Abstract
A local axis connects the apical ectoplasmic specialization (ES) at the Sertoli-spermatid interface, the basal ES at the blood–testis barrier (BTB), and the basement membrane across the seminiferous epithelium functionally in rat testes. As such, cellular events that take place simultaneously across the epithelium such as spermiation and BTB remodeling that occur at the apical ES and the basal ES, respectively, at stage VIII of the cycle are coordinated. Herein, laminin α2, a structural component of the basement membrane, was found to regulate BTB dynamics. Sertoli cells were cultured in vitro to allow the establishment of a tight junction (TJ) barrier that mimicked the BTB in vivo. Knockdown of laminin α2 by transfecting Sertoli cells with laminin α2-specific short hairpin RNA vs the nontargeting negative control was shown to perturb the Sertoli cell TJ barrier, illustrating laminin α2 was involved in regulating BTB dynamics. This regulatory effect was mediated through mammalian target of rapamycin complex 1 (mTORC1) signaling because the two mTORC1 downstream signaling molecules ribosomal protein S6 and Akt1/2 were activated and inactivated, respectively, consistent with earlier findings that mTORC1 is involved in promoting BTB remodeling. Also, laminin α2 knockdown induced F-actin and microtubule (MT) disorganization through changes in the spatial expression of F-actin regulators actin-related protein 3 and epidermal growth factor receptor pathway substrate 8 vs end-binding protein 1 (a MT plus-end tracking protein, +TIP). These laminin α2 knockdown-mediated effects on F-actin and MT organization was blocked by exposing Sertoli cells to rapamycin, an inhibitor of mTORC1 signaling, and also SC79, an activator of Akt. In summary, laminin α2-mediated regulation on Sertoli cell BTB dynamics is through mTORC1 signaling.
Basement membrane laminin α2 chain is a regulator of blood–testis barrier (BTB) dynamics in the rat testis, confirming the presence of short regulatory loop between the basement membrane and the BTB.
In the mammalian testis, cellular events that take place across the seminiferous epithelium during the epithelial cycle are tightly coordinated to support spermatogenesis [for reviews, see (1–4)]. For instance, at stage VIII of the epithelial cycle in the rat or mouse testis, spermiation that takes place at the luminal edge of the adluminal compartment to release the sperm into the tubule lumen and restructuring of the blood–testis barrier (BTB) to facilitate the transport of preleptotene spermatocytes across the immunological barrier occur simultaneously, but at the opposite ends of the epithelium [for reviews, see (1, 5–7)]. These cellular events must be tightly coordinated to support spermatogenesis. It is of interest to note that the ultrastructure that supports and regulates elongated spermatid adhesion and its degeneration at spermiation vs the one that supports Sertoli cell–cell adhesion and its restructuring called apical vs basal ectoplasmic specialization (ES) are virtually identical when examined by electron microscopy—a testis-specific, actin-rich anchoring junction [for reviews, see (8–11)]. Nonetheless, the apical and basal ES do have some dissimilarities. First, the apical ES has only a single array of bundled actin microfilaments found in the Sertoli cell at the Sertoli–spermatid interface, but the basal ES has two arrays of bundled actin microfilaments at the Sertoli–Sertoli cell interface with one on each side of the adjacent Sertoli cells. Second, the constituent proteins between the two are different. For instance, adhesion complexes α6β1-integrin/laminin-α3β3γ3, nectin 3/afadin, and JAM-C are specific to the apical ES, whereas occludin/zonula occludens 1 (ZO-1), JAM-A/ZO-1, and N-cadherin/β-catenin are mostly at the basal ES [for reviews, see (4, 6)]. Studies have shown that there is a local autocrine-based functional axis between the apical and basal ES to coordinate cellular events that take place at these two ultrastructures across the epithelium during the epithelial cycle [for reviews, see (12, 13)]. In brief, biologically fragments of laminin chains, such as F5-peptide from domain IV of laminin-γ3 chain, released from degenerating apical ES through the action of matrix metalloprotease 2 (14, 15) were shown to induce BTB remodeling, making the barrier “leaky” both in vitro and in vivo (16–18). This thus coordinates cellular events that take place at the opposite ends of the seminiferous epithelium. The presence of this functional axis has also been confirmed using the phthalate-induced Sertoli cell injury model (15, 19). During these earlier studies, it was shown that laminin fragments generated at the apical ES also perturbed the expression of β1-integrin found at the hemidesmosome at the Sertoli cell-basement membrane interface (16). We thus sought to investigate if there is a local feedback (or crosstalk) loop between the basement membrane and the BTB, as these two ultrastructures are intimately related and lie adjacent to each other. In short, we sought to examine if a loss-of-function of laminin α2 chain, a major constituent component of the basement membrane which deletion was shown to cause infertility in male mice (20), by RNA interference (RNAi) would perturb the Sertoli cell BTB function. Also, recent studies have shown that mammalian target of rapamycin complex 1 (mTORC1) signaling complex is working in concert with its downstream signaling proteins ribosomal protein S6 (rpS6) and Akt1/2 to modulate Sertoli cell BTB both in vitro and in vivo through changes in the organization of actin microfilaments at the ES by making the BTB “leaky” both in vitro and in vivo (21, 22). Herein, we examined if the laminin α2 knockdown-induced BTB restructuring was mediated through F-actin organization at the basal ES, via mTORC1, rpS6, and Akt1/2. We envisioned that this information, if known, would provide crucial insights to better understand the molecular mechanism(s) that regulate BTB dynamics during the epithelial cycle of spermatogenesis.
Materials and Methods
Animals and antibodies
Sprague-Dawley rats, male pups at 16 to 19 days of age with a foster mother for every 10 pups, were purchased from Charles River Laboratories (Kingston, NY). The use of rats for experiments reported herein was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol Numbers 12-506 and 15-780-H. Antibodies were obtained commercially and listed in Table 1.
Table 1.
Antibodies Used for Different Experiments in This Report
| Antibody | Host Species | Vendor | Catalog Number |
Working Dilution |
|
|---|---|---|---|---|---|
| IB | IF/IHC | ||||
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | 1:300 | |
| Akt | Rabbit | Cell Signaling Technology | 9272 | 1:1000 | |
| p-Akt1 S473 | Rabbit | Cell Signaling Technology | 4060 | 1:1000 | |
| p-Akt2 S474 | Rabbit | Cell Signaling Technology | 8599 | 1:1000 | |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | 1:3000 | 1:50 |
| α-tubulin | Mouse | Abcam | ab7291 | 1:1000 | 1:300 |
| β-catenin | Rabbit | Thermo Fisher Scientific | 71-2700 | 1:250 | 1:100 |
| β-tubulin | Rabbit | Abcam | ab6046 | 1:1000 | |
| CAR | Rabbit | Santa Cruz Biotechnology | sc-15405 | 1:200 | 1:50 |
| Detyrosinated α-tubulin | Rabbit | Abcam | ab48389 | 1:1000 | |
| Dia1 | Goat | Santa Cruz Biotechnology | sc-10885 | 1:200 | |
| EB1 | Mouse | BD Biosciences | 610534 | 1:200 | |
| EB1 | Rabbit | Santa Cruz Biotechnology | sc-15347 | 1:200 | |
| Eps8 | Mouse | BD Biosciences | 610143 | 1:5000 | 1:50 |
| Laminin α2 | Mouse | Millipore | MAB1922 | 1:300 | 1:300 (tissue) 1:100 (cell) |
| MARK2 | Rabbit | Proteintech | 15492-1-AP | 1:2000 | |
| MARK4 | Rabbit | Proteintech | 20174-1-AP | 1:2000 | |
| mTOR | Rabbit | Cell Signaling Technology | 2983 | 1:2000 | |
| N-cadherin | Mouse | Thermo Fisher Scientific | 33-3900 | 1:200 | 1:100 |
| Raptor | Rabbit | Cell Signaling Technology | 2280 | 1:1000 | |
| rpS6 | Rabbit | Cell Signaling Technology | 2217 | 1:1000 | |
| p-rpS6 S235/236 | Rabbit | Cell Signaling Technology | 4858 | 1:1000 | |
| p-rpS6 S240/244 | Rabbit | Cell Signaling Technology | 5364 | 1:1000 | |
| ZO-1 | Rabbit | Thermo Fisher Scientific | 61-7300 | 1:250 | 1:100 |
| Goat IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2350 | 1:3000 | |
| Rabbit IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2370 | 1:3000 | |
| Mouse IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2371 | 1:3000 | |
| Rabbit IgG-Alexa Fluor 555 | Goat | Thermo Fisher Scientific | A-21429 | 1:250 | |
| Mouse IgG-Alexa Fluor 488 | Goat | Thermo Fisher Scientific | A-11029 | 1:250 | |
| Mouse IgG-Alexa Fluor 555 | Goat | Thermo Fisher Scientific | A21422 | 1:250 | |
Abcam, Cambridge, MA; Cell Signaling Technology, Danvers, MA; Santa Cruz Biotechnology, Santa Cruz, CA; Sigma-Aldrich, St. Louis, MO; Invitrogen, Life Technologies, Carlsbad, CA; Proteintech, Chicago, IL; BD Biosciences, San Jose, CA; Millipore Corp., Billerica, MA.
Abbreviations: Arp3, actin-related protein 3; EB1, end-binding protein 1; Eps8, epidermal growth factor receptor pathway substrate 8; IHC, immunohistochemistry; MARK, microtubule affinity-regulating kinase.
Primary Sertoli cell cultures
Sertoli cells were isolated from the testes of 20-day-old rats as described (23). Cells were seeded on Matrigel-coated [1:5∼1:7, diluted in F12/Dulbecco's modified Eagle medium (DMEM); BD Biosciences, San Jose, CA] culture plates [for immunoblotting (IB) and microtubule (MT) polymerization assay], coverslips [for immunofluorescence (IF) microscopy], or bicameral units [Millipore, Billerica, MA; for transepithelial electrical resistance (TER) measurement to assess tight junction (TJ)-permeability barrier function] at a cell density of 0.5, ∼0.03 to 0.04, or 1.0 × 106 cells/cm2, respectively. Sertoli cells were cultured in serum-free F12/DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with growth factors and gentamicin (23) in a CO2 incubator with a humidified atmosphere of 95% air/5% CO2 (volume-to-volume ratio) at 35°C. On day 2, Sertoli cells were subjected to a brief hypotonic treatment with 20 mM Tris (pH 7.4) for 2.5 minutes (24) to lyse residual germ cells (24). These Sertoli cell cultures contained ∼98% Sertoli cells, with negligible contaminations of Leydig cells, peritubular myoid cells, or germ cells when assessed by specific markers of these cells using IB and/or reverse transcription polymerase chain reaction as described (25). Sertoli cells cultured in vitro were shown to establish a functional TJ-permeability barrier with ultrastructures of TJ, basal ES, gap junction, and desmosome that mimic the Sertoli cell BTB in vivo as described earlier (26, 27).
Knockdown of Sertoli cell laminin α2 by short hairpin RNA
SureSilencing short hairpin RNA (shRNA) plasmids were obtained from Qiagen (Chatsworth, CA), and the shRNAs including both negative control and specific to laminin α2 knockdown were each cloned into the pGeneClipTM hMGFP vector, which contained a GFP marker to monitor successful transfection. The sequence of shRNA that specifically targeted laminin α2 (Clone ID 4) was 5′-ACAGGAAGCTGATCGGCTAAT-3′. The sequence of the nontargeting negative control was 5′-GGAATCTCATTCGATGCATAC-3′. Based on results of pilot experiments, Clone IDs 1 to 3 of laminin α2 shRNAs were found to be less effective in silencing laminin α2 in Sertoli cells which were not used in subsequent experiments. All plasmids were prepared by ZymoPURE™ Plasmid Midiprep Kit (Zymo Research, Irvine, CA). Sertoli cells were cultured in vitro for 3 days as described earlier with an established functional TJ-permeability barrier. Thereafter, Sertoli cells were transfected with plasmid DNA at 0.5 μg (for IF on coverslips placed in 12-well dishes), 1.5 μg (for IB in 12-well dishes), 0.75 μg (for TER in bicameral units which were placed in 24-well dishes), or 2.7 μg (for MT polymerization assay in six-well plates) using LipojetTM In Vitro Transfection Reagent (SignaGen Laboratories, Rockville, MD) using a 3-μL transfection medium:1-μg plasmid DNA ratio according to the manufacturer’s instructions. After 24 hours, cells were rinsed with F12/DMEM twice and then cultured in fresh F12/DMEM supplemented with growth factors and gentamicin. Cells were terminated 48 hours posttranfection for IF analysis. Protein lysates were obtained from these cultures 72 hours (for IB or MT polymerization assay) posttransfection for analysis.
Treatment of Sertoli cells with rapamycin
Rapamycin readymade solution [2.5 mg/mL in dimethyl sulfoxide (DMSO)] was purchased from Sigma-Aldrich (St Louis, MO). On day 3, Sertoli cells were transfected with laminin α2 shRNA vs negative control shRNA. After 24 hours, transfected cells were rinsed with F12/DMEM twice. For IF, cells were treated with 100 ng/mL rapamycin for 24 hours, and on day 5, cells were fixed for IF analysis. For IB and MT polymerization assay, transfected cells were cultured in fresh F12/DMEM supplemented with growth factors and gentamicin for an additional 24 hours, and on day 5, cells were treated with 100 ng/mL rapamycin for 24 hours; thereafter, cells were terminated on day 6. Same volume of DMSO was used for vehicle controls.
Treatment of Sertoli cells with SC79
SC79, [2-amino-6-chloro-α-cyano-3-(ethoxycarbonyl)-4H-1-benzopyran-4-acetic acid ethyl ester, Mr 364.78], was obtained from Millipore (Billerica, MA). SC79 is a specific pan-Akt activator, capable of activating Akt1, 2, and 3 (28). However, because we had used antibodies against only Akt1 and Akt2 (Akt1/2) to assess changes in the expression of their activated forms p-Atk1-S473 and p-Akt-S474, and given the fact that Akt3, unlike Akt1/2 (which are crucial to maintain spermatogenesis in rodent males), is not essential to support male mouse fertility, based on studies using genetic models of single-, double-, and triple-knockout mice for the three isoforms of Akt (29), we envisioned that Akt1/2 were the major players in our study. A solution of SC79 was freshly prepared at 25 μg/μL in DMSO. To assess the involvement of Akt1/2 signaling in laminin α2 knockdown-induced Sertoli cell TJ barrier function disruption, Sertoli cells subjected to laminin α2 knockdown by RNAi using laminin α2 shRNA were coincubated with 2 μg/mL SC79 (5.5 µM) (and with the same amount of DMSO without SC79 used in controls) during transfection for 24 hours vs SC79 alone or laminin α2 shRNA alone. The selected concentration of SC79 used in our studies was similar to earlier studies on mammalian cells and tissues both in vitro and in vivo without cytotoxicity (28, 30). Besides monitoring the effects of SC79 to recue laminin α2 knockdown-mediated Sertoli TJ barrier disruption through a downregulation of p-Akt1/2, we also examined if SC79 recued laminin α2-induced MT and F-actin disruption in Sertoli cells.
Lysate preparation and IB
Protein lysates from primary Sertoli cells were obtained using IP lysis buffer [50 mM Tris, 0.15 M NaCl, 1% NP-40, 2 mM EGTA, and 10% glycerol (volume-to-volume ratio), pH 7.4 at 22°C] supplemented with protease inhibitor mixture (Sigma-Aldrich) and phosphatase inhibitor mixture II (Sigma-Aldrich) prior to its use. Protein concentration was determined using a DC Protein Assay Kit from Bio-Rad (Hercules, CA). Equal amount of protein lysates (either 25 or 40 µg protein per lane in specific experiments) were resolved by SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad) for immunoblot analysis using the corresponding primary and secondary antibody (Table 1). Target proteins were visualized by chemiluminescence using a kit prepared in-house as described (31). Images were acquired and quantified using a Fujifilm LAS-4000 mini-Luminiscent Image Analyzer and Multi Gauge software package (Version 3.1) from Fujifilm Corp (Valhalla, NY).
Dual-labeled IF analysis
Dual-labeled IF was performed using frozen sections of testes (7 μm thickness) obtained in a cryostat at –22°C or Sertoli cells cultured on coverslips at a density of ∼0.03 to 0.04 × 106 cells/cm2. Sections and/or cells were fixed with 4% PFA or ice-cold methanol, permeabilized with 0.1% Triton X-100 in PBS, and subsequently blocked with 1% BSA in PBS as described (32). Sections and/or cells were then incubated with primary antibodies (Table 1) at 4°C overnight, followed by Alexa Fluor 488 (green) or Alexa Fluor 555 (red)-conjugated secondary antibodies (Thermo Fisher Scientific) for 1 hour. To visualize F-actin, cells were incubated with rhodamine-conjugated phalloidin (Thermo Fisher Scientific) at 1:50 dilution for 1 hour. Slides were mounted in Prolong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies) to visualize cell nuclei. Fluorescence images were examined and acquired using a Nikon Eclipse 90i Fluorescence Microscope system equipped with a Nikon DS-Qi1Mc and a Nikon DS-Fi1 digital cameras, with the Nikon NIS Elements Imaging Software (Nikon Instruments, Inc.). Image files were analyzed using Photoshop in Adobe Creative Suite (Version 3.0; San Jose, CA) for image overlay to assess protein colocalization. All sections of testes or Sertoli cells in a study were processed simultaneously including treatment vs control groups in a single experimental session to eliminate interexperimental variations. Whereas images shown were representative findings of a single experiment, each experiment was repeated at least three times using different rat testes or Sertoli cell preparations and yielded similar observations.
Assessment of Sertoli cell TJ-permeability barrier function in vitro
Sertoli cells were plated on Matrigel (1:5)–coated bicameral units (Millipore Millicell units, diameter, 12 mm; pore size, 0.45 μm; effective surface area, 0.6 cm2) at 1 × 106 cells/cm2. Bicameral units were then placed in 24-well plates with 0.5 mL F12/DMEM in the apical and basal compartment in each well. To assess Sertoli cell TJ-permeability barrier, both the silencing and the control group had triplicate bicameral units and TER across the cell epithelium was recorded daily. It is noted that TER monitored the ability of the Sertoli cell TJ barrier to resist conductivity of electrical current that was sent across the electrodes of the Millipore Millicell ERS system (23). Each experiment was repeated at least three times using different batches of Sertoli cells which yielded similar results.
MT polymerization assay
MT polymerization assay was performed as described (33) with minor modifications. Primary Sertoli cells were seeded on six-well plates at 0.4 × 106 cells/cm2. On day 3, cells were transfected with 2.7 μg plasmid DNA containing laminin α2 shRNA vs negative control shRNA. After 24 hours, cells were rinsed with F12/DMEM twice and then cultured in fresh F12/DMEM supplemented with growth factors and gentamicin for an additional 24 hours. On day 5, cells were treated with 100 ng/mL rapamycin for 24 hours. In this assay, taxol [also known as paclitaxel, a MT stabilizing agent (34)] at 30 µM vs CaCl2 at 4 mM [known to induce MT depolymerization in its presence (35)] was added onto cell lysates before centrifugation (in triplicates) and used as the corresponding positive and negative controls. All samples used were done in triplicates in this experiment including control vs treatment groups including positive and negative controls. Thereafter, cells were harvested in prewarmed lysis and MT stabilization buffer (100 mM PIPES, 5 mM MgCl2, 1 mM EGTA, 0.1% NP-40, 0.1% Triton X-100, 0.1% Tween 20, 0.1% β-mercaptoethanol, 30% glycerol, pH 6.9). Cells were then homogenized with a 22-gauge syringe needle and centrifuged at 100,000 g for 30 minutes at 35°C to separate free tubulin monomers (supernatant) from MTs/polymerized tubulin (pellet). Supernatant was collected, 40 μL supernatant of each sample were analyzed by IB for β-tubulin. The pellet was resuspended in 250 μL of 4 mM CaCl2, and 30 μL of each sample were used for IB.
Image analysis
For in vitro experiments that assessed the effects of laminin α2 silencing on target protein distribution in Sertoli cells at the cell-cell interface, at least 200 cells were randomly selected and examined in experimental vs control groups with n = 3 to 5 experiments using different batches of cell preparations. Fluorescence intensity of a target protein in Sertoli cells was quantified using ImageJ 1.45 software (National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij) as described (32).
Statistical analysis
For studies using Sertoli cell cultures, triplicate coverslips, dishes, or bicameral units were used. Each data point (or bar graph) is a mean ± standard deviation (SD) of three to five experiments. Statistical analysis was performed using the GB-STAT software package (Version7.0; Dynamic Microsystems, Silver Spring, MD) with two-way analysis of variance followed by Dunnett’s test. In selected experiments, Student t test was used for paired comparisons.
Results
Knockdown of laminin α2 perturbs Sertoli cell TJ-permeability barrier function, likely involving mTORC1 signaling
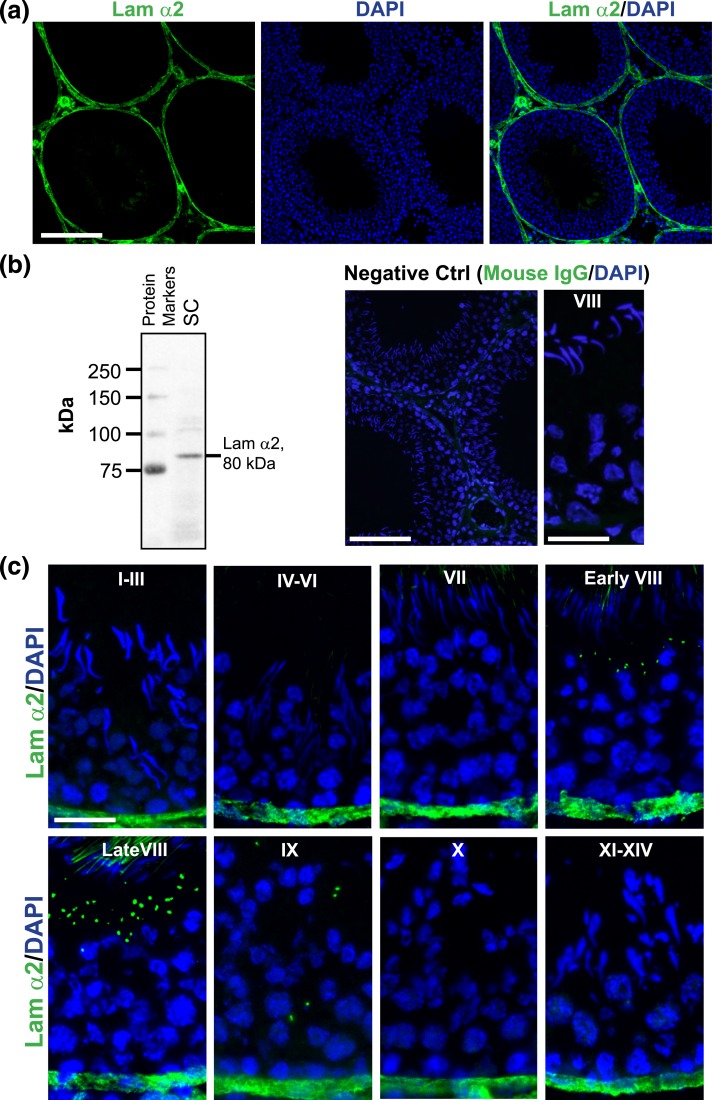
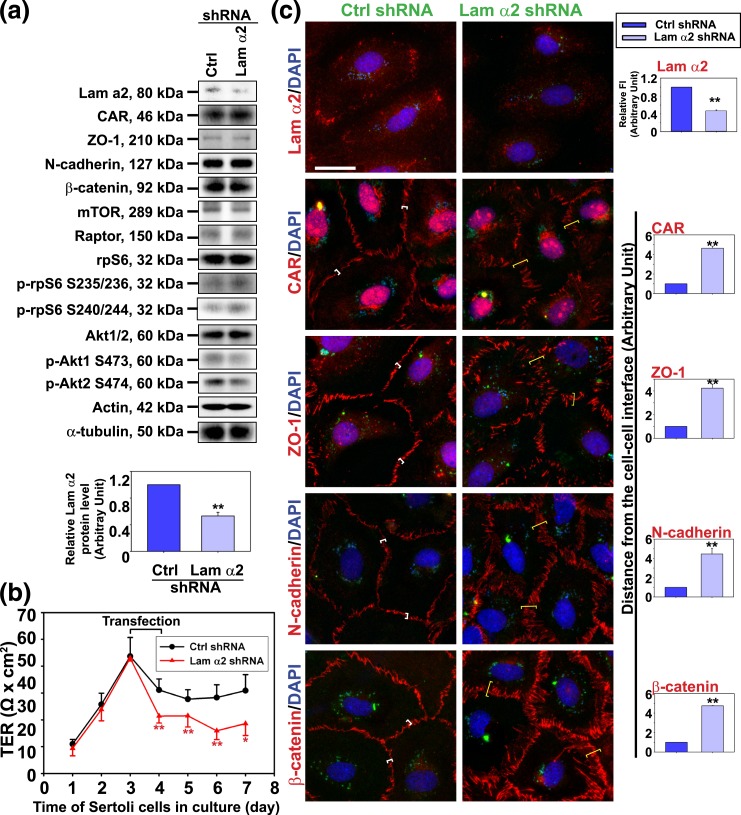
Using an antibody specific to the 80-kDa fragment of laminin α2 [Fig. 1(a) and 1(b); Table 1], laminin α2 was found to localize at the base of the seminiferous tubule, consistent with its localization at the basement membrane of the tunica propria in virtually all stages of the epithelial cycle [Fig. 1(a) and 1(b)]. This staining was specific because no laminin α2 staining was seen when the primary antibody was substituted with normal mouse IgG as shown in the negative control [Fig. 1(b)]. These findings are also consistent with earlier (20, 36–39) and recent reports (40) that laminin α2 is a component of the basement membrane and a product of Sertoli and germ cells in the mammalian testis. Interestingly, laminin α2 was also detected in the adluminal compartment of the seminiferous epithelium from early to late stage VIII and appeared as discrete aggregates in the Sertoli cell cytosol, but considerably diminished at stage IX, and not found in any other staged tubules, besides its robust expression at the basement membrane [Fig. 1(b)]. Thus, the appearance of laminin aggregates at stage VIII of the epithelial cycle coincides with the time when BTB undergoes remodeling to facilitate the transport of preleptotene spermatocytes at the immunological barrier [for reviews, see (2, 41)]. Collectively, these findings suggest that laminin α2 at the basement membrane may be involved in maintaining the BTB integrity in nonstage VIII tubules, but possibly involved in BTB restructuring in stage VIII tubules when aggregates of laminin α2 were detected when they were being transported to the basal and adluminal compartment. To explore this possibility, a loss-of-function approach was used wherein laminin α2 was silenced by RNAi using laminin α2 shRNA vs nontargeting negative control shRNA. Indeed, a knockdown of laminin α2 by RNAi caused its expression to be reduced by ∼50% [Fig. 2(a)], which was associated with a considerable disruption of the Sertoli cell TJ-permeability barrier function [Fig. 2(b)]. However, the steady-state levels of several BTB-associated proteins was not affected [Fig. 2(a)], illustrating there was no apparent off-target effects using the laminin α2-specific shRNA for its knockdown through transfection in Sertoli cell epithelium. This disruptive effect on the Sertoli cell TJ-permeability barrier following laminin α2 knockdown was associated with changes in the localization of TJ (e.g., CAR and ZO-1) and basal ES (e.g., N-cadherin, β-catenin) proteins in Sertoli cells based on analysis by fluorescence microscopy [Fig. 2(c)]. For instance, these proteins no longer tightly localized to the Sertoli cell–cell interface, but were rapidly internalized by moving into the cell cytosol following laminin α2 knockdown [Fig. 2(c)]. It was noted that the steady-state protein levels of mTOR, raptor (the binding partner of mTOR that created the mTORC1 complex), total rpS6 and Akt1/2, were not altered following the knockdown of laminin α2 [Fig. 2(a)]. However, the downstream signaling proteins of the mTORC1 complex, such as the activated rpS6, p-rpS6 S235/236 and p-rpS6 S240/244, vs the activated Akt, p-Akt1 S473 and p-Akt2 S474, recently shown to be involved in mTORC1-mediated regulation on BTB dynamics (22, 42), were considerably up- and downregulated, respectively, following laminin α2 knockdown [Fig. 2(a), Supplemental Fig. 1 (157.3KB, pdf) ]. These findings are consistent with earlier findings that overexpression of a constitutively active quadruple phosphomimetic mutant of rpS6 by inducing an increase in p-rpS6 steady-state protein level in Sertoli cells perturbed Sertoli cell TJ-permeability barrier through a downregulation of p-Akt1/2 (42). More important, these disruptive effects on the Sertoli cell TJ-function and a disruption of F-actin organization caused by p-rpS6 overexpression could be reproduced by a specific knockdown of Akts (22, 42). These findings thus support the notion that laminin α2 exerts its effects on Sertoli cell BTB function through mTORC1/rpS6/Akt signaling.
Figure 1.
Stage-specific expression of laminin α2 in the rat testis. (a) Localization of laminin α2 in the seminiferous epithelium of adult rat testes. Laminin α2 (green fluorescence) was predominantly expressed at the base of seminiferous tubules, consistent with its localization at the basement membrane of the tunica propria, as well as the basal lamina of microvessels in the interstitial space. Cell nuclei were stained by DAPI (blue). Scale bar, 200 μm. (b) The specificity of laminin α2 antibody was supported by IB using lysates of Sertoli cells (SC) that yielded a prominent band with an apparent Mr corresponding to the C-terminal 80-kDa laminin fragment. Negative control (Ctrl) was shown on the right panel where cross-sections of adult rat testes were stained with normal mouse IgG instead of the antilaminin α2 antibody (see Table 1) at low and high (a stage VIII tubule) magnification. Scale bar, 100 µm (left panel) and 60 µm (right panel). (c) Stage-specific expression of laminin α2 (green fluorescence) in the seminiferous epithelium of adult rat testis. Laminin α2 was exclusively expressed in the basement membrane of seminiferous epithelium in all stages. However, laminin α2 was also prominently expressed in the adluminal compartment near the tubule lumen and appeared as discrete aggregates in stage VIII tubules only, which was considerably diminished at stage IX, and not found in the adluminal compartment in all other stages. Cell nuclei were visualized by DAPI. Scale bar, 60 μm.
Figure 2.
Knockdown of laminin α2 disrupts the Sertoli cell TJ-permeability barrier function through mTORC1-Akt signaling. Primary Sertoli cells cultured for 3 days were transfected with laminin α2 (Lam α2) vs nontargeting negative control (Ctrl) shRNA for 24 hours. Cells were rinsed and cultured for additional 24 hours (for IF) or 48 hours (for IB). (a) Studies by IB illustrated laminin α2 was silenced by ∼50%. Knockdown of laminin α2 had no effect on the protein levels of TJ (e.g., CAR and ZO-1) and basal ES proteins (e.g., N-cadherin and β-catenin). Laminin α2 knockdown, however, induced an upregulation of p-rpS6 S235/236 and p-rpS6 S240/244 (the activated form of rpS6) and downregulated p-Akt1 S473 and p-Akt2 S474, but not the total rpS6 or total Akt. Each bar in the histogram is a mean ± SD of 6 independent experiments. ** P < 0.01 by Student t test. Changes in the steady-state level of p-rpS6 S235/236 and p-rpS6 S240/244 vs p-rpS6, and also p-Akt1 S473 and pAkt2 S474 vs total Akts, were shown in Supplemental Fig. 1 (157.3KB, pdf) . (b) Knockdown of laminin α2 perturbed the Sertoli cell TJ-permeability barrier function. Each data point is a mean ± SD of triplicate bicameral units of a representative experiment from a total of n = 3 independent experiments which yielded similar results. *P < 0.05; **P < 0.01 by Student t test. (c) Studies by IF confirmed laminin α2 expression in Sertoli cell cytosol but laminin α2 also prominently localized at the cell-matrix interface, consistent with the notion that laminin α2 is a basement membrane protein. Knockdown of laminin α2 by using specific shRNA considerably reduced its expression (red fluorescence) in Sertoli cells. Laminin α2 knockdown also caused mislocalization of BTB-associated proteins (e.g., CAR, ZO-1, N-cadherin, and β-catenin), such that these proteins no longer localized tightly at the cell-cell interface (see white brackets in cells transfected with control shRNA), but diffusely localized at the site (yellow brackets in cells transfected with laminin α2-specific shRNA), illustrating these proteins were being redistributed from the near the cell surface to the cell cytosol. Sertoli cell nuclei were visualized by DAPI (blue). The expression of GFP (green) illustrated successful transfection because GFP is an integrated component of the vector DNA. Scale bar, 30 μm. Histograms on the right panel summarized IF results for each target protein shown on the two left panels with each bar represents a mean ± SD of three experiments. Fluorescence at two opposite sides of two adjacent cells from 50 randomly selected cells were scored and analyzed from each experiment as described in Materials and Methods, and a total of three independent experiments were done, which yielded similar results. **P < 0.01 by Student t test.
Laminin α2 regulates Sertoli cell F-actin organization at the BTB through mTORC1 signaling
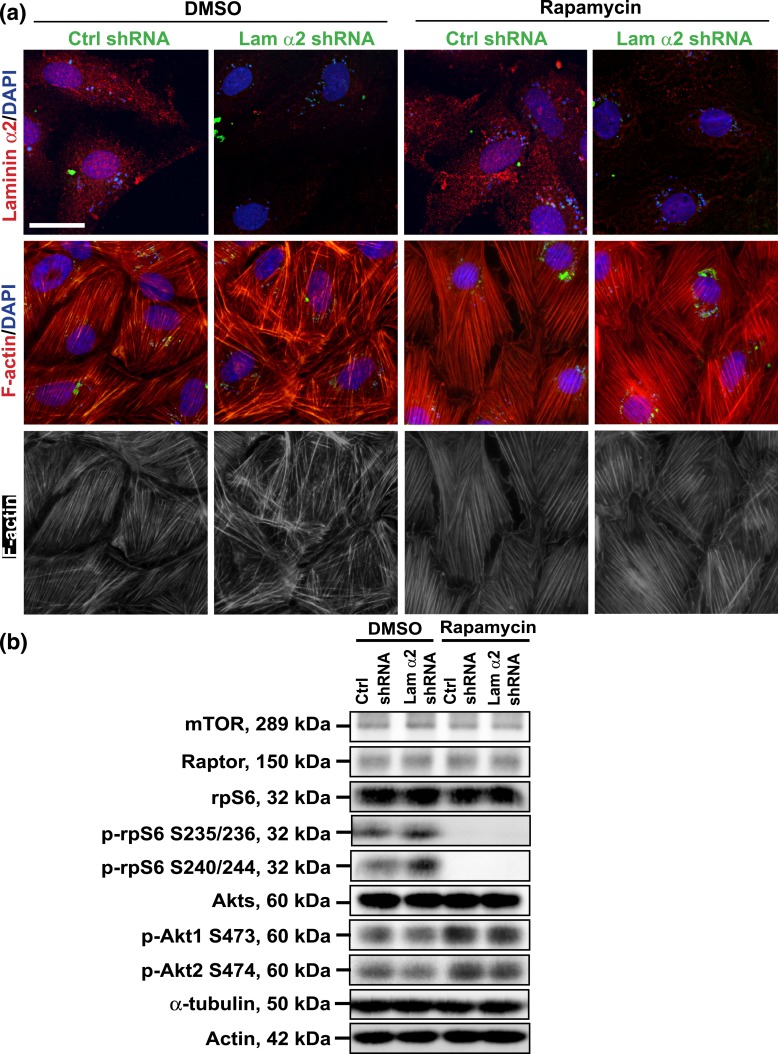
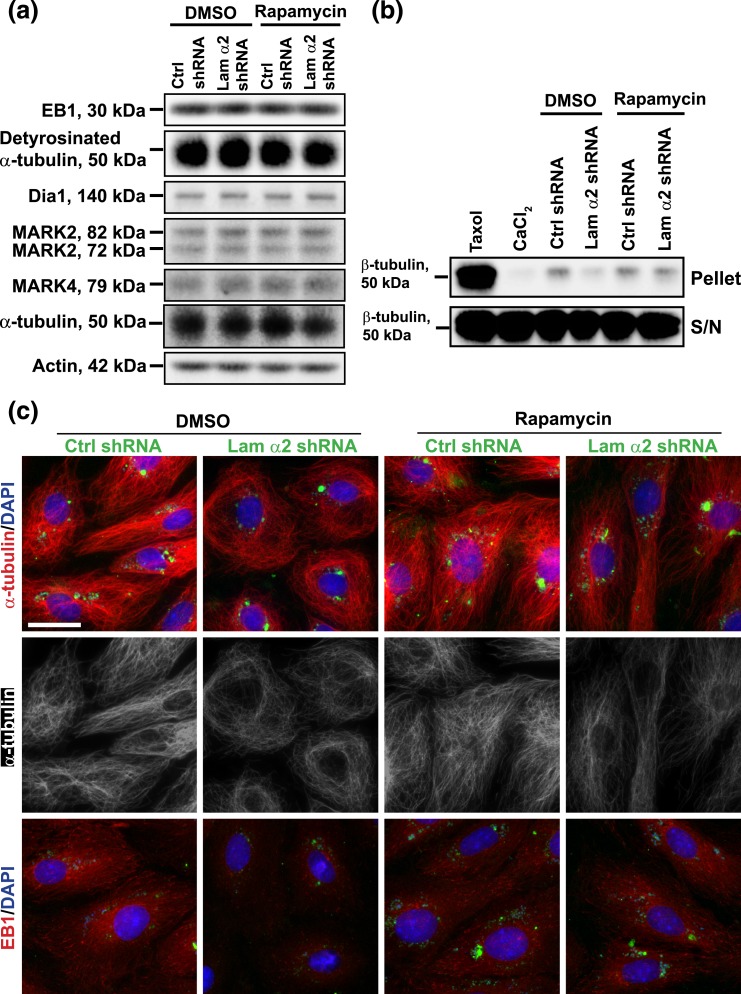
Treatment of mammalian cells with rapamycin that inactivates mTORC1 signaling is the hallmark of mTORC1 mechanism of action [for reviews, see (43–46)], we thus examined the involvement of mTORC1 signaling in laminin α2-mediated changes in Sertoli cell BTB dynamics as shown in Fig. 2, wherein Sertoli cell laminin α2 expression was silenced by RNAi using laminin α2-specific shRNA. The efficacy of laminin α2 knockdown was illustrated by considerable reduction in laminin α2 fluorescence signals in both groups of cells treated with or without rapamycin [Fig. 3(a)]. Indeed, treatment of Sertoli cells with rapamycin blocked the expression of p-rpS6, as well as its downstream inactivation of p-Akt. However, rapamycin had no effect on the steady-state levels of mTOR, raptor, total rpS6 and total Akt [Fig. 3(b)]. It is of interest to note that knockdown of laiminin α2 activated p-rpS6 S235/236 and p-rpS6 S240/244, and down-regulated p-Akt1 S473 and p-Akt2 S474 in DMSO-treated control group [Fig. 3(b)], which was consistent with Fig. 2(a). Furthermore, treatment of Sertoli cells with rapamycin per se had no apparent effects on the organization of F-actin in Sertoli cells, but rapamycin blocked the laminin α2 knockdown-induced disruptive effects on F-actin organization [Fig. 3(a), column 4 vs column 2)]. Collectively, these findings further support the notion regarding the involvement of mTORC1 signaling pathway in the laminin α2-mediated regulatory effects on F-actin organization in Sertoli cells.
Figure 3.
Knockdown of laminin α2 disrupts the Sertoli cell F-actin organization through mTORC1-rpS6-Akt signaling. Primary Sertoli cells cultured for 3 days were transfected with laminin α2 (Lam α2) vs nontargeting negative control (Ctrl) shRNA for 24 hours. Cells were rinsed and treated with rapamycin (100 ng/mL) for 24 hours (for IF). For IB, transfected cells were cultured for additional 24 hours and then treated with rapamycin (100 ng/mL) for 24 hours before cells were harvested for lysate preparation. (a) Studies by IF confirmed that transfection of Sertoli cells with laminin α2 shRNA successfully knockdown laminin α2, as the expression of laminin α2 was considerably diminished in the laminin α2 silenced cells. However, rapamycin blocked the disruptive effect of laminin α2 knockdown on F-actin organization. Knockdown of laminin α2 caused truncation and defragmentation of F-actin network, whereas rapamycin rescued this disruptive effect, supporting the notion that laminin α2 exerts its effects through mTORC1 signaling. GFP expression (green fluorescence) illustrated successful transfection. Sertoli cell nuclei were visualized by DAPI. Scale bar, 30 μm. (b) Studies by IB confirmed that a knockdown of laminin α2 activated mTORC1-rpS6-Akt signaling by upregulating p-rpS6 S235/236 and p-rpS6. S240/244 and downregulating p-Akt1 S473 and p-Akt2 S474, but not total rpS6 and Akts (i.e., Akt1, 2, and 3). Rapamycin inactivated p-rpS6/p-Akt pathway by abolishing the laminin α2 knockdown-induced up- and downregulation of p-rpS6 and p-Akt1/2, but rapamycin had no effect on mTOR, raptor, total rpS6, and Akt levels. Actin and α-tubulin served as the protein loading control.
Laminin α2 regulates Sertoli cell TJ dynamics involving F-actin regulatory proteins actin-related protein 3 and epidermal growth factor receptor pathway substrate 8
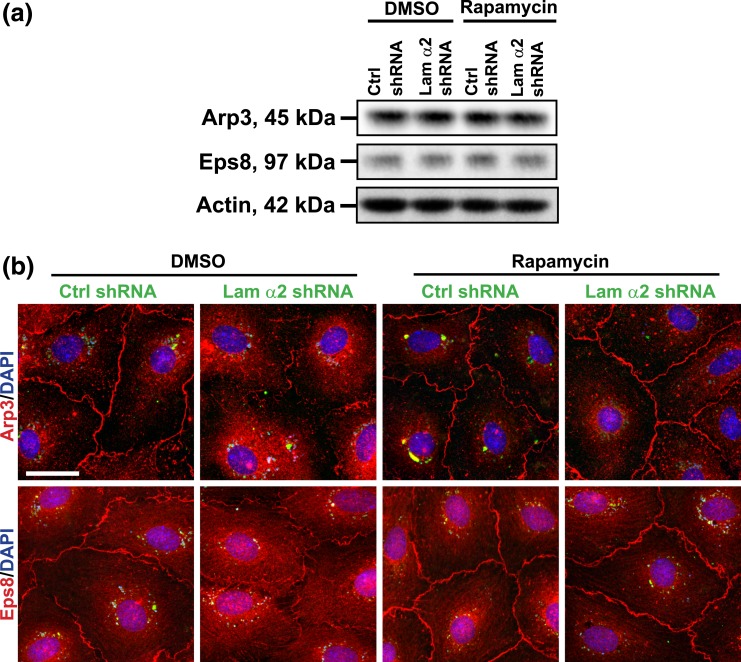
Based on findings shown in Figs. 2 and 3, it was noted that changes in F-actin organization following laminin α2 knockdown (Fig. 3) caused the disruptive localization of TJ and basal ES proteins at the Sertoli cell-cell interface (Fig. 2). As shown in Fig. 4, laminin α2 knockdown induced changes in the spatial expression, but not the steady-state protein level, of branched actin polymerization protein actin-related protein 3 (Arp3) and actin barded-end capping and bundling protein epidermal growth factor receptor pathway substrate 8 (Eps8) in Sertoli cells. Based on the intrinsic activity of these two proteins, Arp3 and Eps8 would confer actin filaments to assume a branched/unbundled and bundled configuration, respectively. Thus, their changes in the spatial expression in Sertoli cells, such as by redistributing from the cell-cell interface, moved into the cell cytosol, thereby perturbing the organization of actin microfilaments across the Sertoli cell as noted in Fig. 3(a). It was also noted that laminin α2 knockdown and/or rapamycin treatment had no apparent effect on the steady-state protein levels of Arp3 and Eps8 protein [Fig. 4(a)]. However, rapamycin treatment that inactivated mTORC1 effectively blocked the disruptive effects mediated by laminin α2 knockdown regarding the spatial expression of Arp3 and Eps8, but rapamycin alone without laminin α2 knockdown had no apparent effect on the spatial expression of either Arp3 or Eps8 [Fig. 4(b)]. These findings support the notion that mTORC1 signaling complex was involved in laminin α2-mediated effects on F-actin organization in Sertoli cells.
Figure 4.
Rapamycin rescues laminin α2 knockdown-mediated changes in the spatial expression of actin binding and regulatory proteins. Sertoli cells cultured for 3 days were transfected with laminin α2 (Lam α2) vs negative control (Ctrl) shRNA for 24 hours. Cells were rinsed and treated with rapamycin (100 ng/mL) for 24 hours (for IF). For IB, transfected cells were cultured for an additional 24 hours and were then treated with rapamycin (100 ng/mL) for 24 hours. (a) A study by IB showed that laminin α2 knockdown and/or rapamycin had no apparent effect on the Arp3 and Eps8 steady-state protein levels. β-actin served as a protein-loading control. (b) A study by IF illustrated that laminin α2 knockdown caused redistribution of branched actin polymerization protein Arp3 and actin barbed end capping and bundling protein Eps8. These two proteins no longer prominently localized at the cell-cell interface to maintain proper organization of actin filament bundles to support the Sertoli cell TJ barrier function following laminin α2 knockdown. These proteins were mostly internalized after laminin α2 knockdown. However, rapamycin blocked laminin α2 knockdown-induced redistribution of Arp3 and Eps8 in Sertoli cells. GFP expression (green) illustrated successful transfection. Sertoli cell nuclei were visualized by DAPI. Scale bar, 30 μm.
Laminin α2 regulates Sertoli cell MT organization through the mTORC1 signaling complex
Because it was noted that laminin α2 knockdown also perturbed the organization of MTs in Sertoli cells in preliminary experiments, we examined if laminin α2 knockdown had any effects on MT regulatory proteins. Interestingly, laminin α2 knockdown failed to perturb the steady-state levels of several MT regulatory proteins, such as end-binding protein 1 (EB1) (a +TIP protein), detyrosinated α-tubulin [i.e., an endogenous generated MTs in which the C-terminal Tyr was removed by exposing Glu at the newly formed C-terminus, causing MT stabilizing by rendering MT less dynamic (47–49)], Dia1, microtubule affinity-regulating kinase (MARK) 2, and MARK4 [known to be involved in MT stabilization; for reviews, see (50–53); Fig. 5(a)]. Also, the use of rapamycin that blocked the binding of raptor to mTOR to generate the mTORC1 complex had no apparent effect on the expression of these MT regulatory proteins with or without laminin α2 knockdown [Fig. 5(a)]. Furthermore, a knockdown of laminin α2 considerably reduced MT polymerization, but this laminin α2 knockdown-mediated reduction in MT polymerization was found to be blocked in Sertoli cells treated with rapamycin [Fig. 5(b)], illustrating mTORC1 was indeed involved in laminin α2-mediated MT disorganization following its knockdown. Interestingly, laminin α2 knockdown induced disorganization of MTs in Sertoli cells in which MTs no longer stretched across the Sertoli cell cytosol as noted in control cells; instead, MTs retracted from cell peripheries and rounded up close to the cell nuclei [Fig. 5(c)]. This laminin α2 knockdown-induced MT disorganization apparently was the result of changes in the distribution of EB1 [Fig. 5(c)], but not on the steady-state protein level of EB1 [Fig. 5(a)], which was earlier shown to be involved in MT dynamics by promoting MT stabilization but also catastrophe depending on cellular status (54–56). For instance, EB1 no longer was found to localize along the MTs. Interestingly, treatment of Sertoli cells with rapamycin was found to block the laminin α2 knockdown-induced mislocalization of EB1 along the MTs, rendering these cells similar to control cells transfected with negative control shRNA [Fig. 5(c)]. Thus, treatment of rapamycin was found to rescue laminin α2 knockdown-induced disorganization of MTs in Sertoli cells.
Figure 5.
Laminin α2 knockdown by shRNA that causes Sertoli cell MT disorganization is mediated through mTORC1 signaling. Sertoli cells cultured for 3 days were transfected with laminin α2 (Lam α2) vs negative control (Ctrl) shRNA for 24 hours. Cells were rinsed and treated with rapamycin (100 ng/mL) for 24 hours before harvested for IF analysis. For IB and MT polymerization assay, transfected cells were cultured for an additional 24 hours and were then treated with rapamycin (100 ng/mL) for 24 hours. (a) Studies by IB illustrated that laminin α2 knockdown without or with rapamycin treatment had no apparent effect on the expression of MT regulatory proteins EB1, MARK2 and MARK4 vs detyrosinated α-tubulin (the stabilized form of MTs, rendering MTs less dynamic). (b) MT polymerization assay was performed to quantify the ability of cell lysates to induce MT polymerization in Sertoli cells. Laminin α2 knockdown reduced MT polymerization because considerably less polymerized MTs were detected. However, treatment of Sertoli cells with rapamycin that blocked the function of mTORC1 abolished the laminin α2 knockdown-induced downregulation of MT polymerization (see pellet). The supernatant contained the monomers of MTs which were similar in all groups. Taxol (paclitaxel) and CaCl2 served as the corresponding positive and negative controls, which promoted and inhibited MT polymerization, respectively. (c) Studies by IF showed that laminin α2 knockdown led to MT disorganization as well as mislocalization of EB1 [a +TIP (plus-end tracking protein)] in which EB1 no longer prominently localized with MTs appeared to discrete dots along the MTs. In control cells, α-tubulin, building blocks of MTs, stretched across the entire cell cytosol, whereas MTs retracted from cell cytosol but rounded up to enclose the Sertoli cell nuclei after laminin α2 knockdown. On the other hand, EB1 associated with long stretches of MTs in control cells, but it no longer prominently found to associate with MTs after laminin α2 knockdown, likely dispersed in cell cytosol because the EB1 level was not perturbed based on IB [see (a)]. Rapamycin treatment that blocked the mTORC1 signaling rescued laminin α2 knockdown induced MT disorganization. GFP expression (green) illustrated the successful transfection. Sertoli cell nuclei were visualized by DAPI. Scale bar, 30 μm.
Laminin α2 regulates Sertoli cell MT and F-actin organization through the mTORC1/rpS6/Akt signaling pathway
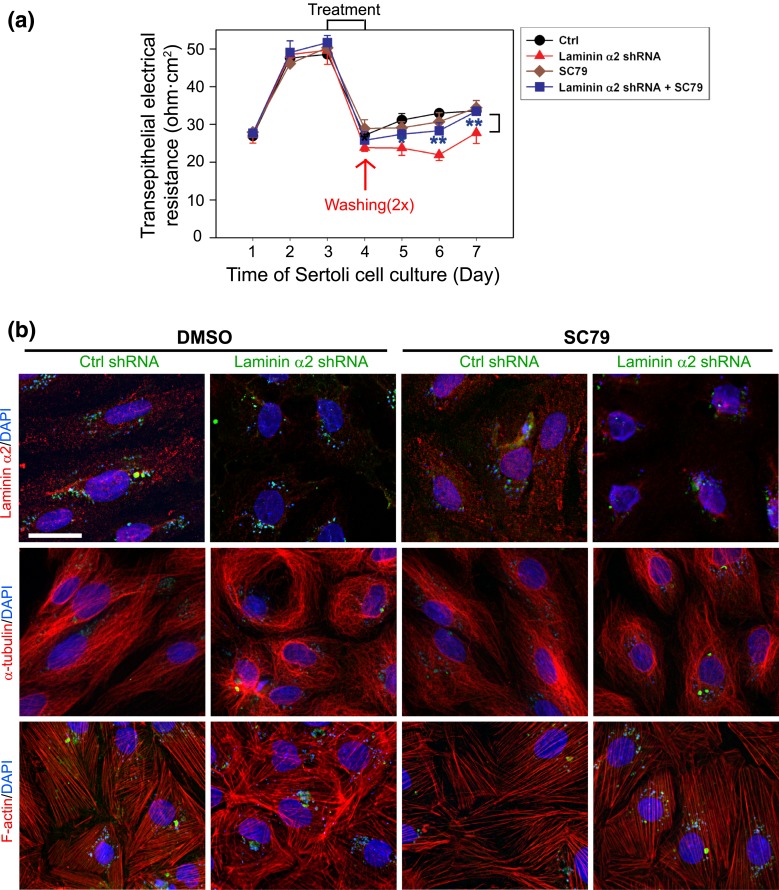
Studies have shown that mTORC1 promotes Sertoli cell TJ barrier disruption, making it “leaky,” is mediated through p-rpS6 and p-Akt signaling in which overexpression of a constitutively active rpS6 mutant that perturbs the Sertoli cell TJ-permeability barrier function is associated with a downregulation of p-Akt1/2 (22, 42). Findings reported herein support the notion that laminin α2 regulates Sertoli cell BTB dynamics through mTORC1/rpS6/Akt1/2 signaling. To further confirm this possibility, a known Akt activator SC79 was used. Indeed, SC79 that activated Akt1/2 rescued Sertoli cells from the disruptive effects mediated by laminin α2 knockdown when the Sertoli cell TJ-permeability barrier function was monitored [Fig. 6(a)]. More important, SC79 also rescued laminin α2 knockdown-induced disorganization of MTs (visualized by α-tubulin staining, which is the building block of MTs) and F-actin because SC79 maintained the proper organization of MTs and F-actin across the Sertoli cell cytosol [Fig. 6(b)].
Figure 6.
The use of SC79, an Akt activator, rescues laminin α2 knockdown-induced Sertoli cell TJ barrier disruption through reorganization of MT- and actin-based cytoskeletons. Sertoli cells cultured for 3 days were transfected with laminin α2 (Lam α2) and coincubated with or without SC79 vs negative control (Ctrl) shRNA for 24 hours. Cells were rinsed and incubated for 24 hours before used for IF, whereas the TJ-permeability barrier was quantified by measuring the TER across the Sertoli cell epithelium daily where Sertoli cells were cultured on bicameral units in parallel experiments. (a) Knockdown of laminin α2 perturbed the Sertoli cell TJ-permeability barrier function but SC79 (at 5.5 μM) was found to rescue the laminin α2 knockdown-induced TJ barrier disruption. Each data point is a mean ± SD of triplicate bicameral units of a representative experiment from a total of n = 3 independent experiments which yielded similar results. **P < 0.01 by Student t test by comparing the laminin α2 shRNA+SC79 and laminin α2 shRNA group. (b) Efficacy of laminin α2 knockdown was shown in the first row in both DMSO vs SC79 cotreatment group. The presence of SC79 successfully rescued laminin α2 knockdown-induced MT- and F-actin disruption. GFP expression (green fluorescence) illustrated successful transfection. Sertoli cell nuclei were visualized by DAPI. Scale bar, 30 μm.
Discussion
Findings reported herein have supported the notion that basement membrane, a modified form of extracellular matrix, in the seminiferous epithelium of testes [for reviews, see (38, 57)] regulates the BTB function. Earlier studies have also provided compelling evidence regarding the presence of this basement membrane-BTB functional axis. First, studies have shown that the basement membrane [or its constituent protein(s)] regulates Sertoli cell function by affecting Sertoli cell morphology, differentiation and migration [for reviews, see (38, 39)], as well as Sertoli cell TJ-permeability barrier function (58, 59). Second, the basement membrane is constituted by extracellular matrix proteins, such as laminins, type IV collagen [a network-forming collagen type (60)], heparin sulfate proteogylcans, entactin, fibulins and fibronectin, which are produced by either Sertoli cells or pertibubular myoid cells, or contributed by both cell types in the testis [for a review, see (39)]. A disruption of the basement membrane function by passive transfer of antibodies to rats raised against seminiferous tubule basement membrane (61) or noncollagenous fraction of seminiferous tubule basement membrane (62) led to focal sloughing of the seminiferous epithelium. More important, a specific anticollagen (type IV) antibody (58) or purified recombinant noncollagenous domain 1 peptide derived from type IV collagen α3 chain (59) was each shown to perturb the Sertoli cell TJ barrier function. Thus, a disruption of the basement membrane function perturbs spermatogenesis, likely the result of a disruption of BTB integrity. Third, laminin α1, α2, β1, β2, and γ1 are the major components of the basement membrane in rodent testes (20). Deletion of laminin α2 (also known as merosin) led to muscular dystrophy (36) because laminin α2 is also a component of basement membrane in muscle cells (63). Interestingly, male mice lacking laminin α2 were infertile, and elongating/elongated spermatids were not found in their seminiferous tubules due to a severe disruption of meiosis and an arrest in spermiogenesis (20). However, the mechanism by which an inactivation of laminin α2 that led to male infertility in mice remains unknown. Studies have shown that a functional BTB correlates with the onset of meiosis I/II in rodents (64, 65). For instance, treatment of neonatal rats with diethylstilbestrol to delay the establishment of a functional BTB also delays the onset of meiosis and spermiation (66). Thus, laminin α2 chain, being in such close proximity to the BTB, may play a role in modulating BTB dynamics. Indeed, studies reported herein have demonstrated unequivocally that laminin α2 chain is a BTB regulator because its silencing by RNAi through an overexpression of a laminin α2-specific shRNA was found to perturb the Sertoli cell TJ barrier function. Furthermore, we have provided compelling evidence that laminin α2 exerts its regulatory effects at the BTB through mTORC1/rpS6/Akt1/2 signaling pathway following its knockdown by shRNA, involving changes in the organization of actin- and MT-based cytoskeletal function. These findings thus support the concept regarding a local functional axis between the basement membrane and the BTB as depicted in Fig. 7.
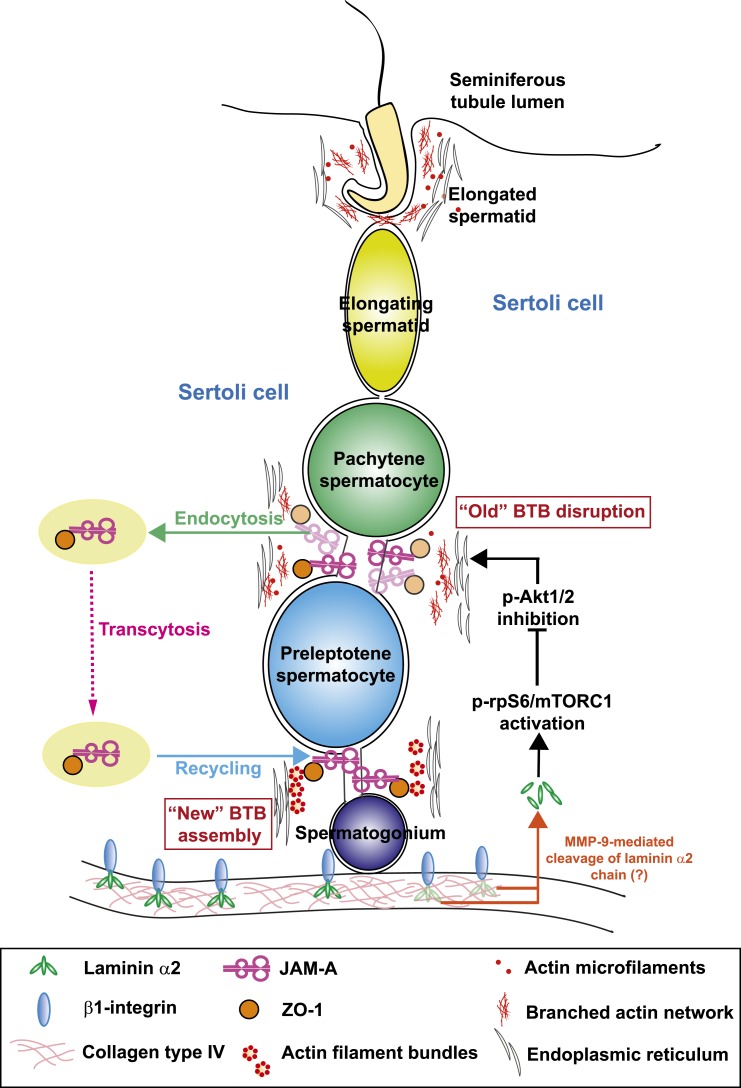
Figure 7.
A schematic drawing that illustrates the likely signaling pathway by which laminin α2 at the basement membrane regulates Sertoli cell BTB dynamics in the seminiferous epithelium of rat testes. Based on the findings reported herein, it is likely that an 80-kDa fragment is released from laminin α2 chain in the basement membrane, mediated by MMP-9 earlier reported in the rat testis (58). Once released, this fragment is being transported across the seminiferous epithelium. When the level of this 80-kDa laminin fragment is reduced—such as following its knockdown by RNAi as reported herein or mediated by its intrinsic downregulation mediated by miRNAs (e.g., endogenouosly produced siRNA) or its reduced spatiotemporal expression during the epithelial cycle—rpS6 is also activated through an induction of p-rpS6, which, in turn, downregulates p-Akt1/2. The activation of this mTORC1/rpS6/Akt1/2 signaling pathway induces BTB remodeling, causing a gradual disassembly of the “old” BTB above the preleoptotene spermatocytes connected in clones. Both TJ and basal ES proteins at the “old” BTB undergo endocytosis, transcytosis and recycling as earlier reported (67–69) to facilitate the assembly of the “new” BTB behind the preleptotene spermatocytes. This thus facilitates remodeling of the BTB at stage VIII of the epithelial cycle to support the transport of preleptotene spermatocytes across the immunological barrier, illustrating the physiological significance of laminin α2 chain in this series of cellular event.
Studies have shown that BTB integrity is modulated by mTORC1 and mTORC2, in which mTORC1 promotes BTB remodeling, making the BTB “leaky” (21), whereas mTORC2 promotes BTB integrity, making it “tighter” (70). Subsequent studies have shown that mTORC1 exerts its effects by activating the Arp3-mediated F-actin organization, causing the actin microfilaments at the BTB to assume a branched/unbundled configuration to promote BTB restructuring (22) to accommodate the transport of preleptotene spermatocytes across the immunological barrier. These changes are mediated through the p-rpS6/p-Akt1/2–induced MMP-9 activation, involving an increase in p-rpS6 steady-state level and a downregulation of p-Akt1/2 expression (42). These changes, in turn, lead to restructuring of the adhesion protein complexes, such as occludin-ZO-1, at the BTB to support TJ barrier remodeling (42). Furthermore, an increase in the expression of p-rpS6 and a downregulation of p-Akt1/2 also lead to a disorganization of actin microfilament across the Sertoli cell cytosol, failing to support the Sertoli cell TJ barrier function (22). Collectively, these two studies (22, 42) thus illustrate mTORC1 exerts its disruptive effects on the Sertoli cell BTB, making it “leaky,” as mediated through an upregulation and a concomitant downregulation of p-rpS6 and p-Akt1/2, respectively. As shown herein, laminin α2 is utilizing this mTORC1/rpS6/Akt12 signaling pathway to modulate the Sertoli cell BTB function after its knockdown.
It is known that mTOR associates with its partner adaptor protein raptor (regulatory-associated protein of mTOR) to generate the mTORC1 signaling complex which is sensitive to rapamycin because this antibiotic effectively blocks mTORC1 signaling function [for reviews, see (71, 72)]. Herein, laminin α2 induced disruption of the Sertoli cell TJ barrier function following its knockdown was shown to be blocked by rapamycin. Rapamycin also reversed the laminin α2 knockdown-induced disruption on F-actin organization. Furthermore, these changes in F-actin organization at the Sertoli cell BTB caused by laminin α2 knockdown were shown to be mediated by changes in the spatiotemporal expression of branched actin polymerization protein Arp3 and actin barbed end capping and bundling protein Eps8. These changes thus disrupted the orderly organization of actin microfilaments at the BTB to support the barrier integrity, leading to BTB disruption following laminin α2 knockdown. However, rapamycin treatment was found to reverse such disorderly spatiotemporal expression of Arp3 and Eps8 induced by laminin α2 knockdown. Additionally, the use of rapamycin that blocks the action of mTORC1 was found to promote actin organization to support the TJ integrity in laminin α2-silenced Sertoli cells. Furthermore, these effects were associated with an up-regulation of p-rpS6 and a downregulation of the p-Akt1/2 which are the signaling partners of mTORC1. More important, the use of SC79, a specific pan-Akt activator, was also shown to rescue laminin α2-knockdown induced Sertoli TJ barrier disruption and F-actin disorganization. Collectively, these findings support the notion that laminin α2 mediates its effects through mTORC1/rpS6/Akt as its downstream signaling complex.
An unusual observation made in this study is that a knockdown of laminin α2 in Sertoli cells was found to perturb the organization of MTs in which MTs failed to stretch out across the entire cell epithelium. This observation suggests that laminin α2 also exerts its regulatory effects at the Sertoli cell BTB through changes in MT organization. The involvement of laminins in MT dynamics has been studied in the growth cone at the tip of the developing axon [for a review, see (73)]. For instance, laminin-2 (also known as laminin-211 or laminin-α2β1γ1) is known to work in concert with integrin-linked kinase to modulate oligodendrocyte growth cone and adhesion dynamics (74). Binding of laminin to integrin receptors serves as a cue, involving Rac 1 to accelerate the growth of neuronal processes from various types of neurons with the participation of F-actin and MTs (75). Furthermore, studies have shown MTs provide the tracks to support spermatid transport (76), as well as intracellular organelles (e.g., residual bodies, phagosomes) and/or protein trafficking (e.g., endosomes) including Sertoli cells [for reviews, see (50, 77)].
In summary, while the underlying molecular mechanism(s) by which laminin α2 modulates MT and F-actin organization remains to be explored, the fact that the use of rapamycin or SC79 could reverse the laminin α2 knockdown-mediated MT and/or F-actin disorganization suggests that laminin α2 is working in concert with mTORC1/rpS6/Akt1/2 signaling pathway to modulate Sertoli cell MT- and actin-based cytoskeletal dynamics as depicted in Fig. 7.
Acknowledgments
This work was supported by grants from the National Institutes of Health, NICHD R01 HD056034 and U54 HD029990 Project 5, to C.Y.C.; Hong Kong Research Grants Council (RGC; General Research Fund GRF774213 and GRF17100816) to W.-y.L.; RGC/National Natural Science Foundation of China Joint Research Scheme (N_HKU 717/12) to W.M.L.; and Hong Kong University Seed Funding (to W.-y.L. and W.M.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Arp3
- actin-related protein 3
- BTB
- blood–testis barrier
- DAPI
- 4′,6-diamidino-2-phenylindole
- DMEM
- Dulbecco's modified Eagle medium
- DMSO
- dimethyl sulfoxide
- EB1
- end-binding protein 1
- Eps8
- epidermal growth factor receptor pathway substrate 8
- ES
- ectoplasmic specialization
- IB
- immunoblotting
- IF
- immunofluorescence
- MARK
- microtubule affinity-regulating kinase
- MT
- microtubule
- mTORC1
- mammalian target of rapamycin complex 1
- RNAi
- RNA interference
- rpS6
- ribosomal protein S6
- shRNA
- short hairpin RNA
- TER
- transepithelial electrical resistance
- TJ
- tight junction
- ZO-1
- zonula occludens 1.
References
- 1.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. [DOI] [PubMed] [Google Scholar]
- 2.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3(4):404–417. [DOI] [PubMed] [Google Scholar]
- 3.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52(1):198–236. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82(4):825–874. [DOI] [PubMed] [Google Scholar]
- 5.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64(1):16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1(1):14–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. [DOI] [PubMed] [Google Scholar]
- 9.Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol. 2013;303:319–355. [DOI] [PubMed] [Google Scholar]
- 10.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. [DOI] [PubMed] [Google Scholar]
- 11.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778(3):692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6(7):380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44(5):245–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70(4):945–964. [DOI] [PubMed] [Google Scholar]
- 15.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82(3):516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105(26):8950–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun 2012;3:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7(39):64203–64220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao PL, Lin YC, Richburg JH. TNF α-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80(3):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Häger M, Gawlik K, Nyström A, Sasaki T, Durbeej M. Laminin α1 chain corrects male infertility caused by absence of laminin α2 chain. Am J Pathol. 2005;167(3):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 Regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 2012;153(10):5036–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok KW, Chen H, Lee WM, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat sertoli cells: an in vitro study. Endocrinology. 2015;156(5):1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;2:249–254. [Google Scholar]
- 25.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25(2):200–215. [DOI] [PubMed] [Google Scholar]
- 26.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41(11):2302–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280(26):25029–25047. [DOI] [PubMed] [Google Scholar]
- 28.Jo H, Mondal S, Tan D, Nagata E, Takizawa S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, Tejeda AO, Man H, Rigby AC, Luo HR. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA. 2012;109(26):10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26(21):8042–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Zhang H, Hao S, Yan H, Zhang Z, Hu Y, Zhuang Z, Li W, Zhou M, Li K, Hang C. Akt specific activator SC79 protects against early brain injury following subarachnoid hemorrhage. ACS Chem Neurosci. 2016;7(6):710–718. [DOI] [PubMed] [Google Scholar]
- 31.Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1(2):121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Lui WY, Lee WM, Cheng CY. Polarity protein Crumbs homolog-3 (CRB3) regulates ectoplasmic specialization dynamics through its action on F-actin organization in Sertoli cells. Sci Rep. 2016;6:28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: an in vitro study. Endocrinology. 2015;156(2):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)). J Biol Chem. 1997;272(4):2534–2541. [DOI] [PubMed] [Google Scholar]
- 35.Acharya BR, Espenel C, Kreitzer G. Direct regulation of microtubule dynamics by KIF17 motor and tail domains. J Biol Chem. 2013;288(45):32302–32313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415(1):33–39. [DOI] [PubMed] [Google Scholar]
- 37.Schlatt S, de Kretser DM, Loveland KL. Discriminative analysis of rat Sertoli and peritubular cells and their proliferation in vitro: evidence for follicle-stimulating hormone-mediated contact inhibition of Sertoli cell mitosis. Biol Reprod. 1996;55(2):227–235. [DOI] [PubMed] [Google Scholar]
- 38.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15(1):102–115. [DOI] [PubMed] [Google Scholar]
- 39.Siu MKY, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004;71(2):375–391. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Mruk D, Chen H, Lui WY, Lee WM, Cheng CY. Regulation of the blood-testis barrier by a local axis in the testis: role of laminin α2 in the basement membrane. FASEB J. 2017; 31(2): 584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda). 2014;29(4):286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mok KW, Mruk DD, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J Cell Sci. 2014;127(22):4870–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mok KW, Mruk DD, Cheng CY. Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the “Yin” and “Yang” effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Int Rev Cell Mol Biol. 2013;301:291–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–162. [DOI] [PubMed] [Google Scholar]
- 45.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14(3):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N, Cheng CY. Mammalian target of rapamycin complex (mTOR) pathway modulates blood-testis barrier (BTB) function through F-actin organization and gap junction. Histol Histopathol. 2016;31(9):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000;113(22):3907–3919. [DOI] [PubMed] [Google Scholar]
- 48.Dunn S, Morrison EE, Liverpool TB, Molina-París C, Cross RA, Alonso MC, Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci. 2008;121(7):1085–1095. [DOI] [PubMed] [Google Scholar]
- 49.Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987;6(9):2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217(2):R13–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowne-Anderson H, Hibbel A, Howard J. Regulation of microtubule growth and catastrophe: unifying theory and experiment. Trends Cell Biol. 2015;25(12):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grimaldi AD, Zanic M, Kaverina I. Encoding the microtubule structure: allosteric interactions between the microtubule +TIP complex master regulators and TOG-domain proteins. Cell Cycle. 2015;14(9):1375–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011;23(1):94–101. [DOI] [PubMed] [Google Scholar]
- 55.Kumar P, Wittmann T. +TIPs: SxIPping along microtubule ends. Trends Cell Biol. 2012;22(8):418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su X, Ohi R, Pellman D. Move in for the kill: motile microtubule regulators. Trends Cell Biol. 2012;22(11):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26(9):978–992. [DOI] [PubMed] [Google Scholar]
- 58.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144(1):371–387. [DOI] [PubMed] [Google Scholar]
- 59.Wong EWP, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis 2013;3(2):e25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. [DOI] [PubMed] [Google Scholar]
- 61.Lustig L, Denduchis B, González NN, Puig RP. Experimental orchitis induced in rats by passive transfer of an antiserum to seminiferous tubule basement membrane. Arch Androl. 1978;1(4):333–343. [DOI] [PubMed] [Google Scholar]
- 62.Denduchis B, Satz ML, Sztein MB, Puig RP, Doncel G, Lustig L. Multifocal damage of the testis induced in rats by passive transfer of antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7(1):59–75. [DOI] [PubMed] [Google Scholar]
- 63.Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48(3-4):181–191. [DOI] [PubMed] [Google Scholar]
- 64.Mok KW, Mruk DD, Lee WM, Cheng CY. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis. 2011;1(3):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergmann M, Dierichs R. Postnatal formation of the blood-testis barrier in the rat with special reference to the initiation of meiosis. Anat Embryol (Berl). 1983;168(2):269–275. [DOI] [PubMed] [Google Scholar]
- 66.Toyama Y, Ohkawa M, Oku R, Maekawa M, Yuasa S. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22(3):413–423. [PubMed] [Google Scholar]
- 67.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22(6):1945–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316(17):2945–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107(25):11399–11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 2013;27(3):1137–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95(4):1157–1187. [DOI] [PubMed] [Google Scholar]
- 73.Kahn OI, Baas PW. Microtubules and Growth Cones: Motors Drive the Turn. Trends Neurosci. 2016;39(7):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michalski JP, Cummings SE, O’Meara RW, Kothary R. Integrin-linked kinase regulates oligodendrocyte cytoskeleton, growth cone, and adhesion dynamics. J Neurochem. 2016;136(3):536–549. [DOI] [PubMed] [Google Scholar]
- 75.Grabham PW, Reznik B, Goldberg DJ. Microtubule and Rac 1-dependent F-actin in growth cones. J Cell Sci. 2003;116(18):3739–3748. [DOI] [PubMed] [Google Scholar]
- 76.Tang EI, Lee WM, Cheng CY. Coordination of actin- and microtubule-based cytoskeletons supports transport of spermatids and residual bodies/phagosomes during spermatogenesis in the rat testis. Endocrinology. 2016;157(4):1644–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711–726. [DOI] [PubMed] [Google Scholar]