Abstract
The filamentous fungus Aspergillus nidulans grows by polarized extension of hyphal tips. The actin cytoskeleton is essential for polarized growth, but the role of microtubules has been controversial. To define the role of microtubules in tip growth, we used time-lapse microscopy to measure tip growth rates in germlings of A. nidulans and in multinucleate hyphal tip cells, and we used a green fluorescent protein-α-tubulin fusion to observe the effects of the antimicrotubule agent benomyl. Hyphal tip cells grew ≈5 times faster than binucleate germlings. In germlings, cytoplasmic microtubules disassembled completely in mitosis. In hyphal tip cells, however, microtubules disassembled through most of the cytoplasm in mitosis but persisted in a region near the hyphal tip. The growth rate of hyphal tip cells did not change significantly in mitosis. Benomyl caused rapid disassembly of microtubules in tip cells and a 10× reduction in growth rate. When benomyl was washed out, microtubules assembled quickly and rapid tip growth resumed. These results demonstrate that although microtubules are not strictly required for polarized growth, they are rate-limiting for the growth of hyphal tip cells. These data also reveal that A. nidulans exhibits a remarkable spatial regulation of microtubule disassembly within hyphal tip cells.
INTRODUCTION
Polarized cellular growth is important for many organisms and is particularly important for filamentous fungi in which nearly all growth occurs at hyphal tips. Tip growth in the genus Aspergillus is particularly significant because some species (e.g., A. niger and A. oryzae) produce economically important products, whereas others (e.g., A. fumigatus) are serious pathogens, particularly in immune-compromised patients. Still other species (e.g., A. flavus) cause contamination of foods with toxic and carcinogenic aflatoxins.
In spite of the importance of tip growth in Aspergillus, the mechanisms by which it occurs are incompletely understood. It is now clear that actin and myosin are essential for tip growth. McGoldrick et al. (1995) placed the myoA myosin of Aspergillus nidulans under the control of the alcA promoter and demonstrated that when myoA expression was repressed, polarized tip growth was inhibited. Torralba et al. (1998b) demonstrated further that when actin filament assembly was inhibited with cytochalasin A, hyphal tips swelled, but polarized growth was inhibited. The importance of actin in hyphal tip growth has been established in other fungi as well (reviewed by Heath, 1990, 1994).
The role of microtubules in tip growth in Aspergillus is more controversial. A number of inhibitor studies suggest that microtubules are important for tip growth in other fungi (Howard and Aist, 1977; That et al., 1988; Temperli et al., 1991; Akashi et al., 1994; Raudaskoski et al., 1994; Rupes et al., 1995; Pedregosa et al., 1995; Steinberg et al., 2001). On the other hand, conidia (asexual spores) of A. nidulans can germinate in the presence of the antimicrotubule agent benomyl and exhibit polarized growth, forming germ tubes (Oakley and Morris, 1980). An added complication has emerged recently. Although it has been shown that cytoplasmic microtubules disassemble in mitosis in A. nidulans (Oakley et al., 1990; Jung et al., 2001), Riquelme et al. (2003) found that hyphal tip growth did not slow during mitosis. If microtubules are, indeed important for tip growth, one might expect that tip growth would slow when microtubules disassemble. These data raise a number of important questions. Are microtubules unimportant for tip cell growth in Aspergillus? Could they be less important for polarized growth in germlings than in hyphal tip cells? If they are important for growth of tip cells, why does growth not slow in mitosis?
The development of A. nidulans strains expressing green fluorescent protein (GFP)-α-tubulin (Han et al., 2001) has made it possible to observe microtubules in living cells and to investigate the effects of antimicrotubule agents on microtubules. We have used time-lapse microscopy of strains expressing GFP-α-tubulin to investigate polarized cell growth in A. nidulans. We find that hyphal tip cells grow much more rapidly than germlings and that growth of hyphal tip cells is greatly slowed by microtubule depolymerization. Cytoplasmic microtubules disassemble completely during mitosis in germlings. In rapidly growing hyphal tip cells, most cytoplasmic microtubules disassemble in mitosis, but some cytoplasmic microtubules near the growing tip remain intact. These data resolve many of the unanswered questions about the role of microtubules in tip growth in A. nidulans.
MATERIALS AND METHODS
Strains and Media
A GFP-α-tubulin–expressing diploid strain of A. nidulans, LO1052, was used for live imaging of microtubules. LO1052 was constructed as follows. A plasmid carrying the wild-type pyrG gene and GFP fused to the N terminus of the tubA (α-tubulin) gene (Osmani et al., 2003) was transformed into strain SO6 (nimA5, wA2, pyrG89, cnxE16, choA1, yA2, chaA1, sC12). It is worth noting that in this construction, the GFP-tubA fusion is under control of the tubA promoter rather than the alcA promoter that has been used with previous constructs. Spores from two separate transformants (L01015 and LO1016) that showed GFP-positive microtubules were spread on 5-fluoro-orotic acid plates (Dunne and Oakley, 1988) to evict the wild-type copy of tubA. Two GFP-positive evictants (LO1015-30a and LO1016-18a) were crossed to LO635 (mipAD159, pabaA1, fwA1). From the LO1015-30a–LO635 cross, LO1022 (GFP-tubA, wA2, pabaA1, cnxE16, sC12) was obtained. From the LO1016-18a–LO635 cross, we obtained LO1029 (GFP-tubA, fwA1, pabaA1, choA1, pyrG89). LO1052 is a stable vegetative diploid formed from LO1022 and LO1029. LO1052 grew at a rate indistinguishable from control strains, and colony morphology and conidiation were normal. The fluorescence images of microtubules in living LO1052 were very similar to those from immunofluorescence microscopy (Oakley et al., 1990; Jung et al., 2001). From these observations, we conclude GFP-α-tubulin expression is not harmful and does not significantly alter microtubule behavior. During observations, germlings and hyphae were grown in liquid minimal medium [6 g/l NaNO3, 0.52 g/l KCl, 0.52 g/l MgSO4·7H2O, 1.52 g/l KH2PO4, 10 g/l d-glucose, 1 ml/l trace element solution (Cove, 1966), pH adjusted to 6.5 with NaOH before autoclaving] supplemented with 1 mg/l para-aminobenzoic acid.
Microscopy
Four-dimensional (4D) (time lapse with Z-series stacks acquired at each time point) GFP images were taken using the following two microscope systems. The first system was a Nikon Eclipse TE3000 inverted microscope equipped with a PerkinElmer Ultraview spinning-disk confocal system controlled by Ultraview software. Images were acquired with a 1.4 numerical aperture (N.A.) 100× Planapochromatic objective and Hamamatsu Orca ER cooled charge-coupled device (CCD) camera. Z-axis position was controlled with a Physik Instrumente piezoelectric positioner. Images were acquired, imported into ImageJ (NIH freeware version 1.30 for Macintosh), and analyzed or cropped for exporting either as TIFF files or QuickTime movies. The second system was an Olympus IX71 inverted microscope equipped with a mercury light source as well as a Uniblitz electronic shutter, a Prior Z-axis drive, and a Hamamatsu Orca ER cooled CCD camera. Images were acquired with a 1.3 N.A. planfluor objective by using Slidebook software (Intelligent Imaging Innovations, Denver, CO) on an Apple PowerMac G4 computer. With both microscope systems, the images were acquired using 2 × 2 binning, which gives a field size of 672 × 512 pixels and a pixel size of 0.129 μm. The pixel size was well under the limit of resolution in each system.
Cells were grown and observed using four-chamber Lab-Tek chambered coverglasses (Nalge Nunc International, Naperville, IL). These consist of plastic growth chambers, with a removable cover, attached to a #1 coverslip. Aeration is excellent in these chambers, and germlings and hyphae grow vigorously for long periods. Imaging was through the coverslips at the bottom of the chambers. During benomyl addition and washout experiments, the chambers were clamped to the microscope stage. For benomyl addition, the chamber cover was removed, benomyl was added, and the chamber cover was replaced. For benomyl washout, the benomyl-containing medium was removed and replaced immediately with benomyl-free medium. This was repeated twice, the cells were left in benomyl-free medium, and the chamber cover was replaced. The benomyl washout process generally required ≈2 min to complete. Because the hyphae adhered tightly to the coverslip at the bottom of the chamber, it was often possible to image the same hyphal tip before benomyl addition, after benomyl addition, and after benomyl washout.
Measurements of Tip Growth
Hyphal tip positions were plotted from maximum intensity projections generated by Slidebook or Ultraview software. The projection images were imported into ImageJ version 1.30, and the XY coordinates (in pixels) of the extreme tip of the hyphae were obtained. To control for stage drift, the position of a static reference point (often fluorescent debris or an ungerminated spore) was taken at each time point. These coordinates were exported into Microsoft Excel along with the time point at which each Z-stack was taken. In Excel, the pixel values were converted to micrometers, and the hyphal tip position (after any necessary correction for stage drift) was plotted relative to time. Tip growth was defined as the change in hyphal tip position. The rate of growth was the slope of the curve when growth was plotted with respect to time (Figures 1, 2, 3 and 5). The slope over the desired interval was determined by linear regression using the slope function of Excel.
Figure 1.

Growth and microtubule distribution in an early germling. (A) Time-lapse images (Fig 1.mov) of a binucleate GFP-α-tubulin–expressing germling growing and going through mitosis. Images are projections of Z-axis stacks acquired using a wide-field microscope. Note that microtubules are less brightly fluorescent in young germlings than in hyphal tip cells, presumably because the GFP has had longer to mature in the older hyphal tip cells. The time, in seconds, after the start of observation is indicated in the lower left corner of each panel. At the 4322-s time point, the germling is entering mitosis. Two small spindles have formed and only a few very faint cytoplasmic microtubule fragments remain (arrows). At the 4502-s time point, all cytoplasmic microtubules have disassembled. Cytoplasmic microtubules reassemble after mitosis (5582-s time point). (B) Profile of tip growth. Growth of the germling tip (in micrometers) is plotted with respect to time (in seconds). Each open diamond represents a single time point. The slope (growth rate) over two intervals was determined by regression analysis and is shown with black lines. Thus, for the interval from 1801 to 4502 s, the growth rate was 0.015 μm/min, and for the interval from 8180 to 14147 s, the growth rate was 0.194 μm/min. This germling went through mitosis during the observation period (gray region). The spindle length is shown with filled circles. Note that for this germling tip growth rates varied greatly over time. However, growth rates were generally faster after nuclear division when there were four nuclei in the germling instead of two.
Figure 2.

Effects of benomyl on hyphal tip growth. (A) Time-lapse images of a GFP-α-tubulin–expressing strain from Fig 2.mov. Images are projections of Z-stacks obtained with a wide-field microscope and the time (in seconds) after the start of observation is indicated in the upper right corner of each panel. Benomyl was added 900 s after the start of observations. The hypha at the left was in interphase and the hypha at the right was in anaphase when benomyl was added. A mitotic spindle is visible in the hypha at the right at the 840-s time point. The growth of both hyphae dramatically slowed after the addition of benomyl. Bulging of tips after benomyl addition is indicated by arrows, and bulging of a medial region of the hypha at the left is indicated by an arrowhead. (B) Profile of the growth of the left hypha in A. The time of benomyl addition is designated with the filled arrow and the completion of cytoplasmic microtubule disassembly is designated by the open arrow. The rates of tip growth before and after the addition of benomyl were determined by regression analysis (gray lines) and are indicated.
Figure 3.

Growth recovery after benomyl washout. A rapidly growing hypha was treated with benomyl for 15 min. The benomyl was then washed out and tip growth was monitored. (A) The hyphal tip just before benomyl addition. (B) The same hyphal tip after 15 min in benomyl and immediately before benomyl washout. The microtubules have disassembled. (C) Time course images (Fig 3.mov) of the same hypha after benomyl washout. Imaging was restarted as soon as possible after benomyl washout (time 0). Microtubules are clearly visible at 540 s, and there is a visible bulge in the hyphal tip (arrowhead). The bulge is larger than before the benomyl washout. Normal tip growth has resumed by the 1440-s time point, and the hyphal bulge is still visible (arrowhead). (D) The growth of the hyphal tip was plotted against time after the completion of benomyl washout. Tip growth accelerated after the benomyl washout and reached a consistent rate of 0.458 μm (gray line, determined by regression analysis).
Figure 5.

Cytoplasmic microtubules persist during mitosis near the tips of rapidly growing hyphae. (A and C) Time-lapse images of the hyphal tip regions of a GFP-α-tubulin–expressing hyphae. The images in A are from Fig 5A.mov, and the images in C are from Fig 5C.mov. All images are maximum intensity projections of Z-stacks acquired using a spinning-disk confocal microscope. The time (in seconds) after the start of observation is indicated in the upper right corner of each panel. The series begins in mitosis and ends in interphase. Schematic drawings of microtubule distributions at the zero time points are shown at the top of each panel. Cytoplasmic microtubules are present near the hyphal tip throughout mitosis. (B) Profile of tip growth. Elongation of the hyphal tip (filled squares) and the length of the spindle closest to the hyphal tip in A (open circles) were plotted. The growth rate of the hypha (gray lines, determined by regression analysis) was the same in mitosis and the period afterward.
RESULTS
Hyphal Tip Cells Grow Much More Rapidly than Germlings
When A. nidulans conidia (uninucleate asexual spores) germinate, the first nuclear division occurs at about the time the germ tube begins to extend. The first three rounds of nuclear division occur in a single cytoplasmic compartment before the formation of the first septum. This septum forms near the conidium, dividing the cell asymmetrically, and leaving more nuclei in the tip cell than in the basal cell. As growth continues, additional rounds of nuclear division and septation occur, and these result in a hypha tipped by a multinucleate tip cell. Most hyphal growth and nuclear divisions occur in this tip cell or in side branches that form with growing tip cells of their own. Many of the observations on tip growth, mitosis, and the microtubule cytoskeleton have been made on relatively young germlings, and it is possible that these processes are qualitatively or quantitatively different in hyphal tip cells.
We first investigated rates of tip growth in germlings and hyphal tip cells. We used strain LO1052, a diploid strain that expresses GFP-α-tubulin. For consistency, our measurements of germling growth rates were on germlings with two nuclei at the beginning of observation (Figure 1 and Fig 1.mov), although they often underwent division during our observations. At 25 ± 1°C, the mean rate of tip growth in these germlings was 0.094 ± 0.060 μm/min (n = 11, >47 h of total observation), but for individual hyphae the rate of growth fluctuated over time from almost 0–0.194 μm/min. These germlings underwent repeated slow but steady growth periods punctuated by pauses in growth (Figure 1B). Tip growth in hyphal tip cells was steadier and much more rapid (0.506 ± 0.21 μm/min, n = 37). These data reveal that growth of hyphal tip cells is much faster than the growth of germlings.
Benomyl Inhibits Growth of Hyphal Tip Cells
Previous data on the effects of microtubule inhibitors on tip growth of filamentous fungi have been contradictory. In A. nidulans, germination and significant germ tube growth occur at concentrations of benomyl that cause disassembly of the microtubule cytoskeleton (Oakley and Morris, 1980; Ovechkina et al., 2003). These data demonstrate that microtubules are not an absolute requirement for tip growth. Data from other fungi, however, suggest that microtubules are important for tip growth. One possible resolution of these data is that tip growth in A. nidulans is mechanistically different from that of other fungi. Another explanation, however, is that microtubules are unimportant for the slow growth of germlings, but important for the much more rapid growth of hyphal tip cells. The development of strains expressing GFP-α-tubulin, moreover, should allow us to observe the effects of antimicrotubule agents on the microtubules (to see whether and when they disassemble) while we simultaneously observe tip growth.
We observed microtubules and apical growth of hyphal tip cells in LO1052 at 25 ± 1°C. The growth chambers we used allowed us to add the antimicrotubule agent benomyl to the chamber without disturbing the specimen. After an initial period of 4D microscopy (typically 15 min), benomyl was added to the medium to a final concentration of 2.4 μg/ml and observations were continued. Figure 2 shows a typical result. Complete disassembly of microtubules occurred quickly after benomyl addition. Microtubules disappeared by 6.3 ± 2.1 min after benomyl addition, and tip growth slowed, gradually reaching a steady slower growth rate 10.9 ± 2.9 min after benomyl addition (n = 12). The growth rate was reduced more than 10-fold, from 0.61 ± 0.27 to 0.054 ± 0.058 μm/min. We did not see a dramatic change of growth rate in controls in which ethanol (which was used as a solvent to dissolve benomyl) was added to the medium. Bulging of the hyphal tip was observed in 71% of the tips (10 of 14 tips observed) of benomyl-treated cells. The hyphal tip started to bulge about the time of microtubule disassembly (Figure 2A, indicated by white arrows) and bulging became more pronounced over time. Although the addition of benomyl caused a more than 10-fold decrease in tip growth rate, growth of the tip never stopped completely. It is also worth noting that growth was observed in benomyl-treated hyphae at locations other than the tip (e.g., formation of side branches or bulging in the middle of hyphae, indicated by arrowhead in Figure 2A). These experiments demonstrate that although microtubules are not absolutely required for tip growth, they are critical for the rapid growth of hyphal tip cells.
Benomyl Inhibition of Tip Growth Is Reversible
To determine whether the effects of benomyl are reversible, we added benomyl, waited 15 min, verified that microtubule disassembly had occurred, and then removed the benomyl with three washes of benomyl-free growth medium. We then captured 4D image series to determine whether microtubules reassembled and tip growth recovered. Microtubule assembly was rapid. Microtubules were visible as soon as we could begin capturing images after benomyl washout (≈1 min after the final wash), and an apparently normal microtubule array was present 6–9 min after benomyl washout. Tip growth accelerated after benomyl washout and microtubule reassembly (Figure 3 and Fig 3.mov) reaching a rate of 0.43 ± 0.18 μm/min (n = 12), which is similar to the rate of untreated material. There was often, but not always, a pause between benomyl removal and the resumption of rapid growth. As hyphal growth resumed, the hyphal tip often visibly bulged (indicated by arrowheads in Figure 3C) and a normal, thinner hyphal tip then grew from the bulge.
Hyphal Tip Growth Persists in Mitosis
Riquelme et al. (2003) have reported that hyphal tip growth persists during mitosis in A. nidulans. The criterion of Riquelme et al. (2003) for mitotic entry and exit was indirect (change in phase density of nuclei), so we thought it would be worthwhile to examine the rate of tip growth during mitosis by using the direct criterion of mitotic spindle formation and disassembly as direct criteria for mitosis. We captured 4D image series starting in very early mitosis and continuing until well after the completion of mitosis. Plotting the positions of the cell tip against time revealed no significant difference in the tip growth rate between mitosis (0.464 ± 0.095 μm/min) and the period after mitosis (0.417 ± 0.125 μm/min) (n = 13).
Microtubules Persist through Mitosis at Rapidly Growing Hyphal Tips
Extensive immunofluorescence observations indicate that cytoplasmic microtubules disassemble during mitosis in germlings (Oakley et al., 1990; Jung et al., 2001). Our data, in combination with those of Riquelme et al. (2003), indicate, however, that microtubules are required for rapid tip growth and that rapid tip growth persists during mitosis. To resolve these seemingly contradictory data, we carried out time-lapse microscopy of microtubules in mitosis in germlings and hyphal tip cells.
We first observed the germlings undergoing the second nuclear division. The organization of the microtubules in these cells corresponded very well with earlier immunofluorescence microscopy data. Cytoplasmic microtubules disappeared completely upon the onset of mitosis, initial spindle elongation took place in the absence of cytoplasmic microtubules, and cytoplasmic (astral) microtubules reassembled during anaphase (Figure 1 and Fig 1.mov). As mentioned, the growth rates of these germlings fluctuated, and there was no obvious slowing of growth as germlings went through mitosis (Figure 1B).
It is well established that the nuclei in the same cytoplasmic compartment go into mitosis in semisynchronous manner (Robinow, 1969; Doonan, 1992). Time-lapse observations of hyphal tip cells revealed that mitosis typically initiated in the nucleus farthest from the hyphal tip and proceeded rapidly in a wave toward the hyphal tip (38 cases of 42 hyphae observed). As in germlings, cytoplasmic microtubules disassembled as nuclei distal to the hyphal tips entered mitosis (Figure 4 and Fig 4.mov). Near the hyphal tip, however, microtubules generally persisted through mitosis (Figure 5 and Fig 5A.mov and Fig 5C.mov). We found that at least one (generally more) microtubule persisted at the hyphal tip throughout mitosis in 88% (37 of 42) of mitotic tip cells. In some cases, the cytoplasmic microtubules extended beyond the second spindle in the cell to near the third spindle (Figure 5C). In other cases, microtubules persisted only in a region from near the tip-most spindle to the hyphal tip, and fewer microtubules persisted. In all cases, the persistent microtubules reached very close to the tip of the cell. Tracing each persistent microtubule (Figure 5, A and C, top drawings) indicated that these microtubules arise near the first spindle, most likely from the spindle pole bodies of the nucleus nearest the cell apex.
Figure 4.
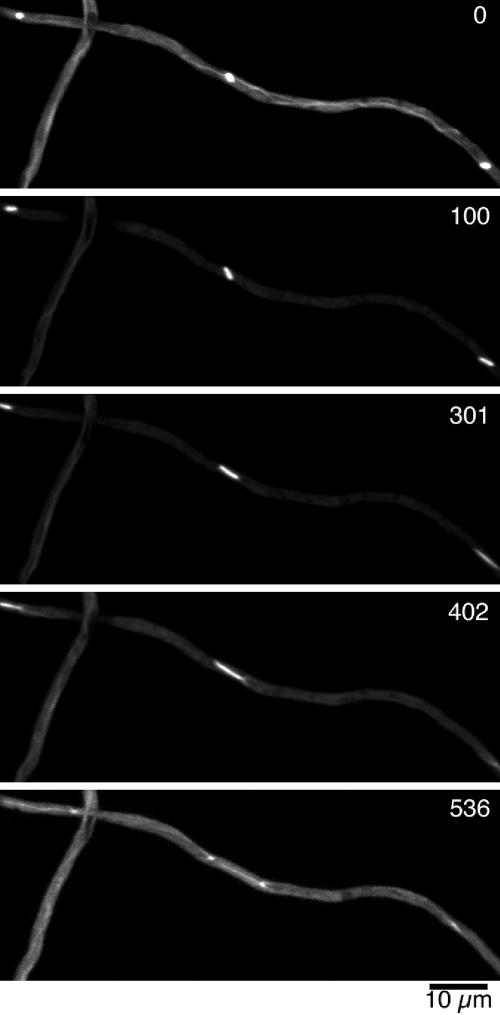
Time-lapse images of GFP-α-tubulin in the medial region of a hyphal tip cell undergoing mitosis from Fig 4.mov. Images are maximum intensity projections of Z-stacks acquired with a spinning-disk confocal microscope. The time (in seconds) after the start of observation is indicated in the upper right corner of each panel. Three spindles go into anaphase successively. Note that the cytoplasmic microtubules disassemble completely in the early stages of spindle formation (100 s).
DISCUSSION
Microtubules Are Essential for Rapid Hyphal Growth
Our data demonstrate that the cytoplasmic microtubule array is necessary for rapid hyphal growth of A. nidulans. By observing the microtubules in living cells, we were able to follow the events that occurred in rapidly growing hyphae in response to the addition of the antimicrotubule agent benomyl. For the first few minutes after the addition of benomyl, the cytoplasmic microtubules remained intact, presumably because this period of time was required for benomyl to diffuse through the medium and penetrate into cells. The microtubule array then disassembled rapidly and was gone ≈6 min after the addition of benomyl. Tip growth initially continued at an unreduced rate after microtubule disassembly but slowed down rapidly, reaching a steady, slower rate ∼11 min after benomyl addition. It should be noted, however, that although microtubules are essential for rapid tip growth, they are not absolutely required for polarized growth. Tip growth continued slowly even when microtubules were depolymerized, and it has been shown previously that conidia germinate and polarized tip growth occurs in concentrations of benomyl that completely inhibit microtubule assembly (Oakley and Morris, 1980).
The present and previous data suggest a model for the roles of the microtubule and actin cytoskeletons in tip growth. We suggest that the main function of microtubules in tip growth is to transport vesicles containing cell wall materials to the vicinity of the hyphal tip. This transport system is not essential for tip growth but is certainly rate limiting for the growth of hyphal tip cells. The vesicles transported by the microtubule cytoskeleton are captured by a system involving actin and the myoA myosin, and this system is required for the fusion of these vesicles into the membrane at the growing tip. This acto-myosin system is essential for tip growth under all circumstances. When microtubules are disassembled by benomyl, there is initially enough material in the tip vicinity to allow rapid growth, but these materials become depleted quickly in the absence of microtubules and growth slows dramatically. The residual, slow growth that persists afterward is presumably due to wall materials reaching the tip area by nonmicrotubule-dependent mechanisms such as Brownian motion. In germlings, tip growth is slow (less than twice the growth rate in benomyl-treated tip cells), and, consequently, microtubule-based movement of wall materials is relatively unimportant for tip growth.
Our data are also consistent with the possibility that microtubules play a secondary role in focusing actin at the tip of growing hyphae. This would explain why tips bulge slightly after benomyl addition (Figure 2). It also would explain the larger bulges that are often seen when benomyl is washed out. By our model, when microtubules reassemble, they transport wall materials to the vicinity of the tips. These are captured by the poorly focused actomyosin system and used to synthesize new wall, and this creates a larger bulge. However, the microtubules quickly focus the actomyosin system and normal growth is resumed. It has previously been shown that microtubules are required for the apical localization of actin in Candida albicans germlings (Akashi et al., 1994); Torralba et al., (1998a) have reported, however, that treatment of A. nidulans hyphae with 0.25 μg/ml antimicrotubule agent methyl benzimidazole-2-yl carbamate actually causes actin to move closer to the hyphal tip. However, it should be noted that microtubules were not disassembled completely under these conditions, and, consequently, the role of microtubules in the localization of actin to hyphal tips in A. nidulans remains unresolved.
The role of cytoskeletal elements in tip-growing systems has been studied in a variety of organisms. In all cases, the actin cytoskeleton is absolutely required for tip extension. In growing neurites, microtubules are also essential (reviewed by Gordon-Weeks, 1991; Dent and Gertler, 2003). In plants, the role of microtubules has been evaluated in root hair cell growth and pollen tube extension. The growth rate of root hair cells was not significantly affected by the presence of antimicrotubule agents, but the cells no longer grew straight and started to exhibit waviness and branching (Bibikova et al., 1999; Ketelaar et al., 2003). In angiosperm pollen tubes, microtubules are essential for tip elongation and, in addition, loss of microtubules leads to tip swelling and bifurcation (Anderhag et al., 2000; Justus et al., 2004). In angiosperms, the available data suggest that microtubules are relatively unimportant in pollen tube tip growth (Heslop-Harrison et al., 1988; Ålström et al., 1995), although it would be useful to repeat these experiments with current techniques and reagents. These observations, and our data, suggest that the importance of microtubule-dependent transport varies among tip growth systems. In some systems it is critical but in others it is relatively unimportant. These data also suggest that microtubules often play a role in positioning and organizing the site of tip growth.
A Remarkable Spatial Regulation of Microtubule Disassembly Facilitates Tip Growth during Mitosis
We have confirmed the finding of Riquelme et al. (2003) that hyphal tip growth is continuous during mitosis. Our data also resolve a paradox with the Riquelme et al. (2003) data. If cytoplasmic microtubules are required for rapid tip growth as we have shown, and they disassemble in mitosis as reported previously (Oakley et al., 1990; Jung et al., 2001), the obvious expectation is that tip growth would slow during or shortly after mitosis. We have found, however, that, in hyphal tip cells, microtubules near the cell apex generally do not depolymerize completely during mitosis (although they do depolymerize completely in germlings as previously reported). We hypothesize that the remnant microtubules transport adequate amounts of cell tip precursors to the vicinity of the tip to allow continuous growth. It also should be noted that enough cell wall precursors are normally at the tip to allow growth for some time after microtubule disassembly by benomyl, so the remnant microtubules do not need to be as efficient in supplying cell wall precursors as the interphase microtubule cytoskeleton. They only need to supply enough materials to tide the growing tip over until the interphase microtubule cytoskeleton reassembles at the end of mitosis.
The maintenance of microtubules near the hyphal tip during mitosis constitutes a remarkable spatial regulation of microtubule disassembly. Cytoplasmic microtubules disassemble rapidly throughout most of the cytoplasm but, in the same cell, they remain intact near the hyphal tip. Cytoplasmic microtubules disassemble in many types of cells as they enter mitosis, and the regulation of microtubule assembly and disassembly is generally thought to involve a balance between the activities of proteins that stabilize microtubules (e.g., microtubule-associated proteins) and proteins that promote microtubule disassembly (catastrophe factors). Because relatively little is known about microtubule stabilizers and catastrophe factors in A. nidulans, it is premature to speculate on the mechanism by which disassembly is inhibited near the hyphal tip. Whatever the mechanism, it would seem to require a system for sensing tip proximity (e.g., release of factors by the growing tip), and the tip proximity signal must act directly or indirectly on the factors that regulate microtubule disassembly. Because the regulation occurs over a very short time span, it is more likely to involve protein modification than protein synthesis.
Rapid Tip Growth, Asymmetric Division, and the Coenocytic Growth Form
We have found that hyphal tip cells grow 5.4 times as rapidly as germlings. It is obviously advantageous for tip growth to be rapid because it allows the organism to find new sources of nutrients quickly, but why are hyphal tip cells able to grow so much more rapidly than germlings? We suggest that a part of the answer has to do with the ratio of nuclei to cytoplasmic volume. It is likely that there is an optimal nucleus-to-cytoplasm ratio (Clutterbuck, 1969; Fiddy and Trinci, 1976). Certainly, some variation in the nucleus-to-cytoplasm volume ratio will be tolerated, and this ratio might vary to some extent in different growth conditions. However, the general principle must apply that tip growth cannot significantly outpace nuclear division because the nucleus-to-cytoplasm ratio would decrease and the nuclei would eventually be unable to supply the cytoplasm with mRNAs sufficient to support maximum growth. Because the diameters of hyphae are relatively consistent under a given set of growth conditions, it follows that if there is an optimal nucleus-to-cytoplasm ratio, there must be an optimal hyphal length per nucleus. Fiddy and Trinci (1976) have found that for diploid hyphae such as the ones we have used for our experiments, the average hyphal length per nucleus is 22 ± 7 μm. Thus, binucleate germlings would average 44 μm in length, and such a germling would grow in one cell cycle to be a germling with four nuclei and an average length of 88 μm (i.e., would grow 44 μm in one cell cycle). A tip cell with 10 nuclei would be ∼220 μm in length and in one cell cycle it would become a cell with 20 nuclei and a length of 440 μm. It would, thus, grow 220 μm in one cell cycle (5 times as much as a binucleate germling) with no alteration in the nucleus to cytoplasm ratio.
We suggest that maintaining an appropriate nucleus-to-cytoplasm ratio while maintaining a maximal growth rate is an important reason that A. nidulans cells divide asymmetrically and that A. nidulans has a coenocytic growth form. When A. nidulans spores germinate, three to four rounds of nuclear division occur before the first septum forms (Fiddy and Trinci, 1976). The septum divides the cell asymmetrically creating a tip cell that contains the majority of the nuclei. Additional rounds of asymmetric septation follow successive rounds of nuclear division and these eventually result in multinucleate tip cells. We suggest having many nuclei in the tip cell is necessary for maximal tip growth and that the delay of septation until at least the third nuclear division coupled with asymmetric division at this and subsequent stages are adaptations that allow tip cells to have many nuclei. We also note that A. nidulans must have mechanisms that govern the transition from slow germling growth to rapid growth of hyphal tip cells.
Supplementary Material
Acknowledgments
We thank Mary Forget for performing a portion of the videomicroscopy, Elizabeth Oakley for technical assistance, and Dr. Stephen Osmani for use of the spinning disk confocal microscope. This work was supported by National Institutes of Health grant GM-31837 (to B.R.O.) and grant for basic science research projects from Sumitomo Foundation (to T.H.).
Article published online ahead of print in MBC in Press on November 17, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0798).
Abbreviations used: 4D, four-dimensional.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Akashi, T., Kanbe, T., and Tanaka, K. (1994). The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology 140, 271-280. [DOI] [PubMed] [Google Scholar]
- Ålström, H., Sorri, O., and Raudaskoski, M. (1995). Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex. Plant Reprod. 8, 61-69. [Google Scholar]
- Anderhag, P., Hepler, P. K., and Lazzaro, M. D. (2000). Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway spruce). Protoplasma 214, 141-157. [Google Scholar]
- Bibikova, T. N., Blancaflor, E. B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657-665. [DOI] [PubMed] [Google Scholar]
- Clutterbuck, A. J. (1969). Cell volume per nucleus in haploid and diploid strains of Aspergillus nidulans. J. Gen. Microbiol. 55, 291-299. [DOI] [PubMed] [Google Scholar]
- Cove, D. J. (1966). The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113, 51-56. [DOI] [PubMed] [Google Scholar]
- Dent, E. W., and Gertler, F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209-227. [DOI] [PubMed] [Google Scholar]
- Doonan, J. H. (1992). Cell division in Aspergillus. J. Cell Sci. 103, 599-611. [DOI] [PubMed] [Google Scholar]
- Dunne, P. W., and Oakley, B. R. (1988). Mitotic gene conversion, reciprocal recombination and gene replacement at the benA, beta-tubulin, locus of Aspergillus nidulans. Mol. Gen. Genet. 213, 339-345. [DOI] [PubMed] [Google Scholar]
- Fiddy, C., and Trinci, A. P. (1976). Mitosis, septation, branching and the duplication cycle in Aspergillus nidulans. J. Gen. Microbiol. 97, 169-184. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks, P. R. (1991). Control of microtubule assembly in growth cones. J. Cell Sci. Suppl. 15, 45-49. [DOI] [PubMed] [Google Scholar]
- Han, G., Liu, B., Zhang, J., Zuo, W., Morris, N. R., and Xiang, X. (2001). The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 11, 719-724. [DOI] [PubMed] [Google Scholar]
- Heath, I. B. (1990). The role of actin in tip growth of fungi. Int. Rev. Cytol. 123, 95-127. [Google Scholar]
- Heath, I. B. (1994). The cytoskeleton in hyphal growth, organelle movement. In The Mycota I Growth, Differentiation and Sexuality, ed. J.G.H. Wessels and F. Meinhardt, Springer, Berlin, 43-65.
- Heslop-Harrison, J., Heslop-Harrison, Y., Cresti, M., Tiezzi, A., and Moscatelli, A. (1988). Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J. Cell Sci. 91, 49-60. [Google Scholar]
- Howard, R. J., and Aist, J. R. (1977). Effects of MBC on hyphal tip organization, growth, and mitosis of Fusarium acuminatum, and their antagonism by D2O. Protoplasma 92, 195-210. [DOI] [PubMed] [Google Scholar]
- Jung, M. K., Prigozhina, N., Oakley, C. E., Nogales, E., and Oakley, B. R. (2001). Alanine-scanning mutagenesis of Aspergillus γ-tubulin yields diverse and novel phenotypes. Mol. Biol. Cell 12, 2119-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus, C. D., Anderhag, P., Goins, J. L., and Lazzaro, M. D. (2004). Microtubules and microfilaments coordinate to direct a fountain streaming pattern in elongating conifer pollen tube tips. Planta 219, 103-109. [DOI] [PubMed] [Google Scholar]
- Ketelaar, T., de Ruijter, N.C.A., and Emons, A.M.C. (2003). Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15, 285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoldrick, C. A., Gruver, C., and May, G. S. (1995). myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J. Cell Biol. 128, 577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B. R., and Morris, N. R. (1980). Nuclear movement is β-tubulin dependent in Aspergillus nidulans. Cell 19, 255-262. [DOI] [PubMed] [Google Scholar]
- Oakley, B. R., Oakley, C. E., Yoon, Y., and Jung, M. K. (1990). γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 61, 1289-1301. [DOI] [PubMed] [Google Scholar]
- Osmani, A. H., Davies, J., Oakley, C. E., Oakley, B. R., and Osmani, S. A. (2003). TINA interacts with the NIMA kinase in Aspergillus nidulans and negatively regulates astral microtubules during metaphase arrest. Mol. Biol. Cell 14, 3169-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovechkina, Y., Maddox, P., Oakley, C. E., Xiang, X., Osmani, S. A., Salmon, E. D., and Oakley, B. R. (2003). Spindle formation in Aspergillus is coupled to tubulin movement into the nucleus. Mol. Biol. Cell 14, 2192-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa, A., Riso, S., Monistrol, I., and Laborda, F. (1995). Effect of the microtubule inhibitor methyl benzimidazol-2-yl carbamate (MBC) on protein secretion and microtubule distribution in Cladosporium cucumerinum. Mycol. Res. 99, 43-48. [Google Scholar]
- Raudaskoski, M., Mao, W. Z., and Yli-Mattila, T. (1994). Microtubule cytoskeleton in hyphal growth. Response to nocodazole in a sensitive and a tolerant strain of the homobasidiomycete Schizophyllum commune. Eur. J. Cell Biol. 64, 131-141. [PubMed] [Google Scholar]
- Riquelme, M., Fisher, R., and Bartnicki-García, S. (2003). Apical growth and mitosis are independent processes in Aspergillus nidulans. Protoplasma 222, 211-215. [DOI] [PubMed] [Google Scholar]
- Robinow, C. F. (1969). Mitosis in Aspergillus nidulans. J. Cell Sci. 5, 403-431. [DOI] [PubMed] [Google Scholar]
- Rupes, I., Mao, W., Astrom, H., and Raudaskoski, M. (1995). Effect of nocodazole and brefeldin-A on microtubule cytoskeleton and membrane organization in the homobasidiomycete Shizopyllum commune. Protoplasma 185, 212-221. [Google Scholar]
- Steinberg, G., Wedlich-Soldner, R., Brill, M., and Schulz, I. (2001). Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J. Cell Sci. 114, 609-622. [DOI] [PubMed] [Google Scholar]
- Temperli, E., Roos, U.-P., and Hohl, H. (1991). Germ tube growth and the microtubule cytoskeleton in Phytophthora infestans. Effects of antagonists of hyphal growth, microtubule inhibitors, and ionophores. Mycol. Res. 95, 611-617. [Google Scholar]
- That, T. C., Rossier, C., Barja, F., Turian, G., and Roos, U. P. (1988). Induction of multiple germ tubes in Neurospora crassa by antitubulin agents. Eur. J. Cell Biol. 46, 68-79. [PubMed] [Google Scholar]
- Torralba, S., Pedregosa., A., Lucas, J. D., Dias, M., Monistrol, I., and Laborda, F. (1998a). Effect of the microtubule inhibitor methyl benzimidazole-2-yl carbamate (MBC) on production and secretion of enzymes in Aspergillus nidulans. Mycol. Res. 100, 1375-1382. [Google Scholar]
- Torralba, S., Raudaskoski, M., Pedregosa, A. M., and Laborda, F. (1998b). Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology 144, 45-53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


