Abstract
Hydrogen sulfide (H2S) is a third known gasotransmitter. Most of the time it was knows as a poisonous gas. In last 30 years, we are seeing change in its perception. Scientists have discovered its major role in different organ systems. It is endogenously produced in various tissues and its production is influenced by many factors. In normal, physiological conditions only 20% of H2S is in its free form. The role of H2S is very wide. It acts as a signaling molecule, has influence on vascular tone, inflammatory response, scavenges reactive oxygen species, can be cytoprotective and can even reduce the extent of myocardial ischemia. Different studies have shown H2S has considerable influence in pathology of sepsis and its outcome. High free plasma levels of H2S are predictor of unfavorable outcome. Findings show that moderate free plasma levels of H2S have protective effect. Paradoxical very low free plasma levels of H2S, seen in patients with chronic heart failure, are also predictor of severity of disease and poor outcome. We presume that relationship between morbidity/mortality and concentration of H2S has a wide U-shape curve dependence. New researches with discovery of H2S agonists and antagonists could open new ways in understanding different pathologies and ability to treat them. Recent advances in the identification of H2S agonists and antagonists may help in forwarding our understanding of pathomechanisms and hence their treatment.
Key words: hydrogen sulfide, shock, sepsis
INTRODUCTION
Hydrogen sulfide (H2S) is a long-known substance. In normal conditions, it is a gas with a very characteristic odor of rotten eggs (1,2). In the 16th century it was the cause to eye inflammation and bacterial infection in sewer workers. The toxic effect of H2S is in its ability to bind to cytochrome C oxidase. As such, inhibiting the mitochondrial respiratory chain and consequently inhibiting cell energy production. A change in perspection came in 1989, when H2S was detected in mammalian brain. At the end of the century it was discovered that hydrogen sulfide has ability to modulate vascular tone, neuronal function and also has cryoprotective abilities during ischemia (3). With these discoveries, the hydrogen sulfide, beside nitric oxide (NO) and carbon monoxide (CO), became a member of so called gasotransmitters (4). In the last decade, numerous studies have investigated the role of H2S at different diseases, ranging from chronic heart failure to different types of shock. In one of this studies, Goslar and colleagues demonstrated that total plasma sulfide is a marker of shock severity in non-surgical, critically ill adult patients admitted into the medical ICU (5). Other animal and human studies confirm their findings.
ENDOGENOUS PRODUCTION AND METABOLISM OF H2S
To date we know four different pathways of H2S production (Figure 1). Two of them are pyridoxal 5’-phosphate (vitamin B6) dependent enzymatic pathway with cystathionine β-synthase (CBS) and cystathionine γ-lyse (CSE). The substrate in both cases is L-cysteine (6). CBS is the main H2S synthase in the nervous system, but is also present in kidney, liver, brain, ileum, uterus, placenta and pancreatic islets. The production of H2S is suppressed by NO and CO and enhanced via CSB by S-adenosyl methionine. CSE is widely present in different peripheral organs (kidney, liver, thoracic part of aorta, ileum, portal vein, uterus, pancreatic islets, placenta), but it was not detected in the brain. The activity of CSE is regulated by concentration of Ca2+ (7).
Figure 1.
Pathways of endogenous production of H2S (simplified scheme)

Legend
CSE: cystathionine γ-lyse; CSB: cystathionine ß-synthase; CAT: cysteine aminotransferase; 3-MST: 3-mercaptopyruvate sulfurtransferase; DAO: D-aminoacid oxidase
The third pathway consists of two enzymes, cysteine aminotransferase (CAT – aspartate aminotransferase) and 3-mercaptopyruvate sulfurtransferase (3-MST). This way of producing H2S is present mainly in mitochondria. The base ingredient is L-cysteine. The activity of both enzymes is presumed to be suppressed under oxidative stress conditions, like in mitochondria. Other way to regulate the production of H2S via CAT/3-MST is with Ca2+ ions. In the absence of Ca2+ the production of H2S is maximal and lowered in concentration depended manner (6,7).
The fourth way of H2S synthesis is synthesis from D-cysteine via D-aminoacid oxidase (DAO) and 3-MST. This is happening in kidney and cerebellum. D-cysteine is not endogenous produced molecule and can be only provided from food – exogenous pathway. Because of this, it is thought, that D-cysteine has a therapeutic potential to increase H2S production in cerebellum and kidney (6,7).
As already mentioned H2S can stop (inhibit) mitochondrial respiratory chain by inhibiting cytochrome c oxidase. This happens when H2S concentration is high. Similar happens with NO, CO and cyanide. On the other side, it is very interesting that it can also be enzymatically metabolized in mitochondria and as that can be a source for generating ATP. This can only happen in very low concentrations (less than 10 µM) of H2S. In that conditions, it also increases mitochondrial O2 consumption and ATP production. High concentrations have opposite effect (6).
PHYSIOLOGICAL ROLE OF H2S
Hydrogen sulfide is known to have a large number of pharmacological effects in various cell types and tissues. Its effect mostly depends on plasma level of H2S. Higher plasma levels have pros and cons, which will be described in following lines. Also, low levels, below than normal present in healthy individual, can indirectly predict increased morbidity and mortality. This was shown in study by Kovačić and colleagues where they have demonstrated that total plasma sulfide in patients with chronic heart failure (CHF) vary with NYHA stages.
Lowest sulfide levels were present in NYHA class IV (2.67 [2.22-4.31] µM) compared to NYHA class II (5.84 [4.33-8.00] µM), along with negative correlation with pro-BNP and pulmonary artery systolic pressure (8). High levels are often associated with cytotoxic mechanisms, which are associated with generation of free radicals, depletion of glutation, release of intracellular iron and proapoptotic actions (8,9). Based on different data we can assume, that morbidity/mortality, among other factors, depends also on plasma concentration of hydrogen sulfide. Correlation can be illustrated with wide U shape curve (Figure 2).
Figure 2.
Graph presenting allegedly correlation between total plasma sulfide and morbidity/mortality
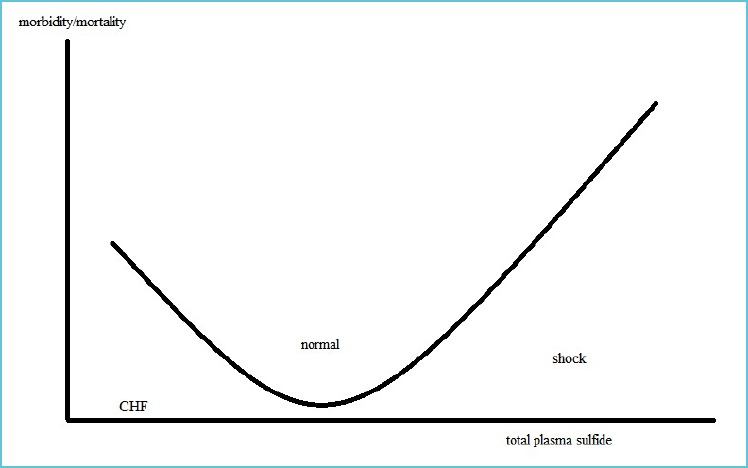
CHF: chronic heart failure
Because of the topic of the article we will focus on the cytoprotective and vascular effects (dysfunction). This is crucial to understanding the H2S effect during septic shock.
Cytoprotective effect
Evidence shows that H2S has wide cytoprotective effect in various tissues and organs. Low levels of H2S are presumed to exert antiapoptotic and antinecrotic mechanisms (9). In nervous system, it increases the levels of glutathione (a major intracellular antioxidant) and, as such, protects cells from oxidative stress. Similar effect is seen in kidney and heart (7). During heart ischemia the energy levels drop. Because there is lack of oxygen the anaerobic respiration starts and lactic acid starts to accumulate. The consequence is reduction of intracellular pH (10). Na/K-ATPase cannot function and there is accumulation of sodium in the cell. This leads to reversal of Na/Ca antiporter and intracellular and mitochondrial calcium accumulation. At the reperfusion, oxygen delivery restores, but the respiratory chain complexes are in reduced state and a large quantity of reactive oxygen species is observed. All these events lead to promotion of cell death by necrosis (11). With studies where endogenous production of H2S was enhanced or exogenous H2S donors were administered, scientists observed that H2S was successful in attenuating myocardial infarction following ischemic-reperfusion injury, promoted angiogenic responses and inhibited fibrosis during heart failure. H2S also acts as a ROS scavenger (7). A study by Elrod JW et al. from 2007 demonstrated that exogenous administration of H2S or overexpression of CSE, which enhance H2S production, reduced myocardial infarct size and preserved left ventricular function after ischemic-reperfusion injury. H2S can reduce mitochondrial respiration to induce a state known as a “suspended-animation” in which cellular respiration and oxygen demand are reduced. Consequently, the oxidative stress is reduced and mitochondrial function is preserved (3).
Vasodilatory effect
Hydrogen sulfide has also vasodilatory effect, which is concentration-dependent. H2S induces the relaxation of vascular smooth muscle via activation of KATP channels. The consequence is hyperpolarization of the cell and the effect is relaxation of the cell (4). Although the effect of H2S is not only dependent on KATP channels. Its function can also be modified by local oxygen concentration (5). High expression of KATP channels leads to vascular dysfunction (hypotension) during sepsis.
One other pathway has also been demonstrated to induce vasodilatation with H2S. H2S can also increase intracellular concentration of cyclic adenosine monophosphate (cAMP). This causes activation of protein kinase A and relaxation of smooth muscle (12).
Evidence suggest that there is a synergistic action of NO and H2S in the modulation of vascular tone (13). H2S can activate nitric oxide synthase located on endothelia (eNOS) and augment NO bioavailability (3). NO donor was shown to increase the expression of CSE and by that increase the conversion of L-cysteine to H2S (14).
HYDROGEN SULFIDE AND ITS ROLE IN SEPTIC SHOCK
By definition, since 2016, sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock should be defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone (15). During this time, many endogenous factors contribute to development of symptoms we usually see in septic patient. There is substantial production of cytokines, eicosanoids, reactive oxygen species and nitrogen species. All this overproduction leads to severe systemic inflammatory response with hypotension and vital organs hypoperfusion and the end result can be multiorgan failure. Secondary to that there is also loss of vascular responses to endogenous and exogenous catecholamines.
In 2011, Goslar and colleagues reported that during the state of shock plasma sulfide values are very elevated. Based on our results it can be concluded that total plasma sulfide is equivalent to lactate as a predictor of ICU survival. As described above we confirmed the findings that H2S is involved in regulation of vascular tone and the association between plasma H2S concentration and extent of tissue damage. Higher total plasma sulfide was inversely correlated with blood pressure and cardiac function. Patients with higher total plasma sulfide during ICU admission had higher mortality compared to patients with lower levels. According to our findings an increase in total plasma sulfide of 1µM resulted in a 5,8% higher probability of ICU mortality (5). In 2008, Zhang found that endogenous production of H2S is time-dependent during sepsis. Over production was only present in early stages and peaked 4-8 hours after CLP (coecal ligation and puncture) (16).
Correlation between plasma H2S levels and sepsis was also shown in a different study by Zhang and coworkers, where they showed significant increase in plasma H2S levels in mouse models of LPS (bacterial endotoxin lipopolysaccharide) induced sepsis along with increased CSE gene expression and activity of the enzyme (17). This increase in gene expression, enzymatic activity and consequently H2S levels was reduced with administration of dexamethasone. Dexamethasone also reduced activity of inducible nitric oxide synthase (iNOS) (18).
The importance of H2S in pathogenesis of sepsis is also reported in studies where propargylglycine (PAG), a CSE inhibitor, was administered. There was a significant decrease in plasma H2S levels with better survival rates. Furthermore, high H2S levels during sepsis may be responsible for hemodynamic collapse in septic shock (4).
Increased endogenous production of H2S starts in the acute phase of shock. During septic shock, there is also increase of NO levels because of upregulation of iNOS. This higher NO levels can induce CSE expression, which leads to higher H2S levels – a positive feedback loop (4). Further studies are needed to elucidate the role of H2S synthesis, where it may mediate inflammation or an anti-inflammatory mechanism.
Contrary to the data above there are some reports which are suggesting that higher (probably moderately high?) H2S levels act as cytoprotective and may prevent multiorgan failure (4). It is known that H2S acts as a ROS scavenger in vitro. The reduction of oxidative stress can be result of inactivation of ROS producing enzymes or by direct “rummaging” of ROS (4, 19).
H2S can also act as an endogenous modulator of inflammation. As already described during sepsis there is an increase in H2S production. Inhibition of H2S production decreased systemic inflammatory response and reduced multi-organ failure in sepsis. Suggesting that H2S has proinflammatory properties (16). Leukocytes and their interaction with endothelium have a main role in pathogenesis of sepsis. Studies utilizing H2S donors (sodium hydrogen sulfide and sodium sulfide) showed reduced infiltration and adherence of leukocytes to the endothelium through the activation of KATP channels. H2S donors also reduced edema formation. Suppression of endogenous H2S production have the opposite effect. Suppression can be achieved with NSAID, which reduce expression of CSE (14).
MEASUREMENT OF ENDOGENOUS H2S AND ITS DIFFICULTIES
There are several methods to measure sulfide concentrations in live biological systems – head space gas analysis, derivatization methods (pentafluorobenzyl bromide or N,N-dimethyl-p-phenylenediamine sulfate), spectrophotometry, direct measurement in solution with a silver sulfide or polarographic sensor and most recently developed colorimetric system using a silver-embedded Nafion/polyvinylpyrrolidone (PVP) membrane (20, 21). Different methods give very variable results and there is no consensus which method is the most accurate and best represent the true value of sulfide in living system. Direct and precise measurement in living cells and fluids still remains a challenge.
Sulfide is naturally subjected to oxidation, it has lipophilic property, is slightly soluble in water and acts as a weak acid. Beside free plasma H2S there are also other forms of sulfide in the body – mostly sulfane sulfur (elemental sulfur, thiosulfate, persulfide, thiosulfonate, polysulfides, polythionates) and acid-labile sulfur. The latter is mostly present in iron-sulfur clusters in proteins. The level of H2S is also influenced by pH and temperature – at physiological pH and 37°C, around 20% of sulfide is present as H2S, and if the temperature drops to 25°C 40% of sulfide is present as H2S. Main problem today is how to measure the gaseous form of H2S (5, 20, 21). Measurement of plasma H2S is still a challenge and new methods have to be developed.
Most widely used method is indirect measurement of H2S in plasma. In this case plasma is mixed with distilled water, trichloroacetic acid, zinc acetate, N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCI and FeCI3 in 1.2 M HCI. This results in a blue color reaction – methylene blue formation. The supernatant is then spectrophotometrically measured. The calibration curve of NaHS (3.125-100 µM) or Na2S (0.699-139.86 µM) are used to calculate the sulfide concentration (5, 16, 21).
A recently developed method enables selective detection of H2S in living cells using a silver embedded Nafion/polyvinylpyrrolidone (PVP) membrane and a colorimetric detection method. Silver and H2S form Ag2S which is brown in color. Then absorbance at 310 nm is measured (22).
With gas chromatography, we are able to detect sulfide at physiological levels, but it can falsely rise sulfide levels by liberating loosely-bound sulfide because of irreversible sulfide binding or shifts in phase transition equilibria (21).
Hartman and Dcona have described a method for direct measuring of H2S. It is based on conversion of profluorescent 8-azidopyrene-1,3,6-trisulfonic acid (N3-PTS) to 8-aminopyrene-1,3,6-trisulfonic acid (APTS) by H2S. Then the fluorescence at 435 nm is measured (23).
CONCLUSION
The knowledge about role of hydrogen sulfide in sepsis is still fragmental. High endogenous (over)production of H2S during sepsis has multiple implications. It contributes to smooth muscle cell dysfunction, which leads to hypotension. This causes circulatory failure, myocardial dysfunction, organ injury and at the end multi organ failure. Contrary to all negative effect, higher H2S production also has some beneficial effects. It improves endothelial function, prevents adhesion of leukocytes and thrombocytes, stimulates the host defense system. H2S acts as reactive oxygen species scavenger and reduces oxidative stress, preserves mitochondrial function consequently leading to less apoptotic cells. H2S has U-shaped effects curve; in moderate elevation of H2S production during (early stages of) sepsis can be beneficial in term of host defense and other protective effect; on the other hand, the extensive overproduction has harmful effects.
All physiological stimulations which influence H2S production and maintain a certain level of substance in the tissue are not known. Next step can be discovering and synthesis of H2S agonists and antagonists, which will influence the pathogenesis of several diseases where the role of H2S apparently has been implicated.
REFERENCES
- 1.Szabo C. Hydoregen sulfide and its therapeutic potential. Nac Rev Drug Discov. 2007; 6(11): 917-935. [DOI] [PubMed] [Google Scholar]
- 2.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Toxicol. 1992; 32: 109-134. [DOI] [PubMed] [Google Scholar]
- 3.Polhemus DJ, Calvert JW, Butler J, Lefler DJ. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica (Cairo). 2014; 2014: 768607. Published online 2014 Jun 22. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coletta C, Szabo C: Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Current Vascular Pharmacology. 2013; 11(2): 208-221. [PubMed] [Google Scholar]
- 5.Goslar T, Marš T, Podbregar M. Total plasma sulfide as a marker of shock severity in nonsurgical adult patients. Shock. 2011; 36(4): 350-355. doi: 10.1097/SHK. 0b013e31822bcfd0. [DOI] [PubMed] [Google Scholar]
- 6.Bełtowski J. Hydrogen sulfide in pharmacology and medicine - An update. Pharmacol Rep. 2015; 67(3): 647-658. doi: 10.1016/j.pharep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxidants & Redox signaling. 2015; 22(5): 362-376. doi: 10.1089/ars.2014.5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovačić D, Glavnik N, Marinšek M. Total plasma sulfide in congestive heart failure. Journal of Cardiac Failure. 2012; 18(7): 541-548. doi: 10.1016/j.cardfail.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008; 295(2): 801-806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM. Myocardial ischemia – reperfusion injury: a neglected therapeutic target. Journal of clinical investigation. 2013; 123(1): 92-100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salloum FN. Hydrogen sulfide and cardioprotection – Mechanistic insights and clinical translatability. Pharmacology & Therapeutics. 2015; 152: 11-17. doi: 10.1016/j. pharmthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000; 267:129-133. [DOI] [PubMed] [Google Scholar]
- 13.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium dependent vasorelaxation. Proc Natl Acad Sci USA. 2012; 109(23): 9161-9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006; 20(12): 2118-2120. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315(8): 801-810. doi:10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Moochhala SM, Bhatia M. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. J Immunol. 2008; 181(6): 4320-4331. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006; 290(6): 1193-1201. doi: 10.1152/ajplung.00489.2005 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Whiteman M, Moore PK. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulfide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med. 2009; 13(8B):2684-2692. doi: 10.HH/j.1582-4934.2008.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA. 2011; 108(33):13829-13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulfide species in blood. Br J Pharmacol. 2010; 160(4):941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X, Kolluru GK, Yuan S, Kevil CG. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015; 554:31-45. doi: 10.1016/bs.mie.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn YJ, Lee YJ, Lee J, Lee D, Park HK, Lee GJ. Colorimetric detection of endogenous hydrogen sulfide production in living cells utilizing silver-embedded polymer membrane. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2017; 177: 118-124. doi: 10.1016/j. saa.2017.01.040 [DOI] [PubMed] [Google Scholar]
- 23.Hartman MCT, Dcona M. A new, highly water-soluble, fluorescent turn-on chemodosimeter for direct measurement of hydrogen sulfide in biological fluids. Analyst. 2012; 137(21): 4910-4912. doi: 10.1039/c2an35870k. [DOI] [PMC free article] [PubMed] [Google Scholar]


