Abstract
Loss-of-function mutations in the X-linked immunoglobulin superfamily, member 1 (IGSF1) gene cause central hypothyroidism. IGSF1 is a transmembrane glycoprotein of unknown function expressed in thyrotropin (TSH)-producing thyrotrope cells of the anterior pituitary gland. The protein is cotranslationally cleaved, with only its C-terminal domain (CTD) being trafficked to the plasma membrane. Most intragenic IGSF1 mutations in humans map to the CTD. In this study, we used CRISPR-Cas9 to introduce a loss-of-function mutation into the IGSF1-CTD in mice. The modified allele encodes a truncated protein that fails to traffic to the plasma membrane. Under standard laboratory conditions, Igsf1-deficient males exhibit normal serum TSH levels as well as normal numbers of TSH-expressing thyrotropes. However, pituitary expression of the TSH subunit genes and TSH protein content are reduced, as is expression of the receptor for thyrotropin-releasing hormone (TRH). When challenged with exogenous TRH, Igsf1-deficient males release TSH, but to a significantly lesser extent than do their wild-type littermates. The mice show similarly attenuated TSH secretion when rendered profoundly hypothyroid with a low iodine diet supplemented with propylthiouracil. Collectively, these results indicate that impairments in pituitary TRH receptor expression and/or downstream signaling underlie central hypothyroidism in IGSF1 deficiency syndrome.
Igsf1-deficient mice have reduced pituitary TRH receptor expression and are, therefore, less sensitive to TRH stimulated TSH synthesis and secretion.
Hypothyroidism is a common endocrine disorder affecting an estimated 5% of the US population (1). Alhough the disease most often derives from thyroid gland dysfunction (i.e., primary hypothyroidism), it is more rarely caused by perturbations at the hypothalamic or pituitary levels [collectively called central hypothyroidism (CeH)] (2). CeH is characterized clinically by low to low-normal thyroid hormone levels in the presence of nonelevated thyrotropin (TSH) levels (3, 4). CeH can occur spontaneously due to pituitary adenoma, interruption of blood flow from the hypothalamus to the pituitary, surgery or radiotherapy, and/or other lesions (3–5). More rarely, the disorder is congenital and presents as isolated CeH with mutations in the TSHβ subunit, thyrotropin-releasing hormone (TRH) receptor, or TBL1X genes; or as a component of combined pituitary hormone deficiency associated with mutations in developmentally important transcription factors (e.g., HESX1, OTX2, PROP1, POU1F1, LHX3, LHX4, SOX2, SOX3, and GLI2) (6–9).
More recently, loss-of-function mutations in the immunoglobulin superfamily, member 1 (IGSF1) gene, with an estimated prevalence of 1 in 100,000 (2), have emerged as a common cause of congenital CeH (2, 10–16). As the gene is X-linked, IGSF1 deficiency syndrome (Online Mendelian Inheritance in Man no. 300888) primarily affects males (10). Although CeH is a hallmark of the syndrome, carriers can also present with hypoprolactinemia and/or growth hormone deficiency, delayed pubertal testosterone rise, and, often, macroorchidism (10, 17). IGSF1 is expressed in developing pituitary (Rathke’s pouch) as well as in thyrotropes, lactotropes, and somatotropes of the differentiated gland (11, 18). Thus, deficits in pituitary hormone production/secretion may represent cell-autonomous effects of impaired IGSF1 function. IGSF1/Igsf1 messenger RNA (mRNA) expression has been reported in rat, human, and mouse testes, as has IGSF1 protein expression in rat testes (11, 18, 19). However, it is presently unclear whether testicular enlargement results from loss of protein function in the testis or from indirect endocrinopathies (20–22).
The IGSF1/Igsf1 gene encodes a 12 Ig-loop (C2-type) transmembrane glycoprotein that is cotranslationally cleaved in the endoplasmic reticulum (ER) into N- and C-terminal domains (CTDs) (11, 23–25). The CTD traffics to the plasma membrane, whereas the N-terminal domain appears to be retained in the ER (25). Intragenic mutations in IGSF1 cluster in the part of the gene encoding the IGSF1-CTD (Supplemental Fig. 1 (622.7KB, pdf) ) and inhibit the protein’s expression at the cell surface (11, 13–15, 17, 26). Because the phenotypes of individuals with intragenic mutations (whether missense, frame-shift, or nonsense) are comparable to those with entire gene deletions, it appears that the IGSF1-CTD is the functional part of the protein and must reach the cell surface to perform its normal cellular activities. These activities, however, are presently unresolved.
Some insight was gleaned from preliminary investigations of a knockout mouse model in which exon 1 of the 20-exon Igsf1 gene was replaced by a positive selection marker (Igsf1Δex1) (19). Although exon 1 is noncoding (translation of the full-length protein starts in exon 2), pituitary expression of at least three major Igsf1 mRNA isoforms is abrogated in these mice. Moreover, they exhibit CeH, although with variable penetrance (11, 19). The initial report indicated low to normal serum TSH levels, reduced pituitary TSH content, and low to normal serum T3 and T4 levels, perhaps due to a reduction in pituitary Trhr1 mRNA expression (11).
A possible explanation for the heterogeneity of the CeH phenotype is the retention of at least one mRNA isoform in Igsf1Δex1 mice, Igsf1 isoform 4 (Igsf1-4). Igsf1-4 derives from an alternate promoter in intron 9 of the Igsf1 gene and is therefore intact in these animals (19). At present, there is no evidence that Igsf1-4, which encodes the entirety of the CTD, is upregulated in Igsf1Δex1/y mice. Moreover, the IGSF1-CTD protein is not observed in their pituitaries. It is still possible, however, that it may be expressed at levels too low to be detected but may retain functionality in some mice under some conditions (19, 25).
To address this possibility and completely abrogate IGSF1-CTD function, we used CRISPR-Cas9 to generate a novel Igsf1 loss-of-function mouse model. Strikingly, these mice exhibit phenotypes highly similar to the original Igsf1Δex1 model. Results from these two strains converge to indicate that the principal impairment in IGSF1 deficiency syndrome is attenuated TRH actions in pituitary thyrotropes.
Materials and Methods
CRISPR guide RNA construction
DNA oligonucleotides encoding the CRISPR RNA (crRNA) sequence were purchased from Integrated DNA Technologies (Coralville, IA) (Supplemental Table 1 (17.5KB, docx) ). The oligonucleotides were annealed and 5′ phosphorylated in the same reaction. Briefly, 10 µM of each primer was mixed with 1× T4 ligase buffer (Promega, Madison, WI) and 5 U of T4 polynucleotide kinase (New England Biolabs, Whitby, ON, Canada) and incubated at 37°C for 30 minutes. The reaction was then incubated at 95°C for 5 minutes before the temperature was ramped down to 25°C at a rate of 5°C/min. The annealed oligonucleotides were ligated into the pX330 vector (42230; Addgene, Cambridge, MA) in a digestion–ligation reaction. Briefly, the pX330 vector was digested with 10 U of BbsI (FastDigest; Thermo Fisher, Burlington, ON, Canada). In the same reaction, the annealed/phosphorylated oligonucleotides were ligated into the digested vector using 10 U of T4 DNA ligase (Promega) in the presence of 1× FastDigest buffer, 1 mM dithiothreitol, and 1 mM adenosine triphosphate. The reaction was cycled six times for 5 minutes at 37°C and 5 minutes at 23°C. The resulting plasmid (11 µL) was treated with 10 U of PlasmidSafe exonuclease (Epicentre, Madison, WI) in the presence of 1× PlasmidSafe buffer and 1 mM adenosine triphosphate at 37°C for 30 minutes, and transformed into NEB10 competent bacteria (New England BioLabs, Ipswich, MA). Single colonies were selected and grown in 3-mL cultures of lysogeny broth supplemented with 100 µg/mL ampicillin. Plasmid DNA was extracted using a plasmid DNA extraction kit (BS614; Bio Basic, Markham, ON, Canada ) and analyzed by Sanger sequencing (Génome Québec, Montreal, QC, Canada). Part of the plasmid including the T7 promoter and guide RNA, which comprises crRNA and transactivating crRNA, were amplified by polymerase chain reaction (PCR) using oligonucleotides from Integrated DNA Technologies (Supplemental Table 1 (17.5KB, docx) ). The PCR product was gel purified with the Bio Basic gel extraction kit (BS654) following the manufacturer’s instructions, and transcribed to RNA using the MAXIscript T7 kit (AM1312; Thermo Fisher, Burlington, ON, Canada) following the manufacturer’s instructions.
Development of Igsf1-deficient mice
Mice were generated by cytoplasmic microinjections of the guide RNA described above and Cas9 mRNA (CAS9MRNA; Sigma-Aldrich, Oakville, ON, Canada) into single-cell mouse C57BL6/N zygotes (Harlan, Indianapolis, IN). The microinjections were performed at the Goodman Cancer Research Centre Mouse Transgenic Facility at McGill University. The injected zygotes were cultured overnight in KSOM (ZEKS-050; Zenith Biotech, Guilford, CT) droplets under mineral oil (ZSCO-100; Zenith Biotech) in a 35-mm dish at 37°C in a 5% CO2 incubator. The 36 embryos that developed to the two-cell stage were transferred to the oviducts of two pseudopregnant CD1-Elite females (Charles River Laboratories, Wilmington, MA). Five pups were born from only one female and were genotyped by PCR amplifying genomic DNA extracted from tail biopsies using 0.5 mL of lysis buffer [100 mM Tris-HCl (pH 8.5), 5 mM EDTA (pH 8.0), 200 mM NaCl, 0.2% (v/v) sodium dodecyl sulfate (SDS), and 100 µg/mL proteinase K]. The targeted region of Igsf1 was amplified by PCR using primers in Supplemental Table 1 (17.5KB, docx) , resolved on a 1% agarose gel, and gel purified. The product was sent for direct Sanger sequencing (Génome Québec) using the primers in Supplemental Table 1 (17.5KB, docx) . A founder female had 6-bp and 312-bp deletions in her two Igsf1 alleles, both of which were germline transmissible. As described in Results, the 312-bp mutation, subsequently called Igsf1Δ312, was pursued.
Animal housing and collection
Igsf1Δ312 mice (Igsf1em1Djb; Mouse Genome Informatics no. 5779502) are described above and Igsf1Δex1 mice (Igsf1tm1Zuk; Mouse Genome Informatics no. 2671049) were previously described (19). All animals were housed on a 12:12-hour light/dark cycle and were given ad libitum access to food and water. The diet consisted of standard rodent chow (2020X, Teklad diets; Envigo, Toronto, ON, Canada) unless otherwise specified. All animal work was conducted in accordance with federal and institutional guidelines and with the approval of the Goodman Cancer Centre Facility Animal Care Committee at McGill University (protocol no. 5204).
Eight- to 10-week-old animals were euthanized by CO2 asphyxiation. Blood was collected by cardiac puncture, and pituitary glands were extracted and either flash frozen in liquid nitrogen, or frozen in liquid nitrogen in 500 µL of TRIzol. Blood was allowed to coagulate at room temperature (RT) for 30 minutes and spun down for 10 minutes at 3000 rpm. The serum was collected and stored at −20°C until measurements were taken. The pituitaries were stored at −80°C until processed.
Constructs
pcDNA3.0 was purchased from Invitrogen (Burlington, ON, Canada). The IGSF1-4 construct was generated by Dr. Thalia Robakis (Columbia University, New York, NY). Briefly, complementary DNA (cDNA) encoding the CTD of murine Igsf1 was obtained from American Type Culture Collection (Manassas, VA, clone no. 3289361). The sequence was amplified with primers containing BamHI and NotI sites and ligated into the same sites in the pNice vector (Dr. Peter Scheiffele, now at Biozentrum, Basel, Switzerland). The IGSF1-4–HA, IGSF1-4–Δ6bp, and IGSF1-4–Δ9bp constructs were derived from the parental IGSF1-4 vector. The N-terminal HA tag was added by reverse PCR amplification of the IGSF1-4 plasmid with phosphorylated primers (Supplemental Table 1 (17.5KB, docx) ) following the QuikChange mutagenesis cycling conditions. The resulting linear PCR product was ligated with 10 U of T4 DNA ligase at 4°C overnight. The 6- and 9-bp deletions were introduced using the QuikChange mutagenesis protocol with the primers indicated in Supplemental Table 1 (17.5KB, docx) . The murine IGSF1-1 expression plasmid was generated by amplifying the full-length Igsf1-1 from wild-type mouse pituitary cDNA with primers containing XhoI and HindIII restriction sites (Supplemental Table 1 (17.5KB, docx) ). The PCR product was then ligated into pcDNA3.0 digested with the same enzymes. All plasmids were transformed into NEB10 component bacteria and confirmed by sequencing (Génome Québec).
Cell transfection
HEK293 cells (provided by Dr. Terry Hébert, McGill University) were cultured in Dulbecco’s modified Eagle medium (319-005Cl; Wisent, Saint-Jean-Baptiste, QC, Canada) supplemented with 10% FBS (12483-020; Gibco, Burlington, Ontario). The cells were seeded at 800,000 cells per well in six-well plates (3516; Costar, Corning, NY) and were transfected with the indicated plasmids the following day. Two micrograms of plasmid were incubated in serum-free Dulbecco’s modified Eagle medium with 6 µg of polyethylenimine for 15 minutes. The complex was then added to the cells in serum-free medium and incubated for 2 hours, before the medium was changed to complete medium.
Immunoblotting and deglycosylation assays
SDS–polyacrylamide gel electrophoresis (SDS-PAGE), immunoblots, and protein extraction were performed essentially as in Sun et al. (11). Briefly, for lysates from tissue, pituitary glands were dissected from 8-week-old males, immediately frozen in liquid nitrogen, and kept at –80°C until analysis. The protein lysates were extracted by adding 50 to 100 µL of RIPA buffer (150 mM NaCl, 50 mM sodium fluoride, 10 mM NaPO4, 2 mM EDTA, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitors [2 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin A, and 0.2 M phenylmethylsulfonyl fluoride (PMSF)] directly in the tube containing the frozen tissue. A handheld homogenizer was used to dissociate the tissue. To extract lysates from cell culture, cells were washed twice with 1 mL of phosphate-buffered saline (PBS) and then incubated for 20 minutes with 300 µL of IP lysis buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100] containing protease inhibitors (2 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin A, and 0.2 M PMSF). The cells were then transferred to 1.5-mL tubes. Lysates from cells and pituitaries were sonicated with a microtip probe sonicator (Sonicator 3000; Misonix, Farmingdale, NY) for 15 seconds at 3 W and then spun down for 20 minutes at 13,000 rpm at 4°C. Protein concentrations were measured using BCA assays (Thermo Fisher) following the manufacturer’s instructions. Where indicated, 20 µg of each protein lysate was denatured at 95°C for 10 minutes and then deglycosylated with 50 U of EndoH (P0702S), PNGaseF (P0704S; New England Biolabs), or left untreated for 2 hours at 37°C prior to being resolved by SDS-PAGE. In all other cases, 10 µg of each protein lysate was denatured at 70°C for 10 minutes in Laemmli buffer and resolved by SDS-PAGE on 8% Tris-glycine gels. Proteins were transferred to Protran nitrocellulose membranes (NBA083C001EA; Perkin Elmer, Waltham, MA), blocked in 5% nonfat milk in Tris-buffered saline [TBS; 150 mM NaCl, 10 mM Tris (pH 8.0)] containing 0.05% Tween 20 (TBST), and incubated in the indicated primary Ab in 5% milk in TBST overnight at 4°C with agitation. The polyclonal rabbit antimouse IGSF1 primary Ab (1:1000) was first described in Robakis et al. (25), and the mouse anti–β-actin Ab was purchased from Sigma-Aldrich (St. Louis, MO; 1:40,000, A5441) (Supplemental Table 2 (17.5KB, docx) ). The next day, the membranes were washed in TBST and subsequently incubated in horseradish peroxidase–conjugated rabbit or mouse secondary Ab (1:5000, goat anti-mouse 170-6516, goat anti-rabbit 170-6515; Bio-Rad Laboratories, Mississauga, ON, Canada) in 5% milk in TBST. Blots were washed in TBST, incubated with ECL Western Lightning Plus (NEL105001EA; PerkinElmer, Boston, MA), and exposed to HyBlot CL autoradiography film (E3018; Denville Scientific, Metuchen, NJ).
TRH stimulation
Adult male Igsf1+/y and Igsf1Δ312/y mice were injected with TRH (P1319; Sigma-Aldrich, 10 μg/kg body weight) by intraperitoneal (i.p.) injection with ∼200 μL of 1 ng/μL TRH diluted in PBS containing 0.002% bovine serum albumin (BSA). Half of the mice were injected at 8 weeks and the other half were injected at 9 weeks. Prior to injection, blood was collected from the tail vein by creating a small incision with a scalpel on either side of the tail, ∼1.5 to 3 cm from the base. Thirty microliters of blood was collected using EDTA-coated Microvettes (20.1278.100; Sarstedt, Nümbrecht, Germany) and immediately kept on ice. Blood samples were also collected 15 minutes, 1 hour, and 2 hours following TRH injection by rubbing the scab off of the previous incision with a sterile gauze soaked in PBS and proceeding as with the first sample. The samples were spun at 3000 rpm for 10 minutes at 4°C and the plasma was collected and stored at −20°C until analyzed. To minimize stress, the mice were handled daily for 2 weeks prior to the experiment and were not restrained during blood collection.
Adult 8-week-old male Igsf1+/y and Igsf1Δex1/y mice were injected with TRH as described previously. Blood was collected by submandibular venipuncture prior to the injection, and animals were killed by CO2 asphyxiation 15 minutes postinjection. Blood was then collected by cardiac puncture and serum was collected as described previously.
Diet-induced hypothyroidism
Eight- or 10-week-old Igsf1+/y and Igsf1Δ312/y mice were fed a low iodine (LoI) diet supplemented with 0.15% propylthiouracil (PTU; TD.95125, Teklad diets; Envigo, Toronto, ON, Canada) for 3 weeks ad libitum. The diet was changed every Monday, Wednesday, and Friday to replace the PTU. Blood samples were collected from the animals prior to starting the diet, and after 1 week and 2 weeks on the diet, by performing submandibular venipuncture (∼100 to 200 µL). At the end of the third week, the animals were killed, their blood was collected by cardiac puncture (∼500 to 800 µL), and their pituitary glands were collected in 500 µL of TRIzol (Invitrogen) and immediately frozen in liquid nitrogen. Serum was collected from the blood samples as described previously. Eight-week-old male Igsf1+/y and Igsf1Δex1/y mice were fed an LoI/PTU diet or a regular diet for 3 weeks. Blood and pituitary glands were collected as described above after 3 weeks on the indicated diet.
Hormone measurements
TSH was measured by radioimmunoassay (RIA) [Fig. 6(b); see Supplemental Figs. 8A, 8B, and 12A (622.7KB, pdf) ] or the Milliplex MAP mouse pituitary magnetic bead panel (MPTMAG-49K; EMD Millipore, Darmstadt, Germany) [Figs. 3(a), 3(d), 4(a), and 6(a); see Supplemental Fig. 10A] (622.7KB, pdf) . The TSH RIA was performed as previously described (27) with the following modification. 125I-labeled rat TSH was obtained from the Institute of Isotopes (Budapest, Hungary) and precipitation used in-house produced sheep anti-rat IgG. The TSH Milliplex assay was performed following the manufacturer’s instructions with some modifications. In particular, serum samples collected from animals on the LoI/PTU diet were diluted 1:5 in serum matrix provided in the kit prior to measurement, as pilot experiments indicated that undiluted samples were out of the assay range. Ten microliters of each dilution was used in the assays. Proteins were extracted from frozen pituitary using lysis buffer [50 mM HEPES, 150 mM NaCl, 10 mM EDTA, 1% (v/v) Triton X-100, 1 mM Na3NO4, 30 mM NaF, 10 mM Na4P2O7, 1 µg/mL pepstatin A, 1 µg/mL leupeptin, 1 µg/mL aprotinin, 1 mM PMSF]. To measure pituitary TSH content, lysates were diluted 1:10,000 in assay buffer and 10 µL was used in the assay. The MAGPIX instrument with xPONENT software was used for the measurements, and the analysis was performed using the Milliplex Analyst 5.1 software. The range of detection was between 12.21 and 50,000 pg/mL. The intrassay coefficient of variation (CV) was 2.68%, and the interassay CV was 4.38%.
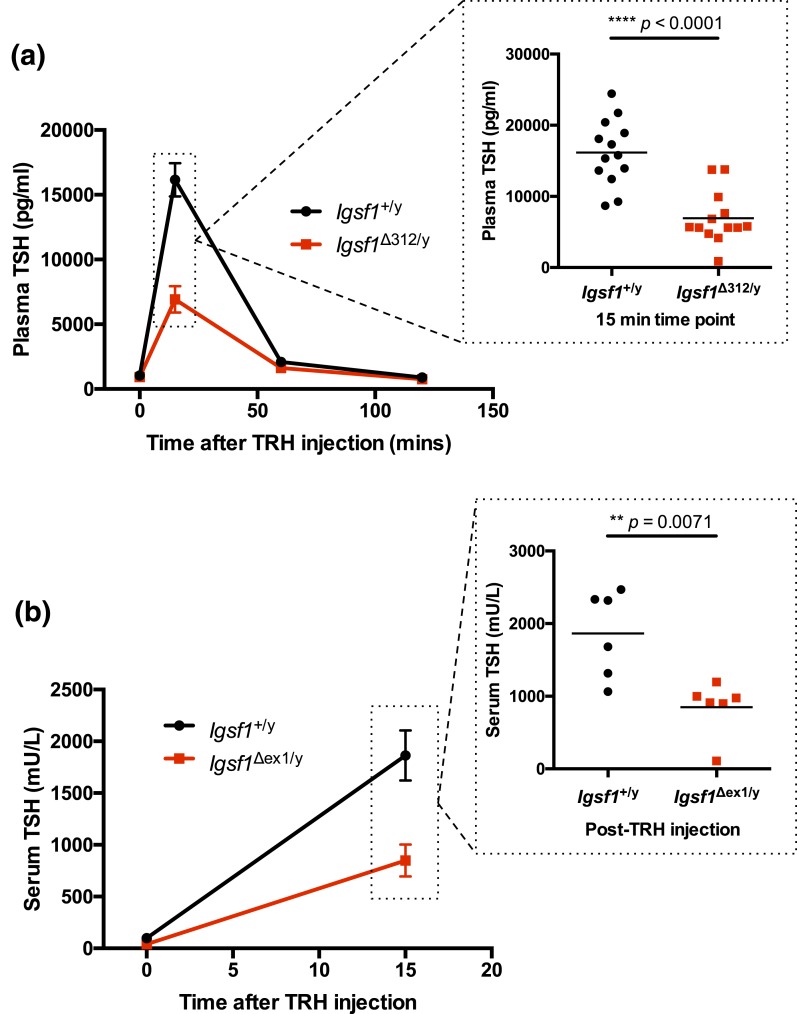
Figure 6.
TRH-induced TSH release is blunted in Igsf1-deficient mice. (a) Eight-week-old male wild-type and Igsf1Δ312/y mice were injected with TRH (i.p. 10 μg/kg). Blood samples were collected before the TRH injection as well as 15 minutes, 1 hour, and 2 hours after injection. Plasma TSH levels were measured by ELISA. Data were analyzed by two-way ANOVA [interaction: F(3, 99) = 30.31, ****P < 0.0001; time after injection: F(3, 99) = 160.9, ****P < 0.0001; genotype: F(1, 99) = 37.67, ****P < 0.0001] and post hoc t test, n = 13 for each genotype. The data from the 15-minute time point were expanded to show individual data points (two-tailed t test: t = 5.627, df = 22.72, ****P < 0.0001). (b) Male 8-week-old wild-type and Igsf1Δex1/y mice were injected with TRH (i.p. 10 μg/kg). Blood samples were collected prior to and 15 minutes after TRH injection. Serum TSH levels were measured by RIA. Data were analyzed by two-way ANOVA [interaction: F(1, 20) = 11.01, **P = 0.0034; time: F(1, 20) = 79.65, ****P < 0.0001; genotype: F(1, 20) = 13.88, **P = 0.0013] and post hoc t test (t = 3.531, df = 8.466, **P = 0.0071), n = 6 per genotype.
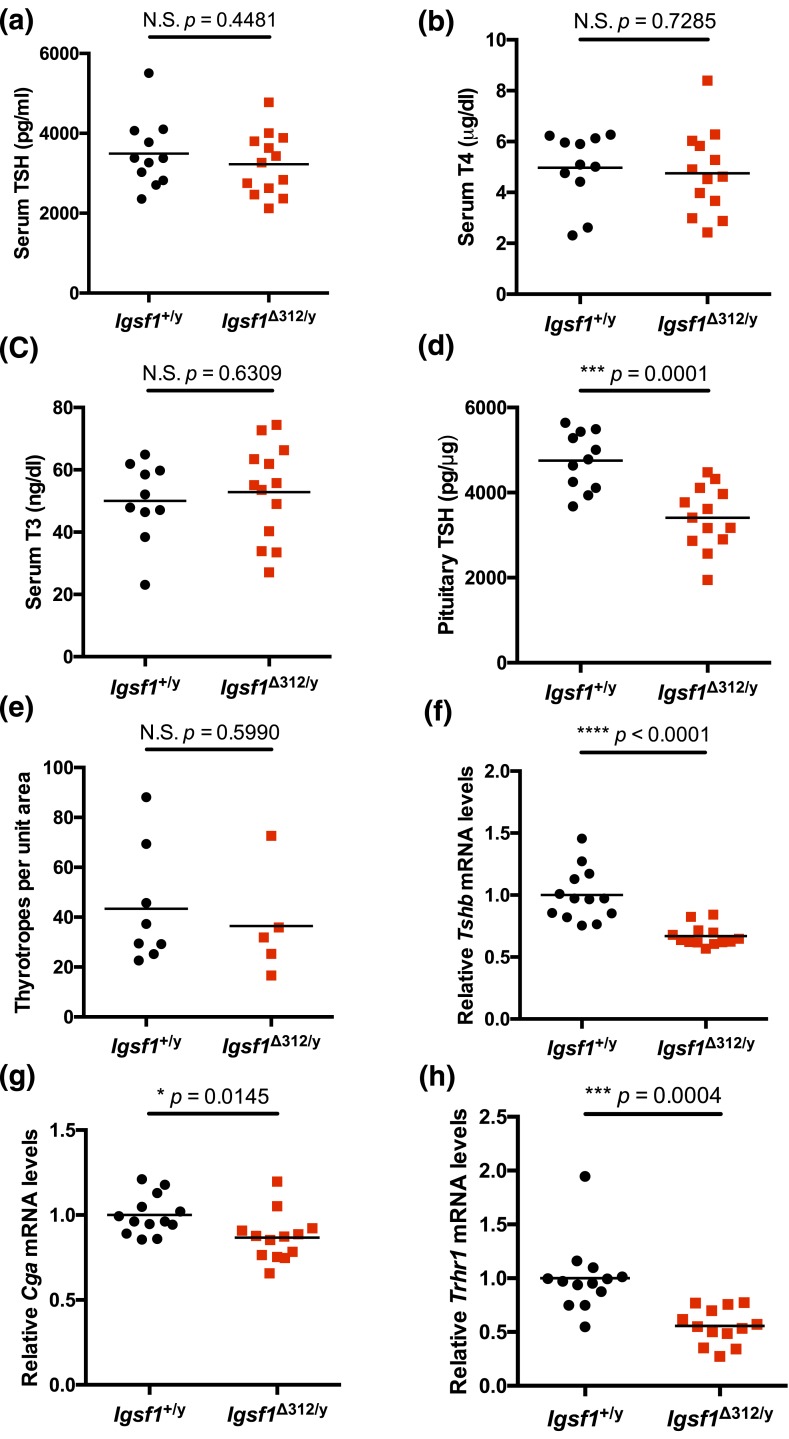
Figure 3.
TSH synthesis is reduced in Igsf1Δ312/y mice. Serum (a) TSH, (b) T4, (c) T3 levels, and (d) pituitary TSH content in 8-week-old wild-type and Igsf1Δ312/y males. (e) Number of thyrotrope cells counted per unit area on immunohistochemistry-stained sections. (f–h) Tshb, Cga, and Trhr1 mRNA levels in pituitaries of 10-week-old wild-type and Igsf1Δ312/y males. The data were analyzed by a two-tailed unpaired t test: (a) t = 0.7735, df = 20.40, N.S. P = 0.4481; (b) t = 0.3515, df = 22.00, N.S. P = 0.7285; (c) t = 0.4876, df = 20.88, N.S. P = 0.6309; (d) t = 4.645, df = 21.78, ***P = 0.0001; (e) t = 0.5445, df = 9.263, N.S. P = 0.5990; (f) t = 5.316, df = 15.68, ****P < 0.0001; (g) t = 2.644, df = 23.04, *P = 0.0145; (h) t = 4.353, df = 17.82, ***P = 0.0004. N.S., not significant.
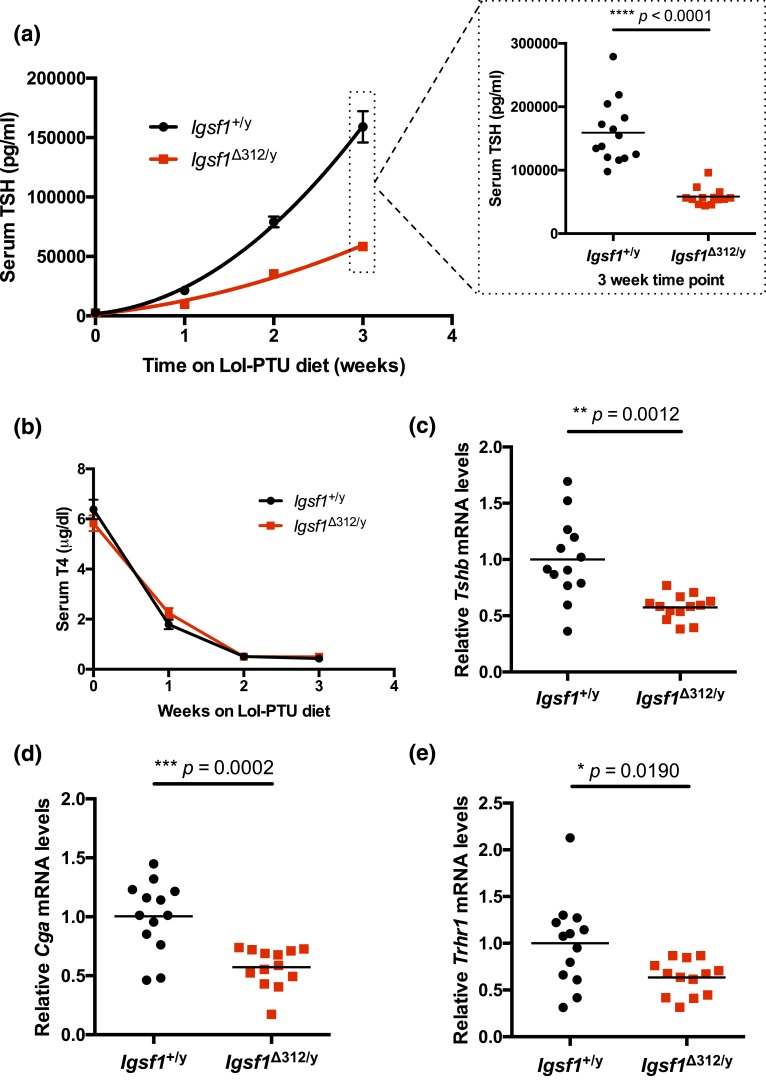
Figure 4.
TSH release is blunted in Igsf1Δ312/y mice. Ten-week-old male wild-type (black line with circles) and Igsf1Δ312/y (black line with squares) mice were placed on a LoI/PTU diet for 3 weeks. (a) Serum TSH was measured immediately before, and then at weekly intervals after, mice were placed on the diet. The data were analyzed by hybrid two-way ANOVA [interaction: F(3, 75) = 40.6, ****P < 0.0001; time on diet: F(3, 75) = 184.0, ****P < 0.0001; genotype: F(1, 25) = 77.68, ****P < 0.0001] and post hoc t tests. n = 14 for wild-type and n = 13 for Igsf1Δ312/y. Data at the 3-week time point are expanded to show individual data points (two-tailed t test: t = 7.322, df = 15.16, ****P < 0.0001). (b) T4 levels were measured from the same serum samples as in (a). The data were analyzed by two-way ANOVA: interaction: F(3, 97) = 2.148, N.S. P = 0.0992; time on diet: F(3, 97) = 349.2, ****P < 0.0001; genotype: F(1, 97) = 0.004213, N.S. P = 0.9484). (c–e) Pituitary RNA from the same animals as in (a) was extracted after 3 weeks on the LoI/PTU diet and (c) Tshb, (d) Cga, and (e) Trhr1 mRNA levels were measured by reverse transcriptase qPCR. The data were analyzed by two-tailed t tests: (c) t = 4.039, df = 14.31, **P = 0.0012; (d) t = 4.531, df = 18.73, ***P = 0.0002; (e) t = 2.614, df = 15.66, *P = 0.0190. N.S., not significant.
T4 levels were measured by RIA [Fig. 3(b); see Supplemental Fig. 8E and 8F (622.7KB, pdf) ] or by enzyme-linked immunosorbent assay (ELISA) [Fig. 4(b); see Supplemental Fig. 10B (622.7KB, pdf) ]. The T4 RIA was performed as described previously (28). The T4 ELISA was performed using a commercially available ELISA kit purchased from MP Biomedicals (Cedarlane Laboratories, Burlington, ON, Canada, catalog no. 07BC-1007) following the manufacturer’s instructions. The data were collected using a SpectraMax Plus 384 (Molecular Devices) plate reader and analyzed using SoftMax Pro v5.0.1. The range of detection was between 0.5 and 25 μg/dL. The intraassay CV was 1.4%, and the interassay CV was 6.8%. T3 levels were measured by RIA [Fig. 3(c); see Supplemental Figs. 8C, 8D, and 12B (622.7KB, pdf) ] as described previously (28).
Reverse transcription quantitative PCR
Total RNA was extracted from frozen pituitary glands in TRIzol following the manufacturer’s instructions and using a Polytron PT 10-35 homogenizer. One hundred nanograms of RNA was treated with 1 U of RQ1 DNase (M6101; Promega) and reverse transcribed with 100 U of Moloney murine leukemia virus reverse transcriptase enzyme (M170B; Promega) in the presence of 20 U of RNAsin (N2111; Promega). Two microliters of the resulting cDNA was analyzed per reaction by quantitative PCR (qPCR) with the EvaGreen 2× qPCR MasterMix-S from Applied Biological Materials (Richmond, BC, Canada) and 0.4 pmol Tshb, Trhr1, Cga, Igsf1, Lhb, Fshb, or Rpl19 primers (Supplemental Table 3 (17.5KB, docx) ). All primer sets were designed to span introns. Single amplicons were confirmed by melting curve analyses and gel electrophoresis. Amplicon identity was validated by DNA sequencing. Primer set efficiency was determined by analyzing serially diluted pituitary cDNA. Data from primer sets with efficiencies that did not fall between between 90% and 110% were corrected as previously described (29). The reactions were performed on a Corbett Rotor-Gene 6200 HRM (Corbett Life Science, Concord, NSW, Australia) according to the EvaGreen cycling conditions. Each sample was run in duplicate and normalized to Rpl19 using the 2−ΔΔCt method (30).
3′ Rapid amplification of cDNA ends
RNA was extracted from pituitaries as described previously. One microgram of RNA was reverse transcribed with reagents in the FirstChoice RLM-RACE kit from Ambion (AM1700; Thermo Fisher). Gene-specific primers (outer, 5′-GAGATCTGGGTGACTGGTAAG-3′, inner, 5′-GATAAGCTTCCTAAACCCTCTCTGTCAGCC-3′) were used with the kit’s outer and inner primers, respectively, to amplify the region of interest by nested PCR. The inner PCR product, flanked with BamHI and HindIII restriction sites, was ligated into pBluescript II KS (+) (X52328; Stratagene, La Jolla, CA) digested with the same enzymes using 10 U of T4 DNA ligase in the presence of 1× T4 DNA ligase buffer at 4°C overnight. The plasmid was transformed into NEB10 competent bacteria, white colonies were selected, and the plasmid DNA was extracted and sequenced (Génome Québec).
Immunohistochemistry
Mice were injected with an overdose of tribromoethanol (Avertin, 20 µL/g body weight, T48402; Sigma-Aldrich) followed by isoflurane inhalation. The anesthetized mice were perfused intracardially with 20 mL of PBS, followed by 20 mL of 4% paraformaldehyde (PFA) in PBS. The pituitary glands were dissected and fixed further in 4% PFA in PBS for 1 hour at RT, followed by an overnight incubation in a 30% sucrose solution in PBS. The pituitary glands were frozen in OCT (4583; Tissue-Tek, Sakura Finetek, Torrance, CA), sectioned at 5 µm with a Leica cryostat, and mounted on glass slides. The sections were washed with PBS containing 0.5% Triton X-100. They were then incubated with 0.5% H2O2 solution in PBS for 30 minutes at RT. Following three 10-minute washes in PBS, the sections were blocked for 1 hour at RT in 3% normal donkey serum in PBS and incubated overnight at RT with a goat anti-TSHβ Ab (1:500, sc-7815; Santa Cruz Biotechnology, Dallas, Texas) (Supplemental Table 2 (17.5KB, docx) ). The next day, the sections were washed in PBS, incubated with rabbit anti-goat biotinylated secondary Ab (1:200, BA-5000; Vector Laboratories, Burlingame, CA) in 3% donkey serum for 1 hour at RT, washed three times for 10 minutes in PBS, incubated with Vectastain ABC reagents for 30 minutes at RT (PK6100; Vector Laboratories), and washed three times for 10 minutes in PBS. The sections were then incubated in a diaminobenzidine solution [0.05% diaminobenzidine, 0.015% H2O2, in PBS (pH 7.2)] for 5 minutes, followed by washing three times for 10 minutes in PBS. Coverslips were then mounted using Permount. Sections were examined using a Leica DM 1000 LED microscope. The TSHβ+ cells (i.e., brown cells) were counted in two sections per animal. For each section, the number of TSHβ+ cells was normalized to the area of the anterior pituitary analyzed.
In situ hybridization
All in situ hybridization (ISH) experiments were carried out as previously described (31). Briefly, brains dissected from male 12-week-old Igsf1+/y and Igsf1Δex1/y mice and 10-week-old Igsf1+/y and Igsf1Δ312/y mice were flash frozen in isopentane cooled to −30°C to −50°C on dry ice and stored at −80°C. Coronal 20-μm slices were cut on a cryostat (Leica CM 3050S), thaw-mounted on silane-treated slides (Thermo Scientific, Darmstadt, Germany), air-dried and stored at −80°C until further processing. Radiolabeled riboprobes were generated by in vitro transcription using the linearized Bluescript SK II (+) plasmid containing a cDNA fragment corresponding to nucleotides 1251 to 1876 of mouse Trh (NM_009426.2) and [35S]uridine triphosphate as labeled substrate (Hartmann Analytic, Braunschweig, Germany). The [35S]-labeled Trh riboprobe was diluted in hybridization buffer [50% formamide, 10% dextran sulfate, 0.6 M NaCl, 10 mM Tris/HCl (pH 7.5), 1× Denhardt’s solution, 100 µg/mL sonicated salmon sperm DNA, 1 mM EDTA-di-Na, 0.5 mg/mL transfer RNA, and 10 mM dithiothreitol] to a final concentration of 1 × 104 cpm/µL. Additionally, unlabeled TRH riboprobe (final concentration: 0.5 ng/µL) was added to the hybridization mix. Prior to application, frozen sections were fixed with 4% PFA in PBS for 60 minutes at RT, rinsed three times with PBS, and then permeabilized with 0.4% Triton X-100 in PBS for 10 minutes. After a washing step with PBS, sections were incubated in 0.1 M triethanolamine (pH 8), supplemented with 0.25% (v/v) acetic anhydride for 10 minutes, and rinsed three times with PBS. Sections were dehydrated with successive ethanol washes of increasing concentrations, followed by air-drying. Coverslips were then overlayed and sections incubated in a humidified chamber at 58°C for 16 hours. Two times standard saline citrate [SSC; 0.3 M NaCl, 0.03 M sodium citrate (pH 7.0)] was used to remove coverslips after hybridization and sections were treated with RNase A (20 μg/mL) and RNase T1 (1 U/mL) at 37°C for 30 minutes. Sections were then washed at RT with decreasing concentrations of SSC (1×, 0.5×, and 0.2×) for 20 minutes each and then incubated in 0.2× SSC at 65°C for 1 hour. Sections were dehydrated and exposed to x-ray film (BioMax MR; Kodak, Rochester, NY) for 24 hours and then dipped in Kodak NTB2 nuclear emulsion and stored at 4°C for 3 days. Autoradiograms were developed in Kodak D19 for 7 minutes and fixed in Kodak Rapid Fixer for 14 minutes. Sections were viewed under darkfield illuminations. Images were quantified using ImageJ, where background signal was subtracted from the signal intensity and divided by the area of the image.
T3/T4 replacement
Eight-week-old male Igsf1+/y and Igsf1Δex1/y mice were subjected to a LoI/PTU diet (as described above). During the last 4 days, mice were injected i.p. with Dulbecco’s PBS containing 0.002% BSA (control), 5 ng of L-T3/g body weight per day (low T3), 25 ng of L-T3/g body weight per day (high T3), or 100 ng of L-T4/g body weight per day (T4). L-T3 and L-T4 were dissolved in 1 M NaOH/EtOH (1:10) at 1 mg/mL and diluted in Dulbecco’s PBS with 0.002% BSA for working concentrations of 1 µg/mL (low T3), 200 µg/mL (high T3), and 100 µg/mL (T4), allowing injection of ∼200 µL per animal regardless of treatment. Twelve hours after the last injection, animals were killed via CO2 asphyxiation and blood, brains, and pituitaries were collected as described above.
Statistics
Body weight, testis weight, thyrotrope cell number, and hormone levels were compared using unpaired two-tailed t tests. Statistical comparisons of mRNA levels were made using unpaired t tests, with Welch’s correction when the standard deviations (SDs) were significantly different as assessed by F test. The mRNA levels from Igsf1Δ312/y animals were normalized relative to those of Igsf1+/y littermates for each gene analyzed. In the case of the mRNA analysis of the mice on the standard and LoI/PTU diets, Tshb, Cga, and Trhr1 data were square root transformed to make the distributions normal. Data from TRH injection experiments with the Igsf1Δ312/y mice, from the LoI/PTU experiment with the Igsf1Δex1/y mice, and from Trh ISH with the Igsf1Δex1/y mice were analyzed by two-way analysis of variance (ANOVA). The TSH measurements from the LoI/PTU diet experiment with the Igsf1Δex1/y mice were log transformed. The T3 and T4 data from the same experiment were analyzed by a one-sample t test. The data from the LoI/PTU diet experiment with Igsf1Δ312/y mice, as well as from the TRH injection experiment with the Igsf1Δex1 mice, were analyzed with a hybrid repeated measures two-way ANOVA. Genotype was a between-subject factor and time was a within-subject factor. In the experiment comparing Igsf1Δ312/y and control mice on the LoI/PTU diet vs normal diet, the Trhr1 mRNA levels were log transformed. The TSH levels after 3 weeks on the diet and 15 minutes after TRH injections were also compared by unpaired two-tailed t tests post hoc. Datasets with potential outliers were tested with ROUT (Q = 1.0%). Outliers were not removed from figures, but alternative statistical tests without outliers are presented in the figure legends (see Supplemental Figs. 8A and 10A (622.7KB, pdf) ) when outliers were present. The number of replicates and results of the statistical tests are found in the text and figure legends. The statistical analyses were made using GraphPad Prism 6. Significance was assessed relative to P < 0.05.
Results
Generation of a novel Igsf1 loss-of-function mouse model
A potential concern with Igsf1Δex1 mice is that they continue to express the Igsf1-4 mRNA isoform in their pituitary glands (19). Igsf1-4 mRNA encodes the entirety of the IGSF1-CTD (Supplemental Fig. 2 (622.7KB, pdf) ) and could, in principle, compensate for the loss of the protein derived from the larger, more abundant Igsf1-1 transcript. To address this concern, we generated a new loss-of-function mouse model that directly disrupts the IGSF1-CTD, regardless of the transcript from which it is derived.
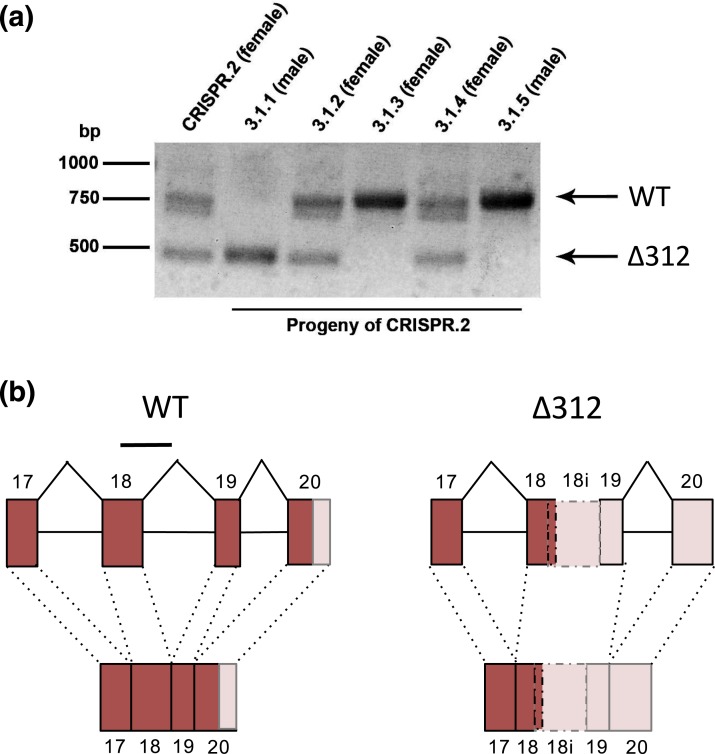
There are no consensus mutations in IGSF1-deficient patients; however, there is a relatively high density of frame-shift and nonsense mutations in exon 18, which encodes the terminal 12th Ig loop (Supplemental Fig. 1 (622.7KB, pdf) ). We, therefore, used CRISPR-Cas9 technology to introduce deletions in Igsf1 exon 18 of one-cell murine zygotes (32, 33). An initial litter of five pups contained one male and one female without any observable Igsf1 mutations (CRISPR.1 and CRISPR.5 in Supplemental Fig. 3 (622.7KB, pdf) ); one male with a 9-bp deletion (recall that Igsf1 is X-linked; CRISPR.3); one female with a 6-bp deletion on one allele and no mutation on the other (CRISPR.4); and a female (hereafter CRISPR.2) with a 6-bp deletion on one allele and a 312-bp deletion on the other [Fig. 1(a), lane 1; Supplemental Fig. 3 (622.7KB, pdf) ].
Figure 1.
Generation of Igsf1 loss-of-function mice using CRISPR-Cas9. (a) PCR amplification of the targeted region in exon 18 of Igsf1 in the founder female (CRISPR.2, lane 1) and five of her progeny (3.1.1 to 3.1.5, lanes 2 to 6). Arrows at the right indicate the wild-type (top) or Δ312 (bottom) alleles. (b) Genomic organization (top) and RNA splicing (bottom) around exons 17 to 20 of the wild-type (left) and Δ312 alleles (right). Exons are presented as boxes and are numbered. Intervening introns are shown as lines. The deleted parts of exon 18 and intron 18 in the Δ312 allele are shown with a line at the top of the wild-type schematic (left). The novel exon in the Δ312 allele, which is derived from the 5′ end of exon 18, the 3′ end of intron 18 (labeled 18i), and all of exon 19, is shown with broken lines (right). Dark boxes denote translated sequences, whereas the pale boxes reflect untranslated regions. Genomic structure 5′ of exon 17 is not pictured. WT, wild-type.
As the 6- and 9-bp deletions were in-frame, we examined whether they were damaging in a heterologous in vitro expression system. Neither markedly affected expression of the IGSF1-CTD (Supplemental Fig. 4 (622.7KB, pdf) , compare lanes 4 and 5 to lanes 2 and 3). In contrast, the 312-bp deletion removed the 3′ end of exon 18 (139 bp) and the 5′ end of intron 18 (173 bp) of the Igsf1 gene [Fig. 1(b)]. This deletion, which we now refer to as Igsf1Δ312, was predicted to cause a frame-shift and premature termination of the CTD (more below). We therefore pursued analyses using this allele.
To address concerns about off-target effects of the Cas9 enzyme, we examined the three noncoding and three coding regions most likely to be targeted by the guide RNA, according to Hsu et al. (34). None of these regions was altered in Igsf1Δ312/y mice (data not shown).
The Igsf1Δ312 allele encodes a truncated IGSF1-CTD
Igsf1 mRNA expression was altered in pituitaries of Igsf1Δ312/y males (please note that as this is an X-linked gene and a male-specific disorder, our analysis was limited to male mice). In contrast to the splicing of exons 18 and 19 seen in wild-type mice, the mutants expressed an mRNA harboring a novel hybrid exon comprising the 5′ end of exon 18 (140 bp) and the 3′ end of intron 18 (215 bp) spliced to exon 19 [Fig. 1(b), right]. As a result, 47 amino acids normally encoded by exon 18 were lost and six novel amino acids followed by a premature stop codon were introduced from the retained intron 18 sequence. Amino acids encoded by exons 19 and 20, including the transmembrane domain and intracellular C-terminal tail (C-tail), were lost (Supplemental Fig. 5 (622.7KB, pdf) ). No Igsf1Δ312/y mutants expressed the wild-type Igsf1 mRNA (Supplemental Fig. 6A (622.7KB, pdf) ), and no wild-type mice expressed the mutant Igsf1 mRNA in their pituitary glands (Supplemental Fig. 6B (622.7KB, pdf) ).
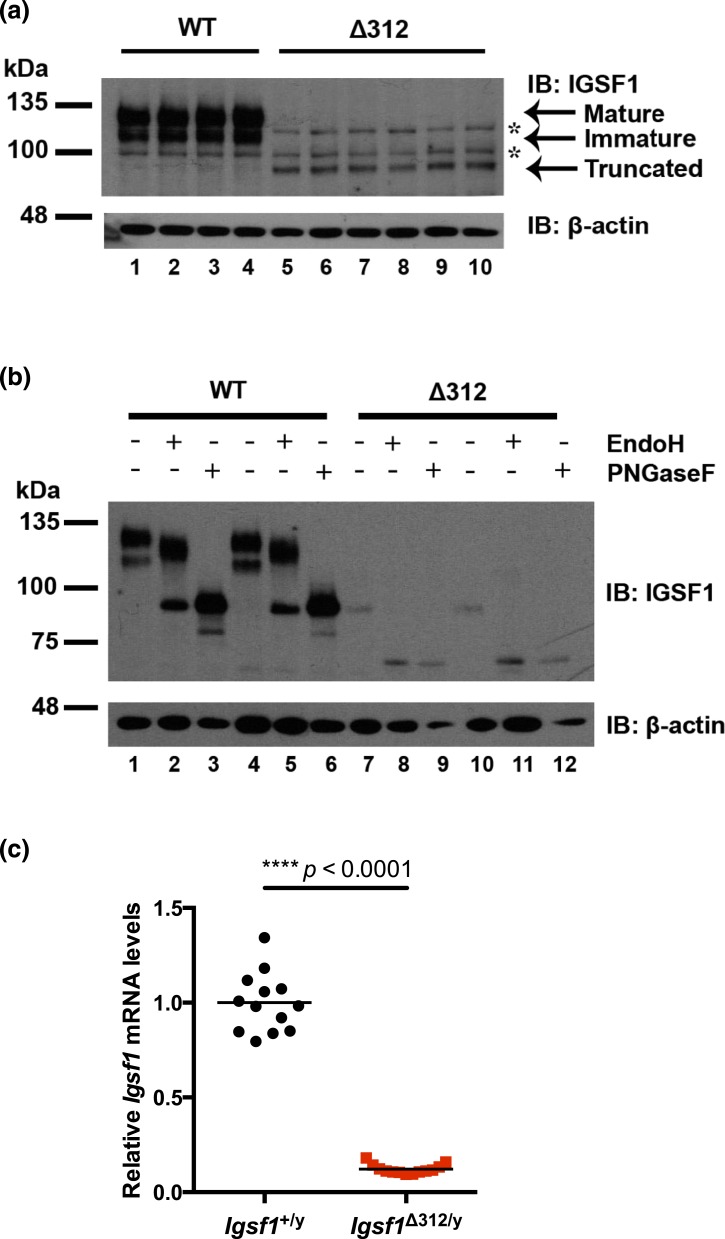
Premature truncation of the human protein in Ig loop 12 prevents membrane trafficking of the IGSF1-CTD (10, 11, 13, 15, 17, 35). This appeared to be the case in Igsf1Δ312/y mice as well. As expected, the IGSF1-CTD in wild-type mouse pituitary migrated as a doublet on SDS-PAGE [Fig. 2(a), lanes 1 to 4). These represent mature (top band, EndoH-resistant) and immature glycoforms (bottom band, EndoH sensitive) of the protein [Fig. 2(b), lanes 2 and 5]. In contrast, neither of these protein glycoforms was expressed in pituitaries of Igsf1Δ312/y males [Fig. 2(a), lanes 5 to 10]. Rather, they expressed a novel, truncated protein of ∼90 kDa. This protein was EndoH sensitive [Fig. 2(b), lanes 8 and 11], indicating that it was a glycoprotein that failed to transit from the ER to the Golgi. IGSF1-CTD protein expression in pituitaries of Igsf1Δ312/y mice was reduced relative to wild-type [Fig. 2(a) and 2(b)]. The lower abundance protein expression derived, at least in part, from markedly reduced Igsf1 mRNA expression and/or stability in Igsf1Δ312/y mice [Fig. 2(c)].
Figure 2.
Reduced pituitary IGSF1 protein and mRNA expression in Igsf1Δ312/y mice. (a) Proteins extracted from four adult wild-type (lanes 1 to 4) and six Δ312 males (lanes 5 to 10) were subjected to SDS-PAGE and immunoblotting using an anti–IGSF1-CTD antibody. Arrows mark the mature and immature glycoforms in wild-type and the truncated protein in Δ312 males. *, nonspecific bands. (b) Protein extracts from two wild-type (lanes 1 to 6) and two Δ312 males (lanes 7 to 12) were treated with EndoH or PNGaseF prior to analysis as in (a). (c) RNA was extracted from pituitaries of adult wild-type (circles) or Δ312 males (squares). mRNA expression of Igsf1 was analyzed by reverse transcription qPCR using primers common to both transcripts. Group means are indicated with solid horizontal lines. The data were analyzed by a two-tailed t test (t = 19.95, df = 12.64, ****P < 0.0001). IB, immunoblotting; WT, wild-type.
Igsf1Δ312/y mice exhibit reduced TSH production
Having established impaired IGSF1-CTD protein expression and trafficking in pituitaries of Igsf1Δ312/y mice, we next characterized their phenotypes. Adult Igsf1Δ312/y males had significantly increased body mass compared with their wild-type littermates (Supplemental Fig. 7A (622.7KB, pdf) ), consistent with what we reported previously in Igsf1Δex1/y mice (11). Some Igsf1Δ312 males also had increased testis mass, even when correcting for the difference in body mass (Supplemental Fig. 7B (622.7KB, pdf) ); however, we did not observe enlarged testes in a second cohort of animals (data not shown). Therefore, it is not yet clear whether these mice have true macroorchidism.
We previously reported that Igsf1Δex1/y males had suppressed TSH and T3 levels on a control diet relative to wild-type controls (11). However, follow-up analyses in the same strain yielded variable results, with TSH, T4, and T3 levels differing or not between genotypes depending on the experiment (Supplemental Fig. 8 (622.7KB, pdf) ). In this study, in the new model, serum TSH, T4, and T3 levels were statistically equivalent between Igsf1Δ312/y males and their wild-type littermates [Fig. 3(a–c)], although there was a nonsignificant trend for TSH levels to be reduced in the former (P = 0.4481). TSH protein content, however, was significantly reduced in the pituitaries of Igsf1Δ312/y males [Fig. 3(d)]. Reduced TSH production did not appear to result from a reduction in the number of thyrotropes in Igsf1Δ312/y mice [Fig. 3(e)].
In light of these results, we measured pituitary Tshb, Cga, and Trhr1 mRNA levels in a second cohort of mice (as pituitaries in the previous cohort were used in the protein content analysis). We observed significant decreases in pituitary expression of all three genes in Igsf1Δ312/y relative to wild-type littermates [Fig. 3(f–h)]. In contrast, Fshb or Lhb mRNA levels were normal in pituitaries of Igsf1Δ312/y males (Supplemental Fig. 9B–E (622.7KB, pdf) ). Notably, there was again a nonsignificant trend for serum TSH to be reduced in this cohort of Igsf1Δ312/y males relative to controls; their T4 levels were equivalent to wild-type (Supplemental Fig. 10 (622.7KB, pdf) ).
TSH release is blunted in Igsf1Δ312/y mice
Although Igsf1Δ312/y males demonstrated a clear impairment in pituitary TSH synthesis, they were nonetheless able to maintain an apparently euthyroid state under normal laboratory conditions. We next asked how they would respond to a physiological challenge. Specifically, we rendered mice profoundly hypothyroid by placing them on an LoI/PTU diet for a period of 3 weeks. Serum T4 levels were significantly reduced within 1 week and maximally by 2 weeks in both genotypes on the LoI/PTU diet [Fig. 4(b)]. Serum TSH levels increased markedly across the 3 weeks of the experiment, from 2725.0 to 159,100.0 pg/mL [Fig. 4(a)] in wild-type males. TSH levels also increased in Igsf1Δ312/y mice, but the response was significantly blunted, from 2410.25 to 58,412.5 pg/mL [Fig. 4(a)]. Associated with the impaired TSH secretion, Tshb, Cga, and Trhr1 mRNA levels were also reduced in pituitaries of Igsf1Δ312/y males relative to controls after 3 weeks on the LoI/PTU diet [Fig. 4(c–e)]. In a second, but smaller cohort of animals, we directly compared pituitary gene expression on the control and LoI/PTU diets. Pituitary Tshb, Cga, and Trhr1 mRNA levels increased in response to the LoI/PTU diet in both genotypes, but the response was blunted in Igsf1Δ312/y relative to control mice (Supplemental Fig. 11 (622.7KB, pdf) ). Consistent with these data in Igsf1Δ312/y mice, the previously described Igsf1 knockout strain (Igsf1Δex1/y) also secreted less TSH on the LoI/PTU diet than did controls (Supplemental Fig. 12 (622.7KB, pdf) ).
TRH-induced TSH release is blunted in Igsf1-deficient mice
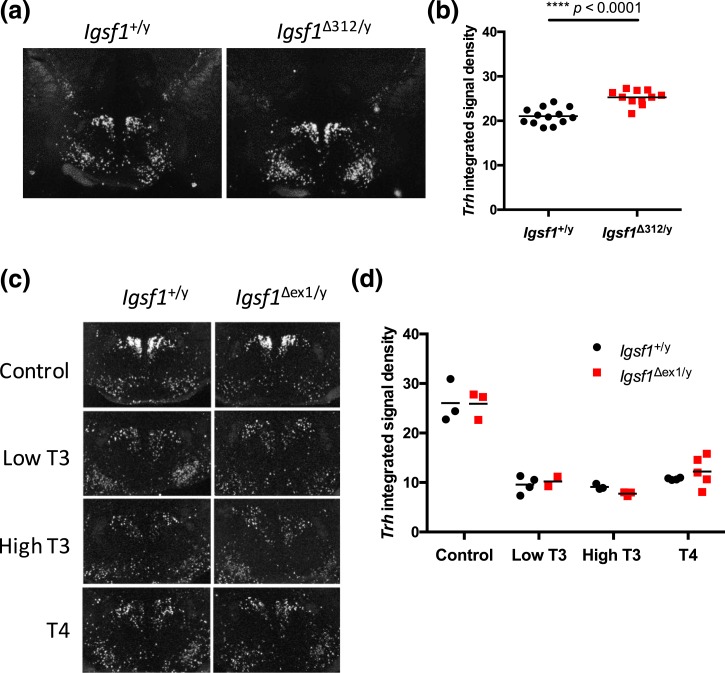
As Igsf1 is expressed in both the pituitary and brain (11, 18, 19, 36), blunted TSH release in Igsf1-deficient mice on the LoI/PTU diet could be explained by dysfunction in thyrotrope cells, the hypothalamus, or both. We reported previously that hypothalamic Trh mRNA levels were increased in Igsf1Δex1/y relative to control mice (11). Similarly, in this study we observed a small, but significant increase in Trh mRNA levels in the paraventricular nuclei (PVN) of Igsf1Δ312/y mice relative to littermate controls on standard laboratory chow [Fig. 5(a)]. Circulating T4 levels were normal in these particular animals (Supplemental Fig. 10B (622.7KB, pdf) ). It is therefore unclear whether the increased Trh mRNA levels reflected a response to peripheral hypothyroidism that we failed to detect or a hypothalamic defect. That being said, Trh mRNA levels were equally high in the PVN of wild-type and Igsf1Δex1/y mice on a LoI/PTU diet, and they were equivalently downregulated in response to exogenous T3 or T4 (Fig. 5B). Thus, increased Trh mRNA levels in Igsf1-deficient mice likely reflect mild hypothyroidism (i.e., reduced feedback) that our assays failed to detect.
Figure 5.
Thyroid hormone feedback mechanisms appear to be intact in Igsf1-deficient mice. (a) Representative ISH images of Trh mRNA expression in the PVN of brains from 8-week-old Igsf1+/y and Igsf1Δ312/y mice on a normal diet. (b) Quantification of average Trh ISH signal in Igsf1+/y and Igsf1Δ312/y brains. The data were analyzed by two-tailed t test: t = 5.861, df = 21.87, ****P < 0.0001. (c) Representative ISH images of Trh mRNA expression in the PVN of brains from 11-week-old Igsf1+/y and Igsf1Δex1/y mice on a LoI/PTU diet for 3 weeks. On the last 4 days of the experiment the mice were injected with one of four treatments (saline, low T3, high T3, high T4). (d) Quantification of average Trh ISH signal in Igsf1+/y and Igsf1Δex1/y brains in each condition. The data were analyzed by two-way ANOVA [interaction: F(3, 19) = 0.4753, N.S. P = 0.7032; treatment: F(3, 19) = 72.83, ****P < 0.0001; genotype: F(1, 19) = 0.02785, N.S. P = 0.8692]. N.S., not significant.
We therefore focused on pituitary responsiveness to TRH, in particular because of the reduced pituitary Trhr1 mRNA expression in both Igsf1Δ312/y [Figs. 3(h) and 4(e); Supplemental Fig. 11C (622.7KB, pdf) ) and Igsf1Δex1/y mice (11). In response to exogenous TRH, wild-type males showed a robust increase in TSH secretion (from 1033.75 to 16,152.5 pg/mL) within 15 minutes [Fig. 6(a)]. Plasma TSH levels were still elevated, but well below peak, after 1 hour (2090.25 pg/mL), and returned to baseline 2 hours after TRH injection (896.25 pg/mL). The TSH response in Igsf1Δ312/y mice was comparatively blunted (921.5 to 6925.0 pg/mL) 15 minutes after TRH injection [Fig. 6(a)]. We observed a similar impairment in TRH-induced TSH release in Igsf1Δex1/y mice compared with Igsf1+/y littermates [Fig. 6(b)]. Note that the experiments in Fig. 6 used different TSH assays (ELISA vs RIA). However, we showed in independent analyses that the two assays yielded highly similar results when applied to the same samples (data not shown).
Discussion
Loss of IGSF1 function results in central hypothyroidism in humans and mice (11). Until now, the underlying disease mechanism was unresolved. Based on the results of analyses in two distinct, but complementary mouse models, we conclusively demonstrate that IGSF1 deficiency causes a reduction in pituitary responsiveness to TRH. Specifically, pituitary Trhr1 expression is downregulated, resulting in impairments in TRH induction of TSH synthesis and secretion. Consistent with this model, TRH-induced TSH release is also impaired in nearly all neonatal IGSF1-deficient patients, and it is impaired or low-normal in children and adults (11, 12). Therefore, central hypothyroidism in IGSF1 deficiency syndrome stems principally from a defect in pituitary (and specifically thyrotrope), rather than brain, function.
Although IGSF1 is expressed centrally (18, 36, 37), the available data do not suggest a hypothalamic defect. First, Trh mRNA expression is modestly elevated, not reduced, in the PVN of Igsf1-deficient mice. Assuming that increased expression translates into enhanced TRH release, one would expect increased TSH levels in these animals. Rather, TSH synthesis and/or secretion are either normal or reduced, further supporting the proposed mechanism of impaired TRH action. Second, Trh mRNA expression is appropriately reduced in response to exogenous T3 or T4 in the PVN of Igsf1Δex1/y mice, indicating that thyroid hormone feedback regulation in the brain is intact. Admittedly, we cannot definitively rule out hypothalamic defects until we measure TRH release at the level of the pituitary portal vessels. However, these are technically challenging experiments, particularly in mice, and may require analyses in larger animal models of Igsf1 deficiency.
Although Trhr1 mRNA levels are reduced in pituitaries of Igsf1-deficient mice, we do not yet know whether this alone explains their CeH. Mice with global deletions of Trhr1 have greater reductions in thyroid hormone levels (38, 39) than we observe in either Igsf1-deficient strain. However, the more apt comparison would be with heterozygous Trhr1 knockout mice, as the animals analyzed in the present study have reduced, but not absent, Trhr1 expression. Unfortunately, to our knowledge, the phenotypes of Trhr1+/− mice have not been investigated systematically. It is also possible that, in addition to reductions in Trhr1 expression, Igsf1-deficient mice might exhibit alterations in hormone metabolism at the pituitary level. For example, increases in expression of the TRH degrading enzyme (Trhde) could reduce pituitary sensitivity to TRH (exogenous or endogenous). However, we did not observe alterations in pituitary Trhde mRNA levels in either Igsf1-deficient strain (data not shown). Alternatively, increased sensitivity of the pituitary to thyroid hormone feedback (e.g., through alterations in deiodination or action) could contribute to reduced TSH production or release (40–45). However, Trhr1, Tshb, and Cga mRNA levels, as well as TSH release, were attenuated in Igsf1-deficient animals on the LoI/PTU diet, suggesting that their regulation is at least in part thyroid hormone–independent. Therefore, the available data point principally to a defect in expression of and/or signaling by the TRH receptor in thyrotropes.
The molecular basis for reduced Trhr1 expression in Igsf1-deficient mice is presently unresolved. This stems from our relatively limited understanding of mechanisms controlling Trhr1 mRNA expression in thyrotropes (46–48) coupled with our ignorance of IGSF1 function. The data suggest that IGSF1 may be a positive regulator of Trhr1 mRNA expression, but they provide no insight into the basis for this regulation. The IGSF1-CTD is a transmembrane protein with a large extracellular domain of seven immunoglobulin loops and a short intracellular C-tail. Other transmembrane immunoglobulin proteins function as both ligands and receptors (49, 50), but molecules (proteins or otherwise) interacting with the extracellular domain of IGSF1-CTD have not been described. Moreover, the IGSF1-CTD C-tail lacks known functional domains. Therefore, we cannot yet determine whether or how IGSF1-CTD participates in intercellular or intracellular signaling, or how alterations in this signaling lead (directly or indirectly) to reductions in Trhr1 mRNA expression.
A major strength of the present study was our use of two independent mouse models of Igsf1 deficiency. Not only do the two models converge to indicate a major impairment in TRH action, but they each help address potential concerns about the other. First, we can conclude that the phenotypes of the newly generated Igsf1Δ312 mice result from loss of IGSF1-CTD function and do not reflect off-target effects of the Cas9 enzyme (51–53). Second, the variably penetrant phenotypes of the previously generated Igsf1Δex1 model are unlikely to reflect compensatory actions of IGSF1-CTD derived from the residual Igsf1-4 transcript. In fact, both models show variable CeH phenotypes under standard laboratory conditions. It is only when increased demands are placed on the TRH signaling system that the full extent of CeH can be appreciated. One can imagine, therefore, that interindividual variation that we observe in these congenic strains may derive from perturbations in the laboratory environment that are imperceptible to or unnoticed by the investigators, such as temperature fluctuations or other stressors (54–56). It is notable that individuals within a given family carrying the same IGSF1 mutation can similarly exhibit considerable variation in their relative extents of hypothyroidism (2, 10–15, 17, 57). Whether this derives from epigenetic effects, modifier genes, or acute environmental variables has yet to be determined.
In summary, central hypothyroidism in IGSF1 deficiency syndrome derives in full or in part from deficiencies in TRH action in pituitary thyrotrope cells. It is not yet clear whether Trhr1 downregulation alone explains the phenotype or whether there are also impairments in TRH receptor signaling. The mechanisms underlying the decrease in receptor expression as well as the normal functions of IGSF1 are still unresolved. What is clear, however, is that individuals with IGSF1 deficiency are likely to show impaired TSH synthesis and secretion under conditions that increase TRH drive from the hypothalamus to the pituitary gland.
Acknowledgments
The authors thank Dr. Kristen Vella for expert advice regarding the TRH stimulation assays, the Goodman Cancer Research Centre Mouse Transgenic Facility for assisting with the production of the new mouse model and for performing cytoplasmic microinjections, Séverine Audusseau in the laboratory of Dr. Qutayba Hamid (McGill University Health Centre) for help with the Luminex assay, Dr. Beata Bak for contributing the data in Supplemental Fig. 2, and Monique Losekoot in the laboratory of Clinical Genetics of the Leiden University Medical Center for providing results from Sanger sequencing of IGSF1 in affected patients.
Acknowledgments
This work was supported by National Institutes of Health Grant R37DK15070 (to S.R.), Canadian Institutes for Health Research Grant MOP-133557 and Natural Sciences and Engineering Research Council Grants 2015-05178 (to D.J.B.), as well as by a Canada Graduate Scholarships–Master’s Program Natural Sciences and Engineering Research Council training grant and Réseau Québécois en reproduction FONCER grant (to M.O.T.). H.H. and D.D. were supported by Deutsche Forschungsgemeinschaft Grant SPP1629/HE3418/8-1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- BSA
- bovine serum albumin
- cDNA
- complementary DNA
- CeH
- central hypothyroidism
- crRNA
- CRISPR RNA
- C-tail
- C-terminal tail
- CTD
- C-terminal domain
- CV
- coefficient of variation
- ELISA
- enzyme-linked immunosorbent assay
- ER
- endoplasmic reticulum
- IGSF1
- immunoglobulin superfamily, member 1
- IGSF1-4
- immunoglobulin superfamily, member 1 isoform 4
- i.p.
- intraperitoneal
- ISH
- in situ hybridization
- LoI
- low iodine
- LoI/PTU
- low iodine diet supplemented with propylthiouracil
- mRNA
- messenger RNA
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PFA
- paraformaldehyde
- PMSF
- phenylmethylsulfonyl fluoride
- PTU
- propylthiouracil
- PVN
- paraventricular nuclei
- qPCR
- quantitative polymerase chain reaction
- RIA
- radioimmunoassay
- RT
- room temperature
- SD
- standard deviation
- SDS
- sodium dodecyl sulfate
- SDS-PAGE
- sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SSC
- standard saline citrate
- TBS
- Tris-buffered saline
- TBST
- Tris-buffered saline containing 0.05% Tween 20
- TRH
- thyrotropin-releasing hormone
- Trhde
- thyrotropin-releasing hormone degrading enzyme
- TSH
- thyrotropin.
References
- 1.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 2.Joustra SD, van Trotsenburg AS, Sun Y, Losekoot M, Bernard DJ, Biermasz NR, Oostdijk W, Wit JM. IGSF1 deficiency syndrome: a newly uncovered endocrinopathy. Rare Dis. 2013;1:e24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lania A, Persani L, Beck-Peccoz P. Central hypothyroidism. Pituitary. 2008;11(2):181–186. [DOI] [PubMed] [Google Scholar]
- 4.Persani L. Clinical review: Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97(9):3068–3078. [DOI] [PubMed] [Google Scholar]
- 5.Yamada M, Mori M. Mechanisms related to the pathophysiology and management of central hypothyroidism. Nat Clin Pract Endocrinol Metab. 2008;4(12):683–694. [DOI] [PubMed] [Google Scholar]
- 6.Alatzoglou KS, Dattani MT. Genetic forms of hypopituitarism and their manifestation in the neonatal period. Early Hum Dev. 2009;85(11):705–712. [DOI] [PubMed] [Google Scholar]
- 7.Castinetti F, Reynaud R, Saveanu A, Jullien N, Quentien MH, Rochette C, Barlier A, Enjalbert A, Brue T. Mechanisms in Endocrinology: an update in the genetic aetiologies of combined pituitary hormone deficiency. Eur J Endocrinol. 2016;174(6):R239–R247. [DOI] [PubMed] [Google Scholar]
- 8.Fang Q, Benedetti AF, Ma Q, Gregory L, Li JZ, Dattani M, Sadeghi-Nejad A, Arnhold IJ, Mendonca BB, Camper SA, Carvalho LR. HESX1 mutations in patients with congenital hypopituitarism: variable phenotypes with the same genotype. Clin Endocrinol (Oxf). 2016;85(3):408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinen CA, Losekoot M, Sun Y, Watson PJ, Fairall L, Joustra SD, Zwaveling-Soonawala N, Oostdijk W, van den Akker EL, Alders M, Santen GW, van Rijn RR, Dreschler WA, Surovtseva OV, Biermasz NR, Hennekam RC, Wit JM, Schwabe JW, Boelen A, Fliers E, van Trotsenburg AS. Mutations in TBL1X are associated with central hypothyroidism. J Clin Endocrinol Metab. 2016;101(12):4564–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joustra SD, Schoenmakers N, Persani L, Campi I, Bonomi M, Radetti G, Beck-Peccoz P, Zhu H, Davis TM, Sun Y, Corssmit EP, Appelman-Dijkstra NM, Heinen CA, Pereira AM, Varewijck AJ, Janssen JA, Endert E, Hennekam RC, Lombardi MP, Mannens MM, Bak B, Bernard DJ, Breuning MH, Chatterjee K, Dattani MT, Oostdijk W, Biermasz NR, Wit JM, van Trotsenburg AS. The IGSF1 deficiency syndrome: characteristics of male and female patients. J Clin Endocrinol Metab. 2013;98(12):4942–4952. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Bak B, Schoenmakers N, van Trotsenburg AS, Oostdijk W, Voshol P, Cambridge E, White JK, le Tissier P, Gharavy SN, Martinez-Barbera JP, Stokvis-Brantsma WH, Vulsma T, Kempers MJ, Persani L, Campi I, Bonomi M, Beck-Peccoz P, Zhu H, Davis TM, Hokken-Koelega AC, Del Blanco DG, Rangasami JJ, Ruivenkamp CA, Laros JF, Kriek M, Kant SG, Bosch CA, Biermasz NR, Appelman-Dijkstra NM, Corssmit EP, Hovens GC, Pereira AM, den Dunnen JT, Wade MG, Breuning MH, Hennekam RC, Chatterjee K, Dattani MT, Wit JM, Bernard DJ. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet. 2012;44(12):1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes JN, Aubert M, Heatlie J, Gardner A, Gecz J, Morgan T, Belsky J, Thomas PQ. Identification of an IGSF1-specific deletion in a five-generation pedigree with X-linked central hypothyroidism without macroorchidism. Clin Endocrinol (Oxf). 2016;85(4):609–615. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura A, Bak B, Silander TL, Lam J, Hotsubo T, Yorifuji T, Ishizu K, Bernard DJ, Tajima T. Three novel IGSF1 mutations in four Japanese patients with X-linked congenital central hypothyroidism. J Clin Endocrinol Metab. 2013;98(10):E1682–E1691. [DOI] [PubMed] [Google Scholar]
- 14.Van Hulle S, Craen M, Callewaert B, Joustra S, Oostdijk W, Losekoot M, Wit JM, Turgeon MO, Bernard DJ, De Schepper J. Delayed adrenarche may be an additional feature of immunoglobulin super family member 1 deficiency syndrome. J Clin Res Pediatr Endocrinol. 2016;8(1):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakover YT, Turgeon MO, London S, Hermanns P, Pohlenz J, Bernard DJ, Bercovich D. Familial central hypothyroidism caused by a novel IGSF1 gene mutation. Thyroid. 2016;26(12):1693–1700. [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1089/thy.2016.0005. Nishigaki S, Hamazaki T, Fujita K, Morikawa S, Tajima T, Shintaku H. A Japanese family with central hypothyroidism caused by a novel IGSF1 mutation. Thyroid. 2016;26(12):1701–1705. doi:10.1089/thy.2016.0005. [DOI] [PubMed] [Google Scholar]
- 17.Joustra SD, Heinen CA, Schoenmakers N, Bonomi M, Ballieux BE, Turgeon MO, Bernard DJ, Fliers E, van Trotsenburg AS, Losekoot M, Persani L, Wit JM, Biermasz NR, Pereira AM, Oostdijk W; IGSF1 Clinical Care Group . IGSF1 deficiency: lessons from an extensive case series and recommendations for clinical management. J Clin Endocrinol Metab. 2016;101(4):1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joustra SD, Meijer OC, Heinen CA, Mol IM, Laghmani H, Sengers RM, Carreno G, van Trotsenburg AS, Biermasz NR, Bernard DJ, Wit JM, Oostdijk W, van Pelt AM, Hamer G, Wagenaar GT. Spatial and temporal expression of immunoglobulin superfamily member 1 in the rat. J Endocrinol. 2015;226(3):181–191. [DOI] [PubMed] [Google Scholar]
- 19.Bernard DJ, Burns KH, Haupt B, Matzuk MM, Woodruff TK. Normal reproductive function in InhBP/p120-deficient mice. Mol Cell Biol. 2003;23(14):4882–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke PS, Meisami E. Early hypothyroidism in rats causes increased adult testis and reproductive organ size but does not change testosterone levels. Endocrinology. 1991;129(1):237–243. [DOI] [PubMed] [Google Scholar]
- 21.De França LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec. 1995;242(1):57–69. [DOI] [PubMed] [Google Scholar]
- 22.Holsberger DR, Kiesewetter SE, Cooke PS. Regulation of neonatal Sertoli cell development by thyroid hormone receptor α1. Biol Reprod. 2005;73(3):396–403. [DOI] [PubMed] [Google Scholar]
- 23.Frattini A, Faranda S, Redolfi E, Allavena P, Vezzoni P. Identification and genomic organization of a gene coding for a new member of the cell adhesion molecule family mapping to Xq25. Gene. 1998;214(1–2):1–6. [DOI] [PubMed] [Google Scholar]
- 24.Mazzarella R, Pengue G, Jones J, Jones C, Schlessinger D. Cloning and expression of an immunoglobulin superfamily gene (IGSF1) in Xq25. Genomics. 1998;48(2):157–162. [DOI] [PubMed] [Google Scholar]
- 25.Robakis T, Bak B, Lin SH, Bernard DJ, Scheiffele P. An internal signal sequence directs intramembrane proteolysis of a cellular immunoglobulin domain protein. J Biol Chem. 2008;283(52):36369–36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joustra SD, Wehkalampi K, Oostdijk W, Biermasz NR, Howard S, Silander TL, Bernard DJ, Wit JM, Dunkel L, Losekoot M. IGSF1 variants in boys with familial delayed puberty. Eur J Pediatr. 2015;174(5):687–692. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1089/thy.1999.9.1265. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9(12):1265–1271. doi:10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara AM, Liao XH, Gil-Ibáñez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154(7):2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dzidic A, Mohr A, Meyer K, Bauer J, Meyer HH, Pfaffl MW. Effects of mycophenolic acid (MPA) treatment on expression of Fc receptor (FcRn) and polymeric immunoglobulin receptor (pIgR) mRNA in adult sheep tissues. Croat Med J. 2004;45(2):130–135. [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 31.Heuer H, Schäfer MK, O’Donnell D, Walker P, Bauer K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J Comp Neurol. 2000;428(2):319–336. [PubMed] [Google Scholar]
- 32.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajima T, Nakamura A, Ishizu K. A novel mutation of IGSF1 in a Japanese patient of congenital central hypothyroidism without macroorchidism. Endocr J. 2013;60(2):245–249. [DOI] [PubMed] [Google Scholar]
- 36.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA. 2002;99(7):4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabeler R, Mittag J, Geffers L, Rüther U, Leitges M, Parlow AF, Visser TJ, Bauer K. Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol Endocrinol. 2004;18(6):1450–1460. [DOI] [PubMed] [Google Scholar]
- 39.Zeng H, Schimpf BA, Rohde AD, Pavlova MN, Gragerov A, Bergmann JE. Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol Endocrinol. 2007;21(11):2795–2804. [DOI] [PubMed] [Google Scholar]
- 40.Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155(10):4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29(7):898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65(4):570–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astapova I, Vella KR, Ramadoss P, Holtz KA, Rodwin BA, Liao XH, Weiss RE, Rosenberg MA, Rosenzweig A, Hollenberg AN. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25(2):212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vella KR, Ramadoss P, Costa-E-Sousa RH, Astapova I, Ye FD, Holtz KA, Harris JC, Hollenberg AN. Thyroid hormone signaling in vivo requires a balance between coactivators and corepressors. Mol Cell Biol. 2014;34(9):1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JW, Bain M, Lindley K, Muntoni F, Vargha-Khadem F, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366(3):243–249. [DOI] [PubMed] [Google Scholar]
- 46.Gershengorn MC, Osman R. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol Rev. 1996;76(1):175–191. [DOI] [PubMed] [Google Scholar]
- 47.Oron Y, Straub RE, Traktman P, Gershengorn MC. Decreased TRH receptor mRNA activity precedes homologous downregulation: assay in oocytes. Science. 1987;238(4832):1406–1408. [DOI] [PubMed] [Google Scholar]
- 48.Yamada M, Monden T, Satoh T, Iizuka M, Murakami M, Iriuchijima T, Mori M. Differential regulation of thyrotropin-releasing hormone receptor mRNA levels by thyroid hormone in vivo and in vitro (GH3 cells). Biochem Biophys Res Commun. 1992;184(1):367–372. [DOI] [PubMed] [Google Scholar]
- 49.Barclay AN. Membrane proteins with immunoglobulin-like domains—a master superfamily of interaction molecules. Semin Immunol. 2003;15(4):215–223. [DOI] [PubMed] [Google Scholar]
- 50.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. [DOI] [PubMed] [Google Scholar]
- 51.Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabriel R, von Kalle C, Schmidt M. Mapping the precision of genome editing. Nat Biotechnol. 2015;33(2):150–152. [DOI] [PubMed] [Google Scholar]
- 53.O’Geen H, Yu AS, Segal DJ. How specific is CRISPR/Cas9 really? Curr Opin Chem Biol. 2015;29:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joseph-Bravo P, Jaimes-Hoy L, Uribe RM, Charli JL. 60 Years of Neuroendocrinology: TRH, the first hypophysiotropic releasing hormone isolated: control of the pituitary-thyroid axis. J Endocrinol. 2015;227(3):X3. [DOI] [PubMed] [Google Scholar]
- 55.Sotelo-Rivera I, Jaimes-Hoy L, Cote-Vélez A, Espinoza-Ayala C, Charli JL, Joseph-Bravo P. An acute injection of corticosterone increases thyrotrophin-releasing hormone expression in the paraventricular nucleus of the hypothalamus but interferes with the rapid hypothalamus pituitary thyroid axis response to cold in male rats. J Neuroendocrinol. 2014;26(12):861–869. [DOI] [PubMed] [Google Scholar]
- 56.Zoeller RT, Kabeer N, Albers HE. Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology. 1990;127(6):2955–2962. [DOI] [PubMed] [Google Scholar]
- 57.Tenenbaum-Rakover Y, Grasberger H, Mamanasiri S, Ringkananont U, Montanelli L, Barkoff MS, Dahood AM, Refetoff S. Loss-of-function mutations in the thyrotropin receptor gene as a major determinant of hyperthyrotropinemia in a consanguineous community. J Clin Endocrinol Metab. 2009;94(5):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]