Abstract
It has been shown that dendritic cells (DCs) are able to present glycolipids to natural killer (NK) T cells in vivo. However, the essential role of DCs, as well as the role of other cells in glycolipid presentation, is unknown. Here, we show that DCs are the crucial antigen-presenting cells (APCs) for splenic NK T cells, whereas Kupffer cells are the key APCs for hepatic NK T cells. Both cell types stimulate cytokine production by NK T cells within 2 h of glycolipid administration, but only DCs are involved in the systemic, downstream responses to glycolipid administration. More specifically, CD8α+ DCs produce IL-12 in response to glycolipid presentation, which stimulates secondary IFN-γ production by NK cells in different organs. Different APCs participate in glycolipid presentation to NK T cells in vivo but differ in their involvement in the overall glycolipid response.
Keywords: dendritic cell, Kupffer cell
Natural killer (NK) T cells are unique lymphocytes defined by their coexpression of surface markers associated with NK cells along with a T cell antigen receptor. NK T cells recognize amphipatic ligands such as glycolipids or phospholipids presented in the context of the nonpolymorphic, MHC class I-like molecule CD1d (1).
To date, very few compounds have been found that stimulate NK T cells. The best-characterized is the glycolipid α-galactosylceramide (α-GalCer), which stimulates the production of large quantities of IFN-γ and IL-4 by these cells within 2 h of injection (2, 3). In addition, bystander activation of NK cells (4, 5), conventional T cells (6), and B cells (7) also occurs subsequent to α-GalCer injection in mice, as does enhanced cytolytic activity and proliferation by the NK T cells themselves (2, 8–10). Because of these myriad functional responses after injection, α-GalCer exhibits powerful therapeutic activity against a number of infectious, neoplastic, and autoimmune diseases (11).
Another molecule that stimulates NK T cells is α-C-galactosylceramide (α-C-GalCer), a C-glycoside analog of α-GalCer. Previously, we found that in vivo α-C-GalCer stimulated prolonged production of IFN-γ and IL-12 and diminished production of IL-4 when compared with α-GalCer (12).
Although it is evident that the responses to both α-GalCer and α-C-GalCer in mice require CD1d-mediated presentation of the glycolipids to Vα14+ NK T cells, it is still unclear in vivo what antigen-presenting cells (APCs) are required for stimulating the full range of responses induced by either compound. Given that CD1d is expressed by all leukocytes, as well as by various other cells, including hepatocytes, many potential candidates exist (13). Previous data suggest that dendritic cells (DCs) are the relevant APCs that present glycolipids to NK T cells. Fujii et al. (14) showed that DCs loaded with α-GalCer and adoptively transferred into naove mice stimulated a better NK T cell response in vivo than that stimulated by injection of free compound. Other studies have demonstrated the ability of α-GalCer to induce maturation of both splenic and hepatic DCs in vivo (15, 16), and others have shown the importance of DCs as sources of α-GalCer-induced IL-12 (17–19).
Even though these studies clearly demonstrate the ability of DCs to present glycolipids to NK T cells, the possibility that other cell types might act as APCs for NK T cells has yet to be fully assessed. To better understand what cell types act as APCs for NK T cells, we used in vivo cell ablation techniques to deplete DCs and Kupffer cells and see the effect on NK T cell responses after glycolipid injection. Using these techniques, our study provides unique information regarding glycolipid presentation to NK T cells in vivo.
Materials and Methods
Chemicals. α-GalCer [(2S,3S,4R)-1-O-(α-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol] was synthesized by Kirin Brewery (Gumma, Japan). The stock solution was dissolved in a 0.5% polysorbate-20 (Nikko Chemicals, Tokyo)/0.9% NaCl solution at a concentration of 200 μg/ml and diluted in PBS to the desired concentration just before injection into mice.
α-C-GalCer [(2S,3S,4R)-1-CH2-(α-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol] was synthesized as described in ref. 20. The stock solution, originally dissolved to a concentration of 1 mg/ml in 100% DMSO, was diluted to a desired concentration in PBS just before being injected into mice.
Diphtheria toxin (DT) was purchased from MP Biomedicals (Aurora, OH) and dissolved in water to a concentration of 1 mg/ml. Before injection into mice, the stock solution was diluted in PBS to the appropriate concentration.
Clodronate (dichloromethylene-bisphosphonate) was a gift of Roche Diagnostics. Phosphatidylcholine (Lipoid E PC, Lipoid, Ludwigshaften, Germany) and cholesterol (Sigma) were used to prepare the liposomes as described in ref. 21.
Mice. Six- to 8-week-old female BALB/c and C57BL/6 mice were purchased from the National Cancer Institute (Bethesda). Breeding pairs of BALB/c and C57BL/6 CD11c-DT receptor (DTR) transgenic mice were purchased from The Jackson Laboratory and bred in our pathogen-free animal facility. These mice express a transgene expressing a simian DTR–EGFP fusion protein under the control of a CD11c promoter element (22).
Injections. For DC depletion, transgene-positive mice were injected i.p. with 100 ng of DT (≈4 ng of DT per gram of body weight) 12–24 h before further manipulation. For Kupffer cell depletion, WT mice were injected i.p. with 50 μl of a standard suspension of clodronate-loaded liposomes diluted to a final volume of 200 μl in PBS 48 h before further manipulation. For glycolipid treatments, mice were injected i.p. or i.v. with 1 μg of either α-GalCer or α-C-GalCer.
Determination of Serum Cytokine Concentrations. The serum concentrations of IFN-γ and IL-12p70 in mice were measured at indicated time points after treatment with α-GalCer or α-C-GalCer by way of a sandwich ELISA kit for IFN-γ (eBioscience, San Diego, CA) or a sandwich ELISA kit for IL-12 (Pharmingen) according to the manufacturer's protocol.
Flow Cytometry and Intracellular Cytokine Staining (ICCS). For IFN-γ ICCS, freshly isolated splenocytes or liver lymphocytes from C57BL/6 mice were surface-stained for NK and NK T cells by first incubating the cells for 15 min at 4°C with unlabeled anti-mouse Fcγ III/II receptor mAb clone 2.4G2 (Pharmingen) in staining buffer (PBS containing 1% FBS and 0.1% NaN3) to block Fc receptors. Next, the cells were surface-stained with phycoerythrin (PE)-labeled anti-NK1.1 mAb clone PK136 (Pharmingen) and FITC-labeled anti-CD3ε mAb clone 145-2C11 (Pharmingen) in staining buffer for 30 min at 4°C. After two washes in staining buffer, the cells were fixed and permeablized by using Cytofix/Cytoperm Plus (Pharmingen) and stained for intracellular IFN-γ by using allophycocyanin-labeled anti-IFN-γ mAb clone XMG1.2 (Pharmingen) according to the manufacturer's protocol. The stained cells were then analyzed by using a FACSCalibur instrument (BD Biosciences) with cellquest software (BD Biosciences). For IL-4 ICCS, all of the procedures followed those for IFN-γ ICCS, except that cells were stained by using PE-labeled anti-IL-4 mAb clone BVD4-1D11 (Pharmingen).
For IL-12 ICCS, freshly isolated splenocytes from C57BL/6 or BALB/c mice were surface-stained for CD8α+ and CD8α–DCs with PE-conjugated anti-CD11c mAb clone HL3 (Pharmingen) and FITC-conjugated anti-CD8α mAb clone 53-6.7 (Pharmingen) and then were fixed and permeablized in the manner indicated above. PE-conjugated anti-CD11b mAb clone M1/70, FITC-conjugated anti-CD19 mAb clone 1D3, and FITC-labeled anti-CD3ε mAb clone 145-2C11 (all from Pharmingen) were used to surface-stain macrophages, B cells, and T cells, respectively. Intracellular IL-12 was then probed by using allophycocyanin-conjugated anti-IL-12 p40/p70 mAb clone C15.6 (Pharmingen), followed by FACS analysis as indicated above. As an isotype control, we used allophycocyanin-conjugated rat IgG1 clone R3-34 (Pharmingen).
Kupffer cells in liver and macrophages in spleen were stained with FITC-conjugated anti-F4/80 mAb clone A3-1 (Serotec). For marginal zone B cell detection, splenocytes were surface-stained with allophycocyanin-conjugated anti-B220 mAb clone RA3-6B2, FITC-conjugated anti-CD21 mAb clone 7G6, and PE-conjugated anti-CD23 mAb clone B3B4, and we looked for the CD21hi, CD23low population of gated B220+ cells by FACS. Finally, for CD1d detection, FITC-conjugated anti-CD1d mAb clone 1B1 was used, and, as an isotype control, FITC-conjugated rat IgG2b clone A95-1 (Pharmingen) was used.
Results
DCs Act as APCs for Splenic, but Not Hepatic, NK T Cells. To examine the role of DCs in the in vivo response to NK T cell ligands, we used a recently developed CD11c-DTR transgenic (Tg) mouse model that allows for the inducible depletion of DCs in vivo upon DT injection (22). We found that DCs were depleted in the spleens and livers of Tg mice 24 h after DT injection, whereas NK and NK T cells were preserved (Fig. 5 and Table 1, which are published as supporting information on the PNAS web site). We also found that the percentages and absolute numbers of conventional T cells, macrophages, and B cells, including marginal zone B cells, were not markedly affected by DT injection (Table 1), nor was CD1d expression by macrophages in the spleen (Fig. 6A, which is published as supporting information on the PNAS web site).
Because the Tg mouse model for in vivo DC ablation allows for DC depletion but preserves NK and NK T cells, we used this model to assess the role of DCs as APCs for NK T cells. To gauge antigen presentation to NK T cells, we took advantage of α-GalCer's ability to stimulate IFN-γ and IL-4 production in high percentages of NK T cells 1–2 h after injection into mice (3). This early cytokine production by NK T cells depends on T cell antigen receptor interaction with CD1d–α-GalCer complexes and not on secondary cytokine signaling (23). As a consequence, the relative numbers of IFN-γ- and IL-4-positive NK T cells at 2 h post-α-GalCer treatment is indicative of the degree of antigen presentation taking place in vivo.
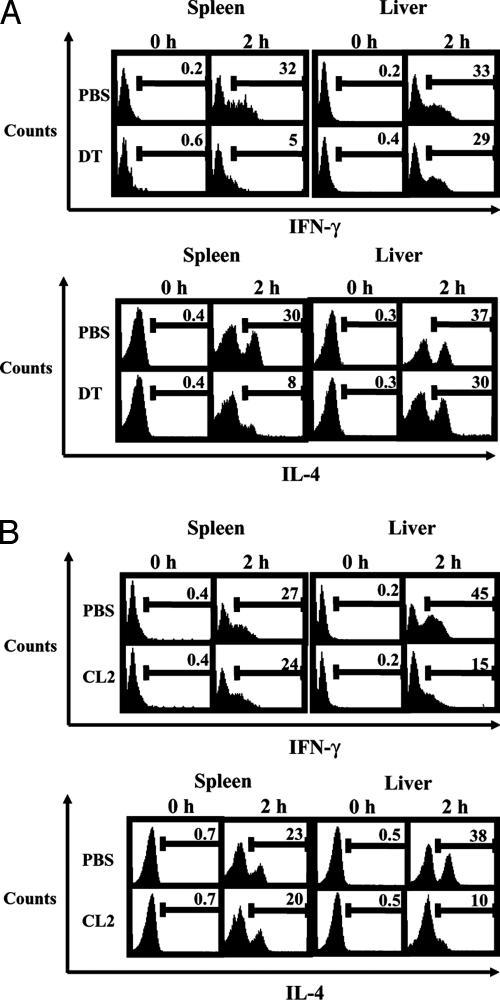
We injected groups of C57BL/6 Tg mice with DT or PBS and, 24 h later, treated the mice with α-GalCer. Two hours later, we obtained splenocytes and liver lymphocytes and analyzed them for the number of IFN-γ- and IL-4-positive NK T cells by way of ICCS. In the spleens of PBS-treated control mice, we found that 32% of NK T cells stained positive for IFN-γ 2hafter α-GalCer treatment, whereas 30% stained positive for IL-4. In contrast, only ≈5% of NK T cells stained positive for IFN-γ in the spleens of DC-depleted mice 2 h after α-GalCer treatment; 8% stained positive for IL-4 (Fig. 1A).
Fig. 1.
DC depletion results in diminished reactivity of splenic NK T cells, whereas Kupffer cell depletion results in diminished reactivity of liver NK T cells to α-GalCer. (A) Groups of three C57BL/6 Tg mice were injected with DT or PBS and, 24 h later, were treated with 1 μg of α-GalCer. Two hours after glycolipid treatment, splenocytes and hepatic lymphocytes were isolated and subjected to ICCS. NK1.1+ and CD3+ NK T cells were gated from both cell populations. (B) Groups of three WT C57BL/6 mice were injected with either clodronate-loaded liposomes (CL2) or PBS-loaded liposomes (PBS) and, 48 h later, were treated with 1 μg of α-GalCer. Two hours after glycolipid treatment, splenocytes and hepatic lymphocytes were isolated and subjected to ICCS. NK1.1+ and CD3+ NK T cells were gated from both cell populations. The data shown come from one of three independent experiments with similar results.
As for the liver, we found that similar percentages of NK T cells stained positive for both IFN-γ andIL-42hafter α-GalCer injection, regardless of DC depletion. In PBS-treated mice, 33% of hepatic NK T cells stained positive for IFN-γ, compared with 29% in DT-treated animals (Fig. 1 A). Similarly, 37% of liver NK T cells stained positive for IL-4, compared with 30% in DC-depleted mice. Based on these results, DCs appear to be important cells, responsible for presenting glycolipid to NK T cells in the spleen but not to NK T cells in the liver.
Kupffer Cells Act as APCs for Liver NK T Cells. Our finding that liver NK T cells are not affected by DC depletion at 2 h post-α-GalCer treatment suggests that DCs are not the primary APCs for NK T cells in the liver. An alternative APC for liver NK T cells might be Kupffer cells. Kupffer cells comprise the major phagocyte population in the liver, and they are known to be involved in various immune and inflammatory responses (24, 25). They also express CD1d and thus have the capacity to present α-GalCer (13). To assess the role of this cell population in presenting glycolipid antigens to liver NK T cells in vivo, we used clodronate-loaded liposomes, which are known to deplete Kupffer cells 24–48 h after injection into mice (21, 26, 27).
We treated groups of WT mice with clodronate-loaded liposomes (clodronate liposomes) or with PBS-loaded liposomes (PBS liposomes) as controls and, 2 days later, injected the mice with α-GalCer. We found that the dose of clodronate liposomes we used depleted >90% of Kupffer cells 2 days after injection (Fig. 7 and Table 2, which are published as supporting information on the PNAS web site) but preserved the relative and absolute numbers of NK cells, NK T cells, conventional T cells, B cells, and DCs in the liver (Table 2, which is published as supporting information on the PNAS web site). In contrast, the dose of clodronate liposomes we used depleted only a small subset of splenic macrophages (Fig. 7 and Table 2) but preserved the relative and absolute numbers of DCs, NK cells, NK T cells, conventional T cells, and B cells, including marginal zone B cells (Table 2). We also found that clodronate liposome injection had no effect on CD1d expression by DCs in the liver (Fig. 6B).
Two hours after α-GalCer treatment, we obtained splenocytes and liver lymphocytes from the mice for ICCS analysis of IFN-γ- and IL-4-producing NK T cells. In the spleens of PBS liposome-treated mice, we found that ≈27% of NK T cells stained positive for IFN-γ, whereas 23% stained positive for IL-4. Similarly, in the spleens of clodronate liposome-treated mice, 23% of splenic NK T were IFN-γ-positive, whereas 20% were IL-4-positive (Fig. 1B). In sharp contrast, clodronate liposome treatment greatly reduced the percentages of liver NK T cells secreting both IFN-γ and IL-4 2 h after α-GalCer injection. Whereas ≈45% of liver NK T cells stained positive for IFN-γ and 38% stained positive for IL-4 in mice receiving PBS liposomes, in mice injected with clodronate liposomes, only 15% of liver NK T cells stained positive for IFN-γ and 10% stained positive for IL-4 (Fig. 1B). In all, these results indicate that Kupffer cells act as important APCs for NK T cells in the liver.
DCs, but Not Kupffer Cells, Are Required for Optimal Th1 Cytokine Production Induced by NK T Cell Ligands. In addition to stimulating cytokine production by NK T cells 1–2 h after injection, α-GalCer also stimulates secondary production of IFN-γ by NK cells at later time points (4, 12, 23). Similarly, α-C-GalCer is able to stimulate secondary production of IFN-γ by NK cells even though it is a weak stimulus for primary cytokine production by NK T cells (12). The downstream IFN-γ stimulated by α-GalCer or α-C-GalCer requires the concomitant production of IL-12 (12, 23), which a number of studies suggest comes from DCs (17–19). Our finding that both DCs and Kupffer cells act as APCs for NK T cells raises the possibility that both of these cell types play a role in coordinating the downstream Th1 cytokine storm induced by α-GalCer or α-C-GalCer.
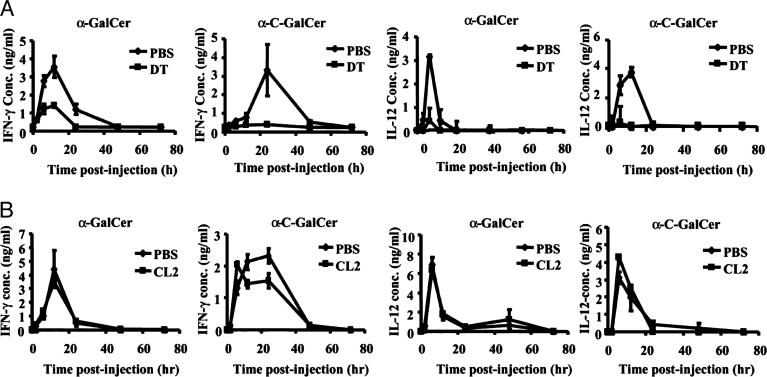
To assess the importance of DCs in downstream Th1 cytokine production induced by glycolipid administration, we injected groups of Tg mice with DT or PBS and, 12 h later, treated them with identical doses of α-GalCer or α-C-GalCer. We then obtained blood samples from the mice 2, 6, 12, 24, 48, and 72 h after glycolipid treatment and analyzed the IFN-γ and IL-12 concentrations in the serum by way of ELISA. First, we observed a significant difference in the serum levels of IFN-γ upon injection of α-GalCer and α-C-GalCer in Tg mice receiving DT compared with those receiving PBS. Tg mice injected with PBS produced IFN-γ in response to α-GalCer and α-C-GalCer in a comparable manner to that observed previously (12): α-C-GalCer stimulated prolonged IFN-γ production compared with α-GalCer (Fig. 2A). However, when Tg mice were injected with DT, the α-GalCer- and α-C-GalCer-stimulated IFN-γ levels in the sera were markedly reduced (Fig. 2 A). Interestingly, we were able to detect some IFN-γ in DT-treated Tg mice injected with α-GalCer but were unable to detect the cytokine in such mice treated with α-C-GalCer (Fig. 2 A).
Fig. 2.
DC but not Kupffer cell depletion results in diminished Th1 cytokine production in response to α-GalCer or α-C-GalCer administration. (A) Groups of five DTR Tg mice were injected with DT or PBS and, 12 h later, were treated with 1 μgof α-GalCer or α-C-GalCer. Blood samples were taken for ELISA analyses of IFN-γ and IL-12 concentrations in the sera of the mice 2, 6, 12, 24, 48, and 72 h after glycolipid injection. One representative experiment of three is shown. (B) Groups of five WT mice were injected with clodronate-loaded liposomes (CL2) or PBS-loaded liposomes (PBS) and, 48 h later, were treated with 1 μgof α-GalCer or α-C-GalCer. Blood samples were taken for ELISA analyses of IFN-γ and IL-12 concentrations in the sera of the mice 2, 6, 12, 24, 48, and 72 h after glycolipid injection. The data are expressed as the average ± SD of two different dilutions of pooled sera. One representative experiment of two is shown.
When we measured the serum levels of IL-12 after glycolipid treatment, we found that depletion of DCs in Tg mice had a very striking effect on the serum levels of IL-12. In Tg mice injected with PBS, the α-GalCer- and α-C-GalCer-induced IL-12 production profiles followed the same pattern observed previously (12). In these mice, α-C-GalCer stimulated a more prolonged IL-12 production than did α-GalCer (Fig. 2 A). In contrast, Tg mice injected with DT produced virtually no detectable IL-12 after injection of either α-GalCer or α-C-GalCer (Fig. 2 A).
Next, to assess the importance of Kupffer cells in downstream Th1 cytokine production induced by glycolipid administration, we injected groups of WT mice with clodronate liposomes or PBS liposomes and, 2 days later, treated them with identical doses of α-GalCer or α-C-GalCer. We then obtained blood samples from the mice 2, 6, 12, 24, 48, and 72 h after glycolipid treatment and analyzed the IFN-γ and IL-12 concentrations in the serum by way of ELISA. In contrast to what we observed in DC-depleted mice, we found that both the IFN-γ and IL-12 production profiles remained the same, regardless of whether mice were depleted of Kupffer cells (Fig. 2B). Overall, these results indicate an important role for DCs, but not Kupffer cells, in coordinating the systemic Th1-type response that follows from injection of α-GalCer or α-C-GalCer. However, it is possible that Kupffer cells produce some IL-12 locally in the liver in response to glycolipid administration.
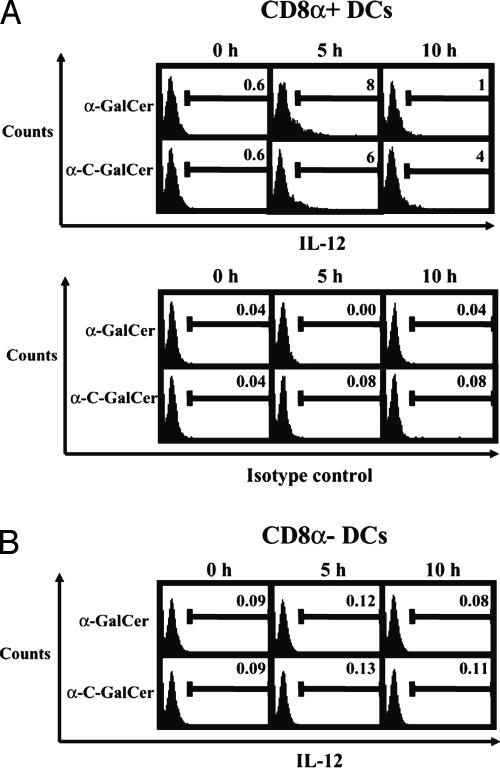
CD8α+ DCs Are the Principal in Vivo IL-12 Producers After α-GalCer orα-C-GalCer Injection. Our observation that DC-depleted Tg mice produce virtually no IL-12 in response to α-GalCer or α-C-GalCer treatment, whereas Kupffer cell-depleted mice do, strongly suggests that the main IL-12 producers after glycolipid injection come from the CD11c+ DC population. Because DCs act as important APCs for NK T cells in the spleen, we injected WT mice with α-GalCer or α-C-GalCer and, 5 and 10 h later, obtained spleen cells for ICCS analysis of IL-12-positive DCs. More specifically, we looked for the presence of IL-12 in CD8α+ DCs and CD8α–DCs because recent studies suggest that different DC subsets possess different capacities to produce cytokines (28–30).
We found that, in response to α-GalCer injection, ≈8% of CD8α+ DCs stained positive for IL-12 5 h after glycolipid injection, whereas ≈6% of CD8α+ DCs stained positive for IL-125hafter α-C-GalCer injection (Fig. 3A). At this same time point, CD8α–DCs did not stain positive for IL-12 at levels above background (Fig. 3B), nor did macrophages, B cells, or T cells, which we also checked (Fig. 8, which is published as supporting information on the PNAS web site). At 10 h postglycolipid treatment, the percentage of IL-12-positive CD8α+ DCs in α-GalCer-treated mice fell to 1%, whereas the percentage of CD8α+ DCs positive for IL-12 fell to 4% in mice treated with α-C-GalCer (Fig. 3A). Again, at this time point, CD8α–DCs did not stain positive for IL-12 (Fig. 3B), nor did macrophages, B cells, or T cells (Fig. 8). Overall, these results show that CD8α+ DCs are the primary producers of IL-12 stimulated by NK T cell ligands.
Fig. 3.
CD8α+ DCs produce IL-12 in vivo after α-GalCer or α-C-GalCer injection. Groups of three WT mice were injected with 1 μgof α-GalCer or α-C-GalCer, and, 5 or 10 h later, splenocytes were obtained and subjected to ICCS. (A) CD11c+ and CD8α+ DCs were gated and analyzed for the presence of IL-12. Also shown are isotype controls for each time point. The numbers shown represent the percentage of IL-12-positive cells in the gated population. (B) CD11c+ and CD8α–DCs were gated and analyzed for the presence of IL-12. The data shown come from one of four experiments with similar results.
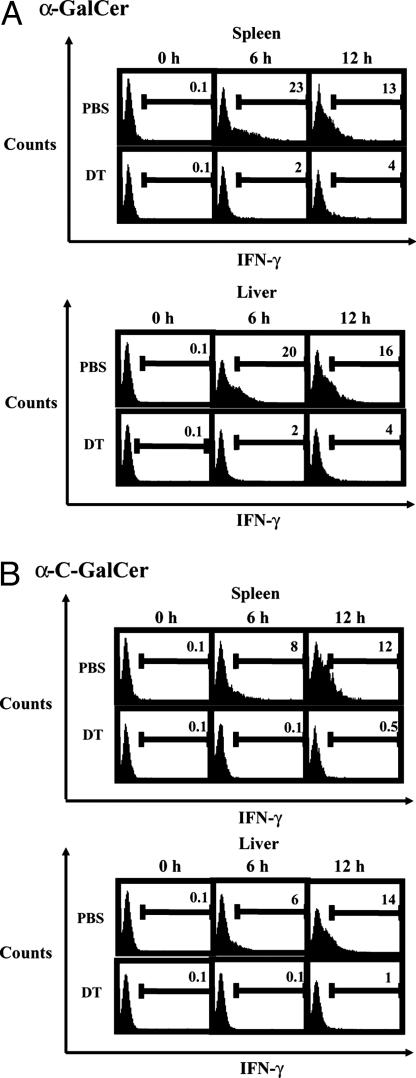
DCs Are Required for Downstream IFN-γ Production by NK Cells upon α-GalCer or α-C-GalCer Treatment. Because CD8α+ DCs produce IL-12 in response to α-GalCer or α-C-GalCer injection and because downstream IFN-γ production in response to glycolipid injection depends on IL-12-mediated stimulation of NK cells (12), we predicted that the NK cell IFN-γ response to α-GalCer and α-C-GalCer would be markedly attenuated in DC-depleted mice. To test this hypothesis, we injected groups of C57BL/6 Tg mice with DT or PBS and, 24 h later, treated these mice with α-GalCer or α-C-GalCer. We then obtained splenocytes and liver lymphocytes from the mice 6 and 12 h later for ICCS analysis of IFN-γ-positive NK cells. In both the spleens and livers of PBS-treated Tg mice, we found that NK cells produced IFN-γ in response to α-GalCer or α-C-GalCer in a manner comparable to that observed previously in WT mice (12): α-GalCer-treated mice possessed peak levels of IFN-γ-producing NK cells at 6 h posttreatment; these levels fell by 12 h (Fig. 4A). α-C-GalCer-treated mice possessed lower levels of IFN-γ-positive NK cells at 6 h, with peak levels occurring 12 h after treatment (Fig. 4B).
Fig. 4.
DC depletion results in attenuated downstream IFN-γ production by NK cells after α-GalCer or α-C-GalCer injection. Groups of three C57BL/6 Tg mice were injected with DT or PBS and, 24 h later, were treated with 1 μg of α-GalCer (A) or α-C-GalCer (B). Hepatic lymphocytes and splenocytes were isolated and subjected to ICCS 6 or 12 h after glycolipid treatment. NK1.1+ and CD3–NK cells were gated from both cell populations. One representative experiment of two is shown.
In contrast to these nondepleted controls, Tg mice depleted of DCs exhibited far fewer IFN-γ-positive NK cells 6 and 12 h after injection of α-GalCer or α-C-GalCer. At 6 h post-α-GalCer treatment, only 2% of NK cells isolated from either spleen or liver stained positive for IFN-γ in DC-depleted mice, down from the 20–23% found in nondepleted controls (Fig. 4A). Similarly, at 12 h post-α-GalCer treatment, only 4% of spleen and liver NK cells produced IFN-γ, compared with 13–16% in nondepleted mice (Fig. 4A).
In DC-depleted Tg mice treated with α-C-GalCer, <1% of NK cells from both the spleen and liver stained positive for IFN-γ 6 h after treatment, compared with the 6–8% of such cells that produced IFN-γ in these organs in nondepleted controls (Fig. 4B). Likewise, at 12 h post-α-C-GalCer treatment, only 0.5–1% of NK cells stained positive for IFN-γ in the spleens and livers of DC-depleted mice, whereas 12–14% of such cells were IFN-γ-positive in nondepleted controls (Fig. 4B). In all, these data closely resemble those observed previously in IL-12-deficient mice (12) and strongly suggest that IL-12 produced by CD8α+ lymphoid DCs is responsible for the downstream IFN-γ production of NK cells stimulated by α-GalCer or α-C-GalCer.
Discussion
In the current study, we addressed the question of what cells act as APCs for NK T cells in vivo after injection of glycolipid ligands into mice. Based on prior work showing a potent ability of DCs to stimulate NK T cells (14–19, 31), we first focused on these cells. To evaluate the role of DCs as APCs for NK T cells in vivo, we used a Tg mouse model that allows for transient depletion of DCs (22). The expression of simian DTR on the surface of CD11c+ DCs in the Tg mice allows for the depletion of these cells upon injection of DT (32, 33). Importantly, DT injection into Tg mice does not affect the percentages or absolute numbers of NK or NK T cells.
To evaluate the importance of DCs as APCs for NK T cells, we injected α-GalCer into Tg mice depleted of DCs and checked for NK T cell responsiveness 2 h later by ICCS analysis of IFN-γ and IL-4 production. Because the cytokine production that occurs in NK T cells 1–2 h after α-GalCer treatment depends on T cell antigen receptor interaction with CD1d–α-GalCer complexes and not on secondary cytokine signaling (23), measurement of NK T cell-derived IFN-γ and IL-4 2 h after α-GalCer injection indicates the degree of functional antigen presentation taking place in vivo. Our finding that the number of IFN-γ- and IL-4-producing NK T cells is greatly reduced in the spleens of DC-depleted Tg mice injected with α-GalCer strongly suggests that DCs are the important cells presenting glycolipids to NK T cells in the spleen. In contrast, our finding that hepatic NK T cell responsiveness to α-GalCer was unaffected by DC depletion indicates that DCs are not important APCs for liver NK T cells.
That the depletion of DCs has no effect on the responsiveness of liver NK T cells is not surprising in light of a recent study showing that liver DCs are far less immunogenic and far fewer in number than those found in the spleen (34). Because DCs do not seem to be important in hepatic NK T cell antigen presentation, we considered Kupffer cells as an alternative cell type that might act as APCs for liver NK T cells. Kupffer cells are liver macrophages that express CD1d (13) and that partake in various immunoregulatory functions in the liver (24, 25, 35–38). To assess the role of these cells as APCs for liver NK T cells, we used clodronate-loaded liposomes, which deplete Kupffer cells 24–48 h after injection into mice (21, 26, 27). When loaded into liposomes, clodronate preferentially gains access into Kupffer cells and accumulates to the point where it triggers cell death.
In mice depleted of Kupffer cells, we found that far fewer hepatic NK T cells responded to α-GalCer at 2 h than did such cells in nondepleted controls. In contrast, we observed no difference in the splenic NK T cell response to α-GalCer 2 h after injection, regardless of whether clodronate liposomes were administered beforehand. This latter result is consistent with our earlier findings that showed the importance of DCs as APCs for splenic NK T cells. It still is possible that because clodronate liposome injection depleted only a small subset of splenic macrophages, the remaining macrophages could have APC function for splenic NK T cells. However, in view of our DC depletion data, these remaining splenic macrophages would have to belong to the CD11c+ population. Regardless, that clodronate liposome injection had no effect on the ability of splenic NK T cells to produce cytokines in response to α-GalCer indicates that these liposomes do not adversely affect the function of NK T cells in vivo. Thus, the diminished NK T cell response we observed in the livers of mice treated with clodronate liposomes stems from the depletion of Kupffer cells and indicates that Kupffer cells act as important APCs for liver NK T cells. Interestingly, although the liver NK T cell response to α-GalCer is reduced in mice injected with clodronate liposomes, there is still some detectable NK T cell activity present. This partial activity may stem from incomplete depletion of Kupffer cells by clodronate liposomes. Alternatively, the residual NK T cell response after Kupffer cell depletion could be due to other cell types, including hepatocytes, B cells, endothelial cells, and even liver DCs. More research is needed to clarify this issue.
Even though both DCs and Kupffer cells act as APCs for NK T cells in vivo, we found that DCs played the major role in coordinating the downstream Th1 cytokine response stimulated by NK T cell ligands. In mice depleted of DCs, we observed reduced levels of IFN-γ and IL-12 after α-GalCer or α-C-GalCer injection. In contrast, we observed no difference in the serum levels of these cytokines in mice depleted of Kupffer cells. Previously, we showed that downstream IFN-γ production after α-GalCer or α-C-GalCer administration depends on IL-12-mediated activation of NK cells (12). Our finding that IL-12 production in mice is abrogated in the absence of DCs, but not in the absence of Kupffer cells, implies that DC-derived IL-12 is responsible for the later IFN-γ produced by NK cells, a conclusion that is supported by our additional finding that NK cell IFN-γ production in response to α-GalCer or α-C-GalCer is markedly attenuated in DC-depleted mice. This DC-derived-IL-12-mediated stimulation of downstream IFN-γ appears to be distinct from the IFN-γ produced within the first 2 h of α-GalCer administration, which depends on the interaction of T cell antigen receptors expressed by NK T cells with CD1d-glycolipid complexes expressed by APCs. The fact that a small amount of IFN-γ is detectable in the serum of DC-depleted α-GalCer-treated mice at early time points, but not at later ones, supports this scenario. The early IFN-γ in these mice probably comes from liver NK T cells stimulated by α-GalCer-bearing Kupffer cells. Later on, however, no IFN-γ is made, because there are no DCs present to produce IL-12, which is needed to stimulate NK cells to produce downstream IFN-γ. A similar process most likely occurs in DC-depleted α-C-GalCer-treated mice, but because α-C-GalCer is a weaker stimulus for early IFN-γ production by NK T cells, the amount of IFN-γ in the sera of these mice was undetectable. Despite the noninvolvement of Kupffer cells in supplying the systemic IL-12 needed for downstream IFN-γ production by NK cells, they may produce smaller amounts of IL-12 locally in the liver.
That DCs are the cell type responsible for making IL-12 in response to α-GalCer or α-C-GalCer injection was verified by our finding that CD8α+ DCs stain positive for IL-12 5–10 h after glycolipid administration. No other cell types analyzed, including CD8α–DCs, macrophages, B cells, and T cells, were found to stain positive for IL-12 above background levels. This result agrees with a number of previous studies that show the predilection of CD8α+ DCs to act as the primary IL-12 producers in response to different microbial stimuli or to T cell-associated CD40 ligand (CD40L) (28–30). It also agrees with a very recent study by Fujii et al. (39) that shows that CD8α+ DCs are the main IL-12 producers after in vivo administration of α-GalCer. The study by Fujii et al. also shows that CD40–CD40L interaction between DCs and NK T cells is required for downstream production of IL-12 in response to α-GalCer, an observation that has been reported by a number of other groups (15, 17, 18, 23, 40). This finding helps explain why α-GalCer- and α-C-GalCer-induced IL-12 is not affected by Kupffer cell depletion. Kupffer cells are capable of producing IL-12, but their ability to do so optimally requires ligation of toll-like receptors by microbial stimuli, not CD40–CD40L (24, 25, 35–38).
Overall, this report reveals the organ-dependent fashion of glycolipid presentation to NK T cells in vivo. In particular, it shows that DCs are the crucial APCs for splenic NK T cells, whereas Kupffer cells are the key APCs for hepatic NK T cells. Despite this compartmentalization of NK T cell antigen presentation, the report also demonstrates the primacy of DCs in coordinating the systemic Th1-type response that follows from injection of the NK T cell ligands α-GalCer and α-C-GalCer. This activity involves CD8α+ DC production of IL-12, which stimulates downstream IFN-γ production by NK cells in different organs.
Supplementary Material
Acknowledgments
We thank C. Palavacino for technical assistance and G. Eberl for critical review of the manuscript. This work was supported by National Institutes of Health Grants GM-60271 (to R.W.F.) and AI-47840 (to M.T.) and a grant from the Department of Chemistry at Hunter College/City University of New York (to R.W.F.). M.T. also was supported by New York University School of Medicine and The Aaron Diamond AIDS Research Center.
Author contributions: J.S. and M.T. designed research; J.S. performed research; J.S. and M.T. analyzed data; J.S. and M.T. wrote the paper; and G.Y., R.W.F., and N.V.R. contributed new reagents/analytic tools.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: α-C-GalCer, α-C-galactosylceramide; α-GalCer, α-galactosylceramide; APC, antigen-presenting cell; DC, dendritic cell; DT, diphtheria toxin; DTR, DT receptor; ICCS, intracellular cytokine staining; NK, natural killer; Tg, transgenic; PE, phycoerythrin.
References
- 1.Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. (2000) Immunol. Today 21, 573–583. [DOI] [PubMed] [Google Scholar]
- 2.Kawano, T., Cui, J., Koezuka, Y., Toura, I., Kaneko, Y., Motoki, K., Ueno, H., Nakagawa, R., Sato, H., Kondo, E., et al. (1997) Science 278, 1626–1629. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda, J. L., Naidenko, O. V., Gapin, L., Nakayama, T., Taniguchi, M., Wang, C., Koezuka, Y. & Kronenberg, M. (2000) J. Exp. Med. 192, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnaud, C., Lee, D., Donnars, O., Park, S., Beavis, A., Koezuka, Y. & Bendelac, A. (1999) J. Immunol. 163, 4647–4650. [PubMed] [Google Scholar]
- 5.Eberl, G. & MacDonald, H. R. (2000) Eur. J. Immunol. 30, 985–992. [DOI] [PubMed] [Google Scholar]
- 6.Eberl, G., Brawand, P. & MacDonald, H. R. (2000) J. Immunol. 165, 4305–4311. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura, H., Ohta, A., Sekimoto, M., Sato, M., Iwakabe, K., Nakui, M., Yahata, T., Meng, H., Koda, T., Nishimura, S., et al. (2000) Cell Immunol. 199, 37–42. [DOI] [PubMed] [Google Scholar]
- 8.Wilson, M. T., Johansson, C., Olivares-Villagomez, D., Singh, A. K., Stanic, A. K., Wang, C. R., Joyce, S., Wick, M. J. & Van Kaer, L. (2003) Proc. Natl. Acad. Sci. USA 100, 10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe, N. Y., Uldrich, A. P., Kyparissoudis, K., Hammond, K. J., Hayakawa, Y., Sidobre, S., Keating, R., Kronenberg, M., Smyth, M. J. & Godfrey, D. I. (2003) J. Immunol. 171, 4020–4027. [DOI] [PubMed] [Google Scholar]
- 10.Harada, M., Seino, K., Wakao, H., Sakata, S., Ishizuka, Y., Ito, T., Kojo, S., Nakayama, T. & Taniguchi, M. (2004) Int. Immunol. 16, 241–247. [DOI] [PubMed] [Google Scholar]
- 11.Wilson, M. T., Singh, A. K. & Van Kaer, L. (2002) Trends Mol. Med. 8, 225–231. [DOI] [PubMed] [Google Scholar]
- 12.Schmieg, J., Yang, G., Franck, R. W. & Tsuji, M. (2003) J. Exp. Med. 198, 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigl, M. & Brenner, M. B. (2004) Annu. Rev. Immunol. 22, 817–890. [DOI] [PubMed] [Google Scholar]
- 14.Fujii, S., Shimizu, K., Kronenberg, M. & Steinman, R. M. (2002) Nat. Immunol. 3, 867–874. [DOI] [PubMed] [Google Scholar]
- 15.Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R. M. (2003) J. Exp. Med. 198, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomantaite, I., Dikopoulos, N., Kroger, A., Leithauser, F., Hauser, H., Schirmbeck, R. & Reimann, J. (2004) Eur. J. Immunol. 34, 355–365. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura, H., Iwakabe, K., Yahata, T., Nishimura, S., Ohata, A., Ohmi, Y., Sato, M., Takeda, K., Okumura, K., Van Kaer, L., et al. (1999) J. Exp. Med. 189, 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, Y., Tomura, M., Ono, S., Hamaoka, T. & Fujiwara, H. (2000) Int. Immunol. 12, 1669–1675. [DOI] [PubMed] [Google Scholar]
- 19.Trobonjaca, Z., Leithauser, F., Moller, P., Schirmbeck, R. & Reimann, J. (2001) J. Immunol. 167, 1413–1422. [DOI] [PubMed] [Google Scholar]
- 20.Yang, G., Schmieg, J., Tsuji, M. & Franck, R. W. (2004) Angew. Chem. Int. Ed. 43, 3818–3822. [DOI] [PubMed] [Google Scholar]
- 21.Van Rooijen, N. & Sanders, A. (1994) J. Immunol. Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- 22.Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K., Sparwasser, T., Wu, S., Vuthoori, S., Ko, K., Zavala, F., et al. (2002) Immunity 17, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda, J. L., Gapin, L., Baron, J. L., Sidobre, S., Stetson, D. B., Mohrs, M., Locksley, R. M. & Kronenberg, M. (2003) Proc. Natl. Acad. Sci. USA 100, 8395–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki, E., Tsutsui, H., Tsuji, N. M., Hayashi, N., Adachi, K., Nakano, H., Futatsugi-Yumikura, S., Takeuchi, O., Hoshino, K., Akira, S., et al. (2000) Immunol. Rev. 174, 35–46.10807505 [Google Scholar]
- 25.Naito, M., Hasegawa, G., Ebe, Y. & Yamamoto, T. (2004) Med. Electron Microsc. 37, 16–28. [DOI] [PubMed] [Google Scholar]
- 26.Van Rooijen, N. & Van Nieuwmegen, R. (1984) Cell Tissue Res. 238, 355–358. [DOI] [PubMed] [Google Scholar]
- 27.Van Rooijen, N. & Sanders, A. (1996) Hepatology 23, 1239–1243. [DOI] [PubMed] [Google Scholar]
- 28.Reis e Sousa, C., Hieny, S., Scharton-Kersten, T., Jankovic, D., Charest, H., Germain, R. N. & Sher, A. (1997) J. Exp. Med. 186, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalod, M., Salazar-Mather, T. P., Malmgaard, L., Lewis, C., Asselin-Paturel, C., Briere, F., Trinchieri, G. & Biron, C. A. (2002) J. Exp. Med. 195, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochrein, H., Shortman, K., Vremec, D., Scott, B., Hertzog, P. & O'Keeffe, M. (2001) J. Immunol. 166, 5448–5455. [DOI] [PubMed] [Google Scholar]
- 31.Burdin, N., Brossay, L., Koezuka, Y., Smiley, S. T., Grusby, M. J., Gui, M., Taniguchi, M., Hayakawa, K. & Kronenberg, M. (1998) J. Immunol. 161, 3271–3281. [PubMed] [Google Scholar]
- 32.Pappenheimer, A. M., Jr., Harper, A. A., Moynihan, M. & Brockes, J. P. (1982) J. Infect. Dis. 145, 94–102. [DOI] [PubMed] [Google Scholar]
- 33.Holmes, R. K. (2000) J. Infect. Dis. 181, 156–167. [Google Scholar]
- 34.Pillarisetty, V. G., Shah, A. B., Miller, G., Bleier, J. I. & DeMatteo, R. P. (2004) J. Immunol. 172, 1009–1017. [DOI] [PubMed] [Google Scholar]
- 35.Salkowski, C. A., Neta, R., Wynn, T. A., Strassmann, G., van Rooijen, N. & Vogel, S. N. (1995) J. Immunol. 155, 3168–3179. [PubMed] [Google Scholar]
- 36.Takahashi, M., Ogasawara, K., Takeda, K., Hashimoto, W., Sakihara, H., Kumagai, K., Anzai, R., Satoh, M. & Seki, S. (1996) J. Immunol. 156, 2436–2442. [PubMed] [Google Scholar]
- 37.Matsui, K., Yoshimoto, T., Tsutsui, H., Hyodo, Y., Hayashi, N., Hiroishi, K., Kawada, N., Okamura, H., Nakanishi, K. & Higashino, K. (1997) J. Immunol. 159, 97–106. [PubMed] [Google Scholar]
- 38.Seki, E., Tsutsui, H., Tsuji, N. M., Hayashi, N., Adachi, K., Nakano, H., Futatsugi-Yumikura, S., Takeuchi, O., Hoshino, K., Akira, S., et al. (2002) J. Immunol. 169, 3863–3868. [DOI] [PubMed] [Google Scholar]
- 39.Fujii, S., Liu, K., Smith, C., Bonito, A. J. & Steinman, R. M. (2004) J. Exp. Med. 199, 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomura, M., Yu, W., Ahn, H., Yamashita, M., Yang, Y., Ono, S., Hamaoka, T., Kawano, T., Taniguchi, M., Koezuka, Y., et al. (1999) J. Immunol. 163, 93–101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.