Abstract
Background:
Early diagnosis of autism spectrum disorder is critical, because early intensive treatment greatly improves its prognosis.
Methods:
We review studies that examined vocalizations of infants with autism spectrum disorder and mouse models of autism spectrum disorder as a potential means to identify autism spectrum disorder before the symptomatic elements of autism spectrum disorder emerge. We further discuss clinical implications and future research priorities in the field.
Results:
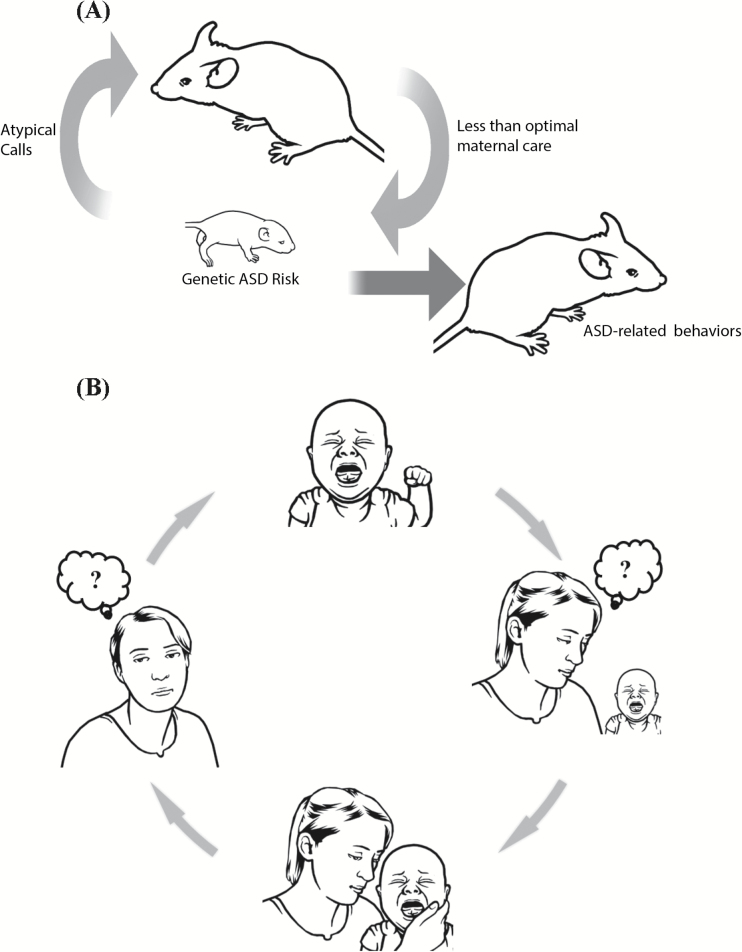
Atypical early vocal calls (i.e., cry) may represent an early biomarker for autism spectrum disorder (or at least for a subgroup of children with autism spectrum disorder), and thus can assist with early detection. Moreover, cry is likely more than an early biomarker of autism spectrum disorder; it is also an early causative factor in the development of the disorder. Specifically, atypical crying, as recently suggested, might induce a “self-generated environmental factor” that in turn, influences the prognosis of the disorder. Because atypical crying in autism spectrum disorder is difficult to understand, it may have a negative impact on the quality of care by the caregiver (see graphical abstract).
Conclusions:
Evidence supports the hypothesis that atypical vocalization is an early, functionally integral component of autism spectrum disorder.
Keywords: cry, autism spectrum disorder, early biomarkers, animal models of ASD, ultrasonic vocalizations
Significance Statement
A hypothetical causative chain of events involving atypical vocalizations in mice and humans. Striking similarities have emerged from human and mouse data indicating how genetic components may lead to the production of atypical distress vocalizations, which in turn jeopardizes effective neonatal social communication between infants and mothers. A) Atypical vocal call sequences of pups with an autism spectrum disorder (ASD) risk gene evoke less maternal approach in mothers in mice. The suboptimal maternal care acts as a self-generated environmental factor to exacerbate the phenotypic expression of ASD-related behaviors in the risk carriers, resulting in ASD-related behaviors later. B) In humans, atypical vocalization in infants with an ASD risk factor diminishes mothers’ ability to appropriately respond to babies’ specific needs because such cries are not well understood or are negatively perceived by mothers. This might lead to a vicious cycle of confusion and negative perception as to how to appropriately respond to baby’s atypical cries.
Introduction
Since Kanner’s description of autism (Kanner, 1943), impairment in social communication and social skills have been considered core symptoms of the disorder. Over the last decade, a large body of work has been produced to ontologically define the disorder, now defined as autism spectrum disorder (ASD) in the latest version of the Diagnostic and Statistical Manual of Mental Disorders, which especially highlights its heterogeneity. However, a clear understanding of the etiopathogenesis of ASD and its heterogeneity is still lacking.
There is an intense interest in identifying very early signs of ASD. Although it is possible to diagnose ASD by 2 years of age in humans, earlier identification of signs is critical, because early intervention is highly effective (Wallace and Rogers, 2010; Rogers et al., 2014; Wetherby et al., 2014; Green et al., 2015). Many anatomical, functional, and behavioral features predictive of later ASD diagnosis have been examined (Elsabbagh and Johnson, 2016; Varcin and Jeste, 2017). Brain imaging studies have identified alterations of brain structures and regional activities among infants in their first year of life who are later diagnosed with ASD (Varcin and Nelson, 2016; Varcin and Jeste, 2017). Moreover, atypical development of behavioral features serve as markers of later diagnosis of ASD in infants (Ozonoff et al., 2010; 2014; Zwaigenbaum et al., 2015), including a reduced attention to eyes (Jones and Klin, 2013; Moriuchi et al., 2017), poor postural control (Flanagan et al., 2012), early motor asymmetry (Teitelbaum et al., 1998; Esposito et al., 2009, 2011), increased perceptual sensitivity (Clifford et al., 2013), and atypical preverbal vocalizations (Esposito and Venuti, 2010b; Ozonoff et al., 2010; 2014).
Among these behavioral features, neonatal vocalization has been examined in mice as a potential proxy of one aspect of ASD. In mice, pup vocal calls are thought to be a form of social communication similar in function to the nonverbal calls of infants (Scattoni et al., 2009). When separated from dams, rodent pups emit vocal calls that the dams use to locate and retrieve the pups (Ehret and Bernecker, 1986; D’Amato et al., 2005; Uematsu et al., 2007; Wöhr et al., 2010). Atypical neonatal calls have been identified in a number of genetic mouse models of ASD, some of which show ASD-related behaviors (i.e., lack of reciprocal social interaction and repetitive behavioral traits) during adolescence and adulthood (Michetti et al., 2012; Lai et al., 2014; Nishi and Hiroi, 2016). More importantly, a recent observation indicates that pups with an ASD risk gene variant emit atypical vocal sequences, which in turn induce less than optimal maternal care, thereby establishing atypical vocal call sequences of a genetic ASD mouse model as a functionally integral phenotype of ASD-associated gene variants (Takahashi et al., 2016; Kikusui and Hiroi, 2017).
Here, we argue that atypical early cry may represent an early biomarker for a subgroup of children with ASD (Esposito, 2016) and, moreover, may also represent an early causative factor in the development of the disorder. Specifically, atypical crying likely induces a “self-generated environmental factor” in caregivers (Kikusui and Hiroi, 2017).
Cry as an Early Biomarker
Forcing air through the vocal tract and over the larynx produces cry. The process of controlling the air passing through the larynx is regulated, through the cranial nerves, by the brainstem and limbic system, functions of which are thought to be compromised in individuals with ASD (Amaral et al., 2008). Assessment of the spectrographic characteristics of crying can thus give investigators important information about function of those brain areas that are involved in the pathogenesis of ASD.
The cry sound can be described by a variety of parameters, including loudness (i.e., amplitude), the timing of onset, and inter-utterance intervals. Other aspects of cry can be represented acoustically by fundamental frequency (F0), voicing, and formant frequencies. F0 is the base frequency of a cry and is perceived as pitch. Voicing is a phonation that results from harmonic vibration of the vocal folds (Sheinkopf et al., 2012). Formant frequency is a cluster of sound waves at a particular frequency and is produced by resonance of the vocal tract. ASD is associated with atypical vocal quality (i.e., atypical pitch production and shorter duration) and different developmental trajectories (Esposito and Venuti, 2010a).
These vocal parameters may also help to identify incipient ASD infants, who are likely to be diagnosed in the future with ASD (Sheinkopf et al., 2000, 2012; Esposito et al., 2014a). Sheinkopf et al. (2012) investigated the acoustic properties of cries of 6-month-old infants at risk for autism and of a typically developing low-risk group. The at-risk group produced pain-related cries with higher pitch with greater variability than low-risk infants; those at-risk infants later diagnosed with ASD produced the widest frequency range and most poorly phonated cries. Thus, differences in cries may be an early manifestation of an atypical affective state, which may play a role in the development of poor social communication.
A subsequent study (Esposito et al., 2014a) examined the acoustic characteristics of cries extracted from the separation phase of the Strange Situation Procedure, a procedure devised to observe attachment relationships between a caregiver and a child, in child siblings of an older child with ASD defined as high-risk (HR, the incidence of ASD is higher than in the general population) and low-risk (LR) for ASD. Higher fundamental frequency (f0) and maximum f0 (highest pitch) of the first utterance of crying was found in the HR compared with the LR toddlers. HR toddlers had shorter duration of crying than LR toddlers. Finally, HR toddlers who were later diagnosed with ASD had the highest f0 and the shortest cry duration. Since the onset of crying is thought to be under direct neural control, these findings suggest poor central control of crying in HR toddlers.
As early as the first months of age, infants later diagnosed with autism show a different pattern of cry compared with those with other types of developmental delays and typically developing infants (Esposito and Venuti, 2010a). Specifically, episodes of cry in ASD showed higher fundamental frequencies, higher formant frequencies, and a decreased number of pauses.
Mouse Models
Over the last decade, researchers interested in the mechanisms of early distress vocalization in ASD have focused on mouse models. When separated from mother during the first few weeks of life, mouse and rat pups emit distress calls as ultrasonic vocalizations (USVs), ranging from 30 to >100 kHz (Scattoni et al., 2009). Mouse pups increase the number of ultrasonic vocal calls during the first week and decrease calls thereafter. This rodent period corresponds to a period up to 2 years old in humans (Semple et al., 2013).
A compromised ability to emit USVs during the neonatal period has been seen in various animal models of autism. This bears resemblance to observations made in infants at high risk for ASD (Leonard et al., 2013; Mody et al., 2016), as well as those diagnosed with ASD (Mody et al., 2016). An altered number of neonatal USVs has been reported for mouse models with various genetic variants (Michetti et al., 2012; Lai et al., 2014; Nishi and Hiroi, 2016). In addition, mouse models of ASD exhibit spectrographic deviations (Schmeisser et al., 2012; Michetti et al., 2012; Belagodu et al., 2016) and altered vocal repertoire (Scattoni et al., 2008; Young et al., 2010; Hiramoto et al., 2011; Ey et al. 2013; Hiroi et al., 2013; Romano et al., 2013; Burkett et al., 2015; Yang et al., 2015; Fraley et al., 2016).
One interpretative caution here is that the genetic background of mutant and wild-type littermates is not controlled (i.e., noncongenic mice) in some studies, and alleles surrounding the targeted gene are expected to systematically differ between mutant and their wild-type littermates. As different inbred mouse lines have distinct vocal patterns (Scattoni et al., 2008), any phenotypic difference in vocal calls between noncongenic mutant and wild-type littermates might not genuinely reflect the impact of the targeted ASD-linked gene mutation.
Crying As a Modulator of the Environment
Crying is evolutionarily shaped to elicit parental responses. Parental responses to infant cries are the results of cultural norms, caregiver characteristics, and characteristics of the infant cry (Gustafson and Green, 1989; Bakeman et al., 1990; Zeifman, 2003). Over the last several decades, many studies have focused on how the frequency and duration of crying episodes modulates adult responses. Cry pitch can influence caregivers’ perception: higher frequency cries are often perceived as more aversive and distressing than lower frequency cries.
Parents often report they had great difficulty decoding the emotional signals of their infants with ASD diagnosis, especially during the first year of the child’s life. They report, in particular, problems understanding the causes of crying episodes (Esposito and Venuti, 2008; Esposito and Venuti, 2010b; Bornstein et al., 2016). Such a lack of understanding regarding why their infants are crying can initiate a vicious cycle; the mother fails to recognize the infant’s needs, which in turn results in an inadequate feedback to the infant.
To understand the precise acoustic parameters that negatively influence perception of crying, some studies (Esposito et al., 2013, 2014b) deconstructed ratings of distress, as perceived by parents, in cries of infants with ASD. These studies identified that pause lengths (duration of inhalation), compared with the number of utterances or fundamental frequency, had the strongest impact on the perception of distress in 2 contrasting cultural groups. These findings underscore the importance of acoustic parameters other than the commonly researched fundamental frequency in analyzing caregiver perception of infant cry.
Episodes of crying in infants with ASD are interpreted by adults as high levels of distress in the infant and thus cause increased distress in adult listeners (Esposito et al., 2013). Overestimated levels of distress expressed in an episode of crying may jeopardize caregiver responsiveness, because accurate evaluation of infant’s vocalizations can be critical for appropriate bonding and for offspring well-being and survival (Seifritz et al., 2003).
The adult brain responds to the acoustic characteristics of infant cries. An fMRI-based study measured brain activity during adult processing of cries of infants with ASD and of matching control infants. For ASD infant cries, in addition to higher activations in the primary and secondary auditory cortex, higher levels of activation were observed in the inferior frontal gyrus (Venuti et al., 2012), a region known to play a critical role in processing emotional information and evaluating the affective salience of speech. This suggests that listening to the cries of infants with ASD calls for deeper and more effortful auditory attention and comprehension, and in particular comprehension of “emotional content,” which may be compromised in the cries of infants with ASD.
Consistent with the notion that ASD cries are more distressful, ASD cries elicited higher activations in the left inferior frontal gyrus/anterior insula, which are known to mediate brain responses to aversive and arousing stimuli. Additionally, activity in the supplementary motor cortex and precentral gyri emerged, suggesting preparation for care behaviors. ASD cries appear to activate adults’ intentions to reduce infant distress.
A more recent study (Esposito et al., 2015) assessed activation of the autonomic nervous system of fathers of typically developing children and non-fathers in response to typical and atypical (i.e., those of ASD) infant vocalizations. Galvanic Skin Response, cardiac dynamics via Inter-Beat Interval, and right hand temperature change were measured. Both groups showed greater negative responses (i.e., increased Galvanic Skin Response) while listening to cries of infants with ASD compared with typical cries and laughter. In contrast, fathers showed higher Inter-Beat Interval and greater temperature increases in right hand temperature change than non-fathers while listening to typical and atypical ASD cries. These findings point to similarities and differences in fathers’ and non-fathers’ physiological responsiveness to cries of infants with ASD and might guide specific intervention programs for parents of children at risk for ASD.
Expression of distress in ASD is not only evident in the vocal domain. Indeed, when female adults were made to judge the distress and typicality (expected normality) of isolated vocal, facial, and bodily cues of 18-month-old ASD developmentally delayed or typically developing infants, vocal cries of ASD and developmentally delayed infants were perceived as equally distressing, but cries were perceived as more atypical for infants who were later diagnosed with ASD as they had higher frequency cries (Esposito and Venuti, 2008). Facial cues of ASD infants were perceived as less typical and less distressed, consistent with the lack of emotional expressivity. ASD children’s bodily movements were perceived as more distressed and less typical compared with those of infants with other types of developmental delay or with typical development (Esposito et al., 2011).
All of these differences in distress cues may account for adults’ bias in interpreting their infant’s states of uneasiness. This bias may in turn jeopardize adequate parental responsiveness. Indeed, qualitatively different responses were shown in response to crying episodes. While mothers of typically developing infants or those with other types of developmental delays were more likely to use tactile or vestibular stimulation to soothe their infants, mothers of infants with ASD used more verbal soothing (Esposito and Venuti 2010c). A possible explanation of this is that when parents cannot understand the meaning of a crying episode, they use more verbal interactions.
Mouse Models
Pups’ distress vocalizations have some communicative value for mothers. Pups’ USVs are thought to be essential for the development of social bonds between the mother and young, making USVs vital for the pup’s survival (Zippelius and Schleidt, 1956). Pups’ calls alone are demonstrated to elicit maternal approach behaviors (Uematsu et al., 2007; Okabe et al. 2013). A more recent study (Takahashi et al., 2016) demonstrated that USVs of pups that carry heterozygous deletion of the gene Tbx1, an ASD risk gene encoded in the 22q11.2 region (Hiramoto et al., 2011; Hiroi et al., 2013) and itself a monogenic ASD risk gene (Gong, 2001; Paylor et al., 2006; Ogata et al., 2014), elicits less maternal approach compared with those of wild-type pups. The USVs of Tbx1 heterozygous pups were also characterized by less individually variable call sequences and less complex call types compared with those of wild-type littermate pups. Randomized sequences of calls of wild-type pups induced less maternal approach behaviors, indicating that mutation of this ASD risk gene alters the neonatal call sequence, which renders the pup’s social communication with mothers ineffective and maternal care less efficient (Takahashi et al., 2016; Kikusui and Hiroi, 2017).
Where Should We Head Next?
To date, human and mouse studies have shown that assessment of early distress vocalization can offer insights into the behavioral and brain mechanisms behind the earliest stages of development in infants with ASD. Striking similarities have emerged from human and mouse data pointing to how genetic components may create a specific physiological deficit that leads to the production of atypical distress vocalizations, which in turn jeopardizes the effective communicative power of the distress vocalization and finally hinders caregiver-infant interaction. In other words, a genetic component plays a major role in producing a specific phenotype (atypical crying) that modulates the environment in which the phenotype is expressed (hindering caregiver understanding and responsiveness, see also graphical abstract). This can thus be seen as a complex dynamic of gene × environment effect in which the environmental factor is positively created, instead of being passively perceived (e.g., accidental environmental insults), by risk carriers (Kikusui and Hiroi, 2017). Here, the environmental factor results from the behavioral phenotype of a genetic risk carrier and at the same time results in the augmentation of the genetic impact.
Finally, we would like to highlight 3 directions for research studies in these fields. First, considering that ASD is a spectrum of disorders characterized by wide heterogeneity, a biomarker detectable during early development (such as the acoustic features of cries) in incipient infants with ASD and their siblings could potentially inform ASD subtype identification and clinical population stratification. Second, training of the caregiver to interpret atypical crying episodes is likely to improve social communication between incipient ASD infants and their parents. Third, both human and rodent studies should quantitatively evaluate the predictive prognostic value of early vocal abnormalities in incipient ASD. Infant cries in both humans and mouse models of ASD can be capitalized on to advance our understanding of the developmental origin of ASD and to devise effective therapeutic options.
Statement of Interest
N.H. received a research grant from Astellas for a project not directly related to the subject of this article.
Acknowledgments
We gratefully acknowledge Bindiya Lakshmi and Nur Atiqah Binte Azhari (Nanyang Technological University, Singapore), Andrea Bonassi, and Tommaso Ghilardi (University of Trento, Italy) for their support and Paola Rigo and Tommaso Sega for the drawing of the graphical abstract. This research was supported by grants from the FP7 PEOPLE-Marie Curie Career Integration Grants (GA-2013–630166) and the NAP SUG of the Nanyang Technological University to G.E. and NIH (R21HD053114, R01MH099660 and U54HD090260) to N.H.
References
- Amaral DG, Schumann CM, Nordahl CW. (2008) Neuroanatomy of autism. Trends Neurosci 31:137–145. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Bakeman R, Adamson LB, Konner M, Barr RG. (1990) !Kung infancy: the social context of object exploration. Child Dev 61:794. [PubMed] [Google Scholar]
- Belagodu AP, Johnson AM, Galvez R. (2016) Characterization of ultrasonic vocalizations of fragile X mice. Behav Brain Res 310:76–83. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Costlow K, Truzzi A, Esposito G. (2016) Categorizing the cries of infants with ASD versus typically developing infants: a study of adult accuracy and reaction time. Res Autism Spectr Disord 31:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett ZD, Day NF, Peñagarikano O, Geschwind DH, White SA. (2015) VoICE: a semi-automated pipeline for standardizing vocal analysis across models. Sci Rep 5:10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford SM, Hudry K, Elsabbagh M, Charman T, Johnson MH. (2012). Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. J Aut Dev Dis 43:673–686. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. (2005) Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet 35:103–112. [DOI] [PubMed] [Google Scholar]
- Ehret G, Bernecker C. (1986) Low-frequency sound communication by mouse pups (mus musculus): wriggling calls release maternal behaviour. Animal Beh 34:821–830. [Google Scholar]
- Elsabbagh M, Johnson MH. (2016) Autism and the social brain: the first year puzzle. Bio Psychiatry 80:94–99. [DOI] [PubMed] [Google Scholar]
- Esposito G. (2016) Atypical infant cries among incipient ASDs, developmentally delayed individuals, and language-impaired individuals. Int J Neuropsychopharmacol 19:35–35. [Google Scholar]
- Esposito G, Del Carmen Rostagno M, Venuti P, Haltigan JD, Messinger DS. (2014a) Brief report: atypical expression of distress during the separation phase of the strange situation procedure in infant siblings at high risk for ASD. J Autism Dev Disord 44:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Nakazawa J, Venuti P, Bornstein MH. (2013) Componential deconstruction of infant distress vocalizations via tree-based models: a study of cry in autism spectrum disorder and typical development. Res Dev Disabil 34:2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Nakazawa J, Venuti P, Bornstein MH. (2014) Judgment of infant cry: the roles of acoustic characteristics and sociodemographic characteristics. Jpn Psychol Res 57:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Valenzi S, Islam T, Bornstein MH. (2015) Three physiological responses in fathers and non-fathers’ to vocalizations of typically developing infants and infants with Autism Spectrum Disorder. Res Dev Disabil 43–44:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Venuti P. (2008) How is crying perceived in children with Autistic Spectrum Disorder. Res Autism Spectr Disord 2:371–384. [Google Scholar]
- Esposito G, Venuti P. (2010a) Developmental changes in the fundamental frequency (f0) of infants’ cries: a study of children with Autism Spectrum Disorder. Early Child Dev Care 180:1093–1102. [Google Scholar]
- Esposito G, Venuti P. (2010b) Understanding early communication signals in autism: a study of the perception of infants’ cry. J Intellect Disabil Res 54:216–223. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Bornstein MH. (2011) Assessment of distress in young children: a comparison of autistic disorder, developmental delay and typical development. Res Autism Spectr Disord 5:1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Venuti P. (2010c) Comparative analysis of crying in children with autism, developmental delays, and typical development. Foc Autism Ot Dev Dis 24:240–247. [Google Scholar]
- Esposito G, Venuti P, Apicella F, Muratori F. (2011) Analysis of unsupported gait in toddlers with autism. Brain Dev 33:367–373. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Maestro S, Muratori F. (2009) An exploration of symmetry in early autism spectrum disorders: analysis of lying. Brain Dev 31:131–138. [DOI] [PubMed] [Google Scholar]
- Ey E, Torquet N, Le Sourd A-M, Leblond CS, Boeckers TM, Faure P, Bourgeron T. (2013) The autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res 256:677–689. [DOI] [PubMed] [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. (2012). Head lag in infants at risk for autism: a preliminary study. Am J Occup Ther 66:577–585. [DOI] [PubMed] [Google Scholar]
- Fraley ER, Burkett ZD, Day NF, Schwartz BA, Phelps PE, White SA. (2016) Mice with Dab1 or Vldlr insufficiency exhibit abnormal neonatal vocalization patterns. Sci Rep 6:25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W. (2001) Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet 38:45e–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, Johnson MH. (2015) Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. Lancet Psychiatry 2:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson GE, Green JA. (1989) On the importance of fundamental frequency and other acoustic features in cry perception and infant development. Child Dev 60:772–780. [PubMed] [Google Scholar]
- Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y, Hiroi N. (2011) Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet 20:4775–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. (2013) Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry 18:1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. (2013) Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. (1943) Autistic disturbances of affective contact. Pathology 2:217–250. [PubMed] [Google Scholar]
- Kikusui T, Hiroi N. (2017) A self-generated environmental factor as a potential contributor to atypical early social communication in autism. Neuropsychopharmacology 42:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JKY, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA. (2014) Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res 259:119–130. [DOI] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Charman T, Elsabbagh M, Johnson MH, Hill EL, Baron-Cohen S, Bolton P, Chandler S, Garwood H, Holmboe K, Hudry K, The BASIS team (2013) Motor development in children at risk of autism: a follow-up study of infant siblings. Autism 18:281–291. [DOI] [PubMed] [Google Scholar]
- Michetti C, Ricceri L, Scattoni ML (2012) Modeling social communication deficits in mouse models of autism. Autism S1:007.
- Mody M, Shui AM, Nowinski LA, Golas SB, Ferrone C, O’Rourke JA, McDougle CJ. (2016) Communication deficits and the motor system: exploring patterns of associations in autism spectrum disorder (ASD). J Autism Dev Disord 47:155–162 [DOI] [PubMed] [Google Scholar]
- Moriuchi JM, Klin A, Jones W. (2017) Mechanisms of diminished attention to eyes in autism. Am J Psychiatry 174:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Hiroi N. (2016) Genetic mechanisms emerging from mouse models of CNV-Associated neuropsychiatric disorders. In: The neurobiology of schizophrenia (Abel T, Nickl-Jockschat T, eds), pp397–417. New York: Academic Press/Elsevier. [Google Scholar]
- Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R, Nakayama K, Nakashima S, Kato F, Fukami M, Aoki Y, Matsubara Y. (2014) TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One 9:e91598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N, Mogi K, Kikusui T. (2013) Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav Neurosci 127:432–438. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS (2010) A prospective study of the emergence of early behavioral signs of autism. J Am Ac Child Ado Psy 49:256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, Johnson S, Miller M, Rogers SJ, Schwichtenberg AJ, Steinfeld M, Iosif AM (2014) The broader autism Phenotype in infancy: When does it emerge? J Am Ac Child & Ado Psy 53:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi C-H, Oghalai J, Curran S, Murphy KC, Monks S, Williams N, O’Donovan MC, Owen MJ, Scambler PJ, Lindsay E. (2006) Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A 103:7729–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. (2014) Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Aut Dev Dis 44:2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Michetti C, Caruso A, Laviola G, Scattoni ML. (2013) Characterization of neonatal vocal and motor repertoire of reelin mutant mice. PLoS One 8:e64407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. (2008) Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One 3:e3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. (2009) Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev 33:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, et al. (2012) Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486:256–260. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Lüthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. (2003) Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry 54:1367–1375. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Iverson JM, Rinaldi ML, Lester BM. (2012) Atypical cry acoustics in 6-month-old infants at risk for autism spectrum disorder. Autism Res 5:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Mundy P, Oller DK, Steffens M. (2000) Vocal atypicalities of preverbal autistic children. J Autism Dev Disord 30:345–354. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Okabe S, Broin PÓ, Nishi A, Ye K, Beckert MV, Izumi T, Machida A, Kang G, Abe S, Pena JL, Golden A, Kikusui T, Hiroi N. (2016) Structure and function of neonatal social communication in a genetic mouse model of autism. Mol Psychiatry 21:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. (1998) Movement analysis in infancy may be useful for early diagnosis of autism. PNAS 95:13982–13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Kikusui T, Kihara T, Harada T, Kato M, Nakano K, Murakami O, Koshida N, Takeuchi Y, Mori Y. (2007) Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res 1163:91–99. [DOI] [PubMed] [Google Scholar]
- Varcin KJ, Jeste SS. (2017) The emergence of autism spectrum disorder. Curr Op Psychiatry 30:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcin KJ, Nelson CA. (2016) A developmental neuroscience approach to the search for biomarkers in autism spectrum disorder. Curr Op Neurology 29:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, de Falco S. (2012) Differential brain responses to cries of infants with autistic disorder and typical development: an fMRI study. Res Dev Disabil 33:2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KS, Rogers SJ. (2010) Intervening in infancy: implications for autism spectrum disorders. J Child Psy Psychiatry 51:1300–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L, Lord C. (2014) Parent-implemented social intervention for toddlers with autism: an RCT. Pediatrics 134:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Oddi D, D’Amato FR. (2010) Effect of altricial pup ultrasonic vocalization on maternal behavior. In: Handbook of behavioral neuroscience (Brudzynski SM, ed), pp159–166. Burlington, MA: Elsevier B.V. [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. (2002) Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci 25, 336–340. [DOI] [PubMed] [Google Scholar]
- Yang M, Mahrt EJ, Lewis F, Foley G, Portmann T, Dolmetsch RE, Portfors CV, Crawley JN. (2015) 16p11.2 Deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res 8:507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang S-B, Jan YN, Jan LY. (2010) Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. PNAS 107:11074–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeifman DM. (2003) Predicting adult responses to infant distress: adult characteristics associated with perceptions, emotional reactions, and timing of intervention. Infant Ment Health J 24:597–612. [Google Scholar]
- Zippelius H- M, Schleidt WM. (1956) Ultraschall-Laute bei jungen Mäusen. Naturwissenschaften 43:502–502. [Google Scholar]
- Zwaigenbaum L, et al. (2015) Early identification of autism spectrum disorder: recommendations for practice and research. Pediatrics 136:S10–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]