Abstract
The SET domain is an evolutionarily conserved motif present in histone lysine methyltransferases, which are important in the regulation of chromatin and gene expression in animals. In this study, we searched for SET domain–containing genes (SET genes) in all of the 147 arthropod genomes sequenced at the time of carrying out this experiment to understand the evolutionary history by which SET domains have evolved in insects. Phylogenetic and ancestral state reconstruction analysis revealed an arthropod-specific SET gene family, named SmydA, that is ancestral to arthropod animals and specifically diversified during insect evolution. Considering that pseudogenization is the most probable fate of the new emerging gene copies, we provided experimental and evolutionary evidence to demonstrate their essential functions. Fluorescence in situ hybridization analysis and in vitro methyltransferase activity assays showed that the SmydA-2 gene was transcriptionally active and retained the original histone methylation activity. Expression knockdown by RNA interference significantly increased mortality, implying that the SmydA genes may be essential for insect survival. We further showed predominantly strong purifying selection on the SmydA gene family and a potential association between the regulation of gene expression and insect phenotypic plasticity by transcriptome analysis. Overall, these data suggest that the SmydA gene family retains essential functions that may possibly define novel regulatory pathways in insects. This work provides insights into the roles of lineage-specific domain duplication in insect evolution.
Keywords: insects, domain, gene duplication, histone modification
Background
Protein domains are functional and structural units that are evolutionarily well conserved across species [1]. Specific protein domains are often linked to discrete biological function; therefore, the frequent duplication, gain, and loss of protein domains play substantial roles in functional novelty [2]. Domain duplication can be achieved via frequent domain-containing gene family expansion. Thus, the member number of a gene family that contains domains can be expanded, representing a common method by which divergence to domain sequences can lead to the evolutionary novelty of domain-containing genes [3]. Rapid domain diversification in particular lineages is important for the adaptation of lineage-specific ecological specializations [4].
Histones are highly alkaline proteins in cell nuclei that package and order the nuclear DNA into nucleosomes, which are the main components of chromatin. Histone modifications are a major epigenetic regulatory mechanism for phenotypic plasticity in insects. Inhibition of histone deacetylation affects developmental plasticity both in ants (Camponotus floridanus) and honeybees (Apis mellifera) [5, 6]. Genome-wide profiling of histone modifications revealed an important role of histone H3 lysine 27 acetylation in the caste differentiation of ants [7]. Methylations of histone H3 lysine 27 and histone H3 lysine 36 are more abundant in queen ovaries than in larvae, implying that histone methylation plays a specific role in honey bees [8]. In recent years, an increasing number of publications have established histone lysine methylation as a central epigenetic modification in the regulation of chromatin and transcription. The SET domain, which is observed in many histone lysine methyltransferases, is widely and probably universally distributed in metazoan species. This protein family typically comprises an approximately 130 amino acid–long SET domain, which was identified in the strongest PEV suppressor gene Su(var)3-9, in the Pc-G gene Enhancer of zeste [E(z)], and in the activating trx-G gene Trithorax of Drosophila [9]. The SET domain possesses a catalytic activity that transfers a methyl group to the amino group of lysine residues of nuclear histones from S-adenosyl-L-methionine. Based on their biochemical characteristics, SET domain is capable of catalyzing mono-, di-, or tri-methylation of their lysine substrates. SET domain–dependent methylation has been identified in a wide range of lysine residues in different histones: K4 (K is the abbreviation for lysine), K9, K27, K36, and K79 in histone H3; K20 in histone H4; K59 in the globular domain of histone H4; and K26 in histone H1B [10]. Methylation of lysine residues in histone proteins is an important post-translational epigenetic event that regulates gene expression by serving as an epigenetic marker for the recruitment of complexes that participate in the organization of chromatin structure [11]. The importance of SET domain–containing genes is strongly supported by the involvement of this protein family in diverse biological mechanisms, such as transcriptional activation, transcriptional repression, enhancer function, mRNA splicing, and DNA replication [12]. Therefore, expectedly, the regulation of various SET domain–containing genes is increasing, correlated with diverse epigenetic phenomena that, e.g., include epigenetic control in plants, centromeric gene silencing in yeasts, repeat-induced point mutations in fungi, DNA elimination in Tetrahymena, germline chromatin silencing in worms, and heterochromatin formation in flies [13].
Insects constitute a remarkably diverse group of organisms that make up a vast majority of known species, with their importance including biodiversity, agricultural, and human health concerns. The insect lineage comprises species that are both cosmopolitan distributed and geographically restricted, showing a broad range of adaptation diversity. The evolutionary history of gene families is not confounded by whole-genome duplication, and the major topology of insect species is well resolved [14]. Therefore, the insect lineage offers an excellent model to study domain/gene evolution in the context of gene family dynamics [15–19]. Insect SET domain–containing genes (SET genes) have been identified in a limited number of representative insect species without complicated analysis [20–22]. The Smyd subfamilies of SET genes have expanded in a few insects from Diptera and Hymenoptera, and several members of the Smyd subfamilies show significant changes in gene expression in response to phenotypic plasticity in ants [23, 24]. However, the evolutionary history of insect SET genes remains largely unknown because the SET genes from a broad range of insect species have not been combined in a single evolutionary framework. Therefore, a comprehensive study of the origin and diversification of the SET gene family in insects is required. Accurate classification of SET domain–containing genes can pave the fundamental way to further understanding the epigenetic basis of gene regulation in insects.
In the present study, we aimed to ascertain the origin and diversification of SET genes in insects. We searched for SET genes in the 130 insect genomes and the 17 other arthropod genomes as outgroups. These 130 insect species include both hemimetabolous and holometabolous insects and cover all the insect species for which genome data have been fully available and annotated so far. Our phylogenetic analysis revealed that an important diversification of arthropod-specific SET genes, SmydA, occurred during insect evolution. Experimental evidence of the important functions of SmydA genes in insects was obtained through fluorescence in situ hybridization, in vitro methyltransferase activity assay, and survival assay after expression knockdown. Furthermore, we compared the gene expression patterns and examined the selection signatures of SmydA genes in the four representative insects exhibiting phenotypic plasticity. These results provide insights into the regulatory roles of lineage-specific domain duplication in insect evolution.
Results
Identification and phylogenetic classification of SET genes
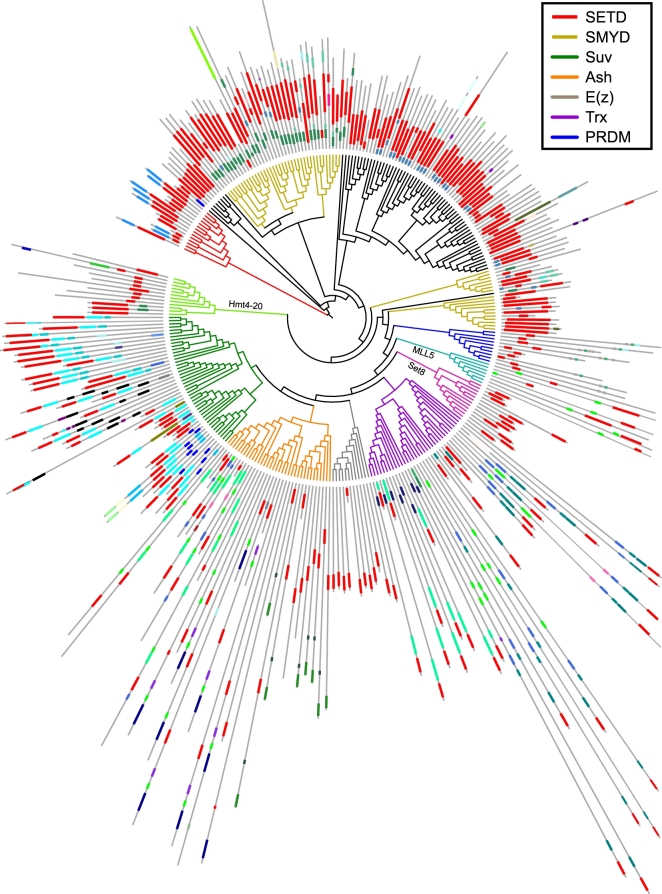
We comprehensively searched for SET genes in a wide range of sequenced insect species, which included 130 insect species from 14 insect orders (Supplementary Table S1). The SET genes were defined by the presence of the SET domain as predicted by the HMMER search, and their gene models were manually improved. Seventeen non-insect arthropods were also included to achieve ancestral status along with insect evolution. In total, 4498 SET genes were identified in the 147 arthropod genomes (Supplementary Table S2). The genes showing potential pseudogene signals were removed in these identified SET genes. A database webserver (http://159.226.67.242:8080/) has been constructed to select, retrieve, and analyze the data in this study. In insects, the number of SET genes found per species ranges from 16 in the scuttle fly Megaselia scalaris to 81 in the mosquito Culex quinquefasciatus (Table 1; see Supplementary Table S3 for the full list/summary of SET genes in the 147 arthropod genomes). This observation suggests that the size of SET genes varies significantly among different insect lineages Although the genome size of the migratory locust Locusta migratoria is approximately 30-fold that of the fruit fly Drosophila melanogaster [25], the number of SET genes in locusts is comparable with that of flies. The specificity of certain substrates is reflected by the classification of SET genes, and SET genes can be classified into seven major conserved groups, namely Suv, Ash, Trx, E(z), PRDM, SMYD, and SETD [20]. We performed phylogenetic analysis of the SET genes for representative species to obtain insights into the evolution of insect SET genes. Multiple sequence alignments of complete proteins could not accurately determine the homologous sites of SET genes because of the considerably different sequence lengths and domain architectures of these genes. Thus, alignment-based methods using Bayesian inferences for SET domain sequences and alignment-free methods based on feature frequency profiles for complete protein sequences were conducted to infer phylogenetic relationships. The overall tree topologies (Fig. 1) inferred using the two methods were generally consistent. Based on the previous nomenclature system [20], the phylogenetic tree topology allows the grouping of insect SET genes into seven major conserved groups, generally showing slight fluctuation in the member sizes in each conserved group. The protein domains for each SET gene were annotated using the InterProScan package. In general, the SET genes in the same conserved group exhibited a similar domain composition, suggesting that the domain architectures support the conserved group classification inferred through the phylogenetic analysis. In addition to the SET genes in the conserved groups, a large number of SET genes could not be classified into known conserved groups on the basis of the phylogenetic analysis. These unclassified genes act as potential “arthropod-specific” genes. Indeed, a large number of these SET genes are homologous to the arthropod-specific SmydA genes described in the previous study [24]. The lineage specificity was further verified through reciprocal BLAST search against known SET genes of nematodes and humans.
Table 1:
Summary of SET genes in insect genomes.
| Order | Genus | SMYD | SETD | PRDM | Ash | Suv | Trx | Ez | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Coleoptera | Agrilus (1) | 4 | 1 | 2 | 3 | 3 | 3 | 1 | 9 | 26 |
| Coleoptera | Anoplophora (1) | 7 | 1 | 2 | 3 | 3 | 3 | 2 | 7 | 28 |
| Coleoptera | Dendroctonus (1) | 5 | 1 | 1 | 3 | 3 | 3 | 1 | 12 | 29 |
| Coleoptera | Leptinotarsa (1) | 10 | 1 | 1 | 2 | 5 | 3 | 1 | 9 | 32 |
| Coleoptera | Onthophagus (1) | 4 | 1 | 1 | 3 | 4 | 3 | 1 | 10 | 27 |
| Coleoptera | Oryctes (1) | 6 | 1 | 1 | 3 | 3 | 1 | 1 | 9 | 25 |
| Coleoptera | Tribolium (1) | 6 | 2 | 1 | 3 | 3 | 3 | 1 | 15 | 34 |
| Phthiraptera | Pediculus (1) | 6 | 1 | 1 | 3 | 4 | 3 | 1 | 9 | 28 |
| Blattodea | Blattella (1) | 4 | 2 | 2 | 4 | 3 | 2 | 1 | 7 | 25 |
| Diptera | Aedes (2) | 11–12 | 1 | 2 | 3–4 | 2–3 | 3–4 | 1–2 | 11–12 | 34–38 |
| Diptera | Anopheles (19) | 6–19 | 1 | 1–2 | 1–3 | 2–3 | 2–3 | 1 | 4–11 | 20–37 |
| Diptera | Bactrocera (2) | 4–5 | 1 | 1–2 | 3–4 | 4 | 3–6 | 1–2 | 13–22 | 31–45 |
| Diptera | Ceratina (1) | 5 | 1 | 1 | 2 | 4 | 3 | 1 | 11 | 28 |
| Diptera | Ceratitis (1) | 5 | 1 | 1 | 3 | 3 | 3 | 1 | 14 | 31 |
| Diptera | Culex (1) | 40 | 1 | 1 | 13 | 2 | 9 | 1 | 14 | 81 |
| Diptera | Drosophila (22) | 4–5 | 1 | 1 | 3–4 | 3–5 | 2–4 | 1 | 7–14 | 24–31 |
| Diptera | Glossina (6) | 4–5 | 1 | 1 | 3–4 | 2–5 | 3–4 | 1 | 12–15 | 29–34 |
| Diptera | Lucilia (1) | 5 | 1 | 1 | 3 | 3 | 3 | 1 | 12 | 29 |
| Diptera | Lutzomyia (1) | 6 | 1 | 1 | 3 | 3 | 2 | 1 | 10 | 27 |
| Diptera | Mayetiola (1) | 13 | 1 | 1 | 9 | 6 | 4 | 1 | 25 | 60 |
| Diptera | Megaselia (1) | 2 | 1 | 1 | 3 | 2 | 1 | 1 | 5 | 16 |
| Diptera | Musca (1) | 5 | 1 | 1 | 3 | 3 | 3 | 1 | 20 | 37 |
| Diptera | Phlebotomus (1) | 5 | 1 | 1 | 4 | 3 | 3 | 1 | 6 | 24 |
| Diptera | Belgica (1) | 27 | 2 | 1 | 3 | 5 | 4 | 1 | 12 | 55 |
| Diptera | Stomoxys (1) | 5 | 1 | 1 | 3 | 2 | 3 | 1 | 16 | 32 |
| Ephemeroptera | Ephemera (1) | 18 | 1 | 1 | 3 | 2 | 2 | 1 | 12 | 40 |
| Hemiptera | Acyrthosiphon (1) | 14 | 1 | 0 | 2 | 10 | 4 | 1 | 31 | 63 |
| Hemiptera | Cimex (1) | 4 | 1 | 2 | 3 | 5 | 3 | 1 | 5 | 24 |
| Hemiptera | Diaphorina (1) | 3 | 1 | 1 | 4 | 4 | 3 | 2 | 11 | 29 |
| Hemiptera | Gerris (1) | 6 | 1 | 1 | 3 | 3 | 3 | 1 | 8 | 26 |
| Hemiptera | Halyomorpha (1) | 5 | 1 | 1 | 2 | 5 | 3 | 1 | 8 | 26 |
| Hemiptera | Homalodisca (1) | 5 | 2 | 2 | 2 | 5 | 4 | 1 | 8 | 29 |
| Hemiptera | Nilaparvata (1) | 4 | 1 | 6 | 2 | 4 | 4 | 1 | 7 | 29 |
| Hemiptera | Oncopeltus (1) | 6 | 1 | 1 | 2 | 5 | 4 | 1 | 7 | 27 |
| Hemiptera | Pachypsylla (1) | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 9 | 20 |
| Hemiptera | Rhodnius (1) | 6 | 1 | 1 | 2 | 2 | 2 | 1 | 6 | 21 |
| Hymenoptera | Acromyrmex (1) | 7 | 2 | 1 | 3 | 3 | 3 | 1 | 7 | 27 |
| Hymenoptera | Apis (3) | 6–7 | 1 | 1 | 3 | 3–4 | 1–3 | 1 | 7–9 | 22–29 |
| Hymenoptera | Athalia (1) | 7 | 1 | 2 | 2 | 3 | 2 | 1 | 8 | 26 |
| Hymenoptera | Atta (1) | 8 | 1 | 1 | 3 | 4 | 3 | 1 | 7 | 28 |
| Hymenoptera | Bombus (2) | 7–8 | 1 | 1 | 3 | 4 | 3 | 1 | 8–10 | 29–30 |
| Hymenoptera | Camponotus (1) | 8 | 2 | 1 | 2 | 3 | 2 | 1 | 8 | 27 |
| Hymenoptera | Cardiocondyla (1) | 7 | 2 | 1 | 3 | 4 | 3 | 1 | 10 | 31 |
| Hymenoptera | Cephus (1) | 6 | 1 | 1 | 2 | 3 | 2 | 1 | 6 | 22 |
| Hymenoptera | Cerapachys (1) | 5 | 1 | 1 | 2 | 3 | 3 | 1 | 6 | 22 |
| Hymenoptera | Ceratosolen (1) | 8 | 1 | 1 | 3 | 3 | 2 | 1 | 9 | 28 |
| Hymenoptera | Copidosoma (1) | 17 | 1 | 1 | 3 | 4 | 2 | 1 | 16 | 45 |
| Hymenoptera | Dufourea (1) | 7 | 2 | 1 | 3 | 4 | 3 | 1 | 7 | 28 |
| Hymenoptera | Eufriesea (1) | 6 | 2 | 1 | 3 | 4 | 3 | 1 | 8 | 28 |
| Hymenoptera | Fopius (1) | 9 | 1 | 1 | 3 | 4 | 1 | 1 | 9 | 29 |
| Hymenoptera | Habropoda (1) | 8 | 2 | 1 | 3 | 4 | 3 | 1 | 8 | 30 |
| Hymenoptera | Harpegnathos (1) | 8 | 2 | 0 | 1 | 2 | 1 | 1 | 8 | 23 |
| Hymenoptera | Linepithema (1) | 7 | 2 | 1 | 3 | 4 | 3 | 1 | 8 | 29 |
| Hymenoptera | Megachile (1) | 7 | 2 | 1 | 3 | 3 | 3 | 1 | 8 | 28 |
| Hymenoptera | Melipona (1) | 7 | 2 | 1 | 3 | 4 | 3 | 1 | 8 | 29 |
| Hymenoptera | Microplitis (1) | 18 | 1 | 1 | 3 | 4 | 3 | 2 | 8 | 40 |
| Hymenoptera | Monomorium (1) | 6 | 1 | 1 | 2 | 3 | 2 | 1 | 5 | 21 |
| Hymenoptera | Nasonia (1) | 17 | 1 | 1 | 3 | 4 | 2 | 1 | 23 | 52 |
| Hymenoptera | Orussus (1) | 11 | 2 | 1 | 2 | 3 | 3 | 1 | 7 | 30 |
| Hymenoptera | Pogonomyrmex (1) | 5 | 2 | 1 | 2 | 4 | 3 | 1 | 8 | 26 |
| Hymenoptera | Polistes (1) | 6 | 1 | 1 | 1 | 4 | 2 | 1 | 6 | 22 |
| Hymenoptera | Solenopsis (1) | 2 | 1 | 1 | 3 | 3 | 3 | 1 | 7 | 21 |
| Hymenoptera | Trichogramma (1) | 15 | 1 | 1 | 3 | 4 | 1 | 1 | 26 | 52 |
| Hymenoptera | Vollenhovia (1) | 6 | 1 | 1 | 3 | 4 | 2 | 1 | 3 | 21 |
| Hymenoptera | Lasioglossum (1) | 9 | 1 | 1 | 3 | 3 | 3 | 1 | 8 | 29 |
| Hymenoptera | Wasmannia (1) | 7 | 1 | 1 | 3 | 3 | 3 | 1 | 6 | 25 |
| Isoptera | Zootermopsis (2) | 6 | 1 | 2 | 2 | 4 | 3 | 1 | 10 | 29 |
| Lepidoptera | Bombyx (1) | 4 | 2 | 1 | 3 | 4 | 3 | 1 | 8 | 26 |
| Lepidoptera | Danaus (1) | 5 | 1 | 1 | 3 | 5 | 3 | 1 | 10 | 29 |
| Lepidoptera | Heliconius (1) | 5 | 1 | 1 | 2 | 4 | 3 | 1 | 6 | 23 |
| Lepidoptera | Papilio (2) | 6 | 1 | 1 | 3 | 2–4 | 2 | 1 | 9–11 | 26–27 |
| Lepidoptera | Lerema (1) | 4 | 1 | 2 | 3 | 3 | 3 | 1 | 10 | 27 |
| Lepidoptera | Melitaea (1) | 5 | 1 | 1 | 3 | 1 | 3 | 1 | 8 | 23 |
| Lepidoptera | Manduca (1) | 6 | 2 | 7 | 7 | 5 | 5 | 2 | 29 | 63 |
| Lepidoptera | Plutella (1) | 5 | 4 | 1 | 4 | 5 | 6 | 0 | 13 | 38 |
| Odonata | Ladona (1) | 3 | 2 | 2 | 3 | 4 | 3 | 1 | 9 | 27 |
| Orthoptera | Locusta (1) | 9 | 1 | 1 | 3 | 4 | 3 | 1 | 7 | 29 |
| Phasmatoptera | Timema (1) | 3 | 1 | 1 | 3 | 5 | 3 | 1 | 6 | 23 |
| Thysanoptera | Frankliniella (1) | 6 | 2 | 8 | 3 | 5 | 3 | 1 | 21 | 49 |
| Trichoptera | Limnephilus (1) | 3 | 1 | 0 | 2 | 3 | 2 | 1 | 6 | 18 |
The numbers in parentheses indicate the number of the species in each genus. The dash is used to represent the range of SET gene numbers in each genus. The exact gene numbers for different groups in a species are shown in the Supplementary Table 3. Other, arthropod-specific and unclassified SET genes.
Figure 1:
Phylogenetic analysis of SET genes in insects. A phylogeny using Bayesian inference is generated from the domain protein sequence of SET genes. One representative is elected for each order. The protein domains, which are labeled with different colors based on the domain type, are shown in the exterior circle of the phylogenetic tree. The length of the grey long line after each terminal is directly proportional to the length of the corresponding SET gene. The branch colors of the phylogenetic tress indicate the established SET gene classification that divides SET genes into seven major conserved groups, namely Suv, Ash, Trx, E(z), PRDM, SMYD, and SETD. The SET genes labeled in black branches cannot be classified into the seven major conserved groups, suggesting their arthropod origin. The representative species include Apis mellifera, Daphnia pule, Drosophila melanogaster, Ixodes scapularis, Locusta migratoria, Pediculus humanus, Plutella xylostella, Rhodnius prolixus, Tetranychus urticae, Timema cristinae, and Tribolium castaneum.
Ancestral states of the SET gene family in insects
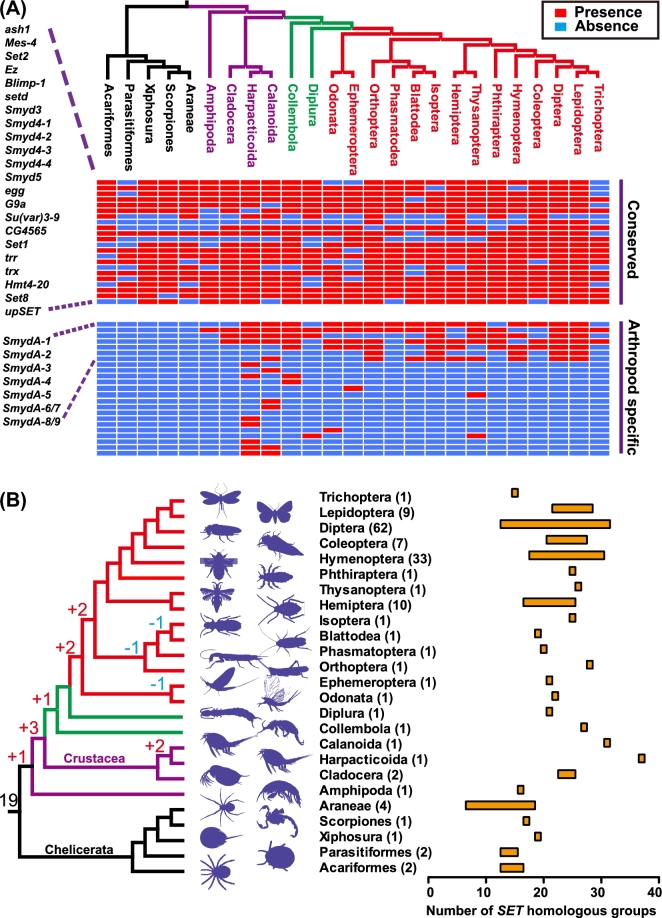
A character matrix that represents the present/absent states for each SET homologous group (a OrthoMCL-based homolog set including both putative orthologs and paralogs) was constructed to infer the ancestral states of interior nodes along with the species tree using the Mesquite program. The ancestral states at different nodes could infer the emergences/losses of the SET homologous group that occurred at and above the level of orders (Fig. 2). The grouping of SET homologous genes for each species was inferred using the OrthoMCL program with the corresponding orthologous SET gene in D. melanogaster, and the grouping reliability was supported by the phylogenetic analysis (Supplementary Figs S1–S5). The putative ancestral state was composed of 19 SET homologous groups present in the last common ancestor (LCA) of the studied arthropod species. Generally, the insect species possessed more SET homologous groups than the chelicerata species studied, suggesting that SET homologous groups considerably expanded during insect evolution. At the interior clades, novel SET homologous groups emerged several times. Few losses of SET homologous groups, such as the loss of SmydA-3, were observed at the interior clades. The large fluctuation of SET homologous groups in each species indicates that these groups experienced rapid lineage-specific expansion/contraction within insect orders. For example, in Hymenoptera, the number of SET homologous groups ranged from 18 (covering 23 SET genes) in the jumping ant Harpegnathos saltator to 30 (covering 52 SET genes) in the parasitoid wasp Nasonia vitripennis. In Diptera, 13 SET homologous groups (covering 14 SET genes) were found in M. scalaris, and the oriental fruit fly Bactrocera dorsalis possessed only 31 SET homologous groups (covering 45 SET genes). A large number of arthropod-specific SET homologous groups cannot be classified into the seven major conserved groups, which revealed their origin after the emergence of main arthropod lineages. Nevertheless, at least six of these groups were present among insect species belonging to different orders, indicating their broad conservation in insects (Fig. 2A).
Figure 2:
Diversification of arthropod-specific SET genes. (A) Distribution pattern of SET genes in arthropod orders. One representative is elected for each order. Red color indicates the presence of SET genes, and blue color indicates the absence of SET genes. (B) Inference of ancestral sets of SET homologous groups along the evolution of insects. The gains and losses of SET homologous groups are indicated in the internal nodes of the phylogenetic tree. The number in parentheses indicates the number of species in each order. The bars indicate the number ranges of SET homologous groups in each order.
SET domains do not just function as an independent unit, as in many proteins it co-occurs with multiple other protein domains to regulate their target specificity and catalysis [12]. We surveyed the gene ontology (GO) classification of proteins by integrating biological knowledge into three hierarchies, namely biological process, molecular function, and cellular component, to assess the function innovation of domain acquisition globally. The common GO categories included histone lysine methylation (GO:0034968), regulation of transcription (GO:0006355), protein binding (GO:0005515), nucleic acid binding (GO:0003676), and metal ion binding (GO:0046872) (Fig. 3A). Partitioning of SET gene families between the conserved and arthropod-specific groups revealed that GO categories could be shared between the two groups or assigned exclusively to one group. The GO categories, which were only exclusive in the arthropod-specific groups, included RNA methyltransferase activity (GO:0008173), metallocarboxypeptidase activity (GO:0004181), lysozyme activity (GO:0003796), homophilic cell adhesion (GO:0007156), sulfotransferase activity (GO:0008146), and so on.
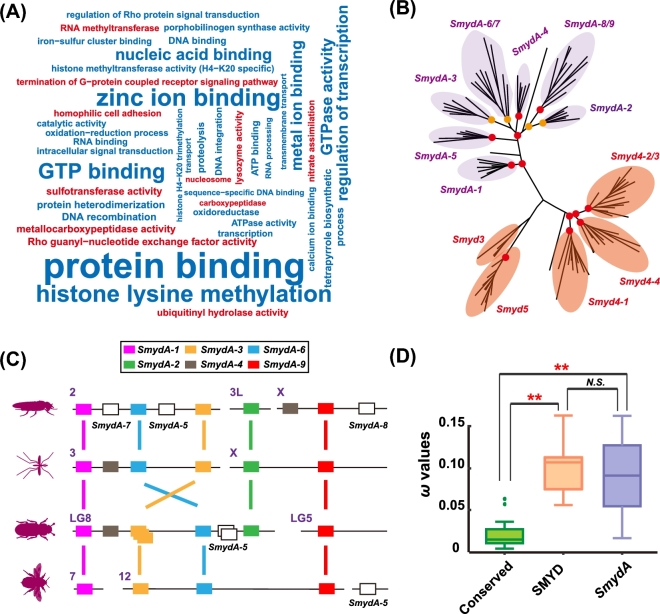
Figure 3:
Evolution of SmydA genes in insects. (A) Gene ontology categories of the conserved and arthropod-specific groups of SET genes. The gene ontology categories, which are only present in the arthropod-specific group, are highlighted in red. (B) Phylogenetic tree of the SMYD gene family of the representative species selected from each order. The representative species include Apis mellifera, Daphnia pule, Drosophila melanogaster, Ixodes scapularis, Locusta migratoria, Pediculus humanus, Plutella xylostella, Rhodnius prolixus, Tetranychus urticae, Timema cristinae, and Tribolium castaneum. The phylogenetic tree is constructed using the Bayesian inference method. The Bayesian posterior probability (PP) values are indicated only for the internal nodes to improve clarity; consequently, the SET genes are grouped into different monophyletic clades (SMYD subfamilies). Red and orange circles indicate PP > 90% and PP > 70%, respectively. (C) Conserved syntenies for SmydA genes in four holometabolous species. Shown from top to bottom are Drosophila melanogaster, Anopheles gambiae, Tribolium castaneum, and Apis mellifera. (D) Distributions of ω (ω = dN/dS ratio) values of the conserved SMYD and SmydA groups of SET genes.
Emergence of arthropod lineage-specific SET gene families
Pairwise BLAST search against all the SET genes indicated that the arthropod-specific SET genes showed considerable amino acid similarity to the SMYD groups, which contain a conserved core consisting of a SET domain and an MYND (myeloid translocation protein, Nervy, Deaf) zinc finger domain [26]. The arthropod-specific SET genes also contain the SET and MYND domains and were named SmydA [24]. We performed the phylogenetic analysis of the SMYD genes through Bayesian inferences. The majority of the SMYD genes could be classified into 11 monophyletic clades, which exhibited similar high Bayesian posterior probability values (Fig. 3B). In a global view, these SMYD genes fell into two distinct branches, which correspond with the conserved SMYD and SmydA groups. These results could exclude the possibility that the SmydA groups have raised from multiple independent gain events by duplications from deeply diverged SMYD genes of insects. Indeed, as shown in Fig. 2A, SmydA genes were absent from all Chelicerata species investigated but present in the genomes of crustacean species and insect species, suggesting that SmydA genes may have originated prior to the divergence of Crustacea and Insecta. SmydA-1, SmydA-2, SmydA-3, and SmydA-6 were already present before the split of Crustacea with other insects, showing clues for their ancient duplication events. The strong support for distinct individual lineages of paralogous genes implied that multiple duplications occurred within the order level; the most notable case was the detection of three copies of SmydA-3 in the red flour beetle Tribolium castaneum (Supplementary Table 2). SmydA-1/SmydA-4 and SmydA-6 were subjected to additional rounds of duplication in Lepidoptera and Orthoptera, respectively. The genes annotated as SmydA-8 and SmydA-9 in D. melanogaster previously formed a single clade alone with a high Bayesian posterior probability value (0.99), suggesting a specific duplication event in Drosophila. Therefore, the SmydA groups differed considerably in the number of genes in each insect order, implying the complexity of their evolutionary histories.
To shed light into the evolutionary history of SmydA genes, we determined the location and gene order of SmydA genes in the four holometabolous species with available chromosome-level genome assemblies or genome-scale genetic linkage maps (Fig. 3C). In Diptera, the syntenic gene orders could be inferred from the four ancient SmydA genes, namely SmydA-1, SmydA-2, SmydA-3, and SmydA-6, all of which may have been present in the ancestor of insects and crustaceans. An insect-specific SmydA-9 could be observed in the majority of insect orders, including both hemimetabolous and holometabolous insects. SmydA-9 showed syntenic conservation with the four ancient genes. This gene order was also conserved when SmydA genes in insects distantly related from other insect orders were examined. Almost all of the five synteny-anchoring genes were maintained in both the coleopteran species T. castaneum and hymenoptera species A. mellifera, with an exception of SmydA-2 that was missed in A. mellifera. In contrast to those in T. castaneum and A. mellifera, the reversed order of SmydA-3 and SmydA-6 in Dipteran species implies that an intrachromosome transfer event of genomic segments occurred before the emergence of Diptera. Duplication events could also occur in the early diversification of arthropod species. No orthologous SmydA-4 gene was detected the chelicerata species, indicating that that duplication event contributes to the emergence of the SmydA-4 gene in Pancrustacea species. SmydA-4 was present in all the hemimetabolous insect orders studied, as well as in the holometabolous insect orders Lepidoptera, Coleoptera, and Diptera. The absence of SmydA-4 in all the 32 hymenopteran species suggested that subsequent loss of SmydA-4 could be traced back to the ancestor of the hymenopteran lineage before the divergence of wasps, ants, and bees. In the SMYD phylogenetic tree, the Bayesian inferences supported the grouping of SmydA-3, SmydA-4, and SmydA-6. Three of the four species exhibited an accordant location of SmydA-3/SmydA-4/SmydA-6 in the syntenic regions. In addition to the old duplication events that categorized the divergent duplicates into distinct SmydA subfamilies (e.g., SmydA-3 and SmydA-4), recent duplications within an insect order were also observed. The three copies of SmydA-3 in T. castaneum, which spanned a 4.2 kb genomic region, were observed in tandem array between the two syntenic genes SmydA-1 and SmydA-6. The closeness in protein sequence and genomic location implies an evolutionary origin of these three copies of SmydA-3 via local duplication. Overall, our data suggest that the order of SmydA genes was conserved over a remarkable wide range of holometabolous insect orders.
Selective pressures acting on SmydA genes
Functional differentiations or mutations leading to pseudogene formation are the two major causes for sequence divergence between new duplicates and their orthologous counterpart. Synonymous substitutions are assumed to accumulate at a constant rate; hence, the ratios of nonsynonymous substitution per nonsynonymous site (dN) to synonymous substitution per synonymous site (dS) are deemed to be an indicator to measure the relative rates of evolution for protein sequences. The four genes (ACYPI26757 and ACYPI55839 in Acyrthosiphon pisum; Px015362.1 and Px001029.1 in Plutella xylostella) showing signals of recombination were removed from the further selection analysis. We estimated a global dN/dS ratio (one ratio, model M0) for these SET genes to determine whether the SmydA genes have been under different selection pressures than the other conserved SET genes. The dN/dS ratios (ω = dN/dS ratio) of SET genes varied from low (0.0007, Ez, CG6502) to high (0.1627, Smyd4-1, CG1868), indicating a variance in the rates of protein evolution on different SET genes (Table 2). The ω values among the conserved SET genes (excluding the SMYD genes) ranged from 0.0007 to 0.0624 (mean ω = 0.0185). The conserved SMYD and SmydA groups showed ω values in the ranges of 0.055–0.1627 (mean ω = 0.1020) and 0.0052–0.1623 (mean ω = 0.0884), respectively. Overall, both the conserved SMYD and SmydA (P = 0.0003 and P = 0.0178, Wilcoxon signed-rank tests with Bonferroni correction, respectively) groups exhibited significantly higher ω values than the conserved SET genes (Fig. 3D). However, the distributions of ω values of the conserved SMYD and SmydA groups were statistically indistinguishable (P = 1.0000, Wilcoxon signed-rank tests with Bonferroni correction).
Table 2:
Tests of rate heterogeneity acting on SET genes in insects.
| Gene | One Ratio Likelihood | One Ratio ω | Free Ratio Likelihood | df | P | |
|---|---|---|---|---|---|---|
| Smyd3 | −4833.870633 | 0.055 | −4833.870633 | 16 | <0.001 | |
| Smyd4-1 | −17270.854806 | 0.1627 | −17140.293096 | 58 | <0.001 | |
| SMYD | Smyd4-2 | −13187.367961 | 0.1125 | −13112.105983 | 44 | <0.001 |
| Smyd4-3 | −20488.963155 | 0.1069 | −20364.991393 | 66 | <0.001 | |
| Smyd4-4 | −15552.366084 | 0.1112 | −15475.97917 | 44 | <0.001 | |
| Smyd5 | −21495.435476 | 0.0633 | −21329.013029 | 64 | <0.001 | |
| upSET(MLL5) | −7286.598116 | 0.0103 | −7247.800191 | 62 | 0.087 | |
| Set8 | −6450.096636 | 0.0321 | −6386.997507 | 60 | <0.001 | |
| Hmt4-20 | −3523.660744 | 0.0079 | −3478.339497 | 56 | <0.001 | |
| SETD | SETD | −9030.115692 | 0.033 | −9009.972504 | 34 | 0.212 |
| PRDM | Blimp-1 | −2679.981724 | 0.0051 | −2664.129882 | 52 | 0.988 |
| Mes-4 | −5530.425067 | 0.0163 | −5504.225668 | 56 | 0.612 | |
| Ash | ash1 | −4995.315864 | 0.0122 | −4947.987993 | 60 | <0.001 |
| Set2 | −5636.021533 | 0.0118 | −5570.266003 | 60 | <0.001 | |
| Su(var)3-9 | −4351.473377 | 0.0212 | −4308.872564 | 32 | <0.001 | |
| Suv | egg | −15308.272711 | 0.0624 | −15214.544773 | 54 | <0.001 |
| CG4565 | −7168.675146 | 0.056 | −7114.254055 | 46 | <0.001 | |
| G9a | −4641.585219 | 0.0091 | −4604.810574 | 54 | 0.040 | |
| trx | −3897.22035 | 0.0031 | −3877.624919 | 58 | 0.972 | |
| Trx | Set1 | −3733.003015 | 0.0026 | −3700.07484 | 60 | 0.281 |
| trr | −4549.712 | 0.0114 | −4471.116449 | 60 | <0.001 | |
| E(z) | Ez | −3368.302419 | 0.0007 | −3355.922925 | 61 | 1.000 |
| SmydA-1 | −10066.858829 | 0.0904 | −9995.276076 | 34 | <0.001 | |
| SmydA-2 | −11858.796558 | 0.0052 | −11812.616411 | 30 | <0.001 | |
| SmydA-3 | −13902.688419 | 0.0817 | −13842.811542 | 56 | <0.001 | |
| SMYDA | SmydA-4 | −9602.742487 | 0.0254 | −9583.599425 | 26 | 0.057 |
| SmydA-5 | −13748.769161 | 0.1179 | −13656.268493 | 50 | <0.001 | |
| SmydA-6 | −12142.197791 | 0.1623 | −12043.993185 | 42 | <0.001 | |
| SmydA-9 | −13258.406279 | 0.1357 | −13193.536113 | 52 | <0.001 |
Accounting for the unequal genome sequencing efforts between different insect families, we selected one species within each genus to be representative of the genus.
Function approval of SmydA genes
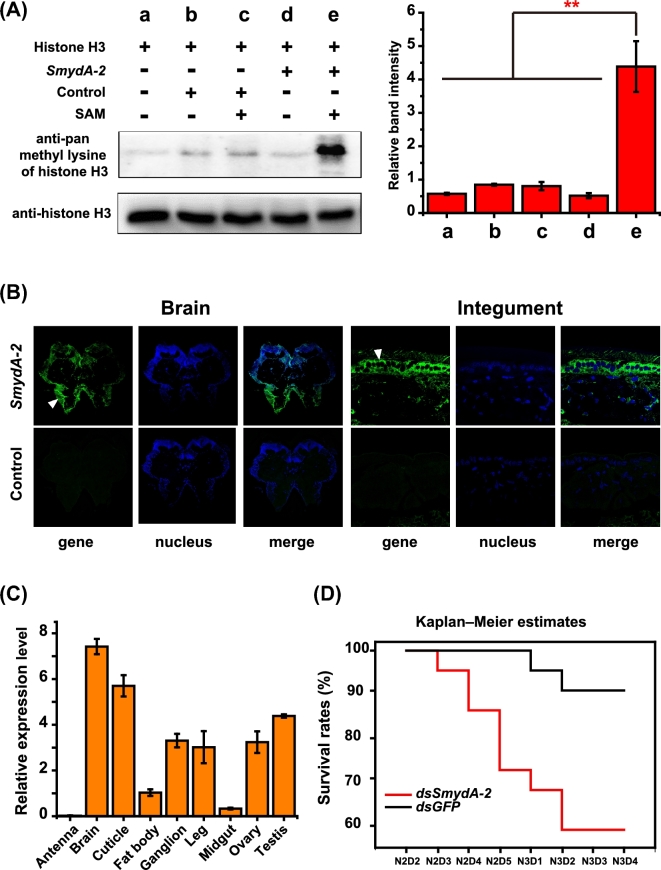
We attempted to determine whether the SmydA genes retained histone methylation activities to approve the non-pseudogenization process of these genes. We expressed SmydA-2 as a randomly selected representative and performed in vitro histone methylation activity assays using histones as substrates in the migratory locust. As shown in Fig. 4A, western blot analysis detected increased lysine methylation on histone H3 compared with the controls, indicating that SmydA-2 possesses methyltransferase activity on histones. Similar to that of the other conserved SMYD genes, the methyltransferase activity of SmydA-2 was also dependent on S-adenosyl methionine. Fluorescence in situ hybridization analysis provided further tissue expression evidence to support the reliability of the SmydA-2 gene function. Obvious fluorescence signals were observed in the brain and epidermal cells of cuticles in the locusts (Fig. 4B). These cells did not show any hybridization signal for the negative controls. The origin and evolution of new emerging genes undergo an increased expression breadth of new duplicated genes over evolutionary time [27, 28]. Thus, we determined the expression levels of the SmydA-2 gene using quantitative real-time polymerase chain reaction (qPCR) analysis in the different tissues. qPCR data showed that the SmydA-2 gene was expressed in a broad range of tissues, including brains, testes, ovaries, cuticles, and legs (Fig. 4C). The broad expression pattern suggests that the SmydA-2 gene is less tissue specific and may serve as a functional gene in multiple tissues [28].
Figure 4:
Function approval of SmydA-2 genes through experimental evidence. (A)In vitro methyltransferase assay of histone H3 of SmydA-2 in locusts. Anti-pan methyl lysine antibody recognizes histone H3 in vitro methylated with SmydA-2. Anti-histone H3 serves as endogenous control for protein samples. The analyses were carried out in three replicates. **P < 0.01. (B) Expression evidence of SmydA-2 in the brain and cuticle of locusts via fluorescence in situ hybridization analysis. Green signals indicate the expression of SmydA-2/control, and blue signals indicate nuclear staining with Hoechst. (C) Relative gene expression of SmydA-2 in the different tissues. mRNA levels are quantified using the SYBR Green expression assays on a LightCycler 480 instrument. The qPCR data are shown as the mean ± SEM (n = 6). (D) Survival analysis of the locusts after SmydA-2 double-strand RNA injection. Data are analyzed through the Kaplan–Meier survival curve comparison of the dsSmydA-2 and dsGFP groups for three replicates.
Essential genes are often considered conserved and functionally important [29], whereas pseudogenes have been considered to be more dispensable and to have minor influence on survival and phenotype. To determine whether the SmydA-2 gene plays an essential role during development [30], we knocked its expression down by using RNA interferences in the locusts. Compared with the controls, the relative mRNA level of the SmydA-2 gene decreased by approximately 70% after injecting double-strand RNAs (Supplementary Fig. S6). After injection of dsSmydA-2, we observed large numbers of dead locusts, which did not display obvious defect phenotype. As shown in Fig. 4D, Kaplan–Meier survival estimates indicate that injection of locusts with dsSmydA-2 significantly increased mortality when compared with the controls (χ2 = 6.260, df = 1, P = 0.0123, chi-square tests).
Expression and selection analysis of SmydA genes in response to phenotypic plasticity
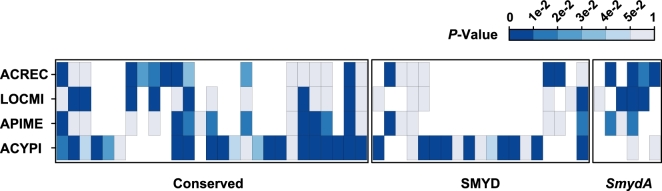
Epigenetic reprogramming that modifies chromatin structure through histone modifiers contributes to the orchestration of the generation and maintenance of phenotypic plasticity, which is a key trait for the success of insects. Therefore, we compared the expression patterns of histone-modifier SET genes in four representative insects exhibiting phenotypic plasticity, namely locust density-dependent behavior, aphid seasonal morphs, and dietary-mediated interactions of bees and ants. Specially, we performed differential expression analysis between gregarious and solitary locusts, between asexual and sexual morphs in A. pisum, between queens and workers in A. mellifera, and between large workers and queens in Acromyrmex echinatior. In all the four species, a number of differentially expressed genes (DEGs) were detected between the two alternative phenotypes using the criteria of a false discovery rate (FDR)–corrected P < 0.05. In terms of DEG number, a large portion of SET genes showed significant changes in gene expression (12 in 29, 41%, in A. mellifera; 23 in 62, 37%, in A. pisum; 11 in 29, 38%, in L. migratoria; and 10 in 27, 37%, in A. echinatior). Compared with that of the DEGs observed at the genome-wide level (DEGs in total), the number changes of the DEGs in SET genes in the four insects were even more prominent, emphasizing the important regulatory role of SET genes in phenotypic transition (Ps < 0.05, chi-square tests). Overlapping of the differentially expressed SET genes derived from the same ortholog could provide a clue of their convergent function in phenotypic transition. We found that two SET genes, namely Set2 and SmydA-5, showed significant changes in gene expression simultaneously in three of the four insect species studied.
Assuming that a non-pseudogene gene should not be randomly expressed, we compared the expression pattern of the duplication-derived SmydA genes to their derived ancestral SMYD genes in response to environment-dependent phenotypic plasticity (Fig. 5). The majority of SET genes from the conserved SMYD (33 in 34 in total, 97%) and SmydA (13 in 17 in total, 76%) groups were expressed in at least one insect. No significant differences (P = 0.749, chi-square tests) in the number of expressed genes were observed between the two groups. A number of DEGs were detected in both the conserved SMYD and SmydA groups in the four-insect species. All the four SmydA genes in A. echinatior were also differentially expressed. We also obtained significant results in three of the six SmydA genes of L. migratoria and in two of the five SmydA genes of A. mellifera between the two alternative phenotypes. The DEG number in the SmydA groups did not show significant deviation from those in the conserved SMYD group in the four insects (Ps > 0.2, Fisher's exact tests). This result suggests that the SmydA genes might not be randomly expressed and that they did not represent pseudogenes or transcriptional byproducts. Thus, the SmydA genes may preserve a regulatory role, indicating the function similarity to their ancestral SMYD genes.
Figure 5:
Differential expression analysis in insects showing phenotype plasticity. Alternative phenotype includes gregarious and solitary phases in Locusta migratoria (LOCMI), asexual and sexual morphs in Acyrthosiphon pisum (ACYPI), queens and workers in Apis mellifera (APIME), and large workers and queens in Acromyrmex echinatior (ACREC).
The free ratio model of SmydA genes fitted the data significantly better than the one model (model M0) using likelihood ratio tests (Ps < 0.001), indicating heterogeneous rates of sequence evolution along the gene tree of SmydA genes. Therefore, we tested whether the differentially expressed SmydA genes between alternative phenotypes (foreground branches) evolved under different selective pressures than those in the remaining branches (background branch) (Supplementary Fig. S7). The branch model was much better supported by the data than the model M0 for SmydA-5 in A. mellifera and SmydA-1 in L. migratoria (Table 3). Fixing ω = 1 for the foreground branch did not result in an improved fit over the branch model with the unconstrained foreground branch (the null neutral model and the alternative model). This result suggests that the ω values in the external branch were smaller than 1 for SmydA-3 and SmydA-5 in A. mellifera, SmydA-1 in L. migratoria, and SmydA-3 in A. echinatior. Only SmydA-1 in L. migratoria exhibited elevated ω values, and a branch-site model allowing heterogeneous ω values across sequences and branches identified four sites (5M, 11K, 93P, and 105C) under positive selection.
Table 3:
Signatures of selection acting on differentially expressed SET genes in response to phenotypic plasticity.
| APIME | LOCMI | ACREC | ||||
|---|---|---|---|---|---|---|
| Model Parameters | SmydA-3 | SmydA-5 | SmydA-1 | SmydA-3 | SmydA-5 | SmydA-9 |
| Basic models | ||||||
| M0: ω | 0.082 | 0.118 | 0.090 | 0.082 | 0.118 | 0.136 |
| Branch models | ||||||
| B0: lnL | −13914.741 | −13749.007 | −10088.904 | −13905.140 | −13749.047 | −13259.370 |
| B0: ω0 (ω1 = 1) | 0.077 | 0.113 | 0.090 | 0.081 | 0.117 | 0.135 |
| BA: lnL | −13901.138 | −13745.405 | −10056.182 | −13901.922 | −13748.719 | −13258.338 |
| BA: ω0, ω1 | 0.080, 0.142 | 0.115, 0.313 | 0.095, 0.003 | 0.081, 0.177 | 0.118, 0.181 | 0.135, 0.186 |
| Branch-site models | ||||||
| A0: p2a (ω2 = 1) | 0.078 | 0.059 | 0.111 | 0.082 | 0.155 | 0.096 |
| AA: p2a΄, ω2 | 0.078, 1.000 | 0.025, 3.102 | 0.109, 8.895 | 0.082, 1.000 | 0.155,1.000 | 0.011, 19.742 |
| Positively selected sites (BEB) | 5 M 11 K 93 P 105 C | |||||
| LRT, P | ||||||
| M0 versus BA | 0.078 | 0.009 | <0.001 | 0.216 | 0.752 | 0.712 |
| BA versus B0 | <0.001 | 0.007 | <0.001 | 0.011 | 0.418 | 0.151 |
| A0 versus AA | 1.000 | 0.802 | 0.022 | 1.000 | 1.000 | 0.082 |
Ω: the ratios of nonsynonymous substitution per nonsynonymous site to synonymous substitution per synonymous site; ω0, ω1: background and foreground ω values, respectively; APIME: Apis mellifera; ACREC: Acromyrmex echinatior; LOCMI: Locusta migratoria.
Discussion
In this study, the phylogenetic analyses allowed the subdivision of the insect SET genes into seven major conserved groups and one arthropod-specific SmydA group. We inferred many SmydA gene duplication events along insect evolution, suggesting that an important diversification of the SmydA genes occurred during insect evolutionary processes. With the SmydA-2 genes in locusts as representatives, the maintenance of essential gene function was confirmed from the experimental evidence of in vitro methyltransferase activity, in situ mRNA expression, and phenotypes after expression knockdown. Based on the examination of distribution pattern and selection signatures across insects, our data indicated that extensive pseudogenization unlikely occurred for the SmydA genes. Finally, the transcriptome analyses of the four insects showed that several SmydA genes are involved in insect phenotype plasticity, suggesting that SmydA genes contributed novelties for insect adaptive evolution. These data suggest a role of diverged regulatory functions after their duplication in insects.
A recent study has provided a framework for understanding the evolution history of the SMYD gene family in representative animal phyla [24]. The phylogenetic results show that the metazoan SMYD genes can be classified in three main classes, Smyd3, Smyd5, and Smyd4. Two sub-classes of SMYD genes, namely Smyd4-4 and SmydA, are absent in vertebrates; the former one is insect specific, and the later one is arthropod specific. Within Chelicerata, we detected Smyd4-4 in Acariform mites (non-insect arthropods), suggesting that our evidence did not support the point that Smyd4-4 is specific to insects. Since Chelicerata represents an out-group branch for this study, further studies covering more basal branches of arthropod phylogeny are required to ascertain the origin of Smyd4-4. SmydA genes represent a class of arthropod-specific genes that are only present in the LCA of insect species and crustacean species, suggesting their origin after the split of chelicerates from Pancrustacea species. Conservation of five ancient SmydA genes in a wide range of species suggests that they probably originated from duplication events of conserved SMYD genes predating the diversification of insects. Although a few cases of whole-genome duplication have been documented in chelicerates, evidence that whole-genome duplication occurs widely in arthropod evolution remains lacking [31]. Therefore, gene duplication rather than whole-genome duplication possibly leads to the emergence of multiple copies of ancient SmydA genes in the LCA of the Pancrustacea species. The clear split of conserved SMYD and SmydA genes excluded the possibility that multiple independent duplication events from conserved SMYD genes resulted in the current repertoire of SmydA genes in insects. This result suggests that the five ancient SmydA genes were first produced from a single ancestral gene, which was derived from conserved SMYD genes. The five ancient SmydA genes were thus the source from which insect-specific SmydA duplications were subsequently produced in insects. Determining the location and order of multiple gene members at the genomic scale sheds light on the evolutionary history of the gene family. The closely linked manner in genomic location suggests that homologous recombination and functional differentiation may be a major force to shape the evolution of SmydA genes in insects. For instance, in dipteran and lepidopteran insects, homologous recombination may give rise to SmydA-6 via the duplication events of SmydA-3 because SmydA-3 and SmydA-6 were in close proximity to each other in both genomic location and phylogenetic trees. The tandem organization of three SmydA-3 copies in T. castaneum may also result from species-specific duplications via homologous recombination. Retrotransposition events may represent another contributing force for generating unlinked SmydA genes; these events can also generate intronless retroposed gene copies [32]. However, the retrotransposition events could not be inferred from the presence of the signature of intron–exon structure because of the subsequent insertion in deeply diverged duplicates, such as SmydA-5. Conserved gene orders between species from Lepidoptera, Coleoptera, and Diptera revealed a high degree of macrosyntenic gene order of the five ancient SmydA genes during approximately 348 million years of evolutions splitting these insects [33]. This observation implies strong constraints for preserving the conserved gene order of SmydA genes in insects. Currently, whether this macro-syntenic gene order is preserved outside holometabolous insects cannot be determined because chromosome-level genome assemblies or genome-scale genetic linkage maps are not available in hemimetabolous insects. This issue would be addressed when the genome assembly is considerably improved in the future.
Selective pressures were significantly weaker for the SMYD genes than for the six conserved groups (Suv, Ash, Trx, E(z), PRDM, and SETD). Compared with the six conserved groups, SMYD genes were the least conserved gene group and, concordantly, the least constrained one. Nevertheless, the ω values of SMYD genes ranged from 0.0052 for SmydA-2 to 0.1627 for Smyd4-1. ω < 1 was consistent with their broad conservation across insects, implying their essential functional roles. This observation suggests that purifying selection is the main force governing the evolution of SMYD genes. The distributions of ω values of the conserved SMYD and SmydA gens were statistically indistinguishable, indicating a symmetrical rate of sequence evolution. Thus, purifying selection is subject to the conserved SMYD and SmydA genes, but their intensity may be relaxed compared with other SET genes. Both the GO analysis and the in vitro methyltransferase activity assay suggest that SmydA genes, similar to their conserved SMYD ancestors, are sufficient to perform the original function relating to histone methylation [34]. GO ontology analysis implied that the SmydA genes have developed to acquire novel functions. These functions were absent in the conserved SMYD genes, indicating that the SmydA genes may have undergone functional differentiation. Gene duplications that occurred in specific lineages are important in contributing to lineage-specific adaptive processes [35]. After gene duplication, purifying selection is expected in both gene copies if duplication can confer a selective advantage [36]. By contrast, one of the two copies can evolve either under relaxed purifying selection when no immediate advantage is shown from gene duplication or under positive selection when a new function is acquired via advantageous mutations [37]. Overall, these data suggest that the SmydA genes may not represent redundant gene copies that are under pseudogenization.
Several members of the SMYD family of histone methyltransferases have undergone a dramatic expansion in the insect lineage [23]. These SMYD genes were identified as caste-specific genes in ants (Harpegnathos saltator), suggesting that these histone modifiers play dedicated regulatory roles in insect phenotypic plasticity. However, the biological significance of the differential expressions of these genes remains unknown [38]. Our study further verified the presence of the differential expression patterns of the SMYD genes in the four other insects that also possessed adaptive phenotypic plasticity. Consequently, the understanding of the convergent regulatory roles of the SMYD genes in insect phenotypic plasticity was extended. Histone lysine methyltransferase catalyzes methyl group transfer to the amino group of lysine residues of histones by means of the SET domain, a domain presented within many proteins that regulate diverse development processes [39]. Histone lysine methylation on specific residues is associated with distinct signatures of gene expression, thereby serving as a chromatin modulator for epigenetic regulation [40]. Future studies should understand how the expanded SMYD gene family can quickly become essential and identify the roles of the duplicated SMYD genes in insects, despite the expectation of redundant functionality at the beginning of new duplicated gene evolution [30].
Materials and Methods
Identification of insect SET genes
Genome assemblies and official gene sets of 130 insect species, including 62 dipteran insects, 33 hymenopteran insects, 10 hemipteran insects, 7 coleopteran species, 9 lepidopteran insects, and representatives from Orthoptera, Phthiraptera, Phasmatoptera, Trichoptera, Thysanoptera, Isoptera, Blattodea, Ephemeroptera, and Odonata, were obtained from their respective genome databases (Supplementary Table S1). Among the basal arthropod species, we included 17 arthropod genomes from 10 chelicerate species, five crustacean species, and two non-insect hexapod species.
The hidden Markov model–based HMMER program was used to identify the SET domain containing proteins using PF00856 in the Pfam database with a conditional E-value cutoff of 1e-5 [41, 42]. Despite that the SET domain can be detected in their homologs in closely related species, the genes lacking the SET domain were considered deprived of lysine methylation capacity and were excluded for further analysis. The resulting genes with stop codons or frameshift mutations were subsequently manually checked. The obvious incorrect gene models were improved with transcriptome data through the GeneWise version 2.2.0 program [43]. The PSILC version 1.21 program was used to identify the potential pseudogenes [44]. Gene ontology (GO) categories were determined via scanning protein sequences against Interpro member databases using various profile-based and hidden Markov models in the InterProScan version 5.13-52.0 package [45]. The member database binaries and models include TIGRFAM, ProDom, Panther, SMART, PrositePatterns, SuperFamily, PRINTS, Gene3d, PIRSF, PfamA, and PrositeProfiles.
Phylogenetic analysis, ancestral state reconstructions, and tests for selection
Alignment-based methods using Bayesian inferences for SET domain sequences and alignment-free methods based on feature frequency profiles for complete protein sequences were used to infer phylogenetic relationships of SET genes across insects. Multiple alignments were generated using the MAFFT alignment software package [46]. According to the Akaike information criterion, the model of molecular evolution with the best fit to the data was determined by using the ProtTest 3.4.2 software package [47]. Bayesian reconstruction of phylogeny was conducted using the MrBayes 3.2.1 software package for 10 000 000 generations [48]. The first 25% of the trees were discarded as burn-in. The alignment-free and distance-based methods for phylogenetic tree building were implemented by means of the feature frequency profile method with the FFP version 3.19 suite (http://sourceforge.net/projects/ffp-phylogeny/), utilizing the FFPaa program for amino acid sequences with a word length of L = 5. The FFPboot program was used for bootstrap analysis of the tree generated for 100 replicates.
We constructed a character matrix that represents present/absent states for each SET homologous group to reconstruct the ancestral states of interior clades. We did not consider member number variation and considered only the binary state, presence or absence, of a given SET homologous group in any given node. The grouping of the SET genes was inferred from the OrthoMCL software package with the corresponding orthologous SET gene in D. melanogaster. Ancestral state reconstruction was implemented in the Mesquite program (http://mesquiteproject.org/) under maximum likelihood optimization using the Markov k-state 1 parameter model. After ancestral reconstruction, we measured emergence and loss events of the SET homologous group along each branch in the phylogenetic tree. The emergence event of the SET homologous group was defined as: the SET homologous group was absent at the ancestral nodes of a given node and either of the out-groups. This process requires a phylogeny tree of all the species studied. Single-copy orthologous gene families were inferred from the benchmarking universal single-copy ortholog BUSCO gene sets from each species [49]. The resulting 527 single-copy orthologous (completed genes in BUSCO) gene families were used to construct the neighbor-joining species tree, which is consistent with the phylogenomic tree recently inferred from transcriptome data [14]. The neighbor-joining species tree was constructed from amino acid sequences of single-copy orthologs using the Phylip version 3.69 package. The bootstrap values were calculated from 100 replicates using the seqboot, protdist, neighbor, and consense programs of Phylip package.
Expression of SMYD family genes in response to phenotypic plasticity
The transcriptome data for gregarious and solitary locusts in L. migratoria, asexual and sexual morphs in A. pisum, queens and workers in A. mellifera, and minor and major workers in A. echinatior were retrieved from the NCBI database under accession numbers PRJNA79681, GSE56830, GSE61253, and GSE51576, respectively. The raw reads were preprocessed to remove adapters and low-quality bases using the Trimmomatic software package; these reads were then mapped to the genome assembly (genome assembly version: v2.4 for L. migratoria, v1.0 for A. pisum, Amel_2.0 for A. mellifera, and Aech_v2.0 for A. echinatior, respectively) using Tophat2 version 2.0.14 software [50, 51]. Raw counts of each gene were calculated and annotated using the HT-seq version 0.6.1 package in Python, and the trimmed mean of M value normalization method was used to normalize raw counts [52]. Differential expression analysis was performed using the edgeR version 3.8.0 package at an FDR cut-off of 0.05 [53].
Function approval of SmydA-2 genes via experimental evidence
Fluorescence in situ analysis of SmydA-2 was performed on the brains and integuments of locust nymphs. Biotin-labeled antisense and sense probes (Supplementary Table S4) of SmydA-2 were produced from pGEM-T Easy plasmids (Promega) by using the T7/SP6 RNA transcription system (Roche) following the manufacturer's protocol. The PCR parameters were a preincubation 94°C for five minutes, followed by 30 cycles of 94°C for 10 seconds, 58°C for 30 seconds, 72°C for 30 seconds, and a final extension at 72°C for 10 minutes. The brains and integuments were fixed in 4% paraformaldehyde overnight. The paraffin-embedded slides (5 μm thick) were deparaffinized in xylene, rehydrated with an ethanol gradient, digested with 20 μg/mL proteinase K (Roche) at 37°C for 15 minutes, and then incubated with SmydA-2 probe at 60°C for five minutes. The slides were hybridized for seven to 15 hours at 37°C and washed in 0.2 × SSC and 2% BSA at 4°C for five minutes. The biotin-labeled probes of SmydA-2 were detected with a streptavidin horseradish peroxidase conjugate and fluorescein tyramide substrate using a TSA kit (Perkin Elmer). Images for fluorescence signals were acquired using an LSM 710 confocal fluorescence microscope (Zeiss).
The recombinant proteins for SmydA-2 and the negative controls of the translation system were produced using the TNT protein expression system (Promega) following the manufacturer's protocol. In brief, 3 μg PCR-generated DNA templates (Supplementary Table S4) were added to 30 μl TNT master mix, and the translation reactions were incubated at 25°C for two hours. The recombinant proteins were verified by western blotting using His-tag antibodies. For in vitro methyltransferase assay, 2 mg of unmodified histone H3 peptides (Sino Biological) were incubated with 1 mg of recombinant protein and 0.1 mM S-adenosyl-methionine (SAM, NEB) in a reaction buffer containing 50 mM Tris-HCl (pH 8.0), 10% glycerol, 20 mM KCl, 5 mM MgCl2, 1 mM DTT, and 1 mM PMSF at 30°C for two hours. The reaction mixtures were subjected to electrophoresis on SDS-PAGE, and the methylation activities were detected in western blotting using anti-pan methyl lysine antibody (Abcam Cat# ab7315, RRID:AB_305840). Anti-histone H3 (Abcam Cat# ab176877, RRID:AB_2637011) was used as an endogenous control for protein samples.
Locusts (the migratory locust, Locusta migratoria) were reared in large, well-ventilated cages (40 cm × 40 cm × 40 cm) at a density of 500–1000 insects per container. These colonies were reared under a 14:10 light/dark photo regime at 30°C and were fed fresh wheat seedlings and bran. Double-stranded RNAs of SmydA-2 and green fluorescent protein (GFP) were prepared using the T7 RiboMAX Express RNAi system (Promega) in accordance with the manufacturer's protocols. Second-instar locusts were injected with double-stranded RNAs in the second ventral segment of the abdomen. Total RNAs were isolated using TRIzol reagent (Thermo Fisher Scientific) and then reverse-transcribed into cDNA using M-MLV reverse transcriptase (Promega). The mRNA levels were quantified using the SYBR Green expression assays on a LightCycler 480 instrument (Roche). The parameters were a pre-incubation 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 seconds, 58°C for 20 seconds, and a single acquisition when 72°C for 20 seconds. The ribosomal protein 49 gene was used as reference control, and the quantification was based on the requirement of PCR cycle number to cross or exceed the fluorescence intensity level; the 2−ΔΔCt method was used to analyze mRNA expression levels. Survival data were analyzed using the Kaplan–Meier method [54], and survival curves were compared using log-rank testing for the dsSmydA-2 and dsGFP curves.
Signature of selection detected through likelihood ratio tests
Protein sequences of SET genes were aligned with the MAFFT alignment software package [46] and then back-translated into corresponding nucleotide sequences. Gene conversion was detected using the recombination detection program GENECONV version 1.81a. To assess the contribution of natural selection during the diversification of the SET gene family in insects, the ratios of nonsynonymous substitution per nonsynonymous site (dN) to synonymous substitution per synonymous site (dS) across the phylogenetic tree of the species were calculated using the software package PAML version 4.48a [55]. The basic model M0 (null model) assumes that the ratio ω = dN/dS is invariable (one-ratio model) among all branches examined, whereas the alternative model allows the ω ratio to vary in different tree branches in the phylogenetic tree [56, 57]. Likelihood ratio tests were applied to compare the null and alternative models, which estimated ω ratio separately for different branches, assuming a priori and the background branches. A significantly higher likelihood of the alternative model than the null model indicates a better fit to the data, indicating a variation of selective pressures in different tree branches [56, 57].
Additional files
Additional file 1: Table S1: The arthropod genome data involved in this study.
Additional file 2: Table S2: SET genes in the 147 arthropod genomes.
Additional file 3: Table S3: Summary of SET genes in the 147 arthropod genomes.
Additional file 4: Table S4: Primers used in the study.
Additional file 5: Figure S1: Phylogenetic analysis of the SET genes in Lepidoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 6: Figure S2: Phylogenetic analysis of the SET genes in Diptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 7: Figure S3: Phylogenetic analysis of the SET genes in Hemiptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 8: Figure S4: Phylogenetic analysis of the SET genes in Hymenoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 9: Figure S5: Phylogenetic analysis of the SET genes in Coleopteran using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 10: Figure S6: Effects of RNA interference of the mRNA expression levels of SmydA-2 in locust brains. The locusts are injected with double-stranded RNAs into the second ventral segment of the abdomen. Due to the systemic RNA interference in locusts, the brain, which is spatially distant from the abdomen, is used in qPCR assays to guarantee effective expression knockdown. qPCR data are shown as the mean ± SEM (n = 6). **P < 0.01.
Additional file 11: Figure S7: Tree topology and branch labeling for tests of selection on SET genes. APIME: Apis mellifera; ACREC: Acromyrmex echinatior; LOCMI: Locusta migratoria. Supplementary Table S1 presents the abbreviation of insect species.
Abbreviations
DEGs: differentially expressed genes; E(z): Enhancer of zeste; FDR: false discovery rate; GFP: green fluorescent protein; GO: gene ontology; LCA: last common ancestor; MYND: myeloid translocation protein; PP: posterior probability; qPCR: quantitative real-time polymerase chain reaction; SAM: S-adenosyl-methionine; SET genes: SET domain-containing genes.
Supplementary Material
Additional file 1: Table S1: The arthropod genome data involved in this study.
Additional file 2: Table S2: SET genes in the 147 arthropod genomes.
Additional file 3: Table S3: Summary of SET genes in the 147 arthropod genomes.
Additional file 4: Table S4: Primers used in the study.
Additional file 5: Figure S1: Phylogenetic analysis of the SET genes in Lepidoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 6: Figure S2: Phylogenetic analysis of the SET genes in Diptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 7: Figure S3: Phylogenetic analysis of the SET genes in Hemiptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 8: Figure S4: Phylogenetic analysis of the SET genes in Hymenoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 9: Figure S5: Phylogenetic analysis of the SET genes in Coleopteran using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 10: Figure S6: Effects of RNA interference of the mRNA expression levels of SmydA-2 in locust brains. The locusts are injected with double-stranded RNAs into the second ventral segment of the abdomen. Due to the systemic RNA interference in locusts, the brain, which is spatially distant from the abdomen, is used in qPCR assays to guarantee effective expression knockdown. qPCR data are shown as the mean ± SEM (n = 6). **P < 0.01.
Additional file 11: Figure S7: Tree topology and branch labeling for tests of selection on SET genes. APIME: Apis mellifera; ACREC: Acromyrmex echinatior; LOCMI: Locusta migratoria. Supplementary Table S1 presents the abbreviation of insect species.
Acknowledgments
The computational resources were provided by the Research Network of Computational Biology and the Supercomputing Center at Beijing Institutes of Life Science, Chinese Academy of Sciences.
Availability of supporting data and materials
Snapshots of the data set supporting the conclusions of this article are available in the GigaScience database (GigaDB) [58], as well as on our website, which also has a BLAST server (http://159.226.67.242:8080).
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All animal procedures were licensed under the Institutional Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Authors' contributions
F.J., X.W., and L.K. conceived and designed the experiments. F.J. and Q.L. analyzed and interpreted the data. F.J., Q.L., Y.W., J.Z., H.W., T.S., and M.Y. performed the experiments. F.J., Q.L., and L.K. wrote the paper.
Funding
This research was supported by grants from the National Natural Science Foundation of China (Nos. 31430023, 31672353, 31472047, 31301915, and 31472051). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Elofsson A, Sonnhammer EL. A comparison of sequence and structure protein domain families as a basis for structural genomics. Bioinformatics 1999;15(6):480–500. [DOI] [PubMed] [Google Scholar]
- 2. Itoh M, Nacher JC, Kuma K et al. Evolutionary history and functional implications of protein domains and their combinations in eukaryotes. Genome Biol 2007;8(6):R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakarya O, Conaco C, Egecioglu O et al. Evolutionary expansion and specialization of the PDZ domains. Mol Biol Evol 2010;27(5):1058–69. [DOI] [PubMed] [Google Scholar]
- 4. Moore AD, Bornberg-Bauer E. The dynamics and evolutionary potential of domain loss and emergence. Mol Biol Evol 2012;29(2):787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simola DF, Graham RJ, Brady CM et al. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science 2016;351(6268):aac6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spannhoff A, Kim YK, Raynal NJ et al. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep 2011;12(3):238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simola DF, Ye C, Mutti NS et al. A chromatin link to caste identity in the carpenter ant Camponotus floridanus. Genome Res 2013;23(3):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickman MJ, Kucharski R, Maleszka R et al. Extensive histone post-translational modification in honey bees. Insect Biochem Mol Biol 2013;43(2):125–37. [DOI] [PubMed] [Google Scholar]
- 9. Jenuwein T, Laible G, Dorn R et al. SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol Life Sci 1998;54(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dillon SC, Zhang X, Trievel RC et al. The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol 2005;6(8):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boros IM. Histone modification in Drosophila. Brief Funct Genomics 2012;11(4):319–31. [DOI] [PubMed] [Google Scholar]
- 12. Herz HM, Garruss A, Shilatifard A. SET for life: biochemical activities and biological functions of SET domain-containing proteins. Trends Biochem Sci 2013;38(12):621–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jenuwein T. The epigenetic magic of histone lysine methylation. FEBS J 2006;273(14):3121–35. [DOI] [PubMed] [Google Scholar]
- 14. Misof B, Liu S, Meusemann K et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014;346(6210):763–7. [DOI] [PubMed] [Google Scholar]
- 15. Ferguson LC, Green J, Surridge A et al. Evolution of the insect yellow gene family. Mol Biol Evol 2011;28(1):257–72. [DOI] [PubMed] [Google Scholar]
- 16. Helmkampf M, Cash E, Gadau J. Evolution of the insect desaturase gene family with an emphasis on social Hymenoptera. Mol Biol Evol 2015;32(2):456–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanaka K, Diekmann Y, Hazbun A et al. Multispecies analysis of expression pattern diversification in the recently expanded insect Ly6 gene family. Mol Biol Evol 2015;32(7):1730–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urena E, Pirone L, Chafino S, Perez C et al. Evolution of SUMO function and chain formation in insects. Mol Biol Evol 2016;33(2):568–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benton R. Multigene family evolution : perspectives from insect chemoreceptors. Trends Ecol Evol 2015;30(10):590–600. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Ma H. Complex evolutionary history and diverse domain organization of SET proteins suggest divergent regulatory interactions. New Phytologist 2012;195(1):248–63. [DOI] [PubMed] [Google Scholar]
- 21. Vidal NM, Grazziotin AL, Iyer LM et al. Transcription factors, chromatin proteins and the diversification of Hemiptera. Insect Biochem Mol Biol 2016;69:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rider SD Jr, Srinivasan DG, Hilgarth RS. Chromatin-remodelling proteins of the pea aphid, Acyrthosiphon pisum (Harris). Insect Mol Biol 2010;19(suppl 2):201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonasio R, Zhang G, Ye C et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 2010;329(5995):1068–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calpena E, Palau F, Espinos C, Galindo MI. Evolutionary history of the Smyd gene family in metazoans: a framework to identify the orthologs of human Smyd genes in Drosophila and other animal species. PLoS One 2015;10(7):e0134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Fang X, Yang P et al. The locust genome provides insight into swarm formation and long-distance flight. Nat Commun 2014;5:2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson EC, Travers AA. A Drosophila Smyd4 homologue is a muscle-specific transcriptional modulator involved in development. PLoS One 2008;3(8):e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaessmann H. Origins , evolution, and phenotypic impact of new genes. Genome Res 2010;20(10):1313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Assis R, Bachtrog D. Neofunctionalization of young duplicate genes in Drosophila. Proc Natl Acad Sci U S A 2013;110(43):17409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krylov DM, Wolf YI, Rogozin IB et al. Gene loss, protein sequence divergence, gene dispensability, expression level, and interactivity are correlated in eukaryotic evolution. Genome Res 2003;13(10):2229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kemkemer C, Long M. New genes important for development. EMBO Rep 2014;15(5):460–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenny NJ, Chan KW, Nong W et al. Ancestral whole-genome duplication in the marine chelicerate horseshoe crabs. Heredity (Edinb) 2016;116(2):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaessmann H, Vinckenbosch N, Long M. RNA-based gene duplication: mechanistic and evolutionary insights. Nat Rev Genet 2009;10(1):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 2006;22(23):2971–2. [DOI] [PubMed] [Google Scholar]
- 34. Tan X, Rotllant J, Li H et al. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci U S A 2006;103(8):2713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Domazet-Loso T, Tautz D. An evolutionary analysis of orphan genes in Drosophila. Genome Res 2003;13(10):2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Innan H, Kondrashov F. The evolution of gene duplications: classifying and distinguishing between models. Nat Rev Genet 2010;11(2):97–108. [DOI] [PubMed] [Google Scholar]
- 37. Pegueroles C, Laurie S, Alba MM. Accelerated evolution after gene duplication: a time-dependent process affecting just one copy. Mol Biol Evol 2013;30(8):1830–42. [DOI] [PubMed] [Google Scholar]
- 38. Bonasio R. The role of chromatin and epigenetics in the polyphenisms of ant castes. Brief Funct Genomics 2014;13(3):235–45. [DOI] [PubMed] [Google Scholar]
- 39. Leinhart K, Brown M. SET/MYND lysine methyltransferases regulate gene transcription and protein activity. Genes (Basel) 2011;2(1):210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sims RJ 3rd, Nishioka K, Reinberg D. Histone lysine methylation: a signature for chromatin function. Trends Genet 2003;19(11):629–39. [DOI] [PubMed] [Google Scholar]
- 41. Finn RD, Mistry J, Tate J et al. The Pfam protein families database. Nucleic Acids Res 2010;38(database issue):D211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 2011;39(web server issue):W29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res 2004;14(5):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coin L, Durbin R. Improved techniques for the identification of pseudogenes. Bioinformatics 2004;20(suppl 1):i94–100. [DOI] [PubMed] [Google Scholar]
- 45. Jones P, Binns D, Chang HY et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 2014;30(9):1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Katoh K, Asimenos G, Toh H. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 2009;537:39–64. [DOI] [PubMed] [Google Scholar]
- 47. Darriba D, Taboada GL, Doallo R et al. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 2011;27(8):1164–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ronquist F, Teslenko M, van der Mark P et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 2012;61(3):539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simao FA, Waterhouse RM, Ioannidis P et al. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015;31(19):3210–2. [DOI] [PubMed] [Google Scholar]
- 50. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30(15):2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25(9):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 2010;11(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26(1):139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc 1958;53(282):457–81. [Google Scholar]
- 55. Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 2007;24(8):1586–91. [DOI] [PubMed] [Google Scholar]
- 56. Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol 1998;15(5):568–73. [DOI] [PubMed] [Google Scholar]
- 57. Yang Z, Nielsen R. Synonymous and nonsynonymous rate variation in nuclear genes of mammals. J Mol Evol 1998;46(4):409–18. [DOI] [PubMed] [Google Scholar]
- 58. Jiang F, Liu Q, Wang Y et al. Supporting data for “Comparative genomic analysis of SET-domain family reveals the origin, expansion, and putative function of the arthropod-specific SmydA genes as histone modifiers in insects.” GigaScience Database 2017; http://dx.doi.org/10.5524/100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: The arthropod genome data involved in this study.
Additional file 2: Table S2: SET genes in the 147 arthropod genomes.
Additional file 3: Table S3: Summary of SET genes in the 147 arthropod genomes.
Additional file 4: Table S4: Primers used in the study.
Additional file 5: Figure S1: Phylogenetic analysis of the SET genes in Lepidoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 6: Figure S2: Phylogenetic analysis of the SET genes in Diptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 7: Figure S3: Phylogenetic analysis of the SET genes in Hemiptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 8: Figure S4: Phylogenetic analysis of the SET genes in Hymenoptera using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch. The representative species are selected to improve clarity.
Additional file 9: Figure S5: Phylogenetic analysis of the SET genes in Coleopteran using maximum-likelihood inferences with PhyML. The SET gene families labeled with different colors are shown in the exterior circle of the phylogenetic tree. The insect species involved are represented with different colors of the external branch.
Additional file 10: Figure S6: Effects of RNA interference of the mRNA expression levels of SmydA-2 in locust brains. The locusts are injected with double-stranded RNAs into the second ventral segment of the abdomen. Due to the systemic RNA interference in locusts, the brain, which is spatially distant from the abdomen, is used in qPCR assays to guarantee effective expression knockdown. qPCR data are shown as the mean ± SEM (n = 6). **P < 0.01.
Additional file 11: Figure S7: Tree topology and branch labeling for tests of selection on SET genes. APIME: Apis mellifera; ACREC: Acromyrmex echinatior; LOCMI: Locusta migratoria. Supplementary Table S1 presents the abbreviation of insect species.