Abstract
No successful therapies are available for pulmonary fibrosis, indicating the need for new treatments. Lipoxins and their 15-epimers, aspirin-triggered lipoxins (ATL), present potent antiinflammatory and proresolution effects (Martins et al., J Immunol 2009;182:5374–5381). We show that ATLa, an ATL synthetic analog, therapeutically reversed a well-established pulmonary fibrotic process induced by bleomycin (BLM) in mice. We investigated the mechanisms involved in its effect and found that systemic treatment with ATLa 1 week after BLM instillation considerably reversed the inflammatory response, total collagen and collagen type 1 deposition, vascular endothelial growth factor, and transforming growth factor (TGF)-β expression in the lung and restored surfactant protein C expression levels. ATLa also inhibited BLM-induced apoptosis and cellular accumulation in bronchoalveolar lavage fluid and in the lung parenchyma as evaluated by light microscopy and flow cytometry (Ly6G+, F4/80+, CD11c+, CD4+, and B220+ cells) assays. Moreover, ATLa inhibited the lung production of IL-1β, IL-17, TNF-α, and TGF-β induced by BLM-challenged mice. ATLa restored the balance of inducible nitric oxide synthase–positive and arginase-positive cells in the lungs, suggesting a prevalence of M2 versus M1 macrophages. Together, these effects improved pulmonary mechanics because ATLa treatment brought to normal levels lung resistance and elastance, which were clearly altered at 7 days after BLM challenge. Our findings support ATLa as a promising therapeutic agent to treat lung fibrosis.
Keywords: lipoxin, ATLa, fibrosis, inflammation, lung
Clinical Relevance
Our work demonstrates a new possible therapeutic approach for lung fibrosis. Aspirin-triggered lipoxin A4, a synthetic analog of lipoxin A4, has an antifibrotic effect and was able to reverse the lung fibrosis in a mouse model. We believe that our research contributes significantly to basic and clinical science.
Pulmonary fibrosis is a devastating disease that has a prevalence of 7 to 10:100,000 (increasing with age) and a mean survival of 3 to 4 years (decreasing with age) (1, 2) and is characterized by diffuse chronic interstitial inflammation, increased fibroblast proliferation, and enhanced extracellular matrix synthesis and deposition (3–7). Fibrosis is an interstitial disorder of the parenchyma that is a common end-stage condition of a number of lung diseases, resulting in the disruption of lung architecture that renders gas exchange difficult (3–5). Despite the increase in knowledge about the mediators and the mechanisms involved in fibrotic processes, especially in lung fibrosis (8), the basic therapeutic strategy is still the use of corticosteroids, alone or in combination with other immunosuppressive agents, with little impact on long-term survival (1, 7, 9–11). In the present study, we used a well-established mouse model of pulmonary fibrosis induced by bleomycin (BLM) (12). BLM is an antibiotic agent with antitumor activity that presents fibrosis as an important side effect, depending on the dose and the age of the patient (13, 14).
Lipoxins (LXs) are endogenous eicosanoids with potent antiinflammatory and proresolution biological activities (15–17). These lipid mediators can be produced by different pathways. The original pathways identified for LX formation were via lipoxygenase–lipoxygenase interactions. Later, another pathway for LX synthesis was described that involves aspirin-triggered acetylation of cyclooxygenase-2, 5-lipoxygenase, resulting in the formation of 15-epimer LX or aspirin-triggered LX (ATL) (18). Data in the literature support the proposal that changes in LX production can drive the evolution of several diseases, such as asthma, fibrosis, cancer, atherosclerosis, and scleroderma interstitial fibrotic lung disease (19, 20). In a murine model of asthma, the administration of a stable analog of LXA4 blocked inflammation and airway hyperresponsiveness (21). Furthermore, diminished levels of LXA4 are detected in the sputum of patients with severe asthma, of which the hallmark is the presence of irreversible airway remodeling, including increased collagen deposition and smooth muscle proliferation in the bronchial wall (22).
LXs, as trihydroxytetraene-containing products of arachidonic acid metabolism, act in an autocrine and/or a paracrine manner and are rapidly enzymatically inactivated (23). The development of metabolically stable synthetic analogs represents useful tools to evaluate their potential pharmacological and therapeutic activities on pathological processes (24, 25). Among the current ATL analogs studied, 15-epi-16-(para-fluoro)-phenoxy-LXA4 (ATLa) and related substances have been shown to be active in vivo in a number of inflammatory models (26, 27), including cystic fibrosis (28).
In the present study, we hypothesized that ATLa may revert the established pulmonary fibrosis induced by BLM. Different from our previous report (29), in this study we initiated ATLa treatment after BLM challenge for 7 days and expanded the mechanisms of action of ATLa, evaluating its effect on the inflammatory infiltration, cytokine production, the balance between M1 versus M2 macrophages, apoptosis, specific markers of fibrosis (e.g., collagen 1 and transforming growth factor [TGF]-β expression), and markers of tissue repair, surfactant protein C (SP-C), and vascular endothelial growth factor (VEGF) expression.
Materials and Methods
Fibrosis Model
C57BL/6 mice (8–10 wk of age) were provided by the Oswaldo Cruz Foundation Breeding Unit (Rio de Janeiro, Brazil). Fibrosis was induced by intratracheal instillation of BLM (0.06 U/mouse) (Sigma Aldrich, Saint Louis, MO). The control group received sterile saline at Day 0 and 0.2% ethanol (vehicle) at Days 7 and 10. ATLa (1 and 0.1 μg/mouse) was instilled intravenously 7 and 10 days after BLM injection. BOC-2 (Phoenix Pharmaceuticals, Burlingame, CA) was coinjected (100 μg and 10 μg/mouse) with ATLa at the same time points. All experimental procedures were performed according to guidelines of the Committee on Ethical Use of Laboratory Animals of the Federal University of Rio de Janeiro (DFBCICB028).
Histological and Morphometric Analysis
Lungs were collected and fixed with buffered formalin (10%) at 18 to 22 cm H2O for 1 to 2 minutes. The tracheas were clamped at end-expiration, and lungs were removed en bloc and immersed in buffered formalin (10%) for 48 hours. The left lung was paraffin embedded and stained with hematoxylin and eosin and Sirius red as previously described (30).
Hydroxyproline Assay
Lung hydroxyproline levels were determined spectrophotometrically, and results were expressed as nanograms of OH-proline/lung tissue (30).
Immunohistochemistry
Sections were immunostained with Vectastain ABC (Vector Laboratories, Burlingame, CA). Antibodies for collagen 1A, TGF-β, arginase-1, VEGF, and SP-C (Santa Cruz Biotechnology, Santa Cruz, CA); inducible nitric oxide synthase (iNOS) (Cayman Chemical, Ann Arbor, MI); and TUNEL KIT (Millipore, Billerica, MA) were used at 1/300 dilution in TBS/Tween buffer overnight at 4°C (31).
Leukocyte Analysis
Mononuclear cells and neutrophils were quantified in Giemsa-stained sections in 30 fields of 26,000 μm2 (10 random fields of three different sections) in each lung (30).
Bronchoalveolar Lavage
Bronchoalveolar lavage was performed with PBS (3 × 1 ml) containing EDTA (10 mM) and was analyzed as described elsewhere (32).
Flow Cytometry Analysis
Lungs were collected and digested with collagenase I (0.2% solution; Sigma-Aldrich). Cells were stained with antibodies anti-Ly6G-FITC, GR-1-PE, F4/80-FITC, CD11c-PE-Cy5, CD11b-PE, CD4-PE-Cy5, CD8-PE, and B220-PerCP Cy5.5 (BD Biosciences Pharmingen, San Jose, CA) as described elsewhere (1).
ELISA
IL-1β, IL-17, TNF-α, and TGF-β in lung samples were measured by ELISA (Becton Dickinson, Piscataway, NJ and R&D Systems, Minneapolis, MN) (33).
Respiratory Mechanics
Animals were sedated with diazepam (1 mg/kg, intraperitoneally), anesthetized with thiopental sodium (20 mg/kg intraperitoneally), tracheotomized, and paralyzed with vecuronium bromide (0.005 mg/kg, intravenously). Airflow and transpulmonary pressure were recorded with a pulmonary mechanics system (Buxco Electronics, Wilmington, NC). Resistance (R) and dynamic elastance (Edyn) were measured (34) at baseline (aerosolized PBS) and after increasing concentrations of methacholine (3, 9, and 27 mg/m/ aerosolized for 5 min each).
Statistical Analysis
One-way ANOVA, followed by Bonferroni post hoc test, was used. Mechanical data were evaluated using two-way ANOVA followed by the Bonferroni post hoc test. Data were expressed as mean ± SEM and are representative of at least three separate experiments. GraphPad Prism 5 statistical software package (GraphPad Software, La Jolla, CA) was used. A P value < 0.05 was considered significant.
Results
ATLa Treatment Reverses BLM-Induced Lung Fibrosis
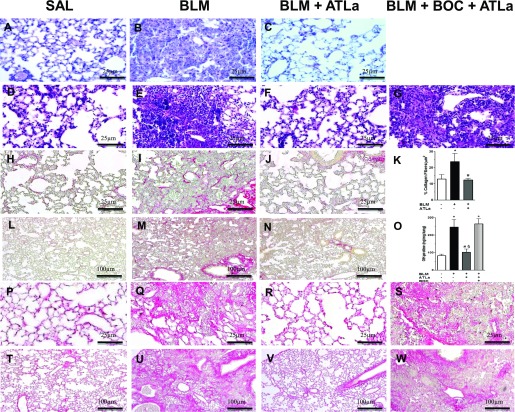
Figure 1 presents representative photomicrographs of slides stained with hematoxylin and eosin on Days 14 (Figures 1A–1C) and 21 (Figures 1D–1G). The administration of ATLa 7 days after BLM challenge reduced lung cell infiltration and edema, and the effect was completely reversed by the administration of BOC-2, an ALX receptor antagonist, suggesting that ATLa acts via an ALX receptor (Figure 1G). BLM led to collagen deposition, observed by Sirius red staining on Days 14 (Figures 1I and 1M) and 21 (Figures 1Q and 1U). After ATLa treatment, the amount of collagen fibers in the lung was similar to the amount observed in saline-injected animals (Figure 1K) on Days 14 and 21 (Figures 1J and 1N and Figures 1R and 1V, respectively), and treatment with BOC-2 prevented the action of ATLa (Figures 1S and 1W). BLM induced an increase in OH-proline content, whereas ATLa therapy reduced it to control levels (Figure 1O). Treatment with BOC-2 abolished the protective effect of ATLa on collagen deposition, confirming ATLa’s dependence on the ALX receptor (Figures 1O, 1S, and 1W) for these effects.
Figure 1.
Aspirin-triggered lipoxin A4 (ATLa) treatment reversed bleomycin (BLM)-induced lung damage and fibrosis. Lungs were removed from animals on the Days 14 and 21 after treatment with saline (SAL), BLM (0.06 U/mouse), BLM plus ATLa late treatment (1 μg/mouse and boosted 0.1 μg/mouse on Days 7 and 10, respectively), or BLM plus BOC-2 with ATLa (100 μg/mouse and 10 μg/mouse of BOC-2 on Days 7 and 10, respectively). Histological changes were demonstrated by hematoxylin and eosin and on Days 14 (A–C) and 21 (D–G) and by Sirius Red staining on Day 14 (H–K) on higher (×40) and lower magnification (L–N) (–10), OH-proline quantification on Day 21 (O), and Sirius red staining on Day 21 (P–S) on higher (×40) and lower magnification (T–W) (×10) as described in Materials and Methods (n = 6 for each experimental group). Pictures are representative of each group. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group; §P < 0.05 compared with BLM+BOC-2+ATLa group.
ATLa Treatment Ameliorates Tissue Repair Markers
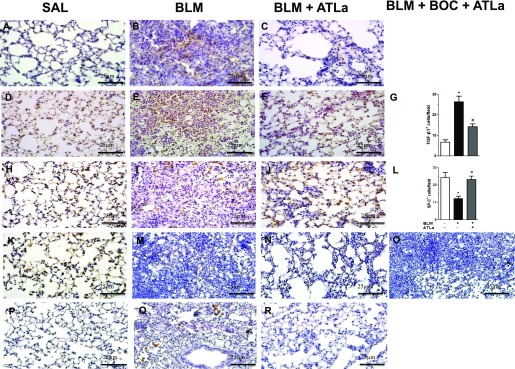
Representative photomicrographs in Figure 2 show reduced collagen 1 content after treatment with ATLa when compared with BLM (Figures 2A–2C). Furthermore, BLM induced an increase in TGF-β expression, which was reduced to control levels by ATLa therapy (Figures 2D–2G). The protection conferred by ATLa administration on BLM-induced lung fibrosis was confirmed by SP-C expression analysis, showing the restoration of SP-C levels on Days 14 and 21 (Figures 2J and 2N, respectively) to similar levels detected in the control group (Figures 2H and 2K); treatment with BOC-2 prevented this effect (Figure 2O). The antiangiogenic effect of ATLa was also shown by the decrease in VEGF expression (Figure 2R) compared with BLM (Figure 2Q).
Figure 2.
ATLa treatment ameliorates markers of tissue repair. Lungs were removed from animals on Days 14 and 21 after treatment with saline, BLM (0.06 U/mouse), BLM plus ATLa late treatment (1 μg/mouse and boosted 0.1 μg/mouse on Days 7 and 10, respectively), or BLM plus BOC-2 with ATLa (100 μg/mouse and 10 μg/mouse of BOC-2 on Days 7 and 10). Immunolabeling for collagen 1 (A–C), TGF-β (D–G), SP-C (H–L, Day 14), and SP-C on Day 21 (K–O) and vascular endothelial growth factor (P–R) as described in Materials and Methods (n = 6 for each experimental group). Pictures are representative of each group. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group.
ATLa Treatment Restores Cell Content in the Lungs after BLM Challenge
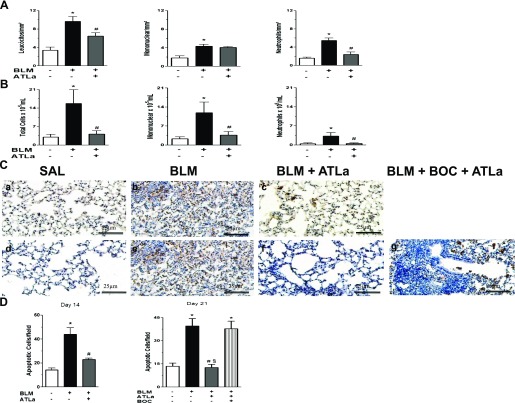
We also investigated whether ATLa treatment was able to reverse leukocyte infiltration evoked by BLM. For this purpose, lung morphometry and the cells in BALF were analyzed. Figure 3 shows that BLM injection induced the accumulation of leukocytes in the lung parenchyma (Figure 3A) and in bronchoalveolar lavage fluid (BALF) (Figure 3B). The administration of ATLa 7 days after BLM-induced fibrosis decreased the number of total leukocytes in parenchyma and BALF, resulting in an important reduction in the number of neutrophils in the alveolar septa and in BALF and in a significant decrease in the mononuclear cells into the BALF.
Figure 3.
ATLa treatment reduced the BLM-induced inflammatory cell infiltration in lung tissue (A), bronchoalveolar lavage (BAL) (B), and apoptosis (C and D). Leukocytes were quantified 14 days after the administration of saline, BLM (0.06 U/mouse), or BLM plus ATLa late treatment (added on Days 7 and 10). Apoptosis was evaluated by TUNEL assay on Days 14 and 21 after saline, BLM, BLM plus ATLa, and BLM plus BOC-2 with ATLa (100 μg/mouse and 10 μg/mouse of BOC-2 on Days 7 and 10, respectively) as described in Materials and Methods. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group; §P < 0.05 compared with BLM+BOC-2+ATLa group (n = 6 for each experimental group for lung tissue cells, n = 12 for each experimental group for BAL cells, and n = 6 for each experimental group in the TUNEL staining). Each value represents the mean ± SE of two different experiments.
The presence of apoptotic cells was investigated by TUNEL assay. The number of positive cells (apoptotic cells) after BLM was higher on Day 14 (Figure 3C, panel b; Figure 3D) and 21 (Figure 3C, panel e; Figure 3D), and the treatment with ATLa decreased cellular apoptosis at both time points (Figures 3C, panel c; 3C, panel f; 3D). Treatment with BOC-2 blocked the effect of ATLa (Figure 3C, panel g; Figure 3D).
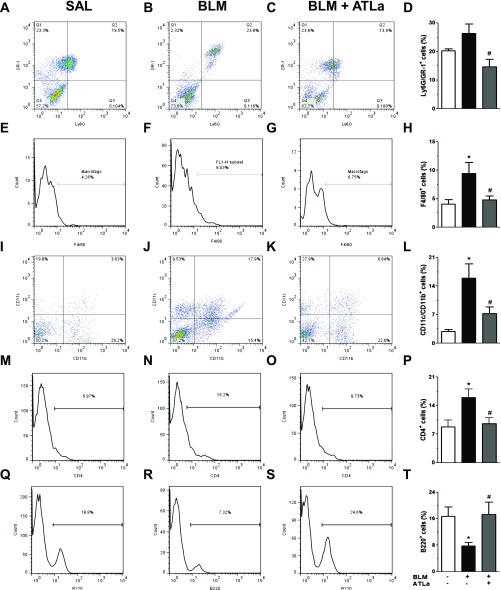
In another set of experiments, lungs obtained 14 days after saline, BLM, and BLM plus ATLa treatment were digested, and lung total cell content was analyzed by flow cytometry. Under these experimental conditions, there was an increase in the populations of Ly6G+GR-1+ (Figures 4A–4D), F4/80+ (Figures 4E–4H), CD11c+CD11b+ (Figures 4I–4L), and CD4+ (Figures 4M–4P) cells and a decrease in B220+ (Figures 4Q–4T) cells after BLM challenge. ATLa treatment restored all the cell populations evaluated to levels observed in saline-injected mice (Figure 4; control group).
Figure 4.
ATLa treatment modulated the content of lung immune cells. Lungs removed from the animals on Day 14 after treatment with saline, BLM (0.06 U/mouse), and BLM plus ATLa late treatment (administered on Days 7 and 10) were prepared as described in Materials and Methods. Lung cells were marked for Ly6G+/GR1+ cells (A–D), F4/80+ (E–H), CD11c+/CD11b+ (I–L), CD4+ (M–P), and B220+ (Q–T). *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group (n = 8 for each experimental group). Each value represents the mean ± SE of two different experiments.
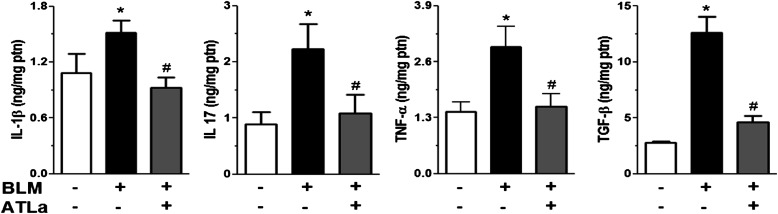
Regulation of BLM-Induced Cytokine Production by ATLa Treatment
We evaluated whether ATLa treatment could modulate the profile of cytokine production induced by BLM challenge. BLM administration enhanced the protein levels of IL-1β, IL-17, TNF-α, and TGF-β in the lungs when compared with the values observed in the control group as assessed by ELISA (Figure 5). Once again, ATLa administration, even 7 days after the BLM injection, was able to decrease cytokine production and reestablish the basal levels observed in control groups (Figure 5), reinforcing the modulatory capacity of this substance.
Figure 5.
ATLa reduced the production of cytokines related to fibrosis in lung tissue. Cytokines were measured using ELISA. The lungs were removed 14 days after treatment with saline, BLM (0.06 U/mouse), or BLM plus ATLa late treatment (added on Days 7 and 10), homogenized, and processed as described in Materials and Methods. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group (n = 5 for each experimental group). Data are representative of three different experiments.
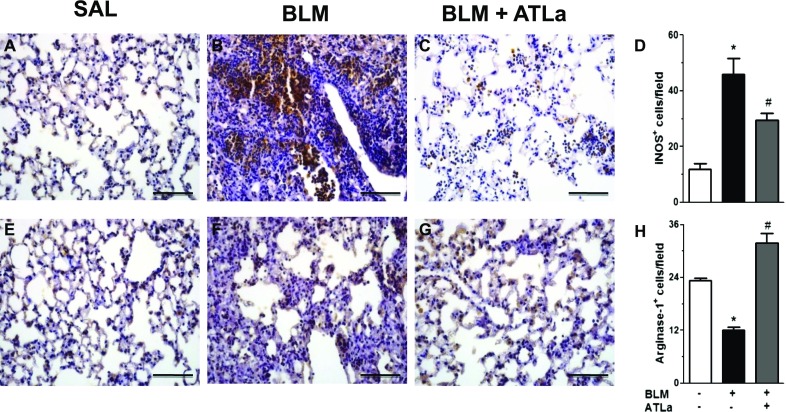
Restoration of M1 (iNOS+)/M2 (Arginase+) Macrophages Balance by ATLa
The existence of different macrophages populations is well recognized. Macrophages play important roles in physiological and pathophysiological processes (35). We demonstrated that BLM-challenged mice presented an increase in the iNOS+ cells (Figures 6A–6D) and a reduction in arginase+ cells (Figures 6E–6H) in lung parenchyma. However, when the animals were treated with ATLa, this compound selectively decreased the number of iNOS+ and increased the arginase+ cells to the same level observed in saline-injected mice, suggesting that ATLa treatment modulates the macrophage subtypes in the lungs (Figure 6), favoring the resolution of the fibrotic process.
Figure 6.
ATLa restored the M1 (iNOS+) versus M2 (arginase+) macrophage balance. Lungs were removed from animals on Day 14 after treatment with saline, BLM (0.06 U/mouse), or BLM plus ATLa late treatment (1 μg/mouse and boosted 0.1 μg/mouse on Days 7 and 10, respectively). Immunostaining was performed for iNOS (A–D) and arginase-1 (E–H) as described in Materials and Methods (n = 6 for each experimental group). Original magnification, ×40. Pictures are representative of each group. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group.
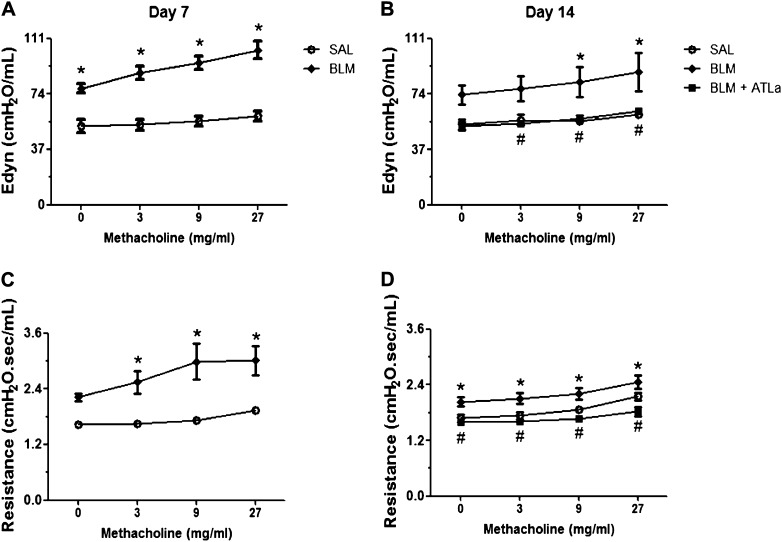
ATLa Treatment Promotes Lung Mechanics Improvement
Seven days after BLM administration, Edyn and R were higher than in saline-injected mice (Figures 7A and 7C, respectively). The increase in Edyn and R evoked by methacholine was significantly higher in BLM- than in saline-injected mice. At Day 14 (Figures 7B and 7D), the mechanical profile obtained was similar to that observed at Day 7, in which BLM-injected mice showed an increase in Edyn and R compared with the control group. At Day 7, when the animals injected with BLM already presented impairment in lung function, two intravenous injections of ATLa, the first at 7 days and a boost 10 days after BLM, reduced Edyn to values comparable to those observed in the saline-injected control group. The resistance observed was smaller than that noted in the control group (Figures 7B and 7D).
Figure 7.
ATLa restored lung function after BLM challenge. Lung function was analyzed by the use of methacholine dose–response. The animals were analyzed 7 and 14 days after the instillation of saline, BLM (0.06 U/mouse), or BLM plus ATLa late treatment (added on Days 7 and 10, only to the animals analyzed at Day 14). Graphics represent the dynamic elastance (A and B) and resistance (C and D), at 7 (A–C) and 14 days (B–D) after BLM challenge, respectively. *P < 0.05 compared with saline group; #P < 0.05 compared with BLM group (n = 5 for each experimental group, representative of three different experiments).
Discussion
The process of tissue healing after an injury involves several steps in two distinct stages: 1) a regenerative phase, with the replacement of injured cells), and 2) a fibrotic phase, in which connective tissue replaces normal parenchyma (3, 9, 36). Depending on the intensity, magnitude, time, and individual response to the injury, an inappropriate healing process can lead to the formation of scar tissue (3), which can compromise the function of the organ. Regarding the lung compartment, fibrotic diseases are characterized by tissue destruction and dysfunction (37). The mechanisms involved in these processes are complex and not fully understood, but it is well recognized that fibroblast activation, alterations in the immune response, and chronic inflammation underlie the process. The stimulatory pathways are counteracted by natural regulatory mechanisms, which limit inflammation and fibrotic responses. In this view, there is evidence that chronic inflammation can result from insufficient production of antiinflammatory and proresolving mediators (38).
The therapeutic approach used to treat pulmonary fibrosis is based on corticosteroids and immunosuppressive agents, which are ineffective and have significant side effects. Although there has been an increase in the past years in the knowledge of fibrosis development and the need of new therapeutics for this condition, no significant improvement in treatment options has occurred (39). In this scenario, LXs emerge as powerful substances, capable of regulating the inflammatory response and participating in the resolution of inflammation, preventing an overexuberant inflammatory response, and limiting damage to the host (1, 16, 17).
LX production was reported to occur during a wide range of respiratory illnesses, and in vitro and in vivo studies have shown that LX and ATL display strong antiinflammatory actions in a variety of respiratory diseases (40–42). Reduced levels of LX have also been found in the airways of patients suffering from cystic fibrosis, a condition in which these lipids appear to play a role (20, 27). In this study, we show that lung cell infiltration and edema induced by BLM were significantly reduced by systemic ATLa treatment (Figure 1). Furthermore, cellular analysis indicates that the treatment with the analog can modulate the efflux of several cells types from lung parenchyma and BAL, including Ly6G+GR-1+ (neutrophils), F4/80+ (macrophages), CD11c+CD11b+, and CD4+ cells, and induce the influx of B220+ (Figures 3 and 4). The mechanism by which ATLa decreases cellular infiltration does not seem to occur by increasing cellular death though apoptosis (Figures 2C, panel e; 2D). The analog could be working by inhibiting the inflammatory influx and enhancing the efflux because all inflammatory cells analyzed in this report express ALX, LX, and ATLa receptor (43). Other studies have demonstrated that LX can modulate cytokine production in the kidney (39), and our data corroborate these findings; in our study, ATLa treatment reduced the levels of several proinflammatory cytokines, perhaps as a consequence of the modulation of cellular infiltration or even by a direct effect. Our data are insufficient to determine the mechanism involved in such cytokine reduction, and further experiments are necessary to clarify this point.
An excessive deposition of extracellular matrix, mainly collagen, is a hallmark of fibrosis. Herein, we present evidence that ATLa posttreatment inhibited BLM-induced matrix protein and collagen 1 deposition and decreased the levels of TGF-β, as observed in situ and by ELISA. Among several mediators, TGF-β is the major cytokine associated with pulmonary fibrosis, involved in the transition of fibroblasts into myofibroblasts (44), the synthesis of matrix proteins, and collagen degradation inhibition (45). This result is in agreement with previously reported evidence that LXA4 attenuates TGF-β–driven collagen synthesis in fibroblasts in vitro (22, 39). Regarding the reduction of VEGF expression observed after ATLa treatment, antiangiogenic effect could be beneficial for the reversal of pulmonary fibrosis induced by BLM. Our findings are in agreement with literature data, which demonstrate that the angiogenesis is important for pulmonary fibrosis progression (46).
Alveolar type II epithelial cells produce and secrete surfactant lipids and surfactant-associated proteins (i.e., SP-A, SP-B, SP-C, and SP-D) that enhance alveolar compliance and host defense. SP-C is selectively synthesized by type II epithelial cells in the lung and is secreted into the airspace along with other surfactant lipids (47). After BLM instillation, it was observed that the expression of SP-C was reduced (Figure 1) and that ATLa treatment restored the hallmark of pneumocyte type II, showing for the first time another reparative effect of ATLa. We believe that ATLa effect on SP-C levels restoration is dependent on TGF-β reduction because it has previously been demonstrated that pneumocyte type II stimulated with TGF-β decreases SP-C production and increases the extracellular matrix proteins deposited, such as fibronectin (48).
In the past few years, a large body of evidence has emerged pointing to the existence of a dichotomy in the macrophage population, M1 (classical activated macrophages) and M2 (antiinflammatory macrophages, now also subdivided into different classes), whose balance can drive the fate of the inflammatory reaction to a chronic state or to resolution (49). We observed that BLM administration expanded the M1 population but decreased the M2 subtype, favoring an inflammatory milieu. On the other hand, when the animals were treated with ATLa, a less proinflammatory and proresolution environment was observed, with the predominance of M2 subtype over M1 (Figure 5). More experiments are necessary to better characterize the macrophage markers and the subpopulations modulated by LX. Another important cell type in the physiopathology of pulmonary fibrosis is the myofibroblast. In a previous paper by our group, we demonstrated that the increased number of myofibroblasts induced by BLM was reduced by ATLa treatment 4 days after fibrosis induction (1). In the present work, ATLa administered at Day 7 inhibited the myofibroblast presence in the lung, reinforcing the antifibrotic effect of ATLa as therapeutic tool.
Because ATLa reversed several inflammatory and fibrotic processes, we investigated whether ATLa treatment was also able to restore BLM-induced pulmonary function impairment. ATLa after treatment recovered an established BLM-induced increase in dynamic elastance and resistance of the lung to normal values, reinforcing the promising therapeutic approach for ATLa. The antiinflammatory and proresolution role of LXs in lung diseases has already been shown (50), although not particularly in fibrosis.
The ATLa protective effect in all parameters evaluated was observed with very low doses of the analog (1 μg with a booster of 0.1 μg). It will be interesting to learn the lowest dose of ATLa capable of reverting the fibrotic process. Additional studies are necessary to evaluate the potential protective action of ATLa regarding dose–effect experimentation and to determine the effect of ATLa in the chronic long-term pulmonary fibrosis model, which is under investigation at the moment. However, the availability limitation of the analog is the major concern.
In the present study, we showed that ATLa effects the improvement of lung function in a model of pulmonary fibrosis, encouraging future studies to better understand LX/ATL mechanisms. Using a relevant in vivo model of lung fibrosis, the present results elucidate the antifibrotic effect of an aspirin-triggered LX. Furthermore, the therapeutic effects of ATLa on the fibrotic processes of various etiologies are apparent. These data have important implications for future efforts in developing an efficient therapeutic strategy for the treatment of lung fibrosis by targeting LX/ATL actions.
Acknowledgments
Acknowledgments
The authors thank Dr. J.F. Parkinson for the generous gift of ATLa.
Footnotes
This work was supported by Conselho Nacional de Pesquisa, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro.
Originally Published in Press as DOI: 10.1165/rcmb.2012-0462OC on July 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dempsey OJ. Clinical review: idiopathic pulmonary fibrosis: past, present and future. Respir Med. 2006;100:1871–1885. doi: 10.1016/j.rmed.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, Kollias G, Aidinis V. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS ONE. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 4.Kawanami O, Ferrans VJ, Crystal RG. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982;46:39–53. [PubMed] [Google Scholar]
- 5.Kuhn C. The pathogenesis of pulmonary fibrosis. Monogr Pathol. 1993;36:78–92. [PubMed] [Google Scholar]
- 6.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 7.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 8.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dury S. Treatment of idiopathic pulmonary fibrosis. Rev Mal Respir. 2006;23:5S87–5S91. [PubMed] [Google Scholar]
- 10.Mapel DW, Samet JM, Coultas DB. Corticosteroids and the treatment of idiopathic pulmonary fibrosis: past, present, and future. Chest. 1996;110:1058–1067. doi: 10.1378/chest.110.4.1058. [DOI] [PubMed] [Google Scholar]
- 11.Bouros D, Antoniou KM. Current and future therapeutic approaches in idiopathic pulmonary fibrosis. Eur Respir J. 2005;26:693–702. doi: 10.1183/09031936.05.00145004. [DOI] [PubMed] [Google Scholar]
- 12.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaunois LM. Mechanisms in pulmonary toxicology. Clin Chest Med. 2004;25:1–14. doi: 10.1016/S0272-5231(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 14.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- 15.Fierro IM, Serhan CN. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz J Med Biol Res. 2001;34:555–566. doi: 10.1590/s0100-879x2001000500002. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 18.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowal-Bielecka O, Kowal K, Distler O, Rojewska J, Bodzenta-Lukaszyk A, Michel BA, Gay RE, Gay S, Sierakowski S. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: an imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis Rheum. 2005;52:3783–3791. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- 20.McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Renal Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- 21.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 22.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Clish CB, Levy BD, Chiang N, Tai HH, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation: a novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J Biol Chem. 2000;275:25372–25380. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- 24.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4) J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 26.Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy BD, Serhan CN. Exploring new approaches to the treatment of asthma: potential roles for lipoxins and aspirin-triggered lipid mediators. Drugs Today (Barc) 2003;39:373–384. doi: 10.1358/dot.2003.39.5.740217. [DOI] [PubMed] [Google Scholar]
- 28.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 29.Martins V, Valença SS, Farias-Filho FA, Molinaro R, Simões RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel SL, Fierro IM, et al. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol. 2009;182:5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 30.Valença SS, da Hora K, Castro P, Moraes VG, Carvalho L, Porto LC. Emphysema and metalloelastase expression in mouse lung induced by cigarette smoke. Toxicol Pathol. 2004;32:351–356. doi: 10.1080/01926230490431466. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-β signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6:2080–2088. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 32.Komura K, Yanaba K, Horikawa M, Ogawa F, Fujimoto M, Tedder TF, Sato S. CD19 regulates the development of bleomycin-induced pulmonary fibrosis in a mouse model. Arthritis Rheum. 2008;58:3574–3584. doi: 10.1002/art.23995. [DOI] [PubMed] [Google Scholar]
- 33.Trujillo G, O’Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2008;172:1209–1221. doi: 10.2353/ajpath.2008.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonseca BP, Olsen PC, Coelho LP, Ferreira TP, Souza HS, Martins MA, Viola JP. NFAT1 transcription factor regulates pulmonary allergic inflammation and airway responsiveness. Am J Respir Cell Mol Biol. 2009;40:66–75. doi: 10.1165/rcmb.2007-0102OC. [DOI] [PubMed] [Google Scholar]
- 35.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meneghin A, Hogaboam CM. Infectious disease, the innate immune response, and fibrosis. J Clin Invest. 2007;117:530–538. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardo A, Selman M. Molecular mechanisms of pulmonary fibrosis. Front Biosci. 2002;7:d1743–d1761. doi: 10.2741/pardo. [DOI] [PubMed] [Google Scholar]
- 38.Izumo T, Kondo M, Nagai A. Cysteinyl-leukotriene 1 receptor antagonist attenuates bleomycin-induced pulmonary fibrosis in mice. Life Sci. 2007;80:1882–1886. doi: 10.1016/j.lfs.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Börgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A4 and benzo-lipoxin A4 attenuate experimental renal fibrosis. FASEB J. 2011;25:2967–2979. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- 40.Lee TH, Crea AE, Gant V, Spur BW, Marron BE, Nicolaou KC, Reardon E, Brezinski M, Serhan CN. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990;141:1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 41.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- 43.Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc. 2008;5:305–310. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A, Vancheri C. TGF-β1 targets the GSK-3β/β-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Wan YY, Tian GY, Guo HS, Kang YM, Yao ZH, Li XL, Liu QH, Lin DJ. Endostatin, an angiogenesis inhibitor, ameliorates bleomycin-induced pulmonary fibrosis in rats. Respir Res. 2013;14:56–68. doi: 10.1186/1465-9921-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madala SK, Maxfield MD, Davidson CR, Schmidt SM, Garry D, Ikegami M, Hardie WD, Glasser SW.Rapamycin regulates bleomycin-induced lung damage in SP-C-deficient mice Pulm Med2011;2011. 653524–653535. [DOI] [PMC free article] [PubMed]
- 48.Beers MF, Solarin KO, Guttentag SH, Rosenbloom J, Kormilli A, Gonzales LW, Ballard PL. TGF-beta1 inhibits surfactant component expression and epithelial cell maturation in cultured human fetal lung. Am J Physiol. 1998;275:L950–L960. doi: 10.1152/ajplung.1998.275.5.L950. [DOI] [PubMed] [Google Scholar]
- 49.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Planagumà A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]