Abstract
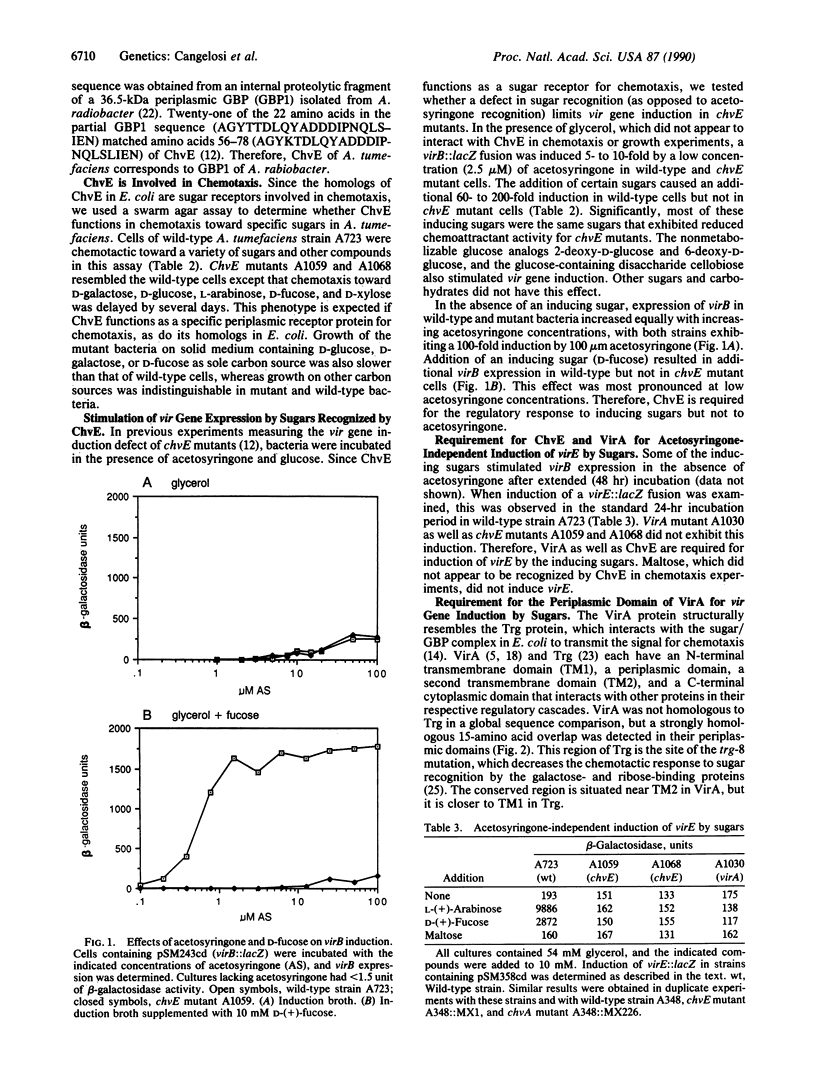
Phenolic plant metabolites such as acetosyringone induce transcription of the virulence (vir) genes of Agrobacterium tumefaciens through the transmembrane VirA protein. We report here that certain sugars induce the vir genes synergistically with phenolic inducers by way of a distinct regulatory pathway that includes VirA and a chromosomally encoded virulence protein, ChvE. Sequence comparison showed that ChvE is a periplasmic galactose-binding protein corresponding to the GBP1 protein isolated from Agrobacterium radiobacter. Like homologous sugar-binding proteins in Escherichia coli, ChvE was required for chemotaxis toward galactose and several other sugars. These sugars strongly induced vir gene expression in wild-type cells when acetosyringone was absent or present in low concentrations. Mutations in chvE abolished vir gene induction by sugars and resulted in a limited host range for infection but did not affect vir gene induction by acetosyringone. A mutant lacking the periplasmic domain of VirA exhibited the same regulatory phenotype and limited host range as chvE mutants. These data show that the vir genes are regulated by two separate classes of plant-derived inducers by way of distinct regulatory pathways that can be separated by mutation. Induction by sugars is essential for infection of some but not all plant hosts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollinger J., Park C., Harayama S., Hazelbauer G. L. Structure of the Trg protein: Homologies with and differences from other sensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3287–3291. doi: 10.1073/pnas.81.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Tait R. C., Kado C. I. Regulation of Ti plasmid virulence genes by a chromosomal locus of Agrobacterium tumefaciens. J Bacteriol. 1985 Nov;164(2):774–781. doi: 10.1128/jb.164.2.774-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish A., Greenwood J. A., Jones C. W. Binding-protein-dependent glucose transport by Agrobacterium radiobacter grown in glucose-limited continuous culture. J Gen Microbiol. 1988 Dec;134(12):3099–3110. doi: 10.1099/00221287-134-12-3099. [DOI] [PubMed] [Google Scholar]
- Cornish A., Greenwood J. A., Jones C. W. Binding-protein-dependent sugar transport by Agrobacterium radiobacter and A. tumefaciens grown in continuous culture. J Gen Microbiol. 1989 Nov;135(11):3001–3013. doi: 10.1099/00221287-135-11-3001. [DOI] [PubMed] [Google Scholar]
- Das A., Pazour G. J. Delineation of the regulatory region sequences of Agrobacterium tumefaciens virB operon. Nucleic Acids Res. 1989 Jun 26;17(12):4541–4550. doi: 10.1093/nar/17.12.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Hogg R. W. L-Arabinose- and D-galactose-binding proteins from Escherichia coli. Methods Enzymol. 1982;90(Pt E):463–467. doi: 10.1016/s0076-6879(82)90172-0. [DOI] [PubMed] [Google Scholar]
- Huang Y., Morel P., Powell B., Kado C. I. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. doi: 10.1128/jb.172.2.1142-1144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk T. J., Bourret R. B., Sedee N. J., Schilperoort R. A., Hooykaas P. J. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J. 1989 Jul;8(7):1919–1925. doi: 10.1002/j.1460-2075.1989.tb03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. W., Morris R. O. Identification of an Agrobacterium tumefaciens virulence gene inducer from the pinaceous gymnosperm Pseudotsuga menziesii. Proc Natl Acad Sci U S A. 1990 May;87(9):3614–3618. doi: 10.1073/pnas.87.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Hazelbauer G. L. Mutations specifically affecting ligand interaction of the Trg chemosensory transducer. J Bacteriol. 1986 Jul;167(1):101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Das A. virG, an Agrobacterium tumefaciens transcriptional activator, initiates translation at a UUG codon and is a sequence-specific DNA-binding protein. J Bacteriol. 1990 Mar;172(3):1241–1249. doi: 10.1128/jb.172.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Das A. virG, an Agrobacterium tumefaciens transcriptional activator, initiates translation at a UUG codon and is a sequence-specific DNA-binding protein. J Bacteriol. 1990 Mar;172(3):1241–1249. doi: 10.1128/jb.172.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Veluthambi K., Krishnan M., Gould J. H., Smith R. H., Gelvin S. B. Opines stimulate induction of the vir genes of the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1989 Jul;171(7):3696–3703. doi: 10.1128/jb.171.7.3696-3703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Ebert P. R., Stachel S. E., Gordon M. P., Nester E. W. A gene essential for Agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8278–8282. doi: 10.1073/pnas.83.21.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Nester E. W. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988 Sep;170(9):4047–4054. doi: 10.1128/jb.170.9.4047-4054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Ward J. E., Nester E. W. A protein required for transcriptional regulation of Agrobacterium virulence genes spans the cytoplasmic membrane. J Bacteriol. 1989 Mar;171(3):1616–1622. doi: 10.1128/jb.171.3.1616-1622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]


