Abstract
Context:
Obesity is associated with poor bone mineralization and quality. Fibroblast growth factor 23 (FGF23) plays an important role in skeletal physiology.
Objective:
To test hypothesis that greater adiposity results in higher FGF23 levels among individuals with normal estimated glomerular filtration rate (eGFR).
Design, Setting, Participants:
Cross-sectional analyses among participants with eGFR ≥60 mL/min/1.73m2. We assessed the association between crude [body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR); n = 5610] and refined (abdominal adipose tissue area by computed tomography; n = 1313) measures of adiposity and FGF23 using multivariable linear regression.
Main Outcome Measure:
Serum FGF23.
Results:
FGF23 was higher across BMI categories (BMI <25: 37.7; BMI 25 to 29.99: 38.7; BMI 30 to 39.99: 39.8; BMI ≥40: 40.9 pg/mL, unadjusted P trend < 0.0001). The association between BMI and FGF23 was independent of known confounders of FGF23 (adjusted β = +7.2% higher FGF23 per 10 kg/m2; P < 0.0001). Similar results were observed using WC and WHR. Abdominal adipose tissue area was also independently associated with higher FGF23 (P < 0.01). Notably, the positive associations between FGF23 and adiposity were observed despite the fact that eGFR did not decline and serum phosphate levels did not increase with adiposity.
Conclusion:
In a large cohort with normal kidney function, adiposity was associated with higher FGF23 levels independent of known confounders, including eGFR and phosphate. Further studies are needed to evaluate the causes of higher FGF23 in settings of greater adiposity and the potential impact on skeletal health.
In this cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis, higher adiposity was associated with higher fibroblast growth factor 23 levels, independent of phosphate and eGFR.
There is evidence that greater adiposity may be a risk factor for poor skeletal health. This supposition is in contrast to prior evidence that obesity may impart protective skeletal effects as a result of factors such as increased mechanical loading (1–3). In this regard, recent data have shown that obese individuals, compared with normal weight individuals, may be at increased risk for low-trauma extremity fractures, inferior bone quality, and lower bone formation (4–9). The reason for these findings is not well understood and is likely multifactorial; one key element likely involves secondary hyperparathyroidism and subsequent abnormal bone mineralization that stems from a higher prevalence of vitamin D deficiency (10, 11).
Several humoral factors that may link adipose tissue to deleterious impacts on the skeleton have been noted, including proinflammatory cytokines and adipocytokines (12, 13). It is notable that adipocytes and osteoblasts share a common precursor, the pluripotent mesenchymal stem cell, thereby providing a cellular link between adiposity and skeletal homeostasis (14). Fibroblast growth factor 23 (FGF23) is a key regulator of phosphate and vitamin D metabolism and is a known predictor of metabolic bone disease in chronic kidney disease (CKD) (15–17). Although there is no direct evidence that FGF23 is produced in adipose tissue, other members of the FGF family (factors 19 and 21) are expressed by adipocytes and may have roles in energy regulation (18, 19). In addition, higher parathyroid hormone (PTH) levels, which are commonly observed in obesity, may promote higher FGF23 levels via direct PTH action and/or indirectly via upregulation of 1,25-dihydroxyvitamin D (1,25OH2D) (20, 21). The actions of FGF23 include inhibition of 1,25OH2D synthesis and increase of phosphate excretion; therefore, excess FGF23 may interfere with bone mineralization by decreasing the availability of calcium and phosphate substrates.
In non-CKD populations, higher FGF23 levels have been associated with higher fracture risk (22), and two relatively small studies have observed an independent association between adiposity and higher FGF23 levels in non-CKD individuals (23, 24). These studies have raised the question of whether adipose tissue may promote an increase in FGF23 levels. A major limitation of the aforementioned studies was the incomplete assessment of notable regulators of bone and mineral metabolism that may have affected FGF23 and/or skeletal health, including PTH, vitamin D, and serum and urinary calcium and phosphate.
In this context, we hypothesized that FGF23 may be oversecreted in obesity and states of greater adiposity, even in the setting of normal kidney function. We tested this hypothesis by performing a cross-sectional study to assess whether greater adiposity was significantly associated with higher FGF23 levels, independent of other known regulators of FGF23 and mineral metabolism. We used crude measures of adiposity [body-mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR)], as well as more sensitive assessments of adiposity [abdominal adipose tissue area measured by computed tomography (CT)], and accounted for the influences of estimated glomerular filtration rate (eGFR), calcium and phosphate balance, 25-hydroxyvitamin D (25OHD), and PTH.
Materials and Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of 6814 community-living adults ages 45 to 84 years, which was established to study risk factors and progression of subclinical cardiovascular disease. Details of this cohort have been previously published (25). Subjects were recruited from six centers across the United States (New York, New York; Baltimore, Maryland; Forsyth County, North Carolina; Chicago, Illinois; St. Paul, Minnesota; and Los Angeles, California). Institutional review boards approved the study at all sites, and participants provided informed consent.
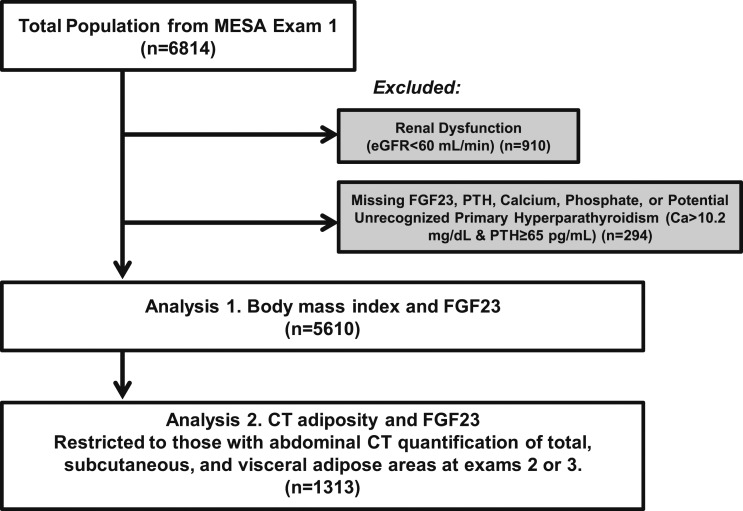
At the first MESA examination, conducted from 2000 to 2002, 6814 participants were included and underwent assessments of demographic data, anthropometrics, medication use, and assessments of serum and urinary calcium and phosphate, 25OHD, PTH, and FGF23. For our current analyses, we included only those participants with data available for all these relevant mineral metabolites and excluded those with key data missing. Furthermore, we excluded any participant who may have had potentially unrecognized primary hyperparathyroidism (Ca >10.2 mg/dL with PTH >65 pg/mL). Given that chronic kidney disease is a strong predictor of FGF23, we excluded participants with an eGFR <60 mL/min/1.73m2 to reduce confounding. After exclusions, the final sample size for this study was 5610 participants (Fig. 1).
Figure 1.
Selection of study participants.
Among this population of 5610, a subset of 1313 participants underwent abdominal CT with assessment of total, visceral, and subcutaneous adipose tissue areas, as previously described (26). CT scans were performed during MESA examinations 2 or 3, which occurred between 2002 and 2005. We excluded any participant who had missing data for the assessments of total, subcutaneous, or visceral adipose area, leaving a final sample size of 1313 participants who had CT measurement and quantification of adipose tissue areas (Fig. 1).
Assessment of demographic and anthropometric variables
Questionnaires and standardized interviews administered by study coordinators were completed by all participants to obtain demographic, medical history, and medication use data. Anthropometric protocols were standard across sites (27). BMI was calculated from weight and height measurements at examination 1, with participant BMI categorized by World Health Organization categories 1 to 4 (1, BMI <25; 2, BMI 25 to 29.99; 3, BMI 30 to 39.99; 4, BMI ≥40). WC was measured at the umbilicus, and WHR was calculated with the addition of hip measurement, which was measured at the widest circumference of the buttock.
Assessment of abdominal adipose tissue area by CT
Adipose tissue was identified as being between −190 and −30 Hounsfield units. Measurement of specific adipose depot areas was obtained by semiautomated segmentation of the body compartments using Medical Image Processing, Analysis, and Visualization software (MIPAV 4.1.2) provided by the National Institutes of Health (Bethesda, MD). Adipose tissue areas were defined as the mean area (in cm2) of two CT slices, one at the L3/L4 junction and the second just superior to the L3/L4 junction. Subcutaneous and visceral adipose tissue areas were calculated separately, as previously described with respect to the MESA cohort (26). Total abdominal adipose tissue (TAAT) area included subcutaneous adipose tissue (SAT) area, visceral adipose tissue (VAT) area, and a small amount of intermuscular adipose, collected during the same scan.
Laboratory measurements
All serum and urine samples at examination 1 were collected after an overnight fast, in the morning before 10 am, and stored at −80°C prior to analysis. Serum intact FGF23 was measured using the Kainos sandwich immunoassay (Kainos, Tokyo, Japan), which measures the full-length (intact) FGF23 molecule (28). The interassay coefficients of variation of standardized high- and low-control samples were 6.7% and 12.4%, respectively (29). Intact serum PTH concentrations were measured at the University of Washington using the Beckman-Coulter Dx1 automated two-site immunoassay (Beckman-Coulter, Brea, CA), with coefficients of variation of 6.1% at 30.1 pg/mL and 3.4% at 94.5 pg/mL (30). Annualized 25OHD measurements were estimated using a cosinor model from single measurements to adjust for seasonal variations in 25OHD to estimate year-long 25OHD status. This model has been validated using longitudinal 25OHD measurements within the MESA population, as previously described (31), with low sample coefficient of variation of 11.8% at 24.8 ng/mL and 8.5% at 7.0 ng/mL (31). Serum creatinine was used to estimate eGFR by the CKD Epidemiology Collaboration (CKD-EPI) Equation (32). Serum and urinary calcium and serum and urinary phosphate were measured as previously described (33). Fractional excretion of phosphate was calculated from the serum and urinary measures of phosphate and creatinine.
Statistical analysis
Our primary analysis assessed the association between BMI and FGF23 levels (n = 5610). Secondary analyses using WC and WHR, instead of BMI, were also performed; in addition, we assessed the association of the more specific measure of adiposity by CT measurements with FGF23 in the subset of participants who had undergone CT examination (n = 1313).
We describe all study variables as means with standard deviations or counts with percents. We compared the mean values of FGF23 and other markers of mineral metabolism across BMI categories and quartiles of WC, WHR, and abdominal adiposity area using unadjusted trend analysis. Multivariable linear regression was used to assess the independent association between the predictors (BMI, WC, WHR, and adipose tissue area as continuous variables) and the outcome (FGF23) with adjustments for relevant demographic and socioeconomic covariates, as well as notable regulators of bone and mineral metabolism that may represent confounders or predictors of FGF23. Model A included adjustment for age, ethnicity, sex, eGFR, urine albumin-to-creatinine ratio, hypertension status [yes (Y)/no (N), defined by guidelines from the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (34)], use of antihypertensive medications (Y/N), diabetes status [defined as normal, impaired fasting glucose, or diabetes on the basis of the 2003 American Diabetes Association fasting criteria (35)], use of antidiabetic medications (Y/N), education level (no schooling though grade 11, or completed high school/through some college completed, or technical school certificate through graduate/professional school degree), cigarette smoking status (never, former, current), and physical activity (reported physical activity hours per week). We adjusted for both eGFR and urine albumin-to-creatinine ratio to account for mild forms of kidney dysfunction that may be present even with eGFR ≥ 60. Model B included adjustment for variables in model A, as well as the biochemical parameters of mineral metabolism: PTH, 25OHD, serum calcium, and urinary calcium-to-creatinine ratio. Model C adjusted for variables in models A and B, as well as serum phosphate and urinary fractional excretion of phosphate. We considered models B and C to be exploratory, given that some of these biochemical parameters may be involved as mediators in a potential causal pathway linking FGF23 with adiposity (especially phosphate metabolism). Univariate relationships between covariates and FGF23 are shown in Supplemental Table 1 (264.8KB, pdf) . We also compared the mean values of FGF23 and other markers of mineral metabolism by race/ethnicity, and tested the interaction of both race/ethnicity and sex on the association of adiposity with FGF23. FGF23 was not normally distributed and therefore was log-transformed in linear regression models. Beta coefficients were exponentiated and reported as percent difference in FGF23 per specified unit of adiposity measure.
We used SAS version 9.4 (SAS Institute, Cary, NC) for all statistical analyses. A two-tailed P value of <0.05 was used to indicate statistical significance.
Results
Study population
The mean age of the total study population was 60.8 (9.9) years, 52.8% were women, and the mean BMI was 28.2 (5.5) kg/m2 (Table 1). The mean concentrations were as follows: FGF23 was 38.8 (16.6) pg/mL, 25OHD was 25.3 (10.9) ng/mL, PTH was 43.2 (18.1) pg/mL, calcium and phosphate were 9.6 (0.4) mg/dL and 3.7 (0.5) mg/dL, respectively, and eGFR was 82.0 (13.1) mL/min/1.73 m2. The characteristics of the subset of participants who underwent CT with abdominal adipose tissue area measurements were similar to those of the total study population (Table 1), although notably, there were minor differences in racial distribution, diabetes status, and values of BMI, WC, and WHR.
Table 1.
Demographic and Biochemical Characteristics of the Total Study Population and the Subset That Underwent CT Abdominal Adipose Tissue Area Quantification
| Characteristics | Total Cohort (N = 5610) | CT Subset (n = 1313) |
|---|---|---|
| Age, y | 60.8 (9.9) | 60.6 (9.4) |
| Women, No. (%) | 2963 (52.8) | 680 (51.8) |
| Race/ethnicity, No. (%) | ||
| White | 2109 (37.6) | 522 (39.8) |
| Chinese American | 682 (12.2) | 205 (15.6) |
| African American | 1551 (27.7) | 274 (20.9) |
| Hispanic | 1268 (22.6) | 312 (23.8) |
| BMI, kg/m2 | 28.2 (5.5) | 27.1 (4.6) |
| WC, cm | 97.7 (14.4) | 94.9 (13.0) |
| WHR | 0.93 (0.08) | 0.92 (0.08) |
| Systolic blood pressure, mm Hg | 125.1 (21.0) | 124.9 (21.2) |
| Diastolic blood pressure, mm Hg | 71.9 (10.2) | 72.3 (10.3) |
| With hypertension, No. (%) | 2294 (40.9) | 523 (39.8) |
| Receiving antihypertensive medications, No. (%) | 1867 (33.3) | 403 (30.7) |
| No. of antihypertensive medications (%) | ||
| 1 | 1150 (20.5) | 252 (19.2) |
| 2 | 522 (9.3) | 113 (8.6) |
| ≥3 | 182 (3.2) | 32 (2.4) |
| Diabetes status, No. (%) | ||
| Normal | 4194 (74.8) | 1026 (78.1) |
| Impaired fasting glucose | 741 (13.2) | 163 (12.4) |
| Diabetes | 675 (12.0) | 124 (9.5) |
| Receiving antidiabetic medications, No. (%) | 512 (9.1) | 88 (6.7) |
| Fasting glucose, mg/dL | 97.1 (30.4) | 94.5 (25.0) |
| eGFR, mL/min/1.73m2 | 82.0 (13.1) | 81.3 (12.6) |
| Annualized 25OHD, ng/mL | 25.3 (10.9) | 26.3 (11.1) |
| PTH, pg/mL | 43.2 (18.1) | 43.5 (18.5) |
| Serum calcium, mg/dL | 9.6 (0.4) | 9.64 (0.39) |
| Urine calcium/Cr ratio, mg/g | 0.10 (0.07) | 0.10 (0.07) |
| Serum phosphate, mg/dL | 3.7 (0.5) | 3.7 (0.5) |
| Fractional excretion of phosphate, % | 11.4 (5.2) | 11.2 (4.5) |
| FGF23, pg/mL | 38.8 (16.6) | 39.7 (23.9) |
Values are mean (standard deviation), unless otherwise specified.
Abbreviation: Cr, creatinine.
Crude measures of adiposity, FGF23, and mineral metabolism
Higher BMI was associated with lower 25OHD, lower serum calcium and urinary calcium excretion, and higher PTH levels (Table 2). FGF23 levels were significantly higher across BMI categories despite the fact that eGFR did not decline (36) and serum phosphate did not increase. These findings were recapitulated when examined across WC and WHR quartiles; higher WC and WHR were associated with higher FGF23 despite lower serum phosphate, higher fractional excretion of phosphate, and no difference in eGFR (Supplemental Tables 2 and 3 (264.8KB, pdf) ).
Table 2.
Biochemical Characteristics by BMI WHO Categories (N = 5610)
| BMI < 25 | BMI 25–29.99 | BMI 30–39.99 | BMI ≥ 40 | P Value | |
|---|---|---|---|---|---|
| No. of patients | 1631 | 2214 | 1564 | 201 | |
| BMI, kg/m2 | 22.5 (1.8) | 27.4 (1.4) | 33.4 (2.6) | 43.9 (3.3) | <0.0001 |
| FGF23, pg/mL a | 37.7 (17.1) | 38.7 (14.0) | 39.8 (19.6) | 40.9 (14.5) | <0.0001 |
| 35.5 (29.0–43.8) | 36.8 (29.7–44.7) | 37.4 (30.4–46.0) | 38.7 (30.6–48.2) | ||
| eGFR, mL/min/1.73m2 | 81.8 (12.5) | 81.5 (12.8) | 82.7 (13.9) | 85.0 (14.6) | 0.0014 |
| Annualized 25OHD, ng/mL | 28.3 (11.1) | 26.0 (11.1) | 22.1 (9.3) | 18.3 (9.0) | <0.0001 |
| PTH, pg/mL | 38.8 (16.6) | 42.5 (16.3) | 47.4 (20.0) | 55.0 (21.6) | <0.0001 |
| Serum calcium, mg/dL | 9.64 (0.37) | 9.65 (0.38) | 9.62 (0.37) | 9.52 (0.38) | 0.0065 |
| Urine calcium/Cr ratio, mg/g | 0.11 (0.08) | 0.10 (0.07) | 0.08 (0.06) | 0.09 (0.08) | <0.0001 |
| Serum phosphate, mg/dL | 3.70 (0.5) | 3.63 (0.5) | 3.66 (0.5) | 3.68 (0.5) | 0.11 |
| Fractional excretion of phosphate, % | 11.0 (4.5) | 11.7 (5.8) | 11.5 (4.9) | 11.6 (5.1) | 0.0021 |
Values are mean (standard deviation), unless otherwise specified. P values are linear trend across categories. Abbreviations: Cr, creatinine; WHO, World Health Organization.
Reported as mean with standard deviation and median with interquartile range (25th to 75th percentile).
We assessed the independent association between BMI (as a continuous variable) and FGF23 levels. BMI was positively associated with FGF23 levels, independent of notable demographic and socioeconomic covariates, but also independent of other regulators of FGF23 and bone and mineral metabolism (Table 3). The fully adjusted effect estimate suggests that for a 10 kg/m2 increase in BMI, there is a 7.2% higher FGF23. The fully adjusted (model C) R2 was 9%, and BMI accounted for 1.1% of this variance. We observed a similar independent association between higher WC and WHR and higher FGF23 levels (Supplemental Table 4 (264.8KB, pdf) ).
Table 3.
Association Between BMI (as Continuous Variable) and FGF23 Levels (N = 5610)
| β (95% CI) | P Value | |
|---|---|---|
| Univariate | +5.0% (3.3%–6.7%) | <0.0001 |
| Multivariable Aa | +6.3% (4.5%–8.2%) | <0.0001 |
| Multivariable Bb | +7.4% (5.5%–9.3%) | <0.0001 |
| Multivariable Cc | +7.2% (5.3%–9.1%) | <0.0001 |
Effect estimates were calculated using log-transformed FGF23, which was exponentiated and reported as percent difference per 10 kg/m2 of BMI.
Multivariable A: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, and urine albumin/creatinine.
Multivariable B: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, urine albumin/creatinine, plus PTH, 25OHD, serum calcium, and urinary calcium/creatinine.
Multivariable C: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, urine albumin/creatinine, PTH, 25OHD, serum calcium, urinary calcium/creatinine, plus serum phosphate and fractional excretion of phosphate.
White ethnicity was associated with higher FGF23, lower eGFR, and highest fractional excretion of phosphate than other ethnic groups (Supplemental Table 5 (264.8KB, pdf) ). However, there was no significant heterogeneity between BMI and FGF23 levels by race/ethnicity (Supplemental Table 6 (264.8KB, pdf) ). In addition, there was no significant heterogeneity between BMI and FGF23 levels by sex (Supplemental Table 7 (264.8KB, pdf) ).
Abdominal adipose tissue area, FGF23, and mineral metabolism
We repeated our investigations using the more refined measures of abdominal adiposity obtained via CT. Higher areas of TAAT were associated with higher BMI (correlation r = 0.81) (Table 4). Notably, there were large overlaps in the BMI ranges for each quartile of TAAT, underscoring the discrepancy between abdominal adiposity measures by CT and the crude measure of BMI. Higher TAAT was associated with lower 25OHD, lower urinary calcium excretion, and higher PTH levels, again suggesting a vitamin D deficiency and secondary hyperparathyroidism with higher adipose tissue burden. FGF23 was again significantly higher with adiposity, and this observation was despite the fact that serum phosphate levels were lower, fractional excretion of phosphate was higher, and eGFR was no different with higher abdominal adiposity (Table 4).
Table 4.
Biochemical Characteristics by TAAT Area Quartiles (N = 1313)
| TAAT Q1 (33.2–265.3 cm2) | TAAT Q2 (265.4–353.5 cm2) | TAAT Q3 (353.7–461.6 cm2) | TAAT Q4 (462.0–1175.3 cm2) | P Value | |
|---|---|---|---|---|---|
| No. of patients | 329 | 327 | 328 | 329 | |
| TAAT, cm2 | 193.1 (50.9) | 308.4 (25.4) | 403.4 (30.9) | 592.5 (116.1) | <0.0001 |
| BMI, kg/m2 | 22.8 (2.5) | 25.7 (2.5) | 27.7 (2.7) | 32.2 (4.4) | <0.0001 |
| BMI range, kg/m2 | 16.1–31.4 | 19.3–38.2 | 21.5–38.8 | 23.2–49.0 | |
| FGF23, pg/mLa | 37.2 (12.4) | 39.7 (28.6) | 39.6 (13.6) | 42.4 (33.4) | 0.0092 |
| 35.7 (29.0–42.2) | 37.8 (30.0–45.0) | 37.9 (30.8–46.0) | 38.4 (31.8–47.0) | ||
| eGFR, mL/min/1.73m2 | 81.4 (12.1) | 81.7 (12.9) | 81.4 (12.7) | 80.7 (12.9) | 0.42 |
| Annualized 25OHD, ng/mL | 27.8 (10.6) | 26.4 (13.4) | 25.8 (10.0) | 25.0 (10.1) | 0.0013 |
| PTH, pg/mL | 39.1 (15.9) | 42.3 (15.5) | 44.5 (17.5) | 48.2 (23.1) | <0.0001 |
| Serum calcium, mg/dL | 9.66 (0.39) | 9.65 (0.37) | 9.61 (0.35) | 9.65 (0.50) | 0.47 |
| Urine calcium/Cr ratio, mg/g | 0.12 (0.09) | 0.10 (0.07) | 0.10 (0.07) | 0.09 (0.06) | <0.0001 |
| Serum phosphate, mg/dL | 3.74 (0.5) | 3.66 (0.5) | 3.65 (0.5) | 3.64 (0.5) | 0.0083 |
| Fractional excretion of phosphate, % | 10.3 (4.1) | 11.0 (4.3) | 12.0 (4.8) | 11.5 (4.7) | <0.0001 |
Values are mean (standard deviation), unless otherwise specified. P values are linear trend across categories.
Abbreviation: Cr, creatinine.
Reported as mean with standard deviation and median with interquartile range (25th to 75th percentile).
The independent association between TAAT (as a continuous variable) and FGF23 levels was evaluated using adjusted linear regression models. A positive association between TAAT and FGF23 remained statistically significant following adjustment for important confounders of FGF23 and bone and mineral metabolism. In the fully adjusted model, there was a 3.1% higher FGF23 per standard deviation of TAAT area (Table 5). We also examined whether specific depots of abdominal adipose tissue (SAT or VAT) may play a specific role in this relationship and observed no substantial differences by adipose tissue type (Table 5).
Table 5.
Association Between Abdominal Adipose Tissue Area (TAAT, SAT, and VAT as Continuous Variables) and FGF23 Level (N = 1313)
| TAAT |
SAT |
VAT |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Univariate | +3.5% (1.6%–5.5%) | 0.0003 | +1.8% (−0.08% to 3.8%) | 0.061 | +4.1% (2.1%–6.1%) | <0.0001 |
| Multivariable Aa | +3.4% (1.4%–5.4%) | 0.0008 | +2.5% (0.5%–4.6%) | 0.013 | +3.6% (1.5%–5.8%) | 0.0009 |
| Multivariable Bb | +3.2% (1.2%–5.2%) | 0.002 | +2.3% (0.2%–4.4%) | 0.029 | +3.5% (1.3%–5.7%) | 0.0016 |
| Multivariable Cc | +3.1% (1.1%–5.1%) | 0.0025 | +2.3% (0.3%–4.4%) | 0.027 | +3.2% (1.1%–5.5%) | 0.003 |
Effect estimates were calculated using log-transformed FGF23, which was exponentiated and reported as percent difference per standard deviation (cm2) of adipose tissue area.
Multivariable A: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, and urine albumin/creatinine.
Multivariable B: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, urine albumin/creatinine, plus PTH, 25OHD, serum calcium, and urinary calcium/creatinine.
Multivariable C: adjusted for age, ethnicity, education level, cigarette smoking, physical activity, hypertension status, use of antihypertensive medications, diabetes status, use of antidiabetic medications, eGFR, urine albumin/creatinine, PTH, 25OHD, serum calcium, urinary calcium/creatinine, plus serum phosphate and fractional excretion of phosphate.
Discussion
In a large community-based cohort with normal eGFR, we observed significantly higher FGF23 levels with adiposity, as assessed by crude measures such as BMI, WC, and WHR, as well as by more refined measurements with abdominal CT. These observations were independent of known determinants and confounders of FGF23. Low eGFR and high phosphate levels are traditionally considered to stimulate FGF23; however, we observed that eGFR did not decline and serum phosphate levels tended to be lower with higher abdominal adiposity, suggesting that higher FGF23 levels with greater degrees of adiposity may represent a novel regulation of FGF23 that is unique from the traditional influences of kidney function and phosphate balance.
FGF23 is a phosphaturic hormone that was initially identified as a key regulator of phosphate metabolism (37) and is most extensively understood and studied within the context of CKD. FGF23 increases significantly with declining eGFR and subsequent increases in serum phosphate; its phosphaturic effect may be considered as serving as a physiological “brake” on serum phosphate accumulation. In addition to increasing renal phosphate excretion, FGF23 also inhibits 1-α hydroxylase and stimulates 24-hydroxlyase, acting to reduce activated vitamin D (i.e., 1,25OH2D) concentrations. Given that 1,25OH2D enhances intestinal phosphate absorption, FGF23 suppression of 1,25OH2D production leads to further reductions in serum phosphate. Our observation of normal eGFR and stable to lower serum phosphate with higher adiposity would be expected to be associated with lower FGF23. Therefore, our contrary finding of higher FGF23 levels, despite these physiologic suppressants, may suggest that the high adipose tissue milieu, or adipose tissue itself, may induce increased secretion (or decreased degradation) of FGF23. It is important to note that in our non-CKD population, the observed changes in FGF23 with adiposity were of a much smaller magnitude than those seen in CKD (38), suggesting that the mechanisms for higher FGF23 with adiposity may be distinct from those stimulating FGF23 in the setting of declining eGFR.
There are several hypotheses for why FGF23 may be higher with higher adiposity in a non-CKD population. Higher PTH levels as a result of secondary hyperparathyroidism may stimulate 1-α hydroxylase, thereby increasing 1,25OH2D production. FGF23 expression is upregulated by 1,25OH2D (20); therefore, secondary hyperparathyroidism in obesity may indirectly increase FGF23 via its stimulation of 1,25OH2D production. Moreover, there is evidence that PTH may directly stimulate FGF23 production via PTH 1 receptor signaling in whole bone and osteocytes, although these data are limited to mouse models (21, 39).
Adipose tissue expresses and secretes numerous other hormones that have been linked to FGF23 production (40), providing other potential explanations for our findings. It is notable that the other members of the FGF family to which FGF23 belongs have been shown to be produced in adipose tissue and have important roles in energy metabolism; FGF21 is produced by adipocytes, among other tissues (18), and contributes to thermogenesis and fatty acid storage (19). There are also potential immunological roles of FGF23; its expression has been noted in mouse thymus, as well as dendritic cells and macrophages (41), which are increased in proinflammatory visceral adipose tissue. Furthermore, higher levels of aldosterone have been described in overweight individuals (42) and those with higher VAT (43). In a mouse model, administration of aldosterone increased FGF23 transcription by osteoblasts (44); thus, higher FGF23 with adiposity may also be related to renin-angiotensin-aldosterone system dysregulation. It is notable that we did not find a significant difference in the association of FGF23 with adiposity by specific adipose depot (i.e., SAT vs VAT), in contrast with other studies that have shown a more defined correlation between skeletal disease and VAT (45). Lastly, leptin, which is typically increased in obesity as a result of leptin resistance, may play a role as well; in ob/ob mice, infusion of leptin markedly increased bone FGF23 expression (46).
Whether higher FGF23 levels associated with adiposity have a clinical impact on skeletal health in obesity is yet to be determined. It is plausible that higher FGF23 levels in obesity negatively affect bone health via inhibition of 1,25OH2D activity and promotion of phosphaturia, two actions that can result in a deficiency in calcium and phosphate substrates that are needed for bone mineralization. This, compounded by the mineralization defects related to higher PTH in obesity, may provide a mechanism that explains adverse skeletal health in this population.
Beyond skeletal health, evidence linking higher FGF23 levels with cardiovascular disease and mortality (29, 47, 48) is growing, even in the absence of CKD. Given the already increased cardiovascular risk in obesity, FGF23 may play a role as dual offender in this population, negatively affecting not only skeletal health, but also cardiovascular health.
Strengths and limitations
Our study sample was relatively large, and included detailed measurements of bone and mineral metabolism and adiposity. In particular, we had specific measurements of nearly every known determinant of FGF23 to assess independent relationships between adiposity and FGF23 levels. In addition to BMI, WC, and WHR, which are relatively crude measures of adiposity, we were able to use the refined measure of abdominal adipose tissue areas derived from CT to strengthen and validate our observations.
Our study did have several important limitations that should be noted. The cross-sectional nature of this study design precludes us from concluding causality or directionality. Although significant and independent of numerous potential confounders, the effect estimates for the absolute relationships between adiposity and FGF23, as well as the variance in FGF23 explained by adiposity in the models, were relatively small. However, because these differences were in a population with normal kidney function, in which the deleterious impact of FGF23 is rarely studied, it may be premature to discount the size of these observed effects. Furthermore, the cross-sectional nature of the effect estimates prevents us from assessing the consequences of small but chronically higher FGF23 levels over time. We have presented potential explanations for the positive association of adiposity with FGF23 as being caused by excess adiposity. However, it is possible that FGF23 itself may promote adiposity, via increasing insulin resistance, for example, although evidence for this is currently lacking. Longitudinal studies may help to differentiate the direction of causality of our findings. In addition, all of our study measurements were performed at examination 1, except for the measurements of abdominal adiposity by CT, which were performed at examinations 2 or 3. However, we used the CT measurements as a cross-sectional snapshot to validate our findings with BMI, and in this regard, our two analyses were complementary and consistent. We did not have measures of serum 1,25OH2D or detailed information regarding dietary intake; however, the availability of PTH, as well as serum and urinary calcium and phosphate levels, should have accounted for the actions of 1,25OH2D and integrated dietary intake. Other studies have investigated the relationship between dietary intake and FGF23 levels (49). Last, we cannot extend these findings to clinical outcomes related to skeletal health, such as bone mineral density and fracture; further study is needed to do so.
In summary, we observed that higher adiposity, as measured by BMI and abdominal adipose area according to CT, was independently associated with higher FGF23 levels in a large cohort of participants with normal eGFR. Higher FGF23 activity with greater adipose tissue burden may provide an additional explanation for the increased adverse skeletal outcomes seen in obesity. Further studies are needed to better understand the causes and consequences of high FGF23 in states of greater adiposity.
Acknowledgments
The authors thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01 HL096875, N01 HC95159, N01 HC95166, N01 HC95169, R01 HL071739, R01 HL072403, and F32 HL132477 (S.Z.). A.V. was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number R01 DK107407, by the National Heart, Lung, and Blood Institute under award number K23 HL111771, and by grant number 2015085 from the Doris Duke Charitable Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 1,25OH2D
- 1,25-dihydroxyvitamin D
- 25OHD
- 25-hydroxyvitamin D
- BMI
- body mass index
- CKD
- chronic kidney disease
- CT
- computed tomography
- eGFR
- estimated glomerular filtration rate
- FGF23
- fibroblast growth factor 23
- MESA
- Multi-Ethnic Study of Atherosclerosis
- PTH
- parathyroid hormone
- SAT
- subcutaneous adipose tissue
- TAAT
- total abdominal adipose tissue
- VAT
- visceral adipose tissue
- WC
- waist circumference
- WHR
- waist-to-hip ratio.
References
- 1.Greco EA, Fornari R, Rossi F, Santiemma V, Prossomariti G, Annoscia C, Aversa A, Brama M, Marini M, Donini LM, Spera G, Lenzi A, Lubrano C, Migliaccio S. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010;64(6):817–820. [DOI] [PubMed] [Google Scholar]
- 2.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92(5):1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Díez-Pérez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, Lacroix AZ, Roux C, Sambrook PN, Siris ES; Glow Investigators . Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukumar D, Schlussel Y, Riedt CS, Gordon C, Stahl T, Shapses SA Obesity alters cortical and trabecular bone density and geometry in women. Osteoporosis Int 2011;22(2):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Müller R, Zhao B, Guo X, Lang T, Saeed I, Liu XS, Guo XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98(6):2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25(2):292–297. [DOI] [PubMed] [Google Scholar]
- 8.Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. 2014;99(9):3290–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. [DOI] [PubMed] [Google Scholar]
- 11.Kamycheva E, Sundsfjord J, Jorde R Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur J Endocrinol 2004;151(2):167–172. [DOI] [PubMed] [Google Scholar]
- 12.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermeo S, Gunaratnam K, Duque G. Fat and bone interactions. Curr Osteoporos Rep. 2014;12(2):235–242. [DOI] [PubMed] [Google Scholar]
- 14.Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem. 2012;45(12):874–879. [DOI] [PubMed] [Google Scholar]
- 15.Kanda E, Yoshida M, Sasaki S. Applicability of fibroblast growth factor 23 for evaluation of risk of vertebral fracture and chronic kidney disease-mineral bone disease in elderly chronic kidney disease patients. BMC Nephrol. 2012;13:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. [DOI] [PubMed] [Google Scholar]
- 17.Manghat P, Fraser WD, Wierzbicki AS, Fogelman I, Goldsmith DJ, Hampson G Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporosis Int. 2010;21(11):1853–1861. [DOI] [PubMed] [Google Scholar]
- 18.Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol (Oxf). 2013;78(4):489–496. [DOI] [PubMed] [Google Scholar]
- 19.Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko I, Saini RK, Griffin KP, Whitfield GK, Haussler MR, Jurutka PW. FGF23 gene regulation by 1,25-dihydroxyvitamin D: opposing effects in adipocytes and osteocytes. J Endocrinol. 2015;226(3):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza MA, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, Ljunggren O, Larsson TE. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res. 2011;26(4):857–864. [DOI] [PubMed] [Google Scholar]
- 23.Mirza MA, Alsiö J, Hammarstedt A, Erben RG, Michaëlsson K, Tivesten A, Marsell R, Orwoll E, Karlsson MK, Ljunggren O, Mellström D, Lind L, Ohlsson C, Larsson TE. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31(1):219–227. [DOI] [PubMed] [Google Scholar]
- 24.Holecki M, Chudek J, Wiecek A, Titz-Bober M, Dulawa J The serum level of fibroblast growth factor-23 and calcium-phosphate homeostasis in obese perimenopausal women. Int J Endocrinol 2011;2011:707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 26.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christoph MJ, Allison MA, Pankow JS, Decker PA, Kirsch PS, Tsai MY, Sale MM, de Andrade M, Sicotte H, Tang W, Hanson NQ, Berardi C, Wassel CL, Larson NB, Bielinski SJ. Impact of adiposity on cellular adhesion: The Multi-Ethnic Study of atherosclerosis (MESA). Obesity (Silver Spring). 2016;24(1):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91(6):2055–2061. [DOI] [PubMed] [Google Scholar]
- 29.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, de Boer IH. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR Jr, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf). 2013;79(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, Kestenbaum B, de Boer IH. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN, Tracy R, Laughlin GA, Criqui MH, Budoff MJ, Li D, Ix JH. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab. 2013;98(12):4890–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The National Heart, Lung, and Blood Institute. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Institutes of Health; 1997. NIH Publication No. 98-4080. [PubMed] [Google Scholar]
- 35.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemoine S, Guebre-Egziabher F, Sens F, Nguyen-Tu MS, Juillard L, Dubourg L, Hadj-Aissa A. Accuracy of GFR estimation in obese patients. Clin J Am Soc Nephrol. 2014;9(4):720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20(5):230–236. [DOI] [PubMed] [Google Scholar]
- 38.Chathoth S, Al-Mueilo S, Cyrus C, Vatte C, Al-Nafaie A, Al-Ali R, Keating BJ, Al-Muhanna F, Al Ali A. Elevated fibroblast growth factor 23 concentration: prediction of mortality among chronic kidney disease patients. Cardiorenal Med. 2015;6(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Y, Bi R, Densmore MJ, Sato T, Kobayashi T, Yuan Q, Zhou X, Erben RG, Lanske B. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2016;30(1):428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. [DOI] [PubMed] [Google Scholar]
- 41.Masuda Y, Ohta H, Morita Y, Nakayama Y, Miyake A, Itoh N, Konishi M. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull. 2015;38(5):687–693. [DOI] [PubMed] [Google Scholar]
- 42.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35(6):1270–1277. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Umbach AT, Chen H, Yan J, Fakhri H, Fajol A, Salker MS, Spichtig D, Daryadel A, Wagner CA, Föller M, Lang F. Up-regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun. 2016;470(2):384–390. [DOI] [PubMed] [Google Scholar]
- 45.Jankowska EA, Rogucka E, Medraś M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia. 2001;33(6):384–389. [DOI] [PubMed] [Google Scholar]
- 46.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25(8):1711–1723.20200981 [Google Scholar]
- 47.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83(1):160–166. [DOI] [PubMed] [Google Scholar]
- 48.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosk D, Kramer H, Luke A, Camacho P, Bovet P, Rhule JP, Forrester T, Wolf M, Sempos C, Melamed ML, Dugas LR, Cooper R, Durazo-Arvizu R. Dietary factors and fibroblast growth factor-23 levels in young adults with African ancestry [published online ahead of print December 9, 2016]. J Bone Miner Metab. doi: 10.1007/s00774-016-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]