Synopsis
The approach to breast cancer screening has changed over time from a blanket approach to a more personalized, risk-based approach. Women with dense breasts, one of the most prevalent risk factors, are now being informed that they are at increased risk of developing breast cancer and to consider supplemental screening beyond mammography. This article reviews the current evidence regarding the impact of breast density relative to other known risk factors, the evidence regarding supplemental screening for women with dense breasts, a description of supplemental screening options, and recommendations for physicians having shared decision-making discussions with women who have dense breasts.
Keywords: risk-based screening, mammography, breast density, supplemental screening
Introduction
With the recently revised recommendations for routine mammography screening from both the U.S. Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS),1,2 there is growing consensus in the medical community that screening regimens should be tailored to patient risk. Overall, routine mammography screening has been shown to reduce mortality and, for those at average risk, routine screening should begin around age 45 or 50 years. However, for those at increased risk, the start age and screening interval remain uncertain. Moreover, the increasing availability of new screening modalities beyond mammography further complicates the landscape of breast cancer screening.
Patient advocacy groups have brought personalized, risk-based screening to the forefront, focusing on breast density as a common risk factor for developing breast cancer. Given the widespread press coverage regarding breast density and a growing number of U.S. state-level density reporting laws, women are increasingly bringing their questions about density as a risk factor to primary care physicians. Common concerns include the accuracy of screening mammography and whether they should have ultrasound or other supplemental screening. To inform these discussions, we aim to describe the current state of risk-based breast cancer screening with a focus on breast density. We review the evidence regarding its impact relative to other known risk factors for developing breast cancer, the evidence for and against supplemental screening for women with dense breasts, a description of current modalities for supplemental screening beyond mammography, and recommendations for physicians having shared decision-making discussions with women who have dense breasts.
Breast Cancer Risk Factors
The strongest risk factors for breast cancer include age and genetic mutations. Additional known risk factors include breast density, family history, and reproductive history. These risk factors are outlined in Table 1, along with their associated relative risks. Factors such as age and genetic mutation status have a larger relative risk than other factors, including breast density.3 Even though breast density is a lower risk than other risk factors such as family history, it is more common in the general population. Thus, some researchers have suggested that breast density alone accounts for a considerable proportion of cancer risk at the population level.3 This was supported by Canadian screening program data which suggested that increased breast density accounts for 16% of all breast cancers diagnosed, 40% of interval cancers diagnosed, and 12% of screen-detected cancers.4
Table 1.
Relative Risks of Developing Breast Cancer for Women Age 40–49
| Risk Factor | Breast Cancer Risk Ratio (95% CI) |
|---|---|
| Two 1st degree relatives with breast cancer | 3.84 (2.37–6.22) |
| 1st degree relative with breast cancer at age <40 | 3.0 (1.8–4.9) |
| 1st degree relative with breast cancer at age <50 | 2.17 (1.86–2.53) |
| One 1st degree relative with breast cancer | 2.14 (1.92–2.38) |
| Extremely dense breasts on mammography | 2.04 (1.84–2.26) |
| Prior benign breast biopsy | 1.87 (1.64–2.13) |
| 2nd degree relative with breast cancer | 1.7 (1.4–2.0) |
| Heterogeneously dense breasts on mammography | 1.62 (1.51–1.75) |
| Current oral contraceptive use | 1.30 (1.13–1.49) |
| Nulliparity | 1.25 (1.08–1.46) |
| Age at first birth ≥ 30 years | 1.20 (1.02–1.42) |
Adapted from Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women age 40 to 49: a systematic review and meta-analysis. Ann Intern Med 2012; 156(9):635–48; with permission.
Risk-Based Screening Protocols
In the absence of randomized controlled trials evaluating the efficacy of risk-based screening protocols (e.g., different intervals or the use of supplemental screening modalities for women at increased risk), simulation modeling studies have provided insight regarding the likely balance between the benefits and risks of different risk-based screening protocols. These modeling studies suggest that screening regimens should be personalized based on a woman’s age, breast density, and other risk factors.5
One study used several established NCI-funded Cancer Intervention and Surveillance Modeling Network (CISNET) simulation models to determine the most efficient screening strategies based on breast cancer risk. Taking into account advances in imaging and treatment, biennial screening strategies were the most efficient for the majority of women who are at average-risk for breast cancer. However, for women with two- to four-fold increase in risk (e.g., family history of breast cancer or extremely dense breasts – defined as those with the highest of four categories for breast density as described in the next section), annual screening beginning at age 40 had comparable risks and benefits to women at average risk undergoing biennial screening between ages 50 and 74.6 Another CISNET study found that women aged 40–49 with a two-fold increased risk for developing breast cancer (e.g., women with extremely dense breasts) have similar harm-benefit risk ratios compared to average risk women aged 50–74 undergoing biennial screening.7
Mammographic Breast Density
Given the importance of breast density as a risk factor for breast cancer and increasing attention to screening strategies based on density, a brief background on breast density is provided. Breast density is defined based on a subjective estimate made by an interpreting radiologist of the relative amount of radiopaque breast parenchyma in relation to radiolucent fatty tissue comprising each breast. This measure does not correlate with physical breast examination findings of breast firmness.8 Additionally, mammographic breast density has been associated with both a masking effect on mammography, where dense tissue can obscure cancers, as well as an inherent, independent higher risk for the development of breast cancer.3,4
According to the American College of Radiology (ACR), breast density should be subjectively classified into one of four categories by interpreting radiologists under the Breast Imaging Reporting and Data System (BI-RADS): almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense (Figure 1). There is inter- and intra-reader variability in radiologists’ interpretation of breast density. Nevertheless, women who fall into the latter two categories (heterogeneously dense and extremely dense) are commonly lumped together and considered to have “dense breasts.” About 43% of women aged 40–74 in the U.S. have heterogeneously or extremely dense breasts by mammography (Table 2).
Figure 1. Density Categories by Mammography.

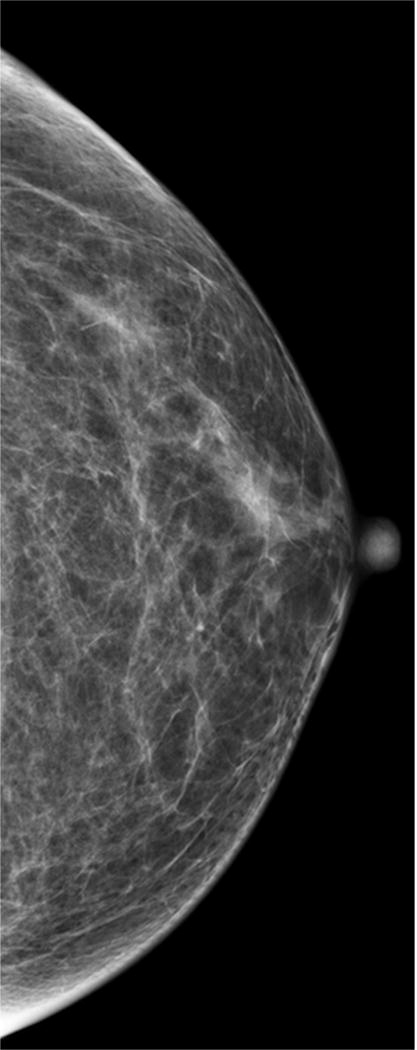
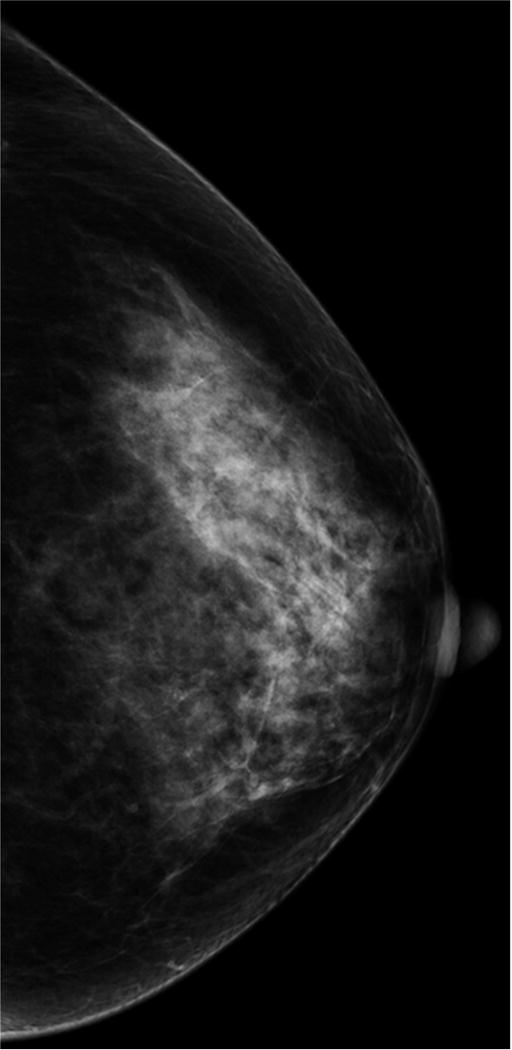
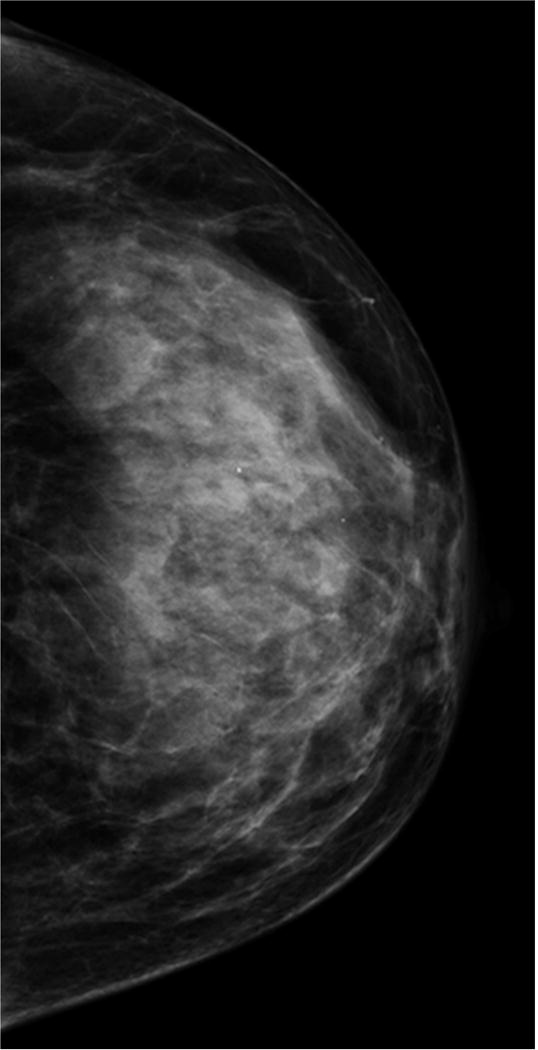
Multiple craniocaudal views of the left breast in four different patients. Approximately 10% of the screening population has almost entirely fatty breasts (A), 40% have scattered fibroglandular densities (B), 40% have heterogeneously dense breasts (C), and 10% have extremely dense breasts (D).
Table 2.
Density Prevalence by Age
| Distribution of BI-RADS Breast Density Categories | ||||
|---|---|---|---|---|
| Age | Almost Entirely Fatty | Scattered Fibroglandular Densities | Heterogeneously Dense | Extremely Dense |
| 40–44 years | 8% | 36% | 44% | 13% |
| 45–49 years | 8% | 37% | 43% | 12% |
| 50–54 years | 12% | 42% | 38% | 8% |
| 55–59 years | 15% | 47% | 33% | 5% |
| 60–64 years | 18% | 49% | 29% | 4% |
| 65–69 years | 18% | 50% | 28% | 3% |
| 70–74 years | 20% | 53% | 24% | 2% |
| 75–79 years | 20% | 53% | 25% | 3% |
| 80–84 years | 18% | 54% | 26% | 2% |
| 85+ years | 19% | 54% | 25% | 3% |
Adapted from Sprague BL, Gangnon RE, Trentham-Dietz A, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014; 106(10):dju255; with permission.
Breast density is influenced by a variety of factors; it usually decreases with increasing age, increases with hormone replacement therapy, and decreases with increasing body mass index (BMI). Other modifiable factors influencing breast density include tamoxifen therapy for chemoprevention, and diet changes.9 Given the moving target of mammographic breast density and its subjective nature, automated software has been developed in an attempt to decrease inter-reader variability, moving towards more quantitative rather than qualitative measures.10 While automated quantitative density measurement software is approved by the U.S. Food and Drug Administration (FDA), existing technology is currently limited in accurately estimating three-dimensional density and is sensitive to breast positioning. Thus, none are currently in wide use for general screening in the U.S.11,12
Breast Density as a Double-Pronged Risk
Increased breast density can mask cancers in dense tissue on mammography leading to lower accuracy of mammography. A study on 329,495 women undergoing screening mammography in the U.S. between 1996 and 1998, found that mammography had a sensitivity of 87% and specificity of 97% for women with fatty breasts (with 12 month follow-up data to ensure capture of interval cancers). In comparison, sensitivity was 63% and specificity was 89% for women with extremely dense breasts.13 In fact, mammographic breast density, along with age, remains a primary predictor for the accuracy of screening mammography.13 Given this, radiologists now frequently reference having heterogeneously or extremely dense breasts as a limitation in their final reports as it lowers the sensitivity of mammography.
The masking effect caused by dense breast tissue has been reduced with the adoption of digital mammography. The Digital Mammographic Imaging Screening Trial (DMIST) demonstrated that the overall diagnostic accuracy of digital mammography is similar to that of screen-film mammography, but that digital mammography is more accurate for women under age 50, women with dense breasts, and women who are premenopausal or perimenopausal.14 A large prospective cohort study involving over 300,000 women in the U.S., found that digital mammography had higher sensitivity for women with extremely dense breasts compared to screen-film mammography (83.6% vs. 68.1%; p=0.05), confirming DMIST results in U.S. community practice.15 Digital mammography now comprises more than 95% of mammography units used in the U.S. and is the standard primary screening modality of choice for women with dense breasts.
Beyond the masking effect, mammographic breast density is also an independent risk factor for the development of breast cancer. It is hypothesized that the greater proportion of epithelial and non-epithelial cells in areas of high breast density, and the greater cumulative exposure to hormones and growth factors, may stimulate more cell division which increases breast cancer risk.16 A systematic review and meta-analysis of 42 studies evaluating breast cancer risk related to breast density found that the relative risk of incident breast cancer is 2.92 for women with heterogeneously dense breasts and 4.64 for women with extremely dense breasts compared to women with almost entirely fatty breasts.17 These figures, however, may be misleading since they are comparing relative risks for women with dense breasts to women with almost entirely fatty breasts, the lowest classification for breast density that affects only about 10% of the screening population. Instead, when compared to women with scattered fibroglandular densities, relative breast cancer risk associated with breast density is much smaller and is estimated to be about 1.2 for women with heterogeneously dense breasts and 2.1 for women with extremely dense breasts.18 The variable density comparison groups used in different analyses has led to confusion regarding the true magnitude of cancer risk associated with dense breasts.
Patients have voiced concerns about the increased risk of breast cancer and the potential masking effect on mammography from dense breasts. Fueled by patient advocacy groups, more than half of U.S. states have now adopted legislation requiring radiology facilities to disclose mammography breast density directly to women, many with language recommending that patients discuss options for supplemental screening with their physicians.19,20 Connecticut was the first state to enact density reporting legislation in 2011, with some clinicians referring all patients with dense breasts for supplemental ultrasound in the first year after adoption.21 National legislation is also currently under consideration, including a proposed amendment to the Public Health Service Act requiring notification of breast density to patients and stating that they may benefit from supplemental screening.22 With mandatory reporting and greater patient and physician awareness regarding dense breasts, there are increasing opportunities for shared decision-making and personalized screening regimens.23 However, the challenges of classifying density and the limited evidence base need to be considered and are described below.
Tomosynthesis
In 2011, the FDA approved digital breast tomosynthesis for all clinical indications accepted for mammography, including screening. In contrast to digital mammography, tomosynthesis obtains multiple mammographic images with the x-ray source traveling in an arc over the compressed breasts, allowing for a three-dimensional reconstruction of the breast.24 This technology is now a built-in feature of newer generation digital mammography units and can be obtained during the same compression required for standard digital mammography views. By allowing radiologists the ability to scroll through breasts slice-by-slice, tomosynthesis can further mitigate the masking effect of dense breasts and allow visualization of small breast cancers.
Early evidence from prospective cohort studies regarding tomosynthesis for population-based screening are limited.25,26 Interim results from a Norwegian study, involving 12,631 women aged 50–69, found that adding tomosynthesis to digital mammography screening resulted in a 31% increase in cancer detection rate and a 15% decrease in recall rate.25 An Italian study demonstrated similar results, with the detection of 8.1 cancers per 1,000 screens when tomosynthesis was added, compared to 5.3 cancers per 1,000 screens for digital mammography alone, with false-positive recalls decreasing by 17% with the addition of tomosynthesis.26 The Italian study also demonstrated similar improvements in cancer detection rate beyond digital mammography for women with dense and non-dense breasts alike, suggesting that tomosynthesis could be beneficial for all women. However, reports from both European studies were limited by small size and the lack of multi-year follow-up data.
A multicenter, retrospective U.S. cohort study found improvements in cancer detection and recall rates comparable to those of European prospective studies after adoption of tomosynthesis.27 This same study, however, suggested increased breast biopsy rates after tomosynthesis adoption, reflecting the unknown nature of the balance between benefits and harms of tomosynthesis screening. One CISNET modeling study examined the potential of adding tomosynthesis to biennial digital mammography screening for U.S. women with dense breasts aged 50–74.28 Researchers found that tomosynthesis screening could avert 0.5 additional breast cancer deaths and 405 false-positive screening examinations among 1,000 women screened.28
Among most facilities that have adopted tomosynthesis, 3D acquisition is obtained in addition to standard 2D acquisition. Thus, since 3D acquisition confers a dose comparable to standard digital mammography, a patient receives about two times the usual radiation dose. Increased radiation dose may be mitigated with adoption of FDA-approved software that provides a synthetic 2D mammogram created from 3D acquisition during tomosynthesis, negating the need for both 2D and 3D image acquisition.29
Tomosynthesis is increasingly being used in clinical practices in the U.S. despite this limited evidence base. From 2013 to 2015, tomosynthesis availability in U.S. imaging facilities participating in the Breast Cancer Surveillance Consortium increased from 0% to 50%.30 Beginning in January 2015, the U.S. Centers for Medicare and Medicaid Services began reimbursing for tomosynthesis for all women regardless of breast density, likely accelerating its rapid adoption. No randomized trial data or long-term follow-up data from any tomosynthesis study are currently available; such studies are necessary to determine the true improvements in accuracy and patient outcomes associated with tomosynthesis.
Challenges of Risk-Based Screening Based on Breast Density
The classification of a woman’s breast density is itself complicated by the fact that there is considerable intra- and inter-reader variability.31 Most of the inter-reader agreement is in the two classifications at the extreme ends of the spectrum (almost entirely fatty and extremely dense), with considerable variation in agreement between the two middle density classes; it is these two middle density classes that separate women as having or not having dense breasts according to the U.S. state legislation efforts.32 In one study of 30 U.S. facilities, assessments of dense breast made by 83 radiologists ranged from 6.3% to 84.5% of all mammograms interpreted by the radiologist, with multivariable adjustments for patient characteristics having little effect on the range of variation.19 Moreover, from one screening mammogram to the next, 17% of women had discordant assessments with regards to having dense versus non-dense breasts, even though breast density likely changes much more gradually and over a longer period of time.19
Potential Supplemental Screening Modalities for Women with Dense Breasts
Over the last several years, multiple imaging modalities have been proposed as supplemental screening tools for women at increased risk of breast cancer, including women with dense breasts. The potential benefits and harms of different supplemental screening modalities currently available to women with dense breasts are summarized in Table 3 and described in more detail below.
Table 3.
Supplemental Screening Options for Women with Dense Breasts
| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| Digital breast tomosynthesis |
|
|
| Screening ultrasound |
|
|
| Magnetic resonance imaging |
|
|
| Contrast-enhanced spectral mammography |
|
|
| Molecular breast imaging |
|
|
Ultrasound
The few states that do mandate insurance coverage for supplemental screening require reimbursement for screening ultrasound.33 Ultrasound has the advantage of not emitting ionizing radiation, being well tolerated by patients, and already being widely available at the majority of radiology practices (Figure 2). The disadvantages include the high operator-dependence, a slowdown in radiologist workflow, and a lower specificity compared to mammography.
Figure 2. Supplemental Screening Breast Ultrasound and MRI.
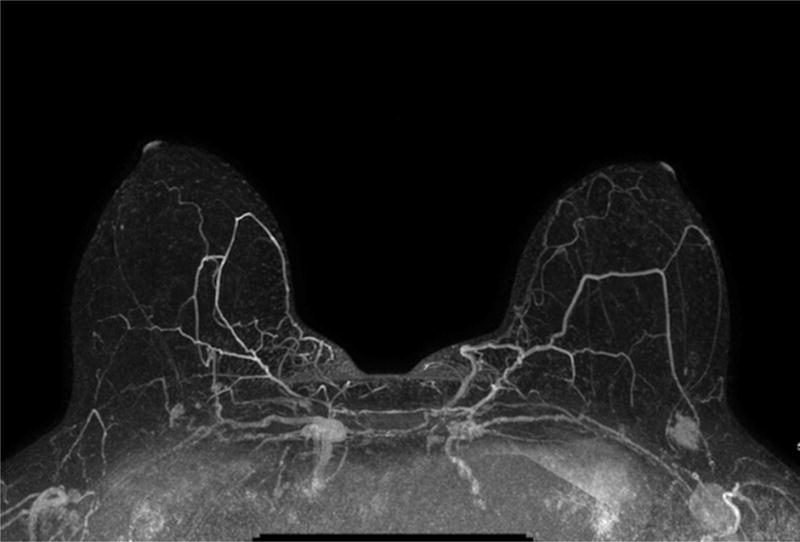
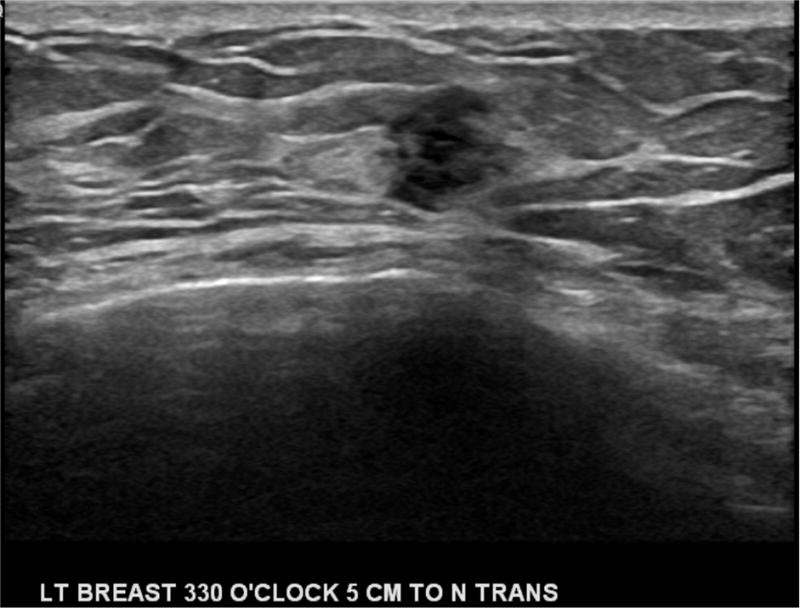
A) Example of a maximum intensity projection image from a screening MRI demonstrating a suspicious mass in the left breast that was subsequently biopsied and found to be early-stage invasive ductal carcinoma. B) Handheld screening ultrasound of the left breast with an irregular hypoechoic mass identified and found to be early-stage invasive ductal carcinoma after ultrasound-guided biopsy.
The highest quality data available regarding supplemental ultrasound for women with dense breasts come from ACRIN 6666, a study of U.S. women with heterogeneously or extremely dense breasts and at least one other risk factor (e.g., family history). This large, prospective, multi-institutional trial demonstrated that supplemental ultrasound screening led to incremental cancer detection increases of 3–4 per 1,000.34 The majority of these additional cancer cases were early invasive cancers with a lower stage and higher likelihood of survival. However, adding ultrasound after a negative mammogram also increased false-positives and benign biopsies, and decreased overall screening PPV.34 A recent systematic review conducted for the USPSTF found that ultrasound after a negative mammogram had a sensitivity of 80–83%, specificity of 86–95%, and PPV of 3–8%.35 Real-world data regarding supplemental ultrasound for women with dense breasts suggests that the incremental cancer detection rate is less than that seen in ACRIN 6666.36
Several CISNET models were used to evaluate supplemental screening ultrasound after negative mammography for women with dense breasts aged 50–74.37 In a comparative modeling study, researchers found that adding ultrasound averted 0.36 additional breast cancer deaths but resulted in 354 unnecessary benign biopsies per 1,000 women screened.37 Even though the comparative weighting of relatively rare benefits of deaths prevented and more common risks such as benign biopsies is somewhat subjective, researchers concluded that adding ultrasound to digital mammography screening likely causes more harm relative to benefits gained.
Handheld ultrasound exams suffer from being highly operator-dependent. To offer reduced operator dependence, automated whole breast ultrasound (ABUS) was approved as a new screening modality by the FDA in 2012. ABUS involves automated sweeps through the entirety of both breasts and can be administered by a technologist. Early studies demonstrate promise for ABUS for detecting additional cancers.38–40 However, its limitations include relatively high false-positive rates, the need to obtain additional handheld ultrasound data for indeterminate findings, and radiologists’ interpretive time for reviewing the hundreds of images obtained.41
MRI
MRI is considered the most sensitive imaging modality for detecting breast cancer (Figure 2), but has low specificity, requires intravenous gadolinium injection, and is associated with a higher false-positive rate and higher costs. The American Cancer Society and other medical societies consider MRI an effective adjunct screening tool for BRCA mutation carriers and other women with > 20% lifetime risk of developing breast cancer;42 however, these recommendations are based on added incidence risk, rather than added mortality risk. Moreover, there is little evidence to support MRI for women with dense breasts and no other risk factors. A systematic review found that, among women with dense breasts, MRI has a sensitivity of 75–100%, specificity of 78–94%, and a PPV of 3–33%.35 When added to mammography screening for women with dense breasts, MRI detects 3.5–28.6 additional cancers per 1,000 women, but is associated with a recall rate of 12–24%.35
A subset of patients in ACRIN 6666, with breast density and at least one other risk factor (thus, intermediate risk), had an additional MRI added to mammography and ultrasound screening. This subanalysis demonstrated that women who had a supplemental MRI had a higher cancer yield and lower false-positive rate than women who had a supplemental ultrasound.43 Thus, for women who have dense breasts and other risk factors that push them over the >20% lifetime risk threshold, supplemental MRI is preferred over ultrasound. To address the lengthy MRI imaging protocol that decreases the level of patient tolerance (30–45 minutes in length), a fast MRI is currently being developed. This abbreviated protocol takes about 3 minutes to acquire images. In early studies for screening women with dense breasts and negative screening mammograms and ultrasounds, the fast MRI has been associated with negative predictive value of 99.8% and a cancer detection rate of 18.3 per 1,000 women.44
Alternative Functional Imaging
MRI is more effective than ultrasound for detecting breast cancers since its interpretation is not hindered by breast density and provides more functional information for breast masses, including lesion vascularity. Dual-energy contrast-enhanced spectral mammography takes advantage of widely available iodinated contrast to detect enhancing lesions within the breast by comparing pre- and post-contrast mammography images. Early studies suggest that this technique is faster and cheaper than MRI, while having the ability to detect nearly all invasive cancers with fewer false positives than MRI.45 Similarly, nuclear medicine techniques can provide information about metabolic activity more suggestive of breast cancers using available radiotracers and are effective in cancer detection independent of breast density.46 Newer breast-specific gamma cameras can achieve improved spatial resolution, allowing for improved detection of smaller invasive cancers and acquisition of images in projections mimicking those of a standard mammogram.47,48
Molecular breast imaging (MBI) techniques, which use intravenous Tc99m-sestamibi, are not currently in widespread use. Early studies suggest increased sensitivity and an improved PPV for MBI plus mammography, versus mammography alone, among women with dense breasts.47,48 However, the additional systemic radiation dose from MBI poses an increased lifetime radiation-induced cancer risk if used repeatedly as an adjunct screening tool. In addition, MBI requires nearby production and storage of radioactive tracers, limiting patient access.
National Group Recommendations
Most national groups agree that the primary screening modality for women with dense breasts is routine digital mammography (Table 4). Even though early studies on tomosynthesis are promising, most authoritative bodies claim that there is insufficient evidence to recommend routine tomosynthesis screening.1 The American College of Physicians goes a step further and currently advises against screening women at average risk for breast cancer with tomosynthesis, citing a current lack of evidence for improved value in care.49
Table 4.
Current National Group Positions on Screening Women with Dense Breasts
| National Group | Year | Recommendation Statement on Primary Screening | Recommendation Statement on Supplemental Screening |
|---|---|---|---|
| U.S. Preventive Services Task Force (USPSTF) | 2009/2016 | “For younger women and women with dense breast tissue, overall detection is somewhat better with digital mammography (2009).” | “[T]he current evidence is insufficient to assess the balance of benefits and harms of adjunctive screening for breast cancer using breast ultrasonography, MRI, DBT, or other methods in women identified to have dense breasts on an otherwise negative screening mammogram (2016).” |
| American Cancer Society (ACS) | 2015 | “Although overall the sensitivity of digital and screen-film mammography is similar, digital mammography is more sensitive in younger women and women with mammographically dense breasts.” | “Accumulating data on digital breast tomosynthesis (DBT) appear to demonstrate further improvements in accuracy (both sensitivity and specificity), and DBT is steadily increasing in prevalence in mammography facilities.” “The [guideline development group (GDG)] also did not include in this review evidence on the effectiveness of supplemental breast imaging for women with mammographically dense breasts.” |
| American College of Radiology (ACR) | 2013 | Annual screening mammography is indicated for high-risk and intermediate-risk women. Additionally, for high-risk women, contrast-enhanced MRI is indicated as well. Ultrasound can be considered as an alternative for those with contraindications to MRI. | “[M]ammography alone does not perform as well as mammography plus supplemental screening in certain subsets of women, particularly those with genetic predispositions to the disease and those with dense breasts.” “Supplemental screening with ultrasound for women with intermediate risk and dense breasts is an option to increase cancer detection.” |
| National Comprehensive Cancer Network (NCCN) | 2016 | “Digital mammography appears to benefit young women and women with dense breasts.” | “Although there are some studies supporting the use of ultrasound for breast cancer screening as an adjunct to mammography for high risk women with dense breast tissue, the NCCN Panel however cautions that there is insufficient evidence to support routine supplemental screening in women with dense breasts and no other risk factors.” |
Moreover, no supplemental screening modalities are currently recommended by most national organizations for women with dense breasts (Table 4).1,50 Currently, only the American College of Radiology considers supplemental ultrasound an appropriate supplemental screening option for women who have dense breasts and at least one additional risk factor.51 Even though supplemental screening ultrasound is covered by insurance in a few U.S. states due to mandatory density reporting laws, there is currently no evidence to support routine use of supplemental screening ultrasound in patients with dense breasts and no other risk factors.52 There are currently several ongoing large-scale trials aimed at addressing the efficacy of multi-modality screening for women with dense breasts.53,54
Shared Decision-Making Discussions
Currently, over half of the U.S. states have implemented some sort of breast density notification law. In response to this legislation, and the likely increase in patient awareness and inquiry around supplemental screening, several efforts have taken place to help provide guidance for shared decision-making conversations between primary care physicians and their patients. Clear communication between physicians and women with dense breasts following these mandatory notifications is especially critical given the fact that the wording used in the dense breast notifications scores low on readability and understandability, meaning that these reports are likely difficult for women to interpret.55 Below, we outline several recommendations for physicians engaging in these discussions.
First, physicians must understand the patient’s risk profile. While breast density is an independent risk factor for the development of breast cancer, the actual risk of developing breast cancer when high breast density is the patient’s only risk factor is quite low (Table 5).56 Additionally, the current evidence suggests that breast density should not be the sole factor for pursuing supplemental screening. This has been supported by a recent prospective cohort study from the U.S. that found that not all women with dense breasts have high interval cancer rates.57 Researchers found that five-year cancer risk was low to average for 51.0% of women with heterogeneously dense breasts and 52.5% of women with extremely dense breasts in a cohort of 365,426 women aged 40–74 years. Thus, women whose only risk factor is high breast density are unlikely to benefit from supplemental screening.
Table 5.
Absolute 5-Year Risk of Breast Cancer by Age and Density
| 5- Year Risk for Breast Cancer by Density (%) | ||||
|---|---|---|---|---|
| Age | Almost Entirely Fatty | Scattered Fibroglandular Densities | Heterogeneously Dense | Extremely Dense |
| 40–44 years | 0.2 | 0.5 | 0.7 | 1.0 |
| 45–49 years | 0.4 | 0.8 | 1.2 | 1.6 |
| 50–54 years | 0.5 | 1.0 | 1.6 | 2.1 |
| 55–59 years | 0.7 | 1.4 | 2.2 | 3.0 |
| 60–64 years | 0.8 | 1.7 | 2.6 | 3.4 |
| 65–69 years | 1.3 | 2.0 | 2.9 | 3.0 |
| 70–74 years | 1.4 | 2.1 | 3.1 | 3.3 |
Adapted from Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 2008; 148(5):337–47; with permission. Table 5
Second, in order to determine a woman’s overall risk profile, physicians should use an established risk model or calculator. Models such as BRCAPRO, Tyrer-Cuzick, and Claus offer estimates of lifetime risk, while the Breast Cancer Surveillance Consortium (BCSC) risk calculator offers estimates of risk for the next five and ten years. It is important that formal risk assessment should take all of a patient’s risk factors into account when determining a personalized screening approach. Online and easily accessible risk assessment tools include the National Cancer Institute’s Breast Cancer Risk Assessment Tool (https://www.cancer.gov/bcrisktool/) and the BCSC’s Risk Calculator (https://tools.bcsc-scc.org/bc5yearrisk/calculator.htm). Only the latter incorporates a woman’s mammographic breast density into its risk calculations.
For women at average risk (i.e., lifetime risk < 15%), regardless of breast density, no supplemental screening beyond digital mammography is recommended. For women at high risk (i.e., lifetime risk >20%), regardless of breast density, supplemental screening is recommended in the form of breast MRI.58 The addition of supplemental ultrasound is unnecessary for these patients as a large multi-institutional trial demonstrated that no additional cancers detected with ultrasound beyond those found by mammography and MRI.43 For those with dense breasts and intermediate risk (i.e., 15–20% lifetime risk), a discussion about the supplemental screening options, such as screening ultrasound, and their associated benefits and risks is suggested. For women at intermediate to high risk, shared decision-making conversations about screening can also involve discussion about potential risk reducing therapies, such as tamoxifen.
These shared decision-making conversations should cover risks, benefits, alternatives, and patient preferences regarding supplemental screening. A Cochrane review of trials examining personalized risk communication on informed decision making suggested that such communications related to breast cancer screening led to increased knowledge and accuracy in personal risk perception among patients.59 While physicians can serve as experts regarding the clinical evidence, the final choice for supplemental screening should be based on the patient’s values. For those women at low or average risk that choose supplemental screening for dense breasts against clinical recommendations, ultrasound is the cheaper and more accessible option. Patients should be warned that, except for a few U.S. states that have mandated insurance coverage for additional supplemental screening, most women will incur additional out-of-pocket expenses to undergo supplemental screening.
Summary
Fueled by patient advocacy groups, there is growing interest in better understanding the increased cancer risk and decreased mammography accuracy associated with breast density. In comparison to screen-film mammography, digital mammography demonstrates higher accuracy for screening women with dense breasts and represents the primary screening modality of choice. Digital breast tomosynthesis, or 3D mammography, shows early promise for mitigating the masking effect of dense breast tissue though evidence regarding the effectiveness of tomosynthesis is lacking. Screening ultrasound likely improves cancer detection, but also raises recall and biopsy rates. While awaiting results from ongoing trials that will help women with dense breasts decide on the most appropriate multi-modality screening regimens, physicians should be able to understand and communicate the absolute and relative risk of dense breasts and review the available options with patients. In addition, physicians should remember to consider breast density as only one factor when determining risk-based screening approaches for individual women.
Key Points.
Breast density is just one factor that should be considered when physicians discuss risk-based breast cancer screening options with women.
Digital mammography remains the primary screening tool for women with dense breasts.
Based on early evidence, digital breast tomosynthesis, or 3D mammography, may hold promise for improving screening accuracy among women with dense breasts, though studies are ongoing.
Most women with dense breasts and no other risk factors would likely experience more harms than benefits with supplemental screening ultrasound.
Women with dense breasts and additional risk factors that place them at high lifetime risk for developing breast cancer (>20%) should undergo breast MRI rather than supplemental screening ultrasound.
Acknowledgments
C.I. Lee is supported by grants from the National Institutes of Health (grant P01CA154292) and American Cancer Society (grant 126947-MRSG-14-160-01-CPHPS). Dr. Elmore is supported by a grant from the National Cancer Institute (R01 CA172343).
Disclosure Statement: Dr. Lee previously received research grants from GE Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siu AL, Force USPST Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 5.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215–225. doi: 10.7326/M15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med. 2012;156(9):609–617. doi: 10.1059/0003-4819-156-9-201205010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swann CA, Kopans DB, McCarthy KA, White G, Hall DA. Mammographic density and physical assessment of the breast. AJR Am J Roentgenol. 1987;148(3):525–526. doi: 10.2214/ajr.148.3.525. [DOI] [PubMed] [Google Scholar]
- 9.Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9(2):151–160. [PubMed] [Google Scholar]
- 10.Spayne MC, Gard CC, Skelly J, Miglioretti DL, Vacek PM, Geller BM. Reproducibility of BI-RADS breast density measures among community radiologists: a prospective cohort study. Breast J. 2012;18(4):326–333. doi: 10.1111/j.1524-4741.2012.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciatto S, Bernardi D, Calabrese M, et al. A first evaluation of breast radiological density assessment by QUANTRA software as compared to visual classification. Breast. 2012;21(4):503–506. doi: 10.1016/j.breast.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Alonzo-Proulx O, Jong RA, Yaffe MJ. Volumetric breast density characteristics as determined from digital mammograms. Phys Med Biol. 2012;57(22):7443–7457. doi: 10.1088/0031-9155/57/22/7443. [DOI] [PubMed] [Google Scholar]
- 13.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 15.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 17.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 18.Sickles EA. The use of breast imaging to screen women at high risk for cancer. Radiol Clin North Am. 2010;48(5):859–878. doi: 10.1016/j.rcl.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Sprague BL, Conant EF, Onega T, et al. Variation in Mammographic Breast Density Assessments Among Radiologists in Clinical Practice: A Multicenter Observational Study. Ann Intern Med. 2016;165(7):457–464. doi: 10.7326/M15-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol. 2013;10(12):913–917. doi: 10.1016/j.jacr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09–41. Radiology. 2012;265(1):59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 22.Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics. 2015;35(2):302–315. doi: 10.1148/rg.352140106. [DOI] [PubMed] [Google Scholar]
- 23.Lee CI, Bassett LW, Lehman CD. Breast density legislation and opportunities for patient-centered outcomes research. Radiology. 2012;264(3):632–636. doi: 10.1148/radiol.12120184. [DOI] [PubMed] [Google Scholar]
- 24.Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol. 2011;18(10):1298–1310. doi: 10.1016/j.acra.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 26.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- 28.Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology. 2015;274(3):772–780. doi: 10.1148/radiol.14141237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuley ML, Guo B, Catullo VJ, et al. Comparison of two-dimensional synthesized mammograms versus original digital mammograms alone and in combination with tomosynthesis images. Radiology. 2014;271(3):664–671. doi: 10.1148/radiol.13131530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houssami N, Miglioretti DL. Digital Breast Tomosynthesis: A Brave New World of Mammography Screening. JAMA Oncol. 2016;2(6):725–727. doi: 10.1001/jamaoncol.2015.5569. [DOI] [PubMed] [Google Scholar]
- 31.Kerlikowske K, Grady D, Barclay J, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst. 1998;90(23):1801–1809. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson BT, LoRusso AP, Smolkin M, Bovbjerg VE, Petroni GR, Harvey JA. Accuracy of assigned BI-RADS breast density category definitions. Acad Radiol. 2006;13(9):1143–1149. doi: 10.1016/j.acra.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Dehkordy SF, Carlos RC. Dense breast legislation in the United States: state of the states. J Am Coll Radiol. 2013;10(12):899–902. doi: 10.1016/j.jacr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(4):268–278. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parris T, Wakefield D, Frimmer H. Real world performance of screening breast ultrasound following enactment of Connecticut Bill 458. Breast J. 2013;19(1):64–70. doi: 10.1111/tbj.12053. [DOI] [PubMed] [Google Scholar]
- 37.Sprague BL, Stout NK, Schechter C, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742. doi: 10.1007/s00330-009-1588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly KM, Dean J, Lee SJ, Comulada WS. Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol. 2010;20(11):2557–2564. doi: 10.1007/s00330-010-1844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin Imaging. 2013;37(3):480–486. doi: 10.1016/j.clinimag.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Chang JM, Moon WK, Cho N, Park JS, Kim SJ. Breast cancers initially detected by hand-held ultrasound: detection performance of radiologists using automated breast ultrasound data. Acta Radiol. 2011;52(1):8–14. doi: 10.1258/ar.2010.100179. [DOI] [PubMed] [Google Scholar]
- 42.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 43.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 45.Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266(3):743–751. doi: 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalkhali I, Baum JK, Villanueva-Meyer J, et al. (99m)Tc sestamibi breast imaging for the examination of patients with dense and fatty breasts: multicenter study. Radiology. 2002;222(1):149–155. doi: 10.1148/radiol.2221010237. [DOI] [PubMed] [Google Scholar]
- 47.Brem RF, Floerke AC, Rapelyea JA, Teal C, Kelly T, Mathur V. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology. 2008;247(3):651–657. doi: 10.1148/radiol.2473061678. [DOI] [PubMed] [Google Scholar]
- 48.Rhodes DJ, Hruska CB, Phillips SW, Whaley DH, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology. 2011;258(1):106–118. doi: 10.1148/radiol.10100625. [DOI] [PubMed] [Google Scholar]
- 49.Wilt TJ, Harris RP, Qaseem A, High Value Care Task Force of the American College of P Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 50.Bevers T, Bibbins-Domingo K, Oeffinger KC, Smith ML. Controversies in Breast Cancer Screening Strategies. J Natl Compr Canc Netw. 2016;14(5 Suppl):651–653. doi: 10.6004/jnccn.2016.0183. [DOI] [PubMed] [Google Scholar]
- 51.Mainiero MB, Lourenco A, Mahoney MC, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol. 2013;10(1):11–14. doi: 10.1016/j.jacr.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 52.Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632. doi: 10.1002/14651858.CD009632.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts: Interim Report of a Prospective Comparative Trial. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.63.4147. [DOI] [PubMed] [Google Scholar]
- 54.Emaus MJ, Bakker MF, Peeters PH, et al. MR Imaging as an Additional Screening Modality for the Detection of Breast Cancer in Women Aged 50–75 Years with Extremely Dense Breasts: The DENSE Trial Study Design. Radiology. 2015;277(2):527–537. doi: 10.1148/radiol.2015141827. [DOI] [PubMed] [Google Scholar]
- 55.Kressin NR, Gunn CM, Battaglia TA. Content, Readability, and Understandability of Dense Breast Notifications by State. JAMA. 2016;315(16):1786–1788. doi: 10.1001/jama.2016.1712. [DOI] [PubMed] [Google Scholar]
- 56.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–681. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freer PE, Slanetz PJ, Haas JS, et al. Breast cancer screening in the era of density notification legislation: summary of 2014 Massachusetts experience and suggestion of an evidence-based management algorithm by multi-disciplinary expert panel. Breast Cancer Res Treat. 2015;153(2):455–464. doi: 10.1007/s10549-015-3534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2:CD001865. doi: 10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]


