Abstract
Diabetic retinopathy (DR) is a frequent cause of acquired blindness worldwide. Various studies have reported the effects of body mass index (BMI) on the risk of DR, but the results remain controversial. Therefore, a meta-analysis was performed to evaluate the relationship between BMI and the risk of DR.
A systematic search was performed using the Cochrane Library, PubMed, and Embase databases to obtain articles published through December 2016. Articles regarding the association between BMI and the risk of DR were retrieved. The adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were included and then pooled with a random effects model.
A total of 27 articles were included in this meta-analysis. When BMI was analyzed as a categorical variable, neither being overweight (OR = 0.89, 95% CI 0.75–1.07; P = .21; I2 = 65%) nor obesity (OR = 0.97, 95% CI 0.73–1.30; P = .86) were associated with an increased risk of DR when compared with normal weight. When BMI was analyzed as a continuous variable, a higher BMI was not associated with an increased risk of DR (OR = 0.99, 95% CI 0.97–1.01; P = .25; I2 = 79%). The pooled results did not significantly change after the sensitivity analysis.
Based on the current publications, neither being overweight nor obesity is associated with an increased risk of DR. Further studies should confirm these findings.
Keywords: body mass index, diabetic retinopathy, risk factor
1. Introduction
Diabetic retinopathy (DR), the most common visual complication of diabetes, is a frequent cause of acquired blindness. In 2010, it was reported that nearly 285 million people worldwide had been plagued with diabetes, of which over one-third had signs of DR.[1] Recent epidemiological studies have identified that the duration of diabetes, blood pressure, and glycemic control are several key risk factors for the development of DR.[2] However, evidence from the Action to Control Cardiovascular Risk in Diabetes (ACCORD-Eye) and Action in Diabetes and Vascular Disease (ADVANCE) revealed that limited effects had been obtained despite better glucose and blood pressure control.[3,4] Thus, exploring modifiable risk factors has become increasingly important.
Body mass index (BMI), the most commonly used index of body mass, is calculated by dividing the weight in kilograms by the square of the height in meters.[5] According to the World Health Organization BMI classification system, BMI is categorized into the following four grades: underweight (<18.5 kg/m2), normal weight (18.5 kg/m2–24.9 kg/m2), overweight (25.0 kg/m2–29.9 kg/m2), and obese (≥30.0 kg/m2). It has been shown that being overweight and obesity are 2 risk factors for diabetes mellitus.[6] Thus, overweight and obese people are more vulnerable to DR. However, the results from previous studies were equivocal, with some studies[7–9] observing a decreased incidence of DR in higher BMI individuals, while other studies[10–13] detected a null association between high BMI and the incidence of DR. Moreover, other studies[14,15] demonstrated that a significant decrease in glycated hemoglobin (HbA1c) and a significant increase in high-density lipoprotein cholesterol (HDL-C) and blood pressure were observed in higher BMI individuals, all of which are the risk factors for DR. Motivated by these equivocal results, we conducted a meta-analysis to assess the association between BMI and the risk of DR.
2. Methods
In performing this meta-analysis, we adhered to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines.[16] No ethical approval was warranted since it was a meta-analysis of available studies.
2.1. Literature search and study selection
We electronically searched the Cochrane Library, PubMed, and Embase databases up to December 2016 for articles evaluating the effects of BMI on DR in patients with type 1 or type 2 diabetes. The key words related to body mass (“body mass index” or “body mass” or “BMI” or “body weight” or “obesity” or “overweight’” or “adiposity”) and “diabetic retinopathy” were used to search for the relevant articles published in English. Moreover, reference lists and conference abstracts were also examined to retrieve potential relevant studies.
Studies were included if they fulfilled the following criteria: (a) observational study type; (b) participants with type 1 or 2 diabetes or both; (c) outcomes included DR; and (d) BMI was analyzed as a categorical or continuous variable. The categorical levels of BMI were assessed according to the WHO-recommended BMI classifications.[17] BMI levels within 2 kg/m2 of standard categories were also considered to be acceptable in case losing studies were available. The exclusion criteria were as follows: (a) the adjusted risk estimates were unavailable; (b) certain publication types (e.g., reviews, letters, case reports, comments, conference abstracts, and editorials); and (c) studies with duplicate or insufficient data.
2.2. Data extraction and quality assessment
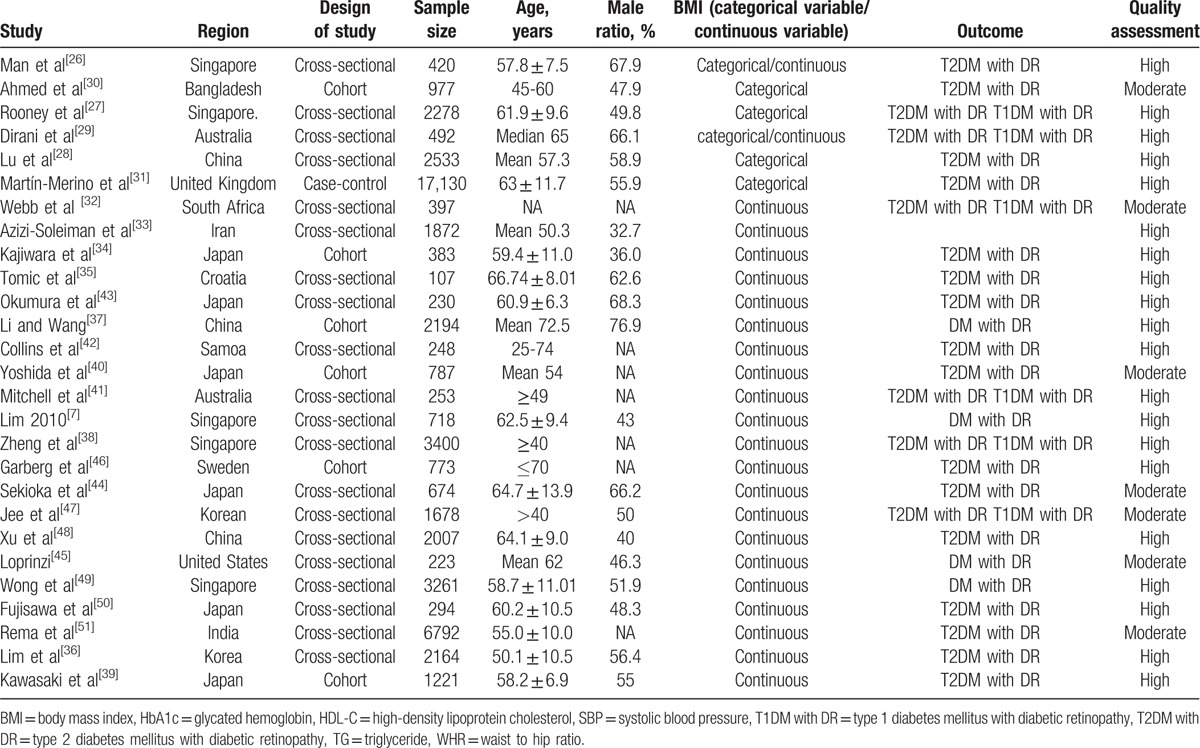
Two reviewers (Z-Y and Z-YZ) independently screened all the identified articles, and any disagreements were resolved through discussion or through a consultation with the senior researcher (W-CY). For the included studies, the basic characteristics including the first author, publication year, study location, age range, design of study, sample size, diagnostic basis, grading standard, and the outcome were extracted. Additionally, if one study had several risk estimates (e.g., different periods of follow-up or different multivariate statistical analysis models), the risk estimate with the longest follow-up or the estimate that was most completely adjusted was extracted.
The Newcastle–Ottawa scale (NOS) score was used to evaluate the quality of the observational studies,[18,19] which gave with a maximum score of 9 for the cohort or case-control study, and 8 for the cross-sectional study. NOS scores of 0–3, 4–6, and ≥7 were defined as low, moderate, and high quality, respectively.
2.3. Statistical analysis
All the statistical analyses were completed with the Revman Manager 5.3 (the Nordic Cochrane Center, Rigshospitalet, Denmark; http://ims.cochrane.org/revman). We assessed the association between BMI and the risk of DR when BMI was analyzed as a categorical or continuous variable. For each study, the adjusted odds ratio (OR) and its 95% confidence interval (CI) were regarded as the common risk estimate and pooled by the random-effects model with inverse variance weighting. The random-effects model was more conservative and could provide better estimates with wider confidence intervals than the fixed-effect model for any heterogeneity.[20] The consistency of included studies was evaluated using the Cochrane Q test complemented with I2 values, where I2 values <25% indicated no heterogeneity, 25%≤I2<50% indicated low heterogeneity, 50%≤I2<75% indicated moderate heterogeneity, and I2≥75% indicated high heterogeneity. In view of the heterogeneity among the included studies, we performed a sensitivity analysis by removing the included articles one by one. A funnel plot was used to assess the publication bias when more than 10 studies were included. In the presence of potential publication bias, visual asymmetry would be observed in the funnel plot.
3. Results
3.1. Study selection
As shown in Fig. 1, a total of 1544 articles were initially identified the Cochrane Library (n = 34), PubMed (n = 572), and Embase (n = 938) databases, of which 836 duplicate articles were removed. Among the remaining 708 articles, we excluded another 672 articles after screening the titles and abstracts. The potential 36 relevant articles thoroughly assessed for eligibility, and 9 of them were excluded for different reasons. Two articles[10,12] calculated the total effect of overweight and obesity together instead of their separate effects. A case-control study[13] only reported the unadjusted ORs at a 95% CI. Two studies[21,22] treated the nonobese group as the control. Four studies[23–25,57] did not adhere to the BMI classification we adopted. Finally, 27 articles[7,26–51] were included in this meta-analysis. All of the basic characteristics and the reporting qualities of the included studies are shown in Table 1.
Figure 1.
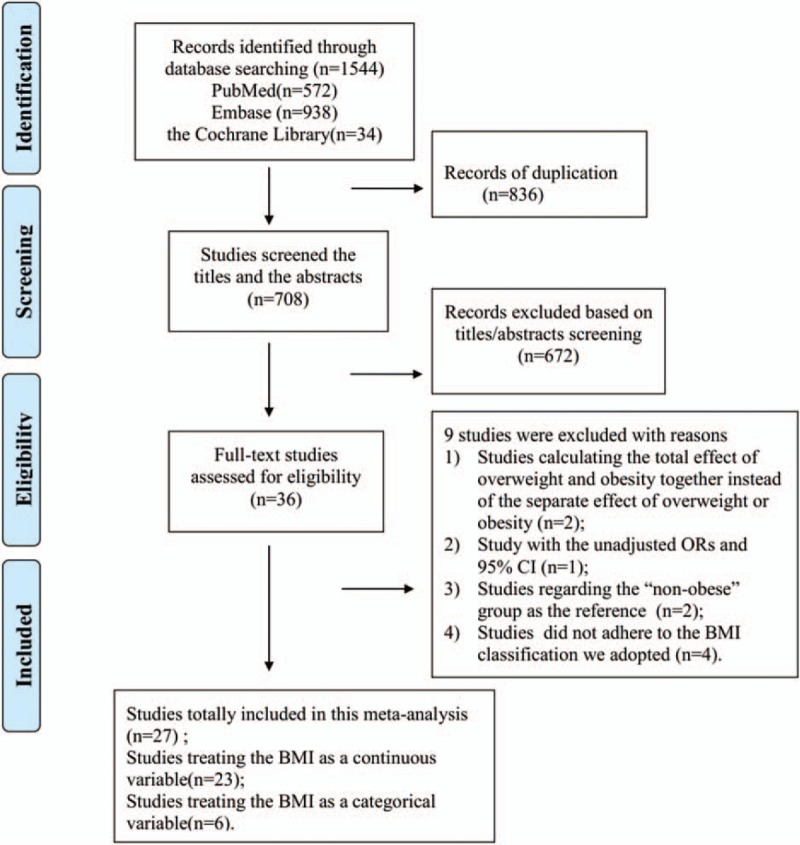
Overview of the research strategy. BMI = body mass index, OR = odds ratio.
Table 1.
Characteristics of the 27 included studies in this meta-analysis.
3.2. Meta-analysis of BMI and DR
When BMI was analyzed as a categorical variable, 6 studies compared the risk of DR between ‘overweight or obese BMI’ and ‘normal BMI.’ As shown in Fig. 2, neither being overweight (OR = 0.89, 95% CI 0.75–1.07; P = .21; I2 = 65%) nor obesity (OR = 0.97, 95% CI 0.73–1.30; P = .86; I2 = 72%) were associated with an increased risk of DR when compared with normal weight. When BMI was analyzed as a continuous variable, 23 studies reported an association between BMI and the risk of DR. As shown in Fig. 3, a higher BMI was not associated with an increased risk of DR (OR = 0.99, 95% CI 0.97–1.01; P = .25; I2 = 79%). In view of the heterogeneity among the included studies, we performed a sensitivity analysis to explore the source of heterogeneity. After we removed the included articles one by one, the I2 values were still more than 50%, which indicated moderate-high heterogeneity. However, the sensitivity analysis demonstrated that the stability of the overall treatment effects was good.
Figure 2.
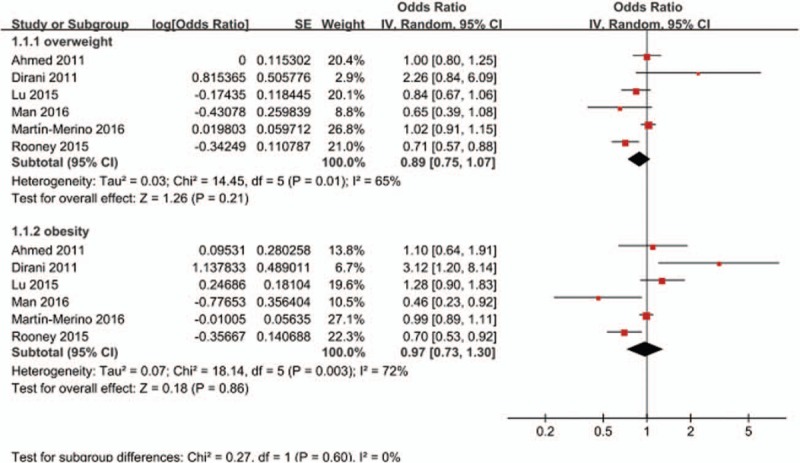
Forest plot for the association between BMI and the risk of DR when BMI was analyzed as a categorical variable. BMI = body mass index, CI = confidence interval, DR = diabetic retinopathy, SE = standard error.
Figure 3.
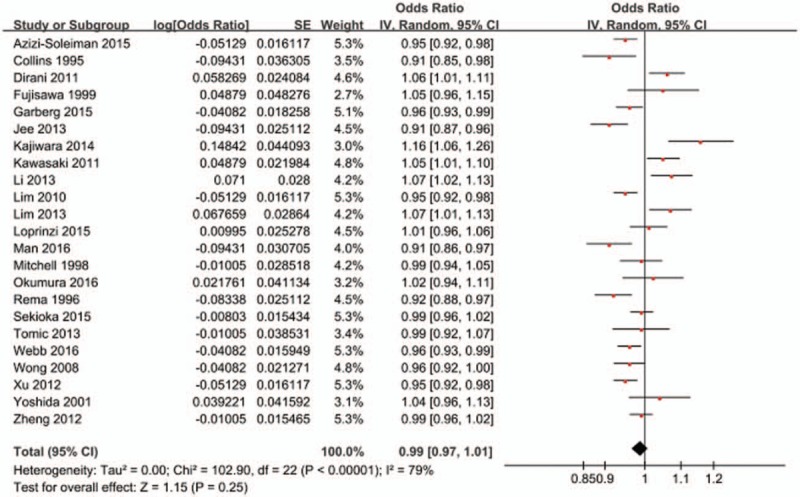
Forest plot for the association between BMI and the risk of DR when BMI was analyzed as a continuous variable. BMI = body mass index, DR = diabetic retinopathy, SE = standard error, CI = confidence interval.
3.3. Publication bias
As shown in Fig. 4, the largest studies plotted near the average and the smaller studies were spread evenly on both sides of the average, indicating that a possible absence of publication bias was observed when the BMI was analyzed as a continuous variable. However, when BMI was analyzed as a categorical variable, only 6 studies were included. We therefore did not display the funnel plot in this part, as the analyses are likely underpowered.
Figure 4.
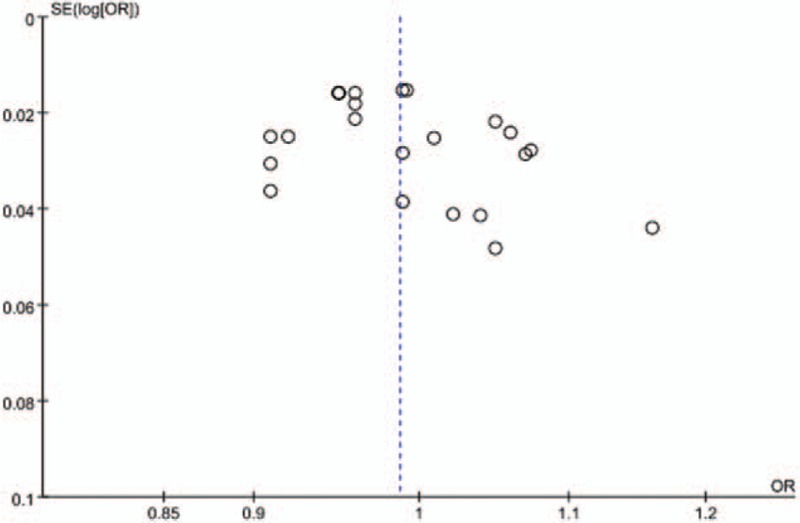
Funnel plot of all the included studies for the bias analysis when BMI was analyzed as a continuous variable. BMI = body mass index, SE = standard error.
4. Discussion
As shown in a systematic analysis,[52] obesity has become a major global health challenge because of the increasing prevalence of obesity worldwide. From 1980 to 2013, the proportion of overweight and obese people increased to 36.9% for men and 38.0% for women. Consequently, increased morbidity and decreased life expectancy were also observed in the obese.[53] Obesity has also been observed to have detrimental effects on multiple eye diseases such as glaucoma,[54] late age-related maculopathy,[55] and cataracts.[56] However, although an increasing number of epidemiologic studies have been performed to analyze the association between BMI and DR, the results are still inclusive. To our knowledge, this was the first meta-analysis to evaluate the association between BMI and DR. In our meta-analysis, neither being overweight nor obesity conferred an increased risk of DR. This was consistent with the previous studies[57] where BMI was analyzed as a continuous variable. Notably, significant heterogeneity was observed in our analysis, which may be attributed to differences in study design, participant characteristics, and race or ethnicity, suggesting that our results should be treated with great caution.
To date, very few mechanisms have accounted for the neutral association between BMI and DR. Perhaps obesity has both protective and adverse effects on the risk of DR. Elevated BMI may confer a protective effect on DR through many ways. First, increased C-peptide levels were found in higher BMI individuals,[58] which could reduce the risk of DR.[59] Moreover, a higher BMI may be a reflection of better glycemic control or shorter diabetes duration. Obese individuals were also more vulnerable to suffer from comorbid conditions; consequently, aggressive treatments have been taken, and reduced the development of DR. In contrast, a higher BMI may have adverse effects on DR. First, an elevated BMI is often correlated with hypertension and dyslipidemia, both of which are risk factors for DR.[1] Additionally, hyperleptinemia in obese individuals[60] may increase blood pressure and oxidative stress levels, which may partly be responsible for the development of DR. Moreover, higher vascular endothelial growth factor levels were observed in obese individuals,[61] which has been shown to be involved in the pathogenesis of proliferative DR.[62] We hypothesize that the neutral effects of BMI on DR may counteract its adverse and protective effects.
In contrast to BMI, which is an index for measuring generalized obesity, WHR is used to assess abdominal obesity. There may be some differences in the associations between WHR or BMI and DR. A study[27] evaluating the risk of DR in an obese population based on BMI and WHR showed an increased risk in the abdominal obesity group but not in the generalized obesity group. The mechanisms underlying the detrimental WHR-DR association were not defined; however, the high levels of inflammation[63] and insulin resistance[64] in the abdominal area of obese people may be responsible for the development of DR.
4.1. Limitations
There were several limitations in our meta-analysis, so the results should be interpreted with great caution. First, most of the studies we included were cross-sectional; thus, a causal relationship could not be conferred. Second, BMI is not an accurate parameter to reflect body adiposity since it also includes bone mass and muscle. For example, low BMI individuals may have a higher WC or metabolically obese normal weight.[65] Third, significant heterogeneity existed between the studies. Regretfully, we failed to explore the source of heterogeneity because of the limited number of included studies. Fourth, several studies included in this meta-analysis evaluated the association between BMI and DR without differentiating the types of diabetes; therefore, we could not separately evaluate the effects of BMI on DR in patients with type 1 or type 2 diabetes.
5. Conclusions
Our meta-analysis demonstrated that elevated BMI did not increase the risk of DR. However, since being overweight and obesity are risk factors for multiple diseases, it is still imperative to maintain a healthy weight. Notably, since BMI is an index to assess generalized obesity, other anthropometric measurement indexes (e.g., WHR and WC) should also be used to explore the association between obesity and DR. Furthermore, longitudinal studies based on different anthropometric measurement indexes are warranted to determine the association between being overweight or obese and DR.
Acknowledgments
We gratefully thank Z-WG (Department of Cardiology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330000, China) for helping us design the search strategy.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DR = diabetic retinopathy, HbA1c = glycated hemoglobin, HDL-C = high-density lipoprotein cholesterol, NOS = Newcastle–Ottawa scale, OR = odds ratio, WC = waist circumference, WHO = World Health Organization, WHR = waist-to-hip ratio.
Ethical approval: Ethical approval was not sought, as it was not required for conducting a meta-analysis.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–63. [DOI] [PubMed] [Google Scholar]
- [3].Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beulens JW, Patel A, Vingerling JR, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia 2009;52:2027–36. [DOI] [PubMed] [Google Scholar]
- [5].Criqui MH, Klauber MR, Barrett-Connor E, et al. Adjustment for obesity in studies of cardiovascular disease. Am J Epidemiol 1982;116:685–91. [DOI] [PubMed] [Google Scholar]
- [6].Luczynski W, Szypowska A, Bossowski A, et al. [Overweight, obesity and metabolic syndrome in children with type 1 diabetes melllitus]. Pediatr Endocrinol Diabetes Metab 2010;16:83–8. [PubMed] [Google Scholar]
- [7].Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010;51:4458–63. [DOI] [PubMed] [Google Scholar]
- [8].Maberley DA, King W, Cruess AF, et al. Risk factors for diabetic retinopathy in the Cree of James Bay. Ophthalmic Epidemiol 2002;9:153–67. [DOI] [PubMed] [Google Scholar]
- [9].Nguyen HT, Luzio SD, Dolben J, et al. Dominant risk factors for retinopathy at clinical diagnosis in patients with type II diabetes mellitus. J Diabetes Complications 1996;10:211–9. [DOI] [PubMed] [Google Scholar]
- [10].Araki A, Ito H, Hattori A, et al. Risk factors for development of retinopathy in elderly Japanese patients with diabetes mellitus. Diabetes Care 1993;16:1184–6. [DOI] [PubMed] [Google Scholar]
- [11].Manaviat MR, Rashidi M, Afkhami-Ardekani M. Four years incidence of diabetic retinopathy and effective factors on its progression in type II diabetes. Eur J Ophthalmol 2008;18:572–7. [DOI] [PubMed] [Google Scholar]
- [12].Bener A, Al-Laftah F, Al-Hamaq AO, et al. A study of diabetes complications in an endogamous population: an emerging public health burden. Diabetes Metab Syndr 2014;8:108–14. [DOI] [PubMed] [Google Scholar]
- [13].Lima VC, Cavalieri GC, Lima MC, et al. Risk factors for diabetic retinopathy: a case-control study. Int J Retina Vitreous 2016;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katusic D, Tomic M, Jukic T, et al. Obesity—a risk factor for diabetic retinopathy in type 2 diabetes? Coll Antropol 2005;29(suppl 1):47–50. [PubMed] [Google Scholar]
- [15].Kastelan S, Tomic M, Gverovic AA, et al. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm 2013;2013:436329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [17].Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1–253. [PubMed] [Google Scholar]
- [18].van Dijk GM, Maneva M, Colpani V, et al. The association between vasomotor symptoms and metabolic health in peri- and postmenopausal women: a systematic review. Maturitas 2015;80:140–7. [DOI] [PubMed] [Google Scholar]
- [19].G. Wells SBOC. The Newcastle Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 20, 2016, 2014. [Google Scholar]
- [20].Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res 2012;21:409–26. [DOI] [PubMed] [Google Scholar]
- [21].Pang C, Jia L, Jiang S, et al. Determination of diabetic retinopathy prevalence and associated risk factors in Chinese diabetic and pre-diabetic subjects: Shanghai diabetic complications study. Diabetes Metab Res Rev 2012;28:276–83. [DOI] [PubMed] [Google Scholar]
- [22].Raman R, Rani PK, Gnanamoorthy P, et al. Association of obesity with diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8). Acta Diabetol 2010;47:209–15. [DOI] [PubMed] [Google Scholar]
- [23].Janghorbani M, Amini M, Ghanbari H, et al. Incidence of and risk factors for diabetic retinopathy in Isfahan, Iran. Ophthalmic Epidemiol 2003;10:81–95. [DOI] [PubMed] [Google Scholar]
- [24].van Leiden HA, Dekker JM, Moll AC, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care 2002;25:1320–5. [DOI] [PubMed] [Google Scholar]
- [25].Dowse GK, Humphrey AR, Collins VR, et al. Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol 1998;147:448–57. [DOI] [PubMed] [Google Scholar]
- [26].Man RE, Sabanayagam C, Chiang PP, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol 2016;134:251–7. [DOI] [PubMed] [Google Scholar]
- [27].Rooney D, Lye WK, Tan G, et al. Body mass index and retinopathy in Asian populations with diabetes mellitus. Acta Diabetol 2015;52:73–80. [DOI] [PubMed] [Google Scholar]
- [28].Lu J, Hou X, Zhang L, et al. Association between body mass index and diabetic retinopathy in Chinese patients with type 2 diabetes. Acta Diabetol 2015;52:701–8. [DOI] [PubMed] [Google Scholar]
- [29].Dirani M, Xie J, Fenwick E, et al. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci 2011;52:4416–21. [DOI] [PubMed] [Google Scholar]
- [30].Ahmed KR, Karim MN, Bukht MS, et al. Risk factors of diabetic retinopathy in Bangladeshi type 2 diabetic patients. Diabetes Metab Syndr 2011;5:196–200. [DOI] [PubMed] [Google Scholar]
- [31].Martin-Merino E, Fortuny J, Rivero-Ferrer E, et al. Risk factors for diabetic retinopathy in people with Type 2 diabetes: A case-control study in a UK primary care setting. Prim Care Diabetes 2016;10:300–8. [DOI] [PubMed] [Google Scholar]
- [32].Webb EM, Rheeder P, Roux P. Screening in primary care for diabetic retinopathy, maculopathy and visual loss in South Africa. Ophthalmologica 2016;235:141–9. [DOI] [PubMed] [Google Scholar]
- [33].Azizi-Soleiman F, Heidari-Beni M, Ambler G, et al. Iranian risk model as a predictive tool for retinopathy in patients with type 2 diabetes. Can J Diabetes 2015;39:358–63. [DOI] [PubMed] [Google Scholar]
- [34].Kajiwara A, Miyagawa H, Saruwatari J, et al. Gender differences in the incidence and progression of diabetic retinopathy among Japanese patients with type 2 diabetes mellitus: a clinic-based retrospective longitudinal study. Diabetes Res Clin Pract 2014;103:e7–10. [DOI] [PubMed] [Google Scholar]
- [35].Tomic M, Ljubic S, Kastelan S, et al. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm 2013;2013:818671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lim S, Kim KM, Kim MJ, et al. The association of maximum body weight on the development of type 2 diabetes and microvascular complications: MAXWEL study. PLoS One 2013;8:e80525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li X, Wang Z. Prevalence and incidence of retinopathy in elderly diabetic patients receiving early diagnosis and treatment. Exp Ther Med 2013;5:1393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zheng Y, Lamoureux EL, Lavanya R, et al. Prevalence and risk factors of diabetic retinopathy in migrant Indians in an urbanized society in Asia: the Singapore Indian eye study. Ophthalmology 2012;119:2119–24. [DOI] [PubMed] [Google Scholar]
- [39].Kawasaki R, Tanaka S, Tanaka S, et al. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 2011;54:2288–94. [DOI] [PubMed] [Google Scholar]
- [40].Yoshida Y, Hagura R, Hara Y, et al. Risk factors for the development of diabetic retinopathy in Japanese type 2 diabetic patients. Diabetes Res Clin Pract 2001;51:195–203. [DOI] [PubMed] [Google Scholar]
- [41].Mitchell P, Smith W, Wang JJ, et al. Prevalence of diabetic retinopathy in an older community. The Blue Mountains Eye Study. Ophthalmology 1998;105:406–11. [DOI] [PubMed] [Google Scholar]
- [42].Collins VR, Dowse GK, Plehwe WE, et al. High prevalence of diabetic retinopathy and nephropathy in Polynesians of Western Samoa. Diabetes Care 1995;18:1140–9. [DOI] [PubMed] [Google Scholar]
- [43].Okumura A, Unoki-Kubota H, Yoshida-Hata N, et al. Reduced serum level of leukocyte cell-derived chemotaxin 2 is associated with the presence of diabetic retinopathy. Clin Chim Acta 2016;463:145–9. [DOI] [PubMed] [Google Scholar]
- [44].Sekioka R, Tanaka M, Nishimura T, et al. Serum total bilirubin concentration is negatively associated with increasing severity of retinopathy in patients with type 2 diabetes mellitus. J Diabetes Complications 2015;29:218–21. [DOI] [PubMed] [Google Scholar]
- [45].Loprinzi PD. Concurrent healthy behavior adoption and diabetic retinopathy in the United States. Prev Med Rep 2015;2:591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garberg G, Lovestam-Adrian M, Nasic S, et al. The prognosis of diabetic retinopathy in patients with type 2 diabetes since 1996-1998: the Skaraborg Diabetes Register. Int Ophthalmol 2015;35:503–11. [DOI] [PubMed] [Google Scholar]
- [47].Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008–2011. Invest Ophthalmol Vis Sci 2013;54:6827–33. [DOI] [PubMed] [Google Scholar]
- [48].Xu J, Wei WB, Yuan MX, et al. Prevalence and risk factors for diabetic retinopathy: the Beijing Communities Diabetes Study 6. Retina 2012;32:322–9. [DOI] [PubMed] [Google Scholar]
- [49].Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology 2008;115:1869–75. [DOI] [PubMed] [Google Scholar]
- [50].Fujisawa T, Ikegami H, Yamato E, et al. Association of plasma fibrinogen level and blood pressure with diabetic retinopathy, and renal complications associated with proliferative diabetic retinopathy, in Type 2 diabetes mellitus. Diabet Med 1999;16:522–6. [DOI] [PubMed] [Google Scholar]
- [51].Rema M, Ponnaiya M, Mohan V. Prevalence of retinopathy in non insulin dependent diabetes mellitus at a diabetes centre in southern India. Diabetes Res Clin Pract 1996;34:29–36. [DOI] [PubMed] [Google Scholar]
- [52].Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA 2003;289:187–93. [DOI] [PubMed] [Google Scholar]
- [54].Bengtsson B, Heijl A. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J Glaucoma 2005;14:135–8. [DOI] [PubMed] [Google Scholar]
- [55].Delcourt C, Michel F, Colvez A, et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol 2001;8:237–49. [DOI] [PubMed] [Google Scholar]
- [56].Ye J, Lou LX, He JJ, et al. Body mass index and risk of age-related cataract: a meta-analysis of prospective cohort studies. PLoS One 2014;9:e89923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang J, Zhang RY, Chen RP, et al. Prevalence and risk factors for diabetic retinopathy in a high-risk Chinese population. BMC Public Health 2013;13:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ahren J, Ahren B, Wierup N. Increased beta-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets 2010;2:353–6. [DOI] [PubMed] [Google Scholar]
- [59].Cai X, Han X, Zhang S, et al. Age at diagnosis and C-peptide level are associated with diabetic retinopathy in Chinese. PLoS One 2014;9:e91174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–5. [DOI] [PubMed] [Google Scholar]
- [61].Miyazawa-Hoshimoto S, Takahashi K, Bujo H, et al. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia 2003;46:1483–8. [DOI] [PubMed] [Google Scholar]
- [62].Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–7. [DOI] [PubMed] [Google Scholar]
- [63].Panagiotakos DB, Pitsavos C, Yannakoulia M, et al. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis 2005;183:308–15. [DOI] [PubMed] [Google Scholar]
- [64].Fujimoto WY, Abbate SL, Kahn SE, et al. The visceral adiposity syndrome in Japanese–American men. Obes Res 1994;2:364–71. [DOI] [PubMed] [Google Scholar]
- [65].Ruderman N, Chisholm D, Pi-Sunyer X, et al. The metabolically obese, normal-weight individual revisited. Diabetes 1998;47:699–713. [DOI] [PubMed] [Google Scholar]


