Abstract
Multiple copies of the mitochondrial genome in eukaryotic cells are organized into protein–DNA complexes called nucleoids. Mitochondrial genome repair mechanisms have been reported, but they are less well characterized than their nuclear counterparts. To expand our knowledge of mitochondrial genome maintenance, we have studied the localization of the BRCA1 protein, known to be involved in nuclear repair pathways. Our confocal and immunoelectron microscopy results show that BRCA1 is present in mitochondria of several human cancer cell lines and in primary breast and nasal epithelial cells. BRCA1 localization in mitochondria frequently overlapped that of nucleoids. Small interfering RNA-mediated knockdown of BRCA1 in human cancer cells (confirmed by Western blot) results in decreased nuclear, cytoplasmic, and mitochondrial staining after immunofluorescence microscopy, establishing the specificity of the BRCA1 immunolabeling. Furthermore, using cell fractionation, dephosphorylation, and enzyme protection experiments, we show that a 220-kDa phosphorylated isoform of BRCA1 is enriched in mitochondrial and nuclear fractions but reduced in cytoplasmic subcellular fractions. Submitochondrial fractionation confirmed the presence of BRCA1 protein in isolated mitoplasts. Because phosphorylation of BRCA1 and subsequent changes in subcellular localization are known to follow DNA damage, our data support a universal role for BRCA1 in the maintenance of genome integrity in both mitochondria and nucleus.
INTRODUCTION
Mitochondria synthesize their own DNA and multiply semi-autonomously. In humans, the mitochondrial matrix contains multiple copies of circular 16.5-kb DNA that encodes 13 polypeptides required for oxidative phosphorylation, 22 tRNA, and two rRNA species (Anderson et al., 1981). Multiple mitochondrial DNA (mtDNA) molecules are organized into discrete protein–DNA complexes called nucleoids within the mitochondrial matrix. Nucleoids are well characterized in Saccharomyces cerevisiae (Miyakawa et al., 1987), where each individual nucleoid is estimated to contain three to four copies of mtDNA and several specific nucleoid proteins.
It has been suggested that mtDNA displays a high susceptibility to mutations (Richter et al., 1988), partly because of limited repair mechanisms. mtDNA has available base excision repair (Driggers et al., 1993), direct damage reversal (Myers et al., 1988; Yasui et al., 1992), and mismatch repair and recombinational repair mechanisms (Reenan and Kolodner, 1992). However, both nucleotide excision repair (Clayton et al., 1974) and transcription-coupled repair for correcting oxidative DNA damage (Driggers et al., 1997; Anson et al., 1998) seem to be absent from mitochondria. The existence of mtDNA repair mechanisms is strengthened by the fact that DNA maintaining proteins have been isolated from nucleoids in yeast such as DNA binding proteins Abf2p and Mgm101 (Diffley and Stillman, 1992; Zuo et al., 2002), the latter also functioning in the repair of oxidatively damaged mtDNA (Meeusen et al., 1999) and Ntg1p, a base excision repair enzyme (O'Rourke et al., 2002). Recently, it was shown in mammalian cells that mtDNA molecules also are clustered and organized in discrete protein-rich complexes (Spelbrink et al., 2001; Garrido et al., 2003) within the mitochondrial network and are able to divide and redistribute in this network. Some human nucleoid proteins have been identified such as the Twinkle protein, a helicase that displays structural similarity to phage T7 gene 4 primase/helicase and other hexameric ring helicases; TFAM, a mitochondrial transcription factor; mitochondrial single-stranded DNA-binding protein; and mtDNA polymerase POLG (Spelbrink et al., 2001; Garrido et al., 2003).
During tumor progression, an increased nuclear mutation rate is observed and is thought to be in part due to failures of DNA repair mechanisms. Although the role of mtDNA mutations in tumor development is still unclear, several studies have reported that tumor formation, including breast cancer (Parrella et al., 2001), is often associated with increased mtDNA mutations (Bianchi et al., 2001; Penta et al., 2001). This raised the question whether these elevated mtDNA mutations are also due to the failure of the nuclear DNA repair mechanisms, i.e., whether nuclear and mtDNA are maintained and repaired by the same conserved mechanisms. Such a hypothesis is plausible because the mitochondrial genome is dependent upon the nuclear genome for transcription, translation, replication, and repair. The majority of mitochondrial proteins are indeed nuclear encoded and posttranslationally imported to mitochondria by a specific import machinery (Neupert, 1997).
A protein known to play a crucial role in nuclear DNA maintenance is BRCA1, a tumor suppressor that, when harboring germline mutations, confers susceptibility to breast and ovarian cancer. This breast cancer gene encodes a 200- to 220-kDa nuclear phosphoprotein with a RING domain (Miki et al., 1994), two BRCA1 C-terminal domains (Koonin et al., 1996), nuclear localization signals (Chen et al., 1996), and a nuclear export signal (Rodriguez and Henderson, 2000). Since this gene was identified and cloned in 1994 (Miki et al., 1994), multiple functions have been revealed all of which control genomic stability in the nucleus such as cell cycle regulation and checkpoint activation (Hakem et al., 1997; Somasundaram et al., 1997; Wang et al., 1997; Xu et al., 1999), regulation of a set of specific transcriptional pathways (Monteiro, 2000), and several highly specialized DNA repair processes and recombination (Jasin, 2002). BRCA1 also has been implicated in regulation of centrosomes (Deng, 2002), apoptosis (Yan et al., 2002), DNA binding (Yamane et al., 2000; Paull et al., 2001), and chromatin remodeling (Ye et al., 2001). Therefore, BRCA1 is thought to function as a caretaker. Many of these functions are linked to protein–protein interactions (Deng and Brodie, 2000) or binding to DNA. BRCA1 function in cell cycle progression and the DNA damage response seems to be regulated by distinct and specific phosphorylation events (Scully et al., 1997; Venkitaraman, 2002).
Here, we studied the subcellular localization of human BRCA1 by a combination of confocal and immunoelectron microscopy (EM), small interfering RNA (siRNA) expression knockdown, and cell fractionation on several human cancer cell lines and primary breast and nasal epithelial cells, by using antibodies against different epitopes of the BRCA1 protein. Subcellular fractionation demonstrates that the 220-kDa phosphorylated BRCA1-species is detectable in whole homogenate, enriched in a nuclear fraction (NF), and reduced in a cytoplasmic fraction (CF). Further fractionation and dephosphorylation experiments on human cells and isolated rat liver mitoplasts show that mitochondria contain phosphorylated BRCA1. Moreover, a frequent colocalization of BRCA1 with the mtDNA was seen. The presence of BRCA1 protein in both sites in the cell containing DNA supports a universal role in maintenance of genome integrity.
MATERIALS AND METHODS
Primary Antibodies
The following antibodies were used: rabbit polyclonal anti-BRCA1 antibodies Ab-C (exon 11, aa 768–793) and Ab-D (aa 1847–1863; BD Biosciences PharMingen, San Diego, CA); mouse monoclonal anti-BRCA1 antibodies Ab-1 (clone MS110, aa 1–304), Ab-4 (clone SD118; exon 11, aa 1005–1313), and Ab-5 (clone AP16, aa 1313–1863; Oncogene Science, Cambridge, MA); goat polyclonal antibodies anti-Lamin A/C, anti-Lamin B1 (C-20), anti-p-BRCA1 Ser 988 (short aa sequence containing phosphorylated Ser-988), anti-p-BRCA1 Ser 1497 (short aa sequence containing phosphorylated Ser-1497; Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal antibodies anti-cytokeratin broad spectrum (CKBS) and anti-Ki67 (Biogenex, San Ramon, CA); and rabbit polyclonal serum anti-F1 ATPase (kindly provided by Dr. C. Galloway, Yale University, New Haven, CT) also were used. As a mitochondrial marker we applied the mitochondrion-selective probe MitoTracker RedCMXRos (Molecular Probes, Eugene, OR) and for visualizing mtDNA, we applied a mouse monoclonal anti-DNA IgM antibody (Sir William Dunn School of Pathology, Oxford, United Kingdom).
Cells
HBL-100 cell line is a human epithelial breast carcinoma cell line obtained from Vanderbilt University Medical Center (Nashville, TN) and was grown in McCoy's 5A medium with 2 mM l-glutamine, supplemented with 10% heat-inactivated fetal calf serum (FCS) and insulin-transferrin-selenium-A (100× stock solution; Invitrogen, Paisley, United Kingdom). Human mammary epithelial cells (HMECs) were obtained from Cambrex Bio Science Walkersville (Walkersville, MD) and were cultured in mammary epithelium cell basal medium supplemented with 10 ng/ml human recombinant epidermal growth factor, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamicin, and 50 ng/ml amphotericin-B and 30 mg/ml bovine pituitary extract (Cambrex Bio Science Walkersville). Human cervical carcinoma HeLa cells, obtained from American Type Tissue Collection (Manassas, VA) (CCL-2) and human vascular endothelial ECV 304 cells (The European Collection of Cell Cultures ref no. 92091712), were grown in Dulbecco's minimal essential medium, supplemented with 10% heat inactivated FCS, 50 U/ml penicillin-50 μg/ml streptomycin mixture, and 2 mM l-glutamine (Cambrex Bio Science Walkersville). SK-BR-3 breast adenocarcinoma cells obtained from American Type Tissue Collection (HTB-30) were grown in Earl's minimal essential medium (Cambrex Bio Science Walkersville), supplemented with 10% heat inactivated FCS, 50 U/ml penicillin-50 μg/ml streptomycin mixture, and 2 mM l-glutamine. Normal primary human cells were obtained from volunteers by nasal brushing.
Light Microscopy
To analyze BRCA1 presence in mitochondria HeLa, ECV 304 and SK-BR-3 cells were grown in six-well plates onto glass coverslips until well spread. Living cells were incubated with 25 nM MitoTracker (Molecular Probes) in medium for 10 min, washed, and fixed in different ways: methanol fixation at -20°C for 5 min, followed by acetone at 4°C for 2 min or 4% paraformaldehyde (PFA)/250 mM HEPES, pH 7.4, fixation for 10 min on ice followed by 8% PFA/250 mM HEPES, pH 7.4, for 50 min at room temperature. The PFA-fixed coverslips were quenched with 50 mM NH4Cl for 10 min, permeabilized with 0.2–0.5% Triton X-100 (TX100) in phosphate-buffered saline (PBS) for 5 min, and blocked using 0.2–0.4% fish skin gelatin/PBS for 30 min, followed by primary antibodies Ab-1 1:10, Ab-4 1:50, Ab-5 1:50, Ab-C 1:200, and Ab-D 1:250 and secondary antibodies Alexa Fluor 488 goat anti-mouse IgG (H+L) (Molecular Probes) 1:500 and goat anti-rabbit-fluorescein isothiocyanate (FITC) (H+L) (Vector Laboratories, Burlingame, CA) 1:50 diluted in 0.2–0.4% fish skin gelatin/phosphate-buffered saline for 1 h each. Negative controls were obtained by omission of the primary antibody step. The coverslips were mounted in Mowiol supplemented with 4,6-diamidino-2-phenylindole (DAPI). The localization of BRCA1 protein and mitochondria was evaluated using a Bio-Rad Radiance 2000 confocal laser scanning microscope; for visualization and recording of the mtDNA DAPI signal, we used an epifluorescence Zeiss Axioplan 2 microscope equipped with a Princeton Instruments charge-coupled device (CCD) Micromax camera. Nuclear and mtDNA also were visualized by staining the cells with anti-DNA IgM mouse monoclonal antibody 1:400 and goat anti-mouse IgM specific-Texas Red (Vector Laboratories) 1:50. Double labeling with anti-BRCA1 antibodies was performed as described previously. The coverslips were mounted in Mowiol and analyzed using a Bio-Rad Radiance 2000 confocal laser scanning microscope.
To confirm that the anti-DNA IgM antibody specifically detected DNA in mitochondria, ECV 304 cells were 4–8% PFA/250 mM HEPES fixed, quenched, and permeabilized as mentioned previously, with and without DNAse (0.1 mg/ml), and diluted in PBS and 4.2 mM MnCl2 for 30 min at room temperature, before blocking. Cells were then labeled with a mouse monoclonal anti-DNA IgM 1:400 as described previously and analyzed with confocal and epifluorescence microscopy.
For the siRNA experiment, ECV 304 cells were fixed 72 h after transfection in 4% PFA/250 mM HEPES, pH 7.4, fixation for 10 min on ice followed by 8% PFA/250 mM HEPES, pH 7.4, for 50 min at room temperature. Coverslips were similarly processed for triple immunofluorescence as described above using goat anti-Lamin B1 1:100, mouse anti-BRCA1 Ab-5 1:40, and rabbit anti-F1 ATPase 1:50 as primary antibodies and donkey anti-goat Alexa 488 1:400 (conjugated in house from Molecular Probes labeling kit and Jackson ImmunoResearch Laboratories UK secondary antibodies [distributed by Stratech Scientific, Cambridge, United Kingdom]), donkey anti-mouse Cy3 1:100, and donkey anti-rabbit Cy5 1:100 (Jackson ImmunoResearch Laboratories UK) as secondary antibodies. The results were evaluated using a Bio-Rad MRC 1024 confocal laser scanning microscope.
Electron Microscope Immunocytochemistry
For all the thawed frozen ultrathin section immunocytochemistry, the pellets of HBL-100, HMEC, HeLa, and normal primary human cells obtained by nasal brushing were fixed in 4 and 8% PFA in 250 mM HEPES adjusted to pH 7.4 as described above. Fixed cells were repelleted, embedded in gelatin, and snap frozen in liquid nitrogen with sucrose as a cryoprotectant. Sections of 80-nm nominal thickness were cut using a Reichert Ultracut with the FCS attachment, picked up on Formvar-coated grids, and processed using the Tokuyasu procedure with uranyl acetate methylcellulose stabilization (Tokuyasu, 1983). Antibody-1 1:1 and Ab-5 1:5 were detected using a rabbit anti-mouse secondary antibody 1:50 (MP Biomedicals, Irvine, CA), and this was visualized using protein A 1:100 (Pfizer, Täby, Sweden) on 9-nm gold. Antibody-C 1:10 was detected using protein A 1:100 on 9-nm gold. Deletion of the first antibody resulted in a clean background.
The enriched rat liver mitochondrial preparations also were assessed by EM. The mitochondrial pellet was processed for thawed frozen ultrathin section immunocytochemistry as described above; stained for F1 ATPase by using a rabbit antiserum diluted 1:200; and detected with goat anti-rabbit 1:50 on 10-nm gold (British BioCell International, Cardiff, United Kingdom). The ultrathin (70-nm-thick) sections were examined on a Leo 912 electron microscope.
Small Interfering RNA
siRNAs specific for BRCA1 and Lamin B1 were synthesized (Dharmacon RNA Technologies, Lafayette, CO) and transfected into ECV 304 cells by using Lipofectamine 2000 and Opti-MEM medium (Invitrogen) according to the manufacturer's instructions. Briefly, 24 h before transfection at 50–80% confluence, the ECV 304 cells were trypsinized, transferred to 24-well plates, and seeded in fresh medium without antibiotics. Transfection was performed on 30–50% confluent cells by using LipofectAMINE 2000 and 0.5 μg of siRNA duplex was applied per well. After 72 h of incubation, cells were fixed and stained for light microscope analysis as described above. For biochemical analysis, the cells were seeded in six-well plates, similarly transfected, and incubated for 48 h. The cells were harvested by scraping, centrifuged at 2000 rpm, and the pellet was resuspended in sample buffer. Equal amounts of protein per lane were loaded on 6% polyacrylamide gels and further processed for Western blot analysis as described below.
The siRNA sequence used for human BRCA1 was 5′-UCA CAG UGU CCU UUA UGU A dTdT-3′. The siRNA sequence used for human Lamin B1 was 5′-CGC GCU UGG UAG AGG UGG A dTdT-3′.
Cell Fractionation
The HeLa fractionation of nucleus and cytoplasm was obtained by means of the NE-PER nuclear and cytoplasmic extraction reagents kit (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. The mitochondrial fraction was obtained by sequential ultracentrifugation in mitochondrial medium buffer consisting of 10 mM Tris-HCl, 0.25 M sucrose, and 2 mM EDTA dehydrate, pH 7.4, according to (Scholte et al., 1992) with minor modifications. Briefly, HeLa cells were washed with ice-cold PBS, scraped, and pelleted. The pellet was resuspended in mitochondrial medium buffer with protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany), thoroughly homogenized with 20 strokes at 800 rpm on ice by using a motor-driven Potter Dounce homogenizer, and centrifuged at 5600 × g for 3 min at 4°C. The supernatant was kept and pellet was dissolved in mitochondrial medium buffer and homogenized as described above, followed by centrifugation step at 5600 × g for 3 min at 4°C. Both supernatants were combined and centrifuged at 5600 × g for 3 min at 4°C to remove the remaining nuclei. The supernatant was centrifuged at 37,000 × g for 3 min at 4°C. The pellet containing the enriched mitochondrial fraction (MitF) was dissolved in a small volume of mitochondrial medium buffer, centrifuged for 15 min at 12,000 rpm, and the pellet was further processed for Western blotting, dephosphorylation, and enzyme protection assays.
Mitoplast Preparation
An adult rat was killed and the liver quickly removed and placed in ice-cold HIM buffer containing 220 mM d-mannitol, 70 mM sucrose, 10 mM HEPES buffer, 1 mM EGTA, and 2 mg/ml delipidated bovine serum albumin (BSA). The pH of the medium was adjusted to 7.5 with KOH. The liver was minced and rinsed to remove the blood and transferred to 20 ml of HIM buffer + BSA + protease inhibitors in a 50-ml tube. All subsequent steps were conducted at 4°C. The liver was homogenized with a Polytron homogenizer operating for 4 × 1 s at setting of 6.5. At this stage, a “whole homogenate” (WH) sample was taken for Western blot analysis. Nuclei and unbroken cells were pelleted at 3000 rpm for 10 min. The supernatant was collected and kept on ice, whereas the pellet was resuspended in another volume of HIM buffer + BSA and again subjected to a second round of Polytron homogenization. Both supernatants were combined and centrifuged for 10 min at 3000 rpm, and the mitochondria were collected at 7000 × g for 15 min. This pellet represents the enriched MitF, and samples were taken for Western blot and EM analysis. These liver mitochondria were then suspended in HIM buffer without BSA, and mitoplasts were obtained using digitonin fractionation according to Schnaitman and Greenawalt (1968) with minor modifications. Stock 5% digitonin solution was prepared by heating, cooling down, and filtering, and BSA was added after the digitonin had been dissolved. The stock solution was diluted with HIM buffer + BSA to obtain the desired ratio of 0.11 mg of digitonin to 1 mg of mitochondrial protein. Aliquots of ice-cold digitonin were added, and the mixture was rocked in an ice bath for 15 min. The resulting solution was diluted with 3 volumes of HIM buffer + BSA, homogenized gently by hand, and centrifuged for 10 min at 9500 × g. Equal amounts of WH, enriched MitF, and MP protein were loaded for Western blotting as described below. To further purify the enriched MitF, we applied self-forming Percoll gradient centrifugation. Briefly, the enriched MitF pellet is dissolved in 6 ml of HIM buffer + BSA and mixed with 20 ml of 30% Percoll in HIM + BSA buffer. The mixture was equally divided over two Quick-Seal Ultra-Clear centrifuge tubes and centrifuged at 95,000 × g for 2 × 30 min at 4°C by using a 70Ti rotor. The brownish intact mitochondrial (IM) band was collected via syringe aspiration. To remove the Percoll, the purified MitF was washed twice with HIM + BSA and centrifuged at 7080 × g for 10 min. The pellet was washed twice with 150 mM KCl to remove attached proteins and finally washed twice with HIM buffer without BSA. Samples were taken for Western blot and enzyme activity assays. To obtain MP fractions, Percoll-purified IM were osmotically broken by diluting the mitochondrial pellet in 4 volumes of distilled water and centrifuged for 10 min at 12,300 × g to give an MP pellet. The MP pellet was dissolved in HIM buffer without BSA, and samples were taken for Western blot and enzyme activity assays.
Western Blotting
Gel electrophoresis of the NF (∼2 × 105 cells, except for CKBS and Lamin A/C, where ∼1 × 105 cells were loaded), CF (∼1 × 105 cells), MitF (∼1 × 107 cells), and WH (∼1 × 105 cells) HeLa fractions was carried out using 3–8% Tris-acetate gels and Tris-acetate SDS running buffer (Invitrogen). For low-molecular-weight proteins, i.e., CKBS and Lamin A/C, a 7% Tris-acetate gel was used and for the siRNA and mitoplast analysis, we used 6% polyacrylamide gels. Equal amounts of siRNA Lamin B1- and siRNA BRCA1-transfected cells were loaded for the siRNA experiment. For the first mitoplast experiment, WH, enriched MitF, and untreated MP (∼230 μg of protein/lane) and the dephosphorylated MP sample (∼180 μg of protein/lane) were loaded (Ab-5 detection), whereas for the detection with the p-BRCA1 Ser 1497 antibody, the loadings were ∼120 μg of protein/lane for WH, enriched MitF, and MP-untreated and ∼180 μg of protein/lane for the dephosphorylated MP sample. For the second MP experiment ∼125 μg protein/lane for both Percoll-purified IM and MP was loaded. The proteins were blotted onto a nitrocellulose membrane; blocked for 2–3 h in 10% milk powder/0.1% TX100/PBS at room temperature; incubated with the primary antibodies Ab-1 1:30, Ab-4 1:50, Ab-5 1:30, Ab-C 1:800–1:1000, p-BRCA1 Ser 988 1:100, p-BRCA1 Ser 1497 1:100–1:400, anti-Lamin A/C antibody 1:100, anti-CKBS antibody 1:100, anti-Ki67 1:400, anti-Lamin B1 1:2500, and anti-F1 ATPase 1:500; diluted in 10% milk powder/0.1% TX100/PBS overnight at 4°C on a rocking platform; washed, blocked; and finally detected with secondary antibodies sheep anti-rabbit IgG peroxidase (POD) and rabbit anti-goat POD (Chemicon International, Temecula, CA), sheep anti-mouse IgG POD, donkey anti-sheep/goat IgG POD (Roche Diagnostics), donkey anti-mouse horseradish peroxidase (HRP) 1:7500, donkey anti-rabbit HRP 1:7500, and donkey anti-goat HRP 1:7500 (Jackson ImmunoResearch Laboratories UK) by using the biochemiluminescence technique and Hyperfilm ECL development.
Dephosphorylation and Enzyme Protection
BRCA1 proteins of the different subcellular HeLa fractions were dephosphorylated by λ protein phosphatase (12,000–24,000 U/ml; New England Biolabs, Beverly, MA), dissolved in 1× λ phosphatase buffer (from a 10× NEB phosphatase buffer stock), 1× MnCl2 (10× stock 20 mM), and BSA. After incubation of the samples for 60 min at 30°C, dephosphorylated and nondephosphorylated samples were subjected to Western blotting with Ab-C and Ab-4 as described above. For the mitoplast dephosphorylation, 8000 U/ml λ protein phosphatase was used in an incubation for 30 min at 30°C. Untreated WH, enriched MitF, MP fractions and dephosphorylated MP samples were subjected to Western blotting with Ab-5 and p-BRCA1 Ser 1497 antibodies as described above. For the Western blot with Ab-C in the enzyme protection assay, MitF were obtained as described above, divided into equal aliquots, and single treated with 2% TX100 for 5 min, treated with proteinase K (5 ng/ml) with and without TX100 for 1 h at 25°C, and stopped with 10 mM phenylmethylsulfonyl fluoride for 10 min, or treated with λ protein phosphatase with and without TX100 as described above.
Enzyme Activity Assay
A cytochrome c oxidase (CCO) kit (Sigma-Aldrich, St. Louis, MO) was used to monitor the presence of endogenous CCO activity in the IM and MP fractions. The solutions were freshly prepared and used promptly. 1× assay buffer, 1× enzyme dilution buffer, and sample were combined according to the manufacturer's instructions. Before reading the absorbance at 550 nm, the ferrocytochrome c substrate was added and the absorbance was measured with a 5-s delay, for six readings at 20-s intervals. Initial reaction rates were calculated and calibrated to a positive control enzyme stock. For the malate dehydrogenase (MLDH) assay, 0.1 M phosphate buffer, pH 7.5, was mixed with both freshly prepared oxaloacetate and NADH. Just before reading the absorbance at 340 nm, the sample or enzyme (positive control) was added. Absorbance readings were taken at 340 nm every 20 s for 1 min. Initial reaction rates were calculated and calibrated to a positive control enzyme stock.
Intramitochondrial Distribution of BRCA1
Enriched intact rat liver mitochondria were prepared, fixed, frozen in cryoprotectant, cryosectioned into ultrathin sections, and used for immunocytochemistry as described above. Antibody-1 (1:1), IgM anti-DNA (1:5) and rabbit anti-F1 ATPase (1:200) were detected using goat anti-mouse IgG+IgM 1:50 on 10-nm gold and goat anti-rabbit 1:50 on 10-nm gold (British BioCell International) respectively. Deletion of the first antibody resulted in a clean background. The sections were examined on a Leo 912 electron microscope. Images were acquired using a 2k × 2k 16-bit cooled CCD camera, and quantitation was carried out by counting gold particles blind on 50 images for each label; this represents a substantial oversampling compared with recent recommendations for quantitation of immunogold label distribution on thin sections (Lucocq et al. (2004)).
RESULTS
Light Microscopy
The subcellular localization of the BRCA1 protein was investigated by immunocytochemistry by using both confocal laser scanning and wide-field light microscopy. To ensure the specificity of the BRCA1 labeling, a panel of primary antibodies recognizing different regions on the BRCA1 molecule was selected (Figure 1). In particular, we focused on the extranuclear BRCA1 distribution by means of double labeling of HeLa, ECV 304, and SK-BR-3 cells with MitoTracker probe and Ab-1, Ab-4, Ab-5, Ab-C, and Ab-D anti-BRCA1 antibodies (Figure 2). Both nuclear and cytoplasmic staining was seen in all cell lines with all anti-BRCA1 antibodies. The nucleolus was always excluded from the nuclear BRCA1 staining pattern (our unpublished data). BRCA1 protein was present in the cytoplasm as a granular staining pattern; moreover in ECV 304 cells, which have an endothelial cell morphology and linear extended mitochondria, we found pronounced linear BRCA1 staining patterns colocalizing with similar linear MitoTracker staining with all of the anti-BRCA1 antibodies, suggesting that BRCA1 is associated with mitochondria (Figure 2, A–G, J–L). Similar results were obtained with HeLa (Figure 2H) and SK-BR-3 cells (Figure 2I) by using Ab-5 (as shown) and the other primary antibodies (our unpublished data), although less easily discerned because of the more condensed mitochondria.
Figure 1.

Schematic diagram of the BRCA1 gene and polypeptide representing the multiple BRCA1 epitopes against which the different antibodies were raised. Exons are drawn to scale.
Figure 2.
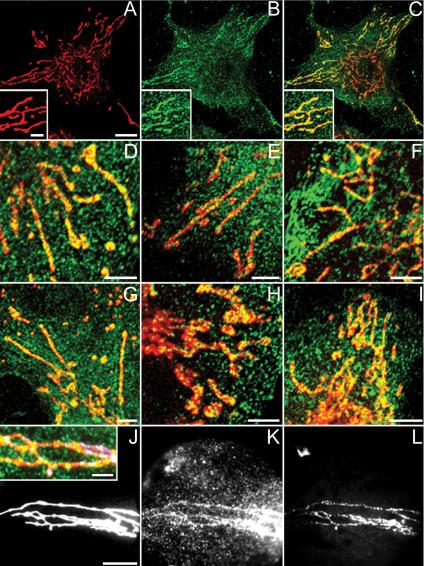
Confocal and wide-field microscopical data of ECV 304, HeLa, and SK-BR-3 cells labeled with MitoTracker (red) and anti-BRCA1-FITC/Alexa Fluor 488 antibodies (green). PFA-fixed ECV 304 cells stained with Ab-5 (B and C) and Ab-1 (E) and methanol-fixed ECV 304 cells stained with Ab-4 (D), Ab-C (F), and Ab-D (G) show granular BRCA1 cytoplasmic staining and colocalization of linear MitoTracker staining with linear BRCA1 staining results in yellow. (A) Single MitoTracker staining, overview. (B) Single Ab-5 staining, overview. (C) Merge of A and B. Insets in A–C represent a higher magnification. Double labeling of PFA-fixed HeLa (H) and SK-BR-3 (I) cells with MitoTracker and Ab-5 again shows yellow colocalization of both labels. Wide-field microscopical data shows colocalization of MitoTracker (J), Ab-5 (K), and mtDNA DAPI (L) on PFA-fixed ECV 304 cells, resulting in white foci (J, inset: higher magnification merge of J–L). Bars, 3 μm in insets; 5 μm in the other panels.
mtDNA staining was visualized with DAPI (excitation 365 nm/emission 420 nm), by using a CCD camera on a wide-field microscope. mtDNA foci were distributed within structures defined by the MitoTracker signal. After staining ECV 304 cells with MitoTracker (Figure 2J), Ab-5 (Figure 2K) and DAPI (Figure 2L), an overlapping linear staining pattern for each label was seen. In particular, although we found mitochondrial BRCA1 foci with and without DNA signal and mtDNA clusters with and without BRCA1, white (triple labeled) foci (Figure 2J, inset) were also common. This apparent partial colocalization of all three labels suggests a possible BRCA1 association with mtDNA. We also found that the mtDNA DAPI staining pattern was identical with an anti-DNA IgM antibody staining pattern and that both labels were exclusively located within the linear mitochondrial structures seen in the phase contrast image (Supplementary Figure A, A–C). To demonstrate the DNA specificity of this anti-DNA IgM antibody, we treated ECV 304 cells with DNAse. After DNAse treatment of the cells, the staining with both DAPI and the anti-DNA IgM antibody was absent from mitochondria, suggesting that this reagent is indeed specifically detecting DNA and therefore also can be used to visualize mtDNA (Supplementary Figure A, D–F). Double labeling of ECV 304 cells with Ab-5 and anti-DNA IgM (Supplementary Figure A, G–I) showed a similar linear colocalization of BRCA1, DNA and mitochondrial structures in the phase contrast transmission image.
Electron Microscope Immunocytochemistry
We performed cryoimmuno-EM on HBL-100, HMEC, HeLa, and normal primary human cells obtained by nasal brushing with Ab-1, Ab-5, and Ab-C anti-BRCA1 antibodies (Figures 3 and 4). Strong focal nuclear labeling was seen in all cases confirming the confocal data (Figure 3). In each cell type, most of the nuclear signal is seen in the form of widely distributed gold clusters ∼70–80 nm in diameter. We did not see any evidence for association of gold clusters with any discernable nuclear feature. In particular, the clusters were not associated with the nuclear envelope. The clusters were very often seen at the boundary between heterochromatin and euchromatin (Figure 3, 4) and excluded from nucleoli (Figure 3, 2 and 3).
Figure 3.
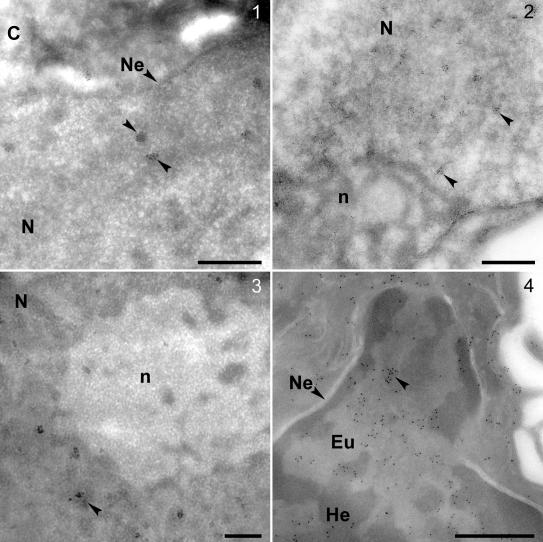
Thawed frozen section EM-immunocytochemistry by using antibodies Ab-1 and Ab-5 on human breast cells, HeLa cells, and normal primary human cells obtained by nasal brushing. BRCA1 gold clusters (arrowheads) in nuclei of HBL100 (1), HeLa (2), HMEC (3), and normal primary human cells (4) by using anti-BRCA1 antibodies Ab-1 (1–3) and Ab-5 (4). In normal primary human cells the clusters are only visible in the lighter euchromatin regions or at the boundary of eu- and heterochromatin (4). Bars, 500 nm (1–4). C, cytoplasm; Eu, euchromatin; He, heterochromatin; Ne, nuclear envelope; N, nucleus; and n, nucleolus.
Figure 4.

Thawed frozen section EM-immunocytochemistry by using antibodies Ab-C, Ab-1, and Ab-5 on human breast cells and normal primary human cells obtained by nasal brushing. BRCA1 gold clusters in mitochondria of normal primary human cells (1), HBL100 (2), HMEC (3), and HeLa (4 and 5) by using anti-BRCA1 antibodies Ab-C (2), Ab-1 (3 and 4), and Ab-5 (1 and 5). Panel 6 shows a schematic representation of a higher mitochondrial magnification of 5; the selected region is depicted by a box. Bars, 100 nm. C, cytoplasm; Cr, cristae; IM, inner membrane; and M, matrix; circles emphasize gold clusters located in the matrix; OM, outer membrane.
In agreement with our biochemical and confocal data, cytoplasmic labeling was seen with all of the antibodies in all cell types. This labeling also took the form of gold particle clusters. A number of examples of an apparent association between such cytoplasmic clusters and bundled cytoskeletal filaments were found for each cell line. In addition to nuclear and cytoplasmic labeling, a strong focal labeling within mitochondria of HBL-100, HMEC, HeLa, and normal primary human cells obtained by nasal brushing was observed with Ab-1, Ab-5, and Ab-C (Figure 4). The clusters of gold particles were found predominantly over the mitochondrial matrix (see below and Figure 9). The clusters were indistinguishable from the clusters seen within the nucleus. These results were consistent with the light microscopical data and confirmed the presence of BRCA1 within mitochondria.
Figure 9.
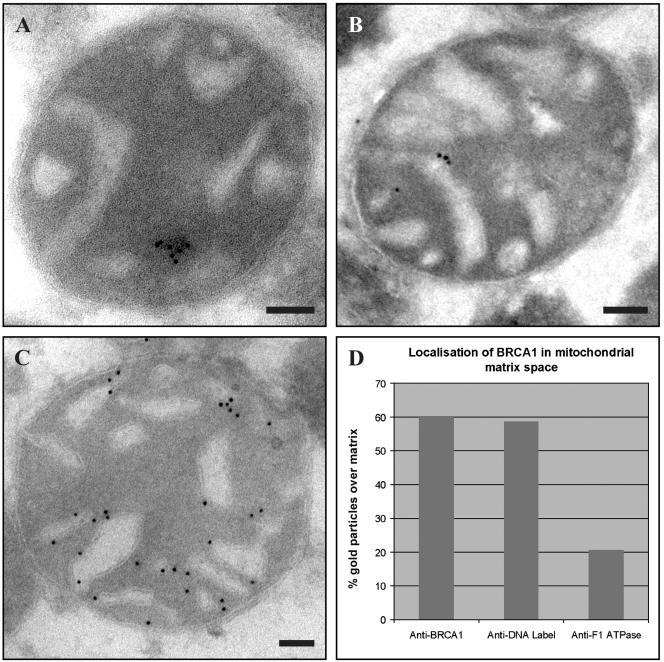
Intramitochondrial localization of BRCA1. (A) EM analysis of rat liver mitochondria with anti-BRCA1 Ab-1 shows BRCA1 gold clusters in the matrix. (B) EM analysis of rat liver mitochondria with anti-DNA IgM shows IgM signal in the matrix. (C) EM analysis of rat liver mitochondria with anti-F1 ATPase shows that F1 ATPase is associated with the mitochondrial membrane. Bars, 100 nm. (D) Table shows localization of BRCA1 in mitochondrial matrix space. BRCA1 (60%) and IgM (59%) are both predominantly located in the mitochondrial matrix space; F1 ATPase is predominantly associated with the cristae and therefore only a minority (20%) is located over the matrix space.
Small Interfering RNA
siRNA-mediated depletion of mRNA specific for BRCA1 and Lamin B1 using previously validated siRNA sequences (Ganesan et al., 2002) was used to inhibit synthesis of these proteins in cultured cells. Biochemical study of the Lamin B1 protein levels after single transfection and 48-h incubation of ECV 304 cells with Lamin B1-specific RNAi (Figure 5A, 1) or BRCA1-specific RNAi (Figure 5A, 2) showed that Lamin B1 protein was knocked down in the cells transfected with specific Lamin B1 duplex, whereas the level remained high in the cells transfected with specific BRCA1 duplex. The latter served as an internal control to exclude any nonspecific Lamin B1 protein knockdown due to the transfection procedure itself. Similarly, the BRCA1 protein levels after single transfection with Lamin B1-specific (Figure 5A, 1) or BRCA1-specific RNAi (Figure 5A, 2) showed that the BRCA1 protein was only knocked down in the cells transfected with specific BRCA1 duplex, whereas the level remained high in the cells transfected with the Lamin B1 duplex alone. Similar amounts of Lamin B1-specific (Figure 5A, 1) and BRCA1-specific (Figure 5A, 2) RNAi-transfected cells were loaded, and this was confirmed by monitoring the expression of the housekeeping protein F1 ATPase. Quantitation of Western blots (NIH Image 1.62) confirmed the selective reduction in Lamin B1 and BRCA1 protein levels (40% reduction for Lamin B1 and 52.4% reduction for BRCA1 in the experiment shown).
Figure 5.

Biochemical and confocal analysis of siRNA-mediated knockdown of BRCA1/Lamin B1 in ECV 304 cells. (A) Lamin B1 protein was knocked down in the cells transfected with specific Lamin B1 duplex (1), whereas the level remained high in the cells transfected with specific BRCA1 duplex (2). BRCA1 protein was knocked down in the cells transfected with specific BRCA1 duplex (2), whereas the level remained high in the cells transfected with specific Lamin B1 duplex (1) (arrowhead). Equal amounts of cells transfected with specific Lamin B1 duplex (1) and with specific BRCA1 duplex (2) were loaded, which was confirmed by a similar F1 ATPase expression in both lanes. (B) siRNA Lamin B1-only knockdown shows a diminished Lamin B1 expression at the nuclear envelope; the nuclear, cytoplasmic, and mitochondrial BRCA1 staining is not affected nor is the mitochondrial F1 ATPase staining pattern. (C) siRNA Lamin B1 and BRCA1 double knockdown shows a diminished expression of both nuclear, cytoplasmic, and mitochondrial BRCA1 and Lamin B1 proteins in the transfected cells. Mitochondrial F1 ATPase staining remains present. The untransfected cells still show Lamin B1 and BRCA1 nuclear, cytoplasmic, and mitochondrial staining as well as mitochondrial F1 ATPase expression. Bar, 10 μm.
After confocal analysis, we can conclude that transfection of ECV 304 cells with Lamin B1-specific RNAi led to marked suppression of Lamin B1 synthesis after 72 h without affecting either BRCA1 or F1 ATPase expression (Figure 5B). After double transfection of ECV 304 cells with both BRCA1 and Lamin B1-specific RNAi, we observed reduced expression of both BRCA1 and Lamin B1 proteins (Figure 5C); mitochondria remained intact and the F1 ATPase signal was unchanged. In addition, we note that the reduction in BRCA1 signal occurs in both nuclear and mitochondrial pools. No reduction in Lamin B1 or BRCA1 protein expression was observed in control mock-treated cells (our unpublished data).
Cell Fractionation and Western Blotting
To examine the localization of BRCA1 in different compartments, we first separated HeLa cells into NF and CF (Figure 6A). These fractions were shown to be well separated by control Western blots by using anti-Lamin A/C and anti-Ki67 for the nucleus and anti-CKBS antibodies for the cytoplasm. CKBS reactivity was present in the CF but not the NF, whereas Lamin A/C and Ki67 were present in the NF but not the CF fraction. After Western blotting of the NF with Ab-1, Ab-4, and Ab-C, we found a doublet of bands at ∼200 and ∼220 kDa present with all of the antibodies. The cytoplasmic pattern was slightly different. The top band (220 kDa) was weaker (Ab-1 and Ab-C) or absent (Ab-4). These results already suggest that there may be differences in the distribution of two forms of the protein between the nucleus and the rest of the cell.
Figure 6.

Western blotting with anti-BRCA1 antibodies Ab-1, Ab-4, Ab-5, and Ab-C, and anti-CKBS, anti-Lamin A/C, and anti-Ki67 antibodies on NF, CF, WH, and MitF HeLa fractions. (A) Western blotting with Ab-1, Ab-4, and Ab-C on NF and CF HeLa fractions. A 220- and 200-kDa double BRCA1 band is visualized, the top 220-kDa band being strongly reduced or absent in the CF. Control Western blots with anti-CKBS, anti-Lamin A/C, and anti-Ki67 on NF and CF show only Lamin A/C and Ki67 present in NF and cytokeratin in CF. Control Western blots with anti-Ki67 on MitF versus WH show only Ki67 present in WH. Arrowheads, left, 210 kDa; top right, 41 kDa; middle right, 71 kDa; and two bottom right, 340 kDa. (B) Western blotting with antibodies Ab-1, Ab-4, Ab-5, and Ab-C on WH and MitF HeLa fractions detecting the BRCA1 protein.
Because differences in distribution were obvious even in these simple separations, a more elaborate fractionation by ultracentrifugation was carried out on HeLa cells to obtain an enriched mitochondrial fraction. BRCA1 protein was detected in both WH and MitF (Figure 6B) with Ab-1, Ab-4, Ab-5, and Ab-C. Multiple BRCA1 immunoreactive species were seen in the WH with each of the antibodies (Figure 6B). For the MitF, BRCA1 was seen as an obvious 200- to 220-kDa doublet with Ab-C, a strong top band and fainter bottom band for Ab-5, whereas the bottom band only was seen with Ab-4 and Ab-1 antibodies (Figure 6B). Ki67 was positive in WH (∼1 × 105 cells; Figure 6A) but negative in the MitF (∼1 × 107 cells; Figure 6A), suggesting that there was no cross-contamination of nuclear proteins.
Dephosphorylation and Enzyme Protection Assay
The NF, CF, and MitF fractions from HeLa cells were subjected to Western blotting with anti-p-BRCA1 Ser 988 and anti-p-BRCA1 Ser 1497 antibodies to detect the presence of phosphorylated BRCA1 in these fractions (Figure 7A). Both antibodies stained the top (220-kDa) band in the NF and CF fractions (our unpublished data) and also in MitF (1) and WH (2).
Figure 7.

Western blotting of both untreated and treated NF, CF, MitF, and WH HeLa samples with p-BRCA1 Ser 988, p-BRCA1 Ser 1497, Ab-4, and Ab-C. (A) p-BRCA1 Ser 988 and p-BRCA1 Ser 1497 on MitF HeLa fractions (1) and WH (2) give a 220-kDa BRCA1 band in both lanes. (B) Western blotting with Ab-C and Ab-4 on NF and CF before (1) and after (2) dephosphorylation with λ protein phosphatase. The 220-kDa band disappeared after λ protein phosphatase treatment, showing that it is this species that is phosphorylated. (C) Western blotting with Ab-C on mitochondrial fractions treated for enzyme protection assay. Mitochondrial HeLa untreated control fraction (1), treated with TX100 (2), treated with λ protein phosphatase (3), and treated with λ protein phosphatase and TX100 (4) are presented (top). The 220-kDa BRCA1 band only disappears after combined λ protein phosphatase and TX100 treatment. Mitochondrial HeLa untreated control fraction (1), treated with proteinase K (2), and treated with proteinase K and TX100 (3) are presented (bottom). The 200-kDa band disappears after proteinase K treatment alone, whereas the 220-kDa band only becomes vulnerable after combined proteinase K and TX100 treatment.
In another approach to show that phosphorylated BRCA1 was present in these fractions, we subjected the subcellular NF and CF fractions (1) to enzymatic dephosphorylation by using λ protein phosphatase (2) (Figure 7B) followed by Western blotting with Ab-4 and Ab-C. In the NF and CF fractions, dephosphorylation resulted in essentially complete disappearance of the top band, suggesting that it is this species that is phosphorylated. This finding is consistent with the findings of Scully et al. (1997). Moreover, the enzyme protection assay (Figure 7C) shows that when the dephosphorylation experiments were repeated on the mitochondrial fraction, we found that the 220-kDa band was resistant to λ protein phosphatase enzyme until the sample also was treated with the detergent TX100. This protection suggests that phosphorylated BRCA1 is present within intact mitochondria and therefore protected from the λ protein phosphatase enzyme. After TX100 treatment, solubilization of the mitochondrial membranes occurs and gives access to the matrix and the BRCA1 protein, allowing the enzyme to dephosphorylate BRCA1. This results in a complete disappearance of the top 220-kDa band (Figure 7C, top). Similar results were obtained using proteinase K treatment with and without TX100 (Figure 7C, bottom) showing that only the lower band disappeared after treatment with proteinase K alone, whereas the top 220-kDa band became vulnerable after combined treatment with protease and detergent. These results suggest that the 200-kDa protein is accessible on the surface of isolated mitochondria and therefore is more easily degraded after proteinase K treatment, whereas the phosphorylated BRCA1 is protected inside mitochondria and is only degraded after combined TX100 and proteinase K treatment.
Analysis of Intact Mitochondrial and Submitochondrial Mitoplast Fraction
EM analysis of the intact mitochondrial pellet confirmed that this mitochondrial preparation was highly enriched for mitochondria and not heavily contaminated with other organelles (Supplementary Figure C). Western blot analysis of the different rat liver fractions with Ab-5 and p-BRCA1 Ser 1497 anti-BRCA1 antibodies showed that BRCA1 was not only present in the WH and MitF but also in the subsequent MP-enriched fraction (Figure 8A). This MP-enriched fraction was rich in the most highly phosphorylated BRCA1 species (Figure 8A, arrowhead), but it contained reduced amounts of more rapidly migrating hypophosphorylated forms compared with the original mitochondrial fraction. After dephosphorylation of the MP-enriched fraction with 400 U of λ protein phosphatase and detection on Western blot with anti-BRCA1 antibody Ab-5, we found that the top BRCA1 band (Figure 8A, arrowhead) was partly dephosphorylated, resulting in the appearance of an extra, more rapidly migrating, lower band (Figure 8A, arrow). This lower band was not present in the untreated MP-enriched fraction. Western blotting of the same dephosphorylated sample with the p-BRCA1 Ser 1497 antibody gave a complete loss in signal, suggesting that the serine 1497 BRCA1 epitope is highly accessible and therefore completely dephosphorylated.
Figure 8.

Biochemical analysis of rat liver mitoplasts. (A) Antibody-5 anti-BRCA1 antibody detects the protein in WH, MitF, and a MP-enriched fraction (arrowhead). After dephosphorylation, the top BRCA1 band is strongly reduced, whereas a lower band occurs (arrow). P-BRCA1 Ser 1497 anti-BRCA1 antibody detects the same protein in WH, MitF, and the MP-enriched fraction. After dephosphorylation, all p-BRCA1 Ser 1497 signal is absent. (B) Western blot of Percoll-purified IM and MP stained with Ab-5 showed an increase in signal for two hyperphosphorylated isoforms (* and **) after MP preparation from IM. Arrowhead, 160 kDa. Table of enrichment shows the MP/IM ratio for CCO and MLDH enzymes and the two asterisked BRCA1 protein isoforms. The graph displays the intensity of the BRCA1 protein forms versus the relative migration. Two hyperphosphorylated BRCA1 proteins (* and **) are enriched in the MP fraction versus IM fraction.
In a further experiment, we used an additional Percoll-gradient purification step during mitochondrial isolation and subsequently applied a hypotonic lysis method for mitoplast isolation, Western blot analysis of the Percoll-purified IM and MP fractions with Ab-5 showed that BRCA1 was present in both samples. Furthermore, the slowly migrating hyperphosphorylated BRCA1 protein isoforms were clearly enriched in the MP fraction versus IM fraction (Figure 8B, * and **). Enzyme activity assays were performed on IM and MP fractions, and both CCO and MLDH activity was detected (Figure 8B, table). The MP/IM ratio showed an increase in enzyme activity in the MP fraction versus IM. There was a 90.4% recovery of CCO activity and a 102.3% recovery of BRCA1 protein in the MP fraction compared with the IM fraction. The intensity of the IM and MP Western blot bands were measured using Quantity One Bio-Rad software and the band detection tools option. The MP/IM ratio for the first two BRCA1 protein bands is shown in the table (Figure 8B, * and **). After subtracting the background using rolling disk calculation, the intensity values were plotted in a graph (Figure 8B). These data show that the first two hyperphosphorylated BRCA1 bands are clearly enriched in the MP fraction.
Intramitochondrial Distribution of BRCA1
To define a more precise location of the BRCA1 gold clusters in mitochondria, we immunolabeled intact rat mitochondria with Ab-1 (Figure 9A), IgM anti-DNA (Figure 9B) and anti-F1 ATPase (Figure 9C) and counted the individual gold particles over matrix or over inner membrane and intermembrane space in 50 micrographs for each label. A recent analysis suggests that quantitation of immunogold labeling in individual subcompartments of the cell requires counting until 100–200 gold particles per compartment have been scored for a coefficient of error of 10–20% to be achieved across up to 16 subcompartments (Lucocq et al. 2004). Our analysis sought to distinguish only two subcompartments (matrix space vs. inner membrane and intermembrane space) and was based on total counts of >1500 gold particles, which represents a considerable oversampling. Anti-IgM gold particles, representing the mitochondrial DNA, were predominantly present over the matrix space (58%), whereas the gold clusters decorating F1 ATPase were predominantly associated with the inner membranes and intermembrane space (80%; Figure 9D). In this analysis, we found that 60% of the BRCA1-associated gold particles were located over the matrix space (Figure 9D). This result confirms that mitochondrial BRCA1 has a location profile indistinguishable from that of mtDNA, strongly suggesting a submitochondrial location within the matrix. This is consistent with the observed presence of hyperphosphorylated BRCA1 in isolated mitoplast preparations (see above).
DISCUSSION
This study provides evidence for nuclear, cytoplasmic, and mitochondrial localization of the BRCA1 protein in human cells. We used several antibodies directed against different epitopes within the BRCA1 protein on several cell types and found them to give essentially identical results. The experiments were carried out on five human cell lines and one primary cell population. HMEC cells were chosen because they are primary human breast cells, and HBL-100 cells were chosen as a breast cell line with a high expression of BRCA1 protein, whereas HeLa cells are a highly malignant, extensively dedifferentiated human cervical carcinoma derivative, known also to express BRCA1 protein. ECV 304 cells were selected for their flat endothelial morphology and widely dispersed mitochondria and SK-BR-3 cells as a second representative human breast cancer cell line. Nasal brushing was used as a source of normal primary human cells. The intracellular expression pattern was evaluated by two biochemical methods, namely, subcellular fractionation of cultured cells and mitoplast isolation from fresh tissue followed by Western blotting, and by two morphological methods, namely, immunofluorescence microscopy and EM immunocytochemistry on thawed frozen thin sections. The specificity of these findings is confirmed by siRNA-mediated knock-down of BRCA1 expression, validated by Western blotting; the nuclear, cytoplasmic, and mitochondrial signals are all coordinately reduced.
The initial results show a distribution of BRCA1 protein in the cytoplasm as well as in the nucleus. The relative distribution between nucleus and cytoplasm varies between cell types and from cell to cell in an unsynchronized population of any one cell type, in line with previous reports using a wide range of antibodies on these and other cell lines. Furthermore, the results from Western blotting after subcellular fractionation convincingly confirm the morphological findings at light and EM level. A nuclear localization of the BRCA1 protein would be expected from sequence analysis alone; the protein contains a RING finger motif, two BRCA1 C-terminal domains, three potential nuclear localization signals, and a nuclear export signal. Biochemical data confirming interactions between BRCA1 and known nuclear proteins such as RAD51 and BARD-1 would support this expectation, as would the demonstration that the C-terminal domain of BRCA1 is a transcriptional activator. The phosphorylation of BRCA1 in response to DNA damage adds further strength to a model in which more slowly migrating phosphorylated BRCA1 species act in the nucleus as part of a DNA maintenance and repair pathway. Previous results from many groups have demonstrated BRCA1 protein in the nucleus at the light microscope level. In the experiments we report here, both confocal microscope immunofluorescence and staining of thawed frozen sections in the electron microscope reveal a focal or clustered pattern of BRCA1 labeling within the nucleus. The nuclear foci of BRCA1 protein are widely distributed within the nucleoplasm, but they are excluded from nucleoli. The termination of a subset of invaginations of the nuclear envelope adjacent to nucleoli (Bourgeois et al., 1979; Fricker et al., 1997) together with exclusion of BRCA1 foci from within nucleoli may account for a previous interpretation that BRCA1 foci associated with invaginations of the nuclear envelope (Coene et al., 1997). However, at the ultrastructural level, it is clear that the BRCA1 foci do not show obvious association with the nuclear envelope, either at the nuclear margin or on the walls of nuclear invaginations.
In contrast to increasing evidence for a nuclear role for BRCA1, evidence for a cytoplasmic function of this protein is lacking. Neither sequence analysis nor initial studies on protein–protein interactions suggested a cytoplasmic function. Nonetheless, several groups have reported an apparent cytoplasmic pool of BRCA1 protein. A previous report links BRCA1 with the centrosome and goes on to demonstrate that γ-tubulin interacts with BRCA1 and does so preferentially with a hypophosphorylated form of the protein (Hsu and White, 1998). A possible role for BRCA1 in a G2/M checkpoint or in the completion of mitosis is suggested (Larson et al., 1997; Xu et al., 1999). The data presented here confirm that each of the cell types examined has a cytoplasmic pool of BRCA1 and that this pool mainly consists of rapidly migrating hypophosphorylated forms of the protein. Confocal and EM immunocytochemistry both suggest that much of the BRCA1 in the cytoplasmic pool is not diffuse, but it is focally distributed in the cytoplasm. A highly variable association of focal BRCA1 labeling with cytoskeletal elements in EM immunocytochemistry is now under active investigation.
Previous studies have identified the BRCA1 protein in the nucleus, the Golgi apparatus, the secretory pathway, and the cytoplasm (Chen et al., 1995; Jensen et al., 1996; Coene et al., 1997). Variations in the distribution have been described for normal versus transformed cells (Chen et al., 1995) and at different stages of the cell cycle. In this study, we demonstrate by Western blotting after subcellular fractionation and by light and EM immunocytochemistry that the BRCA1 protein also may be found within mitochondria. The mitochondrial pool (and indeed, the mitoplast pool) of BRCA1 is enriched in the slower migrating hyperphosphorylated forms of the protein, similarly to the NF and in contrast to the CF. Thus, mitochondrial BRCA1 is hyperphosphorylated like nuclear BRCA1, and it is the hyperphosphorylated form of the protein that has been implicated in DNA maintenance (Scully et al., 1997).
The molecular interactions contingent upon these phosphorylation events are complex and only beginning to be unraveled. BRCA1 phosphorylation itself is a complex and poorly understood process; BRCA1 is a large protein (1863 aa) with 90 potential phosphorylation sites (7 cAMP-dependent kinase sites; 34 protein kinase C sites; 47 casein kinase II sites; and 2 tyrosine phosphorylation sites, based on Prosite sequence motif analysis). Although specific phosphorylation has been detected at only a very small number of these sites, multisite phosphorylation of BRCA1 correlates with functional changes to the protein. For example, Scully et al. (1997) have demonstrated a correlation between BRCA1 nuclear dot dispersal after DNA damage and progressive changes in phosphorylation of the protein. This increasing BRCA1 phosphorylation seems to be dose, time course, and cell cycle phase dependent, as well as dependent on the mechanism of DNA damage. All these different factors contribute to shifts in protein mobility, resulting in the appearance of a multiplicity of species with different electrophoretic mobilities detectable by Western blotting, as reported previously (Scully et al., 1997). The detection of posttranslationally modified BRCA1 is further complicated by differences in accessibility to phosphorylated sites and/or diversity in protein folding due to different degrees of phosphorylation. Nonetheless, a combination of phospho-specific antibodies and phosphatase treatment as described here will permit these effects to be more fully dissected. We can, for example, already conclude that intramitochondrial BRCA1 is phosphorylated at Ser 1497 and at least one other site, because phosphatase treatment quantitatively removes the phosphate at Ser1497 but still leaves detectable slowly migrating, phosphorylated protein detectable with other antibodies.
Our combined light microscope and quantitative EM immunocytochemical data show that the mitochondrial BRCA1 label is found in small foci widely distributed throughout the mitochondrial matrix space. These labeled foci are indistinguishable from the abundant nuclear foci. Moreover, a subset of mitochondrial BRCA1 foci were found to colocalize with mtDNA clusters by light microscopy. Interestingly, this BRCA1–mtDNA association shows striking similarities with some of the mitochondrial nucleoid proteins. Recently, it was shown that in mammalian cells, mtDNA molecules are organized in discrete protein-rich complexes within the mitochondrial network and some nucleoid human proteins such as Twinkle, TFAM, mitochondrial single-stranded DNA-binding protein, and mtDNA polymerase POLG were identified (Spelbrink et al., 2001; Garrido et al., 2003). Both TFAM and mitochondrial single-stranded DNA-binding protein colocalize with Twinkle in these intramitochondrial foci. Furthermore, mtDNA polymerase POLG and various other as yet unidentified proteins copurify with mtDNA nucleoids by using a biochemical isolation procedure, as does TFAM. Because the nucleoid distribution resembles very closely the mitochondrial BRCA1 distribution, together with the fact that BRCA1 has been shown to bind nuclear DNA in the past and colocalize with mtDNA in our results, it is tempting to speculate that BRCA1 may be a component of these mitochondrial nucleoid structures. The results presented here are thus consistent with a model in which mitochondrial BRCA1 can associate with mitochondrial genome clusters and provide a similar maintenance function for mtDNA as for nuclear DNA.
Regardless of function, the presence of BRCA1 in mitochondria raises the question of how the protein is targeted to this site. A clue to one possible mechanism may come from recent studies of another enzyme involved in DNA metabolism, topoisomerase Ib. This normally nuclear gene product can be generated in a mitochondrially targeted form by alternative splicing of a single gene to give an N-terminal mitochondrial import signal (Zhang et al., 2001). Although this is a possible mechanism for BRCA1 targeting to mitochondria, there is no evidence to date for alternative splicing generating a novel BRCA1 N terminus capable of acting as a mitochondrial targeting sequence. Recently, an alternative, posttranslational, phosphorylation-dependent, mitochondrial import mechanism has been suggested for the protein GSTA4-4 (Robin et al., 2003) based on the observations that mitochondrial GSTA4-4 is more heavily phosphorylated than its cytosolic counterpart and that the Hsp70 chaperone is required for the efficient translation of GSTA4-4 as well as its translocation to mitochondria. A possible analogous phosphorylation-dependent targeting of BRCA1 to mitochondria could be comparable with the selective nuclear migration of BRCA1 after phosphorylation in response to DNA damage.
Considering that both replication and repair of mtDNA are carried out by proteins encoded by nuclear genes and imported in mitochondria by selective import machinery, it may be less surprising that BRCA1 also was found within the mitochondrial matrix. This localization would be consistent with a similar function for BRCA1 in DNA repair in the mitochondria as in the nucleus, suggesting a significant additional role for BRCA1 as a caretaker of mitochondrial genomic integrity.
Supplementary Material
Acknowledgments
It is with great regret that we announce the death of our colleague and mentor, Prof. Dr. C. De Potter, which occurred during the latter part of this investigation. His contribution to this article cannot be underestimated and his loss to the scientific community at large is immeasurable. We thank Dr. John van Emmelo for providing the Beckmann ultracentrifuge, Jan Meuleman and Lance Tomlinson for help with the photography, Professor Peter Cook (Sir William Dunn School of Pathology) for the anti-DNA IgM antibody, and Dr. R. Jensen (Vanderbilt University Medical Center, Nashville, TN) for providing the HBL-100 cells. This work was funded in part by a Medical Research Council project grant to D.J.V. and a joint Anglo-Flemish grant from the British Council to C.R.D.P. and D.J.V. Parts of this work were made possible by a major equipment grant from the Wellcome Trust to D.J.V. and financial support of the Departments of Pathology and Uro-Gynaecology (University Hospital Ghent, Belgium). E.D.C. is a Postdoctoral Researcher employed by the Fund of Scientific Research Flanders.
Article published online ahead of print in MBC in Press on December 9, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0895).
Abbreviations used: CCO, cytochrome c oxidase; CF, cytoplasmic fraction; CKBS, cytokeratin broad spectrum; DAPI, 4,6-diamidino-2-phenylindole; EM, electron microscopy; HRP, horseradish peroxidase; MitF, mitochondrial fraction; MLDH, malate dehydrogenase; MP, mitoplasts; mtDNA, mitochondrial DNA; NF, nuclear fraction; PFA, paraformaldehyde; POD, peroxidase; siRNA, small interfering RNA; TX100, Triton X 100; WH, whole homogenate.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Anderson, S., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290, 457-465. [DOI] [PubMed] [Google Scholar]
- Anson, R. M., Croteau, D. L., Stierum, R. H., Filburn, C., Parsell, R., and Bohr, V. A. (1998). Homogenous repair of singlet oxygen-induced DNA damage in differentially transcribed regions and strands of human mitochondrial DNA. Nucleic Acids Res. 26, 662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, N. O., Bianchi, M. S., and Richard, S. M. (2001). Mitochondrial genome instability in human cancers. Mutat. Res. 488, 9-23. [DOI] [PubMed] [Google Scholar]
- Bourgeois, C. A., Hemon, D., and Bouteille, M. (1979). Structural relationship between the nucleolus and the nuclear envelope. J. Ultrastruct. Res. 68, 328-340. [DOI] [PubMed] [Google Scholar]
- Chen, C. F., Li, S., Chen, Y., Chen, P. L., Sharp, Z. D., and Lee, W. H. (1996). The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. J. Biol. Chem. 271, 32863-32868. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Chen, C. F., Riley, D. J., Allred, D. C., Chen, P. L., Von Hoff, D., Osborne, C. K., and Lee, W. H. (1995). Aberrant subcellular localization of BRCA1 in breast cancer. Science 270, 789-791. [DOI] [PubMed] [Google Scholar]
- Clayton, D. A., Doda, J. N., and Friedberg, E. C. (1974). The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc. Natl. Acad. Sci. USA 71, 2777-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coene, E., Van Oostveldt, P., Willems, K., van Emmelo, J., and De Potter, C. R. (1997). BRCA1 is localized in cytoplasmic tube-like invaginations in the nucleus. Nat. Genet. 16, 122-124. [DOI] [PubMed] [Google Scholar]
- Deng, C. X. (2002). Roles of BRCA1 in centrosome duplication. Oncogene 21, 6222-6227. [DOI] [PubMed] [Google Scholar]
- Deng, C. X., and Brodie, S. G. (2000). Roles of BRCA1 and its interacting proteins. Bioessays 22, 728-737. [DOI] [PubMed] [Google Scholar]
- Diffley, J. F., and Stillman, B. (1992). DNA binding properties of an HMG1-related protein from yeast mitochondria. J. Biol. Chem. 267, 3368-3374. [PubMed] [Google Scholar]
- Driggers, W. J., Holmquist, G. P., LeDoux, S. P., and Wilson, G. L. (1997). Mapping frequencies of endogenous oxidative damage and the kinetic response to oxidative stress in a region of rat mtDNA. Nucleic Acids Res. 25, 4362-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers, W. J., LeDoux, S. P., and Wilson, G. L. (1993). Repair of oxidative damage within the mitochondrial DNA of RINr 38 cells. J. Biol. Chem. 268, 22042-22045. [PubMed] [Google Scholar]
- Fricker, M., Hollinshead, M., White, N., and Vaux, D. (1997). Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136, 531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, S., et al. (2002). BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell 111, 393-405. [DOI] [PubMed] [Google Scholar]
- Garrido, N., Griparic, L., Jokitalo, E., Wartiovaara, J., van der Bliek, A. M., and Spelbrink, J. N. (2003). Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell 14, 1583-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakem, R., de la Pompa, J. L., Elia, A., Potter, J., and Mak, T. W. (1997). Partial rescue of BRCA1 (5–6) early embryonic lethality by p53 or p21 null mutation. Nat. Genet. 16, 298-302. [DOI] [PubMed] [Google Scholar]
- Hsu, L. C., and White, R. L. (1998). BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA 95, 12983-12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin, M. (2002). Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene 21, 8981-8993. [DOI] [PubMed] [Google Scholar]
- Jensen, R. A., Thompson, M. E., Jetton, T. L., Szabo, C. I., van der Meer, R., Helou, B., Tronick, S. R., Page, D. L., King, M. C., and Holt, J. T. (1996). BRCA1 is secreted and exhibits properties of a granin. Nat. Genet. 12, 303-308. [DOI] [PubMed] [Google Scholar]
- Koonin, E. V., Altschul, S. F., and Bork, P. (1996). BRCA1 protein products. Functional motifs. Nat. Genet. 13, 266-268. [DOI] [PubMed] [Google Scholar]
- Larson, J. S., Tonkinson, J. L., and Lai, M. T. (1997). A BRCA1 mutant alters G2-M cell cycle control in human mammary epithelial cells. Cancer Res. 57, 3351-3355. [PubMed] [Google Scholar]
- Lucocq, J. M., Habermann, A., Watt, S., Backer, J. M., Mayhew, T. M., and Griffiths, G. (2004). A rapid method for assessing the distribution of gold labeling on thin sections. J. Histochem. Cytochem. 52, 991-1000. [DOI] [PubMed] [Google Scholar]
- Meeusen, S., Tieu, Q., Wong, E., Weiss, E., Schieltz, D., Yates, J. R., and Nunnari, J. (1999). Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA. J. Cell Biol. 145, 291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, Y., et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66-71. [DOI] [PubMed] [Google Scholar]
- Miyakawa, I., Sando, N., Kawano, S., Nakamura, S., and Kuroiwa, T. (1987). Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. J. Cell Sci. 88, 431-439. [DOI] [PubMed] [Google Scholar]
- Monteiro, A. N. (2000). BRCA 1, exploring the links to transcription. Trends Biochem. Sci. 25, 469-474. [DOI] [PubMed] [Google Scholar]
- Myers, K. A., Saffhill, R., and O'Connor, P. J. (1988). Repair of alkylated purines in the hepatic DNA of mitochondria and nuclei in the rat. Carcinogenesis 9, 285-292. [DOI] [PubMed] [Google Scholar]
- Neupert, W. (1997). Protein import into mitochondria. Annu. Rev. Biochem. 66, 863-917. [DOI] [PubMed] [Google Scholar]
- O'Rourke, T. W., Doudican, N. A., Mackereth, M. D., Doetsch, P. W., and Shadel, G. S. (2002). Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol. Cell. Biol. 22, 4086-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrella, P., et al. (2001). Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 61, 7623-7626. [PubMed] [Google Scholar]
- Paull, T. T., Cortez, D., Bowers, B., Elledge, S. J., and Gellert, M. (2001). From the cover: direct DNA binding by BRCA1. Proc. Natl. Acad. Sci. USA 98, 6086-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penta, J. S., Johnson, F. M., Wachsman, J. T., and Copeland, W. C. (2001). Mitochondrial DNA in human malignancy. Mutat. Res. 488, 119-133. [DOI] [PubMed] [Google Scholar]
- Reenan, R. A., and Kolodner, R. D. (1992). Isolation and characterization of two Saccharomyces cerevisiae genes encoding homologs of the bacterial HexA and MutS mismatch repair proteins. Genetics 132, 963-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, C., Park, J. W., and Ames, B. N. (1988). Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 85, 6465-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin, M. A., Prabu, S. K., Raza, H., Anandatheerthavarada, H. K., and Avadhani, N. G. (2003). Phosphorylation enhances mitochondrial targeting of GSTA4–4 through increased affinity for binding to cytoplasmic Hsp70. J. Biol. Chem. 278, 18960-18970. [DOI] [PubMed] [Google Scholar]
- Rodriguez, J. A., and Henderson, B. R. (2000). Identification of a functional nuclear export sequence in BRCA1. J. Biol. Chem. 275, 38589-38596. [DOI] [PubMed] [Google Scholar]
- Schnaitman, C., and Greenawalt, J. W. (1968). Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J. Cell Biol. 38, 158-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte, H. R., et al. (1992). Assessment of deficiencies of fatty acyl-CoA dehydrogenases in fibroblasts, muscle and liver. J. Inherit. Metab. Dis. 15, 347-352. [DOI] [PubMed] [Google Scholar]
- Scully, R., Chen, J., Ochs, R. L., Keegan, K., Hoekstra, M., Feunteun, J., and Livingston, D. M. (1997). Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90, 425-435. [DOI] [PubMed] [Google Scholar]
- Somasundaram, K., Zhang, H., Zeng, Y. X., Houvras, Y., Peng, Y., Wu, G. S., Licht, J. D., Weber, B. L., and El-Deiry, W. S. (1997). Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature 389, 187-190. [DOI] [PubMed] [Google Scholar]
- Spelbrink, J. N., et al. (2001). Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 28, 223-231. [DOI] [PubMed] [Google Scholar]
- Tokuyasu, K. T. (1983). Visualization of longitudinally-oriented intermediate filaments in frozen sections of chicken cardiac muscle by a new staining method. J. Cell Biol. 97, 562-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman, A. R. (2002). Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108, 171-182. [DOI] [PubMed] [Google Scholar]
- Wang, H., Shao, N., Ding, Q. M., Cui, J., Reddy, E. S., and Rao, V. N. (1997). BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene 15, 143-157. [DOI] [PubMed] [Google Scholar]
- Xu, X., Weaver, Z., Linke, S. P., Li, C., Gotay, J., Wang, X. W., Harris, C. C., Ried, T., and Deng, C. X. (1999). Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3, 389-395. [DOI] [PubMed] [Google Scholar]
- Yamane, K., Katayama, E., and Tsuruo, T. (2000). The BRCT regions of tumor suppressor BRCA1 and of XRCC1 show DNA end binding activity with a multimerizing feature. Biochem. Biophys. Res. Commun. 279, 678-684. [DOI] [PubMed] [Google Scholar]
- Yan, Y., Haas, J. P., Kim, M., Sgagias, M. K., and Cowan, K. H. (2002). BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. J. Biol. Chem. 277, 33422-33430. [DOI] [PubMed] [Google Scholar]
- Yasui, A., Yajima, H., Kobayashi, T., Eker, A. P., and Oikawa, A. (1992). Mitochondrial DNA repair by photolyase. Mutat. Res. 273, 231-236. [DOI] [PubMed] [Google Scholar]
- Ye, Q., Hu, Y. F., Zhong, H., Nye, A. C., Belmont, A. S., and Li, R. (2001). BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J. Cell Biol. 155, 911-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Barcelo, J. M., Lee, B., Kohlhagen, G., Zimonjic, D. B., Popescu, N. C., and Pommier, Y. (2001). Human mitochondrial topoisomerase I. Proc. Natl. Acad. Sci. USA 98, 10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X. M., Clark-Walker, G. D., and Chen, X. J. (2002). The mitochondrial nucleoid protein, Mgm101p, of Saccharomyces cerevisiae is involved in the maintenance of rho(+) and ori/rep-devoid petite genomes but is not required for hypersuppressive rho(-) mtDNA. Genetics 160, 1389-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


