Abstract
Background
Definitive radiation therapy (RT) (with or without cisplatin-based chemotherapy) is one of the most effective treatments for cervical squamous cell carcinoma (CSCC), but efficacy is limited due to resistance. In the present study, we investigated the relationship between the expression of Aurora kinase A (Aurora-A, AURKA)and response to RT in patients with CSCC.
Methods
The expression of Aurora-A in biopsy specimens of untreated primary tumors in 129 Uyghur patients with CSCC was investigated immunohistochemically. Primary treatment in these patients was definitive radical RT, which consisted of pelvic RT plus brachytherapy (total point A dose:70–85 Gy) (with or without cisplatin-based chemotherapy). The prognostic value of tumoral Aurora-A expression and patients’ clinical outcomes were evaluated.
Results
Aurora-A expression was significantly associated with lymph node metastasis (P<0.001), large tumor size (P<0.001), low hemoglobin (Hb) level (P=0.011) and recurrence (P<0.001), but not other clinicopathological factors. Definitive RT was unfavorable in patients with high Aurora-A expression (P < 0.001). In 129 enrolled patients, lymph node metastasis, large tumor size, low Hb level, and AURKA overexpression were prognostic factors for both recurrent free survival (RFS) and overall survival (OS) in univariate analysis. However, only high AURKA expression was an adverse independent risk factor for both RFS (hazard ratio, 3.953; 95% CI, 1.473-10.638; P = 0.006) and OS (hazard ratio 9.091; 95%CI 2.597-32.258; P<0.001) in multivariate analyses.
Conclusions
Aurora-A may serve as a predictive biomarker of radiation response and a therapeutic target to reverse radiation therapy resistance.
Keywords: Aurora kinase A, cervical squamous cell carcinoma, radiation, Uyghur, cell cycle
INTRODUCTION
Cervical cancer (CC) is the fourth most common malignant tumor in women worldwide, with 528,000 new cases in 2012 [1]. Approximately 87% of CC cases occur in developing countries. Furthermore, the morbidity rate due to CC in China is among the highest in the world [2]. In particular, Uyghur women who live in the southern region of Xinjiang Province, China, have the highest morbidity (590/100,000) [3] due to CC in the country. Squamous cell carcinoma (SCC) accounts for approximately 95% of all CCs [4]. Furthermore, CC tends to develop in Uyghur women at a younger age.
Concurrent chemoradiation therapy (CCRT) with cisplatin is generally the primary treatment of choice for stage IB2 to IVA disease based on the results of five randomized clinical trials [5, 6]. These five trials showed that CCRT resulted in a 30% to 50% decrease in the risk of death compared with radiation therapy (RT) alone [7–11]. Although RT can achieve a good outcome in patients with early-stage disease, treatment failure occurs in patients with advanced-stage disease. Studies have shown that the failure rate in patients with stage I–IVA CSCC after definitive RT is 29% [12]. In addition, although chemoradiation is tolerated, acute and long-term side effects have been reported [13]. For locally advanced CC, several criteria have been proposed to predict the risk of recurrence. These include age [14], adenocarcinoma [14], stage [14], tumor size [15, 16], and pretreatment anemia [16]. However, even in patients with similar sized tumors, the same stage of CSCC and receiving the same dose of radiotherapy, treatment response can be different. Thus, there is an urgent need to identify new biomarkers and/or therapeutic targets that can be used to treat these patients.
Aurora-A, an important member of the Aurora kinase family, is mainly located in the central body at prophase, near the pole spindle at the medium-term, and located at pole microtubules at anaphase and telophase [17]. Aurora-A regulates the functions of centrosomes, spindles and kinetochores required for correct mitotic progression. Aurora-A has been observed to positively regulate the G2 to M phase of the cell cycle, and activation of Aurora-A in late G2 is inhibited by DNA damage [18]. The cell cycle significantly influences radiosensitivity. In the present study, we determined the expression of Aurora-A in Uyghur CSCC patients treated with definitive radical RT and determined its correlation with clinical characteristics. We also investigated the relationship between the expression of Aurora-A and the response to RT or CCRT in patients with CSCC. In addition, we identified the expression of Aurora-A and its correlation with prognosis in CSCC.
RESULTS
Aurora-A staining and its association with clinicopathological characteristics
AURKA expression was analyzed by immuno-histochemical (IHC) staining on tissues. Aurora-A expression was mainly found in the cytoplasm of tumor cells (Figure 1), which was similar to a previous report [19]. High expression of Aurora-A was detected in 73 out of 129 (56.6%) selected CSCC tissues and low in the remaining 56 (43.4%) tissue specimens. Basic clinicopathological characteristics of the 129 patients are shown in Table 1. Fifty-eight patients (44.96%) experienced recurrence, including 14 locoregional relapses, 29 distant metastases, and 15 multiple site recurrences. Approximately 50% of patients had lymph node metastasis and more than half had large tumors (≥5.7 cm). Approximately 30% of these patients had pretreatment anemia. AURKA expression was significantly correlated with lymph node metastasis (P<0.001), large tumor size (P<0.001), low Hb level (P=0.038), and recurrence (P<0.001).
Figure 1. Expression of AURKA in CSCC.
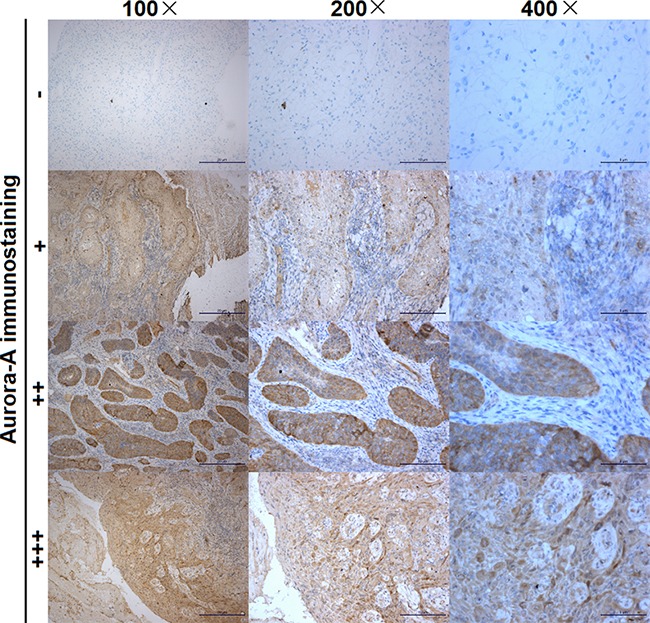
The intensity of the dye color was graded as 0 (no color), 1 (light yellow), 2 (light brown) or 3 (brown), and the number of positive cells was graded as 0 (no staining), 1 (<30%), 2 (30%-60%) and 3 (>60%); the two grades were added together and the specimens were assigned to one of four levels as follows: a score of 0-1 (-), a score of 2 (+), a score of 3-4 (++), a score of more than 5 (+++). Immunostaining was considered to be low (-, +) or high (++, +++).
Table 1. Clinicopathological characteristics of 129 CSCC patients and their association with Aurora kinase A (AURKA) IHC intensity.
| Clinicopathological features | NO | AURKA IHC | χ2 | P | |
|---|---|---|---|---|---|
| High(++,+++) | Low(-,+) | ||||
| ECOG performance status | |||||
| (0,1) | 87 | 49 | 38 | 0.010 | 0.919 |
| (2,3) | 42 | 24 | 18 | ||
| Age (y) | |||||
| <50 | 55 | 29 | 26 | 0.340 | 0.560 |
| ≥50 | 74 | 44 | 30 | ||
| Stage | |||||
| II | 61 | 37 | 24 | 0.497 | 0.481 |
| III | 68 | 36 | 32 | ||
| Lymph nodes metastases | |||||
| No | 70 | 28 | 42 | 15.701 | 0.000 |
| Yes | 59 | 45 | 14 | ||
| Differentiation | |||||
| Well-moderate | 62 | 39 | 23 | 1.474 | 0.225 |
| Poor | 67 | 34 | 33 | ||
| Tumor size | |||||
| ≥5.7cm | 83 | 64 | 19 | 39.894 | 0.000 |
| <5.7cm | 46 | 9 | 37 | ||
| SCC-ag level (ng/ml) | |||||
| <2 | 48 | 24 | 24 | 1.352 | 0.509 |
| 2-10 | 53 | 32 | 21 | ||
| <10 | 28 | 17 | 11 | ||
| Hb level (g/dl) | |||||
| <10 | 35 | 25 | 10 | 4.306 | 0.038 |
| ≥10 | 94 | 48 | 46 | ||
| Treatment | |||||
| Radiation | 55 | 34 | 21 | 0.728 | 0.393 |
| Concurrent Chemoradiation | 74 | 39 | 35 | ||
| Recurrence events | |||||
| Yes | 58 | 49 | 9 | 31.345 | 0.000 |
| No | 71 | 24 | 47 | ||
Relationships between Aurora-A expression and response to RT
The percentage of patients with a complete response (CR), partial response (PR)/stable disease (SD) or progressive disease (PD) following RT was: 61.24% (79 out of 129), 33.33% (43 out of 129) and 5.43% (7 out of 129), respectively. Analysis of the relationship between the expression of Aurora-A and clinical response to RT indicated that RT was more favorable in patients who had low-Aurora-A expression in tumors (P< 0.001) (Table 2).
Table 2. correlation of aurora-A expression with clinical response to RT.
| AURKA IHC | Clinical response to RT (n=129) | total | χ2 | P | ||
|---|---|---|---|---|---|---|
| CR | PR+SD | PD | ||||
| high | 33 | 34 | 6 | 73 | 18.324 | P<0.001 |
| low | 46 | 9 | 1 | 56 | ||
Relationship between Aurora-A expression and RFS or OS
The 5-year RFS and OS were 23.26% and 27.91%, respectively.
Univariate analysis showed that the RFS in all 129 cases was significantly influenced by lymph node metastasis (P<0.001), large tumor size (P<0.001), low Hb level (P=0.011), and AURKA expression level (P<0.001) (Table 3). The Kaplan-Meier RFS curves according to AURKA expression are shown in Figure 2. However, only AURKA overexpression (hazard ratio, 3.953; 95% CI, 1.473-10.638; P = 0.006) was identified as an independent unfavorable prognostic factor for RFS in multivariate analysis (Table 3). Univariate analysis showed that the OS of all 129 cases was significantly influenced by lymph node metastasis (P<0.001), large tumor size (P<0.001), low Hb level (P=0.004), and AURKA expression (P<0.001) (Table 4). The Kaplan-Meier OS curves according to AURKA expression level are shown in Figure 2. However, only AURKA overexpression (hazard ratio 9.091; 95%CI 2.597-32.258; P<0.001) was identified as an independent unfavorable prognostic factor for OS in multivariate analysis (Table 4).
Table 3. Univariate and multivariate analyses of the prognostic influence of clinicopathological factors on recurrence-free survival.
| Clinicopathological features | median RFS (month) | Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RFS (%) | χ2 | P | HR | 95.0% CI for HR | P | |||||
| 1year | 3year | 5year | low | high | ||||||
| ECOG performance status | ||||||||||
| (0,1) | 72.00 | 81.61 | 58.62 | 19.54 | 0.637 | 0.627 | ||||
| (2,3) | 45.72 | 95.24 | 50.00 | 30.95 | ||||||
| Age (y) | ||||||||||
| <50 | 72.00 | 83.63 | 63.64 | 27.27 | 3.715 | 0.054 | ||||
| ≥50 | 44.19 | 87.84 | 50.00 | 20.27 | ||||||
| Stage | ||||||||||
| II | 72.00 | 86.89 | 62.30 | 22.95 | 0.532 | 0.466 | ||||
| III | 72.00 | 85.29 | 50.00 | 23.53 | ||||||
| Lymph nodes metastases | ||||||||||
| No | 72.00 | 92.85 | 77.14 | 40.00 | 31.566 | 0.000 | 0.658 | 0.316 | 1.368 | 0.262 |
| Yes | 22.31 | 77.97 | 30.51 | 3.39 | ||||||
| Differentiation | ||||||||||
| Well-moderate | 72.00 | 90.32 | 50.00 | 20.97 | 0.002 | 0.961 | ||||
| Poor | 72.00 | 82.09 | 61.19 | 25.37 | ||||||
| Tumor size | ||||||||||
| ≥5.7cm | 32.42 | 80.72 | 39.76 | 13.25 | 16.056 | 0.000 | 1.508 | 0.72 | 3.165 | 0.276 |
| <5.7cm | 72.00 | 95.65 | 84.78 | 41.30 | ||||||
| SCC-ag level (ng/ml) | ||||||||||
| <2 | 54.41 | 89.58 | 52.08 | 25.00 | 0.365 | 0.833 | ||||
| 2-10 | 72.00 | 79.25 | 60.38 | 22.64 | ||||||
| >10 | 72.00 | 92.86 | 53.57 | 21.43 | ||||||
| Hb level (g/dl) | ||||||||||
| <10 | 23.25 | 80.00 | 45.71 | 20.00 | 4.283 | 0.038 | 0.879 | 0.504 | 1.531 | 0.647 |
| ≥10 | 72.00 | 88.30 | 59.57 | 24.47 | ||||||
| Treatment | ||||||||||
| Radiation | 52.78 | 90.91 | 54.55 | 29.09 | 0.307 | 0.580 | ||||
| Concurrent Chemoradiation | 72.00 | 82.43 | 56.76 | 18.92 | ||||||
| Aurora-A IHC | ||||||||||
| High | 27.60 | 80.82 | 34.25 | 4.11 | 35.878 | 0.000 | 3.953 | 1.473 | 10.638 | 0.006 |
| Low | 72.00 | 92.86 | 83.93 | 48.21 | ||||||
Figure 2. Recurrence-free survival (RFS) and overall survival (OS) curves, and the association between AURKA expression and survival.
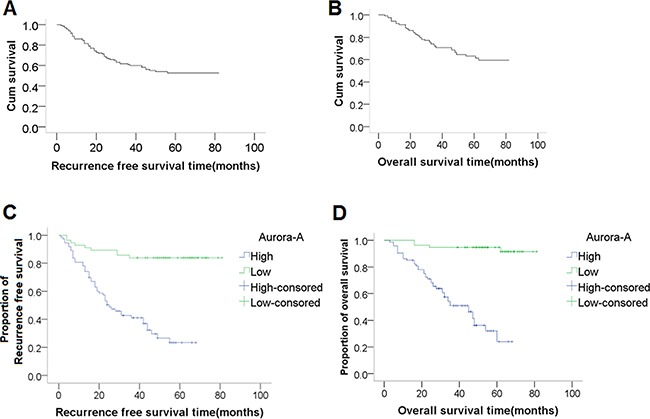
RFS curve (A) and OS curve (B), respectively, and the 5-year RFS and OS were 23.26% and 27.91%, respectively. (C-D) Kaplan-Meier plots of (C) RFS and (D) OS in 129 patients with CSCC according to AURKA expression. The P values for survival comparison, which were obtained by log-rank test, were all less than 0.05.
Table 4. Univariate and multivariate analyses of the prognostic influence of clinicopathological factors on overall survival.
| Clinicopathological features | median OS (month) | Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OS (%) | χ2 | P | HR | 95.0% CI for HR | P | |||||
| 1year | 3year | 5year | low | high | ||||||
| ECOG performance status | ||||||||||
| (0,1) | 72.00 | 90.80 | 65.52 | 24.14 | 0.019 | 0.891 | ||||
| (2,3) | 72.00 | 95.24 | 64.28 | 35.71 | ||||||
| Age (y) | ||||||||||
| <50 | 72.00 | 94.55 | 69.09 | 29.09 | 2.375 | 0.123 | ||||
| ≥50 | 70.89 | 90.54 | 62.16 | 27.03 | ||||||
| Stage | ||||||||||
| II | 72.00 | 96.72 | 68.85 | 26.23 | 3.210 | 0.073 | ||||
| III | 72.00 | 88.24 | 61.76 | 29.41 | ||||||
| Lymph nodes metastases | ||||||||||
| No | 72.00 | 100.00 | 85.71 | 48.57 | 31.257 | 0.000 | 0.869 | 0.411 | 1.835 | 0.712 |
| Yes | 32.77 | 84.75 | 40.68 | 3.39 | ||||||
| Differentiation | ||||||||||
| Well-moderate | 72.00 | 93.55 | 58.06 | 25.81 | 0.028 | 0.867 | ||||
| Poor | 72.00 | 91.04 | 71.64 | 29.85 | ||||||
| Tumor size | ||||||||||
| ≥5.7cm | 53.24 | 87.95 | 48.19 | 16.87 | 23.076 | 0.000 | 2.646 | 0.987 | 7.092 | 0.053 |
| <5.7cm | 72.00 | 100.00 | 95.65 | 47.83 | ||||||
| SCC-ag level (ng/ml) | ||||||||||
| <2 | 70.99 | 97.92 | 62.50 | 31.25 | 1.101 | 0.577 | ||||
| 2-10 | 72.00 | 84.91 | 66.04 | 24.53 | ||||||
| >10 | 72.00 | 96.43 | 67.86 | 28.57 | ||||||
| Hb level (g/dl) | ||||||||||
| <10 | 72.00 | 91.49 | 71.28 | 29.79 | 6.466 | 0.004 | 0.704 | 0.389 | 1.276 | 0.247 |
| ≥10 | 48.95 | 94.29 | 48.57 | 22.86 | ||||||
| Treatment | ||||||||||
| Radiation | 72.00 | 90.54 | 62.16 | 22.97 | 0.009 | 0.925 | ||||
| Concurrent Chemoradiation | 72.00 | 94.55 | 69.09 | 34.55 | ||||||
| AURKA IHC | ||||||||||
| High | 38.44 | 86.30 | 42.47 | 5.48 | 44.402 | 0.000 | 9.091 | 2.597 | 32.258 | 0.001 |
| Low | 72.00 | 100.00 | 94.64 | 57.14 | ||||||
DISCUSSION
Aurora-A plays a key role in the regulation of cell cycle progression and those relating to cell cycle and mitosis control are associated with worse clinical outcomes in early-stage ovarian cancer [20]. Aurora-A protein is overexpressed in many tumors and this overexpression is associated with unfavorable prognosis and low survival [21–24]. At present, definitive radical radiotherapy is the main treatment for patients with locally advanced CC. Considering radiation response is one of the most important factors in predicting the prognosis of these patients, it is very important to identify predictive biomarkers in clinical practice. To date, the expression of Aurora-A and its prognostic significance in CC has been poorly investigated. In 2008, a study [25] showed that Aurora A expression was significantly increased in carcinoma and cervical intraepithelial neoplasia 3, compared with normal cervix, and this overexpression was a relatively early phenomenon in the genesis of malignant epithelial tumorigenesis. In 2009, another study [26] showed that the expression of Aurora-A mRNA and protein was significantly higher in cervical carcinoma cells than in normal cervical epithelial cells. Patients with high Aurora-A expression had poorer disease-free survival and OS rates than patients with low Aurora-A expression. However, there are no data on its potential role in predicting CC radiotherapy response.
In this study, we determined tumoral Aurora-A expression using 129 tissue specimens from CSCC patients treated with definitive radical RT. High Aurora-A expression was found in 56.57% (73/129) of patients, this percentage is very similar to that previously reported by Zhang et al. who showed that 51.3% (38/74) of CC tissues examined demonstrated mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR) [26]. Our results showed that increased expression of Aurora-A was significantly associated with aggressive tumor variables, including lymph node metastasis, large tumor size, low Hb level and disease recurrence. These findings are consistent with those in previous reports, as one study found that Aurora-A was overexpressed in laryngeal squamous cell carcinoma and was associated with advanced tumor stage [27]. Another report [28] showed that Aurora-A mRNA and protein up-regulation was significantly associated with tumor stage and the occurrence of regional lymph node metastasis, as well as distant metastasis.
In multivariate analysis, high Aurora-A expression was an independent adverse risk factor for both RFS and OS in CSCC patients treated with definitive radical RT. In addition, our study suggested that patients with high Aurora-A expression may benefit less from RT treatment, as it was associated with poorer treatment response and shorter RFS and OS. Thus, we believe Aurora-A is a potential biomarker for predicting unfavorable radiation response and prognosis in CSCC patients.
Consistent with our findings, a recent randomized controlled trial, semiquantitatively evaluated Aurora-A expression in 144 cases with locally advanced naso-pharyngeal carcinoma (NPC) by immunohistochemistry staining. Of these patients, 69 received neoadjuvant chemotherapy plus CCRT, and 75 cases were treated with neoadjuvant chemotherapy plus RT. It was found that Aurora-A was highly expressed in NPC, but was deficient in normal adjacent epithelia; Aurora-A overexpression predicted a shorter 5-year OS, progression-free survival, and distant metastasis-free survival, and multivariate regression analysis confirmed that Aurora-A was an independent prognostic factor for death, recurrence, and distant metastasis; these results confirmed that Aurora-A was an independent prognostic factor for NPC patients who underwent RT [29]. Another report showed that Aurora-A overexpression was an independent prognostic factor for LSCC and was responsible for the relative tumor resistance to radiation therapy [27]. However, the results of another study [30] were in contrast to those of our study and showed that patients with Aurora-A overexpression had better clinical and histological response to CCRT. In some in vitro studies, inhibition of Aurora-A potently inhibited proliferation of atypical teratoid/rhabdoid tumor cells [31], glioblastoma neurosphere tumor cells [32], canine mast cell tumor cells [33] and sensitized these cells to radiation. In both in vitro and in vivo models of human cancers, including hepatocellular carcinoma[34], androgen-insensitive prostate cancer [35], oral squamous cell carcinoma [36] and lung cancer [37], some novel small molecule Aurora-A inhibitors showed radiation sensitization. However, to date, it has not been confirmed that Aurora-A overexpression results in a better response to RT in both in vivo and in vitro tumor models.
As for the reasons, we consulted a lot of related literature. Some reports have described a correlation between a better RT effect and mitotic catastrophe, which was caused by dysfunction of G2/M checkpoint regulation [38]. It is also known that Aurora-A is a key regulator of cell-cycle events from late S phase through to M phase [39], and a 2- to 6-fold increase in G2/M phase in Aurora A inhibitor-treated cells was reported compared with untreated control cells [40], whereas, the G2/M phase was most effective in radiotherapy. In addition, Aurora A inhibitors induced mitotic entry delay [41], prolonged mitotic duration [41, 42], induced mitotic spindle disassembly defects [41, 43], and cytokinesis defects [44, 45], leading to multiple centrosomes [41], and polyploid formation [23–25, 36, 41–49]. One report showed that long-term G2-arrested cells undergo senescence via G2 slippage and this cellular process of G2 slippage is the mechanism responsible for senescence of cells under long-term G2 arrest [50]. Other studies have shown that Aurora-A through a non-cell cycle-dependent method causes radiotherapy sensitization. One study [51] showed that Aurora-A enhanced the binding of NF-kappaB to DNA, thereby increasing the gene transcription by NF-kappaB and decreasing the radiosensitivity of the cells. Another study [52] showed that Aurora-A and BRCA1/2 inversely controlled the sensitivity of cancer cells to radiotherapy through the ATM/Chk2-mediated DNA repair networks.
This study demonstrated the importance of lymph node metastasis, large tumor size, and low Hb level as prognostic factors in patients with CSCC who underwent primary RT. These findings are consistent with previous reports [14, 15, 53]. However, tumor stage and treatment method failed to influence prognosis. The RTOG90-01 study [54] showed that there was no significant difference in 5-year OS and disease-free survival rates between stage III and IVA patients treated with RT compared with CCRT, similar to our results.
In the present study, we noted that the cure rate in patients with advanced CSCC who underwent definitive RT (with or without cisplatin-based chemotherapy) was not ideal as the 5-year survival rate was not high at just 27.91%. This was much lower than that reported in other studies [12, 16] at the same stage, which was approximately 50%–60%. We consider that this may have been due to the following factors: the proportion of advanced cancer patients was relatively large [stage IIB and above 92.86% (91/98) vs 46.71% (412/882)] [12], tumor volume was relatively large, and there may be a difference in Uygur patients with regard to genotype. The recurrence rate was as high as 44.96%, this percentage was much higher than that in the aforementioned studies [12, 16], which was approximately 30%. This may be due to the reasons outlined previously.
This study has several limitations. First, the relatively low number of patients and recurrences or deaths may have reduced the probability of identifying significant prognostic factors in multivariate analysis. Second, in this study, we only explored the association between survival and Aurora-A IHC staining score and did not evaluate Aurora-A expression using other techniques. Recently, Zhang et al [26] performed RT-PCR, western blot and IHC assays to determine the gene expression of Aurora-A and showed that patients with high Aurora-A expression had poorer RFS and OS rates than patients with low Aurora-A expression; multivariate analysis showed that high Aurora-A expression was an independent prognostic factor (risk ratio: 2.88; P=0.005). More importantly, both their study and our study showed that the Aurora-A expression level (either by RT-PCR or IHC) was an independent prognostic factor for OS.
In conclusion, although further experiments are needed to confirm these phenomena, these findings suggest that unfavorable responses to RT can be predicted based on Aurora-A overexpression in tumor cells of CSCC patients. Aurora-A overexpression was a significant prognostic factor for CSCC recurrence and was shown to be correlated with poor RFS and OS.
MATERIALS AND METHODS
Patients
Between January 2009 and December 2012, 129 Uyghur patients with CSCC in the People's Hospital of Xinjiang Uygur Autonomous Region were included in this study. All patients followed the principle of National Comprehensive Cancer Network definitive radical radiotherapy: radiotherapy (EBRT) combined with brachytherapy (ICRT), the prescription dose of EBRT (6 MV X-rays) was approximately 45 Gy (40–50 Gy), the prescription dose of ICRT (252Cf neutron) was approximately 40 Gy (30–40 Gy), for a total point A dose of approximately 80 Gy (70–85 Gy). Only Uyghur patients with histologically confirmed CSCC were eligible. Initially, 174 Uyghur women met the inclusion criteria, but 41 patients were excluded due to insufficient paraffin-embedded tissue and 4 were excluded due to death from a non-tumor disorder. The median age was 51.0 years (range, 33–73 years). The general physical status score was assessed according to the Eastern Cooperative Oncology Group performance status (ECOG). Classification of disease stage according to the International Federation of Gynecology and Obstetrics (FIGO) (2009) was as follows: 13 patients had stage IIA1, 5 patients had IIA2, 43 patients had IIB, 4 patients had IIIA, and 64 patients had IIIB. 74 patients were treated with CCRT (weekly cisplatin).
Ethics and informed consent
The study protocol for the collection of tumor samples and clinical information was approved by the institutional review board, and patients provided written informed consent authorizing the collection and use of their tumor samples for research purposes. This retrospective study was carried out according to the principles set out in the Declaration of Helsinki 1964 and all subsequent revisions and was approved by the Institutional Review Board of our hospital.
Follow up
All patients had follow-up records for over 3 years. After completion of therapy, patients were observed at 3-month intervals during the first 3 years and at 6-month intervals thereafter. OS was defined as the time from diagnosis to the date of death or when censored at the latest date if patients were still alive. RFS was defined as the length of time after diagnosis without signs or symptoms of CSCC or death. The median follow-up period was 47.0 months (range, 3–81 months). Tumor size was measured in at least five target lesions; the sum of the largest dimension was used as an initial size measurement as well as an indicator of response, as recommended by Response Evaluation Criteria In Solid Tumors criteria (RECIST) [55]. CR was defined as the complete disappearance of all measurable lesions for one month after completion of treatment. PR was defined as more than a 30% reduction in measurable lesions. PD was defined as more than a 20% increase in measurable lesions or the appearance of one or more new lesions. SD was defined as neither sufficient lesion shrinkage for PR, nor a sufficient increase for PD. Pelvic and para-aortic lymph node enlargement was defined as enlargement over a short-axis diameter of 1 cm assessed by pretreatment computed tomography or magnetic resonance imaging.
IHC analysis and evaluation
A total of 129 formalin-fixed and paraffin-embedded tumor blocks from biopsies (collected before treatment) were obtained from the Department of Pathology, in our Hospital. To determine the expression of Aurora-A, a 4-μm section of each tumor specimen was subjected to IHC analysis. The slides were deparaffinized, rehydrated, and treated with 3% H2O2 in methanol for 15 min to inhibit endogenous peroxidase. Pretreatment was carried out in a pressure-cooker with Tris/EDTA buffer solution (pH 9.0). Following transfer to a humidified chamber, the slides were blocked with 10% normal goat serum at room temperature for 30 min and incubated with rabbit anti-Aurora-A polyclonal antibody, 1:500 (ab1287, Abcam®, Cambridge, UK) overnight at 4°C (the positive control sample was a colonic mucosal section known to express Aurora-A). In the negative controls, primary antibodies were omitted and were then incubated for 30 min at 37°C with a ready to use two-step assay kit (PV-6001, ZSGB-Bio®, BeiJing, China), followed by a DAB IHC detection kit (ZAI9017, ZSGB-Bio®, BeiJing, China) according to the manufacturer's instructions. Finally, the samples were counterstained with hematoxylin, dehydrated, and mounted. The specificity of staining was tested by selective substitution of the primary antibody by nonimmunogenic serum, and was confirmed by western blot.
Each section was rated according to the scale of intensity of staining score in addition to the area of staining. At least 10 high-power fields were randomly chosen, and >1,000 cells were counted in each section. Two independent pathologists, blinded to the follow-up data, evaluated IHC staining. A third pathologist arbitrated when discrepancies arose between these two pathologists. The intensity of the dye color was graded as 0 (no color), 1 (light yellow), 2 (light brown) or 3 (brown), and the area of staining was evaluated as follows: 0, no staining of cells in any of the microscopic fields; 1+, <30% of tissue stained positive; 2+, between 30% and 60% stained positive; 3+, >60% stained positive [38]. The two grades were added together and specimens were assigned to one of four levels as follows: a score of 0–1 (-), a score of 2 (+), a score of 3–4 (++), and a score more than 5 (+++). Immunostaining was considered to be low (-, +), or high (++, +++) (Supplementary Figure 1).
Statistical and survival analysis
Receiver operating characteristic (ROC) curves were used to determine the best cutoff points for pretreatment Hb level, and tumor size for predicting disease recurrence. Statistical analysis of group differences was performed using the χ2 test or Fisher's exact test. Survival was estimated using the Kaplan–Meier method and the log-rank test was used to compare the survival curves. Univariate and multivariate (step-wise forward conditional method) Cox regression analyses were carried out to determine the prognostic significance of clinicopathological factors and AURKA expression. P <0.05 was regarded as statistically significant in two-sided tests. SPSS software (version 19.00, SPSS, Chicago, IL, USA) was used for all statistical analyses.
Acknowledgments
We thank the Clinical Laboratory Center of Xinjiang Uygur Autonomous Region People's Hospital to provide experimental platform.
Footnotes
CONFLICTS OF INTEREST
The authors declared no potential conflict of interest with respect to the authorship and/or publication of this article.
GRANT SUPPORT
This work was supported in part by grants from the Natural Science Foundation of China Xinjiang Uygur Autonomous Region (No. 2015211C211, Yuhua Ma) and the International Cooperation Projects of Ministry of Science and Technology (No. 2012DFA31560,Ruozheng Wang).
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Tusong A, Zhou J, Mairuimu N, BiJia N, Ruxian G, Shala M, Xu X, Hair N, Aimu R. Investigation of cervical cancer in Cele county of Xinjiang, China [Article in Chinese] Maternal and Child Health Care of China. 2006;21:524–26. [Google Scholar]
- 4.Lalai S, Peng Y, Zhou K, Fang X, Wang L. The analysis of pathogenetic tendency of cervical cancer in various ethnic women in Xinjiang [Article in Chinese] Journal of XinJiang Medical University. 2006. pp. 569–571.
- 5.Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, Rao GG, Small W, Jr, Varia MA, Wolfson AH, Yashar CM, Yuh W, Cardenes HR. ACR Appropriateness criteria® on advanced cervical cancer expert panel on radiation oncology-gynecology. Int J Radiat Oncol Biol Phys. 2011;81:609–14. doi: 10.1016/j.ijrobp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007(25):2952–65. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 8.Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W, Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, Clarke-Pearson DL, Liao SY. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a gynecologic oncology group and southwest oncology group study. J Clin Oncol. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 10.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GM. Improved treatment for cervical cancer--concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–200. doi: 10.1056/NEJM199904153401509. [DOI] [PubMed] [Google Scholar]
- 12.Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, Lee SP, Hsueh S. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:249–57. doi: 10.1016/j.ijrobp.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 13.Takekuma M, Kasamatsu Y, Kado N, Kuji S, Tanaka A, Takahashi N, Abe M, Hirashima Y. Reconsideration of postoperative concurrent chemoradiotherapy with fluorouracil and cisplatin for uterinecervical cancer. J Obstet Gynaecol Res. 2015;41:1638–43. doi: 10.1111/jog.12754. [DOI] [PubMed] [Google Scholar]
- 14.Wong FC, Tung SY, Leung TW, Sze WK, Wong VY, Lui CM, Yuen KK, SK O. Treatment results of high-dose-rate remote after loading brachytherapy for cervical cancer and retrospective comparison of two regimens. Int J Radiat Oncol Biol Phys. 2003;55:1254–64. doi: 10.1016/s0360-3016(02)04525-x. [DOI] [PubMed] [Google Scholar]
- 15.Beriwal S, Kannan N, Sukumvanich P, Richard SD, Kelley JL, Edwards RP, Olawaiye A, Krivak TC. Complete metabolic response after definitive radiation therapy for cervical cancer: patterns and factors predicting for recurrence. Gynecol Oncol. 2012;127:303–6. doi: 10.1016/j.ygyno.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Kudaka W, Nagai Y, Toita T, Inamine M, Asato K, Nakamoto T, Wakayama A, Ooyama T, Tokura A, Murayama S, Aoki Y. Long-term results and prognostic factors in patients with stage III-IVA squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy from a single institution study. Int J Clin Oncol. 2013(18):916–21. doi: 10.1007/s10147-012-0457-x. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, et al. A homologue of drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998(17):3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 19.Cho MK, An JM, Kim CH, Kang SG. Elevated aurora kinase a protein expression in diabetic skin tissue. Arch Plast Surg. 2014;41:35–9. doi: 10.5999/aps.2014.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ocaña A, Pérez-Peña J, Alcaraz-Sanabria A, Sánchez-Corrales V, Nieto-Jiménez C, Templeton AJ, Seruga B, Pandiella A, In Amir E. silico analyses identify gene-sets, associated with clinical outcome in ovarian cancer: role of mitotic kinases. Oncotarget. 2016;7:22865–72. doi: 10.18632/oncotarget.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borges KS, Moreno DA, Martinelli CE, Jr, Antonini SR, de Castro M, Tucci S, Jr, Neder L, Ramalho LN, Seidinger AL, Cardinalli I, Mastellaro MJ, Yunes JA, Brandalise SR, et al. Spindle assembly checkpoint gene expression in childhood adrenocortical tumors (ACT): overexpression of Aurora kinases A and B is associated with a poor prognosis. Pediatr Blood Cancer. 2013(60):1809–16. doi: 10.1002/pbc.24653. [DOI] [PubMed] [Google Scholar]
- 22.Zeng B, Lei Y, Zhu H, Luo S, Zhuang M, Su C, Zou J, Yang L, Luo H. Aurora-A is a novel predictor of poor prognosis in patients with resected lung adenocarcinoma. Chin J Cancer Res. 2014;26:166–73. doi: 10.3978/j.issn.1000-9604.2014.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh CN, Yen CC, Chen YY, Cheng CT, Huang SC, Chang TW, Yao FY, Lin YC, Wen YS, Chiang KC, Chen JS, Yeh TS, Tzeng CH, et al. Identification of aurora kinase A as an unfavorable prognostic factor and potential treatment target for metastatic gastrointestinal stromal tumors. Oncotarget. 2014;5:4071–86. doi: 10.18632/oncotarget.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Yue CF, Zhou WH, Qian YM, Zhang Y, Wang SW, Liu AW, Liu Q. Aurora-A contributes to cisplatin resistance and lymphatic metastasis in non-small cell lung cancer and predicts poor prognosis. J Transl Med. 2014;12:200. doi: 10.1186/1479-5876-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twu NF, Yuan CC, Yen MS, Lai CR, Chao KC, Wang PH, Wu HH, Chen YJ. Expression of Aurora kinase A and B in normal and malignant cervical tissue: high Aurora A kinase expression in squamous cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2009;142:57–63. doi: 10.1016/j.ejogrb.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Wang J, Liu SJ, Hua W, Xin XY. Correlation between Aurora-A expression and the prognosis of cervical carcinoma patients. Acta Obstet Gynecol Scand. 2009(88):521–7. doi: 10.1080/00016340902835927. [DOI] [PubMed] [Google Scholar]
- 27.Guan Z, Wang XR, Zhu XF, Huang XF, Xu J, Wang LH, Wan XB, Long ZJ, Liu JN, Feng GK, Huang W, Zeng YX, Chen FJ, et al. Aurora-A, a negative prognostic marker, increases migration and decreases radiosensitivity in cancer cells. Cancer Res. 2007;67:10436–44. doi: 10.1158/0008-5472.CAN-07-1379. [DOI] [PubMed] [Google Scholar]
- 28.Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Aurora kinases in head and neck cancer. Lancet Oncol. 2013(14):e425–35. doi: 10.1016/S1470-2045(13)70128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan XB, Fan XJ, Huang PY, Dong D, Zhang Y, Chen MY, Xiang J, Xu J, Liu L, Zhou WH, Lv YC, Wu XY, Hong MH, et al. Aurora-A activation, correlated with hypoxia-inducible factor-1?, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci. 2012;103:1586–94. doi: 10.1111/j.1349-7006.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamotsu K, Okumura H, Uchikado Y, Kita Y, Sasaki K, Omoto I, Owaki T, Arigami T, Uenosono Y, Nakajo A, Kijima Y, Ishigami S, Natsugoe S. Correlation of Aurora-A expression with the effect of chemoradiation therapy on esophageal squamous cell carcinoma. BMC Cancer. 2015(15):323. doi: 10.1186/s12885-015-1329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkataraman S, Alimova I, Tello T, Harris PS, Knipstein JA, Donson AM, Foreman NK, Liu AK, Vibhakar R. Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012(107):517–26. doi: 10.1007/s11060-011-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown SL, Mikkelsen T, Lehman NL. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014(73):983–90. doi: 10.1007/s00280-014-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiomitsu K, Sajo E, Rubin C, Sehgal I. The radiosensitizing effect of the aurora kinase inhibitors, ENMD-2076, on canine mast cell tumours in vitro. Vet Comp Oncol. 2016;14:13–27. doi: 10.1111/vco.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin ZZ, Chou CH, Cheng AL, Liu WL, Chia-Hsien Cheng J. Radiosensitization by combining an aurora kinase inhibitor with radiotherapy in hepatocellular carcinoma through cell cycle interruption. Int J Cancer. 2014;135:492–501. doi: 10.1002/ijc.28682. [DOI] [PubMed] [Google Scholar]
- 35.Moretti L, Niermann K, Schleicher S, Giacalone NJ, Varki V, Kim KW, Kopsombut P, Jung DK, Lu B. MLN8054, a small molecule inhibitor of Aurora kinase A, sensitizes androgen-resistant prostate cancer toradiation. Int J Radiat Oncol Biol Phys. 2011;80:1189–97. doi: 10.1016/j.ijrobp.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Nakashiro K, Iwamoto K, Tokuzen N, Fujita Y, Shirakawa R, Oka R, Goda H, Hamakawa H. Targeting Aurora kinase A suppresses the growth of human oral squamous cell carcinoma cells in vitro and in vivo. Oral Oncol. 2013;49:551–9. doi: 10.1016/j.oraloncology.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Woo JK, Kang JH, Shin D, Park SH, Kang K, Nho CW, Seong JK, Lee SJ, Oh SH. Daurinol enhances the efficacy of radiotherapy in lung cancer via suppression of aurora kinase A/B expression. Mol Cancer Ther. 2015;14:1693–704. doi: 10.1158/1535-7163.MCT-14-0960. [DOI] [PubMed] [Google Scholar]
- 38.Okumura H, Natsugoe S, Matsumoto M, Yokomakura N, Uchikado Y, Takatori H, Ishigami S, Takao S, Aikou T. Predictive value of p53 and 14-3-3sigma for the effect of chemoradiation therapy on esophageal squamous cell carcinoma. J Surg Oncol. 2005;91:84–9. doi: 10.1002/jso.20279. [DOI] [PubMed] [Google Scholar]
- 39.Reiter R, Gais P, Jütting U, Steuer-Vogt MK, Pickhard A, Bink K, Rauser S, Lassmann S, Höfler H, Werner M, Walch A. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival inhead and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–41. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- 40.Görgün G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, Nahar S, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–13. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asteriti IA, Di Cesare E, De Mattia F, Hilsenstein V, Neumann B, Cundari E, Lavia P, Guarguaglini G. The Aurora-A inhibitor MLN8237 affects multiple mitotic processes and induces dose-dependent mitotic abnormalities and aneuploidy. Oncotarget. 2014;5:6229–6242. doi: 10.18632/oncotarget.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marxer M, Ma HT, Man WY, Poon RY. p53 deficiency enhances mitotic arrest and slippage induced by pharmacological inhibition of Aurora kinases. Oncogene. 2014;33:3550–3560. doi: 10.1038/onc.2013.325. [DOI] [PubMed] [Google Scholar]
- 43.Mannino M, Gomez-Roman N, Hochegger H, Chalmers AJ. Differential sensitivity of glioma stem cells to Aurora kinase A inhibitors: implications for stem cell mitosis and centrosome dynamics. Stem Cell Res. 2014;13:135–143. doi: 10.1016/j.scr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldini E, Tuccilli C, Prinzi N, Sorrenti S, Antonelli A, Fallahi P, Mian C, Barollo S, Catania A, Morrone S, Tartaglia F, Mascagni D, Coccaro C, et al. Selective inhibitors of aurora kinases inhibit proliferation, reduce cell viability and impair cell cycle progression in papillary thyroid carcinoma cells. J Biol Regul Homeost Agents. 2015;29:793–803. [PubMed] [Google Scholar]
- 45.Zullo KM, Guo Y, Cooke L, Jirau-Serrano X, Mangone M, Scotto L, Amengual JE, Mao Y, Nandakumar R, Cremers S, Duong J, Mahadevan D, O’Connor OA. Aurora A kinase inhibition selectively synergizes with histone deacetylase inhibitor through cytokinesis failure in T-cell lymphoma. Clin Cancer Res. 2015;21:4097–4109. doi: 10.1158/1078-0432.CCR-15-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldini E, Tuccilli C, Prinzi N, Sorrenti S, Antonelli A, Gnessi L, Morrone S, Moretti C, Bononi M, Arlot-Bonnemains Y, D’Armiento M, Ulisse S. Effects of selective inhibitors of Aurora kinases on anaplastic thyroid carcinoma cell lines. Endocr Relat Cancer. 2014;21:797–811. doi: 10.1530/ERC-14-0299. [DOI] [PubMed] [Google Scholar]
- 47.Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH, He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, Yang T, Chen XW, Qiu JX, et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther. 2015;9:1555–1584. doi: 10.2147/DDDT.S74197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X, Wang D, Yang T, Wang NJ, Zhao RJ, Zhou SF. Inhibition of mitotic Aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther. 2015;9:487–508. doi: 10.2147/DDDT.S74127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tentler JJ, Ionkina AA, Tan AC, Newton TP, Pitts TM, Glogowska MJ, Kabos P, Sartorius CA, Sullivan KD, Espinosa JM, Eckhardt SG, Diamond JR. P53 family members regulate phenotypic response to aurora kinase A inhibition in triple-negative breast cancer. Mol Cancer Ther. 2015;14:1117–1129. doi: 10.1158/1535-7163.MCT-14-0538-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye C, Zhang X, Wan J, Chang L, Hu W, Bing Z, Zhang S, Li J, He J, Wang J, Zhou G. Radiation-induced cellular senescence results from a slippage of long-term G2 arrested cells into G1 phase. Cell Cycle. 2013(12):1424–32. doi: 10.4161/cc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh ET, Byun MS, Lee H, Park MT, Jue DM, Lee CW, Lim BU, Park HJ. Aurora-a contributes to radioresistance by increasing NF-kappaB DNA binding. Radiat Res. 2010(174):265–73. doi: 10.1667/RR2017.1. [DOI] [PubMed] [Google Scholar]
- 52.Sun H, Wang Y, Wang Z, Meng J, Qi Z, Yang G. Aurora-A controls cancer cell radio- and chemoresistance via ATM/Chk2-mediated DNA repair networks. Biochim Biophys Acta. 2014(1843):934–44. doi: 10.1016/j.bbamcr.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Tseng JY, Yen MS, Twu NF, Lai CR, Horng HC, Tseng CC, Chao KC, Juang CM. Prognostic nomogram for overall survival in stage IIB-IVA cervical cancer patients treated with concurrentchemoradiotherapy. Am J Obstet Gynecol. 2010(202):174, e1–7. doi: 10.1016/j.ajog.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 54.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01.J. Clin Oncol. 2004(22):872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 55.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009(45):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]


