Abstract
Atrial fibrillation (AF) in the elderly occurs as a consequence of cardiovascular aging and an age related increase of comorbidity. Several predisposing factors for AF have been identified for the overall AF population. Most of them, cardiovascular disease in particular, play a role in younger and older patients. The longer time period during which these risk factors can cause structural changes that ultimately lead to AF may, at least in part, explain the association between age and AF. In addition, less well defined age-related changes in cellular electrophysiologic properties and structure predispose to AF in the elderly.
Keywords: Atrial fibrillation, Atrial fibrosis, Mechanisms, Risk factors, Stroke, The elderly
1. Introduction
Atrial fibrillation (AF) is the most frequent clinically significant arrhythmia and its incidence is continuously rising.[1] This increase is at least in part due to the aging of the population. Studies have also demonstrated an increase in AF incidence after age adjustment, which is probably a reflection of comorbidities and cardiovascular risk factors, in addition to other factors such as lifestyle changes.[1],[2] It is expected that in the European Union the number of subjects age ≥ 55 years with AF will more than double between 2010 and 2060, from 8.8 to 17.9 million.[3] Likewise, it is expected that in the US more than 5.6 million people will have AF in 2050, and half of them will be older than 80 years.[4] The anticipated increase in AF may also be related to an earlier and more frequent diagnosis across all age groups due to better awareness of the arrhythmia and its complications and more frequent ECG monitoring over longer periods of time. In addition, improved treatment and subsequently improved survival of patients with cardiovascular disease may lead to an increase in individuals with AF compared to the past.
The association of AF with increasing age is well recognized and has been shown by several epidemiological studies.[5]–[7] The risk of developing AF doubles with each progressive decade of gaining and exceeds 20% by age 80 years.[8] Although important and well established, age is not the only risk factor associated with AF. The arrhythmia shows wide heterogeneity regarding comorbidities and age.[9] While it is observed frequently in older patients, it is also observed in young people and those without any comorbidity.
AF etiology in the elderly likely differs from younger patients.[10] The EORP-AF general pilot registry was designed to gain information regarding AF management in Europe. Based on these registry data, Fumagalli, et al.,[11] analyzed differences in presentation, co-morbidities and treatment of AF according to age. One third of patients in this registry, more than 1000 were ≥ 75 years of age. Older patients more often had persistent or permanent AF compared with younger patients. They also had a higher prevalence of comorbidities, including coronary artery disease, chronic heart failure including a substantial proportion of patients with heart failure with preserved ejection fraction, chronic kidney disease, chronic obstructive pulmonary disease (COPD), valvular heart disease and hypertension. Previous hemorrhagic events and transient ischemic attack (TIA) were reported more often in older patients. All these comorbidities led to higher CHA2DS2-VASc and HAS-BLED scores. With regard to symptoms, older patients reported palpitations less frequently, but reported dyspnea more often. They appeared to do better with rate rather than rhythm control,[12] which was also the preferred management in older patients.[11]
This article summarizes risk factors for AF and associated pathophysiological changes, both in the general AF population, and with respect to the elderly patient.
2. Risk factors for development of AF
For the overall population, several risk factors for AF development have been identified. They all contribute to AF, each factor individually, and in combination with each other.[13] Risk factors for incident AF (after age-adjustment) in the Framingham cohort were cigarette smoking, diabetes mellitus, hypertension, and prevalent CAD. Combined, they explained 44% of the burden of AF in men and 58% in women.[14] The absolute and attributable risks of AF in relation to optimal and borderline risk factors were studied in The Atherosclerosis Risk in Communities (ARIC) Study.[15] Overall, 56.5% of AF cases could be explained by one borderline or elevated risk factor of which elevated blood pressure was the most important contributor.[16] Thus, in a high number of cases, AF is associated with acquired risk factors that should be amenable to preventive measures.
While some risk factors are well established, others are rather new. Because different risk factors may cause the same electrical and structural changes that predispose for AF and several factors are frequently present in an individual patient, quantification of a given factor's specific impact on AF development is a futile effort.
Across all age groups, the incidence of AF is higher in men than in women.[5] It is unknown whether this difference is due to sex itself or just a phenomenon of under-diagnosed AF in women and/or over-diagnosed AF in men due to different symptom intensity or/and differing medical attention. Hypertension is the number one risk factor globally.[2] The higher the blood pressure, the greater is the risk for incident AF.[13],[17]
Like hypertension, heart failure is associated with an increase in atrial pressure and/or volume overload and diastolic ventricular dysfunction, which may lead to atrial dilatation and fibrosis, the electrical and structural changes providing the basis for AF development. While hypertension is an important and well established risk factor for AF, heart failure is less well defined and has not been studied as extensively. Valvular heart disease, particularly left sided disease, leads to atrial pressure and volume overload and is associated with AF development. Both passive atrial stretch and an increase in atrial pressure during atrial contraction have been found to stimulate the release of atrial natriuretic factor. The level of this neurohormonal activation was found to be a predictor of paroxysmal AF. Diabetes mellitus and hyperthyroidism have also been recognized as independent risk factors for AF. Coronary artery disease and chronic kidney disease are risk markers for AF as well.[14],[18] High body mass index (BMI) which is often associated with sleep apnea ranks sixth in the global list.[2] High BMI is also associated with increased left atrial volume. COPD appears to be associated with progression from paroxysmal to persistent and permanent AF.
Less well-established risk factors are tall stature, increased epicardial fat, high birth weight, alcohol consumption, smoking and high-level endurance training. Obesity has recently received increasing attention as a risk factor for AF based on epidemiological, mechanistic and clinical evidence.[19] It carries a strong link to metabolically active atrial epicardial fat tissue.[20]
Genetic factors, both monogenic and polygenic, have recently been identified as risk factors for AF. A positive family history of AF nearly doubles the risk of developing AF.[21] Early-onset AF in particular appears to have a strong heritable component that is independent of concomitant cardiovascular conditions.[22],[23] Up to one third of AF patients carry common genetic variants that predispose to AF.
Inflammation has been suggested as pathophysiological mechanism in AF development and perpetuation.[24] The causal role of inflammation in structural atrial damage has been reinforced by experimental studies.[23] Inflammation has been studied predominantly in post-operative AF, and its role is less well established for other forms of AF.
Left atrial (LA) enlargement has also been described as risk factor for AF.[18] In a study by Tsang, et al.,[25] LA volume was confirmed to be independent of both clinical risk factors and diastolic function profile for the prediction of AF. Whether LA enlargement is the hen or the egg with regard to AF is not known, only patients with new onset of AF were included in the study. Still it is possible that patients already had asymptomatic episodes of AF over some time that led to left atrial enlargement.
There is also an association between sick sinus syndrome (SSS) and AF. Like AF, SSS is diagnosed more often in men, increases with age and is associated with several cardiovascular risk factors like hypertension, diabetes and higher body mass index.[26] In addition, SSS can have a genetic background. It can manifest as bradycardia with or without tachycardia-bradycardia syndrome.[27] Both forms of SSS, with and without tachycardia-bradycardia syndrome, are correlated with severe structural and electrical remodeling, thereby predisposing for AF.
Recently, advanced interatrial block, first described by Bayes de Luna., et al.,[28] has been shown in the ARIC study to be associated with an increased risk for AF after adjustment for socio-demographics, cardiovascular risk factors, and potential confounders.[29]
Cardiovascular comorbidities and other risk factors as well as AF itself induce a slow but progressive process of structural remodeling in the atria.[22] Activation of fibroblasts, enhanced connective tissue deposition, and fibrosis are the hallmarks of this process. In addition, atrial fatty infiltrations, inflammatory infiltrates, myocyte hypertrophy, necrosis and amyloidosis are found in patients with concomitant conditions predisposing to AF. In many patients, the structural remodeling process occurs before the onset of AF.[22] In addition to structural changes, AF induces electrical and autonomic tone remodeling. The relative contribution of underlying primary conditions versus AF itself to the clinical progression of AF is presently unclear.[30]
In the German Atrial Fibrillation Network (AFNET) registry, the likelihood that patients had persistent or permanent AF increased with the number of risk factors in a given patient.[31] The “HATCH” score, including the risk factors heart failure, age, previous TIA or stroke, COPD and hypertension, was proposed to identify patients with AF progression.[32] This emphasizes the fact that several risk factors typically coexist and act in combination.
The impact of each risk factor may vary by age. Genetic factors, obesity and endurance sports likely play a larger role in younger patients, while other factors are more prevalent and relevant in older patients. Their contribution to AF in an individual patient, especially in combination with the risk factor “age” which discussed below, is unknown, but likely depends on disease severity and consequent treatment.
3. “Age” as a risk factor for AF
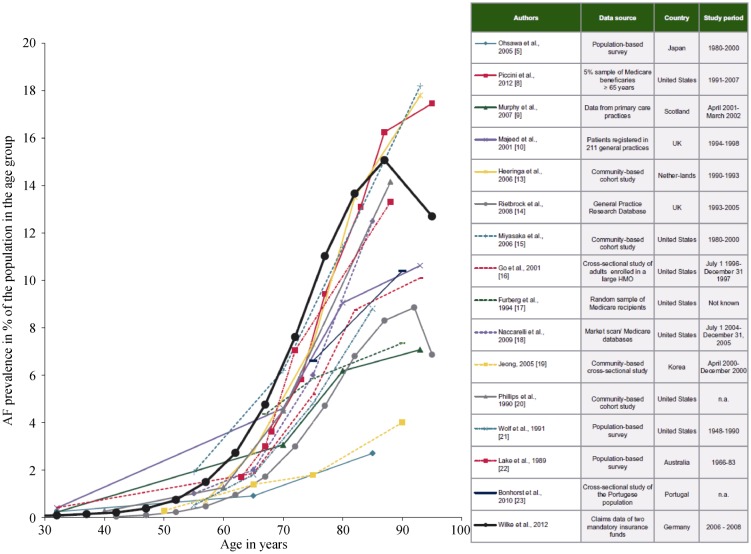
Aging involves a relentless and systematic process of degeneration in living organisms leading to attenuation of most biochemical and physiological functions.[33] “Age” as risk factor remains to be defined and the rate at which aging occurs is different between individuals. Most studies define “elderly” as those patients older than 65 years.[18] At the same time, it is generally accepted that calendar age and biological age are not synonymous. At least from an epidemiological standpoint, there is a marked increase in AF between 60 and 65 years (Figure 1).[33]
Figure 1. Depiction of the AF prevalence in different studies.
There is a steep increase in AF prevalence across all studies between 60 and 65 years of age (red line). Adjusted from citation [31] with permission. AF: atrial fibrillation.
It is well known that aging increases the propensity for occurrence of AF.[34],[35] The pathophysiologic mechanisms, though, by which aging increases the likelihood for AF development remain poorly understood.[36] A longer time period during which the atrial myocardium is exposed to external stressors, i.e., risk factors, likely plays a role in the association of age and AF as well. Most elderly patients present with one or more comorbidities. It appears very difficult if not impossible to distinguish the impact of these comorbidities from true “age” related factors.
There are only limited experimental and even less clinical data that relate to “age” as a predisposing factor for AF despite the overwhelming evidence of the close association between increasing age and AF. While AF generally is more prevalent in men than in women in the AFNET registry, women outnumber men in the age group above 80years,[31] likely because they live longer than men. Since men typically acquire more cardiovascular risk factors, it could be hypothesized, that theses comorbidities play a larger role for AF than the risk factor “age”.
The atrial myocardium undergoes electrical and structural remodeling with age, both of which may play an important role in the initiation and/or perpetuation of atrial arrhythmias.[34],[37] Changes in atrial tissue structure appear to be of major importance in providing a substrate for AF.
Structurally aged atrial bundles are characterized by enhancement of the fibrous tissue that is interspersed between myocytes.[34] Cardiac fibrosis is characterized by excessive accumulation of fibrillary collagen in the extracellular space. It may result from age-dependent cardiomyocyte loss (replacement fibrosis),[38] or it may be an interstitial response to chronic diseases such as hypertension, myocarditis, and congestive heart failure (reactive fibrosis).[34] Fibrosis is ubiquitous in the atria of the aging heart and the hallmark of the structural AF substrate.[30] Both, increased non-uniform atrial interstitial fibrosis[39] and atrial conduction slowing have been shown in patients with AF.[40]
Age-related electrical changes due to ionic current alterations include modifications in the cellular action potential shape and duration as well as an enhanced dispersion of cardiac repolarization.[34] This has been shown in canine models and in human tissue. Anyukovsky, et al.,[41] showed that conduction velocity for premature beats was reduced in older canine hearts and occurred during a wider time window. Again, they found a twofold increase in the amount of fibrous tissue. In a rat model, Hayashi, et al.,[42] showed that old rats had significantly longer interatrial conduction time and P wave durations compared with young rats. AF was inducible in old rats, but not young rats. As reported by others, histology revealed a significant increase in interstitial atrial fibrosis, atrial cell size, and heart weight.
Arterial stiffness increases with age and predicts coronary artery disease, stroke and mortality.[43] Fumagalli, et al.,[44] reported a significant association between arterial stiffness (measured as cardio-ancle vascular index on both upper and lower extremities) and age in a small group of patients. Of note, this index was not affected by hypertension, coronary artery disease, cerebrovascular disease, chronic renal failure and heart failure, all known to be risk factors for AF. Arterial stiffness was also associated with increased left atrial diameters, but the exact interaction between arterial stiffness, left atrial and left ventricular properties (i.e., stiffness) could not be fully explained by their observations. The majority of AF cases may be the consequence of risk factors causing increased arterial stiffness, diastolic dysfunction, and atrial volume overload.[45],[46] Recently, the association between age-related arterial stiffness and persistence or recurrence of AF has been studied.[46] For each one-unit increase in cardio-ancle vascular index, the risk of finding AF at the control visit was 2.31 times higher. When using pulse pressure as marker of arterial stiffness, participants of the Framingham Heart Study were found to have an increased risk for AF as well.[47]
Diastolic left ventricular dysfunction with abnormal left ventricular relaxation is very common in the elderly and has been regarded as part of the normal aging process.[48] Although this form of left ventricular relaxation abnormality is considered the mildest one, patients with this form of diastolic dysfunction have a greater risk for AF independent of the effects of age. Pathophysiologically, LV relaxation abnormalities may lead to the development of higher atrial pressures during atrial diastole by reducing passive LA emptying. Over time, LA and pulmonary veins could dilate and potentiate electrical and structural remodeling thereby increasing vulnerability to AF. In a study by Tsang, et al.,[25] the diastolic dysfunction profile was incremental to clinical risk factors and left atrial volume. Furthermore, the gradient of risk appeared to be related to the severity of diastolic dysfunction.
4. AF progression
AF is typically seen as a progressive arrhythmia, where structural and electrophysiological changes lead to persistent and permanent AF over time. This progression, though, differs widely between patients. Progression of AF from paroxysmal to persistent is faster in older patients and those with underlying heart disease.[30],[49] Saksena, et al.,[50] analyzed data of patients (mean age of 70 ± 10 years) with paroxysmal AF and a dual chamber pacemaker. AF progression was observed in 24% of patients and associated with a progressive increase in AT (atrial tachyarrhythmia)/AF burden. This increase was highly correlated with the presence of structural heart disease, which was interpreted as a relation between AF progression and substrate rather than trigger-based progression. Still it remains unclear, why paroxysmal AF may remain self-terminating for decades in some patients, but progresses to persistent AF within weeks in others.[50]
Footnotes
This article is part of a Special Issue “Atrial fibrillation in the elderly”.
Guest Editors: Manuel Martínez-Sellés & Antoni Bayés de Luna
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Ohlmeier C, Mikolajczyk R, Haverkamp W, Garbe E. Incidence, prevalence, and antithrombotic management of atrial fibrillation in elderly Germans. Europace. 2013;15:1436–1444. doi: 10.1093/europace/eut048. [DOI] [PubMed] [Google Scholar]
- 6.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 8.Magnani JW, Wang N, Benjamin EJ, et al. Health, aging, and body composition study.atrial fibrillation and declining physical performance in older adults: the health, aging, and body composition study. Circ Arrhythm Electrophysiol. 2016;9:e003525. doi: 10.1161/CIRCEP.115.003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boriani G, Diemberger I, Martignani C, et al. The epidemiological burden of atrial fibrillation: a challenge for clinicians and health care systems. Eur Heart J. 2006;27:893–894. doi: 10.1093/eurheartj/ehi651. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Kirkwood G, Dibb K, Garratt CJ. Comparison of atrial fibrillation in the young versus that in the elderly: a review. Cardiol Res Pract. 2013;2013:976976. doi: 10.1155/2013/976976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fumagalli S, Said SAM, Laroche C, et al. Age-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe. J Am Coll Cardiol EP. 2015;1:326–334. doi: 10.1016/j.jacep.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Shariff N, Desai RV, Patel K, et al. Rate-control versus rhythm-control strategies and outcomes in septuagenarians with atrial fibrillation. Am J Med. 2013;126:887–893. doi: 10.1016/j.amjmed.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options--a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2012;14:8–27. doi: 10.1093/europace/eur241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 15.Norby FL, Soliman EZ, Chen LY, et al. Trajectories of cardiovascular risk factors and incidence of atrial fibrillation over a 25-year follow-up: the ARIC Study (Atherosclerosis Risk in Communities) Circulation. 2016;134:599–610. doi: 10.1161/CIRCULATIONAHA.115.020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conen D, Tedrow UB, Koplan BA, et al. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 19.Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2016;37:1565–1572. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]
- 20.Haemers P, Hamdi H, Guedj K, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2017;38:53–61. doi: 10.1093/eurheartj/ehv625. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Parise H, D'Agostino RB, Sr, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P, Breithardt G, Aliot E, et al. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15:1540–1556. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 24.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor for the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 26.Jensen PN, Gronroos NN, Chen LY, et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–538. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung H, Uhm JS, Sung JH, et al. The type of sinus node dysfunction might predict the severity of atrial remodeling and clinical outcome after catheter ablation of atrial fibrillation. Int J Cardiol. 2014;172:487–489. doi: 10.1016/j.ijcard.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 28.Bayés de Luna A, Cladellas M, Oter R, et al. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–1118. doi: 10.1093/oxfordjournals.eurheartj.a062407. [DOI] [PubMed] [Google Scholar]
- 29.O'Neal WT, Zhang ZM, Loehr LR, et al. Electrocardiographic advanced interatrial block and atrial fibrillation risk in the general population. Am J Cardiol. 2016;117:1755–1759. doi: 10.1016/j.amjcard.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattel S, Guasch E, Savelieva I, et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J. 2014;35:1448–1456. doi: 10.1093/eurheartj/ehu028. [DOI] [PubMed] [Google Scholar]
- 31.Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 33.Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 34.Pandit SV, Jalife J. Aging and atrial fibrillation research: where we are and where we should go. Heart Rhythm. 2007;4:186–187. doi: 10.1016/j.hrthm.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61. doi: 10.1111/j.1076-7460.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 36.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 37.Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 38.Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study. Heart Rhythm. 2007;4:175–185. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 40.Papageorgiou P, Monahan K, Boyle NG, et al. Site-dependent intra-atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation. 1996;94:384–389. doi: 10.1161/01.cir.94.3.384. [DOI] [PubMed] [Google Scholar]
- 41.Anyukhovsky EP, Sosunov EA, Plotnikov A, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi H, Wang C, Miyauchi Y, et al. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13:801–808. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 43.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Fumagalli S, Gabbai D, Nreu B, et al. Age, left atrial dimension and arterial stiffness after external cardioversion of atrial fibrillation. A vascular component in arrhythmia maintenance? Results from a preliminary study. Aging Clin Exp Res. 2014;26:327–330. doi: 10.1007/s40520-013-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman JE, Gersh BJ. Atrial fibrillation and stroke prevention in aging patients: what's good can be even better. Circulation. 2014;130:129–131. doi: 10.1161/CIRCULATIONAHA.114.010873. [DOI] [PubMed] [Google Scholar]
- 46.Fumagalli S, Giannini I, Pupo S, et al. Atrial fibrillation after electrical cardioversion in elderly patients: a role for arterial stiffness? Results from a preliminary study. Aging Clin Exp Res. 2016;28:1273–1277. doi: 10.1007/s40520-016-0620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell GF, Vasan RS, Keyes MJ, et al. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 48.Tsang TS, Barnes ME, Gersh BJ, et al. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 50.Saksena S, Hettrick DA, Koehler JL, et al. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884–892. doi: 10.1016/j.ahj.2007.06.045. [DOI] [PubMed] [Google Scholar]