Abstract
Triglycerides are stored in specialized organelles called lipid droplets. Numerous proteins have been shown to be physically associated with lipid droplets and govern their function. Previously, the protein hypoxia-inducible lipid droplet–associated (HILPDA) was localized to lipid droplets and was suggested to inhibit triglyceride lipolysis in hepatocytes. We confirm the partial localization of HILPDA to lipid droplets and show that HILPDA is highly abundant in adipose tissue, where its expression is controlled by the peroxisome proliferator–activated receptor γ and by β-adrenergic stimulation. Levels of HILPDA markedly increased during 3T3-L1 adipocyte differentiation. Nevertheless, silencing of Hilpda using small interfering RNA or overexpression of Hilpda using adenovirus did not show a clear impact on 3T3-L1 adipogenesis. Following β-adrenergic stimulation, the silencing of Hilpda in adipocytes did not significantly alter the release of nonesterified fatty acids (NEFA) and glycerol. By contrast, adenoviral-mediated overexpression of Hilpda modestly attenuated the release of NEFA from adipocytes following β-adrenergic stimulation. In mice, adipocyte-specific inactivation of Hilpda had no effect on plasma levels of NEFA and glycerol after fasting, cold exposure, or pharmacological β-adrenergic stimulation. In addition, other relevant metabolic parameters were unchanged by adipocyte-specific inactivation of Hilpda. Taken together, we find that HILPDA is highly abundant in adipose tissue, where its levels are induced by peroxisome proliferator–activated receptor γ and β-adrenergic stimulation. In contrast to the reported inhibition of lipolysis by HILPDA in hepatocytes, our data do not support an important direct role of HILPDA in the regulation of lipolysis in adipocytes in vivo and in vitro.
We found, using in vivo and in vitro gene silencing and overexpression, that the lipid droplet–associated protein HILPDA is not a physiological regulator of adipocyte lipolysis.
In humans and many other animal species, excess energy is efficiently stored in white adipose tissue (WAT) as triglycerides, providing a buffer against periods of low energy intake. Triglycerides are stored in the adipose tissue in lipid droplets, which are dynamic intracellular organelles that are regulated by the organism’s nutritional status. Lipid droplets consist of a core of triglycerides and cholesteryl esters surrounded by a monolayer of phospholipids and proteins (1). Many lipid droplet–associated proteins are involved in the release of nonesterified fatty acids (NEFA) from lipid droplets or the incorporation of triglycerides into lipid droplets (1–4). For example, comparative gene identification-58 (ABHD5) is a lipid droplet–associated protein that potently stimulates the activity of adipose triglyceride lipase (ATGL, PNPLA2), the rate-limiting enzyme of lipolysis (5, 6). A better understanding of the function of lipid droplet–associated proteins is important to gain more insight into the regulation of lipid storage and mobilization.
The peroxisome proliferator–activated receptor (PPAR) family, consisting of PPARα, PPARβ/δ, and PPARγ, represents an important subgroup within the nuclear receptor superfamily. An intricate relationship exists between lipid storage and PPARs. On the one hand, changes in intra- and extracellular lipid metabolism impact the activation of PPARs by influencing the availability of ligands in the form of fatty acids and fatty acid derivatives (7–10). In contrast, PPARs influence lipid metabolism by activating the transcription of numerous target genes, including many genes encoding lipid droplet–associated proteins such as ATGL, G0/G1 switch gene 2 (G0S2), perilipin 2 (PLIN2), and cell death–inducing DFF45-like effector C (8, 11–13). Functional studies of PPAR-target genes may help to further uncover the intricate regulation of lipid storage and mobilization in the adipose tissue.
Recently, hypoxia-inducible lipid droplet–associated (HILPDA) was identified as a lipid droplet–associated protein in HeLa cells and hepatocytes (14, 15). We and others have identified Hilpda as a target gene of PPARα in liver involved in hepatic triglyceride metabolism (14, 16). HILPDA overexpression resulted in a fatty liver due to a reduction in very-low-density lipoprotein triglyceride secretion, whereas Hilpda inactivation lowered hepatic lipid accumulation (14, 16). More specifically, loss of HILPDA was found to lead to enhanced triglyceride lipolysis and triglyceride turnover in isolated primary hepatocytes (14). Considering the reported localization of HILPDA to lipid droplets and the importance of lipolysis in adipocytes, we examined the role and regulation of HILPDA in adipose tissue (15, 16). In particular, we investigated the potential role of HILPDA in adipocyte lipolysis using in vitro and in vivo tools of HILPDA overexpression and inactivation. We find that Hilpda is a sensitive PPARγ target gene in adipocytes and is highly responsive to β-adrenergic stimulation. In contrast to the reported inhibition of lipolysis by HILPDA in hepatocytes, our data do not support an important direct role of HILPDA in the regulation of lipolysis in adipose tissue.
Materials and Methods
All animal experiments were performed in accordance with Directive 2010/63/EU from the European Union. All animal studies were reviewed and approved by the Animal Ethics Committee of Wageningen University.
Animal experiments
Mice were housed in temperature- and humidity-controlled specific pathogen-free conditions. Mice had ad libitum access to food (chow) and water, except when otherwise specified. To generate mice with adipocyte-specific deletion of Hilpda, heterozygous floxed Hilpda animals on a mixed C57BL/6 and Sv129 background were purchased from The Jackson Laboratory (Bar Harbor, ME). LoxP sites were introduced to flank the second exon of Hilpda, followed by the open reading frame for membrane-tethered human placental alkaline phosphatase (ALPP) after the second loxP site. Following Cre recombination, ALPP is expressed under control of the Hilpda promoter. Floxed Hilpda mice were crossed with Adiponectin-Cre mice (The Jackson Laboratory) on a C57BL/6 background. After initial crosses, Hilpdaflox/flox mice were crossed with Hilpdaflox/flox mice heterozygous for Adiponectin-Cre, yielding 50% wild-type and 50% adipocyte-specific Hilpda null animals (HilpdaΔAT), equally distributed among males and females. For the first experiment, in which the adipocyte-specific inactivation of Hilpda was verified by separation of the adipose tissue into adipocytes and stromal vascular fractions (SVF), four 20-week-old female Hilpdaflox/flox and four 20-week-old female HilpdaΔAT mice were killed by cervical dislocation, and epididymal adipose tissue depots were excised and placed in Dulbecco’s modified Eagle medium (DMEM; Lonza, Verviers, Belgium) supplemented with 1% fatty acid–free bovine serum albumin (BSA) until further isolation, as described later.
For the second experiment, in which mice were injected with the β-adrenergic agonist CL316,243, 15- to 18-week-old male Hilpdaflox/flox (n = 16) and HilpdaΔAT (n = 16) littermates were fasted for 3 hours and subcutaneously injected with 1 mg/kg CL316,243 (n = 8 per genotype) or saline solution (n = 8 per genotype). During the experiment, all mice were housed individually. At 10 minutes, 30 minutes, and 60 minutes after injection, blood was drawn via the tail vein. Three hours after injection, the animals were anesthesized with isoflurane, blood was collected, and, following cervical dislocation, several tissues were excised and either snap frozen or placed in DMEM + 1% BSA for separation into adipocytes and SVF. The animals remained fasted until euthanasia.
For the third experiment, 9- to 13-week-old male Hilpdaflox/flox (n = 6) and HilpdaΔAT (n = 8) littermates were fasted for 24 hours and euthanized by cervical dislocation, after which several tissues were excised and snap frozen. During the fasting period, all mice were housed individually.
For the fourth experiment, 15- to 18-week-old male Hilpdaflox/flox and HilpdaΔAT littermates were exposed to a cold environmental temperature (4°C) (n = 10 per genotype) or a thermoneutral temperature (28°C) (n = 8 per genotype) for 10 days. After 10 days, animals were anesthesized with isoflurane, blood was collected, and, following cervical dislocation, several tissues were excised and snap frozen. Food intake, body weight, and body temperature were monitored daily. Body temperature of cold-exposed mice was monitored via readout of transponders (IPTT-300) that were injected subcutaneously prior to the experiment (Bio Medic Data Systems, Seaford, Delaware).
For the fifth experiment, in which the rates of lipolysis of WAT explants were examined in vitro, adipose tissue of male Hilpdaflox/flox (n = 3) and HilpdaΔAT (n = 3) mice was excised and minced with scissors. Adipose tissue explants were plated, serum starved for 30 minutes in DMEM (Lonza) with 1% fatty acid–free BSA, and subsequently treated with 10 μM isoproterenol for 3 hours. Medium samples were assayed for NEFA, as described later, and corrected for total protein.
For the sixth experiment, in which the rates of lipolysis of primary adipocytes were examined in vitro, inguinal white adipose tissue (iWAT) of male Hilpdaflox/flox (n = 3) and HilpdaΔAT (n = 3) mice was excised and minced with scissors. SVF were isolated and differentiated to mature adipocytes, as described previously (17). Mature adipocytes were serum starved for 2 hours in DMEM with 1% fatty acid–free BSA and subsequently treated with 10 μM isoproterenol or 10 μM CL316,243 for 3 hours. Medium samples were assayed for NEFA, as described later, and corrected for total protein.
Recombinant adenoviruses
Adenoviruses (AVs) were generated by cloning Gfp or Hilpda complementary DNA (cDNA) in human AV type 5 (dE1/E3). Expression was under the control of the cytomegalovirus promotor. Viruses were produced and titrated by Vector Biolabs (Philadelphia, PA).
Chemicals
Isoproterenol, norepinephrine, 3-isobutyl-1-methylxanthine, insulin, dexamethasone, fatty acid–free BSA, and rosiglitazone were purchased from Sigma-Aldrich (Schnelldorf, Germany). Forskolin, procaterol, and CL316,243 were from Tocris Bioscience (Bristol, United Kingdom).
Separation stromal vascular fractions and adipocytes
Dissected adipose tissue depots from the different experiments were kept on ice in DMEM supplemented with 1% fatty acid–free BSA (Sigma-Aldrich). Samples were minced into small 1- to 2-mm3 pieces and incubated with collagenase solution [DMEM, 3.2 mM CaCl2, 15 mM HEPES, 0.5% BSA, 10% fetal calf serum (FCS), and 1.5 mg/mL collagenase type II (Sigma-Aldrich; C6885)] at 37°C for 45 minutes. Mixtures were strained through a 100-µm cell strainer and centrifuged at 300g for 10 minutes at room temperature. Floating adipocytes were collected and snap frozen for RNA isolation. The pelleted SVF were resuspended in TRIzol (Thermo Fisher Scientific, Landsmeer, The Netherlands) and snap frozen for RNA isolation.
Cell culture
The 3T3-L1 fibroblasts [Research Resource Identifier (RRID): CVCL_0123] were maintained in DMEM supplemented with 10% newborn calf serum (Lonza) and 1% penicillin/streptomycin (Lonza) and differentiated, as described previously (18). The day at which differentiation was started is indicated as day 0. Dharmacon ON-TARGETplus SMARTpool small interfering RNAs (siRNAs) against Hilpda, G0s2, or Pparg and non-targeting control small interfering RNAs (siCtrls) were purchased from Thermo Fisher Scientific. siRNAs were diluted in Dharmacon 1× siRNA buffer [final concentration 20 mM KCl, 6 mM HEPES (pH 7.5), and 0.2 mM MgCL2]. Transfections were performed with Lipofectamine RNAiMAX transfection reagent (Life Technologies, Bleiswijk, The Netherlands). All siRNA transfections were carried out at a concentration of 40 nM siRNA and 2 µL transfection reagent for a 12-well plate. To study the effect of Hilpda silencing on 3T3-L1 differentiation, siRNAs were added 2 days before initiation of differentiation, at the start of differentiation, and subsequently every third day. A similar protocol was used to study the effect of Pparg silencing on Hilpda messenger RNA (mRNA) during 3T3-L1 and Simpson-Golabi-Behmel syndrome (SGBS) adipocyte differentiation (19). To silence Hilpda, G0s2, or Pparg in mature 3T3-L1 adipocytes, cells were washed with phosphate-buffered saline (PBS), trypsinized, and collected in PBS or DMEM. After centrifugation at 400g for 5 minutes, cells were strained over a 70 µM cell strainer and plated to 70% confluency. siRNAs were added 2 hours later, and experiments were carried out after an additional 48 to 72 hours of incubation. siCtrl-, siHilpda-, and siG0s2-treated adipocytes were incubated in DMEM with 1% fatty acid–free BSA for 2 hours and subsequently exposed, as described in the figure legends. Medium samples were assayed for NEFA, as described later.
For overexpression experiments in differentiating 3T3-L1 adipocytes, recombinant viruses were diluted in DMEM (low glucose, 1 g/L), supplemented with 0.5 µg/mL poly-l-lysine, and incubated at room temperature for 100 minutes. Mixtures were added to cells and allowed to incubate for 90 minutes, followed by the addition of normal culture medium. Differentiation was initiated 2 days later. AVs were used at a multiplicity of infection of 500. To study isoproterenol-induced NEFA release, mature 3T3-L1 adipocytes were replated at 70% confluency, as described earlier, and subsequently starved in DMEM (low glucose) plus 1% FCS for 12 hours, followed by DMEM (low glucose) plus 0.1% FCS for an additional 12 hours. AVs diluted in 0.5 µg/mL poly-l-lysine were added to serum-starved cells at a multiplicity of infection of 750. Regular culture medium containing 10% FCS was added after 90 minutes. Three days later, cells were incubated in DMEM plus 1% BSA for 2 hours and subsequently exposed, as described in the figure legends. Medium samples were assayed for NEFA, as described later.
SVF derived from epididymal white adipose tissue (eWAT) or iWAT of wild-type male C57BL/6 mice (n = 3/type of WAT) were differentiated, as described previously, and treated, as described in figure legends (17). SGBS cells (RRID: CVCL_GS28) and human multipotent adipose-derived stem (hMADS) cells were cultured and differentiated according to published methods (20, 21). For microarray analysis, cells were exposed to 0.5 μM rosiglitazone or dimethylsulfoxide control for 6 hours, as described previously (22).
T37i cells (P31–36; kind gift of M. Lombès INSERM UMR-S 1187, Paris Sud, France) were cultured in DMEM/F-12 (Gibco, Life Technologies, Blijswijk, The Netherlands), supplemented with 10% FBS and 1% penicillin/streptomycin. Two days postconfluency, cell culture medium was supplemented with 112 ng/mL insulin and 2 nM T3 (Sigma-Aldrich) to induce differentiation. After 7 days of differentiation, cells were switched back to regular medium and used for experiments 2 to 3 days thereafter.
For isolation of primary brown adipocytes, brown adipose tissue (BAT) from 1-month-old pups was used. Tissues of 5 to 10 pups were pooled, minced with scissors, and digested for 30 minutes in collagenase-containing medium at 37°C (DMEM without serum, 2 mg/mL collagenase type II, 2% BSA, and 25 mM HEPES). After digestion, cells were passed through a 70 or 100 μM filter, mature adipocytes were discarded, and cells were centrifuged at 800g for 5 minutes. Cells were resuspended in differentiation medium (DMEM, 10% FBS, 20 nM insulin, and 1 nM T3) and plated. Upon confluence, cells were treated with induction medium (differentiation medium supplemented with 0.5 mM 3-isobutyl-1-methylxanthine, 0.5 μM dexamethasone, and 0.125 mM indomethacin) for 2 days. After washing, cells were incubated in differentiation medium for another 5 to 7 days.
Triglyceride quantification
For triglyceride quantification, 3T3-L1 preadipocytes were differentiated in the presence of siCtrl or siHilpda, or Gfp or Hilpda adenoviral constructs, as described earlier. At day 9 of differentiation, cells were washed twice with PBS, incubated with Solution 1 [25 mM Tris-HCl (pH 7.5) and 1 mM EDTA], and frozen at −20°C overnight. Upon defrosting, a tertiary butanol/methanol (4:1) mixture was added to the cells, followed by incubation for 10 minutes. Subsequently, all liquid was evaporated, and triglyceride liquicolor reagent (Human, Wiesbaden, Germany) was added, incubated for 20 minutes, and assayed at 490 nm.
Oil-Red-O staining
The 3T3-L1 adipocytes were fixed with 4% formaldehyde in PBS for 20 minutes at room temperature. Cells were subsequently washed with PBS and incubated with filtered Oil-Red-O solution (30 mg/mL in 60% isopropanol) for 10 minutes. Cells were washed two to three times with ddH2O twice before taking pictures.
Serum analyses
Triglycerides were determined using a triglyceride liquicolor mono kit (Human). NEFA were measured with the NEFA-HR (2) kit (Wako, Neuss, Germany). Cholesterol and glucose were measured with commercially available kits from Diasys (Holzheim, Germany). Glycerol was measured with a kit from Sigma-Aldrich. Plasma adiponectin was determined using the mouse ultrasensitive insulin enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH). Plasma insulin levels were quantified using the Quantikine enzyme-linked immunosorbent assay (R&D Systems, Abingdon, United Kingdom).
ChIP-seq data
ChIP-seq data sets from 3T3-L1 cells (GSE13511) (23), hMADS cells (GSE59703) (24), and mouse eWAT and BAT, either whole tissue (GSE43763) (25) or primary in vitro differentiated adipocytes (GSE41481) (26), were obtained from the Gene Expression Omnibus database. PPARγ peaks in mouse and human adipocytes were identified with default settings and scanned for the presence of known motifs using HOMER (27). The location of PPARγ superenhancers was identified using annotatePeaks.pl-style super in HOMER. Conservation of PPARγ binding sites was determined using the University of California at Santa Cruz (Santa Cruz, CA) LiftOver tool from the mm9 to hg19 genome assemblies. The University of California, Santa Cruz, genome browser (28) was used for visualization.
Western blot
Proteins were extracted from adipose tissue using homogenization buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 1% v/v/ Nonidet P40, 0.5% v/v sodium deoxycholate, and 0.1% sodium dodecyl sulfate] supplemented with Complete EDTA–free protease inhibitor cocktail and phosSTOP tablets (Roche Diagnostics, Almere, The Netherlands) and the Qiagen Tissuelyser II (Qiagen, Venlo, The Netherlands). Lysates were rotated for 15 minutes at 4°C and subsequently centrifuged at 11,000 rpm for 15 minutes at 4°C. For extraction of protein from cells, homogenization buffer was added to PBS-washed cells and the cells were subsequently scraped. Equal amounts of protein were diluted with 2× or 5× Laemmli sample buffer, boiled, and separated on 8% to 16% or 4% to 20% Criterion gradient gels (Bio-Rad, Veenendaal, The Netherlands). Proteins were transferred to polyvinylidene difluoride membrane using a Transblot turbo system (Bio-Rad). Antibodies to detect HILPDA (1:1000; Santa Cruz Biotechnology; sc-137518, RRID: AB_2011522), β-tubulin (1:1000; Santa Cruz Biotechnology; sc-23949, RRID: AB_628413), actin (1:2000; Sigma-Aldrich; A2066, RRID: AB_476693), HSP90 (1:2000; Cell Signaling Technology; 4874, RRID: AB_2121214), hormone-sensitive lipase (HSL; 1:5000; Cell Signaling Technology; 4107, RRID: AB_2296900), ATGL (1:1000; Cell Signaling Technology; 2138, RRID: AB_2167955), phospho-HSL Ser660 (1:2000; Cell Signaling Technology; 4126, RRID: AB_490997) (see Table 1 for an overview), and corresponding secondary antibodies were diluted in PBS with 0.1% Tween20 or Tris-buffered saline with 0.1% Tween20 containing 5% weight-to-volume skimmed milk powder. Quantification of Western blots was performed with the ChemiDoc MP system (Bio-Rad) and either Pierce ECL plus (Thermo Fisher Scientific) or Clarity ECL substrate (Bio-Rad).
Table 1.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| HILPDA/HIG2 | C terminus of HIG2 of human origin | HIG2 (C-14) antibody | Santa Cruz Biotechnology; sc-137518 | Rabbit; polyclonal | 1:1000 | AB_2011522 |
| β-Tubulin | β-tubulin (2-28-33) antibody | Santa Cruz Biotechnology; sc-23949 | Mouse; monoclonal | 1:1000 | AB_628413 | |
| Actin | Ser-Gly-Pro-Ser-Ile-Val-His-Arg-Lys-Cys-Phe | Anti-actin antibody | Sigma-Aldrich; A2066 | Rabbit; polyclonal | 1:2000 | AB_476693 |
| HSP90 | HSP90 antibody | Cell Signaling Technology; 4874 | Rabbit; polyclonal | 1:2000 | AB_2121214 | |
| LIPE | HSL antibody | Cell Signaling Technology; 4107 | Rabbit; polyclonal | 1:5000 | AB_2296900 | |
| ATGL | Synthetic peptide corresponding to a sequence around Pro186 of human ATGL | ATGL antibody | Cell Signaling Technology; 2138 | Rabbit; polyclonal | 1:1000 | AB_2167955 |
| Phospho-HSL (Ser660) | Synthetic phosphopeptide corresponding to residues surrounding Ser651 of mouse HSL | Phospho-HSL (Ser660) antibody | Cell Signaling Technology; 4126 | Rabbit; polyclonal | 1:2000 | AB_490997 |
RNA isolation and quantitative polymerase chain reaction
RNA was isolated from tissues and cells using TRIzol (Thermo Fisher Scientific). Homogenization of tissues was performed using a Qiagen Tissue Lyser II, and cultured cells were lysed by pipetting up and down several times. Isolated RNA and RNA from the FirstChoice Human Total RNA Survey Panel (Ambion, via Thermo Fisher Scientific) were reverse transcribed using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) or iScript cDNA Synthesis Kit (Bio-Rad). Gene expression analysis was performed on a CFX384 real-time polymerase chain reaction platform (Bio-Rad). SensiMix polymerase chain reaction mix was purchased from Bioline (GC Biotech, Alphen aan de Rijn, The Netherlands). Gene expression values were normalized with expression values for 36B4 (housekeeping gene).
Microarray analysis
RNA from SGBS and hMADS (22) cells was purified with an RNAeasy Minikit (Qiagen). RNA quality was verified with the RNA 6000 Nano assay on an Agilent 2100 Bioanalyzer (Agilent Technologies, Amsterdam, The Netherlands). Hybridization, washing, and scanning of the Affymetrix Human Gene 1.1 ST array plate were performed according to standard protocols on an Affymetrix GeneTitan platform. Bioconductor packages were used to analyze the scans of the arrays (29). Robust multiarray normalization was applied to obtain raw signal intensities. Probe sets were defined using remapped chip definition file based on the Entrez gene database. Fold changes were calculated by dividing expression values of rosiglitazone-treated adipocytes by expression values of dimethylsulfoxide-treated adipocytes.
Confocal microscopy
A HILPDA-Turquoise2 plasmid and a HILPDA-mCherry plasmid were constructed by fusing full-length HILPDA DNA with the 3′ end of enhanced green fluorescent protein (eGFP) in pEGFP-N2 (Clontech). A cell death-inducing DFF45-like effector B (CIDEB)-mCherry plasmid was constructed by fusing full-length CIDEB cDNA with 3′ end of eGFP in pEGFP-N2 (Clontech). Next, the eGFP region was substituted with cDNA encoding the fluorescent protein mTurquoise2 or the fluorescent protein mCherry (30). The 3T3-L1 fibroblasts were transfected with HILPDA-mTurquoise2 plasmid DNA and CIDEB-mCherry plasmid DNA or HILPDA-mCherry plasmid DNA with Fugene (Promega). Twenty-four hours posttransfection, 3T3-L1 cells were lipid loaded with 400 µM oleic acid. Lipid droplets were stained with BODIPY® 493/503(4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene) (Molecular Probes, Leiden, The Netherlands). BODIPY was diluted in PBS at a concentration of 1 mg/mL and incubated for 45 minutes. Confocal laser-scanning microscopy imaging was performed using a confocal laser-scanning microscope (LSM510; Carl Zeiss, Jena, Germany). Cells were maintained in medium for cellular imaging [20 mM HEPES (pH 7.4), 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 20 mM d-glucose]. For mTurquoise2, an excitation light of 458 nm was used and a band-pass filter of 470 to 500 nm was used for detection. mCherry was excited using a He/Ne diode laser (543 nm), and a band-pass filter 560 to 615 nm was used for detection. A PlanNeofluar 63× oil immersion objective with a numeric aperture of 1.25 was used. The pinhole setting was set at 1 to 1.3 Airy units. Images were collected as 2048 × 2048 pixel scans at 12-bit intensity resolution. Pixel saturation was avoided using the range indicator view during acquisition.
Statistical analyses
Student t tests or two-way analyses of variance with Tukey’s post hoc test were performed in GraphPad Prism (GraphPad Software, La Jolla, CA). The significance level was set at P < 0.05.
Results
HILPDA is highly abundant in human and mouse adipose tissue and localizes to lipid droplets
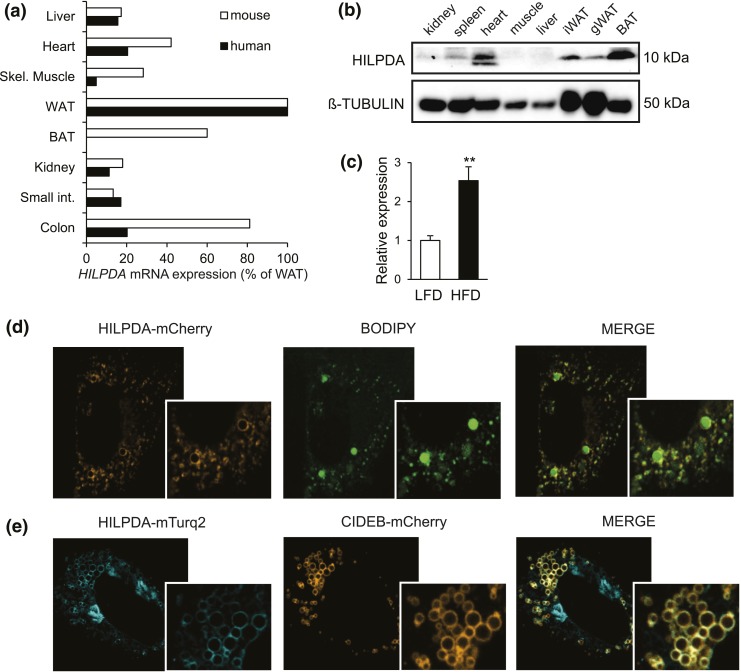
First, we determined the distribution of HILPDA expression across various human and mouse tissues. At the mRNA level, the highest HILPDA expression was seen in WAT in mice and humans, with lower levels in liver, heart, muscle, colon, and murine BAT [Fig. 1(a)]. At the protein level in mice, HILPDA was found to be particularly abundant in BAT, and to a lesser extent in WAT and heart [Fig. 1(b)]. Diet-induced obesity increased Hilpda mRNA levels in murine adipose tissue [Fig. 1(c)]. In agreement with previous reports, fluorescently labeled HILPDA localized primarily, although not exclusively, to the lipid droplets in lipid-loaded 3T3-L1 preadipocytes, as demonstrated by the colocalization with BODIPY and CIDEB, an established lipid droplet protein [Fig. 1(d) and 1(e)] (14, 15, 31).
Figure 1.
HILPDA is abundant in adipose tissue and localizes to lipid droplets. (a) HILPDA mRNA levels across various human (n = 1) and mouse tissues (n = 4). From top to bottom: liver, heart, skeletal muscle, WAT, BAT, kidney, small intestine, and colon. Expression levels in WAT were set at 100%. (b) Immunoblot for HILPDA in various mouse tissues (n = 1). (c) Hilpda mRNA in adipose tissue of C57BL/6 mice fed a low-fat diet (LFD) or high-fat diet (HFD) for 20 weeks. (d) The 3T3-L1 preadipocytes were transfected with HILPDA-mCherry plasmid, loaded with 400 μM oleic acid and, 48 hours posttransfection, stained with BODIPY and analyzed by confocal microscopy. (e) The 3T3-L1 preadipocytes were transfected with HILPDA-Turquoise2 plasmid (HILPDA-mTurq2) and CIDEB-mCherry plasmid, loaded with 400 μM oleic acid, and analyzed by confocal microscopy 48 hours after transfection. Asterisks indicate significant differences according to Student t test relative to (c) LFD; **P < 0.01. gWAT, gonadal white adipose tissue.
HILPDA is a PPARγ target gene in adipocytes
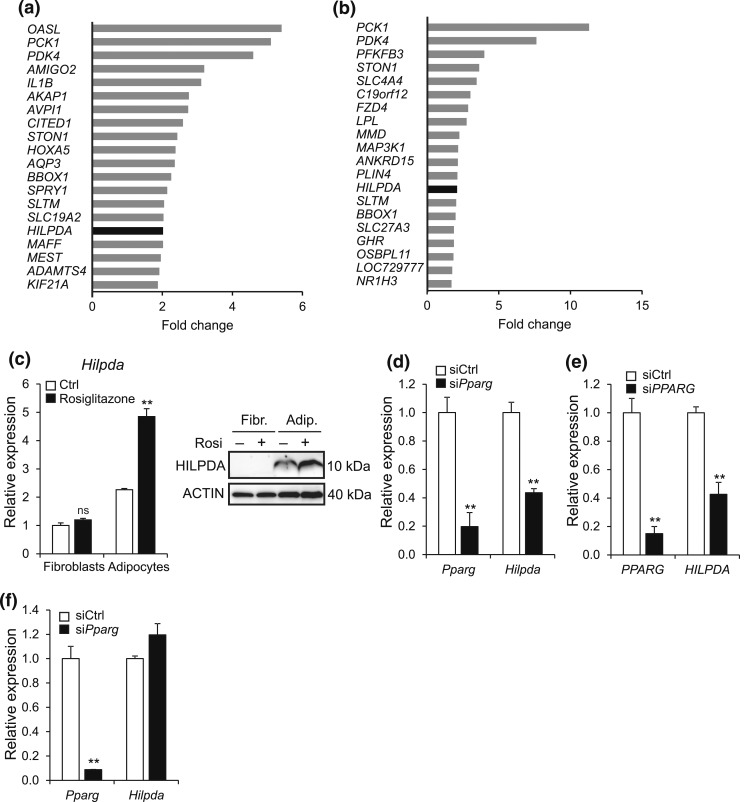
Hilpda was recently identified as a target gene of PPARα in liver (16). To explore whether PPARγ impacts HILPDA expression in human adipocytes, we treated different types of adipocytes with the PPARγ agonist rosiglitazone. Interestingly, microarray analyses indicated that HILPDA was among the 20 most significantly upregulated genes by rosiglitazone in differentiated human SGBS adipocytes and differentiated hMADS, together with the well-known PPARγ target genes PCK1, PDK4, and LPL [Fig. 2(a) and 2(b)]. Rosiglitazone also significantly induced Hilpda mRNA and HILPDA protein in mature 3T3-L1 adipocytes, but not in undifferentiated 3T3-L1 fibroblasts [Fig. 2(c)]. Consistent with PPARγ-dependent regulation during adipogenesis, Hilpda expression in 3T3-L1 and human SGBS adipocytes was markedly decreased when Pparg was silenced using siRNA [Fig. 2(d) and 2(e)]. By contrast, silencing Pparg in differentiated 3T3-L1 adipocytes did not influence Hilpda expression [Fig. 2(f)].
Figure 2.
HILPDA is a PPARγ target gene in adipocytes. (a and b) Top 20 of most highly induced genes following gene expression profiling of (a) SGBS and (b) hMADS adipocytes treated with 0.5 μM rosiglitazone or dimethylsulfoxide control for 6 hours. Fold change was calculated by dividing gene expression values of rosiglitazone-treated adipocytes by values of control-treated adipocytes. (c) Undifferentiated 3T3-L1 fibroblasts and fully differentiated 3T3-L1 adipocytes were exposed to 10 μM rosiglitazone or dimethylsulfoxide control for 24 hours and subsequently analyzed for Hilpda mRNA (left) and HILPDA protein (right). Gene expression levels of control-treated fibroblasts were set at one. (d) Pparg and Hilpda mRNA expression in 3T3-L1 adipocytes that had been treated with siCtrl or siRNA against Pparg (siPparg) from the start of differentiation and analyzed at day 5. (e) PPARG and HILPDA mRNA expression in SGBS adipocytes that had been treated with siCtrl or siRNA against Pparg (siPPARG) from the start of differentiation and analyzed at day 5. (f) Fully differentiated 3T3-L1 adipocytes were trypsinized, replated at 70% confluency, incubated with siCtrl or siPparg, and analyzed for the gene expression levels of Pparg and Hilpda after 72 hours. Gene expression levels of siCtrl-treated adipocytes were set at one. Data are mean ± standard error of the mean. Asterisks indicate significant differences according to Student t test relative to (c) control-treated fibroblasts or adipocytes or relative to (d–f) siCtrl-treated adipocytes; **P < 0.01. Adip., adipocytes; Fibr., fibroblasts.
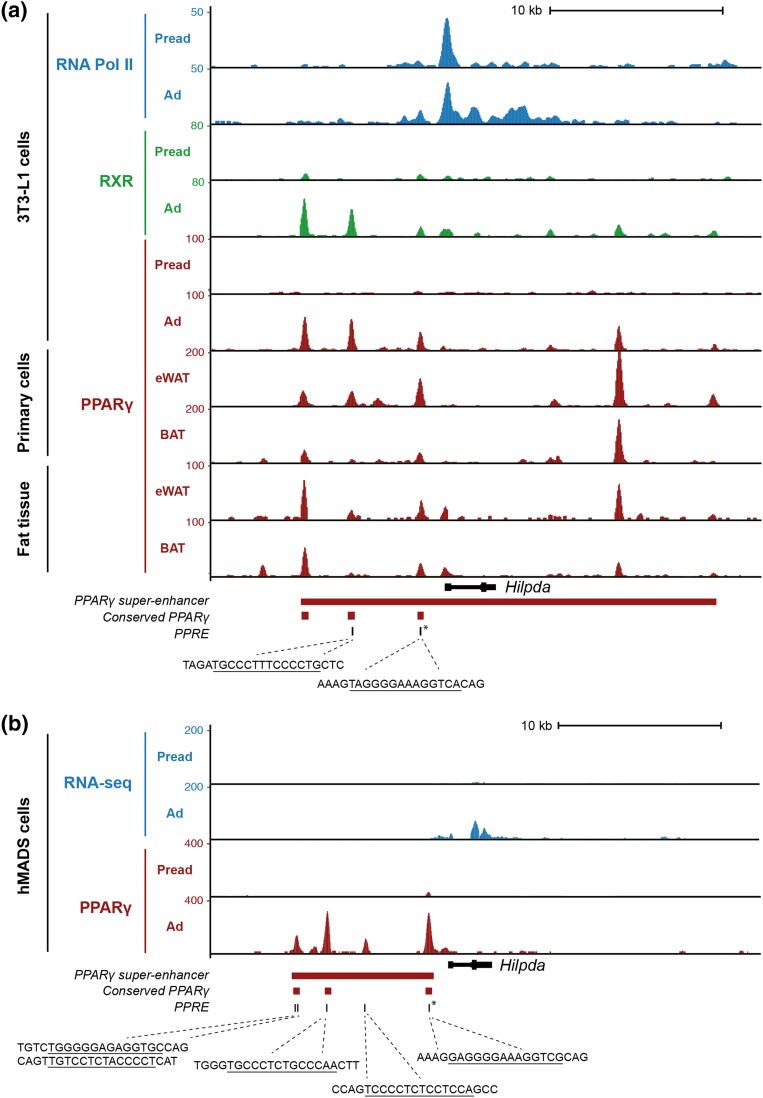
Previously, a functional PPAR response element was identified ∼1200 bp upstream of the transcriptional start site (TSS) of the Hilpda gene (16). Investigation of PPARγ ChIP-seq data from 3T3-L1, primary eWAT- and BAT-derived adipocytes, and hMADS adipocytes demonstrated that PPARγ binds to three conserved sites upstream of the TSS (Fig. 3). The PPARγ binding site closest to the TSS overlaps with the location of a highly conserved PPAR response element that was previously shown to mediate PPARγ-dependent transcriptional activation in vitro (Fig. 3) (16). Because previous studies have shown that clusters of nearby enhancers (i.e., superenhancers) (32) control important cell type–defining genes, we next asked whether the HILPDA locus is associated with a PPARγ superenhancer. Interestingly, in both mouse and human adipocytes, a prominent PPARγ superenhancer is located near HILPDA (Fig. 3), suggesting that HILPDA is an important PPARγ target gene that might control key adipocyte functions.
Figure 3.
PPARγ is associated with the HILPDA locus in mouse and human adipocytes. (a) Screenshot of the mouse Hilpda locus showing ChIP-seq profiles of retinoid X receptor (RXR), PPARγ, and RNA polymerase II in 3T3-L1 preadipocytes and adipocytes, and PPARγ ChIP-seq profiles from eWAT and BAT (primary adipocytes and whole tissue). (b) Screenshot of the human HILPDA locus showing RNA sequencing (RNA-seq) and PPARγ ChIP-seq profiles in hMADS preadipocytes and adipocytes. PPARγ binding sites that are conserved between mouse and human and the position of PPAR response elements within the PPARγ binding sites are highlighted. The sequences indicate conserved PPAR response elements (PPREs) in the respective binding sites. The red bar indicates the position of adipocyte-specific PPARγ superenhancers in 3T3-L1 and hMADS adipocytes. The PPARγ binding site that has also been reported to bind PPARα in liver and that harbors a highly conserved and experimentally confirmed PPAR response element (16) is marked by an asterisk. Ad, adipocytes; Pread, preadipocytes.
HILPDA does not affect 3T3-L1 adipogenesis
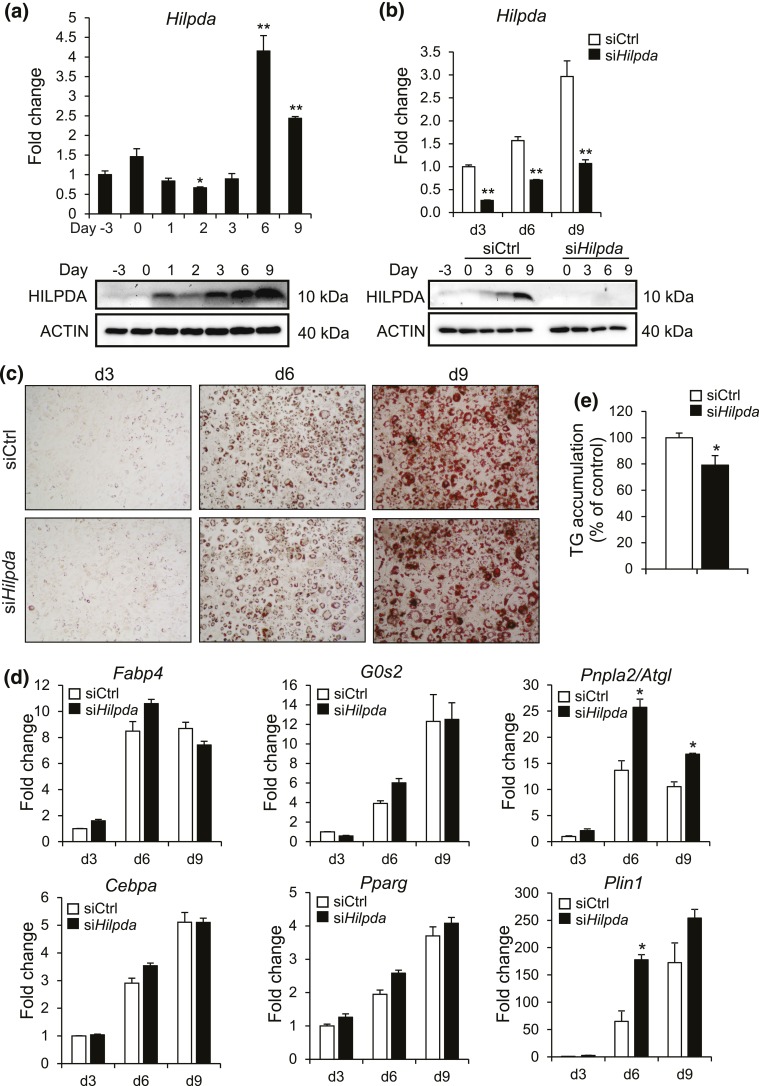
With Hilpda being a PPARγ target gene, we explored a potential role for HILPDA in adipocyte differentiation. Expression of Hilpda mRNA significantly increased during mouse 3T3-L1 adipocyte differentiation, which was confirmed at the protein level [Fig. 4(a)]. To assess the role of HILPDA in 3T3-L1 adipocyte differentiation, we used siRNA-mediated silencing of Hilpda. When compared with control siRNA, siRNA-targeting Hilpda effectively reduced Hilpda mRNA and HILPDA protein levels [Fig. 4(b)]. However, silencing of Hilpda did not substantially influence 3T3-L1 adipogenesis, as shown by Oil-Red-O staining and the expression of several adipogenic marker genes [Fig. 4(c) and 4(d)]. Interestingly, however, expression of Plin1 and Pnpla2/Atgl was significantly higher in Hilpda siRNA-treated adipocytes, especially at day 6. In addition, a slight but statistically significant decrease in triglyceride accumulation was observed in Hilpda siRNA-treated adipocytes [Fig. 4(e)].
Figure 4.
siRNA-mediated silencing of Hilpda does not affect adipogenesis. (a) Hilpda mRNA (top) and HILPDA protein (bottom) levels during 3T3-L1 adipogenesis. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. Gene expression level of day −3 was set at 1. (b) Hilpda mRNA (top) and HILPDA protein (bottom) levels during 3T3-L1 adipogenesis. siCtrl or Hilpda siRNAs (siHilpda) were added 2 days before initiation of adipogenesis, at the start of differentiation, and on day 3 and day 6 of differentiation. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. Gene expression levels of siCtrl-treated adipocytes at day 3 of differentiation were set at one. (c) Oil-Red-O staining of 3T3-L1 adipocytes treated with siCtrl or siHilpda. siCtrl or siHilpda was added 2 days before initiation of adipogenesis, at the start of differentiation, and on day 3 and day 6 of differentiation. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. (d) Fabp4, G0s2, Cebpa, Pparg, Pnpla2/Atgl, and Plin1 mRNA levels during adipogenesis of 3T3-L1 adipocytes treated with siCtrl or siHilpda at 2 days before initiation of adipogenesis, at the start of differentiation, and on day 3 and day 6 of differentiation. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. Gene expression levels of siCtrl-treated adipocytes at day 3 of differentiation were set at one. (e) Triglyceride (TG) quantification of 3T3-L1 adipocytes treated with siCtrl or siHilpda at 2 days before initiation of adipogenesis, at the start of differentiation, and on day 3 and day 6 of differentiation. Adipocytes were harvested at day 9 of differentiation. Asterisks indicate significant differences according to Student t test relative to (a) day −3 or relative to (b, d, and e) siCtrl-treated adipocytes; **P < 0.01; *P < 0.05.
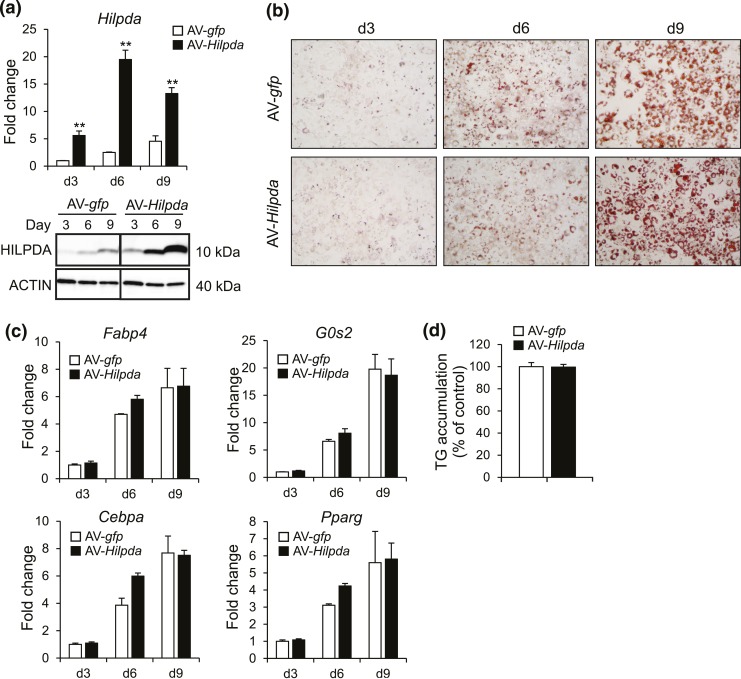
As an alternative approach, we used adenoviral-mediated transduction of Hilpda, which effectively raised Hilpda mRNA and HILPDA protein [Fig. 5(a)]. However, Hilpda overexpression did not influence 3T3-L1 adipogenesis, as revealed by Oil-Red-O staining [Fig. 5(b)], the expression of several adipogenic marker genes [Fig. 5(c)], and triglyceride quantification [Fig. 5(d)]. Together, these data do not indicate that HILPDA directly regulates 3T3-L1 adipogenesis, but do suggest that HILPDA may influence specific lipid droplet-related genes.
Figure 5.
Adenoviral-mediated overexpression of Hilpda does not affect adipogenesis. (a) Hilpda mRNA (top) and HILPDA protein (bottom) during 3T3-L1 adipogenesis of AV-gfp– or AV-Hilpda–treated cells. Recombinant AVs expressing gfp or Hilpda were added at a multiplicity of infection of 500 two days before initiation of differentiation. Cells were harvested at indicated days, with day (d) 0 as the day at which differentiation was started. Gene expression levels of AV-gfp–treated adipocytes at day 3 of differentiation were set at one. (b) Oil-Red-O staining of AV-gfp– or AV-Hilpda–treated cells at indicated days during the process of differentiation. Recombinant AVs expressing gfp or Hilpda were added at a multiplicity of infection of 500 two days before initiation of differentiation. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. (c) Fabp4, G0s2, Cebpa, and Pparg mRNA levels during adipogenesis of 3T3-L1 adipocytes treated with AV-gfp or AV-Hilpda. Recombinant AVs expressing gfp or Hilpda were added at a multiplicity of infection of 500 two days before initiation of differentiation. Cells were harvested at indicated days, with day 0 as the day at which differentiation was started. Gene expression levels of AV-gfp–treated adipocytes at day 3 of differentiation were set at one. (d) Triglyceride quantification of 3T3-L1 adipocytes treated with AV-gfp or AV-Hilpda. Recombinant AVs expressing gfp or Hilpda were added at a multiplicity of infection of 500 two days before initiation of differentiation. Adipocytes were harvested at day 9 of differentiation. Data are mean ± standard error of the mean. (a, c, and d) Asterisks indicate significant differences according to Student t test relative to AV-gfp–treated adipocytes; **P < 0.01.
HILPDA is highly induced upon activation of β-adrenergic signaling
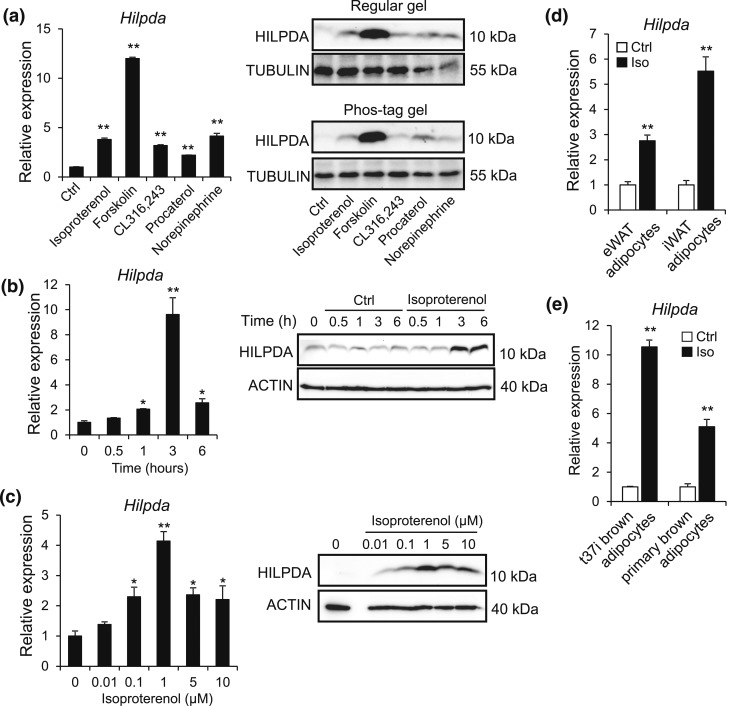
Several lipid droplet proteins are responsive to β-adrenergic signaling, the key signaling pathway that activates adipocyte lipolysis (33, 34). To assess whether β-adrenergic signaling regulates HILPDA levels, 3T3-L1 adipocytes were treated with various β-adrenergic receptor agonists. Hilpda mRNA and HILPDA protein levels were significantly induced upon treatment with the nonselective β-receptor agonist isoproterenol, the β3-receptor agonist CL316,243, the β2-receptor agonist procaterol, the nonselective adrenergic receptor agonist norepinephrine, and the adenylate cyclase activator forskolin, suggesting that HILPDA is responsive to β-adrenergic stimulation [Fig. 6(a)]. In subsequent experiments, isoproterenol treatment induced Hilpda mRNA and HILPDA protein levels in a time- and concentration-dependent manner, with HILPDA levels peaking after 3 hours of stimulation and an isoproterenol concentration of 1 μM [Fig. 6(b) and 6(c)]. Induction of Hilpda mRNA by isoproterenol was confirmed in primary mouse gonadal and inguinal adipocytes [Fig. 6(d)], as well as in brown adipocytes [Fig. 6(e)].
Figure 6.
HILPDA is highly induced upon activation of β-adrenergic signaling. (a) Hilpda mRNA (left) and HILPDA protein (right) levels in fully differentiated 3T3-L1 cells exposed to control (Ctrl) medium, 10 μM isoproterenol (Iso), 5 μM forskolin, 10 μM CL316,243, 10 μM procaterol, and 10 μM norepinephrine for 3 hours. Protein levels of HILPDA were assessed on a regular gel and on a Phos-tag gel to assess HILPDA phosphorylation. Gene expression levels of control-treated adipocytes were set at one. (b) Hilpda mRNA (left) and HILPDA protein (right) levels in fully differentiated 3T3-L1 cells exposed to 10 μM isoproterenol for the indicated duration. Gene expression levels at time point 0 hours were set at one. (c) Hilpda mRNA (left) and HILPDA protein (right) levels in fully differentiated 3T3-L1 cells incubated with control medium or indicated concentrations of isoproterenol for 6 hours. Gene expression levels of control-treated adipocytes were set at one. (d) Hilpda mRNA levels in primary adipocytes differentiated from the SVF of eWAT or iWAT and exposed to 10 μM isoproterenol (Iso) for 3 hours. Gene expression levels of control-treated adipocytes were set at one. (e) Hilpda mRNA levels in fully differentiated T37i adipocytes and primary brown adipocytes exposed to 10 μM isoproterenol (Iso) for 3 hours. Gene expression levels of control-treated adipocytes were set at one. Data are mean ± standard error of the mean. (a–e) Asterisks indicate significant differences according to Student t test relative to control-treated adipocytes; **P < 0.01; *P < 0.05.
siRNA-mediated silencing of Hilpda in adipocytes does not affect lipolysis
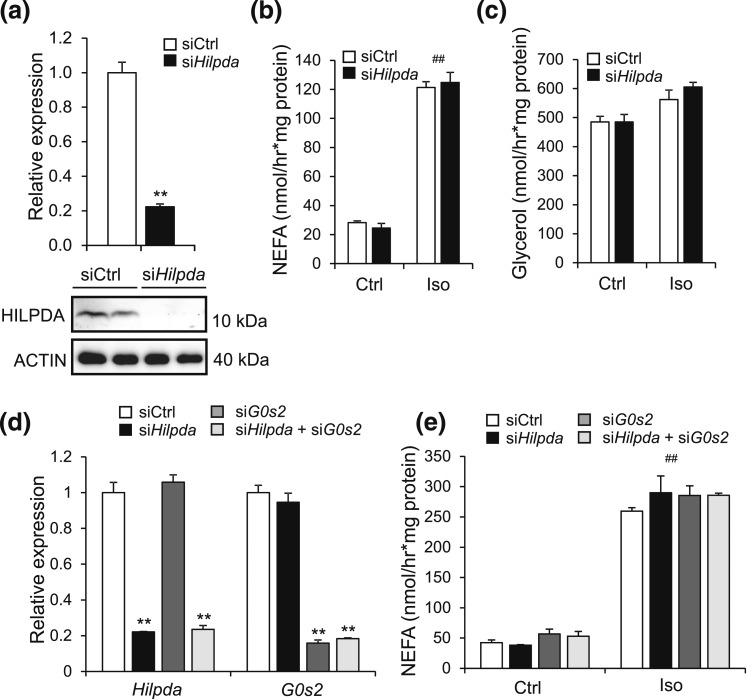
Inactivation of Hilpda was previously shown to increase triglyceride hydrolysis in primary hepatocytes (14). Also, the protein that shares the highest sequence homology with HILPDA is G0S2, an inhibitor of intracellular lipolysis (14) (unpublished data). To explore a possible role of HILPDA in adipose tissue lipolysis, we studied the effect of siRNA-mediated Hilpda silencing on intracellular lipolysis in fully differentiated 3T3-L1 adipocytes (day 9). Although treatment with siRNA-targeting Hilpda effectively reduced Hilpda mRNA and HILPDA protein levels in the adipocytes [Fig. 7(a)], it did not influence the release of NEFA and glycerol upon stimulation with isoproterenol [Fig. 7(b) and 7(c)]. To determine whether the reduction in HILPDA may be functionally compensated by G0S2, we assessed the impact of siRNA-mediated silencing of Hilpda and/or G0s2 on intracellular lipolysis in 3T3-L1 adipocytes. Again, whereas treatment with siRNA-targeting Hilpda and/or G0s2 effectively reduced RNA levels of Hilpda and G0s2 [Fig. 7(d)], it did not affect NEFA release upon stimulation with isoproterenol [Fig. 7(e)]. Together, these results indicate that knockdown of Hilpda in adipocytes—alone or in combination with G0s2—does not have a major impact on the in vitro lipolytic response to pharmacologic β3-adrenergic stimulation.
Figure 7.
Silencing of Hilpda in adipocytes does not affect lipolysis. (a) Hilpda mRNA (top) and HILPDA protein levels (bottom) in fully differentiated 3T3-L1 adipocytes that were trypsinized, replated at 70% confluency, and treated with Hilpda RNA (siHilpda) or siCtrl for 48 hours. Gene expression levels of siCtrl-treated adipocytes were set at one. (b) NEFA and (c) glycerol release in medium of fully differentiated 3T3-L1 adipocytes that were trypsinized, replated at 70% confluency, and treated with siHilpda or siCtrl for 48 hours. After 48 hours, adipocytes were serum starved for 2 hours and incubated with 5 µM isoproterenol or control medium for 2 hours. NEFA and glycerol levels were corrected for protein levels in cell lysates. (d) Hilpda and G0s2 mRNA levels in fully differentiated 3T3-L1 adipocytes that were trypsinized, replated at 70% confluency, and treated with siCtrl, siHilpda, or siG0s2 for 48 hours. After 48 hours, adipocytes were serum starved for 2 hours and incubated with 5 µM isoproterenol or control medium for 2 hours. Gene expression levels are for isoproterenol-treated cells. Gene expression of siCtrl-treated adipocytes was set at one. (e) NEFA release in medium of fully differentiated 3T3-L1 adipocytes treated with siCtrl, siHilpda, or siG0s2 for 48 hours, followed by isoproterenol treatment of 2 hours. NEFA levels were corrected for protein levels in cell lysates. Asterisks and hashtags indicate significant differences according to two-way analysis of variance (#) followed by Tukey's honest significant difference post-hoc test (*) relative to control-treated adipocytes (#) or siCtrl-treated adipocytes (*). ** or ##P < 0.01. Ctrl, control; Iso, isoproterenol.
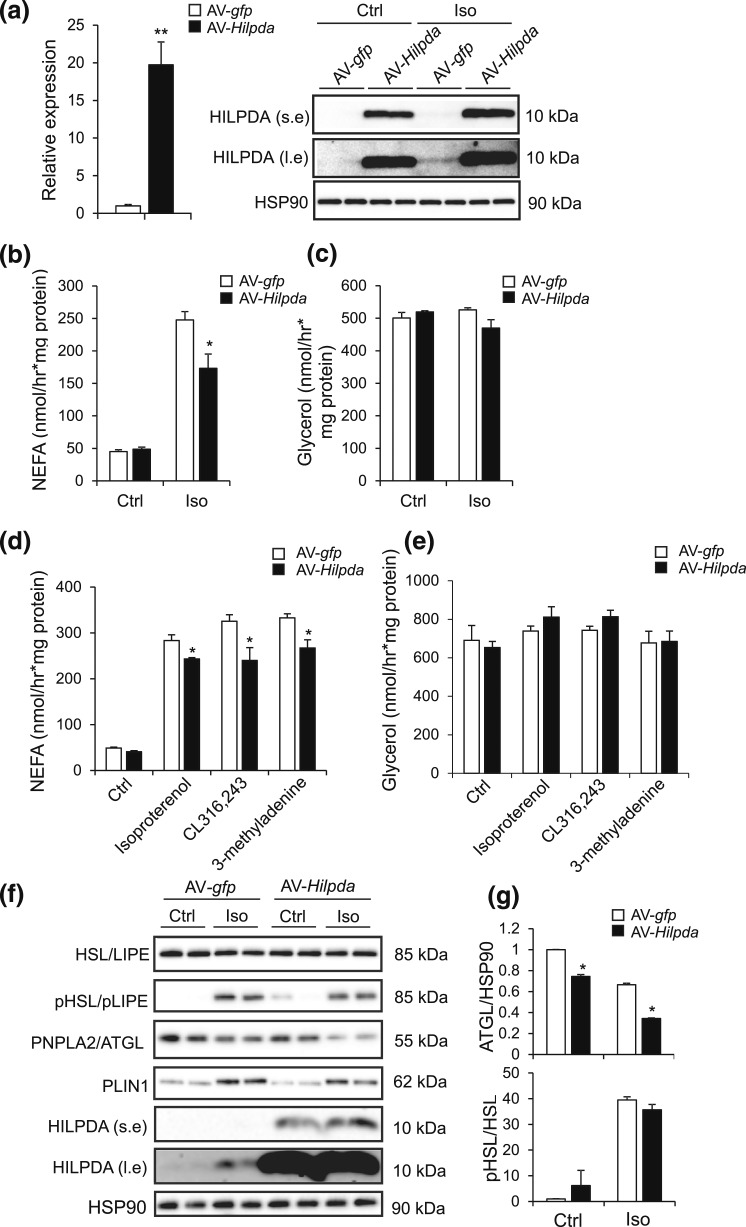
Overexpression of Hilpda in adipocytes in vitro mildly reduces NEFA release
We reasoned that a possible effect of HILPDA on lipolysis might be more evident when Hilpda is overexpressed as opposed to silenced. Accordingly, we studied the effect of adenoviral-mediated Hilpda overexpression on lipolysis in differentiated 3T3-L1 adipocytes (day 9). Control experiments using AV-gfp verified the effectiveness of the transduction procedure in 3T3-L1 adipocytes (Supplemental Fig. 1 (694.2KB, pdf) ). Transduction of the adipocytes with AV-Hilpda led to a pronounced increase in Hilpda mRNA and HILPDA protein [Fig. 8(a)]. Remarkably, Hilpda overexpression modestly but significantly reduced NEFA release induced by isoproterenol [Fig. 8(b)], whereas glycerol release remained unchanged [Fig. 8(c)]. The inhibitory effect of Hilpda overexpression on NEFA release and the lack of effect on glycerol release were reproduced with the β3-adrenergic agonist CL316,243 and the phosphatidylinositol 3-kinase inhibitor 3-methyladenine [Fig. 8(d) and 8(e)]. To explore the potential mechanism underlying the observed antilipolytic effect of HILPDA, we studied the effect of AV-mediated Hilpda overexpression on the relative abundance and activation status of key lipolytic enzymes, including ATGL/PNPLA2 and HSL/LIPE. Interestingly, Hilpda overexpression modestly decreased the abundance of ATGL, but did not have a clear effect on unphosphorylated or phosphorylated HSL [Fig. 8(f) and 8(g)]. These data suggest that overexpression of Hilpda has a mild attenuating effect on isoproterenol-induced lipolysis in 3T3-L1 adipocytes, possibly via reduced ATGL expression.
Figure 8.
Overexpression of Hilpda in adipocytes suppresses NEFA release. (a) Hilpda mRNA (left) and HILPDA protein levels (right) in fully differentiated 3T3-L1 adipocytes transduced with AV-gfp or AV-Hilpda. Fully differentiated 3T3-L1 adipocytes were trypsinized, replated at 70% confluency, serum starved for 24 hours, and transduced with recombinant AVs expressing gfp or Hilpda at a multiplicity of infection of 750 for 72 hours. Gene expression levels of AV-gfp–treated adipocytes were set at one. (b) NEFA and (c) glycerol release in fully differentiated 3T3-L1 adipocytes that were trypsinized, replated at 70% confluency, serum starved for 24 hours, and transduced with recombinant AVs expressing gfp or Hilpda at a multiplicity of infection of 750 for 72 hours. Transduced differentiated 3T3-L1 adipocytes were serum starved for 2 hours and incubated with 5 µM isoproterenol for 3 hours. (d) NEFA and (e) glycerol release in fully differentiated 3T3-L1 adipocytes that were transduced with recombinant AVs expressing gfp or Hilpda and incubated either with 10 µM isoproterenol, 5 mM 3-methyladenine, or 10 µM CL316,243 for 3 hours. (f) Representative immunoblots for HSL (LIPE), phospho-HSL, ATGL (PNPLA2), perilipin 1 (PLIN1), and HILPDA in fully differentiated 3T3-L1 adipocytes that were transduced with recombinant AVs expressing gfp or Hilpda and incubated with 10 µM isoproterenol for 3 hours. (g) Quantification of phospho-HSL and ATGL immunoblots in differentiated 3T3-L1 adipocytes, transduced as described earlier. Data are mean ± standard error of the mean. Asterisks indicate significant differences according to Student t test relative to AV-gfp–treated adipocytes; **P < 0.01; *P < 0.05. Ctrl, control; Iso, isoproterenol; l.e., long exposure; s.e., short exposure.
Adipocyte-specific inactivation of Hilpda has minimal effects on metabolic parameters following pharmacological β-adrenergic stimulation, fasting, and cold
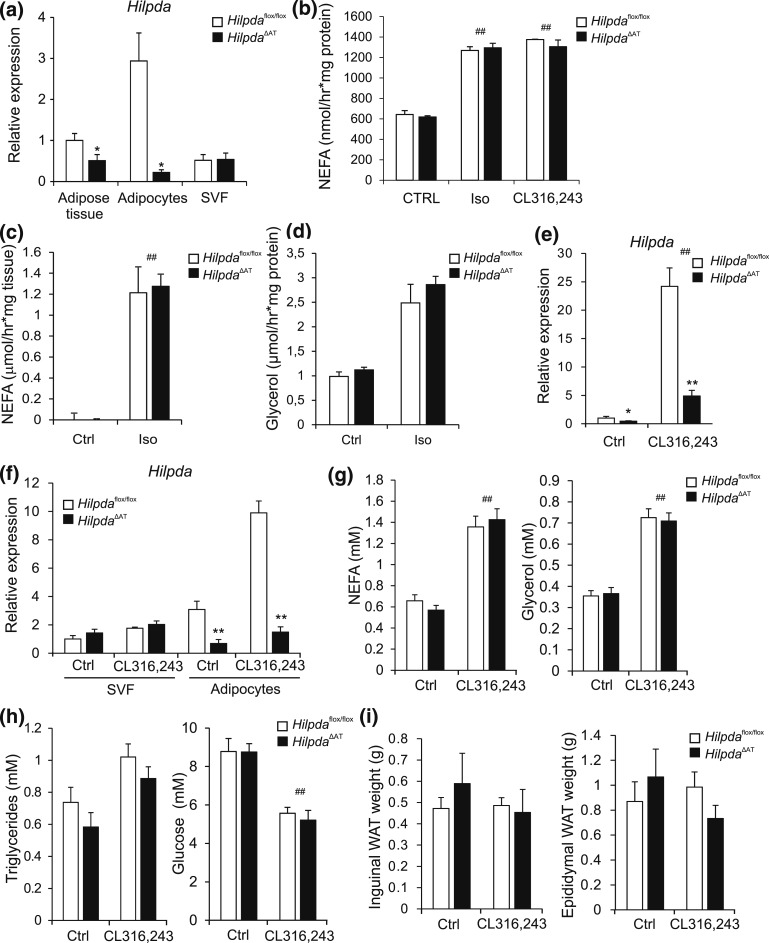
To examine a potential role for HILPDA in adipocyte lipolysis in vivo, we generated adipocyte-specific Hilpda mutant mice (HilpdaΔAT) by crossing floxed Hilpda mice (Hilpdaflox/flox) with Adiponectin-Cre mice. Hilpda mRNA expression was ∼50% lower in adipose tissue of HilpdaΔAT mice as compared with Hilpdaflox/flox mice, which was accounted for by an almost complete loss of Hilpda expression in adipocytes [Fig. 9(a)]. Upon examination of the ex vivo NEFA release from primary adipocytes and adipose tissue explants stimulated with isoproterenol, we found that NEFA release was comparable between HilpdaΔAT mice and Hilpdaflox/flox mice, in line with our data of in vitro silencing of Hilpda [Fig. 9(b) and 9(c)]. Glycerol release was also not different between adipose tissue explants of HilpdaΔAT mice and Hilpdaflox/flox mice [Fig. 9(d)]. To examine a potential role of HILPDA in the in vivo lipid metabolic response to β-adrenergic stimulation, we injected HilpdaΔAT and Hilpdaflox/flox mice with the β3-adrenergic agonist CL316,243 to induce lipolysis. In agreement with our in vitro data, injection with CL316,243 potently induced Hilpda mRNA expression in WAT of Hilpdaflox/flox mice [Fig. 9(e)]. Induction of Hilpda mRNA expression upon CL316,243 injection was specifically observed in the adipocytes and not in the SVF [Fig. 9(f)]. Although injection with CL316,243 significantly increased plasma NEFA and glycerol levels—suggesting that CL316,243 effectively induced lipolysis—no differences were observed between HilpdaΔAT and Hilpdaflox/flox mice [Fig. 9(g)]. Plasma levels of other metabolites [Fig. 9(h)] and weight of iWAT and eWAT [Fig. 9(i)] were also not different between HilpdaΔAT and Hilpdaflox/flox mice.
Figure 9.
Adipocyte-specific inactivation of Hilpda has no effect on indicators of lipolysis in mice injected with CL316,243. (a) Hilpda mRNA levels in whole gonadal adipose tissue, and in freshly separated adipocytes and SVF of Hilpdaflox/flox (n = 4) and HilpdaΔAT mice (n = 4). Gene expression levels of Hilpda in adipose tissue from Hilpdaflox/flox mice were set at one. (b) NEFA release in medium of primary adipocytes differentiated from the SVF of iWAT from HilpdaΔAT mice (n = 3) and Hilpdaflox/flox mice (n = 3), serum starved for 2 hours, and incubated with 10 µM isoproterenol or 10 µM CL316,243 for 3 hours. (c) NEFA and (d) glycerol release in medium of WAT explants of HilpdaΔAT mice (n = 3) and Hilpdaflox/flox mice (n = 3), serum starved for 30 min, and incubated with 10 µM isoproterenol for 3 hours. (e) Hilpda mRNA levels in eWAT of Hilpdaflox/flox (n = 8/treatment) and HilpdaΔAT (n = 8/treatment) mice excised and snap frozen 3 hours after subcutaneous injection with 1 mg/kg CL316,243 or saline control. Gene expression levels of saline-treated Hilpdaflox/flox mice were set at one. (f) Hilpda mRNA levels in freshly isolated adipocytes or SVF isolated from eWAT of Hilpdaflox/flox (n = 8/treatment) and HilpdaΔAT mice (n = 8/treatment) 3 hours after subcutaneous injection with 1 mg/kg CL316,243 or saline control. Gene expression levels of the SVF of saline-treated Hilpdaflox/flox mice were set at one. Plasma levels of (g) NEFA and glycerol and (h) triglycerides and glucose of Hilpdaflox/flox (n = 8/treatment) and HilpdaΔAT (n = 8/treatment) mice 3 hours after subcutaneous injection with 1 mg/kg CL316,243 or saline control. (i) Tissue weights of iWAT and eWAT of Hilpdaflox/flox mice (n = 8/treatment) and HilpdaΔAT mice (n = 8/treatment) 3 hours after subcutaneous injection with 1 mg/kg CL316,243 or saline control. Data are mean ± standard error of the mean. (a and e) Asterisks indicate significant differences according to Student t test (*) relative to adipose tissue or SVF of saline-treated Hilpdaflox/flox mice. Asterisks or hashtags indicate significant differences according to two-way analysis of variance (#), followed by Tukey’s honest significant difference post hoc test (*) relative to (b and c) control-treated adipocytes or (d–h) saline-treated mice (#) and relative to (d and f–h) Hilpdaflox/flox mice (*); ** or ##P < 0.01; *P < 0.05. Ctrl, control; Iso, isoproterenol.
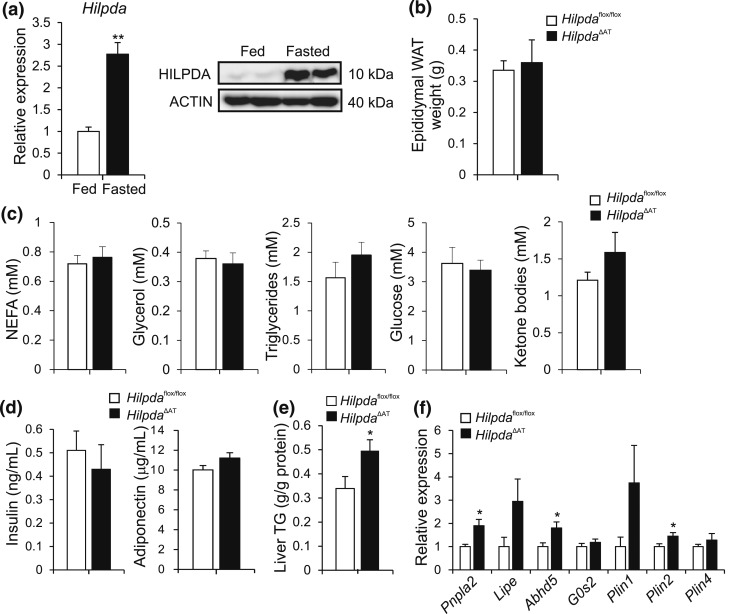
A physiological condition that is associated with the activation of β-adrenergic signaling and adipose tissue lipolysis is fasting. Indeed, fasting caused a marked increase in Hilpda mRNA and HILPDA protein levels in WAT of wild-type mice [Fig. 10(a)]. After a 24-hour fast, however, WAT weight was not different between HilpdaΔAT and Hilpdaflox/flox mice [Fig. 10(b)]. No major differences in plasma NEFA and glycerol as well as other metabolites were observed between the two genotypes [Fig. 10(c)]. Also, plasma insulin and adiponectin levels and adipose tissue morphology were similar between HilpdaΔAT and Hilpdaflox/flox mice [Fig. 10(d); Supplemental Fig. 2 (694.2KB, pdf) ]. Interestingly, levels of hepatic triglycerides were nearly 50% higher in HilpdaΔAT compared with Hilpdaflox/flox mice [Fig. 10(e)]. Finally, we observed an increase in the expression of several genes involved in lipolysis and lipid droplet morphology in WAT of HilpdaΔAT compared with Hilpdaflox/flox mice, including Pnpla2/Atgl [Fig. 10(f)].
Figure 10.
Adipocyte-specific inactivation of Hilpda has no effect on tissue weights or plasma parameters following a 24-hour fast. (a) Hilpda mRNA (left) and HILPDA protein levels (right) in eWAT from C57BL/6 mice fed or fasted for 18 hours (n = 6/group). Gene expression levels of fed mice were set at one. (b) Weight of eWAT of male Hilpdaflox/flox (n = 7) and HilpdaΔAT (n = 8) fasted for 24 hours. (c) Plasma levels of NEFA, glycerol, triglycerides, glucose, and ketone bodies of male Hilpdaflox/flox (n = 7) and HilpdaΔAT (n = 8) mice fasted for 24 hours. (d) Plasma levels of insulin and adiponectin of Hilpdaflox/flox (n = 7) and HilpdaΔAT (n = 8) mice fasted for 24 hours. (e) Triglyceride (TG) levels in livers of Hilpdaflox/flox (n = 7) and HilpdaΔAT (n = 8) mice fasted for 24 hours. (f) mRNA levels of genes involved in lipolysis or lipid droplet morphology (Pnpla2, Lipe, Abhd5, G0S2, Plin1, Plin2, Plin4) in eWAT of Hilpdaflox/flox (n = 7) and HilpdaΔAT (n = 8) mice fasted for 24 hours. Gene expression levels for Hilpdaflox/flox mice were set at one for each gene. Data are mean ± standard error of the mean. Asterisks indicate significant differences according to Student t test relative to (a) fed mice or (b–e) Hilpdaflox/flox mice ; **P < 0.01; *P < 0.05.
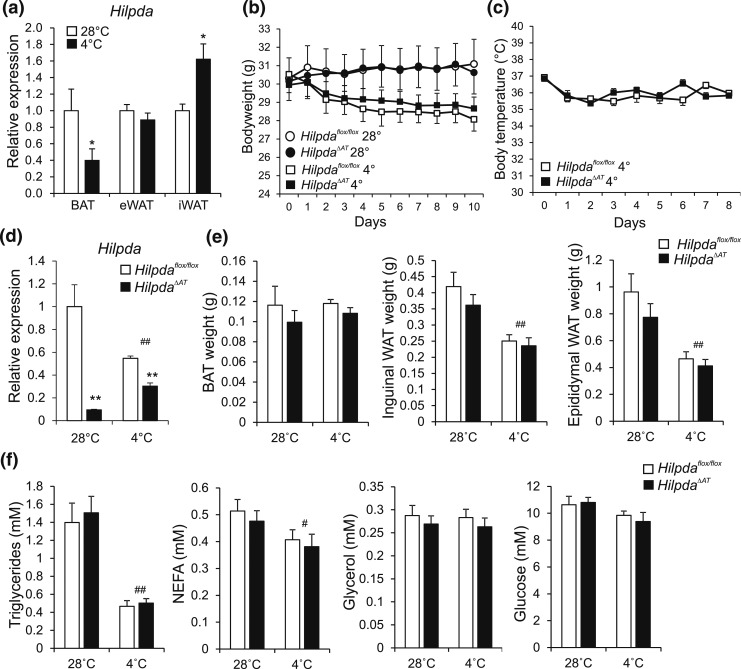
Another physiological condition that is accompanied by activation of β-adrenergic signaling and adipose tissue lipolysis is cold exposure. In wild-type mice, cold exposure induced Hilpda mRNA in iWAT, but not gonadal WAT, and suppressed Hilpda mRNA in BAT [Fig. 11(a)]. To study the role of HILPDA during cold, HilpdaΔAT and Hilpdaflox/flox mice were exposed to a cold (4°C) or a thermoneutral (28°C) environment for 10 days. No differences between HilpdaΔAT and Hilpdaflox/flox mice were observed in body weight [Fig. 11(b)] and body temperature [Fig. 11(c)] throughout the study, despite a marked reduction in Hilpda mRNA in BAT of HilpdaΔAT mice [Fig. 11(d)]. Intriguingly, the reduction in Hilpda mRNA in HilpdaΔAT was much less pronounced at 4°C. Furthermore, weight of interscapular BAT, iWAT, and eWAT after 10 days of cold or thermoneutral temperature was not different between HilpdaΔAT and Hilpdaflox/flox mice [Fig. 11(e)], nor were there any differences in plasma metabolites, including NEFA [Fig. 11(f)]. Finally, no differences in the morphological appearance of BAT and WAT, as assessed by hematoxylin and eosin staining, could be observed between HilpdaΔAT and Hilpdaflox/flox mice (Supplemental Fig. 3 (694.2KB, pdf) ).
Figure 11.
Adipocyte-specific inactivation of Hilpda has no effect on tissue weights or plasma parameters in mice exposed to thermoneutral temperatures or cold. (a) Hilpda mRNA levels in BAT, eWAT, and iWAT of C57BL/6 mice exposed to 4°C or 28°C for 10 days (n = 8 to 10 animals per group). Gene expression levels of mice exposed to 28°C were set at one. (b) Body weight of Hilpdaflox/flox and HilpdaΔAT mice exposed to 4°C or 28°C for 10 days (n = 8 to 10 animals per group). (c) Body temperature of Hilpdaflox/flox and HilpdaΔAT mice exposed to 4°C or 28°C for 10 days (n = 10 animals per group). (d) Hilpda mRNA levels in BAT of Hilpdaflox/flox and HilpdaΔAT mice exposed to 4°C or 28°C for 10 days (n = 8 to 10 animals per group). Gene expression levels of Hilpdaflox/flox mice exposed to 28°C were set at one. (e) Tissue weights of BAT, eWAT, and iWAT of Hilpdaflox/flox and HilpdaΔAT mice exposed to 4°C or 28°C for 10 days (n = 8 to 10 animals per group). (f) Triglycerides, NEFA, glucose, and glycerol levels in plasma of Hilpdaflox/flox and HilpdaΔAT mice exposed to 4°C or 28°C for 10 days (n = 8 to 10 animals per group). Data are mean ± standard error of the mean. (a) Asterisks indicate significant differences according to Student t test (*) relative to mice exposed to thermoneutrality. Asterisks or hashtags indicate significant differences according to two-way analysis of variance (#), followed by Tukey’s honest significant difference post hoc test (*) relative to mice exposed to (d–h) thermoneutrality (#) and relative to (d–h) Hilpdaflox/flox mice (*); ** or ##P < 0.01; * or #P < 0.05.
Discussion
Our data show that HILPDA is abundant in adipocytes, where its expression is controlled by PPARγ and β-adrenergic signaling. Whereas adenoviral overexpression of Hilpda modestly lowered NEFA release from adipocytes, the absence of an effect of Hilpda inactivation on NEFA release in vitro and plasma NEFA and glycerol levels in vivo suggests that HILPDA is not a direct physiological regulator of lipolysis in adipocytes, at least under conditions of β-adrenergic stimulation, cold exposure, or fasting. However, our studies do provide a number of indications for an indirect suppressive effect of HILPDA on adipocyte lipolysis. Also, based on our data, we cannot exclude that HILPDA may be involved in the regulation of adipose tissue lipolysis under different conditions, such as obesity or physical exercise.
The reason for the apparent discrepancy between the effect on lipolysis of in vitro Hilpda overexpression vs in vivo/in vitro Hilpda inactivation or silencing remains unclear. It can be hypothesized that the lack of effect of Hilpda inactivation and silencing on any of the parameters studied may be because the loss of HILPDA is functionally compensated by other proteins. One protein that might be suspected to be able to functionally compensate for HILPDA is G0S2, a small ∼11-kDa protein that is known to inhibit ATGL activity and influence ATGL localization (33, 35). Remarkable similarities exist between HILPDA and G0S2: HILPDA and G0S2 are both highly sensitive PPAR targets, share striking sequence homology in their N-terminal domains, and increase hepatic lipid accumulation when overexpressed in liver (11, 14, 16, 33, 36). In our studies, however, silencing of G0s2 did not significantly alter isoproterenol-induced NEFA release in 3T3-L1 adipocytes, whether alone or in combination with silencing of Hilpda, suggesting that G0S2 does not functionally compensate for HILPDA upon Hilpda silencing.
Because HILPDA does not seem to be a direct inhibitor of adipocyte lipolysis—which is supported by our previous studies indicating that HILPDA does not directly inhibit HSL or ATGL—the modest inhibitory effect of Hilpda overexpression on NEFA release by adipocytes might reflect an indirect mechanism (19). In particular, the concurrent lack of effect of Hilpda overexpression on glycerol release might suggest that HILPDA promotes fatty acid re-esterification, an interesting hypothesis that should be explored further. Additionally, it may be speculated that HILPDA, similar to other lipid droplet–associated PPAR targets such as cell death–inducing DFF45-like effector C and PLIN2, plays a role in lipid droplet coating, lipid droplet growth, or the regulation of interactions of lipid droplets with other organelles, such as the endoplasmic reticulum (37–40). An important future research strategy should be to further examine the exact localization of HILPDA in adipocytes and hepatocytes and to study the potential influence of relevant stimuli on HILPDA localization. Our efforts to use cellular fractionation to locate HILPDA to lipid droplets in 3T3-L1 adipocytes have to date been unsuccessful, in contrast to hepatocytes, possibly because the very high concentration of lipids in the lipid droplet fraction interferes with the migration or detection of HILDPA. Immunofluorescence studies on endogenous HILPDA in 3T3-L1 adipocytes would also be very helpful. However, the available antibodies do not seem to detect HILPDA via immunofluorescence. Finally, efforts should be undertaken to identify binding partners of HILPDA. One possible binding partner for HILPDA is PLIN2, as PLIN2 and HILPDA are both sensitive PPAR targets, have a similar tissue expression profile, and colocalize on the surface of some, but not all lipid droplets (13, 15, 16).
Our data on the role of HILPDA in adipocytes add to the current literature on HILPDA function. The observation that HILPDA is physically associated with lipid droplets in 3T3-L1 cells is in line with previous findings in lipid-loaded HeLa cells and primary hepatocytes (14, 15). In those studies, overexpression of Hilpda in HeLa cells promoted cellular lipid accumulation, whereas inactivation of Hilpda in hepatocytes reduced lipid accumulation and resulted in more, yet smaller lipid droplets (14, 15). The reduced lipid accumulation was attributed to increased triglyceride lipolysis and triglyceride turnover (14). Similarly, overexpression of Hilpda in mouse liver increased hepatic lipid accumulation, whereas liver-specific inactivation of Hilpda lowered hepatic lipid accumulation, which was observed specifically in mice fed a chow diet, but not in mice fed a high-fat diet (14, 16). By contrast, we did not observe any effect of adipocyte-specific inactivation of Hilpda in mice on adipocyte lipolysis, weight of adipose tissue depots, or other relevant metabolic parameters. Although the collective data thus point toward a role for HILPDA in lipid droplet morphology and lipid storage/mobilization, the exact molecular function of HILPDA remains to be elucidated.
An intriguing observation is that Hilpda is rapidly and very potently upregulated upon β-adrenergic stimulation. β-adrenergic signaling generally leads to activation of proteins that promote lipolysis (34). As discussed previously, our data do not support such a function for HILPDA. The exact biological rationale and mechanisms underlying the upregulation of Hilpda mRNA by β-adrenergic stimulation require further investigation. Besides β-adrenergic stimulation, our data show that HILPDA expression is potently stimulated by treatment with PPARγ agonists in human and mouse adipocytes. Previously, we demonstrated that Hilpda expression in liver is under transcriptional control of PPARα (16). In the current work, we show the presence of several conserved PPARγ binding sites in the vicinity of the HILPDA gene, indicating that HILPDA is a bona-fide PPARγ target gene in mouse and human adipocytes. The preservation of three conserved PPARγ sites, one of which is also conserved at the sequence level, is remarkable given that only ∼17% of all mouse adipocyte PPARγ binding sites (and 28% of PPARγ sites in the vicinity of adipocyte-selective genes) are preserved in human adipocytes (41). Furthermore, we demonstrate the existence of a conserved PPARγ superenhancer in association with the Hilpda locus in both mouse and human adipocytes.
Only a few genes and pathways are induced by both β-adrenergic signaling and PPARγ. Interestingly, a shared downstream effect of activation of PPARγ and β-adrenergic signaling is the regulation of adipocyte lipolysis (34, 42). Whereas β-adrenergic signaling stimulates lipolysis mainly by promoting the phosphorylation of key lipolytic proteins, the role of PPARγ in lipolysis is a lot more complicated (34, 42–44). Whether β-adrenergic signaling and PPARγ interact in the regulation of Hilpda mRNA remains unclear. Using Phos-tag gels, we did not find any evidence that HILPDA becomes phosphorylated upon β-adrenergic stimulation. Overall, the combined regulation of HILPDA by β-adrenergic signaling and PPARγ in adipocytes hints at an important, yet not fully understood role of HILPDA in adipocyte biology.
In conclusion, we demonstrate that Hilpda is well expressed in adipocytes, where it is potently induced by PPARγ and β-adrenergic signaling. Although overexpression of Hilpda modestly inhibited NEFA release in adipocytes, adipocyte-specific inactivation of Hilpda did not affect plasma NEFA, glycerol, and other relevant metabolic parameters upon pharmacological β-adrenergic stimulation, cold exposure, and fasting. Taken together, in contrast to the purported inhibition of lipolysis by HILPDA in hepatocytes, HILPDA does not appear to be a major direct physiological regulator of adipocyte lipolysis. Future studies should reveal the exact molecular function of HILPDA in adipocytes.
Acknowledgments
We thank Karin Mudde, Georgia Lenihan-Geels, Nicole Hamers, Zhen Xue, and Haibo Sha for excellent technical assistance.
Acknowledgments
This work was supported by Fondation Leducq Grant 12CVD04, The Netherlands Cardiovascular Research Initiative/Dutch Heart Foundation Grant CVON2014-ENERGISE, The Netherlands Organization for Scientific Research Grant NWO-ALW (to S.K.), and National Institutes of Health Grant R01DK105393 (to L.Q.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATGL
- adipose triglyceride lipase
- AV
- adenovirus
- BAT
- brown adipose tissue
- BSA
- bovine serum albumin
- cDNA
- complementary DNA
- CIDEB
- cell death-inducing DFF45-like effector B
- DMEM
- Dulbecco’s modified Eagle medium
- eGFP
- enhanced green fluorescent protein
- eWAT
- epididymal white adipose tissue
- FCS
- fetal calf serum
- G0S2
- G0/G1 switch gene 2
- HILPDA
- hypoxia-inducible lipid droplet–associated
- hMADS
- human multipotent adipose-derived stem
- HSL
- hormone-sensitive lipase
- iWAT
- inguinal white adipose tissue
- mRNA
- messenger RNA
- NEFA
- nonesterified fatty acids
- PBS
- phosphate-buffered saline
- PLIN2
- perilipin 2
- PPAR
- peroxisome proliferator–activated receptor
- RRID
- Research Resource Identifier
- siCtrl
- control small interfering RNA
- siRNA
- small interfering RNA
- SGBS
- Simpson-Golabi-Behmel syndrome
- SVF
- stromal vascular fraction
- TSS
- transcriptional start site
- WAT
- white adipose tissue.
References
- 1.Walther TC, Farese RV Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149(3):942–949. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. [DOI] [PubMed] [Google Scholar]
- 4.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng J-X, Graham M, Christiano R, Fröhlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV Jr, Walther TC. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24(4):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279(45):46835–46842. [DOI] [PubMed] [Google Scholar]
- 6.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3(5):309–319. [DOI] [PubMed] [Google Scholar]
- 7.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem. 2012;287(30):25038–25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber R, Hofer P, Taschler U, Voshol PJ, Rechberger GN, Kotzbeck P, Jaeger D, Preiss-Landl K, Lord CC, Brown JM, Haemmerle G, Zimmermann R, Vidal-Puig A, Zechner R. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc Natl Acad Sci USA. 2015;112(45):13850–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulsen Ll, Siersbæk M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23(6):631–639. [DOI] [PubMed] [Google Scholar]
- 10.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zandbergen F, Mandard S, Escher P, Tan NS, Patsouris D, Jatkoe T, Rojas-Caro S, Madore S, Wahli W, Tafuri S, Müller M, Kersten S. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem J. 2005;392(Pt 2):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langhi C, Baldán Á. CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting- and diet-induced hepatosteatosis. Hepatology. 2015;61(4):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvardsson U, Ljungberg A, Lindén D, William-Olsson L, Peilot-Sjögren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006;47(2):329–340. [DOI] [PubMed] [Google Scholar]
- 14.DiStefano MT, Danai LV, Roth Flach RJ, Chawla A, Pedersen DJ, Guilherme A, Czech MP. The lipid droplet protein hypoxia-inducible gene 2 promotes hepatic triglyceride deposition by inhibiting lipolysis. J Biol Chem. 2015;290(24):15175–15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimm T, Wiese M, Teschemacher B, Deggerich A, Schödel J, Knaup KX, Hackenbeck T, Hellerbrand C, Amann K, Wiesener MS, Höning S, Eckardt K-U, Warnecke C. Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. FASEB J. 2010;24(11):4443–4458. [DOI] [PubMed] [Google Scholar]
- 16.Mattijssen F, Georgiadi A, Andasarie T, Szalowska E, Zota A, Krones-Herzig A, Heier C, Ratman D, De Bosscher K, Qi L, Zechner R, Herzig S, Kersten S. Hypoxia-inducible lipid droplet-associated (HILPDA) is a novel peroxisome proliferator-activated receptor (PPAR) target involved in hepatic triglyceride secretion. J Biol Chem. 2014;289(28):19279–19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MKC, Reue K, van Marken Lichtenbelt WD, Olivecrona G, Rensen PCN, Heeren J, Kersten S. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. eLife. 2015;4:e08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S, Roelofsen H, Houtman R, van der Burg B, Mandrup S, Bonvin AMJJ, Kalkhoven E, Müller M, Hooiveld GJ, Kersten S. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol Cell Biol. 2013;33(7):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Beekum O, Brenkman AB, Grøntved L, Hamers N, van den Broek NJF, Berger R, Mandrup S, Kalkhoven E. The adipogenic acetyltransferase Tip60 targets activation function 1 of peroxisome proliferator-activated receptor γ. Endocrinology. 2008;149(4):1840–1849. [DOI] [PubMed] [Google Scholar]
- 20.Wabitsch M, Brenner RE, Melzner I, Braun M, Möller P, Heinze E, Debatin KM, Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25(1):8–15. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez A-M, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri E-Z, Dani C, Ailhaud G. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315(2):255–263. [DOI] [PubMed] [Google Scholar]
- 22.Jeninga EH, Bugge A, Nielsen R, Kersten S, Hamers N, Dani C, Wabitsch M, Berger R, Stunnenberg HG, Mandrup S, Kalkhoven E. Peroxisome proliferator-activated receptor gamma regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81). J Biol Chem. 2009;284(39):26385–26393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Børgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22(21):2953–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loft A, Forss I, Siersbæk MS, Schmidt SF, Larsen ASB, Madsen JGS, Pisani DF, Nielsen R, Aagaard MM, Mathison A, Neville MJ, Urrutia R, Karpe F, Amri E-Z, Mandrup S. Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes Dev. 2015;29(1):7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajakumari S, Wu J, Ishibashi J, Lim H-W, Giang A-H, Won K-J, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17(4):562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siersbæk MS, Loft A, Aagaard MM, Nielsen R, Schmidt SF, Petrovic N, Nedergaard J, Mandrup S. Genome-wide profiling of peroxisome proliferator-activated receptor γ in primary epididymal, inguinal, and brown adipocytes reveals depot-selective binding correlated with gene expression. Mol Cell Biol. 2012;32(17):3452–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JYH, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedhart J, von Stetten D, Noirclerc-Savoye M, Lelimousin M, Joosen L, Hink MA, van Weeren L, Gadella TWJ Jr, Royant A. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat Commun. 2012;3:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9(2):177–190. [DOI] [PubMed] [Google Scholar]
- 32.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Lu X, Lombès M, Rha GB, Chi Y-I, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11(3):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweiger M, Paar M, Eder C, Brandis J, Moser E, Gorkiewicz G, Grond S, Radner FPW, Cerk I, Cornaciu I, Oberer M, Kersten S, Zechner R, Zimmermann R, Lass A. G0/G1 switch gene-2 regulates human adipocyte lipolysis by affecting activity and localization of adipose triglyceride lipase. J Lipid Res. 2012;53(11):2307–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zhang Y, Qian H, Lu J, Zhang Z, Min X, Lang M, Yang H, Wang N, Zhang P. The g0/g1 switch gene 2 is an important regulator of hepatic triglyceride metabolism. PLoS One. 2013;8(8):e72315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Q, Goodman JM. The lipid droplet: a well-connected organelle. Front Cell Dev Biol. 2015;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frühbeck G, Méndez-Giménez L, Fernández-Formoso J-A, Fernández S, Rodríguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27(1):63–93. [DOI] [PubMed] [Google Scholar]
- 39.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195(6):953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580(23):5484–5491. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt SF, Jørgensen M, Chen Y, Nielsen R, Sandelin A, Mandrup S. Cross species comparison of C/EBPα and PPARγ profiles in mouse and human adipocytes reveals interdependent retention of binding sites. BMC Genomics. 2011;12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Festuccia WT, Laplante M, Berthiaume M, Gélinas Y, Deshaies Y. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49(10):2427–2436. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Cuenca S, Carobbio S, Vidal-Puig A. Ablation of Pparg2 impairs lipolysis and reveals murine strain differences in lipolytic responses. FASEB J. 2012;26(5):1835–1844. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Cuenca S, Carobbio S, Velagapudi VR, Barbarroja N, Moreno-Navarrete JM, Tinahones FJ, Fernandez-Real JM, Orešic M, Vidal-Puig A. Peroxisome proliferator-activated receptor γ-dependent regulation of lipolytic nodes and metabolic flexibility. Mol Cell Biol. 2012;32(8):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]