Abstract
In the adult mammalian brain, new neurons are continuously generated from a proliferating population of neural progenitor/stem cells and become incorporated into the existing neuronal circuitry via a process termed adult neurogenesis. The existence of active functional adult neurogenesis raises the exciting possibility that manipulating endogenous neural progenitors, or transplanting the progeny of exogenously expanded neural progenitors, may lead to successful cell replacement therapies for various degenerative neurological diseases. Significant effort is being made to decipher the mechanisms regulating adult neurogenesis, which may allow us to translate this endogenous neuronal replacement system into therapeutic interventions for neurodegenerative diseases. This review focuses on adult neurogenesis as a strategy to derive potential therapies, and discusses future directions in the field.
Keywords: adult neurogenesis, development, neurodegenerative disease, progenitor, regeneration, stem cell, transplantation
1. Introduction
A long-standing dogma in neuroscience, since the time of Ramon y Cajal, declares that the adult mammalian brain is unable to generate new neurons and is fated to degrade with time, incapable of regeneration after injury [1]. In the last decade, repeated demonstration of active adult neurogenesis in almost all mammals, including humans, has put this dogma to rest [2–4]. Now it is clear that a population of neural progenitor/stem cells (NSCs) exists in the mature central nervous system (CNS), and the adult CNS can integrate these differentiated NSCs as new neurons into the established circuitry. This striking regenerative capacity of the adult CNS holds significant promise for developing novel strategies to treat various neurodegenerative diseases.
NSCs have the basic properties of all stem cells, namely the capacity for extensive self-renewal and giving rise to differentiated cell types [5]. By manipulating the endogenous NSCs or by transplanting cells derived from embryonic or adult NSCs, it may be possible to repair degenerated populations of neurons in Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS) [6,7]. A deep understanding of these disease mechanisms, in conjunction with deciphering the mechanisms regulating endogenous adult neurogenesis, will be necessary to develop effective neurodegenerative disease therapies that guide transplanted or endogenously coaxed new cells to migrate, differentiate, integrate and survive in the existing circuitry of the adult CNS to promote functional recovery.
This review summarises the existing body of research on the basic mechanisms of adult neurogenesis, and discusses recent studies aimed at enhancing endogenous populations of adult NSCs and investigating how they respond to various physiological and disease processes. The authors also review transplantation studies in both normal and diseased environments using various cell types, including neural progenitors and their progeny, and engineered cells designed to secrete various factors to enhance the survival of existing cell populations. Finally, the authors propose future directions for the advancement of NSC-based therapies for neurodegenerative diseases. Interested readers can consult several comprehensive reviews on a similar topic [6–10].
2. Neurodegenerative diseases as potential targets for neural stem cell therapy
Progressive degeneration of specific neuronal types and deterioration of local neuronal circuitry are the hallmarks of degenerative neurological diseases, such as PD, AD, HD and ALS. As these diseases are lethal with limited therapeutic options, replacement of degenerated populations with NSC therapy may be essential.
Some degenerative neurological diseases involve loss of specific neuronal subtypes within restricted regions, such as HD, ALS and PD. HD is an autosomal dominant inherited disease causing degeneration of striatal medium spiny neurons and cortical neurons, with symptoms of involuntary jerking movements, balance impairment, cognitive disability and a projected survivability of 15 – 30 years after diagnosis [11]. HD is caused by inherited CAG trinucleotide repeats that are amplified over generations [12]. There is no known cure for HD, with treatment limited to alleviating symptoms and providing genetic counselling for individuals and their family members. ALS is a neurodegenerative disease of the upper corticospinal tract and lower motor neurons, causing gradual weakness, muscle atrophy and eventual respiratory failure [13]. More than 90% of ALS patients have a sporadic form of the disease and the remaining 10% of the population have various autosomally inherited dominant mutations, with 20% being due to mutations in the gene for superoxide dismutase-1. The aetiology of ALS is still not fully understood. PD occurs when dopaminergic neurons in the substantia nigra degenerate, causing dopamine levels to drop significantly in their target area, the striatum [14]. PD occurs in > 1% of the population over the age of 65, with symptoms of muscular rigidity, resting tremor, gait abnormalities and slow, laboured movement. Existing treatments focus on sustaining dopamine levels or stimulating the striatum with surgically implanted deep brain electrodes [15]. Other degenerative neurological diseases, such as AD, involve degeneration of multiple neuronal types with diffuse localisations. AD selectively causes neuronal degeneration in the cortex and hippocampus where β-amyloid plaques form intracellularly with neurofibrillary tangles [16]. AD afflicts 10% of persons over the age of 65 and presents with gradual, uncompromising decline in cognition and memory function until death.
In the late 1980s, human fetal mesencephalic tissue transplantation into the striatum of human PD patients led to significant functional recovery with evidence of increased dopamine production [15,17–19]. Similar recovery was seen with fetal tissue transplantation into HD patients [20,21]. These transplantations required a significant amount of fetal tissue, making it unfeasible as a high-capacity therapeutic option. Double-blind clinical trials later failed to show significant benefit with fetal grafts in PD, and dyskinesia side effects were observed in some patients [22]. Variability in the preparation of the tissue and the heterogeneous cell population of the grafts may account for the different outcomes. Despite these setbacks, successful cases of functional recovery after fetal transplants showed the promise of cell replacement therapy in degenerative neurological diseases.
NSCs have distinct advantages over primary tissue preparations as they can be expanded, genetically modified in culture, and enriched to a more distinct and pure neuronal type prior to transplantation [7]. For successful treatment of neurodegenerative diseases, the proper cell populations must be derived, including neuronal subtypes, such as dopaminergic neurons for PD and medium spiny neurons for HD. These neurons must survive and not succumb to the disease mechanism that destroys the degenerating population. Appropriate circuits must be restored, requiring nerve guidance and synapse formation mechanisms to be in place. Most importantly, cell replacement therapies must be superior to existing treatments without causing further harm.
3. Basic biology of neurogenesis in the adult CNS
Significant progress has been made in the last decade in our understanding of the basic processes of neurogenesis in the adult CNS, as well as its regulation under physiological and pathological conditions [4,23]. The prevailing view is that active neurogenesis in the intact adult mammalian CNS is limited to two specialised regions, the dentate gyrus of the hippocampus and the lateral ventricles extending to the olfactory bulb. Adult neurogenesis in these regions appears to recapitulate the embryonic neuronal developmental process, including proliferation and fate specification of neural progenitors, neuronal maturation, migration, targeting and synapse formation (Figure 1). Areas outside these two regions are generally considered ‘non-neurogenic’, where proliferating NSCs contribute largely to gliogenesis under normal conditions with minimal or no neurogenesis. However, under certain pathological conditions, or after isolation and culture, these NSCs seem capable of producing both neurons and glia, suggesting that NSCs with neurogenic potential may be present throughout the adult CNS [8,9].
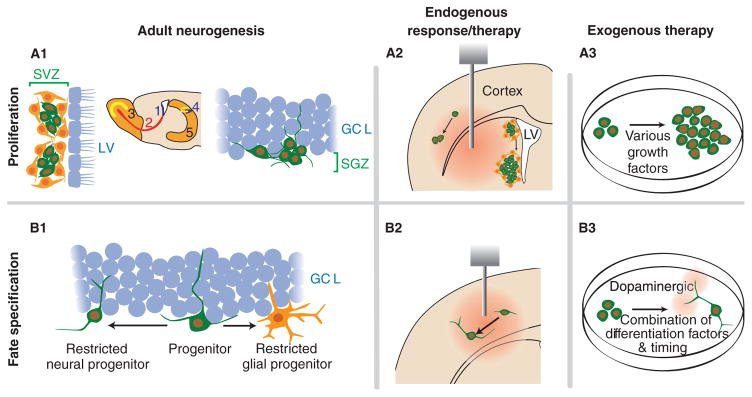
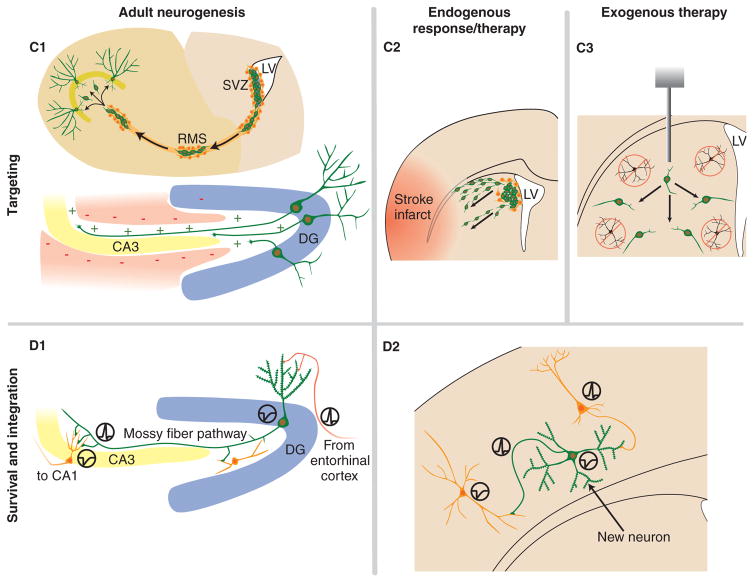
Figure 1. Outline of how adult neurogenesis mechanisms can be translated into endogenous and exogenous NSC replacement therapies for neurodegenerative diseases.
A1. Neurogenesis occurs in the SVZ (1) of the lateral ventricles where the new neurons migrate via the RMS (2) to the olfactory bulb (3). Neurogenesis also occurs in the dentate gyrus (4) of the hippocampus (5) where it is confined to the SGZ of the GCL. A2. Infusion of various growth factors into the parenchyma or ventricles can activate existing NSCs within neurogenic regions and quiescent NSCs in the adult environment. In addition, disease mechanisms may affect proliferation of NSCs. A3. NSCs can be expanded in defined culture conditions for multiple passages before eventually being used for transplantation. B1. Fate specification is regulated by the local environment (the niche). In this example, NSCs in the dentate gyrus differentiate into a restricted neural progenitor that will eventually become a granule neuron, or a restricted glial progenitor that will become an astrocyte. B2. In certain disease states, the existing NSCs generate new neurons in response to changes in the local environment. Infusing differentiation factors may enhance this response. B3. In culture, cells can be induced to differentiate into different cell types, including subtypes, prior to transplantation to insure proper in vivo differentiation without tumour formation. C1. In the SVZ, adjacent to the LV, new neurons migrate a significant distance anteriorly to the olfactory bulb via the RMS. In the hippocampus, the granule cells extend dendrites toward the molecular layer and axons to the CA3 in response to positive and negative guidance cues in the local environment. C2. Certain injuries and diseases induce migration of NSCs or their neuronal progeny to the injury site. C3. Transplanted cells need to migrate away from the injection site, finding the degenerated population and eventually form a three-dimensional network. D1. In the hippocampus, NSCs differentiate into functional granule neurons receiving input via their dendrites from the entorhinal cortex, and relay the signal down their axons to downstream targets in the hilus and CA3 region. D2. Both endogenous and exogenous NSC therapies require the new cells to appropriately incorporate into existing circuits.
DG: Dentate gyrus; GCL: Granule cell layer; LV: Lateral ventricles; NSC: Neural stem cell; RMS: Rostral migratory stream; SGZ: Subgranule zone; SVZ: Subventricular zone.
3.1 Neurogenesis in neurogenic regions of the adult mammalian CNS
In the hippocampal system, a population of NSCs, localised in the subgranule zone (SGZ) between the hilus and the granule cell layer of the dentate gyrus (Figure 1, A1, B1), proliferate and give rise to neuroblasts, which then migrate a short distance into the inner granule cell layer and differentiate into granule neurons [4,23]. These new neurons extend their axonal and dendritic projections, becoming synaptically integrated within 2 – 4 weeks after birth (Figure 1, D1) [24–27]. Approximately half of the newborn neurons survive for 1 month after birth and are maintained for an extended period of time [28].
In the lateral ventricles, adult NSCs in the subventricular zone (SVZ), a region beneath the ependymal cell layer (Figure 1, A1), proliferate to generate neuroblasts [29]. These neuroblasts migrate a significant distance anteriorally, via the rostral migratory stream (RMS), to the olfactory bulb [30] and differentiate into two types of olfactory interneurons: granule and glomerular neurons (Figure 1, C1). Interestingly, migration to the olfactory bulb has been observed in all mammalian species studied except humans, although humans have an actively dividing population of NSCs in the SVZ [31,32].
Adult neurogenesis is a dynamic process influenced by environmental changes, such as various growth factors, pathological conditions, injuries and external stimuli [4,8]. In vivo studies have shown that NSCs are responsive to numerous physiological conditions, including seizures [33,34], ischaemia [35–37], depression [38], environmental enrichment and exercise [39].
The cellular and molecular mechanisms regulating adult neurogenesis are largely unknown [23]. Specific anatomical and cell type characteristics of the neurogenic niches seem to play essential roles for NSCs due to their close proximity with endothelial cells [40] of capillaries [41], astrocytes [42–44] and ependymal cells [45]. In addition to growth factors that serve as mitogens for NSCs, including epidermal growth factor (EGF), fibroblast growth factor (FGF)-2 and Sonic hedgehog (Shh) [9], molecules that regulate fate specification of adult NSCs are beginning to be identified. Bone morphogenic protein (BMP) was shown to promote glial differentiation of NSCs both in vitro and in vivo [45]. Secreted noggin in the SVZ and neurogenesin-1 in the SGZ act as BMP antagonists, causing these factors to shift the niche towards creating new neurons [45,46]. Wnt, expressed by local astrocytes in the adult neurogenic regions, was shown to promote neuroblast proliferation and neuronal fate specification [47]. Knocking down Wnt signalling significantly decreases hippocampal neurogenesis, whereas overexpression of Wnt3 causes an increase. Retinoic acid, a potent NSC neuronal differentiation factor in vitro that has connections to the Wnt signalling pathway, also plays essential roles in adult neurogenesis in vivo [48].
The cellular and molecular mechanisms regulating neuronal maturation, targeting and synaptic integration are less understood. Recent studies have revealed the essential role of GABA, a major inhibitory neurotransmitter, in multiple steps of adult neurogenesis, including proliferation of neural progenitors in the SVZ [49], migration of neuroblasts in the RMS [50], neuronal differentiation [51] and synaptic integration in the dentate gyrus [27].
A driving question of adult neurogenesis asks how this relic of development occurs in the adult brain: is it due to the nature of NSCs themselves, or the environment nurturing them? Most probably, it is a synergistic action with genetic instructions guiding the development of NSCs, the microenvironment providing cell–cell interactions and paracrine factors that control the proliferation rate, instructing the cells to navigate, eventually driving activity-dependent incorporation and the survival or death of the new cells [27,29,43]. Adult neurogenesis demonstrates the extensive plasticity of the newborn neurons, while at the same time this phenomenon demonstrates the tremendous plasticity of the mature CNS to support and guide nerve growth, migration and synapse formation. Understanding this endogenous process of new neuron acceptance by the adult environment will be essential to developing successful NSC-based cell replacement therapies.
3.2 Neurogenesis in non-neurogenic regions of the adult mammalian CNS
Active gliogenesis has been observed throughout the CNS, while neuronal fate specification from NSCs in regions outside of neurogenic niches remains controversial [4,8,52]. There are both technical difficulties as well as theoretical issues in detecting new neurons, as new neuron incorporation could possibly cause destabilisation of the highly organised mature, adult connectivity [53]. The prevailing view is that adult neurogenesis is extremely limited in the non-neurogenic regions of the intact adult mammalian CNS. On the other hand, NSC populations with neurogenic potential appear to exist in these non-neurogenic regions, as their neuronal fate specification in vitro and in vivo has been demonstrated under various conditions [4,8,9].
Specific injury- or disease-induced changes in non-neurogenic regions have demonstrated proliferation and neuronal differentiation of local NSCs. Using a mild, site-specific induction of apoptosis in the cortex of adult mice, Magavi and colleagues demonstrated the activation of a small population of NSCs in the cortex, which eventually differentiated into neurons expressing mature neuronal markers with apparent incorporation [54]. In a later study, the same group induced targeted apoptosis of corticospinal motor neurons and, remarkably, neurogenesis was induced with the long-distance projection of newly formed corticospinal motor neurons to the spinal cord [55].
NSCs have been derived from the striatum, spinal cord and white matter tracts of both rodents and humans [56,57]. NSCs from these regions exhibit in vitro differentiation characteristics quite similar to neurogenic niche-derived cells. When transplanted back into the adult hippocampus, clones of spinal cord-derived NSCs were capable of differentiating into granule neurons in the dentate gyrus, demonstrating the role of environmental cues in fate specification [58]. These in vitro and in vivo studies suggest that NSCs with neurogenic potential are present throughout the adult CNS and their neuronal differentiation could be influenced by their local environment. Identification of these environmental signals could promote neurogenesis in different regions of the adult brain for cell replacement therapy in degenerative neurological diseases (Figure 1, A2 and B2).
4. Endogenous cell replacement in degenerative neurological diseases
A tempting strategy for neurodegenerative therapies involves recruiting endogenous NSC populations in situ to replace degenerating neuron populations. This would require increasing endogenous NSC proliferation, promoting neuronal fate specification and differentiation, luring the migration of new neurons to locations of degeneration and guiding their integration into the adult circuitry (Figure 1, A2, B2 and C2).
At a limited level, the adult brain appears to already have an injury-responsive neuronal replacement mechanism in place. Global or focal cerebral ischaemia causes a significant upregulation of neurogenesis in both the SVZ and dentate gyrus [35,59]. With focal ischaemia, NSC proliferation increases in the SVZ ipsilateral to the infarct, and within the first 2 weeks immature neurons redirect their migration laterally to implant into the damaged striatum (Figure 1, C2) [60,61]. Some of these cells mature and acquire morphological and immunohistochemical characteristics of medium spiny neurons [37]. Whether these neurons become electrically active and synaptically integrate into striatal circuits remains to be determined. Only 20% of the NSC-derived neurons survived beyond 6 weeks, most likely as a result of inadequate tropic or activity-dependent support for integration and survival. In another study, using a growth factor cocktail infusion, Nakatomi and colleagues were able to recruit resident NSCs of the posterior periventricular region, an extension of the SVZ, to migrate to the CA1 hippocampal cell layer, where they replaced lost CA1 pyramidal neurons after global ischaemia [62]. Notably, they observed significant cognitive recovery after 7 weeks.
After inducing temporal lobe epilepsy in rats, Parent and co-workers observed a 5-fold increase in adult NSC proliferation in the dentate gyrus of the hippocampus for the first 2 weeks, which returned to the control level by 4 weeks [33]. Seizures have also been shown to accelerate synaptic integration of newborn neurons [63]. In addition, some SGZ NSCs migrated into the hilus and differentiated into mature granule neurons [34,64]. Due to this aberrant synaptic integration, these ectopic granule neurons could potentially contribute to the recurrent seizures that occur chronically after the initial seizure induction [34]. These findings emphasise the importance of proper neuronal subtype differentiation and targeting (Figure 1, D1 and D2).
Taken together, these studies demonstrated that NSCs outside the SVZ and dentate gyrus, responding to a modified CNS environment, can be activated to migrate into the parenchyma, differentiate into neurons and integrate into the adult circuitry. Understanding the molecular mechanisms that allow the environment to sustain neurogenesis and new cell incorporation will be crucial for successful endogenous cell replacement therapies.
5. Transplantation therapy for degenerative neurological diseases
For maximal benefit in neurodegenerative therapies, it may be necessary to transplant exogenous cells due to the limited proliferation capacity of endogenous NSCs [10]. In addition, recruiting new neurons that are derived from endogenous neural progenitors in the diseased CNS, without neuroprotective therapies, makes these cells prone to the same degeneration mechanisms as the existing mature neurons. At present, there are two therapeutic transplantation strategies: to replace lost neurons or provide tropic support for dying neurons.
5.1 Available cell types for transplantation
Embryonic stem (ES) cells are pluripotent stem cells derived from the inner cell mass of a blastocyst with the capacity to differentiate into all cell types of an organism [65,66]. Newly developed techniques allow long-term expansion of pluripotent stem cells, including human ES cells and embryonic germ (EG) cells (Figure 1, A3) [67,68]. With various in vitro manipulations, the cells can be induced to differentiate into distinct cell types, including specific neuronal subtypes such as dopaminergic neurons (Figure 1, B3) [69,70]. Despite the potential for ES cells, their application still has ethical and technical issues to overcome, such as immune complications and the possibility that undifferentiated ES cells may form teratomas after transplantation [10].
Somatic stem cells, such as mesenchymal and neural stem cells, are multipotent and can give rise to cell types of a particular organ. NSCs can be derived from various CNS regions of fetal and adult mammalian tissue, including adult human resected surgical specimens [71] and postmortem tissue [72]. These multipotent stem cells can be expanded in defined culture media for numerous passages and differentiated into the different neural cell types, including neurons, astrocytes and oligodendrocytes [8,9]. Other types of somatic stem cells, such as mesenchymal stem cells, could potentially provide tropic support for degenerating cells after transplantation, although their ability to give rise to functional neurons remains controversial. Non-stem cells, such as genetically modified fibroblasts, can also be used for this purpose [73,74].
5.2 Transplanting NSCs and their progeny into the normal CNS
Numerous studies have been performed to transplant embryonic, fetal or adult-derived NSCs into various regions of the normal CNS. These studies are essential to understand how the NSCs differentiate, migrate and incorporate into the adult environment both inside and outside the neurogenic niches [29].
Adult rat hippocampal NSCs expanded in vitro with media containing FGF-2 were transplanted into the adult rat hippocampus [75]. These NSCs differentiated into astrocytes as well as mature neurons with phenotypes identical to adjacent mature granule neurons, demonstrating the ability of these cells to retain their characteristics even after in vitro expansion. When these hippocampal NSCs were transplanted into the RMS, the cells migrated like SVZ-derived cells and incorporated into the olfactory bulb as interneurons [76]. This study demonstrates that regional cues drive adult NSC neuronal subtype differentiation and adult NSCs exhibit plasticity to differentiate into neuronal phenotypes other than their normal fate in situ.
Zhang and colleagues differentiated human ES cells into neuronal precursors in vitro and transplanted these cells into the lateral ventricles of newborn mice [77]. The transplanted cells incorporated into the hippocampus and cortex as neurons and astrocytes without the formation of teratomas, a common complication of undifferentiated ES cell transplantation. Lepore and colleagues performed a similar series of experiments in adult rats where undifferentiated neuroepithelial stem cells, differentiated lineage-restricted neuron or glial precursors were isolated and transplanted into the hippocampus, spinal cord and striatum [78]. Interestingly, the undifferentiated, immature neuroepithelial cells did not survive, whereas the predifferentiated glial and neuronal precursors survived, integrated and differentiated into mature neurons and astrocytes in all the transplanted regions. When the authors repeated the transplantation of neuroepithelial cells, but instead predifferentiated the cells to a precursor state, they observed similar survival and integration as seen in the lineage-restricted precursors.
Human-derived NSCs from fetal and adult tissue are also capable of differentiating into neurons and glia in vitro and after transplantation. Human fetal-derived NSCs were enriched by cell sorting, expanded in culture and transplanted into the lateral ventricle of neonatal mice [79]. Seven months later, the cells were found to incorporate into the SVZ and SGZ, where some of the cells differentiated into neurons and glia, while some transplanted cells were still dividing in these germinal niches. Similar results were seen from adult human subcortical white matter-derived progenitor cells after transplantation into rat fetuses [57].
These studies show that the brain can incorporate new populations of cells, even NSCs derived from human tissue, although the germinal niches are more receptive by guiding the fate and incorporation of the new cells compared with other brain regions. In principle, expanding NSCs in culture does not irreversibly alter them to a non-neural progenitor state, but proper pretransplantation differentiation seems essential to ensure the fate of transplanted NSCs.
5.3 Transplanting NSCs and their progeny into the diseased CNS
Studies have also been performed to determine the behaviour of exogenously derived cells when transplanted into various disease models. With endogenous populations of NSCs becoming activated in various neurodegenerative diseases, as outlined above, it seems that the adult CNS may transform and become more receptive to cell replacement for transplanted exogenous NSCs.
In an animal model of PD, Björklund and co-workers injected undifferentiated blastocyst-derived ES cells into the striatum of rats and observed dopaminergic-like tyrosine hydroxylase-positive neurons after 14 – 16 weeks [80]. Within 7 weeks they observed significant behavioural recovery, but 20% of the animals formed lethal teratomas. Instead of undifferentiated ES cells, Kim and colleagues first expressed a transcription factor, nuclear receptor related-1, in mouse ES cells to promote differentiation into tyrosine hydroxylase-positive precursor neurons in culture with the addition of various growth factors [81]. When these immature neurons were injected into the striatum of PD rats, they differentiated into electrically active dopaminergic neurons receiving synaptic inputs within 4 weeks and survived up to 8 weeks without forming teratomas. This study demonstrates the importance of proper differentiation of NSCs before transplantation in order to insure the proper cell fate without forming tumours.
In work concentrating on ALS therapies, adult rats that had an experimental paralysis model were transplanted with human EG cells into the CSF at the lumbar region of the spinal cord [82]. The cells migrated and incorporated into the parenchyma of injured, but not uninjured, animals, and a small population of cells appeared to differentiate into neurons and astrocytes. The behavioural recovery in the animals was probably from tropic effects of transplanted cells rescuing host neurons instead of functional cell replacement. When mouse ES cells that were first partially differentiated into spinal motor neurons in vitro were transplanted into the same paralysis model, ~ 25% survived for > 1 month [83]. After infusing a Rho kinase inhibitor to block the inhibitory effect of myelin on axonal growth, extensive neurite outgrowth was observed compared with rats not receiving the inhibitor, where no neurite outgrowth into the white matter was observed.
More recently, the same group used a multistep treatment to address proper differentiation, neurite outgrowth, survival and synapse formation [84]. Mouse ES cells were differentiated into committed motor neuron progenitors, mixed with a drug to block myelin inhibition, and were transplanted into the spinal cord of injured rats. In addition, glial-derived neurotrophic factor (GDNF)-secreting fetal NSCs were injected into the sciatic nerves, as GDNF has been shown to be an effective motor neuron outgrowth factor, known to attract axons. There was significant behavioural recovery with the multistep treatment, and a portion of the ES-derived cells differentiated into neurons that integrated into the host circuitry, having intact neuron–muscular junctions at skeletal muscles. This elegant study illustrates how complicated future cell therapies may need to be in order to address all the steps of new neuron incorporation.
Human ES cells have also been induced to differentiate into dopaminergic neurons or motor neurons in culture [69,70,85,86]. Dopaminergic differentiation of human ES cells can be induced by either coculture with PA6 cells [69] or a combination of FGF-8 and Shh [86]. When these human dopaminergic neurons were transplanted into animal models of PD, they did not survive and could not maintain their dopaminergic phenotype in the adult brain [87,88], suggesting that additional strategies are needed for human cells. In other studies, McBride and co-workers treated human fetal cortex stem cells in vitro with ciliary neurotrophic factor, which aided in predifferentiating the cells into neurons, and injected the cells into the striatum of HD adult rats [89]. Although the animals had limited behavioural recovery as compared with vehicle-injected animals, the stem cell-transplanted animals had a significant increase in striatal volume, with the transplanted cells differentiating into neurons and astrocytes.
These studies make evident the abilities of NSCs with some preliminary results demonstrating incorporation, albeit at a very limited level. Carefully predifferentiating cells prior to transplantation, purifying the cell population to exclusively contain the correct cellular type(s) and modifying the transplanted region to allow for new neuron incorporation appear to be essential for successful functional recovery. The fate of transplanted cells may be enhanced with environmental stimulation, as seen in animal models [90,91], and for human transplantation, postoperative therapy may be essential to stimulate the cells to incorporate into the adult environment. Even though the diseased environment appears somewhat receptive to new cell populations, it still remains a diseased environment, and NSCs that are transplanted may also be prone to the same fate as the populations they are replacing. Thus, it may be logical to enhance the survival of transplanted NSCs with various tropic factors.
5.4 Transplantation of genetically engineered cells into the diseased CNS
Another strategy for stem cell transplantation involves engineering cells that secrete certain tropic factors and transplanting them into regions early in the disease process to enhance the survival of dysfunctional or degenerating cell populations. These approaches can be described as ex vivo gene therapies that are localised to transplanted regions, minimising the risk of complications associated with virus-mediated gene therapies [73,92].
GDNF has been shown to enhance the survival of dopaminergic neurons in animal models of PD and a few clinical trials [93]. Direct infusion of GDNF into the putamen of human PD patients demonstrated significant recovery of coordination-related activities, stimulated sprouting of tyrosine hydroxylase-positive neurons and increased dopamine storage even 2 years after the start of infusion [94,95]. Rats with experimentally induced PD that were transplanted with normal or encapsulated GDNF-secreting fibroblasts into their striatum showed significant improvement in motor behaviour associated with slower dopaminergic neuron degeneration [96,97].
In a Phase I clinical trial, AD patients had autologous transplantations of fibroblasts that were transduced in vitro to express nerve growth factor (NGF), a growth factor supporting cholinergic neurons that are specifically degenerated in AD [74]. Twenty-two months after transplantation, a large population of cells appeared to be intact and secreting NGF. The mature neurons of the host also appeared to sprout cholinergic axons and there was a 51% improvement in cognitive function. This study demonstrates some significant recovery with tropic enhancement in addition to the ability to self-transplant and safely deliver neuroprotective genes in humans.
6. Expert opinion and conclusion
In summary, the reality of NSC therapy for the treatment of neurodegenerative diseases is becoming feasible, although a significant amount of research needs to be completed before successful human therapies are developed. The adult brain is a staggeringly complex organ with trillions of neuronal connections. Extrinsic manipulation to re-establish the correct circuitry lost in degenerative diseases, without any form of intrinsic guidance, would be hopeless. Fortunately, the adult brain still retains the ability to guide the incorporation of new cells in specific regions via the process of adult neurogenesis. Deciphering the mechanisms of this endogenous system can serve as a blueprint to translate into effective endogenous or exogenous NSC therapies for neurodegenerative diseases.
During the last few years, significant progress has been made in understanding the factors regulating fate specification in the neurogenic niche and how NSCs are affected by various physiological and disease stimuli. On the other hand, we are just beginning to understand how these cells incorporate into the adult circuitry. More research is needed to discover the intrinsic mechanisms that regulate self-renewal of embryonic and adult NSCs, efficient fate determination and, more particularly, specific neuronal subtype differentiation. Conversely, discovering the extrinsic factors and cell–cell interactions of the adult neurogenic niches that cause these microenvironments to nurture NSCs, and discovering how the new cells are accepted into the existing adult circuitry, both with proper neurite targeting and correct synapse formation, seem essential for developing therapies.
Injury-induced migration of NSCs from the ventricular regions of the brain to damaged areas indicates that a cell replacement system or, conversely, an acceptance mechanism can be potentially activated in non-neurogenic regions of the adult CNS. What injury-induced factors are being secreted to cause this migration and lure the cells to the damaged tissue? Stromal cell-derived factor-1α is a potential candidate as it may have a role in luring NSCs from the SVZ to the infarct in stroke-induced neurogenesis [98]. Other factors, such as vascular endothelial growth factor [99] and stem cell factor [100], have also been shown to enhance migration. By discovering more of these motility factors and further enhancing their release with infusions or engineered cell transplants over the course of the disease progression, it may be possible to recruit more endogenous NSCs to fill and incorporate into degenerating regions. These factors are also crucial for transplantation therapies as extensive migration from the injection sites is required to fill the diseased region with new cells (Figure 1, C3).
Modifying the environment to accept new cells, in addition to enhancing targeting and survival, is essential in the diseased environment. Successful cell therapy with unmodified NSCs would depend on the assumption that the newly incorporated cell population from either endogenous NSC recruitment or transplantation will be immune to the disease process of the degenerated population that is being replaced. With the promising results of ex vivo gene therapy, with tropic factor-secreting cell transplantation in humans [74] and combination therapies [84], it seems feasible that the transplanted NSCs themselves could be modified to secrete factors for autocrine and paracrine support. Another potential strategy would be to prime the region with an initial transplantation of support cells, either glial progenitors or fibroblasts, that replicate the germinal niches prior to transplanting NSCs, which could help in the survival and integration of the new cells.
In conclusion, in order for true NSC-based therapies to be successful, the functional incorporation of new cells replacing the degenerated population needs to occur. Differentiating into the correct subcellular phenotype, establishing the correct migration to the region, forming the correct dendritic and axonal connections, in addition to true functional electrophysiological integration into the existing circuitry with observable recovery in the patient, are all requirements for stem cell therapy. Although this seems daunting, taken together with the existing mass of encouraging research and foreseeable work to be performed, NSC-based treatments have high potential for future neurodegenerative disease therapies.
Acknowledgments
The authors thank J Sailor for help with editing the manuscript. The research in the authors’ laboratories is supported by funding from the Packard Center for ALS at Johns Hopkins University, McKnight Scholar Award, NINDS and NIA (to HS), and Klingenstein Fellowship Award, Charles E. Culpeper Scholarship in Medical Science, March of Dimes, Whitehall Foundation, Alfred P. Sloan Foundation and NINDS (to G-LM).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.RAMON Y CAHAL S. In: Degeneration and Regeneration of the Nervous System. Ramon Y, Cahal S, editors. Haffner Publishing Co; New York, NY, USA: 1928. [Google Scholar]
- 2••.ALTMAN J, DAS GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. Early evidence showing neurogenesis in the mammalian postnatal brain. [DOI] [PubMed] [Google Scholar]
- 3••.ERIKSSON PS, PERFILIEVA E, BJORK-ERIKSSON T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. First study demonstrating the presence of neurogenesis in the adult human brain. [DOI] [PubMed] [Google Scholar]
- 4.MING GL, SONG H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 5.GAGE FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 6.LINDVALL O, KOKAIA Z, MARTINEZ-SERRANO A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 7.ROSSI F, CATTANEO E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002;3(5):401–409. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- 8.EMSLEY JG, MITCHELL BD, KEMPERMANN G, MACKLIS JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75(5):321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.LIE DC, SONG H, COLAMARINO SA, MING GL, GAGE FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 10.MARTINO G, PLUCHINO S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7(5):395–405. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 11.VONSATTEL JP, MYERS RH, STEVENS TJ, et al. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 12.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. NO AUTHORS LISTED. [DOI] [PubMed] [Google Scholar]
- 13.CLEVELAND DW, ROTHSTEIN JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2(11):806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 14.GIBB WR. Neuropathology of Parkinson’s disease and related syndromes. Neurol Clin. 1992;10(2):361–376. [PubMed] [Google Scholar]
- 15.LINDVALL O, BJORKLUND A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1(4):382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CUMMINGS JL, COLE G. Alzheimer’s disease. JAMA. 2002;287(18):2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 17••.LINDVALL O, BRUNDIN P, WIDNER H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247(4942):574–577. doi: 10.1126/science.2105529. Fetal transplant human clinical trial showing some recovery of PD symptoms. [DOI] [PubMed] [Google Scholar]
- 18.HAUSER RA, FREEMAN TB, SNOW BJ, et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson’s disease. Arch Neurol. 1999;56(2):179–187. doi: 10.1001/archneur.56.2.179. [DOI] [PubMed] [Google Scholar]
- 19.PICCINI P, BROOKS DJ, BJORKLUND A, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 20.BACHOUD-LEVI AC, REMY P, NGUYEN JP, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356(9246):1975–1979. doi: 10.1016/s0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- 21.FREEMAN TB, CICCHETTI F, HAUSER RA, et al. Transplanted fetal striatum in Huntington’s disease: phenotypic development and lack of pathology. Proc Natl Acad Sci USA. 2000;97(25):13877–13882. doi: 10.1073/pnas.97.25.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OLANOW CW, GOETZ CG, KORDOWER JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 23.LLEDO PM, ALONSO M, GRUBB MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 24••.VAN PRAAG H, SCHINDER AF, CHRISTIE BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. First study to demonstrate the functional incorporation of new neurons into the mammalian brain by measuring electrophysiological properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ZHAO C, TENG EM, SUMMERS RG, JR, MING GL, GAGE FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ESPOSITO MS, PIATTI VC, LAPLAGNE DA, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25(44):10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GE S, GOH EL, SAILOR KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KEMPERMANN G, GAST D, KRONENBERG G, YAMAGUCHI M, GAGE FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 29.ALVAREZ-BUYLLA A, LIM DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 30•.LOIS C, ALVAREZ-BUYLLA A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. First strong evidence showing SVZ cell migration to the olfactory bulb via the RMS. [DOI] [PubMed] [Google Scholar]
- 31.SANAI N, TRAMONTIN AD, QUINONES-HINOJOSA A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 32.QUINONES-HINOJOSA A, SANAI N, SORIANO-NAVARRO M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 33.PARENT JM, YU TW, LEIBOWITZ RT, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PARENT JM, ELLIOTT RC, PLEASURE SJ, BARBARO NM, LOWENSTEIN DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 35.KOKAIA Z, LINDVALL O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13(1):127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 36.LIU J, SOLWAY K, MESSING RO, SHARP FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ARVIDSSON A, COLLIN T, KIRIK D, KOKAIA Z, LINDVALL O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 38.WARNER-SCHMIDT JL, DUMAN RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 39.KEMPERMANN G, KUHN HG, GAGE FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 40.SHEN Q, GODERIE SK, JIN L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 41.PALMER TD, WILLHOITE AR, GAGE FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.SONG H, STEVENS CF, GAGE FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 43.MA DK, MING GL, SONG H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15(5):514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 44.LIM DA, ALVAREZ-BUYLLA A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA. 1999;96(13):7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LIM DA, TRAMONTIN AD, TREVEJO JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 46.UEKI T, TANAKA M, YAMASHITA K, et al. A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci. 2003;23(37):11732–11740. doi: 10.1523/JNEUROSCI.23-37-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.LIE DC, COLAMARINO SA, SONG HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. Identification of the first factor that promotes neuronal fate choice of adult NSCs. [DOI] [PubMed] [Google Scholar]
- 48.JACOBS S, LIE DC, DECICCO KL, et al. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103(10):3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LIU X, WANG Q, HAYDAR TF, BORDEY A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BOLTEUS AJ, BORDEY A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.TOZUKA Y, FUKUDA S, NAMBA T, SEKI T, HISATSUNE T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 52.RAKIC P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3(1):65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 53.RAKIC P. Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 54••.MAGAVI SS, LEAVITT BR, MACKLIS JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. doi: 10.1038/35016083. The first evidence of potential neurogenesis in the adult cortex after selective injury. [DOI] [PubMed] [Google Scholar]
- 55•.CHEN J, MAGAVI SS, MACKLIS JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci USA. 2004;101(46):16357–16362. doi: 10.1073/pnas.0406795101. A striking study showing mammalian neurogenesis with proper long-distance axonal targeting of corticospinal motor neurons after injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.PALMER TD, MARKAKIS EA, WILLHOITE AR, SAFAR F, GAGE FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19(19):8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.NUNES MC, ROY NS, KEYOUNG HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9(4):439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 58••.SHIHABUDDIN LS, HORNER PJ, RAY J, GAGE FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. A study showing evidence of resident NSCs in the adult spinal cord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DEMPSEY RJ, SAILOR KA, BOWEN KK, TUREYEN K, VEMUGANTI R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87(3):586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 60.PARENT JM, VEXLER ZS, GONG C, DERUGIN N, FERRIERO DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 61.ZHANG RL, ZHANG ZG, CHOPP M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11(5):408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 62.NAKATOMI H, KURIU T, OKABE S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 63.OVERSTREET-WADICHE LS, BROMBERG DA, BENSEN AL, WESTBROOK GL. Seizures accelerate functional integration of adult generated granule cells. J Neurosci. 2006;26(15):4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SCHARFMAN HE, SOLLAS AE, BERGER RE, GOODMAN JH, PIERCE JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121(4):1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 65.FUCHS E, SEGRE JA. Stem cells: a new lease on life. Cell. 2000;100(1):143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 66.SMITH AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 67.SHAMBLOTT MJ, AXELMAN J, WANG S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95(23):13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.THOMSON JA, ITSKOVITZ-ELDOR J, SHAPIRO SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. The first successful study to efficiently grow human ES cells in culture. [DOI] [PubMed] [Google Scholar]
- 69.BUYTAERT-HOEFEN KA, ALVAREZ E, FREED CR. Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells. 2004;22(5):669–674. doi: 10.1634/stemcells.22-5-669. [DOI] [PubMed] [Google Scholar]
- 70.YAN Y, YANG D, ZARNOWSKA ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ROY NS, WANG S, JIANG L, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6(3):271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 72.PALMER TD, SCHWARTZ PH, TAUPIN P, et al. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 73.GAGE FH. Cell therapy. Nature. 1998;392(6679 Suppl):18–24. [PubMed] [Google Scholar]
- 74•.TUSZYNSKI MH, THAL L, PAY M, et al. A Phase I clinical trial of nerve growth factor gene therapy for Alzheimer’s disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. A clinical trial using autologous fibroblasts manipulated to express NGF transplantated into the cortex of AD patients with significant recovery, without rejection. [DOI] [PubMed] [Google Scholar]
- 75.GAGE FH, COATES PW, PALMER TD, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92(25):11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.SUHONEN JO, PETERSON DA, RAY J, GAGE FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons. in vivo Nature. 1996;383(6601):624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 77.ZHANG SC, WERNIG M, DUNCAN ID, BRUSTLE O, THOMSON JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 78.LEPORE AC, HAN SS, TYLER-POLSZ CJ, et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1(2):113–126. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.UCHIDA N, BUCK DW, HE D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.BJORKLUND LM, SANCHEZ-PERNAUTE R, CHUNG S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99(4):2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.KIM JH, AUERBACH JM, RODRIGUEZ-GOMEZ JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. A transplantation study where rodent ES-derived dopaminergic cells were injected into PD animals demonstrating electrophysiological incorporation. [DOI] [PubMed] [Google Scholar]
- 82.KERR DA, LLADO J, SHAMBLOTT MJ, et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. 2003;23(12):5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.HARPER JM, KRISHNAN C, DARMAN JS, et al. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101(18):7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84••.DESHPANDE DM, KIM YS, MARTINEZ T, et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60(1):32–44. doi: 10.1002/ana.20901. An elegant multistep transplantation study with significant physiological and behavioural results. [DOI] [PubMed] [Google Scholar]
- 85.LI XJ, DU ZW, ZARNOWSKA ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 86.PERRIER AL, TABAR V, BARBERI T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101(34):12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.SCHULZ TC, NOGGLE SA, PALMARINI GM, et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7):1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 88.BEN-HUR T, IDELSON M, KHANER H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22(7):1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 89.MCBRIDE JL, BEHRSTOCK SP, CHEN EY, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol. 2004;475(2):211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- 90.DOBROSSY MD, DUNNETT SB. The influence of environment and experience on neural grafts. Nat Rev Neurosci. 2001;2(12):871–879. doi: 10.1038/35104055. [DOI] [PubMed] [Google Scholar]
- 91.LAZIC SE, GROTE HE, BLAKEMORE C, et al. Neurogenesis in the R6/1 transgenic mouse model of Huntington’s disease: effects of environmental enrichment. Eur J Neurosci. 2006;23(7):1829–1838. doi: 10.1111/j.1460-9568.2006.04715.x. [DOI] [PubMed] [Google Scholar]
- 92.SOMIA N, VERMA IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 93.ESLAMBOLI A. Assessment of GDNF in primate models of Parkinson’s disease: comparison with human studies. Rev Neurosci. 2005;16(4):303–310. doi: 10.1515/revneuro.2005.16.4.303. [DOI] [PubMed] [Google Scholar]
- 94•.LOVE S, PLAHA P, PATEL NK, et al. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11(7):703–704. doi: 10.1038/nm0705-703. Evidence of tyrosine hydroxylase neuron sprouting in a human patient due to GDNF infusion therapy. [DOI] [PubMed] [Google Scholar]
- 95.PATEL NK, BUNNAGE M, PLAHA P, et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57(2):298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 96.DUAN D, YANG H, ZHANG J, ZHANG J, XU Q. Long-term restoration of nigrostriatal system function by implanting GDNF genetically modified fibroblasts in a rat model of Parkinson’s disease. Exp Brain Res. 2005;161(3):316–324. doi: 10.1007/s00221-004-2075-y. [DOI] [PubMed] [Google Scholar]
- 97.SAJADI A, BENSADOUN JC, SCHNEIDER BL, LO BIANCO C, AEBISCHER P. Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson’s disease. Neurobiol Dis. 2005;22(1):119–129. doi: 10.1016/j.nbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 98.ROBIN AM, ZHANG ZG, WANG L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 99.ZHANG H, VUTSKITS L, PEPPER MS, KISS JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163(6):1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.ERLANDSSON A, LARSSON J, FORSBERG-NILSSON K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp Cell Res. 2004;301(2):201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]