Abstract
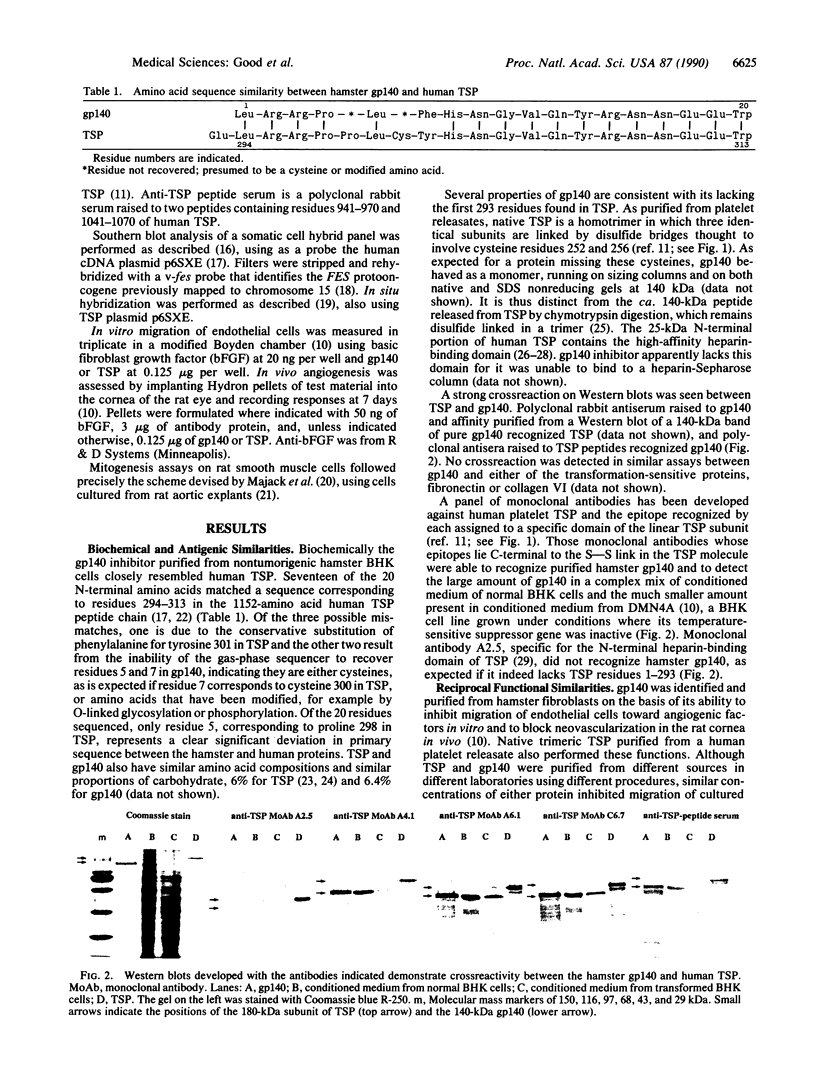
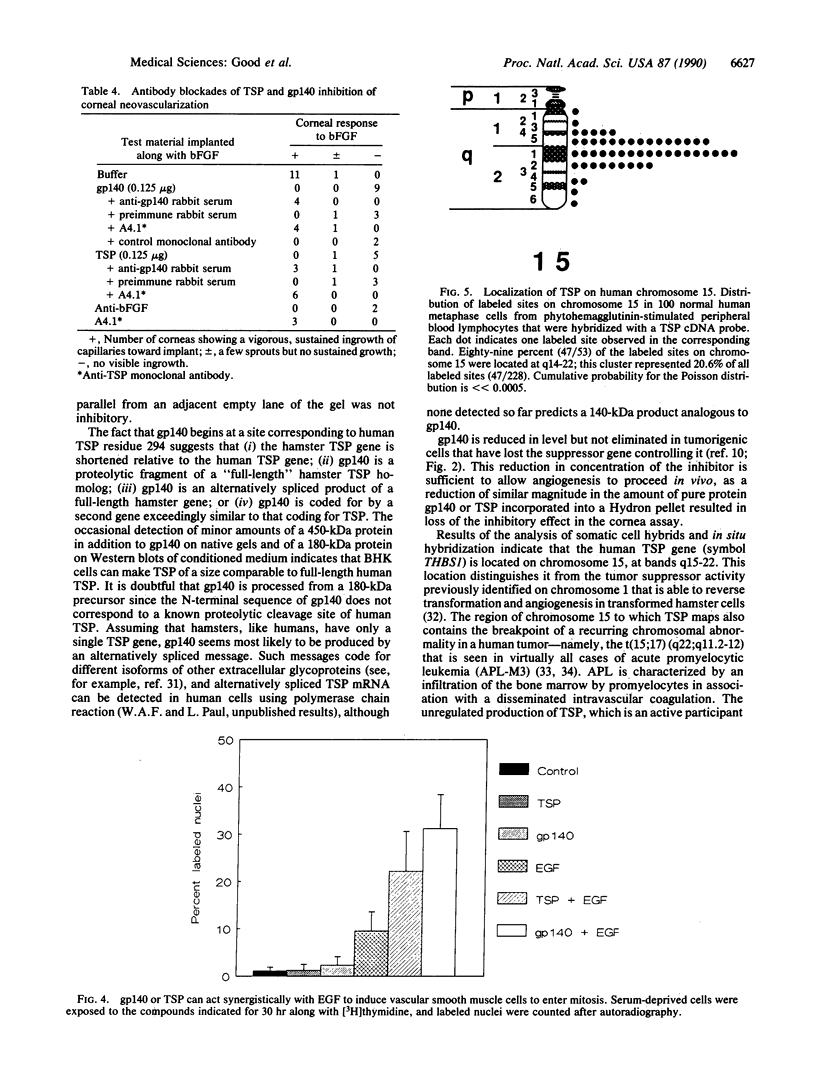
A secreted inhibitor of angiogenesis that is controlled by a tumor suppressor gene in hamster cells has been found to be similar to a fragment of the platelet and matrix protein thrombospondin. The two proteins were biochemically similar and immunologically crossreactive and could substitute for one another in two functional assays. Human thrombospondin inhibited neovascularization in vivo and endothelial cell migration in vitro, as does the hamster protein, gp140. gp140 sensitized smooth muscle cells to stimulation by epidermal growth factor, as does human thrombospondin. The thrombospondin gene has been localized on human chromosome 15. These results demonstrate a function for the ubiquitous adhesive glycoprotein thrombospondin that is likely to be important in the normal physiological down-regulation of neovascularization. In addition, they raise the possibility that thrombospondin may be one of a number of target molecules through which a tumor suppressor gene could act to restrain tumor growth.
Full text
PDF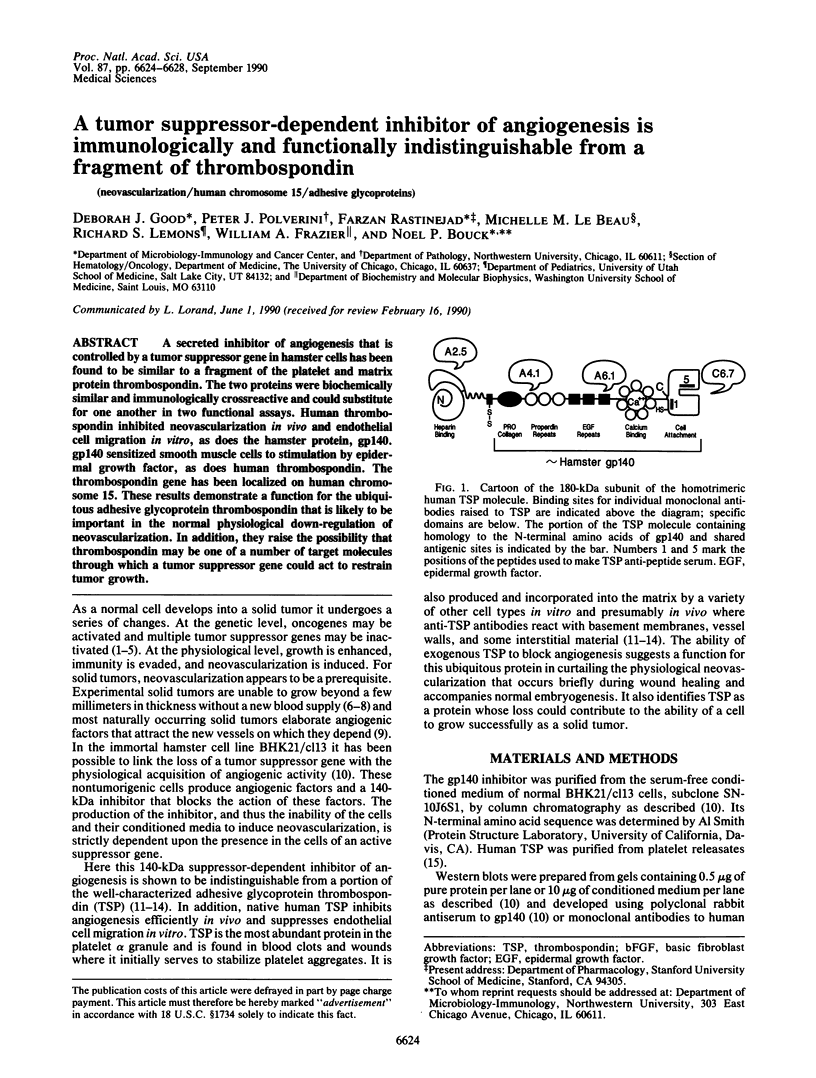


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Nachman R. L. Thrombospondin: phenomenology to function. Prog Hemost Thromb. 1989;9:157–176. [PubMed] [Google Scholar]
- Baenziger N. L., Brodie G. N., Majerus P. W. Isolation and properties of a thrombin-sensitive protein of human platelets. J Biol Chem. 1972 May 10;247(9):2723–2731. [PubMed] [Google Scholar]
- Bautch V. L., Toda S., Hassell J. A., Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell. 1987 Nov 20;51(4):529–537. doi: 10.1016/0092-8674(87)90122-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bouck N. P., Benton B. K. Loss of cancer suppressors, a driving force in carcinogenesis. Chem Res Toxicol. 1989 Jan-Feb;2(1):1–11. doi: 10.1021/tx00007a001. [DOI] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Schor S. L., Grant M. E. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986 Apr 15;235(2):375–383. doi: 10.1042/bj2350375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Galvin N. J., O'Rourke K. M., Frazier W. A. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986 Feb 5;261(4):1962–1968. [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. A monoclonal antibody against human thrombospondin inhibits platelet aggregation. Proc Natl Acad Sci U S A. 1985 May;82(10):3472–3476. doi: 10.1073/pnas.82.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. Effects of anti-thrombospondin monoclonal antibodies on the agglutination of erythrocytes and fixed, activated platelets by purified thrombospondin. Biochemistry. 1985 Jul 30;24(16):4270–4275. doi: 10.1021/bi00337a003. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin N. J., Dixit V. M., O'Rourke K. M., Santoro S. A., Grant G. A., Frazier W. A. Mapping of epitopes for monoclonal antibodies against human platelet thrombospondin with electron microscopy and high sensitivity amino acid sequencing. J Cell Biol. 1985 Oct;101(4):1434–1441. doi: 10.1083/jcb.101.4.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Franchini G., Love J., Simon M. I., Gallo R. C., Wong-Staal F. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983 Jul 14;304(5922):169–171. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Dixit V. M., Grant G. A., Frazier W. A., Santoro S. A. Localization of the hemagglutinating activity of platelet thrombospondin to a 140 000-dalton thermolytic fragment. Biochemistry. 1984 Nov 6;23(23):5597–5603. doi: 10.1021/bi00318a033. [DOI] [PubMed] [Google Scholar]
- Hennessy S. W., Frazier B. A., Kim D. D., Deckwerth T. L., Baumgartel D. M., Rotwein P., Frazier W. A. Complete thrombospondin mRNA sequence includes potential regulatory sites in the 3' untranslated region. J Cell Biol. 1989 Feb;108(2):729–736. doi: 10.1083/jcb.108.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D., Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988 Jul;59(1):44–51. [PubMed] [Google Scholar]
- Larson R. A., Kondo K., Vardiman J. W., Butler A. E., Golomb H. M., Rowley J. D. Evidence for a 15;17 translocation in every patient with acute promyelocytic leukemia. Am J Med. 1984 May;76(5):827–841. doi: 10.1016/0002-9343(84)90994-x. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Le Beau M. M., Pettenati M. J., Lemons R. S., Diaz M. O., Westbrook C. A., Larson R. A., Sherr C. J., Rowley J. D. Assignment of the GM-CSF, CSF-1, and FMS genes to human chromosome 5 provides evidence for linkage of a family of genes regulating hematopoiesis and for their involvement in the deletion (5q) in myeloid disorders. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):899–909. doi: 10.1101/sqb.1986.051.01.103. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. Evidence for two distinct c-src loci on human chromosomes 1 and 20. Nature. 1984 Nov 1;312(5989):70–71. doi: 10.1038/312070a0. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Interactions of thrombospondin with cells in culture: rapid degradation of both soluble and matrix thrombospondin. Semin Thromb Hemost. 1987 Jul;13(3):343–351. doi: 10.1055/s-2007-1003510. [DOI] [PubMed] [Google Scholar]
- O'Shea K. S., Dixit V. M. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988 Dec;107(6 Pt 2):2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Golomb H. M., Dougherty C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet. 1977 Mar 5;1(8010):549–550. doi: 10.1016/s0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- Sage H., Bornstein P. Endothelial cells from umbilical vein and a hemangioendothelioma secrete basement membrane largely to the exclusion of interstitial procollagens. Arteriosclerosis. 1982 Jan-Feb;2(1):27–36. doi: 10.1161/01.atv.2.1.27. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Santoro S. A., Frazier W. A. Isolation and characterization of thrombospondin. Methods Enzymol. 1987;144:438–446. doi: 10.1016/0076-6879(87)44193-1. [DOI] [PubMed] [Google Scholar]
- Stoler A., Bouck N. Identification of a single chromosome in the normal human genome essential for suppression of hamster cell transformation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):570–574. doi: 10.1073/pnas.82.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer P., Beeck H., Voss B. Synthesis, intracellular processing and secretion of thrombospondin in human endothelial cells. Eur J Biochem. 1985 Dec 16;153(3):435–443. doi: 10.1111/j.1432-1033.1985.tb09321.x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Yabkowitz R., Lowe J. B., Dixit V. M. Expression and initial characterization of a recombinant human thrombospondin heparin binding domain. J Biol Chem. 1989 Jun 25;264(18):10888–10896. [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]