Abstract
Densely granulated and sparsely granulated (SG) growth hormone (GH) pituitary adenomas differ in biological behavior, which may be correlated with their known differences in cytoplasmic keratin distribution and E-cadherin expression. We wanted to explore candidate genes that might further explain this behavior. Exon expression microarray was performed on 21 GH tumors (10 SG and 11 densely granulated) and 20 normal control pituitaries from autopsy. Bioinformatic analyses confirmed a differential molecular signature between normal pituitary and GH tumors as well as between the GH tumor subtypes. There was a consistent downregulation of transcripts involved in the structure and function of the desmosome, including desmoplakin (eightfold), desmoglein 2 (sixfold), plakophilin 2 (sevenfold), and p53 apoptosis effector related to PMP-22 (PERP; sixfold) in SG tumors compared with normal pituitary. PERP is lost in more aggressive SG human GH pituitary tumors. PERP re-expression in GH3 rat GH tumor cells resulted in decreased colony formation compared with vector transfectants, confirming the role of PERP as a tumor suppressor with no effects on proliferation. Increased PERP expression was associated with loss of a survival advantage in a hypoxic environment, as assessed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (P < 0.05) and cleaved caspase-3 (P < 0.05). Downregulation of desmosomal formation transcripts including PERP may contribute to the aggressive phenotype seen in SG GH pituitary tumors and their behavior in response to surgery and medical therapy.
PERP and desmosome components are dysregulated in sparsely granulated GH tumors. In vitro studies suggest that downregulation of PERP plays an important role in sparsely granulated tumorigenesis and survival.
Acromegaly is a clinical syndrome due to excess growth hormone (GH) secretion usually from a GH pituitary tumor. Patients with inadequately treated acromegaly have increased morbidity and mortality due to concomitant metabolic, respiratory, and cardiovascular complications resulting in a decreased lifespan of at least 10 years, and these patients have significant increased cost of medical care (1, 2). Transsphenoidal resection of the tumor by an experienced neurosurgeon is the preferred primary therapy, followed by medical therapy with somatostatin analogs (SSAs) and/or GH receptor (GHR) antagonists (3). Despite this stepwise approach to treatment of these tumors, >50% of patients do not achieve remission (4, 5).
We have been interested in defining biomarkers of disease prognosis in patients with GH tumors. Prior work by our laboratory and others has confirmed the importance of histologic subtyping to predict response to surgery and medical therapy (6–14). Densely granulated (DG) GH tumors have keratin filaments distributed throughout the cytoplasm as defined by diffuse immunohistochemistry (IHC) cytoplasmic staining for CAM5.2 [cytokeratin 8/18 (K8/18) stain], and they tend to have high surgical cure rates and an excellent response to SSAs when residual tumor remains postoperatively (6, 8, 10–12). In contrast, sparsely granulated (SG) GH tumors, characterized by rounded aggregates of cytoplasmic keratin filaments (fibrous bodies) on CAM5.2 IHC, are more commonly seen in younger patients, often are larger tumors, and are more locally invasive with a poor response to surgery or medical therapy with SSAs, although SG tumors usually respond to pegvisomant, the GHR inhibitor (6, 8–14). Additionally, it is known that DG GH adenomas show strong E-cadherin cytoplasmic immunostaining, although this is lost in SG GH adenomas (8, 12, 14–16). E-cadherin is known to be involved in epithelial-to-mesenchymal transition (EMT) in a wide variety of tissue and tumor types, underscoring the important role for this protein. Interestingly, in GH adenomas with transitional or intermediate forms of small poorly formed fibrous bodies (8, 12, 14), the E-cadherin immunoreactivity remains strong and the tumors behave akin to the more indolent DG GH pituitary tumor, suggesting that levels of E-cadherin may be even more important for controlling/reflecting biological behavior than the keratin distribution pattern.
To further investigate the molecular differences between the histological GH tumor subtypes, we performed exon expression microarray on individual human GH tumors compared with normal pituitary tissue from individual autopsy samples.
We identified a differential molecular expression pattern between the GH tumors and normal pituitary as well as unique signatures that separate the GH tumor subtypes. We confirmed previously observed upregulation of somatostatin receptor (SSTR)2 in DG tumors as well as low expression of E-cadherin and p27kip in SG tumors, consistent with an EMT-like signature in SG tumors (7, 8, 11, 12, 14–17). Analysis of the expression data demonstrated that SG tumors had a dramatic and consistent loss of multiple critical desmosome components, including p53 apoptosis effector related to PMP-22 (PERP), a tumor suppressor previously identified to act as a docking component of the desmosome and a downstream target of p53 in the apoptosis pathway (18). We examined the functional role of PERP protein in GH tumor cells and confirmed its role in modifying tumor formation in soft agar. The antitumorigenic actions with reintroduction of PERP were dependent on its role to control rates of cell survival in a hypoxic microenvironment, but not on cell proliferation. We hypothesize that dysregulation of PERP in SG GH tumors via effects on tumor cell survival as well as its role with other components of the desmosome underlie the different histologic features and clinically observed increase in local invasion and tumor persistence despite standard surgical and medical therapy.
Materials and Methods
Characterization of human pituitary tumors and normal pituitary
Pituitary tumor samples were obtained from patients at University of Colorado Hospital at the time of transsphenoidal surgery after informed consent. Portions of the specimens were immediately dissected, placed in RNAlater (QIAGEN Bioinformatics, Valencia, CA), and stored at −80°C. Tumors were classified as GH tumors as defined by positive immunostaining for GH in the clinical pathology specimen. GH tumor subtyping was determined using cytoplasmic immunostaining for CAM5.2 (monoclonal, prediluted; Becton Dickinson, Franklin Lakes, NJ). DG GH adenomas were defined as those with diffuse cytoplasmic CAM5.2 immunoreactivity, corresponding to distribution of cytokeratin filaments throughout the cytoplasm and an absence of CAM5.2 IHC-positive fibrous bodies. Conversely, SG GH adenomas showed an IHC pattern with numerous, diffusely distributed fibrous bodies on CAM5.2. De-identified normal pituitary glands were obtained at autopsy within 2 to 18 hours of death from University of Colorado Denver Pathology Department. Demonstration of RNA and protein integrity of all samples was confirmed as previously described (19–21).
Microarray analysis
Our cohort consisted of 21 GH tumors (11 DG and 10 SG) and 20 samples of normal human pituitary. Total RNA was isolated using TRIzol method (Invitrogen, Carlsbad, CA), followed by clean-up with a QIAGEN Bioinformatics RNeasy mini kit (Redwood City, CA). RNA was quantified by spectrophotometry, and RNA integrity was verified using the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). Microarray targets were prepared and labeled from 150 ng of total RNA using the Ambion WT (Thermo Fisher Scientific, Waltham, MA) expression kit following the manufacturer’s instructions. An Affymetrix Human Gene 1.1 ST array plate (Thermo Fisher Scientific) was hybridized with 2.3 µg of fragmented and labeled single-stranded complementary DNA (cDNA). Hybridization and scanning were done on the Affymetrix GeneTitan instrument. Hybridization intensities were quantified and normalized across all arrays using the robust multichip average algorithm available as an array processing tool on Partek Genomics Suite software (Partek, St. Louis, MO). Data are available as a tab-delineated file in Supplemental Tables 1 and 2 (696.3KB, pdf) and have also been deposited, along with the original Affymetrix CEL files used to generate the raw data, in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo).
Biostatistical and bioinformatic analysis
One-way analysis of variance (ANOVA) with Fisher’s least significant difference using the Partek Genomics Suite 6.6 software was used to examine differentially expressed transcripts with a stringent false discovery rate (FDR) of <0.05. An expression change cutoff of more than twofold was applied to generate a set of transcripts with differential expression between GH tumor subtypes and between normal and tumor tissues. Ingenuity Pathway Analysis (IPA) (QIAGEN Bioinformatics, Redwood City, CA) was then used to identify biological functions and diseases associated with differentially expressed genes.
Cell culture and reagents
GH3 cells were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomyocin at 37°C in humidified 5% CO2. The hPERP cDNA was generated by polymerase chain reaction (PCR) using SG tumor cDNA as template and two primers: 5′-CTAGTGAATTCACCAT GATCCGCTGCGGCCTGGCCTGCG-3′ (forward) and 5′-GCATCTCGAGTTAGGCAGATGTGTAGA AGTACCTGGGC-3′ (reverse). The PCR product was digested by EcoRI and XhoI and then inserted to pcDNA3 vector, and the sequence was confirmed through Sanger sequencing. A PERP antibody was purchased from Abcam (Cambridge, MA); caspase-3 antibody was from Cell Signaling Technology (Danvers, MA); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was from Millipore (Billerica, MA) as seen in Table 1.
Table 1.
Antibody Table
| Antibody Name | Company | Catalog No. | RRID No. |
|---|---|---|---|
| PERP | Abcam | Ab48032 | AB_881983 |
| Caspase-3 | Cell Signaling Technology | 9662 | AB_10694681 |
| GAPDH | Millipore | MAB374 | AB_2107445 |
Immunoblot analysis and IHC
For immunoblot analysis, cells were lysed in RIPA buffer [150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris (pH 8.0)] with added protease inhibitor (Sigma-Aldrich, St. Louis, MO) and phenylmethylsulfonyl fluoride (0.5 μM). Protein concentration of the lysates was quantified using a bicinchoninic acid assay (Pierce, Rockford, IL) and electrophoresed through sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Protein transfer was performed using the Mini-Transblot system (Bio-Rad, Hercules, CA) and electrotransferred onto polyvinylidene difluoride membranes. The polyvinylidene difluoride membranes were then blocked using a 3% bovine serum albumin solution [in Tris-buffered saline with Tween 20 (TBST)] for 60 minutes on a shaker. Dilutions of primary antibodies were done using a TBST/0.5% bovine serum albumin/0.1% NaN3 buffer and the membranes were allowed to be incubated in primary antibodies at 4°C overnight on a shaker. Membranes were then washed with TBST three times, 10 min each. Secondary antibodies conjugated to horseradish peroxidase (Amersham Biosciences, Piscataway, NJ) were diluted in TBST and were allowed to incubate with the membranes for 1 hour on a shaker at room temperature. The membranes were washed with TBST for 10 minutes each and visualization was performed using ECL according to the manufacturer’s protocol (Pierce, Rockford, IL).
Quantitative real-time PCR
Total RNA was extracted from tissues using TRIzol (Invitrogen), according to the manufacturer’s protocol, and was purified with an RNeasy mini kit (QIAGEN Bioinformatics), and 2.5 µg was reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR (qPCR) on human tissues was run of five normal pituitaries, five DG tumors, and five SG tumors, which are the same as tissues for microarray. Primer sequences (Thermo Fisher Scientific) used for qPCR were: human PERP (forward, 5′-CATGGAGTACGCGTGGGGTA-3′, reverse, 5′-AAGACAAGCATCTGGGGTCC-3′), rat Perp (forward, 5′-CGCACTGGCTGCTGTATTCC-3′, reverse, 5′-ATCCTCGTAGTTGGGGAGGC-3′), human desmoplakin (DSP; forward, 5′-GCTAAACGCCGCCAGGAT-3′, reverse, 5′-CCGCATGACTGTGTTGGAAT-3′), human plakophilin 2 (PKP2; forward, 5′-ATGGCTCTGTTAGCGACACC-3′, reverse, 5′-AAGCCCTGTTCTGAGTGACG-3′), human desmoglein 2 (DSG2; forward, 5′-AGCACGCCAAGAAAGTACCA-3′, reverse, 5′-AGGAGTCATGCTGTGCTTCC-3′), human GAPDH (forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse, 5′-AGAGGGGGCAGAGATGATGA-3′), rat Gapdh (forward, TGGAGTCTACTGGCGTCTT-3′, reverse, 5′-TGTCATATTTCTCGTGGTTCA-3′).
Colony formation assay
Anchorage-dependent growth of tumor cells was investigated by a standard colony formation assay (20). GH3 cells, transfected with either vector or PERP, were plated in triplicate in a 60-mm dish and incubated at 37°C under humidified 5% CO2. Twenty-four hours later, cells were cultured in serum-replete conditions containing G418 (800 μg/mL). Media were changed every 48 hours. After 14 days, colonies were stained using Crystal Violet for 10 minutes after methanol fixation for 5 minutes. Images of colonies on 60-mm plates were captured using an Olympus camera (Olympus, Center Valley, PA). Images were quantified using ImageJ software on triplicate experiments.
Proliferation assay
Rates of cell proliferation were assessed using direct cell counts. Briefly, 5000 cells (vector control or PERP overexpressed) were plated in a 96-well plate in complete medium (Dulbecco’s modified Eagle medium plus 10% fetal bovine serum) (nine wells per condition). Cells were trypsinized on days 1, 3, and 5 after plating, and viable cells were counted by trypan blue exclusion.
Apoptosis assays in response to hypoxic microenvironment
Cells were incubated in a 1% O2 hypoxic chamber for 17 hours in media that were hypoxia preconditioned for 8 hours prior. For cleaved caspase-3 assay, the cells were harvested at 17 hours and immunoblotting was performed. Additionally, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) was performed at 17 hours to analyze rates of cell death. Briefly, cells were fixed with 4% paraformaldehyde for 25 minutes at 4°C. After three washes with PBS, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 minutes at room temperature. The TUNEL reaction mixture was obtained by adding terminal deoxynucleotidyltransferase to nucleotide mixture as per the manufacturer’s manual (Promega, Madison, WI). Cells were then incubated with 50 μL of the TUNEL reaction mixture in a moist chamber for 1 hour at 37°C. After rinsing with PBS, cell nuclei were visualized using 0.5 μg/mL 4′,6-diamidino-2-phenylindole. The green fluorescence of apoptotic cells was detected by fluorescence microscopy (Nikon Eclipse E600).
Statistical analysis
Data are presented as means ± standard error of the mean from three or more separate experiments. The P value calculations were conducted using an unpaired Student t test for two group comparisons or ANOVA (with Bonferroni posttest analysis for multiple comparisons). All data were analyzed and presented by using GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, CA).
Study approval
All human subjects studies were performed with approval from the Colorado Institutional Review Board of the University of Colorado.
Results
Microarray analysis identifies a unique molecular signature of GH tumor subtypes and normal pituitary
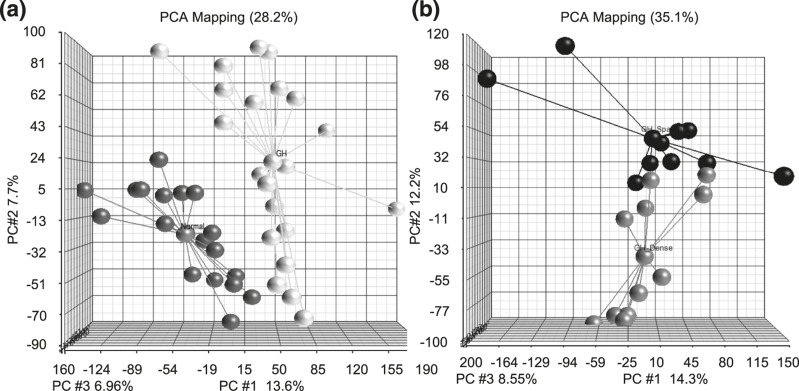
Affymetrix Human Gene 1.1 ST exon expression microarray was performed on 21 GH tumors (11 DG and 10 SG) and 20 normal pituitary tissues. A one-way ANOVA using Partek Genomic Suite 6.6 was performed on 33,297 gene probes to compare the difference in gene expression between individual normal pituitary and SG and DG human GH pituitary tumor samples. Principal component analysis (PCA) plots were generated using one-way ANOVA to visually display the variants of the data in the three-dimension coordinate system. Figure 1(a) shows the PCA plot of normal pituitaries (black) compared with GH tumors (white). This unsupervised analysis shows clear separation between tumor and normal pituitary tumor samples, suggesting a unique molecular expression of GH tumors. Figure 1(b) displays the PCA plot of DG (gray) compared with SG tumors (black) and suggests a differential molecular signature between the GH tumor subtypes.
Figure 1.
PCA graphs showing differential gene expression between normal pituitary, DG, and SG GH tumors. (a) PCA plot displaying the expression profiles of individual GH tumors (white dots) compared with normal pituitary samples (black dots). (b) PCA plot of the global differences in gene expression between SG (black) and DG (gray) GH tumors.
Bioinformatic analysis reveals a large number of differentially expressed transcripts and pathways
Bioinformatic analysis with Partek Genomic Suite using one-way ANOVA identified differentially expressed transcripts with a FDR of <0.05 to control for multiple testing. The analysis revealed that 1026 genes expressed >2.0-fold differential expression between GH tumors and normal pituitaries. Among this list, 251 genes were upregulated in tumors compared with normal, whereas 775 genes were downregulated. A parallel analysis comparing the GH tumor subtypes, using the same parameters for FDR and fold change, identified 891 differentially expressed genes, with 415 upregulated and 476 downregulated in SG compared with DG tumors. Supplemental Tables 1 and 2 (696.3KB, pdf) show the top 100 uniquely expressed transcripts for normal pituitary vs GH tumor and the SG vs DG tumor analyses, respectively. Next, to further identify biological pathways associated with differentially expressed genes, IPA was used and revealed several signaling pathways differentially regulated (at least 1.3-fold) between GH tumors subtypes including the cell junction signaling pathway (P = 2.27 × 10−5) (Supplemental Fig. 1 (696.3KB, pdf) ).
Transcript expression confirms previously reported dysregulations in SG compared with DG tumors
To validate the candidates identified by our bioinformatic analysis, we examined pathways and genes reported to be dysregulated between GH tumor subtypes in prior publications. Downregulation of E-cadherin in SG tumors has been reported by us and others (8, 12, 14–16). The E-cadherin (CDH1) transcript level was 10-fold lower in SG tumors compared with DG tumors (Supplemental Fig. 2 (696.3KB, pdf) ). Downregulation of E-cadherin has been classically associated with EMT in tumorigenesis. Previously, Lekva et al. (22) alluded to a role of EMT in GH tumorigenesis; however, the relationship to the subtype of GH pituitary tumor was not reported. With significant downregulation of E-cadherin in SG tumors, we asked whether SG tumors are more likely to be associated with EMT signature. Interestingly, although some of the EMT signature markers (23), such as CDH1 (−10.4-fold), EPCAM (−2.4-fold), SPINT1 (−4.8-fold), TMEM30B (−10.4-fold), PPAP2B (4.5-fold), ZEB1 (1.7-fold), and IL1R1 (3.7-fold), were dramatically dysregulated in SG tumors (Supplemental Fig. 3 (696.3KB, pdf) ), other known EMT components, such as VMT, SNAIL, SLUG, and TWIST (24), were unchanged. We also examined the transcript levels of SSTR1–5, which are the major target of somatostatin analogs used for treatment of patients with GH tumors. In agreement with previous reports (7, 11, 17), only SSTR2 was significantly downregulated in SG compared with DG tumors (P < 0.001) (Supplemental Fig. 4 (696.3KB, pdf) ).
Desmosome protein PERP is downregulated in SG tumors compared with DG tumors
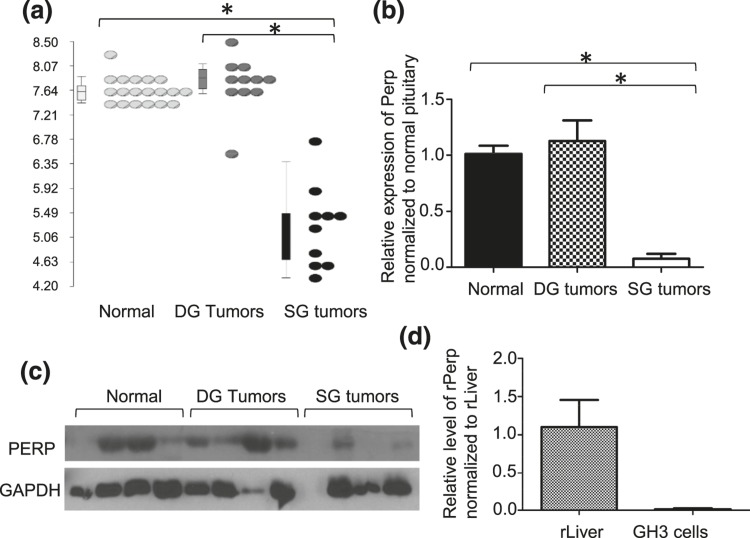
Although adherens junction dysregulation associated with downregulation of E-cadherin has been described in SG tumors (8, 12, 14–16), the role of other components of the cell junction have not been explored. Integrating IPA and Partek analysis, we observed that the PERP transcript, a critical component of the desmosome, was sixfold lower in SG than DG tumors (P < 0.001) [Fig. 2(a)]. This differential expression was then confirmed by quantitative reverse transcription PCR for messenger RNA [mRNA; 13-fold, P < 0.001; Fig. 2(b)] and by immunoblot for protein [Fig. 2(c)]. The tissue used for protein for immunoblot is different from tissues used for microarray.
Figure 2.
PERP is downregulated in SG compared with DG tumors and normal pituitary. (a) Perp transcript levels are significantly repressed in SG tumors compared with DG or normal pituitary samples. *P < 0.001. (b) Downregulation of PERP was confirmed at the mRNA level by qPCR. *P < 0.001. (c) Low PERP protein levels in SG tumors are also seen by immunoblot. (d) Perp is low in GH3 rat pituitary cell model compared with rat liver control by qPCR.
PERP overexpression decreases tumor formation
To examine the potential role of PERP in pituitary tumorigenesis, in the absence of human GH tumor cell lines, we used rat GH3 pituitary tumor cells, which similarly to human SG tumors have low expression of PERP protein compared with liver, which is known to have abundant PERP [Fig. 2(d)]. GH3-PERP transient transfectants were generated to express PERP at levels similar to human DG tumors whereas GH3-vector cells were used as a model of SG tumors [Fig. 2(c); see Fig. 4(b)].
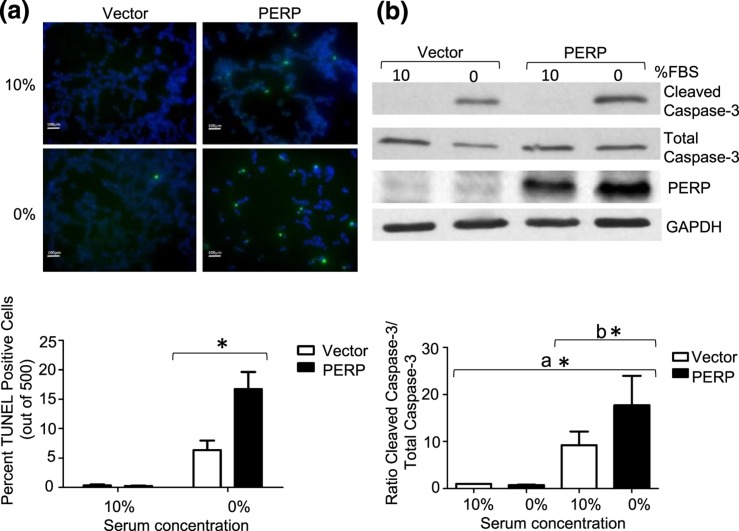
Figure 4.
PERP is associated with increased apoptosis in hypoxic conditions. (a) Overexpression of PERP increased rates of cell death. PERP or vector-transfected cells were exposed to 1% O2 for 17 hours in serum-replete and serum-depleted conditions, and apoptosis was assessed by TUNEL staining. Rates of apoptosis are shown as percentage of TUNEL-positive cells to total cells. Scale bars, 100 μm. *P < 0.05. (b) Increased apoptosis as assessed by caspase 3 cleavage in GH3 cells overexpressing PERP in serum-replete and serum-depleted conditions in 1% O2 for 17 hours in serum-replete and serum-depleted conditions. *P < 0.05, (a) t test normalized to pDNA3 10%, (b) t test normalized to pDNA3 0%.
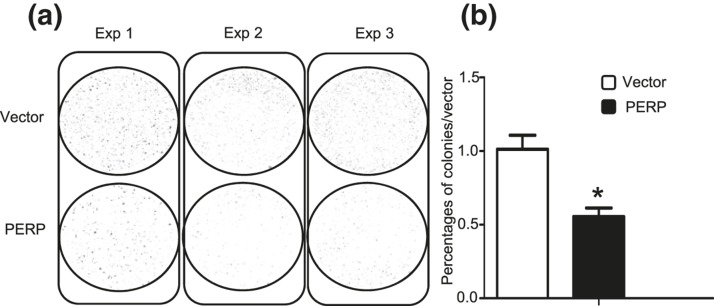
In colony formation assays, GH3 cells were transiently transfected with vector or PERP and selected by G418. GH3-PERP cells demonstrated 2.3-fold fewer colonies than did cells containing vector control at 14 days (P = 0.006; Fig. 3). To understand the mechanism of tumorigenesis in the presence or absence of PERP, effects on rates of cell proliferation and survival were determined. Tumor cell proliferation assays using cell counts were performed for 5 days. No differences in rates of proliferation between the two groups of GH3 tumor cells were observed (Supplemental Fig. 5 (696.3KB, pdf) ), suggesting that the tumorigenic effects of loss of PERP were not dependent on alterations in cell proliferation.
Figure 3.
Increased PERP represses colony formation in GH3 cell. (a) Photomicrograph of colony formation assay of vector and PERP transfectants in serum-replete conditions containing G418 for 14 days. (b) PERP overexpression decreased colony formation. Number of colonies in vector control and PERP transfectants were assessed at 14 days *P = 0.006.
PERP overexpression is associated with increased apoptosis in the hypoxic environment
Progression and maintenance of tumorigenesis characteristically requires strategies to allow tumor cells to evade death. To explore the effects of PERP re-expression on cell survival, cells transiently transfected with vector or PERP were grown in serum replete vs deplete media. No effects of PERP re-expression were observed on cell survival under these conditions (data not shown). Because we and others have previously shown that hypoxic conditions may play an important role in pituitary tumorigenesis (25–28), we tested the impact of differential expression of PERP in a hypoxic microenvironment. GH3-vector and GH-PERP cells were placed in a hypoxic (1% O2) environment in the presence or absence of serum for 17 hours and cell death was assessed by TUNEL. Under hypoxia and serum deprivation, PERP expression was associated with increased rates of apoptosis compared with vector controls [50% vs 19%, respectively; P < 0.05; Fig. 4(a)], suggesting that the lower levels of PERP in human SG pituitary tumors might offer a survival advantage under hypoxic stress. Increased rates of apoptosis were also confirmed by levels of cleaved caspase-3 where tumor cells expressing increased levels of PERP displayed increased rates of apoptosis during 17 hours of hypoxia in serum-depleted media, as shown in Fig. 4(b) (P < 0.05). Taken together, these data suggest that the decrease in rates of colony formation associated with PERP overexpression is mediated by an increase in rates of cell death rather than significant effects on rates of tumor cell proliferation.
Multiple desmosome components are downregulated in SG tumors
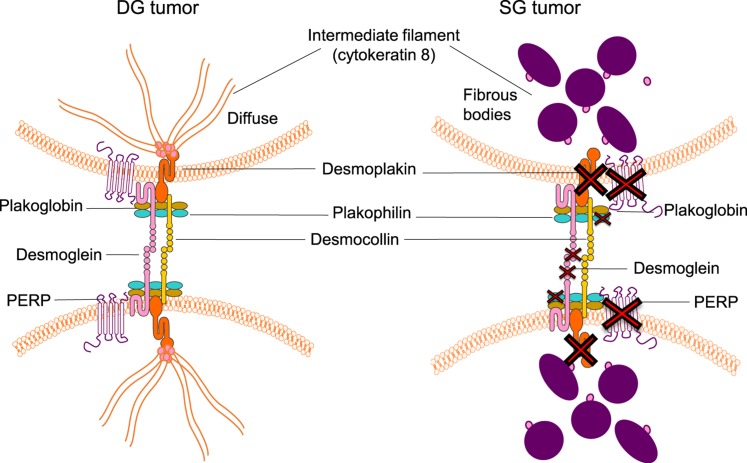
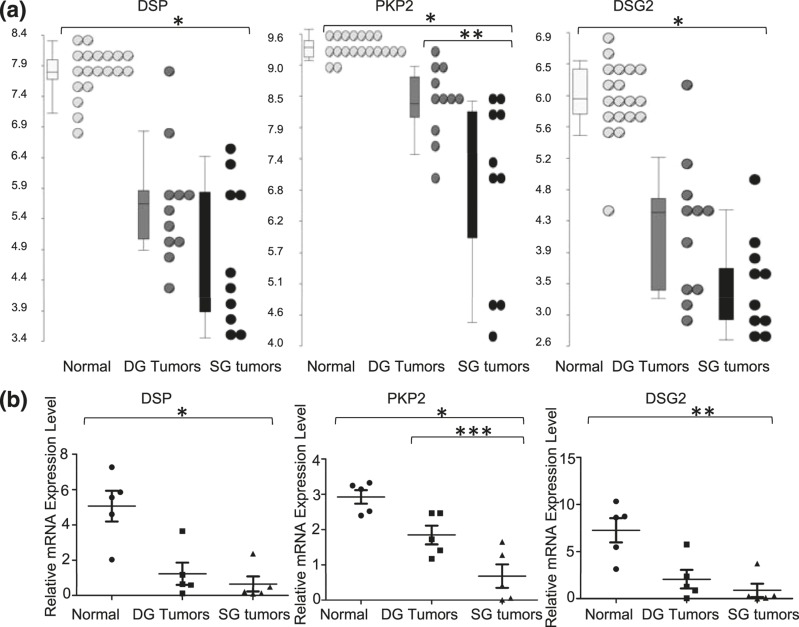
In addition to its potential proapoptotic properties (18), PERP is known to be a critical component of the junctional desmosome structure (see Fig. 6). To further characterize changes in other desmosome components in SG compared with DG tumors, we examined the transcript levels from the microarray data. In addition to loss of PERP, multiple other components of the desmosome were downregulated in SG human pituitary tumors, including DSP (eightfold; P < 0.001), PKP2 (sevenfold, P < 0.001), and DSG2 (sixfold; P < 0.001) compared with normal pituitary as seen in Fig. 5. qPCR confirmed the changes at the mRNA level and demonstrated downregulation in human SG tumors compared with normal pituitary, that is, DSP at sevenfold (P = 0.002), PKP2 at fourfold (P = 0.0004), and DSG2 at eightfold (P = 0.003) (Fig. 5). Whereas the expression of PKP2 is also differential between SG and DG tumors on transcript (2.9-fold, P = 0.0004) and mRNA level (P = 0.03), DSP and DSG are not statistically different, although there is a trend toward loss of these genes with tumor aggressiveness. Antibodies were obtained to examine the protein expression of the various desmosome components, but unfortunately the reagents were not specific and could not be optimized in the positive control tissue by immunoblotting or IHC. Nevertheless, the similar pattern of downregulation of PERP and multiple other desmosomal components on the transcript level via microarray and quantitative reverse transcription PCR strongly supports the hypothesis that the downregulated or complete loss of expression of these proteins may contribute to the histologic staining differences as well as to the aggressive behavior of SG GH pituitary tumors.
Figure 6.
Illustration of desmosome dysregulation in SG compared with DG tumors, showing downregulation of multiple proteins encoded by genes that include PERP, DSG2, PKP2, DSP, and desmocollin. In SG tumors there are also abnormal-appearing cytokeratin 8 fibrous bodies that anchor to desmosome.
Figure 5.
Desmosome components are dysregulated in SG tumors compared with DG and normal pituitary. (a) Dot blots show the transcript expression on log-transformed scale for DSP, PKP2, and DSG2 (white indicates normal pituitary, gray indicates DG tumors, and black indicates SG tumors). (b) mRNA expression of desmosome components, DSP, PKP2, and DSG2, using qPCR. *P < 0.001, **P < 0.01, ***P = 0.03.
Discussion
GH pituitary tumors are associated with significant morbidity and yet are poorly characterized to date regarding their pathogenesis (1). Early observations in the field found that an activating mutation in G protein subunit A leading to constitutively active cyclic adenosine monophosphate was associated with DG tumors (29). Subsequent studies, however, found that G protein subunit A mutations occur in 40% to 65% of GH adenomas, including 23% to 38% of the SG subtype, meaning that these mutations do not correlate with the tumor histological subtypes (8, 12, 29–33). Asa et al. (34) found that 43% of SG tumors had GHR mutations whereas none was found in DG tumors. GHR mutations are associated with impaired GHR processing, ligand binding, and signaling (34). Interestingly, others groups have not detected GHR mutations in their cohorts (30, 35), although GH tumor histologic subtyping was not performed. Others in the field have examined cyclic adenosine monophosphate, cytosolic Ca2+ signaling pathways, and ZAC1 expression in GH tumor subtypes and found no difference for these intracellular signaling mechanisms (36). Studies in our laboratory and those of others have reported upregulation of SSTR2 in DG tumors compared with SG tumors, although the underlying mechanism of this finding remains to be elucidated (7, 11, 17). Previous studies have also reported that E-cadherin is one of the most dysregulated proteins in GH tumors subtypes, with DG tumors having high expression and SG tumors having low or absent expression (8, 12, 14–16). Our group recently showed that the expression of p27kip is also frequently lost in SG tumors, and in vitro experiments suggest that E-cadherin upregulation might be associated with increased p27kip levels (7). A study by Lekva et al. (22) demonstrated that E-cadherin expression in GH tumors correlates with an EMT signature, although histologic subtyping of GH tumors was not reported.
Histological subtyping of GH tumors is becoming a useful prognostic indicator of tumor behavior and response to surgical and medical therapy. SG GH tumors compared with DG tumors occur in younger patients and tend to have aggressive characteristics, such as local invasion and lack of response to surgery and first-line medical therapy with somatostatin analogs (6, 8–14). In this study, genomic profiling of GH tumors and normal pituitary using gene expression microarray technology confirmed a differential molecular signature of GH tumor histological subtypes. Our bioinformatic analysis revealed dysregulation of the cell junction pathways with downregulation of PERP and several other critical components of the desmosome structure. This is interesting in light of the fact that well-developed desmosomes are not an ultrastructural feature of pituitary adenomas (37). However, pituitary cells (termed “follicular cells”), which are poorly granulated, can form follicle-like structures with lumens and contain microvilli, which are observed in normal pituitary and adenomas. These cells also form junctions of the zonula adherens (adhering juctions) and macula adherens (desmosome) type (37). Data from electron microscopy suggest that there may be fluidity in desmosome formation for pituitary cells. Our data would underscore the importance of alterations in specific components of the desmosome in GH tumor subtypes.
We have shown that downregulation of PERP protein has protumorigenic effects in GH pituitary cells that might play a role in aggressiveness of SG tumors seen clinically. PERP is tetraspan membrane protein that was initially described as a transcriptional target of p53 specifically during apoptosis (18). Subsequent studies also identified PERP downstream of p63, a master regulator of stratified epithelia development, where PERP-null mice showed a dramatic blistering in stratified epithelia due to compromised adhesions (38). To characterize the function of PERP in tumorigenesis, Beaudry et al. (39) used conditional Perp knockout mice selectively in stratified epithelia and showed that PERP loss promoted UVB-induced squamous cell cancer by increasing cell survival, desmosome loss, and inflammation. Interestingly, squamous cell cancer tumors in PERP-deficient mice were found to be less differentiated than those arising in control mice, implicating PERP loss in tumor progression. Additionally, Dusek et al. (40) showed that loss of PERP altered mammary gland homeostasis and promoted tumorigenesis in mammary cancer driven by p53 tumor suppressor loss. Downregulation of PERP has also been associated with monosomy 3–type of uveal melanoma where re-expression of PERP had proapoptotic effects on melanoma cells in vitro (41). Our studies in a GH pituitary tumor cell model are in agreement with previous literature (42, 43) where re-expression of PERP decreases tumorigenesis, as evidenced by decreases in colony formation in GH3 pituitary tumor cells. Our data suggest that PERP levels play a role in tumorigenesis not via alteration in the rates of proliferation but via changes in rates of cell survival, similar to its role in other human cancers (44).
Prior literature supports that PERP loss is associated with the impaired desmosome function and downregulation of desmosomal components, as another mechanism of promoting tumorigenesis (39). In the mice where Perp is silenced selectively in the epidermis, instability of multiple desmosmal components was noted (39). However, tumors in these mice displayed strong staining for both E-cadherin and B-catenin with intact adherens junctions. The authors postulated that PERP downregulation can facilitate desmosome downregulation, which can directly lead to tumor development by specific mechanisms distinct from EMT, which promotes change in differentiation status (39). Whereas E-cadherin and subsequent adherens junction loss might occur in late stages of tumor development, PERP loss and desmosomal instability may be an early driver of tumor progression. In SG human pituitary tumors, adherens junction dysregulation occurs with a partial EMT phenotype, as previously observed and confirmed by our expression microarray, which also showed downregulation of critical desmosome components as we noted. Although both of these mechanisms may have implications concerning an aggressive clinical phenotype seen in SG compared with DG tumors, it is surprising that dysregulation of these pathways of tumorigenesis found in human solid cancers does not seem to promote metastatic disease in the SG subtype of GH tumors. The significance of partial EMT signature in SG tumors has not been well understood, and it is a mechanism that should be studied further.
The hallmark of SG tumors is a finding of fibrous bodies on IHC using CAM5.2 staining for K8/18 (45). Abnormal accumulation of intermediate filament has been described in several tumor processes such as neuroendocrine small cell carcinoma and Merkel cell skin cancer, but the role and mechanism of abnormal appearance of cytokeratin have not been elucidated (46, 47). One of the major functions of cytokeratin intermediate filaments is the maintenance of surface membrane integrity through direct interaction with the desmosome (40, 48). The desmosome components, as outlined in Fig. 6, include cadherin desmoglein and desmocollin, members of the armadillo family plakoglobin and plakophilin, and the plakins desmoplakin and plectin (40, 49). The structure of desmosome is organized by forming protein–protein interaction between these components, and the main role of desmoplakin is the anchoring of the cytokeratin intermediate filaments to desmosome. Studies in K8/18-null mouse hepatocytes showed that the intermediated filament loss leads to disruption of desmoplakin at the cell membrane (50). We postulate that the abnormal assembly of K8/18 in SG tumors reflects the disruption of the intermediate filaments and downstream desmosome stability. Compromised desmosome integrity, together with loss of PERP, a critical desmosome protein and tumor suppressor, likely plays a role in human SG GH pituitary tumor invasion and progression.
Acknowledgments
The authors thank Dr. Beatriz Lopes for critical review of the manuscript.
Acknowledgments
This work was supported by Veterans Affairs Merit Review Award 001 (to M.E.W.), National Institutes of Health Grant K12CA086913-12 (to K.K.V.), American Cancer Society ACS IRG #57-001-53 (to K.K.V.), Doris Duke Charitable Foundation Grant 2015212 (to K.K.V), University of Colorado Cancer Center Support Grant P30-CA046934, and by funding from the Cancer Center Genomics Core.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- cDNA
- complementary DNA
- DG
- densely granulated
- DSG2
- desmoglein 2
- DSP
- desmoplakin
- EMT
- epithelial-to-mesenchymal transition
- FDR
- false discovery rate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GH
- growth hormone
- GHR
- growth hormone receptor
- IHC
- immunohistochemistry
- IPA
- Ingenuity Pathway Analysis
- K8/18
- cytokeratin 8/18
- mRNA
- messenger RNA
- PCA
- principal component analysis
- PCR
- polymerase chain reaction
- PERP
- p53 apoptosis effector related to PMP-22
- PKP2
- plakophilin 2
- qPCR
- quantitative polymerase chain reaction
- SG
- sparsely granulated
- SSA
- somatostatin analog
- SSTR
- somatostatin receptor
- TBST
- Tris-buffered saline with Tween 20
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119(11):3189–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broder MS, Neary MP, Chang E, Cherepanov D, Katznelson L. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary. 2014;17(4):333–341. [DOI] [PubMed] [Google Scholar]
- 3.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA; Endocrine Society . Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1097/00006123-200106000-00008. Shimon I, Cohen ZR, Ram Z, Hadani M. Transsphenoidal surgery for acromegaly: endocrinological follow-up of 98 patients. Neurosurgery. Jun 2001;48(6):1239–1243. [DOI] [PubMed] [Google Scholar]
- 5.Donangelo I, Melmed S. Treatment of acromegaly: future. Endocrine. 2005;28(1):123–128. [DOI] [PubMed] [Google Scholar]
- 6.Kiseljak-Vassiliades K, Carlson NE, Borges MT, Kleinschmidt-DeMasters BK, Lillehei KO, Kerr JM, Wierman ME. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine. 2015;49(1):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiseljak-Vassiliades K, Xu M, Mills TS, Smith EE, Silveira LJ, Lillehei KO, Kerr JM, Kleinschmidt-DeMasters BK, Wierman ME. Differential somatostatin receptor (SSTR) 1–5 expression and downstream effectors in histologic subtypes of growth hormone pituitary tumors. Mol Cell Endocrinol. 2015;417:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakhtiar Y, Hirano H, Arita K, Yunoue S, Fujio S, Tominaga A, Sakoguchi T, Sugiyama K, Kurisu K, Yasufuku-Takano J, Takano K. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrinol. 2010;163(4):531–539. [DOI] [PubMed] [Google Scholar]
- 9.Bando H, Sano T, Ohshima T, Zhang CY, Yamasaki R, Matsumoto K, Saito S. Differences in pathological findings and growth hormone responses in patients with growth hormone-producing pituitary adenoma. Endocrinol Jpn. 1992;39(4):355–363. [DOI] [PubMed] [Google Scholar]
- 10.Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S. The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab. 2005;90(11):6290–6295. [DOI] [PubMed] [Google Scholar]
- 11.Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary. 2013;16(4):490–498. [DOI] [PubMed] [Google Scholar]
- 12.Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol (Oxf). 2012;76(1):96–102. [DOI] [PubMed] [Google Scholar]
- 13.Mazal PR, Czech T, Sedivy R, Aichholzer M, Wanschitz J, Klupp N, Budka H. Prognostic relevance of intracytoplasmic cytokeratin pattern, hormone expression profile, and cell proliferation in pituitary adenomas of akromegalic patients. Clin Neuropathol. 2001;20(4):163–171. [PubMed] [Google Scholar]
- 14.Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, Rayhan N, Mustafizur Rahman M, Yamada S. Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol. 2008;19(2):82–91. [DOI] [PubMed] [Google Scholar]
- 15.Sano T, Rong QZ, Kagawa N, Yamada S. Down-regulation of E-cadherin and catenins in human pituitary growth hormone-producing adenomas. Front Horm Res. 2004;32:127–132. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Sano T, Yoshimoto K, Yamada S. Downregulation of E-cadherin and its undercoat proteins in pituitary growth hormone cell adenomas with prominent fibrous bodies. Endocr Pathol. 2002;13(4):341–351. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Inoshita N, Sugiyama T, Tani Y, Shichiri M, Sano T, Yamada S, Hirata Y. Differential expression of genes related to drug responsiveness between sparsely and densely granulated somatotroph adenomas. Endocr J. 2012;59(3):221–228. [DOI] [PubMed] [Google Scholar]
- 18.Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000;14(6):704–718. [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelis KA, Knox AJ, Xu M, Kiseljak-Vassiliades K, Edwards MG, Geraci M, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45β) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152(10):3603–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M, Knox AJ, Michaelis KA, Kiseljak-Vassiliades K, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME. Reprimo (RPRM) is a novel tumor suppressor in pituitary tumors and regulates survival, proliferation, and tumorigenicity. Endocrinology. 2012;153(7):2963–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shorts-Cary L, Xu M, Ertel J, Kleinschmidt-Demasters BK, Lillehei K, Matsuoka I, Nielsen-Preiss S, Wierman ME. Bone morphogenetic protein and retinoic acid-inducible neural specific protein-3 is expressed in gonadotrope cell pituitary adenomas and induces proliferation, migration, and invasion. Endocrinology. 2007;148(3):967–975. [DOI] [PubMed] [Google Scholar]
- 22.Lekva T, Berg JP, Fougner SL, Olstad OK, Ueland T, Bollerslev J. Gene expression profiling identifies ESRP1 as a potential regulator of epithelial mesenchymal transition in somatotroph adenomas from a large cohort of patients with acromegaly. J Clin Endocrinol Metab. 2012;97(8):E1506–E1514. [DOI] [PubMed] [Google Scholar]
- 23.Gröger CJ, Grubinger M, Waldhör T, Vierlinger K, Mikulits W. Meta-analysis of gene expression signatures defining the epithelial to mesenchymal transition during cancer progression. PLoS One. 2012;7(12):e51136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong W, Knox AJ, Xu M, Kiseljak-Vassiliades K, Colgan SP, Brodsky KS, Kleinschmidt-Demasters BK, Lillehei KO, Wierman ME. Mammalian Ste20-like kinase 4 promotes pituitary cell proliferation and survival under hypoxia. Mol Endocrinol. 2015;29(3):460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristof RA, Aliashkevich AF, Hans V, Haun D, Meyer B, Thees C, Schramm J. The regional oxygen saturation of pituitary adenomas is lower than that of the pituitary gland: microspectrophotometric study with potential clinical implications. Neurosurgery. 2003;53(4):880–885. [DOI] [PubMed] [Google Scholar]
- 27.Vidal S, Scheithauer BW, Kovacs K. Vascularity in nontumorous tuman pituitaries and incidental microadenomas: a morphometric study. Endocr Pathol. 2000;11(3):215–227. [DOI] [PubMed] [Google Scholar]
- 28.Vidal S, Horvath E, Kovacs K, Kuroki T, Lloyd RV, Scheithauer BW. Expression of hypoxia-inducible factor-1α (HIF-1α) in pituitary tumours. Histol Histopathol. 2003;18(3):679–686. [DOI] [PubMed] [Google Scholar]
- 29.Spada A, Arosio M, Bochicchio D, Bazzoni N, Vallar L, Bassetti M, Faglia G. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990;71(6):1421–1426. [DOI] [PubMed] [Google Scholar]
- 30.Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol. 2013;168(4):491–499. [DOI] [PubMed] [Google Scholar]
- 31.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340(6236):692–696. [DOI] [PubMed] [Google Scholar]
- 32.Adams EF, Brockmeier S, Friedmann E, Roth M, Buchfelder M, Fahlbusch R. Clinical and biochemical characteristics of acromegalic patients harboring gsp-positive and gsp-negative pituitary tumors. Neurosurgery. 1993;33(2):198–203. [DOI] [PubMed] [Google Scholar]
- 33.Yang I, Park S, Ryu M, Woo J, Kim S, Kim J, Kim Y, Choi Y. Characteristics of gsp-positive growth hormone-secreting pituitary tumors in Korean acromegalic patients. Eur J Endocrinol. 1996;134(6):720–726. [DOI] [PubMed] [Google Scholar]
- 34.Asa SL, Digiovanni R, Jiang J, Ward ML, Loesch K, Yamada S, Sano T, Yoshimoto K, Frank SJ, Ezzat S. A growth hormone receptor mutation impairs growth hormone autofeedback signaling in pituitary tumors. Cancer Res. 2007;67(15):7505–7511. [DOI] [PubMed] [Google Scholar]
- 35.Kola B, Korbonits M, Diaz-Cano S, Kaltsas G, Morris DG, Jordan S, Metherell L, Powell M, Czirják S, Arnaldi G, Bustin S, Boscaro M, Mantero F, Grossman AB. Reduced expression of the growth hormone and type 1 insulin-like growth factor receptors in human somatotroph tumours and an analysis of possible mutations of the growth hormone receptor. Clin Endocrinol (Oxf). 2003;59(3):328–338. [DOI] [PubMed] [Google Scholar]
- 36.Mayr B, Buslei R, Theodoropoulou M, Stalla GK, Buchfelder M, Schöfl C. Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur J Endocrinol. 2013;169(4):391–400. [DOI] [PubMed] [Google Scholar]
- 37.Horvath E, Kovacs K, Penz G, Ezrin C. Origin, possible function and fate of “follicular cells” in the anterior lobe of the human pituitary. Am J Pathol. 1974;77(2):199–212. [PMC free article] [PubMed] [Google Scholar]
- 38.Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, Mills AA, Attardi LD. Perp is a p63-regulated gene essential for epithelial integrity. Cell. 2005;120(6):843–856. [DOI] [PubMed] [Google Scholar]
- 39.Beaudry VG, Jiang D, Dusek RL, Park EJ, Knezevich S, Ridd K, Vogel H, Bastian BC, Attardi LD. Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS Genet. 2010;6(10):e1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dusek RL, Bascom JL, Vogel H, Baron S, Borowsky AD, Bissell MJ, Attardi LD. Deficiency of the p53/p63 target Perp alters mammary gland homeostasis and promotes cancer. Breast Cancer Res. 2012;14(2):R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies L, Spiller D, White MR, Grierson I, Paraoan L. PERP expression stabilizes active p53 via modulation of p53-MDM2 interaction in uveal melanoma cells. Cell Death Dis. 2011;2:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan IA, Yoo BH, Masson O, Baron S, Corkery D, Dellaire G, Attardi LD, Rosen KV. ErbB2-dependent downregulation of a pro-apoptotic protein Perp is required for oncogenic transformation of breast epithelial cells. Oncogene. 2016;35(44):5759–5769. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Luo Z, Li Z, Liu Y, Zhao Q. PERP gene therapy attenuates lung cancer xenograft via inducing apoptosis and suppressing VEGF. Cancer Biol Ther. 2011;12(12):1114–1119. [DOI] [PubMed] [Google Scholar]
- 44.Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, Attardi LD. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol. 2003;13(22):1985–1990. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Aiba T, Sano T, Kovacs K, Shishiba Y, Sawano S, Takada K. Growth hormone-producing pituitary adenomas: correlations between clinical characteristics and morphology. Neurosurgery. 1993;33(1):20–27. [DOI] [PubMed] [Google Scholar]
- 46.Wick MR. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17(3):194–203. [PubMed] [Google Scholar]
- 47.Scott MP, Helm KF. Cytokeratin 20: a marker for diagnosing Merkel cell carcinoma. Am J Dermatopathol. 1999;21(1):16–20. [DOI] [PubMed] [Google Scholar]
- 48.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127(11):2499–2515. [DOI] [PubMed] [Google Scholar]
- 49.Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res. 2005;7(5):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loranger A, Gilbert S, Brouard JS, Magin TM, Marceau N. Keratin 8 modulation of desmoplakin deposition at desmosomes in hepatocytes. Exp Cell Res. 2006;312(20):4108–4119. [DOI] [PubMed] [Google Scholar]