main steps (or phases). Activation of the Protein Kinase RNA-like Endoplasmic Reticulum Kinase (PERK), which phosphorylates eIF2alpha, results in general inactivation of CAP-dependent translation, despite some specific exceptions such as the transcription factor ATF4. Then, ER stress activates the inositol requiring element 1 (IRE1) – which splices the mRNA of XBP1 – and the transcription factor ATF6. ATF4, ATF6 and XBP1 induce the expression of genes involved in protein folding and degradation in order to reestablish proper protein folding and to remove protein debris. In the later ER stress response, pro-apoptotic proteins are upregulated such as CHOP in order to activate programmed cell death if the cell cannot resume physiological function.
ROS act especially on the phosphorylation status of eIF2alpha since protein phosphatase 1 (PP1), which controls together with GADD34 the dephosphorylation of eIF2alpha, is redox sensitive. H2O2 is able to oxidize a cysteine close to the catalytic core in a reversible manner resulting in the inactivation of PP1. Sources for ROS in ER stress are Ero1 in the ER or mitochondria, but also NADPH oxidases play a role. NOX1, NOX4 and p22phox were shown to interact with ER proteins such as PDI [98], and NADPH oxidase derived ROS were involved in the ER stress response [99], [100], [101], [102]. In response to ER stress due to inhibition of N-glycosylation, NOX4 associated with GADD34 thereby oxidizing PP1, extending eIF2alpha phosphorylation and promoting survival of cardiomyocytes [101]. However, other reports showed that 7-keto-cholesterol-induced ER stress in human arterial smooth muscle cells induced NOX4, resulting either directly in cell death [100] or in increased autophagy [99], which demonstrated a pro-apoptotic role for NOX4. In addition, NOX1 and NOX2 can play a pro-apoptotic role in cardiomyocytes [102] and renal cells [103] in response to ER stress. In summary, ROS derived from NADPH oxidases participate in the ER stress response. However, the subsequent outcome seems to be dependent on the cellular context, the stimuli used, the spatial distribution and the type of ROS. It is important to take these parameters into account when analyzing the role of ROS in ER stress and other pathophysiological conditions.
Another enzyme connected to ER stress is neutral sphingomyelinase (N-SMase). Sphingomyelinases (SMases) are categorized into neutral, acid and alkaline subtypes and hydrolyze sphingomyelin to ceramide [104]. Mutay Aslan discusses the accumulating evidence that ceramide induces oxygen species production via the mitochondrial respiratory chain [105], [106] and stimulates inducible nitric oxide synthase (NOS2) expression [107]. Indeed, activation of neutral sphingomyelinase (N-SMase) is reported to be involved in various disease pathologies connected to “oxidative stress”. Studies have shown that ischemia reperfusion (IR) injury leads to activation of N-SMase in rat cardiac myocytes [108] and rat liver [109]. Repletion of glutathione (GSH) via N-acetylcysteine (NAC) treatment in post-myocardial infarction rat hearts resulted in inhibition of N-SMase and decreased oxidative stress resulting in improved left ventricular function [110]. Similarly, inhibition of N-SMase reduced elevated levels of nitrative and oxidative stress markers in liver IR injury [111].
Neutral SMase is localized in sphingolipid-rich membrane fractions [111], establishing the structural basis for its functional interaction with NOS [112]. Cellular stress responses, which increase N-SMase activity, also affect NOS2 expression and nitric oxide bioavailability. It has been reported that ceramide production by N-SMase is a key mediator in the induction of NOS2. Other studies showed that exogenous ceramide induces NOS2 expression in rat primary astrocytes [113] and reported positive modulation of NOS2 gene expression by SMase and/or ceramide in vascular smooth muscle cells and microglia [114], [115]. Neutral SMase inhibition decreased both NOS2 and nitrotyrosine levels in an experimental model of glaucoma [116] and liver IR injury [109]. In summary, studies reveal that cellular stress responses significantly increase N-SMase activity and sphingomyelin/ceramide levels, leading to increased nitrative and oxidative modifications. Inhibition of N-SMase significantly reduced oxidant and nitrative stress markers, which emphasizes the need for future studies evaluating agents blocking N-SMase activity that can facilitate the development of treatment strategies to alleviate inflammation and oxidative injury.
The close relationship between bacteria and host is evident in bacterial toxin production that alters redox regulation in the host and in the response of bacteria to host-derived secondary oxygen metabolites generated as an innate immune response by post-translationally modifying bacterial proteins for protection and survival. Amanda J. Edson and Kari E. Fladmark discuss how cyanotoxins induce oxidative modifications in the host. The overabundance of cyanobacteria in aquatic ecosystems may result in a large increase in the manufacture and release of cyanotoxins into the aquatic environment, which cause human health implications through biomagnification [117]. A variety of cyanotoxins are produced by a wide range of cyanobacteria including: microcystins [118], [119] (MC), nodularin [120] (NOD), and β-N-methylamino-L-alanine [121], [122] (BMAA). Microcystins and nodularin are potent inhibitors of Ser/Thr protein phosphatases (PP), specifically PP1 and PP2A [118], [120] through direct interaction at the catalytic site [123]. Both toxins are cyclic peptides containing several non-proteinogenic amino acids [118], [120]. BMAA has been shown to indirectly target PP2A by inhibitory phosphorylation mediated through BMAA activation of the glutamate receptor mGluR5 [124]. However, unlike MC and NOD, BMAA is a non-proteinogenic amino acid with a high degree of structural similarity to simple amino acids allowing it to be misincorporated into proteins leading to an additional toxic effect [121]. MC, NOD, and BMAA exposure of cells has been found to promote protein hyperphosphorylation, increase ROS production, and induce apoptosis [118], [120], [121], [124].
Phosphorylation of proteins including ROS scavenging enzymes may be one of the major mechanisms of ROS induction by cyanotoxins. PP inhibitory action of cyanotoxins will result in broad protein hyperphosphorylation. In addition to ROS induction through PP inhibition, BMAA may also increase ROS through a non-glutamatergic mechanism [122]. H2O2 accumulation occurs within minutes in MC and NOD-exposed hepatocytes followed by cytoskeletal rearrangement and apoptosis [119], [120]. Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been shown to be essential in MC- and NOD-induced apoptosis and acts up-stream of H2O2 accumulation. Inhibition of PP by toxin exposure prevents the dephosphorylation of CaMKII autophosphorylated on Thr287/286 [123] leading to kinase hyper-activation. Rather surprisingly, CaMKII seems to be responsible for all observed MC and NOD-induced phosphorylation supporting its role as a key actor in cytotoxicity. Toxin-induced H2O2 generation may also amplify CaMKII activity and thereby also H2O2 generation through specific methionine oxidation of CaMKII [123]. The role of CaMKII in BMAA-induced toxicity remains to be elucidated, however CaMKII is shown to bind to and regulate the directly BMAA targeted mGluR5 receptor [125]. The converging signaling pathways of BMAA, MC and NOD suggest that co-existence of these toxins may increase the environmental risk factor to human health.
2.5. Oxidative stress and adaptation processes
Hypochlorous acid (HOCl) is a strong oxidant produced by activated neutrophils that kills pathogenic bacteria. Thus, bacteria have to defend against hypochlorite to maintain the reduced state of their cytoplasm. Gram-negative bacteria utilize glutathione (GSH) as their major thiol-redox buffer, but most Gram-positive bacteria do not produce GSH. Melanie Hillion and Haike Antelmann study redox modifications in Gram-positive bacteria under oxidative stress. Actinomycetes utilize mycothiol (MSH; AcCys-GlcN-myoinositol), which functions in protection against reactive oxygen, nitrogen and electrophilic species, antibiotics and heavy metals [126], [127]. Firmicutes bacteria, including Bacillus and Staphylococcus species produce the redox buffer bacillithiol (BSH; Cys-GlcN-malate). BSH-deficient Staphylococcus aureus isolates were more sensitive to antibiotics (fosfomycin and rifampicin) and showed reduced survival in macrophage infection assays [127]. Thus, BSH biosynthesis genes could be drug targets for the development of novel antibiotics to treat S. aureus infections. Under conditions of NaOCl stress, BSH plays an important role in the protection and redox regulation of proteins by formation of BSH mixed protein disulfides, termed S-bacillithiolation [127]. In Bacillus subtilis, S-bacillithiolation controls the activity of the organic hydroperoxide repressor OhrR and the methionine synthase MetE [127], [128]. S-bacillithiolation of the OhrR repressor leads to derepression of the OhrA peroxiredoxin that confers NaOCl resistance. S-bacillithiolation of MetE in its Zn-binding active site leads to methionine auxotrophy under oxidative stress. Two bacilliredoxins (BrxA and BrxB) with unusual CGC active site motifs were characterized that function in the reduction of S-bacillithiolated OhrR and MetE [129]. De-bacillithiolation results in the formation of bacillithiolated Brx (Brx-SSB) that requires BSH and an uncharacterized BSSB reductase for recycling. However, the bacilliredoxin pathway is redundant with the thioredoxin pathway in vivo for reduction of BSH mixed protein disulfides.
In Corynebacterium glutamicum, about 25 S-mycothiolated proteins were identified under hypochlorite stress conditions [130]. These include many metabolic enzymes, such as the methionine synthase MetE, the glycogen phosphorylase MalP, the myoinositol-1-phosphate synthase Ino1 and antioxidant enzymes (Tpx, Gpx, MsrA). S-mycothiolation of MalP is required for the oxidative stress resistance of C. glutamicum and could prevent glycogen degradation under NaOCl stress to save the energy and carbon source. Redox regulation of the thiol peroxidase Tpx, the MSH peroxidase Mpx and the methionine sulfoxide reductase MsrA was recently studied. The mycoredoxin (Mrx1) and thioredoxin (Trx) pathways are both involved in reduction and regeneration of S-mycothiolated Mpx and MsrA to restore their enzyme activities for detoxification of peroxides and reduction of methionine sulfoxides [131], [132]. In conclusion, S-bacillithiolation and S-mycothiolation are widespread redox modifications in Gram-positive bacteria that function in redox regulation and thiol-protection under oxidative stress conditions.
Acclimatization of an organism to stress or so-called preconditioning is a modulating response that prevents pathophysiological damage. In plants, waves of calcium and ROS are generated as response to abiotic stress, pathogen infection and mechanical injury. These waves mediate long-distance signaling and systemic cell-to-cell communication [133]. Khrystyna Semen and Olha Yelisyeyeva propose mitochondrial ROS oscillations as a balancing factor in mammalian metabolism. The development of a hormetic reaction (preconditioning or mild stress) represents one approach to increase resistance to oxidative damage and eliminate signs of oxidative stress (OS), which is involved in multiple pathological processes and aging [134], [135]. A hormetic reaction is triggered by a mild energy deficit, which precludes some increase in ADP/ATP and NAD+/NADH ratios leading to activation of redox processes and energy function of mitochondria. As a result, mobilization of various metabolic pathways, especially PUFA oxidation, takes place which promotes the flow of succinate into the Krebs cycle, activation of succinate oxidation and monopolization of the respiratory chain by succinate dehydrogenase. Such a metabolic state promotes more diverse shunting of the Krebs cycle with activation of glutamine/glutamate metabolism, the glyoxalate cycle, peroxisomal oxidation and accumulation of succinate. At the same time, activation of transaminase and alcohol dehydrogenase (ALDH2) reactions causes an increased flow of alpha-ketoglutarate and its more efficient oxidation [30], [136], [137]. Simultaneous activation of pathways related to these two reciprocal mitochondrial substrates serves as an important mechanism involved in the hormetic response. At the cell level that is reflected by the improved interactions between catabolism and anabolism, while excessive flow of electrons in the respiratory chain can be controlled by several mechanisms of “mild uncoupling” including reverse electron transport from Complex II to Complex I, activation of sirtuins (especially SIRT1) and uncoupling proteins. The fine-tuned leak of electrons that occurs with an increase in membrane and ATP potentials is further involved in production of a certain amount of superoxide, hydrogen peroxide and nitric oxide [30], [60]. Subsequently these reactive oxygen/nitrogen species function as signaling molecules for multiple pathways, mainly HIF/NRF2/NF-κB and their crosstalk, rather than promote excessive oxidative damage to macromolecules.
Mild prooxidant activity accompanying the formation of a hormetic response gives rise to the production of metabolic “endogenous” oxygen, which can be readily used either for oxygenase or oxidase reactions, maintenance of pO2 and elimination of hypoxia. Under such circumstances the activity of oxygen dependent enzymes, including those involved in H2S/NO/CO synthesis, is optimized, which promotes activation of K+ATP, BK and TRP channels. In fact, more frequent oscillation of ROS and metabolism dependent O2 promote sustained efficient function of mitochondria, which in turn leads to prolongation of free radical reactions (FRR) and membrane lipid peroxidation in the cell. The oscillatory nature of ROS/O2 and related regulatory substances is the least studied aspect in free radical biology. In our opinion, the most important outstanding questions include the mechanisms of fluctuations of ROS/O2, transcriptional factors and other regulatory substances derived from oxidative modifications of lipid and proteins, their pattern and localization in cell compartments. A better understanding of these mechanisms will promote therapeutic manipulation of ROS generation and thus, the dynamic association of respiratory complexes (supercomplex formation) to maintain optimal energy function of the mitochondria. Energy produced during FRR may play a crucial regulatory role to achieve better self-organization of the metabolism, improved stress resistance and sustained hormetic reactions.
For some diseases, including cardiovascular disease, cancer, or metabolic and neurological pathologies, the involvement of redox deregulation is unquestionable. Fernando Antunes and Paula M. Brito discuss how systems quantitative redox biology may bring us closer to an in depth understanding of “ROS”, their effects and therapeutic intervention in disease. Deregulation of reactive oxygen, nitrogen, and sulfur species (RONSS) affects cellular and organismal well being. RONSS target selected proteins either by binding to, or oxidizing metal centers or cysteine residues at the level of specific organs, tissues, cell types, and cellular organelles. These selected proteins constitute redox switches. Most of them are controlled by reversible oxidation and are key players in many signal transduction pathways [138]. The identification of hundreds of redox switches by large scale proteomic analysis [138] has provided new potential therapeutic targets and biomarkers that are yet to be explored. Thus, a critical mass has been reached to implement an ambitious translational redox biomedicine program. Perhaps the most important barrier to the development of such a program is the complexity of RONSS effects, including biphasic curves with narrow concentration ranges that often lead to contradictory conclusions. Such complex behavior is caused by the highly interactive molecular network formed by redox switches. A promising approach to deal with this issue is systems quantitative redox biology [139], [140] that integrates the network of redox-regulated pathways and has the potential to predict the behavior of redox networks when interrogated with a pharmaceutical drug. This will help identify redox circuits that are amenable to redox therapeutic interventions without triggering undesired responses by pathways that share the same redox mediators. RONSS generation is tightly controlled with respect to kinetics, concentration and subcellular location [141]; the implications of these observations are that highly targeted redox therapies, as opposed to systemic antioxidant interventions, are key for a viable pharmacological strategy. Recent advances in nanotechnology have provided new tools to deliver drugs to specific therapeutic target locations and have the potential to generate new classes of drugs that contain redox-active molecules or molecules that modulate the levels of RONSS as active pharmaceutical ingredients.
2.6. Microparticles and reactive oxygen species
According to Rhian M. Touyz, microparticles, also termed microvesicles, are cell membrane-derived fractions, which are generated from activated cells that undergo stress or injury [37]. Theoretically all cell types are able to generate microparticles, which are detectable in biological fluids. In the circulation the major microparticle subtypes are derived from platelets, neutrophils, erythrocytes and endothelial cells and are measured and phenotyped by specific markers and flow cytometry [37], [142]. Microparticles have distinct characteristics: they are 100–1000 nm in diameter, contain features of their parent cells and do not contain nuclear material. Microparticles were first considered as ‘plasma dust’ representing cell debris; however, it is now clear that these elements are biologically functional and that they are signatures of the cells from which they are derived. Accordingly, microparticles have been considered as biomarkers of various pathologies [37], [142], [143]. Many pathological states including cardiovascular diseases, kidney disease, cancer, diabetes, thrombosis among others are associated with increased levels of circulating microparticles. In addition, increasing evidence indicates that microparticles themselves can contribute to pathophysiological processes, because they contain proteins, enzymes, nucleic acids, cytoskeletal machinery, specialized lipids and microRNAs, that can be transferred to other cells, thereby initiating signaling events and changes in target cell function [144], [145], [146]. Through this mechanism, microparticles also play an important role in cell-cell communication and cross-talk. As such microparticles are now considered not only biomarkers of diseases, but also biovectors. In addition, because microparticles transfer their ‘cargo’, they have been considered as novel delivery systems for therapeutics [147]. Increased circulating levels of microparticles are found in conditions associated with oxidative stress, including hypertension, diabetes, kidney disease and cancer. Moreover, many of the mechanisms underlying the generation of microparticles involve ROS and many of the effects of microparticles are redox sensitive [148], [149]. Formation of microparticles involves lipid oxidation, mitochondrial activation, caspases, calcium signaling and Rho kinase activation, processes linked to increased intracellular ROS bioavailability. Microparticles can possess the enzymatic machinery responsible for ROS formation, including NOX subunits and/or nitric oxide synthase (NOS) and there is growing evidence that microparticles can generate ROS and nitric oxide (•NO) [148], [149]. Microparticles are supposed to transfer O2•-, H2O2 and •NO to neighboring cells, and they can stimulate production of ROS in target cells by stimulating cellular oxidases [150]. These processes can be inhibited by ROS scavengers, NOX inhibitors, NOS inhibitors and antioxidants. Hence microparticles are influenced by redox processes and can themselves modulate redox-sensitive signaling pathways [37], [148], [149]. While it is clear that oxidative stress and microparticle formation are closely linked and that microparticles can act as biovectors influencing cellular redox processes, many of the studies were performed under in vitro conditions in cell-based systems. The pathophysiological significance of these processes in vivo awaits further clarification.
2.7. Conclusions
To tackle these questions a multipronged approach is necessary. In depth understanding of the spatiotemporal role of a particular ROS source in vivo can only be achieved by scientific endeavors that combine basic discovery with appropriate animal models. But these studies depend on the development of improved tools for visualization and quantification of the oxygen metabolite generated. In addition, a combination of the expertise of redox biologists, systems biologists, nanotechnologists and clinical scientists will be required to support the successful translation of redox biology knowledge into viable pharmacological treatments and novel diagnostic biomarkers of diseases, which will have a major scientific and socio-economic impact.
3. ROS as signaling molecules
Agnes Görlach (goerlach@dhm.mhn.de) and Andreas Petry (petry@dhm.mhn.de).
3.1. Introduction
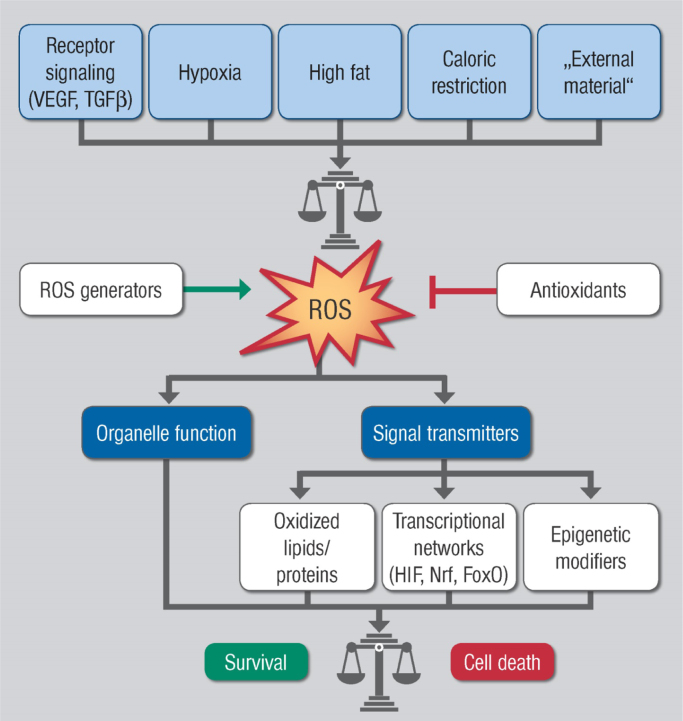
Reactive oxygen species (ROS) in high concentrations have damaging actions, but in lower concentrations they can act as signaling molecules (Fig. 3.1). ROS generated by the activation of enzymes such as NOX, xanthine oxidases, uncoupled NO synthases and other sources such as arachidonic acid metabolizing enzymes, lipoxygenases and cycloxygenases, the cytochrome P450s, peroxidases and other hemoproteins, as well as ROS generated by mitochondria seem to play various roles in the cellular signaling network under different physiological and pathophysiological conditions. Various cellular antioxidant systems oppose ROS load thereby limiting not only cellular damage, but also contributing to ROS-dependent signaling.
Fig. 3.1.
Summary scheme of ROS acting as signaling molecules in different disease settings but also in physiological processes.
3.2. Role of ROS in VEGF and TGF signaling
The dual role of ROS as either damaging or signaling molecules is well illustrated in the vascular system. For example, as discussed by María Monsalve and Ignacio Prieto, ROS have been demonstrated to be central in the control of angiogenesis and are required for the induction of endothelial cell migration and proliferation. VEGF-A is the most potent and primary endothelial cell specific angiogenic growth factor and stimulates vascular permeability, endothelial cell proliferation, migration and tube formation, primarily through the VEGF-A receptor 2 (VEGFR-2). ROS work both upstream and downstream of VEGF-A. Exogenous ROS administration induces VEGF-A levels and promotes endothelial migration and proliferation, which can lead to diabetic retinopathy, development of vasa vasorum in atherosclerosis and tumor angiogenesis [151]. ROS-sensitive transcription factors and coactivators have been identified that can directly regulate VEGF-A mRNA levels, such as the hypoxia-inducible factors 1α and 2α (HIF-1α, HIF-2α) [152], [153], [154], the transcriptional coactivator peroxisome proliferator γ coactivator 1α (PGC-1α) [155], but also Ref-1, p53, NF-κB and Ets-1 [156].
ROS have also been shown to regulate VEGFR-2 activity and signaling [157]. The antioxidant protein peroxiredoxin II (PrxII) has been found associated with VEGFR-2 and is necessary to prevent its oxidation. VEGFR-2 oxidation renders the receptor insensitive to VEGF-A stimulation [158]. Following VEGFR-2 stimulation and tyrosine phosphorylation, the receptor can be dephosphorylated by several phosphatases such as protein tyrosine phosphatases (PTPs) and density-enhanced phosphatase-1(DEP-1)/CD148 which can be inactivated by ROS [159], [160]. Downstream of VEGFR-2, several signaling nodes have also been shown to be ROS sensitive such as PI3K/AKT [161]. Importantly, VEGFR-2 activation can induce ROS production by endothelial cells (EC), and abrogation of VEGFR-2-dependent induction of ROS levels abolishes VEGF-A effects on EC migration and proliferation. It has been amply demonstrated that VEGF-A stimulates ROS production through the activation of NOX enzymes in EC [156]. VEGF-A/VEGFR2-dependent activation of NOX1 has been related to increased angiogenic tube formation of EC [162]. NOX2 knockout mice display impaired neovascularization in hind limb ischemia and their ECs have much reduced VEGF-A induced proliferation and migration [163], [164]. In addition, NOX4 siRNA inhibited VEGF-induced EC migration and proliferation [165]. Upon VEGF-A-stimulated receptor phosphorylation, TSAd-dependent Src activation recruits the scaffold protein IQGAP1 that promotes the recruitment of Rac1 and NOX2 to initiate ROS production [166], [167]. Furthermore, inhibition of mitochondrial ROS production was sufficient to abrogate VEGF-A and EC migration independent of NOX enzymes [168]. One possible mechanism could be inactivation and transcriptional downregulation of PGC-1α following PI3K/AKT activation in response to growth factor stimulation that results in the enhanced production of mitochondrial ROS required to promote endothelial cell migration [169]. Furthermore, several lines of evidence suggest that NOX enzymes can function downstream of mitochondria. Hence, depletion of mtDNA reduces NOX activity, while induction of mitochondrial dysfunction increases mitochondrial ROS production resulting in the activation of NOX enzymes [58]. Enhanced mitochondrial ROS production was sufficient to recapitulate most if not all the features of diabetic retinopathy, and the underlying mechanism involved was associated with ROS-dependent constitutive activation of VEGFR-2, and reduced sensitivity to VEGF-A stimulation. Importantly, both a mitochondrial targeted antioxidant and a general NOX inhibitor effectively normalized VEGF-A signaling and endothelial tube formation, indicating that both NOX and mitochondrial ROS are functionally co-regulated in this context [170], [171].
Other factors that have been identified as potent inducers of ROS belong to the TGF-β family. These cytokines play essential roles in the maintenance of tissue homeostasis, regulation of cell growth, migration and invasion, extracellular matrix remodeling and immune suppression [172]. As Isabel Fabregat points out, deregulation of TGF-β signaling is frequently observed in disease states, such as fibrosis, inflammation and cancer, being considered responsible for part of the sequence of events leading to the end-stage of these diseases [173]. Initial studies in the middle of the 1990s indicated that TGF-β mediates ROS production [174], [175] and a NOX activity was suggested to be responsible for the release of H2O2 [174], [176]. Nowadays, NOXs are considered as relevant mediators of TGF-β actions in different cells, contributing to the regulation of growth, death and activation of myofibroblasts, key executers of the fibrotic process. Although different NOXs have been proposed to be modulated by TGF-β, NOX1 and NOX4 appear to be the NOXs most frequently involved in its actions. Under physiological circumstances, NOX4 is transcriptionally upregulated by TGF-β in a Smad-3-dependent manner and is considered a mediator of TGF-β-mediated suppressor effects. In particular, NOX4 is required for TGF-β-induced apoptosis in epithelial cells [177], [178]. In contrast, NOX1, activated by TGF-β in a caveolin-1-dependent manner, plays opposite roles, stimulating anti-apoptotic signals and preventing cell death [179]. Deciphering the specific roles of NOX1 and NOX4 in TGF-β actions during tumorigenesis will require further investigations, but current data indicate that overactivation of growth factor signals favor activation of NOX1 and inhibit NOX4 up-regulation, which would prevent cell death [180]. Once cells overcome apoptosis, NOX4 might promote pro-tumorigenic actions of TGF-β, such as epithelial-mesenchymal transitions (EMT), that induce cell migration and invasion [181]. Strong evidence supports a role of NOX4 in mediating TGF-β-induced myofibroblast activation in different models of fibrosis [182], [183], as well as in the maintenance of the phenotype of myofibroblasts [184]. NOX4 also mediates TGF-β-induced apoptosis in epithelial cells [178], [184], which contributes to the inflammatory process that concurs with increased activation of myofibroblasts and extracellular matrix deposits. These studies provide proof of concept for therapeutic targeting of NOX4 to inhibit TGF-β-induced fibrogenesis. However, further research is required to validate the safety of these inhibitors, at least in those tissues where TGF-β acts as a tumor suppressor factor. In this sense, recent results indicate that knockdown of NOX4 increases proliferation and tumorigenic properties of liver tumor cells [185].
3.3. Redox regulation of fundamental cellular processes such as cell death
In a more general way, the dual role of ROS has been associated with different cancer types. As Guia Carrara and Geoffrey L. Smith explain, elevated levels of ROS have been a well established property of most cancers where they contribute to many aspects of tumor development and progression, including cell proliferation, genomic instability, resistance to apoptosis, cell adhesion and motility, and a metabolic shift to glycolysis [186], [187]. On the other hand, excess ROS is deleterious to the survival and proliferation of cancer cells. Hence, endogenous antioxidants are also upregulated to detoxify the cell and maintain a delicate balance of elevated intracellular ROS that is beneficial to malignant cells [186], [187]. Recently, the Transmembrane Bax Inhibitor-1 Motif-containing (TMBIM) protein family has received increasing attention in relation to its role in cancer, which is supported, for instance, by dysregulation of expression being associated with many cancer types and by the characterization of its multiple functions that constitute important hallmarks of malignancy [188]. The Golgi anti-apoptotic protein (GAAP) is a member of the TMBIM family and is projected by phylogenetic analyses to have originated before the divergence of plants and protozoa about 2000 million years ago [189]. Orthologues of the human GAAP are remarkably conserved at the protein level (e.g. amino acid sequence, length and hydrophobicity profile) throughout eukaryotes, prokaryotes and some poxviruses, in agreement with a highly conserved ancestral structure and function [190], [191], [192]. Since the first discovery of this gene in 2002 in camelpox virus [193], several cellular functions and structural properties of GAAPs from various origins have been described. Within eukaryotes, GAAPs regulate Ca2+ levels and fluxes from the principal intracellular stores (Golgi and ER), confer resistance to a broad range of apoptotic stimuli and promote cell adhesion and migration via the activation of store-operated Ca2+ entry (SOCE) [193], [194], [195]. Importantly, these multi-transmembrane proteins were shown recently to form cation-selective ion channels, potentially forming the basis for the modulation of the diverse functions of GAAPs [191]. In view of these functions as important hallmarks of cancer, the effects of human GAAP on ROS homeostasis in the context of cancerous cells were investigated. Significantly greater overall basal ROS levels, and more specifically basal H2O2, were detected intracellularly in cells over-expressing GAAP. In addition, cells over-expressing GAAP displayed greater invasive capabilities in tissue culture, which was confirmed by the opposite effect upon GAAP knock down by siRNA. Furthermore, the activity of secreted matrix metalloproteinases 2 and 9, which are sensitive to regulation by ROS and play a key role in migration and invasion, was dysregulated in these cells. Although both the mechanistic links between these observations and the contribution of Ca2+ remain to be established, ROS appear to be a common factor of importance and relevance in understanding the contribution of GAAP in cancer development. Furthermore, the diverse multifunctional properties of GAAP provide a useful common starting point from which the complex interplay between ROS homeostasis with other important hallmarks of cancer such as cell invasion, migration, and resistance to apoptosis can be studied.
Other cellular structures with a putative relation to ROS are centrosomes. Centrosomes are the microtubule organizing centers (MTOCs) that nucleate and organize microtubules. They have critical roles in various processes, including cell division and polarity. Over 100 years ago, it was claimed that centrosome aberrations may lead to genomic instability and consequently to cancer [196]. As Lokman Varisli and Serap Ilikay summarize, it was reported that genetic manipulations that lead to centrosome amplification can cause tumor development [197]. Consistently, centrosome abnormalities have been observed in many human cancers and in premalignant lesions [198]. Increased centrosome numbers can arise from various mechanisms, such as centrosome overduplication, cell fusions or failures during cytokinesis [199]. However, the causes and mechanisms leading to these effects are not fully understood. In recent years, it was suggested that ROS may be involved in the regulation of centrosome organization. Indeed, various researchers reported that oxidative stress may lead to increases in the number of centrosomes. However, there are contradicting reports in this area. While some researchers reported that oxidative stress can trigger hyperamplification of centrosomes and consequently may promote progression of cancer [200], others suggested that centrosomal abnormalities may contribute to the entry of the cells into senescence, thus preventing proliferation of damaged cells. Consequently, centrosome abnormalities are a part of the defense mechanism that inhibits carcinogenesis [201], [202]. On the other hand, it is known that centrosomes are shielded from oxidative stress through their association with peroxiredoxin I (PRX1) during interphase, while this enzyme is inhibited by cyclin-dependent kinase 1 (Cdk1) in mitosis [203]. Moreover, it was reported that the local concentration of H2O2 around centrosomes is involved in the regulation of centrosomal levels of some cell/centrosome cycle related proteins and also in the regulation of mitotic entry [203]. In concordance, it was shown that reduction of peri-centrosomal H2O2 by centrosome-targeted catalase inhibits entry of cells into mitosis [203]. Consistently, treatment of mitotic cells with H2O2 causes mitotic slippage and consequently formation of hypertetraploid cells [204]. Although the mechanisms of H2O2 induced mitotic slippage have not been entirely elucidated, this effect is probably related to exposure of naked centrosomes to H2O2 without a Prx1 (and probably other antioxidants) shield during mitosis.
3.4. Redox regulation involving mitochondria
In mitochondria, the thiol redox conditions are essentially controlled by glutathione and thioredoxin systems. In the latter, a reducing sequence starting with NADPH allows the transfer of electrons to thioredoxin in a process mediated by the mitochondrial selenoenzymes thioredoxin reductases (TrxR2). NADPH is maintained in a reduced form by specific dehydrogenases and by the membrane-bound transhydrogenase. Alberto Bindoli and Maria Pia Rigobello discuss, that TrxR2 is able to reduce, in addition to its specific substrate thioredoxin (Trx2), a large number of different molecules. Thioredoxin is a key component of the thioredoxin system acting as a wide-ranging protein-disulfide reductase and therefore controlling the redox state of different factors [205]. In particular, Trx2 reduces Prx3, which therefore controls the levels of H2O2. The mitochondrial isoform of cyclophilin (CypD) plays a relevant role in regulating the mitochondrial permeability transition pore [206] and is endowed with redox properties due to the presence of specific cysteine residues [207]. In isolated rat heart mitochondria, the inhibition of TrxR2 with the gold compound auranofin leads to a concomitant oxidation of Trx2, Prx3 and CypD as demonstrated by the redox Western blot technique [208]. Similarly, CEM-R cancer cells incubated with auranofin or other inhibitors of thioredoxin reductase such as ATO (arsenic trioxide) and CNDB (1-chloro-2,4-dinitrobenzene) show also a concurrent oxidation of Trx2, Prx3 and CypD. Both in mitochondria and cancer cells, the addition of H2O2 leads to an oxidation pattern similar to that observed after treatment with inhibitors of TrxR2 [208]. In addition, CypD co-immunoprecipitates with both Trx2 and Prx3 [208] indicating a potential cooperation involving these proteins. These results indicate that CypD can act as a redox protein able to modulate the mitochondrial functions such as membrane permeability and that the redox conditions of CypD may be controlled by the thioredoxin system. Of note, Prx3 can act as a sensor of hydrogen peroxide and transduce this oxidation to CypD. This view is further supported by a molecular modeling approach showing a potential interaction of CypD both with Trx2 and Prx3 [208].
As Carlos M. Palmeira and Anabela P. Rolo point out, mitochondria play also an essential role in energy production and cellular homeostasis. Their highly dynamic nature, based on alterations in biogenesis, mitophagy, fusion and fission, allows adjustment of the sequential oxidoreductive reactions in the electron transport chain (ETC) and dissipation of the membrane potential by ATP synthase in response to different environmental cues [209]. Such adaptive processes may involve signaling by ROS and explain how mild levels of mitochondrial-derived ROS trigger a hormetic response resulting in extended lifespan. As ROS are an inevitable by-product of oxidative phosphorylation, alterations in the mitochondrial oxidative rate with a consequent excessive load of ROS, have been traditionally associated with pathological processes such as cancer, diabetes and neurodegeneration. Although in mammals the exact signal released by mitochondria that triggers a hormetic response is still uncertain, more and more studies are addressing ROS as promoters of mitohormesis, as opposed to their pro-aging action due to persistently induced oxidative damage. The concept of mitohormesis proposes that a mild increase in mitochondrial ROS may act as a sublethal trigger of cytoprotective long-lasting metabolic and biochemical changes against larger subsequent stresses [210]. Caloric restriction (CR) has been repeatedly shown to decrease risk- actors for major age-related diseases and to increase lifespan in various organisms. Early on, work in C. elegans [211] has shown that reduced glucose availability was linked to an increase in both ROS and catalase activity, ultimately culminating in increased survival rates. Several other studies have further demonstrated that many strategies promoting longevity share a common downstream aspect, that is: increased mitochondrial ROS. Inhibition of the mitochondrial ETC by certain mutations or inactivation of mitochondrial superoxide dismutase increases C. elegans lifespan, as reviewed by Dancy et al. [212]. Low doses of rotenone, an inhibitor of complex I, have also been shown to extend the lifespan of C. elegans [212] as well as to induce hormesis in primary human fibroblasts, an effect not possible in older cells or with higher concentrations of rotenone [213]. Inhibition of mTORC signaling and the consequent induction of autophagy by caloric restriction or by pharmacological agents has also been found to promote longevity in yeast, worms, flies and mice, as recently reviewed [214]. Further, it has been shown that hearts with impaired mitophagy and consequent accumulation of damaged ROS-forming mitochondria develop cardiomyopathy, which can be surprisingly improved by the ROS-dependent activation of compensatory autophagic pathways of mitochondrial quality control, preventing a vicious cycle of ROS formation and mitochondrial dysfunction [215].
3.5. Role of ROS in hypoxia
When oxygen availability is reduced (hypoxia), eukaryotic cells sense this reduction and trigger a series of cellular and systemic responses that facilitate adaptation to hypoxia, including the optimization of oxygen consumption. As Pablo Hernansanz-Agustín and Antonio Martínez-Ruiz explain, some of these responses are mediated through transcriptional regulation by the stabilization of hypoxia-inducible factors (HIFs); however, this mechanism requires at least several hours for activation, and there is crosstalk with ROS signaling (for a recent review, see [216]). Several acute responses operate in minutes in specialized organs in which local temporal changes in the redox state have been implied. One example is the carotid body, which senses variations in blood oxygen to activate the respiratory center. The search for the molecular mechanisms of oxygen sensing in carotid body cells has recently led Fernández-Agüera et al. to propose a fundamental role for mitochondrial complex I in the production of a ROS signal in response to hypoxia [217]. For a long time there has been a debate on whether hypoxia increases or decreases ROS production, with apparently contradictory reports in the literature. This controversy might arise from the ROS source studied, from the cell type, tissue or organism examined, from the techniques used to measure different ROS, and/or from the duration of hypoxia applied in each study. It has recently been shown that several cell types respond to acute hypoxia with a transient increase in superoxide production at the beginning of hypoxia, which has been called a superoxide burst in acute hypoxia [218]. This may explain in part the apparently divergent results found by different groups that have not taken into account the time frame of acute hypoxic ROS production. Molecular mechanisms in acute hypoxia might involve mitochondrial complex I and the mitochondrial sodium/calcium exchanger (NCLX) (Hernansanz-Agustín et al., manuscript under evaluation). Superoxide production in acute hypoxia seems to be a common mechanism for different cell types, but it would elicit different responses in specialized cells and tissues where the adequate components for signal transduction may be present such as cysteine residues sensitive to reversible oxidation. Examples of this are the carotid body cells where localized ROS production inhibits K+ channels [217], [219]; or the pulmonary arteries where mitochondrial ROS production may elicit a signal cascade including ceramide production, further ROS production by NADPH oxidases and alterations in ion channel activity [220]. In endothelial cells, by using specialized thiol redox proteomics methods, the reversible oxidation of a range of protein cysteine residues has been observed that could mediate acute responses to hypoxia in these cells [221]. Another molecular example of a hypoxia signal transducer is the Na+,K+ ATPase, with cysteine residues that are sensitive to variations in the oxygen concentration, and that alter the function of the protein (recently reviewed in [222]). In conclusion, ROS production is being increasingly considered as a key signaling event in acute hypoxia, and the molecular mechanisms and functional consequences of this event are currently an active field of research, with implications for molecular physiology and pathology.
An enzyme family that requires oxygen for function, and is thus related to oxygen availability, are •NO synthases. As Damir Kračun and Agnes Görlach discuss, all three family members, endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS), catalyze the reaction of •NO production by binding a number of cofactors such as flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme, 5,6,7,8-tetrahydrobiopterin (BH4) and calmodulin to convert L-arginine and O2 to L-citrulline and •NO.
The flow of electrons within NOS is tightly regulated. If disturbed, the chemical reduction of oxygen and the generation of •NO are uncoupled and O2•- is generated from the oxygenase domain. In particular, failure of adequate provision of the cofactor BH4 shifts electrons to molecular oxygen rather than to L-arginine, thus transforming NOS into a pro-oxidant superoxide anion-generating enzyme [223].
Conditions of low oxygen availability have differential effects on •NO levels: decreased, increased and unchanged levels were reported. In some cases, the differences might have been due to different detection methods. Moreover, in several cases, NOS expression levels did not match the •NO levels measured. While several reports showed that NOS expression increased under hypoxia, there was no concomitant increase in •NO levels [224]; recent data indicate that hypoxia leads to uncoupling of NOS, which might explain this observation [225]. Mechanistically, a decrease in BH4 levels observed under hypoxia in vitro and in vivo has been related to this effect. BH4 levels in the endothelium are controlled through de novo BH4 synthesis by GTP cyclohydrolase I (GTPCH-1), loss of BH4 by oxidation to 7,8-dihydrobiopterin (BH2), and regeneration of BH4 from BH2 by dihydrofolate reductase (DHFR) [226]. While GTPCH-1 downregulation under hypoxia was not clearly demonstrated, recent data show that hypoxia can decrease the levels of DHFR, thus diminishing the capacity to recycle BH2 to BH4 [225]. In support of this finding, treatment with folic acid (FA), which has been shown to upregulate DHFR levels under normoxic conditions [227], restored DHFR, BH4, and •NO levels in hypoxic cells [225]. Decreased levels of BH4 and DHFR together with diminished •NO bioavailability were also observed in vivo in mice suffering from chronic hypoxia-induced pulmonary hypertension. While treatment with the biopterin precursor sepiapterin, which needs to be converted to BH4 by DHFR, only marginally affected this disease [228], treatment with FA not only enhanced •NO and BH4 bioavailability but also diminished pulmonary hypertension [225]. Since a decline in DHFR has also been reported to promote angiotensin-II induced hypertension [227], preservation of DHFR levels appears to be an important mechanism to combat uncoupling of NOS and its associated pathologies.
3.6. Redox regulation of gene expression via modulation of transcription factors and epigenetic pathways
Conditions of low oxygen are also favorable for stem cell maintenance. As Holger Steinbrenner and Lars-Oliver Klotz point out, adult mammals harbor multipotent somatic stem cells that are capable of replenishing terminally differentiated functional cells for maintenance and regeneration of tissues as well as for adaptation to metabolic demands. Examples include hematopoietic stem cells (HSCs) located in bone marrow, epithelial stem cells in intestinal crypts, and mesenchymal stem cells in adipose tissue and bone marrow. Somatic stem cells in adults are often quiescent but they may temporarily re-enter the cell cycle and proliferate rapidly in response to the appropriate signals. In recent years, it has become increasingly evident that even slight variations in intra- and extracellular levels of ROS may exert a profound impact on the fate and function of stem cells [229], [230]. ROS serve as signaling molecules that affect the balance between self-renewal and differentiation of stem cells, their commitment, differentiation and maturation to specialized cell types, and their survival and aging. ROS involved in these processes, such as superoxide and hydrogen peroxide (H2O2), derive for the most part from the mitochondrial respiratory chain and from membrane-bound NADPH oxidases [229], [230]. Transcription factors of the forkhead box, class O (FoxO) family participate in regulating and fine-tuning ROS-modulated cellular differentiation processes, as illustrated by the following examples. FoxOs stimulate the biosynthesis of ROS-reducing proteins located in mitochondria [superoxide dismutase 2 (SOD2), peroxiredoxins 3 and 5], in peroxisomes (catalase), and extracellularly [selenoprotein P (SelP)] [231]. Elevated circulating levels of SelP may then result, by means of its function as a plasma selenium transporter, in increased biosynthesis and cellular activity of antioxidant and redox state-modulating selenoenzymes such as glutathione peroxidases and thioredoxin reductases [231]. Thus, FoxOs are crucial for cellular stress resistance and longevity. Tightly controlled increases in FoxO expression and activity may balance ROS generation in differentiating cells through an adaptive up-regulation of antioxidant enzymes, which has been well documented for adipogenesis; committed mesenchymal stem cells (preadipocytes) of the subcutaneous adipose tissue may undergo clonal expansion in response to high nutrient intake. This is followed by growth arrest and terminal differentiation into mature lipid-accumulating adipocytes. Adipogenic differentiation of human adipose tissue-derived stem cells has been shown to be associated with elevated ROS generation, and it was stimulated both by exogenous application of H2O2 and by overexpression of Nox4. During adipogenesis, expression of FoxO1 and FoxO3 genes as well as the FOXO target genes coding for catalase and SOD2 were upregulated; silencing of FoxO1 suppressed adipogenesis [232]. The continuous renewal of the intestinal epithelium represents another example that illustrates the importance of ROS for differentiation processes: stem cells located at the bottom of intestinal crypts are capable of proliferating and differentiating into absorptive and secretory cell types. The switch between cell proliferation and differentiation/growth arrest is typically associated with changes in the intracellular glutathione (GSH/GSSG) and in the extracellular cysteine/cystine redox potentials in the gut: proliferation is fostered in a reducing redox environment, whereas a modest shift towards the oxidation side of the two redox couples favors differentiation. A highly oxidized glutathione redox status, however, associates with cell death [233]. Human intestinal epithelial Caco-2 cells, a commonly used in vitro model for intestinal differentiation, stop proliferation and undergo differentiation into enterocytes/colonocytes following confluency. Differentiation of Caco-2 cells is associated with induction of FoxO1 and SelP [234], an antioxidant protein that was identified as a FoxO1 target gene [235]. Thus, FoxO1-mediated up-regulation of antioxidant enzymes might limit the severity and duration of the redox shift during enterocyte differentiation.
Stem cell differentiation has been also associated with epigenetic alterations. As Alina Hanf and Andreas Daiber summarize, there is a growing body of evidence that ROS/RNS contribute to the regulation of gene expression and genome stability not only by modulation of transcription factors, mRNA stability and DNA damage/repair, but also influence epigenetic pathways by affecting the function or expression of histone and DNA modifying enzymes (Fig. 3.2) [236], [237], [238]. For instance, it was shown that oxidative stress alters global histone methylation by attenuating Fe(II)- and α-ketoglutarate-dependent histone demethylase activity of Jumonji C (JmjC) domain-containing enzymes and TET DNA hydroxylases [237], [238]. Furthermore, it is well established that levels of HDAC2 are decreased due to inflammation-induced ROS/RNS production in chronic obstructive pulmonary disease (COPD) [239]. Another example is the generation of reactive halogen compounds, such as 5-chlorocytosine, by ROS/RNS, leading to inaccurate methylation by DNA methyltransferase (DNMT) [240]. Adverse redox regulation of epigenetic processes may ultimately result in pathological consequences such as dysfunctional energy metabolism or increased oxidative stress that are associated with cardiovascular pathologies. For instance, hydrogen peroxide (H2O2) alters the binding of DNA methyl-transferases (DNMTs) to chromatin leading to hypomethylation in atherosclerosis [241], [242]. Furthermore, epigenetic silencing of superoxide dismutase 2 (SOD2) by selective hypermethylation of CpG islands in the SOD2 gene is associated with pulmonary arterial hypertension [242], [243], and oxidation of HDAC4 by NOX4-mediated ROS production was observed in cardiac hypertrophy [244]. There is experimental evidence that cardiomyocytes of failing hearts undergo significant changes in epigenetic profiles of H3K4 and H3K9 methylation marks that go hand in hand with ROS-induced changes in histone demethylase activity [242], [245], [246]. This has also been observed in humans with heart failure [247]. Not only histone modifying enzymes but also histone proteins themselves can be directly affected by oxidizing and nitrating agents since exposure to peroxynitrite induces dityrosine formation in histone H2A and H2B leading to structural changes by intra-molecular cross-linking [248], [249]. Dityrosine bridge formation represents an irreversible oxidative modification and it may be speculated that histones carrying this modification account for the persistent changes in gene expression observed in various disease states. In conclusion, epigenetic regulation by ROS/RNS in the setting of various oxidative stress-associated disorders may become an attractive target for redox medicine in the near future.
Fig. 3.2.
(A) Reactive oxygen species can display their regulatory effect on the classical gene regulatory machinery and on epigenetic processes. One of the prominent pathways attributed to oxidative stress is thiol oxidation, which is involved in OxyR, NF-κB and KEAP1 signaling. Oxygen sensing prolyl hydroxylases represent another class of redox-dependent enzymes. For example, epigenetic involvement of ROS has been attributed to oxidative conversion of 5-mC to 5-hmC. (B) ROS impact on mRNA stability at the cytosolic level. Reactive oxygen species are involved in GAPDH signaling by directly altering its structure with the help of GSH or S-nitrosoglutathione (GSNO), and thus activate translation of endothelin-1 (ET-1) mRNA (i). AP-1 thiol redox regulation directly affects the gene regulating factor HuR by stability of its target mRNAs (ii). ROS have been implicated in the regulation of miRNA pathways, altering mRNA stability and their transport inside the cytosol. HRE means hormone response element. With permission of Elsevier and the authors. Copyright 2015.
Adapted from [238].
In addition to histone- and DNA-modifying enzymes, microRNAs (miRNAs) have an important impact on the epigenetic landscape. They comprise a large family of conserved, small (19–25 nucleotides (nt) long), non-coding RNAs that regulate gene expression at the post-transcriptional level. miRNAs bind to the 3′-untranslated region (3′-UTR), coding sequences or 5′-untranslated region (5′-UTR) of target messenger RNAs, thus leading to the inhibition of translation or mRNA degradation [250], [251]. As Verónica Miguel and Santiago Lamas explain, it has recently been found that miRNAs are able to “fine-tune” the regulation of redox signaling. This may occur by direct interaction with NRF2, the major transcriptional regulator in the defense against ROS [252], [253], or its co-regulators Kelch-like ECH-associated protein 1 (KEAP1) and CNC homolog 1 (Bach1), or by controlling the generation of ROS [254]. This new subset of miRNAs, that either regulate redox pathways or are themselves regulated by the cellular redox state, has been termed “redoximiRs” [255]. As pointed out above, fibrosis is an aberrant repair process that results from chronic inflammation and leads to excessive extracellular matrix (ECM) deposition that ultimately impairs organ function [256]. Myofibroblasts are considered the quintessential cell type responsible for ECM accumulation [257]. Fibrosis of major organs including liver, lung, heart, skin and kidney share common molecular mechanisms regarding the genesis of the fibrotic process such as the essential role of the TGF-β pathway [258]. However, major differences exist regarding the cellular origin of myofibroblasts and the role of epithelial-mesenchymal transitions (EMT) in each particular organ [259]. The lack of effective and specific therapeutic alternatives for fibrosis prevention or cure remains a fundamental clinical challenge in all organs. In the last decade, miRNAs have been shown to be modulators of pro- and anti-fibrotic processes in human diseases and this subset of miRNAs are called “fibromiRs” [260], [261]. Aberrant expression of fibromiRs drives the initiation and progression of the fibrotic process in response to persistent tissue injury. Moreover, TGF-β signaling through Smad proteins can regulate the transcription of miRNAs by DNA binding or miRNA maturation by associating with the Drosha/DGCR8 complex [262], suggesting a possible link between miRNAs and fibrogenesis. The overlap between the sets of fibromiRs and redoximiRs is now becoming evident and is of potential importance for both mechanistic and translational purposes [263]. Currently several miRNAs pertaining to this intersection have been described and are the objects of intense research, including miR-21 [264], [265], [266], miR-29 [267], [268], miR-9 [269], [270], miR-199 [271] and miR-433 [272]. Of interest, some of these miRNAs, such as miR-21, may regulate metabolic pathways related to fatty acid oxidation, a process that has been proven crucial for the pathogenesis and perpetuation of fibrosis in the kidney [273], [274], [275]. Overall, the crosstalk regulation between redox balance and miRNA function appears to be an exciting avenue of research, which may lead to a more profound comprehension of fibrosis and eventually to provide effective therapeutic alternatives.
3.7. Redox signaling via RONS-derived electrophiles such as oxidized lipids
Fibrosis and inflammatory diseases have been also associated with lipid oxidation. As Catarina B. Afonso and Corinne M. Spickett discuss, oxidized phospholipids (OxPLs) have a known role in several inflammatory diseases such as atherosclerosis and diabetes, but also in neurodegenerative diseases including Alzheimer's and Parkinson's disease. Although they mostly have a pro-inflammatory role, as reviewed recently [276], [277], their capacity as anti-inflammatory agents has also been described. The anti-inflammatory activity of OxPLs has been shown to involve several pathways (reviewed in [278]). The mechanisms by which they act include the inhibition of nitric oxide synthesis in macrophages; activation of peroxisome proliferator activated receptors (PPARs), leading to inhibition of major inflammatory pathways (such as the NF-κB and AP-1 signaling pathways); attenuation of dendritic cell activation and maturation, and inhibition of T-cell proliferation and cytotoxicity, which are important in the adaptive immune system. One of the most studied anti-inflammatory mechanisms by which OxPLs act, however, involves the regulation of Toll-like Receptors (TLRs). These receptors are responsible for innate immune responses by recognizing pathogen-associated molecular patterns (PAMPs). The presence of oxidized phospholipids appears to selectively inhibit some proteins of this family (such as TLR2 and TLR4), possibly through binding to assessory proteins important for their activity [278]. However, much research has been performed using specific oxidized phospholipids, and the different phospholipid species could alter the response observed [279]. Also, their anti-inflammatory activity is known to depend on several factors. For example, low concentrations (<25 µM) of the oxPL are thought to be anti-inflammatory, whereas higher concentrations (>50 µM) tend to be pro-inflammatory and cytotoxic [276]. The effects of cell type [280] and the presence of endotoxin [281] have also been reported. The potential of OxPLs for the diagnosis and treatment of inflammatory diseases is evident, but further research is necessary to characterize the relationships fully.
ROS, RNS and lipid peroxidation (LPO) products are not only crucial in regulating cellular signaling processes under physiological and pathophysiological conditions, they are also crucial in the cellular response to materials, acting in turn as chemo-attractants, signaling molecules and agents of degradation [282]. As Pierre-Alexis Mouthuy and Neven Žarković point out, such interactions between cells, materials (or ‘scaffolds’), and bioactive molecules can be observed in tissue engineering in order to improve or replace biological tissues in regenerative medicine. In this context, modulation of ROS may have the potential to improve the quality of the engineered constructs. So far, approaches in tissue engineering that target ROS in order to improve cell-material interactions have focused on antioxidant therapies to minimize oxidative stress in cells. However, it is challenging to provide antioxidant concentrations that are physiologically relevant and respond to variations of oxidative stress levels, which may occur during new tissue formation. Thus, recent strategies aim to develop polymeric scaffolds that undergo oxidative degradation and/or release bioactive molecules such as antioxidants in response to the oxidant concentrations [283], [284], [285]. On the other hand, considering the multiple roles that oxidants play during the response to biomaterials, pro-oxidant therapies are also becoming attractive for tissue engineering applications. The LPO products, like 4-hydroxynonenal (HNE) known as a "second messenger of free radicals", are particularly interesting candidates to be supplemented as bioactive molecules. Namely, despite being biomarkers of pathological oxidative stress, LPO products have been shown to stimulate cell proliferation and matrix synthesis [286], [287]. Compared to ROS and RNS, the LPO products have the advantage of forming rather stable protein adducts. Moreover, their hydrophobicity suggests that their incorporation into a hydrophobic polymer matrix (such as biodegradable polyesters) could lead to a slow and prolonged release, which is desirable for tissue engineering applications. Overall, the existing evidence supports the idea of providing LPO products during tissue engineering in order to stimulate cell infiltration and new tissue formation. Such a novel approach has been proposed as a supplement for a bone regeneration strategy, which is currently being tested using HNE.
Moreover, proteins can be modified by covalent reactions on the nucleophilic residues cysteine, histidine, arginine and lysine, leading to the formation of a wide variety of adducts with oxidized products of lipids. As Bebiana C. Sousa and Corinne M. Spickett explain, even though protein lipoxidation can occur under basal conditions, it is increased and thought to be relevant in pathophysiological conditions [288]. In order to understand their impact on a complex matrix like cells, tissues or body fluids, potentially important protein-lipid adducts are often generated in vitro under controlled conditions [289]. This approach has the advantage of potentially decoding the reactivity of each specific electrophilic lipid species and specific fragmentation patterns of lipoxidation adducts, allowing a targeted analysis in complex biological samples. On the other hand, this strategy also presents some disadvantages, as previously reported by other authors [290], [291]. Mass spectrometry (MS)-based analytical approaches are one of the most popular methodologies to study biological systems and are used extensively in the study of protein-lipid adducts. The adducts are detected by MS analysis, often involving liquid-chromatography followed by top-down or bottom-up proteomic strategies. Because the top-down approach requires expensive mass analyzers with high mass accuracy and resolution, the bottom-up approach is the most commonly used. However, it also involves disadvantages; for example, when the aldehydes react with lysine and arginine, this leads to additional difficulties since these modifications may interfere with trypsin digestion, which is typically used in proteomics. Furthermore, stabilization of adducts is required prior to the enzymatic digestion and MS analysis due to their labile nature. Certain MS/MS conditions can cleave labile post-translational modifications during peptide fragmentation, resulting in the neutral loss of the modification. For these reasons, it is advantageous to use enrichment procedures and chemical labeling in order to improve sensitivity and selectivity. Western blot immunoassay using specific or non-specific antibodies can also be performed, followed by in-gel digestion and MS analysis for protein identification. However, the cross-reactivity of some antibodies with different lipids is a major disadvantage. The development of new MS approaches could provide tools to identify and quantify lipid-protein adducts and understand their effects upon oxidative stress and related pathophysiological conditions [292].
3.8. Interaction of the gasotransmitter H2S with ROS and RNS
In recent years, H2S biology has attracted a lot of attention by unraveling multiple physiological roles for this gasotransmitter, linking it to disease conditions and offering novel translational opportunities, as summarized by Andreas Papapetropoulos (Chair of the COST Action BM1005 (ENOG)). Hydrogen sulfide is generated in mammalian cells by three distinct enzymes: cystathionine-β synthase (CBS), cystathionine-γ lyase (CSE) and 3-mercaptopyruvate sulfotransferase (3MST) [293], [294]. Although the exact levels of H2S in cells remain unknown, they are most likely in the nM range. In addition to free H2S, acid-labile and protein-bound sulfane sulfur pools exist [295]. H2S is a weak acid and readily ionizes at physiological pH, with 70% of H2S existing in the anionic sulfide (HS-) form [293]. H2S is a weaker reductant than cysteine and glutathione. This, in addition to its much lower concentration compared to glutathione and protein thiols, argues against a role for H2S as a “professional” direct reducing agent in a cellular context. H2S most likely acts as a signaling molecule boosting endogenous antioxidant mechanisms.
Low levels of H2S are neuroprotective, cardioprotective, anti-apoptotic and anti-inflammatory [293]. Many of its biological actions have been attributed to its antioxidant properties [293], [296]. H2S impacts on the cellular redox state as it reacts with ROS, inhibits ROS production, activates antioxidant enzymes and upregulates the expression of antioxidant genes. H2S reacts with ROS/RNS including HOCl, O2•─, H2O2 and ONOO─ [297]. With the exception of HOCl, the reaction rate constants of H2S with the above-mentioned oxidants are low, suggesting that they are most likely of little biological significance [295]. The reaction of H2S with free radicals can give rise to a number of novel species. Of particular interest is the reaction between H2S and •NO, which generates S/N hybrid species and HNO• [298].
NRF2 is a transcription factor that acts as a major regulator of the cellular defense response to oxidative stress since it is the master transcription factor regulating antioxidant genes [299], [300]. NRF2 is found in a complex with Kelch-like ECH-associated protein 1 (KEAP1) in the cytosol that facilitates its degradation through the proteasome pathway. Following exposure to oxidative stress, KEAP1 dissociates from NRF2 allowing it to escape from ubiquitination and enabling translocation to the nucleus where it binds to antioxidant response elements in the promoter region of several genes. Treatment of cells or animals with H2S donors causes persulfidation of KEAP-1 on cysteine-151, which in turn causes a conformational change in KEAP1 allowing it to dissociate from NRF2 [301]. In addition to NRF2 activation, H2S donor administration up regulates NRF2 expression [294].
Treatment with H2S has been shown to be protective against a variety of oxidative stress-related injuries and ROS-induced toxicity [296]. From a mechanistic point of view the protective effects of H2S, in addition to NRF2, have been linked to increased activity of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, inhibition of superoxide generation and lipid peroxidation and reduction of NOX expression [296], [301]. Moreover, treatment with H2S donors increases the cellular concentration of reduced glutathione by promoting cysteine and cystine uptake and by upregulating the level of the GSH biosynthetic enzyme γ-glutamylcysteine synthase and GSH reductase; H2S also increases thioredoxin-1 gene expression [296].
4. Redox biomarkers
Paul Winyard (E-mail: p.g.winyard@exeter.ac.uk) and Pietro Ghezzi (E-Mail: P.Ghezzi@bsms.ac.uk).
4.1. Introduction
This section will deal with selected redox biomarkers. As the causative molecules of oxidative damage are ROS, one might expect most studies would be performed by measuring ROS, such as the superoxide radical or the hydroxyl radical, (among others) in patients’ fluids, just as inflammation researchers measure the level of inflammatory cytokines in disease. However, most ROS are extremely unstable, with half-lives of 10−6 −10−9 s. Also “more long-lived” ROS, such as hydrogen peroxide, have a half-life of less than a millisecond [302]. Therefore, it is difficult to measure them in biological fluids. ROS can be measured during their production in vitro using chemicals (e.g. “spin traps” and “fluorescent probes”) to detect them while they are produced, but they cannot be measured in stored plasma or stored culture supernatants. Therefore, we do what is done in high energy physics. Not being able to detect subatomic particles, we can only have a clue of their presence by the traces they leave. The main feature of ROS is their high reactivity, hence the name, and measurement of oxidized macromolecules that can arise by interaction with endogenous ROS can give us an estimate of the production of ROS (Fig. 4.1) [6]. Likewise, upregulation of ROS enzymatic sources or so-called toxifiers (e.g. myeloperoxidase) can also work as biomarkers of oxidative stress.
Fig. 4.1.
Redox pathways associated with putative biomarkers of oxidative stress. The processes that lead to oxidative modifications of proteins, lipids, and nucleotides are highly complex. Enzymes, such as XO, NOX, and NOS, can produce ROS and RNS. These ROS can furthermore serve as substrates for other enzymes to generate additional types of ROS, such as the generation of HOCl from H2O2 by MPO. Cellular systems and enzymes, including the GSH and thioredoxin system, together with peroxiredoxins (T/Prx), counterbalance the production of ROS. In addition, increased levels of ROS activate NRF2 to transcribe genes that are involved in counteracting these ROS. Oxidative stress affects cGMP signaling through its effects on nitric oxide (•NO) production, scavenging, and on the •NO receptor sGC. cGMP, cyclic guanosine monophosphate; GSH, glutathione; H2O2, hydrogen peroxide; HOCl, hypochlorous acid; MPO, myeloperoxidase; NOS, nitric oxide synthase; NOX, NADPH oxidase; RNS, reactive nitrogen species; ROS, reactive oxygen species; sGC, soluble guanylate cyclase; XO, xanthine oxidase. Adpated from [6]. With permission of Mary Ann Liebert, Inc. Copyright 2015.
Measurement of these oxidized molecules can provide three types of biomarkers. Some can be indicators of the production of ROS. An example is S-thiolated hemoglobin (whose oxidation, to our knowledge, has no pathogenic role). This is similar to the situation with glycated hemoglobin (HbA1c) in diabetes, a good and stable indicator of how much the organism has been exposed to high blood glucose, but which, unlike blood glucose, has limited functional consequences in vivo. Others are, in effect, biomarkers of oxidative stress, that is, they reflect oxidative damage that may have biological consequences. An example of this type is oxidized DNA metabolites, as they may potentially be responsible for oxidative stress-induced mutagenesis and cancer. A third group of biomarkers consists of oxidized products that have an intrinsic biological activity, which may propagate some of the consequences of oxidative stress – such as hydroxynonenal, an aldehyde generated by lipid oxidation [303].
This review will deal with different types of stable redox biomarkers that can be used in biological samples from clinical or population-based studies. The biomarkers have been grouped in the subsections below according to their identity as oxidation products of proteins, lipids, or DNA.
4.2. Protein oxidation products as redox biomarkers
The following section was composed by Serge P. Bottari, Stuart P. Meredith and Corinne M. Spickett. Biological systems are exposed to various endogenous and exogenous oxidants capable of modifying proteins, which, among other processes, may affect cell signaling pathways [304] and contribute to inflammatory diseases [302]. A number of acute and chronic diseases have been reported to cause, be accompanied by, or be due to, "oxidative stress". This stress can be considered as a deregulation of redox signaling resulting in an excess of ROS, which may be responsible for further complications.
Since the major substrate of the primary ROS, O2•-, in terms of kinetics is •NO (the reaction with O2•- has a rate constant which is 3–4 times greater than the rate constant for the dismutation of O2•- catalyzed by SOD), oxidative stress is generally preceded – and accompanied by – an increased generation of RNS, essentially peroxynitrite (ONOO-), which, in the case of excessive concentrations, can cause nitro-oxidative stress [305]. Since RNS can, like ROS, alter proteins, lipids and nucleic acids, RNS may also be involved in pathological mechanisms [306], [307]. Interestingly, however, most posttranslational protein modifications due to RNS appear to often be reversible in vivo. Therefore, nitro-oxidation should be considered as a potential therapeutic target, as the modifications involved are modulated primarily by the redox status, •NO and O2•- fluxes [308], the former being easily manipulable.
The major pathophysiological nitro-oxidative protein modifications elicited by ONOO- are glutathiolation and S-nitrosation of Cys and nitration of Tyr residues. Other modifications are zinc finger oxidation and methionine sulfoxidation. •NO by itself is a major neurotransmitter and an activator of soluble guanylate cyclase. Its reaction with O2•- therefore has dual consequences: primarily the generation of novel messengers (RNS) and a decreased bioavailability of •NO with direct consequences on its targets [305], [307].
Antibody-based methods are among the most common techniques for identifying and quantifying these oxidative post-translational modifications to amino acid residues. The most common techniques (Table 4.1) include ELISA, Western blotting (WB) and immunostaining techniques, such as immunohistochemistry (IHC) and immunocytochemistry (ICC). The accuracy of these techniques relies on the specificity of the available antibodies, but not all antibodies are validated for all procedures [309]. The majority of antibodies against oxidative posttranslational modifications are primarily validated for Western blotting (~75%), with smaller numbers suitable for the other techniques (IHC ~40% and ELISA ~35%), and they are not necessarily interchangeable. Searching on databases such as CiteAb and Biocompare suggests there are more than 500 commercially available antibodies to modified residues (Table 4.1), but on average less than 15% of these have been specifically cited in scientific publications. Some antibodies are more extensively cited and used in different techniques, so selecting the appropriate one is important.
Table 4.1.
Summary of the types of antibodies currently available against oxidatively modified residues.
| Modification | Number of Antibodies | Antibodies Cited | Clonality (mAb: pAb) | Most Common Antigen | Range of Validated Techniques |
|---|---|---|---|---|---|
| 3-Chlorotyrosine | 1 | 1 | 0:1 | 3-Chlorotyrosine (no details) | IA, IHC |
| 3-Nitrotyrosine | 439 | 15 | 129:310 | 3-Nitrotyrosine conjugated to KLH | WB, ELISA, ICC, IF, IHC, IP |
| S-nitrosocysteine | 1 | 0 | 0:1 | Recombinant protein | ELISA, WB |
| Cysteine sulfenic Acid | 3 | 3 | 0:3 | Sulfenic acid-cysteine (2-thiodimedone-specific Ig) | WB |
| Cysteine sulfinic acid | 1 | 0 | 0:1 | Cysteine sulfinic acid adducts | ELISA, IHC |
| Cysteine sulfonate | 3 | 3 | 0:3 | Dimedone-modified cysteine conjugated to KLH | WB |
| 5-Hydroxytryptophan | 17 | 1 | 8:9 | Conjugated to bovine serum albumin via a glutaraldehyde linkage | WB, ELISA, ICC, IHC, IF |
| Methionine sulfoxide | 3 | 2 | 0:2 | Protein with methionine sulfoxide modifications | WB |
| Hydroxyproline | 19 | 3 | 0:19 | Conjugated to bovine serum albumin | ELISA, IHC, ICC, WB |
| Carbonyl | 43 | 41 | 27:16 | Conjugated to bovine serum albumin | ELISA, IHC, ICC, WB |
An important issue is the specificity of the antibodies, which may vary according to the immunogen used to generate them, including the carrier. The most commonly used approach is conjugation of the modified residue to either keyhole limpet hemocyanin (KLH) or bovine serum albumin (BSA), but others were produced by immunizing directly with proteins treated with oxidizing, chlorinating or nitrating agents. A limitation of using modified BSA or other proteins is that it generates antibodies to a variety of epitopes on the antigen that may lead to cross reactivity upon translation, especially as most nitrating and chlorinating agents are also oxidizing, so a wide range of oxidative posttranslational modifications result. Even using synthetic modified residues conjugated to a carrier protein, there are likely to be several antibodies to epitopes that do not include the modification site, so purification is required. Thus the resulting polyclonal sera are semi-specific at best; while monoclonal products are better, some cross-reactivity is still possible. For example, limitations of antibodies and antibody-dependent assays for methionine sulfoxide have been reported [310], [311]. This indicates the importance of understanding the nature of the antibody being used in order to interpret results correctly, but unfortunately, often little information is available from the supplier. It also suggests the benefit of producing better characterized antibodies, possibly with known sequence specificity in addition to modification specificity.
Among the applications of the above antibodies to immunochemical assays, it is of primary interest to be able to detect and quantify nitro-oxidative "stress" in pathophysiological situations before irreversible oxidative cell damage occurs. Among the nitro-oxidative protein modifications, the most stable and therefore easiest one to investigate is Tyr nitration. Indeed, glutathiolation and S-nitrosation, being heavily dependent upon the redox state, can easily be artifactually generated or reduced in biological samples.
In order to achieve monitoring of nitro-oxidative "stress" in pathophysiological situations, Rocha et al. developed an ELISA assay for the quantitative determination of nitrated albumin in plasma. Indeed, assaying free or peptide-bound nitroTyr does not reflect nitro-oxidative stress as most of it is of dietary origin [312].
This test allowed Bottari's group to monitor the occurrence of systemic nitro-oxidative "stress" under two conditions which can lead to severe long-term psychomotor retardation: perinatal asphyxia and neonatal hypoglycemia [313], [314]. Whereas the clinical significance and importance of perinatal asphyxia on neurodevelopmental outcome of severely and even moderately affected newborns is now generally accepted, this has not been the case for neonatal hypoglycemia whose clinical significance is still a matter of intense debate [315], [316], [317], [318], [319]. Interestingly, animal models of these two conditions invariably show cerebral insult associated with increased tyrosine nitration both in vitro and in vivo. These observations have been confirmed in postmortem cerebral and medullar tissues of asphyxiated neonates [320], [321].
Since high peroxynitrite flux generation has been reported to cause neuronal apoptosis, Wayenberg et al. investigated potential correlations between plasma nitroalbumin levels and other biological and clinical parameters in asphyxiated and hypoglycemic neonates [313]. In perinatal asphyxia, it was found that plasma nitroalbumin concentrations at day 1 to be strongly correlated with the severity of neonatal encephalopathy (χ2 =7.23; p<0.05) indicating the occurrence of systemic nitro-oxidative stress in these infants. Nitroalbumin levels were also inversely correlated (p<0.05) with Apgar score (a marker for the health of newborns) and arterial blood pH and directly with creatinemia, arterial base deficit and lactacidemia. The latter correlation indicates a correlation between the degree of hypoxia and albumin nitration, whereas the increase in creatinemia is indicative of a decreased glomerular filtration rate, which may reflect afferent arteriolar vasoconstriction. Nitroalbumin levels were back to control levels at day 4 suggesting that this stress was transient [313].
It has been well documented that moderate and severe neonatal encephalopathy are often associated with subsequent periventricular leucomalacia and cerebral palsy, often leading to impaired neurodevelopmental outcome. The data of Wayenberg et al. therefore suggest that increased RNS generation may play a role in hypoxic-ischemic brain injury. Further studies are required to verify whether plasma nitroalbumin may serve as a marker of nitro-oxidative stress in neuroprotective trials.
In neonatal hypoglycemia [314], Wayenberg et al. found an inverse correlation between glycemia and plasma nitroalbumin as early as the first hour of life (r=−0.35; p<0.02) and through day 1. Lactacidemia was inversely correlated with nitroalbumin suggesting that lactate may serve as an alternate fuel. Another interesting finding is the strong correlation between nitroalbumin levels and the severity and duration of the hypoglycemic events determined as area-under-the-curve. Similarly, in neonates who had more than 2 hypoglycemic episodes during the first 24 h of life, nitroalbumin levels were still significantly elevated at day 4.
Whereas, as mentioned earlier, the impact of neonatal hypoglycemia on neurodevelopmental outcome is still a matter of debate, our data clearly indicate that severe and repeated hypoglycemic events during the first 24 h of life induce an important and long-lasting systemic nitro-oxidative stress both in preterm and term infants, which may be involved in the cerebral insult responsible for the psychomotor retardation reported by several authors. These observations therefore provide grounds for timely and appropriate management of neonatal hypoglycemia and call for further studies on careful metabolic monitoring and long-term follow-up of the neurodevelopmental outcome of these infants.
In conclusion, plasma nitroalbumin determination provides a highly sensitive and robust marker, which may prove useful for monitoring nitro-oxidative stress and for understanding the involvement and role of redox deregulations and RNS in various pathophysiological conditions.
4.3. Lipid oxidation products as redox biomarkers
The following section was composed by Opeyemi S. Ademowo, Irundika Dias and Helen Griffiths. Malondialdehyde (MDA) is the best known and most abundant (10–20 µM in plasma) end-product of the autocatalytic lipid peroxidation chain reaction. In addition to aldehydes, other common cholesterol, phospholipid and polyunsaturated fatty acid peroxidation biomarkers that are formed include ketones such as 4-hydroxynonenal, alcohols such as isoprostanes, hydroperoxides such as 1-palmitoyl-2-(5′-oxo-valeroyl)-sn-glycero-3-phosphocholine (POVPC) and cyclic endoperoxides. Polyunsaturated fatty acids (free or in phospholipids) with methylene interrupted unconjugated olefinic bonds are highly susceptible to free radical oxidation and yield hydroperoxide species in situ, which in the case of membranes, are released by phospholipases. Commonly reported examples of oxidized cholesterols (oxysterols) include 6-cholesten-5α-hydroperoxide, 7-ketocholesterol, 7-dehydrocholesterol and 25-hydroxycholesterol [322]. The suitability of these molecules as biomarkers is dependent on their stability, ease of enrichment or separation and accurate detection methods in the presence of several orders of magnitude higher concentrations of the parent lipid. The commonly used thiobarbituric acid-reactive substances (TBARs) assay lacks specificity for MDA measurement in biological materials and is not a method of choice for MDA quantitation [323].
Lipid peroxidation analysis by absorption spectrometry has been superseded by the accurate determination of unmodified or derivatized lipids using chromatographic separation followed by mass spectrometry (MS) methods. Gas and liquid chromatography have been used successfully according to equipment availability, but LC is easier as it does not require the samples to be volatilized. The precision of such methods is achieved by: 1) stability of the analyte; 2) enrichment of the analyte; 3) specific detection by multiple reaction monitoring transitions; 4) the sensitivity of the MS method including variation in dwell time; and 5) the availability of stable isotope standards for the species of interest.
For MDA, the commercial deuterated standard is available to support stable isotope dilution methods; successful derivatization and stabilization in acetone has been achieved using pentafluorobenzylbromide followed by extraction using toluene to remove matrix followed by GC separation to enrich for species of interest [324], and using 3-nitrophenylhydrazine to derivatize followed by LC separation using stable isotope dilution methods to compensate for matrix effects during electrospray ionization [325].
Quantification is typically achieved by selected ion monitoring (SIM) after MS (e.g. of m/z 251 for MDA and m/z 253 for its stable isotope after neutral loss of PFB) and selected reaction monitoring (SRM) by MS/MS of the mass transition (e.g. m/z 251 → m/z 175 for d0-MDA and m/z 253 → m/z 177 for d2-MDA) [324]. By derivatizing MDA, these methods capture the reactive carbonyl prior to sample processing.
Similar to MDA, 4-HNE is highly reactive and MS methods of analysis typically rely on derivatization using PFB to stabilize the reactive carbonyl. After GC separation and analysis by the SIM method, the deuterated analog, HNE-d11, has been successfully used for quantitation [326]. Plasma concentrations of 4HNE are ~100 nM.
Isoprostanes (IsoPs) are stable products of polyunsaturated fatty acid peroxidation. F2-isoP, the most common, is derived from arachidonic acid with a concentration <0.5 nM in the plasma of healthy human subjects. One LC-MS method using deuterated standardization has been described, despite this method having the potential to be the most sensitive and specific for many different isomeric forms. Solid phase extraction was adopted to remove contaminants and following LC-MS, two MRM transitions were acquired per analyte for quantification and confirmation of IsoPs [327]. The recovery of the spiked standard was 70–120%.
Oxidized cholesterols have biological activity [328] although they are found at very low concentrations in plasma (7-ketocholesterol: <75 nM). They are often esterified. Some methods describe saponification to release the free oxidized cholesterol as an index of oxidative stress whereas other authors prefer to analyze the biologically active free oxidized forms. Solid phase or liquid-liquid extraction and protein precipitation during sample preparation has proved to be important for sample enrichment. The incorporation of deuterated standards using stable isotope dilution enables absolute recovery to be reported. Using a monolithic column, efficient chromatographic separation of isomeric oxysterols was achieved; MRM transitions of m/z 369/287 enabled specific detection for 7-ketocholesterol with 81–108% recovery [329].
In order to highlight the innovations in the field of lipid peroxidation product analysis by LC, a recent approach for detection of the oxidized phospholipid POVPC has been described that relies on a nanoparticle-based strategy for successful trapping and enrichment of aldehyde-containing oxidized phospholipids [330]. After derivatization of carbonyl containing phospholipids with a bifunctional reagent containing a thiol moiety to bind onto nanoparticles, the derivative and enriched moieties can be released by a reducing agent for analysis by LC-MS/MS. The authors highlight a two-fold improvement in the sensitivity of POVPC detection using nanoparticles.
As LC-MS/MS with SRM methods and deuterated standards become more widely available, a new era of reliable lipid peroxidation analysis will dawn enabling researchers to understand the concentration and species produced in different biological contexts. Improved enrichment techniques that minimize the risk of artefactual oxidation and novel chromatographic materials that can offer improved separation of amphipathic molecules are key to further development in this field.
4.4. Nucleic acid oxidation products as redox biomarkers
The following section was composed by Marcus S. Cooke, Mahsa Karbaschi and Henrik E. Poulsen. Nucleic acid oxidation has attracted attention since it was shown that it was one of the most abundant DNA modifications [331]. It also is a pre-mutagenic lesion that induces CG: TA transversion mutations. The guanine moiety is particularly prone to oxidation and is studied as a prototype for both DNA and RNA oxidation. In DNA it can be measured as the nucleoside upon digestion and hydrolysis. The gold standard for measurement is now ultra-high-performance liquid chromatography with tandem mass spectrometry for quantification. The optimum method includes extensive chromatography, isotope dilution with the use of both quantifier and qualified ions [332]. The oxidized nucleosides from both DNA and RNA are excreted into urine and are used as non-invasive biomarkers [6].
Biomonitoring such damage, both in vitro and in vivo, is therefore critical to understanding the mechanisms linking damage with disease, but achieving this can present technical challenges. Blood is a potentially problematic matrix to measure oxidatively generated DNA damage, as it is necessarily invasive (typically ~5 mL is needed); peripheral blood mononuclear cells need to be isolated before analysis or storage, which is a time-consuming process; and artefactual damage occurs when whole blood is frozen without a cryopreservative. To address this, a novel approach has been described that significantly simplifies biomonitoring in vivo: small volumes of whole blood are sufficient (250 µl from a finger prick), which facilitates simple collection and storage without artefacts, together with use in the comet assay without prior isolation of peripheral blood mononuclear cells [333]. Recently, a novel comet assay tank and rack design was described, which exploits holding the slides in a vertical orientation, rather than horizontal [334]. This innovation results in significant improvements in sample throughput (~90% decrease in slide handling time), together with a much smaller footprint, and enhanced cooling. In contrast to blood, urine is non-invasive, although it is also possible to measure biomarkers of oxidative stress in other extracellular matrices [335]. Mass spectrometric approaches remain the gold standard for urinary 8-oxodG analysis, but modifications to a commercially available ELISA for 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) have improved its accuracy [336], [337]. Most recently, the Cooke laboratory has established an approach that allows genome-wide assessments of DNA.
There are many reports of increased excretion of the guanine nucleoside into urine or elevated tissue levels in many diseases, and this has led many researchers to conclude that nucleic acid oxidation is important in many diseases. Oxidative stress-induced damage to nucleic acids (including DNA, RNA and the corresponding nucleotide pools) has been implicated in the pathogenesis of numerous diseases, including cancer, cardiovascular disease and neurodegenerative disease [338]. However, there is a difference between occurrence and importance, and also there is the problem of whether the lesion causes the disease or is a consequence of the disease. The classic criteria for causation are given by the nine Bradford-Hill criteria [339]: strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy. For DNA oxidation there are few publications on the strength (urinary excretion of the oxidized deoxyguaninosine nucleoside (8oxodG)). Consistency is limited and there are only a few existing cohort studies, yet they have shown an association with lung cancer in non-smokers [340] and with breast cancer [341]. These effects are small and probably not important on an individual basis, although they might be important from a public health point of view. There are no important data to evaluate criteria 2,3,4,5. Criterion 6 (plausibility) is provided by the demonstration of GC: TA transversion mutations, even though the quantitative effect is not established. Criterion 7 is partly fulfilled by epidemiological and experimental evidence, and for criterion 8 (experiment) there are data from DNA repair knock-out animals that support the idea that the oxidative modifications are linked to cancer development, or to premature aging [342].
RNA oxidation has only recently attracted attention. The evidence is limited to hemochromatosis [343] and diabetes [344], [345]. The data support criterion 1 in that the effects are considerable, but the rest of the criteria are only supported by hypotheses [346].
Beside the Bradford Hill criteria, there are some additional aspects that need to be considered in the context of clinical utility. If a high level of a biomarker is associated with a disease, are there means to reduce the levels and will this either prevent the disease or improve survival of patients with the disease? There are no data on this. Presently, the quest is to identify non-pharmacological or pharmacological means that are non-toxic and that can reduce markers of DNA or RNA oxidation. Hitherto, the only example is blood-letting for the treatment of hemochromatosis that reduces the biomarker for RNA oxidation and also is well established to reduce the morbidity from high body iron [343]. Still, particularly regarding type 2 diabetes, the clear association between morbidity/mortality and the levels of RNA oxidation is very promising with a potential to identify or develop novel treatments for type 2 diabetes.
4.5. Conclusion
This comprehensive review has provided a summary of some of the protein-, lipid-, and nucleic acid-based biomarkers of oxidative stress, some of the latest developments in the assay methods by which they may be measured, and some of the applications of these methods in clinical studies. As described above, in general, gas/liquid chromatography-mass spectrometry often provides the platform of choice for the determination of all these types of biomolecules, due to the specificity and sensitivity of this platform. However, this type of platform does not necessarily provide a method that is suitable for large-scale clinical studies because of the relatively high cost of mass spectrometry equipment and, frequently, the relatively high time demands for sample preparation. Even mass spectrometry approaches are not without the dangers of artefacts. It should also be borne in mind that good analytical sensitivity and specificity for a particular biomarker does not necessarily translate into good clinical diagnostic sensitivity and specificity when testing the utility of the biomarker in the diagnosis of a specific human disease.
Immunochemical methods such as ELISAs might provide alternative, high-throughput approaches for the analysis of oxidative stress biomarkers (as described above for a range of protein oxidation products, including 3-nitrotyrosine within the albumin polypeptide backbone). However, great caution should be exercised in relation to the specificity of the antibodies used in ELISAs, and it is evident from Table 4.1 that many commercially available antibodies directed against protein oxidation products have received limited validation.
One of the challenges in this area is for the “redox biology community” to work together to validate a group of harmonized assays, which will constitute reference standards for oxidative stress biomarkers in clinical studies. An important series of investigations (the National Institute of Environmental Health Biomarkers of Oxidative Stress Study), addressed the question of which assays provide valid read-outs of oxidative stress induced in animal models. The mass spectrometry-based measurement of F2-isoprostanes was identified as a “gold standard” biomarker of free radical damage caused by carbon tetrachloride in rats [347]. A viewpoint regarding the current status of oxidative stress biomarkers as clinically useful tools was provided by a recent review [6]. Visualization of biomarkers measured in various diseases by cluster analysis (Fig. 4.2) shows that the majority of studies have used ROS-induced modifications as markers of oxidative stress [6]. There is also ongoing discussion on whether traditional assays for ROS and RNS detection are still useful in certain setups or should be replaced by more advanced techniques [348], [349], [350].
Fig. 4.2.
Cluster analysis of ROS biomarkers in disease. Different diseases were clustered according to described ROS biomarkers in Refs. [351], [352], [353] and studies described in this review. Some disease conditions cluster as might be expected, such as ischemia/reperfusion and heart failure, and amyotrophic lateral sclerosis and multiple sclerosis. A comprehensive analysis of ROS markers and pattern analysis in diseases might uncover common disease mechanisms or new measures of disease progression or treatment outcome. Cluster analysis was performed using Genesis software (https://genome.tugraz.at/genesisclient/genesisclient_description.shtml) as described in Mengozzi et al. [354]. Adpated from [6]. With permission of Mary Ann Liebert, Inc. Copyright 2015.
The current review has included some indications as to how oxidative stress biomarkers assays may be applied to medical research studies, but the demonstrated clinical value of oxidative stress measures in diagnosis or monitoring in patients has so far been disappointing. In this respect, the challenge is to validate oxidative stress assays in terms of clinical utility – i.e. clinical diagnostic sensitivity and clinical diagnostic specificity. The development of oxidative stress biomarkers needs the substantial further attention of researchers, particularly in relation to the identification of validated biomarkers for the study of: (i) the pharmacodynamics of novel redox-based therapeutics in animal models of disease, (ii) the clinical diagnosis and/or monitoring of human diseases in which oxidative stress is involved, and (iii) clinical trials of novel redox-based therapeutics. As long as the above three areas remain inadequately addressed, we will continue to struggle to translate our increasing understanding of redox biology into advances in human disease therapies based on redox medicine. The studies described above, in relation to nitrated albumin as a biomarker in perinatal asphyxia and neonatal hypoglycemia, begin to take us in the right direction. But the important challenge of identifying reference methods for oxidative stress assays still has some way to go.
5. Optimizing ROS detection using EPR or fluorescence: in vivo applications to mammalian cells, tissues and plant biology
Yves M. Frappart (E-mail: yves.frapart@parisdescartes.fr).
5.1. Introduction
When oxygen first appeared on Earth, living systems had to adapt to this particular oxidant molecule. This resulted in various reactive oxygen species (ROS: mainly superoxide anion, hydrogen peroxide, but also 1O2, hydroxyl radical) that must be regulated by all aerobic organisms. Due to the regulatory role of ROS and crosstalk between these molecules, their level and localization are therefore critical parameters for biology and medicine. In order for the measurements to be most useful, it often is essential to measure the amount of ROS at particular sites and under appropriate conditions. Specific, sensitive and localized measurements of ROS require special methodologies. As ROS are correlated to oxygen levels, in vivo conditions should be applied to study their biological effects, which is not always possible. Oxygen concentration is therefore a key element in every experiment. There is no gold standard modality to determine, quantify or localize the various ROS species. Scientists who evaluate ROS have to use different modalities, with different qualities and limitations depending on the experimental conditions. Over the last decade, approximately 75% of all reports on ROS have been determined with fluorescence imaging, especially in vitro [355]; almost all the other references have used electron paramagnetic resonance (EPR or electron spin resonance [ESR]) with or without spin trapping for their detection or imaging [356]. Nevertheless other modalities are emerging such as nuclear magnetic resonance imaging (MRI) [357], echography [358] or positron emission tomography [359]. New methodologies such as immuno-spin trapping [360] are also emerging all the time so that it is not possible to give an exhaustive list. Fig. 5.1 shows enzymatic sources of RONS, the reactive species being formed, the potential footprints they leave and imaging techniques that may be applied to measure them [361].
Fig. 5.1.
Spectrum of different ROS imaging techniques. In the upper part, different sources of ROS are shown: Mitochondria (mito), lipoxygenases (LPO), monoamine oxidase (MAO), nicotinamide adenine dinucleotide phosphate oxidase (NOX4 and NOX 1/2/5), xanthine oxidase (XO), functional nitric oxide synthases (NOS) and dysfunctional / uncoupled eNOS (u-eNOS). These result in different types of ROS [including superoxide radical (O2•─), hydrogen peroxide (H2O2), hypochlorous acid (HOCl, not shown in the scheme), peroxynitrite anion (ONOO─), nitric oxide (•NO)] and ROS-induced modifications of GSH, NADPH, proteins, or glucose uptake, which, in turn, are detected by different imaging technologies. Adpated from [361]. With permission of Mary Ann Liebert, Inc. Copyright 2016.
Despite its limitation, EPR is still the most suitable technique to detect ROS. EPR can be used in different methodologies such as EPR coupled to spin-trapping. It is the most specific method, as stated by K. Abbas and F. Peyrot.
5.2. Spin trapping coupled to EPR is an under used specific method to measure oxidative stress in biological systems
Electron paramagnetic resonance (EPR) is the gold standard to detect paramagnetic species. However, when the aim is the detection of short-lived radicals involved in oxidative stress, such as O2•- in living systems, direct detection is not possible due to the low sensitivity of the technique. To overcome this limitation, the spin trapping method was introduced in the 1970s. It relies on the specific single-step addition reaction of the radical of interest on an EPR-silent molecule, named the spin trap, which yields a persistent radical, called the spin adduct, the EPR spectrum of which is characteristic of the trapped radical. The most commonly used spin traps for O2•- in aqueous medium are derivatives of the cyclic nitrone 5,5-dimethyl-1-pyrrolidine N-oxide (DMPO, e.g. DEPMPO) (Fig. 5.2). Since its first application to biological systems in 1979, important contributions have been made to improve spin trap structures based on mechanistic analysis of the spin trapping reaction and of the spin adduct decomposition pathways [362], [363], but also to improve the methodology by characterizing its limitations, artefacts, and sources of misinterpretations [364], [365], [366], [367], [368]. Intracellular detection and quantification are not straightforward, and in vivo detection of O2•- is impossible with spin traps coupled to EPR. However extracellular detection of O2•- produced by cells can now be achieved with excellent specificity and satisfactory sensitivity with CD-DIPPMPO, an extracellular spin trap [369]. Spin trapping of NO is based on its high affinity towards ferrous iron and trapping as stable nitrosyl-iron complexes is achieved by either adding iron-diethyldithiocarbamate (Fe(II)(DETC)2) to tissues or isolated cells [63], [370], or by measurement of endogenously formed nitrosyl-iron complexes such as Hb-NO [371], [372]. Nitrosyl-iron complexes produce typical triplet EPR signals (Fig. 5.2). Though a few spin traps are commercially available, their cost is prohibitive considering that their purity is usually not sufficient for biological studies. Since the quality of the supplies has not improved over the years, there is a need for a greater collaboration between biologists, synthetic chemists, experts in EPR spectroscopy and spin trapping from platforms dedicated to EPR studies of biological systems at national and European levels so that recent advances can be made accessible to all, in order to address questions relative to oxidative stress in biological systems. A few years ago, as Ron Mason was trying to find new accurate ways to use spin trapping in order to determine superoxide levels in living systems, he introduced immuno spin-trapping, which he presented in a recent review [360]. Immuno spin-trapping uses spin-trap antibodies to ensure the detection of the long lived biological species with various methodologies (UV, mass spectrometry, MRI). Spectroscopists can now specifically detect superoxide in vitro; they have therefore moved on to try to overcome the problem in vivo by studying the redox status. Redox status is related to the balance of many biological species (oxidants and reductants or antioxidants).
Fig. 5.2.
(A) Determination of mtROS triggered NADPH oxidase activation in isolated human neutrophils by electron paramagnetic resonance measurement. Freshly isolated human neutrophils (22x106 PMN/mL) were incubated in PBS with 300 µM Ca2+/Mg2+ and 10 mM DEPMPO for 15 min at 37 °C. Activators and inhibitors of phagocytic NOX were added as shown in the figure. The spectrum for phorbol ester (PDBu)-stimulated PMN is displayed at decreased intensity (1/10). All reactions below the red spectrum contained 20 µM myxothiazol. Incubations were with NOX inhibitor (VAS2870), intracellular calcium chelator (BAPTA-AM), cSrc kinase inhibitor (PP2) and PKC inhibitor (chelerythrine). All spectra were recorded at room temperature in 50 µl glass capillaries (Hirschmann Laborgeräte GmbH, Eberstadt, Germany). EPR conditions: B0 =3300 G, sweep =150 G, sweep time =60 s, modulation =3000 mG, MW power =10 mW, gain =9x102 using a Miniscope MS200 from Magnettech (Berlin, Germany). Representative spectra of mixed DEPMPO-OOH• and DEPMPO-OH• adduct for 2 independent experiments. Spectra were recorded as described previously [373]. With permission of Mary Ann Liebert, Inc. Copyright 2014. (B) Whole blood Hb-NO levels were determined by electron paramagnetic resonance spectroscopy as a read-out of iNOS activity in endotoxemic (lipopolysaccharide-treated) rats. The EPR measurements were carried out at 77 K using an X-band table-top spectrometer MS400 (Magnettech, Berlin, Germany). The instrument settings were as follows: 10 mW microwave power, 7000 mG amplitude modulation, 100 kHz modulation frequency, 3300 G center field, 300 G sweep width, 60 s sweep time and 3 scans. With permission of Springer-Verlag Berlin Heidelberg. Copyright 2015. (C) Aortic •NO formation was measured in untreated control and angiotensin-II infused hypertensive mice by EPR spin trapping using Fe(DETC)2. Each spectrum was measured from one murine aorta. The representative spectra below the bar graph are the mean of all measurements. EPR conditions: B0 =3276 G, sweep =115 G, sweep time =60 s, modulation =7000 mG, MW power =10 mW, gain =9x102 using a Miniscope MS400 from Magnettech (Berlin, Germany). The A23187-stimulated •NO signal was absent when the aorta were denuded, L-NAME (200 µM) was added, or when aorta from eNOS-/- mice were used (not shown). With permission of Mary Ann Liebert, Inc. Copyright 2014.
(a) Adapted from [63]. (b) Adapted from [371]. (c) Adapted from [63].
Despite its hazy definition, the redox status can be used as a tool by biologists in many cases. This kind of measurement is based on the aminoxyl (nitroxide), hydroxyamine redox equilibrium. It is suitable for in vivo imaging [374] and often uses soluble molecular probes. When aminoxyl linked to proteins is used, the redox status and conformational changes can be addressed, as presented by A. Pavićević and M. Zatloukalová: the “Assessment of redox status and conformational changes of proteins using EPR spin labeling”, which gives biologists two important sets of information for their studies. Under different physiological and patho-physiological conditions, proteins undergo various conformational changes, which can be studied with a variety of experimental techniques. One of these techniques is EPR, also known as electron spin resonance spectroscopy (ESR), coupled with the use of paramagnetic compounds called spin labels (SLs). SLs, which are commonly employed for such measurements, are derivatives of cyclic aminoxyl (often addressed as nitroxide) radicals, a class of compounds containing an >N-O∙ moiety sterically shielded by methyl groups (or other types of groups) attached to the neighboring carbon atoms. In solutions, these molecules are allowed to rotate freely (short rotational correlation time, τc), and thus the resulting EPR spectrum is composed of three sharp isotropic lines (for 14N nitroxides). However, when molecular motions are restricted (long τc) the overall spectral shapes change, i.e. a broadening of the lines occurs due to the anisotropy of hyperfine splitting constants and g-values. If an appropriate SL is attached to a protein, the anisotropic spectra of the immobilized >N-O∙ moiety provide valuable information about: 1) the hydrophobic/hydrophilic nature of the binding site in the protein; 2) the influence of the local environment induced by various reagents, temperature and pH change, etc.; 3) the binding capacity of the protein; 4) the distances between the active sites of the protein; 5) the folding and unfolding of the protein, which is an especially interesting topic when membrane proteins are studied; 6) the secondary structure of the protein; 7) the dynamics of the protein backbone; and 8) protein-protein and lipid-protein interactions [375], [376]. Numerous SLs have been synthesized for this purpose, a few of which are commercially available. Some of the SLs bind non-covalently to the proteins, while others are intended to covalently bind to specific amino acid residues, such as sulfhydryl groups (-SH). The technique involving the application of the latter is known as site-directed spin labeling (SDSL), and is frequently used in synergy with site-directed mutagenesis. Even though the application of SDSL can be beneficial, the use of conventional site-specific spin labels (SSLs) suffers from a number of drawbacks, so the label has to be chosen prudently, and experiments must be planned thoroughly. For instance, the methanethiosulfonate spin label (MTSSL) binds solely to the sulfhydryl group of free cysteine residues. However, it also tends to form dimers, resulting in dipole-dipole interactions, and consequently in the formation of extra spectral features [377]. On the other hand, maleimido- (MSLs) and 2-iodoacetamido- spin labels (IASLs) need to be incubated with the protein at certain pH values, otherwise they bind not only to –SH, but also to the amino groups.
The protein spin labeling EPR technique has not been used exclusively to study protein conformations and interactions, but has also been applied as a diagnostic tool for various malignancies and benign tumors [378], sepsis [378], diabetes [379], atherosclerosis [379], and cirrhosis [380]. The commonly used methods to detect the aforementioned pathologies include the labeling of blood plasma/serum with 16-doxyl stearic acid (16-DS). 16-DS was chosen due to its high affinity for human serum albumin (HSA). Peptides and metabolites synthesized in the affected organ become attached to HSA, thereby changing its binding capacity and conformation. Such modification is expressed as varied contributions of the unbound, strongly and weakly bound 16-DS to the albumin from the sera of patients, as opposed to healthy individuals. These contributions are calculated using simulation software to decompose EPR spectra, and data are further processed, mainly by neural network software or discriminant analyses. On the other hand, in vitro studies demonstrate that ROS may damage HSA and affect its transport function [381]. Though this methodology offers satisfying accuracy, sensitivity and specificity, and though the procedure seems relatively simple, there are several technical issues that make this diagnostic tool anything but easy. In a recent work by A. Pavićević et al., the analysis of the binding of two spin-labeled fatty acids (SLFAs) to commercial HSA was reported [382]. The obtained data indicate that ethanol, which is extensively used in diagnostic spin labeling protocols, affects the binding of SLFAs to HSA. In order to avoid using ethanol, test tubes should initially be labeled with a small volume of SLFAs dissolved in an organic solvent and left to evaporate. Afterwards, a certain amount of serum should be added and incubated under constant vortexing. The weakness of such an experimental setup is that SLFAs bind to a lower extent to HSA, due to their low water solubility. There are also other difficulties with using 16-DS. One of them is its binding to seven possible sites of HSA; consequently, information about conformational changes cannot be obtained from the specific site. The other issue is that the doxyl group is positioned nearly at the end of the hydrocarbon chain, opposite the carboxylic group. Therefore, anchoring the 16-DS molecule to HSA causes the doxyl moiety to protrude through the albumin's surface [382], [383]. Accordingly, 16-DS appears to be an unreliable reporter to detect local changes in albumin hydrophobic pockets.
Considering all the indicated difficulties, the course of our research was oriented towards estimating the diagnostic potential of other SLs, initially through in vitro studies on purified HSA, and eventually on blood serum samples. The emphasis was on SSLs and SLFAs with a doxyl group attached to the hydrocarbon chain closer to the carboxylic moiety. The preliminary data indicated that the 3-maleimido proxyl (3-MSL) is able to report changes in the cysteine-34 (Cys-34) environment, induced by the binding of fatty acids, warfarin, ibuprofen and benzodiazepines, whose interactions with HSA have been studied exhaustively.
The use of SSLs can also reflect the antioxidant capacity and redox state of HSA (from its free –SH group), since this probe does not bind to the oxidized forms of –SH. The joint electrochemical and EPR study of cytochrome c derivatives indicates that the use of SLs specific for cysteine residues has great potential to estimate the redox state of other proteins bearing free –SH groups as well.
It is very difficult in such innovative methods to reach satisfying accuracy. One way can be to combine multiple methodologies such as “electrochemistry and EPR”. EPR can be completed by electrochemistry to get accurate oxygen concentration information by EPR [384]. Better than adding these methods, one can couple them; this is the proposition of J. Vacek and M. Mojovic that encourages efforts to “couple Electrochemistry and EPR”.
5.3. Electrochemistry and EPR to investigate antioxidant and prooxidant systems
Redox-active and reactive chemical forms directly contribute to homeostasis, the maintenance of a stable internal environment. There is therefore an emphasis on the direct detection and imaging of these chemical entities, among which we classify free radicals, or substances collectively known as RONS as well as an extensive group of antioxidants. Therefore, combining electrochemical methods and EPR spectroscopy represents a highly efficient solution, and both methods can be complementary (thus off-line) or may be applied in situ (i.e. on-line). Electrochemical methods may be used to monitor redox processes; cyclic voltammetry is preferred, or advanced pulse voltammetric techniques, which have a higher sensitivity due to the elimination of capacitive currents [385].
Usually carbon electrodes and anodic voltammetry are used to predict the electron-donor capacity and general reactivity of the examined molecules. The oxidation products can then be identified, which can be further investigated electrochemically, or even isolated and characterized after electrolysis. When interpreting the electrochemical data (voltammograms), we assume that an effective antiradical or antioxidant agent is oxidized at potentials approaching zero, where the electron-donor capacity, and hence its effectiveness, decreases with the increase of anodic potential. Given that many antioxidants are subject to multicomponent (pH-dependent) redox transformations, we always evaluate the potential of the first oxidation peak. Analyses are conveniently performed in an aqueous medium at pH 7.4 in the presence or absence of molecular oxygen, to eliminate artificial effects associated with the spontaneous oxidation of the examined samples. If the redox process is associated with the formation of a radical intermediate, it is practically impossible to detect by electrochemical approaches. For this purpose, EPR spectroscopy, also known as electron spin resonance (ESR) spectroscopy is used [386]. With EPR, we can monitor the absorption of microwave radiation by chemical forms with one or more free electrons, e.g. free radicals, in a strong magnetic field. They can be analyzed using the EPR directly in solution or cell homogenates, in a tissue or even during the anodic reaction on the electrode surface. If the lifetime of the radical forms is too short, the spin-trapping technique is used.
Mojovic et al. present their research on the oxidation of phenolic antioxidants as an example of an effective combination of electrochemical and EPR methods. Although this type of exogenous antioxidant is often perceived sceptically in terms of its biological effect in vivo, a number of these substances are highly effective experimental antioxidants, and in some cases, even chemoprotective agents are fully usable in prophylactic treatments and complementary medicine [387]. One of the most important examples is that of flavonolignans, which are used as hepatoprotectants and antidotes for selected mushroom poisoning. The main example is silybin and its 2,3-dehydroderivative, whose mechanisms of redox transformations and antioxidant effects have been studied [388]. Both of these substances, and also their structural congeners, are characterized by a pleiotropic mode of action, which is partly based on their ability to modulate signaling pathways, or receptor systems in cells (see Section 10.4.2), and also by a combination of their antioxidant vs. prooxidant effects. The antioxidant effect is associated with a relatively high electron-donor capacity, especially for flavonolignan 2,3-dehydroderivatives, and also their metal-chelating capacity. Conversely, pro-oxidant effects are associated with the transition of flavonolignans to reactive aryloxy radical forms or with the formation of highly reactive flavonolignan-metal complexes, which may secondarily generate free radicals through a Fenton-like reaction [389]. Square-wave voltammetry on carbon electrodes [388] was used to investigate the mechanism of redox behavior and analyze the electron-donor capabilities of these substances. EPR spectroscopy was further applied for the direct analysis of aryloxy radicals of flavonolignans that were generated in a strongly alkaline environment according to the methodology developed initially for the purpose of researching flavonol and flavone derivatives [390]. However, aryloxy radicals can also be generated during the electrochemical oxidation of flavonolignans. Their short lifetime, however, makes their identification or further analysis impossible. For this purpose, an in situ spectroelectrochemical technique was developed, where the oxidation of the flavonolignan occurs in the presence of the spin-trap probe 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO). The radicals produced during the electrooxidation therefore associate with BMPO and give rise to a stable radical adduct, which can be subsequently quantified by EPR [391]. In addition to the above, we see EPR as an effective tool to analyze metal flavonoid complexes. EPR techniques were applied to the characterization of the highly reactive oxovanadium(IV)/silybin complex, which induces the formation of RONS in the cellular environment [392].
The main prerequisite to combining electrochemical methods and EPR spectroscopy in the study of antioxidant and prooxidant molecules (or molecules which combine both effects) is based on the fact that EPR enables the visualization of free radicals in situ, which are not detectable by electroanalytical approaches. By contrast, EPR exhibits zero interference with the parent forms of the studied species, nor with the final products formed during their redox transformations. Off-line applications of EPR and electroanalysis have recently started to find applications in research of more complex cellular systems and in vivo monitoring and imaging [393]. In the above, in situ approaches (e.g. [391]) were previously only applied in molecular studies with isolated chemical species, whereas future applications clearly point towards the on-line connection of EPR spectroscopy with electroanalysis under more complex (preferably in vivo) conditions. There is clearly potential in the application of implantable microelectrode systems, in the optimization of new technical solutions for spectroelectrochemical instrumentation and in the development of new hybrid techniques based on electrochemical microscopy in combination with EPR imaging approaches.
Applications of electroanalysis in conjunction with EPR spectroscopy are demonstrated here with low-molecular redox-active substances, monomeric flavonolignans, exhibiting an antioxidant effect while under other specific conditions, exhibiting a prooxidant effect. The applicability of the above techniques in research on biopolymers, specifically proteins, is demonstrated in Section 5.2.
As discussed before, fluorescence is the most used methodology, especially for microscopy. As an example, one can use genetically encoded probes, as proposed by Vsevolod Belousov to detect hydrogen peroxide.
5.4. H2O2 detection using genetically encoded probes and nanoparticles
Out of the many techniques to study redox reactions, imaging using fluorescent genetically encoded biosensors offers the widest set of advantages. First, most importantly, it allows the monitoring of redox events in real time in situ and in vivo. Second, the protein nature of the sensors allows subcellular targeting similar to conventional fluorescent proteins. Recently several technological improvements in the area of redox imaging were achieved.
Since the development of the first genetically encoded redox biosensors, they have all been based on fluorescent proteins with green emission [394]. The development of the first red fluorescent probes for H2O2, HyPerRed, made multiparameter imaging possible by using co-expression of this sensor with green-emitting probes for H2O2, GSH/GSSG and pH. Using HyPerRed, ER stress- induced H2O2 transients in the mitochondrial matrix were detected for the first time within living cells [395]. Interestingly, the increase in H2O2 was not associated with any detectable increase in the mitochondrial matrix GSSG content. Moreover, no H2O2 increase was observed in the inter-membrane space or the cytoplasm of the same cells.
Another important direction in the improvement of genetically encoded redox sensors is the expansion of the concentration limits of detection. For example, for H2O2 signaling studies, it would be important to have a sensor that reflects changes in near-basal concentrations of H2O2. Although it is not yet clear what the basal concentrations of H2O2 are, the first successful attempt to make the H2O2 probes more sensitive has been made recently [396]. The sensor consists of the redox-sensitive fluorescent protein roGFP2 fused to yeast peroxiredoxin Tsa2. This peroxidase has a 100 fold higher reaction rate than OxyR and Orp1 - the domains used in previous H2O2 sensors, HyPer [397] and Orp1-roGFP2 [398]. The Tsa2 part of the sensor is, however, made non-sensitive to H2O2. Instead, it brings roGFP2 in close proximity to endogenous Tsa1 incorporating into Tsa1 decamers. Therefore roGFP2 within the probes form redox relays with endogenous Tsa1 rather than with fused Tsa2 inactivated by point mutagenesis. The resulting probe is half-oxidized under basal conditions and sensitive to both increases and decreases in H2O2. Still some effort has to be made to make roGFP2-Tsp2 functional in cells other than yeast and to improve the reduction speed of the sensor.
One of the basic properties of redox signaling is a high spatio-temporal control over location, and the amounts of the oxidants produced [399]. However, the exact sizes of H2O2 microdomains are not easy to estimate because of the optical limits of fluorescent microscopy. Super-resolution microscopy becomes a more and more popular instrument to study fine structures in cells, however it has never been used before to study dynamic processes, such as second messenger dynamics and enzymatic activities. Recently, the H2O2 sensor HyPer2 was successfully used in sub-diffraction microscopy. The exceptional photo stability of the sensor made its use in Stimulated Emission Depletion (STED) microscopy possible. Fused to cytoskeleton structures, HyPer2 was able to resolve the structures with superior resolution and report H2O2 dynamics in growth factor- stimulated cells. Uneven dynamics of the sensor oxidation between two filaments separated by a distance of 100–200 nm suggests a less than ~100 nm diffusion radius of H2O2 in the cytoplasm of fibroblasts.
On the other hand, developments have been made in the chemical probes used for detection [400], such as near-infrared sensitive probes [401], biphoton probes or nanoparticles (NPs). According to A. Suha Yalçın, NPs are becoming widely used tools in the field of sensing and imaging. Success in developing different luminescence probes has enabled the monitoring of ROS production both in cells and whole animals [402], [403], [404]. Among these are peroxalate-based NPs formulated from peroxalate esters and fluorescent dyes that are used to image H2O2 in vivo with high specificity and sensitivity. Peroxalate NPs were capable of imaging H2O2 in the peritoneal cavity of mice during a lipopolysaccharide-induced inflammatory response [402]. The method was further improved by reducing the size of the NPs and modifying their content to detect H2O2 at physiological concentrations. Chemiluminescent NPs have also been exploited for the in vivo targeting and imaging of tumors and were successfully used to image H2O2 as a tumor signal molecule [405]. Probes that improve the stability of peroxalates in aqueous systems are sensitive to low concentrations of H2O2 within the physiological range. Chen et al. [406] have recently developed a novel upconversion luminescence nanoprobe to detect ROS in aqueous solutions, as well as diagnose rheumatoid arthritis and to bioimage ROS in living cells.
5.5. High throughput assays for superoxide and hydrogen peroxide for rigorous and specific activity of NADPH oxidases
J. Zielonka and B. Kalyanaraman propose that the combination of fluorescence and EPR spin trapping may yield better results. RONS encompass a range of species displaying oxidizing, nitrating, nitrosating and/or halogenating properties. To better understand the pathophysiological mechanisms of ROS/RNS it is crucial to detect and identify the specific species responsible for a given biological effect and to selectively inhibit the source of its formation. NADPH oxidases are a family of enzymes, the only known function of which is transferring electrons from NADPH to molecular oxygen, and the concomitant generation of O2•– and H2O2. NADPH oxidases have been identified as a promising therapeutic target for diseases bearing an oxidative stress component. Despite the wide effort to develop inhibitors of the NOX isoforms, only a limited number of such inhibitors are available. This is due to serious limitations of the assays used to develop NOX inhibitors. Typically, sensitive, but non-selective and artefact-prone assays have been applied for the detection of NOX-derived oxidants, leading to a high rate of false positive hits in high throughput screenings. For example, it has been demonstrated that the L-012 probe to detect superoxide requires one-electron oxidation and may generate superoxide by itself [407]. Also, in many assays horseradish peroxidase (HRP) is used as a catalyst of the oxidation of the probe by NOX-derived H2O2. The lack of probe selectivity for a specific oxidant and the susceptibility of the HTS assays to peroxidase substrates and inhibitors led to the controversy over the NOX-inhibitory potency of the positive hits selected, including apocynin, VAS2870 and 2-acetylphenothiazine [408].
Recently, Zielonka et al. have developed new assays to monitor the activity of the NADPH oxidases [409] and used them to screen a small library of bioactive compounds for potential NOX2 inhibitors [410]. These assays take advantage of the probes, which react directly with O2•– or H2O2 and form easily detectable, fluorescent products. The authors designed and synthesized a cell-impermeable analog of hydroethidine, called hydropropidine (HPr+) to detect NOX2-derived O2•– [411]. Upon its reaction with superoxide, HPr+ undergoes oxidation to red fluorescent 2-hydroxypropidium whose fluorescence quantum yield can be increased further in the presence of DNA. In order to detect H2O2 the authors applied coumarin boronic acid (CBA), which upon oxidation forms the blue fluorescent 7-hydroxycoumarin (umbelliferone). Both assays can be carried out in a 384-well plate format, with rapid measurements using a fluorescence plate reader. For the secondary assays, they applied two probes: hydroethidine (HE) for O2•– and Amplex Red with HRP for H2O2. HE-based assay was coupled to rapid UHPLC-based detection of 2-hydroxyethidium as a specific product of the reaction of HE with O2•–. The Amplex Red-based assay was used in combination with the fluorescence plate reader. Both secondary (orthogonal) assays can be carried out in a 384-well plate format. All four assays can be used for high throughput monitoring of NOX activity, with the HE assay requiring rapid, microwell plate-compatible UHPLC instruments. Rapid UHPLC analyses also enable simultaneous monitoring of O2•– and H2O2 formation, using a mixture of HE and CBA probes [409], [410]. The positive hits identified with these assays can be tested in confirmatory assays, including the measurement of the rates of oxygen consumption by NADPH oxidase in a medium throughput manner using a Seahorse XF96 extracellular flux analyzer and in lower throughput EPR spin trapping of superoxide using DEPPMPO or DIPPMPO cyclic nitrone spin traps.
The newly developed assays provide a framework for reliable measurement of the activity of NADPH oxidases and other cellular sources of O2•– and H2O2. Rapid and rigorous detection and quantitation of O2•– and H2O2 will lead to better understanding of the chemical biology of O2•–/H2O2-producing enzymes (e.g. NOX isoforms) and will also help discover specific inhibitors of NOX isoforms.
Most of the time, ROS detection is applied to biomedical science, but very important results can be obtained in plant science, which helps to understand posttranslational modifications, as proposed by B. De Smet, F. Van Breusegem and J. Huang. The oxidation of crucial cysteines to sulfenic acid (SOH), has emerged as a biologically relevant post-translational modification (PTM) with particular importance in redox-mediated signal transduction [412]. Thus, identifying the sulfenome under oxidative stress would allow us to identify key redox-sensors and –transducers [413], [414].
5.6. Chemical and genetic tools for plant sulfenomics
The oxidation of crucial cysteines to sulfenic acid (SOH), has emerged as a biologically relevant post-translational modification (PTM) with particular importance in redox-mediated signal transduction [412]. Thus, identifying the sulfenome under oxidative stress would allow us to identify key redox-sensors and -transducers [413], [414].
Sulfenomic studies have only recently been applied to plants [414], [415], [416]. As sulfenic acid is often unstable, its identification was mainly examined on a protein-by-protein-basis. At present, both chemical and genetic approaches are used in plants. The majority of the chemical probes are dimedone derivatives (5,5-dimethyl-1,3-cyclohexanedione), that selectively react with sulfenic acid [417]. The bio-DCP1 probe, which was used in Medicago truncatula, is a dimedone analog carrying a biotin affinity tag used for purification [416]. As biotin has many drawbacks, new azido- and alkyne-functionalized dimedone-analogs were developed that allow the addition of biotin post-extraction on the dimedone-tag using click chemistry [415], [418]. Their small size and membrane permeability allow the in vivo tagging of sulfenylated proteins. In Arabidopsis cells, the DYn-2 probe was used to identify the sulfenome under oxidative stress [415].
The yeast AP-1 (YAP1)-based probe offers a genetic way for the in vivo identification of plant sulfenomes [54], [55]. This was first applied in Medicago truncatula to identify the sulfenome upon Medicago truncatula–Sinorhizobium meliloti symbiosis [416]. Recently, a YAP1C-GS probe has been developed and expressed in Arabidopsis cells to reveal the sulfenome under oxidative stress [414].
Using a genetic approach has the advantage that, once the material is transformed, experiments are cost-efficient, allowing multiple repeats and treatments. Additionally, the probe can be targeted to specific tissues or even organelles. On the other hand, chemical probes generate a larger coverage of the sulfenome in a single experiment, as they penetrate whole cells; they do not require transformation and generate stable covalent bonds between the sulfenylated protein and the probes. Another attractive difference is the mode of trapping the sulfenic acid. Whereas the YAP1C genetic probe traps sulfenic acid in its protein context, defined by protein-protein interaction, the chemical probes trap sulfenic acid independently of the protein environment. Another characteristic to consider is that the incubation of the chemical probe can influence the oxidation within a cell, which should not be a problem for the permanently expressed genetic probe, although its expression can act as a scavenger of the oxidized proteins and hence, alter the cell/plant redox state.
As both genetic and chemical probes have specific advantages, the combination of their data obtained with both probes covers the largest part of the plant sulfenome. Current tools are based on protein-identification, rather than on site-identification. Therefore, future research should focus on mapping specific sulfenylation sites. Quantifying the protein sulfenylation, or even the specific cysteine-sulfenylation in response to several elicitors would further improve the sulfenomic approaches and assess the role of cysteine-oxidation upon redox signaling.
5.7. Conclusions
As outlined above, imaging ROS is still very challenging, and the choice of the method will largely depend on the experimental conditions. For the selective in vitro specific detection of superoxide on cells, for example, EPR coupled to spin-trapping is the gold standard method. Abbas et al. have recently developed the detection on living cells under conditions close to in vivo conditions. Less selective, but allowing in vivo EPR imaging and protein folding and mobility, EPR can be coupled to spin labeling to allow redox status determination. Coupling to electrochemistry allows the accurate determination of either the oxygen levels, or the anti-oxidant's ability. Genetically encoded biosensors make it possible to determine hydrogen peroxide and open the way to in vivo luminescence bio imaging. Strong efforts are made in chemistry to allow the selective and easy detection of ROS with fluorescent probes, such as nanoparticles. NPs are versatile, and can be used to detect superoxide anion and hydrogen peroxide. EPR and fluorescence can be coupled, as recently proposed by Zielonka et al.; this yields very accurate detection of NADPH oxidase activity, a key enzyme in oxidative stress. Combining different methods can help understand oxidative stress in a post-transcriptional pathway, as proposed by De Smet, Van Breusegem and Huang in plant biology.
There is still no gold standard for ROS detection and imaging. Many methods are under development, and more collaboration between biologists, methodologists of the modality (e.g. fluorescence, EPR) and chemists is needed to make progress and avoid misinterpretations of experiments leading to false conclusions.
6. Reactive oxygen and nitrogen species in cardiovascular pathologies
Rainer Schulz (E-mail: rainer.schulz@physiologie.med.uni-giessen.de), Andreas Daiber (E-Mail: daiber@uni-mainz.de) and Fabio Di Lisa (E-mail: fabio.dilisa@gmail.com).
6.1. Introduction
In the cardiovascular system, reactive oxygen and nitrogen species (RONS) play an ambivalent role in that small amounts of RONS mediate protective effects (anti-atherosclerotic [Schröder], pro-angiogenesis [Matsui, Bachschmid], endogenous cardioprotection [Andreadou]), while large quantities of RONS cause cell injury eventually leading to loss of viability. RONS-induced cell derangements occur at the level of any organ, including heart [Görbe, Giricz, Ferdinandy] and brain [Casas, Schmidt], especially in the presence of co-morbidities (e.g. diabetes [Schröder; Görbe, Giricz, Ferdinandy]). Furthermore, recent work highlights the contribution of alterations of the intracellular Zn2+ pool [Tuncay, Turan] in RONS-induced cell injury. Importantly, the source of RONS due to its cellular (parenchyma [Casas, Schmidt] vs. vasculature [Schröder]) and subcellular localization might contribute to the beneficial [Schröder] or deleterious [Casas, Schmidt] action of RONS. Among the many sources of RONS, a relevant role is played by NADPH oxidases (NOX), uncoupled nitric oxide synthases (NOS) [Li, Förstermann], and various processes in mitochondria. For instance, the ischemic heart accumulates succinate that upon reperfusion is oxidized causing a burst in ROS formation due to reverse electron transport [Mulvey, Krieg]. Strategies to reduce excessive RONS include administration of antioxidant enzymes (more recently incorporated in endosomes and targeted by antibodies to specific cell types [Muzykantov]) or pharmacological up-regulation of the endogenous antioxidant defense [Lazou]. In addition, an antioxidative effect has been reported for compounds already used in daily practice. This is the case with glucagon-like peptide-1 or DPP4-inhibitors [Steven, Daiber] that abrogate stress-induced blood cell derived RONS. However, antioxidant strategies must be used with caution since they might interfere with endogenous organ protection [Andreadou]. The chapter “Cardiovascular Pathologies” discusses many of the above aspects and is structured according to the importance of RONS in the blood vessels, the heart and the brain (Fig. 6.1).
Fig. 6.1.
Processes contributing to the increase in ROS levels in various tissues. Mitochondrial pathways are highlighted as prominent sources of ROS, especially in the heart. Besides their involvement in tissue injury, ROS have been described also as mediators of cardiac protection against ischemia/reperfusion damage.
6.2. Oxidative stress and redox processes in atherogenesis and angiogenesis
As pointed out by Katrin Schröder, atherosclerosis is a vascular disease characterized by plaque and neo-intima formation, as well as local inflammation of the vessel wall. The latter is, in part, a consequence of endothelial dysfunction. Monocytes are attracted and adhere at sites of endothelial activation, invade the vessel wall and support a vicious cycle of inflammation and cellular recruitment by processes involving the formation of reactive oxygen and nitrogen species (RONS). Especially the superoxide anion (O2•¯) is potentially detrimental for vascular function and promotes atherosclerosis [419]. O2•¯ not only limits •NO-bioavailability, but also gives rise to ONOO¯, which mainly acts as a potent toxic agent. The main source of RONS in the vascular system is the family of NADPH oxidases, whose only known function is the formation of O2•¯ and H2O2. The first NADPH oxidase identified was NOX2, which is the primary NADPH oxidase in macrophages and leukocytes. Its native function is host defense by the massive formation of O2•¯, H2O2 and resulting RONS, in a process termed oxidative burst. Beside the detrimental consequence of massive RONS formation, these species also play an essential role in signal transduction. Recently additional NADPH oxidases have been identified and it is now clear that the number of RONS produced, the site of their formation as well as the type of RONS influences the subsequent signaling. Within the NOX family, NOX4 is an exception as it mainly produces intracellular H2O2 in a constitutive manner at very low concentrations. NOX4-derived H2O2 does not influence •NO bioavailability, rather it can directly react with proteins in signaling pathways. In fact, NOX4 appears to exert a protective role in the vascular system and prevents vascular inflammation [420]. This protection is, among other mechanisms, mediated by the maintenance of NRF2 expression, which eventually leads to the expression of heme oxygenase-1 (HO-1). Through this pathway, NOX4 favors CO production, which contributes to endothelial quiescence and prevents leukocyte adhesion. The protective role of NOX4 is supported by genetic approaches. Nox4 deletion has been reported to promote atherosclerosis in both ApoE-/- mice and in a model of locally defined atherosclerosis through flow restriction (partial carotid artery ligation with high fat diet) [421]. Similar evidence has been obtained in an LDL receptor knockout mouse [422]. On the other hand, endothelial specific overexpression of Nox4 protected ApoE-/- mice against high fat diet induced atherosclerosis [423]. Notably, Nox4 expression is reduced in diabetic patients who develop atherosclerotic plaques when compared to diabetic individuals without atherosclerosis [424]. In conclusion, various forms of stress induce an increased expression of Nox4 that elicits protective mechanisms at least in the vascular system. Importantly, there is evidence that the beneficial role of NOX4 in vascular injury occurs not only in mice, but it may also apply to clinical settings.
Matsui and Bachschmid propose that angiogenesis is redox regulated by the formation of protein glutathione adducts. Oxidative post-translational modification may alter protein functions and mediate cellular signaling. Protein thiols form reversible oxidative modifications including S-sulfenylation (-SOH) and S-nitrosylation (-SNO) [425], which may react with glutathione (GSH), an abundant intracellular thiol, to form more stable GSH-protein adducts (S-glutathionylation) [426]. Protein GSH-adducts can regulate enzyme activity, localization, protein interactions and stability. Various proteins are known to be modified by GSH adducts [427]. GSH adducts can be removed by glutaredoxin-1 (Glrx), a cytosolic thioltransferase that in this way completes the redox signaling cycle. The in vivo role of Glrx and its protein targets in pathophysiology has been explored recently [428], [429], [430]. Oxidants are increasingly recognized as factors that promote angiogenesis, and mouse studies revealed that decreasing oxidants may impair ischemic revascularization after hind limb ischemia [164]. Conversely, increasing oxidants by NOX4 or decreasing the antioxidant response by means of Nrf2 deletion may improve ischemic limb revascularization. These data put forward the concept that oxidants play a protective role in ischemic revascularization, in accordance with multiple reports on ROS conferring beneficial redox signaling in other settings. Glrx is not merely an antioxidant, but obviously also exhibits anti-angiogenic properties. Increased Glrx expression inhibits the angiogenic activity of cultured endothelial cells [431]. Consistent with this finding, Glrx overexpression in vivo also attenuates revascularization after hind limb ischemia [430]. Glrx is known to activate the NF-κB pathway [432]. NF-κB hyper-activation in endothelial cells likely stimulates non-canonical Wnt5a signaling, which induces expression and release of the soluble vascular endothelial growth factor (VEGF) receptor 1 (sFlt-1) into plasma [430]. sFlt-1 is a VEGF decoy receptor, blocking VEGF binding to the proangiogenic VEGF receptor 2. Impaired ischemic revascularization in diabetic mice is associated with elevated levels of sFlt-1 in the ischemic muscle. Because Glrx expression is also NF-κB dependent, proinflammatory conditions such as atherosclerosis [433] and diabetes [432] may increase Glrx leading to impaired or dysregulated angiogenesis. Ablating endogenous Glrx further accelerates ischemic limb revascularization. Hypoxia-inducible factor (HIF-1α), a major angiogenic transcription factor, is stabilized by GSH adducts [428]. In normoxia HIF-1α is hydroxylated and binds to ubiquitin ligase pVHL, which targets HIF-1α to proteasomal degradation. GSH adducts, induced by oxidants or Glrx inhibition, stabilize and activate HIF-1α. As previously reported, nitric oxide-induced S-nitrosylation of HIF-1α [434] may also be converted into more stable GSH adducts in the presence of GSH. Watanabe et al. confirmed a HIF-1α GSH adduct of Cys520 (mouse Cys533) by mass spectrometry and demonstrated increased expression of HIF-1α and VEGF-A in ischemic muscle of Glrx KO mice [428]. Increased GSH protein adducts in ischemic limbs are not a hallmark of oxidative stress, but rather contribute to beneficial responses to ischemia through HIF-1α activation. This may be a mechanism by which oxidants promote ischemic revascularization. In summary, Glrx deletion may facilitate revascularization of ischemic muscles not only by means of HIF-1α stabilization, but also by an increase in GSH adducts of other proteins involved in angiogenesis. Therefore, inhibiting Glrx can be a therapeutic strategy to restore circulation in ischemic limbs.
6.3. RONS formation in ischemic preconditioning, cardiac cycle, ischemia/reperfusion and cardiomyopathy
According to Andreadou, low levels of RONS may be associated with beneficial cardioprotection by interventions known as ischemic pre- and post-conditioning (PC, PostC). Cardiac injury associated with post-ischemic reperfusion is contributed mostly by an increased level of reactive oxygen and nitrogen species (RONS), but also by the reduced availability of “beneficial” reactive species such as nitric oxide and intracellular Ca2+ overload. These deleterious factors synergize in favoring a prolonged opening of the mitochondrial permeability transition pore (mPTP) that is generally considered as a determining factor in ensuing cell death. However, despite the well-established association between RONS elevation and reperfusion injury of the heart, so far antioxidant treatments have hardly provided any therapeutic benefit in clinical studies of cardiac disease [435], [436]. On the other hand, reperfusion injury is greatly reduced by PC and PostC [435], [437]. Although the cardioprotective mechanisms triggered by conditioning protocols are still a matter of debate [438], a general consensus exists that RONS play a crucial role. Indeed, while an excessive formation of RONS contributes to irreversible injury, small amounts of RONS contribute to protection, possibly through a redox-dependent activation of protective cytosolic kinases [439]. In this respect RONS share the same paradox with the conditioning phenomena per se, in that a short ischemia/reperfusion episode protects the same as a mild RONS elevation, whereas a prolonged duration of ischemia followed by reperfusion induces injury that largely depends on lethal levels of intracellular RONS [439]. The role of antioxidant compounds in cardioprotection induced by conditioning strategies is an emerging issue, which needs elucidation in order to provide useful information for the translation of the conditioning phenomena in clinical practice [438]. We should mention that PC is mediated in part by a mild formation of RONS, possibly in response to the opening of mitochondrial KATP channels, and also PostC may lead directly or indirectly to a decrease in RONS [440]. Since the role of antioxidant compounds in these conditioning phenomena might differ to some extent, it is important to distinguish their role in pre-and/or postconditioning separately. Several in vivo studies have thus far shown divergent results concerning the role of widely used antioxidants in the prevention and/or abrogation of the beneficial effects of PC in reducing myocardial infarct size. Skyschally et al. [441] demonstrated that the administration of ascorbic acid in pigs abolished the beneficial effect of PC on infarct size possibly due to ROS scavenging [442]. Accordingly, we also showed that in rabbits the antioxidant action of the acute administration of vitamin C, reflected by a decrease in blood and tissue levels of lipid peroxidation products, abolished PC-induced protection [441]. It is worth pointing out that the use of antioxidants is increasing. This is related especially to the commercialization of numerous nutritional supplements or plant extracts containing antioxidant compounds, such as polyphenols and flavonoids that are marketed for the prevention of cardiovascular diseases [443]. Based upon the dependence of endogenous protective mechanisms on ROS generation, it is essential to know the role of all the antioxidant compounds in different physiological and pathological conditions of the cardiovascular system. The complete understanding of redox mechanisms controlling RONS levels in cardiovascular pathophysiology is likely to allow the design of new clinical trials for the use of antioxidants in cardiac diseases.
According to Tuncay and Turan, Zn2+ release during the cardiac cycle results in increased intracellular free Zn2+([Zn2+]i) levels, and this release is increased in the setting of oxidative stress [444], [445]. However, it is not known whether or not there is a direct relationship between the increased production of RONS and [Zn2+]i changes in cardiomyocytes. Thus, by using confocal microscopy and the specific fluorescence dye FluoZin-3 AM (3 µM), [Zn2+]i changes were monitored in a H9c2 cardiomyoblast cell-line exposed to RONS. Acute increases in hydrogen peroxide (H2O2, 100 μM) induced marked increases in [Zn2+]i, which could be reversed by a thiol reducing agent like dithiothreitol (DTT, 500 μM) (Fig. 6.2A). The NO donor NaNO2 (100 µM) induced similar marked increases in [Zn2+]i, which was not observed in the presence of a selective/potent soluble guanylyl cyclase inhibitor like ODQ and was normalized with a heavy-metal Zn2+ chelator like TPEN (Fig. 6.2B).
Fig. 6.2.
The data show marked increases of [Zn2+]i under either ROS (A) or RNS (B) increases. Bars represent means (±) and *P<0.05 w.r.t. initial value. Inset: Representative electron micrograph images under ZnPT (1-μM) exposure. Magnification: x12,930; bar: 1000 nm; N: nucleus, M: mitochondria, z: Z-line, L: lysosome, arrow: T-tubule, arrow head: sarco/endoplasmic reticulum (SER).
Electron microscopic analysis also demonstrated that increased [Zn2+]i can induce marked alterations in the ultrastructure of rat cardiomyocytes such as clustering of mitochondria, disruption and damage of myofibrils, enlargement in T-tubules and distortion in the T-tubules (TT) and sarcoplasmic reticulum (SR) intersection (Fig. 6.2, inset). Acute increases in RONS can induce marked elevation of [Zn2+]i in cardiomyocytes, which may underlie cardiac dysfunction under oxidative stress. In conclusion, in cardiomyocytes, elevated [Zn2+]i correlates with increased RONS levels.
Mulvey and Krieg highlight the important role of mitochondrial metabolism and RONS formation in myocardial ischemia. RONS have long been known to be key mediators of ischemia/reperfusion (IR) injury, driving not only acute damage but also initiating the pathological cascade that develops over the subsequent weeks and months. This RONS production has generally been assumed to be a non-specific effect of oxygen interacting with a dysfunctional mitochondrial respiratory chain upon its reintroduction to ischemic tissue at reperfusion, a process, which is complex and imprecise. However, recent work from our laboratory shows that contrary to this hypothesis there is in fact a distinct metabolic mechanism responsible [136]. Using an untargeted metabolomic approach, a metabolic hallmark of ischemia was identified from a range of ischemic tissues, which notably included the selective accumulation of succinate. Despite previous descriptions of this in the literature [446], neither the mechanism behind this nor its implications had been characterized. The succinate accumulated during ischemia was found not to originate from normal cardiac metabolism, but rather through aspartate-mediated pathways and reverse action of succinate dehydrogenase (SDH), driven by accumulated ischemic NADH passing electrons through complex I and onto the coenzyme Q (CoQ) pool, which favors the reduction of fumarate by SDH to drive succinate accumulation. Following reperfusion there was then a rapid metabolism of is chemically accumulated succinate, with baseline levels re-established within 5 min [136], [447]. RONS generation from electrons stored in the succinate pool is then thought to occur primarily through the reverse action of complex I in a phenomenon known as reverse electron transport (RET) that has been well characterized in vitro but whose importance in vivo has only been recently understood [448]. Importantly, this model provided several testable predictions with regard to the effect of manipulating succinate levels in ischemic tissues and therefore the downstream extent of RONS production and also infarct size as a clinically relevant indicator of IR injury. Decreasing mitochondrial succinate levels during ischemia using either an infusion of the competitive SDH inhibitor dimethyl malonate [136] or sodium malonate [449] resulted in a decrease in RONS production and reduced infarct size in an in vivo mouse model of myocardial IR injury. These could both be brought back to control levels by an infusion of dimethyl succinate. In addition to modulation of mitochondrial metabolism, recent evidence suggests it is also possible to modulate the quantity of RONS produced at reperfusion from the electrons stored in the ischemic succinate pool through inhibition of the active/deactive transition of Complex I. During ischemia, Complex I enters the deactive state but is rapidly reactivated at reperfusion, enabling it to support RONS production by RET [450]. A growing body of evidence suggests a central role for the Cys39 residue found within the ND3 subunit of Complex I, and indeed it has recently been demonstrated using the mitochondria-targeted S-nitrosothiol MitoSNO that S-nitrosation of this residue can inhibit the active/deactive transition and so allow dissipation of the mitochondrial ischemic succinate pool via alternative pathways to minimize the production of RONS by Complex I [451].
Based on considerations by Görbe, Giricz and Ferdinandy, mitochondrial RONS play an important role in metabolic cardiomyopathies. A growing body of evidence indicates that mitochondrial oxidative stress has a major role in the development of cardiomyopathies in metabolic diseases [452], [453], [454]. Cardiac function relies heavily on intact mitochondrial function including mitochondrial biogenesis, fusion, fission, and autophagy-mitophagy. Disturbances in these processes have been linked to increased mitochondrial oxidative stress and the development of metabolic cardiomyopathies. Several oxidative and nitrative stress-related cellular processes have been shown to be deranged in metabolic disorders. In a rat model of hypercholesterolemia enhanced cellular peroxynitrite formation due to an upregulation of NOX4 has been described and discussed as a potential mechanism of cardiovascular complications [455], [456] – of note, this finding highlights that NOX4 can play different roles in different organs or disease settings depending on whether it confers beneficial redox signaling or contributes to excessive RONS formation. In the same model, mitochondrial expression of connexin43, which may reduce mitochondrial reactive oxygen species production, was decreased [457]. Evidence obtained in models of metabolic syndrome suggests that mitochondrial oxidative stress is linked to cardiac dysfunction. Indeed, in high-fat diet (HFD)-induced mouse models of metabolic syndrome the elevation in myocardial mitochondrial RONS production was associated with reduced diastolic circumferential strain rate assessed by tagged cardiac magnetic resonance imaging [458]. In addition, in mice, diastolic dysfunction induced by a high-fat high-sucrose (HFHS) diet was accompanied by a 3-fold greater rate of mitochondrial H2O2 production along with a decrease in both oxygen consumption and ATP synthesis. In this latter model transgenic expression of mitochondria-targeted catalase alleviated oxidative stress and improved diastolic function [459] indicating that mitochondrial ROS formation is causally linked to contractile impairment. This relationship is further supported by pharmacological approaches suggesting that decreasing mitochondrial RONS levels might have a great therapeutic potential – keeping in mind that RONS can also have beneficial effects as discussed for preconditioning phenomena above [438], and therefore the use of antioxidants is always a two-edged sword that needs to be used with great caution. Diabetic mice treated with a mitochondria-targeted antioxidant, MitoTEMPO, showed preserved heart rates and improved survival after myocardial infarction [460] along with a decrease in mitochondrial RONS generation, apoptosis and myocardial hypertrophy [461]. The latter observations were also obtained ex vivo in hyperglycemic cultured cardiomyocytes [461]. Drug candidates other than direct antioxidants have also shown efficacy in the treatment of metabolic cardiomyopathies. Mitochondrial RONS cause opening of the mPTP channels in which cyclophilin D has a major role. A novel inhibitor of cyclophilin D, NIM811, reduced infarct size in diabetic rats [462]. Mitochondrial aldehyde dehydrogenase-2 (ALDH-2), an enzyme responsible for the removal of cardiotoxic aldehydes, is activated by physiological levels of mitochondrial RONS. ALDH-2 overexpression is reported to reduce diabetic cardiomyocyte hypertrophy and contractile dysfunction, while activation of ALDH-2 by Alda-1 has been shown to alleviate high glucose-induced apoptosis and the reduction in mitochondrial membrane potential in H9C2 cells [463]. Although the above reports connect increased mitochondrial RONS production to metabolic cardiomyopathies, a few studies showed a lack of correlation between mitochondrial RONS formation and diabetic cardiomyopathy [464], [465]. These findings suggest that mitochondrial oxidative stress might not be present in all models and at all stages of metabolic diseases and that it might not be the common underlying mechanism of metabolic cardiomyopathies, again, warranting caution with the use of antioxidants although the targeted modulation of mitochondrial RONS production and its downstream targets might represent applicable future therapeutic strategies.
6.4. RONS formation in cerebral ischemia and stroke
As pointed out by Casas and Schmidt, NADPH oxidase and nitric oxide synthase derived RONS represent potential targets for stroke therapy. Stroke is the leading cause of neurological impairment in industrialized countries, making it the second leading cause of death worldwide and the primary cause of disability. Despite this high medical and social need, no neuroprotective agent is available for clinical therapy and only a single drug reached the market, the anti-thrombolytic drug rt-PA for ischemic stroke. However, due to its multiple contraindications almost 85% of all stroke patients are excluded from receiving this treatment. RONS have been considered as key players in post-stroke neurodegeneration [466]. Targeting pathologically relevant sources of RONS, such as NOX and NOS, may thus provide promising innovative therapeutic approaches. NADPH oxidases, which include the already mentioned NOX4, constitute the only known enzyme family with the sole function to produce O2•¯ and H2O2. The NOX family consists of seven isoforms: NOX1-5 and the dual oxidases (DUOX 1–2). Due to accessory proteins, each isoform has a distinct quaternary structure, activity regulation, tissue expression and product. Therefore, not all isoforms may contribute equally to ischemic injury [32]. Based on a systematic review and meta-analysis, NOX1 seems to play no role in stroke, neither in infarct size reduction nor in neurological outcome. Similarly, the evidence for NOX2 is extremely contradictory and more or less disproven by a pre-clinical randomized controlled trial with the aim to validate NOX2 as a stroke target. This study turned out negative highlighting a persistent publication bias towards positive findings and the lack of statistical power in many studies [467]. Conversely, brain ischemia is one of the best-validated disease indications for NOX4. Under hypoxic conditions, NOX4 is upregulated leading to oxidative damage, cytotoxicity and neuronal death. Therefore, NOX4 inhibition has been recently considered as a promising target for this pathology. Preclinical results show that NOX inhibition by VAS2870 significantly reduces infarct size and post-stroke RONS formation, suggesting indeed a major contribution of this NOX. Further pre-clinical experiments are being currently conducted using specific NOX4 inhibitors and different animal models of stroke [468]. Similarly, nitric oxide is also considered a member of the RONS family, generated by different NOS isoforms. Studies in mice showed that deletion of neuronal NOS (isoform 1) leads to a significant reduction of post-stroke brain damage, suggesting therapeutic targeting of NOS-1. In fact, it has been recently reported that NOS inhibition (L-NAME) significantly reduces tissue damage and infarct size post-stroke using both in vitro and in vivo ischemia models [469]. However, this was not the case for NOS1-PSD-95 interaction inhibitors, possibly by also interfering with other, protective pathways. Pharmacological targeting of NOX and NOS dependent oxidative stress clearly has neuroprotective effects and reduces infarct volume and RONS production after stroke leading to improved neuronal function and survival. Current and future experiments are aimed at validating these findings in phase II and III clinical trials in other rodent and large animal species for further clinical development as first-in-class neuroprotective treatment of stroke.
6.5. Therapeutic targeting of eNOS uncoupling and cardiovascular oxidative stress and inflammation
Based on considerations by Li and Förstermann, pharmacological prevention of eNOS uncoupling is another antioxidant therapeutic strategy that may not interfere with the protective role of redox signaling in the cardiovascular system [9]. Oxidative stress plays a crucial role in the pathogenesis of cardiovascular disease. Among the major producers of RONS, the uncoupled endothelial nitric oxide synthase (eNOS) makes a significant contribution to RONS generation in cardiovascular tissues [470], [471]. Under physiological conditions, eNOS produces •NO. Endothelium-derived •NO has anti-hypertensive, anti-thrombotic and anti-atherosclerotic properties by relaxing blood vessels, inhibiting platelet aggregation/adhesion, preventing leukocyte adhesion/migration, and inhibiting smooth muscle cell proliferation [472]. Under pathological conditions associated with oxidative stress and inflammation, eNOS can be converted from a •NO-producing enzyme to a superoxide-generating molecule, a process termed eNOS uncoupling. All cardiovascular risk factors, such as diabetes, hypertension, hypercholesterolemia and smoking, may induce eNOS uncoupling [472]. A number of mechanisms may contribute to eNOS uncoupling [9], [473], with deficiency of the eNOS cofactor tetrahydrobiopterin (BH4), deficiency of the eNOS substrate L-arginine, and eNOS S-glutathionylation being the most important ones. Peroxynitrite and superoxide can oxidize BH4 to dihydrobiopterin (BH2), leading to BH4 deficiency. A lack of BH4 can also be caused by a reduction of BH4 de novo synthesis (e.g. due to down-regulation of the BH4-synthesizing enzyme GTP cyclohydrolase I) or by a decrease of BH4 regeneration from BH2 (e.g. down-regulation of the recycling enzyme dihydrofolate reductase). A recent study demonstrates that BH4-dependent and S-glutathionylation-induced eNOS uncoupling are mechanistically independent but functionally linked [474]. L-Arginine deficiency can be caused by inflammation-induced up-regulation of arginases, enzymes that compete with eNOS for the same substrate. By producing superoxide, uncoupled eNOS augments the pre-existing oxidative stress and further enhances eNOS uncoupling, creating a vicious circle. Interestingly, some established cardiovascular drugs (and some other compounds) show the potential to prevent eNOS uncoupling. The recoupling of eNOS may represent pleiotropic effects of these drugs and contribute to their therapeutic benefit. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II type-1 (AT1) receptor antagonists, statins, the organic nitrate pentaerythritol tetranitrate (PETN), eNOS transcription enhancers and the red wine polyphenol resveratrol have been shown to reverse eNOS uncoupling in disease models by elevating tissue BH4 levels [475], [476]. PETN [477] and the AT1 receptor blocker telmisartan [478] additionally prevent eNOS S-glutathionylation. Furthermore, arginase inhibition also represents a promising strategy to recouple eNOS [479], [480].
As suggested by Muzykantov, the previously described detrimental but also beneficial effects of RONS warrant careful targeting without interfering with the beneficial signaling pathways by using site- and species-specific approaches [9]. Liposomes and other nanocarriers improve the pharmacokinetics of antioxidants including N-acetyl cysteine (NAC) and antioxidant enzymes (AOE, i.e. catalase and SOD) and thus help alleviate vascular oxidative stress predominantly via detoxification of extracellular RONS [481]. In order to achieve targeted endothelial interventions, these agents can be conjugated with antibodies to endothelial determinants including cell adhesion molecules ICAM [482] and PECAM [483]. In preclinical studies, diverse formulations of such AOE conjugates (Ab/AOE) show binding to endothelium after i.v. injection [482]. Targeting of these formulations qualitatively improves protection of endothelial cells from extracellular RONS [484] and RONS produced inside these cells [485]. As a result, Ab/AOE injected into animals attenuated ischemia-reperfusion injury in lungs of diverse species [486], [487], normalized vasoreactivity in angiotensin II-challenged mice [487], inhibited endothelial pro-inflammatory activation caused by cytokines and potentiated the anti-inflammatory effect of NO donors [485]. Further, an indirect inhibitor of NADPH-oxidase loaded in Ab/liposomes accumulated in endothelial cells, inhibited RONS production and provided more potent protection vs. non-targeted counterparts against oxidative stress in mice [488]. Ab/liposomes loaded with EUK-134, a superoxide dismutase/catalase mimetic, bound to endothelial cells and alleviated endotoxin-induced lung inflammation in mice [489]. Likewise, PECAM-targeted nanocarriers loaded with tocopherol and EUK-134 alleviated endothelial inflammatory activation. Encapsulation in polymeric nanocarriers protects AOE from proteases [490]. Modulation of geometry and affinity features of targeted AOE formulations enables their delivery into endothelial endosomes, quenching RONS produced in these vesicles and intercepts pro-inflammatory endothelial signaling and abnormal activation [485]. Endothelial targeting of antioxidants enables anti-inflammatory mechanisms based on interception of endothelial RONS [9]. This drug delivery strategy may find utility in the management of acute vascular oxidative stress and inflammation (Fig. 6.3).
Fig. 6.3.
Overview on therapeutic options for the improvement of vascular dysfunction. Targeted antioxidant interventions to alleviate pro-inflammatory activation and oxidative stress in endothelial cells. Endothelial ROS from activated NOX2 enzyme in endosomes are formed in response to cytokine binding to the receptors and ignite signaling cascade of transcription factor NFκB. Targeted delivery of antioxidants, antioxidant enzymes (AOE) and inhibitors of ROS production can be achieved using antibodies and other ligands of endothelial surface determinants including cell adhesion molecules PECAM and ICAM. Surface-bound targeted AOE intercept extracellular ROS, whereas targeted formulations using the same ligands configured in a way permitting internalization into the ROS-signaling endosomes allows interception of pro-inflammatory activation manifested among other characteristics by exposure of inducible cell adhesion molecules – E-selectin, VCAM-1, and ICAM-1 - that can be detected using imaging probes conjugated to the ligands of these molecules. With permission of the publisher. Copyright © 1999–2017 John Wiley & Sons, Inc. All Rights Reserved.
Adapted from [9].
As explained by Lazou, accumulating evidence supports a key role for peroxisome proliferator activated receptors (PPARs) in regulating the redox state in the cardiovascular system through transcriptional or post-translational effects and thereby controls the redox balance in cardiac diseases [491]. PPARs are ligand activated transcription factors that belong to the superfamily of nuclear hormone receptors, with well-documented roles in the transcriptional regulation of cardiac lipid metabolism and energy homeostasis. Their non-metabolic, anti-oxidant, anti-ischemic and anti-inflammatory properties have emerged over the past years and are being actively investigated in relation to cardiac dysfunction. All three PPAR isoforms (α, β/δ, γ) have been implicated in the modulation of oxidative stress, although different mechanisms may be employed by each of them. PPARα and PPARγ function in a similar way towards the modulation of oxidative stress, mostly through the transcriptional regulation of target antioxidant enzymes. PPARα activation via clofibrate diminishes RONS production and lipid peroxidation in rat hearts subjected to acute myocardial ischemia through the upregulation of transcription and activity of antioxidant enzymes such as Cu/Zn-SOD (SOD1), Mn-SOD (SOD2), and catalase in the heart tissue as well as the suppression of AT-1 induced NADPH activity [492]. The PPARα agonist, WY14643, ameliorated oxidative stress in a rat model of IR [493], which is reflected by HO-1 down-regulation post IR in WY14643-treated myocardium. PPARγ agonists like troglitazone, rosiglitazone and pioglitazone induce glutathione peroxidase (GPx 3) in human skeletal muscle cells and thioredoxin in neonatal rat cardiac myocytes [491]. RONS generation was also suppressed in cultured cardiac myocytes treated with a PPARβ/δ agonist due to upregulation of catalase [494]. PPARβ/δ also inhibits RONS generation in vascular muscle cells through inhibition of NADPH oxidases [495]. The effect of PPARβ/δ activation on antioxidant defense may also be attributed to improved mitochondrial biogenesis through regulation of target genes such as PGC-1 and mitofusin 2 [496]. Upregulation and activation of PPARs have been also implicated in the endogenous mechanisms of cardioprotection implying that PPAR activation prior to IR could confer preconditioning-like protection to the myocardium. Activation of the PPARα isoform results in significant anti-infarct protection, comparable with the effect of classical ischemic preconditioning that appears to involve survival cascades (ERK1/2 and PI3K/Akt), upregulation of eNOS and the opening of mitochondrial KATP channels [497], [498]. There are several remaining issues that need to be addressed regarding the biological role of PPARs as regulators of the cardiac redox state, especially regarding the translation of in vitro findings in the in vivo setting. However, the pleiotropic activity of these receptors makes them interesting therapeutic targets for the development of antioxidant strategies that aim to control the intracellular redox balance in various cardiac pathologies, especially linked to dyslipidemia, atherosclerosis, and diabetes, that are frequently associated with cardiovascular disorders.
Steven and Daiber emphasize the importance of the pleiotropic antioxidant, anti-aggregatory and anti-inflammatory potential of established drugs as exemplified by DPP-4 inhibitors and GLP-1 analogs [19]. Glucagon-like peptide-1 (GLP-1) is an incretin hormone and released from L-cells in the intestine after food uptake [499], [500]. Its receptor belongs to the family of G-protein-coupled receptors and binding of GLP-1 induces insulin release from beta cells of the pancreas. GLP-1 is involved in glycemic control and due to rapid degradation by the exopeptidase dipeptidyl peptidase-4 (DPP-4) its half-life is below two minutes [501], [502]. Accordingly, inhibition of DPP-4 and supplementation of GLP-1 represent new therapeutic targets for the management of diabetes. Besides this first line indication, there are several reports on beneficial effects of DPP-4 inhibition on cardiovascular disease associated with atherosclerosis [503], [504], but also with psoriasis [505], hepatic steatosis [506] and stroke [507]. All of these diseases have in common, that inflammation and oxidative stress contribute to their pathophysiology. It was previously shown that DPP-4 inhibition suppresses the proinflammatory phenotype of isolated myelomonocytic cells and proinflammatory cascades in endotoxemic rats [508]. Investigation of the effects of DPP-4 inhibition and GLP-1 supplementation on endotoxemia and septic shock revealed improved survival of septic mice (lipopolysaccharide i.p. injection), even when the therapy with the DPP-4 inhibitor linagliptin or the GLP-1 analog liraglutide was started six hours after induction of endotoxemia [371]. The improvement of mortality is based on the control of the initial inflammatory response, which characterizes LPS-induced endotoxemia. The oxidative burst of inflammatory, myelomonocytic cells, oxidative stress measured by dihydroethidium fluorescence, expression of typical inflammatory genes, as well as vascular infiltration of CD11b+ cells was decreased in endotoxemic animals treated with linagliptin and liraglutide. As a consequence, hemodynamic control was recovered by linagliptin and liraglutide treatment preventing the development of lethal hypotension in the endotoxemic animals, all of which was based on activation of the AMP-dependent protein kinase (AMPK) [371]. Our data suggest that DPP-4 inhibitors and GLP-1 analogs have inhibitory effects on myelomonocytic cells, which in the case of DPP-4 inhibition do not entirely rely on the GLP-1 receptor [372]. Inflammation and hemostasis are subject to a complex interplay in the setting of sepsis. Thrombocytopenia and disseminated intravascular coagulation (DIC), characteristic features leading to end organ damage and death in septic shock, were significantly improved by both drugs [372]. Similar results were obtained in an animal model of experimental thrombosis [509]. Further support of this concept was based on the observation that GLP-1 analogs inhibit platelet activation and aggregation directly via the GLP-1 receptor, which is expressed on murine and human thrombocytes, and by cAMP/adenylyl cyclase signaling [372], [509]. In conclusion, sepsis is still a main cause of death all over the world. DPP-4 inhibition and GLP-1 supplementation reduced the mortality in endotoxemic animals by antioxidant, anti-inflammatory and antiaggregatory effects (Fig. 6.4). The potential use of these drugs in patients with sepsis or other inflammatory diseases needs further exploration.
Fig. 6.4.
Proposed mechanisms of lipopolysaccharide (LPS)-induced vascular dysfunction and improvement by linagliptin therapy. LPS treatment activates white blood cells (WBC, envisaged by increased oxidative burst), increases serum levels of xanthine oxidase (XO), increases DPP-4 serum activity and activates vascular cells (detected by expression of endothelial adhesion molecules and inducible cyclooxygenase [COX-2]). This leads to the infiltration of WBC to the vascular wall (detected by aortic FACS analysis for myelomonocytic cells, inducible nitric oxide synthase [NOS2], NOX2 and myeloperoxidase [MPO] expression) and oxidative damage of the vasculature (NOX1 expression, ROS formation, 3-nitrotyrosine levels and lipidperoxidation by malondialdehyde [MDA]). Finally, the tissue damage results in smooth muscle constriction and endothelial dysfunction. With permission by Oxford University Press. Copyright © 2012.
Adopted from [508].
6.6. Conclusion
Evidence derived by experimental approaches exploiting powerful tools of molecular and cell biology is rapidly advancing our understanding of redox reactions involved in RONS formation. These molecules, which were once investigated only for their possible involvement in numerous diseases, are now attracting wide interest for their role as intracellular signals, especially those in response to metabolic or mechanical changes. Therefore, besides the well-established notions that high intracellular levels of RONS impair function and viability of practically any cell type, a large body of evidence indicates that ROS are involved in countless physiological processes and trigger powerful mechanisms of protection against cell injury.
In this review section we summarized established concepts and recent advances supporting the dual role of RONS in cardiovascular pathophysiology. These novel findings might support the therapeutic potential of antioxidant interventions and explain the failure of clinical trials using this approach. Indeed, the issues that remain to be elucidated outnumber those that have been clarified.
7. ROS and the aging process
Jose Vina (E-mail: Jose. Vina@uv.es) and Gloria Olaso-Gonzalez (E-mail: gloria.olaso@uv.es).
7.1. Introduction
The free radical theory of aging postulated by Harman in 1956 has provided a theoretical framework for research oriented toward understanding the mechanisms of aging. This theory states that “aging and the degenerative diseases associated with it are attributed basically to the deleterious side attacks of free radicals (ROS) on cell constituents and on the connective tissue” [510]. Mitochondria have a major role in ROS production [511]. On the other hand, NADPH oxidases (NOXs) are important enzyme systems involved in inducible ROS formation, since they catalyze the partial reduction of O2 to form ROS. It has been shown that the enhanced expression and/or activity of NOX family members, in particular NOX4, plays an important role in age-associated diseases such as cardiovascular disease, fibrosis, cancer and neurodegenerative diseases like Alzheimer's disease [512], [513]. In this situation of misbalance, proteasome-mediated degradation of oxidized proteins is a critical player for protein homeostasis maintenance. Proteasome dysfunction takes place during aging [514]. Proteasome up-regulation in terms of assembly, quantity and function has been achieved through genetic manipulation in animal models. This activation was shown to be successful to decelerate aging progression by enhancing resistance to oxidative stress. However, genetic manipulation is not applicable in humans, so new studies should focus on the identification of nutritional, pharmacological or physiological interventions with proteasome activating properties.
Oxidative stress has been linked with age-associated diseases, but also with a geriatric syndrome characterized by diminished functional reserve and increased vulnerability to low power stressors, i.e. frailty (Fig. 7.1). An association between systemic oxidative stress biomarkers (malondialdehyde, isoprostanes, protein carbonylation and lipoprotein phospholipase A2) and frailty has been reported in the geriatric population [515], [516]. Nevertheless, recent data have shown that lower expression of genes related to antioxidant responses to oxidative stress in older people is associated with a higher risk of being frail independently of age and sex (see El Assar et al. contribution, Section 7.6).
Fig. 7.1.
Scheme summarizing the main concepts of the free radical theory in aging and development of aging associated diseases and syndromes.
In recent years, epidemiological as well as laboratory data have shown that antioxidant supplementation is at least useless if not detrimental in aging [517]. Indeed, antioxidant supplementation did not lower the incidence of many age-associated diseases but, in some cases, increased the risk of death [518].
This has cast doubts on the classical “free radical theory of aging”. We thus proposed “the cell signaling disruption theory of aging”. This theory postulates that “ROS” cause aging inasmuch as they alter—sometimes irreversibly—the signaling network of the cell. If the cell can cope with the stress caused by relatively mild action of ROS, then adaptation takes place and damage does not occur. If, however, the cell is overwhelmed by the action of radicals, subcellular damage and aging will take place”. Indeed, radicals serve as signals and interaction between them is tightly balanced. In this sense, we cannot support the idea of proposing antioxidant supplementation for the general population. It is much better to increase endogenous defenses by nutritional or physiological manipulation than administering antioxidant compounds, such as vitamin C or E. In any case, these considerations do not detract from the free radical theory of aging, which has been extremely useful and has fostered research by providing a general theoretical framework on which many of us have based decades-long experimental research.
7.2. NADPH oxidases in aging and age-associated diseases
Pidder Jansen-Dürr and Rafal Koziel summarize that superoxide anions and other ROS can exert beneficial effects under normal conditions through adaptive cellular signaling responses. On the other hand, ROS are able to induce direct damage to biologically sensitive targets like lipids, proteins and nucleic acids, and their overproduction is involved in both chronic diseases and age-related diseases. To date, a variety of different theories of biological aging have been discussed [519], [520]; however, many of them describe ‘oxidative stress’ as the common cause of aging.
The ‘free radical theory of aging’ predicts the major role of mitochondria-derived ROS in aging [510], however, recent data indicate a key role of other ROS sources in this process. A significant number of enzymatic or chemical processes are capable of producing ROS in vivo; however, the NOXs are the primary enzyme systems involved in inducible ROS formation [65], [521], [522]. The NOX family of membrane-associated enzymes consists of seven isoforms, Nox1-5 and Duox1-2, and catalyzes the reduction of O2 to form ROS [512], [521]. The role and mechanism of the activation of NOXs is isoform type, intracellular localization as well as tissue type specific, and NOX mediated oxidative stress is strongly associated with a variety of human age-related diseases [523](see also below). A growing number of studies indicate that NOX4 is localized to mitochondria in many cell types and promotes aging. We have previously suggested a new pathway by which sustained NOX4 activity decreases mitochondrial function and induces premature senescence in human umbilical vein endothelial cells (HUVEC). NOX4 induced premature senescence by decreasing the concentration and activity of mitochondrial respiratory chain complex I [524], [525]. Accordingly, NOX4 knockdown reduced mitochondrial H2O2 production and markers of oxidative DNA damage, increased the cellular proliferation rate and prolonged the replicative lifespan by more than 2-fold [524], [525]. It remains to be shown if increased NOX4 activity contributes to aging of the vasculature in mammals.
The effector role of NOX4, a constitutively active NOX isoform, to drive cellular senescence is conserved for oncogene-induced senescence, a tumor suppressor pathway that restricts the growth of pre-neoplastic cells in mice and humans. Weyemi et al. found that NOX4 is a critical mediator in the oncogenic H-RasV12-induced DNA-damage response and subsequent senescence [526]. H-RasV12 overexpression correlated with increased NOX4 expression, higher ROS levels, DNA damage, histone H2A.X phosphorylation and p21Cip1 accumulation. Similar conclusions were reached by Kodama et al. who showed that NOX1- and NOX4-generated ROS play an important role in Ras-induced premature senescence, which may involve the DNA damage response and p38MAPK signaling pathways in primary human lung TIG-3 cells. Both NOXs were upregulated by the Ras oncogene. Ablation of Nox1 and Nox4 by small interfering RNAs (siRNAs) blocked the RasV12 senescent phenotype including β-galactosidase activity, growth arrest and accumulation of tumor suppressor proteins such as p53 and p16Ink4a. The involvement of Nox1 in Ras-induced senescence was also confirmed with embryonic fibroblasts derived from Nox1 knockout mice [527]. Another study suggested an important role of NOX4 in oncogene-independent senescence of hepatocellular carcinoma cells [528].
Of interest is compelling evidence that enhanced expression and/or activity of NOX family members, in particular NOX4, plays an important role in age-associated diseases, including cardiovascular disease, fibrosis and cancer. In the vasculature, NOXs are a major source of ROS and are key players in mediating redox signaling under both physiological and pathological conditions. Cardiovascular disease (CVD) is the leading cause of death, and aging is a major risk factor for CVD development. A substantial number of studies describe the role of NOX4 in the age-related pathology of the cardiovascular system. It was shown that NOX4 has an impact on vasoconstriction, atherosclerosis development, vascular cells hypertrophy, apoptosis and differentiation. One of the major age-related arterial phenotypes thought to be responsible for the development of CVD in older adults is endothelial dysfunction. It was speculated that mitochondrial oxidative stress rises when antioxidants are unable to counteract the ROS produced by NOX4, triggering the aging process of the heart [529]. Wang et al. [530] examined the involvement of NOX in age-associated cardiac remodeling in a rodent model of aging and found that age-dependent increases in blood pressure, cardiomyocyte hypertrophy, coronary artery remodeling and cardiac fibrosis were associated with increased myocardial NOX2 activity. Another study indicates that NOX4 and mitochondrial oxidative stress, but not NOX1 or NOX2, are mediators of CVD in aging mice under hyperlipidemic conditions [531]. Whereas initial results obtained with Nox4 KO mice did not reveal striking phenotypical differences relative to wild type mice, there is now solid evidence that the absence of NOX4 alters a number of physiological heart parameters (see also above), and increases susceptibility to tissue damage in a mouse model for stroke [532].
A crucial role of NOX4-derived ROS in age-related diseases was also implicated in the initiation, establishment, and development of tissue fibrosis (reviewed in [533]). In particular, strong experimental evidence suggests that pulmonary fibrosis (also referred to as idiopathic pulmonary fibrosis, IPF, for the lack of a well-defined etiology) is caused by excessive NOX4 activity and can be at least partially reverted by targeting the NOX4-NRF2 redox imbalance [534]. On the other hand, work by the Armanios group has established that genetic deficiencies in telomere maintenance systems cause IPF late in life [535], which therefore has been referred to as a “short telomere disease” [536], and the percentage of genetic lesions affecting telomere maintenance in IPF patients is steadily increasing. From these data, it appears conceivable that telomere shortening and activation of NOX enzymes, in particular NOX4, are mechanistically linked; however such links have so far remained elusive. A potential role of NOX2 has been found in age-related neurodegenerative diseases like Alzheimer's disease [513] and Parkinson's disease [537].
The incidence of most cancers increases dramatically with age, indicating that, with a few exceptions, cancer is primarily an age-associated disease. Concerning the role of NOX family members, a dual role in carcinogenesis has been postulated. On the one hand, the function of NOX to mediate oncogene-induced senescence, leading to tumor suppression (see above), indicates that NOX activity restricts tumor growth in some instances. On the other hand, production of ROS by NOX enzymes is essential for signaling pathways driving cell proliferation and survival, indicating that enhanced NOX activity in epithelial cells favors tumorigenesis. This dual role of NOX-derived ROS is best illustrated in prostate cancer, clearly one of the most relevant age-associated malignancies. Analysis of radical prostatectomy tissue samples and benign and malignant prostate epithelial cell lines identified NOX5 as an abundantly expressed NOX isoform and suggested that NOX5-derived ROS and subsequent depletion of PKCζ and JNK inactivation play a critical role in modulating intracellular signaling cascades driving the proliferation and survival of PCa cells [538]. On the other hand, increased expression of Nox4 in prostate stromal cells (fibroblasts) during their age-associated trans-differentiation to myofibroblasts leads to benign prostate hyperproliferation [539], a condition referred to as “reactive stroma” [540] that is known to favor the emergence of (epithelial) tumor cells in the prostate.
In conclusion, the existing evidence suggests that NOX family members are important drivers of age-associated pathology, and regulating NOX activity/expression and using mitochondrial antioxidants are potential approaches to reducing aging-associated diseases.
7.3. Protein aggregates as redox signaling mediators in aging
The aging process and a number of age-associated diseases are accompanied by the accumulation of high-molecular protein aggregates, as reported by Tilman Grune. In the aging process these protein aggregates are often referred to as lipofuscin, ceroid or age associated fluorophores. This material is largely composed of proteins, often amounting to 60–80%. Some of these proteins are oxidized or modified by various reactive metabolites, e.g. carbohydrates forming advanced glycation end products. Various lipids form another major part of such aggregated material. Interestingly, several authors report also the inclusion of various metals in such protein aggregates.
This raised the question, whether included metals are able to trigger metal-catalyzed redox reactions. It could be demonstrated that iron in protein aggregates is able to catalyze the Fenton-reaction and is only partially chelatable by iron-chelators [541]. Moreover, in the same study it could be demonstrated that in senescent cells protein aggregates contribute to an age-associated shift in the redox-state [541].
Further studies revealed that such protein aggregates do have an active surface. It is likely that due to the reactive surface of the aggregate, e.g. due to reactive hydroperoxides or aldehydes, and due to the hydrophobic patches – a result of the unfolding of the included proteins - protein aggregates can bind cellular proteins. This interactome might either be random or at least partially specific due to the binding of protein-protein-interacting structures. The latter includes certainly the binding of chaperones [542] or proteases designed to degrade unfolded proteins, as in the proteasome [543].
The 20S proteasome is known to be the major intracellular protease responsible for the degradation of damaged and oxidized proteins [543]. On the other hand, it is also the central catalytic part of the ubiquitin-proteasomal-system and, therefore, involved in the degradation of most cytosolic proteins [543]. The proteasome recognizes unfolded protein structures. In the case of protein aggregates this leads to an inhibition of the proteasome upon binding. This was shown in in vitro senescence models [544] or in neurons of Alzheimer's disease patients [545]. Proteasomal inhibition, in turn, leads to a proteasomal mal-performance, leading to disturbances of the regulated turnover of transcription factors, as shown in the case of AP-1 [546], HSF1 and NRF2 [547]. Furthermore, it should be noted that proteasomal inhibition leads also to a vicious cycle in which a reduced level of degradation of newly formed oxidized proteins leads to enhanced protein aggregate formation.
In conclusion, it should be noted that accumulating protein aggregates have multiple pathophysiological effects and cannot be seen as inert waste materials. However, more studies are needed to acquire a more complete scheme of the action of protein aggregates and their contribution to cellular senescence.
7.4. Proteasome activation as an anti-aging and anti-aggregation strategy
From the least to the most complex organisms, aging is a natural inevitable process accompanied by several molecular and biochemical failures. As explained by Niki Chondrogianni and Nikoletta Papaevgeniou, damaged and/or wrongly produced macromolecules tend to accumulate and aggregate, resulting in proteostasis impairment that then triggers a cataract of system deficits. Proteasome-mediated degradation is a critical player for protein homeostasis maintenance; proteasomes are large enzymatic complexes that regulate the cellular protein load equilibrium by degrading the unnecessary peptides and proteins. Proteasomal dysfunction is associated with the progression of aging and protein aggregation further aggravates the problem [514]. Consequently, proteasome activation appears to be a promising anti-aging and anti-aggregation approach.
Proteasome up-regulation in terms of assembly, quantity and function has been achieved through genetic manipulation of various catalytic or regulatory subunits or through treatment with specific activating compounds. This activation has been shown to lead to decelerated aging progression and to enhanced longevity. More specifically, overexpression of β1 and β5 catalytic subunits endowed WI38 human fibroblasts with increased resistance to oxidative stress and elongated cellular lifespan [514]. Overexpression of the pbs-5 subunit (nematode ortholog of β5) in C. elegans resulted in increased proteasome content and function that led to enhanced resistance to oxidative stress and extended lifespan in a daf-16-, skn-1- and hsf-1-dependent manner [548]. In accordance, rpn-6.1 overexpression in C. elegans triggered elevated proteasome activities leading to enhanced longevity under conditions of mild heat stress [549]. The positive effect of proteasome activation on longevity was also shown in the fruit fly where overexpression of the RPN11 19S subunit resulted in enhanced proteasome activities and extended lifespan [550]. With regard to compounds, treatment with a pentacyclic triterpenoid, namely 18α-glycyrrhetinic acid, resulted in increased proteasome content and activity both in cellular (human fibroblasts) and organismal (C. elegans) models [551], [552]. This compound was shown to promote proteasome activation in an NRF2-dependent manner in fibroblasts [551] and a SKN-1-dependent manner in nematodes [552], with elongated lifespan and increased stress resistance as the end result. Finally, rejuvenating and anti-aging properties were attributed to quercetin-mediated proteasome activation in human fibroblasts [553].
Proteasome activation has also been shown to confer protection against the devastating consequences of protein aggregation. Enhancement of proteasome function through pbs-5 overexpression in nematodes endowed animals with increased survival against expanded polyglutamine and Aβ peptide proteotoxicity [548]. Similarly, rpn6.1 overexpression conferred proteotoxic stress resistance in a nematode polyglutamine disease model [549], while RPN11 overexpression suppressed the expanded polyglutamine- induced progressive neurodegeneration in fruit flies [550]. Proteasome activation through 18α-glycyrrhetinic acid treatment led to reduced Aβ peptide deposits both in murine neuronal cells and in an Alzheimer's disease nematode model thus resulting in decelerated progression of the disease's phenotype [552]. Likewise, the polyphenol quercetin was found to induce proteasome activity resulting in inhibition of paralysis in a transgenic C. elegans strain serving as an animal model for Alzheimer's disease [554].
In conclusion, proteasome activation emerges as a promising strategy in the battle against aging and proteotoxicity. Given that genetic manipulation is not applicable in humans, future studies should focus on the identification of compounds with proteasome activating properties as well as in the elucidation of the involved signals and biochemical pathways.
7.5. Free radical theory of aging – dead or alive?
The “free radical theory of aging” was introduced in the early 1950s through the work of Harman [510] and comprises investigations of the role of reactive oxygen species (ROS) formation and mitochondrial function in the aging process and, more recently, the implications of ROS-triggered epigenetic processes and DNA damage for the etiology of aging [555]. Lately, as discussed by Andreas Daiber and Yuliya Mikhed, the field of epigenetics has received considerable attention in the context of aging theories. It has been shown that cellular senescence leads to massive DNA demethylation, causing a state of DNA hypomethylation, particularly in the CpG islands [556] and multiple epigenetic processes are regulated in a redox-dependent manner [238]. Although the lessons learned from animal models of genetic deletion or overexpression of important antioxidant enzymes such as superoxide dismutases, glutathione peroxidases and catalase were rather disappointing, since no clear correlation between life span and the abundance of these antioxidant defense enzymes could be observed, at least some of them (e.g. Sod1-/-) or double gene ablation combinations showed reduced life expectancy, which would be in line with the “free radical theory of aging” [557], [558]. Moreover, the importance of mitochondrial superoxide formation for survival (longevity) is highlighted by the fact that homozygous SOD2 deletion leads to perinatal or neonatal lethality [559] and by the observation that mice deficient in the p66Shc-/- gene, a source of mitochondrial ROS formation, display a 30% longer life span [560]. According to more recent data, oxidative stress in general and mitochondrial ROS formation in particular may play an even more important role for the quality of life, healthy aging, or the so-called “healthspan” than for the lifespan per se [561], [562]. Healthy aging may be an even more important need and challenge in our aging Western societies, also from an economical point of view considering the tremendous costs associated with our public health systems. The cardiovascular system seems to be an excellent example of this concept. From a mechanistic basis the increasing superoxide formation (coming from gradually increased mitochondrial leakage of electrons and NADPH oxidase-derived production due to low grade inflammation) with progressing age will lead to antagonization of nitric oxide, a major vasodilator and antiatherothrombotic signaling molecule of the cardiovascular system [555]. Oxidative depletion of nitric oxide bioavailability by superoxide and the reaction product peroxynitrite will lead to impaired cGMP formation, inhibition of prostacyclin synthase by tyrosine nitration and activation of the renin-angiotensin-aldosterone system as well as endothelin-1 signaling, which will lead to a complete switch from a vasculoprotective to a proatherothrombotic phenotype [9]. In support of this concept, genetic deletion of mitochondrial antioxidant enzymes (Aldh2-/- and Sod2+/-) led to an increase in mitochondrial ROS formation, 8-oxoguanine-dependent mitochondrial DNA strand breaks and, most importantly, an impairment of endothelium-dependent and –independent relaxation (vascular function) (Fig. 7.2A-B) [563]. Likewise, genetic deletion of GPx-1 led to an age-dependent impairment of endothelium-dependent and –independent relaxation, increase in oxidative stress markers and eNOS uncoupling as well as aggravation of inflammatory phenotype of the vasculature (summarized in Fig. 7.2C) [62]. Although these animals showed no obvious decrease in lifespan, it may be assumed that the proatherothrombotic and inflammatory phenotype will ultimately lead to increased prevalence of cardiovascular complications as a consequence of the higher burden of oxidative stress, thereby decreasing the healthspan.
Fig. 7.2.
Correlations between mitochondrial oxidative stress (mtROS), mitochondrial DNA (mtDNA) damage and vascular (endothelial) function (ACh-induced maximal relaxation). (A) mtROS formation was plotted for all age-groups and mouse strains versus the corresponding maximal efficacy in response to acetylcholine (ACh). (B) mtROS was plotted for all age-groups and mouse strains versus the corresponding mtDNA damage. ROS were measured using L-012 (100 µM) enhanced chemiluminescence in isolated cardiac mitochondria upon stimulation with succinate (5 mM). r is the correlation coefficient. (C) Hypothetic scheme of aging-induced vascular dysfunction and the role of mitochondria in this process. Aging-induced mitochondrial dysfunction triggers mitochondrial reactive oxygen species (mtROS) formation from respiratory complexes I, II, and III (Q = ubiquinone). Break-down of mtROS is catalyzed by glutathione peroxidase (GPx, for H2O2) or manganese superoxide dismutase (MnSOD), the latter is in turn inhibited by mitochondrial peroxynitrite (ONOO-) formation. mtROS increase the levels of toxic aldehydes and inhibit the mitochondrial aldehyde dehydrogenase (ALDH-2), the detoxifying enzyme of those aldehydes. Increase in mtROS and toxic aldehydes also leads to mtDNA strand breaks which leads to augmented dysfunction in respiratory chain complexes and further increase in mtROS since mtDNA encodes mainly for those respiratory complexes. mtROS also activates mitochondrial permeability transition pore (mPTP), which upon opening releases mtROS to the cytosol leading to protein kinase C (PKC)-dependent NADPH oxidase activation, eNOS uncoupling and finally to endothelial dysfunction [61]. Cytosolic reactive oxygen and nitrogen species (ROS/RNS) in turn were demonstrated to activate KATP channels, which causes alterations in mitochondrial membrane potential (C) and further augments mtROS levels [564]. Effects of rotenone (Rot), cyclosporine A (CsA), diazoxide (Diaz) and glibenclamide (Glib) have been recently demonstrated in related models of vascular dysfunction and oxidative stress, nitroglycerin-induced nitrate tolerance and angiotensin-II triggered hypertension [61], [564]. With permission of the European Society of Cardiology. All rights reserved. © The Author and Oxford University Press 2008.
Adopted from [563].
In conclusion, there is solid evidence for the “free radical theory of aging” in experimental studies but translation to the clinical situation requires large scale clinical trials, as currently undertaken by the CHANCES consortium (10,622 individuals) reporting on a clear association between oxidative stress markers and all-cause mortality [565] and the MARK-AGE consortium (3337 individuals) the data of which are currently being prepared for final dissemination [566].
7.6. ROS and frailty. Understanding the mechanisms of disability in older people
According to Mariam El Assar and Leocadio Rodríguez-Mañas, frailty is a geriatric syndrome characterized by diminished functional reserve and increased vulnerability to low power stressors. Frailty precedes disability, and, in addition, epidemiological cohorts have demonstrated that the frailty phenotype predicts several outcomes such as falls, hospitalization and mortality [567]. Although the biological determinants of frailty are not well defined, oxidative stress and inflammation have been closely related to aging and seem to be potential drivers of frailty pathogenesis. Inflammation and oxidative stress are closely related processes with crosslinks between their respective signaling pathways. In line with this, observational studies have shown that frailty is associated with different systemic inflammatory biomarkers, including C-reactive protein, and interleukin-6 [568]. Elevated levels of pro-inflammatory cytokines have been shown to be associated with increased risk of morbidity and mortality in frail older subjects. Furthermore, an association between systemic oxidative stress biomarkers (malondialdehyde, isoprostanes, protein carbonylation and lipoprotein phospholipase A2) and frailty has been reported in a geriatric population [515].
The prevalence of chronic diseases such as diabetes, cardiovascular diseases and pulmonary diseases increases with aging. These chronic conditions compromise muscle function, cardiovascular performance and pulmonary function leading to increased vulnerability of the organism when exposed to low intensity stressors. This scenario represents the phenotypic manifestation of frailty. Consistently, all these chronic diseases are associated with a higher risk of frailty in aged populations. The contribution of oxidative stress and reactive oxygen species (ROS) to the decline of different functional systems associated to aging and to age-related chronic diseases has been established [569]. For example, the defective nitric oxide signaling in aged muscle vasculature has been attributed to increased inactivation of this molecule by ROS leading to decreased muscle perfusion. On the other hand, the lung is continuously exposed to oxidative stress while the activity of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase decreases with aging. In addition, the induction of heme oxygenase, which is involved in cellular protection against oxidative stress, is defective in lungs of aged mice, further supporting the involvement of oxidative stress in age-related pulmonary dysfunction.
Since frail older adults have minimal injury resilience and a notably decreased response to stress, the different pathways regulating cellular response to stress (oxidative stress, hypoxia and inflammation among others) stand out as possible players in the development of a frailty phenotype. In this sense, aging, sedentary lifestyle and chronic diseases could cause down-regulation of signaling pathways responsible for the cellular response to oxidative stress, such as NRF2. This results in impaired response to stress and increased oxidative stress leading to a situation of vulnerability to stressors such as frailty. In fact, very recent data obtained by our group have demonstrated that lower expression of genes related to response to oxidative stress in older people is associated with a higher risk of being frail independently of age and sex (El Assar and Rodríguez-Mañas group).
Further investigations are definitely needed to fill the gap of knowledge of this evolving field and provide possible targets for intervention to promote health and independence in the elderly.
8. ROS and inflammation in health and disease
Manuela Garcia Lopez (E-mail: manuela.garcia@uam.es) and Javier Egea (E-mail: javier.egea@inv.uam.es).
8.1. Introduction
Over the past decade, the study of the biological activity and significance of ROS have gained particular interest from both a physiological and pathological perspective. Since ROS are highly unstable and reactive molecules, they interfere with many cellular processes. ROS react with lipids, nucleic acids and proteins, disrupting their cellular functions. Oxidative stress occurs when the damaging effects of ROS exceed the ability of biological systems to neutralize the oxidizing agents and to repair cellular damage. Physiologically, antioxidant defenses are efficient enough to neutralize the damaging effect of oxidizing molecules. In these conditions, the presence of ROS is important for many physiological cellular processes and they participate as signaling molecules in a wide range of cellular functions. Hence, ROS modulate intracellular transduction pathways and transcriptional factors involved in cell proliferation, differentiation, and maturation [570], [571], [572].
Chronic Granulomatous Disease (CGD) is a rare inherited innate immunodeficiency caused by defective NADPH oxidase activity in phagocytes and is recognized as a disease model to understand the pathophysiological consequences of ROS deficiency [22]. CGD patients suffer from life-threatening infections, but an apparent paradox between the absence of ROS production and hyperinflammation is often observed in this disease [32], [33]. The key producers of ROS in many cells are the NOX enzymes, of which there are seven members with different tissue distributions and regulatory mechanisms. ROS produced by the isoform NOX2 is essential to organize the response in host defense against pathogens [573].
In the brain, ROS production regulates neuronal development from neuronal precursors [574]. Redox signaling is also required to trigger neuronal differentiation and axon formation [575]. Nitric oxide (•NO), a diffusible intercellular messenger produced by neuronal nitric oxide synthase (nNOS) exerts a dual regulatory role in neurovascular coupling and neuroenergetics by regulating mitochondrial oxygen consumption [576]. Therefore, ROS act as messengers in the transduction pathways important for synaptic plasticity in the CNS.
The brain is particularly vulnerable to oxidative stress because it consumes a large amount of oxygen, has abundant lipid content, and has little antioxidant activity compared to other organs. The major antioxidants in the brain are ascorbate, glutathione (GSH), and vitamin E in the plasma membrane. GSH production is increased in response to oxidative stress in astrocytes, cells that act as the main supplier of GSH to neurons for antioxidant protection [577]. ROS accumulation in the brain has been associated with the onset of neurodegenerative and psychiatric diseases, whose main consequence is to reduce several neuronal cellular functions. ROS accumulation in neurons and the resulting oxidative stress are responsible for the loss of cognitive and motor functions in several brain diseases.
Oxidative stress and chronic low grade inflammation are interdependent processes that have been implicated in aging and many pathological conditions like cardiovascular disease, neurodegenerative diseases or cancer. Inflammatory cells can release ROS at the site of inflammation increasing oxidative stress, while ROS can initiate intracellular signaling cascades that increase proinflammatory gene expression [47], [578].
Chronic inflammation as a consequence of immune failure is often associated with cancer (known as inflammation-induced tumorigenesis). Hence, in different types of cancers, multiple pathways contribute to chronic cytokine release. Hyperinflammation combined with loss of adhesion and the release of angiogenic factors leads to cellular proliferation.
Here we will put forward some examples of how ROS and inflammation can regulate physiological or pathological conditions in different systems.
8.2. Dual actions of NOX2-derived ROS
As pointed out by Jamel El-Benna and Pham My-Chan Dang, the production of ROS by NOX2 is essential to mount a rapid response to bacterial and fungal invasion; however, excessive or inappropriate ROS production can induce severe tissue injury that participates in the pathophysiology of acute and chronic inflammatory diseases [579], [580]. For several years, NOX2-derived ROS have been considered as pro-inflammatory agents, but recent reports challenged this dogma and suggested that they can also be anti-inflammatory [581].
Under physiological conditions, the objective of ROS production by phagocytes is to kill and eliminate pathogens trapped inside the phagosome [579]. When this goal is successfully achieved, ROS production is terminated and the inflammation is resolved. Thus, the physiological role of transient ROS production is anti-inflammatory as it helps to eliminate inflammation resulting from the infection. In addition to the “direct-ROS-killing effect” during phagocytosis, NOX2-derived ROS can have “paracrine-redox signaling effects” that can modulate the immune response. Indeed, NOX2-derived ROS can dampen T-cell-dependent inflammation through alteration of T-cell membrane oxidation status [582] and of Th17/Treg cell development [583]. The anti-inflammatory role of NOX2-derived ROS is well illustrated in chronic granulomatous disease (CGD) patients who have a genetic defect in one of the NOX2 genes (see Section 8.3). Thus, chronic NOX2 deficiency may cause “ROS-independent” inflammation where ROS are not the causative factor. This “ROS-independent” inflammation is also observed in autoimmune diseases as shown in animal models [581], [582], [584]. In the presence of high levels of pro-inflammatory mediators (cytokines, TLR agonists, lipid mediators…), NOX2 can be hyper-activated, leading to excessive and prolonged ROS production [583]. This high ROS production is undoubtedly deleterious, as evidenced by the multiple antioxidant strategies that have been developed by the organism to protect against ROS [585]. ROS, particularly the diffusible hydrogen peroxide, can oxidize cellular macromolecules (proteins, lipids, DNA), which leads to tissue injury or to the alteration of cellular functions such as imbalance of intracellular signaling pathways, cytokine production, protease activation and release [585], all of which participate in the “ROS-dependent inflammation.” Indeed, inhibition of NOX2 and the use of certain anti-oxidants protect from inflammation in animal models [586].
8.3. Chronic Granulomatous Disease (CGD): implication of NOX2
According to Marie José Stasia, Joe Dan Dunn and Thierry Soldati, CGD is a rare inherited disorder in which phagocytic cells are unable to kill pathogens during an infection. The molecular basis of this disease is the absence of ROS production by the NADPH oxidase complex of phagocytes which is composed of NOX2 and p22phox, also named the membrane flavocytochrome b558, and the cytosolic factors p47phox, p67phox and p40phox. It is a genetically heterogeneous disease with all ethnic groups equally affected. The molecular basis of CGD is characterized by two types of transmission and four main genetic forms. As mentioned earlier, the major genetic form is X-linked CGD caused by mutations in the CYBB gene encoding NOX2. X-CGD accounts for about 70% of the total cases reported to date [587]. The other types of CGD are autosomal recessive forms (AR), characterized by mutations in CYBA, NCF1 and NCF2 encoding p22phox, p47phox and p67phox respectively [588]. Clear information on the severity of CGD according to the genetic forms is difficult to establish. However Kuhns et al. demonstrated a relationship between the presence of residual ROS production and the survival of CGD patients [589]. In addition, mutations affecting the membrane flavocytochrome b558 composed of p22phox and NOX2 seem to be associated with the most severe clinical features of CGD [590]. Indeed, cytochrome b558 is the redox core of the enzyme in which the electron transfer occurs to reduce the molecular oxygen into superoxide.
The soil-dwelling, social amoeba, Dictyostelium discoideum (thereafter Dictyostelium) is an ideal model organism to determine immunity functions of ROS and the consequences of ROS deficiency, i.e., CGD. Dictyostelium amoebae prey on bacteria using phagocytic machinery and intracellular killing mechanisms that are conserved in the immune phagocytes of metazoa, e.g., mammalian macrophages and neutrophils, and are utilized as a model phagocyte to study cell-autonomous immunity [591], [592]. Among the conserved machinery are three NOX2 homologs of the NADPH oxidase catalytic subunit (NOX A, B and C), a p22phox homolog, a single, putative NOX-activating protein (NcfA, a homolog of p67phox), a secreted myeloperoxidase-like enzyme (PoxA), and enzymes for metabolizing ROS such as superoxide dismutases and catalases [593], [594]. Dictyostelium also has homologs of STATs, TRAFs, and guanylate-binding proteins, which are regulated by ROS in macrophages [594], and employs autophagy as a defense mechanism against cytosolic bacteria [595], which is activated downstream of ROS in macrophages.
Dictyostelium undergoes a developmental cycle during which 100,000 amoebae aggregate to form a multicellular slug that ultimately differentiates into a spore-containing fruiting body. ROS scavengers inhibit the initial aggregation of amoebae, and both NOX-deficient mutants and catalase deficient mutants exhibit defects in fruiting body formation [593], [596], [597]. The multicellular slug contains specialized sentinel cells (s-cells) that serve as its patrolling innate immune system [598]. Like neutrophils, S-cells extrude DNA-based extracellular traps (ETs) via a NOX-dependent mechanism: S-cells from NOX-deficient mutants lose the ability to secrete ETs and to clear bacteria from the slug and subsequent fruiting body [599]. Consequently Dictyostelium, on the cusp of multi-cellularity, can be used to model CGD and the influence of NOXs and ETs on the evolution of specialized immune cells and to study the role of ROS in a host-pathogen interaction [600].
CGD patients suffer from recurrent and life threatening infections during early childhood. Phagocytic cells from CGD patients adhere to blood vessels, reach infectious sites by chemotaxis and phagocytose the involved pathogens, but are unable to kill them because of the absence of ROS production by the defective NOX complex. Thus, accumulation of live pathogens in phagocytes, combined with the continuous release of proinflammatory cytokines by these cells, leads to the formation of granulomas in infected tissues. In addition, granuloma formation in hollow organs like the kidneys or the gastrointestinal tract is responsible for obstruction syndromes. Infections can be localized in tissues in direct contact with the environment like the skin, the otorhinolaryngology sphere or, more severely, the lungs. The disease has a more dramatic effect when infections and granulomas are in deep organs such as the brain or the liver. Aspergillosis in the lungs is the first cause of death of CGD patients. One seemingly paradoxical observation that needs to be addressed is: how does the absence of ROS lead to hyperinflammation in CGD? Several hypotheses have been proposed. The oldest one is that efferocytosis by macrophages is reduced in CGD [601]. The overall consequence will be unbalanced neutrophil necrosis, an increase of proteases and toxic oxygen-derived components and release of proinflammatory cytokines, which all contribute to local inflammation. In addition, the absence of ROS production can be responsible for defective activation of genes that regulate NFκB signaling, which is involved in the restriction of the development of inflammatory disorders [602]. Recently, it was shown that ROS deficiency in CGD causes autophagy dysfunction in phagocytes, which contributes to increased production of proinflammatory IL-1β. Indeed, two CGD patients treated with an IL-1 receptor blocker showed rapid and sustained improvement in colitis [603]. Furthermore, defective ATM activation due to the absence of ROS production in a CGD patient was linked to an exacerbation of proinflammatory cytokine release and apoptosis, which might explain the hyperinflammation in this disease [604].
As components of the phagocytic NOX complex are expressed in cells and tissues other than phagocytes, inactivating mutations in these proteins can have pathophysiological consequences unrelated to immunodeficiency syndromes. ROS production by NOX enzymes controls vascular function via modulation of NO bioactivity. Indeed NOX2 deficiency in CGD patients was related to enhanced arterial dilatation [605]. Violi et al. demonstrated that in vivo platelet activation might be directly associated with NOX2 activity. Thus decreased platelet marker activation correlated with the absence of NOX2 expression found in CGD patients. As NOX2-deficient mice demonstrated impaired memory and synaptic deficit, a role of NOX2 in these processes was proposed [606]. However, clinical studies in children with CGD were rather ambiguous [607], [608].
8.4. Neuromodulatory actions of nitric oxide
As summarized by J. Laranjinha and R.M. Barbosa, the brain is bioenergetically exigent and, to optimize neuronal function and survival it is equipped with fine mechanisms for a precise spatial and temporal control of cerebral blood flow (CBF) that provides energy substrates according to cellular activity. This process is achieved through neurovascular coupling, an orchestrated intercellular communication among all components of the neurovascular unit (neurons, astrocytes, perycites and microvessels) that results in a rapid and restricted increase in CBF [609]. Yet neuronal energetics is non-linearly coupled to an activity-dependent increase of CBF, i.e. neuronal activity-induced increases in CBF are not accompanied by proportionate increases in oxygen consumption by neuronal mitochondria [610]. Therefore, an activity-dependent CBF increase and oxygen utilization by active neural cells are inextricably linked and establish a functional metabolic axis in the brain termed the “neurovascular-neuroenergetic coupling axis.” This axis incorporates interdependent processes that need to be coordinated in the normal brain. An impaired functionality of the neurovascular-neuroenergetic coupling axis poses a threat to the healthy brain and can prove particularly detrimental for Alzheimer´s disease and the aged brain.
•NO, a diffusible intercellular messenger synthesized by the neuron-associated synthase isoform (nNOS), coordinates an integrated regulation of this axis by mediating the neurovascular coupling process and by regulating oxygen utilization by mitochondria. Two lines of reasoning support this hypothesis. The first is based on the in vivo dynamic and simultaneous recordings [611] of •NO, oxygen and CBF. It has been shown that upon glutamatergic stimulation of rodent hippocampus, •NO synthesized by nNOS associated with the glutamate NMDA receptor diffuses into neighboring vessels and induces an increase of CBF, which couples to neuronal activity by activating soluble guanylate cyclase in smooth muscle cells [612]. The transitory increases of •NO and CBF from a basal level are temporarily and spatially coupled with oxygen delivered from the vessels, encompassing a sequence of events consisting of glutamatergic neuronal stimulation, •NO transients, CBF increases and oxygen transients. According to the second line of reasoning, the best characterized interaction of •NO with the mitochondria is the inhibition of cytochrome c oxidase by nanomolar concentrations of •NO in competition with oxygen [613]. At physiological oxygen concentrations, NMDA-evoked •NO production inhibits hippocampal oxygen consumption at submicromolar concentrations and induces a small difference in the concentration dynamics of •NO, reflecting that different states of neuronal activation may lead to different outcomes in terms of metabolic rate [614], [615].
8.5. Thiol redox homeostasis in astrocytes
According to Gethin J. McBean, several decades of research have led to the conclusion that astrocytes, the so-called ‘metabolic support’ cells of the brain, act as the central supplier of glutathione (γ-glutamyl-cysteinyl-glycine, GSH) for antioxidant protection of neurons. De novo synthesis of GSH in astrocytes fulfills the antioxidant capacity of those cells and also provides precursors for GSH synthesis in neurons [616]. Cysteine is the rate-limiting substrate for GSH synthesis and is supplied either by transport from the extracellular medium in the form of cystine into astrocytes using the xc- exchanger, or directly into neurons as cysteine via the EAAT3 subtype of the high-affinity glutamate transporter. As the extracellular redox potential dictates a 5:1 ratio of cystine to cysteine, the bulk of uptake takes place into astrocytes. In addition to the xc- cystine-glutamate exchanger, cysteine can also be supplied by transsulfuration (TS) from methionine via homocysteine, as shown in Fig. 8.1. In normal astrocytes, the TS pathway supplies up to one third of the cysteine required for GSH, with the majority coming from the extracellular space [577], [617].
Fig. 8.1.
Cysteine supply pathways in astrocytes. Cysteine is either taken up in its oxidized form, cystine, from the extracellular medium via the xc- cystine-glutamate exchanger, or generated from methionine via the transsulfuration pathway. Cysteine is the immediate precursor for GSH, which is synthesized by the first two enzymes of the γ-glutamyl cycle, as well as taurine and hydrogen sulpfide. CBS, cystathionine-β-synthase; CDO, cysteine dioxygenase; CSA, cysteine sulfinic acid; CSE, cystathionine-γ-lyase; GCS, glutamate cysteine ligase; GS, glutathione synthase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
It is well documented that GSH production in astrocytes is increased in response to oxidative stress. Similarly, upregulation of both the xc- exchanger and the TS pathway (via increased expression of the rate-limiting enzyme, cystathionine-γ-lyase (also known as cystathionase; CSE)) occurs following oxidative stress [617], [618]. Interestingly, experimentally-induced oxidative stress in primary rat cortical astrocytes shows that the relative contribution of the TS pathway to cysteine for GSH increases to 60%, meaning that, under these conditions, the majority of cysteine for GSH comes from this pathway, rather than the xc- exchanger [577]. Pharmacological blockade of the xc- cystine-glutamate exchanger in vitro also acts as a signal to upregulate CSE, leading to enhanced synthesis of GSH [577]. It is not clear at present whether the signal to increase flux through the TS pathway occurs in response to a fall in GSH within astrocytes, or to increased oxidative stress, or both. It has been proposed that upregulation of cystine intake under oxidative stress conditions is decoupled from GSH synthesis and, instead, regulates an intracellular-extracellular cysteine-cystine redox cycle [619]. This proposal fits with our results showing that GSH synthesis under oxidative stress conditions increases reliance on the TS pathway. However, further work is required to fully evaluate the interrelationship between these sources of cysteine for GSH, and other important neuroprotective products, for example, taurine and hydrogen sulfide.
Understanding the origin of cysteine for GSH is important for evaluation of the antioxidant capacity of cells in neurodegenerative disease. There are several examples in the literature of up-regulation of the xc-cystine-glutamate exchanger during neurodegenerative disease [620], [621]. Indeed, at first glance, it would appear that the capacity to increase GSH synthesis during neurodegenerative disease-related oxidative stress would be beneficial. However, the downside is that increased capacity of the xc-cystine-glutamate exchanger is inevitably coupled to the release of glutamate. If this cannot be matched by high-affinity uptake, then the likelihood of glutamate-mediated neurotoxicity threatens neuronal well-being. This situation is well illustrated in the case of glioma/astrocytoma. Here, tumor cells up-regulate the xc-cystine-glutamate exchanger, but down-regulate the high-affinity glutamate transporters [622]. This imbalance generates the capacity for destruction of neurons and increases the likelihood of epileptiform activity. However, strategies to block the xc- exchanger in glioma therapy have been unsuccessful. It is now recognized that drugs that target both the xc- exchanger and the TS pathway have greater potential as effective therapeutics [623]. Research is ongoing to fully understand the beneficial potential of the TS pathway in astrocytes, particularly in the context of astrocyte activation and the immune response.
8.6. Regulation of the oxidative and inflammatory response by microglial α7 cholinergic receptors
As reported by Manuela G Lopez and Javier Egea, dysfunction of the cholinergic system and mitochondria together with increased oxidative stress and neuroinflammation has been reported during aging and diseases related to age like Alzheimer´s and Parkinson´s disease [624], [625]. The α7 acetylcholine nicotinic receptor subtype (α7nAChR) is expressed in neuronal and non-neuronal cells. Wang et al. [626] identified that α7 nAChRs in blood monocytes control inflammation under vagal stimulation and proposed the “cholinergic anti-inflammatory pathway,” which regulates inflammation in the periphery. In the central nervous system, microglia regulate the innate immune response and also express α7 nAChRs; therefore, the role of these receptors in microglial function is being studied.
Primary glial cultures exposed to the α7 nicotinic agonist PNU282987 increased their mitochondrial mass and mitochondrial oxygen consumption without generating oxidative stress. These changes were not seen when the transcriptional factor NRF2 was absent, when the HO-1 enzyme was inhibited and when PGC-1α was silenced. In isolated microglia of adult animals treated with the α7nAChR agonist, a significant increase in mitochondrial mass was also detected. Interestingly, LysMcre-Hmox1Δ/Δ animals, which lack HO-1 in microglial cells, and PGC-1α-/- animals, which do not express PGC-1α protein, showed lower microglial mitochondrial levels, and treatment with PNU282987 did not change the mitochondrial levels. These results indicate the important function of the α7nAChR/NRF2/HO1 and PGC-1α axis to increase mitochondrial biogenesis and thereby improve the bioenergetic status in microglia [627].
The α7 microglial nicotinic receptor also plays a strategic role in controlling neuroinflammation to afford neuroprotection under brain ischemia conditions as shown in in vitro and in vivo models. Organotypic hippocampal cultures (OHC) exposed to oxygen and glucose deprivation (OGD) elicited cell death. However, the selective α7nAChR agonist PNU282987 incubated post-OGD, reduced cell death, ROS production and TNF-α release. The protective effect of PNU282987 was lost in microglia-depleted OHCs as well as in OHCs from Nrf2 deficient mice. Administration of the α7nAChR agonist 1 h after induction of photothrombotic stroke in vivo reduced infarct size and improved motor skills in Hmox1lox/lox mice that express normal levels of HO-1, but not in LysMCreHmox1∆/∆ in which HO-1 expression is inhibited in myeloid cells, including the microglia [628]. These results show that α7 microglial nicotinic receptors play a key role in controlling neuroinflammation and affording neuroprotection under brain ischemia.
Therefore, microglial activation of the α7 nAChR/NRF2/HO-1 axis seems to play an important role in improving microglial bioenergetics and reducing oxidative stress and neuroinflammation [629].
8.7. Regulatory mechanisms of ROS in the motor neuron
According to Anastasia Shakirzyanova and Rashid Giniatullin ROS can also regulate neurotransmission at the neuromuscular junction. Curiously, the actions of ROS are remarkably different in the synapses of newborns, adults and old rats [630]. Thus, it has been reported that the inhibitory effect of the diffusible mild oxidant H2O2 is much stronger in old rats. In newborns tested during the whole first postnatal week, H2O2 did not affect spontaneous transmitter release from nerve endings and even potentiated the end-plate potentials. The resistance of neonates to H2O2 inhibition was associated with higher catalase and glutathione peroxidase activities in skeletal muscle. In contrast, the activities of these enzymes were downregulated in old rats. These results indicate that the vulnerability of transmitter release to oxidative damage strongly correlates with aging and might be used as an early indicator of senescence [630]. Consistent with this, it was shown that the concentration of the redox active amino acid homocysteine increases with aging and in many neurodegenerative diseases significantly aggravates ROS-induced depression of transmitter release from motor nerve terminals. This provides a potential mechanism of peripheral impairment in motor neuron diseases associated with hyperhomocysteinemia [631]. Related to this, is has been reported that the co-transmitter ATP, which operates via a ROS-dependent mechanism, activates presynaptic P2Y12 receptors coupled to NOX to generate endogenous ROS and that this signaling complex likely resides in lipid rafts [632].
The association between ROS and motor neuron disease is exemplified in the case of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease characterized by a progressive loss of motor neurons and degradation of the neuromuscular junctions. In ALS, the decline in synaptic function initiates from the presynaptic terminals. Experimental data have shown that in the neuromuscular junction, a classical model of synaptic transmission, ROS have an inhibitory action on the presynaptic releasing machinery, and it was proposed that the presynaptic sensor of ROS was the SNARE protein Snap25 [633].
8.8. Inflammation and ROS in cancer
As explained by Kemal Sami Korkmaz and Bilge Debelec–Butuner, chronic inflammation subsequent to immune failure or insults such as microbial infections is often associated with cancer. The well-known examples come from Helicobacter pylori and human papillomavirus infections in stomach and cervix cancers respectively, where multiple pathways synergistically contribute to the activation of cytokine release, that combined with the loss of adhesion and the release of angiogenic factors, may eventually contribute to cellular proliferation, differentiation and tumor progression [634]. Thus, the immune defense mechanisms that play important roles in guarding the organism against environmental insults, might induce malignant growth in these tissues. This event is termed inflammation-induced tumorigenesis.
Toll like receptor (TLR) function is critical for regulation of innate and adaptive immune responses in normal cells and usually necessitates distinguishing the defective functionality of the malignant cells that might influence the sensory ability of the immune defense. Since activation of the pro-survival factors such as NF-κB, ERK and JNK kinases as well as increased levels of IL-6 and IL-12 correlate with highly expressed TLR4 levels in malignant cells [635], these findings indicate the important role of TLRs in chronic inflammation and cancer development.
Interestingly, TLR4 has been associated with metastasis in various types of cancer including the prostate. Highly metastatic human prostate cancer cell lines, PC-3 and DU145, express higher levels of TLR4 [636] compared to RWPE and LNCaP prostate cell lines [634], [637], [638]. When these cells are treated in vitro with LPS, TNFα is induced, reaching nM concentrations, and proliferation and migration of prostate cancer cells is observed [637], [638]. Consistently, the depletion of TLR4 expression inhibits the invasion of these cells and also improves the survival of tumor-bearing animals [636], [639], which suggests that the higher TLR4 level or its activation significantly augments tumor cell proliferation upon cytokine exposure. Thus, TLR4 expressed in normal and low-grade tumors could be a contributing factor to chronic inflammation that promotes carcinogenesis.
Nevertheless, the well-known prostate specific and androgen receptor (AR) regulated tumor suppressor NKX3.1 encodes a homeobox protein and facilitates the antioxidant response through transcriptionally upregulating the glutathione peroxidases and peroxiredoxins [640], which leads to subsequent deregulation of the intracellular ROS levels and activation of the DNA damage response.
In addition, the growth rate is metabolically regulated via stress-sensing Sirtuin 1 (SIRT1), an NAD-dependent deacetylase. Under oxidative conditions, such as inflammation, SIRT1 deacetylates a number of transcription factors, including p53, NBS1 and AR, which contribute to the control of the cell cycle, DNA damage response and steroid hormone action in prostate cells respectively. Therefore, SIRT1 has also been linked to tumor cell survival, particularly in prostate cancer, by the deacetylation of AR. Thus, the inflammation induced via TLR4-related cellular mechanisms contributes to the deregulation of the antioxidant response and DNA damage recognition in prostate, where the increased genetic heterogeneity is suppressed via AR, and its deacetylation by activated SIRT1 counteracts the deregulation of cell growth leading to stress tolerance in tumor progression.
8.9. Summary and conclusions
In this section we have seen some examples of how ROS formation has regulatory mechanisms that can be implicated in health and disease. ROS formed from NOX, mitochondria, or NO-producing enzymes are not necessarily toxic, but rather compose a network signaling system, known as redox regulation.
In the case of NOX2, we have seen that under physiological conditions ROS production derived from NOX2 may have an anti-inflammatory role when produced in a limited time and space, while a defect in NOX2 can lead to “ROS-independent inflammation.” However, when they are overproduced, they become pro-inflammatory as they induce oxidative stress and changes in cell homeostasis during a “ROS-dependent inflammation.” While the use of NOX2-deficient mice has advanced our knowledge on the role of NOX2, it is probably not the best approach to determine whether the absence of NOX2 is protective against inflammation, as the deletion of NOX2 inherently leads to a dysregulation of the immune response. A clear example of a human disease related to defects in NOX2 or associated subunits is chronic granulomatous disease [641]; as a consequence of NOX2 deficiency, neutrophil killing is defective due to the extremely low respiratory burst in these cells during phagocytosis [642], [643].
ROS generated by NOX enzymes can control vascular function via modulation of NO bioactivity [644]. In the brain, NO can exert a modulatory role both in the neurovascular coupling and in the functionality of energy formation by the mitochondrial respiratory chain. Therefore, neuronal-derived NO can be considered a master regulator of the neurovascular-neuroenergetic coupling axis. Furthermore, dysfunction of the NO-dependent neurovascular and neuroenergetic coupling has been related to the cognitive decline associated with brain aging and Alzheimer´s disease [576].
Besides the regulatory mechanism of neuronal NO, glial cells also play an active role in maintaining the redox balance in the brain. In line with this, astrocytes act as the central supplier of GSH for antioxidant protection of neurons. Cysteine is required to produce GSH in order to control the antioxidant capacity of cells in neurodegenerative disease; therefore, it is now recognized that drugs that target both the xc- exchanger and the TS pathway have greater potential as effective therapeutics [623]. On the other hand, in microglial cells, cholinergic stimulation of the α7 nicotinic receptor subtype is being recognized as a target to control neuroinflammation and oxidative stress by inducing hemoxygenase-1 to provide neuroprotection [628].
Homocysteine (HCY) is a pro-inflammatory sulfur-containing redox active endogenous amino acid, whose concentration increases in neurodegenerative disorders including ALS. At the neuromuscular junction, the effect of HCY on oxidative stress-induced impairment of transmitter release was shown to be age-dependent [631]; older rats were more sensitive to the inhibitory effect of H2O2 than newborns, most probably because the latter have higher levels of antioxidant enzymes. Furthermore, activation of P2Y12 receptors by ATP, which is co-released with ACh at the neuromuscular junction, can induce ROS production by activating NADPH oxidase [632] and thereby control neurotransmission.
Finally, inflammation and oxidative stress can participate in cancer. Activation of pro-inflammatory cascades via TLR4 has been related to highly metastatic tumors [636]. On the other hand, ROS production via NOX2 can regulate inflammation [645]. In line with these observations, it has been reported that NOX activity and expression is associated with tumorigenesis of lung cancer, and that inhibition of NOX function or mRNA expression can significantly block lung cancer formation and invasion [646].
In conclusion, ROS play a Janus-faced role: they can provide physiological actions or contribute to pathogenesis. We have seen in this review that the regulatory actions of ROS are complex and can differ from one cell system to another. Therefore, understanding the fine regulatory mechanisms of ROS in specific cells and tissues will contribute to the identification of precise targets and thus to the development of more efficient therapeutic strategies.
9. The emerging roles of redox networks in tumor cell proliferation, hearing loss, neuropsychiatric disorders and cardiovascular pharmacology
Isabel Fabregat (E-mail: ifabregat@idibell.cat) and Santiago Lamas (E-mail: slamas@cbm.csic.es).
9.1. Introduction
Our COST Action has invested a significant amount of work and interaction on major biomedical and clinical problems in which ROS have an established role or where significant evidence has been mounted for their participation in the initiation, perpetuation or resolution of the most pressing health challenges in the developed world in the next decade. The focus of this section are the ideas, expertise and studies of the members of the EU-ROS COST Action.
We have grouped the contributions in this sub-section into four major categories: cancer, metabolic diseases, neuropathology and cardiovascular diseases. A summary of these is depicted in Fig. 9.1.
Fig. 9.1.
Summarizing scheme on the contribution of ROS signaling to different physiological and pathophysiological conditions. This section covers an ample spectrum of disorders with the theme of redox signaling and nitroxidative stress as the common modulator of disturbances related to tumor cell migration, fibrogenesis, hearing loss, neuropsychiatric disorders, metabolic syndrome, drug metabolism, renal hemodynamics and lung hypoxia. In the slide the participation of relevant enzymatic pathways (NOXs, SODs, ALDH-2) are indicated. BMI: body mass index. The rest of the abbreviations are defined in the text.
A fundamental problem in cancer biology has to do with the migration of tumor cells to extraneous tissue environments where they may constitute the core of metastatic niches. The role of oxidative stress germane to this phenomenon is far from clear and remains controversial. For example, it has been found that melanoma metastasis may progress by suppressing redox-related effector molecules such as APE1/REF-1 [647] and making melanoma cells less responsive to oxidative-induced DNA damage. In contrast, it has been also reported that oxidative stress may inhibit distant melanoma metastasis in vivo [648]. In a similar vein, the targeting of fundamental endogenous antioxidant systems, such as GSH and thioredoxins, has been proposed as a potential advantageous therapy for cancer and HIV [649]. A more detailed analysis on the molecular mechanisms related to redox responses in the context of cancer cell migration is provided below. Of interest, the cellular and molecular sources responsible for ROS generation have been the object of intense study in pathophysiological settings such as liver fibrosis and carcinogenesis. Here too, the jury is still out regarding the beneficial or detrimental role of NOXs, especially in reference to the apparently opposite effects of NOX1 and NOX4 in liver tumorigenesis, as discussed in the following paragraphs.
The Action has benefited from the work of groups interested in the redox regulation of neurological and psychiatric diseases with a specific emphasis on two relatively unexplored areas: the inner ear and schizophrenia. Of all the NOX isoforms, NOX3 is clearly the most elusive, less studied and even less well understood, primarily due to its restricted topological expression in the inner ear. One of the laboratories involved in this COST action has shed light on the function of NOX3 in balance regulation and hearing function by developing sophisticated mouse models that lend themselves to investigation of this issue. The problem of hearing loss, either genetically based or acquired after environmental exposure to noise or aging, has experienced a recent twist where redox regulation has taken center stage. Indeed, a very captivating report has described that pejvakin, a protein related to peroxisomal function and proliferation, lies at the basis of an inherited sensorineural form of deafness and its lack or malfunction induced by oxidative stress makes the inner ear extremely vulnerable to noise-induced damage [650]. NOX3 is expressed in the cochlea even though mice devoid of it do not show an apparent hearing loss-related phenotype. Whether peroxisomal generation of ROS is connected to NOX3 function is a possibility that remains to be investigated. Another set of diseases where the role of redox regulation is intriguing is in the realm of neuropsychiatry. While a significant amount of evidence regarding the role of oxidative stress has accumulated in neurodegenerative diseases (please see the corresponding section), much less is known in the context of psychoses. In the particular case of schizophrenia, aside from profound alterations in the levels of several neurotransmitters, a disturbed redox homeostasis has been evidenced with a major focus on glutathione regulation. These intriguing findings are discussed below highlighting the major advances and pitfalls in the knowledge of this exciting research avenue.
There is currently little doubt about the relevant role played by oxidative stress in the metabolic syndrome and in particular in type 2 diabetes. Both insulin production, as well as insulin signaling, are redox sensitive processes, highly determined by the levels of O2•- and •NO, and their interplay. Thus, impairment of physiological signaling by ROS/RNS is implicated in the etiopathology of diabetes [651]. Some groups in our COST Action are interested in exploring how the fine tuning of the O2•-/•NO ratio is regulated and how it affects insulin synthesis and degradation. Furthermore, oxidative stress contributes to pancreatic β cell loss, which impairs insulin secretion [652]. Therefore, therapeutic strategies to prevent β cell dysfunction require better knowledge about how to protect them against direct or indirect effects of free radicals and lipid peroxidation. Also relevant is the fact that both insulin sensitivity and metabolic homeostasis depend on the capacity of adipose tissue to take up and utilize excess glucose and fatty acids. This buffering capacity depends on physiological levels of •NO, whose function may be altered by excessive formation of O2•- [653]. Interestingly, recent evidences indicate that redox signaling and oxidative stress may contribute to adipose tissue remodeling and this is a relevant area of research. These new aspects about the relevance of ROS and RNS in the context of metabolic syndromes and diabetes are expanded below highlighting the major challenges addressed by research members within the EU-ROS COST Action.
Cardiovascular physiology and pathophysiology have also been a major object of this COST Action and this is reflected in the present section of this overview. In this section we also include contributions of the COST members related to vascular aspects with a specific focus. One of these is the still unresolved and important problem of toxicity and tolerance derived from the clinical use of organic nitrates. In the last few years new light has been shed upon the molecular mechanisms related to the side effects of nitroglycerin and other nitrates evidencing a major role for mitochondrial dysfunction and disruption of redox homeostasis. A detailed update of the problem is provided in the section that follows. A disturbed vascular redox balance underlies the pathophysiology of important morbid conditions in other organs such as the lung and kidney. In the lung, chronic hypoxic pulmonary hypertension leads to vasoconstriction and vascular remodeling, two phenomena that self-perpetuate the vicious cycle. In the kidney, studies in animals using superoxide dismutase (SOD) mimetics point to an important role of redox dysfunction in the increased vascular resistance associated with chronic kidney disease and open the possibility of therapeutic intervention. Both sets of concepts and data are appropriately discussed below.
9.2. Cancer
The following subsections were composed by Esther Bertrán, Isabel Fabregat, Ana Fernandes, and Nuno Saraiva.
9.2.1. Redox regulation of tumor cell migration
Several cellular and extracellular events are involved in ROS production and can directly or indirectly impact on mechanisms involved in different types of cell migration thus contributing to the invasiveness and poor prognosis of several cancers. These mechanisms include invadopodia formation, MMP activation/expression, focal adhesion dynamics, cell-cell contact, cytoskeleton remodeling, and gene expression regulation (Fig. 9.2) [654], [655], [656]. In turn, many mechanisms involved in cell migration, such as integrin signaling or Ca2+ homeostasis affect ROS production, creating intricate and still poorly understood regulatory mechanisms [656], [657]. ROS can act as second-messengers by oxidizing cysteine residues that impose functional changes on cell migration-relevant kinases, phosphatases and transcription factors [658]. The uneven physical and temporal distribution/activity of the elements involved in ROS production (Fig. 9.2) can lead to heterogeneous ROS accumulation within a migrating cell and during the migration process. Several sources may contribute to the generation of ROS (see figure). Their topological distribution influences the localization and oxidative status of many regulatory elements, thus allowing a redox-dependent spatial and temporal regulation of cell migration/adhesion mechanisms. Mitochondrial ROS are associated with the initial ECM contact while a cytosolic ROS increase is involved in cytoskeleton remodeling and NOX-derived ROS are involved in invadopodia formation [186]. Additionally, the existence of an elevated reductive potential as well as strong redox buffers is essential to limit ROS diffusion and maintain a polarized distribution of oxidant intermediates [657]. The final outcome in cell proliferation, migration and adhesion depends on the activation of a series of effectors regulating gene expression, matrix degradation, cytoskeletal remodeling and cell-cell contact adhesion (see figure).
Fig. 9.2.
ROS and cell migration. (A) There are several sources of reactive species (RS) whose subcellular distribution dictates the fate and direction of cell migration. In this cartoon they are indicated by Arabic numbers. (B) Key mechanisms involved in the redox-regulation of cell migration. The main effectors participating in cell-cell contact adhesion, gene expression activation, matrix degradation, cytoskeletal remodeling and focal adhesion are indicated.
The complexity of mechanisms by which ROS mediate cancer cell migration is still far from being understood. A general pattern of the impact of ROS and antioxidants on tumor metastatic potential is difficult to depict given the inherent differences among cancer types. For example, MnSOD expression may be highly dependent on the primary tissue [659]. Even considering tumors from the same organ of origin, the stage and grade of disease progression may influence intracellular ROS and antioxidant enzyme levels. Most of the previous studies on the influence of ROS in cell migration were performed in cell culture models. However, the activity of antioxidant enzymes varies with the culture conditions and declines after a high number of cell passages. Another aspect that limits the usefulness of cell culture models is their inability to recreate the tumor microenvironment, which includes cancer cells, cancer stem cells and stromal components, such as extracellular matrix, fibroblasts, immune cells, adipocytes, and vascular cells. The analysis of specific subsets of tumor cells is both essential and challenging, since tumors contain different cell types, necrotic material and stroma. Differences between conventional cell culture models and the metastatic microenvironment contribute to the discrepancies observed in the literature, reinforcing the need for more physiologically relevant approaches.
The implementation and standardization of in vivo techniques to measure the levels of specific ROS and their sub-cellular distribution is essential to dissect and confirm several mechanisms by which ROS impact cancer cell migration.
9.2.2. Role of NADPH oxidases in fibrogenesis and cancer
The oxidative stress that takes place during chronic liver diseases is due to increased ROS production, as well as to decreased activity of antioxidant systems. It is not only a consequence of chronic liver injury, but also a significant contributor to excessive tissue remodeling and fibrogenesis. The main source of ROS implicated in liver fibrosis is the NOX family of proteins [523], [660]. NOX proteins are also likely to act as a persistent, endogenous source of ROS during Hepatitis C virus (HCV)-induced pathogenesis [661]. These enzymes are expressed in diverse cells and tissues, and their products are essential in several physiological settings [662]. NOX1, NOX2 and NOX4 have been proposed to play essential roles in liver fibrogenesis. Transforming growth factor-beta (TGF-β)-induced activation of hepatic stellate cells (HSC) to myofibroblasts (MFB) is mediated by NOX4-derived ROS [184]. Moreover, NOX1, activated either by TGF-β or other stimuli, promotes myofibroblast proliferation by PTEN inactivation to positively regulate an AKT/FOXO4/p27 signaling pathway [663]. Furthermore, NOX1 activity might further contribute to the inflammatory process that promotes COX-2 expression and prostaglandin synthesis in hepatocytes [664]. Promotion of hepatocyte apoptosis is another crucial event during fibrogenesis since it triggers Kupffer cell and HSC activation by secreting cytokines, chemokines and microparticles. NOX4 is necessary to mediate apoptosis induced by TGF-β [177], [184]. However, the pro-apoptotic effect of the cytokine can be attenuated when NOX1 is active [179]. NOX2 is expressed in both endogenous liver cells and bone marrow-derived cells, possibly acting in the process of phagocytosis of dead hepatocytes [665]. The role of NOX in hepatocarcinogenesis is more complex and is still a matter of study in different laboratories. NOX4 may play essential roles mediating TGF-β tumor suppressor functions, in particular its effects on cell death [177], [528]. In this sense, recent results indicate that silencing NOX4 in liver tumor cells increases their proliferative and tumorigenic properties [185]. In contrast, NOX1 might control autocrine cell growth of liver tumor cells through regulation of the EGFR pathway [179], [666]. In fact, recent studies indicate that NOX1 and NOX4 proteins have opposite prognostic effects in HCC. High NOX1 and low NOX4 expression were independent predictors of both shorter recurrence-free survival and shorter overall survival [667]. In view of the essential roles played by NOX4 in liver fibrosis, recent preclinical studies with a NOX4/1 inhibitor (GKT137831) have demonstrated its efficiency as a potent inhibitor of fibrosis and hepatocyte apoptosis [668], thus paving the way for future translational studies.
9.3. Neurological and psychiatric disorders
The following subsections were composed by Vincent Jaquet, Karl-Heinz Krause, Francis Rousset, and Tamara Seredenin.
9.3.1. Redox imbalance in the pathogenesis of schizophrenia
The etiology of schizophrenia is unknown, but it is generally thought that a combination of genetic and environmental factors modulates the disease. A popular theory of schizophrenia involves an imbalance between inhibitory and excitatory neurotransmission. This is evidenced at the neuropathological level by a decrease in number of parvalbumin-positive GABAergic interneurons (PV neurons) and alterations of N-methyl-D-aspartate (NMDA) receptor subunits [669]. The molecular mechanisms involved at onset, during psychotic episodes and chronicity of schizophrenia are unknown, but numerous studies in both humans and rodents point towards a contribution of neuroinflammation, increased reactive oxygen species (ROS) generation and dysfunction of redox signaling [670], [671].
Most studies using post-mortem analysis of brains of schizophrenic patients indicate (i) increased markers of oxidative modifications of biomolecules, including lipids (4HNE), nucleic acids (8-hydroxydeoxyguanosine) and proteins (carbonylation); (ii) decreased levels of glutathione and enzymes involved in glutathione metabolism, (iii) microgliosis and astrogliosis and increase of microglia activation markers. Rodent models of social isolation and administration of NMDA receptor antagonists recapitulate several neuropathological features (loss of PV neurons and pro-oxidative phenotypes). Several studies have proposed a key role of specific redox systems in the pathogenesis of diseases of the central nervous system (CNS). Low glutathione (GSH) levels are a hallmark of schizophrenia [672]. GSH is a major regulator of intracellular redox homeostasis. Schizophrenic patients show low levels of GSH in blood and in prefrontal cortex, and mice deficient in the Glutamate-Cysteine Ligase gene, the rate-limiting enzyme for GSH synthesis, display behavioral and neurochemical abnormalities commonly associated with schizophrenia [673]. Clinical trials using the general antioxidant agent N-acetylcysteine (which can act as a GSH precursor) show promise for treating some features of schizophrenia. Behrens et al. observed an increase in NOX2 activity in neurons following repeated administration of subanesthetic doses of the NMDA receptor antagonist ketamine [674]. Importantly, ketamine administration led to the loss of PV neurons, which could be pharmacologically reversed by administration of the antioxidant apocynin. The efficacy of apocynin was further confirmed in another study using a transgenic model of NMDA receptor hypofunction (Ppp1r2-Cre/fGluN1 knockout mice), which endured social isolation. However, the increase of neuronal oxidation was not changed in NOX2-deficient mice, suggesting that NOX2 is not the main source of ROS in this model [675]. Interestingly PGC-1α – a key regulator of mitochondrial biogenesis and antioxidant response – is enriched in GABAergic interneurons [676]. In this social isolation model, PGC-1α downregulation correlates with increased oxidation in PV interneurons, suggesting a role for mitochondrial ROS [675]. Other studies failed to detect NOX2 in mature neurons, but showed that NOX2 is mainly expressed in microglia and adult neural stem cells in the CNS [677], [678]. Microglia are the CNS cell type that expresses the highest levels of proteins regulating redox dynamics suggesting a prominent role in the CNS redox balance [679]. Further studies assessing microglial perturbations in psychiatric diseases may highlight how redox genes are involved in modulating neuronal activity by pruning synapses and regulating neurogenesis. Altogether, there are strong arguments for redox imbalance in schizophrenia; however, the sources of ROS, redox kinetics, contribution of specific redox pathways and the cell types implicated require further studies.
9.3.2. Role of NOX3 in inner ear pathologies
Research over the last decades has identified a major role for ROS in hearing disorders, including overexposure to noise, ototoxic drugs (e.g. cisplatin) and age-related hearing loss [680]. While the sources of ROS are complex and only partially understood (e.g. mitochondria), there is increasing evidence that activation of NOX enzymes, in particular NOX3, plays a key role in hearing loss. High level and specific expression in the inner ear identifies NOX3 as a prime drug target to combat hearing loss [681]. NOX3 is a multi-subunit NADPH oxidase, functionally and structurally closely related to NOX1 and NOX2. NOX3 is active as a multiprotein complex including the membrane-bound NOX3 and p22phox and the cytosolic subunits NOXO1 and NOXA1. NOX3 is crucial for the formation of otoconia, small proteinaceous carbonate crystals involved in balance [682]. Mice carrying a loss of function mutation within the NOX3 complex – including the common NOX1 to NOX4 subunit p22phox, or the cytosolic subunit NOXO1 – have a vestibular phenotype similar to NOX3 mutant mice (referred to as head-tilt mice). However, the role of NOXA1 is less clear and has mostly been addressed in vitro. So far, the physiological relevance of NOX3 expression in the cochlea remains unclear as no hearing phenotype was described for NOX3 loss of function mutant animals. The role of NOX3 in cisplatin induced hearing loss is actually the best documented. An agonist effect of the antineoplastic drug cisplatin on the NADPH oxidase activity of NOX3 was observed in vitro, suggesting a potential role of NOX3 in cochlear damage [683]. Moreover, an increased NOX3 mRNA level has been described in the cochlea of cisplatin treated rats while siRNA against NOX3 were able to prevent cisplatin-mediated hearing loss in this model [684]. Together, it is now accepted that NOX3 is a major source of ROS in the cochlea. However, the role of NOX3 may not be limited to cisplatin-induced ototoxicity. Indeed, oxidative stress is also a major component in other hearing pathologies such as presbycusis, Meniere's disease or noise-induced hearing loss. For these conditions, further experimental confirmation and a deeper confirmation and understanding of the role of NOX3 in hearing loss based on loss of function mutant mice is required. Three mouse models of hearing loss have been successfully developed in one of the laboratories of this COST action – namely noise overexposure, cisplatin and age-related hearing loss models and investigation of the role of NOX3 is currently ongoing through both NOX3 and p22phox mutant mice. In parallel, specific approaches allowing inhibition of pathological NOX3 activity should be developed. At this point, it is difficult to predict whether classical pharmacological approaches, i.e. small molecule NOX3 inhibitors, or rather molecular biology-based therapies, i.e. siRNA-mediated knock-down, will be the ultimate tools for NOX3-targeted therapies.
9.4. Metabolic diseases and diabetes
The following subsections were composed by David Bernlohr, Bato Korac, Irina Milisav, Tatjana Ruskovska, Shlomo Sasson, and Ana Stancic.
9.4.1. Importance of setting superoxide/nitric oxide ratio in diabetes. Role of L-arginine
A hallmark of the diabetic state is the increased level of O2•- in many tissues as a result of hyperglycemia and hyperlipidemia. The high level of O2•-, along with the reduced endogenous synthesis of NO, is responsible for the low bioavailability of NO in diabetic conditions. The product of O2•-/NO interaction, peroxynitrite (ONOO-) and more potent oxidants, deriving from ONOO-, play a significant role in the metabolic complications that accompany diabetes including obesity and insulin resistance. To increase NO bioavailability and/or to simultaneously reduce the O2•- level in order to improve prediabetic and diabetic states, supplementation with L-arginine, the substrate for NO synthases, as well as mimics of superoxide dismutase (SOD) have been proposed as a potential therapeutic strategy. Several beneficial effects of L-arginine have been observed in diabetes. In the pancreas L-arginine induces β-cell regeneration [685] and positively regulates insulin synthesis and secretion [686]. The favorable effects of L-arginine in obesity and insulin resistance are well-established and could be ascribed to the role of L-arginine in improving fat metabolism, by increasing lipolysis and β-oxidation [687] as well as thermogenesis-related energy expenditure [653], [688], [689] and insulin sensitivity [687]. It seems likely that all these effects of L-arginine may be related to improved NO bioavailability. An innovative mechanism-based approach for controlling NO and O2•- levels includes the use of redox-modulating compounds such as selective functional mimics of SOD. SOD mimics were initially designed to scavenge excessive O2•-, but additional data suggest that a decreased formation of peroxynitrite and nitr(os)ative stress and restoration of NO signaling underlie the therapeutic benefits of these agents [690], [691], [692]. These compounds show positive effects on insulin sensitivity and metabolic complications in diabetes, in part through the recovery of mitochondrial function [690], [692], [693]. Altogether, using specific potent redox-active agents to adjust the O2•-/NO ratio seems to have potential in the treatment of this widespread metabolic disorder. The fact that traditional antioxidants failed to show significant benefits in diabetes emphasizes the importance of the aforementioned approaches.
9.4.2. Regulation of insulin degradation by redox-related pathways
S-Nitrosylation (the covalent addition of NO-related species to cysteine residues of target proteins resulting in the formation of nitrosothiols-SNOs) has recently emerged as a relevant mechanism that activates or inhibits protein function, and alters protein conformation, protein aggregation, protein localization and protein-protein interactions [694]. S-nitrosylation can affect other post-translational modifications of cysteine thiol groups (like palmitoylation, which is important for protein-membrane associations), bond formations and cellular signal transduction pathways [695], [696] Only specific cysteine residues are S-nitrosylated, as this depends on the proximity to reactive species, local hydrophobicity and is counterbalanced by denitrosylation enzymes, like the thioredoxin system and protein disulfide isomerases.
Insulin degrading enzyme (IDE) is a zinc metalloprotease that degrades insulin, small peptides and amyloidogenic peptides, like amyloid β peptides, α-synuclein, amylin, and atrial natriuretic peptide (Fig. 9.3) [696]. IDE deficit increases the abundance and signaling of the pancreatic hormones insulin, amylin and glucagon. Increased insulin improves glucose tolerance, increased amylin levels slow postprandial gastric emptying. IDE deficit increases levels of amylin, α-synuclein, and Aβ monomers. These molecules impair the secretory function and survival of pancreatic β cells and neurons through the formation of toxic oligomers. Whereas IDE is expressed in all tissues, the regulation of its levels is an intricate and still unclear process. Cell stress, glucagon and free fatty acids have all been shown to participate [696]. In cells IDE is mostly localized in the cytosol but its presence has also been reported in peroxisomes, mitochondria, cell membranes and extracellular fluids, including cerebrospinal fluid and plasma [696], [697].
Fig. 9.3.
Structure of the insulin degrading enzyme complex with amyloid-β, sites of redox regulation and major functions as a peptidase. The structure was displayed by the Visual Molecular Dynamics program [698] from the structure PDB ID: 2G47 [699]. Yellow: the sulfur atoms of cysteines; orange: Glu111; red/gold: ATP, light green: amyloid-β fragment. We acknowledge the help of Jure Stojan to prepare the crystal structure image.
IDE can be present as a monomer or multimer but it has been shown that dimerization allosterically regulates its catalytic activity [697]. Dimerization and binding with cytoskeletal proteins and ATP enhances the degradation of small peptides, like bradykinin. Aβ-bound IDE structures reveal that the β-strand of amyloidogenic peptides interacts with the door subdomain of IDE, which may explain the affinity of IDE for amyloidogenic peptides.
Exposure of IDE to S-nitrosoglutathione can result in the modification of IDE activity through S-nitrosylation of at least 5 of its 13 cysteine residues: Cys110, Cys178, Cys819, Cys789 and Cys966 [700]. S-nitrosylation of Cys819 promotes nitrosylation of Cys110, which is near the catalytic site that is composed of two glutamates (residues 111 and 189) and a zinc ion coordinated by two histidines (residues 108 and 112). S-nitrosylation of Cys110 results in inactivation of IDE. Modification of Cys178 prevents the nitrosothiol formation on Cys110 and buffers the enzyme inhibition. S-nitrosylations at Cys789 and Cys966 induce a conformational change that triggers the aggregation of IDE and its inhibition. S-nitrosylation of Cys178 protects all of the above mentioned cysteine residues from nitrosylation. The oxidative modifications by H2O2 resulted in the modifications of the same residues with equal effects on IDE activity. Although these results are from in vitro studies, IDE was exposed to 10–100 µM S-nitrosoglutathione or H2O2, which is in the concentration range of ROS/RNS released under pathophysiological conditions [700]. These modifications were tested for inhibition of Aβ hydrolysis. It has been proposed that the cysteines of IDE act as sensors of environmental ROS/RNS levels through subtle alterations of the protein structure and that IDE function is modulated by the levels of oxidative/nitrosative stress. It is likely that the oxidation of cysteine 178 may result in the protection of the active site under moderate amounts of ROS, while severe oxidative stress inhibits the enzyme. The decreased activity of IDE through S-nitrosylation could also reduce the degradation of both insulin and Aβ and contribute to pathological disease conditions in type 2 diabetes and AD. This is supported by findings demonstrating an altered redox state of hippocampal and cortical neurons in both diseases. Indeed, increased levels of glucose result in a significant rise in neuronal reactive nitrogen species similar to the effects of oligomeric Aβ peptides. The high glucose and Aβ oligomers were shown to increase neuronal Ca2+ and NO levels in cortico-hippocampal brain slices and in cortical cultures to the •NO levels that could potentially promote S-nitrosylation of specific protein thiols in IDE resulting in a reduction or inhibition of its activity [701]. Therefore, increased oxidative/nitrosative stress can contribute to increased Aβ levels, an important pathologic hallmark of AD through the S-nitrosylation of IDE. Although IDE is the best characterized enzyme that degrades insulin, reduction in IDE activity in various experimental models does not consistently increase insulin concentrations. Recently the study with the IDE inhibitor that binds to its exosite resulted in increased plasma levels of amylin, but not of insulin [697]. Other regulatory mechanisms and/or IDE substrates may also contribute to glucose homeostasis, development of insulin resistance and diabetes mellitus type 2.
9.4.3. Lipohormesis and lipotoxicity in pancreatic beta cells
Monounsaturated and polyunsaturated fatty acids (MUFA and PUFA, respectively) promote adaptive responses and enhance cell tolerance to lipid overload through induction of cellular defense mechanisms (lipohormesis) that protect cells against lipotoxic effects. Recent lipidomic analysis performed on pancreatic beta cells that were exposed to increasing glucose and palmitic levels revealed extensive remodeling of fatty acids in membrane phospholipids. Particularly, the abundance of PUFA was decreased whereas the content of MUFA increased. These changes were accompanied by non-enzymatic peroxidation of the released PUFA and their transformation to bioactive 4-hydroxyalkenals. This process in beta cells leads to the generation of 4-hydroxynonenal, which activates PPARδ [702], [703], [704]. The latter augments the secretion of insulin, which promotes hepatic and peripheral mechanisms that enhance glucose disposal and the incorporation of free fatty acids into triglycerides and their storage in adipose tissues. Excessive and prolonged nutrient overload often interferes with normal cell functions and dysregulates hormonal-mediated inter- and intra-organ metabolic communication networks. In beta cells, hyperglycemia and hyperlipidemia synergistically compromise insulin synthesis, glucose-stimulated insulin secretion and cell survival. The mechanisms underlying these derangements, which characterize the etiology of type 2 diabetes and other manifestations of the metabolic syndrome, have been thoroughly investigated [705]. A central hormetic mechanism is redox-mediated release of KEAP1 from the KEAP1/NRF2 complex, allowing nuclear translocation of NRF2 and its interaction with Antioxidant Response Elements (ARE) in promoters of target genes encoding antioxidant enzymes, ultimately resulting in their activated transcription. Beyond the modification of dietary habits and life style in addition to treatment of the underlying condition, it would be theoretically sound to propose pharmacological approaches with potent oral antioxidants, which are efficiently absorbed, reach the circulation and affect cells, such as beta cells, and protect them against direct and indirect effects of free radicals and lipid peroxidation. However, the prevailing knowledge favors the notion that these oral antioxidants are only effective when triggering intracellular physiological signals related to endogenous protective pathways such as the aforementioned KEAP1/NRF2-ARE axis.
9.4.4. Redox regulation of white adipose tissue remodeling
The white adipose organ features high metabolic activity and endocrine function and regulates whole-body energy homeostasis through the secretion of macronutrients (fatty acids) and adipokines (e.g., adiponectin, resistin). White adipose tissue (WAT) can be anatomically divided into visceral and subcutaneous. It is found distributed broadly in the body and is subject to regulatory cues throughout development and aging. Evolutionarily developed for effective fat storage in the short periods of food access, as well as for immune surveillance, dysfunctional obese WAT underlies metabolic disorders in the modern era because food is readily and continuously available [706].
In the fed state, insulin mediates the uptake of glucose by adipocytes that is metabolized via the combined actions of glycolysis, TCA cycle and fatty acid biosynthesis to produce precursors utilized for triglyceride biosynthesis. Through these pathways, high nutrient oxidation decreases the adipocyte NAD+/NADH ratio that attenuates the activity of all NAD+ dependent enzymes, including the sirtuins. Sirtuins deacetylate/deacylate both histone and non-histone target proteins regulating their activity and cellular function. SIRT1 is the best studied of all seven mammalian sirtuins. It provides a molecular link between the cellular metabolic status and the adaptive transcriptional response. In the WAT SIRT1 acts through repression of transcriptional activity of the nuclear receptor PPARγ, the master regulator of both lipogenesis and adipogenesis. Calorie restriction increases the adipocyte SIRT1 activity and promotes lipid mobilization, as well as insulin sensitivity and glucose tolerance [707]. Therefore, the adipocyte NAD+/NADH ratio, acts as an important indicator of cellular and whole body energy status, and emerges as one of the key redox regulators of WAT function. SIRT1 also has an important role in the browning of the WAT, a metabolically beneficial WAT remodeling that promotes energy expenditure to combat obesity and diabetes, which might serve as a novel strategy to improve metabolic health. A very recent study reveals that capsaicin (a bioactive compound of red pepper) has the potential to induce increased expression and phosphorylation of SIRT1 in the subcutaneous WAT of mice fed a high fat diet. Activated SIRT1 deacetylates both PPARγ and positive regulatory domain containing 16 (PRDM-16), promotes WAT browning and prevents weight gain. The effect of capsaicin on SIRT1 is mediated by the transient receptor potential cation channel subfamily V 1 (TRPV1), Ca++/calmodulin activated protein kinase II and adenosine monophosphate activated kinase (AMPK). In parallel, an increased expression of mitochondrial uncoupling protein 1 (UCP1) was found in the WAT of mice treated with capsaicin, confirming the key role of UCP1 in the browning of WAT [708].
Evolutionarily designed for effective fat storage (and release), the WAT has developed an extraordinary ability for expansion, thus preventing the metabolically detrimental ectopic fat accumulation. As a consequence of nutrient overload, the existing adipocytes enlarge and accumulate triglycerides (a process known as lipogenesis), and/or new adipocytes differentiate from the multipotent mesenchymal stem cells (MSC) to form mature adipocytes (adipogenesis). Adipogenesis, also known as adipose tissue hyperplasia, leads to a metabolically healthy obese WAT phenotype that is characterized by increased secretion of adiponectin and insulin sensitivity, as well as decreased immune cell recruitment, hypoxia and fibrosis. Among the many factors that initiate and regulate adipogenesis, the contribution of ROS has been recognized in the face of NOX4-generated H2O2. Importantly, ROS depletion with N-acetyl-L-cysteine results in abrogation of in vitro adipogenesis [709]. Whereas adipocytes have an extraordinary capacity for fat accumulation (lipogenesis), their growth above the critical volume (hypertrophy) leads to necrotic death and pro-inflammatory remodeling linked to macrophage infiltration and their phenotypic change from M2 (inflammation resolving) to M1 (pro-inflammatory), appearance of crown-like structures, increased angiogenesis and extracellular matrix production, hypoxia, unbalanced production of pro- and anti-inflammatory cytokines, and insulin resistance. Dysfunctional obese WAT exhibits significantly increased protein carbonylation, indicating the central role of excess ROS and oxidative stress in obesity induced insulin resistance [710].
In contrast to the white adipocytes and their function for fat storage, the adipocytes from the brown adipose tissue (BAT) are responsible for energy dissipation in response to cold, exercise or excess calories. The key player in energy dissipation is the inner mitochondrial membrane protein UCP1 that uncouples the electron transport chain (ETC) from ATP biosynthesis, thus dissipating the proton motive force, generating heat, and increasing the rate of substrate oxidation. The recent discovery of metabolically active BAT in human adults has attracted much interest as a potential target for the development of anti-obesity drugs. In addition to the classical white and brown adipocyte phenotype, beige adipocytes (also known as “brite” – short for brown in white, or “brown-like”) have been identified as an inducible form of thermogenic adipocytes. When induced they exhibit high UCP1 expression and energy expenditure. Beige adipocytes express unique genetic signatures that distinguish them from both white and brown adipocytes. In rodents, the brite adipocytes are most abundant in the subcutaneous WAT and are responsible for its browning [711].
Intriguingly, a recent study reveals that UCP1-mediated thermogenesis depends on mitochondrial ROS production. It has been shown in vivo that acute activation of BAT thermogenesis is associated with a substantial increase in mitochondrial superoxide, lipid hydroperoxides and hydrogen peroxide. Pharmacological depletion of mitochondrial ROS inhibits the UCP1 dependent increase in whole-body energy expenditure, confirming the essential role of ROS in the regulation of adipocyte function and metabolism. At the same time, a substantial oxidation and depletion of the cellular and mitochondrial glutathione pool has been demonstrated, as well as an increased global protein sulfenylation, and sulfenylation of UCP1 Cys253 as a redox sensitive site related to thermogenesis [712]. The possible dependence of WAT UCP1 activity on the cellular pro-oxidant state was studied in Nrf2-/- mice. The NRF2 transcription factor is a critical regulator of genes involved in detoxification and antioxidant defense. Therefore, Nrf2-/- mice are more susceptible to toxicant- and oxidative stress-induced diseases, as well as autoimmune diseases and cancer. Paradoxically, they are also resistant to high fat diet-induced obesity, exhibit improved metabolic profiles, and demonstrate increased energy expenditure in comparison to the wild type mice fed a high fat diet. Such mice also exhibit a concomitant decrease in the GSH/GSSG ratio and up-regulation of UCP1 and other BAT markers in intra-abdominal fat pads [713]. Overall, balanced ROS levels are required for proper WAT function, and both very high and very low levels impede its function and influence whole-body energy homeostasis [714]. Therapeutic control of adipose ROS therefore represents an attractive axis to regulate not only adiposity, but also metabolic healthfulness.
9.5. Cardiovascular pathologies
The following subsections were composed by Andreas Daiber, Vaclav Hampl, Jan Herget, Jaap Joles, Thomas Münzel, Isabel Nguyen, and Olga Vajnerova.
9.5.1. Drug-induced oxidative stress – the case of nitroglycerin
Organic nitrate therapy is still a mainstay in the treatment of patients with acute and chronic congestive heart failure, stable coronary artery disease or acute coronary syndrome [715]. However, despite the fact that members of this drug class have been clinically used for more than a century, chronic organic nitrate therapy (especially in the case of nitroglycerin) is associated with a number of serious side effects, including induction of oxidative stress [716] and endothelial and autonomic dysfunction [717], with incomplete understanding of the underlying pathomechanisms. The induction of reactive oxygen and nitrogen species formation by chronic nitroglycerin therapy was shown to largely depend on mitochondrial oxidative metabolism since partial deficiency in MnSOD aggravated nitrate-induced oxidative stress and also the degree of tolerance to the vasodilator activity of nitroglycerin [718]. This was later shown to rely on complex I dysfunction in response to nitroglycerin therapy [719]. The initial formation of mitochondrial reactive oxygen and nitrogen species can also activate secondary sources of oxidants in a crosstalk fashion involving mitochondrial KATP channels and the permeability transition pore [61]. This leads to the activation of specific enzymatic “redox switches” in NOXs and eNOS, as recently reviewed [60]. The formation of mitochondrial reactive oxygen and nitrogen species may lead to endothelial dysfunction by the activation of the vascular and phagocytic NOXs through protein kinase C [720] as well as to the uncoupling of eNOS by increased S-glutathionylation, inhibitory phosphorylation and oxidative depletion of the eNOS cofactor tetrahydrobiopterin [478]. eNOS uncoupling and oxidative desensitization of the soluble guanylyl cyclase are probably the major enzymatic mechanisms contributing to endothelial dysfunction observed under nitroglycerin therapy, the latter also accounting for impaired nitroglycerin-dependent vasodilation (nitrate tolerance), all of them corrected by co-administration of an activator of soluble guanylyl cyclase [721]. Nitroglycerin-induced endothelial dysfunction but also nitrate tolerance may also rely on direct oxidative break-down of nitric oxide by superoxide leading to the formation of peroxynitrite and significantly increased levels of 3-nitrotyrosine-positive proteins, an effect prevented by the peroxynitrite scavenger hydralazine [722]. Finally, the bioactivation of nitroglycerin largely depends on enzymatic conversion by mitochondrial aldehyde dehydrogenase (ALDH-2) [723], which is a highly redox-regulated enzyme and inactivated by oxidative stress [716]. Hence, nitroglycerin-induced reactive oxygen and nitrogen species formation will ultimately lead to inactivation of the thiol-based enzymatic activity of ALDH-2 contributing to impaired nitroglycerin bioactivation, loss of its vasodilator activity and nitrate tolerance [724]. There is also clinical evidence for nitroglycerin-induced vascular oxidative stress [725] and oxidative DNA damage in patients under chronic nitrate therapy [726]. Very recently, we were able to demonstrate persistent oxidative modifications of DNA and proteins in nitroglycerin-treated rats, even after a 3.5- day nitrate-free interval, which was enough to normalize nitroglycerin responsiveness but not endothelial dysfunction. The latter could be related to endothelial cell death by pro-apoptotic stimuli such as 8-oxoguanine lesions in DNA and 3-nitrotyrosine-positive proteins [727]. Other organic nitrates show similar side effects, such as the activation of NADPH oxidases, increased vascular oxidative stress and severe endothelial dysfunction by chronic isosorbide-5-mononitrate therapy, which was associated with augmented endothelin-1 signaling and eNOS uncoupling [728]. The only exception among the clinically used organic nitrates is pentaerythritol tetranitrate [715], [716], which overcomes the typical side effects of nitrovasodilators by mechanisms that are largely based on NRF2-dependent activation of antioxidant enzymes such as heme oxygenase-1 and superoxide dismutase [729], [730] as well as beneficial effects on other transcription factor and epigenetic pathways [731], [732].
In conclusion, the side effects of chronic therapy with organic nitrates, especially with nitroglycerin, are largely based on oxidative stress and adverse redox regulation. Antioxidant therapy (e.g. by ACE inhibitors or statins) represents a pharmacological tool to overcome these side effects [715], [716], [717]. These and other combination therapies with specific antioxidants, inhibitors of relevant sources of oxidants (e.g. inhibitors of NADPH oxidase isoforms) and drugs for repair of oxidative damage (e.g. activators of soluble guanylyl cyclase) need to be explored in the future to increase the therapeutic window of organic nitrates, the oldest drug class in clinical use today.
9.5.2. Regulation of renal hemodynamics in chronic kidney disease
Hypertension and an increased renal vascular resistance (RVR) are inherent to chronic kidney disease (CKD). There is accumulating evidence that oxidative stress is involved in its pathogenesis [733]. Experimental induction of ROS (superoxide and H2O2) generation in the renal medulla promotes hypertension [734]. Downregulation of the antioxidant enzymes superoxide dismutase (SOD), catalase and glutathione peroxidase in the kidney were found in experimental CKD [735]. Hence, it has been suggested that antioxidant interventions may have beneficial effects on renal hemodynamics in CKD.
Tempol, a SOD-mimetic, has been shown to reduce oxidative injury in cells and animal models. It improved oxidative stress and lowered arterial pressure and RVR in various models of hypertension. However few studies have focused on the effects of Tempol in CKD. Rats undergoing 5/6 renal mass reduction to induce CKD showed an elevated arterial pressure and nitrotyrosine abundance, while urinary NOx excretion was depressed [736]. The administration of Tempol for one week ameliorated hypertension, reduced the nitrotyrosine abundance and increased NOx excretion. In spontaneously hypertensive rats (SHR), two weeks of Tempol administration decreased the elevated mean arterial pressure (MAP) and renal excretion of 8-iso-prostaglandin F2α and increased the glomerular filtration rate (GFR) [737], while it had no significant effect on these variables in the normotensive Wistar-Kyoto (WKY) control rats. These findings suggest that oxygen radicals may be involved in the long-term maintenance of hypertension in SHR. Another study found an elevated RVR in SHR compared to WKY rats [738]. When SHR were treated with Tempol the baseline RVR was no longer significantly different from the WKY rats. The additional use of the NO-synthase inhibitor L-NAME did not influence the effects of Tempol on the RVR, suggesting that it has a renal vasodilator effect and that the elevated RVR is unlikely due to ROS-induced quenching of NO.
The two-kidney one-clip model showed an elevated MAP and 8-iso-prostaglandin F2α excretion, which was significantly reduced by the acute administration of Tempol [739]. In the clipped kidney, adding Tempol increased GFR and effective renal plasma flow and reduced RVR compared to their controls. The latter was also found in the non-clipped kidney. This indicates that Tempol is able to have a vasodilating effect via dismutation of the superoxide resulting from angiotensin signaling that is independent of the local perfusion pressure. This supports the role of superoxide during the early stage of renovascular hypertension. In another study the acute effects of the SOD-mimetic on renal hemodynamics were investigated in rats with long-established CKD induced by bilateral 2/3 nephrectomy [740]. Tempol was not able to reduce the increased MAP in the CKD rats, but did lower MAP in age-matched controls with normal kidneys, suggesting that the maintenance of hypertension in this CKD model does not appear to depend on superoxide. As expected, RVR was higher in the CKD rats compared to controls, but the administration of Tempol did not have any significant effect on RVR. This proposes that renal resistance vessels are not sensitive to the renal vasoconstrictor effects of ROS in this model.
These data suggest that although antioxidant therapy can reduce the elevated arterial pressure in the early stages of experimental CKD, it does not appear to be effective once CKD is established. The same concept could be applied to the effects of Tempol on the RVR. In early stages of experimental CKD, prior to the development of renal fibrosis, Tempol can have a vasodilating effect independent of the local perfusion pressure. However, when structural damage occurs to the vascular beds and in their direct proximity, such as in established CKD, the vasodilating effects of Tempol are lost. This suggests that ROS may not be a driving force in the maintenance of disturbed renal hemodynamics in established CKD.
9.5.3. Vascular effects related to chronic hypoxia of lungs and placenta
Oxidative tissue injury participates in the pathogenesis of chronic hypoxic pulmonary hypertension (HPH) [741], [742]. HPH is caused by persistent lung hypoxia. It results in an increase in pulmonary vascular resistance to blood flow as a consequence of the sustained increase in the tone of the pulmonary vascular smooth muscle, decreased compliance of pulmonary blood vessels due to fibrosis and muscularization of prealveolar pulmonary arterioles. The severity of HPH is dose dependent. After stabilization it does not progress further. It is fully reversible after several weeks in normoxia. HPH typically appears in residents at high altitudes. It is involved in the pathogenesis of pulmonary hypertension in patients with various lung and heart diseases with alveolar hypoxia.
Two basic mechanisms increase pulmonary vascular resistance in HPH: structural remodeling of the peripheral pulmonary vessel walls and vasoconstriction. Vasoconstriction plays a pathogenetic role at the onset of HPH and it is related to hypoxia-induced oxidant vascular stress. Superoxide, hydroxyperoxide and peroxynitrite are important vasoactive factors released at the beginning of exposure to chronic hypoxia [743]. Oxidant tissue injury to the pulmonary vascular wall induced by hypoxia is the primary mechanism that triggers vascular remodeling in HPH. Antioxidant treatment at the beginning of hypoxia is more effective in reducing HPH than antioxidant therapy applied in later stages of developed pulmonary hypertension.
For the fetus, the placenta plays a role similar to that of the pulmonary circulation in postnatal life - blood oxygenation and CO2 removal. For this reason, we speculated that the regulatory and pathophysiological mechanisms of the fetoplacental and pulmonary vessels were similar. Indeed, we showed that both acute and chronic hypoxia elevate vascular resistance in the fetoplacental vascular bed, which is similar to pulmonary and in contrast to systemic vascular beds [744]. We have also demonstrated that a well-known state of high oxidative stress, maternal diabetes, causes an increase similar to chronic hypoxia in fetoplacental vascular resistance (preliminary observations). As is the case in the pulmonary circulation, oxidative stress also appears to be a major mechanism of vascular resistance elevation in the fetoplacental circulation.
9.6. Conclusions
Redox signaling and oxidative stress constitute fundamental regulatory mechanisms not only in highly prevalent pathologies but also in less explored areas of biomedicine such as those related to fibrosis, cell migration and proliferation, hearing loss, schizophrenic disorders, insulin resistance, drug metabolism, lung hypoxia or renal hemodynamics. In some cases, the underlying molecular mechanisms of this redox sensitivity have been deciphered, in others we are just beginning to open the door. It is clear that the pervading nature of ROS reactivity will continue to offer surprises-and explanations- of complex pathologies such as the ones covered in this section.
10. Toxin and bacteria-mediated ROS formation and antioxidant strategies in ROS-related diseases
Thomas Kietzmann (E-mail: Thomas. Kietzmann@oulu.fi) and Kateryna Kubaichuk (E-mail: kateryna.kubaichuk@oulu.fi).
10.1. Introduction
Research during the last decade has shown that ROS are important regulators of physiological and pathophysiological processes and not only simply detrimental due to their chemical nature or by causing oxidative stress. This is further supported by findings indicating that redox active compounds are essential components of living organisms. Thereby, recent advances in genomics and proteomics have led to the identification of a “redoxome” consisting of hundreds of proteins involved in redox systems. It comprises enzymes generating RONS such as NADPH oxidases and nitric oxide synthases, redox relays such as peroxiredoxins, thioredoxins and glutaredoxins, enzymes degrading ROS such as superoxide dismutase or catalase, as well as numerous proteins dependent on redox modifications, which are involved in the defense against oxidant, inflammatory and/or proteotoxic stress [745].
Although a number of substances, factors, products of metabolism, and nutrients are important for normal cell biology and redox signaling of all species, toxins are rather important for causing deleterious effects. Since toxins act universally on almost all species, it is conceivable that their action may, at least in part, involve ROS or oxidative stress and that antioxidant defense mechanisms could help to avoid the deleterious effects of toxins.
10.2. A toxin and its action via ROS
João G. Costa and Nuno G. Oliveira, discuss how the well-known nephrotoxic food contaminant and animal carcinogen, the mycotoxin ochratoxin A (OTA), appears to act at least partially via ROS and induction of oxidative stress, although different mechanisms of its toxicity have also been pointed out. Furthermore, the OTA actions can be overcome by antioxidant based approaches [746], [747], [748] (Fig. 10.1).
Fig. 10.1.
Lines of evidence supporting the induction of oxidative stress by Ochratoxin A (OTA). Alterations in biomarkers related with oxidative stress and several antioxidant-based approaches that provide protection against a plethora of OTA-induced toxic effects are indicated. Abbreviations: SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GST, glutathione S-transferase; GPx, glutathione peroxidase; GR, Glutathione reductase; AP-1, activator protein 1; NRF2, nuclear factor E2-related factor 2; PRDX4, peroxiredoxin-4; VDAC1, voltage-dependent anion channel 1; HO-1, heme-oxygenase 1; DDAH, dimethylarginine dimethylaminohydrolase; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde; 4-HNE, 4-hydroxynonenal; FPG, formamidopyrimidine DNA glycosylase.
OTA increased ROS appear to precede the loss of cell viability particularly in renal cells, indicating that these ROS may contribute to OTA cytotoxicity rather than being a consequence of cell death processes [746], [749]. Concomitant with ROS induction, OTA also increased lipid peroxidation, depleted antioxidant enzymes [746], [747], [748], [749], [750], [751], induced oxidative stress-related proteins, and caused DNA damage. The latter has also been associated with oxidative DNA damage, as assessed by the formation of 8-oxoguanine or using modified versions of the comet assay (e.g. with FPG) [749], [750]. Mechanistically, recent studies propose a pathway in which electrophilic species that directly bind to DNA bases are generated during OTA metabolism [752]. Further, OTA was found to be able to cause nitrosative stress due to stimulation of iNOS-mediated NO formation, which may contribute to the formation of peroxynitrite, nitrogen dioxide, and hydroxyl radicals [753]. Several in vitro and in vivo studies assessed the impact of different antioxidants on the OTA-mediated toxicity and it was found that, in general, antioxidants exerted protective effects [746], [747], [750], [751], [754].
10.3. Are ROS usage and antioxidant reactions conserved?
In order to avoid the life threatening effects of toxins and toxin-induced oxidative stress, both exogenously taken up antioxidants and trace molecules as well as endogenous defense mechanisms contributing to redox regulation with which nature has equipped not only eukaryotes but also prokaryotes and plants, are important to maintain redox status. These systems appear to be quite conserved and, together with the ROS generator sites, they largely contribute to the tightly controlled redox balance.
For example Brandán Pedre and Joris Messens discuss that, Actinomycetes, a genus of gram-positive Actinobacteria, possess a low molecular weight thiol molecule, mycothiol (MSH), which serves as a glutathione surrogate [755], [756], and they express a mycothiol peroxidase (Mpx) - a glutathione peroxidase-like enzyme. Both, MSH and Mpx are involved in reversibly protecting cysteines from oxidation [130] and controlling the level of ROS [131]. Further, MSH in concert with mycoredoxin-1 [757] and the thioredoxin reduction pathways are alternative routes for the reduction of the different oxidative forms of Mpx. Since thioredoxin can de-mycothiolate Mpx, this indicates the interrelationship between the MSH/mycoredoxin-1 and thioredoxin reduction pathways. Interestingly, this is also seen in the pathogenic Actinobacteria family Corynebacterium diphtheriae (Cd), where MSH and thioredoxin can deliver electrons to the methionine sulfoxide reductase A (Cd-MsrA) which stereospecifically reduces Met-S-SO [132], thus controlling sulfur oxidation repair. Thereby, the Cd-MsrA uses a unique intramolecular mycothiol redox relay involving a MSH/mycoredoxin-1 monothiol mechanism, which keeps several MSH-dependent enzymes on track [130], [131], [132], [757], [758]. Interestingly, Mycobacterium tuberculosis 1-Cys peroxiredoxin AhpE is reduced by a mycoredoxin-1 dithiol mechanism in addition to the mycothiol/mycoredoxin-1 monothiol mechanism, again indicating that mycothiol-dependent enzymes are promiscuous in the pathway that they use for receiving electrons from NADPH [758]. Overall, it appears that a number of conserved mechanisms are in place to defend against oxidative stress.
Although a variety of microorganisms have well established defense systems against oxidative stress, the gain of compartmentalization in higher organisms created the need to develop an integrated system allowing redox regulation and protection at the same time in each cell compartment. As indicated by Afroditi Chatzi and Kostas Tokatlidis, an excellent example of such cross-talk is the compartment‐specific import and folding of proteins in the mitochondrial intermembrane space (IMS) in Saccharomyces cerevisiae where potential antioxidant proteins are involved.
The protein import and folding in the IMS relies on the Mia40 pathway [759], [760], [761] where Mia40 and Erv1 are key components. Importantly these mechanisms are not restricted to yeast since Mia40 and Erv1 have homologs in almost all organisms including humans. In that system Mia40 functions as a key import receptor in the IMS where it exerts a chaperone-like role recognizing a specific hydrophobic signal of Cys-rich IMS proteins and transfers a disulfide to the substrate [762]. Erv1, on the other hand, is a FAD-linked oxidoreductase that recycles Mia40 to its oxidized state [763]. It was found that the Mia40 pathway is influenced by hydrogen peroxide and that a fraction of Hyr1/Gpx3, a thiol-peroxidase known to act as a redox-transducer in H2O2 signaling via YAP1 in the cytosol, is localized in the IMS. Additional proteins involved in the antioxidant response, like thioredoxin 1 and thioredoxin reductase1, are also part of the IMS proteome in S. cerevisiae [764]. Although these data indicate that the Mia40 oxidative folding in the IMS [765] is associated with antioxidant proteins, its putative links to redox homeostasis and redox signaling are still elusive. However, it is evident that a cross-talk between redox regulation and the oxidative stress response and the IMS oxidative folding pathway exists in higher eukaryotes.
10.4. Involvement of ROS and antioxidant mechanisms in human diseases
The existence of rather efficient, and conserved systems involved in both the defense and maintenance of cell, tissue, or organism functionality, indicates that an imbalance in ROS homeostasis is connected with the pathogenesis of frequently occurring diseases.
10.4.1. ROS and malignant disease
One example where ROS are considered to be involved is cancer. However, ROS appear to have a dual role; they are often implicated in being the cause of a carcinogenic process, but since the majority of conventional therapies rely also on the increase of ROS they seem to be also of therapeutical benefit causing cancer cell death [766], [767].
The intracellular H2O2 concentration is an important factor for the differential proliferation often observed when comparing cancer and non-cancer cells (Fig. 10.2A) [768]. In general, normal cells do not show high levels of ROS. In contrast, cancer cells with a reduced antioxidant defense and higher instability have usually a higher ROS concentration. This fact can be at least partially be explained by the occurrence of mitochondrial dysfunction, which is associated with an imbalance of the antioxidant defense and an increased production of ROS.
Fig. 10.2.
SODm in cancer therapy. A. Model proposed to describe the opposite effects of intracellular H2O2 concentration on the proliferation of cancer and normal cells. B. SODm generate additional intracellular H2O2, which leads to differential effects in cancer and normal cells. C. Potential applications of SODm in cancer treatment. SODm may protect normal tissues from the adverse side effects of radio and chemotherapy and, conversely, increase the sensitivity of malignant cells to standard radio and chemotherapeutic agents. SODm have also been used as mechanistic tools in the field of redox biology to evaluate the impact of ROS in cancer therapy and in carcinogenesis. (Modified from [768] and book chapter Fernandes et al. Springer International Publishing (2016), Switzerland. DOI 10.1007/978-3-319–30705-3_18).
Among the first defenses against ROS is the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen by SODs; in mitochondria this task is carried out by MnSOD/SOD2. Since mitochondrial dysfunction is a hallmark of cancer, carcinogenesis may be expected to be associated with a dysregulation in the expression of MnSOD. Indeed, numerous reports show that reduced MnSOD contributes to carcinogenesis in many solid tumors [769]. Kateryna Kubaichuk and Thomas Kietzmann discuss the concept that loss of MnSOD leads to a transformed cell type, which was recently supported by findings from cells deficient in MnSOD and in hepatocyte-specific MnSOD knockout mice [770]. The loss of MnSOD in cells caused increased proliferation, reduced apoptosis, decreased contact inhibition and cell adhesion as well as enhanced migration. This was in line with the in vivo findings showing that hepatocyte-specific loss of MnSOD in mice caused liver failure, and initiation of malignant transformation and tumor formation [770].
Mechanistically, these findings could be linked to two pathways: the Wnt/β-catenin pathway and the hypoxia signaling pathway. The Wnt/β-catenin pathway is one of the most important pathways associated with cancer, in particular liver cancer. The Wnt pathway components β-catenin and APC were decreased in MnSOD-deficient HepG2 hepatoma cells as well as in MnSOD deficient mouse livers [770]. This is in line with findings from hepatocyte-specific β-catenin knockout mice, which displayed an increased susceptibility to diethylnitrosamine-induced carcinogenesis [771]. Indeed, induction of HIF-1α, a major regulator of the hypoxia response pathway, was disrupted both in vitro and in vivo due to the loss of MnSOD [770]. Although those data do not unravel all mechanistic aspects of the carcinogenic process in response to MnSOD deficiency, they indicate an intricate interplay between HIF-1α and β–catenin.
Moreover, MnSOD can be regulated by the transcription factor Nuclear factor (erythroid-derived 2)-like 2 (NRF2). As indicated by Natalia Robledinos-Antón and Antonio Cuadrado, NRF2 is of utmost importance and regulates the expression of about 250 genes with homeostatic functions such as those involved in NADPH, glutathione and thioredoxin metabolism, phase II detoxification reactions, inflammation and proteostasis [772], [773].
Considering the fact that cancer development is characterized by an altered hypoxia response and malfunctioning of MnSOD, which both can be affected by NRF2, superoxide dismutase mimics (SODm), as well as NRF2 inhibitors, can be considered to be useful in cancer treatment and in other diseases associated with enhanced ROS including cardiovascular, inflammatory, and neurodegenerative disorders [774].
Ana S. Fernandes and Nuno G. Oliveira point out that SODm are synthetic compounds with low molecular weight that mimic the functional properties of SOD enzymes [774], [775]. Most SODm are considered polyfunctional antioxidants, rather than specific O2•- scavengers [775], [776], [777]. Along with the disproportionation of O2•-, SODm may react with other reactive species (RS), including peroxynitrite, CO3•-, H2O2, •NO2 radicals, peroxyl radicals, and alkoxyl radicals [775], [776]. Although many compounds with SOD-like activity with distinct chemical structures have been reported, the search for SOD mimics has been mostly focused on complexes that contain a redox-active metal center and rich coordination chemistry [776]. This is the case of Mn(III) porphyrins (MnPs), Mn(II) complexes with cyclic polyamines (aza crown ethers), Mn(III) salen derivatives, and different types of iron and copper complexes [775], [776], [778], [779]. Some nonmetallic compounds, such as nitroxides and fullerenes have also been explored as SODm [776], [779].
The rationale underling the use of SODm as prospective drugs in cancer treatment is presented in Fig. 10.2. SODm, which lead to an additional amount of H2O2 (Fig. 10.2B), may reduce cell proliferation and increase the efficacy of chemo- and radiotherapy treatments in cancer cells (pro-oxidative effects).
Conversely, SODm may promote the survival/proliferation of normal cells, protecting them against adverse side effects observed in standard medical treatments for cancer (antioxidant effects) [768], [776], [778], [780]. Finally, SODm can be used to give further mechanistic insights namely to assess the involvement of ROS in carcinogenesis or in cancer therapy (Fig. 10.2).
Mechanistically, SODm can affect redox-based cellular transcriptional activity by affecting reactive thiols of transcription factors (e.g. NF-κB or KEAP1/NRF2) [776], [777], [780]. In line with this and according to Natalia Robledinos-Antón and Antonio Cuadrado, NRF2 inhibitors are being developed for cancer therapy [781], though a wide range of NRF2 activators is already known. Most activators identified so far target KEAP1 by oxidation or adduct formation [782]. These molecules include allyl sulfides, dithiolethiones, flavonoids, isothiocyanates, polyphenols and terpenoids, etc. Moreover, many of these electrophilic compounds may also react with redox sensitive cysteines located in the catalytic center of several phosphatases, thus up-regulating signaling pathways that further impinge on NRF2 activation. The most successful case reported so far of a drug targeting NRF2 is the ester derivative of fumaric acid, dimethyl fumarate, which is used for treatment of multiple sclerosis.
Ana S. Fernandes and Nuno G. Oliveira explain that although the development of SODm started long ago, none has reached clinical use yet, however, some SODm have been entering phase I/II clinical trials, especially in combination with chemotherapy or radiotherapy regimens. Some examples include MnPs, which are currently being studied for radioprotection in late-stage glioma patients treated with concurrent radiotherapy and temozolamide. In addition, both MnPs and Mn macrocycles are being assessed as radioprotectors in head & neck cancer patients. A different SODm, Mangafodipir, was studied as a protector against oxaliplatin neurotoxicity and FOLFOX6 side effects, while its analog calmangafodipir is being studied in combination with FOLFOX6 in advanced metastatic colorectal cancer [http://clinicaltrials.gov, as of 22nd June 2016].
In summary, there are several therapeutic opportunities for SODm in oncology. These compounds can increase the therapeutic index of chemo- and radiotherapy by boosting the efficacy of such treatments in cancer cells, while counteracting major drug and radiation toxicity issues.
10.4.2. ROS and non-malignant disease
Although the above-mentioned SODm may be beneficial in cancer therapy, it is so far unknown whether their antioxidant effect could be of use in other diseases where oxidative stress occurs without proper malignancy. As indicated by Marios Phylactides and Marina Kleanthous, such a disease is β-thalassemia – an autosomal recessive red blood cell disorder characterized by a reduction or complete absence of β-globin chain production, globin chain imbalance and the accumulation of α-globins.
Precipitation and autoxidation of excess α-globins lead to the generation of high levels of ROS as well as the release of free heme and iron. Hemolysis, secondary iron overload, often the result of regular blood transfusions and increased absorption of dietary iron in the gut, as well as ineffective erythropoiesis overwhelm the patients' antioxidant defense systems, giving rise to severe oxidative stress, anemia and secondary complications [783].
Overall, Marios Phylactides and Marina Kleanthous mention that the recurring theme seen in most of the trials involving β-thalassemia patients is an improvement of the oxidant-antioxidant imbalance for the duration of the trial with a decrease of oxidative stress markers (e.g. ROS levels, RBC malondialdehyde concentration, lipid peroxidation) and enhancement of the patients’ antioxidant status (e.g. RBC SOD, glutathione peroxidase, reduced glutathione). However, with some rare exceptions [784], [785], there is no improvement of the patients' anemia, and no effect on hemoglobin levels. Thus, antioxidant supplements undoubtedly have a beneficial role in the management of β-thalassemia; however, they do not substitute for blood transfusions and iron chelation in patients with the most severe anemia, but they can feature more prominently in milder, transfusion-independent forms of the disease.
Given the central role of ROS in the pathophysiology of the disease, Lidija Milković, and Višnja Stepanić indicate that the use of antioxidants as a means of reducing oxidative damage is an attractive therapeutic option. A wide variety of compounds have been tested in this context without, however, becoming established as an indispensable part of the patients’ treatment regime. Some of the antioxidant compounds under investigation are food products or herbal extracts, which are used as supplements or form part of the normal diet (e.g. vitamin E [784], [786], [787], resveratrol [788], curcuminoids [784], [789], N-acetylcysteine [785]), CoQ10 [790], green tea [791], fermented papaya preparation [792], or the thistle Silybum marianum (L.) extract silymarin [793], [794].
The latter appears to be one of the most interesting and, as discussed by Vladimir Kren and Katerina Valentova, silymarin's major active constituent silybin (CAS no. 22888-70-6; syn. silibinin) is a typical radical scavenger per se [795], [796]. However, in addition to its radical scavenging activity, this compound selectively interacts with various specific receptors and signaling molecules such as the estrogen receptor [797]. This effect was found to account for the proapoptotic activity of silybin in MCF-7 breast cancer cells [798]. Silybin also targets p53, NF-κB or Wnt/β-catenin signaling in various cancer cells, thus affecting mitogenesis, cell cycle, apoptosis, autophagy, and angiogenesis. Its ability to reverse the P-glycoprotein mediated multi-drug resistance in small lung carcinoma [799] partially explains its chemopreventive and chemoprotective activity [800], [801]. Moreover, the ability of silybin to bind to the α-amanitin binding site of RNA polymerase II is probably responsible for its antidote effect in Amanita poisoning [802]. Altogether, the role and the mode of action of this traditional and well known chemoprotectant needs to be substantially revised in the future.
10.5. ROS, antioxidants and therapeutic options
In addition to the beneficial effects of antioxidant supplements in mild β-thalassemia, their action may not always be a direct antiradical (scavenging) action, as discussed by Lidija Milković and Višnja Stepanić. Although the ability to reduce the amount of ROS in vitro still remains to be an important stage in the evaluation of the antioxidant potency of any new or known compounds [803], this approach is challenged because: і) many known antioxidants do not show direct antiradical activity in vivo and vice versa [31]; іі) proved antioxidants (e.g. flavonoids, PUFAs as part of fish/plant oils) exhibit a dual role acting as antiradical and prooxidant agents simultaneously [804]; and ііі) effects of the known antioxidants are mostly related to their indirect action [31], [805].
Hence, more specific therapeutic options need to be used and accordingly first generations of specific compounds (e.g. NOX inhibitors, NRF2 activators, myeloperoxidase inhibitors) are being developed for application in a wide range of diseases including metabolic, fibrotic, and neurodegenerative diseases as well as cancer [32]. The validation of the specificity of these drugs is highly dependent on the availability of specific target engagement and functional assays. Since the redox reactions are complex and often redundant, the careful use of tools for measuring ROS is important to select drugs, identify potential non-specific effects and evaluate their mode of action [806].
Tamara Seredenina and Vincent Jaquet indicate that currently, countless chemical probes are used to detect ROS, all with advantages and limitations; it is therefore advisable to use several of them and it is critical to control the types of ROS with which they react [807]. On this matter, it is worth mentioning the innovative use of the cell-permeable small molecule dihydroethidine as it allows detection of specific oxidants and their quantification both in vitro [808] and in vivo [809], [810]. Genetically encoded probes are also great tools for detecting intracellular H2O2 localization and real time monitoring [56] and may prove essential for redox dynamics; they also allow visualization of the redox state directly in paraformaldehyde fixed tissues [811].
The more specific design of redox active compounds together with the selection of appropriate druggable biological targets should encompass different in silico approaches and in vitro assays for prediction and measurement, respectively, of their various redox activities. In addition to standard cell-free assays that allow evaluation of the antioxidant properties of new compounds [e.g. DPPH scavenging method, Trolox equivalent antioxidant capacity (TEAC) method, ABTS radical cation decolorization assay, Ferric reducing-antioxidant power (FRAP) assay or hydroxyl radical averting capacity (HORAC) method], in vitro models of different cancer and normal cells should be used [812]. In vivo, KO mouse models are to be used to prove the specificity of action of a drug on a given target. They may, according to Danylo Kaminskyy, Khrystyna Semen, and Olha Yelisyeyeva, enable evaluation of a compounds’ cytotoxicity and possible selectivity (e.g. toxic to cancer but not to normal cells), pro-oxidative activity (measured as ROS generation), as well as endogenous antioxidative response; whether they are added alone or in combination with known drugs to evaluate any possible synergistic effect.
Despite these advantages and a strong rationale for therapeutic targeting of ROS and redox pathways, it is not known why the antioxidants used so far in clinical trials/therapies did not exert the expected effects [216]. Dose and combination regimen, or duration of treatment, may be partial reasons. Indeed, in those studies high antioxidant doses were usually applied, and some actions such as the prooxidant action of some antioxidants as discussed above have been neglected. Moreover, patients were usually not tested for antioxidant deficiencies and/or compliance as in the EPIC clinical trial [813].
10.6. Conclusion
Altogether, in almost all living organisms ROS cannot simply be considered as detrimental since they contribute to signaling, as well as to maintenance of integrity and homeostasis in different organisms ranging from bacteria to mammals. In the latter, disturbances of ROS homeostasis can contribute to the pathogenesis of several chronic diseases such as atherosclerosis, type II diabetes, and cancer via modulation of diverse processes such as inflammation, and the immune response. The common associated increase in ROS in these diseases points to the therapeutic use of small molecules with antioxidant function; however, so far little or no benefit could be observed from the large scale studies with antioxidant supplementation. Overall, these findings signify that further improvement is needed in our knowledge about ROS, ROS-targets, antioxidants, measurement techniques, and their connection to homeostasis and diseases.
11. Concluding remarks
Andreas Daiber and Fabio Di Lisa.
The last decade has witnessed intense research related to the implications of ROS in cellular pathology with special emphasis on the identification of sources, biochemical reactions and cellular effectors responsible for final pathophysiological outcomes. The recognition of RONS as conveyors of physiological signals has added a new level of understanding to their function and has generated contradictory observations regarding the effects of antioxidant molecules, which we are only beginning to unravel. Studies in the context of whole organs both in their healthy and diseased state have expanded the importance of RONS regulation in unpredicted settings. The previous sections highlight some interesting examples of these aspects and also reflects the major achievements of the EU-ROS consortium, evidencing how redox biology is slowly but inexorably evolving towards redox medicine, a concept also put forward in [30], [814].
Unfortunately, conclusive and precise answers are still needed and the world of redox biology and medicine still has challenges. The notions on the relevant sources might look obvious, yet it is not clear which are the most important ones under physiological and pathological conditions. In addition, it is not clear how much the various RONS are compartmentalized, especially within a given cell. Thirdly, it is still difficult to monitor RONS formation due to limitations that still affect their assessment. Fourthly, the discussion is open on why and how the same molecules might mediate physiological and pathological events. It is likely that a threshold of RONS levels exists separating beneficial effects from contribution to cell injury. The definition of this threshold, that is likely to be different in healthy and diseased cells, requires quantitative methods that are hardly available. The identification of beneficial levels as opposed to detrimental ones should allow defining and/or predicting the consequences of changes in RONS levels, and how “good” RONS can change into “bad” ones. This last issue is obviously crucial and necessary for the proper translation of experimental findings into clinical settings. This applies not only to the development of focused, if not personalized, therapeutic approaches, but also to diagnostic procedures and disease monitoring. Although providing the correct answers to the above defined questions seems daunting, we hope that future studies and technological advances will clarify the unsolved issues leading to an improved control of cardiac, neurodegenerative, metabolic and inflammatory diseases. These concepts have been laid down by members of the EU-ROS consortium within 3 major coordinated collections of position papers, reviews and original articles published by our members [1], [2], [3], [4] (Fig. 11.1).
Fig. 11.1.
Who's the bad guy – or which biological source of RONS formation is the most detrimental one? Likely candidates are mitochondrial RONS formation (mitochondrial superoxide/hydrogen peroxide), NADPH oxidases (Nox1, Nox2, Nox4, in humans also Nox3), uncoupled eNOS (uc-eNOS) or xanthine oxidase (XO). The most challenging task for the future is the discrimination between beneficial and detrimental effects of RONS formation and signaling, which is largely determined by the nature of the involved RONS, as well as the time and place they are formed. This concept was put forward previously [18], [21], [28], [32].
Acknowledgements
The EU-ROS consortium (COST Action BM1203) was supported by the European Cooperation in Science and Technology (COST). The present overview represents the final Action dissemination summarizing the major achievements of COST Action BM1203 (EU-ROS) as well as research news and personal views of its members. Some authors were also supported by COST Actions BM1005 (ENOG) and BM1307 (PROTEOSTASIS), as well as funding from the European Commission FP7 and H2020 programmes, and several national funding agencies.
Footnotes
Throughout this report, the term ROS refers almost exclusively to hydrogen peroxide and superoxide anion radical since both species are involved in redox signaling and are formed by a number of enzymatic sources. Other ROS include the hydroxyl radical or singlet oxygen, which however have most likely no specific roles in redox signaling but rather contribute to unspecific oxidative damage and oxidative stress. A large number of organic peroxides (e.g. lipid peroxides) are also covered by the term ROS but these species are only marginally discussed in the present overview. Other ROS such as hypochlorite are also not in the focus of the present work.
Throughout this report, the term RNS refers almost exclusively to peroxynitrite, peroxynitrous acid and derived free radicals (e.g. hydroxyl radicals and nitrogen dioxide radicals). Peroxynitrite anion has a high specificity for activated thiols and readily reacts with carbon dioxide (the latter supports radical reactions). Peroxynitrous acid also has a high specificity for activated thiols but also reacts with transition metal complexes. RNS also comprises nitric oxide, which upon reaction with oxygen can form nitrogen dioxide radicals or nitrosating species such as N2O3 and has a high affinity for transition metal complexes such as iron(II), all of which contributes to the potent redox signaling properties of nitric oxide. However, in the context of “oxidation of biomolecules” the term RNS does not refer to nitric oxide due to its weak reactivity towards biomolecules in general.
Contributor Information
Fabio Di Lisa, Email: dilisa@bio.unipd.it.
Andreas Daiber, Email: daiber@uni-mainz.de.
References
- 1.Virtual Collection, Emerging concepts in redox biology and oxidative stress, Redox Biol. (18 articles plus editorial), in: Santiago Lamas, Fabio Di Lisa, Andreas Daiber (eds.). 〈https://www.journals.elsevier.com/redox-biology/virtual-collections/emerging-conceptsin-redox-biology-and-oxidative-stress-virt〉. [DOI] [PMC free article] [PubMed]
- 2.Forum Issue, Redox medicine, Antioxid. Redox Signal. (9 articles), in: Harald H.H.W. Schmidt, Fabio Di Lisa (eds.) 〈http://online.liebertpub.com/toc/ars/23/14〉.
- 3.Daiber A., Di Lisa F., Lamas S. Virtual issue by COST action BM1203 (EU-ROS). Emerging concepts in redox biology and oxidative stress. Redox Biol. 2016;8:439–441. doi: 10.1016/j.redox.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Themed issue, Redox biology and oxidative stress in health and disease, Br. J. Pharmacol. (16 articles), in: Peter Ferdinandy and Andreas Daiber (eds.). 〈http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1476–5381/homepage/themed_issues.htm〉.
- 5.Augusto O., Miyamoto S. Oxygen radicals and related species. In: Pantopoulos K., Schipper H.M., editors. Principles of Free Radical Biomedicine. Nova Science Publishers, Inc; 2011. [Google Scholar]
- 6.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D., Knight A.R., Taylor E.L., Oettrich J., Ruskovska T., Gasparovic A.C., Cuadrado A., Weber D., Poulsen H.E., Grune T., Schmidt H.H., Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 8.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 9.Daiber A., Steven S., Weber A., Shuvaev V.V., Muzykantov V.R., Laher I., Li H., Lamas S., Munzel T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giasson B.I., Duda J.E., Murray I.V., Chen Q., Souza J.M., Hurtig H.I., Ischiropoulos H., Trojanowski J.Q., Lee V.M. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 11.Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J. Clin. Investig. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceriello A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006;12(Suppl 1):S60–S62. doi: 10.4158/EP.12.S1.60. [DOI] [PubMed] [Google Scholar]
- 13.Keaney J.F., Jr., Loscalzo J. Diabetes, oxidative stress, and platelet activation. Circulation. 1999;99:189–191. doi: 10.1161/01.cir.99.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kooy N.W., Lewis S.J., Royall J.A., Ye Y.Z., Kelly D.R., Beckman J.S. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit. Care Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Aviello G., Knaus U.G. ROS in gastrointestinal inflammation: rescue or Sabotage? Br. J. Pharmacol. 2016 doi: 10.1111/bph.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E., Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2015 doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steven S., Munzel T., Daiber A. Exploiting the pleiotropic antioxidant effects of established drugs in cardiovascular disease. Int. J. Mol. Sci. 2015;16:18185–18223. doi: 10.3390/ijms160818185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzel P., Kossmann S., Munzel T., Daiber A. Redox regulation of cardiovascular inflammation – Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017 doi: 10.1016/j.freeradbiomed.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D., Daiber A., Cuadrado A. Antioxidants in translational medicine. Antioxid. Redox Signal. 2015;23:1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 23.Gori T., Munzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann. Med. 2011;43:259–272. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 24.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.P. Mustain Antioxidant Supplements: Too Much of a Kinda Good Thing 〈https://blogs.scientificamerican.com/food-matters/antioxidant-supplements-too-much-of-a-kinda-good-thing/〉.
- 26.A. Riley Why vitamin pills don't work, and may be bad for you. 〈http://www.bbc.com/future/story/20161208-why-vitamin-supplements-could-kill-you〉.
- 27.Scudellari M. The science myths that will not die. Nature. 2015;528:322–325. doi: 10.1038/528322a. [DOI] [PubMed] [Google Scholar]
- 28.Chen A.F., Chen D.D., Daiber A., Faraci F.M., Li H., Rembold C.M., Laher I. Free radical biology of the cardiovascular system. Clin. Sci. 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 29.Khaw K.T., Bingham S., Welch A., Luben R., Wareham N., Oakes S., Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European prospective investigation into cancer and nutrition. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 30.Levonen A.L., Hill B.G., Kansanen E., Zhang J., Darley-Usmar V.M. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic. Biol. Med. 2014;71:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casas A.I., Dao V.T., Daiber A., Maghzal G.J., Di Lisa F., Kaludercic N., Leach S., Cuadrado A., Jaquet V., Seredenina T., Krause K.H., Lopez M.G., Stocker R., Ghezzi P., Schmidt H.H. Reactive oxygen-related diseases: therapeutic targets and emerging clinical indications. Antioxid. Redox Signal. 2015;23:1171–1185. doi: 10.1089/ars.2015.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock J.T., Whiteman M. Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- 34.Vile G.F., Basu-Modak S., Waltner C., Tyrrell R.M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson M.C., Hillery C.A., Hogg N. Circulating membrane-derived microvesicles in redox biology. Free Radic. Biol. Med. 2014;73:214–228. doi: 10.1016/j.freeradbiomed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camus S.M., De Moraes J.A., Bonnin P., Abbyad P., Le Jeune S., Lionnet F., Loufrani L., Grimaud L., Lambry J.C., Charue D., Kiger L., Renard J.M., Larroque C., Le Clesiau H., Tedgui A., Bruneval P., Barja-Fidalgo C., Alexandrou A., Tharaux P.L., Boulanger C.M., Blanc-Brude O.P. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsiantoulas D., Perkmann T., Afonyushkin T., Mangold A., Prohaska T.A., Papac-Milicevic N., Millischer V., Bartel C., Horkko S., Boulanger C.M., Tsimikas S., Fischer M.B., Witztum J.L., Lang I.M., Binder C.J. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015;56:440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung T., Hohn A., Grune T. The proteasome and the degradation of oxidized proteins: part II – protein oxidation and proteasomal degradation. Redox Biol. 2014;2:99–104. doi: 10.1016/j.redox.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriegenburg F., Poulsen E.G., Koch A., Kruger E., Hartmann-Petersen R. Redox control of the ubiquitin-proteasome system: from molecular mechanisms to functional significance. Antioxid. Redox Signal. 2011;15:2265–2299. doi: 10.1089/ars.2010.3590. [DOI] [PubMed] [Google Scholar]
- 42.Sigala F., Efentakis P., Karageorgiadi D., Filis K., Zampas P., Iliodromitis E.K., Zografos G., Papapetropoulos A., Andreadou I. Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017;12:70–81. doi: 10.1016/j.redox.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan B., Ezerina D., Amoako T.N., Riemer J., Seedorf M., Dick T.P. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat. Chem. Biol. 2013;9:119–125. doi: 10.1038/nchembio.1142. [DOI] [PubMed] [Google Scholar]
- 44.Dunnill C., Patton T., Brennan J., Barrett J., Dryden M., Cooke J., Leaper D., Georgopoulos N.T. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017;14:89–96. doi: 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheresh P., Kim S.J., Tulasiram S., Kamp D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colgan S.P., Ehrentraut S.F., Glover L.E., Kominsky D.J., Campbell E.L. Contributions of neutrophils to resolution of mucosal inflammation. Immunol. Res. 2013;55:75–82. doi: 10.1007/s12026-012-8350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Neill S., Brault J., Stasia M.J., Knaus U.G. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015;6:135–156. doi: 10.1016/j.redox.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao H., Edirisinghe I., Yang S.R., Rajendrasozhan S., Kode A., Caito S., Adenuga D., Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am. J. Pathol. 2008;172:1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Won H.Y., Jang E.J., Min H.J., Hwang E.S. Enhancement of allergen-induced airway inflammation by NOX2 deficiency. Immune Netw. 2011;11:169–174. doi: 10.4110/in.2011.11.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies M.J. Protein oxidation and peroxidation. Biochem. J. 2016;473:805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brautigam L., Schutte L.D., Godoy J.R., Prozorovski T., Gellert M., Hauptmann G., Holmgren A., Lillig C.H., Berndt C. Vertebrate-specific glutaredoxin is essential for brain development. Proc. Natl. Acad. Sci. USA. 2011;108:20532–20537. doi: 10.1073/pnas.1110085108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brautigam L., Jensen L.D., Poschmann G., Nystrom S., Bannenberg S., Dreij K., Lepka K., Prozorovski T., Montano S.J., Aktas O., Uhlen P., Stuhler K., Cao Y., Holmgren A., Berndt C. Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc. Natl. Acad. Sci. USA. 2013;110:20057–20062. doi: 10.1073/pnas.1313753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prozorovski T., Schneider R., Berndt C., Hartung H.P., Aktas O. Redox-regulated fate of neural stem progenitor cells. Biochim. Biophys. Acta. 2015;1850:1543–1554. doi: 10.1016/j.bbagen.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Gascon S., Murenu E., Masserdotti G., Ortega F., Russo G.L., Petrik D., Deshpande A., Heinrich C., Karow M., Robertson S.P., Schroeder T., Beckers J., Irmler M., Berndt C., Angeli J.P., Conrad M., Berninger B., Gotz M. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Morgan B., Van Laer K., Owusu T.N., Ezerina D., Pastor-Flores D., Amponsah P.S., Tursch A., Dick T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 2016;12:437–443. doi: 10.1038/nchembio.2067. [DOI] [PubMed] [Google Scholar]
- 57.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., Basavarajappa D., Radmark O., Kobayashi S., Seibt T., Beck H., Neff F., Esposito I., Wanke R., Forster H., Yefremova O., Heinrichmeyer M., Bornkamm G.W., Geissler E.K., Thomas S.B., Stockwell B.R., O'Donnell V.B., Kagan V.E., Schick J.A., Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M., Muller J., Schuhmacher S., Hortmann M., Baldus S., Gori T., Brandes R.P., Munzel T., Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid. Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 62.Oelze M., Kroller-Schon S., Steven S., Lubos E., Doppler C., Hausding M., Tobias S., Brochhausen C., Li H., Torzewski M., Wenzel P., Bachschmid M., Lackner K.J., Schulz E., Munzel T., Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 63.Kroller-Schon S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., Xia N., Hausding M., Mikhed Y., Zinssius E., Mader M., Stamm P., Treiber N., Scharffetter-Kochanek K., Li H., Schulz E., Wenzel P., Munzel T., Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jankovic A., Korac A., Buzadzic B., Otasevic V., Stancic A., Daiber A., Korac B. Redox implications in adipose tissue (dys)function – a new look at old acquaintances. Redox Biol. 2015;6:19–32. doi: 10.1016/j.redox.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambeth J.D., Neish A.S. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 66.Sommer F., Backhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8:372–379. doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 67.Corcionivoschi N., Alvarez L.A., Sharp T.H., Strengert M., Alemka A., Mantell J., Verkade P., Knaus U.G., Bourke B. Mucosal reactive oxygen species decrease virulence by disrupting campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez L.A., Kovacic L., Rodriguez J., Gosemann J.H., Kubica M., Pircalabioru G.G., Friedmacher F., Cean A., Ghise A., Sarandan M.B., Puri P., Daff S., Plettner E., von Kriegsheim A., Bourke B., Knaus U.G. NADPH oxidase-derived H2O2 subverts pathogen signaling by oxidative phosphotyrosine conversion to PB-DOPA. Proc. Natl. Acad. Sci. USA. 2016;113:10406–10411. doi: 10.1073/pnas.1605443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pircalabioru G., Aviello G., Kubica M., Zhdanov A., Paclet M.H., Brennan L., Hertzberger R., Papkovsky D., Bourke B., Knaus U.G. Defensive mutualism rescues NADPH oxidase inactivation in gut infection. Cell Host Microbe. 2016;19:651–663. doi: 10.1016/j.chom.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Neish A.S., Jones R.M. Redox signaling mediates symbiosis between the gut microbiota and the intestine. Gut Microbes. 2014;5:250–253. doi: 10.4161/gmic.27917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rimessi A., Previati M., Nigro F., Wieckowski M.R., Pinton P. Mitochondrial reactive oxygen species and inflammation: molecular mechanisms, diseases and promising therapies. Int. J. Biochem. Cell Biol. 2016;81:281–293. doi: 10.1016/j.biocel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Hertzberger R., Arents J., Dekker H.L., Pridmore R.D., Gysler C., Kleerebezem M., de Mattos M.J. H(2)O(2) production in species of the Lactobacillus acidophilus group: a central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014;80:2229–2239. doi: 10.1128/AEM.04272-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ito A., Sato Y., Kudo S., Sato S., Nakajima H., Toba T. The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr. Microbiol. 2003;47:231–236. doi: 10.1007/s00284-002-3993-1. [DOI] [PubMed] [Google Scholar]
- 74.Benisty R., Cohen A.Y., Feldman A., Cohen Z., Porat N. Endogenous H2O2 produced by Streptococcus pneumoniae controls FabF activity. Biochim. Biophys. Acta. 2010;1801:1098–1104. doi: 10.1016/j.bbalip.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Pericone C.D., Overweg K., Hermans P.W., Weiser J.N. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 2000;68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moy T.I., Mylonakis E., Calderwood S.B., Ausubel F.M. Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium. Infect. Immun. 2004;72:4512–4520. doi: 10.1128/IAI.72.8.4512-4520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsh E.K., May R.C. Caenorhabditis elegans, a model organism for investigating immunity. Appl. Environ. Microbiol. 2012;78:2075–2081. doi: 10.1128/AEM.07486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Hoeven R., McCallum K.C., Garsin D.A. Speculations on the activation of ROS generation in C. elegans innate immune signaling. Worm. 2012;1:160–163. doi: 10.4161/worm.19767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balla K.M., Troemel E.R. Caenorhabditis elegans as a model for intracellular pathogen infection. Cell Microbiol. 2013;15:1313–1322. doi: 10.1111/cmi.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mora-Lorca J.A., Saenz-Narciso B., Gaffney C.J., Naranjo-Galindo F.J., Pedrajas J.R., Guerrero-Gomez D., Dobrzynska A., Askjaer P., Szewczyk N.J., Cabello J., Miranda-Vizuete A. Glutathione reductase gsr-1 is an essential gene required for Caenorhabditis elegans early embryonic development. Free Radic. Biol. Med. 2016;96:446–461. doi: 10.1016/j.freeradbiomed.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stenvall J., Fierro-Gonzalez J.C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arner E.S., Persson O.P., Miranda-Vizuete A., Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc Natl. Acad. Sci. USA. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatla N., Horvitz H.R. Light and hydrogen peroxide inhibit C. elegans Feeding through gustatory receptor orthologs and pharyngeal neurons. Neuron. 2015;85:804–818. doi: 10.1016/j.neuron.2014.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olahova M., Veal E.A. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell. 2015;14:558–568. doi: 10.1111/acel.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blackwell T.K., Steinbaugh M.J., Hourihan J.M., Ewald C.Y., Isik M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015;88:290–301. doi: 10.1016/j.freeradbiomed.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lapierre L.R., Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol. Metab. 2012;23:637–644. doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabreiro F., Gems D. Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Mol. Med. 2013;5:1300–1310. doi: 10.1002/emmm.201100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knoefler D., Thamsen M., Koniczek M., Niemuth N.J., Diederich A.K., Jakob U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol. Cell. 2012;47:767–776. doi: 10.1016/j.molcel.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J., Carroll K.S., Liebler D.C. The expanding landscape of the thiol redox proteome. Mol. Cell Proteom. 2016;15:1–11. doi: 10.1074/mcp.O115.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013;48:332–356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westermarck J., Ivaska J., Corthals G.L. Identification of protein interactions involved in cellular signaling. Mol. Cell Proteom. 2013;12:1752–1763. doi: 10.1074/mcp.R113.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arts I.S., Vertommen D., Baldin F., Laloux G., Collet J.F. Comprehensively characterizing the thioredoxin interactome in vivo highlights the central role played by this ubiquitous oxidoreductase in redox control. Mol. Cell Proteom. 2016;15:2125–2140. doi: 10.1074/mcp.M115.056440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartolini D., Galli F. The functional interactome of GSTP: a regulatory biomolecular network at the interface with the Nrf2 adaption response to oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1019:29–44. doi: 10.1016/j.jchromb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Zolotukhin P., Kozlova Y., Dovzhik A., Kovalenko K., Kutsyn K., Aleksandrova A., Shkurat T. Oxidative status interactome map: towards novel approaches in experiment planning, data analysis, diagnostics and therapy. Mol. Biosyst. 2013;9:2085–2096. doi: 10.1039/c3mb70096h. [DOI] [PubMed] [Google Scholar]
- 95.Verrastro I., Tveen-Jensen K., Woscholski R., Spickett C.M., Pitt A.R. Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins. Free Radic. Biol. Med. 2016;90:24–34. doi: 10.1016/j.freeradbiomed.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Petry A., Djordjevic T., Weitnauer M., Kietzmann T., Hess J., Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid. Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 97.Rzymski T., Petry A., Kracun D., Riess F., Pike L., Harris A.L., Gorlach A. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene. 2012;31:3621–3634. doi: 10.1038/onc.2011.522. [DOI] [PubMed] [Google Scholar]
- 98.Janiszewski M., Lopes L.R., Carmo A.O., Pedro M.A., Brandes R.P., Santos C.X., Laurindo F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 99.He C., Zhu H., Zhang W., Okon I., Wang Q., Li H., Le Y.Z., Xie Z. 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am. J. Pathol. 2013;183:626–637. doi: 10.1016/j.ajpath.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., O'Dowd Y., Bens M., Vandewalle A., Gougerot-Pocidalo M.A., Lizard G., Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santos C.X., Hafstad A.D., Beretta M., Zhang M., Molenaar C., Kopec J., Fotinou D., Murray T.V., Cobb A.M., Martin D., Zeh Silva M., Anilkumar N., Schroder K., Shanahan C.M., Brewer A.C., Brandes R.P., Blanc E., Parsons M., Belousov V., Cammack R., Hider R.C., Steiner R.A., Shah A.M. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J. 2016;35:319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li B., Tian J., Sun Y., Xu T.R., Chi R.F., Zhang X.L., Hu X.L., Zhang Y.A., Qin F.Z., Zhang W.F. Activation of NADPH oxidase mediates increased endoplasmic reticulum stress and left ventricular remodeling after myocardial infarction in rabbits. Biochim. Biophys. Acta. 2015;1852:805–815. doi: 10.1016/j.bbadis.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 103.Li G., Scull C., Ozcan L., Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pavoine C., Pecker F. Sphingomyelinases: their regulation and roles in cardiovascular pathophysiology. Cardiovasc. Res. 2009;82:175–183. doi: 10.1093/cvr/cvp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corda S., Laplace C., Vicaut E., Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 106.Lecour S., der Merwe Van, Opie E., Sack L.H., Ceramide M.N. attenuates hypoxic cell death via reactive oxygen species signaling. J. Cardiovasc. Pharmacol. 2006;47:158–163. doi: 10.1097/01.fjc.0000198520.28674.41. [DOI] [PubMed] [Google Scholar]
- 107.Won J.S., Im Y.B., Khan M., Singh A.K., Singh I. The role of neutral sphingomyelinase produced ceramide in lipopolysaccharide-mediated expression of inducible nitric oxide synthase. J. Neurochem. 2004;88:583–593. doi: 10.1046/j.1471-4159.2003.02165.x. [DOI] [PubMed] [Google Scholar]
- 108.Hernandez O.M., Discher D.J., Bishopric N.H., Webster K.A. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ. Res. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- 109.Unal B., Ozcan F., Tuzcu H., Kirac E., Elpek G.O., Aslan M. Inhibition of neutral sphingomyelinase decreases elevated levels of nitrative and oxidative stress markers in liver ischemia-reperfusion injury. Redox Rep. 2016:1–13. doi: 10.1080/13510002.2016.1162431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adamy C., Mulder P., Khouzami L., Andrieu-abadie N., Defer N., Candiani G., Pavoine C., Caramelle P., Souktani R., Le Corvoisier P., Perier M., Kirsch M., Damy T., Berdeaux A., Levade T., Thuillez C., Hittinger L., Pecker F. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J. Mol. Cell Cardiol. 2007;43:344–353. doi: 10.1016/j.yjmcc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Sawai H., Hannun Y.A. Ceramide and sphingomyelinases in the regulation of stress responses. Chem. Phys. Lipids. 1999;102:141–147. doi: 10.1016/s0009-3084(99)00082-1. [DOI] [PubMed] [Google Scholar]
- 112.Perrotta C., Clementi E. Biological roles of Acid and neutral sphingomyelinases and their regulation by nitric oxide. Physiology. 2010;25:64–71. doi: 10.1152/physiol.00048.2009. [DOI] [PubMed] [Google Scholar]
- 113.Pahan K., Sheikh F.G., Khan M., Namboodiri A.M., Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- 114.Katsuyama K., Shichiri M., Marumo F., Hirata Y. Role of nuclear factor-kappaB activation in cytokine- and sphingomyelinase-stimulated inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Endocrinology. 1998;139:4506–4512. doi: 10.1210/endo.139.11.6309. [DOI] [PubMed] [Google Scholar]
- 115.Yang M.S., Jou I., Inn-Oc H., Joe E. Sphingomyelinase but not ceramide induces nitric oxide synthase expression in rat brain microglia. Neurosci. Lett. 2001;311:133–136. doi: 10.1016/s0304-3940(01)02162-0. [DOI] [PubMed] [Google Scholar]
- 116.Aslan M., Basaranlar G., Unal M., Ciftcioglu A., Derin N., Mutus B. Inhibition of neutral sphingomyelinase decreases elevated levels of inducible nitric oxide synthase and apoptotic cell death in ocular hypertensive rats. Toxicol. Appl. Pharmacol. 2014;280:389–398. doi: 10.1016/j.taap.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 117.Masseret E., Banack S., Boumediene F., Abadie E., Brient L., Pernet F., Juntas-Morales R., Pageot N., Metcalf J., Cox P., Camu W., French Network on, A. L. S. C. D. Investigation Dietary BMAA exposure in an amyotrophic lateral sclerosis cluster from southern France. PLoS ONE. 2013;8:e83406. doi: 10.1371/journal.pone.0083406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang X., Chen L., Liu W., Qiao Q., Wu K., Wen J., Huang C., Tang R., Zhang X. Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat. Toxicol. 2015;165:41–50. doi: 10.1016/j.aquatox.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Krakstad C., Herfindal L., Gjertsen B.T., Boe R., Vintermyr O.K., Fladmark K.E., Doskeland S.O. CaM-kinaseII-dependent commitment to microcystin-induced apoptosis is coupled to cell budding, but not to shrinkage or chromatin hypercondensation. Cell Death Differ. 2006;13:1191–1202. doi: 10.1038/sj.cdd.4401798. [DOI] [PubMed] [Google Scholar]
- 120.Hjornevik L.V., Fismen L., Young F.M., Solstad T., Fladmark K.E. Nodularin exposure induces SOD1 phosphorylation and disrupts SOD1 co-localization with actin filaments. Toxins. 2012;4:1482–1499. doi: 10.3390/toxins4121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okle O., Stemmer K., Deschl U., Dietrich D.R. L-BMAA induced ER stress and enhanced caspase 12 cleavage in human neuroblastoma SH-SY5Y cells at low nonexcitotoxic concentrations. Toxicol. Sci. 2013;131:217–224. doi: 10.1093/toxsci/kfs291. [DOI] [PubMed] [Google Scholar]
- 122.Chiu A.S., Gehringer M.M., Braidy N., Guillemin G.J., Welch J.H., Neilan B.A. Excitotoxic potential of the cyanotoxin beta-methyl-amino-L-alanine (BMAA) in primary human neurons. Toxicon. 2012;60:1159–1165. doi: 10.1016/j.toxicon.2012.07.169. [DOI] [PubMed] [Google Scholar]
- 123.Erickson J.R., Joiner M.L., Guan X., Kutschke W., Yang J., Oddis C.V., Bartlett R.K., Lowe J.S., O'Donnell S.E., Aykin-Burns N., Zimmerman M.C., Zimmerman K., Ham A.J., Weiss R.M., Spitz D.R., Shea M.A., Colbran R.J., Mohler P.J., Anderson M.E. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arif M., Kazim S.F., Grundke-Iqbal I., Garruto R.M., Iqbal K. Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc. Natl. Acad. Sci. USA. 2014;111:1144–1149. doi: 10.1073/pnas.1322614111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raka F., Di Sebastiano A.R., Kulhawy S.C., Ribeiro F.M., Godin C.M., Caetano F.A., Angers S., Ferguson S.S. Ca(2+)/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: role of beta-amyloid. Mol. Brain. 2015;8:21. doi: 10.1186/s13041-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fahey R.C. Glutathione analogs in prokaryotes. Biochim. Biophys. Acta. 2013;1830:3182–3198. doi: 10.1016/j.bbagen.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 127.Loi V.V., Rossius M., Antelmann H. Redox regulation by reversible protein S-thiolation in bacteria. Front. Microbiol. 2015;6:187. doi: 10.3389/fmicb.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J.W., Soonsanga S., Helmann J.D. A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. USA. 2007;104:8743–8748. doi: 10.1073/pnas.0702081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gaballa A., Chi B.K., Roberts A.A., Becher D., Hamilton C.J., Antelmann H., Helmann J.D. Redox regulation in Bacillus subtilis: the bacilliredoxins BrxA(YphP) and BrxB(YqiW) function in de-bacillithiolation of S-bacillithiolated OhrR and MetE. Antioxid. Redox Signal. 2014;21:357–367. doi: 10.1089/ars.2013.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chi B.K., Busche T., Van Laer K., Basell K., Becher D., Clermont L., Seibold G.M., Persicke M., Kalinowski J., Messens J., Antelmann H. Protein S-mycothiolation functions as redox-switch and thiol protection mechanism in Corynebacterium glutamicum under hypochlorite stress. Antioxid. Redox Signal. 2014;20:589–605. doi: 10.1089/ars.2013.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pedre B., Van Molle I., Villadangos A.F., Wahni K., Vertommen D., Turell L., Erdogan H., Mateos L.M., Messens J. The Corynebacterium glutamicum mycothiol peroxidase is a reactive oxygen species-scavenging enzyme that shows promiscuity in thiol redox control. Mol. Microbiol. 2015;96:1176–1191. doi: 10.1111/mmi.12998. [DOI] [PubMed] [Google Scholar]
- 132.Tossounian M.A., Pedre B., Wahni K., Erdogan H., Vertommen D., Van Molle I., Messens J. Corynebacterium diphtheriae methionine sulfoxide reductase a exploits a unique mycothiol redox relay mechanism. J. Biol. Chem. 2015;290:11365–11375. doi: 10.1074/jbc.M114.632596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilroy S., Suzuki N., Miller G., Choi W.G., Toyota M., Devireddy A.R., Mittler R. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014;19:623–630. doi: 10.1016/j.tplants.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 134.Calabrese E.J., Iavicoli I., Calabrese V. Hormesis: its impact on medicine and health. Hum. Exp. Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- 135.Yelisyeyeva O., Semen K., Zarkovic N., Kaminskyy D., Lutsyk O., Rybalchenko V. Activation of aerobic metabolism by Amaranth oil improves heart rate variability both in athletes and patients with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2012;118:47–57. doi: 10.3109/13813455.2012.659259. [DOI] [PubMed] [Google Scholar]
- 136.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N., Smith A.C., Eyassu F., Shirley R., Hu C.H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin F., Sancheti H., Liu Z., Cadenas E. Mitochondrial function in ageing: coordination with signalling and transcriptional pathways. J. Physiol. 2016;594:2025–2042. doi: 10.1113/JP270541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Go Y.M., Jones D.P. The redox proteome. J. Biol. Chem. 2013;288:26512–26520. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buettner G.R., Wagner B.A., Rodgers V.G. Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem. Biophys. 2013;67:477–483. doi: 10.1007/s12013-011-9320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pillay C.S., Eagling B.D., Driscoll S.R., Rohwer J.M. Quantitative measures for redox signaling. Free Radic. Biol. Med. 2016;96:290–303. doi: 10.1016/j.freeradbiomed.2016.04.199. [DOI] [PubMed] [Google Scholar]
- 141.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodrigo R., Libuy M., Feliu F., Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers. 2013;35:773–790. doi: 10.1155/2013/974358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leonetti D., Reimund J.M., Tesse A., Viennot S., Martinez M.C., Bretagne A.L., Andriantsitohaina R. Circulating microparticles from Crohn's disease patients cause endothelial and vascular dysfunctions. PLoS One. 2013;8:e73088. doi: 10.1371/journal.pone.0073088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhullar J., Bhopale V.M., Yang M., Sethuraman K., Thom S.R. Microparticle formation by platelets exposed to high gas pressures – an oxidative stress response. Free Radic. Biol. Med. 2016;101:154–162. doi: 10.1016/j.freeradbiomed.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 145.Han W.Q., Chang F.J., Wang Q.R., Pan J.Q. Microparticles from patients with the acute coronary syndrome impair vasodilatation by inhibiting the Akt/eNOS-Hsp90 signaling pathway. Cardiology. 2015;132:252–260. doi: 10.1159/000438782. [DOI] [PubMed] [Google Scholar]
- 146.Pitanga T.N., de Aragao Franca L., Rocha V.C., Meirelles T., Borges V.M., Goncalves M.S., Pontes-de-Carvalho L.C., Noronha-Dutra A.A., dos-Santos W.L. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014;15:21. doi: 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fleury A., Martinez M.C., Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front. Immunol. 2014;5:370. doi: 10.3389/fimmu.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burger D., Kwart D.G., Montezano A.C., Read N.C., Kennedy C.R., Thompson C.S., Touyz R.M. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J. Am. Heart Assoc. 2012;1:e001842. doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burger D., Turner M., Munkonda M.N., Touyz R.M. Endothelial microparticle-derived reactive oxygen species: role in endothelial signaling and vascular function. Oxid. Med. Cell Longev. 2016;2016:5047954. doi: 10.1155/2016/5047954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Safiedeen Z., Rodriguez-Gomez I., Vergori L., Soleti R., Vaithilingam D., Douma I., Agouni A., Leiber D., Dubois S., Simard G., Zibara K., Andriantsitohaina R., Martinez M.C. Temporal cross talk between endoplasmic reticulum and mitochondria regulates oxidative stress and mediates microparticle-induced endothelial dysfunction. Antioxid. Redox Signal. 2017;26:15–27. doi: 10.1089/ars.2016.6771. [DOI] [PubMed] [Google Scholar]
- 151.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 152.Skinner H.D., Zheng J.Z., Fang J., Agani F., Jiang B.H. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J. Biol. Chem. 2004;279:45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- 153.Ryu J.H., Li S.H., Park H.S., Park J.W., Lee B., Chun Y.S. Hypoxia-inducible factor alpha subunit stabilization by NEDD8 conjugation is reactive oxygen species-dependent. J. Biol. Chem. 2011;286:6963–6970. doi: 10.1074/jbc.M110.188706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel S.A., Simon M.C. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arany Z., Foo S.Y., Ma Y., Ruas J.L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S.M., Baek K.H., Rosenzweig A., Spiegelman B.M. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 156.Ushio-Fukai M., Alexander R.W. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol. Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 157.Colavitti R., Pani G., Bedogni B., Anzevino R., Borrello S., Waltenberger J., Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 158.Kang D.H., Lee D.J., Lee K.W., Park Y.S., Lee J.Y., Lee S.H., Koh Y.J., Koh G.Y., Choi C., Yu D.Y., Kim J., Kang S.W. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol. Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 159.Tonks N.K. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 160.Oshikawa J., Urao N., Kim H.W., Kaplan N., Razvi M., McKinney R., Poole L.B., Fukai T., Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abid M.R., Spokes K.C., Shih S.C., Aird W.C. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 162.Kobayashi S., Nojima Y., Shibuya M., Maru Y. Nox1 regulates apoptosis and potentially stimulates branching morphogenesis in sinusoidal endothelial cells. Exp. Cell Res. 2004;300:455–462. doi: 10.1016/j.yexcr.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 163.Ushio-Fukai M., Tang Y., Fukai T., Dikalov S.I., Ma Y., Fujimoto M., Quinn M.T., Pagano P.J., Johnson C., Alexander R.W. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 164.Tojo T., Ushio-Fukai M., Yamaoka-Tojo M., Ikeda S., Patrushev N., Alexander R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 165.Datla S.R., Peshavariya H., Dusting G.J., Mahadev K., Goldstein B.J., Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 166.Yamaoka-Tojo M., Tojo T., Kim H.W., Hilenski L., Patrushev N.A., Zhang L., Fukai T., Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 167.Ikeda S., Yamaoka-Tojo M., Hilenski L., Patrushev N.A., Anwar G.M., Quinn M.T., Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler. Thromb. Vasc. Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y., Zang Q.S., Liu Z., Wu Q., Maass D., Dulan G., Shaul P.W., Melito L., Frantz D.E., Kilgore J.A., Williams N.S., Terada L.S., Nwariaku F.E. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. Cell Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Borniquel S., Garcia-Quintans N., Valle I., Olmos Y., Wild B., Martinez-Granero F., Soria E., Lamas S., Monsalve M. Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol. Cell Biol. 2010;30:4035–4044. doi: 10.1128/MCB.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Quintans N., Prieto I., Sanchez-Ramos C., Luque A., Arza E., Olmos Y., Monsalve M. Regulation of endothelial dynamics by PGC-1alpha relies on ROS control of VEGF-A signaling. Free Radic. Biol. Med. 2016;93:41–51. doi: 10.1016/j.freeradbiomed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 171.Garcia-Quintans N., Sanchez-Ramos C., Prieto I., Tierrez A., Arza E., Alfranca A., Redondo J.M., Monsalve M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1alpha-deficient mice. Angiogenesis. 2016;19:217–228. doi: 10.1007/s10456-016-9502-0. [DOI] [PubMed] [Google Scholar]
- 172.Massague J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fabregat I., Moreno-Caceres J., Sanchez A., Dooley S., Dewidar B., Giannelli G., Ten Dijke P., Consortium I.-L. TGF-beta signalling and liver disease. FEBS J. 2016;283:2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 174.Sanchez A., Alvarez A.M., Benito M., Fabregat I. Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J. Biol. Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- 175.Thannickal V.J., Fanburg B.L. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J. Biol. Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 176.Herrera B., Murillo M.M., Alvarez-Barrientos A., Beltran J., Fernandez M., Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic. Biol. Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 177.Carmona-Cuenca I., Roncero C., Sancho P., Caja L., Fausto N., Fernandez M., Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J. Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 178.Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B., Preynat-Seauve O., Guichard C., Arbiser J.L., Banfi B., Pache J.C., Barazzone-Argiroffo C., Krause K.H. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moreno-Caceres J., Mainez J., Mayoral R., Martin-Sanz P., Egea G., Fabregat I. Caveolin-1-dependent activation of the metalloprotease TACE/ADAM17 by TGF-beta in hepatocytes requires activation of Src and the NADPH oxidase NOX1. FEBS J. 2016;283:1300–1310. doi: 10.1111/febs.13669. [DOI] [PubMed] [Google Scholar]
- 180.Sancho P., Bertran E., Caja L., Carmona-Cuenca I., Murillo M.M., Fabregat I. The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim. Biophys. Acta. 2009;1793:253–263. doi: 10.1016/j.bbamcr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 181.Boudreau H.E., Casterline B.W., Rada B., Korzeniowska A., Leto T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cucoranu I., Clempus R., Dikalova A., Phelan P.J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 183.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sancho P., Mainez J., Crosas-Molist E., Roncero C., Fernandez-Rodriguez C.M., Pinedo F., Huber H., Eferl R., Mikulits W., Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Crosas-Molist E., Bertran E., Sancho P., Lopez-Luque J., Fernando J., Sanchez A., Fernandez M., Navarro E., Fabregat I. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic. Biol. Med. 2014;69:338–347. doi: 10.1016/j.freeradbiomed.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 186.Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Panieri E., Santoro M.M. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rojas-Rivera D., Hetz C. TMBIM protein family: ancestral regulators of cell death. Oncogene. 2015;34:269–280. doi: 10.1038/onc.2014.6. [DOI] [PubMed] [Google Scholar]
- 189.Hu L., Smith T.F., Goldberger G. LFG: a candidate apoptosis regulatory gene family. Apoptosis. 2009;14:1255–1265. doi: 10.1007/s10495-009-0402-2. [DOI] [PubMed] [Google Scholar]
- 190.Carrara G., Saraiva N., Gubser C., Johnson B.F., Smith G.L. Six-transmembrane topology for Golgi anti-apoptotic protein (GAAP) and Bax inhibitor 1 (BI-1) provides model for the transmembrane Bax inhibitor-containing motif (TMBIM) family. J. Biol. Chem. 2012;287:15896–15905. doi: 10.1074/jbc.M111.336149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carrara G., Saraiva N., Parsons M., Byrne B., Prole D.L., Taylor C.W., Smith G.L. Golgi anti-apoptotic proteins are highly conserved ion channels that affect apoptosis and cell migration. J. Biol. Chem. 2015;290:11785–11801. doi: 10.1074/jbc.M115.637306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gubser C., Bergamaschi D., Hollinshead M., Lu X., van Kuppeveld F.J., Smith G.L. A new inhibitor of apoptosis from vaccinia virus and eukaryotes. PLoS Pathog. 2007;3:e17. doi: 10.1371/journal.ppat.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gubser C., Smith G.L. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 2002;83:855–872. doi: 10.1099/0022-1317-83-4-855. [DOI] [PubMed] [Google Scholar]
- 194.de Mattia F., Gubser C., van Dommelen M.M., Visch H.J., Distelmaier F., Postigo A., Luyten T., Parys J.B., de Smedt H., Smith G.L., Willems P.H., van Kuppeveld F.J. Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol. Biol. Cell. 2009;20:3638–3645. doi: 10.1091/mbc.E09-05-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saraiva N., Prole D.L., Carrara G., Johnson B.F., Taylor C.W., Parsons M., Smith G.L. hGAAP promotes cell adhesion and migration via the stimulation of store-operated Ca2+ entry and calpain 2. J. Cell Biol. 2013;202:699–713. doi: 10.1083/jcb.201301016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J. Cell Sci. 2008;121(Suppl 1):S1–S84. doi: 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- 197.Basto R., Brunk K., Vinadogrova T., Peel N., Franz A., Khodjakov A., Raff J.W. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 199.Nigg E.A., Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chae S., Yun C., Um H., Lee J.H., Cho H. Centrosome amplification and multinuclear phenotypes are Induced by hydrogen peroxide. Exp. Mol. Med. 2005;37:482–487. doi: 10.1038/emm.2005.59. [DOI] [PubMed] [Google Scholar]
- 201.Ohshima S. Centrosome aberrations associated with cellular senescence and p53 localization at supernumerary centrosomes. Oxid. Med. Cell Longev. 2012;2012:217594. doi: 10.1155/2012/217594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Manning J.A., Kumar S. A potential role for NEDD1 and the centrosome in senescence of mouse embryonic fibroblasts. Cell Death Dis. 2010;1:e35. doi: 10.1038/cddis.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lim J.M., Lee K.S., Woo H.A., Kang D., Rhee S.G. Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression. J. Cell Biol. 2015;210:23–33. doi: 10.1083/jcb.201412068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ohshima S. Abnormal mitosis in hypertetraploid cells causes aberrant nuclear morphology in association with H2O2-induced premature senescence. Cytom. A. 2008;73:808–815. doi: 10.1002/cyto.a.20604. [DOI] [PubMed] [Google Scholar]
- 205.Bindoli A., Rigobello M.P. Principles in redox signaling: from chemistry to functional significance. Antioxid. Redox Signal. 2013;18:1557–1593. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 206.Biasutto L., Azzolini M., Szabo I., Zoratti M. The mitochondrial permeability transition pore in AD 2016: an update. Biochim. Biophys. Acta. 2016;1863:2515–2530. doi: 10.1016/j.bbamcr.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 207.Linard D., Kandlbinder A., Degand H., Morsomme P., Dietz K.J., Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch. Biochem. Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 208.Folda A., Citta A., Scalcon V., Cali T., Zonta F., Scutari G., Bindoli A., Rigobello M.P. Mitochondrial thioredoxin system as a modulator of cyclophilin D redox state. Sci. Rep. 2016;6:23071. doi: 10.1038/srep23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Palikaras K., Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014;56:182–188. doi: 10.1016/j.exger.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 210.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp. Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 211.Schulz T.J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 212.Dancy B.M., Sedensky M.M., Morgan P.G. Effects of the mitochondrial respiratory chain on longevity in C. elegans. Exp. Gerontol. 2014;56:245–255. doi: 10.1016/j.exger.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 213.Marthandan S., Priebe S., Groth M., Guthke R., Platzer M., Hemmerich P., Diekmann S. Hormetic effect of rotenone in primary human fibroblasts. Immun. Ageing. 2015;12:11. doi: 10.1186/s12979-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lamming D.W. Inhibition of the Mechanistic Target of Rapamycin (mTOR)-rapamycin and beyond. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Song M., Chen Y., Gong G., Murphy E., Rabinovitch P.S., Dorn G.W., 2nd. Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ. Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gorlach A., Dimova E.Y., Petry A., Martinez-Ruiz A., Hernansanz-Agustin P., Rolo A.P., Palmeira C.M., Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fernández-Agüera M.C., Gao L., González-Rodríguez P., Pintado C.O., Arias-Mayenco I., García-Flores P., García-Pergañeda A., Pascual A., Ortega-Sáenz P., López-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 2015;22:825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 218.Hernansanz-Agustin P., Izquierdo-Alvarez A., Sanchez-Gomez F.J., Ramos E., Villa-Pina T., Lamas S., Bogdanova A., Martinez-Ruiz A. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014;71:146–156. doi: 10.1016/j.freeradbiomed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 219.Yuan G., Vasavda C., Peng Y.J., Makarenko V.V., Raghuraman G., Nanduri J., Gadalla M.M., Semenza G.L., Kumar G.K., Snyder S.H., Prabhakar N.R. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci. Signal. 2015;8:ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moreno L., Moral-Sanz J., Morales-Cano D., Barreira B., Moreno E., Ferrarini A., Pandolfi R., Rupérez F.J., Cortijo J., Sánchez-Luna M., Villamor E., Perez-Vizcaíno F., Cogolludo A. Ceramide mediates acute oxygen sensing in vascular tissues. Antioxid. Redox Signal. 2014;20:1–14. doi: 10.1089/ars.2012.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Izquierdo-Álvarez A., Ramos E., Villanueva J., Hernansanz-Agustín P., Fernández-Rodríguez R., Tello D., Carrascal M., Martínez-Ruiz A. Differential redox proteomics allows identification of proteins reversibly oxidized at cysteine residues in endothelial cells in response to acute hypoxia. J. Proteom. 2012;75:5449–5462. doi: 10.1016/j.jprot.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 222.Bogdanova A., Petrushanko I.Y., Hernansanz-Agustin P., Martinez-Ruiz A. "Oxygen sensing" by Na,K-ATPase: these miraculous thiols. Front. Physiol. 2016;7:314. doi: 10.3389/fphys.2016.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. (837a-837d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jeffrey Man H.S., Tsui A.K., Marsden P.A. Nitric oxide and hypoxia signaling. Vitam. Horm. 2014;96:161–192. doi: 10.1016/B978-0-12-800254-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 225.Chalupsky K., Kracun D., Kanchev I., Bertram K., Gorlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid. Redox Signal. 2015;23:1076–1091. doi: 10.1089/ars.2015.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bendall J.K., Douglas G., McNeill E., Channon K.M., Crabtree M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014;20:3040–3077. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gao L., Chalupsky K., Stefani E., Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell Cardiol. 2009;47:752–760. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dubois M., Delannoy E., Duluc L., Closs E., Li H., Toussaint C., Gadeau A.P., Godecke A., Freund-Michel V., Courtois A., Marthan R., Savineau J.P., Muller B. Biopterin metabolism and eNOS expression during hypoxic pulmonary hypertension in mice. PLoS One. 2013;8:e82594. doi: 10.1371/journal.pone.0082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bigarella C.L., Liang R., Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Burgess R.J., Agathocleous M., Morrison S.J. Metabolic regulation of stem cell function. J. Intern. Med. 2014;276:12–24. doi: 10.1111/joim.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Klotz L.O., Sanchez-Ramos C., Prieto-Arroyo I., Urbanek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Higuchi M., Dusting G.J., Peshavariya H., Jiang F., Hsiao S.T., Chan E.C., Liu G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–888. doi: 10.1089/scd.2012.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Circu M.L., Aw T.Y. Redox biology of the intestine. Free Radic. Res. 2011;45:1245–1266. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Speckmann B., Pinto A., Winter M., Forster I., Sies H., Steinbrenner H. Proinflammatory cytokines down-regulate intestinal selenoprotein P biosynthesis via NOS2 induction. Free Radic. Biol. Med. 2010;49:777–785. doi: 10.1016/j.freeradbiomed.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 235.Walter P.L., Steinbrenner H., Barthel A., Klotz L.O. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem. Biophys. Res. Commun. 2008;365:316–321. doi: 10.1016/j.bbrc.2007.10.171. [DOI] [PubMed] [Google Scholar]
- 236.Baird A.-M., O’Byrne K., Gray S. Reactive oxygen species and reactive nitrogen species in epigenetic modifications. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer Berlin Heidelberg; 2014. pp. 437–455. [Google Scholar]
- 237.Niu Y., DesMarais T.L., Tong Z., Yao Y., Costa M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015;82:22–28. doi: 10.1016/j.freeradbiomed.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mikhed Y., Gorlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ito K., Ito M., Elliott W.M., Cosio B., Caramori G., Kon O.M., Barczyk A., Hayashi S., Adcock I.M., Hogg J.C., Barnes P.J. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 240.Valinluck V., Sowers L.C. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 241.O'Hagan H.M., Wang W., Sen S., Destefano Shields C., Lee S.S., Zhang Y.W., Clements E.G., Cai Y., Van Neste L., Easwaran H., Casero R.A., Sears C.L., Baylin S.B. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim G.H., Ryan J.J., Archer S.L. The role of redox signaling in epigenetics and cardiovascular disease. Antioxid. Redox Signal. 2013;18:1920–1936. doi: 10.1089/ars.2012.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Archer S.L., Marsboom G., Kim G.H., Zhang H.J., Toth P.T., Svensson E.C., Dyck J.R., Gomberg-Maitland M., Thebaud B., Husain A.N., Cipriani N., Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang Q.J., Chen H.Z., Wang L., Liu D.P., Hill J.A., Liu Z.P. The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J. Clin. Investig. 2011;121:2447–2456. doi: 10.1172/JCI46277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stein A.B., Jones T.A., Herron T.J., Patel S.R., Day S.M., Noujaim S.F., Milstein M.L., Klos M., Furspan P.B., Jalife J., Dressler G.R. Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J. Clin. Investig. 2011;121:2641–2650. doi: 10.1172/JCI44641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kaneda R., Takada S., Yamashita Y., Choi Y.L., Nonaka-Sarukawa M., Soda M., Misawa Y., Isomura T., Shimada K., Mano H. Genome-wide histone methylation profile for heart failure. Genes Cells. 2009;14:69–77. doi: 10.1111/j.1365-2443.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 248.Khan M.A., Alam K., Dixit K., Rizvi M.M. Role of peroxynitrite induced structural changes on H2B histone by physicochemical method. Int. J. Biol. Macromol. 2016;82:31–38. doi: 10.1016/j.ijbiomac.2015.10.085. [DOI] [PubMed] [Google Scholar]
- 249.Khan M.A., Dixit K., Jabeen S., Moinuddin, Alam K. Impact of peroxynitrite modification on structure and immunogenicity of H2A histone. Scand. J. Immunol. 2009;69:99–109. doi: 10.1111/j.1365-3083.2008.02200.x. [DOI] [PubMed] [Google Scholar]
- 250.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 251.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 252.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 253.Levonen A.L., Hill B.G., Kansanen E., Zhang J., Darley-Usmar V.M. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic. Biol. Med. 2014;71:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cheng X., Ku C.H., Siow R.C. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic. Biol. Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 256.Wynn T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 258.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 259.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am. J. Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pottier N., Cauffiez C., Perrais M., Barbry P., Mari B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol. Sci. 2014;35:119–126. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 261.O'Reilly S. MicroRNAs in fibrosis: opportunities and challenges. Arthritis Res. Ther. 2016;18:11. doi: 10.1186/s13075-016-0929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Davis B.N., Hilyard A.C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fierro-Fernandez M., Miguel V., Lamas S. Role of redoximiRs in fibrogenesis. Redox Biol. 2016;7:58–67. doi: 10.1016/j.redox.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wei C., Li L., Kim I.K., Sun P., Gupta S. NF-kappaB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic. Res. 2014;48:282–291. doi: 10.3109/10715762.2013.865839. [DOI] [PubMed] [Google Scholar]
- 265.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., Castoldi M., Soutschek J., Koteliansky V., Rosenwald A., Basson M.A., Licht J.D., Pena J.T., Rouhanifard S.H., Muckenthaler M.U., Tuschl T., Martin G.R., Bauersachs J., Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 266.Zhong X., Chung A.C., Chen H.Y., Meng X.M., Lan H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol.: JASN. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang B., Komers R., Carew R., Winbanks C.E., Xu B., Herman-Edelstein M., Koh P., Thomas M., Jandeleit-Dahm K., Gregorevic P., Cooper M.E., Kantharidis P. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol.: JASN. 2012;23:252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cushing L., Kuang P., Lu J. The role of miR-29 in pulmonary fibrosis. Biochem. Cell Biol. Biochim. Biol. Cell. 2015;93:109–118. doi: 10.1139/bcb-2014-0095. [DOI] [PubMed] [Google Scholar]
- 269.Fierro-Fernandez M., Busnadiego O., Sandoval P., Espinosa-Diez C., Blanco-Ruiz E., Rodriguez M., Pian H., Ramos R., Lopez-Cabrera M., Garcia-Bermejo M.L., Lamas S. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 2015;16:1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Miguel V., Busnadiego O., Fierro-Fernandez M., Lamas S. Protective role for miR-9-5p in the fibrogenic transformation of human dermal fibroblasts. Fibrogenes. Tissue Repair. 2016;9:7. doi: 10.1186/s13069-016-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Murakami Y., Toyoda H., Tanaka M., Kuroda M., Harada Y., Matsuda F., Tajima A., Kosaka N., Ochiya T., Shimotohno K. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PloS One. 2011;6:e16081. doi: 10.1371/journal.pone.0016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Espinosa-Diez C., Fierro-Fernandez M., Sanchez-Gomez F., Rodriguez-Pascual F., Alique M., Ruiz-Ortega M., Beraza N., Martinez-Chantar M.L., Fernandez-Hernando C., Lamas S. Targeting of gamma-glutamyl-cysteine ligase by miR-433 reduces glutathione biosynthesis and promotes TGF-beta-dependent fibrogenesis. Antioxid. Redox Signal. 2015;23:1092–1105. doi: 10.1089/ars.2014.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chau B.N., Xin C., Hartner J., Ren S., Castano A.P., Linn G., Li J., Tran P.T., Kaimal V., Huang X., Chang A.N., Li S., Kalra A., Grafals M., Portilla D., MacKenna D.A., Orkin S.H., Duffield J.S. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012;4:121ra118. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gomez I.G., MacKenna D.A., Johnson B.G., Kaimal V., Roach A.M., Ren S., Nakagawa N., Xin C., Newitt R., Pandya S., Xia T.H., Liu X., Borza D.B., Grafals M., Shankland S.J., Himmelfarb J., Portilla D., Liu S., Chau B.N., Duffield J.S. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kang H.M., Ahn S.H., Choi P., Ko Y.A., Han S.H., Chinga F., Park A.S., Tao J., Sharma K., Pullman J., Bottinger E.P., Goldberg I.J., Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.L., Binder C.J., Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Greig F.H., Kennedy S., Spickett C.M. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic. Biol. Med. 2012;52:266–280. doi: 10.1016/j.freeradbiomed.2011.10.481. [DOI] [PubMed] [Google Scholar]
- 278.Mauerhofer C., Philippova M., Oskolkova O.V., Bochkov V.N. Hormetic and anti-inflammatory properties of oxidized phospholipids. Mol. Asp. Med. 2016;49:78–90. doi: 10.1016/j.mam.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 279.Leitinger N., Tyner T.R., Oslund L., Rizza C., Subbanagounder G., Lee H., Shih P.T., Mackman N., Tigyi G., Territo M.C., Berliner J.A., Vora D.K. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc. Natl. Acad. Sci. USA. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bretscher P., Egger J., Shamshiev A., Trotzmuller M., Kofeler H., Carreira E.M., Kopf M., Freigang S. Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol. Med. 2015;7:593–607. doi: 10.15252/emmm.201404702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 282.Dziubla T., Butterfield D.A. Academic Press; 2016. Oxidative Stress and Biomaterials. [Google Scholar]
- 283.Napoli A., Valentini M., Tirelli N., Muller M., Hubbell J.A. Oxidation-responsive polymeric vesicles. Nat. Mater. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 284.Wattamwar P.P., Biswal D., Cochran D.B., Lyvers A.C., Eitel R.E., Anderson K.W., Hilt J.Z., Dziubla T.D. Synthesis and characterization of poly(antioxidant beta-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012;8:2529–2537. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 285.Yang J., van Lith R., Baler K., Hoshi R.A., Ameer G.A. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules. 2014;15:3942–3952. doi: 10.1021/bm5010004. [DOI] [PubMed] [Google Scholar]
- 286.G. Svegliati Baroni, L. D’ Ambrosio, G. Ferretti, P. Biondi, A. Casini, A. Di Sario, S. Saccomanno, A.M. Jezequel, A. Benedetti, F. Orlandi, Proliferation of hepatic stellate cells and lipid peroxidation: changes due to polyphenols, in: P. Gentilini, M.U. Dianzani, (eds). New Trends in Hepatology: the Proceedings of the Annual Meeting of the Italian National Programme on Liver Cirrhosis and Viral Hepatitis, San Miniato (Pisa), Italy, 7–9 January 1996. Dordrecht: Springer Netherlands, 1996, pp. 93–103.
- 287.Mrakovcic L., Wildburger R., Jaganjac M., Cindric M., Cipak A., Borovic-Sunjic S., Waeg G., Milankovic A.M., Zarkovic N. Lipid peroxidation product 4-hydroxynonenal as factor of oxidative homeostasis supporting bone regeneration with bioactive glasses. Acta Biochim. Pol. 2010;57:173–178. [PubMed] [Google Scholar]
- 288.Aldini G., Domingues M.R., Spickett C.M., Domingues P., Altomare A., Sanchez-Gomez F.J., Oeste C.L., Perez-Sala D. Protein lipoxidation: detection strategies and challenges. Redox Biol. 2015;5:253–266. doi: 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Magni F., Galbusera C., Tremolada L., Ferrarese C., Kienle M.G. Characterisation of adducts of the lipid peroxidation product 4-hydroxy-2-nonenal and amyloid beta-peptides by liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:1485–1493. doi: 10.1002/rcm.743. [DOI] [PubMed] [Google Scholar]
- 290.Colzani M., Aldini G., Carini M. Mass spectrometric approaches for the identification and quantification of reactive carbonyl species protein adducts. J. Proteom. 2013;92:28–50. doi: 10.1016/j.jprot.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 291.Verrastro I., Pasha S., Jensen K.T., Pitt A.R., Spickett C.M. Mass spectrometry-based methods for identifying oxidized proteins in disease: advances and challenges. Biomolecules. 2015;5:378–411. doi: 10.3390/biom5020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Milic I., Kipping M., Hoffmann R., Fedorova M. Separation and characterization of oxidized isomeric lipid-peptide adducts by ion mobility mass spectrometry. J. Mass Spectrom. 2015;50:1386–1392. doi: 10.1002/jms.3713. [DOI] [PubMed] [Google Scholar]
- 293.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 294.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kabil O., Motl N., Banerjee R. H2S and its role in redox signaling. Biochim. Biophys. Acta. 2014;1844:1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ju Y., Zhang W., Pei Y., Yang G. H(2)S signaling in redox regulation of cellular functions. Can. J. Physiol. Pharmacol. 2013;91:8–14. doi: 10.1139/cjpp-2012-0293. [DOI] [PubMed] [Google Scholar]
- 297.Li Q., Lancaster J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cortese-Krott M.M., Fernandez B.O., Kelm M., Butler A.R., Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 299.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jazwa A., Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 301.Xie Z.Z., Liu Y., Bian J.S. Hydrogen sulfide and cellular redox homeostasis. Oxid. Med. Cell Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Garcia-Garcia A., Rodriguez-Rocha H., Madayiputhiya N., Pappa A., Panayiotidis M.I., Franco R. Biomarkers of protein oxidation in human disease. Curr. Mol. Med. 2012;12:681–697. doi: 10.2174/156652412800792543. [DOI] [PubMed] [Google Scholar]
- 303.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 304.Wall S.B., Oh J.Y., Diers A.R., Landar A. Oxidative modification of proteins: an emerging mechanism of cell signaling. Front. Physiol. 2012;3:369. doi: 10.3389/fphys.2012.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ullrich V., Kissner R. Redox signaling: bioinorganic chemistry at its best. J. Inorg. Biochem. 2006;100:2079–2086. doi: 10.1016/j.jinorgbio.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 306.Calcerrada P., Peluffo G., Radi R. Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications. Curr. Pharm. Des. 2011;17:3905–3932. doi: 10.2174/138161211798357719. [DOI] [PubMed] [Google Scholar]
- 307.Bottari S.P. Protein tyrosine nitration: a signaling mechanism conserved from yeast to man. Proteomics. 2015;15:185–187. doi: 10.1002/pmic.201400592. [DOI] [PubMed] [Google Scholar]
- 308.Daiber A., Frein D., Namgaladze D., Ullrich V. Oxidation and nitrosation in the nitrogen monoxide/superoxide system. J. Biol. Chem. 2002;277:11882–11888. doi: 10.1074/jbc.M111988200. [DOI] [PubMed] [Google Scholar]
- 309.Houee-Levin C., Bobrowski K., Horakova L., Karademir B., Schoneich C., Davies M.J., Spickett C.M. Exploring oxidative modifications of tyrosine: an update on mechanisms of formation, advances in analysis and biological consequences. Free Radic. Res. 2015;49:347–373. doi: 10.3109/10715762.2015.1007968. [DOI] [PubMed] [Google Scholar]
- 310.Wehr N.B., Levine R.L. Wanted and wanting: antibody against methionine sulfoxide. Free Radic. Biol. Med. 2012;53:1222–1225. doi: 10.1016/j.freeradbiomed.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ghesquiere B., Gevaert K. Proteomics methods to study methionine oxidation. Mass Spectrom. Rev. 2014;33:147–156. doi: 10.1002/mas.21386. [DOI] [PubMed] [Google Scholar]
- 312.Rocha B.S., Gago B., Barbosa R.M., Lundberg J.O., Radi R., Laranjinha J. Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic. Biol. Med. 2012;52:693–698. doi: 10.1016/j.freeradbiomed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 313.Wayenberg J.L., Ransy V., Vermeylen D., Damis E., Bottari S.P. Nitrated plasma albumin as a marker of nitrative stress and neonatal encephalopathy in perinatal asphyxia. Free Radic. Biol. Med. 2009;47:975–982. doi: 10.1016/j.freeradbiomed.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 314.Wayenberg J.L., Cavedon C., Ghaddhab C., Lefevre N., Bottari S.P. Early transient hypoglycemia is associated with increased albumin nitration in the preterm infant. Neonatology. 2011;100:387–397. doi: 10.1159/000326936. [DOI] [PubMed] [Google Scholar]
- 315.Kerstjens J.M., Bocca-Tjeertes I.F., de Winter A.F., Reijneveld S.A., Bos A.F. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130:e265–e272. doi: 10.1542/peds.2012-0079. [DOI] [PubMed] [Google Scholar]
- 316.Lucas A., Morley R., Cole T.J. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297:1304–1308. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Stenninger E., Flink R., Eriksson B., Sahlen C. Long-term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch. Dis. Child Fetal Neonatal Ed. 1998;79:F174–F179. doi: 10.1136/fn.79.3.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Deshpande S., Ward Platt M. The investigation and management of neonatal hypoglycaemia. Semin. Fetal Neonatal Med. 2005;10:351–361. doi: 10.1016/j.siny.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 319.McKinlay C.J., Alsweiler J.M., Ansell J.M., Anstice N.S., Chase J.G., Gamble G.D., Harris D.L., Jacobs R.J., Jiang Y., Paudel N., Signal M., Thompson B., Wouldes T.A., Yu T.Y., Harding J.E., Group C.S. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N. Engl. J. Med. 2015;373:1507–1518. doi: 10.1056/NEJMoa1504909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Groenendaal F., Lammers H., Smit D., Nikkels P.G. Nitrotyrosine in brain tissue of neonates after perinatal asphyxia. Arch. Dis. Child Fetal Neonatal Ed. 2006;91:F429–F433. doi: 10.1136/adc.2005.092114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Groenendaal F., Vles J., Lammers H., De Vente J., Smit D., Nikkels P.G. Nitrotyrosine in human neonatal spinal cord after perinatal asphyxia. Neonatology. 2008;93:1–6. doi: 10.1159/000106432. [DOI] [PubMed] [Google Scholar]
- 322.Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids. 2011;164:457–468. doi: 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 323.Griffiths H.R., Moller L., Bartosz G., Bast A., Bertoni-Freddari C., Collins A., Cooke M., Coolen S., Haenen G., Hoberg A.M., Loft S., Lunec J., Olinski R., Parry J., Pompella A., Poulsen H., Verhagen H., Astley S.B. Biomarkers. Mol. Asp. Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 324.Tsikas D., Rothmann S., Schneider J.Y., Suchy M.T., Trettin A., Modun D., Stuke N., Maassen N., Frolich J.C. Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: mda as a biomarker of oxidative stress and its relation to 15(S)−8-iso-prostaglandin F2alpha and nitric oxide (NO) J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1019:95–111. doi: 10.1016/j.jchromb.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 325.Sobsey C.A., Han J., Lin K., Swardfager W., Levitt A., Borchers C.H. Development and evaluation of a liquid chromatography-mass spectrometry method for rapid, accurate quantitation of malondialdehyde in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1029–1030:205–212. doi: 10.1016/j.jchromb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 326.Zelzer S., Mangge H., Oberreither R., Bernecker C., Gruber H.J., Pruller F., Fauler G. Oxidative stress: determination of 4-hydroxy-2-nonenal by gas chromatography/mass spectrometry in human and rat plasma. Free Radic. Res. 2015;49:1233–1238. doi: 10.3109/10715762.2015.1059936. [DOI] [PubMed] [Google Scholar]
- 327.Chafer-Pericas C., Rahkonen L., Sanchez-Illana A., Kuligowski J., Torres-Cuevas I., Cernada M., Cubells E., Nunez-Ramiro A., Andersson S., Vento M., Escobar J. Ultra high performance liquid chromatography coupled to tandem mass spectrometry determination of lipid peroxidation biomarkers in newborn serum samples. Anal. Chim. Acta. 2015;886:214–220. doi: 10.1016/j.aca.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 328.Dias I.H., Polidori M.C., Griffiths H.R. Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem. Soc. Trans. 2014;42:1001–1005. doi: 10.1042/BST20140164. [DOI] [PubMed] [Google Scholar]
- 329.Helmschrodt C., Becker S., Schroter J., Hecht M., Aust G., Thiery J., Ceglarek U. Fast LC-MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin. Chim. Acta. 2013;425:3–8. doi: 10.1016/j.cca.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 330.Haller E., Stubiger G., Lafitte D., Lindner W., Lammerhofer M. Chemical recognition of oxidation-specific epitopes in low-density lipoproteins by a nanoparticle based concept for trapping, enrichment, and liquid chromatography-tandem mass spectrometry analysis of oxidative stress biomarkers. Anal. Chem. 2014;86:9954–9961. doi: 10.1021/ac502855n. [DOI] [PubMed] [Google Scholar]
- 331.Kasai H., Crain P.F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 332.Rasmussen S.T., Andersen J.T., Nielsen T.K., Cejvanovic V., Petersen K.M., Henriksen T., Weimann A., Lykkesfeldt J., Poulsen H.E. Simvastatin and oxidative stress in humans: a randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 2016;9:32–38. doi: 10.1016/j.redox.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Al-Salmani K., Abbas H.H., Schulpen S., Karbaschi M., Abdalla I., Bowman K.J., So K.K., Evans M.D., Jones G.D., Godschalk R.W., Cooke M.S. Simplified method for the collection, storage, and comet assay analysis of DNA damage in whole blood. Free Radic. Biol. Med. 2011;51:719–725. doi: 10.1016/j.freeradbiomed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 334.Karbaschi M., Cooke M.S. Novel method for the high-throughput processing of slides for the comet assay. Sci. Rep. 2014;4:7200. doi: 10.1038/srep07200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lam P.M., Mistry V., Marczylo T.H., Konje J.C., Evans M.D., Cooke M.S. Rapid measurement of 8-oxo-7,8-dihydro-2'-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free Radic. Biol. Med. 2012;52:2057–2063. doi: 10.1016/j.freeradbiomed.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rossner P., Jr., Orhan H., Koppen G., Sakai K., Santella R.M., Ambroz A., Rossnerova A., Sram R.J., Ciganek M., Neca J., Arzuk E., Mutlu N., Cooke M.S. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine analysis by an improved ELISA: an inter-laboratory comparison study. Free Radic. Biol. Med. 2016;95:169–179. doi: 10.1016/j.freeradbiomed.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 337.Rossner P., Jr., Mistry V., Singh R., Sram R.J., Cooke M.S. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine values determined by a modified ELISA improves agreement with HPLC-MS/MS. Biochem. Biophys. Res. Commun. 2013;440:725–730. doi: 10.1016/j.bbrc.2013.09.133. [DOI] [PubMed] [Google Scholar]
- 338.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 339.Hill A.B. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Loft S., Svoboda P., Kawai K., Kasai H., Sorensen M., Tjonneland A., Vogel U., Moller P., Overvad K., Raaschou-Nielsen O. Association between 8-oxo-7,8-dihydroguanine excretion and risk of lung cancer in a prospective study. Free Radic. Biol. Med. 2012;52:167–172. doi: 10.1016/j.freeradbiomed.2011.10.439. [DOI] [PubMed] [Google Scholar]
- 341.Loft S., Olsen A., Moller P., Poulsen H.E., Tjonneland A. Association between 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiol. Biomark. Prev. 2013;22:1289–1296. doi: 10.1158/1055-9965.EPI-13-0229. [DOI] [PubMed] [Google Scholar]
- 342.Hromockyj A.E., Maurelli A.T. Identification of an Escherichia coli gene homologous to virR, a regulator of Shigella virulence. J. Bacteriol. 1989;171:2879–2881. doi: 10.1128/jb.171.5.2879-2881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Broedbaek K., Poulsen H.E., Weimann A., Kom G.D., Schwedhelm E., Nielsen P., Boger R.H. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic. Biol. Med. 2009;47:1230–1233. doi: 10.1016/j.freeradbiomed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 344.Poulsen H.E., Specht E., Broedbaek K., Henriksen T., Ellervik C., Mandrup-Poulsen T., Tonnesen M., Nielsen P.E., Andersen H.U., Weimann A. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 2012;52:1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 345.Broedbaek K., Siersma V., Henriksen T., Weimann A., Petersen M., Andersen J.T., Jimenez-Solem E., Hansen L.J., Henriksen J.E., Bonnema S.J., de Fine Olivarius N., Poulsen H.E. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population-based cohort study. Diabetes Care. 2013;36:669–676. doi: 10.2337/dc12-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Poulsen H.E., Nadal L.L., Broedbaek K., Nielsen P.E., Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim. Biophys. Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 347.Kadiiska M.B., Gladen B.C., Baird D.D., Germolec D., Graham L.B., Parker C.E., Nyska A., Wachsman J.T., Ames B.N., Basu S., Brot N., Fitzgerald G.A., Floyd R.A., George M., Heinecke J.W., Hatch G.E., Hensley K., Lawson J.A., Marnett L.J., Morrow J.D., Murray D.M., Plastaras J., Roberts L.J., 2nd, Rokach J., Shigenaga M.K., Sohal R.S., Sun J., Tice R.R., Van Thiel D.H., Wellner D., Walter P.B., Tomer K.B., Mason R.P., Barrett J.C. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 348.Daiber A., Oelze M., Steven S., Kroller-Schon S., Munzel T. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox Biol. 2017;12:35–49. doi: 10.1016/j.redox.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Principles for integrating reactive species into in vivo biological processes: examples from exercise physiology. Cell Signal. 2016;28:256–271. doi: 10.1016/j.cellsig.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 350.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Going retro: oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016;98:2–12. doi: 10.1016/j.freeradbiomed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 351.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 352.Leiper J., Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat. Rev. Drug Discov. 2011;10:277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- 353.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 354.Mengozzi M., Ermilov P., Annenkov A., Ghezzi P., Pearl F. Definition of a family of tissue-protective cytokines using functional cluster analysis: a proof-of-concept study. Front. Immunol. 2014;5:115. doi: 10.3389/fimmu.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Watanabe H., Kakihana M., Ohtsuka S., Sugishita Y. Randomized, double-blind, placebo-controlled study of ascorbate on the preventive effect of nitrate tolerance in patients with congestive heart failure. Circulation. 1998;97:886–891. doi: 10.1161/01.cir.97.9.886. [DOI] [PubMed] [Google Scholar]
- 356.Takeshita K., Ozawa T. Recent progress in in vivo ESR spectroscopy. J. Radiol. Res. 2004;45:373–384. doi: 10.1269/jrr.45.373. [DOI] [PubMed] [Google Scholar]
- 357.Yu M., Beyers R.J., Gorden J.D., Cross J.N., Goldsmith C.R. A magnetic resonance imaging contrast agent capable of detecting hydrogen peroxide. Inorg. Chem. 2012;51:9153–9155. doi: 10.1021/ic3012603. [DOI] [PubMed] [Google Scholar]
- 358.Perng J.K., Lee S., Kundu K., Caskey C.F., Knight S.F., Satir S., Ferrara K.W., Taylor W.R., Degertekin F.L., Sorescu D., Murthy N. Ultrasound imaging of oxidative stress in vivo with chemically-generated gas microbubbles. Ann. Biomed. Eng. 2012;40:2059–2068. doi: 10.1007/s10439-012-0573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Jørgensen J.T., Persson M., Madsen J., Kjær A. High tumor uptake of 64Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol. 2013;40:345–350. doi: 10.1016/j.nucmedbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 360.Mason R.P. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016;8:422–429. doi: 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Maulucci G., Bacic G., Bridal L., Schmidt H.H., Tavitian B., Viel T., Utsumi H., Yalcin A.S., De Spirito M. Imaging reactive oxygen species-induced modifications in living systems. Antioxid. Redox Signal. 2016;24:939–958. doi: 10.1089/ars.2015.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Frejaville C., Karoui H., Tuccio B., Moigne F.L., Culcasi M., Pietri S., Lauricella R., Tordo P. 5-(Diethoxyphosphoryl)−5-methyl-1-pyrroline N-oxide: a new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J. Med. Chem. 1995;38:258–265. doi: 10.1021/jm00002a007. [DOI] [PubMed] [Google Scholar]
- 363.Villamena F.A., Xia S., Merle J.K., Lauricella R., Tuccio B., Hadad C.M., Zweier J.L. Reactivity of superoxide radical anion with cyclic nitrones: role of intramolecular h-bond and electrostatic effects. J. Am. Chem. Soc. 2007;129:8177–8191. doi: 10.1021/ja0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.K. Abbas, N. Babić, F. Peyrot, Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods,109, 2016, 31–43. [DOI] [PubMed]
- 365.Beziere N., Decroos C., Mkhitaryan K., Kish E., Richard F., Bigot-Marchand S., Durand S., Cloppet F., Chauvet C., Corvol M.-T., Rannou F., Xu-Li Y., Mansuy D., Peyrot F., Frapart Y.-M. First combined in vivo X-ray tomography and high-resolution molecular electron paramagnetic resonance (EPR) imaging of the mouse knee joint taking into account the disappearance kinetics of the EPR probe. Mol. Imaging. 2012;11:220–228. [PubMed] [Google Scholar]
- 366.Bézière N., Hardy M., Poulhès F., Karoui H., Tordo P., Ouari O., Frapart Y.-M., Rockenbauer A., Boucher J.-L., Mansuy D., Peyrot F. Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radic. Biol. Med. 2014;67:150–158. doi: 10.1016/j.freeradbiomed.2013.10.812. [DOI] [PubMed] [Google Scholar]
- 367.Leinisch F., Jiang J., DeRose E.F., Khramtsov V.V., Mason R.P. Investigation of spin-trapping artifacts formed by the Forrester-Hepburn mechanism. Free Radic. Biol. Med. 2013;65:1497–1505. doi: 10.1016/j.freeradbiomed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Pou S., Cohen M.S., Britigan B.E., Rosen G.M. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J. Biol. Chem. 1989;264:12299–12302. [PubMed] [Google Scholar]
- 369.Abbas K., Hardy M., Poulhès F., Karoui H., Tordo P., Ouari O., Peyrot F. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic. Biol. Med. 2014;71:281–290. doi: 10.1016/j.freeradbiomed.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 370.Kleschyov A.L., Munzel T. Advanced spin trapping of vascular nitric oxide using colloid iron diethyldithiocarbamate. Methods Enzymol. 2002;359:42–51. doi: 10.1016/s0076-6879(02)59170-9. [DOI] [PubMed] [Google Scholar]
- 371.Steven S., Hausding M., Kroller-Schon S., Mader M., Mikhed Y., Stamm P., Zinssius E., Pfeffer A., Welschof P., Agdauletova S., Sudowe S., Li H., Oelze M., Schulz E., Klein T., Munzel T., Daiber A. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015;110:6. doi: 10.1007/s00395-015-0465-x. [DOI] [PubMed] [Google Scholar]
- 372.Steven S., Jurk K., Kopp M., Kroller-Schon S., Mikhed Y., Schwierczek K., Roohani S., Kashani F., Oelze M., Klein T., Tokalov S., Danckwardt S., Strand S., Wenzel P., Munzel T., Daiber A. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Deng S., Kruger A., Kleschyov A.L., Kalinowski L., Daiber A., Wojnowski L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 2007;42:466–473. doi: 10.1016/j.freeradbiomed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 374.Kuppusamy P., Li H., Ilangovan G., Cardounel A.J., Zweier J.L., Yamada K., Krishna M.C., Mitchell J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 375.Berliner L.J. From spin-labeled proteins to in vivo EPR applications. Eur. Biophys. J. 2010;39:579–588. doi: 10.1007/s00249-009-0534-x. [DOI] [PubMed] [Google Scholar]
- 376.Klare J.P. Site-directed spin labeling EPR spectroscopy in protein research. Biol. Chem. 2013;394 doi: 10.1515/hsz-2013-0155. [DOI] [PubMed] [Google Scholar]
- 377.Klug C.S., Feix J.B. Methods and applications of site-directed spin labeling EPR spectroscopy. Methods Cell Biol. 2008:617–658. doi: 10.1016/S0091-679X(07)84020-9. [DOI] [PubMed] [Google Scholar]
- 378.Gurachevsky A., Kazmierczak S.C., Jörres A., Muravsky V. Application of spin label electron paramagnetic resonance in the diagnosis and prognosis of cancer and sepsis. Clin. Chem. Lab. Med. 2008;46 doi: 10.1515/CCLM.2008.260. [DOI] [PubMed] [Google Scholar]
- 379.Muravskaya E.V., Lapko A.G., Muravskii V.A. Modification of transport function of plasma albumin during atherosclerosis and diabetes mellitus. Bull. Exp. Biol. Med. 2003;135:433–435. doi: 10.1023/a:1024903006461. [DOI] [PubMed] [Google Scholar]
- 380.Jalan R., Schnurr K., Mookerjee R.P., Sen S., Cheshire L., Hodges S., Muravsky V., Williams R., Matthes G., Davies N.A. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology. 2009;50:555–564. doi: 10.1002/hep.22913. [DOI] [PubMed] [Google Scholar]
- 381.Roy D., Quiles J., Gaze D.C., Collinson P., Kaski J.C., Baxter G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart. 2006;92:113–114. doi: 10.1136/hrt.2004.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pavićević A.A., Popović-Bijelić A.D., Mojović M.D., Šušnjar S.V., Bačić G.G. Binding of doxyl stearic spin labels to human serum albumin: an EPR study. J. Phys. Chem. B. 2014;118:10898–10905. doi: 10.1021/jp5068928. [DOI] [PubMed] [Google Scholar]
- 383.Junk M.J.N., Spiess H.W., Hinderberger D. The distribution of fatty acids reveals the functional structure of human serum albumin. Angew. Chem. Int. Ed. 2010;49:8755–8759. doi: 10.1002/anie.201003495. [DOI] [PubMed] [Google Scholar]
- 384.Boutier-Pischon A., Auger F., Noël J.-M., Almario A., Frapart Y.-M. EPR and electrochemical quantification of oxygen using newly synthesized para-silylated triarylmethyl radicals. Free Radic. Res. 2015:1–8. doi: 10.3109/10715762.2014.995183. [DOI] [PubMed] [Google Scholar]
- 385.Li J., Liu Y., Kim E., March J.C., Bentley W.E., Payne G.F. Electrochemical reverse engineering: a systems-level tool to probe the redox-based molecular communication of biology. Free Radic. Biol. Med. 2017;105:110–131. doi: 10.1016/j.freeradbiomed.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 386.Lund A., Shiotani M., Shimada S. Springer Science & Business Media; 2011. Principles and Applications of ESR Spectroscopy. [Google Scholar]
- 387.Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 388.Pyszková M., Biler M., Biedermann D., Valentová K., Kuzma M., Vrba J., Ulrichová J., Sokolová R., Mojović M., Popović-Bijelić A., Kubala M., Trouillas P., Křen V., Vacek J. Flavonolignan 2,3-dehydroderivatives: preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016;90:114–125. doi: 10.1016/j.freeradbiomed.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 389.Vacek J., Zatloukalová M., Desmier T., Nezhodová V., Hrbáč J., Kubala M., Křen V., Ulrichová J., Trouillas P. Antioxidant, metal-binding and DNA-damaging properties of flavonolignans: a joint experimental and computational highlight based on 7-O-galloylsilybin. Chem.-Biol. Interact. 2013;205:173–180. doi: 10.1016/j.cbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 390.Dimitrić Marković J.M., Marković Z.S., Pašti I.A., Brdarić T.P., Popović-Bijelić A., Mojović M. A joint application of spectroscopic, electrochemical and theoretical approaches in evaluation of the radical scavenging activity of 3-OH flavones and their iron complexes towards different radical species. Dalton Trans. 2012;41:7295. doi: 10.1039/c2dt30220a. [DOI] [PubMed] [Google Scholar]
- 391.Sokolová R., Tarábek J., Papoušková B., Kocábová J., Fiedler J., Vacek J., Marhol P., Vavříková E., Křen V. Oxidation of the flavonolignan silybin. In situ EPR evidence of the spin-trapped silybin radical. Electrochim. Acta. 2016;205:118–123. [Google Scholar]
- 392.Naso L.G., Ferrer E.G., Butenko N., Cavaco I., Lezama L., Rojo T., Etcheverry S.B., Williams P.A.M. Antioxidant, DNA cleavage, and cellular effects of silibinin and a new oxovanadium(IV)/silibinin complex. JBIC J. Biol. Inorg. Chem. 2011;16:653–668. doi: 10.1007/s00775-011-0769-8. [DOI] [PubMed] [Google Scholar]
- 393.Kalamkarov G.P., Bugrova A.E., Konstantinova T.S., Shevchenko T.F. [Endogenous content of the nitric oxide in the cell layers of the eye retina] Ross. Fiziol. Zhurnal Im. I. M. Sechenova/Ross. Akad. Nauk. 2014;100:852–860. [PubMed] [Google Scholar]
- 394.Lukyanov K.A., Belousov V.V. Genetically encoded fluorescent redox sensors. Biochim. Et. Biophys. Acta (BBA) – General. Subj. 2014;1840:745–756. doi: 10.1016/j.bbagen.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 395.Ermakova Y.G., Bilan D.S., Matlashov M.E., Mishina N.M., Markvicheva K.N., Subach O.M., Subach F.V., Bogeski I., Hoth M., Enikolopov G., Belousov V.V. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014;5 doi: 10.1038/ncomms6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Morgan B., Van Laer K., Owusu T.N.E., Ezerina D., Pastor-Flores D., Amponsah P.S., Tursch A., Dick T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 2016;12:437–443. doi: 10.1038/nchembio.2067. [DOI] [PubMed] [Google Scholar]
- 397.Belousov V.V., Fradkov A.F., Lukyanov K.A., Staroverov D.B., Shakhbazov K.S., Terskikh A.V., Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 398.Gutscher M., Sobotta M.C., Wabnitz G.H., Ballikaya S., Meyer A.J., Samstag Y., Dick T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Mishina N.M., Tyurin-Kuzmin P.A., Markvicheva K.N., Vorotnikov A.V., Tkachuk V.A., Laketa V., Schultz C., Lukyanov S., Belousov V.V. Does cellular hydrogen peroxide diffuse or act locally? Antioxid. Redox Signal. 2010;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 400.Pak Y., Swamy K., Yoon J. Recent progress in fluorescent imaging probes. Sensors. 2015;15:24374–24396. doi: 10.3390/s150924374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Guo Z., Park S., Yoon J., Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2013;43:16–29. doi: 10.1039/c3cs60271k. [DOI] [PubMed] [Google Scholar]
- 402.Lee D., Khaja S., Velasquez-Castano J.C., Dasari M., Sun C., Petros J., Taylor W.R., Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007;6:765–769. doi: 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]
- 403.Santra S., Xu J., Wang K., Tand W. Luminescent nanoparticle probes for bioimaging. J. Nanosci. Nanotechnol. 2004;4:590–599. doi: 10.1166/jnn.2004.017. [DOI] [PubMed] [Google Scholar]
- 404.Uusitalo L.M., Hempel N. Recent advances in intracellular and in vivo ROS Sensing: focus on nanoparticle and nanotube applications. Int. J. Mol. Sci. 2012;13:10660–10679. doi: 10.3390/ijms130910660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Choi W.-G., Swanson S.J., Gilroy S. High-resolution imaging of Ca2+, redox status, ROS and pH using GFP biosensors: imaging of Ca2+, redox, ROS and pH using GFP biosensors. Plant J. 2012;70:118–128. doi: 10.1111/j.1365-313X.2012.04917.x. [DOI] [PubMed] [Google Scholar]
- 406.Chen Z., Liu Z., Li Z., Ju E., Gao N., Zhou L., Ren J., Qu X. Upconversion nanoprobes for efficiently in vitro imaging reactive oxygen species and in vivo diagnosing rheumatoid arthritis. Biomaterials. 2015;39:15–22. doi: 10.1016/j.biomaterials.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 407.Zielonka J., Lambeth J.D., Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic. Biol. Med. 2013;65:1310–1314. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Seredenina T., Chiriano G., Filippova A., Nayernia Z., Mahiout Z., Fioraso-Cartier L., Plastre O., Scapozza L., Krause K.-H., Jaquet V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic. Biol. Med. 2015;86:239–249. doi: 10.1016/j.freeradbiomed.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 409.Zielonka J., Cheng G., Zielonka M., Ganesh T., Sun A., Joseph J., Michalski R., O'Brien W.J., Lambeth J.D., Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014;289:16176–16189. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Zielonka J., Zielonka M., VerPlank L., Cheng G., Hardy M., Ouari O., Ayhan M.M., Podsiadły R., Sikora A., Lambeth J.D., Kalyanaraman B. Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J. Biol. Chem. 2016;291:7029–7044. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Michalski R., Zielonka J., Hardy M., Joseph J., Kalyanaraman B. Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic. Biol. Med. 2013;54:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Waszczak C., Akter S., Jacques S., Huang J., Messens J., Breusegem F.V. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 2015;66:2923–2934. doi: 10.1093/jxb/erv084. [DOI] [PubMed] [Google Scholar]
- 413.Cao J., Ying M., Xie N., Lin G., Dong R., Zhang J., Yan H., Yang X., He Q., Yang B. The oxidation states of DJ-1 dictate the cell fate in response to oxidative stress triggered by 4-HPR: autophagy or apoptosis? Antioxid. Redox Signal. 2014;21:1443–1459. doi: 10.1089/ars.2013.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Waszczak C., Akter S., Eeckhout D., Persiau G., Wahni K., Bodra N., Molle I.V., Smet B.D., Vertommen D., Gevaert K., Jaeger G.D., Montagu M.V., Messens J., Breusegem F.V. Sulfenome mining in arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2014;111:11545–11550. doi: 10.1073/pnas.1411607111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Akter S., Huang J., Bodra N., Smet B.D., Wahni K., Rombaut D., Pauwels J., Gevaert K., Carroll K., Breusegem F.V., Messens J. DYn-2 based identification of arabidopsis sulfenomes. Mol. Cell. Proteom. 2015;14:1183–1200. doi: 10.1074/mcp.M114.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Oger E., Marino D., Guigonis J.-M., Pauly N., Puppo A. Sulfenylated proteins in the medicago truncatula–sinorhizobium meliloti symbiosis. J. Proteom. 2012;75:4102–4113. doi: 10.1016/j.jprot.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 417.Benitez L.V., Allison W.S. The inactivation of the acyl phosphatase activity catalyzed by the sulfenic acid form of glyceraldehyde 3-phosphate dehydrogenase by dimedone and olefins. J. Biol. Chem. 1974;249:6234–6243. [PubMed] [Google Scholar]
- 418.Seo Y.H., Carroll K.S. Facile synthesis and biological evaluation of a cell-permeable probe to detect redox-regulated proteins. Bioorg. Med. Chem. Lett. 2009;19:356–359. doi: 10.1016/j.bmcl.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 419.Schroder K., Vecchione C., Jung O., Schreiber J.G., Shiri-Sverdlov R., van Gorp P.J., Busse R., Brandes R.P. Xanthine oxidase inhibitor tungsten prevents the development of atherosclerosis in ApoE knockout mice fed a Western-type diet. Free Radic. Biol. Med. 2006;41:1353–1360. doi: 10.1016/j.freeradbiomed.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 420.Schroder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., Dimmeler S., Shah A.M., Brandes R.P. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 421.Schurmann C., Rezende F., Kruse C., Yasar Y., Lowe O., Fork C., van de Sluis B., Bremer R., Weissmann N., Shah A.M., Jo H., Brandes R.P., Schroder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Langbein H., Brunssen C., Hofmann A., Cimalla P., Brux M., Bornstein S.R., Deussen A., Koch E., Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016;37:1753–1761. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Craige S.M., Kant S., Reif M., Chen K., Pei Y., Angoff R., Sugamura K., Fitzgibbons T., Keaney J.F., Jr. Endothelial NADPH oxidase 4 protects ApoE-/- mice from atherosclerotic lesions. Free Radic. Biol. Med. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Gray S.P., Di Marco E., Kennedy K., Chew P., Okabe J., El-Osta A., Calkin A.C., Biessen E.A., Touyz R.M., Cooper M.E., Schmidt H.H., Jandeleit-Dahm K.A. Reactive oxygen Species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016;36:295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 425.Wang Y., Yang J., Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid. Redox Signal. 2012;16:649–657. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- 426.Klatt P., Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 427.Mieyal J.J., Gallogly M.M., Qanungo S., Sabens E.A., Shelton M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Watanabe Y., Murdoch C.E., Sano S., Ido Y., Bachschmid M.M., Cohen R.A., Matsui R. Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1alpha and improve limb revascularization. Proc. Natl. Acad. Sci. USA. 2016;113:6011–6016. doi: 10.1073/pnas.1524198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.O.G. Miller, J.B. Behring, S.L. Siedlak, S. Jiang, R. Matsui, M.M. Bachschmid, X. Zhu, J.J. Mieyal, Upregulation of glutaredoxin-1 activates microglia and promotes neurodegeneration: implications for parkinson’s disease. Antioxid. Redox Signal., 25, 2016, 967–982. [DOI] [PMC free article] [PubMed]
- 430.Murdoch C.E., Shuler M., Haeussler D.J., Kikuchi R., Bearelly P., Han J., Watanabe Y., Fuster J.J., Walsh K., Ho Y.S., Bachschmid M.M., Cohen R.A., Matsui R. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J. Biol. Chem. 2014;289:8633–8644. doi: 10.1074/jbc.M113.517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Evangelista A.M., Thompson M.D., Weisbrod R.M., Pimental D.R., Tong X., Bolotina V.M., Cohen R.A. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid. Redox Signal. 2012;17:1099–1108. doi: 10.1089/ars.2011.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Hazarika S., Dokun A.O., Li Y., Popel A.S., Kontos C.D., Annex B.H. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ. Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 433.Okuda M., Inoue N., Azumi H., Seno T., Sumi Y., Hirata K., Kawashima S., Hayashi Y., Itoh H., Yodoi J., Yokoyama M. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 434.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Pagliaro P., Moro F., Tullio F., Perrelli M.G., Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 2011;14:833–850. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
- 436.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 438.D.J. Hausenloy, D. Garcia-Dorado, H. Erik Botker, S.M. Davidson, J. Downey, F.B. Engel, R. Jennings, S. Lecour, J. Leor, R. Madonna, M. Ovize, C. Perrino, F. Prunier, R. Schulz, J.P. Sluijter, L.W. Van Laake, J. Vinten-Johansen, D.M. Yellon, K. Ytrehus, G. Heusch, P. Ferdinandy, Novel targets and future strategies for acute cardioprotection: position paper of the european society of cardiology working group on cellular biology of the heart. Cardiovasc. Res., 113, 2017, 564-585. [DOI] [PubMed]
- 439.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 440.Andreadou I., Iliodromitis E.K., Farmakis D., Kremastinos D.T. To prevent, protect and save the ischemic heart: antioxidants revisited. Expert Opin. Ther. Targets. 2009;13:945–956. doi: 10.1517/14728220903039698. [DOI] [PubMed] [Google Scholar]
- 441.Tsovolas K., Iliodromitis E.K., Andreadou I., Zoga A., Demopoulou M., Iliodromitis K.E., Manolaki T., Markantonis S.L., Kremastinos D.T. Acute administration of vitamin C abrogates protection from ischemic preconditioning in rabbits. Pharmacol. Res. 2008;57:283–289. doi: 10.1016/j.phrs.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 442.Skyschally A., Schulz R., Gres P., Korth H.G., Heusch G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H698–H703. doi: 10.1152/ajpheart.00693.2002. [DOI] [PubMed] [Google Scholar]
- 443.Local Food-Nutraceuticals C. Understanding local Mediterranean diets: a multidisciplinary pharmacological and ethnobotanical approach. Pharmacol. Res. 2005;52:353–366. doi: 10.1016/j.phrs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 444.Turan B., Fliss H., Desilets M. Oxidants increase intracellular free Zn2+ concentration in rabbit ventricular myocytes. Am. J. Physiol. 1997;272:H2095–H2106. doi: 10.1152/ajpheart.1997.272.5.H2095. [DOI] [PubMed] [Google Scholar]
- 445.Tuncay E., Bilginoglu A., Sozmen N.N., Zeydanli E.N., Ugur M., Vassort G., Turan B. Intracellular free zinc during cardiac excitation-contraction cycle: calcium and redox dependencies. Cardiovasc Res. 2011;89:634–642. doi: 10.1093/cvr/cvq352. [DOI] [PubMed] [Google Scholar]
- 446.Pisarenko O., Studneva I., Khlopkov V., Solomatina E., Ruuge E. An assessment of anaerobic metabolism during ischemia and reperfusion in isolated guinea pig heart. Biochim. Biophys. Acta. 1988;934:55–63. doi: 10.1016/0005-2728(88)90119-3. [DOI] [PubMed] [Google Scholar]
- 447.Ashrafian H., Czibik G., Bellahcene M., Aksentijevic D., Smith A.C., Mitchell S.J., Dodd M.S., Kirwan J., Byrne J.J., Ludwig C., Isackson H., Yavari A., Stottrup N.B., Contractor H., Cahill T.J., Sahgal N., Ball D.R., Birkler R.I., Hargreaves I., Tennant D.A., Land J., Lygate C.A., Johannsen M., Kharbanda R.K., Neubauer S., Redwood C., de Cabo R., Ahmet I., Talan M., Gunther U.L., Robinson A.J., Viant M.R., Pollard P.J., Tyler D.J., Watkins H. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-Parsy K., Frezza C., Krieg T., Murphy M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 449.Valls-Lacalle L., Barba I., Miro-Casas E., Alburquerque-Bejar J.J., Ruiz-Meana M., Fuertes-Agudo M., Rodriguez-Sinovas A., Garcia-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc. Res. 2016;109:374–384. doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- 450.Gorenkova N., Robinson E., Grieve D.J., Galkin A. Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia. Antioxid. Redox Signal. 2013;19:1459–1468. doi: 10.1089/ars.2012.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Pell V.R., Ding S., James A.M., Cocheme H.M., Reinhold J., Lilley K.S., Partridge L., Fearnley I.M., Robinson A.J., Hartley R.C., Smith R.A., Krieg T., Brookes P.S., Murphy M.P. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Varga Z.V., Giricz Z., Liaudet L., Hasko G., Ferdinandy P., Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta. 2015;1852:232–242. doi: 10.1016/j.bbadis.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Pechanova O., Varga Z.V., Cebova M., Giricz Z., Pacher P., Ferdinandy P. Cardiac NO signalling in the metabolic syndrome. Br. J. Pharmacol. 2015;172:1415–1433. doi: 10.1111/bph.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Frustaci A., Kajstura J., Chimenti C., Jakoniuk I., Leri A., Maseri A., Nadal-Ginard B., Anversa P. Myocardial cell death in human diabetes. Circ. Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 455.Onody A., Csonka C., Giricz Z., Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovasc. Res. 2003;58:663–670. doi: 10.1016/s0008-6363(03)00330-4. [DOI] [PubMed] [Google Scholar]
- 456.Varga Z.V., Kupai K., Szucs G., Gaspar R., Paloczi J., Farago N., Zvara A., Puskas L.G., Razga Z., Tiszlavicz L., Bencsik P., Gorbe A., Csonka C., Ferdinandy P., Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 457.Gorbe A., Varga Z.V., Kupai K., Bencsik P., Kocsis G.F., Csont T., Boengler K., Schulz R., Ferdinandy P. Cholesterol diet leads to attenuation of ischemic preconditioning-induced cardiac protection: the role of connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1907–H1913. doi: 10.1152/ajpheart.01242.2010. [DOI] [PubMed] [Google Scholar]
- 458.Jeong E.M., Chung J., Liu H., Go Y., Gladstein S., Farzaneh-Far A., Lewandowski E.D., Dudley S.C., Jr. Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Sverdlov A.L., Elezaby A., Qin F., Behring J.B., Luptak I., Calamaras T.D., Siwik D.A., Miller E.J., Liesa M., Shirihai O.S., Pimentel D.R., Cohen R.A., Bachschmid M.M., Colucci W.S. Mitochondrial reactive oxygen species mediate cardiac structural, functional, and mitochondrial consequences of diet-induced metabolic heart disease. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Luo M., Guan X., Luczak E.D., Lang D., Kutschke W., Gao Z., Yang J., Glynn P., Sossalla S., Swaminathan P.D., Weiss R.M., Yang B., Rokita A.G., Maier L.S., Efimov I.R., Hund T.J., Anderson M.E. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J. Clin. Investig. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Ni R., Cao T., Xiong S., Ma J., Fan G.C., Lacefield J.C., Lu Y., Le Tissier S., Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Sloan R.C., Moukdar F., Frasier C.R., Patel H.D., Bostian P.A., Lust R.M., Brown D.A. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J. Mol. Cell Cardiol. 2012;52:1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 463.Guo Y., Yu W., Sun D., Wang J., Li C., Zhang R., Babcock S.A., Li Y., Liu M., Ma M., Shen M., Zeng C., Li N., He W., Zou Q., Zhang Y., Wang H. A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetes-induced cardiac dysfunction: role of AMPK-regulated autophagy. Biochim. Biophys. Acta. 2015;1852:319–331. doi: 10.1016/j.bbadis.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 464.Herlein J.A., Fink B.D., Sivitz W.I. Superoxide production by mitochondria of insulin-sensitive tissues: mechanistic differences and effect of early diabetes. Metabolism. 2010;59:247–257. doi: 10.1016/j.metabol.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Essop M.F., Anna Chan W.Y., Valle A., Garcia-Palmer F.J., Du Toit E.F. Impaired contractile function and mitochondrial respiratory capacity in response to oxygen deprivation in a rat model of pre-diabetes. Acta Physiol. 2009;197:289–296. doi: 10.1111/j.1748-1716.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- 466.Radermacher K.A., Wingler K., Langhauser F., Altenhofer S., Kleikers P., Hermans J.J., Hrabe de Angelis M., Kleinschnitz C., Schmidt H.H. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid. Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kleikers P.W., Hooijmans C., Gob E., Langhauser F., Rewell S.S., Radermacher K., Ritskes-Hoitinga M., Howells D.W., Kleinschnitz C., Schmidt H.H. A combined pre-clinical meta-analysis and randomized confirmatory trial approach to improve data validity for therapeutic target validation. Sci. Rep. 2015;5:13428. doi: 10.1038/srep13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Dao V.T., Casas A.I., Maghzal G.J., Seredenina T., Kaludercic N., Robledinos-Anton N., Di Lisa F., Stocker R., Ghezzi P., Jaquet V., Cuadrado A., Schmidt H.H. Pharmacology and clinical drug candidates in redox medicine. Antioxid. Redox Signal. 2015;23:1113–1129. doi: 10.1089/ars.2015.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.C. Kleinschnitz, S. Mencl, P.W. Kleikers, M.K. Schuhmann, G.L. M, A.I. Casas, B. Surun, A. Reif, H.H. Schmidt, NOS knockout or inhibition but not disrupting PSD-95-NOS interaction protect against ischemic brain damage. J. Cereb. Blood Flow Metab., 36, 2016, 1508–12. [DOI] [PMC free article] [PubMed]
- 470.Li H., Horke S., Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 471.Munzel T., Daiber A., Ullrich V., Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 472.Li H., Horke S., Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 473.Li H., Forstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 474.Crabtree M.J., Brixey R., Batchelor H., Hale A.B., Channon K.M. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J. Biol. Chem. 2013;288:561–569. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Li H., Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 476.Li H., Forstermann U. Pharmacological prevention of eNOS uncoupling. Curr. Pharm. Des. 2014;20:3595–3606. doi: 10.2174/13816128113196660749. [DOI] [PubMed] [Google Scholar]
- 477.Schuhmacher S., Oelze M., Bollmann F., Kleinert H., Otto C., Heeren T., Steven S., Hausding M., Knorr M., Pautz A., Reifenberg K., Schulz E., Gori T., Wenzel P., Munzel T., Daiber A. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes. 2011;60:2608–2616. doi: 10.2337/db10-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Knorr M., Hausding M., Kroller-Schuhmacher S., Steven S., Oelze M., Heeren T., Scholz A., Gori T., Wenzel P., Schulz E., Daiber A., Munzel T. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 2011;31:2223–2231. doi: 10.1161/ATVBAHA.111.232058. [DOI] [PubMed] [Google Scholar]
- 479.Kim J.H., Bugaj L.J., Oh Y.J., Bivalacqua T.J., Ryoo S., Soucy K.G., Santhanam L., Webb A., Camara A., Sikka G., Nyhan D., Shoukas A.A., Ilies M., Christianson D.W., Champion H.C., Berkowitz D.E. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol. (1985) 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Xia N., Horke S., Habermeier A., Closs E.I., Reifenberg G., Gericke A., Mikhed Y., Munzel T., Daiber A., Forstermann U., Li H. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 481.Bowler R.P., Arcaroli J., Crapo J.D., Ross A., Slot J.W., Abraham E. Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am. J. Respir. Crit. Care Med. 2001;164:290–294. doi: 10.1164/ajrccm.164.2.2011054. [DOI] [PubMed] [Google Scholar]
- 482.Atochina E.N., Balyasnikova I.V., Danilov S.M., Granger D.N., Fisher A.B., Muzykantov V.R. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am. J. Physiol. 1998;275:L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 483.Dziubla T.D., Shuvaev V.V., Hong N.K., Hawkins B.J., Madesh M., Takano H., Simone E., Nakada M.T., Fisher A., Albelda S.M., Muzykantov V.R. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29:215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Sweitzer T.D., Thomas A.P., Wiewrodt R., Nakada M.T., Branco F., Muzykantov V.R. PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic. Biol. Med. 2003;34:1035–1046. doi: 10.1016/s0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 485.Shuvaev V.V., Tliba S., Pick J., Arguiri E., Christofidou-Solomidou M., Albelda S.M., Muzykantov V.R. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J. Control Release. 2011;149:236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Kozower B.D., Christofidou-Solomidou M., Sweitzer T.D., Muro S., Buerk D.G., Solomides C.C., Albelda S.M., Patterson G.A., Muzykantov V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 487.Shuvaev V.V., Christofidou-Solomidou M., Bhora F., Laude K., Cai H., Dikalov S., Arguiri E., Solomides C.C., Albelda S.M., Harrison D.G., Muzykantov V.R. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Hood E.D., Greineder C.F., Dodia C., Han J., Mesaros C., Shuvaev V.V., Blair I.A., Fisher A.B., Muzykantov V.R. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J. Control Release. 2012;163:161–169. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Howard M.D., Greineder C.F., Hood E.D., Muzykantov V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Dziubla T.D., Karim A., Muzykantov V.R. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J. Control Release. 2005;102:427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 491.Barlaka E., Galatou E., Mellidis K., Ravingerova T., Lazou A. Role of pleiotropic properties of peroxisome proliferator-activated receptors in the heart: focus on the nonmetabolic effects in cardiac protection. Cardiovasc Ther. 2016;34:37–48. doi: 10.1111/1755-5922.12166. [DOI] [PubMed] [Google Scholar]
- 492.Ibarra-Lara L., Hong E., Soria-Castro E., Torres-Narvaez J.C., Perez-Severiano F., Del Valle-Mondragon L., Cervantes-Perez L.G., Ramirez-Ortega M., Pastelin-Hernandez G.S., Sanchez-Mendoza A. Clofibrate PPARalpha activation reduces oxidative stress and improves ultrastructure and ventricular hemodynamics in no-flow myocardial ischemia. J. Cardiovasc Pharmacol. 2012;60:323–334. doi: 10.1097/FJC.0b013e31826216ed. [DOI] [PubMed] [Google Scholar]
- 493.Barlaka E., Ledvenyiova V., Galatou E., Ferko M., Carnicka S., Ravingerova T., Lazou A. Delayed cardioprotective effects of WY-14643 are associated with inhibition of MMP-2 and modulation of Bcl-2 family proteins through PPAR-alpha activation in rat hearts subjected to global ischaemia-reperfusion. Can. J. Physiol. Pharmacol. 2013;91:608–616. doi: 10.1139/cjpp-2012-0412. [DOI] [PubMed] [Google Scholar]
- 494.Barlaka E., Gorbe A., Gaspar R., Paloczi J., Ferdinandy P., Lazou A. Activation of PPARbeta/delta protects cardiac myocytes from oxidative stress-induced apoptosis by suppressing generation of reactive oxygen/nitrogen species and expression of matrix metalloproteinases. Pharmacol. Res. 2015;95–96:102–110. doi: 10.1016/j.phrs.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 495.Lee H., Ham S.A., Kim M.Y., Kim J.H., Paek K.S., Kang E.S., Kim H.J., Hwang J.S., Yoo T., Park C., Kim J.H., Lim D.S., Han C.W., Seo H.G. Activation of PPARdelta counteracts angiotensin II-induced ROS generation by inhibiting rac1 translocation in vascular smooth muscle cells. Free Radic. Res. 2012;46:912–919. doi: 10.3109/10715762.2012.687448. [DOI] [PubMed] [Google Scholar]
- 496.Liu J., Wang P., Luo J., Huang Y., He L., Yang H., Li Q., Wu S., Zhelyabovska O., Yang Q. Peroxisome proliferator-activated receptor beta/delta activation in adult hearts facilitates mitochondrial function and cardiac performance under pressure-overload condition. Hypertension. 2011;57:223–230. doi: 10.1161/HYPERTENSIONAHA.110.164590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Ravingerova T., Carnicka S., Nemcekova M., Ledvenyiova V., Adameova A., Kelly T., Barlaka E., Galatou E., Khandelwal V.K., Lazou A. PPAR-alpha activation as a preconditioning-like intervention in rats in vivo confers myocardial protection against acute ischaemia-reperfusion injury: involvement of PI3K-Akt. Can. J. Physiol. Pharmacol. 2012;90:1135–1144. doi: 10.1139/y2012-052. [DOI] [PubMed] [Google Scholar]
- 498.Ravingerova T., Ledvenyiova-Farkasova V., Ferko M., Bartekova M., Bernatova I., Pechanova O., Adameova A., Kolar F., Lazou A. Pleiotropic preconditioning-like cardioprotective effects of hypolipidemic drugs in acute ischemia-reperfusion in normal and hypertensive rats. Can. J. Physiol. Pharmacol. 2015;93:495–503. doi: 10.1139/cjpp-2014-0502. [DOI] [PubMed] [Google Scholar]
- 499.Baggio L.L., Drucker D.J. Biology of incretins: glp-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 500.Lund P.K., Goodman R.H., Dee P.C., Habener J.F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 502.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 503.Matsubara J., Sugiyama S., Sugamura K., Nakamura T., Fujiwara Y., Akiyama E., Kurokawa H., Nozaki T., Ohba K., Konishi M., Maeda H., Izumiya Y., Kaikita K., Sumida H., Jinnouchi H., Matsui K., Kim-Mitsuyama S., Takeya M., Ogawa H. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J. Am. Coll. Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 504.Shah Z., Kampfrath T., Deiuliis J.A., Zhong J., Pineda C., Ying Z., Xu X., Lu B., Moffatt-Bruce S., Durairaj R., Sun Q., Mihai G., Maiseyeu A., Rajagopalan S. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Nishioka T., Shinohara M., Tanimoto N., Kumagai C., Hashimoto K. Sitagliptin, a dipeptidyl peptidase-IV inhibitor, improves psoriasis. Dermatology. 2012;224:20–21. doi: 10.1159/000333358. [DOI] [PubMed] [Google Scholar]
- 506.Kern M., Kloting N., Niessen H.G., Thomas L., Stiller D., Mark M., Klein T., Bluher M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One. 2012;7:e38744. doi: 10.1371/journal.pone.0038744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Darsalia V., Ortsater H., Olverling A., Darlof E., Wolbert P., Nystrom T., Klein T., Sjoholm A., Patrone C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes. 2013;62:1289–1296. doi: 10.2337/db12-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Kroller-Schon S., Knorr M., Hausding M., Oelze M., Schuff A., Schell R., Sudowe S., Scholz A., Daub S., Karbach S., Kossmann S., Gori T., Wenzel P., Schulz E., Grabbe S., Klein T., Munzel T., Daiber A. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 2012;96:140–149. doi: 10.1093/cvr/cvs246. [DOI] [PubMed] [Google Scholar]
- 509.Cameron-Vendrig A., Reheman A., Siraj M.A., Xu X.R., Wang Y., Lei X., Afroze T., Shikatani E., El-Mounayri O., Noyan H., Weissleder R., Ni H., Husain M. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes. 2016;65:1714–1723. doi: 10.2337/db15-1141. [DOI] [PubMed] [Google Scholar]
- 510.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 511.Miquel J., Economos A.C., Fleming J., Johnson J.E., Jr. Mitochondrial role in cell aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 512.Kleikers P.W., Wingler K., Hermans J.J., Diebold I., Altenhofer S., Radermacher K.A., Janssen B., Gorlach A., Schmidt H.H. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J. Mol. Med. 2012;90:1391–1406. doi: 10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 513.Park L., Zhou P., Pitstick R., Capone C., Anrather J., Norris E.H., Younkin L., Younkin S., Carlson G., McEwen B.S., Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Chondrogianni N., Stratford F.L., Trougakos I.P., Friguet B., Rivett A.J., Gonos E.S. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J. Biol. Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 515.Liu C.K., Lyass A., Larson M.G., Massaro J.M., Wang N., D'Agostino R.B., Sr., Benjamin E.J., Murabito J.M. Biomarkers of oxidative stress are associated with frailty: the Framingham offspring study. Age. 2016;38:1. doi: 10.1007/s11357-015-9864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Ingles M., Gambini J., Carnicero J.A., Garcia-Garcia F.J., Rodriguez-Manas L., Olaso-Gonzalez G., Dromant M., Borras C., Vina J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J. Am. Geriatr. Soc. 2014;62:1324–1328. doi: 10.1111/jgs.12876. [DOI] [PubMed] [Google Scholar]
- 517.Gomez-Cabrera M.C., Ristow M., Vina J. Antioxidant supplements in exercise: worse than useless? Am. J. Physiol. Endocrinol. Metab. 2012;302:E476–E477. doi: 10.1152/ajpendo.00567.2011. (author reply E478-E479) [DOI] [PubMed] [Google Scholar]
- 518.Zhang Y., Ikeno Y., Qi W., Chaudhuri A., Li Y., Bokov A., Thorpe S.R., Baynes J.W., Epstein C., Richardson A., Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Jin K. Modern biological theories of aging. Aging Dis. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- 520.Vina J., Borras C., Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 521.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 522.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 523.Liang S., Kisseleva T., Brenner D.A. The role of NADPH Oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front. Physiol. 2016;7:17. doi: 10.3389/fphys.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Lener B., Koziel R., Pircher H., Hutter E., Greussing R., Herndler-Brandstetter D., Hermann M., Unterluggauer H., Jansen-Durr P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 2009;423:363–374. doi: 10.1042/BJ20090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Koziel R., Pircher H., Kratochwil M., Lener B., Hermann M., Dencher N.A., Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem. J. 2013;452:231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 526.Weyemi U., Lagente-Chevallier O., Boufraqech M., Prenois F., Courtin F., Caillou B., Talbot M., Dardalhon M., Al Ghuzlan A., Bidart J.M., Schlumberger M., Dupuy C. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Kodama R., Kato M., Furuta S., Ueno S., Zhang Y., Matsuno K., Yabe-Nishimura C., Tanaka E., Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 528.Senturk S., Mumcuoglu M., Gursoy-Yuzugullu O., Cingoz B., Akcali K.C., Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 529.Ago T., Matsushima S., Kuroda J., Zablocki D., Kitazono T., Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging. 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Wang M., Zhang J., Walker S.J., Dworakowski R., Lakatta E.G., Shah A.M. Involvement of NADPH oxidase in age-associated cardiac remodeling. J. Mol. Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Vendrov A.E., Vendrov K.C., Smith A., Yuan J., Sumida A., Robidoux J., Runge M.S., Madamanchi N.R. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid. Redox Signal. 2015;23:1389–1409. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., Barit D., Schwarz T., Geis C., Kraft P., Barthel K., Schuhmann M.K., Herrmann A.M., Meuth S.G., Stoll G., Meurer S., Schrewe A., Becker L., Gailus-Durner V., Fuchs H., Klopstock T., de Angelis M.H., Jandeleit-Dahm K., Shah A.M., Weissmann N., Schmidt H.H. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Piera-Velazquez S., Jimenez S.A. Role of cellular senescence and NOX4-mediated oxidative stress in systemic sclerosis pathogenesis. Curr. Rheumatol. Rep. 2015;17:473. doi: 10.1007/s11926-014-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., Meldrum E., Sanders Y.Y., Thannickal V.J. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Stanley S.E., Noth I., Armanios M. What the genetics "RTEL"ing us about telomeres and pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015;191:608–610. doi: 10.1164/rccm.201501-0119ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Zhang W., Wang T., Qin L., Gao H.M., Wilson B., Ali S.F., Zhang W., Hong J.S., Liu B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. FASEB J. 2004;18:589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 538.Holl M., Koziel R., Schafer G., Pircher H., Pauck A., Hermann M., Klocker H., Jansen-Durr P., Sampson N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol. Carcinog. 2016;55:27–39. doi: 10.1002/mc.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Sampson N., Koziel R., Zenzmaier C., Bubendorf L., Plas E., Jansen-Durr P., Berger P. ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol. Endocrinol. 2011;25:503–515. doi: 10.1210/me.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Ayala G., Tuxhorn J.A., Wheeler T.M., Frolov A., Scardino P.T., Ohori M., Wheeler M., Spitler J., Rowley D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- 541.Hohn A., Jung T., Grimm S., Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic. Biol. Med. 2010;48:1100–1108. doi: 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 542.Kastle M., Grune T. Interactions of the proteasomal system with chaperones: protein triage and protein quality control. Prog. Mol. Biol. Transl. Sci. 2012;109:113–160. doi: 10.1016/B978-0-12-397863-9.00004-3. [DOI] [PubMed] [Google Scholar]
- 543.Jung T., Catalgol B., Grune T. The proteasomal system. Mol. Asp. Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 544.Hohn A., Jung T., Grimm S., Catalgol B., Weber D., Grune T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic. Biol. Med. 2011;50:585–591. doi: 10.1016/j.freeradbiomed.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 545.Keck S., Nitsch R., Grune T., Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J. Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 546.Catalgol B., Ziaja I., Breusing N., Jung T., Hohn A., Alpertunga B., Schroeder P., Chondrogianni N., Gonos E.S., Petropoulos I., Friguet B., Klotz L.O., Krutmann J., Grune T. The proteasome is an integral part of solar ultraviolet a radiation-induced gene expression. J. Biol. Chem. 2009;284:30076–30086. doi: 10.1074/jbc.M109.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Kastle M., Woschee E., Grune T. Histone deacetylase 6 (HDAC6) plays a crucial role in p38MAPK-dependent induction of heme oxygenase-1 (HO-1) in response to proteasome inhibition. Free Radic. Biol. Med. 2012;53:2092–2101. doi: 10.1016/j.freeradbiomed.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 548.Chondrogianni N., Georgila K., Kourtis N., Tavernarakis N., Gonos E.S. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J. 2015;29:611–622. doi: 10.1096/fj.14-252189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Vilchez D., Morantte I., Liu Z., Douglas P.M., Merkwirth C., Rodrigues A.P., Manning G., Dillin A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 550.Tonoki A., Kuranaga E., Tomioka T., Hamazaki J., Murata S., Tanaka K., Miura M. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell Biol. 2009;29:1095–1106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Kapeta S., Chondrogianni N., Gonos E.S. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J. Biol. Chem. 2010;285:8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Papaevgeniou N., Sakellari M., Jha S., Tavernarakis N., Holmberg C.I., Gonos E.S., Chondrogianni N. 18alpha-glycyrrhetinic acid proteasome activator decelerates aging and Alzheimer's disease progression in caenorhabditis elegans and neuronal cultures. Antioxid. Redox Signal. 2016;25:855–869. doi: 10.1089/ars.2015.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Chondrogianni N., Kapeta S., Chinou I., Vassilatou K., Papassideri I., Gonos E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010;45:763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 554.Regitz C., Dussling L.M., Wenzel U. Amyloid-beta (Abeta(1)(-)(4)(2))-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014;58:1931–1940. doi: 10.1002/mnfr.201400014. [DOI] [PubMed] [Google Scholar]
- 555.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16:15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Moskalev A.A., Aliper A.M., Smit-McBride Z., Buzdin A., Zhavoronkov A. Genetics and epigenetics of aging and longevity. Cell Cycle. 2014;13:1063–1077. doi: 10.4161/cc.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Perez V.I., Bokov A., Van Remmen H., Mele J., Ran Q., Ikeno Y., Richardson A. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Muller F.L., Lustgarten M.S., Jang Y., Richardson A., Van Remmen H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 559.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 560.Camici G.G., Cosentino F., Tanner F.C., Luscher T.F. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J. Appl. Physiol. 2008;105:1628–1631. doi: 10.1152/japplphysiol.90579.2008. [DOI] [PubMed] [Google Scholar]
- 561.Dai D.F., Chiao Y.A., Marcinek D.J., Szeto H.H., Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Health. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Hamilton R.T., Walsh M.E., Van Remmen H. Mouse models of oxidative stress indicate a role for modulating healthy aging. J. Clin. Exp. Pathol. 2012;Suppl 4 doi: 10.4172/2161-0681.S4-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Wenzel P., Schuhmacher S., Kienhofer J., Muller J., Hortmann M., Oelze M., Schulz E., Treiber N., Kawamoto T., Scharffetter-Kochanek K., Munzel T., Burkle A., Bachschmid M.M., Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 565.Schottker B., Brenner H., Jansen E.H., Gardiner J., Peasey A., Kubinova R., Pajak A., Topor-Madry R., Tamosiunas A., Saum K.U., Holleczek B., Pikhart H., Bobak M. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med. 2015;13:300. doi: 10.1186/s12916-015-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Capri M., Moreno-Villanueva M., Cevenini E., Pini E., Scurti M., Borelli V., Palmas M.G., Zoli M., Schon C., Siepelmeyer A., Bernhardt J., Fiegl S., Zondag G., de Craen A.J., Hervonen A., Hurme M., Sikora E., Gonos E.S., Voutetakis K., Toussaint O., Debacq-Chainiaux F., Grubeck-Loebenstein B., Burkle A., Franceschi C. MARK-AGE population: from the human model to new insights. Mech. Ageing Dev. 2015;151:13–17. doi: 10.1016/j.mad.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 567.Rodriguez-Manas L., Fried L.P. Frailty in the clinical scenario. Lancet. 2015;385:e7–e9. doi: 10.1016/S0140-6736(14)61595-6. [DOI] [PubMed] [Google Scholar]
- 568.Lai H.Y., Chang H.T., Lee Y.L., Hwang S.J. Association between inflammatory markers and frailty in institutionalized older men. Maturitas. 2014;79:329–333. doi: 10.1016/j.maturitas.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 569.Argiles J.M., Campos N., Lopez-Pedrosa J.M., Rueda R., Rodriguez-Manas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J. Am. Med Dir. Assoc. 2016;17:789–796. doi: 10.1016/j.jamda.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 570.Sen C.K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 571.Suzuki Y.J., Forman H.J., Sevanian A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 572.Esposito F., Ammendola R., Faraonio R., Russo T., Cimino F. Redox control of signal transduction, gene expression and cellular senescence. Neurochem. Res. 2004;29:617–628. doi: 10.1023/b:nere.0000014832.78725.1a. [DOI] [PubMed] [Google Scholar]
- 573.Nauseef W.M., Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 574.Forsberg K., Wuttke A., Quadrato G., Chumakov P.M., Wizenmann A., Di Giovanni S. The tumor suppressor p53 fine-tunes reactive oxygen species levels and neurogenesis via PI3 kinase signaling. J. Neurosci. 2013;33:14318–14330. doi: 10.1523/JNEUROSCI.1056-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Wang K., Zhang T., Dong Q., Nice E.C., Huang C., Wei Y. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Lourenco C.F., Ledo A., Dias C., Barbosa R.M., Laranjinha J. Neurovascular and neurometabolic derailment in aging and Alzheimer's disease. Front. Aging Neurosci. 2015;7:103. doi: 10.3389/fnagi.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.McBean G.J. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 578.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Nauseef W.M. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 580.Babior B.M. Phagocytes and oxidative stress. Am. J. Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 581.Sareila O., Kelkka T., Pizzolla A., Hultqvist M., Holmdahl R. NOX2 complex-derived ROS as immune regulators. Antioxid. Redox Signal. 2011;15:2197–2208. doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- 582.Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl. Acad. Sci. USA. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 584.Lee K., Won H.Y., Bae M.A., Hong J.H., Hwang E.S. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc. Natl. Acad. Sci. USA. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 586.Miesel R., Kurpisz M., Kroger H. Suppression of inflammatory arthritis by simultaneous inhibition of nitric oxide synthase and NADPH oxidase. Free Radic. Biol. Med. 1996;20:75–81. doi: 10.1016/0891-5849(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 587.Roos D., Kuhns D.B., Maddalena A., Roesler J., Lopez J.A., Ariga T., Avcin T., de Boer M., Bustamante J., Condino-Neto A., Di Matteo G., He J., Hill H.R., Holland S.M., Kannengiesser C., Koker M.Y., Kondratenko I., van Leeuwen K., Malech H.L., Marodi L., Nunoi H., Stasia M.J., Ventura A.M., Witwer C.T., Wolach B., Gallin J.I. Hematologically important mutations: x-linked chronic granulomatous disease (third update) Blood Cells Mol. Dis. 2010;45:246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Roos D., Kuhns D.B., Maddalena A., Bustamante J., Kannengiesser C., de Boer M., van Leeuwen K., Koker M.Y., Wolach B., Roesler J., Malech H.L., Holland S.M., Gallin J.I., Stasia M.J. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update) Blood Cells Mol. Dis. 2010;44:291–299. doi: 10.1016/j.bcmd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Kuhns D.B., Alvord W.G., Heller T., Feld J.J., Pike K.M., Marciano B.E., Uzel G., DeRavin S.S., Priel D.A., Soule B.P., Zarember K.A., Malech H.L., Holland S.M., Gallin J.I. Residual NADPH oxidase and survival in chronic granulomatous disease. N. Engl. J. Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.van den Berg J.M., van Koppen E., Ahlin A., Belohradsky B.H., Bernatowska E., Corbeel L., Espanol T., Fischer A., Kurenko-Deptuch M., Mouy R., Petropoulou T., Roesler J., Seger R., Stasia M.J., Valerius N.H., Weening R.S., Wolach B., Roos D., Kuijpers T.W. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Boulais J., Trost M., Landry C.R., Dieckmann R., Levy E.D., Soldati T., Michnick S.W., Thibault P., Desjardins M. Molecular characterization of the evolution of phagosomes. Mol. Syst. Biol. 2010;6:423. doi: 10.1038/msb.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Cosson P., Soldati T. Eat, kill or die: when amoeba meets bacteria. Curr. Opin. Microbiol. 2008;11:271–276. doi: 10.1016/j.mib.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 593.Lardy B., Bof M., Aubry L., Paclet M.H., Morel F., Satre M., Klein G. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in Dictyostelium discoideum. Biochim. Biophys. Acta. 2005;1744:199–212. doi: 10.1016/j.bbamcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 594.Basu S., Fey P., Jimenez-Morales D., Dodson R.J., Chisholm R.L. dictyBase 2015: expanding data and annotations in a new software environment. Genesis. 2015;53:523–534. doi: 10.1002/dvg.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Tung S.M., Unal C., Ley A., Pena C., Tunggal B., Noegel A.A., Krut O., Steinert M., Eichinger L. Loss of Dictyostelium ATG9 results in a pleiotropic phenotype affecting growth, development, phagocytosis and clearance and replication of Legionella pneumophila. Cell. Microbiol. 2010;12:765–780. doi: 10.1111/j.1462-5822.2010.01432.x. [DOI] [PubMed] [Google Scholar]
- 596.Bloomfield G., Pears C. Superoxide signalling required for multicellular development of Dictyostelium. J. Cell Sci. 2003;116:3387–3397. doi: 10.1242/jcs.00649. [DOI] [PubMed] [Google Scholar]
- 597.Garcia M.X., Alexander H., Mahadeo D., Cotter D.A., Alexander S. The Dictyostelium discoideum prespore-specific catalase B functions to control late development and to protect spore viability. Biochim. Biophys. Acta. 2003;1641:55–64. doi: 10.1016/s0167-4889(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 598.Chen G., Zhuchenko O., Kuspa A. Immune-like phagocyte activity in the social amoeba. Science. 2007;317:678–681. doi: 10.1126/science.1143991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Zhang X., Zhuchenko O., Kuspa A., Soldati T. Social amoebae trap and kill bacteria by casting DNA nets. Nat. Commun. 2016;7:10938. doi: 10.1038/ncomms10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Zhang X., Soldati T. Of amoebae and men: extracellular DNA traps as an ancient cell-intrinsic defense mechanism. Front. Immunol. 2016;7:269. doi: 10.3389/fimmu.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Rieber N., Hector A., Kuijpers T., Roos D., Hartl D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin. Dev. Immunol. 2012;2012:252460. doi: 10.1155/2012/252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Brown K.L., Bylund J., MacDonald K.L., Song-Zhao G.X., Elliott M.R., Falsafi R., Hancock R.E., Speert D.P. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clin. Immunol. 2008;129:90–102. doi: 10.1016/j.clim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 603.de Luca A., Smeekens S.P., Casagrande A., Iannitti R., Conway K.L., Gresnigt M.S., Begun J., Plantinga T.S., Joosten L.A., van der Meer J.W., Chamilos G., Netea M.G., Xavier R.J., Dinarello C.A., Romani L., van de Veerdonk F.L. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Harbort C.J., Soeiro-Pereira P.V., von Bernuth H., Kaindl A.M., Costa-Carvalho B.T., Condino-Neto A., Reichenbach J., Roesler J., Zychlinsky A., Amulic B. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood. 2015;126:2842–2851. doi: 10.1182/blood-2015-05-645424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Violi F., Sanguigni V., Carnevale R., Plebani A., Rossi P., Finocchi A., Pignata C., De Mattia D., Martire B., Pietrogrande M.C., Martino S., Gambineri E., Soresina A.R., Pignatelli P., Martino F., Basili S., Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 606.Kishida K.T., Hoeffer C.A., Hu D., Pao M., Holland S.M., Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Pao M., Wiggs E.A., Anastacio M.M., Hyun J., DeCarlo E.S., Miller J.T., Anderson V.L., Malech H.L., Gallin J.I., Holland S.M. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- 608.Cole T.S., McKendrick F., Cant A.J., Pearce M.S., Cale C.M., Goldblatt D.R., Gennery A.R., Titman P. Cognitive ability in children with chronic granulomatous disease: a comparison of those managed conservatively with those who have undergone hematopoietic stem cell transplant. Neuropediatrics. 2013;44:230–232. doi: 10.1055/s-0033-1333875. [DOI] [PubMed] [Google Scholar]
- 609.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 610.Raichle M.E., Mintun M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 611.Santos R.M., Lourenco C.F., Piedade A.P., Andrews R., Pomerleau F., Huettl P., Gerhardt G.A., Laranjinha J., Barbosa R.M. A comparative study of carbon fiber-based microelectrodes for the measurement of nitric oxide in brain tissue. Biosens. Bioelectron. 2008;24:704–709. doi: 10.1016/j.bios.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 612.Lourenco C.F., Santos R.M., Barbosa R.M., Cadenas E., Radi R., Laranjinha J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic. Biol. Med. 2014;73:421–429. doi: 10.1016/j.freeradbiomed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 613.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 614.Ledo A., Barbosa R., Cadenas E., Laranjinha J. Dynamic and interacting profiles of *NO and O2 in rat hippocampal slices. Free Radic. Biol. Med. 2010;48:1044–1050. doi: 10.1016/j.freeradbiomed.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Ledo A., Barbosa R.M., Gerhardt G.A., Cadenas E., Laranjinha J. Concentration dynamics of nitric oxide in rat hippocampal subregions evoked by stimulation of the NMDA glutamate receptor. Proc. Natl. Acad. Sci. USA. 2005;102:17483–17488. doi: 10.1073/pnas.0503624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Dringen R., Pfeiffer B., Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Kandil S., Brennan L., McBean G.J. Glutathione depletion causes a JNK and p38MAPK-mediated increase in expression of cystathionine-gamma-lyase and upregulation of the transsulfuration pathway in C6 glioma cells. Neurochem. Int. 2010;56:611–619. doi: 10.1016/j.neuint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 618.Mysona B., Dun Y., Duplantier J., Ganapathy V., Smith S.B. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Muller glial cells. Cell Tissue Res. 2009;335:477–488. doi: 10.1007/s00441-008-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Banjac A., Perisic T., Sato H., Seiler A., Bannai S., Weiss N., Kolle P., Tschoep K., Issels R.D., Daniel P.T., Conrad M., Bornkamm G.W. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 620.Pampliega O., Domercq M., Soria F.N., Villoslada P., Rodriguez-Antiguedad A., Matute C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J. Neuroinflamm. 2011;8:63. doi: 10.1186/1742-2094-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Mesci P., Zaidi S., Lobsiger C.S., Millecamps S., Escartin C., Seilhean D., Sato H., Mallat M., Boillee S. System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain. 2015;138:53–68. doi: 10.1093/brain/awu312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Watkins S., Sontheimer H. Unique biology of gliomas: challenges and opportunities. Trends Neurosci. 2012;35:546–556. doi: 10.1016/j.tins.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Chen L., Li X., Liu L., Yu B., Xue Y., Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-gamma-lyase function. Oncol. Rep. 2015;33:1465–1474. doi: 10.3892/or.2015.3712. [DOI] [PubMed] [Google Scholar]
- 624.Schliebs R., Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J. Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 625.McKinney M., Jacksonville M.C. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem. Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 626.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 627.E. Navarro, L. Gonzalez-Lafuente, I. Perez-Liebana, I. Buendia, E. Lopez-Bernardo, C. Sanchez-Ramos, I. Prieto, A. Cuadrado, J. Satrustegui, S. Cadenas, M. Monsalve, M.G. Lopez, Heme-oxygenase I and PCG-1alpha regulate mitochondrial biogenesis via microglial activation of Alpha7 nicotinic acetylcholine receptors using PNU282987. Antioxid. Redox Signal., 2016. http://dx.doi.org/10.1089/ars.2016.6698. [DOI] [PubMed]
- 628.Parada E., Egea J., Buendia I., Negredo P., Cunha A.C., Cardoso S., Soares M.P., Lopez M.G. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid. Redox Signal. 2013;19:1135–1148. doi: 10.1089/ars.2012.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Egea J., Buendia I., Parada E., Navarro E., Leon R., Lopez M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015;97:463–472. doi: 10.1016/j.bcp.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 630.Shakirzyanova A., Valeeva G., Giniatullin A., Naumenko N., Fulle S., Akulov A., Atalay M., Nikolsky E., Giniatullin R. Age-dependent action of reactive oxygen species on transmitter release in mammalian neuromuscular junctions. Neurobiol. Aging. 2016;38:73–81. doi: 10.1016/j.neurobiolaging.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 631.Bukharaeva E., Shakirzyanova A., Khuzakhmetova V., Sitdikova G., Giniatullin R. Homocysteine aggravates ROS-induced depression of transmitter release from motor nerve terminals: potential mechanism of peripheral impairment in motor neuron diseases associated with hyperhomocysteinemia. Front. Cell. Neurosci. 2015;9:391. doi: 10.3389/fncel.2015.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Giniatullin A., Petrov A., Giniatullin R. The involvement of P2Y12 receptors, NADPH oxidase, and lipid rafts in the action of extracellular ATP on synaptic transmission at the frog neuromuscular junction. Neuroscience. 2015;285:324–332. doi: 10.1016/j.neuroscience.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 633.Giniatullin A.R., Darios F., Shakirzyanova A., Davletov B., Giniatullin R. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. J. Neurochem. 2006;98:1789–1797. doi: 10.1111/j.1471-4159.2006.03997.x. [DOI] [PubMed] [Google Scholar]
- 634.Debelec-Butuner B., Ertunc N., Korkmaz K.S. Inflammation contributes to NKX3.1 loss and augments DNA damage but does not alter the DNA damage response via increased SIRT1 expression. J. Inflamm. 2015;12:12. doi: 10.1186/s12950-015-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Zhao S., Zhang Y., Zhang Q., Wang F., Zhang D. Toll-like receptors and prostate cancer. Front. Immunol. 2014;5:352. doi: 10.3389/fimmu.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Oblak A., Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin. Dev. Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Kundu S.D., Lee C., Billips B.K., Habermacher G.M., Zhang Q., Liu V., Wong L.Y., Klumpp D.J., Thumbikat P. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68:223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 638.Debelec-Butuner B., Alapinar C., Ertunc N., Gonen-Korkmaz C., Yorukoglu K., Korkmaz K.S. TNFalpha-mediated loss of beta-catenin/E-cadherin association and subsequent increase in cell migration is partially restored by NKX3.1 expression in prostate cells. PLoS One. 2014;9:e109868. doi: 10.1371/journal.pone.0109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Yang H., Zhou H., Feng P., Zhou X., Wen H., Xie X., Shen H., Zhu X. Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J. Exp. Clin. Cancer Res. 2010;29:92. doi: 10.1186/1756-9966-29-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Manda G., Isvoranu G., Comanescu M.V., Manea A., Debelec Butuner B., Korkmaz K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015;5:347–357. doi: 10.1016/j.redox.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Winkelstein J.A., Marino M.C., Johnston R.B., Jr., Boyle J., Curnutte J., Gallin J.I., Malech H.L., Holland S.M., Ochs H., Quie P., Buckley R.H., Foster C.B., Chanock S.J., Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 642.Quie P.G., White J.G., Holmes B., Good R.A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J. Clin. Investig. 1967;46:668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Holmes B., Quie P.G., Windhorst D.B., Good R.A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966;1:1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- 644.Forfia P.R., Zhang X., Ochoa F., Ochoa M., Xu X., Bernstein R., Sehgal P.B., Ferreri N.R., Hintze T.H. Relationship between plasma NOx and cardiac and vascular dysfunction after LPS injection in anesthetized dogs. Am. J. Physiol. 1998;274:H193–H201. doi: 10.1152/ajpheart.1998.274.1.H193. [DOI] [PubMed] [Google Scholar]
- 645.Singel K.L., Segal B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016;130:479–490. doi: 10.1042/CS20150660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Han M., Zhang T., Yang L., Wang Z., Ruan J., Chang X. Association between NADPH oxidase (NOX) and lung cancer: a systematic review and meta-analysis. J. Thorac. Dis. 2016;8:1704–1711. doi: 10.21037/jtd.2016.06.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Kaur A., Webster M.R., Marchbank K., Behera R., Ndoye A., Kugel C.H., 3rd, Dang V.M., Appleton J., O'Connell M.P., Cheng P., Valiga A.A., Morissette R., McDonnell N.B., Ferrucci L., Kossenkov A.V., Meeth K., Tang H.Y., Yin X., Wood W.H., 3rd, Lehrmann E., Becker K.G., Flaherty K.T., Frederick D.T., Wargo J.A., Cooper Z.A., Tetzlaff M.T., Hudgens C., Aird K.M., Zhang R., Xu X., Liu Q., Bartlett E., Karakousis G., Eroglu Z., Lo R.S., Chan M., Menzies A.M., Long G.V., Johnson D.B., Sosman J., Schilling B., Schadendorf D., Speicher D.W., Bosenberg M., Ribas A., Weeraratna A.T. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532:250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 648.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Benhar M., Shytaj I.L., Stamler J.S., Savarino A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J. Clin. Investig. 2016;126:1630–1639. doi: 10.1172/JCI85339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Delmaghani S., Defourny J., Aghaie A., Beurg M., Dulon D., Thelen N., Perfettini I., Zelles T., Aller M., Meyer A., Emptoz A., Giraudet F., Leibovici M., Dartevelle S., Soubigou G., Thiry M., Vizi E.S., Safieddine S., Hardelin J.P., Avan P., Petit C. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell. 2015;163:894–906. doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 651.Rochette L., Zeller M., Cottin Y., Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 652.Wang J., Yang X., Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell Signal. 2016;28:1099–1104. doi: 10.1016/j.cellsig.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 653.Jankovic A., Korac A., Buzadzic B., Stancic A., Otasevic V., Ferdinandy P., Daiber A., Korac B. Targeting the nitric oxide/superoxide ratio in adipose tissue: relevance in obesity and diabetes management. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Tochhawng L., Deng S., Pervaiz S., Yap C.T. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013;13:246–253. doi: 10.1016/j.mito.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 655.Diaz B., Courtneidge S.A. Redox signaling at invasive microdomains in cancer cells. Free Radic. Biol. Med. 2012;52:247–256. doi: 10.1016/j.freeradbiomed.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Goitre L., Pergolizzi B., Ferro E., Trabalzini L., Retta S.F. Molecular crosstalk between integrins and cadherins: do reactive oxygen species set the talk? J. Signal Transduct. 2012;2012:807682. doi: 10.1155/2012/807682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Pani G., Galeotti T., Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev. 2010;29:351–378. doi: 10.1007/s10555-010-9225-4. [DOI] [PubMed] [Google Scholar]
- 658.Wu W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastas-. Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 659.Kinnula V.L., Crapo J.D. Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med. 2004;36:718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 660.Crosas-Molist E., Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015;6:106–111. doi: 10.1016/j.redox.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.de Mochel N.S., Seronello S., Wang S.H., Ito C., Zheng J.X., Liang T.J., Lambeth J.D., Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Evsev'eva A.I., Abramenko I.V., Gluzman D.F., Pisniachevskaia G.V., Filatov A.V. Immunophenotypic characteristics of lymphocytes in pleural cavity exudates. Vrachebnoe Delo. 1989:34–37. [PubMed] [Google Scholar]
- 663.Cui W., Matsuno K., Iwata K., Ibi M., Matsumoto M., Zhang J., Zhu K., Katsuyama M., Torok N.J., Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949–958. doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 664.Sancho P., Martin-Sanz P., Fabregat I. Reciprocal regulation of NADPH oxidases and the cyclooxygenase-2 pathway. Free Radic. Biol. Med. 2011;51:1789–1798. doi: 10.1016/j.freeradbiomed.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 665.Jiang J.X., Venugopal S., Serizawa N., Chen X., Scott F., Li Y., Adamson R., Devaraj S., Shah V., Gershwin M.E., Friedman S.L., Torok N.J. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Sancho P., Fabregat I. NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J. Biol. Chem. 2010;285:24815–24824. doi: 10.1074/jbc.M110.114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Ha S.Y., Paik Y.H., Yang J.W., Lee M.J., Bae H., Park C.K. NADPH oxidase 1 and NADPH oxidase 4 have opposite prognostic effects for patients with hepatocellular carcinoma after hepatectomy. Gut Liver. 2016;10:826–835. doi: 10.5009/gnl15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Jiang J.X., Chen X., Serizawa N., Szyndralewiez C., Page P., Schroder K., Brandes R.P., Devaraj S., Torok N.J. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic. Biol. Med. 2012;53:289–296. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Jardri R., Hugdahl K., Hughes M., Brunelin J., Waters F., Alderson-Day B., Smailes D., Sterzer P., Corlett P.R., Leptourgos P., Debbane M., Cachia A., Deneve S. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr. Bull. 2016;42:1124–1134. doi: 10.1093/schbul/sbw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Trepanier M.O., Hopperton K.E., Mizrahi R., Mechawar N., Bazinet R.P. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol. Psychiatry. 2016;21:1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Hardingham G.E., Do K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016;17:125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- 672.Gu F., Chauhan V., Chauhan A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:89–95. doi: 10.1097/MCO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 673.Steullet P., Cabungcal J.H., Kulak A., Kraftsik R., Chen Y., Dalton T.P., Cuenod M., Do K.Q. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Behrens M.M., Ali S.S., Dao D.N., Lucero J., Shekhtman G., Quick K.L., Dugan L.L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 675.Jiang Z., Rompala G.R., Zhang S., Cowell R.M., Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol. Psychiatry. 2013;73:1024–1034. doi: 10.1016/j.biopsych.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Lucas E.K., Markwardt S.J., Gupta S., Meador-Woodruff J.H., Lin J.D., Overstreet-Wadiche L., Cowell R.M. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J. Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Sorce S., Nuvolone M., Keller A., Falsig J., Varol A., Schwarz P., Bieri M., Budka H., Aguzzi A. The role of the NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog. 2014;10:e1004531. doi: 10.1371/journal.ppat.1004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.F. Vilhardt, J. Haslund-Vinding, V. Jaquet, G. McBean, Microglia antioxidant systems and redox signaling. Br. J. Pharmacol., 2016. http://dx.doi.org/10.1111/bph.13426. [DOI] [PMC free article] [PubMed]
- 680.Kopke R., Allen K.A., Henderson D., Hoffer M., Frenz D., Van de Water T. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann. N. Y Acad. Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- 681.Rousset F., Carnesecchi S., Senn P., Krause K.H. Nox3-targeted therapies for inner ear pathologies. Curr. Pharm. Des. 2015;21:5977–5987. doi: 10.2174/1381612821666151029112421. [DOI] [PubMed] [Google Scholar]
- 682.Paffenholz R., Bergstrom R.A., Pasutto F., Wabnitz P., Munroe R.J., Jagla W., Heinzmann U., Marquardt A., Bareiss A., Laufs J., Russ A., Stumm G., Schimenti J.C., Bergstrom D.E. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Banfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 684.Mukherjea D., Jajoo S., Kaur T., Sheehan K.E., Ramkumar V., Rybak L.P. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid. Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Vasilijevic A., Buzadzic B., Korac A., Petrovic V., Jankovic A., Korac B. Beneficial effects of L-arginine nitric oxide-producing pathway in rats treated with alloxan. J. Physiol. 2007;584:921–933. doi: 10.1113/jphysiol.2007.140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Lajoix A.D., Reggio H., Chardes T., Peraldi-Roux S., Tribillac F., Roye M., Dietz S., Broca C., Manteghetti M., Ribes G., Wollheim C.B., Gross R. A neuronal isoform of nitric oxide synthase expressed in pancreatic beta-cells controls insulin secretion. Diabetes. 2001;50:1311–1323. doi: 10.2337/diabetes.50.6.1311. [DOI] [PubMed] [Google Scholar]
- 687.Jobgen W.S., Fried S.K., Fu W.J., Meininger C.J., Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 688.Petrovic V., Korac A., Buzadzic B., Korac B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J. Exp. Biol. 2005;208:4263–4271. doi: 10.1242/jeb.01895. [DOI] [PubMed] [Google Scholar]
- 689.Vasilijevic A., Vojcic L., Dinulovic I., Buzadzic B., Korac A., Petrovic V., Jankovic A., Korac B. Expression pattern of thermogenesis-related factors in interscapular brown adipose tissue of alloxan-treated rats: beneficial effect of L-arginine. Nitric Oxide. 2010;23:42–50. doi: 10.1016/j.niox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 690.Coppey L.J., Gellett J.S., Davidson E.P., Dunlap J.A., Lund D.D., Salvemini D., Yorek M.A. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Br. J. Pharmacol. 2001;134:21–29. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Otasevic V., Korac A., Vucetic M., Macanovic B., Garalejic E., Ivanovic-Burmazovic I., Filipovic M.R., Buzadzic B., Stancic A., Jankovic A., Velickovic K., Golic I., Markelic M., Korac B. Is manganese (II) pentaazamacrocyclic superoxide dismutase mimic beneficial for human sperm mitochondria function and motility? Antioxid. Redox Signal. 2013;18:170–178. doi: 10.1089/ars.2012.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Stancic A., Otasevic V., Jankovic A., Vucetic M., Ivanovic-Burmazovic I., Filipovic M.R., Korac A., Markelic M., Velickovic K., Golic I., Buzadzic B., Korac B. Molecular basis of hippocampal energy metabolism in diabetic rats: the effects of SOD mimic. Brain Res. Bull. 2013;99:27–33. doi: 10.1016/j.brainresbull.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 693.Hoehn K.L., Salmon A.B., Hohnen-Behrens C., Turner N., Hoy A.J., Maghzal G.J., Stocker R., Van Remmen H., Kraegen E.W., Cooney G.J., Richardson A.R., James D.E. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Wojdyla K., Rogowska-Wrzesinska A. Differential alkylation-based redox proteomics– lessons learnt. Redox Biol. 2015;6:240–252. doi: 10.1016/j.redox.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Nakamura T., Tu S., Akhtar M.W., Sunico C.R., Okamoto S., Lipton S.A. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 2013;78:596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Im H., Manolopoulou M., Malito E., Shen Y., Zhao J., Neant-Fery M., Sun C.Y., Meredith S.C., Sisodia S.S., Leissring M.A., Tang W.J. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J. Biol. Chem. 2007;282:25453–25463. doi: 10.1074/jbc.M701590200. [DOI] [PubMed] [Google Scholar]
- 697.Durham T.B., Toth J.L., Klimkowski V.J., Cao J.X., Siesky A.M., Alexander-Chacko J., Wu G.Y., Dixon J.T., McGee J.E., Wang Y., Guo S.Y., Cavitt R.N., Schindler J., Thibodeaux S.J., Calvert N.A., Coghlan M.J., Sindelar D.K., Christe M., Kiselyov V.V., Michael M.D., Sloop K.W. Dual exosite-binding inhibitors of insulin-degrading enzyme challenge its role as the primary mediator of insulin clearance in vivo. J. Biol. Chem. 2015;290:20044–20059. doi: 10.1074/jbc.M115.638205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14(33–38):27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 699.Shen Y., Joachimiak A., Rosner M.R., Tang W.J. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443:870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Ralat L.A., Ren M., Schilling A.B., Tang W.J. Protective role of Cys-178 against the inactivation and oligomerization of human insulin-degrading enzyme by oxidation and nitrosylation. J. Biol. Chem. 2009;284:34005–34018. doi: 10.1074/jbc.M109.030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Akhtar M.W., Sanz-Blasco S., Dolatabadi N., Parker J., Chon K., Lee M.S., Soussou W., McKercher S.R., Ambasudhan R., Nakamura T., Lipton S.A. Elevated glucose and oligomeric beta-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016;7:10242. doi: 10.1038/ncomms10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Cohen G., Riahi Y., Shamni O., Guichardant M., Chatgilialoglu C., Ferreri C., Kaiser N., Sasson S. Role of lipid peroxidation and PPAR-delta in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60:2830–2842. doi: 10.2337/db11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Cohen G., Shamni O., Avrahami Y., Cohen O., Broner E.C., Filippov-Levy N., Chatgilialoglu C., Ferreri C., Kaiser N., Sasson S. Beta cell response to nutrient overload involves phospholipid remodelling and lipid peroxidation. Diabetologia. 2015;58:1333–1343. doi: 10.1007/s00125-015-3566-z. [DOI] [PubMed] [Google Scholar]
- 704.Kahremany S., Livne A., Gruzman A., Senderowitz H., Sasson S. Activation of PPARdelta: from computer modelling to biological effects. Br. J. Pharmacol. 2015;172:754–770. doi: 10.1111/bph.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Maulucci G., Daniel B., Cohen O., Avrahami Y., Sasson S. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol. Asp. Med. 2016;49:49–77. doi: 10.1016/j.mam.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 706.Zimniak P. 4-Hydroxynonenal and fat storage: a paradoxical pro-obesity mechanism? Cell Cycle. 2010;9:3393–3394. doi: 10.4161/cc.9.17.13123. [DOI] [PubMed] [Google Scholar]
- 707.Li X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Baskaran P., Krishnan V., Ren J., Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016;173:2369–2389. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Kanda Y., Hinata T., Kang S.W., Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89:250–258. doi: 10.1016/j.lfs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 710.Ruskovska T., Bernlohr D.A. Oxidative stress and protein carbonylation in adipose tissue – implications for insulin resistance and diabetes mellitus. J. Proteom. 2013;92:323–334. doi: 10.1016/j.jprot.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Kim S.H., Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab. J. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J., Pierce K.A., Laznik-Bogoslavski D., Vetrivelan R., Clish C.B., Robinson A.J., Gygi S.P., Spiegelman B.M. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Schneider K., Valdez J., Nguyen J., Vawter M., Galke B., Kurtz T.W., Chan J.Y. Increased energy expenditure, Ucp1 expression, and resistance to diet-induced obesity in mice lacking nuclear factor-erythroid-2-related transcription factor-2 (Nrf2) J. Biol. Chem. 2016;291:7754–7766. doi: 10.1074/jbc.M115.673756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Castro J.P., Grune T., Speckmann B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016;397:709–724. doi: 10.1515/hsz-2015-0305. [DOI] [PubMed] [Google Scholar]
- 715.Munzel T., Daiber A., Gori T. Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation. 2011;123:2132–2144. doi: 10.1161/CIRCULATIONAHA.110.981407. [DOI] [PubMed] [Google Scholar]
- 716.Daiber A., Munzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid. Redox Signal. 2015;23:899–942. doi: 10.1089/ars.2015.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Munzel T., Daiber A., Gori T. More answers to the still unresolved question of nitrate tolerance. Eur. Heart J. 2013;34:2666–2673. doi: 10.1093/eurheartj/eht249. [DOI] [PubMed] [Google Scholar]
- 718.Daiber A., Oelze M., Sulyok S., Coldewey M., Schulz E., Treiber N., Hink U., Mulsch A., Scharffetter-Kochanek K., Munzel T. Heterozygous deficiency of manganese superoxide dismutase in mice (Mn-SOD+/-): a novel approach to assess the role of oxidative stress for the development of nitrate tolerance. Mol. Pharmacol. 2005;68:579–588. doi: 10.1124/mol.105.011585. [DOI] [PubMed] [Google Scholar]
- 719.Esplugues J.V., Rocha M., Nunez C., Bosca I., Ibiza S., Herance J.R., Ortega A., Serrador J.M., D'Ocon P., Victor V.M. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ. Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- 720.Munzel T., Kurz S., Rajagopalan S., Tarpey M., Freeman B., Harrison D.G. Identification of the membrane bound NADH oxidase as the major source of superoxide anion in nitrate tolerance. Endothelium. 1995;3(Suppl):s14. (abstract) [Google Scholar]
- 721.Jabs A., Oelze M., Mikhed Y., Stamm P., Kroller-Schon S., Welschof P., Jansen T., Hausding M., Kopp M., Steven S., Schulz E., Stasch J.P., Munzel T., Daiber A. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vasc. Pharmacol. 2015;71:181–191. doi: 10.1016/j.vph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 722.Daiber A., Oelze M., Coldewey M., Kaiser K., Huth C., Schildknecht S., Bachschmid M., Nazirisadeh Y., Ullrich V., Mulsch A., Munzel T., Tsilimingas N. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem. Biophys. Res. Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 723.Chen Z., Zhang J., Stamler J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Wenzel P., Hink U., Oelze M., Schuppan S., Schaeuble K., Schildknecht S., Ho K.K., Weiner H., Bachschmid M., Munzel T., Daiber A. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J. Biol. Chem. 2007;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 725.Schulz E., Tsilimingas N., Rinze R., Reiter B., Wendt M., Oelze M., Woelken-Weckmuller S., Walter U., Reichenspurner H., Meinertz T., Munzel T. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- 726.Andreassi M.G., Botto N., Simi S., Casella M., Manfredi S., Lucarelli M., Venneri L., Biagini A., Picano E. Diabetes and chronic nitrate therapy as co-determinants of somatic DNA damage in patients with coronary artery disease. J. Mol. Med. 2005;83:279–286. doi: 10.1007/s00109-005-0634-8. [DOI] [PubMed] [Google Scholar]
- 727.Mikhed Y., Fahrer J., Oelze M., Kroller-Schon S., Steven S., Welschof P., Zinssius E., Stamm P., Kashani F., Roohani S., Kress J.M., Ullmann E., Tran L.P., Schulz E., Epe B., Kaina B., Munzel T., Daiber A. Nitroglycerin induces DNA damage and vascular cell death in the setting of nitrate tolerance. Basic Res. Cardiol. 2016;111:52. doi: 10.1007/s00395-016-0571-4. [DOI] [PubMed] [Google Scholar]
- 728.Oelze M., Knorr M., Kroller-Schon S., Kossmann S., Gottschlich A., Rummler R., Schuff A., Daub S., Doppler C., Kleinert H., Gori T., Daiber A., Munzel T. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J. 2013;34:3206–3216. doi: 10.1093/eurheartj/ehs100. [DOI] [PubMed] [Google Scholar]
- 729.Oberle S., Abate A., Grosser N., Vreman H.J., Dennery P.A., Schneider H.T., Stalleicken D., Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of pentaerythrityl trinitrate. Biochem. Biophys. Res. Commun. 2002;290:1539–1544. doi: 10.1006/bbrc.2002.6379. [DOI] [PubMed] [Google Scholar]
- 730.Oppermann M., Balz V., Adams V., Dao V.T., Bas M., Suvorava T., Kojda G. Pharmacological induction of vascular extracellular superoxide dismutase expression in vivo. J. Cell. Mol. Med. 2009;13:1271–1278. doi: 10.1111/j.1582-4934.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Pautz A., Rauschkolb P., Schmidt N., Art J., Oelze M., Wenzel P., Forstermann U., Daiber A., Kleinert H. Effects of nitroglycerin or pentaerithrityl tetranitrate treatment on the gene expression in rat hearts: evidence for cardiotoxic and cardioprotective effects. Physiol. Genom. 2009;38:176–185. doi: 10.1152/physiolgenomics.00035.2009. [DOI] [PubMed] [Google Scholar]
- 732.Wu Z., Siuda D., Xia N., Reifenberg G., Daiber A., Munzel T., Forstermann U., Li H. Maternal treatment of spontaneously hypertensive rats with pentaerythritol tetranitrate reduces blood pressure in female offspring. Hypertension. 2015;65:232–237. doi: 10.1161/HYPERTENSIONAHA.114.04416. [DOI] [PubMed] [Google Scholar]
- 733.Gamboa J.L., Billings F.T. t., Bojanowski M.T., Gilliam L.A., Yu C., Roshanravan B., Roberts L.J., 2nd, Himmelfarb J., Ikizler T.A., Brown N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016;4 doi: 10.14814/phy2.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Makino A., Skelton M.M., Zou A.P., Roman R.J., Cowley A.W., Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 735.Sindhu R.K., Ehdaie A., Farmand F., Dhaliwal K.K., Nguyen T., Zhan C.D., Roberts C.K., Vaziri N.D. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim. Biophys. Acta. 2005;1743:86–92. doi: 10.1016/j.bbamcr.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 736.Vaziri N.D., Dicus M., Ho N.D., Boroujerdi-Rad L., Sindhu R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63:179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 737.Schnackenberg C.G., Wilcox C.S. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- 738.de Richelieu L.T., Sorensen C.M., Holstein-Rathlou N.H., Salomonsson M. NO-independent mechanism mediates tempol-induced renal vasodilation in SHR. Am. J. Physiol. Ren. Physiol. 2005;289:F1227–F1234. doi: 10.1152/ajprenal.00116.2005. [DOI] [PubMed] [Google Scholar]
- 739.Guron G.S., Grimberg E.S., Basu S., Herlitz H. Acute effects of the superoxide dismutase mimetic tempol on split kidney function in two-kidney one-clip hypertensive rats. J. Hypertens. 2006;24:387–394. doi: 10.1097/01.hjh.0000200511.02700.99. [DOI] [PubMed] [Google Scholar]
- 740.Papazova D.A., van Koppen A., Koeners M.P., Bleys R.L., Verhaar M.C., Joles J.A. Maintenance of hypertensive hemodynamics does not depend on ROS in established experimental chronic kidney disease. PLoS One. 2014;9:e88596. doi: 10.1371/journal.pone.0088596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Herget J., Wilhelm J., Novotna J., Eckhardt A., Vytasek R., Mrazkova L., Ostadal M. A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol. Res. 2000;49:493–501. [PubMed] [Google Scholar]
- 742.Hansen T., Galougahi K.K., Celermajer D., Rasko N., Tang O., Bubb K.J., Figtree G. Oxidative and nitrosative signalling in pulmonary arterial hypertension – implications for development of novel therapies. Pharmacol. Ther. 2016;165:50–62. doi: 10.1016/j.pharmthera.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 743.Hodyc D., Johnson E., Skoumalova A., Tkaczyk J., Maxova H., Vizek M., Herget J. Reactive oxygen species production in the early and later stage of chronic ventilatory hypoxia. Physiol. Res. 2012;61:145–151. doi: 10.33549/physiolres.932206. [DOI] [PubMed] [Google Scholar]
- 744.Jakoubek V., Bibova J., Herget J., Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1638–H1644. doi: 10.1152/ajpheart.01120.2007. [DOI] [PubMed] [Google Scholar]
- 745.Gao B., Doan A., Hybertson B.M. The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin. Pharmacol. 2014;6:19–34. doi: 10.2147/CPAA.S35078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.O'Brien E., Dietrich D.R. Ochratoxin A: the continuing enigma. Crit. Rev. Toxicol. 2005;35:33–60. doi: 10.1080/10408440590905948. [DOI] [PubMed] [Google Scholar]
- 747.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 748.Ringot D., Chango A., Schneider Y.J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006;159:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 749.Schaaf G.J., Nijmeijer S.M., Maas R.F., Roestenberg P., de Groene E.M., Fink-Gremmels J. The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. Biochim. Biophys. Acta. 2002;1588:149–158. doi: 10.1016/s0925-4439(02)00159-x. [DOI] [PubMed] [Google Scholar]
- 750.Costa J.G., Saraiva N., Guerreiro P.S., Louro H., Silva M.J., Miranda J.P., Castro M., Batinic-Haberle I., Fernandes A.S., Oliveira N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: an integrative approach of complementary endpoints. Food Chem. Toxicol. 2016;87:65–76. doi: 10.1016/j.fct.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 751.Marin-Kuan M., Ehrlich V., Delatour T., Cavin C., Schilter B. Evidence for a role of oxidative stress in the carcinogenicity of ochratoxin a. J. Toxicol. 2011;2011:645361. doi: 10.1155/2011/645361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Pfohl-Leszkowicz A., Manderville R.A. An update on direct genotoxicity as a molecular mechanism of ochratoxin a carcinogenicity. Chem. Res. Toxicol. 2012;25:252–262. doi: 10.1021/tx200430f. [DOI] [PubMed] [Google Scholar]
- 753.Cavin C., Delatour T., Marin-Kuan M., Fenaille F., Holzhauser D., Guignard G., Bezencon C., Piguet D., Parisod V., Richoz-Payot J., Schilter B. Ochratoxin A-mediated DNA and protein damage: roles of nitrosative and oxidative stresses. Toxicol. Sci. 2009;110:84–94. doi: 10.1093/toxsci/kfp090. [DOI] [PubMed] [Google Scholar]
- 754.Sorrenti V., Di Giacomo C., Acquaviva R., Barbagallo I., Bognanno M., Galvano F. Toxicity of ochratoxin a and its modulation by antioxidants: a review. Toxins. 2013;5:1742–1766. doi: 10.3390/toxins5101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Newton G.L., Buchmeier N., Fahey R.C. Biosynthesis and functions of mycothiol, the unique protective thiol of actinobacteria. Microbiol. Mol. Biol. Rev. 2008;72:471–494. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Van Laer K., Hamilton C.J., Messens J. Low-molecular-weight thiols in thiol-disulfide exchange. Antioxid. Redox Signal. 2013;18:1642–1653. doi: 10.1089/ars.2012.4964. [DOI] [PubMed] [Google Scholar]
- 757.Van Laer K., Buts L., Foloppe N., Vertommen D., Van Belle K., Wahni K., Roos G., Nilsson L., Mateos L.M., Rawat M., van Nuland N.A., Messens J. Mycoredoxin-1 is one of the missing links in the oxidative stress defence mechanism of Mycobacteria. Mol. Microbiol. 2012;86:787–804. doi: 10.1111/mmi.12030. [DOI] [PubMed] [Google Scholar]
- 758.Hugo M., Van Laer K., Reyes A.M., Vertommen D., Messens J., Radi R., Trujillo M. Mycothiol/mycoredoxin 1-dependent reduction of the peroxiredoxin AhpE from Mycobacterium tuberculosis. J. Biol. Chem. 2014;289:5228–5239. doi: 10.1074/jbc.M113.510248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Chacinska A., Pfannschmidt S., Wiedemann N., Kozjak V., Sanjuan Szklarz L.K., Schulze-Specking A., Truscott K.N., Guiard B., Meisinger C., Pfanner N. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 2004;23:3735–3746. doi: 10.1038/sj.emboj.7600389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Herrmann J.M., Riemer J. Mitochondrial disulfide relay: redox-regulated protein import into the intermembrane space. J. Biol. Chem. 2012;287:4426–4433. doi: 10.1074/jbc.R111.270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Naoe M., Ohwa Y., Ishikawa D., Ohshima C., Nishikawa S., Yamamoto H., Endo T. Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J. Biol. Chem. 2004;279:47815–47821. doi: 10.1074/jbc.M410272200. [DOI] [PubMed] [Google Scholar]
- 762.Sideris D.P., Petrakis N., Katrakili N., Mikropoulou D., Gallo A., Ciofi-Baffoni S., Banci L., Bertini I., Tokatlidis K. A novel intermembrane space-targeting signal docks cysteines onto Mia40 during mitochondrial oxidative folding. J. Cell Biol. 2009;187:1007–1022. doi: 10.1083/jcb.200905134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Bien M., Longen S., Wagener N., Chwalla I., Herrmann J.M., Riemer J. Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione. Mol. Cell. 2010;37:516–528. doi: 10.1016/j.molcel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 764.Vogtle F.N., Burkhart J.M., Rao S., Gerbeth C., Hinrichs J., Martinou J.C., Chacinska A., Sickmann A., Zahedi R.P., Meisinger C. Intermembrane space proteome of yeast mitochondria. Mol. Cell Proteom. 2012;11:1840–1852. doi: 10.1074/mcp.M112.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Banci L., Bertini I., Cefaro C., Ciofi-Baffoni S., Gallo A., Martinelli M., Sideris D.P., Katrakili N., Tokatlidis K. MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat. Struct. Mol. Biol. 2009;16:198–206. doi: 10.1038/nsmb.1553. [DOI] [PubMed] [Google Scholar]
- 766.Milkovic L., Siems W., Siems R., Zarkovic N. Oxidative stress and antioxidants in carcinogenesis and integrative therapy of cancer. Curr. Pharm. Des. 2014;20:6529–6542. doi: 10.2174/1381612820666140826152822. [DOI] [PubMed] [Google Scholar]
- 767.Stepanic V., Gasparovic A.C., Troselj K.G., Amic D., Zarkovic N. Selected attributes of polyphenols in targeting oxidative stress in cancer. Curr. Top. Med. Chem. 2015;15:496–509. doi: 10.2174/1568026615666150209123100. [DOI] [PubMed] [Google Scholar]
- 768.Laurent A., Nicco C., Chereau C., Goulvestre C., Alexandre J., Alves A., Levy E., Goldwasser F., Panis Y., Soubrane O., Weill B., Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 769.Holley A.K., Miao L., St Clair D.K., St Clair W.H. Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxid. Redox Signal. 2014;20:1567–1589. doi: 10.1089/ars.2012.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Konzack A., Jakupovic M., Kubaichuk K., Gorlach A., Dombrowski F., Miinalainen I., Sormunen R., Kietzmann T. Mitochondrial dysfunction due to lack of manganese superoxide dismutase promotes hepatocarcinogenesis. Antioxid. Redox Signal. 2015;23:1059–1075. doi: 10.1089/ars.2015.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Zhang X.F., Tan X., Zeng G., Misse A., Singh S., Kim Y., Klaunig J.E., Monga S.P. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–965. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 773.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 774.Muscoli C., Cuzzocrea S., Riley D.P., Zweier J.L., Thiemermann C., Wang Z.Q., Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br. J. Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Iranzo O. Manganese complexes displaying superoxide dismutase activity: a balance between different factors. Bioorg. Chem. 2011;39:73–87. doi: 10.1016/j.bioorg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 776.Batinic-Haberle I., Reboucas J.S., Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Batinic-Haberle I., Tovmasyan A., Spasojevic I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins – from superoxide dismutation to H2O2-driven pathways. Redox Biol. 2015;5:43–65. doi: 10.1016/j.redox.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Fernandes A.S., Costa J., Gaspar J., Rueff J., Cabral M.F., Cipriano M., Castro M., Oliveira N.G. Development of pyridine-containing macrocyclic copper(II) complexes: potential role in the redox modulation of oxaliplatin toxicity in human breast cells. Free Radic. Res. 2012;46:1157–1166. doi: 10.3109/10715762.2012.695869. [DOI] [PubMed] [Google Scholar]
- 779.Wondrak G.T. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Batinic-Haberle I., Tovmasyan A., Roberts E.R., Vujaskovic Z., Leong K.W., Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014;20:2372–2415. doi: 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 782.Lu M.C., Ji J.A., Jiang Z.Y., You Q.D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med. Res. Rev. 2016;36:924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 783.Voskou S., Aslan M., Fanis P., Phylactides M., Kleanthous M. Oxidative stress in beta-thalassaemia and sickle cell disease. Redox Biol. 2015;6:226–239. doi: 10.1016/j.redox.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Yanpanitch O.U., Hatairaktham S., Charoensakdi R., Panichkul N., Fucharoen S., Srichairatanakool S., Siritanaratkul N., Kalpravidh R.W. Treatment of beta-thalassemia/hemoglobin E with antioxidant cocktails results in decreased oxidative stress, increased hemoglobin concentration, and improvement of the hypercoagulable state. Oxid. Med. Cell. Longev. 2015;2015:537954. doi: 10.1155/2015/537954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Ozdemir Z.C., Koc A., Aycicek A., Kocyigit A. N-Acetylcysteine supplementation reduces oxidative stress and DNA damage in children with beta-thalassemia. Hemoglobin. 2014;38:359–364. doi: 10.3109/03630269.2014.951890. [DOI] [PubMed] [Google Scholar]
- 786.Pfeifer W.P., Degasperi G.R., Almeida M.T., Vercesi A.E., Costa F.F., Saad S.T. Vitamin E supplementation reduces oxidative stress in beta thalassaemia intermedia. Acta Haematol. 2008;120:225–231. doi: 10.1159/000201988. [DOI] [PubMed] [Google Scholar]
- 787.Tesoriere L., D'Arpa D., Butera D., Allegra M., Renda D., Maggio A., Bongiorno A., Livrea M.A. Oral supplements of vitamin E improve measures of oxidative stress in plasma and reduce oxidative damage to LDL and erythrocytes in beta-thalassemia intermedia patients. Free Radic. Res. 2001;34:529–540. doi: 10.1080/10715760100300461. [DOI] [PubMed] [Google Scholar]
- 788.Franco S.S., De Falco L., Ghaffari S., Brugnara C., Sinclair D.A., Matte A., Iolascon A., Mohandas N., Bertoldi M., An X., Siciliano A., Rimmele P., Cappellini M.D., Michan S., Zoratti E., Anne J., De Franceschi L. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99:267–275. doi: 10.3324/haematol.2013.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Kalpravidh R.W., Siritanaratkul N., Insain P., Charoensakdi R., Panichkul N., Hatairaktham S., Srichairatanakool S., Phisalaphong C., Rachmilewitz E., Fucharoen S. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin. Biochem. 2010;43:424–429. doi: 10.1016/j.clinbiochem.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 790.Kalpravidh R.W., Wichit A., Siritanaratkul N., Fucharoen S. Effect of coenzyme Q10 as an antioxidant in beta-thalassemia/Hb E patients. Biofactors. 2005;25:225–234. doi: 10.1002/biof.5520250128. [DOI] [PubMed] [Google Scholar]
- 791.Ounjaijean S., Thephinlap C., Khansuwan U., Phisalapong C., Fucharoen S., Porter J.B., Srichairatanakool S. Effect of green tea on iron status and oxidative stress in iron-loaded rats. Med. Chem. 2008;4:365–370. doi: 10.2174/157340608784872316. [DOI] [PubMed] [Google Scholar]
- 792.Fibach E., Tan E.S., Jamuar S., Ng I., Amer J., Rachmilewitz E.A. Amelioration of oxidative stress in red blood cells from patients with beta-thalassemia major and intermedia and E-beta-thalassemia following administration of a fermented papaya preparation. Phytother. Res. 2010;24:1334–1338. doi: 10.1002/ptr.3116. [DOI] [PubMed] [Google Scholar]
- 793.Darvishi Khezri H., Salehifar E., Kosaryan M., Aliasgharian A., Jalali H., Hadian Amree A. Potential effects of silymarin and its flavonolignan components in patients with beta-thalassemia major: a comprehensive review in 2015. Adv. Pharmacol. Sci. 2016;2016:3046373. doi: 10.1155/2016/3046373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Alidoost F., Gharagozloo M., Bagherpour B., Jafarian A., Sajjadi S.E., Hourfar H., Moayedi B. Effects of silymarin on the proliferation and glutathione levels of peripheral blood mononuclear cells from beta-thalassemia major patients. Int. Immunopharmacol. 2006;6:1305–1310. doi: 10.1016/j.intimp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 795.Biedermann D., Vavrikova E., Cvak L., Kren V. Chemistry of silybin. Nat. Prod. Rep. 2014;31:1138–1157. doi: 10.1039/c3np70122k. [DOI] [PubMed] [Google Scholar]
- 796.Surai P.F. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Pliskova M., Vondracek J., Kren V., Gazak R., Sedmera P., Walterova D., Psotova J., Simanek V., Machala M. Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology. 2005;215:80–89. doi: 10.1016/j.tox.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 798.Zheng N., Zhang P., Huang H., Liu W., Hayashi T., Zang L., Zhang Y., Liu L., Xia M., Tashiro S., Onodera S., Ikejima T. ERalpha down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells. J. Pharmacol. Sci. 2015;128:97–107. doi: 10.1016/j.jphs.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 799.Sadava D., Kane S.E. Silibinin reverses drug resistance in human small-cell lung carcinoma cells. Cancer Lett. 2013;339:102–106. doi: 10.1016/j.canlet.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Agarwal R., Agarwal C., Ichikawa H., Singh R.P., Aggarwal B.B. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 801.Hager H. [Problems in the treatment of ocular circulatory disturbances (author's transl)] Klin. Monbl Augenheilkd. 1974;165:127–136. [PubMed] [Google Scholar]
- 802.Garcia J., Carvalho A.T., Dourado D.F., Baptista P., de Lourdes Bastos M., Carvalho F. New in silico insights into the inhibition of RNAP II by alpha-amanitin and the protective effect mediated by effective antidotes. J. Mol. Graph. Model. 2014;51:120–127. doi: 10.1016/j.jmgm.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 803.Senkiv J., Finiuk N., Kaminskyy D., Havrylyuk D., Wojtyra M., Kril I., Gzella A., Stoika R., Lesyk R. 5-Ene-4-thiazolidinones induce apoptosis in mammalian leukemia cells. Eur. J. Med Chem. 2016;117:33–46. doi: 10.1016/j.ejmech.2016.03.089. [DOI] [PubMed] [Google Scholar]
- 804.Yelisyeyeva O.P., Semen K.O., Ostrovska G.V., Kaminskyy D.V., Sirota T.V., Zarkovic N., Mazur D., Lutsyk O.D., Rybalchenko K., Bast A. The effect of Amaranth oil on monolayers of artificial lipids and hepatocyte plasma membranes with adrenalin-induced stress. Food Chem. 2014;147:152–159. doi: 10.1016/j.foodchem.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 805.Bast A., Haenen G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013;34:430–436. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 806.Hirano K., Chen W.S., Chueng A.L., Dunne A.A., Seredenina T., Filippova A., Ramachandran S., Bridges A., Chaudry L., Pettman G., Allan C., Duncan S., Lee K.C., Lim J., Ma M.T., Ong A.B., Ye N.Y., Nasir S., Mulyanidewi S., Aw C.C., Oon P.P., Liao S., Li D., Johns D.G., Miller N.D., Davies C.H., Browne E.R., Matsuoka Y., Chen D.W., Jaquet V., Rutter A.R. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid. Redox Signal. 2015;23:358–374. doi: 10.1089/ars.2014.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Maghzal G.J., Krause K.H., Stocker R., Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic. Biol. Med. 2012;53:1903–1918. doi: 10.1016/j.freeradbiomed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 808.Kalyanaraman B., Dranka B.P., Hardy M., Michalski R., Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes--the ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta. 2014;1840:739–744. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Seredenina T., Nayernia Z., Sorce S., Maghzal G.J., Filippova A., Ling S.C., Basset O., Plastre O., Daali Y., Rushing E.J., Giordana M.T., Cleveland D.W., Aguzzi A., Stocker R., Krause K.H., Jaquet V. Evaluation of NADPH oxidases as drug targets in a mouse model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2016;97:95–108. doi: 10.1016/j.freeradbiomed.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 810.Talib J., Maghzal G.J., Cheng D., Stocker R. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic. Biol. Med. 2016;97:124–135. doi: 10.1016/j.freeradbiomed.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 811.Fujikawa Y., Roma L.P., Sobotta M.C., Rose A.J., Diaz M.B., Locatelli G., Breckwoldt M.O., Misgeld T., Kerschensteiner M., Herzig S., Muller-Decker K., Dick T.P. Mouse redox histology using genetically encoded probes. Sci. Signal. 2016;9:rs1. doi: 10.1126/scisignal.aad3895. [DOI] [PubMed] [Google Scholar]
- 812.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Agudo A., Cabrera L., Amiano P., Ardanaz E., Barricarte A., Berenguer T., Chirlaque M.D., Dorronsoro M., Jakszyn P., Larranaga N., Martinez C., Navarro C., Quiros J.R., Sanchez M.J., Tormo M.J., Gonzalez C.A. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European prospective investigation into cancer and nutrition (EPIC-Spain) Am. J. Clin. Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 814.Sies H., Berndt C., Jones D.P. Oxidative Stress. Ann. Rev. Biochem. 2017 doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]